Highlights
-
•
Low risk processing predicted increases in risk-taking behaviors (RTB) via stress.
-
•
Insular risk processing predicted increases in perceived stress, but not vice versa.
-
•
Cognitive control moderated links between insular risk processing and RTBs.
-
•
High cognitive control buffered effects of low insular risk processing on RTBs.
Keywords: fMRI, Insula, Cognitive control, Risk-taking behaviors, Stress, Adolescence
Abstract
Adolescence is a critical period for the initiation of risk-taking behaviors. We examined the longitudinal interplay between neural correlates of risk processing and cognitive control in predicting risk-taking behaviors via stress. The sample consisted of 167 adolescents (53% males) who were assessed twice (MAgeTime1 = 14.13, MAgeTime2 = 15.05). Neural risk processing was operationalized as blood-oxygen-level-dependent (BOLD) responses in the anterior insula during a lottery choice task and neural cognitive control as BOLD responses during an inhibitory control task. Adolescents reported on perceived stress and risk-taking behaviors. Structural equation modeling analyses indicated that low insular risk processing predicted increases in perceived stress, while perceived stress did not predict changes in insular risk processing across one year. Moreover, significant moderation by neural cognitive control indicated that low insular risk processing predicted increases in risk-taking behaviors via increases in perceived stress among adolescents with poor neural cognitive control, but not among adolescents with good neural cognitive control. The results suggest that risk processing in the anterior insular cortex plays an important role in stress experience and risk-taking behaviors particularly for vulnerable adolescents with poor neural cognitive control.
1. Introduction
It is well known that adolescence is a developmental period characterized by increases in stressful experiences (Dahl and Gunnar, 2009), and for some, increases in risky behaviors (Kann et al., 2016). Yet, not all adolescents are vulnerable to engaging in risky behaviors. To explain individual differences in risk-taking, we focus on the valuation system that is involved in estimating the incentive value of different options and the control system that is involved in inhibiting prepotent responses (van den Bos et al., 2015). We further propose that the valuation system and the control system interact with each other to contribute to individual differences in risk-taking—processes that may generalize to most or all time points of the adolescent and young adult phase of development (Kim-Spoon et al., 2017b). While prior research has primarily focused on the extent to which adolescents respond to valued rewards, a recent cross-sectional study demonstrated that this valuation system likely involves recruitment of the insular cortex for processing risk or the likelihood of potential reward values, and that signaling in this region interacts with the neural activation related to cognitive control in prefrontal cortex to predict risk-taking behaviors in adolescents (Kim-Spoon et al., 2017a). Given that adolescence is a time of elevated stress and increases in risky behavior, the present study aims to expand on this prior work to better understand the role of stress in linking neural development of valuation and control systems to risk-taking behaviors.
Value-based decision-making research has shown that risky choices are driven by not only neural computations associated with the value of rewards, but also the likelihood of receiving such rewards (d’Acremont and Bossaerts, 2008; Mohr et al., 2010). For instance, an adolescent may be driven to make a risky choice because he or she values the outcome of a decision or because the valued outcome has a high likelihood of occurring. One of the key regions consistently implicated in the processing of risk information is the anterior insular cortex (Mohr et al., 2010; Richards et al., 2013). The insular cortex acts as a signal, guiding adolescents towards or away from risky choices consistent with individual preferences for risk. When making decisions, adolescents recruit the insular cortex more than children or adults, and adolescents’ hypersensitivity of the insular cortex to increasing variance of potential outcomes may be related to making safer choices (van Duijvenvoorde et al., 2015), highlighting the important role of the insula during adolescence (Smith et al., 2014). In addition, consistent with the theoretical perspective emphasizing the interaction between motivational and cognitive control neural systems (Kim-Spoon et al., 2017b), a recent study has shown that adolescents who engaged in risk-taking behaviors experienced blunted reactivity in the insular cortex to risky options in a lottery choice task. However, this relation was found only for adolescents who exhibited high interference-related dorsal anterior cingulate cortex (dACC) reactivity (i.e., poor cognitive control), but not for those adolescents who displayed low interference-related dACC activity (i.e., good cognitive control; Kim-Spoon et al., 2017a). Within the neuroscience literature, previous studies have identified brain regions involved in inhibition, including the basal ganglia (such as caudate, putamen, globus pallidus), that are thought to be involved in inhibition of inappropriate responses, and prefrontal regions (such as inferior, medial, and dorsolateral prefrontal cortices) that receive inputs from the limbic basal ganglia thalamocortical circuit and represent and maintain relevant information for goal directed behaviors (Aron et al., 2014; Booth et al., 2003; Casey et al., 2001). Here, we focus on brain regions that are closely related to cognitive control over interference measured by brain activation during an inhibitory control task primarily involving medial prefrontal cortices.
While adolescence has been marked as a developmental period of increases in risky behavior, it is also known as a time of elevated stress (Dahl and Gunnar, 2009; Fuhrmann et al., 2015). Stress has been described as experiences that present as either psychologically or physiologically taxing (McEwen, 2007). Most prior behavioral research examining the effects of acute stress on decision making used experimentally manipulated stressors and suggested that stress may increase risky decisions and alter neural activity in insula during decision making under risk (Starcke and Brand, 2016). For example, one available study demonstrated that adults who reported higher perceived chronic stress showed less insula blood-oxygen-level-dependent (BOLD) activation when experiencing losses in a monetary incentive delay task (Treadway et al., 2013). However we note that this insula activation was observed during the outcome processing instead of risk-related decision-making phase. Another study indicated that actively making decisions involving monetary reward (as opposed to not making decisions by passively following instructions) under acute stress was accompanied by alterations in the activation of the insula (Lighthall et al., 2012). Though not exactly testing stress effects, one study examined the interact effects between the expected value (EV; the sum of the value of each possible outcome weighted by each associated probability of occurrence) and urgency (manipulated sense of urgency) (Jones et al., 2011). Rationally, options with a higher EV are favored, when all else is equal. The results indicated the effects of urgency on activation in anterior insula as to the difference between positive and neutral EV gambles: insular activation was lower for positive than neutral EV gambles under high urgency, whereas the converse was observed under low urgency. As such, prior studies have not investigated how stress may influence risk processing during decision making—i.e., calculating likelihood of receiving rewards (or variance of potential outcomes). Also, prior studies are limited by their cross-sectional design and can thus not evaluate the direction of effects between stress and insular processing
Based on the literature regarding the effect of stress on insular activation during decision making under risk (e.g., Starcke and Brand, 2016), it is expected that repeated exposure to stress may be an environmental risk factor that influences insular risk processing related to decision making. It is also likely that individual differences in neural risk sensitivity may affect how individuals experience stressful life events. That is, insula activation during risk processing may be a neural risk factor that increases adolescents’ vulnerability to experiencing stress. Indeed, according to the stress generation theory (Hammen, 2006), certain biological and personal vulnerabilities may increase the likelihood to experience stressful life events (Hammen, 2006; Liu, 2013). It follows that susceptible individuals are more likely to create contexts that are stressful, thereby increasing the likelihood of recurrent or chronic stressful experiences. This theory is supported by longitudinal studies in adults showing that more impulsive individuals report experiencing more negative life events at a later time (Iacovino et al., 2016; Liu and Kleiman, 2012). Decades of research has demonstrated that decision-making situations involving lack of predictability or control (i.e., uncertainty) regarding consequences of actions can induce distress (for a review see Koolhaas et al., 2011). Specifically, higher levels of uncertainty (i.e., lack of control or unpredictability over outcomes) predict greater perceived stress, which influences task performance (de Berker et al., 2016). Therefore, decreased risk processing in the insula in response to risk-related cues may also be related to the extent to which adolescents experience episodic stressful life events.
