Abstract
Mindfulness is thought to promote well-being by shaping the way people respond to challenging social-emotional situations. Current understanding of how this occurs at the neural level is based on studies of response to decontextualized emotion stimuli that may not adequately represent lived experiences. In this study, we tested relations between mothers' dispositional mindfulness and neural responses to their own infant in different emotion-eliciting contexts. Mothers (n = 25) engaged with their 3-month-old infants in videorecorded tasks designed to elicit negative (arm restraint) or positive (peekaboo) emotion. During a functional MRI session, mothers were presented with 15-s clips from these recordings, and dispositional mindfulness scores were used to predict their neural responses to arm restraint > peekaboo videos. Mothers higher in nonreactivity showed relatively lower activation to their infants’ arm restraint compared to peekaboo videos in hypothesized regions—insula and dorsal prefrontal cortex—as well as non-hypothesized regions. Other mindfulness dimensions were associated with more limited areas of lower (nonjudgment) and higher (describing) activation in this contrast. Mothers who were higher in mindfulness generally activated more to the positive emotion context and less to the negative emotion context in perceptual and emotion processing areas, a pattern that may help to explain mindfulness-related differences in well-being.
Keywords: Mindfulness, fMRI, Mother, Infant, Emotion
1. Introduction
Mindfulness—“paying attention on purpose, in the present moment, and nonjudgmentally” (Kabat-Zinn, 1990)—has been shown to predict subjective and neural responses to emotional stimuli that in turn map onto well-being (e.g., Hölzel et al., 2011; Keng et al., 2011). Typically, these stimuli consist of simple, decontextualized emotion representations (i.e., words or unfamiliar adult faces) that may not adequately represent the social-emotional experiences shaping well-being. In particular, emotional exchanges within close relationships represent an under-studied area, despite emerging conceptualizations of mindfulness as an interpersonal process (e.g., Duncan et al., 2009). In this study, we seek to determine how mindfulness may help in coping with an important real-life emotional challenge by testing relations between dispositional mindfulness and mothers’ neural responses to their infant in situations that elicit more positive or more negative emotion.
Research on mindfulness has revealed discrepant patterns of neural responsiveness to emotional stimuli. On the one hand, studies have shown that participants higher in dispositional mindfulness exhibit greater prefrontal activation while regulating their response to emotional stimuli (Creswell et al., 2007; Modinos et al., 2010). Similarly, participants instructed to take a mindful approach to emotional stimuli (viewing negative images) have shown heightened activation across several prefrontal areas, including the superior frontal gyrus (SFG) and frontal pole, as well as the insular cortex (Murakami et al., 2015; Smoski et al., 2015). Mapping onto principles of mindfulness, this combination is thought to reflect an experiential awareness of emotion (represented by insula) complemented by a metacognitive awareness (represented by dorsal prefrontal regions). On the other hand, some studies have shown lower insula and/or prefrontal reactivity to negative emotion stimuli related to mindfulness (e.g., Farb et al., 2010; Haase et al., 2016; Taylor et al., 2011). Beyond possible differences related to the operationalization of mindfulness as a state, trait, or practice effect, divergent results may reflect contributions of different mindfulness dimensions. For example, Paul et al. (2013) examined dispositional mindfulness measured by the Five Facet Mindfulness Questionnaire (Baer et al., 2006) and found that one scale in particular—nonreactivity, or the ability to move past difficult experiences without getting caught up in them—predicted lower insula activation to negative images.
Another potentially important factor shaping divergent findings is the emotional response paradigm involved. Mindfulness cultivates a skillful approach to the demands of the situation, which may mean heightened or attenuated activity depending on the situation. Exemplified by the research cited above, knowledge of when and how mindfulness may influence neural activation to emotion cues has typically been limited by a focus on negative impersonal stimuli (though see Desbordes et al., 2012; Lutz et al., 2016; Taylor et al., 2011 for exceptions). In order to fully appreciate how mindfulness may help people to navigate their emotional landscapes, it is necessary to tap their responses to stimuli from their daily lives that vary in their anticipated and perceived emotional valence. One important emotional context that presents a mix of negative and positive stimuli is parenting an infant.
Mothers respond normatively to positive and negative infant emotional stimuli that serve as attachment signals—i.e., behaviors such as smiling and crying that keep the caregiver close by and responsive to the infant’s needs. The nature of this response is complex, encompassing a network of subcortical and cortical brain regions involved in perceptual processing (temporal, parietal, and occipital cortices), emotional responsiveness and empathy (insula, OFC, amygdala), and higher-order attentional and emotion regulation (dorsolateral prefrontal and anterior cingulate cortices) (see Swain, 2011). Recently, the concept of mindful parenting has been introduced to characterize caregivers who bring mindful awareness to the parenting context (Duncan et al., 2009). While behavioral research suggests more mindful parents are able to more skillfully interpret and respond to their child’s emotional signals (e.g., Duncan et al., 2015; Lippold et al., 2015), there is no research to date addressing how this plays out at the neural level.
