Highlights
-
•
5-month-old infants do not show selective cortical responses to affective touch.
-
•
Similar responses observed when infants are stroked with a hand or with a spoon.
-
•
Infants might need additional social cues to be able to identify affective touch.
Keywords: fNIRS, Infants, Affective touch, IFG, STS
Abstract
In adults, affective touch leads to widespread activation of cortical areas including posterior Superior Temporal Sulcus (pSTS) and Inferior Frontal Gyrus (IFG). Using functional Near Infrared Spectroscopy (fNIRS), we asked whether similar areas are activated in 5-month-old infants, by comparing affective to non-affective touch. We contrasted a human touch stroke to strokes performed with a cold metallic spoon. The hypothesis that adult-like activation of cortical areas would be seen only in response to the human touch stroke was not confirmed. Similar patterns of activation were seen in both conditions. We conclude that either the posterior STS and IFG have not yet developed selective responses to affective touch, or that additional social cues are needed to be able to identify this type of touch.
1. Introduction
The sense of touch is crucial for development, and touch deprivation early in life can be highly detrimental. Seminal studies with infant rhesus monkeys demonstrated that the absence of tactile contact, specifically with a soft surrogate mother (made of cloth, in contrast to a mother made of wire), led to impaired social interactions, reduced exploration of new environments and increased psychological stress (Harlow, 1958). For mice and rat pups the lack of a specific type of tactile stimulation, maternal licking and grooming, slows down growth and increases stress responses (for review see Meaney, 2001; Schanberg and Field, 1987). In humans, illuminating research on the role of early tactile contact took advantage of the periods of relative deprivation of contact experienced by very preterm infants. Implementing touch as a daily routine, in the form of massage therapy or skin-to skin contact, showed strikingly long-lasting effects on both physical growth and cognitive measures (Feldman et al., 2013; Field, 1998). A recent study reported that the beneficial effects of Kangaroo Mother Care (i.e. continuous skin-to-skin contact between the mother and the infant paired with exclusive breastfeeding) were still present at a twenty-year follow up (Charpak et al., 2016). Despite striking parallels between human and animal work, it is as yet un-known whether, in humans, beneficial effects are specific to certain kinds of touch, i.e. social vs. non-social touch. For this specificity to occur, infants should be able to discriminate social touch from the multitude of tactile experiences they encounter, just like they discriminate other social signals such as faces and voices, from the variety of visual and auditory stimulation they are exposed to (e.g. Lloyd-Fox et al., 2009; Farroni et al., 2013; Grossmann et al., 2010).
One way in which previous research assessed the discrimination of social and non-social stimulation has been by observing social selective responses in the infant brain. Functional Near Infrared Spectroscopy (fNIRS) has been central to charting the development of specialization to a variety of social stimuli, from early infancy. Notably, this research indicated two areas as consistently engaged for the processing of social stimuli, across modalities: the superior temporal and the inferior frontal cortices. The superior temporal sulcus (STS) runs along the temporal lobe and the banks of this sulcus have been associated with processing faces, voices and biological motion in adults (Deen et al., 2015). Recently, posterior areas around the sulcus have been described as a hub for multisensory integration (Beauchamp et al., 2008, Beauchamp et al., 2004; Dahl et al., 2009), with the suggestion that the close proximity of the STS to all sensory cortices has led to its recruitment for processing highly multimodal social information. Indeed, the STS and the inferior frontal cortex show early specialization for social stimulation, across modalities.
In the visual modality, the posterior STS-temporoparietal junction area (pSTS-TPJ: includes the posterior middle and superior temporal gyri, STS and TPJ) already shows social selectivity in newborn infants (Farroni et al., 2013) and selective responses to a wide range of social visual stimuli (i.e. eye gaze shifts, “Peek-a-boo”, static faces) are consistently reported in this area during early development (Grossmann et al., 2008; Lloyd-Fox et al., 2009, Lloyd-Fox et al., 2011; Biondi et al., 2016; Otsuka et al., 2007). Specialisation to social stimuli has also been observed in some of these studies (Lloyd-Fox et al., 2009, Lloyd-Fox et al., 2011) in the inferior frontal gyrus (IFG). In the auditory domain, while social selectivity has also been reported, it may specialise later in development. Interestingly, the pSTS-TPJ area exhibits non-vocal (i.e. water, bells, rattles) selective responses during the first few months of life before shifting to selectivity for human vocal sounds between 4 and 7 months of age over more anterior STS regions (Blasi et al., 2011; Grossmann et al., 2010; Lloyd-Fox et al., 2011, Lloyd-Fox et al., 2012, Lloyd-Fox et al., 2016), in line with the areas of vocal selectivity seen in adults (Belin et al., 2000).
Despite some consistency in social responsivity across modalities in recent research, this short review highlights obvious differences in developmental trajectories of cortical specialisation. pSTS-TPJ selective responses to visual social stimuli emerge shortly after birth. In contrast, adult-like selectivity to the human voice develops over the first months of life in anterior parts of the STS region, close to auditory sensory cortices. Furthermore, regions around the pSTS show selective activation to non-human sounds over this same period before subsiding, to be replaced by more general responses to auditory stimulation at later ages.
In contrast to the abundant evidence from the visual and auditory domains, only a few studies to date have investigated social selectivity in the tactile domain. Work on social touch has been heavily influenced by the discovery of a particular type of non-myelinated fiber in the hairy human skin - C-Tactile fibers (CT) (Vallbo et al., 1999; Johansson and Vallbo, 1979) - which optimally respond to caress-like touch, i.e. to slow velocity (3–10 cm/s) stroking, with a soft textured instrument (be it a hand or brush), at average skin temperature (Ackerley et al., 2014; Olausson et al., 2010). It was therefore proposed that the CT system encodes affective properties of social touch. Henceforth’ affective touch' will refer to a tactile stimulation optimal for eliciting CT fibers activation. Two studies investigating touch in infancy used a two-channel NIRS system to record responses over the anterior prefrontal cortex. In a study of newborns, an increased bilateral prefrontal response was measured when 3 cm/s strokes were applied with cotton to the forearm or when plastic was applied to the cheek, as compared to stimulation with wood, a rougher material (Saito, 2009). In contrast, a study of 3, 6 and 10 month olds, measuring prefrontal responses to stroking of the palm of the hand, only observed significantly increased responses to velvet (relative to stroking with wood) at 10 months of age (Kida and Shinohara, 2013). A recent study which also used fNIRS, but measured activation over the left somatosensory and right posterior temporal cortices, found no discriminatory response between affective and non-affective touches (slow brush stroking vs. static touch applied with a block of wood) in these regions in 7 month old infants (Miguel et al., 2017). However, a recent study with 2-month old infants, which contrasted the speed of stroking, showed increased temporal lobe responses to slow compared to fast stroking in the left middle temporal gyrus extending into STS (Jönsson et al., 2017).