In the current longitudinal study, we investigated the role of stress in the joint contributions of neural cognitive control and insular risk processing to real-life risk-taking behaviors during early to middle adolescence, a developmental period during which motivational and emotional reactivity is posited to be particularly strong (Casey et al., 2008) and neuroplasticity is heightened (Fuhrmann et al., 2015). Specifically, we examined possible bidirectional effects between stress and neural risk sensitivity: (i) insular risk processing would predict increases in adolescent risk-taking behaviors via its influence on perceived stress, and/or (ii) perceived stress would predict adolescent risk-taking behaviors via its influence on insular risk processing. Additionally, we examined the moderating role of neural cognitive control in these relations. Based on prior findings reporting sex differences in stress effects (e.g., Uy and Galván, 2017), we further explored sex differences in the hypothesized associations among stress, risk related insular activation, and risk-taking behaviors.
2. Methods
2.1. Participants
The current sample included 167 adolescents (53% males). Adolescents were 13 or 14 years of age at Time 1 (M = 14.13, SD = 0.54) and 14 or 15 years of age at Time 2 (M = 15.05, SD = 0.54). Adolescents primarily identified as Caucasian (80%), African-American (13%), and other (7%). Family annual income was relatively low with a mean of $35,000-$ 49,999 at both times. At Time 1, 157 adolescents participated. However, 17 adolescents did not return at Time 2 (approximately one year later) for reasons including: ineligibility for tasks (n = 2), declined participation (n = 7), and lost contact (n = 8). At Time 2, 10 additional adolescents were invited to participate in the study, leading to a final sample of 167 adolescents. Attrition analyses indicated that the 17 adolescents who did not return for Time 2 were not significantly different on demographic (age, income, race, sex) or study variables (perceived stress, risk-taking behaviors, insular risk and neural cognitive control processing Time 1) from the 140 adolescents who did return (all ps > .29). Exclusion criteria included claustrophobia, history of head injury resulting in loss of consciousness for more than 10 min, orthodontia impairing image acquisition, and contraindications to magnetic resonance imaging.
2.2. Procedure
Adolescent participants and their parents were recruited as part of a longitudinal study via email announcements, flyers, notice on the internet, or snowball sampling (word-of-mouth). The current study used data from Time 1 and Time 2, approximately one year apart. Adolescent participants provided written assent and their parents provided written consent for a protocol approved by the university’s institutional review board. Both parents and adolescents received monetary compensation for their time.
2.3. Measures
2.3.1. Perceived stress
Perceived stress was assessed at Time 1 and Time 2 using the 10-item Perceived Stress Scale (Cohen and Williamson, 1988) that has been well validated to assess for perceptions of stress. Adolescents reported about thoughts and feelings they have experienced within the past month (e.g., “In the last month, how often have you felt nervous and ‘stressed’?”) on a 5-point Likert scale (0 = Never to 4 = Very Often). Mean scores were calculated, with higher scores indicating higher perceived stress (α = 0.83 at Time 1 and Time 2).
2.3.2. Risk-taking behaviors
Risk-taking behaviors were assessed at Time 1 and Time 2 using the Things I Do questionnaire (Conger and Elder, 1994). Adolescents reported about things they may have done in the last year (0 = not at all, 1 = once or twice, and 2 = more than twice). A full list of all items can be found in Appendix A. Mean scores were calculated, with higher scores indicated higher risk-taking behaviors (α = 0.74 at Time 1 and α = 0.77 at Time 2).
2.3.3. Imaging acquisition and analysis
For both risk processing and cognitive control tasks, functional neuroimaging data were acquired on a 3T Siemens TIM Trio MRI scanner with a standard 12-channel head matrix coil. Echo-planar images (EPIs) were collected using the following parameters: slice thickness = 4 mm, 34 axial slices, field of view (FoV) = 220 × 220 mm, repetition time (TR) = 2 s, echo time (TE) = 30 ms, flip angle = 90°, voxel size = 3.4375 × 3.4375 × 4 mm (during analysis the images were resliced so that voxels were 3 × 3 × 3 mm), 64 × 64 grid, and slices were hyperangulated at 30 ° from anterior-posterior commissure. The structural scan was acquired using a high-resolution magnetization prepared rapid acquisition gradient echo sequence with the following parameters: TR = 1200 ms, TE = 3.02 ms, FoV = 245 × 245 mm, and 192 slices with the spatial resolution of 1 × 1 × 1 mm. FMRI data were preprocessed and analyzed using SPM8 (Wellcome Trust Neuroimaging Center). For each scan, functional imaging data were corrected for head motion using a six-parameter rigid body transformation and realigned. The mean functional image was co-registered to the anatomical image, then the anatomical image was segmented and registered to the MNI template and functional volumes were normalized using parameters from the segmented anatomical image, and were smoothed using a 6 mm full-width-half-maximum Gaussian filter.