As outlined in the mindfulness-emotional response literature above, greater dispositional mindfulness could predict higher or lower neural activation to emotional situations. In keeping with theorizing about mindfulness more broadly, we would expect a mindful mother to respond flexibly to what the situation demands. For example, even though the maternal brain should allocate resources to processing acute distress signals signaling pain or need, mindful mothers may be less reactive to lower-level expressions of negative emotion and instead may focus more attention on positive exchanges. Mindfulness-related activity may also depend on the aspect of mindfulness under consideration, with some dimensions (such as nonreactivity) playing a more important role in social-emotional responses. The present study was designed to shed light on how mindfulness shapes parent-infant emotional exchanges by relating maternal mindfulness to neural activation to her own infant in situations designed to elicit more negative vs. more positive emotion.
We assessed dispositional mindfulness and functional brain responses to infant videos at 3 months postnatal in a sample of mothers recruited for a larger longitudinal study of mother-infant stress regulation. Videos were taken in the home during tasks designed to elicit low-level frustration (arm restraint) and joy (peekaboo). Based on the theoretical and empirical literature discussed above, we hypothesized that maternal mindfulness—in particular, nonreactivity—would relate to differences in insula and prefrontal activation to the videos. Directional hypotheses were tentative, given conflicting findings in previous mindfulness research, but we generally expected that as mindfulness increased, mothers would show lower activation to the arm restraint emotion video compared to the peekaboo video.
2. Method
2.1. Participants
Mothers (n = 25) of 3-month-old infants were recruited from the Women Infants Children program and other community agencies serving low-income women in a mid-size Pacific Northwest city. Mothers’ ages ranged from 1 to 33 (M = 26.4, SD = 3.8). The majority of mothers were Caucasian (72%; 12% Latina; 8% Asian American; 8% Other) and married or living with a romantic partner (88%). Although most reported some education past high school (84%), only 24% had completed college or received a graduate degree, and the median household income was in the $20,000–$29,999 range. For more than half of mothers (56%) this was their first child (36% second child; 8% third child).1 Most infants were born on time (4% before 37 weeks and 8% after 41 weeks of pregnancy), and none had serious health problems. A vaginal delivery was reported by 56% of mothers, with 88% breastfeeding and 67% bed-sharing with their infant at the time of assessment. Over half of mothers (52%) reported having engaged in a form of contemplative practice (mostly yoga—only 8% indicated some form of meditation), and 31% reported currently engaging in that practice. All women gave informed consent prior to participation, and all study procedures were approved by the University of Oregon Institutional Review Board.
2.2. Dispositional mindfulness
Prior to the home visit described below, mothers completed a number of self-report questionnaires online using Qualtrics, including the Five Facet Mindfulness Questionnaire (FFMQ; Baer et al., 2006). The FFMQ measures dispositional mindfulness traits and is based on a factor analysis of existing mindfulness measures and comprises scales for Observing (e.g., “I pay attention to sensations, such as the wind in my hair or the sun on my face”), Describing (e.g., “Even when I’m feeling terribly upset, I can find a way to put it into words”), Acting with Awareness (e.g., “When I do things, my mind wanders off and I’m easily distracted” [reversed]), Nonjudging (e.g., “I tell myself I shouldn’t be feeling the way I’m feeling” [reversed]), and Nonreactivity (e.g., “When I have distressing thoughts or images, I am able to just notice them without reacting”). Internal consistencies for the scales were adequate (alphas = .79–.93).
2.3. Stimulus collection and presentation
During a home visit scheduled at approximately 12 weeks postnatal, a graduate research assistant conducted a clinical structured interview with the mother and videorecorded her infant during two mother-infant interaction tasks: peekaboo and arm restraint. The infant was placed so that s/he was face to face with the mother for both tasks, and the video focused on the baby’s face with as little background in the shot as possible. For peekaboo, the mother was told to cover her face with her hands and say “baby,” then open her hands and say “peekaboo” (see Montague and Walker-Andrews, 2001). This continued for up to 3 min, or until the infant showed expressions of joy (i.e., smiling, laughing). For the arm restraint task, the mother was asked to change the infant’s diaper and then hold his/her arms to their side for up to two minutes (see Moscardino and Axia, 2006). During that time, the mother was instructed to maintain a neutral expression and not talk to her baby. The same protocol was followed with an additional mother-infant dyad who did not participate in the rest of the study to generate unfamiliar infant peekaboo and arm restraint videos.
The 15 s video segment showing maximum positive (for peekaboo) or negative (for arm restraint) infant emotion was selected for presentation in the scanner. Presentation® software (Version 14.7, Neurobehavioral Systems, Inc. Berkely, CA, www.neurobs.com) was used to present video and rest blocks (each 15s) in counterbalanced order during two 7.5 min runs; each run contained 6 cycles of own infant positive, own infant negative, unfamiliar infant positive, unfamiliar infant negative, and rest. Participants were simply instructed to watch the videos and respond as they normally would without additional task demands in order to allow the most natural range of response to infant stimuli.
