Abstract
Objectives
Previous studies have postulated that the error-related negativity (ERN) may reflect individual differences in impulsivity; however, none have used a longitudinal framework or evaluated impulsivity as a multidimensional construct. The current study evaluated whether ERN amplitude, measured in childhood and adolescence, is predictive of impulsiveness during adolescence.
Methods
Seventy-five children participated in this study, initially at ages 7–9 years and again at 12–18 years. The interval between testing sessions ranged from 5 to 9 years. The ERN was extracted in response to behavioural errors produced during a modified visual flanker task at both time points (i.e. childhood and adolescence). Participants also completed the Barratt Impulsiveness Scale − a measure that considers impulsiveness to comprise three core sub-traits − during adolescence.
Results
At adolescence, the ERN amplitude was significantly larger than during childhood. Additionally, ERN amplitude during adolescence significantly predicted motor impulsiveness at that time point, after controlling for age, gender, and the number of trials included in the ERN. In contrast, ERN amplitude during childhood did not uniquely predict impulsiveness during adolescence.
Conclusions
These findings provide preliminary evidence that ERN amplitude is an electrophysiological marker of self-reported motor impulsiveness (i.e. acting without thinking) during adolescence.
Keywords: Error-related negativity, ERN, Impulsivity, BIS, Development, Adolescence
1. Introduction
A fundamental aspect of human cognition is the ability to monitor ongoing behaviour for errors in performance, thereby fostering continuous adaptation to changing cognitive and environmental demands. Deficits in error monitoring have been associated with clinical symptoms such as inattention (O’Connell et al., 2009; Shiels et al., 2012), poor insight (Lysaker et al., 1998; O’Keeffe et al., 2004), and impulsiveness (Pailing et al., 2002; Ruchsow et al., 2005).
An electrophysiological index of error monitoring is the event-related negativity (ERN; Falkenstein et al., 1990; Gehring et al., 1993). The ERN is an event-related potential (ERP) component with a fronto-central scalp distribution that typically peaks within approximately 100 ms following the commission of an error on speeded reaction time tasks (Dehaene et al., 1994; Falkenstein et al., 1990; Gehring et al., 1993). The onset of the ERN coincides with the commencement of error-correcting activity (Yeung and Summerfield, 2012). It has been postulated that following an error, the mesencephalic dopamine system conveys a negative reinforcement signal to the frontal cortex, which leads to the elicitation of the ERN in the anterior cingulate cortex (ACC; Holroyd and Coles, 2002), and induces error-related source activity within an extended network of neural locations (Brazdil et al., 2002; Buzzell et al., 2017; Padilla et al., 2014). This account is consistent with the ERN’s sensitivity to factors including, but not limited to, response conflict (Yeung et al., 2004), negative affect (Hajcak et al., 2004; Hill et al., 2016), the motivational significance of errors (Hajcak et al., 2005; Maruo et al., 2016; Potts, 2011), and the emphasis of accuracy over speed (Gehring et al., 1993).
Cross-sectional studies indicate the ERN emerges in early childhood (Grammer et al., 2014; Rueda et al., 2004), steadily increases in amplitude throughout adolescence, and reaches maturation in young adulthood (Buzzell et al., 2017; Davies et al., 2004; Downes et al., 2017; Hogan et al., 2005; Wiersema et al., 2007). To our knowledge, however, only two studies have used a longitudinal framework to examine ERN development and both showed an increase in ERN amplitude with age (Anokhin and Golosheykin, 2015; DuPuis et al., 2014). However, each used varying age ranges and different follow-up periods (12, 14, and 16 years; kindergarten [5–7 years], year 1, and year 2 respectively); making it difficult to draw strong conclusions about the development of the ERN. Based on the collective developmental literature, the most notable changes in the ERN are observed from early to late adolescence (Ladoucer et al., 2007; Santesso and Segalowitz, 2008). This corresponds to a period of considerable maturational change in the prefrontal cortex and ACC, which support error monitoring (Adleman et al., 2002; Debener et al., 2005; Gehring et al., 2012; Hermann et al., 2004; van Veen and Carter, 2002), and with theories linking impulsive decisions and actions made by adolescents with decreased activity and developmental change in these brain regions (Gogtay et al., 2004; Jaeger, 2013; Tamnes et al., 2010). Consequently, the ERN has been proposed as a biomarker able to reflect individual differences in impulsivity within the general population (Ruchsow et al., 2005).
Impulsivity is a personality trait that exists along a continuum in the general population (Costa and McCrae 1992; Eysenck and Eysenck 1985; Tellegen 1982). It is a complex construct characterised by a predisposition to respond to internal or external stimuli without forethought or regard of potentially negative consequences (Moeller et al., 2001). Electrophysiological studies examining the relationship between error monitoring and impulsivity in non-clinical populations have typically measured impulsiveness using reaction time tasks. These studies found that individuals with a tendency towards impulsive responding showed reduced ERN amplitudes (Pailing et al., 2002; Ruchsow et al., 2005; Stahl and Gibbons, 2007). Other studies have evaluated impulsivity using self-report measures − such as the Barratt Impulsiveness Scale (BIS) − that capture long-term patterns of behaviour across various contexts. This work has shown that individuals with high self-reported impulsivity, reflected by high BIS total scores, exhibit decreased ERN amplitudes on tasks that are punishment-motivated (Potts et al., 2006), require high-risk choices (Martin and Potts, 2009), and are of moderate and high task difficulty (Takács et al., 2015).
A considerable shortcoming of past ERN studies is that they have evaluated impulsivity as a unitary construct (e.g. using BIS total scores), without considering its multidimensional nature. Ignoring the sub-traits underlying impulsivity may result in omission of important information, such as the subtle differences of varying clinical syndromes (Patton et al., 1995). Patton et al. (1995) asserted impulsivity comprises three core sub-traits: (1) attentional impulsiveness: difficulty focusing on current tasks; (2) motor impulsiveness: acting without thinking; and (3) non-planning impulsiveness: lacking forethought. Although six first-order factors have been proposed to subsume those three sub-traits, most studies tend to focus on the three second-order factors due to their higher reliability and validity (Stanford et al., 2009). This three-factor structure forms the foundation of the BIS and has been widely adopted in the impulsivity literature (Stanford et al., 2009).
Of these impulsivity sub-traits, the evidence associating ERN amplitude with motor impulsiveness is most robust. Recent studies have associated decreases in neural activity and cortical thickness of the ACC with increased motor impulsiveness (Holmes et al., 2016; Huang et al., 2017). Similar findings have been identified in disorders marked by deficits in motor impulsiveness, such as attention deficit hyperactivity disorder (ADHD; Sebastian et al., 2014), substance use disorders (Wilcox et al., 2014), and bipolar disorder (Matsuo et al., 2009; Singh et al., 2013). In turn, errors made by these individuals elicit smaller ERN amplitudes (Bartholow et al., 2012; Groen et al., 2008; Liotti et al., 2005; Marhe et al., 2013; Morsel et al., 2014) relative to controls. That said, not all studies have replicated these results, which may be attributable to varying demographic characteristics, task paradigms, and methods of calculating ERN amplitude (Burgio-Murphy et al., 2007; Kopf et al., 2015; O’Connell et al., 2009; Wiersema et al., 2005).
In addition, to our knowledge, no prior studies have evaluated the relationship between impulsivity and ERN in typically developing children or adolescents. Given the maturational changes that affect both the ACC and ERN, as well as the financial burden impulsivity places on our health and legal systems (Jackson and Webster, 1997; McCown and Vandenbos, 1994; Perna, 2010), it is important to examine the developmental relationship between the ERN and impulsivity. Identification of individuals high in impulsiveness would allow interventions to be implemented at individual and/or societal levels to support them.
To this end, the present study examined a group of typically developing individuals, longitudinally assessed at ages 7–9 years and then again at 12–18 years, to identify whether ERN amplitude in childhood and/or adolescence is predictive of impulsiveness during adolescence. A modified visual flanker task was administered to elicit ERNs during childhood and adolescence. Additionally, the BIS was completed during adolescence to measure the sub-traits underlying impulsivity.
2. Materials and methods
2.1. Ethics statement
Approval for the study was provided by the Human Research Ethics Committee of The University of Western Australia. Written informed consent was obtained from each participant’s parent or legal guardian and informed assent was provided by each participant.