Therefore, the pattern of responses observed from previous research using different textured stimuli, or tactile stimuli applied at different speeds, has not illuminated a clear developmental pathway of specialisation. In contrast, fNIRS and fMRI studies of affective touch in adulthood found consistent patterns of activation in IFG and pSTS (Bennett et al., 2014; Gordon et al., 2013; Voos et al., 2013), but see (Davidovic et al., 2016). Furthermore, a study that looked at the development of these responses from childhood to adulthood found that a region of the middle temporal gyrus (MTG) extending into the pSTS was activated by affective touch as early as 5 years of age, with frontal areas only consistently activated in adulthood (Bjornsdotter et al., 2014).
Given the limited evidence from early development, we set out to clarify the involvement of STS and IFG in social selectivity to touch during early infancy. We aimed to build on previous work in two ways. First, in some studies (Saito, 2009; Kida and Shinohara, 2013) measurements were restricted to a confined region of the anterior prefrontal cortex, or to only the right (Miguel et al., 2017) or the left STS region (Jönsson et al., 2017), which means that inferior frontal and posterior-temporal responses in infants have not been extensively investigated. Second, tactile stimulation in Kida and Shinohara, (2013) was delivered to the palm of the hand, a region that lacks CT fibers (Johansson and Vallbo, 1979; Johnson et al., 2000, Vallbo et al., 1999; Wessberg et al., 2003; Löken et al., 2009). Third, the presentation of touch during these studies was usually concurrent with the infant being embraced or held by their caregiver, with the caregiver and the experimenter administering the touch stimulus within their field of view (i.e. Jönsson et al., 2017). Therefore, in the present study, we delivered stimuli to the upper arm and recorded responses from the inferior frontal and the posterior temporal cortex over both hemispheres. Since we were interested in characterizing the response to the affective touch in isolation of other social cues, we ensured that the infants did not see who was performing the simulation. Furthermore, infants were placed in an infant carrier, on parents’ lap and parents were asked to refrain from touching the infant during the study.
We contrasted responses to affective and non-affective touch, compared to a no tactile stimulation baseline. The affective touch was delivered by a human hand at CT-targeted velocity. We contrasted this with a non-affective stimulus, which was performed at the same speed but with a metal spoon; this was designed to differ from the social affective touch in temperature, the spoon being at room temperature. Recent research had shown that CT firing and pleasantness ratings decreased when tactile stimulation was applied at 18 °C (room temperature) compared to human skin temperature (32 °C; Ackerley et al., 2014). It was suggested that temperature may be one of the key properties of human touch, ensuring thermoregulation early in life when infants themselves poorly regulate their body temperature (Morrison, 2016a). In this way, we sought to tease apart the relative contribution that this factor may have on the social affective response previously observed by manipulating the form of touch in other dimensions. Therefore, we hypothesised that affective touch as delivered through stroking with the hand would lead to increased activation in the pSTS-TPJ region and in IFG, relative to the control stimulation. We chose to investigate these responses at a similar age (5–6 months) to when previous research has shown socially selective responses in the visual and auditory domains. Stronger activation for affective versus non-affective touch in these areas, would allow us to infer that cortical specialization to the affective components of touch is also present in early infancy.
2. Methods
2.1. Participants
Twenty-one five-month-old infants participated in this study (8 female, mean age = 160.19 days, SD = 13.91). A further 8 infants participated but were excluded from the study owing to an insufficient number of valid trials based on behavioural coding (4) or a high level of rejected data due to motion artifact (4). All infants were born full term (37–42 weeks’ gestation) and with normal birth weight (>2500 g). This attrition rate is within the standard range for infant fNIRS studies (see review by Lloyd-Fox et al., 2010). All parents gave written informed consent before the study and the ethics committee at Birkbeck, University of London, approved the study design.
2.2. Stimuli and design
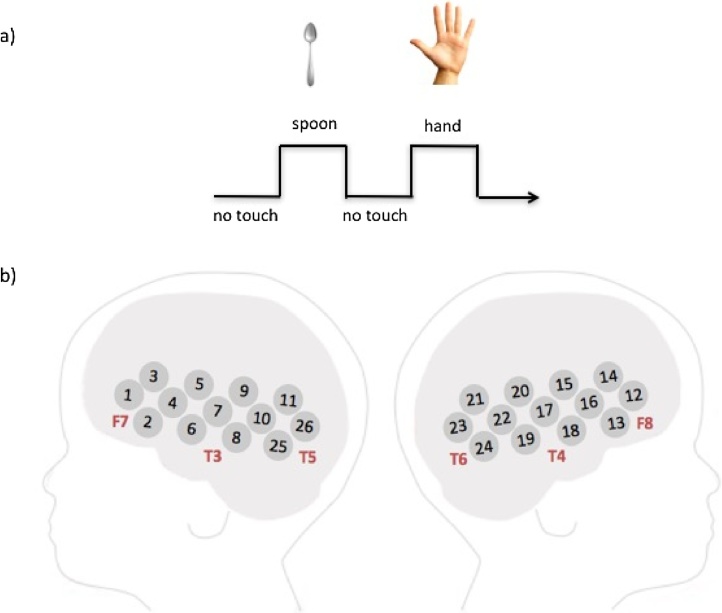
Each stimulus trial was 10 s long (Fig. 1a). The affective touch condition consisted of a gentle stroke, in the velocity range of 3–10 cm/s, performed by the experimenter on the baby’s upper arm, with repeated stroking applied horizontally from the inner arm across to the outer arm. To time the presentation of the stimuli, the experimenter listened to audio cues played in headphones which indicated the beginning and the end of each trial. Each stimulus trial consisted on average of 5 strokes (1 stroke every two seconds), given that the upper arm of the infants in our sample had a length of 10 cm and we administered a stroke velocity which allowed us to cover this length of skin in 2 s. Since infants’ unpredictable movements can induce alterations to this speed (if they move their arm during stimulation) the stroke could vary in speed. Offline coding confirmed that the range of 3–10 cm/s (which is the range in which CT fibers are reported to fire optimally), was not exceeded for any participant as the maximum number of strokes in 10 s was never larger than 6. If the experimenter was halfway through a stroke when the end of the trial was signaled she would complete it, which could add an additional one-two seconds to the duration of the stimulation. In the non-affective touch condition, the arm was stroked by using the back of a spoon at the same speed. Following each 10 s trial there was a period of no-touch baseline which lasted 10 s. Half of the participants received stimulation on the right arm, the other half on the left arm. The order of presentation of the stimuli (hand/spoon) was counterbalanced across participants, with half of the participants receiving the hand stimulation on the first trial, and half the spoon stimulation, with trials alternating in an ABAB sequence thereafter. During the procedure participants watched a colorful screensaver accompanied by music, to avoid them orienting to the tactile stimulation.
