Abstract
The self-concept – the set of beliefs that a person has about themselves – shows significant development from adolescence to early adulthood, in parallel with brain development over the same period. We sought to investigate how age-related changes in self-appraisal processes corresponded with brain network segregation and integration in healthy adolescents and young adults. We scanned 88 participants (46 female), aged from 15 to 25 years, as they performed a self-appraisal task. We first examined their patterns of activation to self-appraisal, and replicated prior reports of reduced dorsomedial prefrontal cortex activation with older age, with similar reductions in precuneus, right anterior insula/operculum, and a region extending from thalamus to striatum. We used independent component analysis to identify distinct anterior and posterior components of the default mode network (DMN), which were associated with the self-appraisal and rest-fixation parts of the task, respectively. Increasing age was associated with reduced functional connectivity between the two components. Finally, analyses of task-evoked interactions between pairs of nodes within the DMN identified a subnetwork that demonstrated reduced connectivity with increasing age. Decreased network integration within the DMN appears to be an important higher-order maturational process supporting the emerging adult self.
Keywords: Adolescent development, Connectivity, Default mode network, Functional MRI, Self
1. Introduction
One of the key developmental tasks during the transition to adulthood is the establishment of a more coherent self-structure (Arnett and Taber, 1994; Harter, 2006). The self-concept – or the set of beliefs that a person has about themselves – becomes more clearly defined during adolescence, supporting a young person’s emerging independence (Harter, 1990; Demo, 1992). The cortical regions that support self-representations develop along a similar timeframe, reaching full maturity in the third decade of life (Gogtay et al., 2004; Lebel and Beaulieu, 2011). Both the self-concept and corresponding brain regions develop together, but in a relationship that remains incompletely understood.
Self-appraisal – one’s reflection on the attributes of the self-concept – activates mainly midline cortical regions in a pattern that overlaps with the default mode network (DMN) (Davey et al., 2016). The DMN comprises a set of regions that shows greater activity and functional coupling when a person is in a task-free ‘resting-state’, but also when engaged in task-oriented forms of self-referential processing (Andrews-Hanna et al., 2014; Raichle et al., 2001; Harrison et al., 2008, 2011). The core DMN regions are the dorsal and ventral medial prefrontal cortex, the posterior cingulate cortex, and the inferior parietal lobule, but other regions are also (inconsistently) included (Buckner et al., 2008). Functional magnetic resonance imaging (fMRI) studies show that anterior components of the DMN, especially the dorsal medial prefrontal cortex (DMPFC), have particular involvement in self-appraisal processes (including those that occur during resting-state introspection); while posterior DMN regions have greater involvement in other resting-state processes, such as low-level surveillance of the external and internal environment (Davey et al., 2016; Whitfield-Gabrieli et al., 2011; D’Argembeau et al., 2005; Gusnard et al., 2001). Model-free analyses of resting-state fMRI data often separates the DMN into anterior and posterior components (Allen et al., 2011; Kalcher et al., 2012; Damoiseaux et al., 2006; Kim and Lee, 2011) – supporting a segregation of their interconnected functions.
While the relationship between development of the brain and psychological aspects of the self remains uncertain, one aspect has been consistently demonstrated (Blakemore, 2012): during the transition from adolescence to early adulthood there is reduced activation in the DMPFC in response to presentation of self-related stimuli (Pfeifer et al., 2007, 2009; Blakemore et al., 2007; Sebastian et al., 2012). It has been speculated that brain maturation leads to greater efficiency of neural processing, such that less DMPFC activity is required to perform self-appraisal tasks over the course of development (Sebastian et al., 2008).
An increased research focus on the interaction of large-scale brain systems has brought a network-based perspective to neurodevelopmental processes (Baker et al., 2015; Power et al., 2010; Kaufmann et al., 2017). Resting-state fMRI studies comparing children (7- to 9-year-olds) to adults (21- to 31-year-olds in one study and 19- to 22-year-olds in another) have shown a pattern of increasing resting-state DMN connectivity with development (Fair et al., 2008; Supekar et al., 2010). In opposition to this, decreased resting-state connectivity between anterior and posterior DMN components has been observed to accompany the transition from adolescence to early adulthood in a cohort of 12- to 30-year-olds (Stevens et al., 2009). While there is, therefore, uncertainty as to whether DMN connectivity shows functional integration or segregation during adolescent development, the connectivity changes are likely to be relevant to the development of the self-concept, and need clarification.
