Abstract
Objectives
Although the majority of the global population lives in developing countries, most of the epidemiological data related to intensive care unit (ICU) acute kidney injury (AKI) comes from developed countries. This systematic review aims to ascertain the methodology of studies on ICU AKI patients in developing and developed countries, to determine whether epidemiological comparisons between these two settings are possible, and to present a summary estimate of AKI incidence.
Methods
A systematic review of published studies reporting AKI in intensive care units (2005–2015) identified in PubMed, LILACS, and IBECs databases was conducted. We compared developed and developing countries by evaluating study methodology, AKI reference serum creatinine definitions, population characteristics, AKI incidence and mortality. AKI incidence was calculated with a random-effects model.
Results
Ninety-two studies were included, one of which reported data from both country categories: 60 from developed countries (1,057,332 patients) and 33 from developing countries (34,539 patients). In 78% of the studies, AKI was defined by the RIFLE, AKIN or KDIGO criteria. Oliguria had 11 different definitions and reference creatinine 23 different values. For the meta-analysis, 38 studies from developed and 18 from developing countries were selected, with similar AKI incidence: 39.3% and 35.1%, respectively. The need for dialysis, length of ICU stay and mortality were higher in developing countries.
Conclusion
Although patient characteristics and AKI incidence were similar in developed and developing countries, main outcomes were worse in developing country studies. There are significant caveats when comparing AKI epidemiology in developed and developing countries, including lack of standardization of reference serum creatinine, oliguria and the timeframe for AKI assessment. Larger, prospective, multicenter studies from developing countries are urgently needed to capture AKI data from the overall population without ICU access.
Introduction
Acute kidney injury (AKI) affects 20 to 50% of intensive care unit (ICU) patients, and it is associated with high mortality, increased ICU length of stay and greater hospitalization cost [1–5]. When renal replacement therapy (RRT) is used, mortality rates can reach up to 80% [2,6].
It is widely accepted that AKI characteristics are different in developed and developing countries due to contrasting socioeconomic patterns, government health expenditures, heath service infrastructure and AKI etiology [7].
Although the vast majority of the global population lives in developing countries, most of the epidemiological data from ICU patients with AKI comes from developed countries. Comparisons of these two different settings are scarce. A multicenter prospective study found higher mortality for ICU AKI patients in developing countries [8], which might be related to an inadequate number of ICU beds in relation to population size and the difficulty of health care access, among other reasons. To determine whether the outcomes in these populations are comparable, it is necessary to evaluate whether there are differences in patient characteristics as well as the methodological aspects among the analyzed studies.
This systematic review covers methodological aspects, including AKI and reference serum creatinine definitions, as well as the main characteristics and outcomes of ICU AKI patients from studies in developed and developing countries. We aim to determine whether epidemiological comparisons between these two country categories are appropriate with the available data and to estimate their AKI incidence using meta-analysis.
Material and methods
Database search
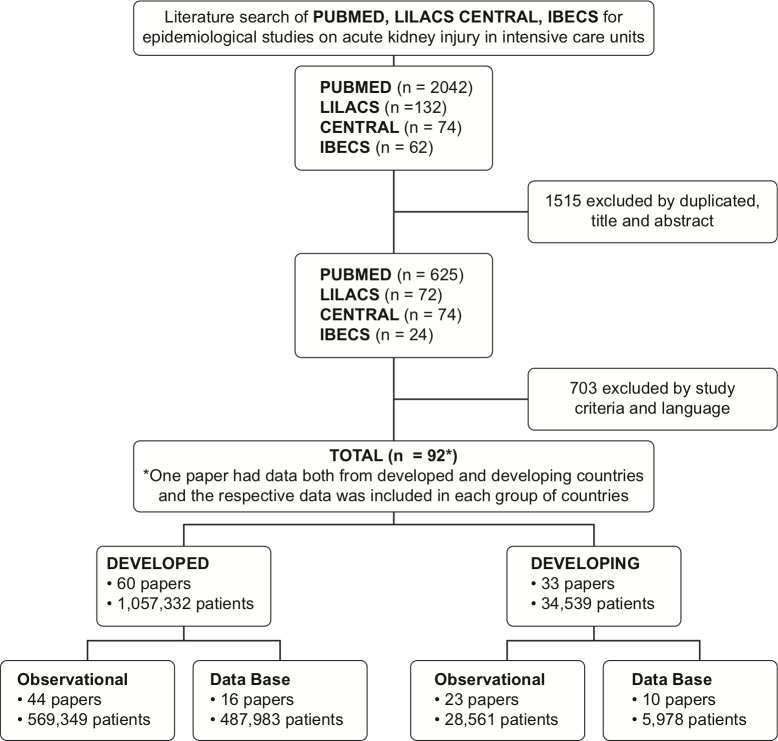
This systematic review was conducted using the recommendations of the “Cochrane Handbook for Systematic Reviews of Interventions” [9] (Fig 1). A systematic electronic search was performed to identify all original studies which might include acute kidney injury patients in intensive care units published from 2005 until 2015, including the keyword terms: “acute kidney injury”, “acute kidney failure”, “acute kidney insufficiency”, “acute renal injury”, “acute renal failure”, “acute renal insufficiency”, “intensive care units”, “critical ill patient and critical ill”. We accessed the PubMed, CENTRAL (Cochrane Controlled Register of Trials), LILACS (Latin American and Caribbean Health Sciences Library), and IBECs (Spanish Bibliographical Index of Health Sciences) databases. The search strategies for each database can be found in S1 File. This search was last updated on July 31, 2015, and the language was limited to English, Spanish, French, Italian and Portuguese. The manuscripts were manually analyzed in order to find additional references. There was no blinding in relation to the author, place of publication, or journal.
Fig 1. Flowchart of study selection.
The studies were classified into two groups: those from developed and those from developing countries, and they were assigned group membership based on the United Nations classification.
Study selection
An initial eligibility screen of all retrieved titles and abstracts was conducted, and only studies reporting AKI in intensive care units in the 10-year surveyed period were selected for further review. Full-text papers were obtained for inspection of each study that potentially fulfilled the inclusion criteria on the basis of title and/or abstract. The following specific criteria were used for final selection: only studies with adult patients and those with reported epidemiological data. Duplicate articles were identified and eliminated using the Endnoteweb [10] software tool.
The final group of manuscripts was selected by the main author and reviewed by a coauthor. Discordances were solved by consensus using the predefined inclusion and exclusion criteria in accordance with the recommendations of the “Cochrane Handbook for Systematic Reviews of Interventions” [9]. When necessary, a final decision was achieved by consulting a third coauthor.
Data collection process
Two authors performed independent data extraction using standardized data extraction forms.
The following data were retrieved:
Research place and study description: country, study type, length of data collection, number of ICUs, type of ICU, number of patients.
AKI characteristics: incidence, definition, criteria used for definition, timeframe for AKI assessment, percentage of oliguric patients, oliguria definition, reference serum creatinine (SCr) defined as the value used for comparing increased SCr to establish the diagnosis of AKI.
AKI patient characteristics: mean age, gender ratio, previous comorbidities, patient source, severity of illness score.
Outcomes: length of ICU stay, length of hospital stay, frequency of RRT use, type of RRT, mortality.
Statistical analysis
The frequencies of studies were calculated by considering those that assessed the respective data. The number of studies used for each calculation is shown after the presented frequency.
Weighted means and percentages of reported data were estimated using the study population as weight.
The pooled point AKI incidence of developed and developing countries´ studies was estimated for studies that used the RIFLE, AKIN or KDIGO criteria for the AKI definition. All estimates and their 95% confidence intervals (95% CI) were calculated using a random-effects model for descriptive data analysis. Subgroup analyses were conducted with studies grouped by each different criterion for the utilized AKI definition. Heterogeneity and consistency were evaluated using Cochran’s Q and the I2 statistics, respectively. Funnel plots were used to evaluate publication bias. The analysis was performed in Microsoft Excel, using the step-by-step approach constructed by Neyeloff et al. [11] to analyze descriptive data. The pooled AKI incidence of both country groups were compared using the estimated 95% confidence interval.
Results
Of 2,459 potential studies, 1,635 were excluded because they did not assess patients, were duplicates, or had titles and abstracts that were not related to the purpose of this review. Of the remaining 824 studies, 17 were excluded for not meeting the language definitions and 714 were excluded as they did not meet the eligibility criteria. Ninety-two studies were included in the final analysis. One study [8] reported data from both developed and developing countries, and its data was reported in both country categories. As a result, the final analysis consisted of 60 studies presenting data from developed countries and 33 studies with data from developing countries (Fig 1 and Table 1). The 92 studies included in the final analysis can be found in S2 File.
Table 1. Frequency of studies in developed and developing countries with data reported.
| Developed countries | Developing countries | |
|---|---|---|
| (N = 60) | (N = 33) | |
| Description of studies (n = 93) | ||
| Retrospective | 46.7% (28) | 42.4% (14) |
| Prospective | 53.3% (32) | 57.6% (19) |
| Definition of AKI by | ||
| RIFLE | 34.4% (20) | 39.3% (13) |
| AKIN | 37.9% (22) | 33.3% (11) |
| KDIGO | 10% (6) | 21.2% (7) |
| sCr* rise | 24.1% (14) | 9% (3) |
| sCr rise + decreases in urinary volume | 73.2% (41) | 78.1% 25 |
| Oliguria definition (n = 81) | 81.6% (49) | 97% (32) |
| Reference SCr definition (n = 60) | 68.3% (41) | 57.5% (19) |
| Timeframe for AKI diagnosis | 58.3% (35) | 69.6% (23) |
| Frequency of AKI > 40% | 54.7% (29) | 82.1% (23) |
| AKI etiology described | 46.6% (28) | 66.6% (22) |
| Patient characteristics | ||
| > 65 years old | 40% (22) | 12.1% (4) |
| Male frequency > 60% | 59.6% (34) | 67.8% (19) |
| Comorbidities | 51.6% (31) | 78.7% (26) |
| Severity scores | ||
| APACHE II | 58.3% (35) | 54.5% (18) |
| SOFA | 25% (15) | 18.1% (6) |
| Outcomes | ||
| Length of ICU stay | 71.6% (43) | 69.6% (23) |
| Length of hospital stay | 38.3% (23) | 21.2% (7) |
| RRT > 30% in AKI patients | 13.3% (8) | 30.3% (10) |
| Mortality | 15.9% (7) | 56% (14) |
*sCr = serum creatinine
Description of studies
Study design
In both developed and developing countries, the majority of studies were from university hospitals [91.6% (55/60) and 72.7% (24/33), respectively] (Tables 2 and 3).