Fig. 1.
a) Experimental design: the stroking was performed using a spoon or a hand; experimental and baseline periods were 10 s long. b) A schematic showing the location of the channels relative to the 10–20 coordinates.
2.3. Apparatus
Infants wore custom-built CBCD NIRS headgear (http://cbcd.bbk.ac.uk/node/165) consisting of two source-detector arrays containing a total of 26 channels (source-detector separations: 20 mm). The arrays were placed over both hemispheres and covered the inferior frontal - temporal lobes (see Fig. 1b). Data was collected with the UCL NIRS system (NTS2; Everdell et al., 2005). This system used 2 continuous wavelengths of source light at 770 and 850 nm. Before the infants began the study, measurements of their head circumference, ear to ear lateral semi circumference, and nasion to inion were taken, and the location of the channels and arrays relative to these anatomical landmarks were recorded (Lloyd-Fox et al., 2014). Measurements from this group of infants showed that the average head circumference was 43.16 cm (SD = 1.81).
2.4. Procedure
The infants were held on their parent’s lap, secured in a baby carrier, and facing outwards towards a 117-cm plasma screen. We chose to use the baby carrier in order to reduce the amount of tactile contact between the parent and the baby, thus isolating the touch delivered by the experimenter. The parent was asked to place their hands on the carrier rather than their infant, and refrain from interacting during the stimuli presentation unless the infant became fussy or sought their attention. The experimenter stood behind the parent and the infant, and delivered the tactile stimulations on the baby’s arm, being careful to remain out of the baby’s sight. Events (trial onset and offset) were marked on-line by a second experimenter observing the first experimenter on a computer monitor. The experiment ended when the infants became fussy. Each session was recorded using a video camera placed just below the screen, and infant behaviour was coded offline.
2.5. Data processing and analysis
The fNIRS system measured changes in the amount of light that was emitted from the sources, and detected by neighbouring detectors. These changes in light attenuation were used to calculate changes in oxy– (HbO2) and deoxy–haemoglobin (HHb) chromophore concentration (μMol) which are haemodynamic indicators of neural activity (Obrig and Villringer, 2003). Prior to conversion to concentration data, the attenuation measurements for each infant were analysed and channels were rejected from further analysis based on the quality of the intensity signals, using artifact detection algorithms (Lloyd-Fox et al., 2010, Lloyd-Fox et al., 2009). In line with previous work, channels were excluded if the coefficient of variation of the attenuation exceeded 10% or if the normalized power was larger than 50% with respect to the total power (Lloyd-Fox et al., 2009). The attenuation signal was low-pass filtered using a cut-off frequency of 1.7 Hz. Following this, the data was segmented into blocks of 24 s of data consisting of 4 s of the baseline prior to the onset of the tactile stimulation, 10 s of tactile stimulation, plus the following 10 s’ baseline. Each block of attenuation data was de-trended with a linear fit between the average of the first and the average of the last 4 s to remove drifts in the signal. The attenuation data was then converted into changes in concentration (μMol) in HbO2 and HHb using the modified Beer–Lambert law (Delpy et al., 1988) and assuming a differential path length factor for infancy (5.13; based on Duncan et al., 1995). Following this, trials were assessed both with motion detection algorithms and offline coding of infant behaviour. Trials were firstly removed if during the 4 s’ baseline prior to the onset of the stimulus trial there were concentration changes greater than +/- 3 μMol, and if during the stimulus trial itself changes exceeded +/- 5 μMol (these thresholds were set at different levels to take into account changes in haemoglobin levels caused by activation during stimulation). In addition, experimental trials were removed following offline coding of infant behaviour. A trial was removed if: a) the infant moved to a degree that it prevented the experimenter from completing a sufficient number of strokes b) the infant turned to look either at the parent or the experimenter c) the parent interfered by either talking to or touching the infant. Not looking at the screen did not constitute a criterion for exclusion. Across the whole group, we rejected individual data on only three occasions because of an infant’s movement, and on one occasion because of parent interference. Further details of the number of presented and valid trials, for those infants included in analysis, can be found in Table 1. For each infant, a channel was included in the statistical analysis if it contained at least three valid artifact-free trials per condition. It follows that at the group level not all infants contributed data to each channel. In addition, to include an infant in the final dataset a minimum of two thirds of the channels within the arrays were required to have valid data (i.e. not rejected during artifact detection algorithms).
Table 1.
Participants’ information. The number of valid trials refers to the number of trials included in the analysis after off-line coding of the infant’s behavior during the study. The first number refers to the mean value across the group and the bracketed number refers to the standard deviation.
| experiment 1 | |
|---|---|
| n | 21 |
| age (days) | 160.19(13.91) |
| female/male | 8:13 |
| head circumference (cm) | 43.16(1.81) |
| number of trials completed | 10.87 (1.90) |
| valid trials | 10.61 (2.01) |
| valid trials in affective touch condition | 5.23 (1.09) |
| valid trials in non-affective touch condition | 5.38 (0.97) |
| number of rejected channels per infant | 0 |
Valid trials for each experimental condition (affective touch, non-affective touch) were averaged together for each infant, and a time course of the mean change in HbO2 and HHb concentration changes was compiled for each channel. A baseline of 1 s of data pre-stimulus onset was subtracted from the signal. Two time windows were selected for analysis, between 1 and 5 s and between 5 and 9 s post-stimulus onset. These periods of time were selected to include the range of maximum concentration changes observed across infants for HbO2 and HHb. Either a significant increase in HbO2 concentration from baseline or a significant decrease in HHb is commonly accepted as an indicator of cortical activation in infant work (Lloyd-Fox et al., 2010).
