Abstract
There is considerable inter-individual variability in the rate at which working memory (WM) develops during childhood and adolescence, but the neural and genetic basis for these differences are poorly understood. Dopamine-related genes, striatal activation and morphology have been associated with increased WM capacity after training. Here we tested the hypothesis that these factors would also explain some of the inter-individual differences in the rate of WM development.
We measured WM performance in 487 healthy subjects twice: at age 14 and 19. At age 14 subjects underwent a structural MRI scan, and genotyping of five single nucleotide polymorphisms (SNPs) in or close to the dopamine genes DRD2, DAT-1 and COMT, which have previously been associated with gains in WM after WM training. We then analyzed which biological factors predicted the rate of increase in WM between ages 14 and 19.
We found a significant interaction between putamen size and DAT1/SLC6A3 rs40184 polymorphism, such that TC heterozygotes with a larger putamen at age 14 showed greater WM improvement at age 19.
The effect of the DAT1 polymorphism on WM development was exerted in interaction with striatal morphology. These results suggest that development of WM partially share neuro-physiological mechanism with training-induced plasticity.
Keywords: Working memory, Development, Dopamine, Striatum, DAT-1, rs40184
1. Introduction
Working memory (WM) capacity increases from early childhood until early adulthood (Gathercole, 1999; Luna et al., 2004), but the rate of development, as well as the maximum WM capacity, differs considerably between individuals. At the lower end of the distribution, WM capacity is associated with several neuropsychiatric disorders, including ADHD (Martinussen et al., 2005; Nikolas and Nigg, 2013), dyslexia (Ahissar, 2007; Kudo et al., 2015) and dyscalculia (von Aster and Shalev, 2007; Mammarella et al., 2015). However, the neural bases for variability in the rate of WM development are poorly understood. The importance of understanding the neural basis of WM variability throughout development is two folded: on one hand it would improve and refine our theoretical knowledge of WM and how it changes over time and development, on the other hand it could be useful to design and test theoretical driven intervention to improve WM, as well as for early identification of children which may need those intervention the most.
In cross-sectional studies, neuroimaging of children and adolescents of different ages show that WM development is related to an increase in the BOLD signal within the intraparietal cortex, superior frontal sulcus, and dorsolateral prefrontal cortex (Klingberg et al., 2002; Kwon et al., 2002; Crone et al., 2006; Olesen et al., 2006) and cortical thinning in the frontal and parietal cortices (Tamnes et al., 2010, Tamnes et al., 2013; Ostby et al., 2011)(13–15). Two longitudinal studies, however, found that BOLD activity in the frontal and parietal cortices is associated with current WM capacity, but not future WM, while BOLD activity in the striatum is related to future WM (Ullman et al., 2014; Darki and Klingberg, 2015).
Dopamine-levels in the brain are associated with WM performance in non-human primates (Sawaguchi and Goldman-Rakic, 1991; Vijayraghavan et al., 2007) and several human polymorphisms within dopamine-related genes (COMT, DAT1, DRD4), are associated with WM capacity. For example, reduced COMT (Val158Met) enzymatic activity in Met carriers is associated with higher WM performance in adults (Barnett et al., 2007; Barnett et al., 2008). Developmental genetic studies suggest that this effect could be age-related and that the Met-allele is not beneficial in younger children (Wahlstrom et al., 2007; Dumontheil et al., 2011).
There is an interaction between DAT-1 polymorphism and WM load that affects the activity in striatum of children (Stollstorff et al., 2010). The DAT1 (SLC6A3) gene codes for a presynaptic transporter for uptake of dopamine, and thus affecting the amount of available dopamine in the synaptic cleft. DAT1 is most densly expressed in the striatum (Ciliax et al., 1999). Heinz et al. showed 22% higher DAT1 availability in putamen of a DAT1 variation (10-repeat homozygotes) compared to those who did not possess this variation (9-repeat carriers) (Heinz et al., 2000).
Recent studies have tested which neural and genetic factors mediate inter-individual differences in the rate of WM improvement after WM training (Brehmer et al., 2009; Söderqvist et al., 2012; Bellander et al., 2015). Training-related improvement can be safely assumed to be experience-dependent. If development during childhood is also (at least partially) driven by experience-dependent processes, than it can be hypothesized that the genes or brain structures associated to training related improvement should also affect development. This is not to say that training and development are just the same process on a different time scale or different extent: natural development take place in the real world and it is a continuous process. Not only, but by nature it is more multisensorial, engaging and motivationally relevant that any training regime can be. In addition, there are likely other neural factors underlying WM capacity, independently of training or plasticity. However, aside from theoretical works, many studies suggest that training and natural development partially share some neurophysiological mechanism. Several WM training-related changes are associated with the dopamine system. WM training gains are associated with changes in density of D1 receptors in the frontal and parietal cortices (McNab et al., 2009) as well as with increased striatal dopamine release (Backman et al., 2011). The latter findings could relate to training-related increases in striatal BOLD signal (Olesen et al., 2004; Dahlin et al., 2008), as increased dopamine release in the striatum is associated with higher BOLD signal (van der Schaaf et al., 2014). Genetic studies of WM training have found associations between the amount of improvement during WM training and polymorphism of DAT1 VNTR (rs27072) (Brehmer et al., 2009), DAT1 polymorphism (rs40184) (Söderqvist et al., 2012), COMT (rs4680) (Bellander et al., 2015) and a polymorphism of DRD2/ANKK1 genes (rs1800497) (Söderqvist et al., 2014). Following up on Söderqvist et al. (Söderqvist et al., 2012), Nymberg et al. showed that the same SNP was related to WM in a large sample of 14-year olds. (Nymberg et al., 2014).
Brain activity in the striatum has thus been linked to relative improvement in WM during development (Ullman et al., 2014; Darki and Klingberg, 2015) and to improvement after WM training (Olesen et al., 2004; Dahlin et al., 2008). However, there has been no longitudinal study focusing on genetic variability and WM development. Furthermore, no study has investigated the interactions between striatal morphology and dopaminergic polymorphisms on the rate of WM development.
