Abstract
Monoclonal antibody (mAb) technology is an excellent tool for the discovery of overexpressed cell surface tumour antigens and the development of targeting agents. Here, we report the development of two novel mAbs against CFPAC-1 human pancreatic cancer cells. Using ELISA, flow cytometry, immunoprecipitation, mass spectrometry, Western blot and immunohistochemistry, we found that the target antigens recognised by the two novel mAbs KU44.22B and KU44.13A, are integrin α3 and CD26 respectively, with high levels of expression in human pancreatic and other cancer cell lines and human pancreatic cancer tissue microarrays. Treatment with naked anti-CD26 mAb KU44.13A did not have any effect on the growth and migration of cancer cells nor did it induce receptor downregulation. In contrast, treatment with anti-integrin α3 mAb KU44.22B inhibited growth in vitro of Capan-2 cells, increased migration of BxPC-3 and CFPAC-1 cells and induced antibody internalisation. Both novel mAbs are capable of detecting their target antigens by immunohistochemistry but not by Western blot. These antibodies are excellent tools for studying the role of integrin α3 and CD26 in the complex biology of pancreatic cancer, their prognostic and predictive values and the therapeutic potential of their humanised and/or conjugated versions in patients whose tumours overexpress integrin α3 or CD26.
Subject terms: Pancreatic cancer, Cancer
Introduction
Pancreatic cancer remains one of the deadliest cancer types. In 2018, there were an estimated 458,918 new cases of pancreatic cancer, and 432,242 deaths as a result of pancreatic cancer in 185 countries worldwide1,2. Pancreatic cancer is predicted to become the second leading cause of cancer death after lung cancer, within the next decade in Western countries3.
At present, the only curative treatment for patients with pancreatic cancer is surgery. However, only a minority of patients are eligible for resection and disease recurrence is a frequent event in many such patients. Historically, gemcitabine-based therapy has been the mainstay for treatment of pancreatic cancer4. More recently the combination of gemcitabine plus capecitabine has been regarded as the new standard of care in the adjuvant setting5. Patients with metastatic disease are treated with either FOLFIRINOX or gemcitabine plus nab-paclitaxel as first-line in patients with good performance status6,7.
In order to reduce the dismal pancreatic cancer mortality rates, it is essential to discover novel biomarkers for use in the early detection of pancreatic cancer, to discover novel therapeutic targets and to develop novel and more effective therapeutic agents8,9. Monoclonal antibodies (mAbs) are excellent tools for the discovery of novel overexpressed cell surface antigens and their specific targeting for diagnostic and therapeutic purposes10,11. To date, 36 mAbs have been approved for cancer treatment in the U.S. and/or European Union, although none for pancreatic cancer yet12,13. As tumour heterogeneity has been reported both between (i.e. inter-tumour heterogeneity) and within tumours (i.e. intra-tumour heterogeneity) in patients with pancreatic cancer, and between primary tumours and their metastatic counterparts, it has not been possible to find a magic and universal drug for the treatment of such patients14,15. As a result, over the past few years, our work has been focused on the discovery of overexpressed cell surface antigens in human pancreatic cancer using a panel of pancreatic cancer cell lines derived from patients at different stages of their disease as the source of tumour immunogen and in the antibody screening and the study of such mAbs for use in cancer diagnosis and therapy. We reported recently the development of two novel antibodies against an antigen with high level of expression in pancreatic cancer (i.e. CD109) using the human pancreatic cancer cell line BxPC-3 (derived from a primary tumour) as the source of tumour immunogen9,16. BxPC-3 is a moderate to poorly differentiated cell line derived from a 61-year-old Caucasian female with a primary body of pancreas adenocarcinoma in whom no metastatic disease was found and who died 6 months later despite chemotherapy and radiation16. Here, we report the development of two novel mouse mAbs using CFPAC-1, a cancer cell line established from liver metastasis of a patient with pancreatic cancer, as the source of tumour immunogen17. As there is no complete concordance between the expression level of some genes and their protein products in the primary pancreatic cancer and the corresponding metastatic lesions, our strategy was to develop other antibodies against antigens with high levels of expression in the primary and/or metastatic pancreatic cancer using both the primary and metastatic pancreatic cancer cell lines as immunogen18–21. Indeed, some of the immunogenic antigens may only be overexpressed in the primary tumour cells (i.e. BxPC-3) and not the metastatic pancreatic tumour cells and vice versa. Using a panel of human pancreatic cancer cell lines established from patients at different stages of their diseases and tumour tissue arrays from patients with pancreatic cancer, we found that the overexpressed target antigens recognised by these two novel antibodies are integrin α3 and CD26. Thus, in addition to their diagnostic and therapeutic potential, these antibodies will be valuable tools for unravelling the role of integrin α3 and CD26 in the progression and complex biology of pancreatic cancer
Results
Development of two novel mouse mAbs KU44.22B and KU44.13A
A panel of hybridomas was generated by fusing lymphocytes from mice immunised with CFPAC-1 human pancreatic cancer cells and SP2 myeloma cells. Flow cytometry and ELISA revealed that the antigen recognised by mAb KU44.22B was widely expressed across pancreatic and other cancer cell lines, particularly in the human pancreatic cancer cells BxPC-3 (MFI = 357), CFPAC-1 (MFI = 283), AsPC-1 (MFI = 265) and Capan-2 (MFI = 107), as well as the ovarian cancer cell lines SKOV-3 (MFI = 687) and CaOV-3 (MFI = 836), the glioblastoma cell line A172 (MFI = 466) and the head and neck cancer cell line HN-5 (MFI = 118; Fig. 1 and Supplementary Figs. 1 and 2). On the other hand, mAb KU44.13A recognised an antigen with overexpression limited to AsPC-1 human pancreatic cancer cells (MFI = 437) and to a lower extent HPAF-II (MFI = 48) and the ovarian cell line SKOV-3 (MFI = 38; Fig. 1 and Supplementary Figs. 1 and 2). While in the ELISA, cancer cell monolayers were treated with the primary antibodies, for flow cytometry tumour cells were treated with trypsin prior to incubation with the primary antibody. As the results of ELISA show, the highest levels of target antigen recognised by mAb KU44.13A were found in human pancreatic cancer cell lines derived from ascites (i.e. AsPC-1 and HPAF-II) and lymph node metastasis (Supplementary Fig. 1). Using the mouse isotyping kits, novel mAbs KU44.22B and KU44.13A were found to be of IgG1κ and IgG2a isotypes respectively (data not shown).
Figure 1.
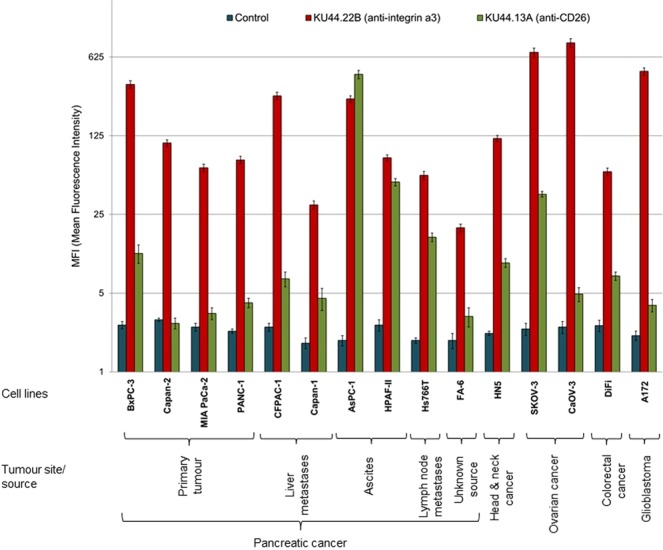
Expression level of the antigens recognised by novel mAbs KU44.22B and KU44.13A on human pancreatic and other cancer cell lines determined by flow cytometry.
