Abstract
Brain imaging studies on academic achievement offer an exciting window on experience-dependent cortical plasticity, as they allow us to understand how developing brains change when children acquire culturally transmitted skills. This contribution focuses on the learning of arithmetic, which is quintessential to mathematical development. The nascent body of brain imaging studies reveals that arithmetic recruits a large set of interconnected areas, including prefrontal, posterior parietal, occipito-temporal and hippocampal areas. This network undergoes developmental changes in its function, connectivity and structure, which are not yet fully understood. This network only partially overlaps with what has been found in adults, and clear differences are observed in the recruitment of the hippocampus, which are related to the development of arithmetic fact retrieval. Despite these emerging trends, the literature remains scattered, particularly in the context of atypical development. Acknowledging the distributed nature of the arithmetic network, future studies should focus on connectivity and analytic approaches that investigate patterns of brain activity, coupled with a careful design of the arithmetic tasks and assessments of arithmetic strategies. Such studies will produce a more comprehensive understanding of how the arithmetical brain unfolds, how it changes over time, and how it is impaired in atypical development.
Keywords: Arithmetic, Children, Development, Brain imaging, Dyscalculia, fMRI, DTI, Fact retrieval, Mathematics
1. Introduction
Brain imaging studies on academic achievement offer an exciting window on experience-dependent cortical plasticity, as they allow us to understand how developing brains change when children acquire culturally transmitted skills, such as reading or arithmetic (Dehaene and Cohen, 2007). This contribution focuses on the learning of arithmetic, i.e. the ability to add, subtract, multiply and divide symbolic whole numbers. This skill constitutes a major element of the mathematics curriculum in primary school (National Mathematics Advisory Panel, 2008) and has a quintessential role in mathematical development (Kilpatrick et al., 2001) for children around the globe. There are large individual differences at the behavioral (Dowker, 2005, Vanbinst and De Smedt, 2016, for a review) and neural levels in this basic competence, even in adulthood (Grabner et al., 2007). On the other hand, persistent deficits in learning arithmetic constitute the hallmark of dyscalculia, a specific neurodevelopmental learning disorder that is characterized by life-long difficulties in calculation that are not merely explained by intellectual disabilities, uncorrected sensory problems, mental or neurological disorders or inadequate instruction (American Psychiatric Association, 2013).
This contribution starts with a succinct discussion of children’s arithmetic development and its supporting cognitive competencies, as well as a brief summary of brain imaging studies in adults. These two sections are short and only provide a lens through which we subsequently discuss the available neural data in children. We systematically review functional brain imaging studies in typically and atypically developing populations and provide an overview of connectivity studies. We also discuss structural brain imaging data that have correlated variability in arithmetic performance with individual differences in white and grey matter properties. This review ends with challenges and outstanding issues that should be considered in future studies.
2. Arithmetic development is characterized by strategy change
Decades of cognitive developmental research have investigated the acquisition of arithmetic and this development involves a change in the mix of strategies that are used to calculate the answer to a particular problem (Geary, 2011, Jordan et al., 2003, Siegler, 1996; for reviews). Already before the start of formal schooling, children use counting to solve simple sums. These counting strategies are initially executed with additional support, such as manipulatives or fingers, yet progressively, children execute these strategies without external aids (verbal counting). The efficiency of these counting strategies increases rapidly with grade, where children move from counting all sets in their entirety to counting from the first (counting-on) or larger (counting-on-larger) number (Geary et al., 1992). The repeated use of these counting routines allows children to develop associations between problems and their answers, arithmetic facts, which are stored in long-term memory. The acquisition of these facts is important because fact retrieval is more efficient and it consumes less working memory than the more cognitively demanding and error-prone procedures, such as counting. The availability of arithmetic facts also allows children to use these facts to decompose problems into smaller problems, such as 7 + 8 =, 7 + 3 = 10, 10 + 5 = 15. These decomposition strategies usually occur in problems with larger numbers (typically when they cross 10) and, evidently, in multi-digit calculations. They are used more often during addition and subtraction − albeit more frequently in subtraction than in addition (Barrouillet et al., 2008) − but they are much less used in multiplication, in which fact retrieval is the most dominant strategy from an early point on in development, i.e. second grade (Imbo and Vandierendonck, 2007, Lemaire and Siegler, 1995). This is because multiplications are typically learned by extensive (rote) training of the multiplication tables rather than by decomposing the problem in its smaller sub-parts, as is often the case in subtraction. On the other hand, multiplication and addition are commutative operations (e.g. 6 × 4 = 4 × 6), in contrast to division and subtraction. This commutativity might facilitate the formation of problem-answer associations in long-term memory for multiplication and addition, for which reason they are more often solved via fact retrieval. Surprisingly little is known about the development of division, but the available evidence suggests that its strategies follow a somewhat different developmental trajectory (Robinson et al., 2006). Because, to the best of our knowledge, there are no developmental brain imaging studies on division, this operation is not considered further.
The development of these strategies is not an abrupt shift from one strategy to the other but rather a change in the frequency distributions of strategies children use, the so-called overlapping waves theory (Siegler, 1996). This theory posits that strategies remain available over development, even in adulthood (LeFevre et al., 1996), but that the frequency in their use changes at different time points, with the more efficient strategies, such as fact retrieval, becoming more dominant. This change is also accompanied by changes in brain activity, as we will review below. Interestingly, similar strategy shifts have been documented in the learning of other academic domains (Siegler, 1996). For example, in word reading, children move towards an increased reliance on efficient orthographic direct recognition coupled with a decreased reliance on phonological decoding (Schlagger and McCandliss, 2007) and the changes in brain structure and function that accompany this (a)typical development have been described (Eden et al., 2016 for a review).
The acquisition of these strategies is supported by additional cognitive competencies that can be characterized as domain-specific, i.e. specifically relevant for learning arithmetic but not for other academic skills, or domain-general, i.e. relevant to learning other academic skills, such as reading, or to learning in general, such as working memory (e.g., Geary and Moore, 2016, Vanbinst and De Smedt, 2016 for a review). A detailed review of these factors is beyond the scope of this paper, but we briefly highlight some of them to frame the subsequent brain imaging data.
One domain-specific factor that has received a lot of attention in studies on individual differences in arithmetic is the ability to process numerical magnitudes (De Smedt et al., 2013, Schneider et al., 2017, for a meta-analysis). It turns out that specifically the ability to process symbolic numerical magnitudes is uniquely related, cross-sectionally (Vanbinst et al., 2012) and predictively (Vanbinst et al., 2016, Vanbinst et al., 2015a, Vanbinst et al., 2015b), to children’s arithmetic strategy use and their increasing reliance on fact retrieval. These associations are not limited to addition and subtraction, but are also observed in multiplication (De Visscher and Noël, 2016, Schleepen et al., 2016).
The fact that symbolic numerical magnitude processing is key to arithmetic development has been connected to the observation that the intraparietal sulcus (IPS) is consistently active whenever people calculate. Indeed, increases in IPS-activity during calculation have been frequently interpreted to reflect the processing of numerical magnitude (e.g., Ansari, 2008; Menon, 2015). On the other hand, atypical IPS structure (e.g., Isaacs et al., 2001) or function (e.g., Price et al., 2007) has been suggested to represent the neural origin of dyscalculia, and these are assumed to reflect poor numerical magnitude processing, which is seen as the core deficit in dyscalculia that cascades into impairments in arithmetic (e.g., De Smedt et al., 2013, Rubinsten and Henik, 2006). This recent emphasis on particularly symbolic numerical magnitude processing has somewhat mistakenly narrowed down the attention to the IPS in studying brain activity during arithmetic (see Fias et al., 2013, Menon, 2015; for critical analyses). Indeed, arithmetic tasks typically recruit a large set of bilateral regions including the dorsolateral (DLPFC) and ventrolateral prefrontal cortex (VLPFC), anterior cingulate (ACC), temporo-parietal cortex (angular (AG) and supramarginal gyri (SMG)), the occipito-ventral cortex (including fusiform gyrus (FG)) and the medial temporal lobe (Arsalidou and Taylor, 2011, Menon, 2016). This suggests the involvement of domain-general processes as well, and behavioral studies have already confirmed that working memory (Peng et al., 2016), executive functions (Bull and Lee, 2014), interference control (De Visscher et al., 2015), phonological processing (De Smedt et al., 2010, Hecht et al., 2001) and retrieval from long-term memory (Garnett and Fleischner, 1983) are uniquely related to individual differences in arithmetic. In all, these data suggest that both domain-specific and domain-general factors should be considered when studying brain activity during arithmetic and that such analysis should not be restricted to the parietal cortex.
We end this section with a brief discussion on how arithmetic strategies are measured (De Smedt, 2016, for a more elaborate discussion). In behavioral research, this mix of strategies has typically been measured through verbal report data (e.g., Campbell and Xue, 2001, Imbo and Vandierendonck, 2007, Siegler, 1996) in which children have to verbally indicate on a trial-by-trial basis which strategy they used to solve the problem. Responses can be reliably and validly classified into categories (Siegler and Stern, 1998), such as retrieval (the child immediately knew the answer with no overt signs of calculations) or procedures (the child counted or decomposed the problem into smaller problems). The collection of verbal report data is quite difficult in brain imaging studies. Some adult studies have analyzed brain activity as a function of verbally reported strategy (Grabner et al., 2009, Grabner and De Smedt, 2011, Tschentscher and Hauk, 2014), but such trial-by-trial strategy data have not been reported in children. For this reason, brain imaging studies have typically used designs in which they compared brain responses of sets of problems on which the use of a particularly strategy was expected on the basis of specific characteristics of the problem, i.e. its size (De Smedt et al., 2011), complexity (Ashkenazi et al., 2012) or operation (Prado et al., 2014). This approach has been criticized (De Smedt, 2016, Siegler, 1987): Not all problems of a given type are solved by the same strategy and aggregating across problem types leads to misleading conclusions (Siegler, 1987). This is especially true for developmental research and studies in atypical populations: Children will differ in their mix of strategies for a given problem type or operation, depending on their age, education or ability level (typical vs. atypical). Therefore, verbal report data may be the most optimal way to study brain activity during arithmetic in developing populations. On a related note, electrophysiological data in adults (Grabner and De Smedt, 2011) have shown that such verbal report data correlate with differences in brain activity, which are not revealed when focusing on problem size or operation alone.
3. Adult brain imaging data
The vast majority of research on the neural correlates of arithmetic has been performed in adults. Studies in adults date back to the description of patients with lesions in the (left) parietal cortex (Gerstmann, 1940, Henschen, 1919) that were accompanied by difficulties in performing arithmetic. Summarizing a series of patients, Dehaene and Cohen (1995) postulated the triple-code model, which included three numerical codes that support different arithmetical processes, including arithmetic fact retrieval (verbal code, located in the left AG) and the execution of (magnitude-based) procedures (magnitude code, situated in the IPS). Conventional non-invasive fMRI methods subsequently allowed to further delineate this arithmetic network, revealing repeatedly that a large, whole-brain network is active when adults perform arithmetic (Arsalidou and Taylor, 2011, Menon, 2015). This network includes the bilateral posterior parietal cortex, inferior and superior prefrontal cortex (PFC), and occipito-temporal regions. Activity in this network is modulated by the arithmetic operation (Rosenberg-Lee et al., 2011), strategy use (Grabner et al., 2009, Tschentscher and Hauk, 2014), expertise (Grabner et al., 2007) and training (Zamarian et al., 2009).
Consistent across these data is the activation of the bilateral IPS during arithmetic, potentially reflecting the role of numerical magnitude processing during calculation (Arsalidou and Taylor, 2011). It also has been suggested that activity in this area is higher for subtractions, large problems and during the execution of procedural strategies. Activity in the temporo-parietal cortex (AG and SMG), has been typically associated with the retrieval of arithmetic facts from long-term memory. Increases in brain activity in this area are usually observed in multiplication and correlate with mathematical expertise (Grabner et al., 2007). Originally, this temporo-parietal activity was thought to reflect the involvement of phonological processes in fact retrieval and multiplication. This interpretation has been questioned (Menon, 2015) and recent data by De Visscher et al. (2015) suggest that it rather reflects the automatic mapping between an arithmetic problem and its answer in long-term memory. Increases in activity in the lateral PFC cortex have been typically attributed to the involvement of auxiliary cognitive functions that are crucial during calculation, such as working memory, inhibitory control, and attentional processes (Arsalidou and Taylor, 2011) and these regions are typically more recruited during more demanding problems, such as larger problems, and during the execution of procedural or back-up strategies, when the answer cannot be retrieved from long-term memory. Finally, occipito-temporal regions, including the FG, are involved in the visual processing of symbolic numerical information, given that arithmetic stimuli represent visual symbols (Arsalidou and Taylor, 2011), but the specific role of this region in arithmetic has not been studied in much detail (Peters et al., 2015).
Training studies in adults that have tried to simulate the above-mentioned developmental process of strategy change arithmetic, in particular the development from procedures to arithmetic fact retrieval (Zamarian and Delazer, 2015 for a review). These studies offer a window to our understanding of how arithmetic networks change across skill acquisition. These data revealed, as a function of training, a decrease in PFC coupled with an increase in activity in the posterior parietal cortex. At the same time, activity in the parietal cortex shifts from the IPS to the AG, potentially reflecting the increasing reliance on retrieval strategies and a decreasing reliance on backup strategies, such as counting or decomposition, which is in line the overlapping waves model of strategy development (Siegler, 1996). These data offer insights in how brain activity changes as a function of learning, but these studies in adults are not necessarily directly transferable to children, because children’s brains are not merely smaller versions of a highly skilled adult brain (Ansari, 2010). Indeed, adult studies miss important changes in the functional organization of brain networks during schooling (Qin et al., 2014, Rosenberg-Lee et al., 2015) and therefore, studies in developmental populations, as we review below, are crucial to understand the neurophysiological changes that are associated with developmental changes in arithmetic strategy use.