Apart from subcortical structures, it has been shown that WM training also affect cortical morphology (e.g. increase in cortical thickness in frontal and parietal regions (Metzler-Baddeley et al., 2016)), cortical activity (e.g. increase in activity in regions of the fronto-parietal networks (Klingberg, 2010; Constantinidis and Klingberg, 2016)) as well as connectivity of specific resting state network (e.g. increase in connectivity at rest between fronto-parietal network regions and lateral occipital complex as well as inferior temporal gyrus (Astle et al., 2015)). Although these results are of interest for the relationship between training and natural development, as for example it has been shown that WM development is associated with increased anatomical connectivity in similar regions of the fronto-parietal network (Klingberg, 2006), we decided to focus on striatal regions, as these are the regions where evidence from genetic and imaging studies converge more convincedly.
In the present study, we tested the hypothesis that genetic variability associated with the relative improvement during WM training (DAT-1, DRD2, DRD4, COMT) would also be associated with the rate of increase in WM capacity during normal development. Moreover, based on the high DRD2 and DAT1 expression in striatum, and the previous association between striatal activity and WM development (Ullman et al., 2014; Darki and Klingberg, 2015), we hypothesized that the morphology of the striatum would also be associated with relative increase in WM capacity during adolescence. We used the CANTAB self-ordered spatial working memory task, which has previously been shown to be sensitive to damage to the prefrontal cortex (38) and to basal ganglia disorders such as Parkinson's and Huntington's diseases (Owen et al., 1992, Owen et al., 1997). Moreover, performance on this task is sensitive to dopaminergic modulation as shown by studies with D2 receptor agents in participants stratified according to the Taq1A polymorphism (Naef et al., 2017), effects of L-Dopa in patients with Parkinson's disease (Lange et al., 1992) and by studies using position emission tomography showing correlations with D2 receptor availability in healthy volunteers (Clatworthy et al., 2009) and patients with Parkinson's disease (Cools et al., 2002). Finally, performance on this task has been shown to measure the gradual improvement in working memory development from childhood to adolescence (De Luca et al., 2003). However, the neural basis for this maturation remains to be elucidated.
2. Method
2.1. Participants
The IMAGEN sample is comprised of >2000 14-years-old adolescents that were tested at one of eight assessment centers (London, Nottingham, Dublin, Berlin, Hamburg, Paris, Dresden, and Mannheim)(Schumann et al., 2010). WM data were collected from 2048 of the participants at age 14 (WM14). Out of this sample 756 participants from six assessment sites (London, Dublin, Berlin, Paris, Dresden and Mannhaim) completed the WM task also at age 19 (WM19) (between visits range = 3–7 years, mean = 4.5 years, sd = 0.7 years). 698 of these individuals had complete genetic data and 487 of these individuals had quality controlled structural MRI data. These latter individuals made up the sample of the present study.
2.2. CANTAB working memory
WM was assessed using the CANTAB self-ordered spatial working memory task (Owen et al., 1990; Robbins et al., 1994) at both time points (Beck et al., 2010). This computerized task displays a number of squares ('boxes') displayed on a touch-sensitive screen. The aim of the test is for the participants to open the boxes to find the single blue token provided in each of a number of boxes (ranging from 4 to 8 over successive blocks) and use them to fill up an empty column. The color and position of the boxes are changed from trial to trial to discourage the use of stereotyped search strategies. If the participant returns to an empty box that has already been opened on that trial this constitutes a ‘between search error’. Hence lower between search errors indicates better performance. The test was completed in the presence of a researcher to ensure instruction compliance.
2.3. Genetics
Polymorphisms were selected on the basis of previously published studies which have found association between genetic variability in dopamine-related genes and WM training (Söderqvist et al., 2012; Bellander et al., 2015). DNA was extracted from blood samples. Five single nucleotide polymorphisms from four genes (rs27072 and rs40184 from DAT1/SLC6A3, rs1800497 from DRD2/ANKK1, rs4680 from COMT and rs11246226 from DRD4; Table 1) were extracted using the software Plink (Purcell et al., 2007). Genotyping procedures have previously been published (Nymberg et al., 2013), further details are provided in the supplemental information.