2.3.4. Insular risk processing
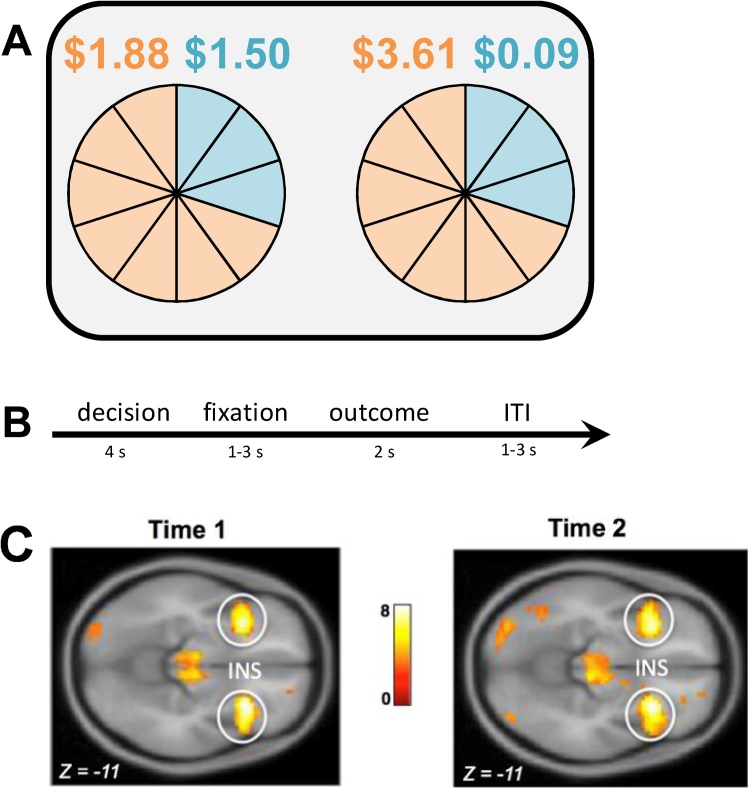
Adolescents engaged in a lottery choice task at Time 1 and Time 2 in which they made choices between pairs of gambles in a modified economic lottery choice task (Holt and Laury, 2002) while their BOLD response was monitored using fMRI (see Fig. 1a and b). For each gamble, there was a high and low monetary outcome, each associated with a specific probability. The associations between outcomes and probabilities were represented with corresponding colors (orange or blue). Pie charts were used to represent probabilities associated with potential payoffs to maximize comprehension of numerical information for adolescent participants. There were 10 slices in each pie, each corresponding to a probability of 10%. Participants were presented with slices representing the probability of receiving a high (orange) or low (blue) monetary outcome (see Fig. 1a). Monetary outcomes and probabilities varied across trials. The associated risk for each gamble was measured using coefficient of variation (CV),1 a scale-free metric calculated by dividing the standard deviation by expected value. Researchers have found that CV is a stronger predictor of choice behavior compared to standard economic measures of risk (e.g., standard deviation or variance) because calculations of risk are often made relative to the average outcome (Bach et al., 2017; Weber et al., 2004). For each pair of gambles, one option was always riskier (higher CV) than the other (lower CV). In order to incentivize performance, participants were compensated based on their actual winnings from 5 randomly selected trials (Smith, 1976). Participants were told that each trial was independent from all other trials and was equally likely to be selected for compensation. Each participant took approximately 30 min to complete a total of 72 trials.
Fig. 1.
a) In the lottery choice task, adolescents were asked to choose between pairs of uncertain gambles. For each gamble, there was a high and low monetary outcome, each associated with a specific probability. The associations between outcomes and probabilities are represented with corresponding colors (orange or blue), b) Each trial consisted of a decision phase, a fixation phase, an outcome phase, and an inter-trial-interval (ITI), c) During the decision phase of the economic lottery choice task, adolescents exhibited increased BOLD responses in the bilateral anterior insular cortex to chosen gambles that were of higher relative to lower levels of risk (i.e., coefficient of variation; CV) at both Time 1 [t(145) = 7.22, p(FWE correction) < .05)] and Time 2 [(t(135) = 7.91, p(FWE correction) < .05]. Figure reprinted from Lauharatanahirun, N., Maciejewski, D., Holmes, C.J., Deater-Deckard, K., Kim-Spoon, J., & King-Casas, B. (accepted). Neural correlates of risk processing among adolescents: Influences of parental monitoring and household chaos. Child Development. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At the subject level of the general linear model (GLM), the decision and outcome events of the task were modeled with a duration of 4 and 2 s, respectively. A parametric regressor of decision phase activity equivalent to the CV for chosen gambles was included in the model. Additionally, a parametric regressor indicating whether subjects received high or low monetary outcomes during the outcome phase was included into the model. At the group level of the GLM, whole brain analysis was conducted to determine how CV for chosen gambles modulated BOLD responses during the decision phase (see Appendix B). Given the consistent and robust results implicating the insular cortex as a key region involved in risk processing (Mohr et al., 2010), we hypothesized that BOLD responses in the bilateral insular cortex would be adjusted by the level of CV (i.e., level of risk). To test a priori hypotheses, region of interest (ROI) analyses were performed using SPM8. Eigenvariate values were extracted for the left and right insular cortex using a 6 mm sphere around the peak voxel coordinates for each region (left: x = −30, y = 17, z = −14; right: x = 30, y = 20, z = −11). See Appendix B for all regions associated with increasing CV during the decision phase. Fig. 1c illustrates activation in the bilateral insular cortex during the lottery choice task for Time 1 and Time 2. We created a latent factor score to operationalize insular risk processing using bilateral insular eigenvariate values, with higher scores indicating higher BOLD responses in the insular cortex (see Appendix D for more information).
