Abstract
Arithmetic facts can be solved using different strategies. Research suggests that some arithmetic problems, particularly those solved by fact retrieval, are related to phonological processing ability and elicit activity in left-lateralized brain regions that support phonological processing. However, it is unclear whether common brain regions support both retrieval-based arithmetic and phonological processing, and if these regions differ across children and adults. This study used activation likelihood estimation to investigate functional neural overlap between arithmetic and phonological processing, separately for children and adults. The meta-analyses in children showed six clusters of overlapping activation concentrated in bilateral frontal regions and in the left fusiform gyrus. The meta-analyses in adults yielded two clusters of concordant activity, one in the left inferior frontal gyrus and one in the left inferior parietal lobule. A qualitative comparison across the two age groups suggests that children show more bilateral and diffuse activation than adults, which may reflect attentional processes that support more effortful processing in children. The present meta-analyses contribute novel insights into the relationship between retrieval-based arithmetic and phonological processing in the brain across children and adults, and brain regions that may support processing of more complex symbolic representations, such as arithmetic facts and words.
Keywords: Arithmetic, Phonological processing, Meta-analysis, Children, Adults
1. Introduction
Arithmetic facts can be solved using different strategies, such as by calculation or retrieving an answer from memory. Small addition and multiplication problems are thought to be solved using direct memory retrieval, whereas subtraction problems are thought to be solved by calculation (Barrouillet et al., 2008, Campbell and Xue, 2001, Dehaene et al., 2003, Siegler, 1988). Retrieval-based arithmetic facts are learned using verbal strategies, are assumed to be stored as verbal codes (Dehaene et al., 2003), and are related to cognitive and neural processes that involve language, including phonological processing. The present study concerns concordant brain activity that supports retrieval-based arithmetic and phonological processing in children and adults.
Broadly, phonological processing encompasses three different sub-processes: phonological awareness, phonological memory, and rapid naming (e.g., De Smedt and Boets, 2010, Hecht et al., 2001, Torgesen et al., 1994). However, studies investigating the neural correlates of phonological processing (e.g., Bitan et al., 2007, Katzir et al., 2005, Tan et al., 2005) or the overlap between arithmetic and phonological processing in the brain (e.g., Andin et al., 2015, Passolunghi et al., 2007, Prado et al., 2011) typically employ tasks such as rhyme judgments, syllable decisions, and phonemic segmentation. These tasks, which necessitate the active analysis or manipulation of speech sounds within words rather than just the recall or retrieval of sounds (unlike sole phonological memory or rapid naming), align most closely with the sub-process of phonological awareness. The present analysis operationalizes phonological processing in accordance with this prior literature. Pseudoword reading tasks are also often used to investigate phonological processing because they involve the transformation of visual word forms to phonology independently of lexical meaning, utilizing a sublexical pathway of accessing phonology rather than the recognition of known words (e.g., Dietz et al., 2005, Georgiewa et al., 1999). Therefore, the present analysis also includes studies that use pseudoword reading tasks.
Evidence suggests that arithmetic and phonological processing are related across development. Phonological awareness is associated with arithmetic ability for children just entering school (Simmons et al., 2008), and with upper elementary school children’s performance on small3 arithmetic problems and those likely solved using retrieval (De Smedt et al., 2010). In adults, phonological processing is positively correlated with multiplication fact retrieval (De Smedt and Boets, 2010) and can interfere with multiplication ability (Lee and Kang, 2002). Further, phonological processing impairments (e.g., dyslexia) are related to arithmetic fact retrieval difficulty in children (Simmons and Singleton, 2008) and adults (De Smedt and Boets, 2010). Taken together, behavioral research suggests that phonological processing is related to performance on arithmetic problems that are likely retrieved from memory (e.g., small, addition and multiplication problems).
Evidence suggests a relationship between arithmetic and phonological processing at the neural level as well. Arithmetic problem solving associated with fact retrieval, such as small addition and multiplication problems, engages brain regions associated with language processing (i.e., angular gyrus [AG], superior temporal gyrus [STG], or middle temporal gyrus [MTG]) (Arsalidou and Taylor, 2011, Dehaene et al., 2003, Evans et al., 2014, Prado et al., 2014). Over development, left-lateralized brain regions, including those that relate to language, become increasingly recruited to support arithmetic processing (Ansari, 2008, Zamarian et al., 2009). For example, in a study of second to seventh graders, Prado et al. (2014) showed that children in higher grades had greater activation in left MTG for single-digit multiplication processing compared to children in lower grades. The authors found that the grade-related change in activity was greater for smaller versus larger multiplication problems (e.g., 3 × 4 = 12 versus 6 × 7 = 42). The relationship between arithmetic and phonological processing also extends to atypical development. Children with dyslexia show atypical brain activation in left temporoparietal areas during addition compared to their typically developing peers (Evans et al., 2014).
Further, neuroimaging in adults has consistently shown that arithmetic processing recruits left-lateralized brain regions involved in phonological processing. Several arithmetic studies have implicated the left AG (for a review see Zamarian et al., 2009), which is also involved in phonological processing and word meaning (Booth et al., 2004, Price, 2000). This region has shown greater activity for exact addition compared to approximate addition (Dehaene et al., 1999) and for more difficult compared to less difficult multiplication problems (Grabner et al., 2013). The left AG is thought to support efficient retrieval of overlearned arithmetic problems in adults (Delazer et al., 2003, Delazer et al., 2005, Grabner et al., 2007, Grabner et al., 2009a, Grabner et al., 2009b, Ischebeck et al., 2006, Stanescu-Cosson et al., 2000, Tschentscher and Hauk, 2014; but also see Rosenberg-Lee et al., 2011). Arithmetic processing for retrieval-based facts in adults may recruit additional left-lateralized frontal and temporal brain structures that are involved in verbal processing, including the inferior frontal gyrus (IFG), STG, and MTG (Prado et al., 2011, Zhou et al., 2007). Across children and adults, research suggests that retrieval-based arithmetic problems likely recruit left-lateralized brain areas, and that activation in these areas is driven by increased fluency with arithmetic facts that occurs with learning.
However, even if arithmetic and phonological processing both recruit left-lateralized brain areas, specific areas that support these two processes may be regionally differentiated. The few studies that have examined direct neural overlap between arithmetic and phonological processing have been done in adults and have yielded conflicting results. Simon et al. (2002) investigated neural overlap in adults for calculation and phonological processing tasks and found a region in left intraparietal sulcus (IPS) mesial to the AG that was active for both tasks. However, Andin et al. (2015) found that multiplication tasks recruited posterior AG (i.e., PGp) while phonological processing recruited anterior left AG (i.e., PGa).
Taken together, the behavioral and neuroimaging research show a relationship between the cognitive and neural mechanisms that support retrieval-based arithmetic and phonological processing. Yet, few neuroimaging studies have examined this in children, and as illustrated above, more research is needed to inform whether common brain regions support both arithmetic and phonological processing in adults. Examining this relationship in children can provide first insight into brain regions that support both processes, shedding light on the extant behavioral relationship. Additionally, examining brain regions that support both arithmetic and phonological processing in adults can speak to the conflicting findings in the literature.
2. The present study
In the present study, we examine the convergence of brain regions that show reliable activity across arithmetic and phonological processing, separately in children and adults, using neuroimaging meta-analysis. We first conduct individual meta-analyses that identify concordant areas of activation across a set of empirical studies for each domain and age group. Second, we conduct conjunction analyses that identify areas of concordant activity across arithmetic and phonological processing, separately for each age group. We then qualitatively compare clusters common to arithmetic and phonological processing across the developmental and adult samples. For the developmental sample, we expect clusters of reliable activation in prefrontal regions, reflecting domain-general attention-related processes necessary for solving arithmetic problems or completing phonological tasks. Based on the behavioral relationship between arithmetic and phonological processing across development, we speculate there may be clusters of reliable activation in temporoparietal cortex (i.e., AG, STG, MTG) that reflect engagement of verbal representations. Alternatively, there may not be clusters in this area, in line with a developmental frontal-temporoparietal shift in brain regions that support arithmetic (e.g., Prado et al., 2014). For adults, we expect clusters of reliable activity in prefrontal areas, as above, and in left temporoparietal areas (i.e., AG, STG, MTG), reflecting fluent arithmetic fact retrieval and phonological processing. Alternatively, there may be temporoparietal clusters for arithmetic and phonological processing, but no shared clusters due to regional differentiation (e.g., Andin et al., 2015). Due to the lack of research on concordant brain activity for arithmetic and phonological processing in children, the qualitative comparison of the conjunction analyses is exploratory.
3. Methods
3.1. Literature search and article selection
There were four literature searches, one for each age group (i.e., developmental, adult) and domain (i.e., arithmetic, phonological processing). Each search followed the same two-step process: (1) a search of the PUBMED database and (2) a review of the reference sections of relevant papers for the specified meta-analyses. For brevity, we discuss searches and inclusion-exclusion criteria by domain, rather than age group.
3.1.1. Arithmetic processing
For the developmental sample, we conducted an initial search using the terms “fMRI and arithmetic and (child* or adolescen* or student),” which yielded 113 papers. For the adult sample, the initial search with the search terms “fMRI and arithmetic” yielded 306 papers. In both cases, we included studies published in English that used fMRI and visually-presented stimuli, studies that involved typically-developing participants, conducted whole brain analyses, and reported within-group contrasts between arithmetic processing and baseline conditions in standard Talairach or Montreal Neurological Institute (MNI) space. We included arithmetic processing tasks thought to draw on verbal strategies: single-digit addition and multiplication, small versus large problems (e.g., De Smedt et al., 2011), or retrieval (e.g., De Visscher et al., 2015), as well as studies with mixed arithmetic that included addition or multiplication (e.g., Andres et al., 2012, Price et al., 2013). Studies with participants under 18 were in the developmental meta-analysis and studies with participants over 18 were in the adult meta-analysis. We excluded reviews, clinical trials, case studies, and other meta-analyses. However, we checked the latter (Arsalidou and Taylor, 2011, Kaufmann et al., 2011) for additional potential studies. We also excluded studies with non-symbolic, non-arithmetic, or calculation-focused experimental tasks (e.g., subtraction or two-digit multiplication), and studies with aggregated analyses across children and adults. Table 1, rows 2 and 5, provides the final number of studies, experiments, foci, and participants for each analysis. One arithmetic study (Chen et al., 2006) contributed contrasts for two groups, yielding 17 experiments across the 16 studies in the developmental sample.
Table 1.
Number of studies, experiments, foci, and participants for each meta-analysis.
| Studies | Experiments | Foci | Participants | |
|---|---|---|---|---|
| Developmental | ||||
| Arithmetic | 16 | 17 | 168 | 530 |
| Phonological processing | 16 | 17 | 188 | 356 |
| Adult | ||||
| Arithmetic | 22 | 22 | 285 | 401 |
| Phonological processing | 23 | 23 | 237 | 363 |
3.1.2. Phonological processing
Search terms were selected to capture the operationalization of phonological processing aligned with the literature discussed above. The search phrase for the developmental meta-analysis “fMRI and phono* and (processing or awareness) and (child* or adolescen* or student),” yielded 274 papers. Search terms for the adult meta-analysis were “fMRI and phono* and (processing or awareness)” and yielded 761 papers. For both searches, we included studies published in English that used fMRI and visually-presented tasks, that involved typically-developing participants, conducted whole brain analyses, and reported within-group contrasts involving a phonological processing task and a baseline condition. Studies with participants under 18 were in the developmental meta-analysis and studies with participants 18 and over were in the adult meta-analysis. We excluded reviews, clinical trials, case studies, and other meta-analyses. We also excluded studies that did not meet the criteria for phonological processing (e.g., semantic judgments, word reading, rapid naming, verbal short-term memory) and studies involving non-alphabetic language tasks. However, we included two papers (Bach et al., 2013, Yamada et al., 2011) in the developmental meta-analysis that employed reading and decoding tasks with Kindergarteners, since beginning readers would need to utilize phonological processing during these tasks. We also excluded two papers from the phonological processing meta-analyses (Bitan et al., 2009, Kareken et al., 2000) that duplicated samples and contrasts from other included papers (Bitan et al., 2007, Lurito et al., 2000; respectively). In addition, we included five studies from our prior reading meta-analyses on reading in typical and atypical readers (Ashby and Pollack, 2016, Pollack et al., 2015, Pollack and Ashby, 2016) that met the inclusion criteria. Rows 3 and 6 in Table 1 provide the number of studies, experiments, foci, and participants. One phonological processing study (Hoeft et al., 2006), reported contrasts for two control groups, yielding 17 experiments across the 16 studies in the developmental sample.