Table 2. Country and study features description—developed countries.
| Author | Home country | Study type | Length of data collection | Number of ICUs | Type of ICU | Number of Patients |
|---|---|---|---|---|---|---|
| Abosaif et al, 2005 | England | Retrospective | 24 | 1 | Mixed | 247 |
| Bagshaw et al, 2005 | Canada | Prospective | 36 | 3 | Mixed | 5,693 |
| Chawla et al, 2005 | USA | Prospective | 8 | 1 | Mixed | 194 |
| Ostermann et al, 2005 | England and Germany | Retrospective | 110 | 22 | Mixed | 41,972 |
| Ahlstrom et al, 2006 | Finland | Prospective | 11 | 2 | Mixed | 658 |
| Herrera-Gutiérrez et al, 2006 | Spain | Prospective | 8 | 43 | Mixed | 15,714 |
| Hoste, 2006 | USA | Retrospective | 12 | 7 | Mixed | 5,383 |
| Bagshaw et al, 2007 | Australia | Prospective | 120 | 20 | Mixed | 91,254 |
| Cruz et al, 2007 | Italy | Prospective | 3 | 19 | Mixed | 2,164 |
| Eachempati et al, 2007 | USA | Prospective | 108 | 1 | Mixed | 41,972 |
| Ostermann et al, 2007 | England and Germany | Retrospective | 120 | 11 | Surgery | 8,505 |
| Bagshaw et al, 2008 | Australia | Retrospective | 60 | 57 | Trauma | 124,088 |
| Bagshaw et al, 2008 | Australia | Retrospective | 60 | 57 | Mixed | 120,123 |
| Barrantes et al, 2008 | USA | Retrospective | 12 | 1 | Mixed | 471 |
| Lopes et al, 2008 | Portugal | Retrospective | 36 | 1 | Mixed | 662 |
| Ostermann et al, 2008 | England and Germany | Retrospective | 123 | 22 | Mixed | 23,303 |
| Abelha et al, 2009 | Portugal | Retrospective | 24 | 1 | Mixed | 1,166 |
| Andrikos et al, 2009 | Italy and Greece | Prospective | 4 | 22 | Mixed | 1,076 |
| Cartin-Ceba et al, 2009 | USA | Retrospective | 42 | 3 | Mixed | 11,644 |
| Costantini et al, 2009 | USA | Retrospective | 36 | 1 | Surgery | 571 |
| Joannidis et al, 2009 | Austria and Portugal | Prospective | . . . | 303 | Mixed | 16,784 |
| Thakar et al, 2009 | USA | Retrospective | 60 | 191 | Mixed | 325,395 |
| Aldawood et al, 2010 | Saudi Arabia | Retrospective | 72 | 1 | Mixed | 7,173 |
| Cruz et al, 2010 | USA | Prospective | 6 | 1 | Mixed | 301 |
| Elseviers et al, 2010 | Belgium | Prospective | 35 | 9 | Mixed | 1,303 |
| Park et al, 2010 | Korea | Retrospective | 6 | 1 | Mixed | 378 |
| Clec'h et al, 2011 | France | Retrospective | 149 | 13 | Mixed | 8,639 |
| Darmon et al, 2011 | France | Prospective | 5 | 3 | Mixed | 203 |
| Garzotto et al, 2011 | Italy | Prospective | 7 | 10 | Mixed | 576 |
| Macedo et al, 2011 | USA | Prospective | 2 | 1 | Mixed | 75 |
| Macedo et al, 2011 | USA | Prospective | . . . | 1 | Mixed | 317 |
| Mandelbaum et al, 2011 | USA | Retrospective | 72 | 7 | Mixed | 14,524 |
| Medve et al, 2011 | Hungary | Prospective | 2 | 7 | Mixed | 459 |
| Ostermann et al, 2011 | England and Germany | Retrospective | 123 | 22 | Mixed | 22,303 |
| Piccini et al, 2011 | Italy | Prospective | 7 | 10 | Mixed | 576 |
| Prowle et al, 2011 | Australia, Canada, Japan, USA, Germany, Italy | Prospective | 1 | 7 | Mixed | 239 |
| Clark et al, 2012 | Canada | Prospective | 11 | 11 | Mixed | 119 |
| Han et al, 2012 | Korea | Retrospective | 57 | 1 | Mixed | 1625 |
| Medve et al, 2012 | Hungary | Prospective | . . . | 1 | Surgery | 265 |
| Odutayo et al, 2012 | Canada | Prospective | 12 | 5 | Mixed | 603 |
| Shashaty et al, 2012 | USA | Retrospective | 45 | 1 | Trauma | 400 |
| Sigurdsson et al, 2012 | Iceland | Retrospective | 12 | 2 | Mixed | 1012 |
| Vaara et al, 2012 | Finland | Retrospective | 23 | 9 | Mixed | 30,380 |
| Wohlauer et al, 2012 | USA | Prospective | 192 | 1 | Surgery | 2,158 |
| Allegretti et al, 2013 | USA | Retrospective | 44 | 1 | Mixed | 863 |
| Alsultan et al, 2013 | Saudi Arabia | Prospective | 36 | 2 | Mixed | 2,574 |
| Fuchs et al, 2013 | USA | Retrospective | 80 | 1 | Mixed | 12,339 |
| Legrand et al, 2013 | France | Retrospective | 48 | 1 | Surgery | 137 |
| Nisula et al, 2013 | Finland | Prospective | 6 | 10 | Mixed | 1568 |
| Poukkanen et al, 2013 | Finland | Prospective | 5 | 1 | Infectious diseases | 423 |
| Poukkanen et al, 2013 | Finland | Prospective | 5 | 1 | Infectious diseases | 918 |
| Doi et al, 2014 | Japan | Prospective | 5 | 1 | Mixed | 339 |
| Han et al, 2014 | Korea | Retrospective | 72 | 1 | Mixed | 1,883 |
| Linder et al, 2014 | Canada | Prospective | 108 | 1 | Mixed | 1,844 |
| Shinjo et al, 2014 | Japan | Retrospective | 60 | 1 | Mixed | 2,579 |
| Udy et al, 2014 | Australia, Singapore, Hong Kong and Portugal | Prospective | . . . | 4 | Mixed | 281 |
| Bouchard et al, 2015 | Various | Prospective | 20 | 9 | Mixed | 316 |
| Harris et al, 2015 | USA | Retrospective | 12 | 1 | Surgery | 624 |
| Rimes-Stigiare et al, 2015 | Sweden | Prospective | 72 | 41 | Mixed | 97,782 |
| Vanmassenhove et al, 2015 | Belgium | Prospective | 14 | 1 | Mixed | 195 |
Table 3. Country and study features description—developing countries.
| Author | Home country | Study type | Length of data collection | Number of ICUs | Type of ICU | Number of patients |
|---|---|---|---|---|---|---|
| Mataloun et al, 2006 | Brazil | Prospective | 12 | 1 | Mixed | 221 |
| Silva Junior et al, 2006 | Brazil | Retrospective | 48 | 1 | Mixed | 381 |
| Chow et al, 2007 | Malaysia | Prospective | 6 | 1 | Mixed | 18,697 |
| Daher et al, 2008 | Brazil | Retrospective | 36 | 1 | Infectious diseases | 722 |
| Lima et al, 2008 | Brazil | Retrospective | 36 | 1 | Infectious diseases | 829 |
| Fernandes et al, 2009 | Brazil | Prospective | 10 | 1 | Mixed | 89 |
| Friedericksen et al, 2009 | South Africa | Retrospective | 12 | 1 | Mixed | 198 |
| Chang et al, 2010 | Taiwan | Retrospective | 35 | 1 | Mixed | 291 |
| Maccariello et al, 2010 | Brazil | Prospective | 18 | 11 | Mixed | 244 |
| Balushi et al, 2011 | Oman | Retrospective | 12 | 1 | Mixed | 1,373 |
| Ponce et al, 2011 | Brazil | Prospective | 24 | 1 | Mixed | 564 |
| Fonseca Ruiz et al, 2011 | Colombia | Retrospective | 24 | 1 | Mixed | 794 |
| Samimagham et al, 2011 | Iran | Retrospective | 12 | 1 | Mixed | 235 |
| Alves et al, 2012 | Brazil | Retrospective | 15 | 1 | Mixed | 204 |
| Chen et al, 2012 | Taiwan | Prospective | 12 | 1 | Mixed | 150 |
| Daher et al, 2012 | Brazil | Prospective | 12 | 1 | Mixed | 408 |
| Lai et al, 2012 | Taiwan | Retrospective | 101 | 1 | Surgical | 634 |
| Wahrhaftig et al, 2012 | Brazil | Prospective | 12 | 1 | Mixed | 200 |
| Zhou et al, 2012 | China | Retrospective | 8 | 5 | Mixed | 1,036 |
| Dalboni et al, 2013 | Brazil | Prospective | . . . | 1 | Mixed | 303 |
| Levi et al, 2013 | Brazil | Prospective | 12 | 1 | Mixed | 190 |
| Silva et al, 2013 | Brazil | Prospective | 20 | 6 | Mixed | 366 |
| Singh et al, 2013 | India | Prospective | 17 | 1 | Mixed | 1,504 |
| Daher et al, 2014 | Brazil | Retrospective | 99 | 1 | Infectious diseases | 253 |
| Luo et al, 2014 | China | Prospective | 6 | 30 | Mixed | 3,107 |
| Morales-Buenrostro et al, 2014 | Mexico | Prospective | 3 | 1 | Mixed | 56 |
| Peng et al, 2014 | China | Retrospective | 36 | 1 | Infectious diseases | 211 |
| Wijewickrama et al, 2014 | Sri Lanka | Prospective | 6 | 1 | Mixed | 108 |
| Bentata et al, 2015 | Morocco | Retrospective | 84 | 1 | Obstetric | 186 |
| Bouchard et al, 2015 | Various | Prospective | 20 | 5 | Mixed | 429 |
| Heegard et al, 2015 | Afghanistan | Prospective | 18 | 2 | Trauma | 134 |
| Ralib et al, 2015 | Malaysia | Prospective | 3 | 1 | Mixed | 143 |
| Santos et al, 2015 | Brazil | Prospective | 12 | 1 | Mixed | 279 |
The 92 studies report data from 1,091,871 patients. The number of patients from developed countries was 30 times higher; 1,057,332 vs. 34,539 patients in developed versus developing countries, respectively. Larger cohorts with more than 5,000 patients were more frequent in developed countries [33.3% (20/60)], whereas in developing countries, 87.8% (29/33) of the studies included less than 1,000 patients (Tables 2 and 3). The number of ICUs included in developed countries was significantly higher than in developing countries (990 vs. 86 ICUs). In fact, in developed countries, 41.6% of the studies (25/60) assessed more than five ICUs, while in developing countries, 81.8% of studies (27/33) assessed only one ICU. The majority of studies (82.7%, i.e., 77/93) evaluated patients from ICUs classified as "mixed" (Tables 2 and 3).
Definition of AKI
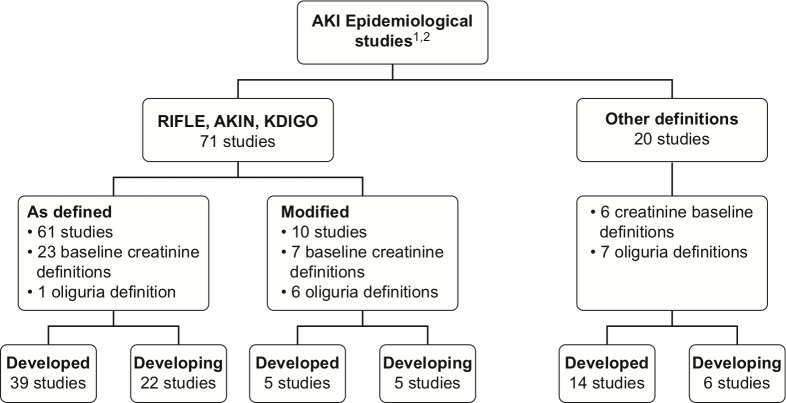
Both developed and developing country studies frequently used RIFLE, AKIN, KDIGO, as defined or modified (Fig 2). In developed countries, AKIN and RIFLE were the most frequently used criteria [37.9% (22/58) and 34.4%, (20/58), respectively], followed by increased SCr (24.1%, 14/58). In developing countries, RIFLE and AKIN were also more often applied [39.3% (13/33) and 33.3% (11/33), respectively], followed by KDIGO at 21.2% (7/33) (Tables 4 and 5). The majority of studies [73.2% (41/56) in developed countries and 78.1% (25/32) in developing countries] reported using both increases in SCr and decreases in urinary volume for the AKI diagnosis.