The two novel mAbs KU44.22B and KU44.13A are directed against integrin α3 and CD26 respectively
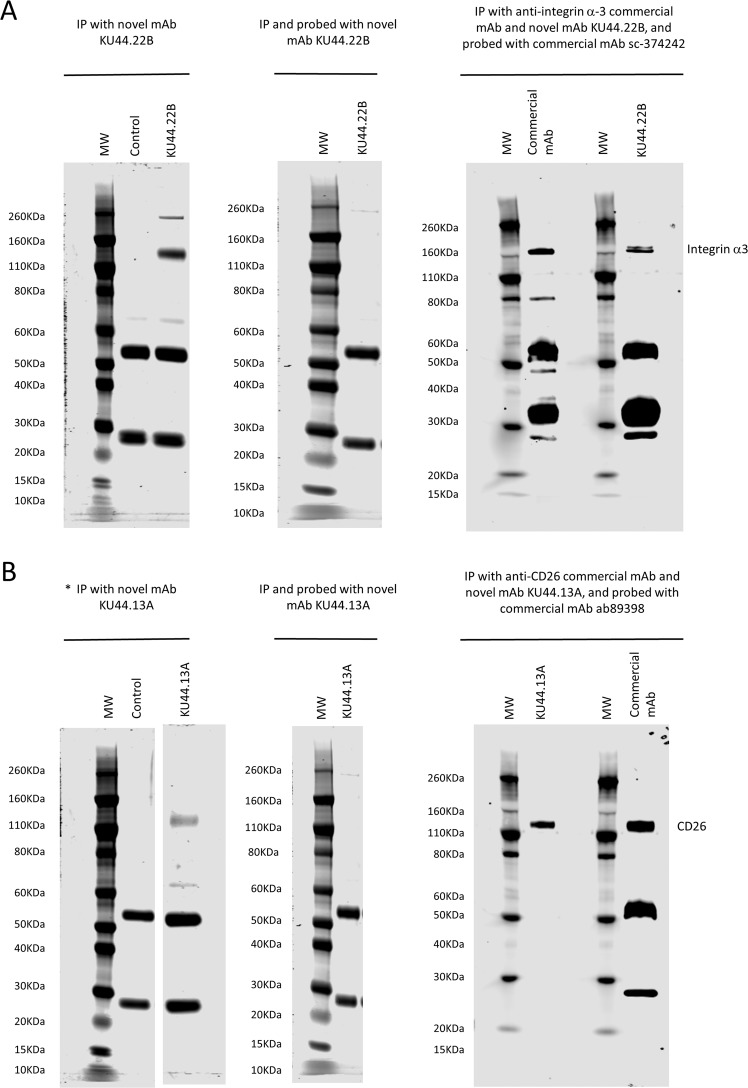
The results of SDS-PAGE stained gels showed that mAb KU44.22B immunoprecipitated a 140 KDa protein identified as integrin α3 by mass spectrometry whereas mAb KU44.13A immunoprecipitated a protein of approximately 110 kDa corresponding to CD26 from the lysates of CaOV3 and ASPC-1 cells respectively (Fig. 2A,B left panel, and Table 1, full-length gels are presented in Supplementary Fig. 3). In addition, using the lysis buffer described in methods, mAb KU44.22B precipitated a protein of approximately 260 kDa that could not be identified by mass spectrometry (Fig. 2A, left panel). However, using RIPA lysis buffer, this additional protein was not immunoprecipitated by mAb KU44.22B (Supplementary Fig. 3C). The faint protein band of ~65 KDa in the antibody samples and controls yielded a single peptide which matched to serum albumin. Neither of the two novel mAbs were able to detect the target antigens by Western blot (Fig. 2A,B, middle panel). To confirm the identity of the target antigens, immunoprecipitation with the novel mAbs and the anti-integrin α3 and anti-CD26 commercial antibodies, followed by immunodetection by Western blot with the same commercial antibodies was performed. As shown in Fig. 2A,B, right panel, both antigens were immunodetected with the commercial mAbs.
Figure 2.
Immunoprecipitation and immunodetection by Western blot of (A) integrin α3 and (B) CD26 antigen with novel mAbs KU44.22B and KU44.13A using lysates from CaOV-3 ovarian cancer cells and AsPC-1 pancreatic cancer cells respectively. Left panel: Immunoprecipitation was performed with novel mAbs (A) KU44.22B and (B) KU44.13A (5 µg) using sheep anti-mouse dynabeads. Protein bands around ~140 KDa and ~ 260KDa were immunoprecipitated with mAb KU44.22B (A; left panel) and ~110 KDa by mAb KU44.13A (B; left panel) respectively and stained with SimplyBlue™ SafeStain. The ~50/25 KDa bands represent heavy and light chains of the anti-mouse antibody. *(B) left panel corresponds to a cropped gel; vertically sliced images of juxtaposed lanes that were non-adjacent in the gel have a clear separation delineating the boundary between the gels. Middle panel: Integrin α3 and CD26 antigen were immunoprecipitated with mAbs (A) KU44.22B and (B) KU44.13A (5 µg) respectively, and probed with the same antibody (30 µg/ml). Target antigens were not immunodetected with either of the mAbs. Right panel: Integrin α3 and CD26 antigen were immunoprecipitated with mAbs (A) KU44.22B and (B) KU44.13A respectively (5 µg) or commercial anti-integrin α3 and anti-CD26 antibodies (2 µg) and immunodetected with commercial mAbs sc-374242 and ab89398 as described in Methods. Immunodetection of target antigens immunoprecipitated by novel mAbs and probed with commercial mAbs confirmed the target identity. MW: molecular weight marker.
Table 1.
Identification of proteins recognised by novel mAbs KU44.13A and KU44.22B by mass spectrometry.
| Band No. | mAb | Protein Hits Matches/Sequences |
||
|---|---|---|---|---|
| 1 | KU44.13A |
P27487 Dipeptidyl peptidase 4/CD26 OS = Homo sapiens GN = DPP4 PE = 1 SV = 2 Mass: 88907 Score: 198 Matches: 5(5) Sequences: 3(3) |
||
| Start-End | Score | Peptide | ||
| 176–184 | 35 | K.IEPNLPSYR.I | ||
| 598–611 | 89 | R.LGTFEVEDQIEAAR.Q | ||
| 659–669 | 73 | R.WEYYDSVYTER.Y | ||
| 2 | KU44.22B |
P26006 Integrin alpha-3 OS = Homo sapiens GN = ITGA3 PE = 1 SV = 5 Mass: 117735 Score: 245 Matches: 4(4) Sequences: 4(4) |
||
| Start-End | Score | Peptide | ||
| 44–60 | 107 | K.EAGNPGSLFGYSVALHR.Q | ||
| 68–76 | 46 | R.YLLLAGAPR.E | ||
| 144–156 | 67 | R.YTQVLWSGSEDQR.R | ||
| 462–471 | 27 | R.ARPVINIVHK.T | ||
MAb KU44.22B inhibits the growth in vitro of Capan-2 cancer cells, increases migration of BxPC-3 and CFPAC-1 cancer cell lines and induces receptor downregulation and internalisation
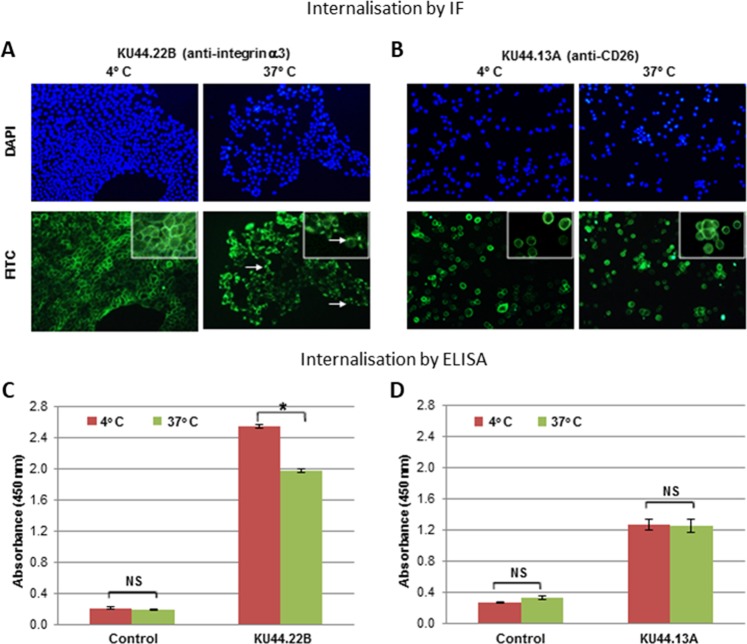
We investigated the effect of treatment with these two novel antibodies on the growth and migration in vitro of a panel of human pancreatic and other cancer cell lines. At 300 nM, mAb KU44.22B inhibited the growth of Capan-2 human pancreatic cancer cells by 94% with an IC50 value of 4.5 nM (Fig. 3) whereas it inhibited the growth of CFPAC-1 cells by 20% (data not shown). Interestingly, treatment with this mAb did not have any effect on the growth of the other cell lines tested including the ovarian cancer cell lines SKOV-3 and CaOV-3, and the glioblastoma cell line A172, despite having higher levels of integrin α3 cell surface expression than Capan-2 cells (data not shown). On the other hand, treatment with mAb KU44.22B increased migration of BxPC-3 and to a lesser extent CFPAC-1 cancer cells (Fig. 4) and induced-receptor downregulation and internalisation (Fig. 5). In contrast, treatment with mAb KU44.13A did not have any effect on the growth or migration of any of the cell lines tested and did not induce receptor downregulation (data not shown, and Fig. 5).