3.1.3. Study overviews by age group
Table 2 presents an overview of the developmental arithmetic (Panel A) and developmental phonological processing (Panel B) studies, including sample size, mean age of participants, contrasts, and statistical thresholds. In line with recent meta-analyses (Sokolowski et al., 2017), we included all applicable contrasts per experiment (see Turkeltaub et al., 2012). For arithmetic, 13 studies (A1-A9, A11, A15, A16) involved single-digit addition. Participants chose between incorrect and correct answers to addition problems, verified addition facts, or added single-digit numbers sequentially. For control tasks, participants matched Greek letters or grayscale patterns, solved simple addition problems of the form x + 1 = y, performed subtraction, or added quantities with non-retrieval approaches (e.g., using counting). Three of the studies (A12-A15) involved single-digit mixed addition and subtraction problems contrasted with performing a digit detection task, a digit matching task, or solving larger addition and subtraction problems. Two studies (A10 and A11) involved verifying multiplication facts, with fixation as the baseline. Almost all studies required a button press; two studies (A11 and A16) required mental calculation only. One study (A16) contributed two experiments because they reported contrasts for two separate participant groups. Note that two studies (A9, A4) report two age groups, but analyses were collapsed across groups.
Table 2.
Details of the studies in the developmental meta-analyses, including sample size, mean age, contrast, and statistical threshold.
| Study | Reference | N | Mean age | Contrast | Statistical threshold |
|---|---|---|---|---|---|
| Arithmetic | |||||
| A1 | Davis et al. (2009a) | 24 | 8.1 years | Addition > Greek letter matching | p < 0.001 uncorrected |
| A2 | Davis et al. (2009b) | 19 | 8.1 years | Addition > Greek letter matching | p < 0.001 uncorrected |
| A3 | Meintjes et al. (2010) | 16 | 10.5 years | Addition > Greek letter matching | p < 0.05 FDR |
| A4 | Kucian et al. (2006) | 10 (3rd grade) 10 (6th grade) |
9.2 years 12.0 years |
Exact addition > Approximate addition Addition > Grayscale matching |
p < .005 FDR |
| A5 | Cho et al. (2011) | 103 | 7–9.9 years | Addition with retrieval > Addition with counting | p < .01 FWE |
| A6 | Rosenberg-Lee et al. (2015) | 20 | 8.44 years | Addition > Subtraction | p < .01 cluster-wise |
| A7 | Ashkenazi et al. (2012) | 17 | 97.41 months | Complex addition > Simple addition | p < .01 FWE |
| A8 | Metcalfe et al. (2013) | 74 | 7.8 years | Complex addition > Simple addition |
p < 0.01 uncorrected; p < 0.05 FWE |
| A9 | Rosenberg-Lee et al. (2015) | 45 (2nd grade) 45 (3rd grade) |
7.67 years 8.67 years |
Complex addition > Simple addition | p < 0.01 FWE |
| A10 | Demir et al. (2014) | 40 | 10.9 years | Multiplication > Fixation | p < .05 cluster-wise |
| A11 | Kawashima et al. (2004) | 8 | 11.6 years | Multiplication > Fixation Addition > Fixation |
p < .05 corrected |
| A12 | Kesler et al. (2006) | 15 | 14.6 years | Mixed addition and subtraction > Digit strings | corrected (unspecified) |
| A13 | Rivera et al. (2002) | 16 | 16.97 years | Mixed addition and subtraction > Digit strings | p < .01 cluster-wise |
| A14 | Price et al. (2013) | 33 | 17 years, 11.5 months | Mixed addition and subtraction > Digit matching | p < .05 FDR |
| A15 | De Smedt et al. (2011) | 18 | 11.77 years | Small addition/subtraction > Large addition/subtraction Addition > Subtraction |
p < .001voxel-wise; p < .05 cluster-wise |
| A16 | Chen et al. (2006) | 8 (abacus experts) 8 (non-experts) |
11.75 years 12.29 years |
Serial addition > Viewing numbers | p < .0001 uncorrected |
| Phonological processing | |||||
| P1 | Booth et al. (2004) | 16 | 10.7 years | Rhyme judgment (words) > Visual matching | p < .01 corrected |
| P2 | Booth et al. (2001) | 5 | 11.1 years | Rhyme judgment (words) > Visual matching | p < .001 uncorrected |
| P3 | Temple et al. (2001) | 15 | 10.5 years | Rhyme judgment (letters) > Letter matching | p < .025 corrected |
| P4 | Bitan et al. (2007) | 36 | 11.7 years | Rhyme judgment (words) > Fixation |
p < .0001 uncorrected; p < .05 corrected |
| P5 | Cao et al. (2008) | 12 | 12.3 years | Rhyme judgment (words) > Fixation | p < .001 uncorrected |
| P6 | Hoeft et al. (2007) | 64 | 10 years | Rhyme judgment (words) > Fixation | p < .01 FDR |
| P7 | Hoeft et al. (2006) | 10 (5th grade) 10 (3rd grade) |
10.95 years 8.75 years |
Rhyme judgment (words) > Fixation | p < .001 uncorrected |
| P8 | Cao et al. (2006) | 14 | 11.5 years | Rhyme judgment (words) > Fixation | p < .001 uncorrected |
| P9 | McNorgan et al. (2011) | 14 (young group) 12 (older group) |
9.3 years 13.5 years |
Rhyme judgment (words) > Fixation | p < .05 FDR |
| P10 | Backes et al. (2002) | 8 | 11.6 years | Rhyme judgment (pseudowords) > Fixation | p < .05 cluster-wise |
| P11 | Georgiewa et al. (1999) | 17 | 14.4 years | Pseudoword reading > Font strings | p < .05 |
| P12 | van der Mark et al. (2009) | 24 | 11.3 years | Pseudoword reading > Fixation | p < .05 FDR |
| P13 | Noble et al. (2006) | 38 | 7 years, 11 months | Pseudoword one-back task > Fixation | p < .0001 uncorrected |
| P14 | Yamada et al. (2011) | 7 | 5.7 years | Letter one-back task > False fonts one-back task | p < .05 uncorrected |
| P15 | Bach et al. (2010) | 18 | 8.3 years | Different letter substitution > Same letter substitution Letter substitution > null |
p < .005 cluster extent threshold |
| P16 | Bach et al. (2013) | 19 | 6.4 years | Word decoding > Symbol identification Word decoding > Null |
p < .005 cluster extent threshold |
For developmental phonological processing, 10 studies (P1-P10) used a rhyming task with pairs of words, pseudowords or letters, with symbol matching, letter matching, or fixation as a baseline. Three studies (P11-P13) utilized a pseudoword reading task, with viewing false font strings or fixation as a baseline. In one study (P14), participants decided whether a letter was the same as a previously presented letter, and performed a similar baseline task using false fonts. In one study (P15) participants read words or pseudowords, mentally substituted a different letter, and decided whether the new word was a real word, making same-letter substitutions during the control condition. In another study (P16), Kindergarten-age participants decoded words; as a control task, they identified asterisks embedded in symbol strings. The participants produced responses by button press in all of the studies. One study (P7) reported contrasts separately for two control groups.
Table 3 presents an overview of the adult arithmetic (Panel A) and adult phonological processing (Panel B) studies including sample demographics, contrasts, and statistical thresholds. For arithmetic, nine studies (A1-A9) used single-digit addition experimental tasks. Baseline tasks were either approximate addition, non-symbolic addition, addition with digit or letter matching, holding digits in mind, number viewing, subtraction, or fixation. One study (A10) used mixed arithmetic operations presented serially, with fixation as a baseline. Two studies (A21-A22) involved mixed addition and subtraction items, with digit identification as a baseline. Ten studies (A11-A20) utilized multiplication tasks with baseline tasks that included digit matching, digit or letter identification, digit ordering, holding digits in mind, subtraction, division, or non-retrieval based multiplication. Most studies required a button press; three studies (A10, A19, A20) involved verbal report and two studies (A6, A13) involved mental calculation only.
Table 3.
Details of the studies in the adult meta-analyses, including sample size, mean age, contrast, and statistical threshold.