The objective of the present study was to investigate the neural correlates of the developing self-concept across adolescence and early adulthood. In particular, we sought to quantify developmental associations with functional network connectivity, with a particular focus on the DMN. We first examined age-dependent changes in brain activation during self-appraisal to test the replicability of previous findings of decreased DMN activation to self-referential stimuli. We then sought to examine the development of DMN connectivity, both at the level of large-scale anterior and posterior DMN sub-networks, and at the finer-grained scale of connectivity between pairs of DMN nodes. To achieve these aims, our analyses thus proceeded across three stages: (i) examination of age-related changes in brain activation during self-appraisal; (ii) identification of DMN components using independent component analysis (ICA), and examination of age-related changes in their interaction; and (iii) examination of age-related changes in connectivity between pairs of DMN nodes.
2. Methods
2.1. Participants
Ninety-six adolescents and young adults, 15–25 years of age, completed the full imaging protocol. Participants were considered eligible if they were (i) without current or past diagnosis of mental illness (Structured Clinical Interview for DSM-IV; First et al., 2002); (ii) competent English speakers; (iii) not taking psychoactive medication; (iv) not pregnant; and (v) had no contraindications to MRI. Participants (and their parents or guardians if they were under 18 years of age) provided their informed consent to participate in the study, which was approved by the Melbourne Health Human Research and Ethics Committee. Of the 96 participants who completed fMRI, we subsequently excluded three participants due to excessive head movement during scanning (see below), and five because of poor task performance on the external attention task (defined as less than 85% accuracy). The final composition of the sample was 88 participants (46 female) with a mean age of 20.1 years (S.D. 2.9 years; see Supplementary Fig. 1 for histograms of the age distributions for each sex). These participants were included in our previously reported study (Davey et al., 2016), which examined the differences and commonalities between brain regions activated during self-appraisal and rest; and were also included as the control group in our examination of self-appraisal in depression (Davey et al., 2017).
2.2. Paradigm design
Participants completed a single run of an fMRI task composed of three alternating experimental conditions: self-appraisal, external attention, and rest-fixation. In the self-appraisal condition, participants were presented with a personality adjective and asked whether or not the word described them. Words were drawn from a frequently used list of personality adjectives (Anderson, 1968), and included words with relatively neutral valence ratings: words such as ‘skeptical’, ‘perfectionistic’, and ‘lucky’ (see Davey et al., 2016). Participants viewed eight blocks of six words, presented for 5 s each, and responded to the question, ‘Does this word describe you?’, by pressing the left or right button on the fiber-optic response pad. In the external attention condition, participants viewed eight blocks of six words, also presented for 5 s each, and responded to the question, ‘Does this word have four or more vowels?’ (Supplementary Fig. 2).
The two lists of 48 words that formed the self-appraisal and external attention conditions were matched on valence ratings and number of vowels, and their presentation was counterbalanced across participants. Each 32 s block (2 s instruction followed by six words presented for 5 s each) was interspersed with a 10 s rest-fixation block in which participants were asked to fixate on a centrally presented cross-hair. Behavioral data (accuracy and response-times) were analyzed with Stata 15 (StataCorp, College Station, USA).
2.3. Image acquisition
A 3 T General Electric Signa Excite system equipped with an eight-channel phased-array head coil was used in combination with ASSET parallel imaging. The functional sequence consisted of a single shot gradient-recalled echo-planar imaging sequence in the steady state (repetition time, 2 s; echo time, 35 ms; and pulse angle, 90°) in a 23-cm field-of-view, with a 64 × 64-pixel matrix and a slice thickness of 3.5 mm (no gap). Thirty-six interleaved slices were acquired parallel to the anterior-posterior commissure line with a 20° anterior tilt to better cover ventral prefrontal cortical brain regions. The total sequence time was 11 min 22 s, corresponding to 341 whole brain echo-planar imaging volumes. The first four volumes from each run were automatically discarded to allow for signal equilibration. A T1-weighted high-resolution anatomical image was acquired for each participant to assist with functional time-series co-registration (140 contiguous slices; repetition time, 7.9 s; echo time, 3 s; flip angle, 13°; in a 25.6 cm field-of-view, with a 256 × 256 pixel matrix and a slice thickness of 1 mm).
2.4. Image preprocessing
Imaging data were processed with Statistical Parametric Mapping software (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK) using MATLAB version 9 (The MathWorks Inc, Natick, USA), and with the FMRIB Software Library 5 (FSL; Analysis Group, FMRIB, Oxford, UK). Motion correction was performed by aligning each participant’s time series to the first image using least-squares minimization and a six-parameter rigid-body spatial transformation. These realigned functional images were then co-registered to each participant’s respective T1 anatomical scans, which were segmented and spatially normalized to the International Consortium for Brain Mapping template using the unified segmentation approach. The functional images were interpolated to 2 mm isotropic resolution (3 mm for independent components analysis to aid computational feasibility), and smoothed with a 5-mm full-width-at-half-maximum (FWHM) gaussian filter.