2.2. Participants
Seventy-five individuals (34 females, 41 males) participated at two time points, as part of a research program investigating the cognitive, emotional, and social development of children. The first wave of testing was conducted between July 2007 and July 2010 when children were aged 7–9 years (mean age = 7.79; SD = 0.95), and the second wave of testing was completed between July and December 2015 when participants were aged 12–18 years (i.e. during adolescence1; mean age = 15.00; SD = 1.37). Consequently, for our participants, the interval between testing sessions was 5–9 years. Exclusion criteria included a history of psychiatric or neurological disorder, as well as hearing and visual impairments that could prevent participants from understanding and following task instructions.
2.3. Materials
The ERN was recorded during childhood and adolescence in response to behavioural errors produced during a modified, child-friendly visual flanker task (based on Richardson et al., 2011; Rueda et al., 2004). Consistent with the method used by Rueda et al. (2004), the task was presented as a game in which the participants had to feed the hungry central fish. Each target display consisted of five fish with arrows on their body (to indicate direction) presented on a blue background. Each fish was separated by 0.2° and subtended 0.9° horizontally and 0.6° vertically. The task consisted of three conditions (See Fig. 1): (1) congruent (0.5 probability), in which the fish were green and all facing the same direction; (2) incongruent (0.25 probability), in which the fish were also green, but the flankers faced the opposite direction to the target; and (3) reversed (0.25 probability), in which the fish all faced the same direction, but all five fish were red, and required a response in the opposite direction to the central fish. The incongruent and reversed conditions were used in this task to increase conflict and the quantity of errors.
Fig. 1.
The six stimuli used in the present study.
Participants were instructed to fixate on the centre of the screen throughout the task and indicate the direction of the central fish in each trial with their index fingers by using the “Z” (left) and “/” (right) keys of a standard QWERTY keyboard. Displays were presented for 300 ms in random order from each condition, and participants were required to respond to each stimulus to continue to the next trial. Emphasis was placed on both speed and accuracy. Visual feedback, indicating whether participants’ responses were correct or incorrect, was provided on the screen at 300 ms (at the initial testing session) or 700 ms (at the follow-up session) after their response. Feedback was delayed at follow-up to avoid contamination of the error positivity − an ERP that occurs approximately 200–500 ms following an incorrect response − as data were collected as part of a broader study. A practice block of 8 trials was administered to ensure participants understood the task requirements. This was followed by an experimental block of 176 trials.
The Barratt Impulsiveness Scale − Version 11 was completed at the follow-up testing session only (BIS-11; Patton et al., 1995). The BIS-11 is considered a valid and reliable measure in adolescents (Nandagopal et al., 2011; Salvo and Castro, 2013). Item 21, “I change residences”, was removed from analyses as the item was considered inappropriate for this sample and missing >5% of responses. For all other items, the proportion of missing values was small (less than 1%) and considered missing completely at random (Little’s MCAR test; X2 (137) 145.56 p = .292), so Expectation Maximisation in IBM SPSS Statistics 22.0 was used to replace them.
2.4. Electrophysiological acquisition
The EEG was continuously recorded using an Easy-Cap™. Electrodes were placed at 33 sites (Fp1, Fp2, F3, F4, F7, F8, Fz, FC1, FC2, FC5, FC6, FCZ, FT9, FT10, C3, C4, Cz, T7, T8, CP1, CP2, CP5, CP6, P3, P4, P7, P8, Pz, PO9, PO10, O1, O2, Iz). Eye movement artefacts were monitored using bipolar leads placed above and below the left eye. A ground electrode was attached to the frontal midline point, AFz, and the right mastoid was set as an online reference. The EEG was amplified using a NuAmps 40-channel amplifier, and digitised at a sampling rate of 250 Hz. Prior to recording, impedances were below 5 kΩ. The ERP processing was conducted offline using Scan 4 software (Compumedics Neuroscan, Charlotte, NC, USA). EEG recordings were re-referenced to an averaged mastoid and filtered offline using a 1–30 Hz zero phase shift band-pass filter (12 dB roll off). The vertical ocular electrodes enabled offline blink reduction according to a standard algorithm.
2.5. EEG analysis
EEG signals were extracted offline and segmented into response-locked epochs of 600 ms prior to response until 1000 ms post response at each of the midline sites (Fz, FCz, and Cz). All epochs were baseline corrected relative to the −600 to −400 ms pre-response interval for consistency with previously published research (Santesso and Segalowitz, 2008). Epochs containing artefacts greater than 150 μV were automatically excluded from processing. Data from three participants were excluded from both time points due to technical difficulties with EEG recordings. Response-locked averages were created for both correct and incorrect trials.
Recent methodological studies have indicated that a minimum of six epochs are required to elicit internally consistent ERNs (Olvet and Hajcak, 2009; Pontifex et al., 2010). Thus, ERP and behavioural data derived from five children and two adolescents who made fewer than six errors were excluded from subsequent analyses. The mean number of errors included in the ERN averaging was 32.12 (SD = 24.53) and 27.52 (SD = 17.93) in childhood and adolescence, respectively. Following data exclusion, the final sample included 67 individuals aged 7–9 years (31 females and 36 males; mean age = 7.72, SD = 0.93) and 69 participants aged 12–18 years (32 females and 37 males; mean age = 15.01, SD = 1.32).
Mean amplitude was used to measure the ERN (for error trials) and the correct response negativity (CRN; for correct trials). This involved subtracting the mean amplitude (±20 ms) around the largest negative peak within the latency window around the response (−50 to 200 ms) from the most positive peak preceding the negative deflection (up to −200 ms), to account for the potential influence of the preceding positivity (Luck, 2005; Olvet et al., 2010). These windows were chosen to ensure the maximum point was identified in each participant’s waveform. Scored in this manner, larger values correspond with greater, more negative ERP amplitudes. As the ERN was maximal at FCz in both children and adolescents (8.45 ± 5.92 μV and 11.39 ± 6.41 μV respectively), compared to Fz (6.31 ± 4.89 μV and 7.93 ± 4.78 μV) and Cz (7.73 ± 5.64 μV and 9.56 ± 5.91 μV), FCz was used for all subsequent analyses. Mean ERN peak latency was 37 ± 44 ms and 62 ± 27 ms in children and adolescents, respectively.
It should also be emphasised that this study explicitly focuses on the relationship between ERN and impulsivity in childhood and adolescence. Thus, other stimulus-locked ERP components (e.g. N2 and P3) were not assessed because they are not directly relevant to the research questions addressed in this paper. Stimulus-locked ERP data from a larger child sample have previously been reported in Richardson et al. (under review).
2.6. Behavioural analysis
Mean accuracy was calculated as the ratio of correct responses relative to the total number of trials in each condition. Mean reaction time was calculated separately for correct responses that immediately followed an error (post-error RT) and for correct responses that immediately followed a hit (post-hit RT). Post-error slowing was then calculated as the difference between post-hit RT and post-error RT on congruent trials.
2.7. Statistical analysis
Behavioural and ERP data were statistically evaluated using IBM SPSS Statistics (Version 22; SPSS Inc., Chicago, IL). Neither accuracy nor RT scores were normally distributed so arcsine and log transformations normalised the distribution of scores within conditions, respectively. Transformed scores have been analysed and the original untransformed values are reported to facilitate interpretation of effects. Repeated measures ANOVAs were used to identify differences as a function of time of testing (childhood, adolescence), and flanker task conditions (congruous, incongruous, reversed). Significant interactions were examined with paired-samples t-tests, adjusting the family-wise error rate using the Bonferroni alpha adjustment. An additional repeated measures factor of response accuracy (correct, incorrect) was included for analyses of the ERN data. Greenhouse-Geisser corrections for violations of sphericity were used when appropriate, and the uncorrected degrees of freedom, p-values, and epsilon are reported. Associations between ERN amplitude, accuracy, RT, and BIS impulsivity data were examined using Pearson bivariate correlations. The influence of gender on ERN amplitude was analysed by means of independent t-tests.
Hierarchical linear regression analyses were used to examine whether the ERN (measured in childhood and adolescence) was uniquely predictive of various facets of impulsiveness (i.e. BIS-11 scores) during adolescence. For these analyses, independent predictors that have previously been identified to influence ERN amplitude and/or impulsiveness (e.g. Davies et al., 2004; Fischer et al., 2017), were entered in Step 1 of the regression model. In Step 2, the component of interest (ERN amplitude) was added to evaluate its unique contribution to explaining variability in BIS-11 scores.