| Study | Reference | N | Mean age | Contrast | Statistical threshold |
|---|---|---|---|---|---|
| Arithmetic | |||||
| A1 | Venkatraman et al. (2005) | 10 | 20–25 years | Addition > Digit matching |
p < .001 uncorrected; p < .05 corrected |
| A2 | Stanescu-Cosson et al. (2000) | 7 | 22–26 years | Exact addition > Approximate addition Addition > Letter matching |
p < .001 uncorrected; p < .05 cluster-wise |
| A3 | van der Ven et al. (2016) | 23 | 21.04 years | Exact addition > Non-symbolic addition |
p < .001 uncorrected; p < .05 FWE |
| A4 | Gullick and Wolford (2014) | 24 | 19 years, 10 months | Addition > Subtraction |
p < .001 uncorrected; p < .05 FDR |
| A5 | Hugdahl et al. (2004) | 12 | 31.0 years | Addition > Digit identification | p < .05 corrected |
| A6 | Kawashima et al. (2004) | 8 | 44.1 years | Addition > Fixation Multiplication > Fixation |
p < .05 corrected |
| A7 | Zhou et al. (2007) | 20 | 22.7 years | Addition > Fixation Multiplication > Fixation |
p < .001 uncorrected |
| A8 | Kuo et al. (2008) | 12 | 21–29 years | Serial addition > Digit maintenance | p < .001 (unspecified) |
| A9 | Sammer et al. (2007) | 20 | 25.4 years | Serial addition > Viewing numbers | p < .05 FWE |
| A10 | De Pisapia et al. (2006) | 20 | 20.3 years | Serial arithmetic > Null | p < .05 uncorrected |
| A11 | Ischebeck et al. (2006) | 12 | 26.8 years | Multiplication > Digit matching |
p < .0001 uncorrected; p < .05 corrected |
| A12 | Delazer et al. (2003) | 13 | 30.5 years | Multiplication > Digit matching | p < .0001 uncorrected |
| A13 | Chochon et al. (1999) | 8 | 20–30 years | Multiplication > Digit ordering Multiplication > Digit identification |
p < .001 uncorrected; p < .05 corrected |
| A14 | Andin et al. (2015) | 17 | 28.6 years | Multiplication > Digit ordering Multiplication > Letter identification |
p < .001 uncorrected; p < .05 FWE |
| A15 | Jost et al. (2009) | 16 | 24.5 years | Multiplication > Digit maintenance Multiplication (retrieval) > Multiplication (non-retrieval) |
p < .001 uncorrected |
| A16 | De Visscher et al. (2015) | 20 | 29 years | Multiplication (retrieval) > Multiplication (non-retrieval) | p < .001 uncorrected |
| A17 | Rosenberg-Lee et al. (2011) | 20 | 23.9 years | Multiplication > Subtraction Multiplication > Division Multiplication > Digit identification |
p < .01 corrected |
| A18 | Zarnhofer et al. (2012) | 42 | 23 years | Multiplication > Subtraction Multiplication (digits) > Multiplication (number words) |
p < .001 uncorrected; p < .05 FWE |
| A19 | Andres et al. (2012) | 18 | 21.3 years | Multiplication > Subtraction Mixed multiplication and subtraction > Letter identification |
p < .05 FDR |
| A20 | Andres et al. (2011) | 10 | 21.0 years | Multiplication > Subtraction Mixed multiplication and subtraction > Letter identification |
p < .001 uncorrected; p < .05 corrected |
| A21 | Keller and Menon (2009) | 49 | 23.99 years | Mixed addition and subtraction > Digit identification |
p < .01 uncorrected p < .001 corrected; |
| A22 | Menon et al. (2000) | 16 | 20.28 years | Mixed addition and subtraction (3 operands) > Digit identification Mixed addition and subtraction (2 operands) > Digit identification |
p < .01 uncorrected |
| Phonological processing | |||||
| P1 | Geva et al. (2012) | 12 19 |
24.6 years 64.1 years |
Rhyme judgment (words) > Visual matching | p < .05 FWE |
| P2 | Hernandez et al. (2013) | 16 | 21.2 years | Rhyme judgment (words) > Visual matching | p < .05 clusterwise |
| P3 | MacSweeney et al. (2009) | 7 | 32 years, 7 months | Rhyme judgment (words) > Visual matching |
p < .05 voxelwise; p < .005 clusterwise |
| P4 | Pecini et al. (2008) | 10 | 27.1 years | Rhyme judgment (words) > Visual matching | p < .05 corrected |
| P5 | Booth et al. (2004) | 16 | 25.2 years | Rhyme judgment (words) > Visual matching | p < .01 corrected |
| P6 | Booth et al. (2003) | 15 | 25.8 years | Rhyme judgment (words) > Visual matching | p < .001 uncorrected |
| P7 | Booth et al. (2001) | 4 | 25.5 years | Rhyme judgment (words) > Visual matching | p < .001 uncorrected |
| P8 | Poldrack et al. (2001) | 8 | 20–29 years | Rhyme judgment (words) > Visual matching | p < .001 uncorrected |
| P9 | Lurito et al. (2000) | 5 | 27 years | Rhyme judgment (words) > Visual matching | t > 6, uncorrected |
| P10 | Cousin et al. (2006) | 11 | 27.5 years | Rhyme judgment (words) > Visual detection | p < .001 uncorrected |
| P11 | Oron et al. (2016) | 37 | 46.3 years | Rhyme judgment (words) > Visual detection | p < .05 FWE |
| P12 | Booth et al. (2002) | 13 | 24.6 years | Rhyme judgment (words) > Word spelling matching | p < .001 uncorrected |
| P13 | McDermott et al. (2003) | 20 | 22.1 years | Rhyme processing (words, silent) > Semantic processing | p < .0012 voxelwise |
| P14 | Taylor et al. (2014) | 22 | 18–20 years | Pseudoword reading > Word reading |
p < .001 uncorrected; p < .05 FWE |
| P15 | Mechelli et al. (2005) | 22 | 36 years | Pseudoword reading (silent) > Word reading | p < .05 corrected |
| P16 | Dietz et al. (2005) | 16 | 31.1 years | Pseudoword reading (silent) > Word reading | p < .001 uncorrected |
| P17 | Joubert et al. (2004) | 10 | 26 years | Pseudoword reading (silent) > Viewing letter strings | p < .0005 voxelwise |
| P18 | Danelli et al. (2013) | 28 | 21 years | Pseudoword reading (silent) > Viewing line patterns | p < .05 FWE |
| P19 | Clark and Wagner (2003) | 20 | 18–33 years | Syllable counting (pseudowords) > Syllable counting (words) | p < .001 uncorrected |
| P20 | Rudner et al. (2013) | 20 | 26.4 years | Final syllable two-back task > Color two-back task |
p < .001 uncorrected; p < .05 corrected |
| P21 | Katzir et al. (2005) | 12 | 18–25 years | Initial sound matching > Object matching | p < .001 uncorrected |
| P22 | Tham et al. (2005) | 6 | 18–23 years | Homophone judgment > Fixation | p < .05 uncorrected |
| P23 | Burton et al. (2005) | 14 | 26.7 years | Rhyme judgment (words/pseudowords) > Visual matching |
p < .01 uncorrected; p < .05 corrected |
For phonological processing, 14 studies (P1-P13, P23) utilized word or pseudoword rhyming. Baseline tasks for these studies included matching or detecting symbols, images, or letter case; matching word spelling; or thinking about word meaning commonalities. Five studies (P14-P18) used pseudoword reading contrasted with reading words, or viewing letter strings or line patterns. One study (P19) contrasted pseudoword and real word syllable counting, and one study (P20) contrasted final syllable matching with color matching. Another study (P21) contrasted matching initial sounds in words with matching objects. One study (P22) contrasted homophone judgments with words with fixation. Two studies (P4, P14) required a verbal response, five (P13, P15-P18) required silent reading, and the remainder required a button press.
3.2. Data analysis
3.2.1. Single-study meta-analyses
All analyses were done using GingerALE version 2.3.6 (Eickhoff et al., 2016, Eickhoff et al., 2012, Eickhoff et al., 2009, Turkeltaub et al., 2012). In light of recent recommendations put forth by Eickhoff et al. (2016), the current meta-analyses followed recommended guidelines for robust and reliable outcomes, which may reveal different patterns of results relative to analyses generated by previous GingerALE algorithms. Prior to analysis, all coordinates were converted into a common space; MNI coordinates were transformed into Talairach space (Talairach and Tournoux, 1988) using the icbm2tal transform native to GingerALE (Laird et al., 2010, Lancaster et al., 2007).
To conduct the analyses, ALE models the foci from each experiment as a three-dimensional Gaussian probability distribution (Eickhoff et al., 2009). It then generates three-dimensional activation maps by taking the maximum of each focus’s Gaussian, a non-additive method that limits within-experiment effects (Turkeltaub et al., 2012). ALE then generates a null distribution for the ALE statistic and probabilities associated with the values of the activation maps (Research Imaging Institute UTHSCSA [RII], 2013). The probabilities can then be compared to the null distribution according to a chosen threshold. In this method, GingerALE simulates random data sets for a chosen number of permutations in which each data set retains the same properties as the original data, such as number of foci and subject sample sizes (RII, 2013). The simulated data is first thresholded with a cluster-forming threshold. Based on the distribution of the cluster sizes, the data are then subject to cluster-level thresholding, which sets a minimum volume cluster size (RII, 2013). The current analysis used 1000 permutations for the simulated data. Due to the two levels of thresholding, we employed the recommended cluster-forming threshold of uncorrected p < .001 with a cluster-level threshold of 0.05 (RII, 2013). GingerALE results were reported in Talairach space, displayed using the anatomical templates native to GingerALE, and were automatically labeled using the Talairach Daemon (talairach.org). We confirmed the labeling from GingerALE using the Talairach Daemon in Mango (RII, 2015) and found no differences.
3.2.2. Conjunction and subtraction analyses
Conjunction analyses (Eickhoff et al., 2011) determined areas of overlap between arithmetic and phonological processing, separately in the developmental and adult samples. ALE uses the meta-analytic results for arithmetic and phonological processing, and a third set of results from the pooled foci from the arithmetic and phonological processing studies acting as an empirical baseline or “null” distribution. The present analysis meets the criterion for adequate power, which is 17–20 experiments for each single-study meta-analysis (Eickhoff et al., 2016, Eickhoff and Etkin, 2016). To determine areas of overlap across the two meta-analyses, GingerALE creates a new ALE map that takes the voxel-wise minimum value from the two original thresholded maps (RII, 2013).
As part of the conjunction analysis, ALE provides subtraction analyses that directly contrast the single-file maps. To calculate significance, GingerALE creates ALE images from randomized simulated data, subtracts the images, and compares them to the real data. This process is iterated to produce p-value images and image statistics that are reported in z-score values (RII, 2013). The current analysis used 5000 permutations with thresholding at p < .01 uncorrected. Because our focus is on regions of overlap in children and adults, we include the subtraction results as supplemental material.
4. Results
4.1. Developmental sample
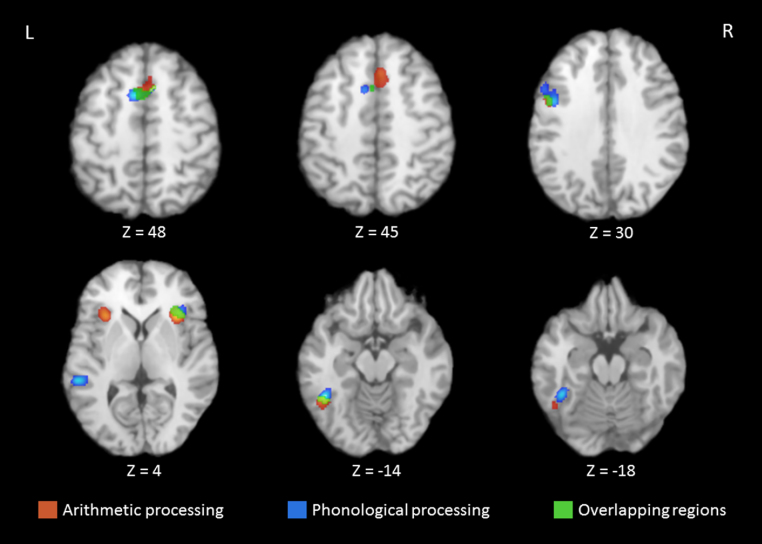
Table 4 displays the results of the three developmental meta-analyses including the cluster location, Talairach coordinates, ALE values, cluster size in mm3, and contributing studies. Panel A displays the single-study meta-analysis for arithmetic processing. Five clusters show reliable activation when participants engage in arithmetic tasks, four of them in frontal regions. The largest cluster is in the left superior frontal gyrus (SFG) (BA 6) with a local extremum in the right cingulate gyrus (BA 32). The second cluster is in the right insula (BA 13). Neighboring gray matter (i.e., within ± 5 mm) to this cluster includes the right claustrum (704 mm3) and the right IFG (56 mm3, BA 45; 48 mm3, BA 13), which suggests this cluster is in anterior right insula. The third cluster is in the left insula (BA 13) with a local extremum in the left IFG (BA 46). Neighboring gray matter outside of the left insula includes the left claustrum (520 mm3) and the left IFG (216 mm3, BA 46; 192 mm3, BA 45), which suggests this cluster is in anterior left insula. The fourth cluster is in the left precentral gyrus (BA 6) and the fifth is in left fusiform gyrus (FFG) (BA 37). Fig. 1 displays the clusters from the arithmetic meta-analysis in red.
Table 4.