2.5. Accounting for head motion
We calculated framewise head motion displacement values for each participant to assess head movement during scanning. Frame-wise displacement measures the head movement between one volume of data to the next, calculated by summing translational and rotational realignment estimates at each time-point (Power et al., 2012; Jenkinson et al., 2002). We found no association between age and mean framewise displacement (r = 0.04, p = 0.70). We excluded participants who had mean framewise displacement greater than 0.2 (removing three participants), and included framewise displacement values as covariates in our connectivity analyses.
2.6. Task-based analyses
2.6.1. Age-related activation differences
Primary regressors for the self-appraisal and external attention conditions were created for each participant by specifying the onset and duration of the task blocks, followed by convolution with a canonical hemodynamic response function, and use of a high-pass filter set at 128 s to remove low-frequency drifts. Parameter estimates were calculated at each voxel using the general linear model and local autocorrelation correction with an AR (Arnett and Taber, 1994) model, and including motion parameters as nuisance covariates. Second-level analysis identified voxels where activation to the self-appraisal compared to external attention conditions were correlated with age, adjusted for mean framewise displacement. As age was associated with task reaction times and general vocabulary (see Results section), these factors were additionally adjusted for in our analyses. Clusters were formed at a height threshold of p < 0.001, and survived whole-brain family-wise error correction (pFWE<0.05).
2.6.2. Independent components analysis & correlational psychophysiological interactions
Tensor-ICA was used to characterize task-locked network components, as implemented in MELODIC (FSL; Analysis Group, FMRIB, Oxford, UK). Tensor-ICA is a data-driven method for estimating the independent spatial components that are time-locked across participants and thus reflect shared responses to a task design. We used tensor-ICA to decompose the group-level data into 30 spatial dimensions – a medium-level dimensionality – repeating the process 10 times with randomized participant order. These component maps were then concatenated, and the resulting 300-component dataset (10 tensor-ICA maps × 30 components) fed into a meta-level ICA decomposition. We used this meta-level approach to identify the components with greater robustness: by concatenating the group-level tensor-ICA outputs and performing further ICA we reduced the variability introduced to single-level ICA by participant order and initial parameter values (Wisner et al., 2013; Smith et al., 2009). Last, we used dual regression to regress each participant’s data against the component spatial maps, deriving individual time-courses for each component. We identified components related to the DMN based on their characteristic appearance and their association with the self-appraisal and rest-fixation model time-courses.
We identified anterior and posterior DMN components – temporally associated with the self-appraisal and resting-fixation parts of the task, respectively – and estimated their interaction using correlational psychophysiological interaction (cPPI) analysis (Fornito et al., 2012). cPPI extends the traditional PPI approach to quantify task-related functional connectivity between regions or networks; in our case, in the self-appraisal compared to the external attention conditions. As in traditional PPI (Friston et al., 1997), this is achieved by modeling the task effects, the physiological signal, and their interaction.
We used a partial correlation framework, as implemented by the cPPI toolbox, to estimate interactions between the anterior and posterior DMN components. Each component time course was first deconvolved (Gitelman et al., 2003), multiplied by the task regressor that defined activity in self-appraisal compared to external attention conditions, and then reconvolved to generate a component-specific PPI term. We then estimated task-related network interaction values, ni, between components as the partial correlation between the two components’ PPI signals, adjusted for the original task regressor (which controls for co-activation effects), the original component time courses (which controls for task-unrelated coupling), time courses of the other seven network components (to isolate specific functional coupling between the anterior and posterior DMN), and nuisance signals (framewise displacement parameters and time-courses from white-matter and cerebrospinal fluid). We determined the presence of age-related effects by correlating the network interaction values with participant age, adjusted for mean framewise displacement, reaction times, and vocabulary.
2.6.3. Pairwise correlations
We used graph theoretic techniques to further characterize the age-related development of functional interactions within the DMN (the combined anterior and posterior DMN components), clarifying whether connectivity changes were present only across components, or were also present within components. We defined nodes within each component (22 anterior DMN and 11 posterior DMN nodes) by generating 4-mm-radius spheres centered on the stereotactic coordinates of local maxima in the statistically thresholded component spatial maps (Fornito et al., 2012), with each maxima separated by at least 12 mm (see Supplementary Table 1 for a list of the nodes and their coordinates). For each participant, we modeled interactions as undirected graphs of 33 nodes connected by all possible edges ([332–33]/2 = 528 edges).
As per prior work (Fornito et al., 2012; Dwyer et al., 2014), we used cPPI to determine network interaction values for each node-pair, using the same approach as for the component interactions, above. We then used the matrices containing the network interaction values for all node-pairs as input to a Network-Based Statistic analysis (NBS 1.2; (Zalesky et al., 2010)) to determine whether task-related functional connectivity in any subnetwork within the broader DMN showed an association with age. We used a height threshold of p < 0.05 for the association of each network-interaction value with age to identify potential subnetworks, generated 5000 permutations to estimate the null distribution of subnetwork size, and then identified subnetworks that showed significant association with age (pFWE < 0.05).
