Abstract
It has been hypothesized that stimulus-aligned brain rhythms reflect predictions about upcoming input. New research shows that these rhythms bias subsequent speech perception, in line with a mechanism of prediction.
There is hardly a term in cognitive neuroscience that encounters as much popularity and debate as ‘the predictive brain’. Indeed, according to some authors, the brain is not much more than a machine that constantly predicts the future [1,2], and our neural architecture seems to be designed for that purpose [3]. It is therefore not surprising that the neural substrates of these predictive processes are a central topic of investigation. A new study reported in this issue of Current Biology by Kösem et al. [4] provides important evidence that neural oscillations, rhythmic fluctuations in neural excitability [5], might represent such a substrate.
It has been shown repeatedly that a rhythmic stimulus, such as speech, evokes a brain response that is also rhythmic and follows the pattern of the input [6]. This alignment between brain response and stimulus input has been termed neural entrainment. It has been suggested that neural entrainment reflects a mechanism of prediction: the brain might align its oscillations so that their high-excitability phase coincides with, and amplifies, predicted important events [7]. Recently, however, it has become more and more evident that most studies might be affected by an important but unresolved issue. Presenting a rhythmic stimulus will inevitably result in brain activity that follows the rhythmic structure of the input — but this brain activity might exclusively consist of evoked neural responses that appear regular only because of the rhythmicity of the input [8]. As a consequence, observing a rhythmic brain response to rhythmic input is not enough to demonstrate ‘genuine’ neural entrainment — involving genuine oscillatory activity — and experimental support for the idea that neural entrainment reflects a mechanism of prediction remained restricted to indirect and sparse evidence [8].
One potential solution to this problem is to measure neural activity (and behaviour) after the rhythmic input. If entrained neural oscillations indeed reflect predictions about the upcoming input, they should persist for some time after the entraining stimulus; evoked neural responses, however, are relatively short and should disappear quickly after stimulus offset. Indeed, previous studies [9–11] have shown that both the probability of detecting a simple target, such as pure tones, and neural activity oscillates briefly after the offset of a rhythmic stimulus. However, evidence is rare, if present at all, that these rhythmic fluctuations in perception and neural activity are linked. Moreover, similar effects had not yet been demonstrated for speech as a stimulus; this is important, as neural entrainment plays a crucial role for current theories of speech perception, based on the finding that rhythmic brain responses to speech are enhanced if the latter is intelligible [12].
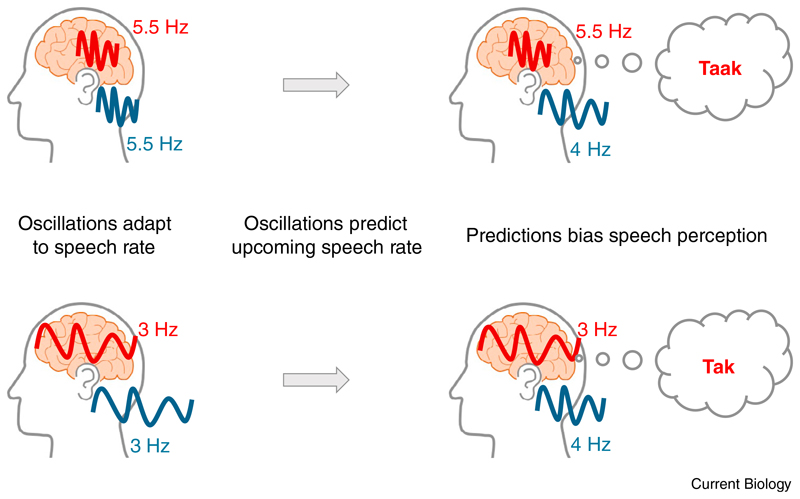
In their elegant new study, Kösem et al. [4] demonstrate that neural entrainment to speech indeed persists after the offset of the entraining speech stimulus, and that this carry-over entrainment has consequences for speech perception. Participants listened to speech sentences at two different rates (~3 Hz or ~5.5 Hz; Figure 1). As explained above, observing ‘oscillations’ with frequencies that differ between these two conditions would not be very surprising, as the difference in speech rate also leads to differences in (the frequency of) evoked neural responses that would inevitably be confounded with any measure of entrainment. The crucial finding, therefore, is what happened in a subsequent time window, in which participants were presented with speech at an intermediate rate in both conditions. According to the authors’ hypothesis (Figure 1), neural oscillations were still operating at the rate of the preceding speech (at 3 Hz or 5.5 Hz), reflecting predictions about the rate of upcoming speech.