Activation likelihood estimation results for arithmetic and phonological processing, and the conjunction analysis in the developmental sample, including cluster, Talairach coordinate, ALE value, volume, and contributing studies. Local extrema are italicized.
| Cluster | Talairach coordinates |
ALE value | Volume (mm3) | Contributing studies | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (A) Arithmetic processing | ||||||
| Left Superior Frontal Gyrus (BA 6) | −2 | 8 | 52 | 0.020871 | 2400 | A1, A2, A3, A7, A9, A11, A14, A13, A16 |
| Right Cingulate Gyrus (BA 32) | 4 | 20 | 42 | 0.020135 | ||
| Right Insula (BA 13) | 32 | 18 | 6 | 0.036845 | 2208 | A1, A2, A3, A4, A8, A9, A13, A14 |
| Left Insula (BA 13) | −30 | 18 | 6 | 0.030595 | 1800 | A4, A5, A7, A8, A9, A14 |
| Left Inferior Frontal Gyrus (BA 46) | −34 | 32 | 10 | 0.016215 | ||
| Left Precentral Gyrus (BA 6) | −46 | −2 | 36 | 0.017363 | 800 | A4, A10, A11, A13, A14, |
| Left Fusiform Gyrus (BA 37) | −46 | −56 | −14 | 0.018369 | 664 | A4, A11, A12, A13 |
| (B) Phonological processing | ||||||
| Left Inferior Frontal Gyrus (BA 6) | −44 | 2 | 32 | 0.022825934 | 2160 | P2, P5, P6, P7, P8, P9, P12, P13 |
| Left Inferior Frontal Gyrus (BA 9) | −52 | 12 | 32 | 0.01523793 | ||
| Left Superior Frontal Gyrus (BA 6) | −6 | 8 | 50 | 0.023172587 | 1904 | P4, P5, P6, P7, P8, P12, P16 |
| Left Middle Temporal Gyrus (BA 22) | −52 | −38 | 2 | 0.023622176 | 1640 | P1, P2, P4, P8, P9, P10, P14, P16 |
| Left Fusiform Gyrus (BA 37) | −42 | −50 | −14 | 0.023692332 | 1624 | P1, P5, P7, P8, P9, P12, P13 |
| Right Insula (BA 13) | 34 | 22 | 4 | 0.018411051 | 1056 | P4, P7, P8, P9, P14 |
| Right Inferior Frontal Gyrus (BA 47) | 34 | 22 | −8 | 0.014701883 | ||
| Left Inferior Frontal Gyrus (BA 46) | −44 | 26 | 14 | 0.022119224 | 960 | P1, P4, P5, P8 |
| (C) Conjunction of Arithmetic and Phonological Processing | ||||||
| Left Superior Frontal Gyrus (BA 6) | −2 | 8 | 52 | 0.020871054 | 720 | A9, A14, A16, P7, P12 |
| Left Precentral Gyrus (BA 6) | −46 | 0 | 36 | 0.017064271 | 568 | A4, A10, A11, A14, P8, P12, P13 |
| Right Insula (BA 13) | 34 | 22 | 4 | 0.018411051 | 488 | A13, A14, P4, P7, P8, P9 |
| Left Fusiform Gyrus (BA 37) | −46 | −54 | −14 | 0.015494634 | 184 | A12 |
| Right Superior Frontal Gyrus (BA 6) | 6 | 12 | 48 | 0.011203893 | 8 | None |
| Right Superior Frontal Gyrus (BA 6) | 8 | 14 | 48 | 0.01048557 | 8 | None |
Fig. 1.
Selection of axial slices showing clusters with significant activation from the arithmetic processing meta-analysis (red), phonological processing meta-analysis (blue), and the conjunction analysis (green) for the developmental sample.
Panel B in Table 4 displays the results of the phonological processing meta-analysis. Six clusters show reliable activation across studies. Clusters are in mostly left-lateralized frontal, temporal, and temporo-occipital regions. The largest cluster is in the left IFG (BA 6), with a local extremum in the left IFG (BA 9). The second cluster is in the left SFG (BA 6). The third and fourth clusters are in the left MTG (BA 22) and left FFG (BA 37), respectively. The fifth cluster is in the right insula (BA 13) with a local extremum in the right IFG (BA 47). Neighboring gray matter in the right IFG (280 mm3, BA 47; 152 mm3 BA 45) suggests this cluster is in anterior right insula. The last cluster is in the left IFG (BA 46). Fig. 1 shows the clusters from the phonological processing meta-analysis in blue.
Panel C in Table 4 presents the results of the conjunction analysis, which quantitatively assesses clusters of concordant activation for arithmetic and phonological processing tasks. There are six clusters of reliable activation, five that are in frontal areas. The largest cluster is in the left SFG (BA 6). The second cluster is in the left precentral gyrus (BA 6). The third cluster is in the right insula (BA 13). Neighboring gray matter outside of the insula includes the right claustrum (72 mm3) and right IFG (40 mm3, BA 45; 24 mm3, BA 13), which suggests this cluster is in anterior right insula. The fourth cluster is in left FFG (BA 37). The fifth and sixth clusters are both in right SFG (BA 6). Even though these clusters have no listed contributing studies (see Table 4, last two rows), they still produce reliable activation across studies. This is because contributing studies have coordinates inside the boundary of the cluster, but additional studies may contribute coordinates that lie on or just outside of the cluster boundary (RII, 2013). Note there is no right SFG cluster per se in the phonological processing meta-analysis (see Table 4). Rather, the cluster in left SFG has neighboring gray matter in the right SFG (424 mm3, BA 6). Fig. 1 displays the clusters from the conjunction analysis in green. We provide the subtraction analyses in Table S1 in the supplemental material.
4.2. Adult sample
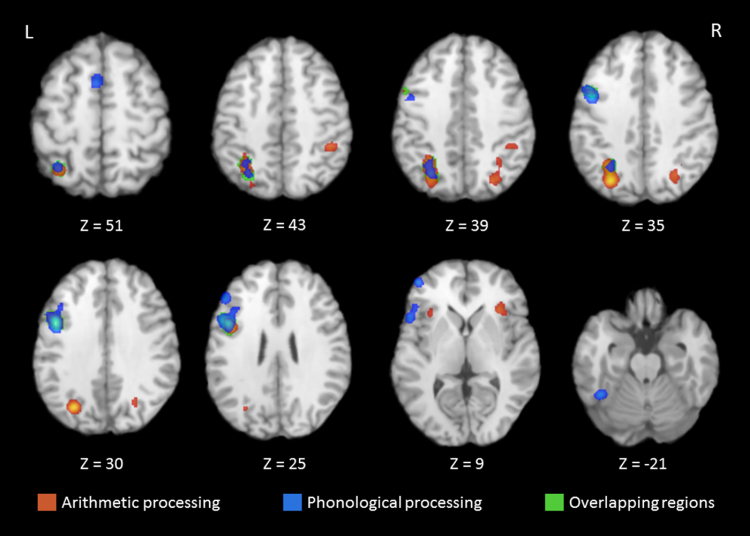
Table 5 displays the results of the three adult meta-analyses. Panel A displays the single-study meta-analysis for arithmetic processing. Six clusters show reliable activation across frontal and parietal regions. The largest cluster is in the left precuneus (BA 19) with local extrema in the precuneus (BA 7), left angular gyrus (BA 39), and left superior parietal lobule (BA 7). The second cluster is in the left inferior frontal gyrus (BA 9). The third cluster is in right precuneus (BA 19) with local extrema in the right precuneus (BA 7) and right superior parietal lobule (BA 7). The fourth cluster is in right insula (BA 13) and has neighboring gray matter in the right claustrum (184 mm3) and right IFG (96 mm3, BA 47; 72 mm3, BA 45), which suggests this cluster is in anterior insula. The fifth cluster is in the right inferior parietal lobule (IPL) (BA 40). The final cluster is in the left insula (BA 13) with a local extremum in left IFG (BA 47), which suggests this cluster is in anterior insula. Fig. 2 shows these six clusters in red.
Table 5.
Activation likelihood estimation results for arithmetic and phonological processing, and the conjunction analysis in the adult sample, including cluster, Talairach coordinate, ALE value, volume, and contributing studies. Local extrema are listed in italics.
| Cluster | Talairach coordinates |
ALE value | Volume (mm3) | Contributing studies | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (A) Arithmetic processing | ||||||
| Left Precuneus (BA 19) | −28 | −72 | 34 | 0.04120283 | 5888 | A1, A6, A7, A8, A9, A10, A11, A12, A13, A14, A15, A17, A19, A20, A21, A22 |
| Left Precuneus (BA 7) | −28 | −64 | 38 | 0.02997227 | ||
| Left Angular Gyrus (BA 39) | −30 | −58 | 38 | 0.029711127 | ||
| Left Superior Parietal Lobule (BA 7) | −32 | −60 | 48 | 0.028536372 | ||
| Left Inferior Frontal Gyrus (BA 9) | −42 | 4 | 28 | 0.036799587 | 2488 | A1, A6, A7, A12, A17, A20, A21, A22 |
| Right Precuneus (BA 19) | 28 | −70 | 38 | 0.022211188 | 1808 | A12, A7, A17, A19, A20, A21, A22 |
| Right Precuneus (BA 7) | 28 | −68 | 34 | 0.020805586 | ||
| Right Superior Parietal Lobule (BA 7) | 32 | −54 | 40 | 0.015481527 | ||
| Right Insula (BA 13) | 32 | 22 | 4 | 0.021382697 | 944 | A7, A19, A20, A21, A22 |
| Right Inferior Parietal Lobule (BA 40) | 42 | −42 | 42 | 0.020873273 | 904 | A1, A9, A11, A19 |
| Left Insula (BA 13) | −32 | 16 | 6 | 0.017058335 | 832 | A7, A12, A18, A21 |
| Left Inferior Frontal Gyrus (BA 47) | −32 | 20 | −2 | 0.016205575 | ||
| (B) Phonological processing | ||||||
| Left Inferior Frontal Gyrus (BA 6) | −44 | 2 | 32 | 0.03733236 | 12096 | P1, P2, P3, P4, P5, P7, P8, P9, P10, P11, P13, P14, P15, P16, P17, P18, P19, P20, P21, P22 |
| Left Middle Frontal Gyrus (BA 46) | −46 | 30 | 22 | 0.027697794 | ||
| Left Inferior Frontal Gyrus (BA 46) | −48 | 28 | 14 | 0.026974306 | ||
| Left Inferior Frontal Gyrus (BA 44) | −52 | 4 | 18 | 0.025123559 | ||
| Left Inferior Frontal Gyrus (BA 10) | −42 | 46 | 2 | 0.024867302 | ||
| Left Superior Temporal Gyrus (BA 22) | −50 | 12 | 0 | 0.024489433 | ||
| Left Middle Frontal Gyrus (BA 9) | −40 | 18 | 26 | 0.018028196 | ||
| Left Inferior Frontal Gyrus (BA 45) | −46 | 22 | 2 | 0.015826639 | ||
| Left Inferior Frontal Gyrus (BA 46) | −40 | 38 | 8 | 0.013467827 | ||
| Left Inferior Parietal Lobule (BA 7) | −32 | −58 | 48 | 0.024964614 | 2888 | P1, P5, P11, P13, P14, P16, P19, P22 |
| Left Precuneus (BA 19) | −28 | −66 | 40 | 0.01947619 | ||
| Left Angular Gyrus (BA 39) | −28 | −60 | 36 | 0.017271489 | ||
| Left Culmen | −40 | −52 | −20 | 0.024182009 | 1112 | P5, P6, P14, P18 |
| Left Superior Frontal Gyrus (BA 6) | −2 | 8 | 50 | 0.020853365 | 960 | P14, P17, P19, P21 |
| (C) Conjunction of Arithmetic and Phonological Processing | ||||||
| Left Inferior Parietal Lobule (BA 7) | −32 | −58 | 48 | 0.024964614 | 2384 | A1, A7, A9, A6, A13, A17, A19, A20, A21, P1, P11, P13, P14, P19, P22 |
| Left Precuneus (BA 19) | −28 | −66 | 40 | 0.01947619 | ||
| Left Angular Gyrus (BA 39) | −28 | −60 | 36 | 0.017271489 | ||
| Left Inferior Frontal Gyrus (BA 9) | −44 | 6 | 28 | 0.03553766 | 2272 | A1, A6, A7, A17, A20, A12, A21, A22, P1, P3, P4, P7, P9, P11, P14, P16, P18, P19, P22 |
Fig. 2.