Fig 2. Flowchart of AKI definition criteria.
Footnote: 1 One study contained data from both (developed and developing) country groups, and the respective data were included in each country group for the analysis. 2 Two studies did not mention the criteria used for the definition of IRA.
Table 4. AKI characteristics—developed countries.
| Author | AKI Frequency (%) | AKI definition | Criteria used for AKI definition | Timeframe for AKI assessment | Oliguric patients (%) | Oliguria definition | Reference creatinine definition |
|---|---|---|---|---|---|---|---|
| Abosaif et al, 2005 | . . . | RIFLE | Cr | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lower Cr on hospital admission |
| Bagshaw et al, 2005 | 4.2 | Cr rise/Oliguria | Cr/Diuresis | . . . | 77.0 | < 500 ml x 24 h | . . . |
| Chawla et al, 2005 | 18.0 | Cr rise/Oliguria | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 48 h | . . . |
| Ostermann et al, 2005 | 17.9 | Cr rise/Oliguria | Cr/Diuresis | . . . | . . . | BUN > 8 mmol/l | Cr > 120 mmol/l |
| Ahlstrom et al, 2006 | 51.9 | RIFLE | Cr | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | MDRD |
| Herrera-Gutiérrez et al, 2006 | 5.7 | Cr rise/Oliguria | Cr/Diuresis | . . . | . . . | < 400 ml x 24 h | . . . |
| Hoste et al, 2006 | 67.0 | RIFLE | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lower Cr on hospital admission, or MDRD |
| Bagshaw et al, 2007 | 5.2 | Cr rise/Oliguria | Cr/Diuresis | . . . | . . . | . . . | . . . |
| Cruz et al, 2007 | 10.8 | RIFLE | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | MDRD |
| Eachempati et al, 2007 | 6.2 | Cr rise | Cr | . . . | . . . | Cr > 2.4 mg/dl | |
| Ostermann et al, 2007 | 35.8 | RIFLE | Cr | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Bagshaw et al, 2008 | 36.1 | RIFLE modified | Cr/Diuresis | Since ICU discharge or death, after 24 h from ICU admission | . . . | < 35 ml/kg/h | MDRD |
| Bagshaw et al, 2008 | 36.1/37.1 | RIFLE modified/AKIN modified | Cr/Diuresis | Since ICU discharge or death, after 24 h from ICU admission | . . . | < 35 ml/kg/h | MDRD |
| Barrantes et al, 2008 | 42.9 | AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Lopes et al, 2008 | 43.8/50.4 | RIFLE modified/AKIN modified | Cr/Diuresis | Until 48 h | . . . | < 7.2 ml/kg X 24 h | MDRD |
| Ostermann et al, 2008 | 35.4 | Cr rise | Cr | . . . | . . . | . . . | . . . |
| Abelha et al, 2009 | 7.5 | AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Andrikos et al, 2009 | 16.0 | RIFLE | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Cartin-Ceba et al, 2009 | 50.0 | RIFLE | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | Lower Cr 3 months before ICU admission |
| Costantini et al, 2009 | 29.8 | AKIN | Cr/Diuresis | Since ICU discharge or death after 48 h from admission | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Joannidis et al, 2009 | 35.5/28.5 | RIFLE/AKIN | Cr/Diuresis | 7 days | . . . | < 0.5 ml/kg/h x 6 h | MDRD |
| Thakar et al, 2009 | 60.8 | AKIN modified | Cr | Since ICU discharge or death | . . . | . . . | Lower Cr 24 h before hospital admission |
| Aldawood et al, 2010 | 24.4 | . . . | . . . | Since hospital discharge or death | . . . | . . . | . . . |
| Cruz et al, 2010 | 44.0 | Cr rise | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lower Cr 3 months before ICU admission or MDRD |
| Elseviers et al, 2010 | . . . | Cr rise | Cr | . . . | . . . | . . . | . . . |
| Park et al, 2010 | 41.3 | RIFLE | Cr/Diuresis | 7 days | . . . | < 0.5 ml/kg/h x 6 h | Lower Cr 3 months before hospital admission, or MDRD |
| Clec’h et al, 2011 | 32.9 | RIFLE | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | MDRD |
| Darmon et al, 2011 | 67.0 | AKIN | Cr/Diuresis | 12 hours | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Garzotto et al, 2011 | 42.7 | RIFLE | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lower Cr 3 months before ICU admission |
| Macedo et al, 2011 | 60.0 | AKIN | Cr/Diuresis | Since ICU discharge or death | 55.0 | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Macedo et al, 2011 | 52.0 | AKIN | Cr/Diuresis | Since hospital discharge or death | 47.0 | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Mandelbaum et al, 2011 | 57.0 | AKIN | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Medve et al, 2011 | 24.4 | AKIN | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Ostermann et al, 2011 | 35.4 | AKIN | Cr/Diuresis | Since ICU discharge or death | . . . | . . . | First Cr on ICU admission |
| Piccini et al, 2011 | 42.7 | RIFLE | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 3 months before ICU admission |
| Prowle et al, 2011 | 9.6 | AKIN modified/ RIFLE modified | Cr/Diuresis | 4 weeks | 38.0 | < 0.5 ml/kg | Cr before disease or estimated using GRF 75 ml/min |
| Clark et al, 2012 | 7.8 | AKIN | Cr | . . . | . . . | < 0.5 ml/kg/h x 6 h | Newest Cr 6 months before ICU admission, or MDRD |
| Han et al, 2012 | 57.0 | AKIN | Cr/Diuresis | 7 days | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 7 days before hospital admission |
| Medve et al, 2012 | 18.1 | AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Odutayo et al, 2012 | 26.7 | AKIN | Cr | 90 days | . . . | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Shashaty et al, 2012 | 36.8 | AKIN | Cr/Diuresis | 5 days | . . . | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Sigurdsson et al, 2012 | 21.7 | RIFLE | . . . | . . . | . . . | < 0.5 ml/kg/h x 6 h | Cr 1 year before ICU admission or the lowest after hospital discharge or MDRD |
| Vaara et al, 2012 | 26.6 | RIFLE | Cr | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr during ICU stay |
| Wohlauer et al, 2012 | 2.1 | Cr rise | Cr | Since hospital discharge or death | . . . | . . . | . . . |
| Allegretti et al, 2013 | . . . | Cr rise | Cr | . . . | . . . | . . . | First Cr on ICU admission |
| Alsultan et al, 2013 | 65.0 | . . . | . . . | . . . | . . . | . . . | . . . |
| Fuchs et al, 2013 | 54.3 | AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr on hospital admission |
| Legrand et al, 2013 | 50.3 | AKIN | Cr/Diuresis | 5 days | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr before ICU admission (time not informed), or MDRD |
| Nisula et al, 2013 | 40,5 | KDIGO | Cr/Diuresis | 5 days after 24 h from admission | . . . | < 0.5 ml/kg/h x 6 h | Newest Cr from last year, excluding the last week before ICU admission, or MDRD |
| Poukkanen et al, 2013 | 36.2 | KDIGO | Cr/Diuresis | 5 days | . . . | < 0.5 ml/kg/h x 6 h | Newest Cr from last year, excluding the week before ICU admission |
| Poukkanen et al, 2013 | 53.2 | KDIGO | Cr/Diuresis | 5 days | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 1 year before ICU admission |
| Doi et al, 2014 | 38.6 | RIFLE | Cr | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 3 months before ICU admission or MDRD |
| Han et al, 2014 | 78.7 | KDIGO | Cr/Diuresis | . . . | 31.8 | < 0.5 ml/kg/h x 6 h | . . . |
| Linder et al, 2014 | 57.0 | KDIGO | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 3 months before ICU admission |
| Shinjo et al, 2014 | 29.5/38.4 | AKIN/KDIGO | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 3 months before ICU admission |
| Udy et al, 2014 | 65.1 | Cr rise | Cr/Diuresis | Since ICU discharge or death, after 48 h from admission | . . . | . . . | . . . |
| Bouchard et al, 2015 | 19.1 | AKIN | Cr/Diuresis | 7 days | 31.5 | < 0.5 ml/kg/h x 6 h | Cr from 3 to 12 months before ICU admission |
| Harris et al, 2015 | 47.0 | RIFLE | Cr | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 1 year before ICU admission or MDRD |
| Rimes-Stigiare et al, 2015 | 5.4 | Cr/Oliguria | Cr/Diuresis | . . . | . . . | < 400 ml x 24 h | . . . |
| Vanmassenhove et al, 2015 | 63.0 | Cr rise | Cr | . . . | . . . | . . . | MDRD |
* Cr = serum creatinine
Table 5. AKI characteristics—developing countries.
| Author | AKI Frequency (%) | AKI definition | Criteria used for AKI definition | Timeframe for AKI assessment | Oliguric patients (%) | Oliguria definition | Reference creatinine definition |
|---|---|---|---|---|---|---|---|
| Mataloun et al, 2006 | 19.0 | Cr rise | Cr | Since ICU discharge or death | . . . | . . . | . . . |
| Silva Junior et al, 2006 | 33.5 | Cr rise | Cr | . . . | . . . | < 600 ml x 24 h | . . . |
| Chow et al, 2007 | 1.1 | Oliguria | Diuresis | . . . | . . . | < 400 ml x 24 h | |
| Daher et al, 2008 | 20.3 | RIFLE modified | Cr/Diuresis | Since ICU discharge or death | 34.0 | < 400 ml x 24 h | Lowest Cr on hospital admission, or MDRD |
| Lima et al, 2008 | 17.7 | RIFLE | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Fernandes et al, 2009 | . . . | ATN-ISS | Diuresis | Since ICU discharge or death | 62.0 | < 400 ml x 24 h | Lowest Cr on hospital admission |
| Friedericksen et al, 2009 | 23.2 | Cr rise | Cr | . . . | 50.0 | < 400 ml x 24 h | Lowest Cr on hospital admission |
| Chang et al, 2010 | . . . | RIFLE/AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Maccariello et al, 2010 | 39.8 | RIFLE | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Balushi et al, 2011 | 0.5 | Cr / Oliguria | Cr/Diuresis | . . . | . . . | < 500 ml x 24 h | . . . |
| Ponce et al, 2011 | 25.5 | AKIN | Cr/Diuresis | Since ICU discharge or death | 68.5 | < 0.5 ml/kg/h x 6 h | . . . |
| Fonseca Ruiz et al, 2011 | 31.1 | AKIN | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Samimagham et al, 2011 | 31.1 | AKIN | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Alves et al, 2012 | 11.8 | RIFLE modified/AKIN modified | Cr/Diuresis | Since ICU discharge or death | . . . | < 400 ml x 6 h | Last Cr 6 months before ICU admission or MDRD |
| Chen et al, 2012 | 28.7 | AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Cr on hospital admission |
| Daher et al, 2012 | 15.3 | RIFLE modified | Cr/Diuresis | Since ICU discharge or death, after 24 h from admission | . . . | < 400 ml x 24 h | Lowest Cr before hospital admission or MDRD |
| Lai et al, 2012 | . . . | RIFLE | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | Last Cr within 1 month to 1 year before admission |
| Wahrhaftig et al, 2012 | 36.0 | RIFLE modified | Cr/Diuresis | Since ICU discharge or death | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr before hospital admission or MDRD |
| Zhou et al, 2012 | 34.1 | AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr 2 days before ICU admission |
| Dalboni et al, 2013 | 45.6 | RIFLE | Cr | 48 h | . . . | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Levi et al, 2013 | . . . | KDIGO | Cr/Diuresis | Since ICU discharge or death, after 24 h from admission | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Silva et al, 2013 | 13.3 | RIFLE | Cr | Since ICU discharge or death, after 48 h from admission | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Singh et al, 2013 | 2.2 | RIFLE modified | Cr/Diuresis | Since hospital discharge or death, after 48 h from admission | 61.0 | < 400 ml x 24 h | Lowest Cr on hospital admission |
| Daher et al, 2014 | . . . | RIFLE modified | Cr/Diuresis | Since ICU discharge or death | . . . | < 400 ml x 24 h | Lowest Cr before hospital admission or MDRD |
| Luo et al, 2014 | 46.9/38.4/51 | RIFLE/AKIN/KDIGO | Cr/Diuresis | 10 days | . . . | < 0.5 ml/kg/h x 6 h | Lower Cr 3 month before ICU admission |
| Morales-Buenrostro et al, 2014 | 30.3 | AKIN | 30 days | . . . | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission | |
| Peng et al, 2014 | 47.9 | KDIGO | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Wijewickrama et al, 2014 | 60.2 | AKIN | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | . . . |
| Bentata et al, 2015 | 34.4 | KDIGO | Cr/Diuresis | 30 days | 17.4 | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission |
| Bouchard et al, 2015 | 19.9 | AKIN | Cr/Diuresis | 7 days | 26.1 | < 0.5 ml/kg/h x 6 h | Cr from 3 to 12 months before hospital admission |
| Heegard et al, 2015 | 34.3 | KDIGO | Cr/Diuresis | 14 days | . . . | < 0.5 ml/kg/h x 6 h | Cr 1 year before hospital admission or MDRD |
| Ralib et al, 2015 | 65.0 | KDIGO | Cr/Diuresis | Since ICU discharge or death, after 48 h from admission | 61.0 | < 0.5 ml/kg/h x 6 h | First Cr on ICU admission, or MDRD |
| Santos et al, 2015 | 32.9 | KDIGO | Cr/Diuresis | . . . | . . . | < 0.5 ml/kg/h x 6 h | Lowest Cr before hospital admission |
* Cr = serum creatinine
Oliguria definition
Although the oliguria criteria were reported in the majority of the studies, only 10% of developed and 21.2% of developing country studies reported the frequency of oliguric patients.
