Abstract
Sertraline is an (SSRI-)antidepressant metabolized by the polymorphic CYP2C19 enzyme. The aim of this study was to investigate the impact of CYP2C19 genotype on the serum concentrations of sertraline in a large patient population. Second, the proportions of patients in the various CYP2C19 genotype-defined subgroups obtaining serum concentrations outside the therapeutic range of sertraline were assessed. A total of 2190 sertraline serum concentration measurements from 1202 patients were included retrospectively from the drug monitoring database at Diakonhjemmet Hospital in Oslo. The patients were divided into CYP2C19 genotype-predicted phenotype subgroups, i.e. normal (NMs), ultra rapid (UMs), intermediate (IMs), and poor metabolisers (PMs). The differences in dose-harmonized serum concentrations of sertraline and N-desmethylsertraline-to-sertraline metabolic ratio were compared between the subgroups, with CYP2C19 NMs set as reference. The patient proportions outside the therapeutic concentration range were also compared between the subgroups with NMs defined as reference. Compared with the CYP2C19 NMs, the sertraline serum concentration was increased 1.38-fold (95% CI 1.26–1.50) and 2.68-fold (95% CI 2.16–3.31) in CYP2C19 IMs and PMs, respectively (p < 0.001), while only a marginally lower serum concentration (−10%) was observed in CYP2C19 UMs (p = 0.012). The odds ratio for having a sertraline concentration above the therapeutic reference range was 1.97 (95% CI 1.21–3.21, p = 0.064) and 8.69 (95% CI 3.88–19.19, p < 0.001) higher for IMs and PMs vs. NMs, respectively. CYP2C19 IMs and PMs obtain significantly higher serum concentrations of sertraline than NMs. Based on the relative differences in serum concentrations compared to NMs, dose reductions of 60% and 25% should be considered in PMs and IMs, respectively, to reduce the risk of sertraline overexposure in these patients.
Subject terms: Genetics research, Enzyme mechanisms
Introduction
Sertraline was among the first selective serotonin reuptake inhibitors (SSRIs) to be approved for treatment of depression in the early 1990s [1]. Indications for the clinical use of sertraline include major depressive, obsessive-compulsive, panic, post-traumatic stress and social anxiety disorders [https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019839s091lbl.pdf#page=27]. In a meta-analysis comparing clinical response and tolerability of 21 antidepressants and placebo in adult patients with major depressive disorder, sertraline was found to be significantly more efficient than placebo with a higher response rate and lower dropout rate than many other antidepressants [2]. Sertraline was also found to be among the most tolerable antidepressants [2].
Despite the convincing efficacy and tolerability data of sertraline on population basis, the individual variability in clinical response during treatment of depression is substantial [2–5]. The extensive individual variability in sertraline pharmacokinetics, as shown in previous studies [3, 6], which could relate to differences in activities of drug-metabolizing enzymes, may represent an important source for the inter-patient differences in the clinical response at standard dosing [2–5]. Sex and age have been reported to be of importance for the pharmacokinetic variability of sertraline [7].
In vitro studies suggest that CYP2C19, CYP2B6, CYP2C9, CYP2D6 and CYP3A4 are all involved in the metabolism of sertraline [8], but CYP2C19 and CYP2B6 are regarded as the most relevant enzymes [9]. While pharmacogenetic differences have a moderate impact on the individual variability in CYP2B6 metabolism, genetic differences are of major importance for the individual variability in CYP2C19-mediated metabolism. CYP2C19 gene variants may encode absent, decreased, normal or increased metabolism. The known variants encoding non-functional enzyme activity comprise CYP2C19*2, *3 and *4, while the variant allele CYP2C19*17 leads to increased metabolism [10]. Based on CYP2C19 genotype, the population can be divided into the following genotype-predicted metabolizer phenotype subgroups: poor metabolizers (PMs), intermediate metabolizers (IMs), normal metabolizers (NMs), and ultra rapid metabolizers (UMs) [11] (www.pharmvar.org). In a general European population, the frequencies are 18% and 22% for the CYP2C19 non-functional alleles and the increased expression allele CYP2C19*17, respectively [12].
Previous studies have investigated the impact of CYP2C19 genotype on serum concentration of sertraline, but the reported findings were not consistent in terms of the quantitative effects of the various CYP2C19 metabolizer subgroups on sertraline exposure [9, 13, 14]. In a study including healthy Chinese volunteers, Wang et al. found a 1.4-fold higher systemic exposure of sertraline in six CYP2C19 PMs vs. six IMs/NMs (merged group) [13]. The study of Wang et al. was carried out before the discovery of CYP2C19*17, but analysis of this allele in the study by Rudberg et al. reported no difference in the steady-state concentration of sertraline between homozygous CYP2C19*17 carriers (UM, n = 9) and CYP2C19*1/*1 carriers (NM, n = 42) in a Caucasian patient population [14]. In the latter study, a 3.2-fold higher concentration was observed in the CYP2C19 PMs (n = 5) vs. the NMs. Recently, Saiz-Rodriguez et al. found no difference between a mixed group of CYP2C19*1/*17 and *17*17 carriers (total n = 9) and CYP2C19 NMs (n = 28) in a healthy Spanish population without including any CYP2C19 PMs [9].