2.4. Scanning
MR imaging was carried out at the University of Oregon Robert and Beverly Lewis Center for Neuroimaging with a 3T Siemens Allegra 3 magnet. A standard 32-channel phase array birdcage coil was used to acquire data from the whole brain. Sessions began with a shimming routine to optimize signal-to-noise ratio, followed by a fast localizer scan (FISP) and Siemens Autoalign routine, then the 2 functional runs and anatomical scan.
2.4.1. Functional
T2*-weighted gradient echo sequence, TR = 2000 ms, TE = 30 ms, flip angle = 90°, 32 contiguous slices acquired ascending and interleaved, thickness = 4 mm, 64 × 64 voxel matrix; 226 vols per run.
2.4.2. Structural
T1-weighted 3D MP-RAGE sequence, TI = 1100 ms, TR = 2500 ms, TE = 3.41 ms, flip angle = 7°, 176 sagittal slices 1.0 mm thick, 256 × 176 matrix FOV = 256 mm
2.4.3. Post-scan ratings
After scanning, mothers watched all of the infant videos and rated both the infant’s and their own emotional valence during each video from −100 (maximum negative) to +100 (maximum positive), with 0 as the neutral point. On average, mothers’ ratings of infant emotion (M = 70.16) were significantly more positive than neutral for the peekaboo videos, t(24) = 9.53–11.92, p < .001, but average ratings for the arm restraint videos (M = 2.96) did not differ significantly from neutral, t(24)=.26–.72, ns. At the same time, mothers did rate both the infant’s and their own emotions as significantly more positive during the peekaboo compared to the arm restraint, t(24) = 4.46–7.04, p < .001, confirming the expected difference in valence—i.e., moving from more negative during arm restraint to more positive during peekaboo—across videos.
2.5. Data analysis
Functional imaging data were analyzed with tools from the fMRIB Software Library (FSL v.5.0.9). Preprocessing steps included motion correction with MCFLIRT, nonbrain structure removal with BET, spatial smoothing using Gaussian kernel 5-mm FWHM, intensity normalization using grand mean scaling, and high-pass temporal filtering (sigma = 65s). Within-subject time series data were analyzed using FILM with local autocorrelation correction, and boxcar models describing onset/offset of each sound stimulus were convolved with a double-gamma basis function. Functional data were registered to the participant’s own high-resolution structural image (6 DOF) and to a standard brain (Montreal Neurological Institute template; 12 DOF) using FLIRT. All data were checked for excessive motion (>1 mm) and artifacts.
Within-participant and group-level analyses were carried out using FEAT v.6.0. For each participant, four explanatory variables (EVs) modeled signal associated with own infant peekaboo, own infant arm restraint, unfamiliar infant peekaboo, and unfamiliar infant arm restraint videos; zero for all four stimulus EVs corresponded to rest. The contrast of parameter estimates (COPE) for own infant arm restraint > peekaboo was used to test primary hypotheses regarding response to own infant in a more negative vs. more positive emotional context2 (own infant peekaboo and arm restraint > rest contrasts were also tested to describe signal change associated with each stimulus relative to baseline). First-level COPE images were averaged across runs using fixed-effects analysis. These served as inputs to higher-level group analyses, conducted using FLAME to model random-effects components of mixed-effects variance. Group statistic images were thresholded using clusters determined by Z > 2.6 and a corrected cluster significance threshold of p = .05 (FWE) in the whole brain.
At the group level, centered mindfulness (FFMQ scale) scores were entered as continuous predictors of brain response. To visualize the data driving continuous mindfulness effects, spherical ROI’s (r = 3 mm) centered on activation peaks were used to compute percent signal change associated with own infant stimuli compared to rest and generate illustrative figures.
3. Results
Descriptive statistics and correlations among FFMQ mindfulness dimensions are shown in Table 1.
Table 1.
Five Facet Mindfulness Dimensions: Descriptives and Correlations.
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. Observing | – | ||||
| 2. Describing | .64* | – | |||
| 3. Acting with Awareness | .30 | .095 | – | ||
| 4. Nonjudging | −.17 | .13 | .28 | – | |
| 5. Nonreactivity | .36 | .41* | .08 | −.03 | – |
| M, SD | 3.26, .87 | 3.79, .95 | 4.25, .55 | 4.00, .71 | 2.96, .83 |
*p < .05.