4. Review method
We performed a search of the literature in September 2016 in the Web of Science database with the keywords “arithmetic*”, “children”, and “*MRI”, “DTI”, “EEG”, “NIRS”, or “MEG”. Studies were only included if they reported original empirical data, comprised multiple participants, collected brain imaging measures and if they were reported in an peer reviewed English-language journal. Relevant articles were further added by screening the references and by reviewing citation lists of the articles obtained from the Web of Science search that met our inclusion criteria. This resulted in a final set of studies that summarized in Table 1, Table 2, Table 3, Table 4, Table 5.
Table 1.
Summary of studies on brain activity during arithmetic in children.
| Study | Method | N | Mage | Age range | Sample | Analysis | Task | Conditions | Additional covariates | Main findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Kawashima et al. (2004) | fMRI | 8 8 |
11.6 44.1 |
9–14 40–49 |
TD | Uni WB |
+, −, x | Children ≈ adults PFC, IPS and OT |
||
| 2. | Rivera et al. (2005) | fMRI | 17 | 13.7 | 8–19 | TD | Uni WB |
+, − | Age | SMG, IPS ↑with age PFC, HC ↓ with age |
|
| 3. | Rocha et al. (2005) | EEG | 20 24 20 |
7.6 8.4 28 |
? ? ? |
TD | Uni | Arithmetic | Distinct patterns of neuronal recruitment for different operations | ||
| 4. | Xuan et al. (2007) | ERP | 38 26 |
9 23 |
8–9 22–25 |
TD | Uni | Arithmetic | Age-related differences in frontal negativity and parietal ERPs | ||
| 5. | Kucian et al. (2008) | fMRI | 10 10 20 |
9.2 12.0 27.2 |
? | TD | Uni WB |
+ | Exact Approximate |
Lowered IFG and IPS activity in children | |
| 6. | Simos et al. (2008) | MEG | 25 14 16 |
10.4 9.7 10.2 |
8–14 8–11 8–12 |
TD DC DLDC |
Uni WB |
+ | DC had ↑ parietal and PFC than DLDC and TD | ||
| 7. | Davis et al. (2009a) | fMRI | 27 10 |
8.1 30.7 |
7–9 25–49 |
TD | Uni WB |
+ | Exact Approximate |
↑ fronto-parietal areas Adults > children |
|
| 8. | Davis et al. (2009b) | fMRI | 24 24 |
8.2 | 8–9 | TD DC |
Uni WB |
+ | Exact Approximate |
Frontal and parietal hyper-activation in DC | |
| 9. | Dresler et al. (2009) | NIRS | 46 44 |
10.0 13.9 |
9–10 13–14 |
TD | Uni WB |
+ | Digits Word problems |
↑ fronto-parietal areas No age- or format effects |
|
| 10. | Meintjes et al. (2010) | fMRI | 14 | 10.5 | 8–12 | TD | Uni WB |
+ | ↑ fronto-parietal areas | ||
| 11. | Prieto-Corona et al. (2010) | ERP | 16 18 |
10.3 26.11 |
9–12 ? |
TD | Uni | x | Differences in N400 and LPC in adults vs. children during retrieval | ||
| 12. | Cho et al. (2011) | fMRI | 19 17 |
8.2 8.6 |
7–9 7–9 |
Retrievers Counters |
Uni WB Multi |
+ | Retrievers had ↑ L-VLPFC, Distinct neural patterns for retrievers vs. counters in HC, L-VLPFC and PPC |
||
| 13. | De Smedt et al. (2011) | fMRI | 8 10 |
11.9 11.7 |
10–12 10–12 |
LAF TAF |
Uni WB |
+, − | Small Large |
Large: ↑ fronto-parietal Small: ↑ L-HC LAF had ↑ R-IPS for small |
|
| 14. | Kesler et al. (2011) | fMRI | 15 | 10.9 | 7–14 | Turner Syndrome | Uni WB |
+, −, x | Single-digit Two-digit |
Number sense training | Training effects: ↑ superior parietal ↓ in PFC and HC |
| 15. | Mondt et al. (2011) | fMRI | 24 | 9.6 | 7–11 | Bilinguals | Uni WB |
+, − | Language instruction | Instruction in school-language: ↑ left-lateralized and more focal activation | |
| 16. | Rosenberg-Lee et al. (2011) | fMRI | 45 45 |
7.7 8.7 |
7-8 7-9 |
TD | Uni WB |
+ | Simple (+1) Complex |
↑ with grade: Parietal, DLPFC ↓ with grade: Ventro-medial PFC |
|
| 17. | Zhou et al. (2011) | ERP | 22 22 |
7.8 21.5 |
7–8 18–26 |
TD | Uni | +, x | Both groups showed frontal and parietal N400 Adults vs. children: ↑ left frontal N400 Children vs. adults: ↑ right parietal N400 |
||
| 18. | Ashkenazi et al. (2012) | fMRI | 17 17 |
8.12 8.17 |
7–9 7–9 |
TD DC |
Uni WB Multi |
+ | Simple (+ 1) Complex |
Hypo-activation in DC in PFC, right PPC and OT Activation patterns between complex and simple less differentiated in DC in IPS |
|
| 19. | Cho et al. (2012) | fMRI | 86 | 8.2 | 7–9 | TD | Uni WB |
+ | Retrieval fluency | ↑ retrieval fluency correlates ↑ HC, lingual gyri, FG, superior parietal | |
| 20. | Price et al. (2013) | fMRI | 33 | 17.9 | 17–18 | TD | Uni WB |
+, − | Math competence (PSAT) | R-IPS is negatively correlated with PSAT L-SMG is positively correlated with PSAT |
|
| 21. | Berteletti et al. (2014) | fMRI | 20 | 11.6 11.5 |
8–13 8–13 |
TD DC |
Uni ROI |
x | Hypo-activation in DC in left IFG, MTG and right PSPL and IPS | ||
| 22. | Evans et al. (2014) | fMRI | 20 | 10.2 10.4 |
9–11 9–11 |
TD DL |
Uni WB |
+, − | ↓ L-SMG in DL Atypical right inferior parietal modulation in DL |
||
| 23. | Moore et al. (2014) | ERP | 20 | 9.9 10.1 |
9–10 9–10 |
High fit Low fit |
Uni | + | Small Large |
Effects of problem size on P1, N170, P3, N400 Fitness modulates ERPs |
|
| 24. | Prado et al. (2014) | fMRI | 34 | 11.1 | 8–13 | TD | Uni ROI |
−, x | Small Large |
Age-related increases: L MTG in multiplication R PSPL in subtraction Age-related decreases: L IFG in multiplication |
|
| 25. | Qin et al. (2014) | fMRI | 28 20 20 |
8.3 15.61 20.50 |
7–9 14–17 19–20 |
TD | Uni WB |
+ | Two time points in children | Children had higher HC than adolescents and adults Longitudinal data: ↑ in HC ↓ in DLPFC, SPL and OT |
|
| 26. | Van Beek et al., 2014a, Van Beek et al., 2014b | ERP | 22 | 11.9 | 11–12 | TD | Uni | + | Small Large |
Problem size effect on N2 and LPC | |
| 27. | Vourkas et al. (2014) | EEG | 20 31 |
9.9 9.7 |
8–9 8–9 |
LAF HAF |
Uni | +, − | Task difficulty effect in EEG patterns Theta band shows more distributed processing Alpha band had greater network integration |
||
| 28. | Berteletti et al. (2015) | fMRI | 39 | 11.3 | 8–13 | TD | Uni WB |
− | Number line estimation | Negative association between IPS activity and number line estimation | |
| 29. | Chang et al. (2015) | fMRI | 28 28 |
8.9 20.4 |
7–10 19–22 |
TD | Uni WB Multi |
+, − | Adults had larger representational similarity between problems in IPS, PSPL, DLPFC and ventral OT | ||
| 30. | Dimitriadis et al. (2015) | EEG | 20 25 |
? ? |
8–12 21–26 |
TD | Uni | Arithmetic comparison | Increased functional segregation of network organization younger children | ||
| 31. | Iuculano et al. (2015) | fMRI | 15 15 |
8.5 8.7 |
7–9 7–9 |
TD DC |
Uni WB Multi |
+ | Math tutoring | Pre-tutoring: Hyper-activation in DC in PFC, parietal and HC Classifier discriminates TD and DC Post-tutoring: DC = TD Classifier does not discriminate |
|
| 32. | Rosenberg-Lee et al. (2015) | fMRI | 20 16 |
8.44 8.34 |
7–9 7–9 |
TD DC |
Uni WB |
+, − | Simple (+ 1) Complex |
Hyper-activation in DC in PFC, parietal and OT | |
| 33. | Van Beek et al. (2015) | ERP | 16 16 |
10.7 10.8 |
7–12 7–12 |
TD mTBI |
Uni | + | Small Large |
Smaller LPC in mTBI | |
| 34. | Chang et al. (2016) | fMRI | 25 19 26 |
8.8 15.7 20.6 |
7–10 13–17 19–22 |
TD | Uni ROI |
− | IPS activity increases with age Posterior AG shows no age effects |
||
| 35. | Demir-Lira et al. (2016) | fMRI | 33 | 10.9 | 8–13 | TD | Uni ROI |
− | SES | SES moderates effect of math fluency on R-IPS and L-MTG activity | |
| 36. | Evans et al. (2016) | fMRI | 30 | ? | 7–29 | TD | Uni WB |
+, − | Age | Age–related increases in R-HC and L-MFG for + but not − Age-related increases in R-temporo-parietal |
|
| 37. | Kim et al. (2016) | EEG | 44 53 |
? ? |
6–17 6–17 |
TD ADHD |
Uni | − | Theta-phase-gamma-amplitude coupling was reduced in ADHD vs. TD | ||
| 38. | Peters et al. (2016) | fMRI | 22 | 10.7 | 9–12 | TD | Uni WB |
− | Subtraction with dots, digits and number words | Dots ↑ Fronto-parietal Digits/Number words ↑ AG, SMG |
Note: ADHD = attention deficit hyperactivity disorder. DC = dyscalculia. DL = dyslexia. DLDC = comorbid dyslexia/dyscalculia. HAF = High aritmetical fluency; LAF = low arithmetical fluency. L = Left. LPC = late positive component. mTBI = mild traumatic brain injury. Multi = Multivariate fMRI analysis. OT = occipito-temporal areas. PPC = Posterior Parietal Cortex. PSAT = Preliminary Scholastic Aptitude test. R = Right. ROI = region of interest approach. TAF = typical arithmetic fluency. TD = Typically developing children. Uni = Univariate fMRI analysis. WB = whole brain analysis.
Table 2.
Summary of studies on task-based functional connectivity during arithmetic in children.
| Study | N | Mage | Age range | Sample | Task | Main findings | |
|---|---|---|---|---|---|---|---|
| 1. | Rosenberg-Lee et al. (2011) | 45 45 |
7.7 8.7 |
7–8 7–9 |
TD | + | ↑ connectivity between PFC, parietal, OT and parahippocampal areas in older children |
| 2. | Cho et al. (2012) | 86 | 8.2 | 7–9 | TD | + | ↑ connectivity between R-HC and PFC with increasing age and ability level |
| 3. | Qin et al. (2014) | 28 20 20 |
8.3 15.61 20.50 |
7–9 14–17 19–20 |
TD | + | ↑HC connectivity with PFC with increasing age HC connectivity with frontal and parietal areas correlates with growth in arithmetic |
| 4. | Rosenberg-Lee et al. (2015) | 20 16 |
8.44 8.34 |
7–9 7–9 |
TD DC |
+, − | Hyper-connectivity between IPS and PFC in DC |
| 5. | Chang et al. (2016) | 25 19 26 |
8.8 15.7 20.6 |
7–10 13–17 |
TD | − | Connectivity between left anterior SMG and PFC shows a non-linear association with age |
Note: DC = dyscalculia.
Table 3.
Summary of studies on resting-state functional connectivity and its association with arithmetic in children.
| Study | N | Mage | Age range | Sample | Behavioral measures | Main findings | |
|---|---|---|---|---|---|---|---|
| 1. | Supekar et al. (2013) | 24 | 8.5 | 8–9 | TD | Gains in on single-digit addition after math tutoring | HC- PFC connectivity at pretest shows positive association intervention gains |
| 2. | Evans et al. (2015) | 20 | ? | 7–9 | TD | WIAT– Arithmetic | Connectivity between DLPFC, posterior parietal, OT and anterior temporal regions predicts arithmetic ability |
| 3. | Jolles et al. (2016a) | 19 19 |
8.8 8.9 |
7–9 7–9 |
TD DC |
Hyper-connectivity in DC between IPS and other parietal, frontal and occipito-temporal areas, Increased connectivity between left and right IPS in DC, Connectivity patterns differentiate TD and DC |
|
| 4. | Jolles et al. (2016b) | 21 21 |
8.6 9.1 |
7–9 7–9 |
TD | Gains in on single-digit addition after math tutoring | Intervention increases IPS intrinsic connectivity with PFC, ventral OT and HC. Increases in IPS connectivity were positively correlated with performance gains in the intervention. |
Note: DC = dyscalculia. WIAT = Wechsler Individual Achievement Test.
Table 4.