A preliminary channel-by-channel analysis was run to identify those channels that responded to touch, irrespective of condition. This was achieved by comparing the response to the experimental trials to the pre-stimulus signal across all infants, using the valid data for each channel. Statistical comparisons (two tailed t-tests) were performed, to compare the maximum signal change during the specified experimental trial time windows, with the averaged pre-stimulus signal (4 s pre-onset). To account for errors due to multiple comparisons, p-values were corrected using a MATLAB false discovery rate (FDR) function (Benajmini and Hochberg, 1995).
Channels that survived FDR corrections together with the homologous channel in the opposite hemisphere were analysed using linear mixed models (LMM) to account for side of stimulation and hemispheric effects. For each pair of channels, a linear mixed model was run, with hemisphere (right, left), stimulus (hand, spoon) and time-window (1–5 s, 5–9 s) as repeated measures factors, and side of stimulation (right, left) as between-subjects factor. We chose to use the LMM approach because of missing values occurring due to subjects not contributing data to some of the channels. LMMs use maximum likelihood estimation to handle missing values as compared to standard factorial analysis, where any subject not contributing data to all channels would be excluded from the analysis.
3. Results
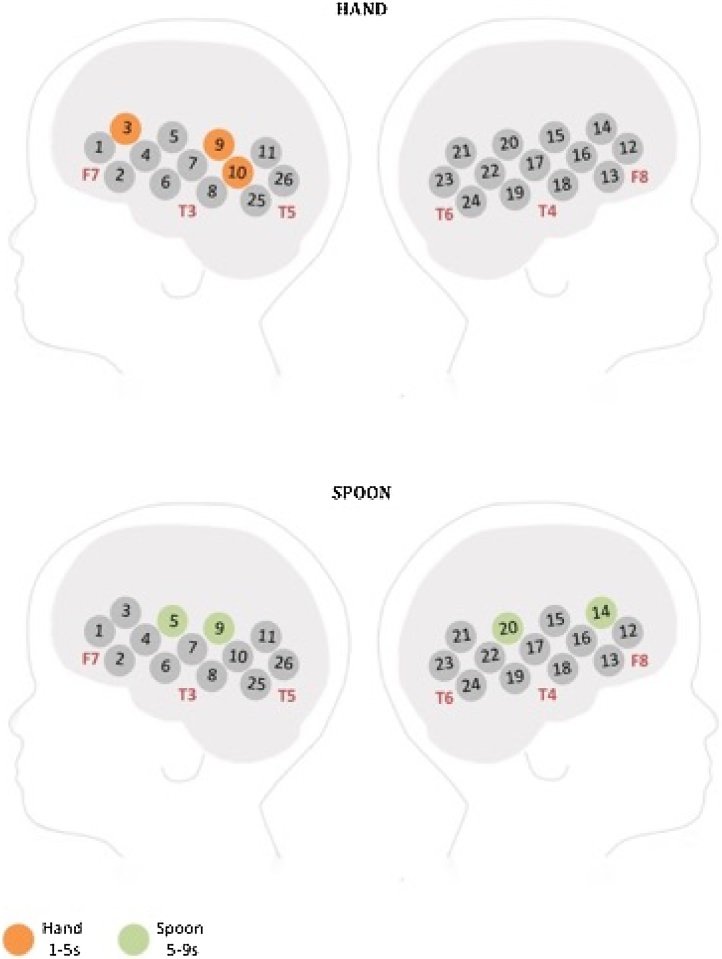
In an initial channel-by-channel analysis of the fNIRS data, t-tests compared the averaged hemodynamic peak changes in HbO2 and HHb (during the time windows of activation described in the methods) evoked by the hand and spoon conditions to a baseline consisting of the 4 s preceding the stimulus, in which no touch was applied. Here we report only the channels that showed significant increases in HbO2; HHb results can be found in the supplementary materials (SOM, Fig. 1). The hand condition revealed significant increases in HbO2 in three channels (ch. 3, 9, 10) in the left hemisphere, in the first time-window post stimulus onset (1–5 s), while the spoon condition revealed significant increases in HbO2 in four channels (ch. 5, 9, 20, 14) bilaterally in the second time-window post stimulus onset (5–9 s) (see Fig. 2). All channels reported here survived FDR corrections (see Table 2 for a complete list of channels). It is worth noting that whilst no channels survived FDR corrections for the hand condition in the second time-window (from 5 to 9 s), six channels showed an uncorrected significant (p < 0.05) increase in response to the hand relative to baseline, including channel 3 and 9 (see Table 2).
Fig. 2.
A schematic view of the NIRS arrays showing HbO2 responses to the hand (top panel) and to the spoon (bottom panel). Channels marked in bright orange revealed a significant response in the 1–5 s time-window to the hand versus baseline. Channels marked in pale green revealed a significant response in the 5–9 s time-window to the spoon versus baseline.
Table 2.