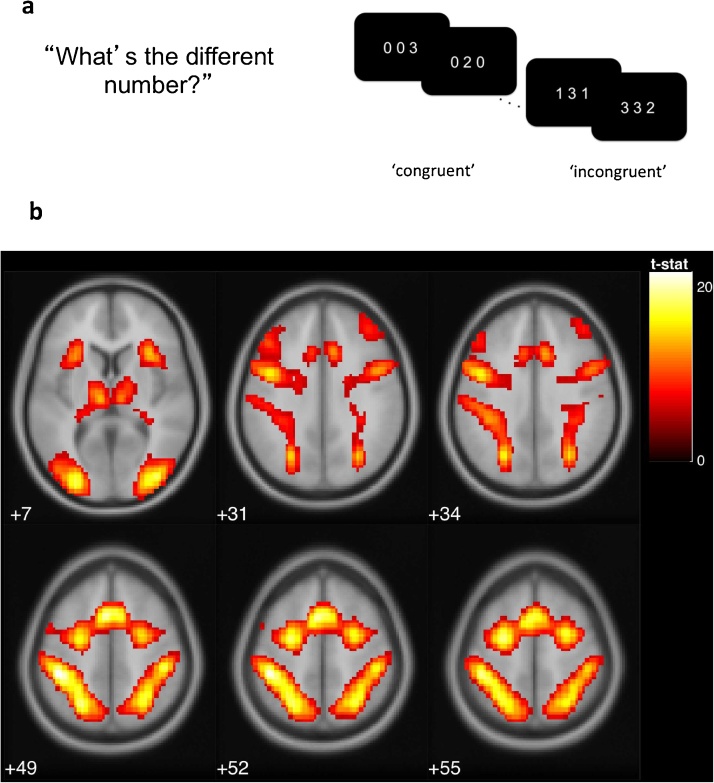
2.3.5. Neural cognitive control
Adolescents engaged in a multi-source interference task (MSIT; Bush et al., 2003) at Time 1 during which adolescents’ BOLD response was recorded (see Fig. 2a). During the task, adolescents were presented with sequences of three digits, two of which were identical. Subjects were required to indicate the identity—but not the position—of the oddball digit. In neutral trials, the identity of the target digit was congruent with the digit’s presented location. In interference trials, the identity of the target digit was incongruent with the digit’s presented location. In line with previous studies (Bush et al., 2003), we found a significant MSIT interference effect in reaction time for correct responses, t(153) = 69.58, p < .001 and accuracy between conditions, t(153) = −15.47, p < .001 (i.e., accuracy was lower and reaction time was higher for interference compared to neutral trials).
Fig. 2.
a) In the multi-source interference task (MSIT), adolescents were asked to identify the digit that differed from two other concurrently presented digits, ignoring its position in the sequence. b) Adolescents exhibited greater activation for interference relative to neutral conditions in the regions of left posterior-medial frontal cortex, right and left inferior frontal gyrus, left and right inferior parietal lobules, right insula, right superior frontal gyrus, and left middle frontal gyrus, displayed at p(FWE) < .001 (see Appendix B). Reprinted from Kim-Spoon, J., Maciejewski, D., Lee, J., Deater-Deckard, K., & King-Casas (2017). Longitudinal associations among family environment, neural cognitive control, and social competence among adolescents. Developmental Cognitive Neuroscience, 26, 69–76. doi: 10.1016/j.dcn.2017.04.009.
To derive activation in cognitive control areas, individual-level ROI values were extracted for each participant at coordinates corresponding to peak activations in the interference minus neutral second-level contrast (see Appendix C). Eigenvariate values of the contrast images were extracted using spherical masks of 6 mm surrounding MNI coordinates, thresholded at p < .001, family-wise error corrected (see Fig. 2b). We created a latent factor score to operationalize neural cognitive control, and this neural cognitive control factor score correlated significantly with accuracy and reaction time difference scores (−0.40 and 0.36, ps < .001), indicating that higher interference-related BOLD activation in these regions was associated with lower cognitive control (see Appendix D for more information).
2.4. Statistical analyses
We conducted longitudinal moderated mediation analyses using structural equation modeling (SEM) following recommendations by Hayes (2013) in Mplus 7.4 (Muthén and Muthén, 1998–2015). The missing data pattern resembled a Completely at Random pattern (Little's MCAR test on study variables: χ2 = 73.78, df = 63, p = .17). Therefore, we used Full Information Maximum Likelihood estimation with robust standard errors (MLR) to account for missing data and non-normal distributions. We calculated bias-corrected bootstrap confidence intervals (CIs) for indirect effects using 10,000 bootstrapping samples.
In the first step (i.e., the main effect model), we tested two competing models. First, we tested the indirect effect of perceived stress Time 1 on risk-taking behaviors Time 2 via insular risk processing Time 2 (Model a, Fig. E1, Appendix E). Second, we tested the indirect effect of insular risk processing Time 1 on risk-taking behaviors Time 2 via perceived stress Time 2 (Model b, Fig. E2, Appendix E). In both models, we controlled for earlier levels of the mediator and the outcome variables by regressing Time 2 scores on Time 1 scores. Thus, we predict the residualized change. Such a measure is preferred over simple difference scores, because difference scores can have high measurement error and do not adjust for baseline differences. In contrast, residualized scores mitigate some of the problems of simple difference scores by for instance adjusting for baseline differences (MacKinnon et al., 2013). Although the main effects of neural cognitive control were not part of our hypotheses, we estimated the main effects of neural cognitive control Time 1 on risk-taking behaviors Time 2 to test subsequent interaction effects. In the second step (i.e., the interaction effect model), we tested the moderating effects of neural cognitive control on the direct and indirect effects (see Figs. E1 and E2 in Appendix E). For model parsimony, only significant interaction effects were retained. To explore sex differences, we conducted multiple group SEM. Here, we compared a model in which main and interaction effects were constrained to be equal between boys and girls to a model where main and interaction effects were freely estimated between boys and girls using the Satorra-Bentler chi-square difference test (Satorra and Bentler, 2001). A significant worse fit of the model with equality constraints compared to a model allowing estimates to vary between the two sex groups indicates presence of sex differences. An α level of 0.05 was used for the significance of all statistical tests.
3. Results
3.1. Descriptive statistics
Exploration of the data revealed one extreme low insular risk processing score at Time 1 and two extreme high risk insular processing scores at Time 2. These scores were winsorized to the next value that was not an outlier (i.e., within 3 SD) to retain statistical power and attenuate bias resulting from elimination (Ghosh and Vogt, 2012). Table 1 depicts the descriptive statistics and correlations for all study variables. Frequency distributions of adolescent perceived stress and risk-taking behaviors can be found in Appendix F. Both perceived stress and risk-taking behaviors significantly increased from Time 1 to Time 2 (t(138) = −2.84, p = .005 and t(134) = −2.22, p = .03, respectively). Multivariate general linear modeling (GLM) analyses indicated that none of the demographic variables had a significant effect on the outcome variable (risk-taking behaviors Time 2), including gender (p = .53), ethnicity (contrasting white vs. non-white, p = .33), family income (p = .13), and age (p = .31).
Table 1.
