Abstract
Nonverbal Learning Disability (NVLD) is characterized by deficits in visual-spatial, but not verbal, reasoning. Nevertheless, the functioning of the neural circuits supporting spatial processing have yet to be assessed in children with NVLD. We compared the resting state functional connectivity of a spatial brain network among children with NVLD, children with reading disorder (RD), and typically developing (TD) children. Seventy-five participants (7–15 years old) were included in the study (20 TD, 24 NVLD, and 31 RD). Group differences in global efficiency and functional connectivity among 12 regions comprising a previously defined spatial network were evaluated. Associations with behavior were explored. Global efficiency of the spatial network associated positively with spatial ability and inversely with socioemotional problems. Within the spatial network, associations between left posterior cingulate (PCC) and right retrosplenial cortical activity were reduced in children with NVLD relative to those without spatial deficits (RD and TD). Connectivity between left PCC and right posterior cerebellum (Crus I and II) was reduced in both groups of children with learning disabilities (NVLD and RD) relative to TD children. Functional connectivity of the spatial network was atypically associated with cognitive and socioemotional performance in children with NVLD. Identifying a neurobiological substrate for NVLD provides evidence that it is a discrete clinical entity and suggests targets for treatment.
Subject terms: Spatial memory, Social behaviour, Human behaviour
Introduction
Nonverbal Learning Disability (NVLD) is a neurodevelopmental disorder characterized by deficits in spatial, but not verbal, reasoning. Children with NVLD frequently have accompanying impairments in socioemotional functioning, mathematical skills, executive function, and fine motor control1–3 that may derive from their core deficit in spatial processing. Understudied is if NVLD is discrete from other neurodevelopmental disorders. For example, the social deficits associated with the disorder are often thought to overlap with Autism Spectrum Disorders (ASD). However, prior findings point to differing social deficits in the two disorders1,2 that are subserved by distinct circuit alterations4. A deeper understanding of the neural correlates of NVLD could provide evidence for recognizing NVLD as a discrete clinical entity.
Spatial reasoning is a complex cognitive skill that relies on perception, memory, attention, and object recognition5. The spatial deficit in NVLD encompasses problems in visuospatial awareness (e.g., awareness of own body in space), visuospatial construction (e.g., copying visually presented materials), visuospatial memory (e.g., remembering patterns and designs), spatial estimation (e.g., judging distance), three-dimensional thinking (e.g., imagining how things will look when rotated), interpreting information presented pictorially (e.g., reading maps) or visuospatial attention (e.g., visual scanning)6–10. Spatial function is also known to associate with social function11,12, suggesting that the social impairment observed in NVLD may derive from core deficits in spatial dysfunction. For example, children with NVLD might have difficulty in social situations due to their inability to comprehend nonverbal communication or cues, or to judge interpersonal space13,14. Despite the documented spatial deficits in NVLD, the functioning of the neural circuits that support spatial processing have yet to be assessed in children with NVLD. Prior findings using task- and resting state functional connectional connectivity point to the existence of a spatial orientation decision network15. In the current study, we examined resting state connectivity of this spatial network in children with NVLD. To avoid behavioral confounds associated with differential group performance in spatial tasks, we elected to study the spatial network using resting state functional connectivity rather than task-fMRI.
Herein, we compared resting state functional connectivity in children with NVLD to typically developing (TD) children and a clinical control group, children with reading disorder (RD). Children with RD have strengths in spatial reasoning16–19 despite other learning deficits20. Contrasting these groups thus allowed us to isolate functional abnormalities specific to NVLD from those associated with learning disabilities more generally. We constructed a spatial network using previously identified regions of interest (ROIs) that were activated during a spatial orientation decision task in healthy adults; resting state functional connectivity between these ROIs predicted spatial task performance in these same individuals15. Meta-analyses of spatial task fMRI studies have identified similar regions to those in the selected network21,22, providing strong evidence for their involvement in spatial reasoning. We first attempted to demonstrate the existence of the spatial network in children, extending prior work in adults15. We then assessed spatial network global efficiency, a graph theoretical measure of network efficiency. We hypothesized that spatial ability (as measured by Performance Intelligence Quotient [PIQ], composed of Block Design and Matrix Reasoning subtests) would be positively associated with resting state functional connectivity of the spatial network. Second, we evaluated group differences and hypothesized that children with NVLD would show altered global efficiency and region-to-region connectivity within the spatial network relative to RD and TD children, consistent with the spatial deficits that define NVLD. Last, we explored associations of socioemotional function (Child Behavior Checklist [CBCL], Total Problems and Total Competence subtests) with spatial ability (PIQ), and associations of both of these processes with spatial network connectivity in children with NVLD.
Results
Participants and behavioral test performance
All children were 7–15 years old; children with NVLD were older on average than those with RD and TD children (Table 1). NVLD and RD children had lower full-scale IQ (FSIQ) than TD children. As expected, those with NVLD had lowered spatial performance (PIQ Mean Difference = 20.74 vs. RD and 31.96 vs. TD), more parent-reported socioemotional problems (CBCL Total Problems Mean Difference = 11.99 vs. RD and 19.14 vs. TD), and lowered parent-rated competence (CBCL Total Competence Mean Difference = 13.40 vs. TD). During the resting state runs, no differences in mean head motion or number of useable images were detected between groups (Table 1).
Table 1.