3. Results
3.1. Behavioural analysis
Participants’ accuracy across each condition is displayed in Table 1. There was a main effect of condition on accuracy (F (2, 122) = 71.33, p < .001, ε = 0.93, ηp2 = 0.54), such that accuracy was poorer in the incongruent (t (66) = 3.23, p = 0.002; t (69) = 7.98, p < 0.001) and reversed (t (66) = 5.76, p < 0.001; t (69) = 12.63, p < 0.001) conditions, compared to the congruent condition, in both childhood and adolescence. Participants were more accurate in the incongruent, than the reversed, condition in both childhood and adolescence (t (66) = 2.56, p = 0.013; t (69) = 6.50, p < 0.001). Additionally, a significant interaction between condition and participant age was present (F (2, 122) = 12.91, p < 0.001, ε = 0.95, ηp2 = 0.18). Participants were significantly more accurate in the congruent condition during adolescence than in childhood (t (61) = 4.27, p < 0.001), but no significant differences were apparent in participants’ accuracy in the incongruent (t (61) = 1.35, p = 0.184) and reversed (t (61) = 0.39, p = 0.701) conditions across age groups. Notably, the number of errors made on the flanker task in childhood and adolescence were not significantly correlated (r = −0.08, p = 0.521).
Table 1.
Mean accuracy and reaction times for correct responses in each condition, as well as post-error slowing, across time points.
| Children | Adolescents | |
|---|---|---|
| M (SD) | M (SD) | |
| Percentage Correct | ||
| Congruent | 85.67 (12.17) | 92.96 (5.34) |
| Incongruent | 81.79 (14.98) | 86.74 (8.51) |
| Reversed | 78.57 (14.75) | 79.58 (13.34) |
| Reaction Time (ms) | ||
| Congruent | 988 (335) | 458 (80) |
| Incongruent | 1152 (412) | 517 (88) |
| Reversed | 1166 (361) | 573 (90) |
| Post-error Slowing (ms) | −159 (361) | −37 (78) |
M: Mean; SD: Standard Deviation.
Mean reaction times for correct responses during each condition are also displayed in Table 1. Overall there was a main effect of condition on reaction times (F (2, 122) = 154.69, p < 0.001, ε = 0.93, ηp2 = 0.72), such that reaction times were significantly faster in the congruent condition, than the incongruent (t (66) = 9.37, p .001 < 0.001; t (69) = 15.29, p .001 < 0.001) and reversed (t (66) = 7.32, p .001 < 0.001; t (69) = 22.16, p .001 < 0.001) conditions, in both childhood and adolescence. There was also a significant interaction between participant age and condition (F (2, 122) = 9.81, p .001 < 0.001, ε = 0.81, ηp2 = 0.14): reaction times were significantly faster in adolescence than in childhood in the congruent (t (61) = 17.17 p .001 < 0.001), incongruent (t (61) = 17.26 p .001 < 0.001), and reversed (t (61) = 16.88, p .001 < 0.001) conditions. Reaction times in each condition were not significantly correlated across time points (congruent: r = −0.06, p = 0.673; incongruent: r = −0.02, p = 0.899; reversed: r = 0.05, p = 0.682).
Post-error slowing was exhibited in childhood and adolescence (see Table 1). Specifically, participants slowed their response speed following incorrect trials, in comparison to correct trials, at both time points (t (60) = −3.42, p = 0.001; t (60) = −3.59, p = 0.001). Participants were significantly slower following errors in childhood than adolescence (t (60) = −2.66, p = 0.010). Post-error slowing across time points was not significantly correlated (r = 0.17, p = 0.190)
3.2. Descriptive statistics for BIS-11 scores
Table 2 provides a detailed summary of the descriptive statistics for each of the BIS-11 subscales. Data were highly consistent with those reported by Stanford and colleagues (2009). Each subscale was also examined for associations with age, gender, and behavioural measures (see Table 2). Notably, age in adolescence was significantly associated with the attentional impulsiveness subscale (r = 0.26, p = 0.033) and total score (r = 0.27, p = 0.024), however these associations did not survive Bonferroni correction for the number of BIS subscales examined. There was a significant relationship between gender and the non-planning impulsiveness scale (r = −0.29, p = 0.011). Furthermore, the BIS-11 scores did not significantly correlate with any behavioural measures.
Table 2.
Descriptive statistics of BIS-11 scores.
| BIS-11 Factors | Range | M (SD) | Correlation Coefficients (r) |
|||||
|---|---|---|---|---|---|---|---|---|
| Age in adolescence | Gendera | Correct RT | Incorrect RT | Accuracy | PES | |||
| Attentional | 9–25 | 18.05 (3.32) | 0.26* | 0.01 | 0.02 | 0.06 | −0.06 | −0.08 |
| Motor | 14–29 | 21.33 (3.43) | 0.18 | −0.02 | 0.01 | −0.10 | 0.09 | −0.16 |
| Non-planning | 13–41 | 24.91 (4.81) | 0.20 | −0.29* | 0.11 | 0.05 | −0.14 | −0.20 |
| Total Score | 45–92 | 64.29 (9.03) | 0.27* | −0.16 | 0.07 | 0.14 | −0.06 | −0.20 |
M: Mean; SD: Standard Deviation; aGender: 1 = male, 2 = female; RT: reaction time; PES: post-error slowing; *: p < 0.05.
3.3. Error processing
There was a main effect of accuracy on amplitudes (F (1, 60) = 220.32, p < 0.001, ε = 1.00, ηp2 = 0.79), such that ERN amplitude was significantly larger than CRN amplitude during childhood (t (66) = 10.04, p .001 < 0.001) and adolescence (t (66) = 12.44, p .001 < 0.001). The ERN and CRN were not significantly correlated at either time point (r =0.22, p = 0.070; r = −0.02, p = 0.902). Additionally, a significant interaction between accuracy and participant age was present (F (1, 60) = 6.20, p = 0.016, ε = 1.00, ηp2 = 0.09). Whilst the ERN was significantly larger in adolescence compared to childhood (t (60) = 2.39, p = 0.020), there was no significant difference in CRN amplitude across time points (t (60) = 1.18, p = 0.242).