Table 1.
Single nucleotide polymorphisms included in analyses.
| Gene | SNP | Position | Location |
|---|---|---|---|
| DAT1 | rs40184 | Chr5:1395077 | intron 14 |
| DAT1 | rs27072 | Chr5: 1394522 | in 3'-UTR |
| DRD2/ANKK1 | rs1800497 | Chr11: 113270828 | Exon 8 |
| COMT | rs4680 | Chr22: 18331271 | Exon 4 |
| DRD4 | rs11246226 | Chr11: 641191 | 500 bp downstream of 3'-UTR |
2.4. MRI data acquisition and analysis
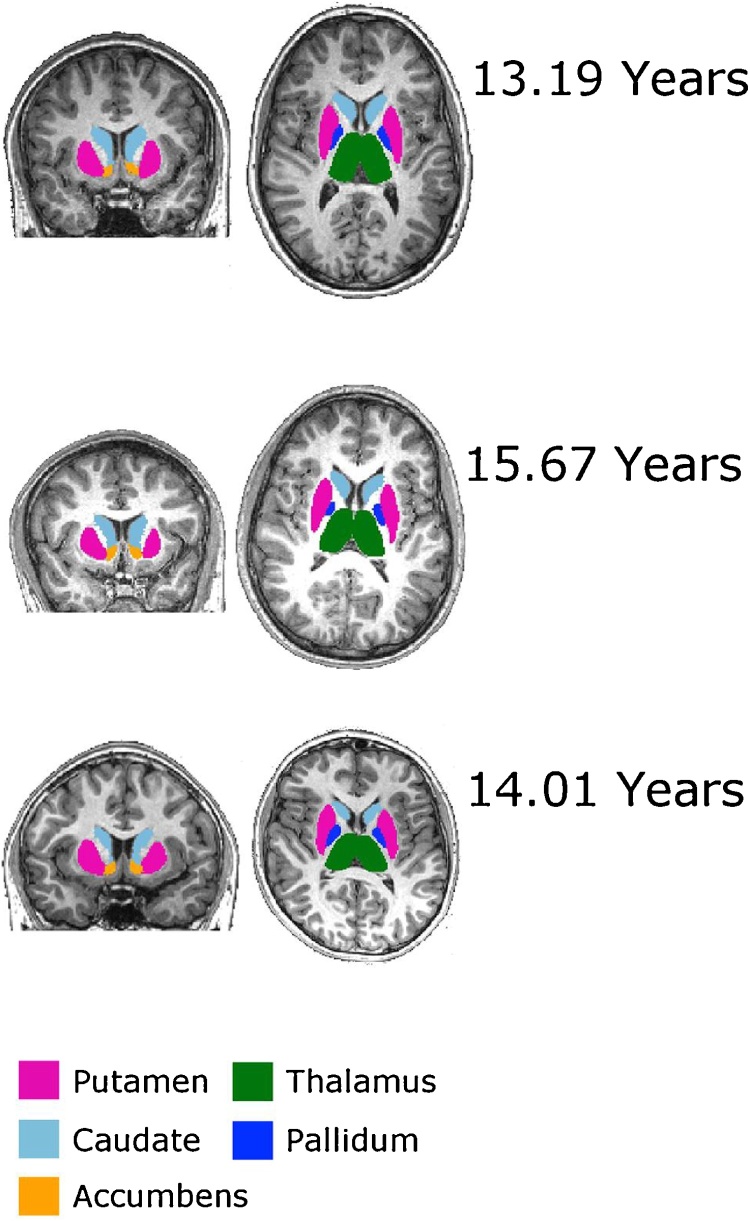
Structural MRI data was acquired at six IMAGEN assessment sites with 3T MRI scanners of different manufacturers (Siemens, Philips, General Electric, Bruker). The scanning variables were specifically chosen to be compatible with all scanners. The same scanning protocol was used at all sites. High-resolution T1-weighted 3D structural images were acquired for each subject (TR = 2300 ms, TE = 2.8 ms, 256 × 256 × 160 matrix, voxel size 1.1 × 1.1 × 1.1). T1-3D images were skull-stripped using the Brain Extraction Tool (BET) (Smith, 2002). Subcortical nuclei (caudate nucleus, putamen, nucleus accumbens) were automatically segmented from T1 images using the FMRIB imaging registration and segmentation tool (FIRST) (http://www.fmrib.ox.ac.uk/fsl/first/index.html). All of the free parameters were set at their default values, based on prior optimization of these parameters (Patenaude, 2007; Patenaude et al., 2011). FIRST generated several coronal, axial and sagittal slices with the superimposed segmented structure displayed, which enabled us to perform quality control of the segmentation of dubious outliers. Fig. 2 shows the results of the segmentation for the oldest and the youngest subjects, as well as for the subject that was closer to the average age of the sample at 14.
Fig. 2.
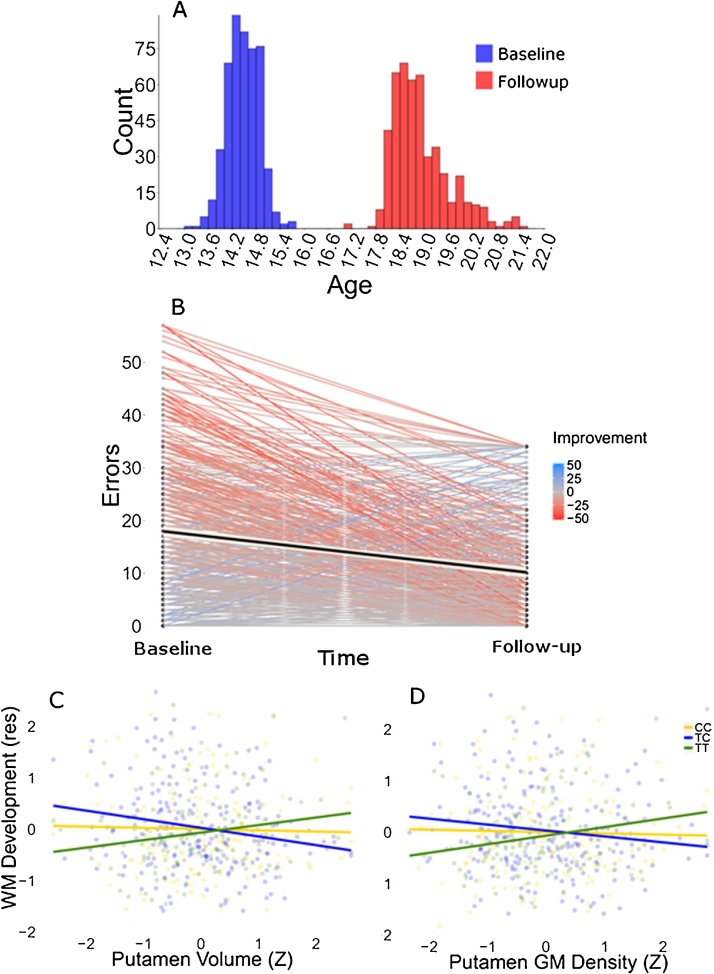
Behavioral finding. A) Age distribution at baseline and follow-up. B) Number of errors at baseline and follow up, the lines connect the performance of the same subject at both time point and are color coded according to the decrease in number of errors. C) Scatterplot illustrating the interaction between rs40184 and putamen volume on WM development. The x axis reports the Z-transformed putamen volume while y axis reports the residuals of WM performance at follow-up over WM performance at baseline, acquisition site and sex. D) Scatterplot illustrating the interaction between rs40184 and putamen volume on WM development. The x axis reports the Z-transformed putamen volume while y axis reports the residuals of WM performance at follow-up over WM performance at baseline, acquisition site and sex.
T1 images were segmented in grey matter, white matter and CSF partitions by means of SPM. A study-specific template was then created using SPMs diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) toolbox (Ashburner, 2007). The same toolbox was used to warp the single grey and white matter density images onto the template while modulating density values in order to correct for expansion and contraction introduced by the warping.
For the grey matter density analysis we extracted the grey matter density using as ROIs the left and right putamen as segmented by FIRST in the previous step.
2.5. Statistical analyses
Statistical analyses were performed in SPSS version 22 and R (https://www.r-project.org/). We computed the total size of the striatum by adding the volumes from both left and right hemisphere of the nucleus accumbens, caudate and putamen and tested 5 models for the 5 different SNPs. WM performance at age 19 (WM19), WM performance at age 14 (WM14) and striatal volume were mean centered and then outliers (defined as point below 1.5 times the lower interquartile distance and above 1.5 times the upper interquartile distance) were set to 1.5 time the value of the relevant interquartile distance. All the SNPs except two were coded for an additive effect: the minor allele was coded as 0, the heterozygote was codes as 1 and the major allele was coded as 2. In coding rs27072 (DAT1) and rs1800497 (DRD2) the minor allele was lumped with the heterozygotes as our sample contained less than 5% of the former, so that in the model the minor allele and the heterozygote were coded as 1 and the major allele as 2.