Select axial slices showing clusters with significant activation from the arithmetic processing meta-analysis (red), phonological processing meta-analysis (blue), and the conjunction analysis (green) for the adult sample.
Panel B of Table 5 displays the clusters resulting from the phonological processing meta-analysis. There are four clusters of reliable activation spanning left lateralized frontal, temporal, and parietal regions. The largest cluster is in left IFG (BA 6) with eight left-lateralized local extrema (see Table 5, rows 15–22). These include IFG (BA 46, BA 44, BA 10), middle frontal gyrus (BA 46, BA 9), and STG (BA 22). The second cluster is in the left IPL (BA 7) and contains local extrema in the left precuneus (BA 19) and left AG (BA 39). The third and fourth clusters are in the left culmen and the left SFG (BA 6), respectively. Fig. 2 displays these clusters in blue.
Table 5 Panel C displays clusters from the conjunction analysis of arithmetic and phonological processing in adults. There are two clusters of overlapping activity. The first is in the left IPL (BA 7) with local extrema in the left precuneus (BA 19) and left AG (BA 39). Additional neighboring gray matter includes the superior parietal lobule (1128 mm3, BA 7) and IPL (48 mm3, BA 40; 40 mm3, BA 39). The second cluster is in the left IFG (BA 9). We display these clusters in Fig. 2 in green. We provide results of the subtraction analyses for the adult sample in Table S2 of the supplemental material.
5. Discussion
The present study examined neural functional overlap for arithmetic and phonological processing in developmental and adult samples. For each age group, we conducted separate meta-analyses and a subsequent conjunction analysis. Each meta-analysis produced clusters that are reliably activated across studies. For each age group, we briefly discuss results for the individual meta-analyses. We focus on clusters common to both arithmetic and phonological processing and a qualitative comparison of the conjunction analyses across the two age groups.
5.1. Developmental sample
5.1.1. Single-study meta-analyses
The arithmetic meta-analysis yielded clusters in bilateral frontal and occipito-temporal regions that are in line with prior work on numerical and arithmetic processing. Prior meta-analyses of numerical abilities and arithmetic in children found reliable activation in bilateral insula, premotor cortex, left IFG, and inferior temporal gyrus (Kaufmann et al., 2011), and left superior frontal gyrus (Houdé et al., 2010). An arithmetic-specific role for anterior insula and the left SFG/right cingulate is unclear. Activity in these regions may relate to the insula-cingulate salience network associated with cognitive control that supports arithmetic processing (Menon, 2015, Supekar and Menon, 2012), or switching between the executive control and default mode networks (Craig, 2009, Menon and Uddin, 2010). Recruitment of frontal regions during arithmetic may reflect the role of attentional processes (Houdé et al., 2010, Owen et al., 2005). Indeed, children engage frontal regions more and temporoparietal regions less than adults, and this difference in activity reflects a developing fluency with arithmetic (Ansari, 2008, Rivera et al., 2005, Zamarian et al., 2009). This could also partially account for the absence of clusters in parietal and temporoparietal regions, which have been present in some meta-analyses with children (Kaufmann et al., 2011), but not others (Houdé et al., 2010). This discrepancy may be due to differences in study contrasts. For example, Kaufmann et al. (2011) was limited to seven studies (included in the present analysis); almost all involved contrasts with non-numeric baselines. The present analysis included a majority of contrasts with numeric or non-retrieval based baseline tasks (see Table 2), both of which would subtract out number specific activity. Because fluency with arithmetic retrieval increases over developmental time, children may not be fluent enough to reliably engage temporoparietal regions across studies.
The phonological processing meta-analysis produced clusters of reliable activation in left frontal, temporal, and occipital regions, in addition to the right anterior insula. These results are in line with models of reading and phonological processing in children that outline a left-lateralized fronto-temporo-occipital network (Houdé et al., 2010, Martin et al., 2015). Specifically, the clusters largely replicate a well-known network of left-lateralized brain regions for typical readers that includes the IFG, MTG, and FFG (Jobard et al., 2003, Sebastian et al., 2014, Vigneau et al., 2006). As mentioned above, activation in the insula could be due to attentional processes or shifting between attention and the default mode network (Craig, 2009, Menon and Uddin, 2010).
5.1.2. Clusters common to arithmetic and phonological processing
5.1.2.1. Left precentral and bilateral SFG
Four of the six clusters common to arithmetic and phonological processing were in left precentral gyrus and bilateral SFG. A prior meta-analysis in children showed reliable activation in left SFG for number abilities, but not reading (Houdé et al., 2010). Both regions were also found in a prior meta-analysis on calculation in children (Kaufmann et al., 2011). Reliable activation in these regions is likely driven by domain-general task demands. For example, activity in the SFG has been associated with selective attention (Anderson et al., 2007). The cluster in precentral gyrus could also reflect different levels of interference in generating motor responses for experimental and control tasks. For example, Kesler et al. (2006) contrasted judging the correctness of addition and subtraction facts (i.e, a true-false judgment) with pressing a button when a ‘0′ was present (i.e., a go/no-go task).
5.1.2.2. Right insula
A cluster in the right anterior insula aligns with prior meta-analyses of number and arithmetic processing in children (Kaufmann et al., 2011). However, right insula activity is found in some reading meta-analyses in children (Houdé et al., 2010), but not others (Martin et al., 2015); this may be due in part to specific contrast selection criteria in prior studies (e.g., contrasts that isolate semantic processing). Reliable activation in the right insula has also been present in some meta-analyses of atypical reading development. Maisog et al. (2008) found hyperactivity in anterior insula for atypical readers, which may have been related to atypical readers’ perception of reading-related stimuli as aversive. Barquero et al. (2014) found underactivation in right insula prior to a reading intervention with children, but found consistent activation in this region after intervention.
The role of the right anterior insula in numeracy or literacy, specifically, is currently unclear. Recruitment of this region may support arithmetic and phonological processing through domain-general functioning. Models of anterior insula function suggest that it supports higher level cognitive processing including task-related attentional capture and control (Craig, 2009, Menon and Uddin, 2010, Nelson et al., 2010), decision-making, or knowing information before recalling it (Craig, 2009). The insula is also thought to direct cognitive and neural resources to internally or externally focused attention (Menon and Uddin, 2010). In the present study, recruitment of the right anterior insula could represent the direction of externally focused task-specific attention toward arithmetic or phonological tasks or the experience of knowing the answer to an arithmetic fact or whether two words rhyme.
5.1.2.3. Left fusiform gyrus
The final cluster common to arithmetic and phonological processing is in the left FFG. Prior arithmetic meta-analyses either did not find activation near this region (Houdé et al., 2010) or showed a cluster in the neighboring inferior temporal gyrus (Kaufmann et al., 2011). Reliable activation in the left FFG or inferior temporal gyrus has also been found in prior meta-analyses related to typically-developing readers (Houdé et al., 2010, Martin et al., 2015, Pollack et al., 2015). This cluster may be common to arithmetic and phonological processing because of its functional role in symbol recognition for words and digits.
The left FFG houses the Visual Word Form Area (VWFA), an area situated near Talairach coordinates −42, −57, −12 that is consistently activated by letters and words (Cohen et al., 2000, Hannagan et al., 2015, McCandliss et al., 2003). Evidence suggests there may be a number form area (NFA) lateral to the VWFA, in (bilateral) ventral inferior temporal gyrus (Grotheer et al., 2016, Hannagan et al., 2015, Shum et al., 2013; however, see Peters et al. (2015) and Price and Ansari (2011) for an alternative view). Whether the left VWFA and NFA are separate or merged is unclear (Hannagan et al., 2015, Starrfelt and Behrmann, 2011). There appears to be functional specialization of both regions in adults compared to children (for reviews, see Menon, 2015, Schlaggar and McCandliss, 2007). Indeed, recent research looking across children and adults suggests that the VWFA and NFA may be merged in children and become functionally distinct areas in adulthood (Cantlon et al., 2011). Thus, it is plausible that the cluster in left FFG found in the present study supports symbolic processing for both number and letter/word identification.
5.2. Adult sample
5.2.1. Individual meta-analyses
The arithmetic analysis yielded clusters that span bilateral precuneus with local extrema in the left AG and bilateral superior parietal lobule. These results align with models of numerical cognition that characterize the superior parietal lobule as a key region for visual attention and number processing and characterize temporo-parietal regions including the AG that support fluent arithmetic fact retrieval (e.g., Dehaene et al., 2003, Zamarian et al., 2009). Additional clusters in frontal regions including bilateral insula and left IFG replicate prior meta-analytic findings in adults related to arithmetic (Arsalidou and Taylor, 2011) and support the notion that temporoparietal and frontal areas both support arithmetic fact retrieval (e.g., Jost et al., 2011).
The phonological processing meta-analysis produced left-lateralized clusters that span frontal regions including superior and inferior frontal gyri, the STG, and the IPL including the AG. Clusters in these regions align with the left-lateralized reading network in adults (Jobard et al., 2003) and specifically with regions known to support phonological processing in adults (Vigneau et al., 2006). Taken together, the results of the individual adult meta-analyses largely replicate well-established regions of brain activity that support arithmetic and phonological processing, respectively.
5.2.2. Clusters common to arithmetic and phonological processing
5.2.2.1. Left inferior parietal lobule
The first cluster common to arithmetic and phonological processing spans the left IPL including the AG. The AG has been implicated separately in fact retrieval (e.g., Grabner et al., 2009a) and phoneme discrimination (Turkeltaub and Coslett, 2010). Studies examining overlap in AG activation for arithmetic and phonological processing have produced inconsistent results. Simon et al. (2002) found shared activation mesial to the left AG for calculation and phoneme detection, but their task (subtraction) does not reflect fact retrieval. Andin et al. (2015) found regional differentiation in the AG for multiplication (i.e., PGp) and phonological processing (i.e., PGa). However, the results of the present meta-analysis support the notion that temporoparietal regions spanning the IPL and including the AG support both arithmetic and phonological processing in adults. Concordant activity in this region may reflect familiarity across symbol sets including letters and digits (Price and Ansari, 2011) or the role of this region as a hub for cross-modal integration (Seghier, 2013). Specifically, activity in the left IPL/AG cluster may support the connection of symbols (i.e., letters, words, and arithmetic facts) to their associated verbal representations.
5.2.2.2. Left IFG
The second cluster common to arithmetic and phonological processing was in left IFG. Prior studies that have investigated an overlap in arithmetic and phonological processing have shown mixed results related to left IFG activation. Andin et al. (2015) found that multiplication was associated with activity in the pars triangularis portion of left IFG (BA 45), whereas phonological processing was associated with posterior activity in the pars opercularis portion of left IFG (BA 44). Similarly, Fedorenko et al. (2012) found an area on the border of BA 44/45 active for language-specific tasks, with brain activity in both anterior and posterior regions bordering BA 44/45 responding to various tasks, including mental arithmetic. Simon et al. (2002) found a common area of activation in left IFG for subtraction and phoneme detection tasks, however this area was also common to other tasks (i.e., grasping). Taken together, these studies do not provide evidence of overlapping brain activation in left IFG that supports retrieval-based arithmetic and phonological processing. However, the results of the current meta-analysis suggest there may be.
One reason for this discrepancy may be a lack of anatomical specificity across studies, as illustrated above. The discrepancy could also be due to variation in tasks across studies. Tasks that place more demands on working memory may be associated with higher left IFG activity. Evidence suggests that arithmetic fact difficulty may vary by operation (Zhou et al., 2007) or by strategy choice (Tschentscher and Hauk, 2014). While the arithmetic problems chosen for the present analysis are thought to rely on retrieval, only a few imaging studies to date explicitly account for strategy choice (e.g., De Visscher et al., 2015, Grabner et al., 2009a, Jost et al., 2009).