Descriptive Statistics and Bivariate Correlations of Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | M | SD | Min | Max | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Neural cognitive control Time 1 | 0.81 | 0.35 | −0.01 | 2.24 | ||||||
| 2. Insular risk processing Time 1 | .02 | 0.03 | 0.81 | −1.84 | 3.45 | |||||
| 3. Insular risk processing Time 2 | .14 | .33*** | −0.05 | 0.74 | −1.73 | 2.26 | ||||
| 4. Perceived stress Time 1 | .07 | −.13 | −.15 | 1.48 | 0.66 | 0.00 | 3.40 | |||
| 5. Perceived stress Time 2 | .21* | −.19* | −.11 | .62*** | 1.60 | 0.63 | 0.20 | 3.10 | ||
| 6. Risk-taking behaviors Time 1 | .03 | −.01 | −.06 | .17* | .12 | 0.24 | 0.19 | 0.00 | 1.00 | |
| 7. Risk-taking behaviors Time 2 | .21* | −.06 | −.04 | .14 | .28*** | .64*** | 0.27 | 0.21 | 0.00 | 0.95 |
3.2. Main effect model
We first fit two competing indirect models to test whether perceived stress predicted changes in risk-taking behaviors via changes in insular risk processing (Model a in Fig. E1 in Appendix E) or whether insular risk processing predicted changes in risk-taking behaviors via changes in perceived stress (Model b in Fig. E2 in Appendix E). Both models showed good model fits (χ2 = 0.67, df = 2, p = .71, CFI = 1.00, RMSEA = 0.00 for Model 1a; and χ2 = 4.81, df = 2, p = .09, CFI = 0.98, RMSEA = 0.09 for Model 1b). In order to formally test whether the path went from perceived stress Time 1 to insular risk processing Time 2 or from insular risk processing Time 1 to perceived stress Time 2, we fitted a cross-lagged model with insular risk processing Time 1 and Time 2 and perceived stress Time 1 and Time 2. We then tested whether including cross-lagged paths would significantly improve model fit. Results showed that including a path from perceived stress Time 1 to insular risk processing Time 2 did not significantly improve model fit (Δχ2 = 1.66, df = 1, p = .20), whereas including a path from insular risk processing Time 1 to perceived stress Time 2 improved model fit (Δχ2 = 3.44, df = 1, p = .06). Therefore, we chose to continue with Model 1b. As shown in Table 2, results of Model 1b indicated that low insular risk processing predicted increases in perceived stress, which in turn predicted increases in risk-taking behaviors. Scatterplots of those main effects can be found in Appendix G. The indirect effect from insular risk processing to increases in risk-taking behaviors via increases in perceived stress was significant (B = −0.005, SE = 0.004, 95% CI [−0.016; −0.0004], b* = −0.021). However, low insular risk processing at Time 1 did not directly predict increases in risk-taking behaviors. In contrast to Model 1b, in Model 1a, none of the paths between insular risk processing, perceived stress, and risk-taking behaviors were significant (see Appendix H for model estimates).
Table 2.
Parameter Estimates for Testing the Moderating Role of Neural Cognitive Control Time 1 on the Indirect Effect from Insular Risk Processing Time 1 to Risk-taking Behaviors Time 2 via Perceived Stress Time 2.
| B | SE | p | b* (beta) | |
|---|---|---|---|---|
| Step 1: Main effects | ||||
| Insular risk processing Time 1 → perceived stress Time 2 | −0.09 | 0.05 | 0.05 | −0.11 |
| Neural cognitive control Time 1 → perceived stress Time 2 | 0.26 | 0.09 | 0.005 | 0.14 |
| Insular risk processing Time 1 → risk-taking behaviors Time 2 | −0.01 | 0.01 | 0.69 | −0.02 |
| Neural cognitive control Time 1 → risk-taking behaviors Time 2 | 0.10 | 0.05 | 0.06 | 0.16 |
| Perceived stress Time 2 → risk-taking behaviors Time 2 | 0.06 | 0.02 | 0.004 | 0.18 |
| Step 2: Interaction effects | ||||
| Insular risk processing Time 1× neural cognitive control Time 1 → perceived stress Time 2 | −0.26 | 0.11 | 0.02 | −0.12 |
| Perceived stress Time 2× neural cognitive control Time 1 → risk-taking behaviors Time 2 | 0.11 | 0.07 | 0.13 | 0.11 |
| Insular risk processing Time 1× neural cognitive control Time 1 → risk-taking behaviors Time 2 | −0.05 | 0.06 | 0.39 | −0.07 |
Note: Perceived stress Time 2 was controlled for perceived stress Time 1 (B = 0.55, SE = 0.06, p < .001, b* = 0.57). Risk-taking behaviors Time 2 were controlled for risk-taking behaviors Time 1 (B = 0.69, SE = 0.08, p < .001, b* = 0.61). Stability coefficients are reported from the final model.
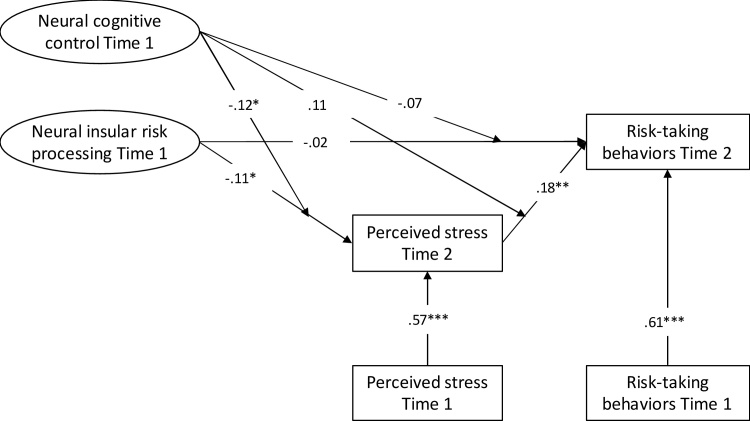
3.3. Interaction effect model
After determining the direction of effects between perceived stress and insular risk processing, we continued to test for the moderating effect of neural cognitive control in Model 1b. The results indicated that neural cognitive control moderated only the path between insular risk processing Time 1 and perceived stress Time 2 (see Table 2 and Fig. 3). This moderation model showed a good model fit (χ2 = 5.93, df = 3, p = .11, CFI = 0.98, RMSEA = 0.08).
Fig. 3.
Final model showing the relation among neural insula processing, neural cognitive control processing, perceived stress and risk-taking behaviors. Arrows pointing on other arrows indicate moderation effects. Standardized estimates are presented. * p ≤ .05, ** p < .01, *** p ≤ .001.