3. Results
3.1. Behavioral results
Mean accuracy on the external attention condition was 97% (S.D. 3%). The mean reaction time for the self-appraisal condition was 1.68 s (range of participant means 1.08–3.09 s) and for the external attention condition 1.92 s (range 1.08–2.87 s). Older age was significantly correlated with faster reaction times for the self-appraisal condition (r=−0.29, p = 0.006); but not for external attention (r=−0.19, p = 0.08). Vocabulary, as measured by the Wechsler Test of Adult Reading (Wechsler, 2001), also had an expected correlation with age (r = 0.37, p < 0.001). Both reaction time and WTAR scores were included as covariates in our analyses (we provide results without inclusion of covariates in Supplementary Table 2). These covariates had minimal effects on reported associations between age and connectivity parameters.
3.2. Age is associated with reduced activation in a distributed self-appraisal network
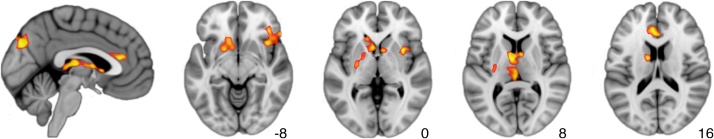
We found that older age was associated with decreased activation to the self-appraisal versus external attention contrast in rostral anterior cingulate cortex (a part of the DMPFC), precuneus, right insula/frontal operculum, and in a subcortical cluster extending from thalamus to striatum (Table 1, Fig. 1, Supplementary Fig. 4). No brain regions showed positive associations between age and activation to the self-appraisal versus external attention contrast.
Table 1.
Brain regions showing a negative association with age.
| Brain region | Cluster size | Peak z-value | Peak z coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| Thalamus extending to caudate | 855 | 4.7 | −6 | 0 | 8 |
| Precuneus | 222 | 4.7 | −2 | −80 | 34 |
| Right anterior insula / frontal operculum | 379 | 4.2 | 34 | 8 | 0 |
| Rostral anterior cingulate cortex | 153 | 4.0 | −4 | 34 | 18 |
Fig. 1.
Activations to self-appraisal versus external attention demonstrating a negative correlation with age. Significant clusters (p < 0.05) were identified at a cluster-forming threshold of p < 0.001.
3.3. Anterior and posterior DMN components associated with self-appraisal and rest-fixation
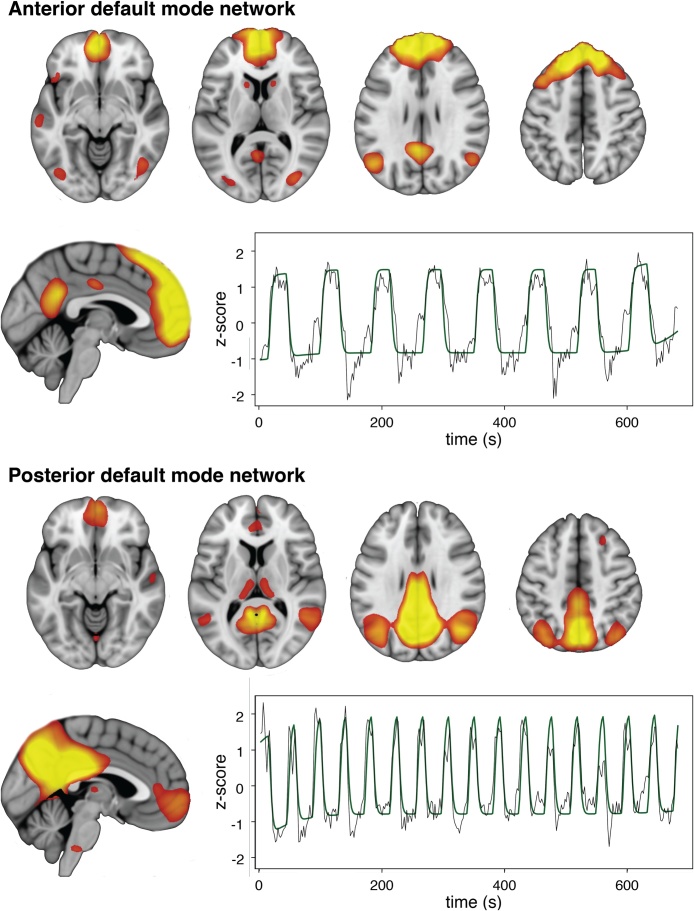
ICA characterized nine components that corresponded to known brain networks: their description and correlation with task components are detailed in Table 2 (and illustrated in Supplementary Fig. 4). The anterior DMN was most robustly correlated with the self-appraisal condition (r = 0.89, p < 0.001) whereas the posterior DMN was correlated with the rest-fixation task (r = 0.89, p < 0.001). The anterior DMN component is composed mainly of DMPFC, a discrete region of ventral posterior cingulate cortex (PCC), caudate, and inferior parietal lobules. The posterior DMN component is composed of ventral medial prefrontal cortex (VMPFC), a large area in posterior midline cortex encompassing PCC and precuneus, and dorsomedial thalamus (Fig. 2). While both components consist of midline regions that show substantial overlap with the conventional DMN, each includes regions outside of it. Of note, the anterior DMN includes a small region of posterior midline cortex (PCC), and the posterior DMN includes a small region of anterior midline cortex (VMPC), highlighting the incomplete anatomical segregation between the components.