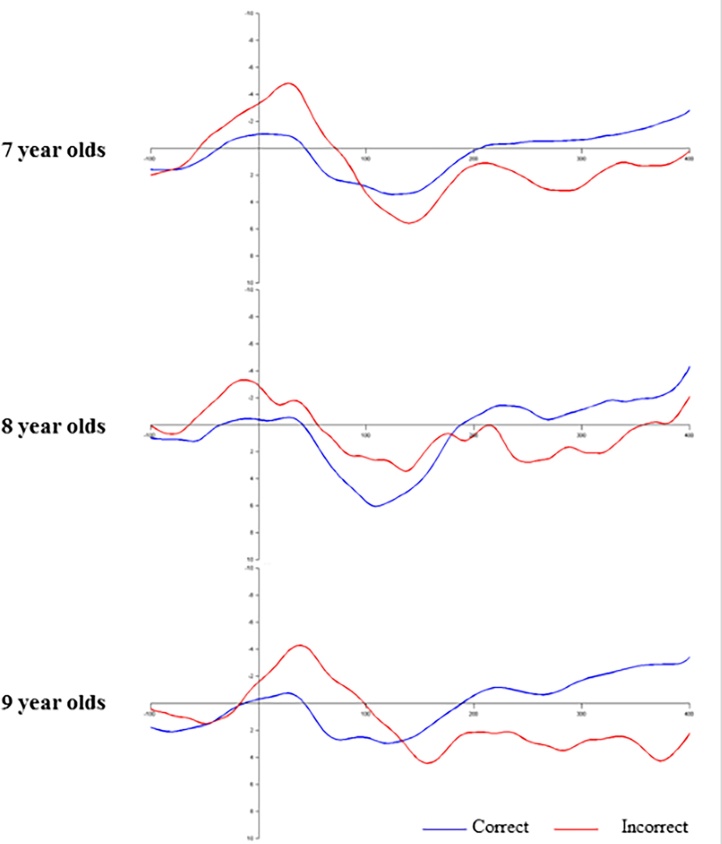
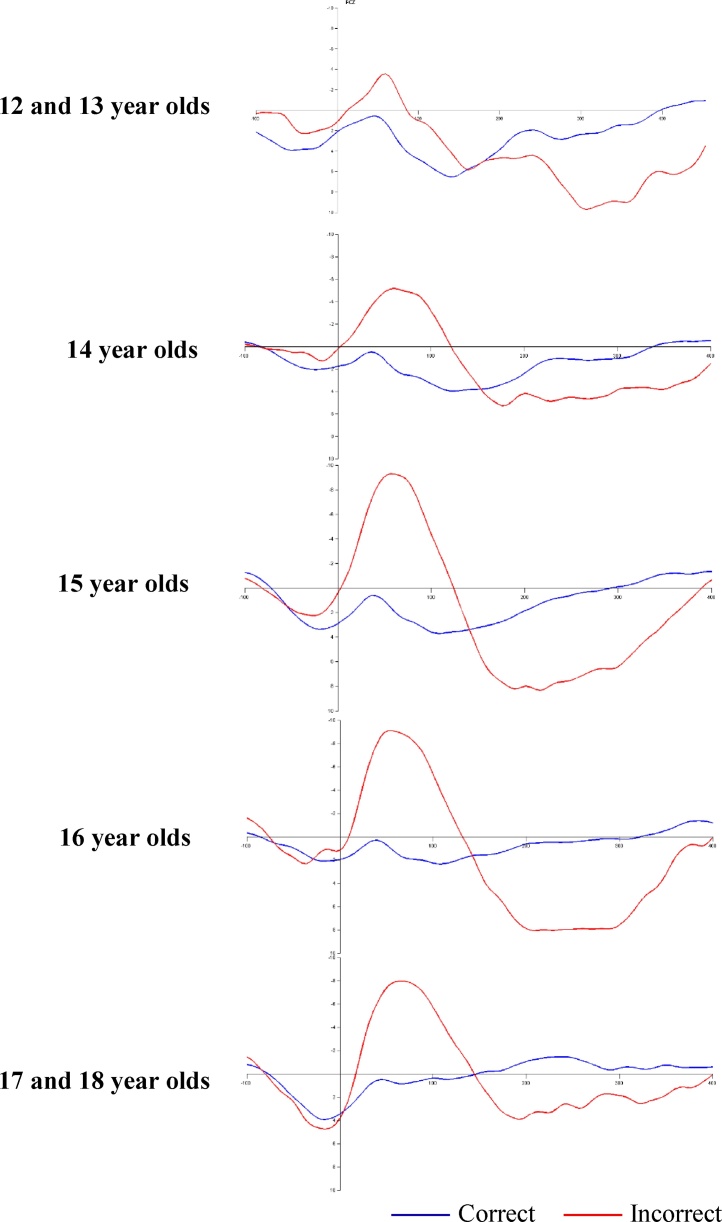
Several factors have been identified in the literature to affect ERN amplitude, including age, gender, and the number of trials included in the ERN. In our data set, there was no statistically significant difference in ERN amplitude between the 7–9 year olds (F (2, 64) = 0.39, p = 0.682, partial η2 = 0.01) (see Fig. 2). In contrast, there was a significant association between age and ERN amplitude during adolescence (r =0.33, p = 0.005; see Fig. 3).2 ERN amplitude in childhood was not significantly associated with ERN amplitude during adolescence (r = 0.24, p = 0.059). Moreover, ERN amplitude did not significantly differ across genders at either time point (t (65) = −0.07, p = 0.946; t (67) = 0.79, p = 0.435). The number of trials included in the ERN was significantly associated with ERN amplitude at each time point (r = −0.48 and −0.40 in children and adolescents respectively, ps < 0.001), and has therefore been included as a covariate in the regression analyses reported below. Nevertheless, no significant difference in the number of errors elicited across time points was apparent (t (60) = 0.93, p = 0.357).
Fig. 2.
Response-locked grand averaged waveforms for correct (blue) and incorrect (red) trials depicted at site FCz for children aged 7 (n = 41), 8 (n = 4), and 9 (n = 22) years old. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Response-locked grand averaged waveforms for correct (blue) and incorrect (red) trials depicted at site FCz for adolescents aged 12 and 13 (n = 7), 14 (n = 16), 15 (n = 24), 16 (n = 11), and 17 and 18 (n = 12) years old. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. ERN amplitude and impulsiveness
Table 3 presents bivariate correlations between the BIS-11 subscales, total score, and ERN amplitude in adolescence. Consistent with Stanford et al. (2009), the BIS-11 subscales were highly inter-correlated. The BIS-11 subscales, however, were not significantly associated with ERN amplitude during adolescence.
Table 3.
Bivariate correlations between the BIS-11 subscales and ERN, both measured in adolescence.
| Variable | 1. | 2. | 3. | 4. | 5. |
|---|---|---|---|---|---|
| 1. Attentional impulsiveness | – | ||||
| 2. Motor impulsiveness | 0.43** | – | |||
| 3. Non-planning impulsiveness | 0.35** | 0.45** | – | ||
| 4. BIS-11 total score | 0.72** | 0.78** | 0.83** | – | |
| 5. ERN in adolescence | −0.08 | −0.17 | −0.10 | −0.15 | – |
**: p < 0.01.
Hierarchical linear regressions were performed to identify whether the relationship between the ERN and impulsiveness differed as a function of age, gender, and/or quantity of errors (see Table 4). Analysis revealed that ERN amplitude during adolescence independently accounted for significant variance in the BIS-11 motor impulsiveness subscale, after controlling for covariates. Specifically, smaller ERN amplitudes during adolescence were associated with larger scores on the motor impulsiveness subscale. Despite ERN amplitude during adolescence not significantly explaining variance across the other subscales, the ERN was identified to significantly account for the variance observed in BIS-11 total scores. However, after partialling out the substantial variance explained by the motor impulsiveness subscale, the contribution of the ERN amplitude to predicting total impulsiveness was negligible (see Table 5).
Table 4.
Hierarchical regressions predicting different facets of impulsiveness from ERN amplitude during adolescence, after controlling for age, gender, and the number of trials included in the ERN.
| BIS-11 | Step 1 |
Step 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression excluding the ERN |
Regression including the ERN |
||||||||
| B | SE B | β | Adj R2 | B | SE B | β | Adj R2 | ΔR2 | |
| Attentional Impulsiveness | 0.06 | 0.06 | 0.01 | ||||||
| Age in adolescence | 0.73 | 0.31 | 0.29* | 0.82 | 0.32 | 0.32* | |||
| Gender a | 0.01 | 0.80 | <0.01 | −0.02 | 0.80 | <−0.01 | |||
| Quantity of errors | 0.04 | 0.02 | 0.19 | 0.03 | 0.02 | 0.14 | |||
| ERN amplitude in adolescence | −0.07 | 0.07 | −0.13 | ||||||
| Motor Impulsiveness | <−0.01 | 0.07 | 0.09* | ||||||
| Age in adolescence | 0.42 | 0.32 | 0.17 | 0.66 | 0.32 | .26* | |||
| Gender | −0.32 | 0.83 | −0.05 | −0.41 | 0.80 | −0.06 | |||
| Quantity of errors | −0.01 | 0.02 | −0.07 | −0.04 | 0.02 | −0.19 | |||
| ERN amplitude in adolescence | −0.18 | 0.07 | −0.34* | ||||||
| Non-planning Impulsiveness | 0.11* | 0.15* | 0.05 | ||||||
| Age in adolescence | 0.59 | 0.43 | 0.16 | 0.85 | 0.44 | 0.23 | |||
| Gender | −3.22 | 1.12 | −0.33** | −3.31 | 1.10 | −0.34** | |||
| Quantity of errors | <−0.01 | 0.03 | −0.01 | −0.03 | 0.03 | −0.10 | |||
| ERN amplitude in adolescence | −0.19 | 0.10 | −0.25 | ||||||
| Total Score | 0.07 | 0.13* | 0.07* | ||||||
| Age in adolescence | 1.75 | 0.82 | 0.25* | 2.33 | 0.83 | 0.34** | |||
| Gender | −3.52 | 2.14 | −0.19 | −3.73 | 2.07 | −0.21 | |||
| Quantity of errors | 0.02 | 0.06 | 0.04 | −0.04 | 0.06 | −0.07 | |||
| ERN amplitude in adolescence | −0.44 | 0.18 | −0.31* | ||||||
Note. B: beta weight; β: standardised beta weight; Adj: adjusted; Δ: change; BIS-11: Barratt Impulsiveness Scale − Version 11; a Gender: 1 = male, 2 = female; *: p < .05; **: p < .01.