3.1. Association of maternal mindfulness with brain response to infant
3.1.1. Primary contrast: arm restraint > peekaboo videos
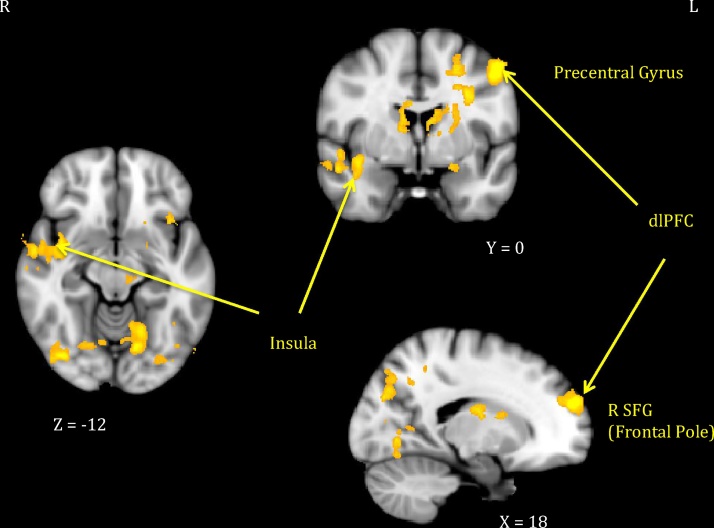
Of the five mindfulness facets entered as predictors of neural response, three related significantly to activation to own infant arm restraint > peekaboo videos (see Table 2). Mothers who reported higher Nonreactivity showed reduced signal in hypothesized regions: bilateral insula and prefrontal cortex (both dorsolateral and ventrolateral regions). They further showed lower signal across a range of cortical areas—bilateral temporal (including auditory regions and temporal pole), occipital (fusiform and lingual gyri), and parietal (including precuneus, supramarginal gyrus)—and subcortical regions (thalamus, right caudate). See Fig. 1 for illustration.
Table 2.
Mothers’ Neural Response to Own Infant Arm Restraint vs. Peekaboo Videos Related to Mindfulness.
| Peak Coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Brain Area | R/L | Volume (mm3) | Cluster p | Peak Z | X | Y | Z |
| Inversely related to Nonreactivity | |||||||
| 1. Temporal lobe (including auditory cortex, planum temporale); | L | 69385 | 1.5 × 10−35 | 5.04 | −39 | −29 | 4 |
| parietal lobe (including supramarginal gyri); | R/L | ||||||
| occipital lobe (including fusiform, lingual gyri); | R/L | ||||||
| thalamus | L | ||||||
| 2. Temporal lobe (including auditory cortex, pole) to insula, frontal operculum | R | 9186 | 2.1 × 10−8 | 4.52 | 43 | −25 | 5 |
| 3. Middle temporal gyrus | L | 4932 | .000036 | 4.79 | −58 | −66 | 1 |
| 4. Superior frontal gyrus – frontal pole | R | 4121 | .00018 | 4.66 | 19 | 60 | 25 |
| 5. Thalamus to caudate | R | 3706 | .00044 | 3.93 | 13 | −5 | 13 |
| 6. Precentral gyrus to superior frontal gyrus | L | 3671 | .00048 | 4.07 | −31 | 3 | 31 |
| 7. Ventrolateral prefrontal cortex to insula | L | 2123 | .018 | 3.61 | −29 | 11 | −15 |
| 8. Precentral gyrus | L | 1914 | .031 | 5.06 | −50 | 0 | 46 |
| 9. Precuneus | L | 1828 | .039 | 3.84 | −10 | −47 | 57 |
| Inversely related to Nonjudging | |||||||
| 1. Angular gyrus | R | 3019 | .0021 | 3.90 | 57 | −56 | 37 |
| 2. Precuneus to posterior cingulate cortex | R/L | 3012 | .0021 | 3.62 | 11 | −84 | 43 |
| 3. Middle to superior frontal gyrus | L | 2053 | .022 | 3.70 | −21 | 25 | 60 |
| Positively related to Describing | |||||||
| 1. Occipital fusiform to lingual gyrus | L | 2682 | .0045 | 3.85 | −17 | −76 | −2 |
| 2. Precuneus | R/L | 2612 | .0053 | 2.27 | −9 | −65 | 32 |
| 3. Superior parietal to supramarginal gyrus | R | 2441 | .0081 | 3.98 | 28 | −41 | 55 |
Fig. 1.
Maternal neural response (own infant arm restraint > peekaboo video) inversely related to nonreactivity.
Note. Activations thresholded at whole-brain FWE = .05 with voxel-level threshold of Z = 2.6. dlPFC = dorsolateral prefrontal cortex; R SFG = right superior frontal gyrus.