Table 2.
Brain networks identified by independent components analysis.
| Brain network | Correlation with task components |
Functional termsa | ||
|---|---|---|---|---|
| Self-appraisal | Ext. attention | Rest-fixation | ||
| Left fronto-parietal | 0.45 | 0.19 | −0.76 | retrieval, semantic, word |
| Right fronto-parietal | −0.80 | 0.77 | 0.04 | response inhibition, working memory, cognitive control |
| Dorsal attention | −0.67 | 0.88 | −0.23 | visual, object, attentional |
| Fronto-temporal | 0.85 | −0.39 | −0.56 | language, sentences, comprehension |
| Sensorimotor | −0.29 | 0.65 | −0.43 | finger, hand, finger movement |
| Visuospatial | −0.86 | 0.34 | 0.64 | moving, action, tactile |
| Primary visual | 0.2 | −0.61 | 0.48 | visual, early visual, primary visual |
| Anterior DMN | 0.89 | −0.78 | −0.15 | social, default, self |
| Posterior DMN | −0.31 | −0.43 | 0.89 | default, deactivation, resting |
The top three functional terms associated with each component according to the Neurosynth database (http://www.neurosynth.org).
Fig. 2.
The anterior and posterior DMN components. The component timecourses are overlaid on the model timecourses for self-appraisal (top) and rest-fixation (bottom).
Network interaction values, as appraised by cPPI, were calculated for the interaction between the anterior and posterior DMN components. As expected, the components showed significant positive connectivity during the self-appraisal compared to the external attention condition (ni = 0.20, t87 = 8.8, p < 0.001).
3.4. Age-related changes in network connectivity
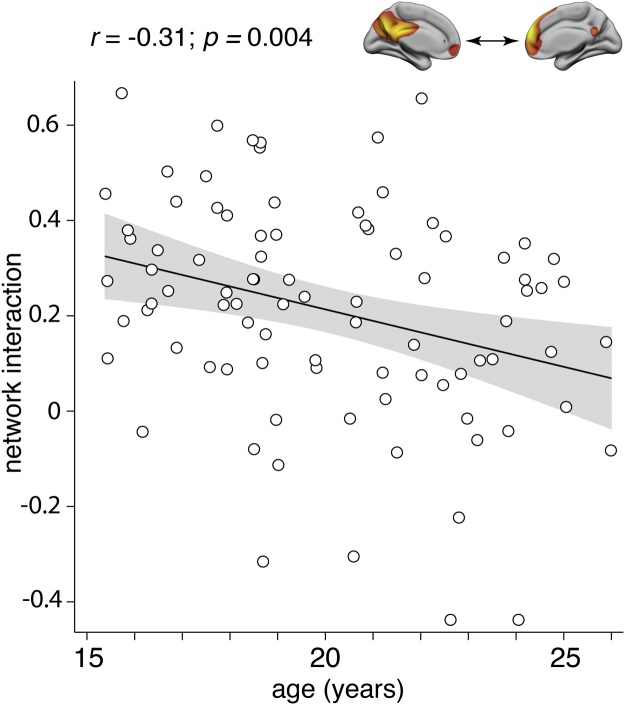
Age-related changes were first examined at the component level, between anterior and posterior DMN components, and then at the level of node-pairs within the extended DMN (formed by the combined components). At the component level, interaction between the anterior and posterior DMN during self-appraisal showed significant reduction with increasing age (r=−0.31, p = 0.004; Fig. 3).
Fig. 3.
Cooperative interaction between the anterior and posterior DMN components during self-appraisal declined over the course of adolescence and early adulthood.