Table 5.
Hierarchical regressions predicting total impulsiveness from ERN amplitude in adolescence, after covarying for current age and partialling out variance explained by the motor impulsiveness subscale.
| BIS-11 | Step 1 |
Step 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression excluding the ERN |
Regression including the ERN |
||||||||
| B | SE B | β | Adj R2 | B | SE B | β | Adj R2 | ΔR2 | |
| Total Score | 0.61** | 0.61** | 0.01 | ||||||
| Age in adolescence | 0.93 | 0.53 | 0.14 | 1.13 | 0.58 | 0.16 | |||
| Motor impulsiveness subscale | 2.01 | 0.21 | 0.75** | 1.96 | 0.21 | 0.73** | |||
| ERN amplitude in adolescence | −0.11 | 0.12 | −0.08 | ||||||
Note. B: beta weight; β: standardised beta weight; Adj: adjusted; Δ: change; BIS-11: BarrattImpulsiveness Scale − Version 11; *: p < .05; **: p < .01.
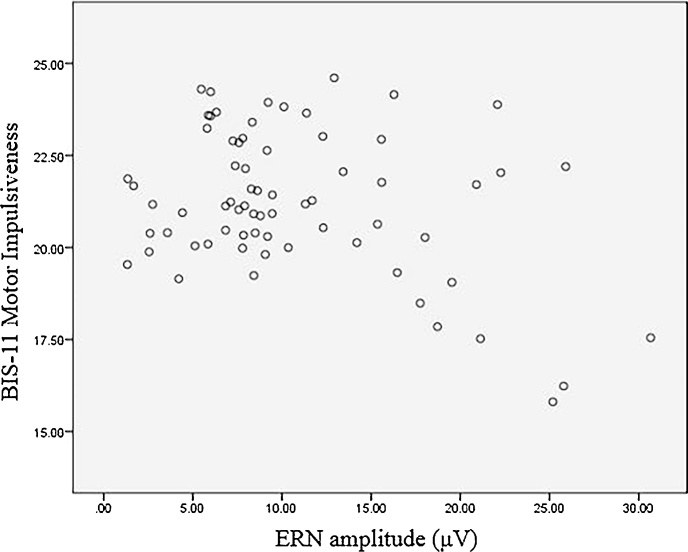
The BIS-11 motor impulsiveness subscale includes items measuring perseverance (Patton et al., 1995), which some authors argue captures ‘a stable lifestyle’ rather than pure motor impulsiveness (e.g. Reise et al., 2013). To identify whether ERN amplitude in adolescence is predictive of pure motor impulsiveness in adolescence (i.e. impetuous action), without being confounded by perseverance, perseverance items (i.e. those comprising the BIS-11 perseverance first-order factor) were partialled out of the analysis (see Table 6). Also, due to post hoc analyses identifying gender and quantity of errors as non-significant predictors of motor impulsiveness, explaining little-to-no variance in the subscale, they were removed from the model. The revised regression model identified ERN amplitude in adolescence continued to account for significant variance in motor impulsiveness after covarying for age, independent of the variance explained by perseverance (see Fig. 4).
Table 6.
Hierarchical regressions predicting motor impulsiveness from ERN amplitude in adolescence, after covarying for current age and partialling out perseverance-related questionnaire items.
| BIS-11 | Step 1 |
Step 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression excluding the ERN |
Regression including the ERN |
||||||||
| B | SE B | β | Adj R2 | B | SE B | β | Adj R2 | ΔR2 | |
| Motor Impulsiveness | 0.25** | 0.29** | 0.05* | ||||||
| Age in adolescence | 0.41 | 0.27 | 0.16 | 0.60 | 0.28 | 0.23* | |||
| BIS-11 perseverance items | 1.20 | 0.26 | 0.49** | 1.15 | 0.25 | 0.48** | |||
| ERN amplitude in adolescence | −0.12 | 0.06 | −0.22* | ||||||
Note. B: beta weight; β: standardised beta weight; Adj: adjusted; Δ: change; BIS-11: Barratt Impulsiveness Scale − Version 11; *: p < .05; **: p < .01.
Fig. 4.
Scatter plot depicting the relationship between ERN amplitude and motor impulsiveness, both measured during adolescence, upon covarying for age and partialling out the variance due to perseverance.
The same analyses were repeated to identify whether ERN amplitude during childhood uniquely predicted impulsiveness during adolescence. The results in Table 7 indicated that ERN amplitude in childhood did not significantly explain the variance in BIS-11 subscales during adolescence, after covarying for age in adolescence, gender, time between testing sessions, and the number of trials included in the ERN at that time point.
Table 7.
Hierarchical regressions predicting different facets of impulsiveness in adolescence from ERN amplitude in childhood, after controlling for age, gender, time between testing sessions, and the number of trials included in the ERN.
| BIS-11 | Step 1 |
Step 2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Regression excluding the ERN |
Regression including the ERN |
||||||||
| B | SE B | β | Adj R2 | B | SE B | β | Adj R2 | ΔR2 | |
| Attentional Impulsiveness | 0.03 | 0.01 | <0.01 | ||||||
| Age in adolescence | 1.05 | 0.50 | 0.39* | 1.06 | 0.50 | 0.40* | |||
| Gender a | 0.26 | 0.89 | 0.04 | 0.26 | 0.90 | 0.04 | |||
| Time between testing sessions (years) | −0.92 | 0.68 | −0.25 | −0.93 | 0.71 | −0.25 | |||
| Quantity of errors | 0.02 | 0.02 | 0.16 | 0.02 | 0.02 | 0.17 | |||
| ERN amplitude in childhood | 0.01 | 0.09 | 0.01 | ||||||
| Motor Impulsiveness | 0.05 | 0.03 | <0.01 | ||||||
| Age in adolescence | 1.05 | 0.47 | 0.41* | 1.04 | 0.48 | 0.40* | |||
| Gender | −0.28 | 0.85 | −0.04 | −0.27 | 0.86 | −0.04 | |||
| Time between testing sessions (years) | −1.05 | 0.65 | −0.30 | −0.99 | 0.67 | −0.28 | |||
| Quantity of errors | 0.03 | 0.02 | 0.19 | 0.02 | 0.02 | 0.16 | |||
| ERN amplitude in childhood | −0.04 | 0.09 | −0.06 | ||||||
| Non-planning Impulsiveness | 0.20** | 0.20** | 0.02 | ||||||
| Age in adolescence | 1.69 | 0.66 | 0.43* | 1.63 | 0.66 | 0.42* | |||
| Gender | −3.31 | 1.19 | −0.33** | −3.26 | 1.18 | −0.33** | |||
| Time between testing sessions (years) | −2.03 | 0.91 | 0.38* | −1.77 | 0.93 | −0.33 | |||
| Quantity of errors | 0.04 | 0.02 | 0.19 | 0.02 | 0.03 | 0.11 | |||
| ERN amplitude in childhood | −0.15 | 0.12 | −0.17 | ||||||
| Total Score | 0.16** | 0.15* | 0.01 | ||||||
| Age in adolescence | 3.79 | 1.25 | 0.53** | 3.72 | 1.26 | 0.52** | |||
| Gender | −3.33 | 2.26 | −0.18 | −3.27 | 2.27 | −0.18 | |||
| Time between testing sessions (years) | −4.00 | 1.72 | −0.40* | −3.68 | 1.78 | −0.37* | |||
| Quantity of errors | 0.09 | 0.05 | 0.23 | 0.07 | 0.05 | 0.18 | |||
| ERN amplitude in childhood | −0.18 | 0.23 | −0.11 | ||||||
Note. B: beta weight; β: standardised beta weight; Adj: adjusted; Δ: change; BIS-11: Barratt Impulsiveness Scale − Version 11; a Gender: 1 = male, 2 = female; *: p < .05; ** p < .01.
4. Discussion
Here we investigated whether ERN amplitude, measured in childhood and adolescence, can predict impulsiveness during adolescence. The current study found ERN amplitude during adolescence, but not in childhood, significantly predicted motor impulsiveness measured in adolescence. This finding suggests that ERN amplitude during adolescence may be an electrophysiological marker for the propensity to act without thinking (i.e. impetuous action), which in turn impacts impulsiveness.