The first analyses focusing on the volume of the whole striatum, the five SNPs and their interaction were exploratory in nature and thus the p values were not corrected for multiple comparisons. In the subsequent analyses (i.e. focusing on rs 40184) p values were corrected for multiple comparisons using Bonferroni correction.
3. Results
Participants were on average 14.47 (±0.39) years old at first testing and 18.92 (±0.71) years old at follow-up (Fig. 1A). There were 2 outliers for the WM measure at first testing and 21 for the WM measure at follow-up. The outliers were set to 1.5 times the value of the relevant interquartile distance. In the first testing subjects committed on average 17.9 (±13.3) errors on the WM task and 10.5 (±10.5) errors at the follow-up. This represented a change of 0.62 standard deviations, which was significant (paired sample t-test: t = −13.65, p < 0.0001, Cohen’s d: 0.62). The correlation of test score between first and second measurement of the CANTAB SWM task was r = 0.5. As shown in Fig. 1B, there was considerable variance in the amount of improvement on the WM tasks, and the subsequent analyses were aimed at finding factors associated with the rate of improvement.
Fig. 1.
Subcortical nuclei segmentation. Axial and coronal view of the subcortical segmentation overlaied onto the anatomical T1-wighted image for the youngest subject (age = 13.19 years), the oldes subject (age = 15.67 years) and the subject clostest to the average (age = 14.01). Striatal nuclei were segmetned together with pallidum, thalmus, hippocampus and amygdala in order to benefit of the automatic overlap correction performed within FIRST.
3.1. Main effects and interactions of dopamine SNPs and striatal volume on WM
In order to evaluate the effect of SNPs and striatal volume on WM development, we performed an analysis with WM capacity at age 19 as the dependent variable. The independent variables were SNP, striatal volume, the interaction of SNP and striatal volume, and WM capacity at age 14:
| WM19 = β0 + β1 SNP + β2 striatum volume + β3SNP × striatum volume + β4WM14 + β5sex + β6site + ε |
By controlling for WM at age 14, this model evaluated factors that contribute to the change in WM capacity between age 14 and 19. In this as in all other models presented throughout the paper we controlled for the effect of acquisition site (parameter site in the model) and for the effect of sex (parameter sex in the model).
Our analyses revealed no main effects of any of the five SNPs or main effect of striatal volume on WM development (see Table 2), but a trend towards an interaction between DAT1 rs40184 and striatal volume (F = 2.61, p = 0.075). Note that these first analyses were exploratory and thus the p values were not corrected for multiple comparisons.
Table 2.
Main effects and interactions of dopamine SNPs and striatal volume on WM development from age 14 to age 19 (based on the model: WM19 = β0 + β1 SNP + β2 striatum volume + β3SNP × striatum volume + β4WM14 + β5sex + β6site + ε). Uncorrected trends denoted in bold.
| Model for SNP: | ANKK1 rs1800497 | DAT1 rs40184 | DAT1 rs27072 | COMT rs4680 | DRD4 rs11246226 |
|---|---|---|---|---|---|
| SNP | F = 0.26, p = 0.61 | F = 0.42, p = 0.66 | F = 1.19, p = 0.28 | F = 0.61, p = 0.55 | F = 2.59, p = 0.11 |
| Striatal Volume | F = 1.84, p = 0.18 | F = 1.35, p = 0.25 | F = 1.96, p = 0.16 | F = 2.45, p = 0.12 | F = 1.07, p = 0.30 |
| SNP*Striatal Volume | F = 0.82, p = 0.37 | F = 2.61, p = 0.075 | F = 0.08, p = 0.77 | F = 0.05, p = 0.96 | F = 2.49, p = 0.12 |
The trend towards significance found for rs40184 when the volume of the whole striatum was entered in the analysis suggested that there could be a spatially specific effect for one of the striatal nuclei. Thus, we divided the striatum into its nuclei, i.e. nucleus accumbens, caudate and putamen in order to investigate main effects and interactions between rs40184 and each of these regions on WM development. The same model was used, but with the subregions (bilateral accumbens, caudate or putamen) instead of the entire striatum in the model. These analyses revealed a significant interaction of rs40184 and putamen volume on WM development (F = 5.22, p = 0.004, p − FWE = 0.012 corrected across the three subcortical nuclei). This interaction remained significant when exact age in months at follow-up was included as a covariate in the model (F = 4.77, p = 0.006, p-FWE = 0.016 corrected across the three subcortical nuclei). The result were also significant when time between measurements were included as a covariate (F = 5.41, p = .005).
A follow-up analysis showed that the effect of putamen volume amongst TT homozygotes was significantly different from the TC heterozygotes (p = 0.0009) and not different from CC homozygotes (p = 0.11) (Fig. 1C). Neither rs40184 nor the rs40184-by-subcortical nuclei interaction were significant for the models testing the effect of accumbens or caudate volume on WM (Table 3).
Table 3.
Main effects and interactions between SNP rs40184 and subcortical nuclei (nucleus accumbens, caudate and putamen) on WM development from age 14 to age 19 (i.e. WM19 = β0 + β1 rs40184 + β2 subcortical nuclei + β3rs40184 × subcortical nuclei + β4WM14 + β5sex + β6site + ε). Significant p-values (p < .05) denoted in bold.
| Model for subcortical nuclei: | L/R Accumbens | L/R Caudate | L/R Putamen |
|---|---|---|---|
| rs40184 | F = 0.45, p = 0.64 | F = 0.53, p = 0.59 | F = 0.34, p = 0.71 |
| Subcortical nuclei | F = 1.76, p = 0.19 | F = 1.48, p = 0.22 | F = 0.33, p = 0.56 |
| Rs40184*Subcortical nuclei | F = 0.44, p = 0.65 | F = 1.08, p = 0.34 | F = 5.22, p = 0.004 |
In order to confirm the interaction effect between rs40184 and putamen volume using a second method we extracted gray matter density in the putamen and fitted the same model as above, but replacing putamen volume with putamen gray matter density. We identified significant interactions between DAT1 rs40184 and putamen gray matter density on WM development (by using WM at age 19 as outcome variable while controlling for WM at age 14) (F = 4.76, p = 0.012; F = 4.72, p = 0.013 when age at follow up was included in the model; Fig. 1D). Supporting the previous results, we found that the effect of putamen grey matter density on improvement was significantly different between TT homozygotes and TC heterozygotes (p = 0.002) while it did not differ between TT and CC homozygotes (p = 0.083) and not different between TC heterozygotes and CC homozygotes (p = 0.31).