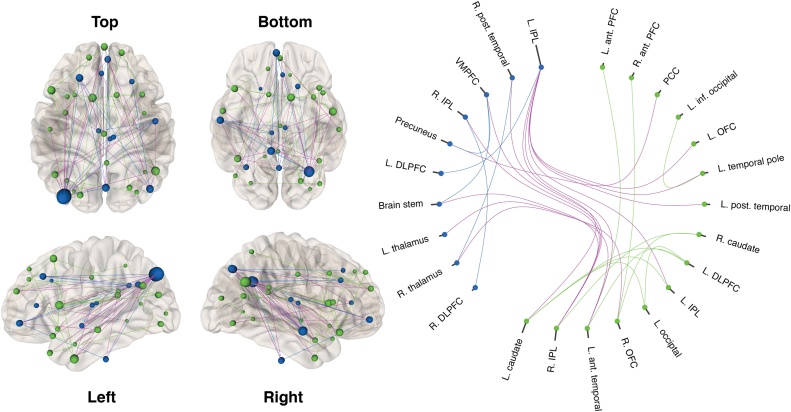
Application of graph theoretic techniques to characterize age-related changes in interactions within the DMN confirmed a subnetwork that showed reduced connectivity with increased age (p = 0.03; Fig. 4). The subnetwork included 32 of the 33 nodes (see Supplementary Table 1 for node coordinates), and 56 edges. The hub nodes (linked to the most edges in this network) were in the inferior parietal lobules (left inferior parietal lobule [15 edges], right inferior parietal lobule, anteriorly [7 edges], and right inferior parietal lobule, posteriorly [5 edges]); and also in precuneus (5 edges) and caudate (7 edges). Most of the age-related changes occurred for edges that spanned across the anterior and posterior DMN nodes (46% of edges), followed by edges within the anterior DMN (32%) and edges within the posterior DMN (21%). No subnetwork was identified that showed increased connectivity with older age.
Fig. 4.
A network of brain regions within the anterior and posterior DMN (represented by blue and green nodes, respectively) showed reduced connectivity during self-appraisal with older age. These included connectivity changes within the anterior DMN (blue lines), within the posterior DMN (green lines), and across components (purple lines). Gray bars indicate the number of network connections for each node. The figures were produced using neuromarvl (http://immersive.erc.monash.edu.au/neuromarvl/) (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
4. Discussion
The self-concept – incorporating notions of self-hood, self-appraisal, independence, and agency – changes markedly with the passage from adolescence to early adulthood, coinciding with substantial changes in brain connectivity. We sought to better understand the links between these developmental processes within the framework of network integration versus segregation, a dynamic balance held to be central to cognitive function (Tononi et al., 1994; Friston, 2009). We first confirmed previous reports that DMPFC showed less activation during self-appraisal with increased age, and also demonstrated similar developmental relationships in precuneus, insula/operculum, and subcortical thalamic and striatal regions. Importantly, we extended previous findings to show that the reduction in self-appraisal activation observed with older age was associated with reduced connectivity between anterior and posterior DMN components, and with a broad pattern of DMN segregation.
The study results are consistent with the notion that self-appraisal is performed more easily as the self becomes more clearly defined, which is one of the primary tasks of adolescent development (Harter, 1990; Kroger, 2005). At a behavioral level, older participants showed faster reaction times to the self-appraisal questions – which may represent greater confidence in their respective responses – and they demonstrated reduced activation and connectivity while responding to the questions.
The division of the DMN into anterior and posterior components has previously been reported in resting-state fMRI studies (Allen et al., 2011; Kalcher et al., 2012; Damoiseaux et al., 2006; Kim and Lee, 2011). Segregation of the DMN in our study was enhanced by our task design, which was able to distinguish between the psychological functions supported by the components. Activity within the anterior DMN component – comprised mainly of DMPFC, but also a small region of PCC, inferior parietal lobule, and the caudate – was highly correlated with self-appraisal. Studies have consistently demonstrated that DMPFC is activated by explicit self-appraisal, suggesting that it supports processing of the more cognitive-reflective aspects of the self-concept (Davey et al., 2016).
Activity within the posterior DMN, on the other hand, was strongly correlated with the periods of rest-fixation. The posterior DMN – comprised mainly of posteromedial cortex, but including also lateral parietal cortex, ventromedial PFC, and thalamus – appears to be responsible for coordinating sensory representations of the self. These include the position of the body in space, its relationship to the external environment, and interoceptive sensations (Shulman et al., 1997; Gilbert et al., 2007). In broad terms, the division of the DMN into anterior and posterior components might represent the hypothesized ‘narrative’ self versus the ‘minimal’ self (Gallagher, 2000). The reduced interaction between the anterior and posterior DMN components across adolescent development, from this perspective, represents the cognitively elaborated self becoming increasingly differentiated from the implicitly experienced self.