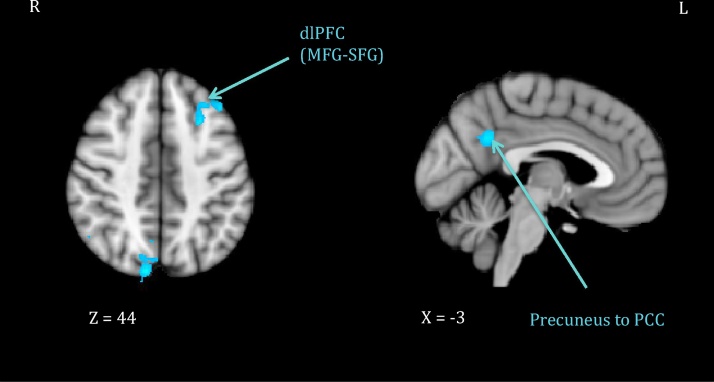
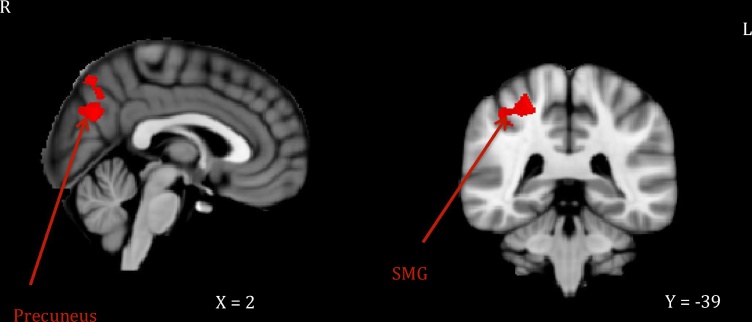
Mothers higher in Nonjudging also showed reduced signal in parietal areas (right angular gyrus, bilateral precuneus extending to posterior cingulate cortex) and in the left dorsolateral prefrontal cortex. Finally, mothers higher in Describing showed increased signal in several occipital (left fusiform to lingual gyrus) and parietal (bilateral precuneus, right superior parietal extending to supramarginal gyrus) areas. See Fig. 2, Fig. 3 for illustration of these effects.
Fig. 2.
Maternal neural response (own infant arm restraint > peekaboo video) inversely related to nonjudgment.
Note. Activations thresholded at whole-brain FWE = .05 with voxel-level threshold of Z = 2.6. dlPFC = dorsolateral prefrontal cortex; MFG = middle frontal gyrus; SFG = superior frontal gyrus; PCC = posterior cingulate cortex.
Fig. 3.
Maternal neural response (own infant arm restraint > peekaboo video) positively related to describing.
Note. Activations thresholded at whole-brain FWE = .05 with voxel-level threshold of Z = 2.6. SMG = supramarginal gyrus.
3.1.2. Follow-up analyses: arm restraint and peekaboo videos > rest
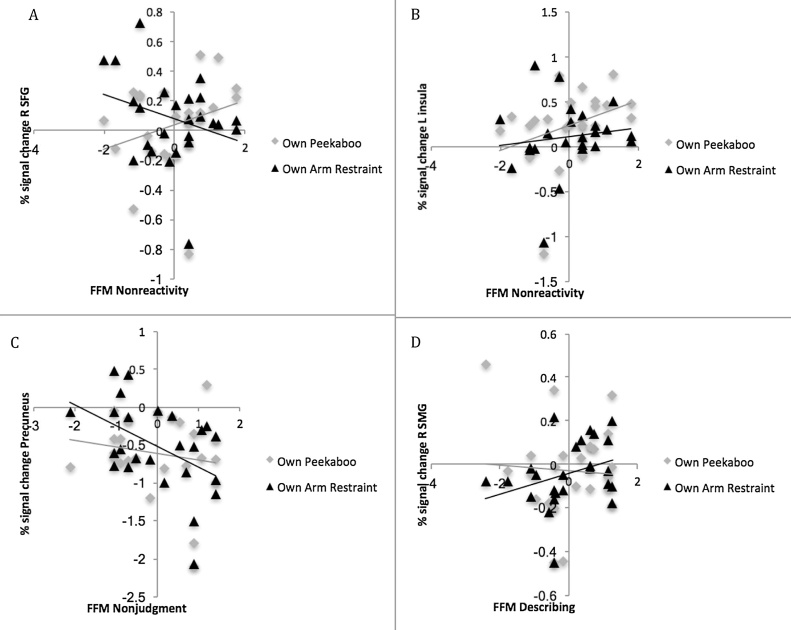
Contrasts of each own infant video > rest were examined to better understand the source of own infant arm restraint > peekaboo effects detected above. Nonreactivity effects were generally driven by increased signal to the peekaboo video, though the right superior frontal gyrus cluster showed a combination of greater activation to peekaboo and deactivation to arm restraint videos. Both the Describing and Nonjudging effects were driven by differential activation to the arm restraint video—increased signal (less deactivation) for the former, and decreased signal (greater deactivation) for the latter. Fig. 4 depicts illustrative signal change plots.
Fig. 4.
Signal change (relative to resting baseline) associated with own infant peekaboo and arm restraint videos by mindfulness facet.
Note. A. Percent change in right superior frontal gyrus related to nonreactivity; B. Percent change in left insula related to nonreactivity; C. Percent change in precuneus related to nonjudgment; D. Percent change in right supramarginal gyrus related to describing. R = right; SFG = superior frontal gyrus; L = left; SMG = supramarginal gyrus.