Owing to the inconsistent findings and a limited number of subjects included in the previous studies, in particular with respect to the extremes of CYP2C19-metabolizer subgroups, the overall effect of CYP2C19 genotype on sertraline metabolism remains to be ascertained. The primary aim of the present study was therefore to investigate the impact of CYP2C19 genotype on the serum concentrations of sertraline and its major metabolite N-desmethylsertraline in a large patient population. Second, the risk of obtaining serum concentrations outside the target therapeutic range of sertraline was assessed in relation to CYP2C19 genotype.
Methods
Patient inclusion
Relevant data from patients who had performed at least one serum concentration measurement of both sertraline and N-desmethylsertraline, as well as CYP2C19 genotyping, were retrospectively retrieved from a therapeutic drug monitoring (TDM) database at the Center for Psychopharmacology, Diakonhjemmet Hospital (Oslo, Norway), during the period 1 January 2007–6 December 2018. The patients were mainly of Caucasian origin, but the ethnicity on an individual level could not be disclosed owing to privacy issues.
Inclusion criteria were a positive serum concentration measured at steady state through conditions, information about the prescribed sertraline daily dose and information about co-medications according to details provided on the requisition forms. Thus the information about co-medications was limited by the list written by the physician on the TDM requisition form, which is generally only complete with respect to drugs acting on the central nervous system. Information about sex and age were also retrieved from the requisition forms.
Exclusion criteria were (i) co-medication with the enzyme inducers phenobarbital, phenytoin and carbamazepine (increasing both CY2C19 and CY3A4 metabolism) and the enzyme inhibitors omeprazole, esomeprazole, lansoprazole, pantoprazole, fluoxetine and fluvoxamine, (ii) measured serum concentration of sertraline and/or metabolite below the lower limit of quantification, (iii) a time interval <10 h or >30 h between the last dose intake of sertraline and blood sampling for TDM, and (iv) prescribed daily doses above >200 mg, due to non-linearity in the dose–concentration relationship of sertraline at higher doses.
The study was approved by the Norwegian Regional Committee for Medical and Health Research Ethics (#2018/1848) and the Investigational Review Board at Diakonhjemmet Hospital. Ethical approval was given without requirement for patient consent as the study was based on existing data retrospectively retrieved from a routine TDM service.
Serum concentration analysis of sertraline and N-desmethylsertraline
Two different liquid chromatography–tandem mass spectrometry (LC-MS/MS) methods were used for routine TDM analysis of sertraline and N-desmethylsertraline at the Center for Psychopharmacology during the inclusion period. The methods were the same in terms of sample preparation (protein precipitation), mobile phase composition (gradient elution) and internal standards. The most recent method was based on ultra-high-performance LC (UHPLC), while the former used ordinary high-performance LC. The methods were cross-validated and showed similar intra- and inter-run imprecision and inaccuracy parameters (<5%).
Briefly, in the latest method, serum samples were purified by protein precipitation mixing 200 µL of serum aliquot with 400 µL of acetonitrile–methanol (90/10 vol/vol), which included the internal standard (sertraline-13C6 and N-desmethylsertraline-13C6), followed by centrifugation for 10 min (4000 rpm at 4 °C), 4 µL of purified sample was then injected into a Vanquish Binary UHPLC system coupled to a Q Exactive Orbitrap HRAM LC-MS/MS with electrospray ionization operated in positive ionization mode (Thermo Scientific, Waltham, MS, USA). Chromatographic separation was performed on a XBridge BEH C18 column (2.5 µm, 2.1 × 75 mm; Waters). The mobile phase gradient comprised a mixture of acetonitrile and ammonium acetate buffer (pH 4.8).
Therapeutic serum concentration range of sertraline
The therapeutic serum concentration range of sertraline is not well defined. The ‘Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie’ (AGNP) suggests a reference range of 30–500 nM [15]. At 30 nM (~10 ng/mL), approximately 80% of serotonin transporter (SERT) is occupied by sertraline, as shown in study from Meyer et al. using positron-emission tomography imaging [16]. Thus the lower boundary of the AGNP guideline seems rational from a therapeutic point of view; the defined upper boundary of 500 nM (~150 ng/mL) may appear too high, taking into account that 80% degree of SERT inhibition is already reached at 30 nM [16]. In the present study, we therefore used 30–250 nM (~10–75 ng/mL) as the target therapeutic range of sertraline, which is equal to the target range defined in our local TDM service at the Center for Psychopharmacology and represents the 95% percentile of sertraline samples in our laboratory.
Genotyping
Analyses of CYP2C19 variant alleles were performed using TaqMan-based real-time PCR assays implemented for routine pharmacogenetic analysis at the Center for Psychopharmacology. The genotyping panel for CYP2C19 included the null alleles CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893) and CYP2C19*4 (rs28399504) and the increased expression allele CYP2C19*17 (rs12248560).