Demographic Information.
| Demographics | TD (n = 20) | RD/RD-ADHD (n = 31) | NVLD (n = 24) | ANOVA F statistic |
|---|---|---|---|---|
| Age – months (SD) | 116.70 (15.06) 86–139 | 120.81 (20.58) 84–155 | 140.83 (29.87) 87–185 | 7.52** |
| Sex – N (%) female | 10 (50%) | 16 (51.6%) | 10 (42.7%) | 0.28 |
| FSIQ | 125.75 (13.09) 88–148 | 111.06 (14.5) 88–134 | 96.38 (10.17) 78–120 | 28.44*** |
| VIQ | 125.00 (11.72) 95–144 | 110.84 (13.63) 74–141 | 105.25 (11.34) 82–122 | 14.43*** |
| PIQ | 120.25 (13.29) 84–143 | 109.03 (16.77) 77–141 | 88.29 (10.05) 70–114 | 30.20*** |
| CBCL Total Problems | 44.95 (8.42) 34–69 | 52.10 (10.96) 25–71 | 64.08 (8.36) 42–82 | 22.49*** |
| CBCL Total Competence | 53.10 (10.03) 37–70 | 43.38 (10.23) 28–70 | 39.71 (7.72) 25–55 | 11.23*** |
| Usable Images | 214.30 (59.22) 106–273 | 186.90 (68.96) 89–278 | 186.92 (55.44) 98–276 | 2.42 |
| Mean Motion | 0.22 (0.21) 0.07–0.94 | 0.26 (0.17) 0.06–0.74 | 0.40 (0.31) 0.08–1.37 | 1.42 |
Displays demographic information for typically developing (TD) children and children with Reading Disorder (RD) or with Nonverbal Learning Disability (NVLD). Means, standard deviations, and ranges are presented for all continuous variables. The ANOVA column indicates ANOVA F-statistics comparing across all three groups. The number and percent of female participants was presented in the sex row and group differences were tested using chi-squared.
FSIQ = Full Scale IQ; VIQ = verbal IQ; PIQ = performance IQ.
*p < 0.05, **p < 0.01, ***p < 0.001.
To investigate whether the socioemotional difficulties observed in NVLD might derive from spatial processing deficits, the hallmark cognitive dysfunction in NVLD, the association between spatial ability (PIQ) and overall socioemotional functioning (CBCL Total Problems and Total Competence, normed t-scores) was evaluated. Across all participants, reduced spatial ability was associated with CBCL Total Problems (b = −0.22, 95% confidence interval [CI]: −0.37, −0.07, t(68) = −2.910, p < 0.005) and Total Competence (b = 0.22, 95% confidence interval [CI]: 0.07, 0.37, t(66) = 2.90, p = 0.005) controlling for age, sex, and NVLD diagnosis.
Spatial network in children
To demonstrate the existence of a spatial processing network in children, we examined average within network connectivity across the 12 nodes of the pre-defined spatial network. The average inter-regional connectivity of the spatial network was non-zero (mean = 0.09; 95% CI 0.083–0.106; t(74) = 16.38).
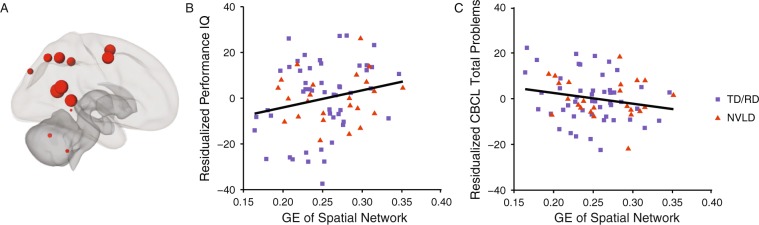
We then probed associations between the global efficiency of the spatial network and behavioral outcomes. GE of the spatial network was associated with PIQ (b = 80.51 [95% CI: −1.42, 162.44], t(69) = 1.96, Fig. 1) and with CBCL total problems (b = −50.93 [95% CI: −107.01, 5.14], t(68) = −1.81, Fig. 1), but not with CBCL Total Competence (b = 26.46 [95% CI: −31.18, 84.11], t(66) = 0.92, p = 0.36).
Figure 1.
Global Efficiency of the Spatial Network. Displays (A) regions of interest comprising the spatial network depicted by red spheres. The relative size of the circle reflects the global efficiency (GE) of the region. Scatter plots show significant associations between residualized GE values (controlling for age, sex, mean motion, and group status) and (B) spatial ability (Performance Intelligence Quotient [PIQ]; b = 80.51, t(69) = 1.96, p = 0.05), and (C) socioemotional impairment (Child Behavior Checklist, Total Problems; b = −50.93, t(68) = −1.81, p = 0.07). Typically developing children (TD) and children with reading disorder (RD) are shown as purple squares, and children with nonverbal learning disability (NVLD) are shown as red triangles.
Group differences in spatial network
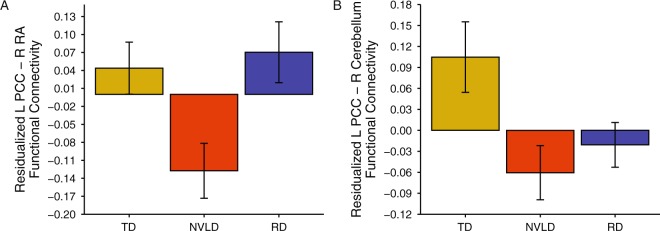
Multivariate analyses of covariance (MANCOVA), one per spatial network ROI, revealed altered spatial network connectivity with the Posterior Cingulate Cortex (PCC). No other spatial network seeds exhibited altered spatial network connectivity. Post-hoc comparisons revealed group differences between left PCC and right RA, deriving from reduced connectivity in children with NVLD relative to the other two groups (F(2, 69) = 6.82; Fig. 2), and between left PCC and right cerebellum, deriving from reduced connectivity in children with NVLD and those with RD relative to TD children (F(2, 69) = 5.15; Fig. 2). No significant group differences in spatial network GE were detected (F(2, 69) = 2.22).