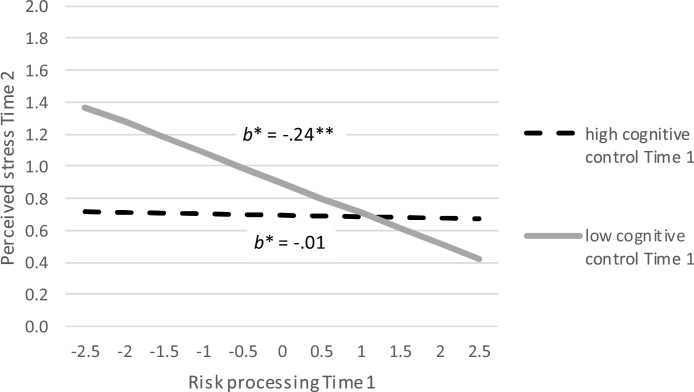
To further evaluate this moderating effect of neural cognitive control, we conducted simple slope analyses to examine the effects of insular risk processing at varying levels of neural cognitive control: low interference-related BOLD responses during the MSIT task (i.e., 1 SD below the mean, good neural cognitive control) versus high interference-related BOLD responses (i.e., 1 SD above the mean, poor neural cognitive control). As shown in Fig. 4, results indicated that the association between insular risk processing at Time 1 and changes in perceived stress was significant for adolescents with poor neural cognitive control (B = −0.19, SE = 0.07, p = .005), but not for adolescents with good neural cognitive control (B = −0.01, SE = 0.05, p = .86). Moreover, the indirect effect from insular risk processing at Time 1 to increases in risk-taking behaviors via increases in perceived stress was significant for adolescents with poor neural cognitive control (B = −0.012, SE = 0.007, 95% CI [−0.029; −0.002], b* = −0.045), but not for adolescents with good neural cognitive control (B = −0.001, SE = 0.004, 95% CI [−0.008; 0.008], b* = −0.002). As such, results indicate a buffering effect of neural cognitive control against the detrimental effects of low insular risk processing on changes in risk-taking behaviors mediated through changes in perceived stress. The multiple SEM for testing sex differences in main and interaction effects revealed that the above mentioned relations did not differ between boys and girls, Δχ2 (6) = 4.90, p = .56. We additionally ran the final model with only Time 1 scores to check for model validity cross-sectionally. However, this model showed a poor fit, χ2 = 2.84, p = .09, CFI = 0.60, RMSEA = 0.11. This corroborates the assumption that the brain effects may take some time to occur, highlighting the necessity to examine neurobiological effects in a longitudinal fashion.
Fig. 4.
Simple slope analyses comparing the relation between insular risk processing and perceived stress for adolescents with high cognitive control (low interference-related BOLD responses) and adolescents with low cognitive control (high interference-related BOLD responses). ** p < .01. Perceived stress Time 2 is controlled for perceived stress Time 1. Standardized estimates (b*) are presented.
4. Discussion
To date, there is limited literature discussing how stress interfaces with neural systems of risk processing and cognitive control to contribute to the development of adolescent real-world risky behavior. Given the key role of the insula in encoding risk during value-based decision-making under uncertainty (Mohr et al., 2010) and the known association between stress and decision making under uncertainty (Starcke and Brand, 2016), we examined the interplay between perceived stress and neural correlates of risk processing and cognitive control as potential contributing factors to the development of adolescent risk-taking behavior. The results show that low risk processing in the insular cortex was related to increases in perceived stress and subsequent increases in risk-taking behaviors only for adolescents with poor neural cognitive control (i.e., high interference-related BOLD responses), but not for adolescents with good neural cognitive control (i.e., low interference-related BOLD responses).
Current neuroscience literature emphasizes the importance of the anterior insula in decision making among adolescents (Smith et al., 2014; Wood and Bechara, 2014), yet research has not examined the ways through which insular risk-related processing may contribute to individual differences in the development of risk-taking behaviors. A large body of behavioral research has found that the presence of uncertainty within decision-making situations often produces stress due to a perceived lack of control or predictability over outcomes (de Berker et al., 2016; Koolhaas et al., 2011 for reviews). The present study offers evidence demonstrating that insular risk-related processes involved in detecting uncertainty within the environment are related to increases in perceived stress, ultimately affecting the development of real-world risk-taking behaviors among adolescents. Importantly, these results indicate a moderating effect of neural cognitive control over insular risk processing supporting theoretical work emphasizing the interaction between motivational and cognitive control systems in predicting risk-taking (Casey et al., 2008; Kim-Spoon et al., 2017b). In particular, although low risk processing in the insular cortex is likely related to increases in risk-taking behaviors via increased perceived stress, this pathway only applied to adolescents with poor neural cognitive control but not to those with good neural cognitive control. This finding is consistent with a recent cross-sectional study showing that BOLD responses in the anterior insula during risk processing interacted with dACC activity during an inhibitory control task to predict risk-taking behaviors among adolescents (i.e., substance abuse and risky sexual behaviors; Kim-Spoon et al., 2017a). Our longitudinal data clarify that the moderating effect of neural cognitive control operates by protecting early adolescents with neural vulnerability during risky decision-making (shown in low insular risk processing) against their increasing perceptions of stress.
The longitudinal findings of the current study further clarify the direction of the link between insular risk processing and stress. Specifically, we examined whether insula activation during risk processing may be a neural risk factor that indicates adolescents’ vulnerability to experiencing increased stress over time. We also examined whether stress exposure may be an environmental risk factor that alters insula activation during risk processing over time. Our data revealed that insular risk processing predicted changes in perceived stress over time, whereas perceived stress did not predict changes in insular risk processing. These findings suggest that low risk processing coupled with poor neural cognitive control may represent a neural vulnerability factor that puts adolescents at risk for experiencing more stress, and subsequently for engaging in risk-taking behaviors. Previous studies have indicated that higher BOLD responses in the anterior insula during decision-making tasks are related to more risk avoidance in adolescents (van Duijvenvoorde et al., 2015). Thus, a blunted engagement of the insula might suggest either a lack of or a difficulty in processing risk, which may lead to decreases in risk aversion. If those adolescents do not possess sufficient neural cognitive control capacities to regulate such risk processing difficulties, then they may be more susceptible to entering risky situations. This in turn might increase perceived stress, perhaps due to the decreased sensitivity of encoding the potential negative outcomes of their actions.