Patients were divided into five phenotype subgroups based on their CYP2C19 genotype: (i) PMs, homozygous carriers of CYP2C19 null alleles, i.e. *2, *3 or *4, (ii) IMs, heterozygous carriers of null alleles, (iii) NMs, absence of variant alleles (CYP2C19 *1/*1), (iv) RMs, heterozygous carriers of the increased expression allele CYP2C19*17 (CYP2C19*1/*17), and (v) UMs, homozygous carriers of CYP2C19*17 (CYP2C19 *17/*17). Patients carrying the CYP2C19null/*17 diplotype were defined as IMs.
Additionally, for the majority of the patients (n = 996) we also retrieved genotyping results for CYP2C9 and CYP2D6 from our laboratory database, as this combination of CYP genotypes (CYP2C9, CYP2C19 and CYP2D6) is usually ordered by the physicians. Genotyping of CYP2C9 included CYP2C9*2 (rs1799853) and CYP2C9*3 (rs1057910), while CYP2D6 genotyping included the null alleles CYP2D6*3 (rs35742686), CYP2D6*4 (rs3892097), CYP2D6*5 (whole gene deletion) and CYP2D6*6 (rs5030655), the reduced-function alleles CYP2D6*9 (rs5030656), CYP2D6*10 (rs1065852) and CYP2D6*41 (rs28371725), as well as copy number analysis to identify multiplication of the CYP2D6 gene giving rise to ultra rapid metabolism. Patients were also divided into phenotype subgroups for both CYP2C9 (NM, IM and PM) and CYP2D6 (UM, NM, IM and PM), according to Supplementary Tables S1 and S2, respectively.
Endpoints and statistics
The primary study endpoint was the effect of genotype-predicted CYP2C19 metabolizer phenotype on the sertraline exposure and exposure of its primary metabolite N-desmethylsertraline. Secondarily, the proportion of patients above or below the defined target concentration range for sertraline, i.e. 30–250 nM, was assessed in relation to CYP2C19 phenotype defining CYP2C19 NMs as the reference group. In preliminary analyses, there were no significant differences (p > 0.96) between the CYP2C19 RMs and UMs, and these subgroups were therefore merged in the statistical analyses.
Prior to the statistical analysis, the measured serum concentrations were adjusted for the daily prescribed doses to enable comparisons of the sertraline exposure between the CYP2C19 genotype subgroups. Further, to provide measures related to the defined target therapeutic range, the serum concentrations were adjusted (harmonized) according to the standard recommended daily dose of 100 mg/day. Harmonization was performed by first dividing the measured serum concentration (nM) by the patient’s prescribed sertraline dose (mg) written on the requisition form, to obtain the concentration-to-dose ratio (C/D ratio) as described by AGNP in the consensus guidelines for TDM [15]. The C/D ratio was then multiplied by 100 mg, i.e. the standard recommended daily dose. To determine the effect of CYP2C19 genotype on N-desmethylsertraline-to-sertraline metabolic ratio, ratios were compared between the CYP2C19 phenotype subgroups (NM was defined as reference).
To account for the variable number of measurements per patient in our study population, the effect of CYP2C19 genotype on exposure and metabolic ratio of sertraline was estimated by multivariate linear mixed model analyses. The multivariate linear mixed-model analyses were performed using sertraline, N-desmethylsertraline concentration or metabolic ratio as the dependent variable, with patient number defined as a mixed-effect (random) variable and CYP2C19 genotype, sex, age and time between last dose and sampling as fixed-effect variables.
Further, a second multivariate linear mixed model analysis of all patients with ‘complete sets’ of genotypes was performed using sertraline concentration or metabolic ratio as the dependent variable, with patient number as random effect and CYP2C19, CYP2C9 and CYP2D6 genotypes, sex, age and time between last dose and sampling as fixed-effect variables.
The linear mixed-model analysis allows for inclusion of multiple measurements per patient. Because of the non-normality of dose-harmonized serum concentrations of sertraline and N-desmethylsertraline, these variables were log(ln)-transformed before linear mixed model analyses. Subgroup estimates were back transformed for data presentation.
The odds ratio (OR) for a patient to have ‘one or more’ TDM sample of measured (actual) sertraline serum concentration below or above the target concentration range of 30–250 nM was analysed by logistic regression with CYP2C19 NMs defined as reference group. In these analyses, the OR estimate were adjusted for age, sex, dose and sampling time.
Statistical analysis was performed using R, version 3.5.1 and RStudio. A p value < 0.05 was considered as statistically significant.
Results
The study included a total of 1202 patients with known CYP2C19 genotype and ≥1 valid TDM measurements of sertraline and N-desmethylsertraline (in total 2190 measurements). A total of 222 patients (264 TDM measurements) were excluded owing to the time interval between the last dose intake of sertraline and blood sampling for TDM and the use of the specific CYP3A4/2C19 enzyme inducers and inhibitors, as indicated on the requisition form. A total of 58 TDM measurements from 39 patients were also excluded owing to sertraline doses >200 mg; however, none of the patients were completely excluded as all patients had multiple TDM measurements. Among these 39 patients, CYP2C19 UMs were overrepresented (n = 13; 33%).