In order to have an index of the strength of the evidences in favor of the effect of the interaction between rs40184 and morphometry we calculated the Bayes Factors (BF) for the full model (i.e. the one including the interaction) and the model without the interaction. The BF were calculated for both volume and grey matter density as index of morphometry. The ratio between the BFs of the full model and of the restricted model was 3.47 for volume and 5.91 for grey matter density. Both of these values indicate moderate evidence in favor of the alternative hypothesis (Lee and Wagenmakers, 2013).
3.2. Main effects and interaction of striatal volume and rs40184 on working memory at age 14 and 19
The prior results suggested a rs40184-by-putamen effect on development of WM. If this is true, one might expect that such an effect would be present also for WM at age 14 and 19, since WM capacity at that these ages are partly dependent on development of WM in the prior years. Therefore, we next tested this hypothesis using the following model:
| WM14/19 = β0 + β1rs40184 + β2 putamen volume + β3rs40184 × putamen volume + β4sex + β5site + ε |
We found a non significant effect of rs40184-by-putamen volume interaction on WM14 (F = 3.15, p = 0.051). However, the interaction reached significance when age at baseline was included in the model (F = 3.33, p = 0.043). Comparing the genotypes pairwise showed that the effect of putamen volume in the TT homozygotes was significantly different than in the CC homozygotes (p = 0.017), but not different from TC heterozygotes (p = 0.21). The effect of putamen volume was not different between TC heterozygotes and CC homozygotes (p = 0.12). When replacing putamen volume with putamen grey matter density we found again that the interaction was not significant (F = 2.85, p = 0.063). However, as for the volume, the interaction reached significance when including age at baseline in the model (F = 3.25, p = 0.041).
Finally, there was a significant rs40184-by-putamen volume interaction also for WM at age 19 (F = 5.94, p = 0.002). The interaction remained significant when age at follow-up was included in the model (F = 5.97, p = 0.002). Pairwise comparisons of the genotypes showed that the effect of putamen volume in the TT homozygotes was significantly different than in both the CC homozygotes (p = 0.012) and the TC heterozygotes (p = 0.0004). The effect was not different between TC heterozygotes and CC homozygotes (p = 0.41). This interaction was also found to be significant when putamen volume was replaced in the model with putamen grey matter density (F = 5.65, p = 0.007; F = 5.91, p = 0.005 when age at follow-up was included in the model). Note that the analyses reported in this paragraph only focused on putamen, as a follow up of the main analysis, and as such we have not applied correction for multiple comparisons.
We have performed additional analyses collapsing TC and CC allele obtaining similar results than in the main analyses. These analyses are reported in the supplemental information.
4. Discussion
This study investigated the hypothesis that variability in spatial WM development and variability in response to WM training partially share genetic and neural underpinnings. Building on the importance of the dopamine system for both spatial WM (Sawaguchi and Goldman-Rakic, 1991, Sawaguchi and Goldman-Rakic, 1994), WM development (Dumontheil et al., 2011) and for response to WM training (McNab et al., 2009; Backman et al., 2011; Bäckman and Nyberg, 2013; Söderqvist et al., 2014) we tested the effect of a subset of dopamine-related genetic polymorphisms, which had been associated with WM improvement after training, on WM development during adolescence. Moreover, in the light of the significant role of the striatum in both SWM development (Owen et al., 1992; Lawrence et al., 1996; Clatworthy et al., 2009; Cools and D’Esposito, 2011) and response to WM training (Dahlin et al., 2008; McNab and Klingberg, 2008; Backman et al., 2011; Kühn et al., 2013), we tested if variability in striatal morphology is related to WM development.
The rate of improvement in WM capacity from age 14–19 differed considerably between individuals (Fig. 2B). Although the variance in improvement can be partially attributed to a regression towards the mean (subjects with the higher number of errors at the first assessment tend to improve more than subjects with lower number of errors), closer inspection of Fig. 2A shows that regression towards the mean is not the only phenomenon observable. Indeed, subjects committing a number of errors close to the group mean showed variable amount of improvement, thus excluding a simple regression toward the mean effect. The analyses we performed tried to identify the source of this variance.
We found that an interaction between rs40184 and putamen volume predicted WM development between the age of 14 and 19. Neither the main effect of rs40184 nor the main effect of putamen volume alone was significant, which suggests that the effect of rs40184 on WM was only related to the interaction with the morphology of the putamen. These results were confirmed using grey matter density in the putamen. Using both volume and GM density measures, we were able to show that the effect of putamen morphology on WM development differed in TT homozygotes relative to TC heterozygotes and the CC homozygotes. Bayes Factors calculated for the model including the interaction between rs40184 and morphometry using two different indexes for morphometry (i.e. overall volume and GM density) converged towards comparable evidence in favor of the alternative hypothesis, thus corroborating our findings.
The putamen is a key structure for WM performance, as it is associated with important functions as filtering of irrelevant stimuli in WM tasks (McNab and Klingberg, 2008; Baier et al., 2010), and updating of item in WM (Yu et al., 2013). It projects not only to the motor cortex, but also to the frontal and parietal lobes (Tziortzi et al., 2011). The fronto-parietal network has been consistently reported as the neural underpinning of visuo-spatial WM development (Crone et al., 2006; Klingberg, 2006; Scherf et al., 2006; Ostby et al., 2009). A direct involvement of the putamen in executive function has been recently suggested by Pauli et al. (2016) by means of a coordinate based meta-analysis of the literature reporting activation within the striatum (Pauli et al., 2016). Noteworthy for our hypothesis, the putamen has also been associated with the amount of improvement after WM training (Kühn et al., 2013) as well as to transfer to untrained skills after WM training (Dahlin et al., 2008).
Variability in the DAT1 gene has previously been associated with both WM development (Sambataro et al., 2015) and WM training (Brehmer et al., 2009), at least in adulthood. Brehmer et al. (2009) found the same SNP associated with the response to WM training (rs40184) as the one we found associated with WM development. To our knowledge this is the first time that an interaction between the DAT1 and volume of putamen on WM development in adolescence has been reported.