Summary of DTI studies that correlate white matter structure and arithmetic.
| Study | N | Mage | Age range | Sample | Behavioral measures | Main findings | |
|---|---|---|---|---|---|---|---|
| 1. | Barnea-Goraly et al. (2005) | 19 19 |
14.4 12.2 |
7–19 7–19 |
TD VCFS |
Arithmetic (WISC) | FA of tracts adjacent to SMG, AG and IPS positively correlated with arithmetic |
| 2. | van Eimeren et al. (2008) | 13 | 8.4 | 7–9 | TD | Numerical operations (WIAT) Mathematical reasoning (WIAT) |
FA in L-SCR and ILF positively associated with arithmetic |
| 3. | Pavlova et al. (2009) | 11 | 14.6 | 13–16 | PPVL | Mental calculation | No association between connectivity and mental calculation |
| 4. | Rykhlevskaia et al. (2009) | 24 23 |
8.8 8.9 |
7–9 7–9 |
TD DC |
Lowered FA of ILF and IFOF in DC | |
| 5. | Tsang et al. (2009) | 25 | 12.6 | 10–15 | TD | Approximate addition | FA of anterior SLF positively correlated with approximate addition |
| 6. | Lebel et al. (2010) | 21 | 9.2 | 5–13 | FASD | Quantitative concepts (Woodcock Johnson–III) |
FA in left parietal SLF positively correlated with math scores |
| 7. | Till et al. (2011) | 31 | 16.4 | 11–19 | MS | Numerical operations (WIAT) |
FA in corpus callosum and in right frontal and parietal areas positively correlated with arithmetic ability |
| 8. | Li et al. (2013) | 47 | 10.5 | 9–11 | TD | Arithmetic (WISC) | FA in left SLF and ILF and bilateral IFOF positively correlated with arithmetic |
| 9. | Matejko et al. (2013) | 30 | 18.0 | 17–18 | TD | PSAT | FA in left parietal areas (SLF, superior CR, cortico-spinal tract) positively correlated with math scores |
| 10. | Ranpura et al. (2013) | 11 11 |
? ? |
8–14 8–14 |
TD DC |
Reduced FA in temporo-parietal areas in DC | |
| 11. | Van Beek et al. (2014a) | 18 | 12.0 | 11–12 | TD | Addition, subtraction, multiplication and division | FA of left anterior AF positively correlated with addition and multiplication but not with subtraction and division |
| 12. | Jolles et al. (2016c) | 18 | ? | 7–9 | TD | Gains in addition and subtraction performance after math tutoring | Changes in left SLF associated with performance gains after tutoring |
Note: DC = Dyscalculia. FA = Fractional Anisotrophy. FASD = Fetal alcohol spectrum disorder. IFOF = Inferior Frontal-Occipital Fasciculus. MS = multiple sclerosis. PPVL = Prematurely born with periventricular lesions. PSAT = Preliminary Scholastic Achievement Test.TD = Typically developing. VCFS = Velocardiofacial syndrome. WIAT = Wechsler Individual Achievement Test. WISC = Wechsler Intelligence Scale for Children.
Table 5.
Summary of structural brain imaging studies that correlate brain structure and arithmetic.
| Study | N | MAge | Age range | Sample | Method | Behavioral measure | Main finding | |
|---|---|---|---|---|---|---|---|---|
| 1. | Isaacs et al. (2001) | 12 12 |
15.7 15.8 |
? ? |
VLBW-NC VLBW-C |
GMV | Reduced GMV in VLBW-C in L IPS | |
| 2. | Rotzer et al. (2008) | 12 12 |
9.7 9.3 |
? ? |
TD DC |
GMV WMV |
Reduced GMV in DC in IPS, MFG Reduced WMV in DC in Left PFC |
|
| 3. | Rykhlevskaia et al. (2009) | 24 23 |
? ? |
7–9 7–9 |
TD DC |
GMV WMV |
Reduced GMV in DC in IPS, FG, HC Reduced WMV in DC in right temporo-parietal cortex |
|
| 4. | Han et al. (2013) | 20 20 |
10.9 10.8 |
? ? |
LA HA |
Anatomical variations | Anatomical differences between LA and HA in OT, orbitofrontal and insular areas | |
| 5. | Li et al. (2013) | 59 | 10.5 | 9–11 | TD | GMV | Arithmetic (WISC) |
GMV in left IPS positively correlated with arithmetic |
| 6. | Ranpura et al. (2013) | 11 11 |
? ? |
8–14 8–14 |
TD DC |
GMV WMV |
Reduced GMV in DC in posterior parietal cortex. | |
| 7. | Supekar et al. (2013) | 24 | 8.5 | 8–9 | TD | GMV | Single–digit addition after math tutoring | Pre-tutoring GMV in right HC predicts intervention gains |
| 8. | Evans et al. (2015) | 43 | ? | 7–9 | TD | GMV | Numerical Operations (WIAT) |
GMV in posterior parietal areas and ventral OT predict gains in arithmetic |
| 9. | Price et al. (2016) | 50 | 7.4 | 6–8 | TD | GMV | Woodcock Johnson −III Math Composite | GMV in left IPS predicts arithmetic |
Note: DC = dyscalculia. GMV = Grey Matter Volume. HA = high arithmetic achievement. LA = low arithmetic achievement. VLBW-NC = very low birth weight − no calculation deficits. VLBW-C = very low birth weight − calculation deficits. WIAT = Wechsler Individual Achievement Test. WISC = Wechsler Intelligence Scale for Children. WMV = White Matter Volume.
5. Brain activity during arithmetic in children
Most of the existing studies have used fMRI to investigate brain activity in children (Table 1; Fig. 1). Only a few studies have used other imaging methods (NIRS: n = 1; MEG: n = 1; EEG: n = 6; ERP: n = 6) and their findings will be only very briefly covered in this section.
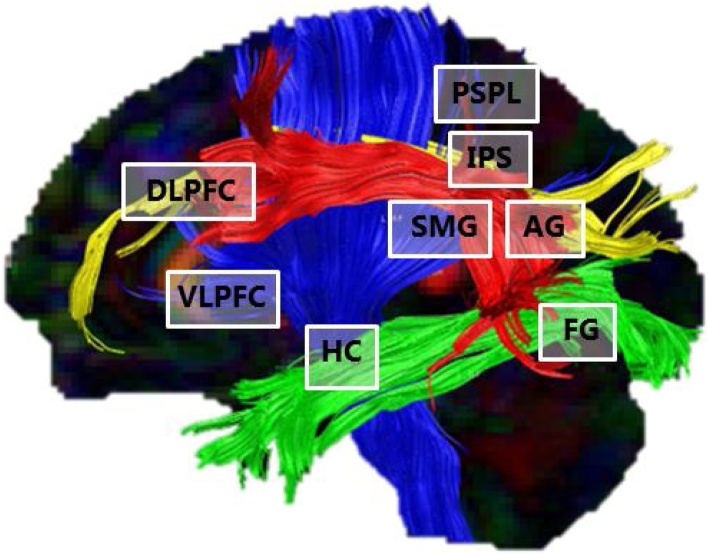
Fig 1.
Sagittal slice showing the arithmetic network. The white boxes indicate the most relevant areas implicated in arithmetic, including DLPFC = dorsolateral prefrontal cortex, VLPFC = ventrolateral prefrontal cortes, HC = hippocampus, PSPL = posterior superior parietal lobe, IPS = intraparietal sulcus, SMG = supramarginal gyrus, AG = angular gyrus and FG = fusiform gyrus. The colored tracts represent the most relevant white matter connections as revealed via spherical deconvolution analysis of DTI data. Yellow = Superior longitudinal fasciculus (SLF); Red = Arcuate fasciculus (AF); Blue = Corona radiata (CR); Green = Inferior longitudinal fasciculus (ILF).
5.1. Typically developing children
fMRI studies in typically developing children have reported that, as in adults, a widespread, bilateral network of areas shows increases in brain activity during arithmetic (Fig. 1; Kaufmann et al., 2011) and such widespread activation has also been observed in ERP (Prieto-Corona et al., 2010, Xuan et al., 2007, Zhou et al., 2011) and EEG (Dimitriadis et al., 2015, Vourkas et al., 2014) studies. Kawashima et al. (2004) reported the first developmental fMRI data and compared children and adults during addition, subtraction and multiplication. Children recruited very similar neural networks, including prefrontal, intraparietal and occipito-temporal areas, as adults, and this was largely comparable across the three operations. However, their sample was very small and coupled with a very broad age range of children, this may have made it very difficult to detect developmental differences.
Subsequent studies largely confirmed the involvement of a bilateral fronto-parietal network during calculation. For example, studies examining addition in children have reported increased activation in bilateral frontal and posterior parietal areas (Kucian et al., 2008, Meintjes et al., 2010, Mondt et al., 2011), but this increase in activity seems to be smaller when directly compared to adults (Davis et al., 2009; Kucian et al., 2008). Similar increases have been observed during subtraction in children (Chang et al., 2016, Mondt et al., 2011, Peters et al., 2016) and adolescents (Chang et al., 2016). These increases in fronto-parietal activity have also been detected with NIRS (Dresler et al., 2009) and MEG (Simos et al., 2008).
These data were a first important step in understanding the brain networks of arithmetic in children, but they did not include systematic task manipulations to unravel how different networks are recruited during various strategies, as is observed in adults (Grabner et al., 2009, Prado et al., 2011, Tschentscher and Hauk, 2014). To address this issue, De Smedt et al. (2011) systematically manipulated problem size (small vs. large) and operation (addition vs. subtraction) in a relatively narrow age range of 10-to-12-year-olds (see Moore et al. (2014) and Van Beek et al., 2014b; for a similar manipulation of the problem size effect with ERPs). De Smedt et al. (2011) reasoned that the large vs. small and subtraction vs. addition contrasts would reveal networks that are more important for procedural strategies, whereas the reverse contrasts would unravel networks that are more relevant to fact retrieval. Their data revealed that, as in adults, subtractions and large problems showed increases in a wide fronto-parietal network that comprised the IPS and PFC (see also Evans et al., 2016). The reverse contrasts, pointing to arithmetic processes that are more related to fact retrieval, however revealed a different pattern than in adults. Instead of the AG, it was the medial temporal lobe, specifically the left hippocampus (HC), that showed increased brain activity during fact retrieval problems. This suggested a specific role of the HC that might be related to the formation of long-term memories of arithmetic facts, a hypothesis that has gained increased attention in the last years (Menon, 2016). These differences between children and adults might be explained by the time-limited role of the hippocampus in long-term memory (Smith and Squire, 2009), with its role being crucial in the early consolidation of (arithmetic) facts, while in later stages of more automatization, posterior parietal systems, including the AG, become more relevant.
The role of the HC in arithmetic fact retrieval has been confirmed in other developmental studies (Menon, 2016; for a review). Cho et al. (2011) studied brain activity during addition in children that were categorized as “counters” or “retrievers” depending on their dominant strategy (i.e. if a child used that strategy on more than 60% of the trials), which was determined via verbal report data prior to scanning. The authors used multivariate pattern analysis (MVPA) techniques (Norman et al., 2006), which allowed them on a much finer scale than univariate fMRI to investigate spatial patterns of activity (in a given region) and compare these patterns between groups. They showed that the counters and retrievers showed distinct patterns of brain activity in the bilateral HC (as well as in medial temporal gyri (MTG), IPS, SMG and AG). This was replicated in children of a similar age range (Cho et al., 2012): Higher frequency of fact retrieval (determined by verbal reports) correlated with increased activity in the bilateral HC (as well as lingual gyri, FG and superior parietal areas). Qin et al. (2014) compared children, adolescents and adults, and further confirmed this time-dependent role of the HC: Children showed increased activity in the bilateral HC, but this activity was significantly lower in adolescents, who in turn, did not differ from adults. MVPA analyses also indicated an increase in inter-problem representational stability in adolescents and adults, compared to children. These data indicate that hippocampal engagement increases in primary school but then decreases and stabilizes in adolescence to reach adult-like levels of activity.
5.2. Developmental changes
The above-mentioned differences between children and adults undoubtedly indicate that these activated brain networks change over developmental time. A handful of studies have aimed to characterize these changes by correlating brain activity with age (Eden et al., 2016, Prado et al., 2014, Rivera et al., 2005), by comparing different age groups (Chang et al., 2015, Dresler et al., 2009, Kawashima et al., 2004, Kucian et al., 2008, Qin et al., 2014, Rosenberg-Lee et al., 2011), and ultimately, but unfortunately scarcely, by following children longitudinally (Qin et al., 2014).
Rivera et al. (2005) reported the first age-related changes in brain activity during small additions and subtractions in 8- to 19-year-old children. They investigated which areas showed positive (i.e. age-related increase) and negative (i.e. age-related decrease) associations with age, and found age-related increases in activity in the left SMG, and the anterior AG and adjoining IPS, reflecting increased specialization in the left parietal cortex, as well as increases in the lateral occipito-temporal cortex. These data probably reflect the neural changes that are accompanied with an increasing reliance on fact retrieval and/or increasing automatization of arithmetic facts, as suggested by the overlapping waves model of strategy development (Siegler, 1996). On the other hand, they reported that activity in DLPFC, VLPFC and ACC, decreased with age, potentially reflecting a decrease in reliance on working memory and attentional resources. Interestingly, they also observed a decrease in hippocampal systems with age.