The allelic frequency of CYP2C19null (16.3%) and CYP2C19*17 (20.3%) observed in this study were in concordance with frequencies observed in Europeans in general [12]. The CYP2C19 genotype distribution in the included patient population is shown in Table 1, and the age, sex, daily dose and blood sampling times in the various genotype-predicted CYP2C19 phenotype subgroups are presented in Table 2.
Table 1.
CYP2C19 genotype (diplotype), predicted phenotype, distribution, and CYP2C19 allelic frequency in the included patient population
| Genotype | Predicted phenotype | n | Frequency (%) |
|---|---|---|---|
| *17/*17 | UM | 58 | 4.8 |
| *1/*17 | RM | 293 | 24.4 |
| *1/*1 | NM | 495 | 41.2 |
| *1/null | IM | 242 | 20.1 |
| *17/null | 79 | 6.57 | |
| null/null | PM | 35 | 2.91 |
| Total | 1202 |
| Allelic frequency | n | Frequency (%) | |
|---|---|---|---|
| *1 | 1525 | 63.4 | |
| *17 | 488 | 20.3 | |
| *2 | 384 | 16.0 | |
| *3 | 5 | 0.21 | |
| *4 | 2 | 0.08 | |
| Total | 2404 |
Null = loss-of-function alleles, i.e. the presence of CYP2C19*2, CYP2C19*3, and CYP2C19*4
Table 2.
Characteristics of the various genotype-predicted CYP2C19 phenotype subgroups
| Measure | Genotype-predicted CYP2C19 phenotype | |||
|---|---|---|---|---|
| UM (N = 351) | NM (N = 495) | IM (N = 321) | PM (N = 35) | |
| Number of samples, n | 604 | 918 | 580 | 88 |
| Females, n (%) | 236 (67) | 317 (64) | 217 (68)a | 22 (63)a |
| Age (years), mean (SD) | 45.6 (22.8)a | 43.2 (20.3) | 46.8 (22.2)a | 31.6 (14.1)a |
| Drug dosage (mg/day), mean (SD) | 106.4 (52.0) | 108.2 (53.4) | 97.0 (49.4)a | 113.4 (49.8) |
| Time between last drug intake and blood sampling (h), mean (SD) | 20.6 (5.6) | 20.3 (5.4) | 20.6 (5.4) | 18.9 (5.7)a |
UMs comprising both CYP2C19*1/*17 and CYP2C19*17/*17
IMs comprising both CYP2C19Null/*1 and CYP2C19Null/*17
aIndicates significant difference (p < 0.05) compared to NM; linear mixed model was used to account for multiple samples per patient
Impact of genotype-predicted CYP2C19 phenotype on sertraline exposure and metabolism
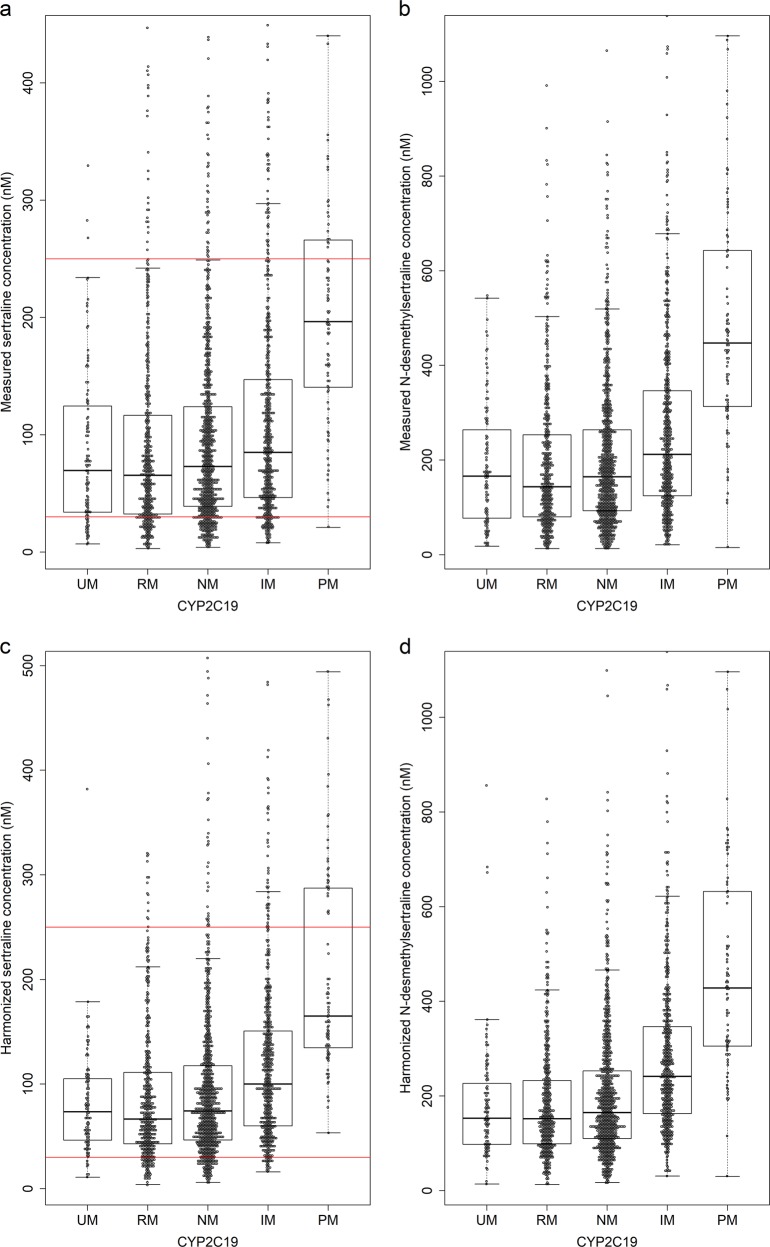
Results of the mixed model analysis of sertraline, N-desmethylsertraline and the metabolic ratio are presented in Table 3. The harmonized sertraline serum concentrations in CYP2C19 PMs and IMs were 2.68-fold (p < 0.001, 95% confidence interval [CI] 2.16–3.31) and 1.38-fold (p < 0.001, 95% CI 1.26–1.50) higher, respectively, compared to CYP2C19 NMs (Fig. 1 and Table 3). A 3.00-fold (p < 0.001, 95% CI 2.46–3.66) and 1.47-fold (p < 0.001, 95% CI 1.35–1.60) higher concentration of N-desmethylsertraline was found in PMs and IMs, respectively, compared to NMs (Fig. 1 and Table 3). Among CYP2C19 UMs, the mean estimated serum concentration of both sertraline and N-desmethylsertraline was 10% lower compared to NMs (p = 0.012 and p = 0.016, respectively) (Table 3). Age and sex were found to be significant covariates explaining individual variability in sertraline serum concentration. A 1.13-fold higher serum concentration of sertraline was detected in women compared to men (p = 0.0017, 95% CI 1.05–1.22), while patients aged >65 years (n = 480) had a 1.12-fold higher serum concentration of sertraline compared to younger patients (p = 0.008, 95% CI 1.03–1.21).