The oliguria definition was stated in 81.6% (49/60) of developed country studies and in 97% (32/33) of developing country studies. In five studies from developed countries and two from developing countries, the oliguria definition was not available in the manuscript and was obtained through contact with the researcher by electronic mail. In total, 11 different definitions for oliguria were used. The most frequent was “urinary volume < 0.5 ml/kg/h for 6 h”, which was found in 81.6% (40/49) of developed and in 68.7% (22/32) of developing country studies (Tables 4 and 5 and Fig 2).
Reference serum creatinine definition
The reference SCr definition was available in 68.3% (41/60) of studies in developed countries and 57.5% (19/33) in developing countries. In 12 studies from developed countries and 8 from developing countries, this information was not available in the manuscript, and it was obtained through contact with the researcher. We found 29 different definitions for reference SCr (Tables 4 and 5 and Fig 2), and there was no particular dominant definition.
Timeframe for AKI assessment
The timeframe for AKI diagnosis was available in 58.3% (35/60) of studies in developed countries and 69.6% (23/33) in developing countries. Among those reporting this information, the most used definition for timeframe was “until ICU discharge or death” found in 42.8% (15/35) of developed and in 39.1% (9/23) of developing country studies (Tables 4 and 5).
Incidence and AKI etiology
The incidence of AKI was reported in 91.3% (85/93) of the analyzed studies (Tables 4 and 5). Most studies of both developed (54.7%, 29/53) and developing countries (82.1%, 23/28) reported an AKI incidence up to 40%. According to different AKI definitions, AKI incidence varied from 2.1% [12] to 78.7% [13] in developed countries and from 0.5% [14] to 65% [15] in developing countries.
AKI etiology was described in 46.6% (28/60) of studies from developed countries and in 66.6% (22/33) of studies from developing countries. Sepsis and shock were the most common causes of AKI in both developed and developing countries (see Tables 6 and 7). The frequency of sepsis as the cause of AKI in developed countries ranged from 4.4% [13] to 100% [16], and half of the studies had frequencies greater than 40% (9/18). In developing countries, the frequency of sepsis as a cause of AKI ranged from 2.9% [17] to 100% [18], and 66.7% of the studies reported a frequency greater than 40% (12/18). In developed countries, shock was less frequently reported as a cause of AKI than in developing countries. Only one study reported tropical diseases (leptospirosis) as contributing to AKI etiology (Tables 6 and 7).
Table 6. Characteristics of patients with AKI—developed countries.
| Author | Median age | Male gender (%) | AKI etiology | Comorbidities | Patient location before ICU | Severity scores |
|---|---|---|---|---|---|---|
| Abosaif et al, 2005 | 65 | 68 | Sepsis 41.2% | CVD* 41.2%, COPD* 41.2%, CKD* 48.4% | Surgery room 47.5%, ward 52.5% | APACHE II 22.3/ SAPS II 51.5 |
| Bagshaw et al, 2005 | 65 | 62 | Shock 15%, drugs 85% | CVD 50%, COPD 34.6%, DM* 30%, CKD 18.8% | . . . | APACHE II 33 |
| Chawla et al, 2005 | 65 | 54 | . . . | CVD 30.4%, DM 36.6%, CKD 18% | . . . | APACHE II 12.5 |
| Ostermann et al, 2005 | 61 | 64 | Surgery 59.4%, medical 40.5% | . . . | Surgery room 57.1%, ward 16.4%, emergency 15.6% | APACHE II 14 |
| Ahlstrom et al, 2006 | 63 | . . . | . . . | . . . | APACHE II 22 | |
| Herrera-Gutiérrez et al, 2006 | 59 | 71 | Shock 36.6%, drugs 38.4%, mixed 21.2% | CVD 84.7%, COPD 18%, CKD 4.4% | Surgery room 18.1%, ward 31.3%, emergency 7.9% | APACHE II 22.5/ SOFA 11.9 |
| Hoste et al, 2006 | 63 | 49 | Sepsis 17.2%, heart failure 33%, neurological 20.7% | . . . | Surgery room 61.5%, ward 38.3% | APACHE II 56 / SOFA 7.8 |
| Bagshaw et al, 2007 | 64 | 61 | . . . | CVD 11.6%, COPD 8.5%, HD* 4.8% | Surgery room 49.6%, ward 50.4% | APACHE II 16.4 |
| Cruz et al, 2007 | 64 | 62 | Sepsis 25.6%, shock 38%, drugs 14.5%, contrast 5.6% | CVD 58.5%, DM 25.6% | Surgery room 27,8%, ward 37.2% | . . . |
| Eachempati et al, 2007 | 63 | 60 | . . . | . . . | Surgery room 100% | APACHE III 81 |
| Ostermann et al, 2007 | 61 | 64 | . . . | . . . | Surgery room 57.1%, ward 16.4%, emergency 15.6% | APACHE II 21 |
| Bagshaw et al, 2008 | 55 | 16 | . . . | CVD 17.7%, COPD 9.7%, HD 3% | Emergency 100% | APACHE II 20.9/APACHE III 71.7 |
| Bagshaw et al, 2008 | 62 | 60 | Sepsis 27.8% | . . . | Surgery room 49.7% | APACHE II 16.9 |
| Barrantes et al, 2008 | 67 | 57 | Sepsis 19.4%, respiratory failure 35.2% | . . . | . . . | APACHE II 15 |
| Lopes et al, 2008 | 59 | 59 | . . . | CVD 53.2% | Ward 76.4%, others 23.6% | SAPS II 46.3 |
| Ostermann et al, 2008 | 61 | 61 | Surgery 45.7%, medical 54.2 | . . . | Surgery room 43.1%, ward 24.2, Emergency 18.6% | APACHE II 18, SOFA 7 |
| Abelha et al, 2009 | 64 | 65 | Emergency 33% | CVD 46% | Emergency 33% | APACHE II 13, SAPS II 33 |
| Andrikos et al, 2009 | 72 | 67 | Sepsis 45.3%, shock 9.4%, Surgery 21.2%, drugs 7.1% | CVD 67.5%, DM 32.9% | Surgery room 31.8%, ward 38.8%, emergency 20% | . . . |
| Cartin-Ceba et al, 2009 | 66 | 54 | . . . | . . . | . . . | APACHE III 11.5 |
| Costantini et al, 2009 | 46 | 71 | Trauma 100% | . . . | Emergency 100% | . . . |
| Joannidis et al, 2009 | 63 | 61 | post surgery 34.5%, emergency 65.3% | CVD 9.1%, COPD 4.2%, DM 8.5%, HD 3.1% | Surgery room 55%, ward 42.2% | SOFA 3, SAPS III 47 |
| Thakar et al, 2009 | 98 | . . . | CVD 51.6%, COPD 22.1%, DM 28.6%, CKD 3.6%, HD 4.5% | Surgery room 22%, ward 45% | . . . | |
| Aldawood et al, 2010 | 60 | 55 | . . . | . . . | Surgery room 3%, ward 84%, others 13% | APACHE II 33 |
| Cruz et al, 2010 | 64 | 69 | . . . | DM 15.6%, CKD 6.6% | Surgery room 8.3%, ward 54.6%, emergency 37.1% | APACHE II 20/ SOFA 5/SAPS II 45 |
| Elseviers et al, 2010 | 64 | 63 | Shock 45.5%, drugs 54.5% | . . . | Surgery room 27.2%, ward 72.8% | APACHE II 23.9/ SOFA 9.2 |
| Park et al, 2010 | 63 | 63 | . . . | . . . | Surgery room 17.5%, ward 82.5% | SOFA 7.4 |
| Clec'h et al, 2011 | 66 | 59 | . . . | CVD 17.9%, COPD 12.9%, HD 6.3%, DM 16.4% | Surgery room 10.9%, ward 71.8%, emergency 17.3% | APACHE II 19.9/ SOFA 5.3/ SAPS II 50.2 |
| Darmon et al, 2011 | 61 | 49 | Sepsis 67.5%, shock 19.8%, Drugs 20.7%, CKD 16.3%, contrast 8.9% | . . . | . . . | SAPS II 46 |
| Garzotto et al, 2011 | 66 | 59 | CVD 12.1%, respiratory failure 27.4%, neurological 17%, trauma 14.4% | . . . | Surgery room 52.7%, ward 47.3% | APACHE 18, SOFA 5, SAPS II 43 |
| Macedo et al, 2011 | 69 | 58 | Sepsis 47% | CVD 70%, DM 30%, CKD 12%, HD 35%, COPD 2.1% | … | . . . |
| Macedo et al, 2011 | 63 | Sepsis 18.5%, shock 34.7%, respiratory failure 52%, drugs 18.5% | CVD 31.7%, DM 30.5%, HD 17.3% | . . . | . . . | |
| Mandelbaum et al, 2011 | 66 | 58 | . . . | . . . | . . . | SOFA 5 |
| Medve et al, 2011 | 65 | 56 | Sepsis 44%, shock 39%, post operatory 16%, drugs 2% | . . . | Surgery room 64.3%, ward 35.7%, | SOFA 6/ SAPS II 47.5 |
| Ostermann et al, 2011 | 62 | 63 | . . . | . . . | Surgery room 38%, ward 30.7%, emergency 14.1%, others 17.2% | APACHE II 18/ SOFA 7 |
| Piccini et al, 2011 | 66 | 59 | CVD 12.1%, respiratory failure 27.4%, neurological 17%, trauma 14.4% | . . . | Surgery room 52.7%, ward 47.3% | APACHE 18, SOFA 5, SAPS II 43 |
| Prowle et al, 2011 | 59 | . . . | . . . | Surgery room 47.7%, ward 52.3% | . . . | |