Figure 1. Rhythmic predictions carried by neural oscillations.
It has been shown before that neural oscillations (red) align to the rhythm of speech (blue), roughly corresponding to the broadband speech envelope. Kösem et al. [4] demonstrate that these stimulus-aligned oscillations persist and bias both the frequency of subsequently recorded oscillatory activity and the perception of an ambiguous word. These oscillations therefore reflect predictions about the rhythm of upcoming speech.
Indeed, Kösem et al. [4] observed oscillatory activity at a frequency corresponding to the preceding speech rate (Figure 1). This finding is important as it demonstrates genuine entrainment to speech: even though the speech presented at an intermediate rate might have evoked neural responses, the rhythm of these responses would not correspond to the frequencies of interest (3 Hz or 5.5 Hz) and is therefore unlikely to have influenced the reported oscillatory activity. Finally, the intermediate speech also contained a target word which could adopt two different meanings, depending on the length of a vowel. Not only were participants more likely to perceive a longer vowel when the preceding speech rate was fast (Figure 1), but, crucially, this perceptual bias was correlated with the strength of the sustained entrainment during this time window. Thus, the authors also showed that the observed carry-over entrainment is correlated with behavioral outcomes of speech perception.
The study by Kösem et al. [4] answers some long-standing questions. For instance, we now have convincing evidence that the entrainment of genuine oscillatory activity reflects a predictive process that modulates speech perception. But the study also raises new questions which need to be answered in future work. For instance, speech is a complex stimulus and individual words or syllables are relatively long — potentially much longer than the high-excitability phase of an oscillation. The brain therefore needs to ‘decide’ which features of speech are important enough to be amplified and which can be ignored (those aligned with the high-excitability or low-excitability phase, respectively). This question cannot be answered from the current study, as speech perception is only inferred from the perceived length of a vowel. Thus, future studies need to reveal how neural entrainment modulates the intelligibility of complex sentences in which the (entrainment-induced) amplification of different speech segments contributes to speech comprehension.
Moreover, in natural language, speech rate varies continuously — indeed, a typical speech rhythm fluctuates between ~2 Hz and 8 Hz [13] — so that neural oscillations might need to adjust not only their phase but also their frequency. This was not observed by Kösem et al. [4]: Neural oscillations seem to operate at a frequency that corresponds to the rate of preceding speech, despite the presentation of speech at a different rate. This apparent inflexibility might be due to a relatively unpredictable change in speech rate, leading to a relatively long time required to adapt oscillatory processes to the new speech rate. In more natural scenarios, the brain might not be able to afford such a delay, as changes in speech rate occur more rapidly. It therefore seems likely that certain cues are necessary to predict upcoming changes in speech rate and adjust the frequency of oscillations accordingly. Although some progress has been made in this respect [14], it is still an open question what these cues are and how we can find them.