Table 3.
Estimated harmonized serum concentration of sertraline and N-desmethylsertraline and metabolic ratio (MR) in different genotype-predicted CYP2C19 phenotype subgroups (NM reference subgroup in statistical analyses)
| Subgroup | Patients (n) | Samples (n) | Sertraline (nM) harmonized to 100 mg/day (95% CI) | Fold changea | p | N-desmethylsertraline (nM) harmonized to 100 mg/day (95% CI) | Fold changea | p | MR (95% CI) | Fold changea | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UM | 351 | 604 | 92.7 (85.1–101.1) | 0.89 | 0.012 | 144.2 (133.1–158.9) | 0.91 | 0.016 | 1.491 (1.374–1.610) | 1.03 | 0.517 |
| NM | 495 | 918 | 103.6 (91.8–116.8) | — | — | 159.2 (142.5–177.9) | — | — | 1.453 (1.270–1.635) | — | — |
| IM | 321 | 580 | 142.9 (130.8–156.2) | 1.38 | <0.001 | 234.3 (215.7–254.4) | 1.47 | <0.001 | 1.658 (1.537–1.779) | 1.14 | <0.001 |
| PM | 35 | 88 | 277.4 (224.1–343.5) | 2.68 | <0.001 | 477.9 (391.8–582.9) | 3.00 | <0.001 | 1.835 (1.547–2.123) | 1.26 | 0.009 |
Linear mixed model analysis was adjusted for age (p < 0.001), gender (<0.001) and time between last dose administration and serum sampling (p < 0.001). To convert nmol/L to ng/mL, multiply by a factor of 3.27 and 3.43 for sertraline and N-desmethylsertraline, respectively
aDifference compared with noncarriers (*1/*1)
Fig. 1.
Measured (actual) serum concentration of a sertraline, red lines indicate the therapeutic reference range (30–250 nM), and b N-desmethylsertraline in CYP2C19 phenotypes and harmonized serum concentration of c sertraline, red lines indicate the therapeutic reference range (30–250 nM), and d N-desmethylsertraline in CYP2C19 phenotypes
Compared with NMs, the N-desmethylsertraline-to-sertraline metabolic ratio was 1.26-fold higher in PMs (p = 0.009, 95% CI 1.07–1.46) and 1.14-fold higher ratio in IMs (p < 0.001, 95% CI 1.06–1.22) (Table 3). No significant difference in metabolic ratio between UMs and NMs was observed (p = 0.517). Age and sex were found to be significant covariates explaining individual variability in sertraline-to-N-desmethylsertraline metabolic ratio. A 1.22-fold higher ratio was detected in patients aged >65 years compared to younger patients (p < 0.001, 95% CI 1.15–1.31), while women had a 1.13-fold higher metabolic ratio compared to men (p < 0.001, 95% CI 1.07–1.21).
Genotype-predicted CYP2C9 or CYP2D6 phenotype groups were not significant predictors of sertraline exposure or metabolism, as shown in the subsequent analysis (Supplementary Table S3).