The interaction between volume of putamen and DAT1 polymorphism on WM development can be interpreted as an interplay between genetic variability and naturally occurring brain development. Both cross-sectional (Ostby et al., 2009) and longitudinal studies (Dennison et al., 2013) have shown that putamen volume decreases with development. Variability in the DAT1 gene (VNTR) is also related to availability of the dopamine transporter, specifically in the putamen (Heinz et al., 2000). A tentative hypothesis invokes the inverted u-shape dose-response curve of dopamine on behavior (Arnsten, 1997; Vijayraghavan et al., 2007; Gjedde et al., 2010; Cools and D’Esposito, 2011). The apex of the u-shaped curve when optimal performance is achieved may not be fixed and may move during brain development. In this study the TT homozygotes with smaller putamen volume at age 14 would be already near the apex of the curve, thus performing better than their counterparts with greater putaminal volumes both at age 14 and 19. On the other hand, TC heterozygotes with greater putaminal volumes would reach the apex later on during development (i.e. when attaining smaller putaminal volume due to occurring brain development). This in turn would lead them to improve more than their TC counterpart with smaller putamen (i.e. those which are already close to the apex of the u-shaped curve). A corollary of this hypothesis is that the observed association between DAT1 and WM performance in children and adolescents with ADHD (Shang and Gau, 2014) may not be a permanent deficit but rather an effect of slower development (i.e. a lagging morphological development of the putamen). This latter hypothesis would be corroborated by finding that older age is associated with smaller putamen volume in healthy participants but not in ADHD (Greven et al., 2015).
The effect of the interaction between rs40184 and volume of the putamen measured at age 14 showed higher significance for future WM performance (i.e. at 19) than for the concurrent one (i.e. at 14), even when the former was corrected for WM performance at 14. This is in line with the proposed hypothesis that the physiological mechanisms related to WM capacity and plasticity are partially dissociated, and that the dopaminergic system involving the striatum would be more related to plasticity than capacity (Klingberg, 2014). This is also in agreement with two previous studies (Ullman et al., 2014; Darki and Klingberg, 2015) suggesting that activity in the striatum but not from the cortex can predict WM development. The difference in the striatal region found to be associated with development (putamen in the current study and caudate nucleus in Darki and Klingberg (2015) and Ullman et al. (2014)) could be due to the different tasks and indexes used to assess WM. While Darki and Klingberg (2015) and Ullman et al. (2014) used a sequence-repetition task and the number of remembered items as index of WM, in the present study a self-ordered spatial working memory task was used, which simulates an optimal foraging task in which participants have to remember not to return to previously rewarded locations. The structure of the CANTAB task, in which the subject has to maintain active the previously visited locations in order not to visit them again, relies on visuomotor monitoring and updating processes to a greater extent than a simple sequence-repetition task, which may thus implicate the putamen to a greater extent (McNab and Klingberg, 2008; Baier et al., 2010; Yu et al., 2013).
We found no significant effects nor interaction with the DRD2/ANKK1 and COMT polymorphisms. As for DRD2/ANKK1, this polymorphism was previously found to be associated with improvement after WM training (Söderqvist et al., 2014) and to influence the effect of ventral striatal BOLD signal on concurrent WM performance (Nymberg et al., 2014). A lack of effect for DRD2/ANKK1 in the presence of a significant effect of variability in DAT1 could reflect a difference in mechanisms between training and development. Furthermore, the effect of DRD2/ANKK1 on concurrent WM found by Nymberg et al. (2014) was exerted through an interaction with BOLD signal in the ventral striatum during a reward anticipation task, a measure that was not included in the present study.
At variance with Dumontheil and colleagues (2011), we did not find a behavioral effect of the COMT gene on WM development nor an interaction between volume of the putamen and COMT SNPs. As for the lack of interaction between COMT SNPs and volume of the putamen, this is expected, as it has been shown that the COMT gene has an effect on cortical, but not striatal dopamine metabolite level (Huotari et al., 2002). Furthermore, Dumontheil and colleagues used a sample ranging from 6 to 20 years old, while the present sample ranged from 14 to 19 years old.
Overall, our results suggest that there is a partial overlap between physiological mechanisms related to WM development and to WM training-related improvement: in particular there would be an interplay between striatal morphology and a dopaminergic related gene that could influence both development and training-improvement. If development and training partially share the same physiological mechanism one can wonder about the difference in the extent of the effect of these two processes: while it has been shown that training is effective in improving WM performance, the extent of this improvement is not as big as that seen during development. Several factors can be invoked to explain this difference: first, the extremely different time-scale involved in the two processes. Moreover, as stated in the introduction; natural development occurs in the natural environment, that is by nature multimodal, multisensorial and more engaging than any training setting can be
One limitation of this study was the use of a single measure in order to characterize WM development. In particular, using different tasks to measure WM capacity could lead to both a more reliable measure, by means of combining different validated measures and a finer grained picture of WM development, allowing to observe potential dissociation between verbal and spatial WM. Indeed, future studies could focus on differential interaction between striatal morphology and genetic variability on different aspect of WM development. A second limitation of the study is the age range of the sample we included: previous studies have shown how fastest rate of WM development happens at younger ages that the ones included in our sample (e.g. between 6 and 11–12 years old; (Luna et al., 2004), between 8 and 20 years old (Roalf et al., 2014) and (Dumontheil et al., 2011)). The IMAGEN study was indeed devised with a focus on factors related to mental health during adolescence, and as such included subjects between 14 and 19 years old. However, the unique nature of the dataset, following hundreds of subjects longitudinally while including imaging, genetic and cognitive measures, allowed us to test relevant and specific hypothesis about the physiological underpinning of WM development. It is worth mentioning that the subjects participating both at baseline and follow-up had higher WM performance at baseline that subjects that did not participate in the follow-up. This means that our results may not generalize to subjects in the lowest spectrum of WM performance. However, our results are still valid for the entire range present in our sample. Finally; the lack of a control task not tapping working memory leave open the possibility that the interaction between putamen morphology and rs40184 that we found to affect WM development could be generally related to cognitive improvement rather than be specific for working memory, future studies including tasks tapping on different cognitive functions will be able to exclude this possibility.