4. Discussion
In this study we demonstrated differential maternal neural responses to their own infant in more negative (arm restraint) vs. more positive (peekaboo) emotional contexts associated with dispositional mindfulness. As hypothesized, mothers higher in the nonreactivity dimension of mindfulness showed relatively lower activation to their infants’ arm restraint compared to peekaboo videos in brain regions involved in the felt experience (insula) and metacognitive awareness (dorsal prefrontal cortex) of emotion. In addition, mothers higher in both nonreactivity and nonjudgment showed lower activation to arm restraint vs. peekaboo videos across regions involved in perceptual, language and memory processing, while mothers higher in describing showed the opposite. Below, we consider how these results fit with and expand on previous knowledge of how mindfulness influences responsiveness to emotion-eliciting situations.
The current findings add to an evolving understanding of when mindfulness contributes to higher vs. lower neural activation to emotional stimuli; we found effects in both directions associated with different aspects of mindfulness. On the one hand, nonreactivity and nonjudgment related to lower brain activity in response to own infant negative compared to positive emotion contexts. These dimensions of mindfulness involve taking a step back from difficult experience, allowing it to happen without engaging in internal struggle. Thus, it makes sense that these characteristics could serve to dampen evaluative and executive control-related functions supported by the precuneus and dorsolateral prefrontal cortex (e.g., Kübler et al., 2006; Smith et al., 2017; Zysset et al., 2002). The describing dimension of mindfulness, on the other hand, related to higher brain activity in this contrast. It is possible that this mindfulness dimension requires a certain type of engagement with negative emotion-eliciting situations in order to generate a useful description of the experience. In this instance, occipital fusiform activity could help mothers to accurately perceive their infant’s facial cues, which are then interpreted in light of autobiographical memories and in-the-moment empathic resonance enabled by the precuneus and right supramarginal gyrus (e.g., Lawrence et al., 2006; Rämä et al., 2001; Rissman et al., 2016). Together, it appears that mindfulness facets may underlie a complex tuning of heightened and dampened neural activation likely to serve the demands of parenting an infant—i.e., allowing the mother to discern what the infant needs without becoming overly entangled or emotionally dysregulated herself.
A closer examination of mothers’ responses to situations meant to induce negative or positive infant emotions helps to further appreciate the nuance of mindfulness-related effects. Based on follow-up analyses of maternal neural response to each video relative to rest, describing effects had to do with enhancing, and nonjudgment effects with dampening, responsiveness specifically to the arm restraint video. By contrast, nonreactivity effects had less to do with downregulating brain response to the arm restraint video, and more to do with upregulating response to the peekaboo video. More nonreactive mothers may allocate more attention and perceptual/emotional processing resources to moments of playfulness and joy with their infants, as opposed to moments of anticipated or actual struggle and frustration, thus improving the overall quality of their emotional landscape. It is also possible that a different pattern would emerge if stimuli involving more severe infant distress were used, in which case the skillful response would involve attention to the negative cues. Still, the present results suggest that mindfulness may exert beneficial effects, at least in part, by reversing the usual attentional bias toward the relatively negative and allowing greater appreciation of the positive.
As in a previous neuroimaging study of dimensions of dispositional mindfulness (Paul et al., 2013), nonreactivity emerged as the dimension most widely predictive of brain response. At least when it comes to emotional functioning, this quality appears to play a decisive role, and future mindfulness research may benefit from distinguishing such dimensional effects. Nonreactivity related to differential activation across a variety of brain regions in this study, some overlapping with those highlighted in prior mindfulness research. The insula has been found less active during exposure to aversive interoceptive stimuli or inhibition of response to negative stimuli in those with mindfulness training or higher dispositional mindfulness (Haase et al., 2016; Paul et al., 2013). This area has also been shown to be more active during mindful observation or acceptance of emotion stimuli (Murakami et al., 2015; Smoski et al., 2015), and during affect labeling in those with higher dispositional mindfulness (Creswell et al., 2007). It may be that with greater mindfulness, the insula becomes more sensitive to the visceral cues associated with positive emotions, and less sensitive—at least, when the skillful response is to move beyond those cues—to those of distress. This would fit with the proposal that changes in insula responsiveness represent a “bottom-up” mechanism by which mindfulness modulates emotional responses.
We also found nonreactivity-related differences in activation of prefrontal regions involved in “top-down” regulation and highlighted in previous mindfulness research. Dorsal and rostral portions of PFC—especially the SFG—have been invoked during mindful observation or acceptance of emotional stimuli (Murakami et al., 2015; Smoski et al., 2015) and during affect labeling in more mindful individuals (Creswell et al., 2007). This may be relevant to the “decentering” skill—i.e., the ability to approach experience from a less ego-centered perspective—highlighted in certain definitions of mindfulness (Lau et al., 2006), which further facilitates skillful engagement with the reality of a situation. In the present context, mothers’ responsiveness to their infant at both the automatic (insula) and intentional (SFG) levels of emotional awareness could support mindful parenting behaviors (see Duncan et al., 2009), a possibility that should be addressed in future research.