Further, our finding provides insight into the role of naturally occurring stress in the development of adolescent risk-taking behaviors by illustrating that increases in perceived stress are related to increases in risk-taking behaviors. This finding largely corresponds with prior longitudinal studies showing that stress increases various risk-taking behaviors among adolescents, including substance use and aggression (Copeland-Linder et al., 2011; Herts et al., 2012). It has been suggested that risk-taking behaviors provide an outlet for relief from uncomfortable and stressful periods or situations (Zillmann and Bryant, 1985). Importantly, our findings suggest specificity in that perceived stress (or naturally occurring stress experiences) may be affected by neural representations of risk that guide risky decision making, whereas acute stress may increase heightened reliance on immediate and potentially high reward (Starcke and Brand, 2016). Also, despite earlier research indicating possible sex differences in acute stress effects (e.g., Uy and Galván, 2017), the demonstrated pathways did not differ between boys and girls as indicated by the multiple group comparison by sex. This finding of non-significant gender moderation is consistent with the recent review on the effects of stress on decision making under uncertainty by Starcke and Brand (2016).
Findings from the present study need to be interpreted in light of its limitations. First, perceived stress and risk-taking behaviors were based on self-report and therefore these associations might have been inflated due to shared method variance. Future studies could benefit from involving multiple informants and observational methods to reduce potential method bias. Moreover, although we had longitudinal data, it was restricted to two time-points. Ideally, future research should involve multiple time points as mediation models assume temporal sequences across the predictor, the mediator, and the outcome (Cole and Maxwell, 2003). Moreover, it is remarkable that some of the relations were not present at Time 1, but present at Time 2 (e.g., the association between perceived stress and risk-taking behaviors). It is possible that such effects are only present at a later developmental stage, when more stress and risk-taking behaviors occur. Indeed, both measures significantly increased over time. Similarly, our model did not fit when only using cross-sectional data. The poor model fit of the cross-sectional model strengthens our argument that the effects of brain variable are panning out over time and are absent in a cross-sectional snap shot. Therefore, had we had run only cross-sectional analyses, we could have missed this important longitudinal effect. This highlights the importance of looking at developmental processes and changes using prospective longitudinal data. Finally, it is also important to note that the interaction effects between insular risk processing and neural cognitive control at Time 1 did not directly predict increases in risk-taking behaviors, but only indirectly by increases in perceived stress. Given the direct associations of interaction effects between insular risk processing and neural cognitive control found among late adolescents (e.g., Kim-Spoon et al., 2017a, Kim-Spoon et al., 2017b), further research is warranted to test whether the direct interaction effects between insular risk processing and neural cognitive control on risk-taking behaviors depend on the developmental stage.
5. Conclusion
Adolescence is thought to be a sensitive period for experiencing stress and engagement in risky behaviors, although more empirical evidence from human studies is warranted (Fuhrmann et al., 2015). Despite the fact that much has been learned from the behavioral work examining how uncertainty is related to stress (see Starcke and Brand, 2016 for a review), research regarding how stress interfaces with both motivational and cognitive control neural systems to affect adolescent risk-taking behaviors is limited. Here, our findings provide critical evidence for the important role of perceived stress in risk-taking behaviors: The combination of low insular risk processing and poor neural cognitive control in prefrontal cortex areas may represent a neural vulnerability that places adolescents at increased risk for experiencing more stress and subsequently for engaging in more risk-taking behaviors. Furthermore, our longitudinal analyses clarify that insular risk processing was predictive of changes in perceived stress, rather than vice versa. Reducing stressful experiences when faced with uncertainty and guiding adolescents toward optimal coping strategies within these situations may be crucial elements to boost the effectiveness of interventions (e.g., cognitive control training) targeting reductions of risk-taking behaviors in the long term.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants awarded to Jungmeen Kim-Spoon and Brooks King-Casas from the National Institute on Drug Abuse (DA036017). We are grateful to the adolescents and parents who participated in this study.
Footnotes
Coefficient of variation (CV) was used to calculate the level of risk associated with each option, with higher values of CV corresponding to increased levels of risk. CV for each option represents the ratio of the standard deviation of potential outcomes associated with an option to the expected value (EV) of that option: (1); (2). Phigh and Plow is the probability of the high and low outcome, respectively, Vhigh and Vlow is the high and low monetary outcome.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.02.005.
Contributor Information
Dominique Maciejewski, Email: d.maciejewski@ggzingeest.nl.
Nina Lauharatanahirun, Email: nina1@vtc.vt.edu.
Toria Herd, Email: tiherd@vt.edu.
Jacob Lee, Email: jacoblee@vtc.vt.edu.
Kirby Deater-Deckard, Email: kdeaterdeck@umass.edu.
Brooks King-Casas, Email: bkcasas@vtc.vt.edu.