Prado et al. (2014) further investigated whether these age-related changes differed depending on the operation (subtraction vs. multiplication) in 8-to-13-year-old children. Such contrast indirectly reveals something about age-related changes in strategy use, as procedural strategies are more common in subtraction (Barrouillet et al., 2008) while retrieval occurs more frequently in multiplication (Imbo and Vandierendonck, 2007). Prado et al. (2014) used two localizer tasks to investigate brain activity in two clusters of areas, i.e. areas related to numerical magnitude processing in the right posterior parietal cortex (including IPS and posterior superior parietal lobule (PSPL)) with a non-symbolic number comparison task as well as areas related to phonological processing in the left MTG and inferior frontal gyrus (IFG) with a phonological rhyming task. Activity in the areas related to numerical magnitude processing showed an age-related decrease during subtraction, but not multiplication. On the other hand, activity in the left MTG was characterized by age-related increases during multiplication, whereas brain activity during subtraction in this region did not change with age. These data suggest that age-related changes in brain activity during arithmetic are operation dependent: Changes in multiplication correlated with language-related areas, while changes in subtraction correlated with numerical magnitude processing areas. Evans et al. (2016) recently reported operation-dependent age-related changes in an even wider age range (7- to 29-year-olds). They observed age-related increases in right HC and left medial frontal gyrus (MFG) for addition, but not for subtraction − they also found age-related increases in addition and subtraction in the right temporo-parietal cortex and these were similar to age-related changes during a reading task. Evans et al. (2016) interpreted these operation differences as reflecting differences in strategy use, assuming a larger amount of fact retrieval in addition than in subtraction, but no study thus far has directly investigated brain activity during different strategies that children use to solve these problems.
While these studies investigated change across very protracted developmental periods, it remained to be determined whether such changes can observed over smaller time intervals. Rosenberg-Lee et al. (2011) addressed this by comparing two groups that only differed in one year of schooling. Second and third graders were carefully matched in terms of IQ, reading, working memory and mathematics, and completed an addition task with two complexity levels during fMRI acquisition. These authors observed that complexity modulated the activity in the PFC differently in the two grades: Second graders showed higher activation differences in the ventromedial PFC, whereas third graders showed larger differences in the left DLPFC. These differences point to a developmental change in the task-appropriate deactivation of the default mode network. The third graders also showed larger activation differences in bilateral IPS and superior parietal lobule, right AG, the right lateral occipital cortex, and the bilateral parahippocampal and lingual gyri. These data suggest that, in addition to the well-established role of the dorsal posterior parietal cortex, also more ventral areas are relevant to calculation, yet their roles have not been studied in detail (Menon, 2015).
Chang et al. (2016) recently aimed to further clarify linear as well as non-linear age-related changes in cytoarchitectonically distinct areas in the posterior parietal cortex, by comparing 7-to-10-year-old children, adolescents and adults during subtraction. They observed linear age-related increases in the bilateral ventral anterior IPS (IPS-hIP1) and neighboring SMG (SMG-PFm) and AG (AG-PGa). However, the posterior AG (AG-PGp) did not show age-related changes, indicating that subdivisions of the AG play distinct roles in the development of arithmetic. Non-linear age-related changes were found in the middle SMG (SMG-PF). These age-related changes were interpreted as reflecting changes in strategy use, but such explanation only remains speculative as strategies were not examined in this study. Furthermore, this study, as well as those described above, only involved a cross-sectional comparison of age groups, and age-cohort differences, rather than developmental differences, might explain the observed differences under study. To exclude this confound, a longitudinal follow-up of the same sample of children is needed in order to study how arithmetic networks change over developmental time.
We only found one study that investigated brain activity at multiple time points in one sample of children (Qin et al., 2014). These authors observed within a one-year time frame an increase in activity in the bilateral HC, coupled with a decrease in activity in bilateral DLPFC, left superior parietal lobule and right posterior occipito-parietal areas. These changes were accompanied by behavioral verbal report data that indicated an increased reliance on fact retrieval strategies over one year time. These findings echo the above-mentioned negative correlations between age and brain activity during arithmetic (Rivera et al., 2005), reflecting a decreasing reliance on working memory and executive control that is probably coupled with a decrease of effortful procedural strategies and an increase in arithmetic fact retrieval, as is predicted by the overlapping waves model (Siegler, 1996).
5.3. Individual differences
Imaging data in adults indicate that activity in the arithmetic network is modulated by individual differences (Grabner et al., 2007). Are such differences also observed in children? De Smedt et al. (2011) compared brain activity in children with low and high levels of arithmetic fluency. De Smedt et al. observed that during small problems, children with low arithmetic fluency showed higher activity in the right IPS than those with high fluency. Berteletti et al. (2014) reported a similar pattern of findings and observed a negative association between number line estimation ability and activity in the IPS during subtraction. Similarly, Price et al. (2013) observed in adolescents that low mathematical competence was associated with increased activity in the right IPS during addition and subtraction. More recently, Demir et al. (2014) observed that such patterns are particularly prominent in children with low SES, who are known to have lower levels of mathematical achievement. These data suggest that individuals with lower mathematical competence show higher activity in numerical magnitude processing related areas of the arithmetic network. It could be that poorer representations of magnitude prevent individuals with low mathematical competence to develop advanced mathematical skills. Alternatively, this increased IPS-activity might reflect a protracted reliance on immature arithmetic procedures, such as counting, that require more numerical magnitude processing. Future studies should further investigate these possibilities.
Price et al. (2013) also showed that adolescents with higher mathematical competence showed higher levels of activity in the left SMG and ACC, as had been observed in adults (Grabner et al., 2007), but such associations have not been found in (younger) children. As reviewed above, HC rather than temporo-parietal regions are more crucial for fact retrieval earlier in development. Interestingly, Cho et al. (2012) reported individual differences in HC activity, showing that children with high retrieval fluency had higher HC-activity.
5.4. Atypical development
Persistent deficits in arithmetic are the key feature of dyscalculia (e.g., Geary, 1993, Geary, 2011) but only a minority of studies examined the neural underpinnings of this neurodevelopmental disorder. Most of available studies focused on numerical magnitude processing as an underlying correlate of the observed mathematical deficits (De Smedt et al., 2013, Kucian and von Aster, 2015; for reviews) but surprisingly few have investigated brain activity during arithmetic in this population (Table 1).
Some studies (Davis et al., 2009b, Rosenberg-Lee et al., 2015, Simos et al., 2008) reported increased activity in a wide-spread network of regions, including parietal, prefrontal and occipito-temporal regions, in dyscalculia during addition and subtraction, and these differences do not merely reflect performance differences (Rosenberg-Lee et al., 2015). These authors also observed that the increases in brain activity were particularly prominent in subtraction, which is more difficult than addition and which relies to a larger extent on procedural strategies, in posterior parietal areas (including bilateral IPS, SPL and AG) and the left FG.
On the other hand, decreased brain activation in children with dyscalculia, coupled with the observation that no brain areas showed increased activity in dyscalculia compared to age-matched controls has also been reported. Ashkenazi et al. (2012) compared the brain responses during simple (i.e., +1) and more complex (single-digit) additions. They found decreased activity in dyscalculia in a wide-spread network of regions, including bilateral PFC, right posterior parietal and occipito-temporal areas. This was further explained by the lack of difficulty-related modulation of neural activity in these regions in dyscalculia: Typically developing children showed increased activity for complex compared to simple problems in these areas, whereas children with dyscalculia did not, a pattern that also has been observed in children with low arithmetical fluency (De Smedt et al., 2011). Multivariate representational similarities further revealed that, in the bilateral IPS, the patterns of brain activity during simple and complex problems were less distinguishable in children with dyscalculia (Ashkenazi et al., 2012), again reflecting a lack of difficulty-related modulation in the neural activation patterns of children with dyscalculia. Berteletti et al. (2014) focused on multiplication and used a localizer approach in which areas related to number processing (i.e., right PSPL and IPS) and to language processing (left IFG and left MTG) were delineated with a non-symbolic number comparison task and rhyming task, respectively. Children with dyscalculia showed reduced activity in these ROIs, leading the authors to conclude that numerical as well as language-related processes in multiplication are impaired in dyscalculia.
Impairments in arithmetic, in particular fact retrieval, have also been described in children with dyslexia (e.g., De Smedt and Boets, 2010, Simmons and Singleton, 2008), a specific neurodevelopmental learning disorder of which persistent deficits in learning to read is the hallmark feature (e.g., Snowling, 2000). The brain networks that support reading and arithmetic are overlapping in the temporo-parietal and inferior frontal cortices (De Smedt et al., 2010, Prado et al., 2011) and individual differences in arithmetic fact retrieval correlate with white matter integrity of the arcuate fasciculus (AF) (Van Beek et al., 2014a), a tract that traditionally has been related to individual differences in language and reading ability, and is impaired in dyslexia (Vandermosten et al., 2012). At the behavioral level, the high correlation between reading and arithmetic fact retrieval is well documented and may reflect the forming of associative relations, for example, between letters and sounds or between arithmetic problems and their answers (e.g., Chu et al., 2016, Fuchs et al., 2016; Koponen et al., 2013). In view of this overlap between reading an arithmetic, one fMRI study has contrasted the brain responses during addition and subtraction in children with and without dyslexia (Evans et al., 2014). These authors observed decreased activation in the left SMG in dyslexia, in line with the decreased activity in language-related areas in these children (Eden et al., 2016). Evans et al. (2014) also observed a group by operation interaction in the right inferior parietal lobe, which echoes the above-mentioned data in children with low arithmetical fluency (De Smedt et al., 2011): Typically developing children recruited this area more during subtraction than addition, while children with dyslexia did not show such modulation.
Findings from children with atypical arithmetical development remain mixed today as both increases and decreases in activity in the arithmetic network have been reported. There are currently too few studies available to draw definitive conclusions and this is further complicated by study differences in criteria used to include participants as well as wide age-ranges, which might mask important developmental changes in children with atypical development. Future studies in narrow age bands are therefore crucial to make headway. It would also be interesting to investigate the differences in brain activity between children with dyscalculia and dyslexia. Both conditions show impairments in arithmetic, but their neural origin might be different. Recent data by Peters et al. (submitted) contrasted brain activity during arithmetic in typically developing children, children with dyscalculia, children with dyslexia and children with both dyslexia and dyscalculia. Surprisingly, the brain activity profiles of these three groups of learning disorders showed high similarity and this suggests that these neural profiles may be more overlapping than originally thought.
Is it possible to change brain activity during arithmetic in atypical populations via targeted interventions? Only two studies investigated this question (Iuculano et al., 2015, Kesler et al., 2011), and this clearly represents an important agenda for future studies. Kesler et al. (2011) ran a pilot study in 7-to-14-year-old children on the effect of a numerical magnitude processing training on brain activity during two-digit calculation. They observed increases in bilateral superior parietal cortex and decreases in frontal, temporal and HC areas following training, indicating that activity in these areas is malleable. The findings are however hard to interpret in the absence of a control group. Recently, Iuculano et al. (2015) offered eight weeks of one-on-one math tutoring intervention, which focused on efficient counting strategies and learning arithmetic facts in children with dyscalculia and age-matched controls. Before training, children with dyscalculia showed increased activation during single-digit addition in frontal, superior parietal, temporo-parietal, and hippocampal areas. After training, their brain activity did not differ from age-matched controls anymore. MVPA further showed that a classifier could reliably discriminate brain activity patterns of children with dyscalculia and age-matched controls before, but not after training, suggesting normalization of brain activity in dyscalculia after intervention.
6. Connectivity between areas of the arithmetic network
The above-reviewed functional imaging data clearly indicate the involvement of multiple distant brain regions in arithmetic. This highlights that in order to fully understand the neural basis of arithmetic and its disorders, one needs to study the connectivity between these regions rather than merely focusing on isolated brain areas (Uddin et al., 2010; for a discussion). We summarize studies that have investigated task-based functional connectivity (Table 2), studies that have correlated resting-state connectivity networks to arithmetic performance (Table 3) and studies of structural connectivity that used diffusion tensor imaging (DTI) to investigate associations between white matter tracts and arithmetic (Table 4; Fig. 1).
6.1. Task-based functional connectivity
Six of the above-mentioned fMRI studies also investigated task-based functional connectivity. This work has revealed the importance of fronto-parietal (Chang et al., 2016; Rosenberg-Lee et al., 2015, Rosenberg-Lee et al., 2011), hippocampal-frontal (Cho et al., 2012, Qin et al., 2014) and hippocampal-parietal (Qin et al., 2014) connectivity.
Rosenberg-Lee et al. (2011) reported increases in the functional connectivity during addition between left DLPFC and posterior parietal areas from second to third grade. Chang et al. (2016) further suggested that this change might be non-linear as they observed increased functional connectivity during addition in adolescents compared to children and adults, who did not differ in their connectivity profiles. Increased parietal-frontal functional connectivity has also been observed in 7-to-9-year-old children with dyscalculia compared to age-matched controls (Rosenberg-Lee et al., 2015). These parietal-frontal circuits have been linked to working memory systems that are recruited during arithmetic (see Menon, 2016; for a review). However, these connectivity differences between ages and ability groups are not so easy to interpret, because increased connectivity might reflect increasingly efficient use of working memory resources or compensatory effects in individuals with lower ability (Menon, 2016). Also, the number of task-based functional connectivity studies is currently too few to draw definitive conclusions. Future research, preferably with longitudinal designs, is needed to further examine this.
Cho et al. (2012) and Qin et al. (2014) reported increases in hippocampal-PFC connectivity during addition with increasing age and retrieval ability. Cho et al. (2012) demonstrated that higher addition-related connectivity between the right HC and PFC was associated with higher fact retrieval fluency. In their longitudinal data, Qin et al. (2014) observed that increases in hippocampal connectivity with PFC and parietal areas during addition were associated with individual gains in arithmetic fact retrieval. Qin et al. (2014) also demonstrated that changes in hippocampal connectivity rather than regional changes in the HC itself, predicted children’s increase of arithmetic facts. This confirms that increases in arithmetic fact retrieval are related to functional connectivity in hippocampal-neocortical circuits, further supporting the role of declarative memory systems in fact retrieval. These interactions between the HC and neocortex are probably not exclusively relevant to learning arithmetic, but play a broader role in memory formation and knowledge acquisition (e.g., McClelland et al., 1995).