Genotype subgroup proportions below/above the sertraline target concentration range
Harmonized and measured (actual) serum concentration of sertraline below or above the target concentration range for the various CYP2C19 genotype-predicted phenotype subgroups are presented in Table 4. In CYP2C19 PMs and IMs, the OR for having one or more TDM measurements above the target concentration range of 250 nM was 8.69 (p < 0.001, 95% CI 3.88–19.19) and 1.97 (p = 0.064, CI: 1.21–3.21), respectively, compared to CYP2C19 NMs. Furthermore, the OR for having ≥1 TDM measurements below the target concentration range of 30 nM in CYP2C19 UMs was 1.31 (p = 0.098, CI: 0.95–1.80) compared to NMs. Among CYP2C19 IMs, the OR was 0.67 (p = 0.032, CI: 0.46–0.96), compared to NMs. Only one patient with the CYP2C19 PM phenotype had sertraline concentration <30 nM; OR = 0.10 (p = 0.026, CI:0.01–0.488).
Table 4.
Odds ratio for TDM measurement below and above the therapeutic target range for measured (actual) and harmonized sertraline concentration in different genotype-predicted CYP2C19 phenotype subgroups
| Genotype | Patients (n) | Sertraline levels <30 nM | Sertraline levels >250 nM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | OR | (95% CI) | p | n | (%) | OR | (95% CI) | p | ||
| Measured concentration | |||||||||||
| UM | 351 | 99 | (28.2) | 1.31 | (0.95–1.80) | 0.098 | 20 | (5.7) | 0.84 | (0.46–1.47) | 0.541 |
| NM | 495 | 111 | (22.4) | — | — | — | 36 | (7.3) | — | — | — |
| IM | 321 | 54 | (16.8) | 0.67 | (0.46–0.96) | 0.032 | 39 | (12.1) | 1.97 | (1.21–3.21) | 0.064 |
| PM | 35 | 1 | (2.9) | 0.10 | (0.01–0.488) | 0.026 | 14 | (40.0) | 8.69 | (3.88–19.19) | <0.001 |
| Harmonized concentration | |||||||||||
| UM | 351 | 52 | (14.8) | 1.15 | (0.77–1.71) | 0.489 | 12 | (3.4) | 0.65 | (0.31–1.27) | 0.221 |
| NM | 495 | 65 | (13.1) | — | — | — | 36 | (5.3) | — | — | — |
| IM | 321 | 19 | (5.9) | 0.41 | (0.24–0.69) | 0.001 | 34 | (10.6) | 2.17 | (1.27–3.74) | 0.004 |
| PM | 35 | 0 | 0 | N/A | (N/A) | N/A | 17 | (48.6) | 17.8 | (8.09–39.16) | <0.001 |
N/A not applicable
Discussion
This is the first study comprising a large number of patients in order to quantify the effect of the CYP2C19 genotype, including the increased expression CYP2C19*17 allele, on sertraline exposure. The results show that CYP2C19*17 is unlikely to have any impact on the therapeutic response of sertraline treatment, while the raised serum concentrations among CYP2C19 PMs and IMs may imply an increased risk of overexposure and consequently adverse drug reactions (ADRs). The latter was supported by the observation that more than ten times as many of the CYP2C19 PMs had serum concentrations above the therapeutic target range compared to CYP2C19 NMs. Thus our results indicate that, in clinical practice, patients with reduced CYP2C19 metabolism would require lower daily doses of sertraline for optimal treatment, while NMs might be underexposed.
CYP2C19 PMs have in preliminary studies been reported to obtain significantly higher serum concentrations of sertraline compared to NMs [8, 13, 14]. In this study, PMs and IMs exhibited a 2.68- and 1.38-fold higher sertraline serum concentration, respectively, compared to NMs, caused by the absence or reduced CYP2C19-mediated sertraline metabolism. This is in line with a previously published smaller study [14]. Clinical guidelines recommend a 50% dose reduction of sertraline in CYP2C19 PMs [11], as high serum concentrations of sertraline may increase the probability of ADRs. A clear relationship between dose and dropout due to ADRs were found in a meta-analysis by Furukawa et al. [17], investigating the optimal target dose and recommended dosing range of SSRIs, venlafaxine and mirtazapine. Saiz-Rodriguez et al. [9] also observed a tendency to more ADRs in individuals with reduced CYP2C19 activity. According to our estimate in this large patient population defining NMs as the reference group, a 60% lower starting dose of sertraline will generally be sufficient in CYP2C19 PMs, while a 25% dose reduction should be considered in CYP2C19 IMs, based on the relative difference between IMs and PMs compared to NMs.
These suggestions in dose-reduction estimates in patients with decreased CYP2C19 metabolism are generally in line with previous recommendations [11]. However, an important point to consider is the fact that the reference group of these estimates are CYP2C19 NMs, and not the ‘average’ metabolizing patient. Thus an interesting observation was that the minimum recommended dose of 50 mg/day is possibly insufficient to reach the lower therapeutic limit of 30 nM in patients being CYP2C19 NM. This may explain the results acquired by a meta-analysis of fixed-dose trials of three SSRIs, where no apparent difference in antidepressant effect was found between sertraline doses of 100, 200 and 400 mg, whereas 50 mg produced a lower response [18]. A superior effect of higher sertraline doses (240–300 mg) compared to medium doses was, however, reported in a previous meta-analysis [19]. Neither of the meta-analyses addressed the relevance of the individual variability in sertraline serum concentration or CYP2C19 genotypes, but the results clearly indicate that rapid sertraline metabolism, which is observed here in NMs and UMs, may cause therapeutic failure due to insufficient exposure.