Figure 2.
Group Differences in Spatial Network Connectivity. Displays differences in spatial network connectivity across the typically developing (TD) children, children with reading disorder (RD), and children with nonverbal learning disability (NVLD). (A) Shows a group difference in residualized connectivity between left posterior cingulate cortex (PCC) and the right retrolimbic area (RA). (B) Shows a group difference in residualized connectivity between left PCC and right cerebellum. Bars indicate mean connectivity values (Fisher r-to-Z transformed correlation values) residualized for age, sex, and mean motion; error bars indicate the standard error of the mean.
Exploratory analyses
To explore brain-behavior associations diagnostic groups were combined when they did not differ in connectivity. For PCC-RA connectivity, children with RD and TD children were combined, representing a group without spatial deficits, and compared to children with NVLD. The group (NVLD vs. TD + RD) by PCC-RA connectivity interaction significantly predicted PIQ (b = 41.13, 95% CI: 7.33, 74.94, t(68) = 2.43), such that PIQ increased as connectivity increased in children with NVLD (b = 23.59, 95% CI: 2.64, 44.53, t(19) = 2.36), but decreased as connectivity increased in TD children and children with RD (b = −12.13, 95% CI: −32.38, 6.12, t(46) = −1.37; Fig. 3). No statistically significant interactions or main effects were detected predicting CBCL effects. To explore brain-behavior associations of PCC-cerebellum connectivity, children with NVLD and RD were combined, representing a learning disability [LD] group, and compared to the TD children. There were no significant interactions or main effects predicting PIQ or CBCL.
Figure 3.
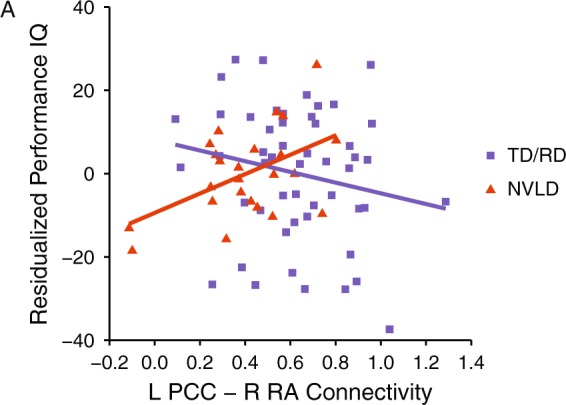
Association between PIQ and Spatial Network Connectivity. Displays the association between residualized spatial ability (Performance IQ) and left PCC - right RA connectivity (controlling for age, sex, mean motion, and group status). Typically developing (TD) children and children with reading disorder (RD) are shown as purple squares, and children with nonverbal learning disorder (NVLD) are shown as red triangles. The group by connectivity interaction predicted PIQ (b = 41.13, t(68) = 2.43, p = 0.02). Specifically, PIQ increased as connectivity increased in children with NVLD (b = 23.59, t(19) = 2.36, p = 0.03), whereas PIQ decreased as connectivity increased in TD children and children with RD (b = −12.13, t(46) = −1.37, p = 0.18). PCC = posterior cingulate cortex; PIQ = Performance IQ; RA = retrolimbic area.
Discussion
This was the first study to examine resting state functional connectivity of a spatial network in children with NVLD, and to compare their connectivity to that in children with RD and TD children. We established a spatial network in 7–15-year-old children, extending prior work in adults, by first identifying functionally connected network regions, and second by showing that GE of the spatial network associated with PIQ across the sample. We further showed that spatial processing and spatial network GE were positively associated with socioemotional functioning, supporting the theory that deficits in spatial processing may underlie the social impairment observed in NVLD13,14. Finally, inter-regional connectivity was altered in children with NVLD. Specifically, cortico-cortical connectivity between left PCC and right RA was reduced in children with NVLD relative to those without spatial deficits (children with RD and TD children) and was associated differentially with spatial ability. In contrast, cortico-cerebellar connectivity between left PCC and right cerebellum (Crus I and II) was reduced in both children with NVLD and children with RD relative to TD children. These findings suggest that the spatial and social deficits in NVLD may derive from underlying alterations of a spatial processing network, providing evidence that NVLD is a discrete clinical entity.
Children with NVLD showed reduced cortico-cortical connectivity within the spatial network relative to children with RD and TD children. Associations between left PCC - right RA connectivity and spatial ability varied with NVLD diagnosis. Although connectivity between these regions increased with better spatial ability in children with NVLD, in those who did not have spatial deficits (children with RD and TD children), connectivity decreased with better ability. The retrosplenial cortex supports allocentric representation and contextual memory, aspects of spatial reasoning that may support performance on tests of spatial ability, such as PIQ23. Our findings may be interpreted as showing that children with RD and TD children require less cortico-cortical connectivity to achieve performance on measures of spatial ability, possibly because a level of automaticity has already been achieved.
Children with both NVLD and RD showed reduced cortico-cerebellar connectivity relative to TD children. That such altered connectivity between left PCC and right posterior cerebellum characterized both groups of children with learning disabilities points to a possible marker of learning disabilities in general. Such findings are consistent with the role of the posterior cerebellum in general learning processes, including spatial learning24–26. Future studies should further investigate the specific contribution of cerebellar Crus I and II functional connectivity to learning in children.