6.2. Resting-state connectivity
Individual differences in arithmetic have also been correlated with connectivity between brain regions during rest (rsfMRI). Evans et al. (2015) found that increased intrinsic connectivity between different areas of the arithmetic network, including DLPFC, posterior parietal regions, ventral occipito-temporal regions and anterior temporal regions at the age of 8, predicted subsequent growth in arithmetic. In their intervention study that investigated the effect of an 8-week one-on-one tutoring focusing on efficient counting and improving retrieval fluency, Supekar et al. (2013) showed that intervention gains in addition were predicted by resting-state hippocampal-PFC connectivity, with individuals with higher intrinsic connectivity showing larger intervention gains. Combining data from various earlier studies (Ashkenazi et al., 2012, Iuculano et al., 2015, Rosenberg-Lee et al., 2015, Supekar et al., 2013), Jolles et al. (2016a) investigated the intrinsic connectivity of the IPS in age-matched typically developing children and children with dyscalculia. The IPS showed strong intrinsic connectivity with multiple areas of the arithmetic network, including bilateral PFC, parietal and ventral occipito-temporal regions. Group comparisons further revealed that children with dyscalculia showed increased interhemispheric IPS connectivity and increased connectivity between the IPS and (dorsal) fronto-parietal regions. Additional analyses showed spontaneous increases in low frequency fluctuations during rest in IPS and other regions in children with dyscalculia. Pattern classification algorithms were able to reliably discriminate children with and without dyscalculia on the basis of their connectivity data, leading the authors to suggest that IPS-connectivity might represent a biomarker for dyscalculia. However, in order to be a reliable biomarker, it remains to be seen whether such hyper-connectivity profiles are specific to dyscalculia or can also be observed in other neurodevelopmental conditions.
Jolles et al., (2016b) recently investigated changes in IPS intrinsic connectivity following the above-mentioned math tutoring intervention (Supekar et al., 2013). Jolles et al., (2016b) observed increases in IPS intrinsic connectivity with PFC, ventral occipito-temporal cortex as well as HC. Increases in IPS connectivity were positively correlated with performance gains in the intervention. These studies are only but a first step to investigate how intrinsic connectivity is related to arithmetic. Future studies are needed in order to carefully specify these individual differences in intrinsic connectivity and how they change as a function of development, ability level and interventions. At least, the available data highlight the need for a multisystem approach rather than an isolated study of specific brain regions.
6.3. Structural connectivity
Structural connections between regions of the arithmetic network can be investigated by means of DTI, which allows one to correlate behavioral performance with the integrity of white matter tracts as indexed by fractional anisotrophy or FA (Matejko and Ansari, 2015, Vandermosten et al., 2012 for excellent reviews on DTI studies in arithmetic and reading, respectively). Studies in typically developing children have revealed significant associations between left temporo-parietal white matter, i.e. the superior corona radiata (CR), and calculation in children (van Eimeren et al., 2008) and adolescents (Matejko et al., 2013). Significant positive associations between arithmetic and the superior longitudinal fasciculus (SLF), which connects the posterior parietal cortex with the PFC have also been observed (Li et al., 2013, Matejko et al., 2013, Tsang et al., 2009). Similar associations were reported for the inferior longitudinal fasciculus (ILF; Li et al., 2013, van Eimeren et al., 2008), which connects PFC with ventral occipito-temporal areas.
Investigating how different operations were related to white matter connections between the frontal and temporo-parietal cortex, Van Beek et al. (2014a) focused on the AF, a tract that has been often investigated in reading research (e.g., Vandermosten et al., 2015). Van Beek et al. (2014a) found that the integrity of the left anterior part of the AF, connecting the frontal and parietal cortex, was positively correlated with addition and multiplication, but not with subtraction and division, suggesting a role for the AF in fact retrieval. Follow-up analyses revealed that this was explained by a close relationship between reading and fact retrieval, as the association between the AF and fact retrieval disappeared when controlling for pseudoword reading. This was interpreted as reflecting a common reliance on phonological codes in (pseudoword) reading and arithmetic (e.g., De Smedt, 2016, Simmons and Singleton, 2008).
Jolles et al., (2016c) recently examined whether different sections of the SLF, which connects frontal to parietal to temporal regions, were related to training-induced changes in arithmetic. The study design was similar to Supekar et al. (2013), and Jolles et al., (2016c) showed that specifically the part of the SLF that connects the frontal and temporal regions, predicted the learning gains in addition and subtraction.
Studies on atypical mathematical development, including children with dyscalculia (Ranpura et al., 2013, Rykhlevskaia et al., 2009), velocardiofacial syndrome (Barnea-Goraly et al., 2005), multiple sclerosis (Till et al., 2011), fetal alcohol syndrome (Lebel et al., 2010) and premature children (Pavlova et al., 2009) have also reported reduced white matter integrity, particularly in the SLF and in temporo-parietal white matter, compared to age-matched controls. These studies further confirm the role of the white matter tracts that connect distinct parts of the arithmetic network.
It is important to emphasize that all the existing DTI studies are cross-sectional and no longitudinal data are available. Such data are really needed to find out how the quality of white matter tracts of the arithmetic network develops as children become more proficient. Furthermore, most of the above-mentioned studies involved samples of children with very wide age ranges (e.g., 7–11 or 10–15 years) but these long developmental periods are characterized by massive changes in white matter (Giedd and Rapoport, 2010) as well as arithmetic, which might confound the observed correlations between white matter and arithmetic. This requires future studies to include children of more narrow age ranges. Finally, white matter in the parietal cortex (but also as in other places throughout the cortex) is characterized by multiple crossing fibers, because many tracts intersect in this cortical area (Fig. 1). For example, white matter in the above-mentioned temporo-parietal areas that correlates with arithmetic, includes intra-hemispheric (AF), inter-hemispheric (posterior part of the corpus callosum) and cortico-spinal (CR) connections. The analysis of DTI data via the classic tensor model, as was done in all reviewed studies, is problematic because it does not allow one to estimate these crossing fibers and as such provides an oversimplification of the underlying anatomy (Dell’Acqua et al., 2012), leaving it unresolved which of these tracts is relevant for individual differences in arithmetic. This can be resolved with non-tensor models, such as spherical deconvolution, which provide a more fine-grained analysis of white matter tracts and their crossing fibers in the brain (Farquharson et al., 2013). To our knowledge, there are no studies that used spherical deconvolution in the studies about arithmetic.
7. Structure of the arithmetic brain network
Voxel-based morphometry studies have investigated how anatomical characteristics (in particular grey matter) of the above-mentioned regions of the arithmetic brain network are correlated with performance. The first structural imaging studies compared children with difficulties in arithmetic to age-matched controls and showed reduced grey matter in distinct areas of the above-mentioned arithmetic network (Han et al., 2013, Isaacs et al., 2001, Ranpura et al., 2013, Rotzer et al., 2008, Rykhlevskaia et al., 2009). The first structural data were reported by Isaacs et al. (2001) and revealed that adolescents with very low birth weight and calculation deficits had smaller grey matter volume in the (left) IPS. Subsequent studies in children with dyscalculia showed that they had reduced grey matter in the posterior parietal cortex (including IPS) (Ranpura et al., 2013, Rotzer et al., 2008), in frontal areas, such as IFG and MFG, (Rotzer et al., 2008), in the parahippocampal gyrus (Ranpura et al., 2013; Ryklehvskaia et al., 2009) and in the occipito-temporal cortex (Han et al., 2013; Ryklehvskaia et al., 2009).
Surprisingly few studies have examined this association between grey matter and arithmetic in typically developing children. Significant positive associations were observed between grey matter volume in the left IPS and arithmetic in 6–7-year-olds (Price et al., 2016) and 9–10-year-olds (Li et al., 2013). Supekar et al. (2013) further showed that the volume of the right HC predicted the learning gains of a one-on-one tutoring intervention that focused on efficient counting and fact retrieval, with larger hippocampal volumes before the intervention predicting larger intervention gains, again confirming the role of the HC in arithmetic fact retrieval. Finally, Evans et al. (2015) reported that grey matter volumes of various parts of the arithmetic network, i.e., posterior parietal areas, and ventral occipito-temporal cortex, predicted the growth in arithmetic across primary school. All these data confirm that the structural integrity of different parts of the arithmetic network is positively correlated with arithmetic performance. On the other hand, studies are needed to investigate how these structural properties of the network and their change as a function of time, ability level and interventions, are related to the brain activity that is occurring when children perform arithmetic.
8. Challenges and future directions
The existing body of brain imaging studies on arithmetic has clearly increased over the last five years. There are emerging trends in our understanding of the functional and structural properties of the arithmetic network in children, but the literature remains scattered to date, particularly in the context of atypical development. Meta-analyses of brain imaging data (see Turkeltaub et al. (2002), for a discussion in the field of reading) are therefore key in order to synthesize the existing studies with inconsistent findings, but this will require a more critical mass of studies than what is currently available. For example, Eickhoff et al. (2016) recommended to include at minimum 17–20 experiments to have enough power to detect a moderate effect. This number obviously increases if one aims to include a test of moderators of the arithmetic network, such as age, ability level or arithmetic task. Such numbers of brain imaging studies are currently not available in the field of arithmetic development.
8.1. Truly understanding development
Many of the above-reviewed studies, whose aim was specifically to investigate age-effects, included samples of very wide age ranges. This is problematic for studying arithmetic, because schooling or instruction, even over a short duration of one year results in changes in brain activity (Rosenberg-Lee et al., 2011), and we can only but speculate on its effects on connectivity and brain structure. If we truly want to understand development, studies should orient their focus to relatively narrow windows of schooling in order to understand how gradual changes occur over time (Karmiloff-Smith, 2010) − note that this window of schooling does not necessarily correspond to a specific chronological age. This should be done in windows during which development is the steepest, or stated differently, instructional attention to a given skill is the largest. In arithmetic, this should be from first to third grade, during which the largest changes in strategies are occurring. The comparison of focused groups, who are comparable in their instructional level, clearly presents a first step in this endeavor, yet longitudinal data are critical to make headway. These questions about development can also be addressed by focused short-term training studies, as has been done in adults (Zamarian and Delazer, 2015), which experimentally manipulate the transition from procedure-based arithmetic to fact retrieval.
8.2. Atypical development
The absence of longitudinal data is particularly problematic in atypical development. The existing data do not allow us to determine whether the functional and structural abnormalities seen in these atypical conditions are the cause or the consequence of their difficulties in arithmetic. It could be that the reported abnormalities are simply due to differences in arithmetic experience and that they do not represent the etiology of a specific learning disorder. For example, reading studies on dyslexia, that compared children with dyslexia to age-matched controls and to children who had a similar reading level and experience but where younger in age (reading-level matched), revealed that some of the observed functional (e.g., Hoeft et al., 2007) and structural (e.g., Krafnick et al., 2014), neurobiological abnormalities in dyslexia are explained by their reduced reading experience and do not represent the etiology of dyslexia (Norton et al., 2015, for a review). Such comparisons with ability-level matched children are non-existent in brain imaging studies on dyscalculia and clearly represent an area for future research.
We also do not know whether these abnormalities in atypical conditions, particularly those related to structure and connectivity, are already present before children learn to calculate, and hence may represent a neurobiological cause of their disability. The availability of child-friendly brain imaging protocols makes it possible to study the brain in children before they learn to calculate. In dyslexia research, there is now an increasing number of brain imaging studies that have investigated pre-readers, including at-risk children (see Vandermosten et al., 2016, for a meta-analysis). Similar studies in the arithmetic domain are crucial in order to further establish the neurobiological cause of atypical arithmetic development. The longitudinal follow-up of these young children is also needed to understand how brain circuits develop when they learn to calculate and how these change when children acquire different arithmetic strategies. Beyond adding to our understanding of atypical development, these studies will inform us about how educational experiences change brain structure and function, further revealing insights on experience-dependent plasticity in children.
8.3. Measures of arithmetic
The measurement of arithmetic skill in future brain imaging studies clearly requires careful attention. The above-reviewed fMRI-studies vary greatly in the arithmetic tasks and baseline conditions they used and in their dependent variables when studying brain-behavior correlations. This makes the current evidence difficult to interpret, because different aspects of the arithmetic task will modulate the arithmetic network. Inconsistencies between studies, particularly in the context of atypical development, might be explained by the selection of an appropriate baseline condition and by how this baseline condition affects activation levels in the parietal cortex (Menon, 2016, for a discussion).
This review started by highlighting that a crucial characteristic in children’s arithmetic development involves a change in the mix of strategies they use (Geary, 2011, Jordan et al., 2003, Siegler, 1996). None of the reviewed brain imaging studies, however, has provided a direct measure of these strategies. Instead, they all relied on indirect measures, such as contrasting different operations or problem sizes, an approach that has been severely criticized (De Smedt, 2016), already for a long time (Siegler, 1987). This is even more problematic in developmental research and atypical development, where there are massive changes in children’s strategic development and problems of a given type (i.e. size or operation) will be solved by different strategies depending on the age, education or ability level. The existing brain imaging data have interpreted differences between operations or problem sizes as reflecting differences in strategies, but they never provided direct measures of these strategies to bolster these conclusions. Studies in adults have shown that it is possible to analyze brain activity on an item level as a function of verbally reported strategies (Grabner et al., 2009, Grabner and De Smedt, 2011, Tschentscher and Hauk, 2014), showing that it is the strategy and not the operation that modulates the arithmetic network. Interestingly, similar data became recently available in children (Polspoel et al., submitted), pointing to very similar conclusions.