Our findings support and extend the developmental literature on the ERN by identifying that the ERN is an electrophysiological marker of motor impulsiveness during adolescence. This is the first study to identify an association between ERN amplitude and a self-reported measure of motor impulsiveness in a non-clinical adolescent sample. As motor impulsiveness has been linked to adverse social (aan het Rot et al., 2014), legal (Constantinou et al., 2011; Warren, 2001), health (Dougherty et al., 2004; Nurmedov et al., 2016), and educational outcomes (Spinella and Miley, 2003), it is important that individuals that have a propensity to engage in impetuous actions are identifiable so that appropriate interventions can be developed and implemented to support them. Future studies may wish to extend our findings by exploring preparatory neural processes of motor readiness, such as the Bereitschaftspotential, in relation to self-reported motor impulsiveness, as this was beyond the scope of our study. The Bereitschaftspotential reflects dynamic changes in motor cortical activity preceding movements (Grosse, 2004; Oken and Phillips, 2009), and therefore might better predict self-reported motor impulsiveness than the ERN.
This study is one of few to implement a longitudinal framework to evaluate developmental changes in ERN. Consistent with Davies et al. (2004) who used a cross-sectional design, our findings suggest minimal difference in ERN amplitudes between the ages of 7 and 9 years. Additionally, analogous to the majority of developmental literature in this field (Buzzell et al., 2017; Davies et al., 2004; Hogan et al., 2005; Wiersema et al., 2007), our results identified a significant increase in ERN amplitude from 12 to 18 years of age. This indicates that early adolescence symbolises a transition from a relatively flat growth curve in ERN amplitude during childhood (7–9 years old) to the rapidly increasing development of this amplitude in adolescence (12–18 years). This pattern of findings may further explain the non-significant correlations identified between the ERN and the CRN, given the CRN is purported to have a cubic developmental trajectory (i.e. an increase from 7 to 9 years, a decrease until age 16, and a leveling off or slight increase thereafter; Davies et al., 2004).
Additionally, this study was the first to evaluate whether ERN amplitude at ages 7–9 years could predict impulsiveness during later adolescence. The results indicated that ERN amplitude in children aged 7–9 years did not significantly predict any facet of impulsiveness during adolescence, over that explained by age and gender. This suggests that motor impulsiveness may manifest differently during adolescence, which might be attributable to puberty, the structural and functional maturation of anterior brain regions, and/or the interplay of developing executive functions (e.g. inhibitory control; Anderson, 2002; Barker, 2016; Horn et al., 2003; Blakemore, 2006; Schachar and Logan, 1990; Spear, 2000). These factors may further explain the lack of correlation between flanker-related measures across time points in our study. Future research should evaluate the effect of these variables on the expression of various facets of impulsivity throughout development.
While our results are interesting, our study nonetheless had several limitations. First, our main finding’s effect size was modest (R2 = 0.09), which suggests that reduced error monitoring is only one of many factors contributing to motor impulsiveness. For instance, it has been proposed that genetics may influence various aspects of impulsiveness (Bevilacqua and Goldman, 2013; Congdon and Canli, 2008; Taylor et al., 2017). Consequently, future research may wish to evaluate whether genetics has an indirect influence on impulsiveness, via error monitoring processes. Second, self-reported impulsiveness was only measured in adolescence. As a result, this study is unable to draw conclusions about the specific nature/direction of the relationship between the ERN and impulsivity. Future longitudinal studies should concurrently track the development of ERN amplitude and impulsiveness to identify whether ERN amplitude at younger ages (i.e. <12 years old) is also predictive of one’s tendency to engage in impetuous actions at that age, or alternatively the age at which ERN amplitude becomes an electrophysiological marker of current motor impulsiveness. Third, future studies should incorporate objective measures and/or informant-reports of impulsivity, in order to establish the generalisability of these findings. Fourth, because we recruited children from primary schools with relatively high levels of socio-educational advantage, our study results may not generalise to broader population bases or to clinical cohorts marked by high levels of impulsivity. Consequently, replication in larger, population-based samples of age-homogenous children and adolescents would be desirable.
In summary, this study has provided the first evidence that the ERN is an electrophysiological marker of current, self-reported motor impulsiveness during adolescence. The ability to identify those at risk of heightened motor impulsiveness is essential, as it has been associated with several maladaptive outcomes. Identification may facilitate the implementation of interventions to support individuals prone to engage in impetuous actions.
Funding
This research was supported by the Australian Research CouncilDP0665616 (http://www.arc.gov.au/) and the School of Psychology, University of Western Australia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
None.
Footnotes
Note. Adolescence starts at the onset of puberty and has been broadly defined as between the ages of 10 and 19 (Barker, 2016; Dumontheil, 2014; Sawyer et al., 2012).
Note. Due to small age subsets, 12 and 13 year olds, as well as 17 and 18 year olds, were combined for a graphical representation of the data.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.01.003.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- aan het Rot M., Moskowitz D.S., Young S.N. Impulsive behaviour in interpersonal encounters: associations with quarrelsomeness and agreeableness. Br. J. Psychol. 2014;106:152–161. doi: 10.1111/bjop.12070. [DOI] [PubMed] [Google Scholar]
- Adleman N.E., Menon V., Blasey C.M., Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., Reiss A.L. A developmental fMRI study of the Stroop color-word. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Anokhin A., Golosheykin S. Neural correlates of error monitoring in adolescents prospectively predict initiation of tobacco use. Dev. Cogn. Neurosci. 2015;16:166–173. doi: 10.1016/j.dcn.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker T.V. The University of Maryland; Washington: 2016. Social Influences of Error Monitoring, Doctor of Philosophy. [Google Scholar]
- Bartholow B.D., Henry E.A., Lust S.A., Saults J.S., Wood P.K. Alcohol effects on performance monitoring and adjustment: affect modulation and impairment of evaluative cognitive control. J. Abnorm. Psychol. 2012;121:173–186. doi: 10.1037/a0023664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L., Goldman D. Genetics of impulsive behaviour. Philos. Trans. R. Soc. Long. B Biol. Sci. 2013;368:20120380. doi: 10.1098/rstb.2012.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J. Development of the adolescent brain: implications for executive function and social cognition. J. Chil. Psychol. Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Brazdil M., Roman R., Falkenstein M., Daniel P., Jurak P., Rektor I. Error processing −evidence from intracerebral ERP recordings. Exp. Brain Res. 2002;146:460–466. doi: 10.1007/s00221-002-1201-y. [DOI] [PubMed] [Google Scholar]
- Burgio-Murphy A., Klorman R., Shaywitz S.E., Fletcher J.M., Marchione K.E., Holahan J., Stuebing K.K., Thatcher J.E., Shaywitz B.A. Error-related event-related potentials in children with attention-deficit hyperactivity disorder oppositional defiant disorder, reading disorder, and math disorder. Biol. Psychol. 2007;75:75–86. doi: 10.1016/j.biopsycho.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzell G.A., Richards J.E., White L.K., Barker T.V., Pine D.S., Fox N.A. Development of the error-monitoring system from ages 9–35: unique insight provided by MRI-constrained source localization of EEG. Neuroimage. 2017;157:13–26. doi: 10.1016/j.neuroimage.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E., Canli T. A neurogenetic approach to impulsivity. J. Pers. 2008;76:1447–1484. doi: 10.1111/j.1467-6494.2008.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou E., Panayiotou G., Konstantinou N., Loutsiou-Ladd A., Kapardis A. Risky and aggressive driving in young adults: personality matters. Accid. Anal. Prev. 2011;43:1323–1331. doi: 10.1016/j.aap.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Costa P.T., McCrae R.R. Psychological Assessment Resources; Odessa (FL): 1992. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEOFFI) Professional Manual. [Google Scholar]