Figure 3.
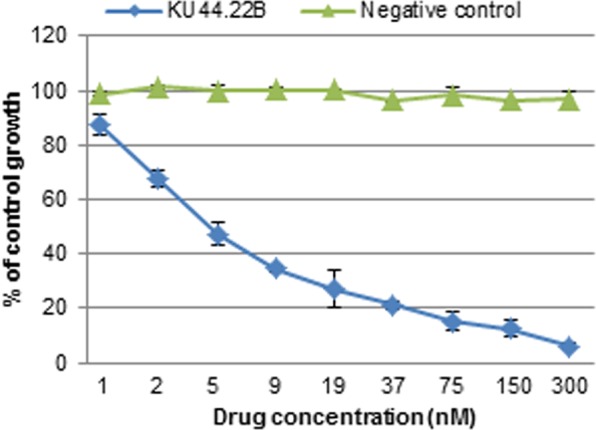
Effect of novel mAb KU44.22B on the growth of Capan-2 human pancreatic cancer cells determined by SRB assay as described in Methods. Novel mAb KU44.22B inhibits the growth of Capan-2 human pancreatic cancer cells with IC50 = 4.5 nM.
Figure 4.
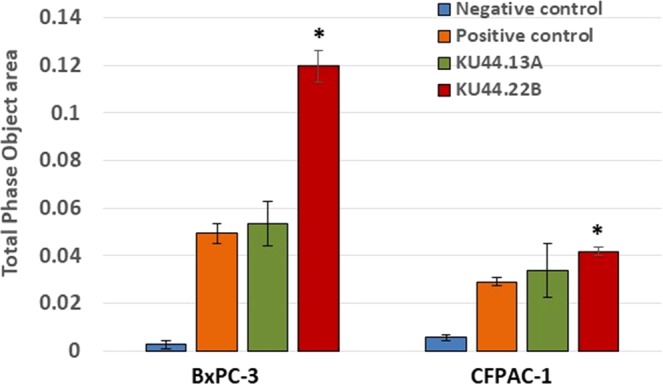
Effect of novel mAbs KU44.22B and KU44.13A on the migration of BxPC-3 and CFPAC-1 human pancreatic cancer cells using the IncuCyte ZOOM® Live-Cell Imaging instrument (Essen Bioscience, UK) as described in Methods. Treatment with mAb KU44.22B (300 nM) significatively increases the migration of BxPC-3 and CFPAC-1 cells.
Figure 5.
Internalisation studies of novel mAbs KU44.22B and KU44.13A in BxPC-3 and AsPC-1 human pancreatic cancer cells determined by (A,B) Immunofluorescence, BxPC-3 and AsPC-1 cancer cells were grown to near confluency and incubated with purified mAbs KU44.22B and KU44.13A respectively (50 μg/ml) or control (PBS/1% BSA) at 4 °C for 1 h and subsequently at 37 °C for extra 30 min to allow internalisation. Cells were then fixed, permeabilised and incubated with anti-mouse secondary antibody (Alexa Fluor 488; 1:200) at 4 °C for 1 h for detection using Nikon eclipse i80 microscope; and (C,D) ELISA, BxPC-3 and AsPC-1 cancer cells were grown to near confluency 96-well plates and incubated with purified mAbs KU44.22B and KU44.13A respectively (50 μg/ml) or control (PBS/1% BSA) at 4 °C for 1 h and subsequently at 37 °C for extra 30 min to allow internalisation. Cells were then fixed, permeabilised and incubated with HRP-conjugated rabbit anti-mouse (1:1000, STAR13B, AbD Serotec) and the absorbance of each sample measured at 450 nm.
Immunohistochemical detection of the antigen recognised by novel mAbs KU44.22B and KU44.13A
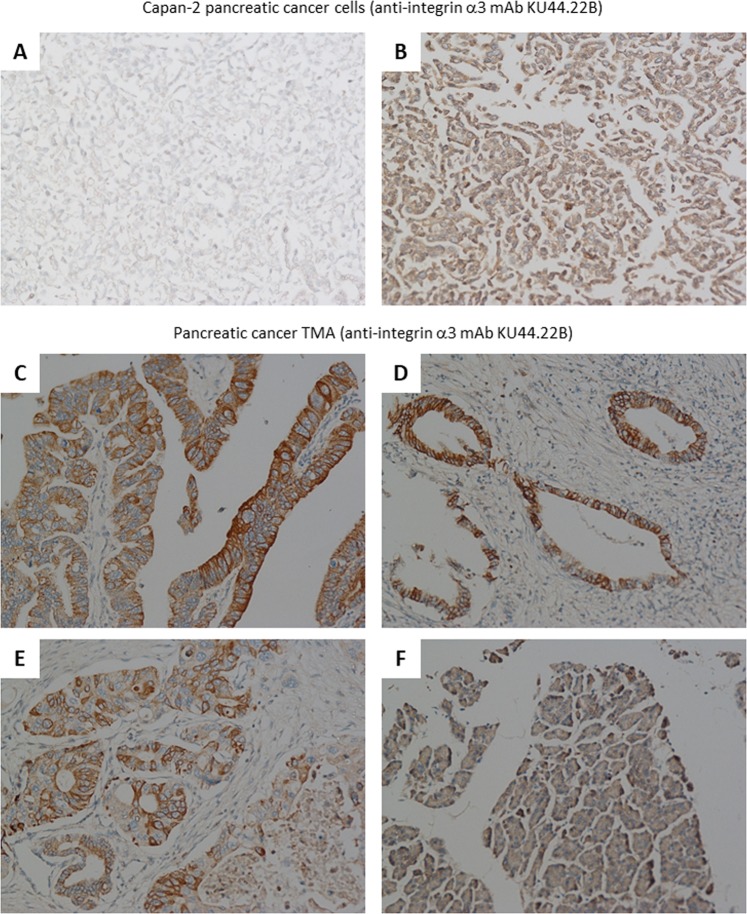
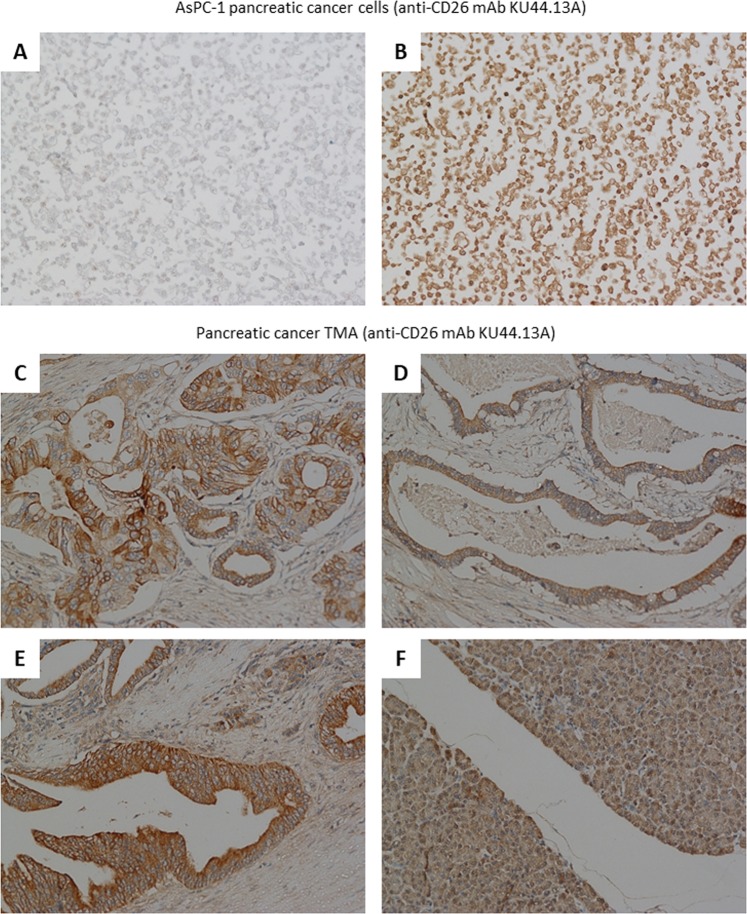
To explore the diagnostic potential of the novel mAbs, immunohistochemical staining was performed in Capan-2 (mAb KU44.22B) and AsPC-1 (mAb KU44.13A) tumour cell pellets. The results showed that both mAbs were able to immunodetect the target antigens in formalin-fixed paraffin-embedded tumour sections (Figs. 6 and 7).
Figure 6.
Examples of immunohistochemical staining of formalin-fixed, paraffin-embedded Capan-2 cancer cell pellets (A-B) and human pancreatic tissue microarrays (C–F) using novel mAb KU44.22B (15 μg/ml). Staining was performed as described in Methods section. (A) Negative control; (B) Staining of Capan-2 cells with mAb KU44.22B; (C) 2/3 + membrane, 2 + cytoplasm (C7); (D) 2 + membrane (B5); (E) 2 + membrane, 1 + cytoplasm (C3); (F) Normal pancreas tissue (F3); magnification 200×.
Figure 7.
Examples of immunohistochemical staining of formalin-fixed, paraffin-embedded AsPC-1 cancer cell pellets (A-B) and human pancreatic tissue microarrays (C–F) using novel mAb KU44.13A (10 μg/ml). Staining was performed as described in Methods section. (A) Negative control; (B) Staining of AsPC-1 cells with mAb KU44.13A; (C) 2 + membrane, 1 + cytoplasm (C3); (D) 1 + membrane/cytoplasm (B3); (E) 2 + cytoplasm/membrane (D4); (F) Normal pancreas tissue (F8); magnification 200×.