In conclusion, this study showed that the DAT1 SNP rs40184, which has been previously associated with training-related improvement in WM (Brehmer et al., 2009) and fluid intelligence (Söderqvist et al., 2012) and that is known to affect the level of dopamine transporter in the putamen (Heinz et al., 2000), is a predictor of development-related plasticity of WM. The association is not between genetic variation and WM per se, but it is exerted through an association with putaminal morphological development.
Conflict of Interest
None.
Acknowledgements
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: BRain Imaging, cognition Dementia and next generation GEnomics) (MR/N027558/1), the FP7 projects IMAGEMEND(602450; IMAging GEnetics for MENtal Disorders) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grant 'c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the Swedish Research Council FORMAS, the Medical Research Council, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1). Further support was provided by grants from: ANR (project AF12-NEUR0008-01 - WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), U.S.A. (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.03.006.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Ahissar M. Dyslexia and the anchoring-deficit hypothesis. Trends Cogn. Sci. 2007;11(11):458–465. doi: 10.1016/j.tics.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. (Oxf.) 1997;11(2):151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Astle D. Cognitive training enhances intrinsic brain connectivity in childhood. J. Neurosci. 2015;35(16):6277–6283. doi: 10.1523/JNEUROSCI.4517-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L., Nyberg L. Dopamine and training-related working-memory improvement. Neurosci. Biobehav. Rev. 2013;37(9):2209–2219. doi: 10.1016/j.neubiorev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Backman L. Effects of working-Memory training on striatal dopamine release. Science. 2011;333(6043):718. doi: 10.1126/science.1204978. [DOI] [PubMed] [Google Scholar]
- Baier B. Keeping memory clear and stable–the contribution of human basal ganglia and prefrontal cortex to working memory. J. Neurosci. 2010;30(29):9788–9792. doi: 10.1523/JNEUROSCI.1513-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett J.H. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol. Psychiatry. 2007;12(5):502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Barnett J.H., Scoriels L., Munafò M.R. Meta-Analysis of the cognitive effects of the catechol-O-Methyltransferase gene Val158/108Met polymorphism. Biol. Psychiatry. 2008;64(2):137–144. doi: 10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Beck S. M. et al. (2010) Primary and secondary rewards differentially modulate neural activity dynamics during working memory, PloS one. Edited by S. Gilbert, 5(2), p. e9251. 10.1371/journal.pone.0009251. [DOI] [PMC free article] [PubMed]
- Bellander M. Lower baseline performance but greater plasticity of working memory for carriers of the val allele of the COMT Val158Met polymorphism. Neuropsychology. 2015;29(2):247–254. doi: 10.1037/neu0000088. [DOI] [PubMed] [Google Scholar]
- Brehmer Y. Working memory plasticity modulated by dopamine transporter genotype. Neurosci. Lett. 2009;467(2):117–120. doi: 10.1016/j.neulet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Ciliax B.J. Immunocytochemical localization of the dopamine transporter in human brain. J. Comp. Neurol. 1999;409(1):38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10363710. (Accessed: 15 February 2018) [DOI] [PubMed] [Google Scholar]
- Clatworthy P.L. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J. Neurosci. 2009;29(15):4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C., Klingberg T. The neuroscience of working memory capacity training. Nat. Revi. Neurosci. 2016;17(7):439–449. doi: 10.1038/nrn.2016.43. [DOI] [PubMed] [Google Scholar]
- Cools R., D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry. 2011;69(12):e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of high-level cognition in Parkinson’s Disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Crone E.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. U. S. A. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E. Transfer of learning after updating training mediated by the striatum. Science. 2008;320(5882):1510–1512. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Darki F., Klingberg T. The role of fronto-parietal and fronto-striatal networks in the development of working memory: a longitudinal study. Cereb. Cortex (New York, N.Y.: 1991) 2015;25(6):1587–1595. doi: 10.1093/cercor/bht352. [DOI] [PubMed] [Google Scholar]
- De Luca C.R. Normative data from the CANTAB. I: development of executive function over the lifespan. J. Clin. Exp. Neuropsychol. 2003;25(2):242–254. doi: 10.1076/jcen.25.2.242.13639. [DOI] [PubMed] [Google Scholar]
- Dennison M. Mapping subcortical brain maturation during adolescence: evidence of hemisphere- and sex-specific longitudinal changes. Dev. Sci. 2013;16(5):772–791. doi: 10.1111/desc.12057. [DOI] [PubMed] [Google Scholar]
- Dumontheil I. Influence of the COMT genotype on working memory and brain activity changes during development. Biol. Psychiatry. 2011;70(3):222–229. doi: 10.1016/j.biopsych.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Gathercole Cognitive approaches to the development of short-term memory. Trends Cogn. Sci. 1999;3(11):410–419. doi: 10.1016/s1364-6613(99)01388-1. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10529796. (Accessed: 15 February 2018) [DOI] [PubMed] [Google Scholar]
- Gjedde A. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc. Natl. Acad. Sci. U. S. A. 2010;107(8):3870–3875. doi: 10.1073/pnas.0912319107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven C.U. Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry. 2015;72(5):490–499. doi: 10.1001/jamapsychiatry.2014.3162. [DOI] [PubMed] [Google Scholar]
- Heinz A. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Huotari M. Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur. J. Neurosci. 2002;15(2):246–256. doi: 10.1046/j.0953-816x.2001.01856.x. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11849292. (Accessed: 21 February 2018) [DOI] [PubMed] [Google Scholar]
- Kühn S. The dynamics of change in striatal activity following updating training. Hum. Brain Mapp. 2013;34(7):1530–1541. doi: 10.1002/hbm.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal-intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44(11):2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Klingberg T.1. Training and plasticity of working memort. Trends Cogn. Sci. 2010;14(7):317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Childhood cognitive development as a skill. Trends Cogn. Sci. 2014;18(11):573–579. doi: 10.1016/j.tics.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Kudo M.F., Lussier C.M., Swanson H.L. Reading disabilities in children: a selective meta-analysis of the cognitive literature. Res. Dev. Disabil. 2015;40:51–62. doi: 10.1016/j.ridd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Kwon H., Reiss A.L., Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. U. S. A. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K.W. L-dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology (Berl) 1992;107(2-3):394–404. doi: 10.1007/BF02245167. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1615139. (Accessed: 15 February 2018) [DOI] [PubMed] [Google Scholar]