| Clark et al, 2012 | 59 | 66 | . . . | CVD 55%, COPD 24%, HD 15%, DM 34% | Surgery room 41%, ward 45%, emergency 14% | APACHE II 27/SOFA 13.4 |
| Han et al, 2012 | 68 | 60 | Sepsis 4.4%, CVD 36.7%, Tumor 16.9% | . . . | Ward 97.9% | APACHE II 19.4 |
| Medve et al, 2012 | 67 | Sepsis 45.8% | . . . | Surgery room 100% | SOFA 5/ SAPS II 40 | |
| Odutayo et al, 2012 | 65 | 71 | . . . | CVD 55%, DM 32% | Ward 86%, emergency 6%, Others 8% | . . . |
| Shashaty et al, 2012 | 40 | 74 | . . . | CVD 16.8%, DM 6% | Emergency 100% | APACHE III 73 |
| Sigurdsson et al, 2012 | 59 | 61 | Sepsis 26%, shock 46.4%, respiratory failure 35.4%, surgery 35% | CVD 46%, DM 14%, COPD 25%, HD 3% | . . . | APACHE II 23 |
| Vaara et al, 2012 | 63 | 63 | . . . | . . . | Surgery room 39.7%, ward 36.7% | SOFA 10/ SAPS II 48 |
| Wohlauer et al, 2012 | 37 | 74 | . . . | . . . | Surgery room 100% | MOF 184 |
| Allegretti et al, 2013 | 63 | . . . | CVD 29%, DM 29%, COPD 20%, HD 18% | Surgery room 45%, ward 55% | Charlson 2 | |
| Alsultan et al, 2013 | 54 | . . . | CVD 22%, DM 20.9% | . . . | APACHE II 29.9 | |
| Fuchs et al, 2013 | 63 | 60 | . . . | CVD 26%, DM 13%, HD 5% | Surgery room 24,3%, ward 75.7% | SOFA 8.9/ SAPS I 16.3 |
| Legrand et al, 2013 | 71 | 60 | . . . | CVD 43%; DM 15%; COPD 9%; HD 7% | Surgery room 100% | SAPS II 57 |
| Nisula et al, 2013 | 65 | 65 | . . . | CVD 77.5%, COPD 10.8%, DM 22.9%, CKD 8.1% | Surgery room 39.4%; emergency 82.1% | SOFA 9/ SAPS II 42 |
| Poukkanen et al, 2013 | 64 | 92 | Sepsis 100% | CVD 19.6%, DM 5.2% | Emergency 96.7% | SOFA 9, SAPS II 43 |
| Poukkanen et al, 2013 | 66 | 66 | Sepsis 31.6% | CVD 10.6%, COPD 12%, DM 24.8% | Surgery room 24.2%, emergency 97% | SAPS II 48, SOFA 11 |
| Doi et al, 2014 | 66 | 61 | . . . | . . . | Surgery room 49.6%, ward 50.4% | APACHE II 15 |
| Han et al, 2014 | 68 | 60 | Sepsis 4.6%, CVD 30.8%, post operatory 1.9% | DM 12.2%, CKD 8.7% | . . . | APACHE II 18.4 |
| Linder et al, 2014 | 61 | 68 | Sepsis 81%, post operatory 27.8% | CVD 8.1%, COPD 12.7%, DM 2.6% | . . . | APACHE II 24.6 |
| Shinjo et al, 2014 | 63 | 66 | . . . | CVD 38.5%, DM 18.4%, COPD 3.5%, HD 3.1% | Surgery room 74.3%, ward 13.2%; emergency 12.5% | APACHE II 9/SOFA 4/ SAPS II 28 |
| Udy et al, 2014 | 54 | 63 | . . . | . . . | Surgery room 44.8%, ward 9.3%; emergency 45.9% | IQR 3 |
| Bouchard et al, 2015 | 62 | 62 | . . . | . . . | . . . | . . . |
| Harris et al, 2015 | 59 | 59 | . . . | CVD 46%, DM 26% | Surgery room 100% | APACHE III 66 |
| Rimes-Stigiare et al, 2015 | 68 | 60 | . . . | . . . | . . . | APACHE II 25/ SAPS II 55 |
| Vanmassenhove et al, 2015 | 66 | 67 | Sepsis 100% | . . . | . . . | APACHE II 27 |
Neurologic etiologies of AKI include polyuria of diabetes insipidus and salt wasting syndrome; CKD refers to AKI on baseline CKD patients. Comorbidities: CVD = cardiovascular disease DM = diabetes mellitus; COPD = chronic obstructive pulmonary disease; HD = hepatic disease; CKD = chronic kidney disease
Table 7. Characteristics of patients with AKI—developing countries.
| Author | Median age | Male gender (%) | AKI etiology | Comorbidities | Patient location before ICU | Severity scores |
|---|---|---|---|---|---|---|
| Mataloun et al, 2006 | 55 | 46 | . . . | CVD 35.7%, DM 14% | Surgery room 44.3%; ward 27.6%; emergency 28.1% | APACHE II 15.2 |
| Silva Junior et al, 2006 | 50 | 62 | Sepsis 40.6%, shock 48.4%, drugs 21.9% | CVD 25.8%, COPD 28.9% | . . . | . . . |
| Chow et al, 2007 | 58 | Sepsis 41%, shock 43.6% | . . . | Surgery room 30.8%, ward 69.2%; obstetrics1% | . . . | |
| Daher et al, 2008 | 45 | 77 | Sepsis 41.5%, shock 40.2%, drugs 10.2% | CVD 13%, COPD 19% | Clinical ward 100% | APACHE II 28 |
| Lima et al, 2008 | 45 | 77 | Infection 100% | CVD 14.2%, HD 40.1% | . . . | APACHE II 27 |
| Fernandes et al, 2009 | 56 | 62 | Sepsis 63% | CVD 63%, HD 22% | Surgery room 44%, ward 51.5%, emergency 45% | APACHE II 25.5 |
| Friedericksen et al, 2009 | 44 | 61 | Sepsis 50%, multiple organ failure 78% | CVD 21.7%, DM 19% | . . . | APACHE II 23.4 |
| Chang et al, 2010 | 62 | 70 | Sepsis 55% | DM 27.5%, HD 42% | . . . | APACHE II 22.8/ SOFA 9.19 |
| Maccariello et al, 2010 | 70 | Sepsis 74%, shock 75%, contrast 33% | CVD 68%, DM 28% | Surgery room 19%, ward 13%; emergency 68% | SOFA 8.3/ SAPS III 70 | |
| Balushi et al, 2011 | 61 | 60 | Shock 53.6%, drugs 46.4% | CVD 53.7%, DM 59.9% | . . . | . . . |
| Ponce et al, 2011 | 57 | 56 | . . . | . . . | . . . | . . . |
| Fonseca Ruiz et al, 2011 | 53 | 53 | Sepsis 19.6% | CVD 44,6%, DM 18,2% | Surgery room 38.2%, ward 57.6%, emergency 4.2% | APACHE II 13/SOFA 4/ SAPS II 27 |
| Samimagham et al, 2011 | 40 | 71 | . . . | . . . | Surgery room 52.3%, ward 11.1% | APACHE II 23.9 |
| Alves et al, 2012 | 50 | 45 | Sepsis 65% | . . . | Surgery room 27.9%, ward 67.4% Obstetrics 4.7% |
SOFA 9.8 |
| Chen et al, 2012 | 69 | 75 | . . . | CVD 73%, DM 61% | Ward 100% | APACHE II 14 |
| Daher et al, 2012 | 55 | Sepsis 40%, drugs 14%, leptospirosis 12% | CVD 24,7%; DM 14,8% | Ward 100% | . . . | |
| Lai et al, 2012 | 64 | 66 | . . . | CVD 58.4%, DM 28.7% | Surgery room 70.7%, emergency 29.3% | . . . |
| Wahrhaftig et al, 2012 | 66 | 47 | Sepsis 74.2%, shock 17% | CVD and DM 99% | Surgery room 13.8%, ward 29.7%, emergency 17.3%, others 35.6% | APACHEII 13 /SOFA 3 |
| Zhou et al, 2012 | 59 | 69 | Sepsis 30.6%, respiratory failure 79.9% | CVD 36%, DM 12.8% | Surgery room 3.7%, ward 79.1%, emergency 7.6%, other 9.6% | APACHE III 45.4 / SOFA 5.1 |
| Dalboni et al, 2013 | 67 | 66 | . . . | CVD 39%, DM 18% | . . . | APACHE II 20 |
| Levi et al, 2013 | 64 | 45 | Sepsis 46.8%, CVD 42% | CVD 46.8%, DM 32.2% | Surgery room 30.5%, ward 30.5%, emergency 22.6% | APACHE II 15 |
| Silva et al, 2013 | 57 | 60 | . . . | COPD 77,3%, HD 49,2% | . . . | SAPS III 69.7 |
| Singh et al, 2013 | 51 | 0 | Sepsis 35.2%, shock 14.2%, drugs 23.5% | CVD 13,7%, COPD 23,5% | . . . | . . . |
| Daher et al, 2014 | 46 | 72 | . . . | HIV/AIDS 30%, Tuberculosis 12% | Ward 100% | APACHE II 50 |
| Luo et al, 2014 | 61 | 65 | Sepsis 32.2 | CVD 5.9%, COPD 6.1%, CKD* 6.1%, DM 18.9%, | Surgery room 57%, | SOFA 6 |
| Morales-Buenrostro et al, 2014 | 52 | 54 | . . . | CVD 16.2%, DM 24.3% | Surgery room 21.6%, ward 64,9%, others 10.8% | . . . |
| Peng et al, 2014 | 52 | 68 | Sepsis 100% | . . . | . . . | APACHE II 20.8 |
| Wijewickrama et al, 2014 | 48 | 62 | Sepsis 28.7%, shock 12.1% | CVD 28%, DM 27% | . . . | SOFA 9 |
| Bentata et al, 2015 | 28 | Obstetrics 100% |
CVD 19.5%, | Emergency 22% | . . . | |
| Bouchard et al, 2015 | 59 | 63 | . . . | . . . | . . . | . . . |
| Heegard et al, 2015 | 26 | 98 | Trauma 100% | . . . | Emergency 100% | . . . |
| Ralib et al, 2015 | 50 | 64 | . . . | CVD 36.4%, DM 28.7% | Surgery room 25.2%, ward 74.8% | APACHE II 19.4/ SOFA 8.7 |
| Santos et al, 2015 | 43 | 66 | Sepsis 2.9%, CVD 3.2%, emergency 48.4% | DM 12.9% | Emergency 48.8% | APACHE II 10 |
Comorbidities: CVD = cardiovascular disease; DM = diabetes mellitus; COPD = chronic obstructive pulmonary disease; HD = hepatic disease; CKD = chronic kidney disease
Patient characteristics
Age
Almost 40% (22/56) of the studies in developed countries described a mean age above 65 years in AKI patients (ranging from 37 [12] to 72 years [19]), while only 12.1% (4/33) of the studies in developing countries reported an age higher than 65 years in AKI patients (ranging from 26 [20] to 70 years [21]) (Tables 6 and 7). The weighted mean ages were 62.0 and 56.8 years for developed and developing country patients, respectively.