Then, we examined the relative expression of integrin α3 and CD26 (immunodetected by mAbs KU44.22B and KU44.13A respectively) in tissue arrays containing 48 specimens from patients with pancreatic cancer. Of these, three samples contained no tumour in the integrin α3 slide and 14 in the CD26 slide and therefore were excluded from the study. Of 45 cases, 64% of tissue samples were integrin α3 positive, with intensity ranging from 1 + weak (n = 17, 37.8%) to 2+ moderate (n = 12, 26.6%). Staining predominated in the membrane and/or the cytoplasm of cancer cells and did not correlate with the disease grade (Fig. 6C–E; Supplementary Table 1). In contrast, normal pancreatic tissue was negative or showed only weak cytoplasmic diffuse staining (Fig. 6F).
On the other hand, of 34 cases, CD26 was overexpressed in 64% of samples, with intensity 1 + weak (n = 16, 47.6%) to 2 + moderate (n = 6, 17.7%; Fig. 7C–E; Supplementary Table 2). Similarly, there was no correlation with tumour grading and weak cytoplasmic staining was seen in normal pancreatic tissue (Fig. 7F).
Discussion
Pancreatic cancer is a highly aggressive and devastating cancer type, responsible for an increasing number of cancer related incidence and deaths1,2,22. The complex biology of pancreatic cancer and its microenvironment, the unavailability of reliable biomarkers for its early detection, the non-specific clinical presentation and the primary and secondary resistance to current therapeutic interventions are some of the attributing factors. Therefore, to improve the poor outcomes for patients diagnosed with pancreatic cancer, it is imperative to develop a screening technique for its early diagnosis, to discover novel therapeutic targets and to develop more effective and less toxic therapeutic agents. Monoclonal antibodies are excellent agents for use in both diagnosis and therapy of human cancer. The exquisite specificity of the antibodies for the target antigen also makes them ideal tools for investigating the biological, diagnostic, prognostic and predictive value of their target antigens and for use in targeted therapy of human cancers10,11,23,24. We have previously reported the generation of two novel anti-CD109 mAbs by means of hybridoma technology using BxPC-3 human pancreatic cancer cells, a cell line derived from a primary tumour, as the source of immunogen9. However, human cancers are heterogeneous in nature, a characteristic feature which contributes to the response of short duration or development of resistance to current therapies21. Both inter-tumour heterogeneity (i.e. heterogeneity between individuals), and intra-tumour heterogeneity (i.e. heterogeneity within the same tumour) as well as heterogeneity between the primary tumour and its metastatic counterparts have been reported in pancreatic cancer14,15,19. Here, using the human pancreatic cancer cell line CFPAC-1, which was isolated from a patient with liver metastasis, we describe the production and characterisation of two other novel mAbs. We have shown that these two novel antibodies are directed against integrin α3 (KU44.22B) and CD26 (KU44.13A).
We found overexpression of the target antigen integrin α3 recognised by the novel mAb KU44.22B in several human pancreatic cancer cell lines as well as human glioblastoma, ovarian and head and neck cancer cell lines as determined by ELISA and flow cytometry (Fig. 1 and Supplementary Figs. 1 and 2). This mAb inhibited the growth in vitro of Capan-2 cells (Fig. 3), increased migration of BxPC-3 and CFPAC-1 cells (Fig. 4) and induced receptor downregulation and internalisation (Fig. 5). While mAb KU44.22B was not able to recognise integrin α3 by Western blot, it proved useful for the immunohistochemical detection of the target antigen in formalin-fixed paraffin-embedded tissue sections from cancer cell pellets and tissue microarrays (Figs. 2 and 6).