- Lawrence A.D. Executive and mnemonic functions in early Huntington’s disease. Brain. 1996;119(Pt 5):1633–1645. doi: 10.1093/brain/119.5.1633. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8931586. (Accessed: 21 February 2018) [DOI] [PubMed] [Google Scholar]
- Lee M., Wagenmakers E. 2013. Bayesian Modeling for Cognitive Science: a Practicla Course. (Cambridge. Cambridge, UK) [Google Scholar]
- Luna B. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Mammarella I.C. Math anxiety and developmental dyscalculia: a study on working memory processes. J. Clin. Exp. Neuropsychol. 2015;37(8):878–887. doi: 10.1080/13803395.2015.1066759. [DOI] [PubMed] [Google Scholar]
- Martinussen R. A meta-Analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- McNab F., Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 2008;11(1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- McNab F. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science (New York, N. Y.) 2009;323(5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C. Task complexity and location specific changes of cortical thickness in executive and salience networks after working memory training. Neuroimage. 2016;130:48–62. doi: 10.1016/j.neuroimage.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef M. Effects of dopamine /D3 receptor antagonism on human planning and spatial working memory. Trans. Psychiatry. 2017;107:D2. doi: 10.1038/tp.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolas M.A., Nigg J.T. Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology. 2013;27(1):107–120. doi: 10.1037/a0030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nymberg C. Neural mechanisms of attention-deficit/hyperactivity disorder symptoms are stratified by MAOA genotype. Biol. Psychiatry. 2013;74(8):607–614. doi: 10.1016/j.biopsych.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Nymberg C. DRD2/ANKK1 polymorphism modulates the effect of ventral striatal activation on working memory performance. Neuropsychopharmacology. 2014;39(10):2357–2365. doi: 10.1038/npp.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen P.J., Westerberg H., Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 2004;7(1):75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Olesen P.J. Brain activity related to working memory and distraction in children and adults. Cereb. Cortex. 2006;17(5):1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Ostby Y. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J. Neurosci. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49(14):3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Owen A.M. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28(10):1021–1034. doi: 10.1016/0028-3932(90)90137-d. Available at: http://www.ncbi.nlm.nih.gov/pubmed/2267054. (Accessed: 15 February 2018) [DOI] [PubMed] [Google Scholar]
- Owen A.M. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115(Pt 6):1727–1751. doi: 10.1093/brain/115.6.1727. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1486458. (Accessed: 15 February 2018) [DOI] [PubMed] [Google Scholar]
- Owen A.M. Spatial and non-spatial working memory at different stages of Parkinson’s disease. Neuropsychologia. 1997;35(4):519–532. doi: 10.1016/s0028-3932(96)00101-7. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9106280. (Accessed: 15 February 2018) [DOI] [PubMed] [Google Scholar]
- Patenaude B. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B. University of Oxford; 2007. Bayesian Statistical Models of Shape and Appearance for Subcortical Brain Segmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli W.M. Regional specialization within the human striatum for diverse psychological functions. Proc. Natl. Acad. Sci. U. S. A. 2016;113(7):1907–1912. doi: 10.1073/pnas.1507610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf D.R. Within-individual variability in neurocognitive performance: age- and sex-related differences in children and youths from ages 8–21. Neuropsychology. 2014;28(4):506–518. doi: 10.1037/neu0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia (Basel Switzerland) 1994;5(5):266–281. doi: 10.1159/000106735. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7951684. (Accessed: 21 February 2018) [DOI] [PubMed] [Google Scholar]
- Söderqvist S. Dopamine, working memory, and training induced plasticity: implications for developmental research. Dev. Psychol. 2012;48(3):836–843. doi: 10.1037/a0026179. [DOI] [PubMed] [Google Scholar]
- Söderqvist S. Polymorphisms in the dopamine receptor 2 gene region influence improvements during working memory training in children and adolescents. J. Cogn. Neurosci. 2014;26(1):54–62. doi: 10.1162/jocn_a_00478. [DOI] [PubMed] [Google Scholar]
- Sambataro F. A variable number of tandem repeats in the 3’-untranslated region of the dopamine transporter modulates striatal function during working memory updating across the adult age span. Eur. J. Neurosci. 2015;42(3):1912–1918. doi: 10.1111/ejn.12956. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P.S. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science (New York, N.Y.) 1991;251(4996):947–950. doi: 10.1126/science.1825731. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1825731. (Accessed: 15 February 2018) [DOI] [PubMed] [Google Scholar]
- Sawaguchi T., Goldman-Rakic P.S. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994;71(2):515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schumann G. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol. Psychiatry. 2010;15(12):1128–1139. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- Shang C.-Y., Gau S.S.-F. Association between the DAT1 gene and spatial working memory in attention deficit hyperactivity disorder. Int. J. Neuropsychopharmacol. 2014;17(1):9–21. doi: 10.1017/S1461145713000783. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollstorff M. Neural response to working memory load varies by dopamine transporter genotype in children. Neuroimage. 2010;53(3):970–977. doi: 10.1016/j.neuroimage.2009.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48(9):2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K. Longitudinal working memory development is related to structural maturation of frontal and parietal cortices. J. Cogn. Neurosci. 2013;25(10):1611–1623. doi: 10.1162/jocn_a_00434. [DOI] [PubMed] [Google Scholar]
- Tziortzi A.C. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Ullman H., Almeida R., Klingberg T. Structural maturation and brain activity predict future working memory capacity during childhood development. J. Neurosci. 2014;34(5):1592–1598. doi: 10.1523/JNEUROSCI.0842-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayraghavan S. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D. Variations in the catechol O-methyltransferase polymorphism and prefrontally guided behaviors in adolescents. Biol. Psychiatry. 2007;61(5):626–632. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Yu Y., FitzGerald T.H.B., Friston K.J. Working memory and anticipatory set modulate midbrain and putamen activity. J. Neurosci. 2013;33(35):14040–14047. doi: 10.1523/JNEUROSCI.1176-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Schaaf M.E. Establishing the dopamine dependency of human striatal signals during reward and punishment reversal learning. Cereb. Cortex. 2014;24(3):633–642. doi: 10.1093/cercor/bhs344. [DOI] [PubMed] [Google Scholar]
- von Aster M.G., Shalev R.S. Number development and developmental dyscalculia. Dev. Med. Child Neurol. 2007;49(11):868–873. doi: 10.1111/j.1469-8749.2007.00868.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.