Significant activations from baseline in Hand and Spoon conditions. * indicates that the response survived the false discovery rate (FDR) correction.
| Hand > Baseline | Spoon > Baseline | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ch | HbO2/HHb | TW | t | p | df | d | Ch | HbO2/HHb | TW | t | p | df | d |
| 1 | HbO2 | 1-5 | 2.45 | 0.025 | 18 | 0.53 | 3 | HbO2 | 1-5 | 2.13 | 0.048 | 18 | 0.46 |
| 3* | HbO2 | 1-5 | 3.07 | 0.007 | 18 | 0.67 | 3 | HbO2 | 5-9 | 2.17 | 0.044 | 18 | 0.47 |
| 3 | HbO2 | 5-9 | 2.32 | 0.032 | 19 | 0.51 | 5 | HbO2 | 1-5 | 3.08 | 0.006 | 18 | 0.67 |
| 5 | HbO2 | 1-5 | 2.33 | 0.031 | 19 | 0.51 | 5* | HbO2 | 5-9 | 3.74 | 0.002 | 18 | 0.82 |
| 5 | HbO2 | 5-9 | 3.53 | 0.002 | 19 | 0.77 | 9 | HbO2 | 1-5 | 2.74 | 0.013 | 20 | 0.60 |
| 9* | HbO2 | 1-5 | 3.42 | 0.003 | 19 | 0.75 | 9* | HbO2 | 5-9 | 4.09 | 0.001 | 20 | 0.89 |
| 9 | HbO2 | 5-9 | 2.67 | 0.015 | 19 | 0.58 | 11 | HbO2 | 5-9 | 2.69 | 0.014 | 19 | 0.59 |
| 10* | HbO2 | 1-5 | 3.62 | 0.002 | 20 | 0.79 | 12 | HbO2 | 5-9 | 2.29 | 0.033 | 20 | 0.47 |
| 11 | HbO2 | 5-9 | 2.18 | 0.041 | 20 | 0.48 | 14 | HbO2 | 1-5 | 2.45 | 0.023 | 20 | 0.67 |
| 15 | HbO2 | 1-5 | 2.38 | 0.028 | 20 | 0.52 | 14* | HbO2 | 5-9 | 3 | 0.007 | 20 | 0.82 |
| 16 | HbO2 | 5-9 | 2.09 | 0.05 | 20 | 0.46 | 15 | HbO2 | 5-9 | 2.38 | 0.027 | 20 | 0.60 |
| 19 | HbO2 | 5-9 | 2.09 | 0.049 | 20 | 0.46 | 20 | HbO2 | 1-5 | 2.83 | 0.01 | 20 | 0.89 |
| 20 | HbO2 | 1-5 | 2.74 | 0.013 | 20 | 0.60 | 20* | HbO2 | 5-9 | 3.7 | 0.001 | 20 | 0.59 |
| 22 | HbO2 | 1-5 | 2.31 | 0.032 | 20 | 0.50 | 21 | HbO2 | 5-9 | 2.83 | 0.01 | 20 | 0.50 |
| 14 | HHb | 1-5 | −2.4 | 0.026 | 20 | −0.52 | 26 | HbO2 | 1-5 | 2.13 | 0.046 | 20 | 0.54 |
| 14 | HHb | 5-9 | −3.3 | 0.004 | 20 | −0.72 | 3* | HHb | 5-9 | −3.01 | 0.008 | 18 | 0.66 |
| 15 | HHb | 5-9 | −2.65 | 0.016 | 20 | −0.58 | 9 | HHb | 5-9 | −2.33 | 0.03 | 20 | 0.52 |
| 21 | HHb | 5-9 | −2.36 | 0.028 | 20 | −0.52 | 10* | HHb | 5-9 | −4.07 | 0.001 | 20 | 0.62 |
| 11 | HHb | 5-9 | −2.26 | 0.036 | 19 | 0.81 | |||||||
| 12 | HHb | 5-9 | −2.4 | 0.026 | 20 | 0.62 | |||||||
| 14* | HHb | 5-9 | −4.73 | <0.001 | 20 | 0.46 | |||||||
| 15* | HHb | 5-9 | −3.26 | 0.004 | 20 | −0.66 | |||||||
| 16* | HHb | 5-9 | −2.65 | 0.015 | 20 | −0.51 | |||||||
| 19 | HHb | 5-9 | −2.23 | 0.038 | 20 | −0.89 | |||||||
| 21* | HHb | 5-9 | −3.09 | 0.006 | 20 | −0.49 | |||||||
| 22* | HHb | 5-9 | −2.92 | 0.008 | 20 | −0.52 | |||||||
| 23 | HHb | 5-9 | −2.42 | 0.025 | 20 | −1.03 | |||||||
| 24* | HHb | 5-9 | −2.85 | 0.01 | 19 | −0.58 | |||||||
We used the standardized scalp surface map of fNIRS channel coordinates within the frontal and temporal lobes specific to 4–7 months old infants (Lloyd-Fox et al., 2014), to identify the most likely cortical regions generating the observed effects in the FDR corrected channels. Channels in which hand elicited a response (versus baseline) are positioned approximately over regions of the left IFG (ch.3) and left pSTS-TPJ (ch. 9, 10), while spoon touch elicited a bilateral response overlaying regions of the right IFG (ch. 14), bilateral pSTS-TPJ (ch. 9, 20) and left precentral gyrus (ch. 5).
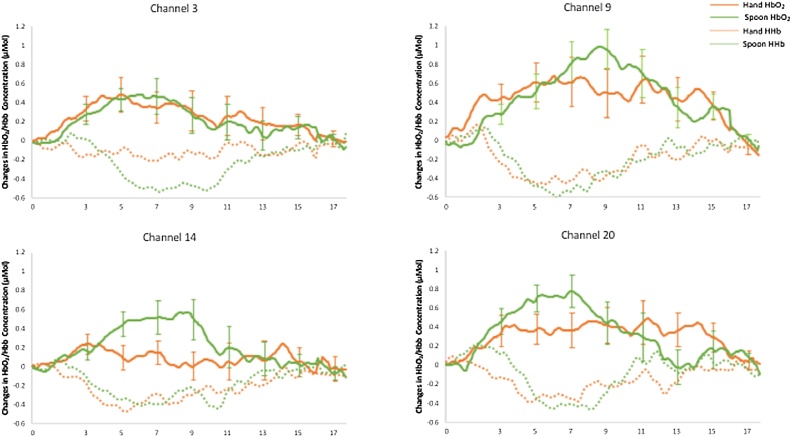
To investigate hemispheric differences between the two conditions, each of the channels showing significant HbO2 response to either stimuli was paired with the homologous channel in the opposite hemisphere, resulting in four pairs: pair1(ch. 14 and 3), pair2 (ch. 20 and 9), pair3(ch. 15 and 5), pair4 (ch. 22 and 10), and analysed using LMMs.