Integrins are a family of heterodimeric (αβ) cell surface receptors that participate in cell-cell and cell-matrix interactions25. Their 18 α and 8 β subunits associate in different combinations and form at least 24 heterodimers with functional and tissue specificity26. Of these, integrin α3 (also known as ITGA3, CD49c, and VLA-3 subunit alpha) is a 140 KDa protein thought to be involved in the hepatocyte growth factor (HGF)/c-Met signal pathway contributing to tumour progression27. The integrin α3 subunit is believed to join a β1 subunit to form a complex that interacts with extracellular matrix proteins including members of the laminin family28. To our knowledge, this is the first report of anti-tumour activity in vitro of an anti-integrin α3 antibody in pancreatic cancer. At 300 nM, mAb KU44.22B inhibited the growth of Capan-2 cells by 94% and with an IC50 value of 4.5 nM (Fig. 3). However, as our hybridomas were grown in medium containing 3% FBS. it may be possible that the mouse antibodies purified on Protein G affinity column contain low level of calf antibodies. If this is the case, the actual IC50 value of mAb KU44.22B in Capan-2 cells could be even lower than 4.5 nM. The adaptation of the mouse hybridoma grown in serum-free medium or the purification of the mouse hybridoma supernatants using anti-mouse IgG affinity column could help increase the purity of the antibodies by eliminating potential contaminant calf antibodies. In another study, a mouse mAb (BCMab1), which was raised against aberrantly glycosylated integrin α3β1 on the human bladder cancer cell line T24, was accompanied by potent antitumor activity in subcutaneous and orthotopic bladder cancer mouse models29. Interestingly, we found that the anti-tumour activity of mAb KU44.22B was limited only to Capan-2 cells despite the high level of expression of its target antigen in other cancer cell lines (Fig. 1), which suggests that Capan-2 cell line is more dependent on integrin α3 for survival than any of the other cell lines examined. Further studies should be conducted to unravel the biological mechanisms that determine response to treatment with anti-integrin α3 mAb KU44.22B and to develop and investigate the therapeutic potential of the humanised version of this antibody in tumours with high levels of anti-integrin α3 in vivo.
Since Capan-2 is a non-migratory pancreatic cell line, the effect mAb KU44.22B on its migration could not be explored. However, treatment of BxPC-3 and CFPAC-1 cells with KU44.22B was accompanied by an increase in the migration of these tumour cells in vitro (Fig. 4). Indeed, integrin α3 has been reported to have both pro-migratory and anti-migratory effects in different tumour types. For example, depletion of integrin α3 was reported to increase the migration of prostate cancer cell lines and has been correlated with the disease grade and stage in prostate cancer30. Similarly, low integrin α3 expression was found to be associated with increased metastasis of certain colon cancers31. In contrast, in other studies integrin α3 was reported to have opposite effect by being overexpressed in glioma stem-like cells and contributing to the invasive nature of such cells32,33. ITGA3 (the protein coding gene for integrin α3) was found to be significantly associated with higher risk of recurrence and lower DFS in patients with colorectal tumours34 and shorter survival in pancreatic cancer35. High levels of ITGA3 correlate with more aggressive phenotypes and poor prognosis in patients with colorectal cancer and pancreatic ductal adenocarcinoma36,27. More recently, in another study overexpression of ITGA3 has been associated with a poor prognosis in patients with pancreatic cancer and ablation of ITGA3 was reported to be accompanied by a significant decrease in the EGFR expression and tumour growth37. ITGA3 expression may therefore be a biomarker of diagnostic and prognostic values in pancreatic cancer38. Our study here demonstrated integrin α3 overexpression in a wide TMA from pancreatic cancer patients by IHC with weak staining of normal pancreatic tissue (Fig. 6). Further studies with mAb KU44.22B are warranted and should help to determine the expression pattern, prognostic significance and predictive value of integrin α3 in patients with pancreatic cancer and other forms of cancer. This antibody is also an excellent tool in helping us understand the complex role of integrin α3 in tumour biology and its potential as target for therapy with monoclonal antibody-based drugs and other forms of therapeutics26.
Using immunoprecipitation followed by mass spectrometry, we found that the antigen recognised by the second mAb KU44.13A is CD26. Of the cell lines examined, CD26 overexpression was found to be limited to those derived from ascites specifically AsPC-1 pancreatic cells and to a lower extent HPAF-II pancreatic cancer cells and SKOV-3 ovarian cancer cells (Fig. 1 and Supplementary Figs. 1 and 2). Treatment with anti-CD26 mAb KU44.13A did not have any effect on cell proliferation, migration or receptor downregulation on the cell lines tested (data not shown and Fig. 5). While this mAb was not able to detect CD26 by Western blot, it was capable of detecting CD26 by immunohistochemistry in formalin-fixed paraffin-embedded tissue sections (Figs. 2 and 7).
CD26 (also known as Dipeptidyl peptidase-4 [DPP4/DPPIV] or ADCP2) is a 110 KDa membrane-associated peptidase. It is expressed in endothelial cells, fibroblasts and lymphocytes, on the apical surfaces of epithelial and acinar cells, and in a soluble form in plasma39. It is believed to play a role in tumour development through its association with intracellular proteins and its effect seems to be dependent on the tumour type and its microenvironment40. While CD26 has been shown to have a tumour suppressor effect in melanoma, ovarian cancer, non-small cell lung cancer, prostate cancer, endometrial cancer, neuroblastoma and glioma cell lines, it has been reported to be a marker of tumour aggressiveness in T-anaplastic large cell lymphoma, T-leukaemia, malignant mesothelioma, colorectal cancer and Ewing sarcoma cell lines40,41. Furthermore, CD26 has been reported to have higher levels of expression in pancreatic cancer tissue than in normal tissue and knockdown of CD26 expression inhibited cell growth, migration, invasion and colony formation, increases cell apoptosis of pancreatic cancer cells in vitro and decreased tumour growth and liver metastasis in vivo42. CD26 has been reported to be secreted in the serum of patients with different cancer types and therefore it has been proposed as a potential tumour biomarker of diagnostic and prognostic value in a wide range of cancers including pancreatic cancer40–42. CD26 has also been suggested as a cancer stem cell marker in different types of cancer and a potential therapeutic target43–45.
Although our anti-CD26 novel mAb KU44.13A did not show any effect on cell proliferation or migration on the panel of cell lines evaluated in this study, treatment with other anti-CD26 monoclonal antibodies have shown to reduce tumour growth in vitro and in vivo and improve survival in malignant mesothelioma, renal cell carcinoma and anaplastic large cell T-cell lymphoma46–49. We found moderate membranous and/or cytoplasmic staining of human pancreatic cancer tissue microarrays and cell pellets with anti-CD26 mAb KU44.13A (Fig. 7). Although there is no published research on the immunohistochemical staining of CD26 in pancreatic cancer, it has been shown that stromal CD26 expression following preoperative chemoradiotherapy has significant association with tumour recurrence and poor prognosis in patients with rectal cancer50. A positive association was also reported between high CD26 expression and tumour stage, development of metastasis and poor outcome in patients with colorectal cancer51. Finally, the results of a recent phase 1 clinical trial of the humanised anti-CD26 mAb YS110 in patients with mesothelioma, renal cell carcinoma and urothelial carcinoma CD26-expressing tumours showed a favourable safety profile and encouraged disease stabilisation in a subset of patients52. Further studies with our novel mAb KU44.13A in a larger panel of patients’ tumour specimens are warranted and should unravel the relative expression, prognostic significance and predictive value of CD26 in patients with pancreatic cancer as well as other cancer types.
In conclusion, in this study, we reported the production of two novel monoclonal antibodies against integrin α3 and CD26 on human pancreatic cancer cells, using the liver metastasis pancreatic cancer cell line CFPAC-1 as immunogen. These antibodies would be excellent tools for investigating the diagnostic, prognostic and predictive value of such antigens in pancreatic cancer, and studying their roles in the complex biology of pancreatic cancer and other cancer types. Further investigation are warranted to determine the therapeutic potential of these mAbs, particularly the conjugated and/or humanised versions with more effector ADCC (antibody-dependent cell-mediated cytotoxicity) and/or CDC (complement-dependent cytotoxicity) functions, for use in antibody-based targeted therapy of tumours with overexpression of these antigens53,54.