5.3. A comparison of overlap of activation across groups
A qualitative comparison of the developmental and adult conjunction analyses shows that there were no common clusters of brain activity that support both arithmetic and phonological processing across the two age groups. The conjunction analyses for both children and adults did reveal clusters in frontal regions. However, for children clusters were in bilateral SFG, left precentral gyrus, and right insula, while for adults there was one cluster in left IFG. This comparison illustrates more diffuse and bilateral concordant activation concentrated in frontal regions for the developmental sample compared to adults. This may reflect children’s greater reliance on domain general processes such as working memory and attention than adults as children develop fluency with arithmetic and phonological processing tasks. Research suggests that across development, reading is associated with an increase in brain activity in left-lateralized frontal and temporal areas, such as IFG, and a decrease in activity in right-lateralized regions (Turkeltaub et al., 2003). Similarly, brain regions that support arithmetic shift over development, reflecting an increase in recruitment of temporal and parietal regions as children become more fluent with arithmetic such as multiplication facts (Prado et al., 2014, Zamarian et al., 2009). The lack of a left temporoparietal cluster in the developmental sample is likely due to absence of concordant left temporoparietal activation in the arithmetic single-file meta-analysis. This suggests that across development, children may not reliably recruit the same temporoparietal regions for arithmetic and phonological processing due to developing fluency with retrieval-based arithmetic. Yet in the adult sample, we see concordant activation in this area for both the single-file arithmetic meta-analysis and the conjunction.
6. Limitations
One important limitation of the present meta-analyses is the heterogeneity in participant ages in the developmental sample, which ranged from 7 to 17 years across arithmetic studies and from about 6 to 14 years across studies involving phonological processing. As a result, areas of common activation in the developmental conjunction analysis likely reflect regions that do not change across developmental time. Therefore, the analysis does not capture, for example, brain regions that support arithmetic during particular points in development. Importantly, this may account for the absence of a temporoparietal cluster for the developmental sample, since recruitment of this regions increases over development (e.g., Ansari, 2008). When additional developmental arithmetic studies are available, future meta-analyses could contrast younger and older children to test this hypothesis.
A second limitation concerns differences in retrieval across arithmetic operations. Retrieval is likely for small addition and multiplication problems but efficiency and use of retrieval may differ by age (Imbo and Vandierendonck, 2008). Further, whether different arithmetic operations recruit different brain regions is still an open question. Several studies have found differentially active brain regions across arithmetic operations (Arsalidou and Taylor, 2011, Chochon et al., 1999, Zhou et al., 2007). However, recent research suggests that neural differences attributed to arithmetic operations per se may be due to surface criteria of problems, and that neural differences may instead reflect differences in strategy use (Tschentscher and Hauk, 2014).
A final limitation concerns the comparison of the arithmetic and phonological contrasts. While we aimed to make the contrasts as similar as possible across domains, many of the developmental phonological processing studies use low-level baselines, such as fixation. While meta-analyses are limited to extant research, they also offer insight into gaps in the literature and provide potential research opportunities. We offer that future neuroimaging studies with developmental samples can also include contrasts with high level control tasks, as a way to better understand the mechanisms that underlie phonological processing.
7. Conclusion
The present study used neuroimaging meta-analysis to investigate whether arithmetic and phonological processing − related but distinct domains − share overlapping areas of brain activity. In the developmental sample, areas of concordant activity were concentrated in frontal regions with an additional cluster in left FFG, regions that may support domain-general and symbol processing, respectively. The adult sample yielded left-lateralized clusters in IPL and IFG, suggesting common regions that support connecting symbols with their verbally-stored referents. Across the two conjunctions, children showed more diffuse and frontal activation compared with adults. Such results highlight the engagement of domain-general attentional processes that support more effortful cognitive processing across domains in children. Investigating brain regions that support both arithmetic and phonological processing in children and adults can inform models of how these two processes are related and how the brain may support processing of higher order symbolic representations, such as arithmetic facts or words. Such work can in turn contribute to a better understanding of the neural correlates of learning throughout development and adulthood.
Conflict of Interest
None.
Acknowledgments
We sincerely thank Gigi Luk, Bert De Smedt, and Jon R. Star for their guidance and mentorship on this project, and their comments on prior versions of this manuscript. We also thank Laura Mesite for assistance with results on a prior version of this manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
For addition and multiplication, De Smedt et al. (2010) defined small problems as problems in which the product of the operands is less than or equal to 25. Small subtraction and division problems were the inverse of the small addition and multiplication problems.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2017.05.003.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Anderson E.J., Mannan S.K., Husain M., Rees G., Sumner P., Mort D.J., McRobbie D., Kennard C. Involvement of prefrontal cortex in visual search. Exp. Brain Res. 2007;180:289–302. doi: 10.1007/s00221-007-0860-0. [DOI] [PubMed] [Google Scholar]
- Andin J., Fransson P., Rönnberg J., Rudner M. Phonology and arithmetic in the language-calculation network. Brain Lang. 2015;143:97–105. doi: 10.1016/j.bandl.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Andres M., Pelgrims B., Michaux N., Olivier E., Pesenti M. Role of distinct parietal areas in arithmetic: an fMRI-guided TMS study. Neuroimage. 2011;54:3048–3056. doi: 10.1016/j.neuroimage.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Andres M., Michaux N., Pesenti M. Common substrate for mental arithmetic and finger representation in the parietal cortex. Neuroimage. 2012;62:1520–1528. doi: 10.1016/j.neuroimage.2012.05.047. [DOI] [PubMed] [Google Scholar]
- Ansari D. Effects of development and enculturation on number representation in the brain. Nat. Rev. Neurosci. 2008;9:278–291. doi: 10.1038/nrn2334. [DOI] [PubMed] [Google Scholar]
- Arsalidou M., Taylor M.J. Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage. 2011;54:2382–2393. doi: 10.1016/j.neuroimage.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Ashby, N.C., Pollack, C. (2016, August). At the intersection of mathematical and reading-related cognition: The meta-analytic convergence of arithmetic fact retrieval and phonological processing in the adult brain. Presentation at the sixth Association of Pacific Rim Universities (APRU) Brain and Mind in the Asia-Pacific research symposium, Auckland, New Zealand.
- Ashkenazi S., Rosenberg-Lee M., Tenison C., Menon V. Weak task-related modulation and stimulus representations during arithmetic problem solving in children with developmental dyscalculia. Dev. Cogn. Neurosci. 2012;2(Suppl. 1):S152–166. doi: 10.1016/j.dcn.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach S., Brandeis D., Hofstetter C., Martin E., Richardson U., Brem S. Early emergence of deviant frontal fMRI activity for phonological processes in poor beginning readers. Neuroimage. 2010;53:682–693. doi: 10.1016/j.neuroimage.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Bach S., Richardson U., Brandeis D., Martin E., Brem S. Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. Neuroimage. 2013;82:605–615. doi: 10.1016/j.neuroimage.2013.05.062. [DOI] [PubMed] [Google Scholar]
- Backes W., Vuurman E., Wennekes R., Spronk P., Wuisman M., van Engelshoven J., Jolles J. Atypical brain activation of reading processes in children with developmental dyslexia. J. Child Neurol. 2002;17:867–871. doi: 10.1177/08830738020170121601. [DOI] [PubMed] [Google Scholar]
- Barquero L.A., Davis N., Cutting L.E. Neuroimaging of reading intervention: a systematic review and activation likelihood estimate meta-analysis. PLoS One. 2014;9:e83668. doi: 10.1371/journal.pone.0083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrouillet P., Mignon M., Thevenot C. Strategies in subtraction problem solving in children. J. Exp. Child Psychol. 2008;99:233–251. doi: 10.1016/j.jecp.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Bitan T., Cheon J., Lu D., Burman D.D., Gitelman D.R., Mesulam M.-M., Booth J.R. Developmental changes in activation and effective connectivity in phonological processing. Neuroimage. 2007;38:564–575. doi: 10.1016/j.neuroimage.2007.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T., Cheon J., Lu D., Burman D.D., Booth J.R. Developmental increase in top-down and bottom-up processing in a phonological task: an effective connectivity, fMRI Study. J. Cogn. Neurosci. 2009;21:1135–1145. doi: 10.1162/jocn.2009.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J.R., Santen F.W.V., Harasaki Y., Gitelman D.R., Parrish T.B., Mesulam M.M., Burman D.D. The development of specialized brain systems in reading and oral-language. Child Neuropsychol. Neuropsychol. Dev. Cogn. Sect. C. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Gitelman D.R., Parrish T.B., Mesulam M.M. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Choy J., Gitelman D.R., Parrish T.B., Mesulam M.M. Modality-specific and −independent developmental differences in the neural substrate for lexical processing. J. Neurolinguistics. 2003;16:383–405. [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Gitelman D.R., Parrish T.B., Mesulam M.M. Development of brain mechanisms for processing orthographic and phonologic representations. J. Cogn. Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton M.W., Locasto P.C., Krebs-Noble D., Gullapalli R.P. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage. 2005;26:647–661. doi: 10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Campbell J.I., Xue Q. Cognitive arithmetic across cultures. J. Exp. Psychol. Gen. 2001;130:299–315. doi: 10.1037//0096-3445.130.2.299. [DOI] [PubMed] [Google Scholar]
- Cantlon J.F., Pinel P., Dehaene S., Pelphrey K.A. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb. Cortex. 2011;21:191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Bitan T., Chou T.-L., Burman D.D., Booth J.R. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J. Child Psychol. Psychiatry. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Bitan T., Booth J.R. Effective brain connectivity in children with reading difficulties during phonological processing. Brain Lang. 2008;107:91–101. doi: 10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Hu Z., Zhao X., Wang R., Yang Z., Wang X., Tang X. Neural correlates of serial abacus mental calculation in children: a functional MRI study. Neurosci. Lett. 2006;403:46–51. doi: 10.1016/j.neulet.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Cho S., Ryali S., Geary D.C., Menon V. How does a child solve 7 + 8? Decoding brain activity patterns associated with counting and retrieval strategies. Dev. Sci. 2011;14:989–1001. doi: 10.1111/j.1467-7687.2011.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chochon F., Cohen L., van de Moortele P.F., Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. J. Cogn. Neurosci. 1999;11:617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- Clark D., Wagner A.D. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S., Naccache L., Lehéricy S., Dehaene-Lambertz G., Hénaff M.A., Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain J. Neurol. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cousin E., Peyrin C., Baciu M. Hemispheric predominance assessment of phonology and semantics: a divided visual field experiment. Brain Cogn. 2006;61:298–304. doi: 10.1016/j.bandc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Craig A.D.(Bud) How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Danelli L., Berlingeri M., Bottini G., Ferri F., Vacchi L., Sberna M., Paulesu E. Neural intersections of the phonological, visual magnocellular and motor/cerebellar systems in normal readers: implications for imaging studies on dyslexia: neural Intersections in Reading. Hum. Brain Mapp. 2013;34:2669–2687. doi: 10.1002/hbm.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Cannistraci C.J., Rogers B.P., Gatenby J.C., Fuchs L.S., Anderson A.W., Gore J.C. Aberrant functional activation in school age children at-risk for mathematical disability: a functional imaging study of simple arithmetic skill. Neuropsychologia. 2009;47:2470–2479. doi: 10.1016/j.neuropsychologia.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Cannistraci C.J., Rogers B.P., Gatenby J.C., Fuchs L.S., Anderson A.W., Gore J.C. The neural correlates of calculation ability in children: an fMRI study. Magn. Reson. Imaging. 2009;27:1187–1197. doi: 10.1016/j.mri.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pisapia N., Slomski J.A., Braver T.S. Functional specializations in lateral prefrontal cortex associated with the integration and segregation of information in working memory. Cereb. Cortex. 2006;17:993–1006. doi: 10.1093/cercor/bhl010. [DOI] [PubMed] [Google Scholar]
- De Smedt B., Boets B. Phonological processing and arithmetic fact retrieval: evidence from developmental dyslexia. Neuropsychologia. 2010;48:3973–3981. doi: 10.1016/j.neuropsychologia.2010.10.018. [DOI] [PubMed] [Google Scholar]
- De Smedt B., Taylor J., Archibald L., Ansari D. How is phonological processing related to individual differences in children’s arithmetic skills? Dev. Sci. 2010;13:508–520. doi: 10.1111/j.1467-7687.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- De Smedt B., Holloway I.D., Ansari D. Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. Neuroimage. 2011;57:771–781. doi: 10.1016/j.neuroimage.2010.12.037. [DOI] [PubMed] [Google Scholar]