In a previous study on escitalopram, CYP2C19 *1/*17 and *17/*17 carriers obtained a 10% and 20% lower serum concentration compared to CYP2C19 NMs, respectively [20]. For sertraline in the current study, patients carrying the CYP2C19*17 allele only had an 8% lower serum concentration compared to NMs. The marginal effect of the CYP2C19*17 allele on CYP2C19 metabolism in this large study population shows that carriers of the CYP2C19*17 allele encodes a close to normal sertraline metabolism without the requirements of a genotype-based dose adjustments.
Currently, the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for sertraline recommend that an alternative SSRI, not predominantly metabolized by CYP2C19, should be considered if the patient is not responding to adequate doses of sertraline [11]. However, this is not supported by our data in CYP2C19 UMs, as CYP2C19*17 did not exhibit a clinically significant effect on the serum concentration of sertraline. TDM analyses could instead be a helpful tool in patient treatment with sertraline in order to disclose potential underexposure and risk of lacking treatment response.
The quantitative impact of CYP2C19 genotype on sertraline metabolism and exposure found in this study suggests that CYP2C19 is responsible for a lower proportion of the clearance of sertraline compared to escitalopram. The small differences in metabolic ratio (sertraline to N-desmethylsertraline) between the different genotype subgroups indicates that other enzymes than CYP2C19 are important for the metabolism of sertraline to N-desmethylsertraline.
It is known that multiple CYP enzymes, and in particular CYP2B6 and CYP3A4, play a role in the N-desmethylation of sertraline to N-desmethylsertraline [8, 21]. The significant effect of CYP2C19 on sertraline and N-desmethylsertraline concentration indicates that CYP2C19 is involved in the metabolism of sertraline; however, the small differences in sertraline-to-N-desmethylsertraline ratio between the different CYP2C19 phenotype groups also indicates that other enzymes might be more important in the N-desmethylation of sertraline. CYP2C19 is also involved in the formation of sertraline ketone [8] and our results indicates that CYP2C19 might contribute more to the formation of sertraline ketone and the subsequent metabolism of N-desmethylsertraline than the N-desmethylation of sertraline to N-desmethylsertraline. Thus it is likely that individual variability in CYP2B6 and/or CYP3A4 metabolism also determine the exposure and metabolism of sertraline. Further assessment of sertraline metabolism should include complete genotype results of these enzymes as well. There is a lack of interaction studies between sertraline and CYP3A4 inhibitors, but a case report describing a serotonin syndrome in a sertraline-treated 12-year-old boy comedicated with the CYP3A4 inhibitor erythromycin supports that CYP3A4 may have an important role in sertraline metabolism [22].
This study has some limitations associated with the use of TDM data. One is that information about comedication rely on the details provided by the physicians when filling out the requisition forms. Another limitation to this study is the potential use of CYP3A4 and CYP2B6 inhibitors. Even though the use of CYP2B6 inhibitors in clinical practice are limited and CYP3A4 inhibitors seems to not affect sertraline metabolism [23], it cannot be excluded as a potential source of variability in the material. Further, other factors potentially affecting the serum concentration of sertraline, e.g. body weight, renal function, liver function, inflammatory state, somatic diseases and/or partial nonadherence, could not be controlled for, although these are probably outweighed by the large cohort of included patients. Finally, we had no access to clinical information about the treatment response, ADRs or the underlying diagnosis (anxiety or depression), which prohibited direct assessments of the clinical impact of CYP2C19 for the response of sertraline treatment.
The definition of the therapeutic range is not definitive and only indicative in this study and should be mentioned as a limitation, especially since there were no data on ADRs or actual treatment outcome. Furukawa et al. [17] showed a clear dose dependency in dropouts due to adverse effects and the overall acceptability of treatments appeared to be optimal towards the lower end of the licensed dosing range. The defined upper target concentration of sertraline of 250 nM represents the 95% percentile of our samples and it is equal to the target range defined by our local TDM service at the Center for Psychopharmacology. Also, as previously mentioned the defined upper boundary of the AGNP guidelines of 500 nM (~150 ng/mL) appear too high taking into account that 80% degree of SERT inhibition is already reached at 30 nM [16].
In conclusion, the significantly increased serum concentration of sertraline in CYP2C19 IM and PM patients in this study indicates that carriers of CYP2C19null alleles are at increased risk of overexposure and potentially adverse effects when treated with standard recommended doses of sertraline. This is supported by the tenfold increased proportion of CYP2C19 PMs vs. NMs with sertraline serum concentration above the target range of maximum occupancy of the SERT in the brain. Thus reduced doses and increased treatment monitoring should be considered when initiating sertraline in CYP2C19 IM and PM patients. The marginal decreased serum concentration in UM compared to NM patients in this study indicates that the CYP2C19*17 allele is of limited clinical relevance in sertraline treatment.