Altered connectivity in the PCC may represent a neural signature of NVLD. The PCC is a hub of the default mode network, which is known to underlie mentalizing and social processing27,28. As children with NVLD often have difficulties with internalizing and social problems6,29, such findings suggest that altered connectivity within and between the spatial and default mode networks may contribute to socioemotional problems that accompany NVLD. This interpretation is consistent with prior findings that altered connectivity between nodes of the spatial (parahippocampal gyrus) and default mode (PCC) networks were associated with social impairments in children with Autism Spectrum Disorder (ASD)30. Specifically, children with ASD showed increased connectivity from PCC associated with social impairment, in contrast to reduced connectivity detected in children with NVLD. These differences in patterns of functional connectivity and social impairment align with prior findings that social deficits in ASD and NVLD derive from altered patterns of connectivity within different regions of the salience network31.
Cortical and cerebellar regions included in the spatial network we studied are purported to support maze navigation, route learning and spatial processing in healthy individuals32–34 and preclinical models24,35,36. Task fMRI studies have shown engagement of the parahippocampal gyrus, retrosplenial cortex, and posterior parietal cortex during a virtual maze navigation task32 and activation of the left medial frontal gyrus and retrosplenial cortex during a route-learning task33. Structural MRI studies have shown that decreased cortical thickness in the precuneus, SOG, and IPL is associated with decreased spatial relative to verbal ability34. Evidence from rodent models also implicates a number of these regions in spatial processing, e.g. the retrosplenial cortex/posterior cingulate cortex35,36 and cerebellum24 in maze navigation. Further support for a spatial processing role involving this network derives from studies of individuals with deficits in spatial reasoning. Altered regional functional activity in this spatial network has been documented in individuals with Turner syndrome37, 22q11 deletion38, and neurofibromatosis39, conditions characterized by spatial impairment. Consistent with our finding of altered PCC connectivity in those with NVLD individuals with 22q11 deletion show reduced PCC activity during spatial working memory tasks40. In contrast to our finding of reduced connectivity between PCC-posterior cerebellum in individuals with LDs, individuals with neurofibromatosis show increased connectivity between PCC and cerebellar regions39. These contrasting findings may point to differences in pathophysiology of the two disorders.
Our study has several limitations. First, the relatively small sample size limits the generalizability of findings. Second, exploratory associations between behavioral outcome measures and functional connectivity values were not corrected for multiple comparisons and therefore require replication. Third, we did not have direct measures of social processing across all of children; future studies should include a broader range of spatial and social tasks to further understand the processing deficits in children with NVLD. In addition, future studies with larger samples may be successful in disentangling functional alterations in spatial versus reading circuits in children with LDs, thereby dissociating such alterations based on behavioral phenotypes.
In sum, the present study investigated, for the first time, the neural underpinnings of the spatial deficit that characterizes NVLD. We provided evidence that the spatial network observed in adults is also present in children, and that efficiency of this network associates with spatial ability and socioemotional functioning. In addition, we demonstrated that children with NVLD have altered functional connectivity within this network that associates with the spatial impairments that characterize the disorder, suggesting that this pattern of aberrant connectivity may represent a neural signature of NVLD. These findings may guide novel opportunities for treatment, e.g. behavioral treatments could target remediation of spatial deficits and assess subsequent improvement in spatial and socioemotional functioning. Additionally, pharmacological targeting of the spatial network may likewise improve function in these areas.
Methods
Participants
One hundred and two children (7–15 years old) enrolled in the current study and were screened for inclusion/exclusion at the New York State Psychiatric Institute, including three groups of children (50 children with NVLD, 63 children with reading disorder, and 22 typically developing children) that were recruited through announcements posted at local schools and clinics, on social media, and in the newsletter of The NVLD Project, a non-profit organization aimed at developing resources for families of children with NVLD. All children were monolingual English speakers. The Institutional Review Board at New York State Psychiatric Institute approved the study; children and their parents and/or legal guardians provided written informed assent and consent, respectively. All research was performed in accordance with the relevant guidelines and regulations.
Of the 50 children evaluated for NVLD (see below), 15 did not meet diagnostic criteria, and five others did not successfully complete an MRI scan (refused to scan, aborted during scan, and/or fell asleep), leaving 30 children with NVLD. Of the 63 children evaluated for RD (see below), eight did not meet diagnostic criteria and 21 did not successfully complete an MRI scan, leaving 34 children with RD. Of the 22 TD children recruited, none met exclusionary criteria and one did not successfully complete an MRI scan, leaving 21 TD children. Of the children who met criteria and completed an MRI scan, 6 children with NVLD, 3 children with RD, and 1 TD children were then excluded from imaging analyses due to head motion (see below). A total of 24 children with NVLD, 31 children with RD, and 20 TD children were included in the final analyses (Table 1).
Diagnostic criteria
A diagnosis of NVLD was established in accord with prior research criteria31,41,42 (Table 2). Children were included in the NVLD group if they had perceptual deficits, intact reading abilities, and deficits in two of the following domains: fine motor, math calculation, visual executive functioning, or social skills.
Table 2.
Criteria for NVLD Diagnosis.
| Criterion | Assessment Measure |
|---|---|
| Child must have: | |
| Perceptual deficit OR a discrepancy between VIQ and PIQ (>15 points) | WISC or WASI: Block Design or Matrix Reasoning ≤ 16th%ile |
| Intact single word reading abilities | WJ-III Letter Word Identification > 16th%ile |
| Absence of autistic traits | ADI-R Interests and Behaviors Module ≤ 4 |
| Child must also have 2 of the following: | |
| Fine motor difficulties | Perdue Pegboard ≤16th%ile |
| Math calculation difficulties | WJ-III Calculation ≤16th%ile |
| Visual executive functioning difficulties | Rey Osterrieth Complex Figure Test Copy ≤16th%ile |
| Social difficulties | Vineland-II Socialization domain ≤16th%ile or CBCL Social Problems ≥95th%ile |
Displays the criteria for NVLD diagnosis. ADI-R = Autism Diagnostic Interview – Revised; CBCL = Child Behavior Checklist; NVLD = Nonverbal Learning Disability; WJ = Woodcock Johnson; WASI = Wechsler Abbreviated Scale of Intelligence; WISC = Wechsler Intelligence Scale for Children.