- De Visscher A., Berens S.C., Keidel J.L., Noël M.-P., Bird C.M. The interference effect in arithmetic fact solving: an fMRI study. Neuroimage. 2015;116:92–101. doi: 10.1016/j.neuroimage.2015.04.063. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Spelke E., Pinel P., Stanescu R., Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Piazza M., Pinel P., Cohen L. Three parietal circuits for number processing. Cogn. Neuropsychol. 2003;20:487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- Delazer M., Domahs F., Bartha L., Brenneis C., Lochy A., Trieb T., Benke T. Learning complex arithmetic—an fMRI study. Cogn. Brain Res. 2003;18:76–88. doi: 10.1016/j.cogbrainres.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Delazer M., Ischebeck A., Domahs F., Zamarian L., Koppelstaetter F., Siedentopf C.M., Kaufmann L., Benke T., Felber S. Learning by strategies and learning by drill—evidence from an fMRI study. Neuroimage. 2005;25:838–849. doi: 10.1016/j.neuroimage.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Demir Ö.E., Prado J., Booth J.R. The differential role of verbal and spatial working memory in the neural basis of arithmetic. Dev. Neuropsychol. 2014;39:440–458. doi: 10.1080/87565641.2014.939182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz N.A.E., Jones K.M., Gareau L., Zeffiro T.A., Eden G.F. Phonological decoding involves left posterior fusiform gyrus. Hum. Brain Mapp. 2005;26:81–93. doi: 10.1002/hbm.20122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Etkin A. Going beyond finding the lesion: a path for maturation of neuroimaging. Am. J. Psychiatry. 2016;173:302–303. doi: 10.1176/appi.ajp.2015.15101350. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Roski C., Caspers S., Zilles K., Fox P.T. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Fox P.M., Lancaster J.L., Fox P.T. Implementation errors in the GingerALE software: description and recommendations: errors in the GingerALE software. Hum. Brain Mapp. 2016 doi: 10.1002/hbm.23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.M., Flowers D.L., Napoliello E.M., Olulade O.A., Eden G.F. The functional anatomy of single-digit arithmetic in children with developmental dyslexia. Neuroimage. 2014;101:644–652. doi: 10.1016/j.neuroimage.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Duncan J., Kanwisher N. Language-selective and domain-general regions lie side by side within Broca’s area. Curr. Biol. 2012;22:2059–2062. doi: 10.1016/j.cub.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiewa P., Rzanny R., Hopf J.M., Knab R., Glauche V., Kaiser W.A., Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Geva S., Jones P.S., Crinion J.T., Price C.J., Baron J.-C., Warburton E.A. The effect of aging on the neural correlates of phonological word retrieval. J. Cogn. Neurosci. 2012;24:2135–2146. doi: 10.1162/jocn_a_00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner R.H., Ansari D., Reishofer G., Stern E., Ebner F., Neuper C. Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage. 2007;38:346–356. doi: 10.1016/j.neuroimage.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Grabner R.H., Ansari D., Koschutnig K., Reishofer G., Ebner F., Neuper C. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009;47:604–608. doi: 10.1016/j.neuropsychologia.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Grabner R.H., Ischebeck A., Reishofer G., Koschutnig K., Delazer M., Ebner F., Neuper C. Fact learning in complex arithmetic and figural-spatial tasks: the role of the angular gyrus and its relation to mathematical competence. Hum. Brain Mapp. 2009;30:2936–2952. doi: 10.1002/hbm.20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner R.H., Ansari D., Koschutnig K., Reishofer G., Ebner F. The function of the left angular gyrus in mental arithmetic: evidence from the associative confusion effect. Hum. Brain Mapp. 2013;34:1013–1024. doi: 10.1002/hbm.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotheer M., Herrmann K.-H., Kovács G. Neuroimaging evidence of a bilateral representation for visually presented numbers. J. Neurosci. 2016;36:88–97. doi: 10.1523/JNEUROSCI.2129-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick M.M., Wolford G. Brain systems involved in arithmetic with positive versus negative numbers: arithmetic with Positive and Negative Numbers. Hum. Brain Mapp. 2014;35:539–551. doi: 10.1002/hbm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannagan T., Amedi A., Cohen L., Dehaene-Lambertz G., Dehaene S. Origins of the specialization for letters and numbers in ventral occipitotemporal cortex. Trends Cogn. Sci. 2015;19:374–382. doi: 10.1016/j.tics.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Hecht S.A., Torgesen J.K., Wagner R.K., Rashotte C.A. The relations between phonological processing abilities and emerging individual differences in mathematical computation skills: a longitudinal study from second to fifth grades. J. Exp. Child Psychol. 2001;79:192–227. doi: 10.1006/jecp.2000.2586. [DOI] [PubMed] [Google Scholar]
- Hernandez N., Andersson F., Edjlali M., Hommet C., Cottier J.P., Destrieux C., Bonnet-Brilhault F. Cerebral functional asymmetry and phonological performance in dyslexic adults: functional asymmetry in dyslexia. Psychophysiology. 2013;50:1226–1238. doi: 10.1111/psyp.12141. [DOI] [PubMed] [Google Scholar]
- Hoeft F., Hernandez A., McMillon G., Taylor-Hill H., Martindale J.L., Meyler A., Keller T.A., Siok W.T., Deutsch G.K., Just M.A., Whitfield-Gabrieli S., Gabrieli J.D.E. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J. Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., Meyler A., Hernandez A., Juel C., Taylor-Hill H., Martindale J.L., McMillon G., Kolchugina G., Black J.M., Faizi A., Deutsch G.K., Siok W.T., Reiss A.L., Whitfield-Gabrieli S., Gabrieli J.D.E. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdé O., Rossi S., Lubin A., Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Dev. Sci. 2010;13:876–885. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Rund B.R., Lund A., Asbjørnsen A., Egeland J., Ersland L., Landrø N.I., Roness A., Stordal K.I., Sundet K. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am. J. Psychiatry. 2004;161:286–293. doi: 10.1176/appi.ajp.161.2.286. [DOI] [PubMed] [Google Scholar]
- Imbo I., Vandierendonck A. Effects of problem size, operation, and working-memory span on simple-arithmetic strategies: differences between children and adults? Psychol. Res. 2008;72:331–346. doi: 10.1007/s00426-007-0112-8. [DOI] [PubMed] [Google Scholar]
- Ischebeck A., Zamarian L., Siedentopf C., Koppelstätter F., Benke T., Felber S., Delazer M. How specifically do we learn? Imaging the learning of multiplication and subtraction. Neuroimage. 2006;30:1365–1375. doi: 10.1016/j.neuroimage.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Jobard G., Crivello F., Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Jost K., Khader P., Burke M., Bien S., Rösler F. Dissociating the solution processes of small, large, and zero multiplications by means of fMRI. Neuroimage. 2009;46:308–318. doi: 10.1016/j.neuroimage.2009.01.044. [DOI] [PubMed] [Google Scholar]
- Jost K., Khader P.H., Burke M., Bien S., Rösler F. Frontal and parietal contributions to arithmetic fact retrieval: a parametric analysis of the problem-size effect. Hum. Brain Mapp. 2011;32:51–59. doi: 10.1002/hbm.21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert S., Beauregard M., Walter N., Bourgouin P., Beaudoin G., Leroux J.-M., Karama S., Lecours A.R. Neural correlates of lexical and sublexical processes in reading. Brain Lang. 2004;89:9–20. doi: 10.1016/S0093-934X(03)00403-6. [DOI] [PubMed] [Google Scholar]
- Kareken D.A., Lowe M., Chen S.H., Lurito J., Mathews V. Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry. Neuropsychol. Behav. Neurol. 2000;13:264–270. [PubMed] [Google Scholar]
- Katzir T., Misra M., Poldrack R.A. Imaging phonology without print: assessing the neural correlates of phonemic awareness using fMRI. Neuroimage. 2005;27:106–115. doi: 10.1016/j.neuroimage.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Kaufmann L., Wood G., Rubinsten O., Henik A. Meta-analyses of developmental fMRI studies investigating typical and atypical trajectories of number processing and calculation. Dev. Neuropsychol. 2011;36:763–787. doi: 10.1080/87565641.2010.549884. [DOI] [PubMed] [Google Scholar]
- Kawashima R., Taira M., Okita K., Inoue K., Tajima N., Yoshida H., Sasaki T., Sugiura M., Watanabe J., Fukuda H. A functional MRI study of simple arithmetic—a comparison between children and adults. Cogn. Brain Res. 2004;18:227–233. doi: 10.1016/j.cogbrainres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Keller K., Menon V. Gender differences in the functional and structural neuroanatomy of mathematical cognition. Neuroimage. 2009;47:342–352. doi: 10.1016/j.neuroimage.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S.R., Menon V., Reiss A.L. Neurofunctional differences associated with arithmetic processing in turner syndrome. Cereb. Cortex. 2006;16:849–856. doi: 10.1093/cercor/bhj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucian K., Loenneker T., Dietrich T., Dosch M., Martin E., von Aster M. Impaired neural networks for approximate calculation in dyscalculic children: a functional MRI study. Behav. Brain Funct. BBF. 2006;2:31. doi: 10.1186/1744-9081-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo B.-C., Yeh Y.-Y., Chen D.-Y., Liang K.C., Chen J.-H. The capacity constraint in the prefrontal and parietal regions for coordinating dual arithmetic tasks. Brain Res. 2008;1199:100–110. doi: 10.1016/j.brainres.2007.12.070. [DOI] [PubMed] [Google Scholar]
- Laird A.R., Robinson J.L., McMillan K.M., Tordesillas-Gutiérrez D., Moran S.T., Gonzales S.M., Ray K.L., Franklin C., Glahn D.C., Fox P.T., Lancaster J.L. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage. 2010;51:677–683. doi: 10.1016/j.neuroimage.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-M., Kang S.-Y. Arithmetic operation and working memory: differential suppression in dual tasks. Cognition. 2002;83:B63–B68. doi: 10.1016/s0010-0277(02)00010-0. [DOI] [PubMed] [Google Scholar]
- Lurito J.T., Kareken D.A., Lowe M.J., Chen S.H.A., Mathews V.P. Comparison of rhyming and word generation with FMRI. Hum. Brain Mapp. 2000;10:99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacSweeney M., Brammer M.J., Waters D., Goswami U. Enhanced activation of the left inferior frontal gyrus in deaf and dyslexic adults during rhyming. Brain. 2009;132:1928–1940. doi: 10.1093/brain/awp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisog J.M., Einbinder E.R., Flowers D.L., Turkeltaub P.E., Eden G.F. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Martin A., Schurz M., Kronbichler M., Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015;36:1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss B.D., Cohen L., Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McDermott K.B., Petersen S.E., Watson J.M., Ojemann J.G. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41:293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- McNorgan C., Alvarez A., Bhullar A., Gayda J., Booth J.R. Prediction of reading skill several years later depends on age and brain region: implications for developmental models of reading. J. Neurosci. 2011;31:9641–9648. doi: 10.1523/JNEUROSCI.0334-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A., Crinion J.T., Long S., Friston K.J., Ralph M.A.L., Patterson K., McClelland J.L., Price C.J. Dissociating reading processes on the basis of neuronal interactions. J. Cogn. Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Meintjes E.M., Jacobson S.W., Molteno C.D., Gatenby J.C., Warton C., Cannistraci C.J., Gore J.C., Jacobson J.L. An fMRI study of magnitude comparison and exact addition in children. Magn. Reson. Imaging. 2010;28:351–362. doi: 10.1016/j.mri.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Rivera S.M., White C.D., Glover G.H., Reiss A.L. Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage. 2000;12:357–365. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- Menon V. Arithmetic in the child and adult brain. In: Cohen Kadosh R., Dowker A., editors. The Oxford Handbook of Numerical Cognition. Oxford University Press; 2015. pp. 502–530. [Google Scholar]
- Metcalfe A.W.S., Ashkenazi S., Rosenberg-Lee M., Menon V. Fractionating the neural correlates of individual working memory components underlying arithmetic problem solving skills in children. Dev. Cogn. Neurosci. 2013;6:162–175. doi: 10.1016/j.dcn.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S.M., Dosenbach N.U.F., Cohen A.L., Wheeler M.E., Schlaggar B.L., Petersen S.E. Role of the anterior insula in task-level control and focal attention. Brain Struct. Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Wolmetz M.E., Ochs L.G., Farah M.J., McCandliss B.D. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev. Sci. 2006;9:642–654. doi: 10.1111/j.1467-7687.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- Oron A., Wolak T., Zeffiro T., Szelag E. Cross-modal comparisons of stimulus specificity and commonality in phonological processing. Brain Lang. 2016;155–156:12–23. doi: 10.1016/j.bandl.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passolunghi M.C., Vercelloni B., Schadee H. The precursors of mathematics learning: working memory, phonological ability and numerical competence. Cogn. Dev. 2007;22:165–184. [Google Scholar]
- Pecini C., Biagi L., Guzzetta A., Montanaro D., Brizzolara D., Cipriani P., Chilosi A., Tosetti M., Cioni G. Brain representation of phonological processing in Italian: individual variability and behavioural correlates. Arch. Ital. Biol. 2008;146:189–203. [PubMed] [Google Scholar]
- Peters L., De Smedt B., Op de Beeck H.P. The neural representation of Arabic digits in visual cortex. Front. Hum. Neurosci. 2015;9:517. doi: 10.3389/fnhum.2015.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Temple E., Protopapas A., Nagarajan S., Tallal P., Merzenich M., Gabrieli J.D. Relations between the neural bases of dynamic auditory processing and phonological processing: evidence from fMRI. J. Cogn. Neurosci. 2001;13:687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Pollack, C., Ashby, N.C. (2016, September). Functional neural overlap between arithmetic and phonological processing in children: A meta-analysis. Poster presented at the 2016 International Mind, Brain, and Education Society (IMBES) Conference, Toronto, ON, Canada.