8.4. Environmental effects
Arithmetic represents a culturally transmitted skill that is obviously not acquired in isolation. Nearly all of studies discussed in this review included children from Western countries (US and Western Europe), the majority of whom were English-speaking, and this needs to be taken into account when evaluating the findings from studies that are currently available. There is massive impact of the home and educational environment on children’s arithmetic development (e.g., Geary, 1996) and consequently on the development of its underlying brain networks. These contextual cross-cultural differences have been ignored in brain imaging studies. For example, there are large differences between Western countries in which arithmetic strategies are instructed, ranging from a continuing emphasis on counting in some countries vs. an early discouraging of counting (even prohibition) in order to promote fact retrieval (De Smedt, 2016, for a discussion). Such instructional differences, combined with the emphasis a curriculum places on arithmetic fact retrieval − the so-called math wars (Schoenfeld et al., 2006) − inevitably will affect the emergence and organization of the arithmetic brain network. In fact, there are adult training data who demonstrate that activity in the arithmetic network is modulated depending on the type of instruction, i.e., learning by strategies or by drill (Delazer et al., 2005). It is also important to emphasize that one year of instruction in a particular culture (e.g., US or Western Europe) does not necessarily result in the same level of expertise as one year of instruction in other cultures (e.g. East Asia), Similarly, instruction that occurs during the maturation of critical white matter tracts, may not be the same as instruction that occurs earlier or later. It would therefore be interesting to run studies to investigate the divergences and communalities between children who are enrolled in different math curricula that differ in their emphasis on arithmetic fact retrieval at different stages of learning arithmetic. At the very least, future brain imaging studies on arithmetic should carefully document the educational context in which the data have been collected as this is crucial to understanding the data. This contextual description makes studies in educational neuroscience also more educationally relevant and allows for more potential bridges between cognitive neuroscience and classroom learning (De Smedt and Grabner, 2015).
8.5. It’s not just the parietal cortex
The present review clearly indicates that arithmetic development and its disorders are not confined to one particular brain area, but rather suggests the involvement of a broad and dynamic network, that changes over time. The interactions between these different components need to be better understood. One example deals with the understanding of the role of the ventral occipito-temporal cortex in calculation. As reviewed above, this area is reliably activated during calculation and structural connectivity in these ventral visual areas is related to individual differences in calculation. The importance of higher-order visual processing in calculation has been highlighted for a long time (Menon et al., 2000), but its role, presumably related to symbolic number representations, has received little attention so far (Menon, 2015). Future studies should therefore determine how these ventral visual circuits interact with other components of the arithmetic network during different arithmetic strategies and how this changes as a function of development and ability level. The controlled manipulation of number representations during the acquisition of brain imaging data and their interaction with arithmetic might allow us to understand the functional role of these ventral areas in calculation and its development (see Peters et al., 2015).
8.6. Methodological advances
The existence of distant but connected nodes of the arithmetic network also requires data-analytic techniques to account for this interconnectivity and as a result, the mere univariate approach that investigates brain activity in (a set of) particular brain regions may not fully capture this complexity. Multivariate approaches, such as MVPA (e.g., Norman et al., 2006) or representational similarity analyses (Kriegeskorte et al., 2008), allow us to study of patterns of brain activity, providing a much more fine-grained understanding of neural representations in a given area or network of areas (Raizada et al., 2010). It might be that there are more subtle differences between different types of strategies in different areas of the brain, for example in IPS, that go undetected if one only uses univariate approaches. These multivariate approaches have been successfully applied in children (Cho et al., 2011), but clearly represent an avenue for future studies. These techniques allow us to investigate how representations change over developmental time (e.g., early stages of fact retrieval vs. fully automated stages; see Qin et al., 2014). They also might capture individual differences between learners, for example by showing less precise representations in one versus the other group.
The vast majority of the reviewed studies have used MRI-techniques and the use of other brain imaging methods to study the arithmetic brain is extremely limited. MRI-data are limited in capturing the temporal dynamics of the calculation process. There is a need for future studies to investigate this precise time course of brain activity during calculation, for example by using EEG/ERP or MEG. Such data could be particularly informative to understand if and when numerical representations are activated during calculation. The combination of these data with those obtained from standard MRI-studies will provide a more fine-grained picture of the arithmetic network and its dynamics.
8.7. Connections between arithmetic and reading
In this review, we have often drawn parallels between the (atypical) development of arithmetic and reading, and their underlying neurocognitive correlates. Until now, these strands of research have been executed in relative isolation of each other, even though both involve the acquisition of symbolic representations (letters vs. numbers), they both show similar developmental changes in strategy use (from time-consuming processing to direct retrieval of information from long-term memory), their disorders often co-occur and parts of their neural networks are overlapping (e.g., Geary, 1993, De Smedt et al., 2010; for a discussion). It is therefore imperative that future studies jointly study both reading and arithmetic in one sample of children to find out similarities and differences in the neural networks of both academic abilities, and such studies are starting to emerge (Evans et al., 2016). These studies will help us to more fully understand how our brains are able to acquire complex culturally transmitted symbolic skills. They will also help us to understand how much of the differences between typically and atypically developing children can be attributed to domain-general differences and how much of these can be explained by domain-specific factors.
9. Conclusion
Arithmetic is a crucial stepping stone in children’s mathematical development and is fundamental in our western everyday life. The study of the brain networks that support this culturally transmitted skill in children is fairly recent, and the existing body of brain imaging studies is only but starting to reveal the functional and structural properties of the arithmetic network in children. This network involves a large set of interconnected areas that include bilateral frontal (DLPFC, VLPFC), parietal (IPS, AG, SMG), occipito-temporal and medial temporal, including HC, areas. This network undergoes developmental changes in its function, connectivity and structure, which are not yet fully understood. There are some parallels of this network with what has been observed in adults, but it is clear that children recruit different networks, particularly during the development of arithmetic facts. Despite these emerging trends, the developmental imaging literature remains scattered and this is particularly the case in the context of atypical development, where there are currently too few studies to make a definitive conclusion. Future studies should therefore include more carefully selected samples of typically and atypically developing children in narrow age bands. The observation that arithmetic involves a wide-spread distributed network of areas highlights the need for connectivity analyses and complex analytic approaches that investigate patterns of brain activity. These methodological advances, coupled with a careful design of the arithmetic tasks and assessments of strategies under study, are likely to produce a more comprehensive understanding of how the arithmetic brain unfolds in children, how it changes over time, and how it is impaired in atypical development.
Conflict of interest
None.
Acknowledgements
This work was supported by grants G.0946.12 and G.0027.16 of the Fund for Scientific Research − Flanders (FWO) and by Grant GOA 2016/010 of the Research Fund KU Leuven.
References
- American Psychiatric Association . 5th ed. Author; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Ansari D. Neurocognitive approaches to developmental disorders of numerical and mathematical cognition: the perils of neglecting the role of development. Learn. Indiv. Differ. 2010;20(2):123–129. [Google Scholar]
- Arsalidou M., Taylor M.J. Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage. 2011;54(3):2382–2393. doi: 10.1016/j.neuroimage.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Ashkenazi S., Rosenberg-Lee M., Tenison C., Menon V. Weak task-related modulation and stimulus representations during arithmetic problem solving in children with developmental dyscalculia. Dev. Cogn. Neurosci. 2012;2(Suppl. 1):S152–S166. doi: 10.1016/j.dcn.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N., Eliez S., Menon V., Bammer R., Reiss A. L.v. Arithmetic ability and parietal alterations: a diffusion tensor imaging study in Velocardiofacial syndrome. Cogn. Brain Res. 2005;25(3):735–740. doi: 10.1016/j.cogbrainres.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Barrouillet P., Mignon M., Thevenot C. Strategies in subtraction problem solving in children. J. Exp. Child Psychol. 2008;99(4):233–251. doi: 10.1016/j.jecp.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Berteletti I., Prado J., Booth J.R. Children with mathematical learning disability fail in recruiting verbal and numerical brain regions when solving simple multiplication problems. Cortex. 2014;57:143–155. doi: 10.1016/j.cortex.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berteletti I., Man G., Booth J.R. How number line estimation skills relate to neural activations in single digit subtraction problems. Neuroimage. 2015;12:011. doi: 10.1016/j.neuroimage.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R., Lee K. Executive functioning and mathematics achievement. Child Dev. Perspect. 2014;8(1):36–41. [Google Scholar]
- Campbell J.I., Xue Q.1. Cognitive arithmetic across cultures. J. Exp. Psychol. Gen. 2001;130(2):299–315. doi: 10.1037//0096-3445.130.2.299. [DOI] [PubMed] [Google Scholar]
- Chang T.-T., Rosenberg-Lee M., Metcalfe A.W.S., Chen T., Menon V. Development of common neural representations for distinct numerical problems. Neuropsychologia. 2015;75:481–495. doi: 10.1016/j.neuropsychologia.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.-T., Metcalfe A.W.S., Padmanabhan A., Chen T., Menon V.v. Heterogeneous and nonlinear development of human posterior parietal cortex function. NeuroImage. 2016;126:184–195. doi: 10.1016/j.neuroimage.2015.11.053. [DOI] [PubMed] [Google Scholar]
- Cho S., Ryali S., Geary D.C., Menon V. How does a child solve 7 + 8? Decoding brain activity patterns associated with counting and retrieval strategies. Dev. Sci. 2011;14(5):989–1001. doi: 10.1111/j.1467-7687.2011.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Metcalfe A.W.S., Young C.B., Ryali S., Geary D.C., Menon V. Hippocampal-prefrontal engagement and dynamic causal interactions in the maturation of children’s fact retrieval. J. Cogn. Neurosci. 2012;24(9):1849–1866. doi: 10.1162/jocn_a_00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F.W., vanMarle K., Geary D. Predicting children's reading and mathematics achievement from early quantitative knowledge and domain-general cognitive abilities. Front. Psychol. 2016;7:775. doi: 10.3389/fpsyg.2016.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Cannistraci C.J., Rogers B.P., Gatenby J.C., Fuchs L.S., Anderson A.W., Gore J.C. The neural correlates of calculation ability in children: an fMRI study. Magn. Reson. Imaging. 2009;27(9):1187–1197. doi: 10.1016/j.mri.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N., Cannistraci C.J., Rogers B.P., Gatenby J.C., Fuchs L.S., Anderson A.W., Gore J.C. Aberrant functional activation in school age children at-risk for mathematical disability: a functional imaging study of simple arithmetic skill. Neuropsychologia. 2009;47(12):2470–2479. doi: 10.1016/j.neuropsychologia.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt B., Boets B. Phonological processing and arithmetic fact retrieval: evidence from developmental dyslexia. Neuropsychologia. 2010;48(14):3973–3981. doi: 10.1016/j.neuropsychologia.2010.10.018. [DOI] [PubMed] [Google Scholar]
- De Smedt B., Grabner R.H. Applications of neuroscience to mathematics education. In: Cohen Kadosh R., Dowker A., editors. The Oxford Handbook of Numerical Cognition. Oxford University Press; Oxford: 2015. [Google Scholar]
- De Smedt B., Taylor J., Archibald L., Ansari D. How is phonological processing related to individual differences in children’s arithmetic skills? Dev. Sci. 2010;13(3):508–520. doi: 10.1111/j.1467-7687.2009.00897.x. [DOI] [PubMed] [Google Scholar]
- De Smedt B., Holloway I.D., Ansari D. Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. Neuroimage. 2011;57(3):771–781. doi: 10.1016/j.neuroimage.2010.12.037. [DOI] [PubMed] [Google Scholar]
- De Smedt B., Noël M.P., Gilmore C., Ansari D. How do symbolic and non-symbolic numerical magnitude processing skills relate to individual differences in children’s mathematical skills? A review of evidence from brain and behavior. Trends Neurosci. Educ. 2013;2(2):48–55. [Google Scholar]