A diagnosis of RD was established by two independent licensed psychologists following the procedure outlined in Davis, et al.43. Children were included if an RD diagnosis was indicated by clinical history and by poor performance (at or below 25th percentile) in at least three domains: word-reading accuracy, pseudoword reading, encoding, rapid naming, or silent or oral reading comprehension. Lifetime diagnosis of neurological or neurodevelopmental disorders (other than Specific Learning Disorder or ADHD) were exclusionary, as determined by clinical interview and administration of the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS)44.
Typically developing children had no current or lifetime diagnoses as determined by the KSADS44. Children in all three groups were excluded if they had an Full-Scale Intelligence Quotient [FSIQ] < 80 based on the Wechsler Abbreviated Scale of Intelligence (WASI)45, any history of major medical conditions, or MRI contraindication.
Neuropsychological and psychosocial outcome measures
A neuropsychological test battery was administered to all participants by a certified school psychologist (Ed.M.) who had formal Autism Diagnosis Interview-Revised (ADI-R)46 and KSADS clinical training. Measures were selected to identify clinical diagnoses. Parents of all participants completed the Child Behavior Checklist (CBCL), a measure of behavioral impairment47. All children completed: WASI, full scale, verbal, and performance intelligence quotient (FSIQ, VIQ, and PIQ, respectively) subscales; Woodcock Johnson Achievement (WJ) 3rd edition, Letter-Word Identification, Word Attack, Spelling, and Reading Fluency subtests48; Comprehensive Test of Phonological Processing 2nd edition, Rapid Letter Naming and Rapid Digit Naming subtests49; Gray Oral Reading Test 5th edition50; Test of Word Reading Efficiency 2nd edition, sight-word efficiency and phonemic decoding efficiency subtests51; and Gates-MacGinitie Reading Tests 4th edition, reading comprehension subtest52. These measures were administered to identify reading problems. Children with NVLD additionally completed Purdue Pegboard53, Rey-Osterieth Complex Figure Test copy54, and WJ Achievement, Math Calculation subtest55. These measures were administered to identify NVLD.
Neuroimaging acquisition
Functional and anatomical MRI data were acquired on a 3 T GE 750 scanner. Structural T1 images were collected with an 8-channel head coil using a 3D FSPGR sequence (flip angle = 11, TE = 2.6 ms, TR = 6.4 ms, 180 slices, 1 mm isotropic resolution). Two runs of resting state data were acquired with a 32-channel head coil using an echo planar imaging (EPI) sequence (flip angle = 77, TE = 30 ms, TR = 2000 ms, 34 slices, 3.5 mm isotropic resolution, 140 acquisition frames per run, 4 minutes and 40 seconds long). During the two resting state runs, participants were instructed to rest quietly with their eyes open without falling asleep. The examiner monitored that participants kept their eyes open and stayed awake during these scans using an in-scanner eye-tracking camera.
Resting state functional connectivity preprocessing
Analysis was performed in the CONN toolbox v17.f (www.nitrc.org/projects/conn)56 for SPM 12. Preprocessing followed a previously published pipeline31 and included realignment, unwarping, centering, slice timing correction, outlier detection, segmentation of cerebral spinal fluid, gray, and white matter, normalization to the Montreal Neurological Institute (MNI) template, and 8 mm full-width half-maximum smoothing for functional images. Structural images were centered, segmented, and normalized to the MNI template. EPI data were band-pass filtered (0.008–0.09 Hz). Denoising was completed with anatomical component-based noise correction (aCompCor)57, regressing ten white matter and ten CSF components (detrended and despiked).
Motion correction
To minimize effects of head motion, image frames exceeding 0.5 mm frame-to-frame displacement or frame-to-frame change in global signal change z > 3 were treated as outliers and in the first level models. In addition, 24 head motion parameters (motion + first-order derivatives + quadradic effects) were included in the first level models. To further adjust for potential effects of motion on functional connectivity measures, mean Euclidian head motion was included as a second level covariate. Participants with less than 81 useable frames were excluded from the analyses (N = 6 NVLD, N = 3 RD, N = 1 TD).
Network selection and connectivity measures
To identify a candidate spatial network, we reviewed studies using either task or resting-state functional magnetic resonance (fMRI) to define circuits associated with spatial navigation and spatial reasoning. We based our network on a study that identified brain regions activated during a spatial orientation decision task. These regions included: bilateral precuneus, posterior cingulate [PCC], and middle frontal gyri [MFG], left inferior parietal [IPL] and superior occipital gyri [SOG], and right posterior cerebellum (crus I/II), parahippocampal gyrus, and retrosplenial cortex [retrolimbic area; RA]). Subsequently functional connectivity of this network during resting state was shown to associate with performance on the spatial orientation task15. Supporting our selection of this network, meta-analyses of spatial task fMRI studies identified regions that overlapped with those in the selected network21,22. We additionally used Neurosynth to extract meta-analytic association maps of brain activation related to the terms “spatial” and “navigation” (thresholded at p-FDR < 0.01); 10/12 seeds in our selected spatial network overlapped these maps (Table 3; www.neurosynth.org; October 3rd 2019)58.
Table 3.