Our network analysis reveals a broader pattern of reduced connectivity with self-development. About half of the connections in this developmental network were between nodes across the components, and half of the connections between nodes within the components, suggesting a generalized pattern of developmental segregation within the DMN. Our results are not consistent with previous reports of increasing DMN connectivity with development (Fair et al., 2008; Supekar et al., 2010). Both of the studies that reported the relationship compared children (7- to 9-year-olds) with adults. However, in a supplementary analysis that also included adolescents, Fair and colleagues reported that the relationship was non-linear, with DMN connectivity peaking in adolescence and then showing some reduction from adolescence to adulthood (Fair et al., 2008). This aligns with the results of our study, and also with Stevens and colleagues’ (Stevens et al., 2009) report of reduced resting-state connectivity between anterior and poster DMN from adolescence to adulthood. An import difference between the aforementioned studies and ours is that they examined DMN connectivity during rest, while we examined task-related changes in connectivity. The decreased DMN connectivity we have demonstrated is therefore contextual: it is evident during self-appraisal compared to external attention. We elaborate on the earlier findings by showing that the reduced connectivity is a correlate of the developing self-concept.
The network analysis indicated that the inferior parietal lobule (IPL) had a coordinating role in the reduced DMN connectivity observed with development. The IPL, which is a core component of the DMN (Davey et al., 2016; Buckner et al., 2008), is activated by tasks involving abstract representations of the self and others: as in self-appraisal, autobiographical memory, mentalization, and theory of mind (Sestieri et al., 2017; Igelström and Graziano, 2017; Mars et al., 2012). The reduction in pairwise connectivity with IPL nodes we observed over the course of adolescent development is consistent with older participants having more ready access to their self-representations.
The idea that the self-concept becomes more easily accessed with older age is also supported by the demonstration of reduced activation in brain regions associated with cognitive effort and accompanying autonomic arousal: in DMPFC and insula (Critchley, 2005; Menon and Uddin, 2010; Shackman et al., 2011). An alternative explanation for our findings might therefore be that older participants were more familiar with the personality adjectives, and thus less cognitive effort was required to process their meaning. We adjusted for vocabulary in our analyses (and also for reaction times), but also note that familiarity with the meanings of the personality adjectives and development of the self-concept are overlapping constructs: as a person learns more about what constitutes their self (and other selves), they become more familiar with the meanings of the words that describe the concepts, and can therefore more easily map one to the other.
The main limitation of our study is one inherent in cross-sectional examination of developmental processes. The participants were not assessed longitudinally: doing so would have allowed us to make stronger inferences about the within-subject nature of the developmental processes. Longitudinal studies will be needed to clarify how the development of the default mode tracks changes in the self-concept at an individual level. The study would also have been stronger had it been able to directly examine the relationship between brain changes and behavioral measures of self-appraisal, which future studies might explore in more detail.
Our study of a cohort of adolescents and young adults has allowed us to examine the brain correlates of self-related developmental processes in more detail than previous studies. We sought to use the lens of functional network integration and segregation to bring more clarity to this issue. Our results expand on previous findings showing reduced DMPFC activation during self-appraisal with older age to demonstrate how these co-occur within a broader pattern of increased network segregation. We have confirmed the increasing functional segregation of the DMN into anterior and posterior components with older age, and demonstrated the significance of a generalized pattern of network segregation for the emerging adult self. These findings suggest an intriguing shift of self-cognition aligning with a dynamic shift in the balance of functional network forces. They raise the questions as to whether this developmental brain network patterning carries over to other aspects of self-related processing; and whether such findings might provide a framework for understanding the disturbances of self-related processes that are common in mental illness.
Competing interests
The authors declare no competing financial interests.