Gender and ethnicity
Male sex was predominant in AKI patients in both groups of countries: 59.6% (34/57) and 67.8% (19/28) of the studies in developed and developing countries, respectively, reported a male frequency above 60%. The weighted male frequencies were 67.1% and 64.5% for developed and developing country patients, respectively.
Only 9.5% (9) of the studies reported the patients’ ethnic background (Tables 6 and 7).
Comorbidities
Comorbidities were assessed in 51.6% (31/60) and 78.7% (26/33) of studies from developed and developing countries, respectively. The most prevalent comorbidities were cardiovascular diseases (CVD), diabetes and chronic respiratory disease.
In developed counties, a CVD frequency greater than 40% was reported in 51.6% (16/31), versus 30.4% (7/23) in developing countries. The weighted CVD frequencies were 41.3% and 32.6% for developed and developing country patients, respectively.
The frequency of diabetes was similar in both groups of countries. In approximately 60% (16/27 in studies of developed and 10/18 of developing countries) of studies, the prevalence of diabetes was over 20% in the studied population (Tables 6 and 7). The weighted diabetes frequencies were 27.3% and 24.5% for developed and developing country patients, respectively.
Severity scores
The most reported severity scores were APACHE II and SOFA. The Apache II score in AKI patients was reported in 32 and 18 studies from developed and developing countries, respectively. The APACHE II score had a similar distribution in the two groups of countries, ranging from 9 [22] to 56 [23] in developed country studies and from 10 [17] to 50 [24] in studies from developing countries. Approximately half of the studies had an APACHE II score up to 20 (16/32) in developed and in developing countries (9/18) (Tables 6 and 7). The weighted APACHE II scores were 18.7 and 21.0 for developed and developing country patients, respectively.
The SOFA score was reported in 21 and 10 studies from developed and developing countries, respectively. The distribution of the SOFA score was similar between groups, ranging from 3 [25] to 13.4 [26] in studies from developed countries and from 3 [27] to 9.8 [28] in developing country studies. In studies where this information was available, a SOFA score over 5 was reported by 71% (15/21) of studies from developed countries and 80% (8/10) of studies in developing countries (Tables 6 and 7). The weighted SOFA scores were 7.6 and 8.2 for developed and developing country patients, respectively.
Patient location before ICU
Most of the AKI patients who were admitted to the ICU came from surgical and clinical wards units. The majority of manuscripts from both developed countries (13/16) and developing countries (9/11) reported that up to 50% of patients with AKI had hospital admission in emergency situations (Tables 6 and 7).
Outcomes
Length of ICU and hospital stay
In developed and in developing countries, 71.6% (43/60) and 69.6% (23/33) of the studies reported the length of ICU stay for AKI patients, which ranged from 1 to 22 days and from 5 to 23 days, respectively. The reported ICU stay was longer than seven days in 38.6% (17/44) and 80% (20/25) of developed and developing country studies, respectively (Tables 8 and 9). The weighted mean ICU stay lengths were 7.2 and 12.2 days for developed and developing country patients, respectively.
Table 8. Outcomes—Developed countries.
| Autor | ICU stay (days) | Hospital stay (days) | RRT in ICU AKI patients (%) | Type of RRT | Mortality in ICU AKI patients (%) |
|---|---|---|---|---|---|
| Abosaif et al, 2005 | . . . | . . . | 38.8 | C 100% | 47.5 |
| Bagshaw et al, 2005 | 8.1 | 22 | 6 | C 61%, I 20% | 50 |
| Chawla et al, 2005 | . . . | . . . | 37.1 | . . . | |
| Ostermann et al, 2005 | . . . | . . . | . . . | C 92%, I 7.1% | 66.7 |
| Ahlstrom et al, 2006 | . . . | . . . | 7 | . . . | 16.6 |
| Herrera-Gutiérrez et al, 2006 | 15.6 | 19.9 | 38 | . . . | 46.8 |
| Hoste et al, 2006 | 3 | 16 | 6 | . . . | |
| Bagshaw et al, 2007 | 4.4 | 14.2 | . . . | . . . | |
| Cruz et al, 2007 | 10 | . . . | 30.3 | C 50.7%, I 14.1% | 36.3 |
| Eachempati et al, 2007 | 16 | 15.9 | 19.8 | I 100% | 45 |
| Ostermann et al, 2007 | . . . | . . . | 12.2 | C 80.2%, I 5.2% | 28.4 |
| Bagshaw et al, 2008 | . . . | . . . | . . . | . . . | 16.7 |
| Bagshaw et al, 2008 | 3.7 | 14.6 | . . . | . . . | 24.2 |
| Barrantes et al, 2008 | 3 | 9 | 15 | . . . | |
| Lopes et al, 2008 | 8.2 | . . . | 27.2 | . . . | 41.3 |
| Ostermann et al, 2008 | 7 | . . . | 23 | C 93.6%, I 0.4% | 31.1 |
| Abelha et al, 2009 | 2.8 | 25 | . . . | . . . | 17.2 |
| Andrikos et al, 2009 | 13 | . . . | 53.5 | C 86%, I 13.2% | 64.7 |
| Cartin-Ceba et al, 2009 | . . . | . . . | 19 | C 39%, I 61% | 20 |
| Costantini et al, 2009 | 13.6 | 25 | 7 | . . . | 15.9 |
| Joannidis et al, 2009 | 2.8 | . . . | . . . | . . . | 16 |
| Thakar et al, 2009 | . . . | . . . | 4.4 | . . . | |
| Aldawood et al, 2010 | 15 | . . . | 9 | . . . | 64 |
| Cruz et al, 2010 | 7 | . . . | . . . | . . . | 17.3 |
| Elseviers et al, 2010 | 16.4 | 34.2 | 49.9 | C 42%, I 58% | 58 |
| Park et al, 2010 | . . . | 17.2 | . . . | . . . | 62.8 |
| Clec'h et al, 2011 | 7 | . . . | 19 | . . . | |
| Darmon et al, 2011 | . . . | . . . | 22.1 | . . . | 36.7 |
| Garzotto et al, 2011 | 5 | . . . | 8 | . . . | 21.7 |
| Macedo et al, 2011 | 4 | 8 | 6 | . . . | |
| Macedo et al, 2011 | 3 | 8 | . . . | . . . | 9.5 |
| Mandelbaum et al, 2011 | 7 | 16 | . . . | . . . | 12.4 |
| Medve et al, 2011 | 4.5 | 13.5 | 15.1 | I 64.8% | 39.3 |
| Ostermann et al, 2011 | 7 | . . . | . . . | . . . | 31.1 |
| Piccini et al, 2011 | 5 | . . . | 8 | . . . | 21.7 |
| Prowle et al, 2011 | . . . | . . . | 39 | . . . | 22.5 |
| Clark et al, 2012 | . . . | . . . | . . . | C 77%, I 17% | |
| Han et al, 2012 | 7 | . . . | 17 | . . . | |
| Medve et al, 2012 | 6 | 18 | . . . | . . . | 33.3 |
| Odutayo et al, 2012 | . . . | . . . | 11.8 | . . . | |
| Shashaty et al, 2012 | . . . | . . . | . . . | . . . | 14.9 |
| Sigurdsson et al, 2012 | 17 | . . . | 17 | . . . | 9 |
| Vaara et al, 2012 | 12.5 | . . . | 6.8 | . . . | 29.1 |
| Wohlauer et al, 2012 | 14 | . . . | 23 | . . . | |
| Allegretti et al, 2013 | 21 | . . . | . . . | . . . | 60.7 |
| Alsultan et al, 2013 | . . . | . . . | 12 | . . . | |
| Fuchs et al, 2013 | . . . | . . . | . . . | . . . | . . . |
| Legrand et al, 2013 | 9 | . . . | . . . | . . . | 23 |
| Nisula et al, 2013 | 2.8 | 9 | 10.3 | . . . | . . . |
| Poukkanen et al, 2013 | 5.7 | 16 | 8 | . . . | . . . |
| Poukkanen et al, 2013 | 4.2 | 14 | . . . | . . . | . . . |
| Doi et al, 2014 | 5 | . . . | . . . | . . . | 12 |
| Han et al, 2014 | 22 | . . . | 38.4 | . . . | 67.4 |
| Linder et al, 2014 | 9.1 | 23.4 | . . . | . . . | 67.1 |
| Shinjo et al, 2014 | 1 | 31 | . . . | . . . | 7.1 |
| Udy et al, 2014 | 5 | . . . | . . . | . . . | 14 |
| Bouchard et al, 2015 | 5 | 11 | 15.5 | . . . | 27.6 |
| Harris et al, 2015 | 4.5 | 19 | 12 | . . . | 33 |
| Rimes-Stigiare et al, 2015 | . . . | . . . | 6 | . . . | 35 |
| Vanmassenhoe et al, 2015 | 12 | . . . | 13.8 | . . . | 23.1 |
RRT: renal replacement therapy; C = continuous RRT, I = intermittent RRT
Table 9. Outcomes in developing countries.
| Autor | ICU stay (days) | Hospital stay (days) | RRT in ICU AKI patients (%) | Type of dialysis | Mortality in ICU AKI patients (%) |
|---|---|---|---|---|---|
| Mataloun et al, 2006 | 16.1 | . . . | 23.8 | I 100% | 76.2 |
| Silva Junior et al, 2006 | 17 | . . . | 32 | I 100% | 62.5 |
| Chow et al, 2007 | 13.7 | . . . | 16.7 | P 69.2%, I 15.3% | . . . |
| Daher et al, 2008 | 11 | . . . | 35 | I 100% | 66.6 |
| Lima et al, 2008 | 12 | . . . | 35 | . . . | 64.6 |
| Fernandes et al, 2009 | 22 | 22.3 | 48 | C 30%, I 10% | 85 |
| Friedericksen et al, 2009 | 10.8 | . . . | 17.3 | C 25%, I 75% | 47.8 |
| Chang et al, 2010 | 11.4 | . . . | . . . | . . . | 60.8 |
| Maccariello et al, 2010 | 23 | 29 | . . . | C 84% | 63 |
| Balushi et al, 2011 | . . . | . . . | 60.7 | . . . | |
| Ponce et al, 2011 | 12.9 | . . . | . . . | . . . | 62.5 |
| Fonseca Ruiz et al, 2011 | 8.4 | . . . | 12.4 | C 30.8%, I 69.2% | 25.4 |
| Samimagham et al, 2011 | 10.6 | . . . | . . . | . . . | 72.6 |
| Alves et al, 2012 | 10.7 | . . . | 2.3 | I 100% | 29 |
| Chen et al, 2012 | . . . | . . . | . . . | . . . | |
| Daher et al, 2012 | . . . | . . . | 68 | I 100% | 33.6 |
| Lai et al, 2012 | . . . | . . . | . . . | . . . | . . . |
| Wahrhaftig et al, 2012 | 12 | . . . | . . . | 53.3 | |
| Zhou et al, 2012 | 11.6 | . . . | 63.5 | . . . | 50 |
| Dalboni et al, 2013 | . . . | . . . | . . . | . . . | 9 |
| Levi et al, 2013 | . . . | . . . | . . . | . . . | 63.1 |
| Silva et al, 2013 | 17 | 20.5 | 28.7 | C 85.3%, I 14.7% | 78.6 |
| Singh et al, 2013 | . . . | . . . | 20.6 | . . . | 73.5 |
| Daher et al, 2014 | . . . | . . . | 27.6 | . . . | 62.8 |
| Luo et al, 2014 | 5 | 27.4 | . . . | . . . | . . . |
| Morales-Buenrostro et al, 2014 | . . . | . . . | . . . | . . . | . . . |
| Peng et al, 2014 | 8.5 | 15.7 | . . . | . . . | . . . |
| Wijewickrama et al, 2014 | 11.6 | . . . | 58.4 | I 97.3%; P 2.7% | 52.3 |
| Bentata et al, 2015 | 6.5 | . . . | . . . | . . . | 10.2 |
| Bouchard et al, 2015 | 6 | 10 | 30.2 | . . . | . . . |
| Heegard et al, 2015 | . . . | . . . | 5.6 | . . . | 21.7 |
| Ralib et al, 2015 | 6.4 | 14.2 | 25 | C 36%, I 58.3% | 91 |
| Santos et al, 2015 | 9.5 | . . . | 71.7 | . . . | 33.3 |
RRT: renal replacement therapy; C = continuous RRT, I = intermittent RRT; P = peritoneal dialysis
In non-AKI patients, ICU stays longer than 7 days were not reported in developed country studies (0/24) but occurred in 44% (4/9) of studies in developing countries. In developed and developing country studies, 38.3% (23/60) and 21.2% (7/33) of the studies reported the length of hospital stay, which ranged from 8 to 31 days and 10 to 29 days, respectively. Hospital stays were longer than 15 days in 58.3% (14/24) and 66.7% (6/9) of developed and developing country studies, respectively (Tables 8 and 9). In non-AKI patients, the hospital stay was longer than 15 days in 25% (4/16) and 33.3% (1/3) in developed and in developing country studies, respectively. The weighted mean lengths of hospital stay were 15.5 and 23.6 days for developed and developing country patients, respectively.