Methods
Cancer cell lines and cell culture
A panel of ten human pancreatic cancer cell lines (BxPC-3 [RRID:CVCL_0186], Capan-2 [RRID:CVCL_0026], MIA PaCa-2 [RRID:CVCL_0428], PANC-1 [RRID:CVCL_0480], AsPC-1 [RRID:CVCL_0152], HPAF-II [RRID:CVCL_0313], CFPAC-1 [RRID:CVCL_1119], Capan-1, Hs766T [RRID:CVCL_0334] and FA-6) were used in this study and cultured as described previously9,55. SP2 myeloma cells (RRID:CVCL_2199) were purchased from European Collection of Cell Cultures (ECACC, UK). HN5 (head and neck cancer cells), SKOV-3 and CaOV-3 (ovarian cancer cells), DiFi (colorectal cancer cells), A172 (glioblastoma cells) and SP2 myeloma cells were grown in Dulbecco’s Modified Eagle’s Medium supplemented with 10% FBS and antibiotics. All culture media and additives were purchased from Sigma Aldrich, UK. MIA PaCa-2, PANC-1, and Capan-1 were authenticated by American Type Culture Collection (ATCC, UK).
Generation and screening of novel monoclonal antibodies
The mice immunisation was performed at St George’s University of London, following ethical approval and under Home Office animal license as described previously9. All experiments were performed in accordance with relevant guidelines and regulations. A group of female BALB/c mice (aged 5–6 weeks) were immunised subcutaneously (s.c) at two sites and intraperitoneally (i.p.) with a total number of 10 million CFPAC-1 human pancreatic cancer cells per immunisation per mouse (3 sites; 100 μl per site). Immunisation was repeated 2 times every 2 weeks and the final injection was administered 3–4 days before collection of lymphocytes from the spleen of immunised mice. B-lymphocytes derived from the spleen of immunised mice were fused with SP2 myeloma cells by 50% polyethylene glycol (PEG; Sigma Aldrich). Cells were cultured in HAT medium (Sigma Aldrich) supplemented with 20% FBS, 10% Hybridoma Cloning Supplement (Santa Cruz Biotechnology, USA) and antibiotics.
Antibodies secreted from novel hybridomas were screened by ELISA as described previously9. Newly formed positive hybridomas were selected, cloned twice by limiting dilution technique, adapted to growth medium containing 3% FBS, grown in roller bottles and the hybridoma supernatants were harvested and purified for further studies as described below.
Flow cytometry
The cell surface expression of target antigens recognised by novel mAbs was determined using flow cytometry as described previously9,56. Briefly, cells were trypsinised and approximately 1 × 106 tumour cells were incubated by rotation for 1 h at 4 °C with novel mouse mAbs KU44.22B or KU44.13A (10 μg/ml) or control (i.e. PBS), followed by incubation with FITC-conjugated goat anti-mouse IgG secondary antibody (1:200; STAR9B, AbD Serotec; RRID:AB_321920) for 45 min at 4 °C. A minimum of 10,000 events were recorded by excitation with an argon laser at 488 nm and analysed using the FL-1 detector (FITC detector; 525 nm) of a BD FACScalibur flow cytometer using CellQuest Pro software (Becton-Dickinson Ltd, UK; RRID:SCR_014489).
Isotyping and purification of novel monoclonal antibodies
Isotyping of novel mAbs was determined using a mouse mAb isotyping kit (AbD Serotec, UK) according to the manufacturer’s protocol and the antibodies were purified by affinity chromatography as described previously9. Briefly, novel mouse mAbs were purified by salt fractionation (solid ammonium sulphate ([NH4]2SO4; 45% of saturation − 270 g/L; Fisher Scientific) followed by affinity chromatography using a 5 ml HiTrap Protein G HP column in an ÄKTAprime plus chromatography system (GE Healthcare, UK), as described previously9. The purified antibodies were filtered through a 0.2 µm syringe filter (Merck Millipore, UK), aliquoted and stored at −20 °C for further studies.
Internalisation studies
Immunofluorescence staining of tumour cells and ELISA were used to determine whether treatment with novel antibodies resulted in down-regulation of the target antigen as described previously9. Briefly, pancreatic cancer cells were grown to near confluency in RPMI/10% FBS in Lab-Tek 8-well chamber slides (VWR, UK) or 96-well plates, respectively. Cells were incubated with mAbs KU44.22B or KU44.13A (50 µg/ml) or control (i.e. PBS/1%BSA alone) for 1 h at 4 °C to allow antibody binding, followed by incubation at 37 °C for 30 min to allow antibody internalisation. A control slide was maintained at 4 °C. Cells were fixed with 4% formaldehyde for 10 min, the cell membrane permeabilised with 0.5% Triton-X 100 for 15 min and non-specific binding blocked with PBS/1%BSA for 1 h at 4 °C. Cells in immunofluorescence slides were then incubated with Alexa Fluor 488 secondary antibody (1:200; Fisher Scientific; RRID:AI_1001) for 1 h at 4 °C, mounted in Vectashield with DAPI (Vector laboratories, UK) and examined using Nikon eclipse i80 microscope and Nikon NIS-Elements software as described previously (RRID:SCR_014329)57. Cells in ELISA plates were incubated under the same conditions above with the primary antibodies and then incubated with HRP-conjugated rabbit anti-mouse (1:1000, STAR13B, AbD Serotec; RRID:AB_321921). Following several washes, the absorbance of each sample was measured at 450 nm as described previously9
Effect of novel mAbs on growth and migration of pancreatic cancer cells
The effect of novel mAbs on the growth of human cancer cell lines was investigated using the Sulforhodamine B (SRB) colorimetric assay9,58. Gen5 software was used to determine the IC50 through non-linear least squares curve fitting, as described previously56,59.
The effect of novel mAbs on the migration of human pancreatic cancer cells BxPC-3, AsPC-1 and CFPAC-1 was investigated using the IncuCyte ZOOM® Live-Cell Imaging instrument (Essen Bioscience, UK) as described previously9. Approximately 1 × 103 cancer cells per well were seeded in duplicate in an IncuCyte™ ClearView 96-well Cell Migration Plate (Essen Bioscience, UK) along with 300 nM mAbs or control (i.e. medium alone). Cells were allowed to settle for 15 min at room temperature before transfer to an incubator at 37 °C for 30 min to pre-incubate the cells in the presence of treatment. Then, 200 μl of chemoattractant (i.e. 10% FBS medium) or control (i.e. 0.5% FBS medium) were added to the appropriate wells of the reservoir plate and the insert plate placed into the pre-filled reservoir plate. The plate was then transferred to the IncuCyte ZOOM® Live-Cell Imaging Instrument (Essen Bioscience) and allowed to warm to 37 °C for 15 min before any condensation accumulated on the plate lid or bottom was wiped away. The plate was imaged at 10x objective using the Chemotaxis Scan Type - Phase channel. The IncuCyte™ Chemotaxis Cell Migration Software Module (Essen Bioscience) was used for data analysis. Whole-well images of cells on both the bottom and the top of the plate membrane were captured every 2 h over 48 h and all images were processed using automatic algorithms to quantify cell area on each side of the membrane.
Immunoprecipitation and mass spectrometry
To identify the target antigens recognised by the novel antibodies, immunoprecipitation and mass spectrometry were performed using protein identification service provided by the University of York (UK) as described previously9. Briefly, novel mAbs (5 μg) were incubated overnight at 4 °C by gentle rotation (14 rpm) with 1 ml AsPC-1 (mAb KU44.13A) or CaOV-3 (mAb KU44.22B) tumour cell lysates (prepared with lysis buffer containing 50 mM Tris-HCl pH 7.2, 150 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 0.1% NaN3, 100 mM DTT, 1% Triton X-100 and 50 mM N-ethylmaleimide), and then incubated with 50 μl pre-washed Dynabeads sheep anti-mouse IgG for 1 h at 4 °C (ThermoFisher Scientific). The immunocomplexes were captured on a DynaMag™-2 for 2 min, the supernatants aspirated, and the samples washed 3 times with PBS. The complexes were then eluted by mixing beads with LDS sample buffer (25% NuPAGE LDS buffer [4 × ], 10% reducing agent [10 × ] and 65% distilled water; Invitrogen, UK), heated to 95 °C for 5 min and analysed by SDS-PAGE. The SDS-PAGE gels were stained with SimplyBlue™ SafeStain (ThermoFisher Scientific) and the protein bands were excised (Supplementary Fig. 3A,B), and in gel digested with trypsin for subsequent mass spectrometry analysis.