- Pollack C., Luk G., Christodoulou J.A. A meta-analysis of functional reading systems in typically developing and struggling readers across different alphabetic languages. Front. Psychol. 2015;6:1–10. doi: 10.3389/fpsyg.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J., Mutreja R., Zhang H., Mehta R., Desroches A.S., Minas J.E., Booth J.R. Distinct representations of subtraction and multiplication in the neural systems for numerosity and language. Hum. Brain Mapp. 2011;32:1932–1947. doi: 10.1002/hbm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J., Mutreja R., Booth J.R. Developmental dissociation in the neural responses to simple multiplication and subtraction problems. Dev. Sci. 2014;17:537–552. doi: 10.1111/desc.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G.R., Ansari D. Symbol processing in the left angular gyrus: evidence from passive perception of digits. Neuroimage. 2011;57:1205–1211. doi: 10.1016/j.neuroimage.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Price G.R., Mazzocco M.M.M., Ansari D. Why mental arithmetic counts: brain activation during single digit arithmetic predicts high school math scores. J. Neurosci. 2013;33:156–163. doi: 10.1523/JNEUROSCI.2936-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: contributions from functional neuroimaging. J. Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Imaging Institute UTHSCSA . 2013. User Manual for GingerALE 2.3. [Google Scholar]
- Research Imaging Institute UTHSCSA . 2015. Mango. [Google Scholar]
- Rivera S.M., Menon V., White C.D., Glaser B., Reiss A.L. Functional brain activation during arithmetic processing in females with fragile X syndrome is related toFMR1 protein expression. Hum. Brain Mapp. 2002;16:206–218. doi: 10.1002/hbm.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera S.M., Reiss A.L., Eckert M.A., Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb. Cortex N. Y. N. 2005;1991(15):1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Lee M., Chang T.T., Young C.B., Wu S., Menon V. Functional dissociations between four basic arithmetic operations in the human posterior parietal cortex: a cytoarchitectonic mapping study. Neuropsychologia. 2011;49:2592–2608. doi: 10.1016/j.neuropsychologia.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M., Ashkenazi S., Chen T., Young C.B., Geary D.C., Menon V. Brain hyper-connectivity and operation-specific deficits during arithmetic problem solving in children with developmental dyscalculia. Dev. Sci. 2015;18:351–372. doi: 10.1111/desc.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner M., Karlsson T., Gunnarsson J., Rönnberg J. Levels of processing and language modality specificity in working memory. Neuropsychologia. 2013;51:656–666. doi: 10.1016/j.neuropsychologia.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Sammer G., Blecker C., Gebhardt H., Bischoff M., Stark R., Morgen K., Vaitl D. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Hum. Brain Mapp. 2007;28:793–803. doi: 10.1002/hbm.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Sebastian R., Gomez Y., Leigh R., Davis C., Newhart M., Hillis A.E. The roles of occipitotemporal cortex in reading, spelling, and naming. Cogn. Neuropsychol. 2014;31:511–528. doi: 10.1080/02643294.2014.884060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum J., Hermes D., Foster B.L., Dastjerdi M., Rangarajan V., Winawer J., Miller K.J., Parvizi J. A brain area for visual numerals. J. Neurosci. 2013;33:6709–6715. doi: 10.1523/JNEUROSCI.4558-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegler R.S. Strategy choice procedures and the development of multiplication skill. J. Exp. Psychol. Gen. 1988;117:258–275. doi: 10.1037//0096-3445.117.3.258. [DOI] [PubMed] [Google Scholar]
- Simmons F.R., Singleton C. Do weak phonological representations impact on arithmetic development? A review of research into arithmetic and dyslexia. Dyslexia Chichester Engl. 2008;14:77–94. doi: 10.1002/dys.341. [DOI] [PubMed] [Google Scholar]
- Simmons F., Singleton C., Horne J. Brief report—phonological awareness and visual-spatial sketchpad functioning predict early arithmetic attainment: evidence from a longitudinal study. Eur. J. Cogn. Psychol. 2008;20:711–722. [Google Scholar]
- Simon O., Mangin J.-F., Cohen L., Le Bihan D., Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Sokolowski H.M., Fias W., Ononye C., Ansari D. Are numbers grounded in a general magnitude processing system? A functional neuroimaging meta-analysis. Neuropsychologia. 2017 doi: 10.1016/j.neuropsychologia.2017.01.019. [DOI] [PubMed] [Google Scholar]
- Stanescu-Cosson R., Pinel P., van De Moortele P.F., Le Bihan D., Cohen L., Dehaene S. Understanding dissociations in dyscalculia: a brain imaging study of the impact of number size on the cerebral networks for exact and approximate calculation. Brain J. Neurol. 2000;123(Pt 11):2240–2255. doi: 10.1093/brain/123.11.2240. [DOI] [PubMed] [Google Scholar]
- Starrfelt R., Behrmann M. Number reading in pure alexia—A review. Neuropsychologia. 2011;49:2283–2298. doi: 10.1016/j.neuropsychologia.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Supekar K., Menon V. Developmental maturation of dynamic causal control signals in higher-Order cognition: a neurocognitive network model. PLoS Comput. Biol. 2012;8:e1002374. doi: 10.1371/journal.pcbi.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. 1st edition. Thieme, Stuttgart; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Tan L.H., Laird A.R., Li K., Fox P.T. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta-analysis. Hum. Brain Mapp. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.S.H., Rastle K., Davis M.H. Interpreting response time effects in functional imaging studies. Neuroimage. 2014;99:419–433. doi: 10.1016/j.neuroimage.2014.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E., Poldrack R.A., Salidis J., Deutsch G.K., Tallal P., Merzenich M.M., Gabrieli J.D. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Tham W.W.P., Rickard Liow S.J., Rajapakse J.C., Choong Leong T., Ng S.E.S., Lim W.E.H., Ho L.G. Phonological processing in Chinese-English bilingual biscriptals: an fMRI study. Neuroimage. 2005;28:579–587. doi: 10.1016/j.neuroimage.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Torgesen J.K., Wagner R.K., Rashotte C.A. Longitudinal studies of phonological processing and reading. J. Learn. Disabil. 1994;27:276–286. doi: 10.1177/002221949402700503. [DOI] [PubMed] [Google Scholar]
- Tschentscher N., Hauk O. How are things adding up? Neural differences between arithmetic operations are due to general problem solving strategies. Neuroimage. 2014;92:369–380. doi: 10.1016/j.neuroimage.2014.01.061. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Coslett H.B. Localization of sublexical speech perception components. Brain Lang. 2010;114:1–15. doi: 10.1016/j.bandl.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P.E., Gareau L., Flowers D.L., Zeffiro T.A., Eden G.F. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eickhoff S.B., Laird A.R., Fox M., Wiener M., Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V., Ansari D., Chee M.W.L. Neural correlates of symbolic and non-symbolic arithmetic. Neuropsychologia. 2005;43:744–753. doi: 10.1016/j.neuropsychologia.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Hervé P.Y., Duffau H., Crivello F., Houdé O., Mazoyer B., Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Stevens C., Dow M., Harn B.A., Chard D.J., Neville H.J. Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: an fMRI study. NeuroImage Special Issue: Educ. Neurosci. 2011;57:704–713. doi: 10.1016/j.neuroimage.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarian L., Ischebeck A., Delazer M. Neuroscience of learning arithmetic—evidence from brain imaging studies. Neurosci. Biobehav. Rev. 2009;33:909–925. doi: 10.1016/j.neubiorev.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Zarnhofer S., Braunstein V., Ebner F., Koschutnig K., Neuper C., Reishofer G., Ischebeck A. The influence of verbalization on the pattern of cortical activation during mental arithmetic. Behav. Brain Funct. 2012;8:13. doi: 10.1186/1744-9081-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Chen C., Zang Y., Dong Q., Chen C., Qiao S., Gong Q. Dissociated brain organization for single-digit addition and multiplication. Neuroimage. 2007;35:871–880. doi: 10.1016/j.neuroimage.2006.12.017. [DOI] [PubMed] [Google Scholar]
- van der Mark S., Bucher K., Maurer U., Schulz E., Brem S., Buckelmüller J., Kronbichler M., Loenneker T., Klaver P., Martin E., Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. Neuroimage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van der Ven F., Takashima A., Segers E., Fernandez G., Verhoeven L. Non-symbolic and symbolic notations in simple arithmetic differentially involve intraparietal sulcus and angular gyrus activity. Brain Res. 2016;1643:91–102. doi: 10.1016/j.brainres.2016.04.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.