For pair1 (IFG) we found a main effect of hemisphere (F(1, 20.951) = 4.926, p = 0.037), with greater activation in the left (M = .640, SE = .137) compared to the right hemisphere (M = .360, SE = .102), and a significant interaction between hemisphere and stimulus (F(1, 21.182) = 5.199, p = .033). Post-hoc t-test revealed that the hand elicited a response in the left but not in the right hemisphere (t = 2.068, p = .053) while there were no hemispheric differences for the response to the spoon, (t = .383, p = .706) (for the time courses of these channels see Fig. 3, left panel). Neither of the other two factors included in the analysis, time-window and side of stimulation, did yield to significant effects (time-window: F(1, 74.797) = .001, p = .889; side of stimulation: F(1,24.498) = .016, p = .91).
Fig. 3.
Grand averages of haemodynamic time courses within channels that showed significant responses, and are centered within two key areas known to respond to affective touch: IFG (Ch. 3 left and Ch. 14 right) and pSTS-TPJ (Ch. 9 left and Ch. 20 right). Error bars represent standard error.
For pair2 (pSTS-TPJ), we found a significant interaction between hemisphere and stimulus (F(1, 34.994) = 6.639, p = .014) with both stimuli eliciting responses in both hemispheres but the hand activated the right hemisphere to a lesser degree than the left. However, this hemispheric difference, which can be observed in Fig. 3 (right panel), did not reach statistical significance (t = 1.46, p = .160). Also in this analysis, neither time-window (F(1, 52.270) = 1.242, p = .270) nor side of stimulation (F(1, 20.901) = .471, p = .5) yielded to significant effects.
Analysis of the remaining two pairs yielded no main effects nor significant interactions (p > .2).
4. Discussion
The aim of the present study was to investigate the development of responses to affective touch in regions of the frontal and temporal cortex in infancy: specifically, we aimed to investigate whether infants exhibited selective cortical responses to the processing of affective components of tactile stimulation by five months of age. Using fNIRS, we focussed on two regions of the cortex known to be selective to visual and auditory social stimuli in infancy - and that have been shown to activate in recent affective touch studies in infancy and adulthood (Gordon et al., 2013; Jönsson et al., 2017; Voos et al., 2013; for a recent meta-analysis see Morrison, 2016a, Morrison, 2016b) - the inferior frontal and posterior superior temporal cortex.
Our choice of stimulus contrast was informed by research suggesting CT-fibers, present in human hairy skin, mediate the perception of affective touch. We hypothesised that the human hand (affective touch stimulus) will generate increased responses in regions of the pSTS-TPJ and IFG, compared to stroking with a metallic spoon, a stimulus with sub-optimal temperature (Ackerley et al., 2014). Contrary to our prediction, we found that both the hand and the spoon stimulation elicited a significant cortical response relative to baseline over these regions.
Exploratory analyses (channel-by-channel t-tests) revealed differences in the latency of the peak response, with only the response to the hand differing from baseline in the early time window; however this was not a significant factor in the main linear mixed model analyses. Rather, an interaction between hemisphere and stimulus was observed. The non-affective stimulus (spoon) elicited IFG and pSTS-TPJ responses bilaterally, while responses to the hand were left lateralized in the inferior frontal (minimal responses observed over right IFG) and posterior temporal regions (with a reduced response observed over right pSTS-TPJ). Note that this hemispheric difference was not a main driver of the results as it was found to have borderline significance. This more localized response to the hand, if replicated, could indicate that at this age specialization and localization of cortical processing of affective touch are ongoing.
The only other two studies to date that measured posterior temporal cortex activation to affective and non-affective touch support our findings. In line with our results, they reported differential activation to affective versus non-affective touch in the left (Jönsson et al., 2017) but not the right hemisphere (Miguel et al., 2017). However, it is hard to draw firm conclusions regarding lateralization from these findings as both studies restricted measurement to one hemisphere. Also, direct comparison of hand and spoon stimulation in our study, did not reveal statistically significance differences in either hemisphere. Thus, although some trend differences were observed between the two stimuli, they remain to be confirmed.
What could explain the differences between our findings and those of Jönsson and colleagues? One difference lies in the nature of the contrast investigated, as Jönsson and colleagues compared slow and fast velocity stroking. It is possible that while cortical specialization to touch velocity is already evident shortly after birth, sensitivity to human body temperature may take more time to develop. Texture, another critical aspect of affective touch, also shows protracted cortical specialisation. Kida and Shinohara (2013) showed increased responses to pleasant touch, over the anterior prefrontal cortex in 10-month-olds, but not in 3 and 6 months-old infants. In the auditory domain as well, selective responses to voice stimuli develop between 4–7 months of age, with non-voice selective responses still present in some infants at 7 months (Grossmann et al., 2010; Blasi et al., 2011; Lloyd-Fox et al., 2012). Moreover, late specialisation of the temporal lobe has already been reported for other aspects of social perception, such as increased specificity for biological motion in the STS and face selectivity in the ventral visual cortex (e.g Carter and Pelphrey, 2006; Scherf et al., 2007).
What may contribute to these differential patterns of social specialization seen within, and across, different sensory modalities? One explanation is that during early development infants are more likely to show cortical specialisation to social stimuli in the presence of multi-modal stimulation. In the study by Jönsson and colleagues, the infants were held in their parents arms and concurrent visual cues were present as they were able to observe the person and their stroking action. This contrasts with the experimental setup of the current study, where we intentionally removed other cues so as to investigate the unique contribtion that temperature has, in the processing of affective touch. In previous fNIRS research investigating temporal lobe activation to communicative cues in a similar live setting, we found higher activation when a combination of visual and auditory ostensive singals were used (Lloyd-Fox et al., 2015). One possibility is that specialization to individual components of social stimuli develops slowly and is facilitated by exposure to multi-modal input. Auditory or tactile stimuli might need to be experienced in conjunction with their visual manifestation (i.e. someone talking to or caressing the child), for enhanced responses to be evident in pSTS, a region described as a multi-modal hub. It may also be that, at least as specialization develops in childhood, the presence of multi-modal information is necessary for selective responses to be observed in experimental situations. Selective responses have been observed in adults to isolated presentation of affective touch (Gordon et al., 2013; Voos et al., 2013). However, it is prescient that this response is highly sensitive to top-down cognitive factors (e.g. who is providing the touch; for a review see Ellingsen et al., 2016).