Funding and disclosure
The project was partly funded by the South-Eastern Norway Regional Health Authority. The authors declare no competing interests.
Supplementary information
Author contributions
LSB retrieved and analysed the data and wrote the manuscript; TH retrieved and analysed the data, MKK and EM designed the study and assisted in the data analyses; MKK, EM, TH, MJ and MI-S assisted in writing the manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0554-x).
References
- 1.Grimsley SR, Jann MW. Paroxetine, sertraline, and fluvoxamine: new selective serotonin reuptake inhibitors. Clin Pharm. 1992;11:930–57. [PubMed] [Google Scholar]
- 2.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundmark J, Reis M, Bengtsson F. Therapeutic drug monitoring of sertraline: variability factors as displayed in a clinical setting. Ther Drug Monit. 2000;22:446–54. doi: 10.1097/00007691-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Hedayati SS, Gregg LP, Carmody T, Jain N, Toups M, Rush AJ, et al. Effect of sertraline on depressive symptoms in patients with chronic kidney disease without dialysis dependence: the CAST randomized clinical trial. JAMA. 2017;318:1876–90. doi: 10.1001/jama.2017.17131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lydiard RB, Stahl SM, Hertzman M, Harrison WM. A double-blind, placebo-controlled study comparing the effects of sertraline versus amitriptyline in the treatment of major depression. J Clin Psychiatry. 1997;58:484–91. doi: 10.4088/JCP.v58n1104. [DOI] [PubMed] [Google Scholar]
- 6.Reis M, Aberg-Wistedt A, Agren H, Hoglund P, Akerblad AC, Bengtsson F. Serum disposition of sertraline, N-desmethylsertraline and paroxetine: a pharmacokinetic evaluation of repeated drug concentration measurements during 6 months of treatment for major depression. Hum Psychopharmacol. 2004;19:283–91. doi: 10.1002/hup.599. [DOI] [PubMed] [Google Scholar]
- 7.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–52. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 8.Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005;33:262–70. doi: 10.1124/dmd.104.002428. [DOI] [PubMed] [Google Scholar]
- 9.Saiz-Rodriguez M, Belmonte C, Roman M, Ochoa D, Koller D, Talegon M, et al. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics and safety of sertraline in healthy volunteers. Basic Clin Pharm Toxicol. 2018;122:501–11. doi: 10.1111/bcpt.12938. [DOI] [PubMed] [Google Scholar]
- 10.Sim SC, Risinger C, Dahl ML, Aklillu E, Christensen M, Bertilsson L, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharm Ther. 2006;79:103–13. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Hicks JK, Bishop JR, Sangkuhl K, Muller DJ, Ji Y, Leckband SG, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharm Ther. 2015;98:127–34. doi: 10.1002/cpt.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Ingelman-Sundberg M, Lauschke VM. Worldwide distribution of cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Clin Pharm Ther. 2017;102:688–700. doi: 10.1002/cpt.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JH, Liu ZQ, Wang W, Chen XP, Shu Y, He N, et al. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin Pharm Ther. 2001;70:42–7. doi: 10.1067/mcp.2001.116513. [DOI] [PubMed] [Google Scholar]
- 14.Rudberg I, Hermann M, Refsum H, Molden E. Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur J Clin Pharmacol. 2008;64:1181–8. doi: 10.1007/s00228-008-0533-3. [DOI] [PubMed] [Google Scholar]
- 15.Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, et al. Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry. 2018;51:9–62. doi: 10.1055/s-0043-116492. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JH, Wilson AA, Sagrati S, Hussey D, Carella A, Potter WZ, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–35. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa TA, Cipriani A, Cowen PJ, Leucht S, Egger M, Salanti G. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6:601–9. doi: 10.1016/S2215-0366(19)30217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hieronymus F, Nilsson S, Eriksson E. A mega-analysis of fixed-dose trials reveals dose-dependency and a rapid onset of action for the antidepressant effect of three selective serotonin reuptake inhibitors. Transl Psychiatry. 2016;6:e834. doi: 10.1038/tp.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, Bloch MH. Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry. 2016;173:174–83. doi: 10.1176/appi.ajp.2015.15030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jukic MM, Haslemo T, Molden E, Ingelman-Sundberg M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: a retrospective study based on 2,087 patients. Am J Psychiatry. 2018;175:463–70. doi: 10.1176/appi.ajp.2017.17050550. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Ishizuka T, Shimada N, Yoshimura Y, Kamijima K, Chiba K. Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metab Dispos. 1999;27:763–6. [PubMed] [Google Scholar]
- 22.Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy. 1999;19:894–6. doi: 10.1592/phco.19.10.894.31561. [DOI] [PubMed] [Google Scholar]
- 23.Lee AJ, Chan WK, Harralson AF, Buffum J, Bui BC. The effects of grapefruit juice on sertraline metabolism: an in vitro and in vivo study. Clin Ther. 1999;21:1890–9. doi: 10.1016/S0149-2918(00)86737-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.