Renal replacement therapy
Sixty-three percent of the analyzed studies reported the use of renal replacement therapy (RRT) in ICU AKI patients. In developed countries, 21% of the 38 studies with available data referred to the use of RRT in ICU AKI patients as being greater than 30%. In developing countries, 48% of the 21 studies with available data showed that the frequency of RRT use was higher than 30% in ICU AKI patients (Tables 8 and 9). The weighted frequencies of RRT were 8.8% and 23.8% for developed and developing country patients, respectively.
Mortality
Reported ICU mortality in AKI patients was greater in developing country studies. AKI mortality greater than 60% was reported in 15.9% (7/44) of studies from developed countries and 56% from developing countries (14/25) (Tables 8 and 9). The weighted frequencies of mortality were 30.8% and 54.8% for developed and developing country patients, respectively.
Synthesis of AKI incidence
Pooled AKI incidence estimates for developed and developing countries in the meta-analysis are presented in Table 10, according to the AKI definition used. The RIFLE, AKIN or KDIGO criteria for AKI definition was used, as defined, by 39 and 21 studies in developed and developing country studies, respectively. One study in developed countries and 3 in developing countries studies did not report AKI incidence, so 38 and 18 studies, respectively, had an AKI incidence estimation included in the meta-analysis. The pooled estimate of AKI incidence in developed and developing countries is shown in Fig 3. There was a tendency towards a greater incidence in developed countries, although this was not significant. When only prospective studies were analyzed, this tendency disappeared (Table 10).
Table 10. Pooled AKI frequency of developed and developing countries’ studies according to AKI Definition.
| Subgroup | Country group | Studies | Patients | AKI frequency | 95% confidence | Test for heterogeneity | |
|---|---|---|---|---|---|---|---|
| (n) | (n) | (%) | interval | I2 Index | Q Test p-value | ||
| RIFLE/AKIN/KDIGO | Developed | 38 | 153,846 | 39.3 | 34.6–43.9 | 27.5 | 0.062 |
| Developing | 18 | 9,174 | 35.1 | 28.4–41.9 | -15.1 | 0.612 | |
| RIFLE | Developed | 14 | 71,954 | 37.4 | 30.0–44.8 | 13.0 | 0.018 |
| Developing | 4 | 1,742 | 26.9 | 15.3–42.0 | 26.9 | 0.250 | |
| AKIN | Developed | 18 | 72,677 | 36.9 | 29.5–44.3 | 22.7 | 0.185 |
| Developing | 8 | 3,372 | 30.8 | 25.5–36.1 | 42.2 | 0.097 | |
| KDIGO | Developed | 6 | 9,215 | 50.7 | 38.3–63.1 | 7.26 | 0.370 |
| Developing | 6 | 4,060 | 43.8 | 34.7–52.8 | 15.2 | 0.316 | |
| RIFLE/AKIN/KDIGO–Prospective studies | Developed | 18 | 29,164 | 37.4 | 30.9–43.9 | 26.4 | 0.145 |
| Developing | 13 | 6,677 | 36.2 | 27.4–44.9 | -16.2 | 0.587 | |
*Included the studies that used the RIFLE, AKIN or KDIGO definition
Fig 3. Forest plot of AKI incidence.
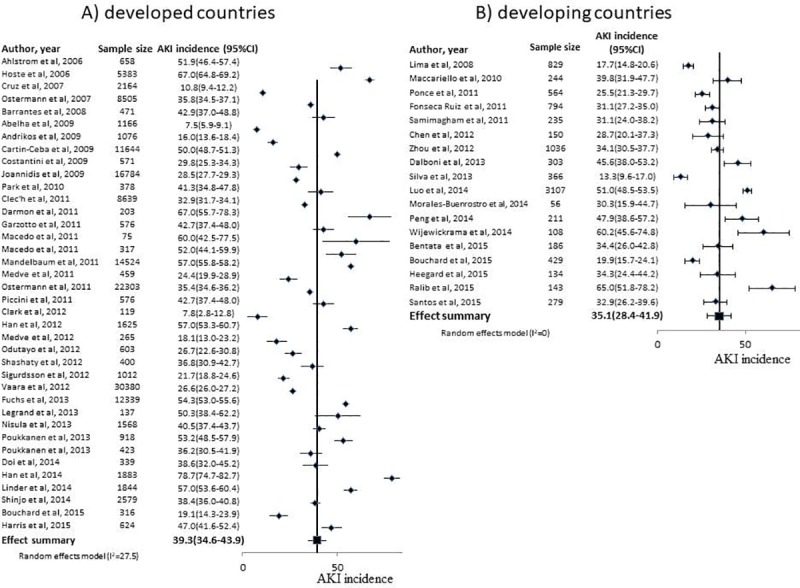
Footnote: The studies shown are those that used the RIFLE, AKIN or KDIGO criteria for AKI definition. A) Developed country studies; B) Developing country studies.
Fig 4 shows the funnel plot for both country groups in which individual study frequency of AKI is a function of their sample size with the pooled incidence of studies that used the RIFLE, AKIN or KDIGO criteria for the AKI definition being depicted as a black line. Note that the Fig 4A (developed countries) had a 10-fold greater sample size than Fig 4B (developing countries). The studies with a greater sample size depart from the polled estimated AKI incidence, suggesting the possibility of publication bias or bias resulting from the lack of standardizing reference creatinine, oliguria, and the timeframe for AKI assessment.
Fig 4. Funnel plot of sample size of studies as a function of AKI incidence.
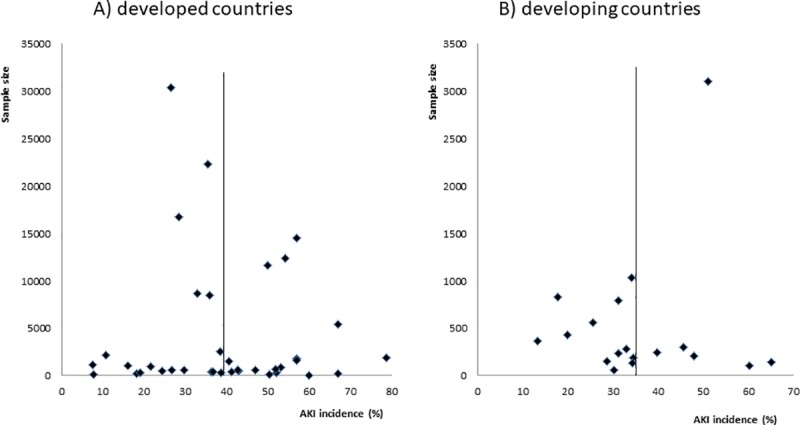
Footnote: The studies shown are those that used the RIFLE, AKIN or KDIGO criteria for AKI definition. A) Developed country studies; B) Developing country studies. Pooled AKI incidence is depicted as a vertical line.
Discussion
We found a high incidence of AKI in both country categories, and a tendency towards a greater incidence in developed countries. Due to the differences in AKI definitions, timeframe and the types of studied population, the reported incidence varied from 0.5% to 78%.
Our review covered a 10-year period. Thus, different AKI definitions were used for AKI assessment, including the RIFLE, AKIN and KDIGO criteria [29,30,31]. Only two-thirds of the studies reported the definition for reference serum creatinine, with 29 different definitions used, which results in high heterogeneity of AKI incidence estimate [32]. In the most recent AKI definitions (RIFLE, AKIN and KDIGO), the reference SCr is the value observed up to seven days or 48 hours before the SCr increase defining the AKI diagnosis. However, we observed that several studies used as reference SCr values obtained months, or even one year before the AKI episode, which is not consistent with the current AKI definitions. When the reference serum creatinine was not available, the MDRD formula has been used for estimation of the missing SCr value, which is a flawed methodology as it can misdiagnose AKI in CKD patients [12,33,34]. The oliguria definition was more uniform, with recent studies correctly using the RIFLE, AKIN and KDIGO oliguria definitions. The addition of urine output criteria was associated with higher and earlier AKI detection and incidences in critically ill patients [32].
Another important caveat to create a valid comparison between developed and developing countries is the striking differences in the number of studies and the sample sizes. Eighty per cent of the world population lives in developing countries, but only one-third of the studies sample reported data from them, with the majority assessing a single center with a relatively small number of patients [35]. Moreover, approximately half of the studies from developing countries were from Brazil, and only two were from Africa. On the other hand, approximately 40% of developed country studies assessed more than five centers. The sample size from developed countries studies was more than 30-fold greater compared to those from developing countries. There is a clear underrepresentation of developing countries that is probably caused by a lack of health resources and electronic medical records, as well as difficulty in gathering epidemiological data and, consequently, conducting adequate large observational studies. These studies can be more capable to determine the true burden of a disease than trials and more valuable in assessing the incidence and prevalence of the disease [36]. A snapshot of worldwide AKI incidence found more severe AKI presentation in patients from developing countries, which was considered to be due to delay in AKI recognition and treatment, adversely affecting the outcomes [7,37].
The incidence of AKI development in the ICUs was similar in both types of countries, with a numeric tendency to be greater in developed country studies. When only prospective studies were analysed, this tendency disappeared. Developing and developed countries have very distinct healthcare patterns. In developing countries, deficiencies in health structure, long distance from the community to the hospital and poor transportation systems limit patient access to healthcare. Lack of universal health coverage and insufficient funding for the health system imposes significant cost of treatment for the patients and family, including high cost procedures such as ICU and renal replacement therapy [38]. Tropical infectious diseases, animal venoms, natural medicine, abortion and eclampsia are known to be important AKI etiological factors in developing countries [39,40]; however, their incidence was extremely low in the ICU population. This is likely due to the limited number of ICUs, which are located mostly in larger urban cities, as well as inadequate recognition of high-risk AKI patients in the primary health system. Furthermore, difficulty transporting patients due to geographical and economic issues may contribute to this situation [39]. As a consequence, developing country’s ICUs reflect tertiary hospitals and university hospitals mostly from an urban population. Thus, patient characteristics were similar in both types of country. Sepsis and shock were the main causes of AKI in both developed and developing countries, but the frequency of sepsis was approximately 50% greater in developing country studies. In developed country studies, AKI patients were older, which likely reflects higher population longevity, better socioeconomic conditions and more structured health services. Overall, cardiovascular diseases were the most frequently reported comorbidity, although they were more frequent in developed country studies.