- De Smedt B. Development of Mathematical Cognition. Elsevier Academic Press; San Diego, CA: 2016. Individual differences in arithmetic fact retrieval; pp. 219–243. [Google Scholar]
- De Visscher A., Noël M.P. Similarity interference in learning and retrieving arithmetic facts. In: Cappelletti M., Fias W., editors. The Mathematical Brain Across the Lifespan2. Elsevier; Amsterdam: 2016. [DOI] [PubMed] [Google Scholar]
- De Visscher A., Berens S.C., Keidel J.L., Noël M.P., Bird C.M. The interference effect in arithmetic fact solving: an fMRI study. Neuroimage. 2015;116:92–101. doi: 10.1016/j.neuroimage.2015.04.063. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. Towards an anatomical and functional model of number processing. Math. Cogn. 1995;1:83–120. [Google Scholar]
- Dehaene S., Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56(2):384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Delazer M., Ischebeck A., Domahs F., Zamarian L., Koppelstaetter F., Siedentopf C.M.…Felber S. Learning by strategies and learning by drill − evidence from an fMRI study. Neuroimage. 2005;25(3):838–849. doi: 10.1016/j.neuroimage.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Dell’Acqua F., Simmons A., Williams S.C.R., Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum. Brain Mapp. 2012;34(10):2464–2483. doi: 10.1002/hbm.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir Ö.E., Prado J., Booth J.R. The differential role of verbal and spatial working memory in the neural basis of arithmetic. Dev. Neuropsychol. 2014;39(6):440–458. doi: 10.1080/87565641.2014.939182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir-Lira O.E., Prado J., Booth J. Neural correlates of math gains vary depending on parental socioeconomic status (SES) Front Psychol. 2016;2016:00892. doi: 10.3389/fpsyg.2016.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis S.I., Laskaris N.A., Micheloyannis S. Transition dynamics of EEG-based network microstates during mental arithmetic and resting wakefulness reflects task-related modulations and developmental changes. Cogn. Neurodyn. 2015;9(4):371–387. doi: 10.1007/s11571-015-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowker A. Taylor & Francis; Sussex, UK: 2005. Individual Differences in Arithmetic: Implications for Psychology, Neuroscience and Education. [Google Scholar]
- Dresler T., Obersteiner A., Schecklmann M., Vogel A.C.M., Ehlis A.C., Richter M.M.…Fallgatter A.J. Arithmetic tasks in different formats and their influence on behavior and brain oxygenation as assessed with near-infrared spectroscopy (NIRS): A study involving primary and secondary school children. J. Neural Transm. 2009;116(12):1689–1700. doi: 10.1007/s00702-009-0307-9. [DOI] [PubMed] [Google Scholar]
- Eden G.F., Olulade O.A., Evans T.M., Krafnick A.J., Alkire D.R. Developmental dyslexia. In: Hickok G., Small S.L., editors. Neurobiology of Language. Academic Press; 2016. pp. 815–826. [Google Scholar]
- Eickhoff S.B., Nichols T.E., Laird A.R., Hoffstaedter F., Amunts K., Fox P.T.…Eickhoff C.R.v. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. 2016;137:70–85. doi: 10.1016/j.neuroimage.2016.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.M., Flowers D.L., Napoliello E.M., Olulade O.A., Eden G.F. The functional anatomy of single-digit arithmetic in children with developmental dyslexia. Neuroimage. 2014;101:644–652. doi: 10.1016/j.neuroimage.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.M., Kochalka J., Ngoon T.J., Wu S.S., Qin S., Battista C., Menon V. Brain structural integrity and intrinsic functional connectivity forecast 6 year longitudinal growth in children’s numerical abilities. J. Neurosci. 2015;35(33):11743–11750. doi: 10.1523/JNEUROSCI.0216-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T.M., Flowers D.L., Luetje M.M., Napoliello E., Eden G.F. Functional anatomy of arithmetic and word reading and its relationship to age. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson S., Tournier J.-D., Calamante F., Fabinyi G., Schneider-Kolsky M., Jackson G.D., Connelly A. White matter fiber tractography: why we need to move beyond DTI. J. Neurosurg. 2013;118(6):1367–1377. doi: 10.3171/2013.2.JNS121294. [DOI] [PubMed] [Google Scholar]
- Fias W., Menon V., Szucs D. Multiple components of developmental dyscalculia. Trends Neurosci. Educ. 2013;2(2):43–47. [Google Scholar]
- Fuchs L.S., Geary D.C., Fuchs D., Compton D.L., Hamlett C.L. Pathways to third-grade calculation versus word-reading competence: are they more alike or different? Child Dev. 2016;87:558–567. doi: 10.1111/cdev.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett K., Fleischner J.E. Automatization and basic fact performance of normal and learning disabled children. Learn. Disabil. Q. 1983;6(2):223–230. [Google Scholar]
- Geary D.C., Moore A.M. Cognitive and brain systems underlying early mathematical development. In: Cappelletti M., Fias W., editors. The Mathematical Brain Across the Lifespan. Elsevier; Amsterdam: 2016. [Google Scholar]
- Geary D.C., Bow-Thomas C.C., Yao Y. Counting knowledge and skill in cognitive addition: a comparison of normal and mathematically disabled children. J. Exp. Child Psychol. 1992;54(3):372–391. doi: 10.1016/0022-0965(92)90026-3. [DOI] [PubMed] [Google Scholar]
- Geary D. Mathematical disabilities − cognitive, neuropsychological and genetic components. Psychol. Bull. 1993;114:345–362. doi: 10.1037/0033-2909.114.2.345. [DOI] [PubMed] [Google Scholar]
- Geary D.C. International differences in mathematical achievement: their nature, causes, and consequences. Curr. Dir. Psychol. Sci. 1996;5(5):133–137. [Google Scholar]
- Geary D. Cognitive predictors of achievement growth in mathematics: a 5-year longitudinal study. Dev. Psychol. 2011;47(6):1539–1552. doi: 10.1037/a0025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstmann J. Syndrome of finger agnosia, disorientation for right and left, agraphia and acalculia. Arch. Neurol. Psychiatry. 1940;44:398–408. [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner R.H., De Smedt B. Neurophysiological evidence for the validity of verbal strategy reports in mental arithmetic. Biol. Psychol. 2011;87(1):128–136. doi: 10.1016/j.biopsycho.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Grabner R.H., Ansari D., Reishofer G., Stern E., Ebner F., Neuper C. Individual differences in mathematical competence predict parietal brain activation during mental calculation. NeuroImage. 2007;38(2):346–356. doi: 10.1016/j.neuroimage.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Grabner R.H., Ansari D., Koschutnig K., Reishofer G., Ebner F., Neuper C. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009;47(2):604–608. doi: 10.1016/j.neuropsychologia.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Han Z., Davis N., Fuchs L., Anderson A.W., Gore J.C., Dawant B.M. Relation between brain architecture and mathematical ability in children: a DBM study. Magn. Reson. Imaging. 2013;31(10):1645–1656. doi: 10.1016/j.mri.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S.A., Torgesen J.K., Wagner R.K., Rashotte C.A. The relations between phonological processing abilities and emerging individual differences in mathematical computation skills: a longitudinal study from second to fifth grades. J. Exp. Child Psychol. 2001;79(2):192–227. doi: 10.1006/jecp.2000.2586. [DOI] [PubMed] [Google Scholar]
- Henschen S.E. Uber Sprach-, Musik- und Rechenmechanismen und ihre Lokalisationen im Grosshirn. Zeitschrift Für Die Desamte Neurologie Und Psychiatrie. 1919;52:273–298. [Google Scholar]
- Hoeft F., Meyler A., Hernandez A., Juel C., Taylor-Hill H., Martindale J.L.…Gabrieli J.D.E. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc. Natl. Acad. Sci. U. S. A. 2007;104(10):4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbo I., Vandierendonck A. The development of strategy use in elementary school children: working memory and individual differences. J. Exp. Child Psychol. 2007;96(4):284–309. doi: 10.1016/j.jecp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Isaacs E.B., Edmonds C.J., Lucas A., Gadian D.G. Calculation difficulties in children of very low birthweight: a neural correlate. Brain. 2001;124(Pt 9):1701–1707. doi: 10.1093/brain/124.9.1701. https://doi.org/11522573 [DOI] [PubMed] [Google Scholar]
- Iuculano T., Rosenberg-Lee M., Richardson J., Tenison C., Fuchs L., Supekar K., Menon V. Cognitive tutoring induces widespread neuroplasticity and remediates brain function in children with mathematical learning disabilities. Nat. Commun. 2015;6:8453. doi: 10.1038/ncomms9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D., Ashkenazi S., Kochalka J., Evans T., Richardson J., Rosenberg-Lee M.…Menon V. Parietal hyper-connectivity, aberrant brain organization, and circuit-based biomarkers in children with mathematical disabilities. Dev. Sci. 2016;19(4):613–631. doi: 10.1111/desc.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D., Supekar K., Richardson J., Tenison C., Ashkenazi S., Rosenberg-Lee M.…Menon V. Reconfiguration of parietal circuits with cognitive tutoring in elementary school children. Cortex. 2016;83:231–245. doi: 10.1016/j.cortex.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D., Wassermann D., Chokhani R., Richardson J., Tenison C., Bammer R.…Menon V. Plasticity of left perisylvian white-matter tracts is associated with individual differences in math learning. Brain Struct. Funct. 2016;221(3):1337–1351. doi: 10.1007/s00429-014-0975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan N.C., Hanich L.B., Kaplan D. Arithmetic fact mastery in young children: a longitudinal investigation. J. Exp. Child Psychol. 2003;85(2):103–119. doi: 10.1016/s0022-0965(03)00032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Neuroimaging of the developing brain: taking development seriously. Hum. Brain Mapp. 2010;31(6):934–941. doi: 10.1002/hbm.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann L., Wood G., Rubinsten O., Henik A. Meta-analyses of developmental fMRI studies investigating typical and atypical trajectories of number processing and calculation. Dev Neuropsychol. 2011;36(6):763–787. doi: 10.1080/87565641.2010.549884. [DOI] [PubMed] [Google Scholar]
- Kawashima R., Taira M., Okita K., Inoue K., Tajima N., Yoshida H.…Fukuda H. A functional MRI study of simple arithmetic − A comparison between children and adults. Cogn. Brain Res. 2004;18(3):225–231. doi: 10.1016/j.cogbrainres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Kesler S.R., Sheau K., Koovakkattu D., Reiss A.L. Changes in frontal-parietal activation and math skills performance following adaptive number sense training: preliminary results from a pilot study. Neuropsychol. Rehabil. 2011;21(4):433–454. doi: 10.1080/09602011.2011.578446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick J., Swafford J., Findell B. National Academies Press; Washington, DC: 2001. Adding it up: Helping Children Learn Mathematics. [Google Scholar]
- Kim J.W., Kim B.N., Lee J., Na C., Kee B.S., Min K.J., Han D.H., Kim J.I., Lee Y.S. Desynchronization of theta-phase gamma-amplitude coupling during a mental arithmetic task in children with attention deficit/hyperactivity disorder. PLoS One. 2016:0145288. doi: 10.1371/journal.pone.0145288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koponen T., Salmi P., Eklund K., Aro T. Counting and RAN: predictors of arithmetic calculation and reading fluency. J. Educ. Psychol. 2013;105:162–175. [Google Scholar]
- Krafnick A.J., Flowers D.L., Luetje M.M., Napoliello E.M., Eden G.F. An investigation into the origin of anatomical differences in dyslexia. J. Neurosci. 2014;34(3):901–908. doi: 10.1523/JNEUROSCI.2092-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Mur M., Bandettini P. Representational similarity analysis − connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008 doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucian K., von Aster M. Developmental dyscalculia. Eur. J. Pediatr. 2015;174(1):1–13. doi: 10.1007/s00431-014-2455-7. [DOI] [PubMed] [Google Scholar]
- Kucian K., von Aster M., Loenneker T., Dietrich T., Martin E. Development of neural networks for exact and approximate calculation: a FMRI study. Dev. Neuropsychol. 2008;33(4):447–473. doi: 10.1080/87565640802101474. [DOI] [PubMed] [Google Scholar]
- LeFevre J., Sadesky G.S., Bisanz J. Selection of procedures in mental addition: reassessing the problem size effect in adults. J. Exp. Psychol.-Learn. Mem. Cogn. 1996;22(1):216–230. [Google Scholar]
- Lebel C., Rasmussen C., Wyper K., Andrew G., Beaulieu C. Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol.: Clin. Exp. Res. 2010;34(2):354–363. doi: 10.1111/j.1530-0277.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Lemaire P., Siegler R.S.1. Four aspects of strategic change: contributions to children’s learning of multiplication. J. Exp. Psychol. 1995;124(1):83–97. doi: 10.1037//0096-3445.124.1.83. [DOI] [PubMed] [Google Scholar]
- Li Y., Hu Y., Wang Y., Weng J., Chen F. Individual structural differences in left inferior parietal area are associated with schoolchildrens’ arithmetic scores. Front. Hum. Neurosci. 2013;7(December):844. doi: 10.3389/fnhum.2013.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matejko A.A., Ansari D. Drawing connections between white matter and numerical and mathematical cognition: a literature review. Neurosci. Biobehav. Rev. 2015;48:35–52. doi: 10.1016/j.neubiorev.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Matejko A.A., Price G.R., Mazzocco M.M.M., Ansari D. Individual differences in left parietal white matter predict math scores on the Preliminary Scholastic Aptitude Test. Neuroimage. 2013;66:604–610. doi: 10.1016/j.neuroimage.2012.10.045. [DOI] [PubMed] [Google Scholar]
- McClelland J.L., McNaughton B.L., O’Reilly R.C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102(3):419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Meintjes E.M., Jacobson S.W., Molteno C.D., Gatenby J.C., Warton C., Cannistraci C.J.…Jacobson J.L. An fMRI study of magnitude comparison and exact addition in children. Magn. Reson. Imaging. 2010;28(3):351–362. doi: 10.1016/j.mri.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Rivera S.M., White C.D., Glover G.H., Reiss A.L. Dissociating prefrontal and parietal cortex activation during arithmetic processing. Neuroimage. 2000;12(4):357–365. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- Menon V. Arithmetic in the child and adult brain. In: Cohen Kadosh R., Dowker A., editors. The Oxford Handbook Of Numerical Cognition. Oxford University Press; Oxford: 2015. [Google Scholar]