Spatial Network Definition and Neurosynth Validation.
| Arnold Network | Neurosynth Validation | ||||
|---|---|---|---|---|---|
| Seed | x | y | z | Neurosynth association with ‘navigation’? | Neurosynth association with ‘spatial’? |
| Left MFG | −26 | −4 | 58 | Yes | Yes |
| Right MFG | 22 | −6 | 50 | No | Yes |
| Right RA | 10 | −44 | 6 | Yes | No |
| Left IPL | −36 | −44 | 46 | No | Yes |
| Right PHG | 32 | −44 | −4 | Yes | No |
| Right Cerebellum Crus II | 48 | −48 | −46 | No | No |
| Right PCC | 20 | −54 | 20 | Yes | No |
| Left Precuneus | −10 | −56 | 50 | Yes | Yes |
| Right Precuneus | 6 | −68 | 50 | Yes | No |
| Left PCC | −16 | −58 | 16 | Yes | No |
| Right Cerebellum Crus I | 34 | −66 | −30 | Yes | No |
| Left SOG | −42 | −86 | 36 | No | No |
Lists the regions of interest (ROI) used in resting state analyses. Each ROI is part of a previously defined spatial network (Arnold et al., 2014). The MNI center coordinates of each ROI are indicated in the x, y, z columns. Neurosynth-based meta-analysis (p-FDR < 0.01) of the terms “navigation” and “spatial” produced association maps of brain regions activated in relevant tasks. Overlap between Arnold ROIs and association maps is indicated in the “Neurosynth Validation” columns. MFG = middle frontal gyrus; RA = retrolimbic area; IPL = inferior parietel lobule; PHG = parahippocampal gyrus; PCC = posterior cingulate cortex; SOG = superior occipital gyrus.
The first principal component of each ROI time series was computed to determine inter-regional temporal associations. The reported functional connectivity values are Fisher r-to-Z transformed correlations between 12 ROIs defining the spatial network15 (Table 3). Spherical ROIs with 6 mm radius were created using Marsbar59 centered on the peak MNI coordinates from the prior task-based results (see Supplemental Methods). GE, a graph theoretical measure of network efficiency, was calculated as the average of the inverse value of the shortest path length from each region to each other region; thus, higher GE values indicate greater network efficiency60,61. To avoid bias associated with selecting only one threshold for adjacency matric calculations, we calculated the GE at three different cost thresholds (0.125, 0.150, and 0.175) and then averaged these GE values62. We selected this range of cost thresholds because cost >0.15 has been shown to have excellent test-retest reliability in children as young as 4 years of age56,63. Within network connectivity strength was assessed by examining all pairwise ROI-ROI connectivity strength values for each participant.
Statistical analyses
Establishing a spatial network
To establish the existence of a spatial network in this group of children, we examined average within network connectivity across the 12 network nodes of the spatial network. To probe associations between network efficiency of the network and behavioral outcomes, the association between GE of the spatial network and spatial ability (indexed by PIQ, composed of Block Design and Matrix Reasoning tests) as well as with overall socioemotional functioning (indexed by CBCL, Total Problems and Total Competence tests) was evaluated with linear regression. Analyses covaried for factors known to associate with diagnosis and functional connectivity: age, sex, mean head motion. To identify associations above and beyond effects of diagnosis, NVLD diagnostic status (NVLD vs. other [RD or TD]) was also included as a covariate.
Group differences
General linear models were used to test group differences (TD, RD, and NVLD children) in GE and within spatial network connectivity strength (ROI-ROI), controlling for age, sex, and mean head motion. Multivariate analyses of covariance (MANCOVA) were used to identify spatial network ROIs with group differences in network connectivity (one for each seed), corrected for multiple comparisons using False Discovery Rate (p-FDR < 0.05). Omnibus protected, post-hoc FDR-corrected F-tests evaluated group differences in every edge associated with any significant ROI. In addition, to explore which ROI pairs differed between groups and how the groups differed from each other, we present bar graphs showing mean and standard error of residualized functional connectivity from significant ROIs across groups (Fig. 2).
Exploratory behavioral associations
Exploratory analyses examined associations between adjacency matrix edge strengths that differed between groups and spatial ability (PIQ) and socioemotional functioning (CBCL Total Problems and Total Competence scores). We used linear regression with group, inter-regional connectivity, and their interaction to test predictors of behavioral outcomes. In these analyses, diagnostic groups that did not differ in connectivity were combined. In this way, children with RD and TD children were combined, representing a group without spatial deficits, or children with NVLD and RD were combined, representing a learning disability [LD] group). The interaction term was dropped from models when it was not significant. All models included group, age, sex, and mean head motion covariates.
Supplementary information
Acknowledgements
This work was supported by The NVLD Project, Promise Project at Columbia, and K23ES026239.
Author contributions
S.M.B. and A.E.M. conceived the experiment. L.T. enrolled participants and acquired data. S.M.B., B.R. A.E.M. and D.P. analyzed the results. S.M.B. drafted the manuscript in consultation with A.E.M. E.R. and A.N.S. assisted with manuscript preparation. R.M. and A.E.M. supervised the project. B.R., T.Z., A.E.M. and S.M.B. revised the document after review. All authors reviewed and contributed to the final manuscript.
Data availability
Data will be made available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56003-y.
References
- 1.Fine JG, Semrud-Clikeman M, Bledsoe JC, Musielak KA. A critical review of the literature on NLD as a developmental disorder. Child Neuropsychol. 2013;19:190–223. doi: 10.1080/09297049.2011.648923. [DOI] [PubMed] [Google Scholar]
- 2.Mammarella IC, Cornoldi C. An analysis of the criteria used to diagnose children with Nonverbal Learning Disability (NLD) Child Neuropsychol. 2014;20:255–280. doi: 10.1080/09297049.2013.796920. [DOI] [PubMed] [Google Scholar]
- 3.Cornoldi, C., Mammarella, I. C. & Fine, J. G. Nonverbal Learning Disabilities (Guilford Press, 2016).