- Davies P.L., Segalowitz S.J., Gavin W.J. Development of response-monitoring ERPs in 7- to 25-year-olds. Dev. Neuropsychol. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Debener S., Ullsperger M., Siegel M., Fiehler K., von Cramon D.Y., Engel A.K. Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci. 2005;25:11730–11731. doi: 10.1523/JNEUROSCI.3286-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Posner M.I., Tucker D.M. Localization of a neural system for error detection and compensation. Psychol. Sci. 1994;5:303–305. [Google Scholar]
- Dougherty D.M., Mathias C.W., Marsh D.M., Papageorgiou T.D., Swann A.C., Moeller F.G. Laboratory measured behavioral impulsivity relates to suicide attempt history. Suicide Life Threat. Behav. 2004;34:374–385. doi: 10.1521/suli.34.4.374.53738. [DOI] [PubMed] [Google Scholar]
- Downes M., Bathelt J., de Haan M. Event-related potential measures of executive functioning from preschool to adolescence. Dev. Med. Child Neurol. 2017;59:581–590. doi: 10.1111/dmcn.13395. [DOI] [PubMed] [Google Scholar]
- DuPuis D., Ram N., Willner C.J., Karalunas S., Segalowitz S.J., Gatzke-Kopp L.M. Implications of ongoing neural development for the measurement of the error-related negativity in childhood. Dev. Sci. 2014;18:452–468. doi: 10.1111/desc.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I. Development of abstract thinking during childhood and adolescence: the role of rostrolateral prefrontal cortex. Dev. Cogn. Neurosci. 2014;10:57–76. doi: 10.1016/j.dcn.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H.J., Eysenck M.W. Plenum Press; New York: 1985. Personality and Individual Differences: A Natural Science Approach. [Google Scholar]
- Falkenstein M., Hohnsbein J., Hoormann J., Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia C.H.M., Gaillard A.W.K., Kok A., editors. Psychophysiological Brain Research. Tilburg University Press; Tilburg: 1990. pp. 192–195. [Google Scholar]
- Fischer A.G., Klein T.A., Ullsperger M. 2017. Comparing the Error-related Negativity Across Groups: The Impact of Error- and Trial-number Differences. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Goss B., Coles M.G.H., Meyer D.E., Donchin E. A neural system for error detection and compensation. Psychol. Sci. 1993;4:385–390. [Google Scholar]
- Gehring W.J., Liu Y., Orr J.M., Carp J. Oxford Handbook of Event-Related Potential Components. 2012. The error-related negativity (ERN/Ne) pp. 231–291. [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer J.K., Carrasco M., Gehring W.J., Morrison F.J. Age-related changes in error processing in young children: a school-based investigation. Dev. Cogn. Neurosci. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen Y., Wijers A.A., Mulder L.J., Waggeveld B., Minderaa R.B., Althaus M. Error and feedback processing in children with ADHD and children with Autistic Spectrum Disorder: an EEG event-related potential study. Clin. Neurophysiol. 2008;119:2476–2493. doi: 10.1016/j.clinph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Grosse P. The bereitschaftspotential. Movement-related cortical potentials. Brain. 2004;127:454–455. [Google Scholar]
- Hajcak G., McDonald N., Simons R.F. Error-related psychophysiology and negative affect. Brain Cogn. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Yeung N., Simons R.F. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–160. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hermann M.J., Rӧmmler J., Ehlis A.C., Heidrich A., Fallgatter A.J. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Brain Res. Cogn. Brain Res. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Hill K.E., Samuel D.B., Foti D. Contextualizing individual differences in error monitoring: links with impulsivity negative affect, and conscientiousness. Psychophysiology. 2016;53:1143–1153. doi: 10.1111/psyp.12671. [DOI] [PubMed] [Google Scholar]
- Hogan A.M., Vargha-Khadem F., Kirkham F.J., Baldeweg T. Maturation of action monitoring from adolescence to adulthood: an ERP study. Dev. Sci. 2005;8:525–534. doi: 10.1111/j.1467-7687.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- Holmes A.J., Hollinshead M.O., Roffman J.L., Smoller J.W., Buckner R.B. Individual differences in cognitive control circuit anatomy link sensation seeking impulsivity, and substance use. J. Neurosci. 2016;36:4038–4049. doi: 10.1523/JNEUROSCI.3206-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Coles M.G.H. The neural basis of human error processing: reinforcement learning: dopamine and the error-related negativity. Psychol. Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Horn N.R., Dolan M., Elliott R., Deakin J.F., Woodruff P.W. Response inhibition and impulsivity. An fMRI study. Neuropsychologia. 2003;41:1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Huang S., Zhu Z., Zhang W., Chen Y., Zhen S. Trait impulsivity components correlate differently with proactive and reactive control. PLoS One. 2017;12:e0176102. doi: 10.1371/journal.pone.0176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M.A., Webster C.D. Introduction. In: Webster C.D., Jackson M.A., editors. Impulsivity: Theory, Assessment, and Treatment. Guilford; New York: 1997. [Google Scholar]
- Jaeger A. Inhibitory control and the adolescent brain: a review of fMRI research. Psychol. Neurosci. 2013;6:23–30. [Google Scholar]
- Kopf J., Volkert J., Heidler S., Dresler T., Kittel-Schneider S., Gessner A., Herrmann M.J., Ehlis A.C., Reif A. Electrophysiological evidence of a typical cognitive distortion in bipolar disorder. Cortex. 2015;66:103–114. doi: 10.1016/j.cortex.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Ladoucer C.D., Dahl R.E., Carter C.S. Development of action monitoring through adolescence into adulthood: ERP and source localization. Dev. Sci. 2007;10:874–891. doi: 10.1111/j.1467-7687.2007.00639.x. [DOI] [PubMed] [Google Scholar]
- Liotti M., Pliszka S.R., Perez R., Kothmann D., Woldorff M.G. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Luck S.J. first ed. MIT Press; Cambridge, MA: 2005. An Introduction to the Event-Related Potential Technique. [Google Scholar]
- Lysaker P.H., Bell M.D., Bryson G., Kaplan E. Neurocognitive function and insight in schizophrenia: support for an association with impairments in executive function but not with impairments in global function. Acta Psychiatr. Scand. 1998;97:297–301. doi: 10.1111/j.1600-0447.1998.tb10003.x. [DOI] [PubMed] [Google Scholar]
- Marhe R., Van de Wetering B.J.M., Franken I.H.A. Error-related brain activity predicts cocaine use after treatment at 3-month follow-up. Biol. Psychiatry. 2013;73:782–788. doi: 10.1016/j.biopsych.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Martin L.E., Potts G.F. Impulsivity in decision-making: an event-related potential investigation. Pers. Individ. Dif. 2009;46:303. doi: 10.1016/j.paid.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo Y., Schacht A., Sommer W., Masaki H. Impacts of motivational valence on the error-related negativity elicited by full and partial errors. Biol. Psychol. 2016;114:108–116. doi: 10.1016/j.biopsycho.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Nicoletti M.A., Peluso M.A., Hatch J.P., Nemoto K., Watanabe Y., Nery F.G., Monkul E.S., Zunta-Soares G.B., Bowden C.L., Soares J.C. Anterior cingulate volumes associated with trait impulsivity in individuals with bipolar. Bipolar Disord. 2009;11:628–636. doi: 10.1111/j.1399-5618.2009.00732.x. [DOI] [PubMed] [Google Scholar]
- McCown W., Vandenbos G.R. Treating the impulsive patient. Hosp. Commun. Psychiatry. 1994;45:1075–1077. doi: 10.1176/ps.45.11.1075. [DOI] [PubMed] [Google Scholar]
- Moeller F.G., Barratt E.S., Dougherty D.M., Schmitz J.M., Swann A.C. Psychiatric aspects of impulsivity. Am. J. Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Morsel A.M., Morrens M., Temmerman A., Sabbe B., de Bruijn E.R. Electrophysiological (EEG) evidence for reduced performance monitoring in euthymic bipolar disorder. Bipolar Disord. 2014;16:820–829. doi: 10.1111/bdi.12256. [DOI] [PubMed] [Google Scholar]
- Nandagopal J.J., Fleck D.E., Adler C.M., Mills N.P., Strakowski S.M., DelBello M.P. Impulsivity in adolescents with bipolar disorder and/or attention-deficit/hyperactivity disorder and healthy controls as measured by the Barratt Impulsiveness Scale. J. Child Adolesc. Psychopharmacol. 2011;21:465–468. doi: 10.1089/cap.2010.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmedov S., Ibadi Y., Noyan O., Yilmaz O., Kesebir S., Dilbaz N., Kose S. Relationship between impulsivity and plasma uric acid levels in patients with substance use disorders. Klin Psikofarmakol B. 2016;26:223–228. [Google Scholar]