- Menon V. Working memory in children’s math learning and its disruption in dyscalculia. Curr. Opin. Behav. Sci. 2016;10:125–132. doi: 10.1016/j.cobeha.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondt K., Struys E., Van den Noort M., Balériaux D., Metens T., Paquier P.…Denolin V. Neural differences in bilingual children’s arithmetic processing depending on language of instruction. Mind Brain Educ. 2011;5(2):79–88. [Google Scholar]
- Moore R.D., Drollette E.S., Scudder M.R., Bharij A., Hillman C.H. The influence of cardiorespiratory fitness on strategic, behavioral, and electrophysiological indices of arithmetic cognition in preadolescent children. Front. Hum. Neurosci. 2014;8(May):258. doi: 10.3389/fnhum.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Mathematics Advisory Panel . U.S. Department of Education; Washington, DC: 2008. Foundations for Success: The Final Report of the National Mathematics Advisory Panel. [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci. 2006;10(9):424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Norton E.S., Beach S.D., Gabrieli J.D.E. Neurobiology of dyslexia. Curr. Opin. Neurobiol. 2015;30:73–78. doi: 10.1016/j.conb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova M., Sokolov A.N., Krägeloh-Mann I. Arithmetic and brain connectivity: mental calculation in adolescents with periventricular lesions. Neuropsychologia. 2009;47(2):439–445. doi: 10.1016/j.neuropsychologia.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Peng P., Namkung J., Barnes M., Sun C. A meta-Analysis of mathematics and working memory: moderating effects of working memory domain, type of mathematics skill, and sample characteristics. J. Educ. Psychol. 2016;108(4):455–473. [Google Scholar]
- Peters L., De Smedt B., Op de Beeck H.P. The neural representation of Arabic digits in visual cortex. Front. Hum. Neurosci. 2015;9(October):517. doi: 10.3389/fnhum.2015.00517. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4585091&tool=pmcentrez&rendertype=abstract Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L., Polspoel B., Op de Beeck H., De Smedt B. Brain activity during arithmetic in symbolic and non-symbolic formats in 9–12 year old children. Neuropsychologia. 2016;86:19–28. doi: 10.1016/j.neuropsychologia.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Prado J., Mutreja R., Zhang H., Mehta R., Desroches A.S., Minas J.E., Booth J.R. Distinct representations of subtraction and multiplication in the neural systems for numerosity and language. Hum. Brain Mapp. 2011;32(11):1932–1947. doi: 10.1002/hbm.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado J., Mutreja R., Booth J.R. Developmental dissociation in the neural responses to simple multiplication and subtraction problems. Dev. Sci. 2014;17(4):537–552. doi: 10.1111/desc.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G.R., Holloway I., Räsänen P., Vesterinen M., Ansari D. Impaired parietal magnitude processing in developmental dyscalculia. Curr. Biol. 2007;17(24):1042–1043. doi: 10.1016/j.cub.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Price G.R., Mazzocco M.M.M., Ansari D. Why mental arithmetic counts: brain activation during single digit arithmetic predicts high school math scores. J. Neurosci. 2013;33(1):156–163. doi: 10.1523/JNEUROSCI.2936-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G.R., Wilkey E.D., Yeo D.J., Cutting L.E. The relation between 1 st grade grey matter volume and 2nd grade math competence. Neuroimage. 2016;124:232–237. doi: 10.1016/j.neuroimage.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Corona B., Rodríguez-Camacho M., Silva-Pereyra J., Marosi E., Fernández T., Guerrero V. Event-related potentials findings differ between children and adults during arithmetic-fact retrieval. Neurosci. Lett. 2010;468(3):220–224. doi: 10.1016/j.neulet.2009.10.094. [DOI] [PubMed] [Google Scholar]
- Qin S., Cho S., Chen T., Rosenberg-Lee M., Geary D.C., Menon V. Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nat. Neurosci. 2014;17(9):1263–1269. doi: 10.1038/nn.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada R.D.S., Tsao F.M., Liu H.M., Holloway I.D., Ansari D., Kuhl P.K. Linking brain-wide multivoxel activation patterns to behaviour: examples from language and math. Neuroimage. 2010;51(1):462–471. doi: 10.1016/j.neuroimage.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranpura A., Isaacs E., Edmonds C., Rogers M., Lanigan J., Singhal A.…Butterworth B. Developmental trajectories of grey and white matter in dyscalculia. Trends Neurosci. Educ. 2013;2(2):56–64. [Google Scholar]
- Rivera S.M., Reiss A.L., Eckert M.A., Menon V. Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cereb. Cortex. 2005;15(11):1779–1790. doi: 10.1093/cercor/bhi055. [DOI] [PubMed] [Google Scholar]
- Robinson K.M., Arbuthnott K.D., Rose D., McCarron M.C., Globa C.A., Phonexay S.D. Stability and change in children’s division strategies. J. Exp. Child Psychol. 2006;93(3):224–238. doi: 10.1016/j.jecp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Rocha F.T., Rocha A.F., Massad E., Menezes R. Brain mappings of the arithmetic processing in children and adults. Brain Res. Cogn. Brain Res. 2005;22(3):359–372. doi: 10.1016/j.cogbrainres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Lee M., Barth M., Menon V. What difference does a year of schooling make? Maturation of brain response and connectivity between 2nd and 3rd grades during arithmetic problem solving. Neuroimage. 2011;57(3):796–808. doi: 10.1016/j.neuroimage.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Lee M., Ashkenazi S., Chen T., Young C.B., Geary D.C., Menon V. Brain hyper-connectivity and operation-specific deficits during arithmetic problem solving in children with developmental dyscalculia. Dev. Sci. 2015;18(3):351–372. doi: 10.1111/desc.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzer S., Kucian K., Martin E., Aster M., von Klaver P., Loenneker T. Optimized voxel-based morphometry in children with developmental dyscalculia. Neuroimage. 2008;39(1):417–422. doi: 10.1016/j.neuroimage.2007.08.045. [DOI] [PubMed] [Google Scholar]
- Rubinsten O., Henik A. Double dissociation of functions in developmental dyslexia and dyscalculia. J. Educ. Psychol. 2006;98(4):854–867. [Google Scholar]
- Rykhlevskaia E., Uddin L.Q., Kondos L., Menon V. Neuroanatomical correlates of developmental dyscalculia: combined evidence from morphometry and tractography. Front. Hum. Neurosci. 2009;3(November):51. doi: 10.3389/neuro.09.051.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagger B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schleepen T.M.J., Van Mier H.I., De Smedt B. The contribution of numerical magnitude comparison and phonological processing to individual differences in fourth graders’ multiplication fact ability. PLoS One. 2016;11(6):1–20. doi: 10.1371/journal.pone.0158335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Beeres K., Coban L., Merz S., Schmidt S.S., Stricker J., De Smedt B. Associations of non-symbolic and symbolic numerical magnitude processing with mathematical competence: a meta-analysis. Dev. Sci. 2017;20:e12372. doi: 10.1111/desc.12372. [DOI] [PubMed] [Google Scholar]
- Schoenfeld A.H., Conner E., Conner E. What doesn’t work: the challenge and failure of the What Works Clearinghouse to conduct meaningful review of studies of mathematics curricula. Educ. Res. 2006;35(2):13–21. [Google Scholar]
- Siegler R.S., Stern E. Conscious and unconscious strategy discoveries: a microgenetic analysis. J. Exp. Psychol. Gen. 1998;127(4):377–397. doi: 10.1037//0096-3445.127.4.377. [DOI] [PubMed] [Google Scholar]
- Siegler R.S. The perils of averaging data over strategies: an example from children’s addition. J. Exp. Psychol. Gen. 1987;116(3):250–264. [Google Scholar]
- Siegler R.S. Oxford University Press; Oxford: 1996. Emerging Minds: The Process of Change in Children’s Thinking. [Google Scholar]
- Simmons F.R., Singleton C. Do weak phonological representations impact on arithmetic development? A review of research into arithmetic and dyslexia. Dyslexia. 2008;14:77–94. doi: 10.1002/dys.341. [DOI] [PubMed] [Google Scholar]
- Simos P.G., Kanatsouli K., Fletcher J.M., Sarkari S., Juranek J., Cirino P.…Papanicolaou A.C. Aberrant spatiotemporal activation profiles associated with math difficulties in children: a magnetic source imaging study. Neuropsychology. 2008;22(5):571–584. doi: 10.1037/0894-4105.22.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.N., Squire L.R. Medial temporal lobe activity during retrieval of semantic memory is related tot the age of the memory. J. Neurosci. 2009;29(4):930–938. doi: 10.1523/JNEUROSCI.4545-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling M.J. 2nd ed. Blackwell Publishing; Malden: 2000. Dyslexia. [Google Scholar]
- Supekar K., Swigart A.G., Tenison C., Jolles D.D., Rosenberg-Lee M., Fuchs L., Menon Vv. Neural predictors of individual differences in response to math tutoring in primary-grade school children. Proc. Natl. Acad. Sci. U. S. A. 2013;110(20):8230–8235. doi: 10.1073/pnas.1222154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till C., Deotto A., Tipu V., Sled J., Bethune A., Narayanan S.…Banwell B.v. White matter integrity and math performance in pediatric multiple sclerosis: a diffusion tensor imaging study. Neuroreport. 2011;22(18):1005–1009. doi: 10.1097/WNR.0b013e32834dc301. [DOI] [PubMed] [Google Scholar]
- Tsang J.M., Dougherty R.F., Deutsch G.K., Wandell B.A., Ben-Shachar M. Frontoparietal white matter diffusion properties predict mental arithmetic skills in children. Proc. Natl. Acad. Sci. 2009;106(52):22546–22551. doi: 10.1073/pnas.0906094106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschentscher N., Hauk O.1. How are things adding up? Neural differences between arithmetic operations are due to general problem solving strategies. Neuroimage. 2014;92:369–380. doi: 10.1016/j.neuroimage.2014.01.061. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K., Amin H., Rykhlevskaia E., Nguyen D.A., Greicius M.D., Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb. Cortex. 2010;20(11):2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek L., Ghesquière P., Lagae L., De Smedt B. Left fronto-parietal white matter correlates with individual differences in children’s ability to solve additions and multiplications: a tractography study. Neuroimage. 2014;90:117–127. doi: 10.1016/j.neuroimage.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Van Beek L., Ghesquiere P., De Smedt B., Lagae L. The arithmetic problem size effect in children: an event-related potential study. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek L., Ghesquiere P., De Smedt B., Lagae L. Arithmetic difficulties in children with mild traumatic brain injury at the subacute stage of recovery. Dev. Med. Child Neurol. 2015:1042–1048. doi: 10.1111/dmcn.12858. [DOI] [PubMed] [Google Scholar]
- Vanbinst K., De Smedt B. Individual differences in children’s mathematics achievement: the roles of symbolic numerical magnitude processing and domain-general cognitive functions. In: Cappelletti M., Fias W., editors. The Mathematical Brain Across the Lifespan. Elsevier; Amsterdam: 2016. pp. 105–130. [DOI] [PubMed] [Google Scholar]
- Vanbinst K., Ghesquiere P., De Smedt B. Representations and individual differences in children’s arithmetic strategy use. Mind Brain Educ. 2012;6(3):129–136. [Google Scholar]
- Vanbinst K., Ceulemans E., Ghesquière P., De Smedt B. Profiles of children’s arithmetic fact development: a model-based clustering approach. J. Exp. Child Psychol. 2015;133:29–46. doi: 10.1016/j.jecp.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Vanbinst K., Ghequière P., De Smedt B. Does numerical processing uniquely predict first graders’ future development of single-digit arithmetic? Learning and Individual Differences. 2015;37:153–160. [Google Scholar]
- Vanbinst K., Ansari D., Ghesquière P., De Smedt B. Symbolic numerical magnitude proceing is as important to arithmetic as phonological awarene is to reading. PLoS One. 2016;11(3):1–11. doi: 10.1371/journal.pone.0151045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Vanderauwera J., Theys C., De Vos A., Vanvooren S., Sunaert S.…Ghesquière P. A DTI tractography study in pre-readers at risk for dyslexia. Dev. Cogn. Neurosci. 2015;14:8–15. doi: 10.1016/j.dcn.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Hoeft F., Norton E.S. How MRI brain imaging studies of pre-reading children inform theories of the etiology of developmental dyslexia and educational practice. Curr. Opin. Behav. Sci. 2016:1–7. doi: 10.1016/j.cobeha.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vourkas M., Karakonstantaki E., Simos P.G., Tsirka V., Antonakakis M., Vamvoukas M.…Micheloyannis S. Simple and difficult mathematics in children: a minimum spanning tree EEG network analysis. Neurosci. Lett. 2014;576:28–33. doi: 10.1016/j.neulet.2014.05.048. [DOI] [PubMed] [Google Scholar]
- Xuan D., Wang S., Yang Y., Meng P., Xu F., Yang W.…Yang Y. Age difference in numeral recognition and calculation: an event-related potential study. Child Neuropsychol. 2007;13(1):1–17. doi: 10.1080/09297040600760465. [DOI] [PubMed] [Google Scholar]
- Zamarian L., Delazer M. Arithmetic learning in adults: evidence from brain imaging. In: Cohen Kadosh R., Dowker A., editors. The Oxford Handbook of Numerical Cognition. Oxford University Press; Oxford: 2015. [Google Scholar]
- Zamarian L., Ischebeck A., Delazer M. Neuroscience of learning arithmetic − Evidence from brain imaging studies. Neurosci. Biobehavi. Rev. 2009;33(6):909–925. doi: 10.1016/j.neubiorev.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Zhou X., Booth J.R., Lu J., Zhao H., Butterworth B., Chen C., Dong Q. Age-independent and age-dependent neural substrate for single-digit multiplication and addition arithmetic problems. Dev. Neuropsychol. 2011;36(3):338–352. doi: 10.1080/87565641.2010.549873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eimeren L., Niogi S.N., McCandliss B.D., Holloway I.D., Ansari D. White matter microstructures underlying mathematical abilities in children. Neuroreport. 2008;19(11):1117–1121. doi: 10.1097/WNR.0b013e328307f5c1. [DOI] [PubMed] [Google Scholar]