- 4.Margolis, A. E., Banker, S. M., Pagliaccio, D., Thomas, L. & Marsh, R. In ACNP 57th Annual Meeting (2018).
- 5.Liu I, Levy RM, Barton JJ, Iaria G. Age and gender differences in various topographical orientation strategies. Brain Res. 2011;1410:112–119. doi: 10.1016/j.brainres.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Semrud-Clikeman M, Walkowiak J, Wilkinson A, Christopher G. Neuropsychological differences among children with Asperger syndrome, nonverbal learning disabilities, attention deficit disorder, and controls. Dev Neuropsychol. 2010;35:582–600. doi: 10.1080/87565641.2010.494747.. [DOI] [PubMed] [Google Scholar]
- 7.Broitman, J. & Davis, J. M. In Treating NVLD in Children: Professional Collaborations for Positive Outcomes (eds. Jessica Broitman & John M. Davis) 9–27 (Springer New York, 2013).
- 8.Mammarella IC, Giofre D, Ferrara R, Cornoldi C. Intuitive geometry and visuospatial working memory in children showing symptoms of nonverbal learning disabilities. Child Neuropsychol. 2013;19:235–249. doi: 10.1080/09297049.2011.640931. [DOI] [PubMed] [Google Scholar]
- 9.Mammarella IC, Lucangeli D, Cornoldi C. Spatial Working Memory and Arithmetic Deficits in Children With Nonverbal Learning Difficulties. Journal of Learning Disabilities. 2010;43:455–468. doi: 10.1177/0022219409355482. [DOI] [PubMed] [Google Scholar]
- 10.Margolis, A. E. & Fisher, P. A New Diagnosis for the DSM? Examining Non-Verbal Learning Disorder. Psychology Today, https://www.psychologytoday.com/us/blog/beyond-disability/201708/new-diagnosis-the-dsm (2017).
- 11.Proulx MJ, Todorov OS, Taylor Aiken A, de Sousa AA. Where am I? Who am I? The Relation Between Spatial Cognition, Social Cognition and Individual Differences in the Built Environment. Frontiers in Psychology. 2016;7:64. doi: 10.3389/fpsyg.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavares RM, et al. A Map for Social Navigation in the Human Brain. Neuron. 2015;87:231–243. doi: 10.1016/j.neuron.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petti VL, Voelker SL, Shore DL, Hayman-Abello SE. Perception of Nonverbal Emotion Cues by Children with Nonverbal Learning Disabilities. Journal of Developmental and Physical Disabilities. 2003;15:23–36. doi: 10.1023/A:1021400203453. [DOI] [Google Scholar]
- 14.Metsala JL, Galway TM, Ishaik G, Barton VE. Emotion knowledge, emotion regulation, and psychosocial adjustment in children with nonverbal learning disabilities. Child Neuropsychology. 2017;23:609–629. doi: 10.1080/09297049.2016.1205012. [DOI] [PubMed] [Google Scholar]
- 15.Arnold AE, Protzner AB, Bray S, Levy RM, Iaria G. Neural network configuration and efficiency underlies individual differences in spatial orientation ability. J Cogn Neurosci. 2014;26:380–394. doi: 10.1162/jocn_a_00491. [DOI] [PubMed] [Google Scholar]
- 16.Diehl JJ, et al. Neural correlates of language and non-language visuospatial processing in adolescents with reading disability. Neuroimage. 2014;101:653–666. doi: 10.1016/j.neuroimage.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard JH, Jr., Howard DV, Japikse KC, Eden GF. Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia. 2006;44:1131–1144. doi: 10.1016/j.neuropsychologia.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 18.von Karolyi C, Winner E, Gray W, Sherman GF. Dyslexia linked to talent: global visual-spatial ability. Brain and language. 2003;85:427–431. doi: 10.1016/S0093-934X(03)00052-X. [DOI] [PubMed] [Google Scholar]
- 19.von Károlyi C. Visual-Spatial Strength in Dyslexia: Rapid Discrimination of Impossible Figures. Journal of Learning Disabilities. 2001;34:380–391. doi: 10.1177/002221940103400413. [DOI] [PubMed] [Google Scholar]
- 20.Stanovich Keith E., Siegel Linda S. Phenotypic performance profile of children with reading disabilities: A regression-based test of the phonological-core variable-difference model. Journal of Educational Psychology. 1994;86(1):24–53. doi: 10.1037/0022-0663.86.1.24. [DOI] [Google Scholar]
- 21.Epstein RA, Patai EZ, Julian JB, Spiers HJ. The cognitive map in humans: spatial navigation and beyond. Nature Neuroscience. 2017;20:1504. doi: 10.1038/nn.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boccia M, Nemmi F, Guariglia C. Neuropsychology of Environmental Navigation in Humans: Review and Meta-Analysis of fMRI Studies in Healthy Participants. Neuropsychology Review. 2014;24:236–251. doi: 10.1007/s11065-014-9247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, A. M. P., Vedder, L. C., Law, L. M. & Smith, D. M. Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition. 8, 10.3389/fnhum.2014.00586 (2014). [DOI] [PMC free article] [PubMed]
- 24.Rochefort C, Lefort JM, Rondi-Reig L. The cerebellum: a new key structure in the navigation system. Frontiers in neural circuits. 2013;7:35. doi: 10.3389/fncir.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang SSH, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babayan BM, et al. A hippocampo-cerebellar centred network for the learning and execution of sequence-based navigation. Scientific Reports. 2017;7:17812. doi: 10.1038/s41598-017-18004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, W., Mai, X. & Liu, C. The default mode network and social understanding of others: what do brain connectivity studies tell us. 8, 10.3389/fnhum.2014.00074 (2014). [DOI] [PMC free article] [PubMed]
- 28.Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Semrud-Clikeman M, Walkowiak J, Wilkinson A, Minne EP. Direct and indirect measures of social perception, behavior, and emotional functioning in children with Asperger’s disorder, nonverbal learning disability, or ADHD. J Abnorm Child Psychol. 2010;38:509–519. doi: 10.1007/s10802-009-9380-7. [DOI] [PubMed] [Google Scholar]