Jungmeen Kim-Spoon, Email: jungmeen@vt.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex: one decade on. Trends Cognit. Sci. 2014;18:177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Bach D.R., Symmonds M., Barnes G., Dolan R.J. Whole-brain neural dynamics of probabilistic reward prediction. J. Neurosci. 2017;37:3789–3798. doi: 10.1523/JNEUROSCI.2943-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Bush G., Shin L.M., Holmes J., Rosen B.R., Vogt B.A. The multi-source interference task: validation study with fMRI in individual subjects. Mol. Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Durston S., Fossella J.A. Evidence for a mechanistic model of cognitive control. Clin. Neurosci. Res. 2001;1:267–282. [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Williamson G. Perceived stress in a probability sample of the United States. In: Spacapam S., Oskamp S., editors. Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Sage; Newbury Park, CA: 1988. pp. 31–68. [Google Scholar]
- Cole D.A., Maxwell S.E. Testing mediational models with longitudinal data: questions and tips in the use of structural equation modeling. J. Abnorm. Psychol. 2003;112:558–577. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- Conger R.D., Elder G.H. Aldine de Gruyter; New York, NY: 1994. Families in Troubled Times: Adapting to Change in Rural America. Social Institutions and Social Change. [Google Scholar]
- Copeland-Linder N., Lambert S.F., Chen Y.-F., Ialongo N.S. Contextual stress and risk-taking behaviors among African American adolescents. J. Youth Adolesc. 2011;40:158–173. doi: 10.1007/s10964-010-9520-y. [DOI] [PubMed] [Google Scholar]
- d’Acremont M., Bossaerts P. Neurobiological studies of risk assessment: a comparison of expected utility and mean-variance approaches. Cognit. Affect. Behav. Neurosci. 2008;8:363–374. doi: 10.3758/CABN.8.4.363. [DOI] [PubMed] [Google Scholar]
- de Berker A.O., Rutledge R.B., Mathys C., Marshall L., Cross G.F., Dolan R.J., Bestmann S. Computations of uncertainty mediate acute stress responses in humans. Nat. Commun. 2016;7:10996. doi: 10.1038/ncomms10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E., Gunnar M.R. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev. Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Fuhrmann D., Knoll L.J., Blakemore S.-J. Adolescence as a sensitive period of brain development. Trends Cogn. Sci. 2015;19:558–566. doi: 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Vogt A. Joint Statistical Meetings. 2012. Outliers: an evaluation of methodologies; pp. 3455–3460. San Diego, CA. [Google Scholar]
- Hammen C. Stress generation in depression: reflections on origins, research, and future directions. J. Clin. Psychol. 2006;62:1065–1082. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Guilford Press; New York, NY: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. [Google Scholar]
- Herts K.L., McLaughlin K.A., Hatzenbuehler M.L. Emotion dysregulation as a mechanism linking stress exposure to adolescent aggressive behavior. J. Abnorm. Child Psychol. 2012;40:1111–1122. doi: 10.1007/s10802-012-9629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C.A., Laury S.K. Risk aversion and incentive effects. Am. Econ. Rev. 2002;92:1644–1655. [Google Scholar]
- Iacovino J.M., Bogdan R., Oltmanns T.F. Personality predicts health declines through stressful life events during late mid-life. J. Pers. 2016;84:536–546. doi: 10.1111/jopy.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.L., Minati L., Harrison N.A., Ward J., Critchley H.D. Under pressure: response urgency modulates striatal and insula activity during decision-making under risk. PLoS One. 2011;6:e20942. doi: 10.1371/journal.pone.0020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann L., McManus T., Harris W.A., Shanklin S.L., Flint K.H., Hawkins J. Youth risk behavior surveillance United States 2015. MMWR Surveill. Summaries. 2016;65:1–174. doi: 10.15585/mmwr.ss6506a1. [DOI] [PubMed] [Google Scholar]
- Kim-Spoon J., Deater-Deckard K., Lauharatanahirun N., Farley J.P., Chiu P.H., Bickel W.K., King-Casas B. Neural interaction between risk sensitivity and cognitive control predicting risk-taking behaviors among late adolescents. J. Res. Adolesc. 2017;27:674–682. doi: 10.1111/jora.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Spoon J., Kahn R.E., Lauharatanahirun N., Deater-Deckard K., Bickel W.K., Chiu P.H., King-Casas B. Executive functioning and substance use in adolescence: neurobiological and behavioral perspectives. Neuropsychologia. 2017;100:79–92. doi: 10.1016/j.neuropsychologia.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas J.M., Bartolomucci A., Buwalda B., de Boer S.F., Flügge G., Korte S.M. Stress revisited: a critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lighthall N.R., Sakaki M., Vasunilashorn S., Nga L., Somayajula S., Chen E.Y. Gender differences in reward-related decision processing under stress. Soc. Cogn. Affect. Neurosci. 2012;7:476–484. doi: 10.1093/scan/nsr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.T., Kleiman E.M. Impulsivity and the generation of negative life events: the role of negative urgency. Personal. Individ. Differ. 2012;53:609–612. [Google Scholar]
- Liu R.T. Stress generation: future directions and clinical implications. Clin. Psychol. Rev. 2013;33:406–416. doi: 10.1016/j.cpr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- MacKinnon D.P., Kisbu-Sakarya Y., Gottschall A.C. Developments in mediation analysis. In: Little T.D., editor. The Oxford Handbook of Quantitative Methods in Psychology: Vol. 2: Statistical Analysis. Oxford University Press; New York, NY: 2013. pp. 338–360. [Google Scholar]
- McEwen B.S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Mohr P.N.C., Biele G., Heekeren H.R. Neural processing of risk. J. Neurosci. 2010;30:6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L.K., Muthén B.O. seventh ed. Muthén & Muthén; Los Angeles, CA: 1998. Mplus User’s Guide. [Google Scholar]
- Richards J.M., Plate R.C., Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci. Biobehav. Rev. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A., Bentler P.M. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.R., Steinberg L., Chein J. The role of the anterior insula in adolescent decision making. Dev. Neurosci. 2014;36:196–209. doi: 10.1159/000358918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V.L. Experimental economics: induced value theory. Am. Econ. Rev. 1976;66:274–279. [Google Scholar]
- Starcke K., Brand M. Effects of stress on decisions under uncertainty: a meta-analysis. Psychol. Bull. 2016;142:909–933. doi: 10.1037/bul0000060. [DOI] [PubMed] [Google Scholar]
- Treadway M.T., Buckholtz J.W., Zald D.H. Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Front. Hum. Neurosci. 2013;7:180. doi: 10.3389/fnhum.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy J.P., Galván A. Acute stress increases risky decisions and dampens prefrontal activation among adolescent boys. Neuroimage. 2017;146:679–689. doi: 10.1016/j.neuroimage.2016.08.067. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde A.C.K., Huizenga H.M., Somerville L.H., Delgado M.R., Powers A., Weeda W.D. Neural correlates of expected risks and returns in risky choice across development. J. Neurosci. 2015;35 doi: 10.1523/JNEUROSCI.1924-14.2015. Retrieved from: http://www.jneurosci.org/content/35/4/1549.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W., Rodriguez C.A., Schweitzer J.B., McClure S.M. Adolescent impatience decreases with increased frontostriatal connectivity. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3765–74. doi: 10.1073/pnas.1423095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E.U., Shafir S., Blais A.-R. Predicting risk sensitivity in humans and lower animals: risk as variance or coefficient of variatio. Psychol. Rev. 2004;111:430–445. doi: 10.1037/0033-295X.111.2.430. [DOI] [PubMed] [Google Scholar]
- Wood S.M., Bechara A. The neuroscience of dual (and triple) systems in decision making. In: Reyna V.F., Zayas V., editors. The Neuroscience of Risky Decision Making. 2014. pp. 177–202. Washington, DC. [Google Scholar]
- Zillmann D., Bryant J. Affect, mood, and emotion as determinants of selective exposure. In: Zillmann D., Bryant J., editors. Selective Exposure to Communication. Lawrence Erlbaum Associates; Hillsdale, NJ: 1985. pp. 157–190. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.