Several other non-hypothesized brain regions showing differential activity related to nonreactivity merit further investigation. The thalamus may play an important role in switching between mind-wandering and mindfulness (Wang et al., 2014), and other mindfulness researchers have suggested that activation of language processing networks reflects verbal labeling of internal experiences (Murakami et al., 2015). More fine-grained research on the sequence of dynamic responses associated with different subjective aspects of mindful awareness will be needed to fully appreciate how each of these regions contributes to an integrated neurocognitive response.
The present findings should be interpreted in light of several limitations that can be used to inform future research in this area. While the nature of the arm restraint and peekaboo tasks (i.e., standard negative and positive emotion elicitors used in previous infant research) and the difference in valence ratings across videos supports their ability to represent a more negative to more positive emotion differential, only the positive videos were rated as significantly different from neutral on average, making it difficult to know if contrast effects reflect emotional salience rather than valence per se. Follow-up tests of correlations between valence ratings and neural activation to videos in mindfulness-related clusters were largely nonsignificant, suggesting a differential effect of the emotion context itself, and not necessarily the degree to which the mother perceived her infant to be negative or positive. Nonetheless, it will be important to conduct further tests of mindfulness-related responses to videos of varying intensities of and contexts for infant emotion to better probe the nature of these effects. Relatedly, we chose to focus on mothers’ naturalistic response to their own infant’s emotional displays, which offered a window into ecologically relevant emotional processes but did not allow for much control. It could be useful to repeat these procedures with mothers who have been instructed to take a more or less mindful approach to the videos, and with more standardized emotion videos. It is worth noting that follow-up tests involving contrasts of own infant with unfamiliar infant videos yielded few mindfulness-related effects; it may be that the effects detected here reflect a broader response set to infant emotion-eliciting situations not specific to one’s own offspring. This possibility should be investigated further at later developmental periods, by which time mothers may have formed more extensive social-emotional repertoires involving their own infant.
Finally, we acknowledge certain complexities related to the interpretation of mindfulness effects. One point is that mindfulness likely influences brain activation to all stimuli, including “rest,” which means there was no stimulus condition we could expect to be completely neutral/uninfluenced by the individual difference predictor of interest. Future work might incorporate resting state fMRI, in addition to social-emotional tasks, to more comprehensively describe the nature of mindfulness effects under different task demands. We also chose to focus on self-reported dispositional mindfulness, which is only one way to operationalize the construct, and existing mindfulness self-report scales have been criticized for limitations on many fronts (see Grossman, 2011). While we might expect a mindfulness intervention—to the extent that such an intervention increases dispositional mindfulness—to enhance the neural response patterns identified here, we cannot be certain of this. There is evidence that mindfulness effects vary according to the type of practice (i.e., open monitoring vs. focused attention) and experience of the practitioner (i.e., novice vs. expert), and it is possible that formal training in different aspects of mindful parenting would bring about distinct suites of changes in maternal response over time (see Lutz et al., 2015). Further study of mindfulness training-related effects will help to clarify when and how mindfulness shapes parental responsiveness at both behavioral and neural levels.
The present study takes an important initial step toward connecting knowledge of mindfulness brain mechanisms with the complex social-emotional context of parenting an infant. We hope that further research in this vein will help to illuminate the ways in which mindfulness helps people meet the challenges and joys in their daily lives.
Conflict of interest
None.
Footnotes
Although parity has been shown to impact neural response in previous research, we found no evidence that activation differences reported below were related to number of children in this sample.
Contrasts of own > unfamiliar infant videos, while not the focus for this study, were also examined for completeness. For the most part, these contrasts were unrelated to maternal mindfulness scores, though Nonreactivity related to own > unfamiliar peekaboo signal in a subset of regions identified in the own arm restraint > peekaboo contrast reported below.