- 30.Lynch CJ, et al. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 2013;74:212–219. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margolis Amy E., Pagliaccio David, Thomas Lauren, Banker Sarah, Marsh Rachel. Salience network connectivity and social processing in children with nonverbal learning disability or autism spectrum disorder. Neuropsychology. 2019;33(1):135–143. doi: 10.1037/neu0000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh R, et al. A virtual reality-based FMRI study of reward-based spatial learning. Neuropsychologia. 2010;48:2912–2921. doi: 10.1016/j.neuropsychologia.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolbers T, Weiller C, Buchel C. Neural foundations of emerging route knowledge in complex spatial environments. Brain research. Cognitive brain research. 2004;21:401–411. doi: 10.1016/j.cogbrainres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Margolis AE, et al. Using IQ Discrepancy Scores To Examine the Neural Correlates of Specific Cognitive Abilities. J Neurosci. 2013;33:14135–14145. doi: 10.1523/JNEUROSCI.0775-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milczarek MM, Vann SD, Sengpiel F. Spatial Memory Engram in the Mouse Retrosplenial Cortex. Current biology: CB. 2018;28:1975–1980.e1976. doi: 10.1016/j.cub.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czajkowski R, et al. Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci USA. 2014;111:8661–8666. doi: 10.1073/pnas.1313222111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Developmental Disabilities Research Reviews. 2009;15:270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodin, M. et al. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genetics in medicine: official journal of the American College of Medical Genetics3, 34–39, doi:10.109700125817-200101000-00008 (2001). [DOI] [PubMed]
- 39.Ibrahim AFA, et al. Spatial working memory in neurofibromatosis 1: Altered neural activity and functional connectivity. NeuroImage: Clinical. 2017;15:801–811. doi: 10.1016/j.nicl.2017.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azuma R, et al. Visuospatial working memory in children and adolescents with 22q11.2 deletion syndrome; an fMRI study. Journal of neurodevelopmental disorders. 2009;1:46–60. doi: 10.1007/s11689-009-9008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine JG, Musielak KA, Semrud-Clikeman M. Smaller splenium in children with nonverbal learning disability compared to controls, high-functioning autism and ADHD. Child Neuropsychol. 2014;20:641–661. doi: 10.1080/09297049.2013.854763. [DOI] [PubMed] [Google Scholar]
- 42.Semrud-Clikeman M, Fine JG, Bledsoe J, Zhu DC. Magnetic resonance imaging volumetric findings in children with Asperger syndrome, nonverbal learning disability, or healthy controls. Journal of clinical and experimental neuropsychology. 2013;35:540–550. doi: 10.1080/13803395.2013.795528. [DOI] [PubMed] [Google Scholar]
- 43.Davis K, Margolis AE, Thomas L, Huo Z, Marsh R. Amygdala sub-regional functional connectivity predicts anxiety in children with reading disorder. Dev Sci. 2018;21:e12631. doi: 10.1111/desc.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman J, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler, D. Wechsler Intelligence Scale for Children-Fourth Edition., (The Psychological Corporation., 2003).
- 46.Rutter, M., Le Couteur, A. & Lord, C. Autism diagnostic interview-revised. (Western Psychological Services, 2003).
- 47.Achenbach, T. & Rescorla, L. Manual for the ASEBA school-age forms & profiles: an integrated system of mult-informant assessment. (University of Vermont, Research Center for Children, Youth & Families, 2001).
- 48.Woodcock, R., McGrew, K. & Mather, N. Woodcock-Johnson III. (Riverside Publishing, 2001).
- 49.Wagner, R., Torgeson, J., Rashotte, C. & Nils, P. Comprehensive Test of Phonological Processing, Second Edition. (WPS, 2013).
- 50.Wiederholt, J. L. & Bryant, B. R. Gray Oral Reading Tests, Fifth Edition. (ProEd).
- 51.Torgeson, J., Rashotte, C. & Waber, D. P. Test of word reading efficiency. (Pro-Ed, 2012).
- 52.MacGintie, W., MacGinitie, R., Maria, K. & Dryer, L. Gates-MacGintie reading test. (Riverside Publishing, 2000).
- 53.Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. The Journal of applied psychology. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 54.Meyers, J. E. & Meyers, K. R. Rey Complex Figure Test and recognition trial professional manual. (Psychological Assessment Resources, 1995).
- 55.Schrank, F., McGrew, K., Mather, N., Wendling, B. & LaForte, E. Woodcock-Johnson IV tests of achievement. (Riverside Publishing Company, 2014).
- 56.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 57.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nature Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brett, M., Anton, J., Valabregue, R. & Poline, J. B. In 8th International Conference on Functional Mapping of the Human Brain. (NeuroImage).
- 60.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Latora V, Marchiori M. Efficient behavior of small-world networks. Physical review letters. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 62.Sheffield JM, et al. Evidence for Accelerated Decline of Functional Brain Network Efficiency in Schizophrenia. Schizophrenia bulletin. 2016;42:753–761. doi: 10.1093/schbul/sbv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paldino MJ, Chu ZD, Chapieski ML, Golriz F, Zhang W. Repeatability of graph theoretical metrics derived from resting-state functional networks in paediatric epilepsy patients. The British journal of radiology. 2017;90:20160656. doi: 10.1259/bjr.20160656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.