Identification of isolated protein was performed by mass spectrometry under contract at the University of York’s protein identification facility, as described previously9. The UniProt_human_SP database was used for protein identification.
Western blotting
The ability of the novel antibodies to recognise the target antigen in Western blot was investigated as described previously9. Briefly, proteins immunoprecipitated with novel mAbs KU44.22B and KU44.13A (5 µg) or commercial mAbs anti-integrin α3 (2 µg; sc-374242; Santa Cruz Biotechnology, UK; RRID:AB_10985868) or anti-CD26 (2 µg; ab89398; Abcam, UK; RRID: AB_2277416) were analysed by SDS-PAGE under reducing conditions, prior to Western blotting. The transfer of proteins from 4–12% Bis-Tris-gels to Immobilon-FL PVDF membranes (Merck Millipore, UK) was performed using the XCell IITM Mini-Cell Blot Module kit (Invitrogen, UK) at a constant voltage of 30 V on ice for 2 h. PVDF membranes were probed with novel mAbs (30 μg/ml), commercial anti-integrin α3 mouse mAb (1:100 dilution) or commercial anti-CD26 rat mAb (1:500 dilution) overnight at 4 °C and subsequently incubated for 1 h at room temperature with secondary goat anti-mouse antibody (1:10,000; RRID:926_32210) or secondary goat anti-rat antibody (1:5,000; RRID:925-68076, both from LI-COR Biosciences, UK) respectively. For visualisation, the blots were analysed using the Oddysey® CLx instrument (LI-COR Biosciences; RRID:SCR_014579).
Immunohistochemical staining
AsPC-1 and Capan-2 cancer cell pellets and human pancreatic cancer tissue microarrays (PA483e, Insight Biotechnology/US Biomax) were used to determine whether novel monoclonal antibodies immunodetect the target antigens in formalin-fixed paraffin-embedded tissue sections using the VENTANA BenchMark ULTRA IHC/ISH System (Roche, UK). Tissue sections were deparaffinised and rehydrated through a series of alcohols followed by heat induced antigen retrieval with standard CC1 (Tris-EDTA buffer pH 7.8) at 95 °C for 36 min and incubation for 1 h with either mAb KU44.22B (15 µg/ml) or mAb KU44.13A (10 µg/ml). An anti-mouse IgG detection system (UltraView Universal DAB Detection Kit, Ventana) was used for amplification and primary antibody detection. The slides were then counterstained with haematoxylin II for 8 min followed by Bluing reagent. The slides were then washed, dehydrated, cleared, mounted in DPX mounting medium (VWR) and coverslipped as described previously60.
Supplementary information
Acknowledgements
This work was supported by Kingston University London towards a PhD project and The Ralph Bates Pancreatic Cancer Research Fund fellowship. We thank Dr. Wai Liu and Dr. Daniel Fowler (St George’s University of London) for conducting the immunisation procedure for this study, and Dr. Soozana Puvanenthiran and Dr. Said Khelwatty for their helps with the staining of the immunohistochemical staining of TMAs on the VENTANA BenchMark Ultra IHC/ISH system.
Author contributions
H.M. conceived and designed the experiments. G.A.P. performed the experiments and data analysis. H.M., A.D., A.W., S.M. and I.B. helped with technical training and data analysis. G.A.P. and H.M. wrote the paper and A.D., A.W., S.M. and I.B. went through the manuscript, edited and approved the final version of the manuscript.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information Files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-57287-w.
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018;15:333–348. doi: 10.1038/s41575-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 9.Arias-Pinilla GA, et al. Development of novel monoclonal antibodies against CD109 overexpressed in human pancreatic cancer. Oncotarget. 2018;9:19994–20007. doi: 10.18632/oncotarget.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012;12:14. [PMC free article] [PubMed] [Google Scholar]
- 11.Modjtahedi H, Ali S, Essapen S. Therapeutic application of monoclonal antibodies in cancer: advances and challenges. Br. Med. Bull. 2012;104:41–59. doi: 10.1093/bmb/lds032. [DOI] [PubMed] [Google Scholar]
- 12.FDA., U. S. Hematology/Oncology (Cancer) Approvals & Safety Notifications, http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm (2019).
- 13.EMA, http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/landing/cancer_disease_area.jsp&mid=WC0b01ac058034ed06 (2019).
- 14.Cros J, Raffenne J, Couvelard A, Pote N. Tumor Heterogeneity in Pancreatic Adenocarcinoma. Pathobiology. 2018;85:64–71. doi: 10.1159/000477773. [DOI] [PubMed] [Google Scholar]
- 15.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nature Reviews. Clin. Oncol. 2017;15:81. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 16.Tan MH, et al. Characterization of a new primary human pancreatic tumor line. Cancer Invest. 1986;4:15–23. doi: 10.3109/07357908609039823. [DOI] [PubMed] [Google Scholar]
- 17.Schoumacher RA, et al. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc. Natl Acad. Sci. USA. 1990;87:4012–4016. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene. 2013;32:5253–5260. doi: 10.1038/onc.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansari D, Urey C, Gundewar C, Bauden MP, Andersson R. Comparison of MUC4 expression in primary pancreatic cancer and paired lymph node metastases. Scand. J. Gastroenterol. 2013;48:1183–1187. doi: 10.3109/00365521.2013.832368. [DOI] [PubMed] [Google Scholar]
- 20.Collisson EA, Maitra A. Pancreatic Cancer Genomics 2.0: Profiling Metastases. Cancer Cell. 2017;31:309–310. doi: 10.1016/j.ccell.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Ansari D, Friess H, Bauden M, Samnegard J, Andersson R. Pancreatic cancer: disease dynamics, tumor biology and the role of the microenvironment. Oncotarget. 2018;9:6644–6651. doi: 10.18632/oncotarget.24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuigan A, et al. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hafeez U, Gan HK, Scott AM. Monoclonal antibodies as immunomodulatory therapy against cancer and autoimmune diseases. Curr. Opin. Pharmacol. 2018;41:114–121. doi: 10.1016/j.coph.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Cruz E, Kayser V. Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics. 2019;13:33–51. doi: 10.2147/BTT.S166310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raab-Westphal Sabine, Marshall John, Goodman Simon. Integrins as Therapeutic Targets: Successes and Cancers. Cancers. 2017;9(12):110. doi: 10.3390/cancers9090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu GH, et al. Expression and prognostic significance of CD151, c-Met, and integrin alpha3/alpha6 in pancreatic ductal adenocarcinoma. Dig. Dis. Sci. 2011;56:1090–1098. doi: 10.1007/s10620-010-1416-x. [DOI] [PubMed] [Google Scholar]
- 28.Hashida H, et al. Integrin alpha3 expression as a prognostic factor in colon cancer: association with MRP-1/CD9 and KAI1/CD82. Int. J. Cancer. 2002;97:518–525. doi: 10.1002/ijc.1625. [DOI] [PubMed] [Google Scholar]
- 29.Li C, et al. BCMab1, a monoclonal antibody against aberrantly glycosylated integrin alpha3beta1, has potent antitumor activity of bladder cancer in vivo. Clin. Cancer Res. 2014;20:4001–4013. doi: 10.1158/1078-0432.CCR-13-3397. [DOI] [PubMed] [Google Scholar]