References
- Baer R.A., Smith G.T., Hopkins J., Krietemeyer J., Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Creswell J.D., Way B.M., Eisenberger N.I., Lieberman M.D. Neural correlates of dispositional mindfulness during affect labeling. Psychosom. Med. 2007;69:560–565. doi: 10.1097/PSY.0b013e3180f6171f. (0033-3174/07/6906-0560) [DOI] [PubMed] [Google Scholar]
- Desbordes G., Negi L.T., Pace T.W., Wallace B.A., Raison C.L., Schwartz E.L. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci. 2012;6:292. doi: 10.3389/fnhum.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L.G., Coatsworth J.D., Greenberg M.T. A model of mindful parenting: implications for parent-child relationships and prevention research. Clin. Child Fam. Psychol. Rev. 2009;12:255–270. doi: 10.1007/s10567-009-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan L.G., Coatsworth J.D., Gayles J.G., Geier M.H., Greenberg M.T. Can mindful parenting be observed? Relations between observational ratings of mother-youth interactions and mothers-self-report mindful parenting. J. Fam. Psychol. 2015;29:276–282. doi: 10.1037/a0038857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A., Anderson A.K., Mayberg H., Bean J., McKeon D., Segal Z.V. Minding one’s emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. Psychol. Assess. 2011;23:1034–1040. doi: 10.1037/a0022713. [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Lazar S.W., Gard T., Schuman-Olivier Z., Vago D.R., Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Haase L., Thom N.J., Shukla A., Davenport P.W., Simmons A.N., Stanley E.A. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Soc. Cogn. Affect. Neurosci. 2016;11:182–190. doi: 10.1093/scan/nsu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler A., Dixon V., Garavan H. Automaticity and reestablishment of executive control-an fMRI study. J. Cogn. Neurosci. 2006;18:1331–1342. doi: 10.1162/jocn.2006.18.8.1331. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Delacourt; New York: 1990. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. [Google Scholar]
- Keng S., Smoski M.J., Robins C.J. Effects of mindfulness on psychological health: a review of empirical studies. Clin. Psychol. Rev. 2011;31:1041–1056. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M.A., Bishop S.R., Segal Z.V., Buis T., Anderson N.D., Carlson L., Shapiro S., Carmody J., Abbey S., Devins G. The Toronto mindfulness scale: development and validation. J. Clin. Psychol. 2006;62:1445–1467. doi: 10.1002/jclp.20326. [DOI] [PubMed] [Google Scholar]
- Lawrence E.J., Shaw P., Giampietro V.P., Surguladze S., Brammer M.J., David A.S. The role of ‘shared representations’ in social perception and empathy: an fMRI study. Neuroimage. 2006;29:1173–1184. doi: 10.1016/j.neuroimage.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lippold M.A., Duncan L.G., Coatsworth J.D., Nix R.L., Greenberg M.T. Understanding how mindful parenting may be linked to mother-adolescent communication. J. Youth Adolescence. 2015;44:1663–1673. doi: 10.1007/s10964-015-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A., Jha A.P., Dunne J.D., Saron C.D. Investigating the phenomenological matrix of mindfulness practices from a neurocognitive perspective. Am. Psychol. 2015;70:632–658. doi: 10.1037/a0039585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J., Brühl A.B., Doerig N., Scheerer H., Achermann R., Weibel A. Altered processing of self-related emotional stimuli in mindfulness meditators. Neuroimage. 2016;124:958–967. doi: 10.1016/j.neuroimage.2015.09.057. [DOI] [PubMed] [Google Scholar]
- Modinos G., Ormel J., Aleman A. Individual differences in dispositional mindfulness and brain activity involved in reappraisal of emotion. Soc. Cogn. Affect. Neurosci. 2010;5:369–377. doi: 10.1093/scan/nsq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague D.P., Walker-Andrews A.S. Peekaboo: a new look at infants’ perception of emotion expressions. Dev. Psychol. 2001;37:826–838. [PubMed] [Google Scholar]
- Moscardino U., Axia G. Infants’ responses to arm restraint at 2 and 5 months: a longitudinal study. Infant Behav. Dev. 2006;29:59–69. doi: 10.1016/j.infbeh.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Murakami H., Katsunuma R., Oba K., Terasawa Y., Motomura Y., Mishima K. Neural networks for mindfulness and emotion suppression. PLoS One. 2015;10:e0128005. doi: 10.1371/journal.pone.0128005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N.A., Stanton S.J., Greeson J.M., Smoski M.J., Wang L. Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Soc. Cogn. Affect. Neurosci. 2013;8:56–64. doi: 10.1093/scan/nss070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rämä P., Martinkauppi S., Linnankoski I., Koivisto J., Aronen H.J., Carlson S. Working memory of identification of emotional vocal expressions: an fMRI study. Neuroimage. 2001;13:1090–1101. doi: 10.1006/nimg.2001.0777. [DOI] [PubMed] [Google Scholar]
- Rissman J., Chow T.E., Reggente N., Wagner A.D. Decoding fMRI signatures of real-world autobiographical memory retrieval. J. Cogn. Neurosci. 2016;28:604–620. doi: 10.1162/jocn_a_00920. [DOI] [PubMed] [Google Scholar]
- Smith R., Alkozei A., Killgore W.D.S. Contributions of self-report and performance-based individual differences measures of social cognitive ability to large-scale neural network functioning. Brain Imaging Behav. 2017;11(June (3)):685–697. doi: 10.1007/s11682-016-9545-2. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Smoski M.J., Keng S., Ji J.L., Moore T., Minkel J., Dichter G.S. Neural indicators of emotion regulation via acceptance vs reappraisal in remitted major depressive disorder. Soc. Cogn. Affect. Neurosci. 2015;10:1187–1194. doi: 10.1093/scan/nsv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain J.E. The human parental brain: in vivo neuroimaging. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:1242–1254. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor V.A., Grant J., Daneault V., Scavone G., Breton E., Roffe-Vidal S. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage. 2011;57:1524–1533. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Wang S., Xu M., Song Y., Li X., Zhen Z., Yang Z. The network property of the thalamus in the default mode network is correlated with trait mindfulness. Neuroscience. 2014;278:291–301. doi: 10.1016/j.neuroscience.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Zysset S., Huber O., Ferstl E., von Cramon D.Y. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15:983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]