4.1. Limitations
An inherent limitation to using fNIRS is the fact that we could only measure responses to touch from the surface of the cortex. Therefore we don’t know whether the posterior insula, a subcortical region involved in processing affective touch in children, adolescents and adults, (Bjornsdotter et al., 2014; Olausson et al., 2010) is selective to affective touch in 5-months-old infants. In infancy, insular activation in response to slow stroking was recently reported both in newborns using fMRI (Tuulari et al., 2017) and in 2-month-old infants using diffuse optical tomography (DOT) (Jönsson et al., 2017). Therefore, it’s possible that a discriminatory response was present at the depth of the insula, but that the technique used for the present study did not allow us to measure it.
Another limitation concerns the degree of control we had over the delivery of the tactile stimulation. Notably, pressure applied through the hand during the affective touch condition might have been different from pressure applied with the spoon. Even though we strived to maintain pressure as consistent as possible across stimuli via gentle application and by checking for deeper skin indentation as a consequence of more pressure, slight differences may have still occurred. Pressure is easy to control when applying stimulation mechanically (Löken et al., 2009; Olausson et al., 2002), but much more difficult to control when using naturalistic stimulation, such as when using the prototypical affective touch stroking with the human hand. We note, however, that increased pressure was previously shown to elicit stronger responses in the somatosensory system, but not in areas that encoded the pleasantness of the stimuli (Francis et al., 1999).
5. Conclusion
We aimed to identify a neural signature of affective touch (defined here as CT-targeted touch) in 5-month-old infants, by contrasting it with a non-CT-optimal tactile stimulus, differing in temperature. However, in contrast with our hypothesis, we found no increased responses for affective versus non-affective touch in pSTS-TPJ or IFG regions. Further studies will aim to clarify whether selectivity of cortical responses emerges later in development, or whether a multi-modal social context is required in order to elicit differential responses at this young age.
Conflict of Interest
None.
Acknowledgements
We are very grateful to the enormous contributions that the families have made to this study. The research was supported by The UK Medical Research Council (G0701484, MR/K021389/1) and the European Project TRIGGER (Transforming Institutions by Gendering Contents and Gaining Equality in Research), Grant agreement no. 661034. LP was supported by the Glover Family Award. We thank Dr. Anna Blasi for her constant and invaluable help with the in-house MATLAb programs used in the present work to perform analysis on the fNIRS data.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.06.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ackerley R., Backlund Wasling H., Liljencrantz J., Olausson H., Johnson R.D., Wessberg J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J. Neurosci. 2014;34:2879–2883. doi: 10.1523/JNEUROSCI.2847-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp M.S., Argall B.D., Bodurka J., Duyn J.H., Martin A. Unraveling multisensory integration : patchy organization within human STS multisensory cortex. Nat. Neurosci. 2004;7:1190–1192. doi: 10.1038/nn1333. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S., Yasar N.E., Frye R.E., Ro T. Touch, sound and vision in human superior temporal sulcus. Neuroimage. 2008;41:1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P., Zatorre R.J., Lafaille P., Ahad P., Pike B. Voice selective areas in human auditory cortex. Nature. 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Benajmini Y., Hochberg Y. Controlling the false discovery rate : a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- Bennett R.H., Bolling D.Z., Anderson L.C., Pelphrey K.A., Kaiser M.D. fNIRS detects temporal lobe response to affective touch. Soc. Cogn. Affect. Neurosci. 2014;9:470–476. doi: 10.1093/scan/nst008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi M., Boas D.A., Wilcox T. On the other hand: increased cortical activation to human versus mechanical hands in infants. Neuroimage. 2016;141:143–153. doi: 10.1016/j.neuroimage.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter M., Gordon I., Pelphrey K.A., Olausson H., Kaiser M.D. Development of brain mechanism for processing affective touch. Behav. Neurosci. 2014;8:1–10. doi: 10.3389/fnbeh.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A., Mercure E., Lloyd-Fox S., Thomson A., Brammer M., Sauter D., Deeley Q., Barker G.J., Renvall V., Deoni S., Gasston D., Williams S.C.R., Johnson M.H., Simmons A., Murphy D.G.M. Early specialization for voice and emotion processing in the infant brain. Curr. Biol. 2011;21:1220–1224. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Carter E.J., Pelphrey K.A. School-aged children exhibit domain-specific responses to biological motion. Soc. Neurosci. 2006;1:396–411. doi: 10.1080/17470910601041382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak N., Tessier R., Ruiz J.G., Hernandez J.T., Uriza F., Villegas J., Nadeau L., Mercier C., Maheu F., Marin J., Cortes D., Gallego J.M., Maldonado D. Twenty-year follow-up of kangaroo mother care versus traditional care. Pediatrics. 2016;139 doi: 10.1542/peds.2016-2063. [DOI] [PubMed] [Google Scholar]
- Dahl C.D., Logothetis N.K., Kayser C. Spatial organization of multisensory responses in temporal association cortex. J. Neurosci. 2009;29:11924–11932. doi: 10.1523/JNEUROSCI.3437-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovic M., Jönsson E.H., Olausson H., Björnsdotter M. Posterior superior temporal sulcus responses predict perceived pleasantness of skin stroking. Front. Hum. Neurosci. 2016;10:1–7. doi: 10.3389/fnhum.2016.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B., Koldewyn K., Kanwisher N., Saxe R. Functional organization of social perception and cognition in the superior temporal sulcus. Cereb. Cortex. 2015;25:4596–4609. doi: 10.1093/cercor/bhv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy D.T., Cope M., van der Zee P., Arridge S., Wray S., Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988;33:1433–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- Duncan A., Meek J.H., Clemence M., Elwell C.E., Tyszczuk L., Cope M., Delpy D.T. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys. Med. Biol. 1995;40:295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- Ellingsen D.M., Leknes S., Løseth G., Wessberg J., Olausson H. The neurobiology shaping affective touch: expectation, motivation, and meaning in the multisensory context. Front. Psychol. 2016;6:1–16. doi: 10.3389/fpsyg.2015.01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everdell N.L., Gibson A.P., Tullis I.D.C., Vaithianathan T., Hebden J.C., Delpy D.T. A frequency multiplexed near-infrared topography system for imaging functional activation in the brain. Rev. Sci. Instrum. 2005;76:93705. [Google Scholar]