Acknowledgements
The research was supported by National Health and Medical Research Council of Australia (NHMRC) Project Grants 1024570 (principal investigator, CGD) and 1064643 (principal investigator, BJH). CGD was supported by an NHMRC Career Development Fellowship (1061757). AF was supported by an Australian Research Council Fellowship (FT130100589). BJH was supported by a NHMRC Career Development Fellowship (1124472).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100626.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Allen E.A., Erhardt E.B., Damaraju E. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N.H. Likableness ratings of 555 personality-trait words. J. Pers. Soc. Psychol. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Saxe R., Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage. 2014;91:324–335. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett J.J., Taber S. Adolescence terminable and interminable: when does adolescence end? J. Youth Adolesc. 1994;23:517–537. [Google Scholar]
- Baker S.T., Lubman D.I., Yücel M. Developmental changes in brain network hub connectivity in late adolescence. J. Neurosci. 2015;35:9078–9087. doi: 10.1523/JNEUROSCI.5043-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., den Ouden H., Choudhury S., Frith C. Adolescent development of the neural circuitry for thinking about intentions. Soc. Cogn. Affect. Neurosci. 2007;2:130–139. doi: 10.1093/scan/nsm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A., Collette F., Van der Linden M. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25:616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey C.G., Pujol J., Harrison B.J. Mapping the self in the brain’s default mode network. Neuroimage. 2016;132:390–397. doi: 10.1016/j.neuroimage.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Davey C.G., Breakspear M., Pujol J., Harrison B.J. A brain model of disturbed self-appraisal in depression. Am. J. Psychiatry. 2017;174:895–903. doi: 10.1176/appi.ajp.2017.16080883. [DOI] [PubMed] [Google Scholar]
- Demo D.H. The self-concept over time: research issues and directions. Annu. Rev. Sociol. 1992;181:303–326. [Google Scholar]
- Dwyer D.B., Harrison B.J., Yücel M. Large-scale brain network dynamics supporting adolescent cognitive control. J. Neurosci. 2014;34:14096–14107. doi: 10.1523/JNEUROSCI.1634-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Dosenbach N.U. The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Zalesky A., Simons J.S. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc. Natl. Acad. Sci. U. S. A. 2012;109:12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J. Modalities, modes, and models in functional neuroimaging. Science. 2009;326:399–403. doi: 10.1126/science.1174521. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: implications for cognitive science. Trends Cogn. Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Dumontheil I., Simons J.S., Frith C.D., Burgess P.W. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317(43) doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E., Raichle M.E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Lopez-Sola M. Consistency and functional specialization in the default mode brain network. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Pujol J., Contreras-Rodriguez O. Task-induced deactivation from rest extends beyond the default mode brain network. PLoS One. 2011;6:e22964. doi: 10.1371/journal.pone.0022964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter S. Developmental differences in the nature of self-representations: implications for the understanding, assessment, and treatment of maladaptive behavior. Cogn. Ther. Res. 1990;14:113–142. [Google Scholar]
- Harter S. The self. In: Lerner R., Damon W., editors. The Handbook of Child Psychology. John Wiley & Sons; Hoboken, NJ: 2006. pp. 505–570. [Google Scholar]
- Igelström K.M., Graziano M.S.A. The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia. 2017;105:70–83. doi: 10.1016/j.neuropsychologia.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kalcher K., Huf W., Boubela R.N. Fully exploratory network independent component analysis of the 1000 functional connectomes database. Front. Hum. Neurosci. 2012;6:301. doi: 10.3389/fnhum.2012.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T., Alnæs D., Doan N.T., Brandt C.L., Andreassen O.A., Westlye L.T. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat. Neurosci. 2017;20:513–515. doi: 10.1038/nn.4511. [DOI] [PubMed] [Google Scholar]
- Kim D.Y., Lee J.H. Are posterior default-mode networks more robust than anterior default-mode networks? Evidence from resting-state fMRI data analysis. Neurosci. Lett. 2011;498:57–62. doi: 10.1016/j.neulet.2011.04.062. [DOI] [PubMed] [Google Scholar]
- Kroger J. Routledge; 2005. Identity in Adolescence: The Balance Between Self and Other; p. 288. [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R.B., Neubert F.X., Noonan M.P., Sallet J., Toni I., Rushworth M.F. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Lieberman M.D., Dapretto M. I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. J. Cogn. Neurosci. 2007;19:1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Borofsky L.A., Dapretto M., Fuligni A.J., Lieberman M.D. Neural correlates of direct and reflected self-appraisals in adolescents and adults: when social perspective-taking informs self-perception. Child Dev. 2009;80:1016–1038. doi: 10.1111/j.1467-8624.2009.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Fair D.A., Schlaggar B.L., Petersen S.E. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian C., Burnett S., Blakemore S.J. Development of the self-concept during adolescence. Trends Cogn. Sci. 2008;12:441–446. doi: 10.1016/j.tics.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., Fontaine N.M., Bird G. Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2012;7:53–63. doi: 10.1093/scan/nsr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C., Shulman G.L., Corbetta M. The contribution of the human posterior parietal cortex to episodic memory. Nat. Rev. Neurosci. 2017;18:183–192. doi: 10.1038/nrn.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., Corbetta M., Buckner R.L. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30:2356–2366. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K., Uddin L.Q., Prater K., Amin H., Greicius M.D., Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G., Sporns O., Edelman G.M. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, Texas: 2001. Manual for the Wechsler Test of Adult Reading (WTAR) [Google Scholar]
- Whitfield-Gabrieli S., Moran J.M., Nieto-Castanon A., Triantafyllou C., Saxe R., Gabrieli J.D. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55:225–232. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Wisner K.M., Atluri G., Lim K.O., Macdonald A.W. Neurometrics of intrinsic connectivity networks at rest using fMRI: retest reliability and cross-validation using a meta-level method. Neuroimage. 2013;76:236–251. doi: 10.1016/j.neuroimage.2013.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.