Kösem et al. [4] manipulated the frequency of entrained oscillations using different speech rates and demonstrated consequences for speech perception. This finding nicely complements previous studies providing evidence that neural entrainment is causally relevant for speech processing and comprehension. Whereas Kösem et al. [4] used sensory input for this experimental manipulation, previous studies applied transcranial alternating current stimulation (tACS) to alter the alignment between neural oscillations and speech rhythm. It was shown that neural responses to speech (measured as blood-oxygen level dependent, BOLD, response) depend on the phase relation between tACS and speech rhythm, but, strikingly, only when the speech is intelligible [15]. In line with these neural findings, two other studies [16,17] confirmed that tACS-induced manipulation of neural entrainment modulates speech comprehension. Nevertheless, an apparent entrainment of neural oscillations by tACS might be affected by similar issues as explained above: Does tACS really align genuine oscillatory activity or merely interact with evoked neural responses? In future studies, it would be interesting to apply the approach designed by Kösem et al. [4] in tACS studies. For instance, does speech perception depend on the frequency (or phase) of preceding tACS? Positive results would represent convincing and important evidence for a modulation of genuine neural oscillations.
To conclude, the study by Kösem et al. [4] represents an exciting change in direction in the field of neural entrainment: Not every rhythmic brain response reflects an oscillation; we need clever paradigms to isolate neural oscillations from other factors that can produce misleadingly similar waveforms. Only then can we demonstrate an important role of neural oscillations for (speech) perception and behaviour, and ultimately reveal one potential substrate of prediction.
References
- 1.Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36:181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- 2.Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 3.Singer Y, Teramoto Y, Willmore BD, King AJ, Schnupp JWH, Harper NS. Sensory cortex is optimised for prediction of future input. eLife. 2018;7:e31557. doi: 10.7554/eLife.31557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kösem A, Bosker HR, Takashima A, Meyer A, Jensen O, Hagoort P. Neural entrainment determines the words we hear. Curr Biol. 2018;28:2867–2875. doi: 10.1016/j.cub.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 6.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoefel B, ten Oever S, Sack AT. The involvement of endogenous neural oscillations in the processing of rhythmic input: more than a regular repetition of evoked neural responses. Front Neurosci. 2018;12 doi: 10.3389/fnins.2018.00095. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathewson KE, Prudhomme C, Fabiani M, Beck DM, Lleras A, Gratton G. Making waves in the stream of consciousness: entraining oscillations in EEG alpha and fluctuations in visual awareness with rhythmic visual stimulation. J Cogn Neurosci. 2012;24:2321–2333. doi: 10.1162/jocn_a_00288. [DOI] [PubMed] [Google Scholar]
- 10.Spaak E, de Lange FP, Jensen O. Local entrainment of α oscillations by visual stimuli causes cyclic modulation of perception. J Neurosci. 2014;34:3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickok G, Farahbod H, Saberi K. The rhythm of perception: entrainment to acoustic rhythms induces subsequent perceptual oscillation. Psychol Sci. 2015;26:1006–1013. doi: 10.1177/0956797615576533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peelle JE, Davis MH. Neural oscillations carry speech rhythm through to comprehension. Front Psychol. 2012;3:320. doi: 10.3389/fpsyg.2012.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding N, Patel AD, Chen L, Butler H, Luo C, Poeppel D. Temporal modulations in speech and music. Neurosci Biobehav Rev. 2017;81:181–187. doi: 10.1016/j.neubiorev.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Seifart F, Strunk J, Danielsen S, Hartmann I, Pakendorf B, Wichmann S, Witzlack-Makarevich A, de Jong NH, Bickel B. Nouns slow down speech across structurally and culturally diverse languages. Proc Natl Acad Sci USA. 2018;115:5720–5725. doi: 10.1073/pnas.1800708115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoefel B, Archer-Boyd A, Davis MH. Phase entrainment of brain oscillations causally modulates neural responses to intelligible speech. Curr Biol. 2018;28:401–408.e5. doi: 10.1016/j.cub.2017.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riecke L, Formisano E, Sorger B, Basxkent D, Gaudrain E. Neural entrainment to speech modulates speech intelligibility. Curr Biol. 2018;28:161–169.e5. doi: 10.1016/j.cub.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Wilsch A, Neuling T, Obleser J, Herrmann CS. Transcranial alternating current stimulation with speech envelopes modulates speech comprehension. NeuroImage. 2018;172:766–774. doi: 10.1016/j.neuroimage.2018.01.038. [DOI] [PubMed] [Google Scholar]