- Farroni T., Chiarelli A.M., Lloyd-Fox S., Massaccesi S., Merla A., Di Gangi V., Mattarello T., Faraguna D., Johnson M.H. Infant cortex responds to other humans from shortly after birth. Sci. Rep. 2013;3:2851. doi: 10.1038/srep02851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Rosenthal Z., Eidelman A.I. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry. 2013;75:56–64. doi: 10.1016/j.biopsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Field T.M. Touch therapy effects on development. Int. J. Behav. Dev. 1998;22:779–797. [Google Scholar]
- Francis S., Rolls E.T., Bowtell R., McGlone F., O’Doherty J., Browning a, Clare S., Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Gordon I., Voos A.C., Bennett R.H., Bolling D.Z., Pelphrey K.A., Kaiser M.D. Brain mechanisms for processing affective touch. Hum. Brain Mapp. 2013;34:914–922. doi: 10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Johnson M.H., Lloyd-Fox S., Blasi A., Deligianni F., Elwell C., Csibra G. Early cortical specialization for face-to-face communication in human infants. Proc. R. Soc. Lond. B Biol. Sci. 2008;275:2803–2811. doi: 10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Oberecker R., Koch S.P., Friederici A.D. The developmental origins of voice processing in the human brain. Neuron. 2010;65:852–858. doi: 10.1016/j.neuron.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow H.F. The nature of love. Am. Psychol. 1958;13:673–685. [Google Scholar]
- Johansson R., Vallbo A. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson E.H., Kotilahti K., Heiskala J., Wasling H.B., Olausson H., Croy I., Mustaniemi H., Hiltunen P., Tuulari J.J., Scheinin N.M., Karlsson L., Karlsson H., Nissilä I. Affective and non-affective touch evoke differential brain responses in 2-month-old infants. Neuroimage. 2017;169:162–171. doi: 10.1016/j.neuroimage.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Kida T., Shinohara K. Gentle touch activates the prefrontal cortex in infancy: an NIRS study. Neurosci. Lett. 2013;541:63–66. doi: 10.1016/j.neulet.2013.01.048. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Volein A., Everdell N., Elwell C.E., Johnson M.H. Social perception in infancy: a near infrared spectroscopy study. Child. Dev. 2009;80:986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;34:269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Everdell N., Elwell C.E., Johnson M.H. Selective cortical mapping of biological motion processing in young infants. J. Cogn. Neurosci. 2011;23:2521–2532. doi: 10.1162/jocn.2010.21598. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Mercure E., Elwell C.E., Johnson M.H. The emergence of cerebral specialization for the human voice over the first months of life. Soc. Neurosci. 2012;7:317–330. doi: 10.1080/17470919.2011.614696. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Richards J.E., Blasi A., Murphy D.G.M., Elwell C.E., Johnson M.H. Coregistering functional near-infrared spectroscopy with underlying cortical areas in infants. Neurophotonics. 2014;1 doi: 10.1117/1.NPh.1.2.025006. 025006–025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Széplaki-Köllod B., Yin J., Csibra G. Are you talking to me? Neural activations in 6-month-old infants in response to being addressed during natural interactions. Cortex. 2015;70:35–48. doi: 10.1016/j.cortex.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Begus K., Halliday D., Pirazzoli L., Blasi A., Papademetriou M., Darboe M.K., Prentice A.M., Johnson M.H., Moore S.E., Elwell C.E. Cortical specialisation to social stimuli from the first days to the second year of life: a rural Gambian cohort. Dev. Cogn. Neurosci. 2016;25:92–104. doi: 10.1016/j.dcn.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Meaney M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miguel H.O., Lisboa I.C., Gonçalves O.F., Sampaio A. Brain mechanisms for processing discriminative and affective touch in 7-month-old infants. Dev. Cogn. Neurosci. 2017;0–1 doi: 10.1016/j.dcn.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I. Keep calm and cuddle on: social touch as a stress buffer. Adapt. Hum. Behav. Physiol. 2016 [Google Scholar]
- Morrison I. ALE meta-analysis reveals dissociable networks for affective and discriminative aspects of touch. Hum. Brain Mapp. 2016;37:1308–1320. doi: 10.1002/hbm.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrig H., Villringer A. Beyond the visible - imaging the human brain with light. J. Cereb. Blood Flow Metab. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., Morin C., Wallin B.G.G., Starck G., Ekholm S., Strigo I., Worsley K., Vallbo a B., Bushnell M.C.C., Vallbo Å.B., Bushnell M.C.C. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Olausson H., Wessberg J., Morrison I., McGlone F., Vallbo Å. The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Otsuka Y., Nakato E., Kanazawa S., Yamaguchi M.K., Watanabe S., Kakigi R. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. Neuroimage. 2007;34:399–406. doi: 10.1016/j.neuroimage.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Saito Y. Does frontal brain activation in response to optimal touch reflect early experience? Bull. Tokai Gakuin Univ. 2009;3:129–134. [Google Scholar]
- Schanberg S.M., Field T.M. Sensory deprivation stress and supplemental stimulation in the rat pup and preterm human neonate. Child Dev. 1987;58:1431–1447. [PubMed] [Google Scholar]
- Scherf K.S., Behrmann M., Humphreys K., Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Dev. Sci. 2007;10 doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Tuulari J.J., Scheinin N.M., Lehtola S., Merisaari H., Saunavaara J., Parkkola R., Sehlstedt I., Karlsson L., Karlsson H., Bjornsdotter M. Neural correlates of gentle skin stroking in early infancy. Dev. Cogn. Neurosci. 2017 doi: 10.1016/j.dcn.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo Å.B., Olausson H., Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J. Neurophysiol. 1999;81:2753–2763. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Voos A.C., Pelphrey K.A., Kaiser M.D. Autistic traits are associated with diminished neural response to affective touch. Soc. Cogn. Affect. Neurosci. 2013;8:378–386. doi: 10.1093/scan/nss009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg J., Olausson H., Fernström K.W., Vallbo Å.B. Receptive field properties of unmyelinated tactile afferents in the human skin. J. Neurophysiol. 2003;89:1567–1575. doi: 10.1152/jn.00256.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.