AKI was associated with poor outcomes, higher length of stay (LOS) and mortality, which is consistent with other studies [41,42]. In developing country studies, AKI had higher LOS and mortality compared to developed countries, although patients were younger, had less CVD and had similar APACHE II scores. Difficulty accessing health services [39,43] and lack of infrastructure, including ICU beds and human resources for care of the critically ill in these countries [44,45], are probably the cause of worse outcomes in such a low resource setting. It is possible that the patients treated in developing countries are transferred to an ICU at a late stage of disease progression and have a reduced change of recovering [45]. The finding of higher frequency RRT use in developing countries supports this hypothesis.
This systematic review highlighted important caveats for the comparison between ICU AKI epidemiology in developed and developing countries. The vast majority of studies assessed university tertiary hospitals, limiting the generalizability of the results. Different AKI definitions were used over time, and even when the new AKI criteria were used, there is an important lack of standardization for reference serum creatinine. Most of the studies from developing countries were single center. The number of patients and ICUs assessed in developed country studies was greater than 30-fold and 10-fold higher than in developing countries, respectively, highlighting the underrepresentation of developing countries.
Conclusion
AKI incidence was high in both types of countries. Patient characteristics were mostly similar, but outcomes were worse for patients in developing country studies. Despite patient´s similarities, the non-inclusion of secondary hospitals and the differences in the number of studies and sample sizes exemplify the challenge of comparing developing and developed country AKI epidemiology. The widespread application of AKI definitions has made it possible to compare AKI epidemiology across different settings. However, an effort to standardize reference serum creatinine, oliguria and the timeframe for AKI assessment is crucial. There is an urgent need for larger, prospective, multicenter studies that assess broader populations from developing countries.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the São Paulo Research Foundation, FAPESP (process number 2014/19286-4) and Fundação de Amparo à Pesquisa do Acre, FAPAC.
References
- 1.Magro MCDS Vattimo MDFF. Avaliação da função renal: creatinina e outros biomarcadores. Rev Bras Ter Intensiva. 2007;19: 182–185. [PubMed] [Google Scholar]
- 2.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114: 5–14. 10.1172/JCI22353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vieira JM, Castro I, Curvello-Neto A, Demarzo S, Caruso P, Pastore L, et al. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35: 184–191. 10.1097/01.CCM.0000249828.81705.65 [DOI] [PubMed] [Google Scholar]
- 4.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76: 422–427. 10.1038/ki.2009.159 [DOI] [PubMed] [Google Scholar]
- 5.Fischer MJ, Brimhall BB, Lezotte DC, Glazner JE, Parikh CR. Uncomplicated acute renal failure and hospital resource utilization: a retrospective multicenter analysis. Am J Kidney Dis. 2005;46: 1049–1057. 10.1053/j.ajkd.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30: 33–37. 10.1007/s00134-003-2078-3 [DOI] [PubMed] [Google Scholar]
- 7.Mehta RL, Burdmann EA, Cerda J, Feehally J, Finkelstein F, Garcia-Garcia G, et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet. 2016;387: 2017–2025. 10.1016/S0140-6736(16)30240-9 [DOI] [PubMed] [Google Scholar]
- 8.Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, et al. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10: 1324–1331. 10.2215/CJN.04360514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011; 2011 [Internet]. Available from: www.cochrane-handbook.org [cited March 2015]. [Google Scholar]
- 10.Thomson Reuters. Endnoteweb; 2016 [Internet]. Available from: http://endnote.com/ [cited March 2016].
- 11.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5: 52 10.1186/1756-0500-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlauer MV, Sauaia A, Moore E, Burlew C, Banerjee A, Johnson J. Acute kidney injury and posttrauma multiple organ failure: the canary in the coal mine. J Trauma Acute Care Surg. 2012;72: 373–378. 10.1097/TA.0b013e318244869b [DOI] [PubMed] [Google Scholar]
- 13.Han SS, Ahn SY, Ryu J, Baek SH, Chin HJ, Na KY, et al. Proteinuria and hematuria are associated with acute kidney injury and mortality in critically ill patients: a retrospective observational study. BMC Nephrol. 2014;15: 93 10.1186/1471-2369-15-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balushi F, Khan S, Riyami D, Ghilaini M, Farooqui M. Acute kidney injury in a teaching hospital in Oman. Saudi J Kidney Dis Transpl. 2011;22: 825–828. [PubMed] [Google Scholar]
- 15.Ralib AM, Nor MBM. Acute kidney injury in a Malaysian intensive care unit: assessment of incidence, risk factors, and outcome. J Crit Care. 2015;30: 636–642. 10.1016/j.jcrc.2015.01.018 [DOI] [PubMed] [Google Scholar]
- 16.Vanmassenhove J, Lameire N, Dhondt A, Vanholder R, Van Biesen W. Prognostic robustness of serum creatinine based AKI definitions in patients with sepsis: a prospective cohort study. BMC Nephrol. 2015;16:112 10.1186/s12882-015-0107-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos PR, Monteiro DLS. Acute kidney injury in an intensive care unit of a general hospital with emergency room specializing in trauma: an observational prospective study. BMC Nephrol. 2015;16: 30 10.1186/s12882-015-0026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Q, Zhang L, Ai Y, Zhang L. Epidemiology of acute kidney injury in intensive care septic patients based on the KDIGO guidelines. Chin Med J (Engl). 2014;127:1820–6. [PubMed] [Google Scholar]
- 19.Andrikos E, Tseke P, Balafa O, Cruz DN, Tsinta A, Androulaki M, et al. Epidemiology of acute renal failure in ICUs: a multi-center prospective study. Blood Purif. 2009;28: 239–244. 10.1159/000231986 [DOI] [PubMed] [Google Scholar]
- 20.Heegard KD, Stewart IJ, Cap AP, Sosnov JA, Kwan HK, Glass KR, et al. Early acute kidney injury in military casualties. J Trauma Acute Care Surg. 2015;78: 988–993. 10.1097/TA.0000000000000607 [DOI] [PubMed] [Google Scholar]
- 21.Maccariello E, Valente C, Nogueira L, Bonomo H, Ismael M, Machado JE, et al. SAPS 3 scores at the start of renal replacement therapy predict mortality in critically ill patients with acute kidney injury. Kidney Int. 2010;77: 51–56. 10.1038/ki.2009.385 [DOI] [PubMed] [Google Scholar]
- 22.Shinjo H, Sato W, Imai E, Kosugi T, Hayashi H, Nishimura K, et al. Comparison of kidney disease: improving global outcomes and acute kidney injury network criteria for assessing patients in intensive care units. Clin Exp Nephrol. 2014;18: 737–745. 10.1007/s10157-013-0915-4 [DOI] [PubMed] [Google Scholar]
- 23.Hoste EA, Kellum JA. Acute kidney dysfunction and the critically ill. Minerva Anestesiol. 2006;72: 133–143. [PubMed] [Google Scholar]
- 24.Daher Ede F, Junior Silva GB, Vieira AP, Souza JB, Falcao Fdos S, Costa CR, et al. Acute kidney injury in a tropical country: a cohort study of 253 patients in an infectious diseases intensive care unit. Rev Soc Bras Med Trop. 2014;47: 86–89. 10.1590/0037-8682-0223-2013 [DOI] [PubMed] [Google Scholar]
- 25.Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009;35: 1692–1702. 10.1007/s00134-009-1530-4 [DOI] [PubMed] [Google Scholar]
- 26.Clark E, Wald R, Levin A, Bouchard J, Adhikari NK, Hladunewich M, et al. Timing the initiation of renal replacement therapy for acute kidney injury in Canadian intensive care units: a multicentre observational study. Can J Anaesth. 2012;59:861–70. 10.1007/s12630-012-9750-4 [DOI] [PubMed] [Google Scholar]
- 27.Wahrhaftig Kde M, Correia LC, de Souza CA. RIFLE Classification: prospective analysis of the association with mortality in critical ill patients. J Bras Nefrol. 2012;34: 369–377. 10.5935/0101-2800.20120027 [DOI] [PubMed] [Google Scholar]
- 28.Alves CMP, Barros MC, Figueiredo PVT. Different approaches in the detection of acute renal dysfunction in serious patients. Rev. Soc. Bras. Clín. Méd. 2012; 10(3). [Google Scholar]
- 29.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204–212 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.KDIGO Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl. 2012; 2: 1–138. [Google Scholar]
- 32.Koeze Koeze J17, Keus F, Dieperink W, van der Horst IC, Zijlstra JG, van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017. February 20;18(1):70) 10.1186/s12882-017-0487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siev Siew ED15, Matheny ME. Choice of Reference Serum Creatinine in Defining Acute Kidney Injury. Nephron. 2015;131(2):107–12 10.1159/000439144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5: 1165–1173. 10.2215/CJN.08531109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerda J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3: 881–886. 10.2215/CJN.04961107 [DOI] [PubMed] [Google Scholar]
- 36.Sawhney S, Fraser SD. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Adv Chronic Kidney Dis. 2017;24:194–204. 10.1053/j.ackd.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607–625. 10.1038/s41581-018-0052-0 [DOI] [PubMed] [Google Scholar]
- 38.Bello AK, Alrukhaimi M, Ashuntantang GE, Bellorin-Font E, Benghanem Gharbi M, Braam B, et al. Global overview of health systems oversight and financing for kidney care. Kidney Int Suppl (2011). 2018;8(2):41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta RL, Cerda J, Burdmann EA, Tonelli M, Garcia-Garcia G, Jha V, et al. International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385: 2616–2643. 10.1016/S0140-6736(15)60126-X [DOI] [PubMed] [Google Scholar]
- 40.Burdmann EA, Jha V. Acute kidney injury due to tropical infectious diseases and animal venoms: a tale of 2 continents. Kidney Int. 2017;91: 1033–1046. 10.1016/j.kint.2016.09.051 [DOI] [PubMed] [Google Scholar]
- 41.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16: 3365–3370. 10.1681/ASN.2004090740 [DOI] [PubMed] [Google Scholar]
- 42.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant. 2008;23: 1970–1974. 10.1093/ndt/gfm908 [DOI] [PubMed] [Google Scholar]
- 43.Lombardi R, Rosa-Diez G, Ferreiro A, Greloni G, Yu L, Younes-Ibrahim M, et al. Acute kidney injury in Latin America: a view on renal replacement therapy resources. Nephrol Dial Transplant. 2014;29: 1369–1376. 10.1093/ndt/gfu078 [DOI] [PubMed] [Google Scholar]
- 44.Prin M, Wunsch H. International comparisons of intensive care: informing outcomes and improving standards. Curr Opin Crit Care. 2012;18: 700–706. 10.1097/MCC.0b013e32835914d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert R, Reignier J, Tournoux-Facon C, Boulain T, Lesieur O, Gissot V, et al. Refusal of intensive care unit admission due to a full unit: impact on mortality. Am J Respir Crit Care Med. 2012;185: 1081–1087. 10.1164/rccm.201104-0729OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.