- O’Connell R.G., Bellgrove M.A., Dockree P.M., Lau A., Hester R., Garavan H., Fitzgerald M., Foxe J., Robertson I.H. The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD) Neuropsychologia. 2009;47:1149–1159. doi: 10.1016/j.neuropsychologia.2009.01.011. [DOI] [PubMed] [Google Scholar]
- O’Keeffe F.M., Dockree P.M., Robertson I.H. Poor insight in traumatic brain injury mediated by impaired error processing? Evidence from electrodermal activity. Brain Res. Cogn. Brain Res. 2004;22:101–112. doi: 10.1016/j.cogbrainres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Oken B.S., Phillips T.S. Evoked potentials: clinical. In: Squire L.R., editor. vol. 4. Academic Press; Oxford: 2009. pp. 19–28. (Encyclopedia of Neuroscience). [Google Scholar]
- Olvet D.M., Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–961. doi: 10.1111/j.1469-8986.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Olvet D.M., Hatchwell E., Hajcak G. Lack of association between the 5-HTTLPR and the error-related negativity (ERN) Biol. Psychol. 2010;85:504–508. doi: 10.1016/j.biopsycho.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Padilla M.L., Pfefferbaum A., Sullivan E.V., Baker F.C., Colrain I.M. Dissociation of preparatory attention and response monitoring maturation during adolescence. Clin. Neurophysiol. 2014;125:962–970. doi: 10.1016/j.clinph.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing P.E., Segalowitz S.J., Dywan J., Davies P.L. Error negativity and response control. Psychophysiology. 2002;39:198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;6:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perna R.B. Impulsivity, shortsighted decisions, and discounting [Review of the book Impulsivity: the behavioral and neurological science of discounting, by G. J. Madden & W. K. Bickel (Eds.)] PsycCritiques. 2010;55(8) (Article 1) [Google Scholar]
- Pontifex M.B., Scudder M.R., Brown M.L., O’Leary K.C., Wu C.T., Themanson J.R., Hillman C.H. On the number of trials necessary for stabilization of error-related brain activity across the life span. Psychophysiology. 2010;47:767–773. doi: 10.1111/j.1469-8986.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- Potts G.F., George M.R.M., Martin L.E., Barratt E.S. Reduced punishment sensitivity in neural systems of behavior monitoring in impulsive individuals. Neurosci. Lett. 2006;397:130–134. doi: 10.1016/j.neulet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Potts G.F. Impact of reward and punishment motivation on behavior monitoring as indexed by the error related negativity. Int. J. Psychophysiol. 2011;81:324–331. doi: 10.1016/j.ijpsycho.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reise S.P., Moore T.M., Sabb F.W., Brown A.K., London E.D. The Barratt Impulsiveness Scale-11: reassessment of its structure in a community sample. Psychol. Assess. 2013;25:631–642. doi: 10.1037/a0032161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C., Anderson M., Reid C.L., Fox A.M. Neural indicators of error processing and intraindividual variability in reaction time in 7 and 9 year-olds. Dev. Psychobiol. 2011;53:256–265. doi: 10.1002/dev.20518. [DOI] [PubMed] [Google Scholar]
- Richardson, C., Anderson, M., Reid, C. L., & Fox, A. M. Development of inhibition and switching: a longitudinal study of the maturation of interference suppression and reversal processes during childhood. Unpublished manuscript under revision. [DOI] [PMC free article] [PubMed]
- Ruchsow M., Spitzer M., Grön G., Grothe J., Kiefer M. Error processing and impulsiveness in normals: evidence from event-related potentials. Brain Res. Cogn. Brain Res. 2005;24:317–325. doi: 10.1016/j.cogbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K., Davis-Stober C.P. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neurosci. 2004;5:39–51. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo G.L., Castro S.A. Reliability and validity of Barratt impulsiveness scale (BIS-11) in adolescents. Rev. Chil. Neuro-Psiquiat. 2013;51:245–254. [Google Scholar]
- Santesso D.L., Segalowitz S.J. Developmental differences in error-related ERPs in middle- to late-adolescent males. Dev. Psychol. 2008;44:205–217. doi: 10.1037/0012-1649.44.1.205. [DOI] [PubMed] [Google Scholar]
- Sawyer S.M., Afifi R.A., Bearinger L.H., Blakemore S.-J., Dick B., Ezeh A.C., Patton G.C. Adolescence: a foundation for future health. Lancet. 2012;379:1630–1640. doi: 10.1016/S0140-6736(12)60072-5. [DOI] [PubMed] [Google Scholar]
- Schachar R., Logan G.D. Impulsivity and inhibitory control in normal development and childhood psychopathology. Dev. Psychol. 1990;26:710–720. [Google Scholar]
- Sebastian A., Jung P., Krause-Utz A., Lieb K., Schmahl C., Tüscher O. Frontal dysfunctions of impulse control − a systematic review in borderline personality disorder and attention-deficit/hyperactivity disorder. Front. Hum. Neurosci. 2014;8:698. doi: 10.3389/fnhum.2014.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K., Tamm L., Epstein J.N. Deficient post-error slowing in children with ADHD is limited to the inattentive subtype. J. Int. Neuropsychol. Soc. 2012;18:612–617. doi: 10.1017/S1355617712000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.K., Chang K.D., Chen M.C., Kelley R.G., Garrett A., Mitsunaga M.M., Bararpour L., Howe M., Reiss A.L., Gotlib I.H. Volumetric reductions in the subgenual anterior cingulate cortex in adolescents with bipolar I disorder. Bipolar Disord. 2013;14:585–596. doi: 10.1111/j.1399-5618.2012.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spinella M., Miley W.M. Impulsivity and educational achievement in college students. Coll. Stud. J. 2003;37:545–549. [Google Scholar]
- Stahl J., Gibbons H. Dynamics of response-conflict monitoring and individual differences in response control and behavioral control: an electrophysiological investigation using a stop-signal task. Clin. Neurophysiol. 2007;118:581–596. doi: 10.1016/j.clinph.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Stanford M.S., Mathias C.W., Dougherty D.M., Lake S.L., Anderson N.E., Patton J.H. Fifty years of the Barratt impulsiveness scale: an update and review. Pers. Individ. Dif. 2009;47:385–395. [Google Scholar]
- Takács Á., Kóbor A., Honbolygó F., Csépe V. Does rare error count in impulsivity? Difference in error-negativity. J. Psychophysiol. 2015;29:64–72. [Google Scholar]
- Tamnes C.K., Walhovd K.B., Torstveit M., Sells V.T., Fjell A.M. Performance monitoring in children and adolescents: a review of developmental changes in the error-related negativity and brain maturation. Dev. Cogn. Neuorsci. 2010;6:1–13. doi: 10.1016/j.dcn.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.B., Cummins T.D.R., Fox A.M., Johnson B.P., Tong J.H., Visser T.A.W., Hawi Z., Bellgrove M.A. Allelic variation in dopamine D2 receptor gene is associated with attentional impulsiveness on the Barratt impulsiveness scale (BIS-11) World J. Biol. Psychiatry. 2017:1–9. doi: 10.1080/15622975.2016.1273549. [DOI] [PubMed] [Google Scholar]
- Tellegen A. University of Minnesota Press; Minneapolis (MN): 1982. Multidimensional Personality Questionnaire Manual. [Google Scholar]
- van Veen V., Carter C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Warren J.I. University of Virginia, Institute of Law, Psychiatry, and Public Policy; Charlottesville: 2001. Baseline Psychopathology in a Women's Prison: Its Impact on Institutional Adjustment and Risk for Violence (NCJ 198621) [Google Scholar]
- Wiersema J.R., van der Meer J.J., Roeyers H. ERP correlates of impaired error monitoring in children with ADHD. J. Neural Transm. 2005;112:1417–1430. doi: 10.1007/s00702-005-0276-6. [DOI] [PubMed] [Google Scholar]
- Wiersema J.R., van der Meer J.J., Roeyers H. Developmental changes in error monitoring: an event-related potential study. Neuropsychologia. 2007;45:1649–1657. doi: 10.1016/j.neuropsychologia.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wilcox C.E., Dekonenko C.J., Mayer A.R., Bogenschutz M.P., Turner J.A. Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev. Neurosci. 2014;25:1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N., Summerfield C. Metacognition in human decision-making: confidence and error monitoring. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:1310–1321. doi: 10.1098/rstb.2011.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N., Botvinick M.M., Cohen J.D. The neural basis of error detection: conflict monitoring and error-related negativity. Psychol. Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.