- 30.Das L, et al. Characterization of Laminin Binding Integrin Internalization in Prostate Cancer Cells. J. Cell Biochem. 2017;118:1038–1049. doi: 10.1002/jcb.25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashida H, et al. The novel monoclonal antibody MH8-4 inhibiting cell motility recognizes integrin alpha 3: inverse of its expression withmetastases in colon cancer. Int. J. Oncol. 2001;18:89–95. doi: 10.3892/ijo.18.1.89. [DOI] [PubMed] [Google Scholar]
- 32.Morini M, et al. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int. J. Cancer. 2000;87:336–342. doi: 10.1002/1097-0215(20000801)87:3<336::AID-IJC5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Nakada M, et al. Integrin alpha3 is overexpressed in glioma stem-like cells and promotes invasion. Br. J. Cancer. 2013;108:2516–2524. doi: 10.1038/bjc.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linhares MM, et al. Genetic and Immunohistochemical Expression of Integrins ITGAV, ITGA6, and ITGA3 As Prognostic Factor for Colorectal Cancer: Models for Global and Disease-Free Survival. PLoS One. 2015;10:e0144333. doi: 10.1371/journal.pone.0144333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, et al. Integrative analysis of gene expression profiles reveals specific signaling pathways associated with pancreatic duct adenocarcinoma. Cancer Commun. (Lond.) 2018;38:13. doi: 10.1186/s40880-018-0289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sa KD, et al. A miR-124/ITGA3 axis contributes to colorectal cancer metastasis by regulating anoikis susceptibility. Biochem. Biophys. Res. Commun. 2018;501:758–764. doi: 10.1016/j.bbrc.2018.05.062. [DOI] [PubMed] [Google Scholar]
- 37.Lee J, Lee J, Choi C, Kim JH. Blockade of integrin alpha3 attenuates human pancreatic cancer via inhibition of EGFR signalling. Sci. Rep. 2019;9:2793. doi: 10.1038/s41598-019-39628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiao Y, Li Y, Liu S, Chen Q, Liu Y. ITGA3 serves as a diagnostic and prognostic biomarker for pancreatic cancer. Onco Targets Ther. 2019;12:4141–4152. doi: 10.2147/OTT.S201675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aliyari Serej Z, Ebrahimi Kalan A, Mehdipour A, Nozad Charoudeh H. Regulation and roles of CD26/DPPIV in hematopoiesis and diseases. Biomed. Pharmacother. 2017;91:88–94. doi: 10.1016/j.biopha.2017.04.074. [DOI] [PubMed] [Google Scholar]
- 40.Beckenkamp A, Davies S, Willig JB, Buffon A. DPPIV/CD26: a tumor suppressor or a marker of malignancy? Tumour Biol. 2016;37:7059–7073. doi: 10.1007/s13277-016-5005-2. [DOI] [PubMed] [Google Scholar]
- 41.Sedo A, Stremenova J, Busek P, Duke-Cohan JS. Dipeptidyl peptidase-IV and related molecules: markers of malignancy? Expert. Opin. Med. Diagn. 2008;2:677–689. doi: 10.1517/17530059.2.6.677. [DOI] [PubMed] [Google Scholar]
- 42.Ye CX, et al. Elevated serum CD26 level is associated with metastasis and post-operation survival in pancreatic cancer patients. Transl. Cancer Res. 2016;5:512–519. doi: 10.21037/tcr.2016.08.38. [DOI] [Google Scholar]
- 43.Beckenkamp A, et al. Differential Expression and Enzymatic Activity of DPPIV/CD26 Affects Migration Ability of Cervical Carcinoma Cells. PLoS One. 2015;10:e0134305. doi: 10.1371/journal.pone.0134305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies S, Beckenkamp A, Buffon A. CD26 a cancer stem cell marker and therapeutic target. Biomed. Pharmacother. 2015;71:135–138. doi: 10.1016/j.biopha.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 45.Nishida H, Hayashi M, Morimoto C, Sakamoto M, Yamada T. CD26 is a potential therapeutic target by humanized monoclonal antibody for the treatment of multiple myeloma. Blood Cancer J. 2018;8:99. doi: 10.1038/s41408-018-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Havre PA, et al. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. 2008;13:1634–1645. doi: 10.2741/2787. [DOI] [PubMed] [Google Scholar]
- 47.Inamoto T, et al. Anti-CD26 monoclonal antibody-mediated G1-S arrest of human renal clear cell carcinoma Caki-2 is associated with retinoblastoma substrate dephosphorylation, cyclin-dependent kinase 2 reduction, p27(kip1) enhancement, and disruption of binding to the extracellular matrix. Clin. Cancer Res. 2006;12:3470–3477. doi: 10.1158/1078-0432.CCR-06-0361. [DOI] [PubMed] [Google Scholar]
- 48.Inamoto T, et al. Humanized anti-CD26 monoclonal antibody as a treatment for malignant mesothelioma tumors. Clin. Cancer Res. 2007;13:4191–4200. doi: 10.1158/1078-0432.CCR-07-0110. [DOI] [PubMed] [Google Scholar]
- 49.Sato T, et al. CD26 regulates p38 mitogen-activated protein kinase-dependent phosphorylation of integrin beta1, adhesion to extracellular matrix, and tumorigenicity of T-anaplastic large cell lymphoma Karpas 299. Cancer Res. 2005;65:6950–6956. doi: 10.1158/0008-5472.CAN-05-0647. [DOI] [PubMed] [Google Scholar]
- 50.Saigusa S, et al. Prognostic relevance of stromal CD26 expression in rectal cancer after chemoradiotherapy. Int. J. Clin. Oncol. 2016;21:350–358. doi: 10.1007/s10147-015-0902-8. [DOI] [PubMed] [Google Scholar]
- 51.Lam CS, et al. Prognostic significance of CD26 in patients with colorectal cancer. PLoS One. 2014;9:e98582. doi: 10.1371/journal.pone.0098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angevin E, et al. First-in-human phase 1 of YS110, a monoclonal antibody directed against CD26 in advanced CD26-expressing cancers. Br. J. Cancer. 2017;116:1126–1134. doi: 10.1038/bjc.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beers SA, Glennie MJ, White AL. Influence of immunoglobulin isotype on therapeutic antibody function. Blood. 2016;127:1097–1101. doi: 10.1182/blood-2015-09-625343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplon H, Reichert JM. Antibodies to watch in 2019. MAbs. 2019;11:219–238. doi: 10.1080/19420862.2018.1556465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ioannou N, et al. Acquired resistance of pancreatic cancer cells to treatment with gemcitabine and HER-inhibitors is accompanied by increased sensitivity to STAT3 inhibition. Int. J. Oncol. 2016;48:908–918. doi: 10.3892/ijo.2016.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khelwatty SA, Essapen S, Seddon AM, Modjtahedi H. Growth response of human colorectal tumour cell lines to treatment with afatinib (BIBW2992), an irreversible erbB family blocker, and its association with expression of HER family members. Int. J. Oncol. 2011;39:483–491. doi: 10.3892/ijo.2011.1054. [DOI] [PubMed] [Google Scholar]
- 57.Khelwatty SA, Essapen S, Seddon AM, Fan Z, Modjtahedi H. Acquired resistance to anti-EGFR mAb ICR62 in cancer cells is accompanied by an increased EGFR expression, HER-2/HER-3 signalling and sensitivity to pan HER blockers. Br. J. Cancer. 2015;113:1010–1019. doi: 10.1038/bjc.2015.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puvanenthiran S, et al. Co-expression and prognostic significance of the HER family members, EGFRvIII, c-MET, CD44 in patients with ovarian cancer. Oncotarget. 2018;9:19662–19674. doi: 10.18632/oncotarget.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ioannou N, et al. Anti-tumour activity of afatinib, an irreversible ErbB family blocker, in human pancreatic tumour cells. Br. J. Cancer. 2011;105:1554–1562. doi: 10.1038/bjc.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andre P, et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175:1731–1743 e1713. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information Files).