Abstract
Chinese is a logographic language that is different from alphabetic languages in visual and semantic complexity. Thus far, it is still unclear whether Chinese children with dyslexia show similar disruption of white matter pathways as in alphabetic languages. The present study focused on the alteration of white matter pathways in Chinese children with dyslexia. Using diffusion tensor imaging tractography, the bilateral arcuate fasciculus (AF-anterior, AF-posterior and AF-direct segments), inferior fronto-occipital fasciculus (IFOF) and inferior longitudinal fasciculus (ILF) were delineated in each individual’s native space. Compared with age-matched controls, Chinese children with dyslexia showed reduced fractional anisotropy in the left AF-direct and the left ILF. Further regression analyses revealed a functional dissociation between the left AF-direct and the left ILF. The AF-direct tract integrity was associated with phonological processing skill, an ability important for reading in all writing systems, while the ILF integrity was associated with morphological processing skill, an ability more strongly recruited for Chinese reading. In conclusion, the double disruption locus in Chinese children with dyslexia, and the functional dissociation between dorsal and ventral pathways reflect both universal and specific properties of reading in Chinese.
Keywords: Dyslexia, Tractography, White matter pathway, Phonology, Morphology, Chinese
1. Introduction
Dyslexia is a neurodevelopmental disorder affecting 3–7% of school-age children, that is characterized by a specific difficulty in reading acquisition not solely accounted for by mental age, visual acuity problems, or inadequate schooling (World Health Organization, 2011). Neuroimaging research has revealed that children and adults with dyslexia showed anomalies in the frontal, temporoparietal and occipitotemporal regions of the left-hemisphere (Fiez and Petersen, 1998; McCandliss and Noble, 2003; Pugh et al., 2001; Richlan et al., 2010). In a recent meta-analysis of 28 functional neuroimaging studies, researchers reported both overlap and differences in dyslexic brain activations between deep and shallow orthographies, suggesting that the dyslexic brain shows both biological unity and orthographic specificity (Martin et al., 2016).
In the past decade, diffusion tensor imaging (DTI) studies have suggested that there is a decrease of fractional anisotropy (FA) in the left temporoparietal and inferior frontal regions in children and adults with dyslexia (Carter et al., 2009; Deutsch et al., 2005; Keller and Just, 2009; Klingberg et al., 2000; Niogi and McCandliss, 2006; Odegard et al., 2009; Richards et al., 2008; Rimrodt et al., 2010; Steinbrink et al., 2008; Vandermosten et al., 2012a). Less is known, however, about the anatomical white matter tracts that are exactly underlying these regions. Considering the most consistent temporoparietal region, for example, some researchers attribute the disruption to the anterior-posterior segment of the arcuate fasciculus (Gold et al., 2007; Klingberg et al., 2000; Nagy et al., 2004), while others attribute it to the corona radiata with an inferior–superior orientation (Beaulieu et al., 2005; Niogi and McCandliss, 2006), and yet others to the corpus callosum (Ben-Shachar et al., 2007). In a meta-analysis of DTI studies that used a voxel-based approach, researchers reported a well-replicated cluster in the left temporoparietal region (x = −29, y = −17, z = 26) (Vandermosten et al., 2012b). However, a more recent meta-analysis reported no reliable FA difference between children and adults with dyslexia and controls (Moreau et al., 2018, in press). Both meta-analyses relied exclusively on voxel-based studies, which have important limitations. Temporoparietal tracts are notoriously difficult to disentangle using voxel-based analysis (VBA). DTI tractography offers the possibility to reliably reconstruct the major fiber tracts in native space (Catani and Thiebaut de Schotten, 2008). It respects inter-individual variability and helps avoiding the problems brought by normalization and reference systems usually encountered in the VBA-approach (Jones et al., 2007; Ramus et al., 2018; Shen et al., 2007). Since this approach typically involves manual or semi-automatic segmentation, it is time-consuming and requires a priori anatomical hypotheses. Until now, only a few studies explored white matter anomalies in children and adults with dyslexia using fiber tractography methods (Vandermosten et al., 2012a, 2015; Yeatman et al., 2012; Zhao et al., 2016). These studies suggest that tracts in both the dorsal (AF and/or SLF) and ventral (IFOF and ILF) pathways are the main candidate neuroanatomical markers for dyslexia. More specifically, findings of reduced FA in the left AF (dorsal pathway, phonology related) are the most consistent across dyslexia studies (Carter et al., 2009; Deutsch et al., 2005; Klingberg et al., 2000; Rimrodt et al., 2010; Steinbrink et al., 2008; Vandermosten et al., 2012a). In contrast, findings of reduced FA in the left IFOF/ILF (ventral pathway, semantic/orthography related) are relatively scarce (Steinbrink et al., 2008; Vandermosten et al., 2015) and have not been replicated in some studies (e.g. Vandermosten et al., 2012a; Zhao et al., 2016). Thus, the most consistent deficit in children and adults with dyslexia in alphabetic languages seems to be in the left dorsal pathway. The correlation between AF integrity and phonological skill is also consistent with the important role of the phonological deficit in dyslexia in alphabetic languages (Vellutino et al., 2004). As an ideographic language, the division of labor between phonology and semantics in Chinese reading is more equitable (Yang et al., 2013; Zhao et al., 2014). This may suggest that not only the phonology-related dorsal pathway but also the semantic/orthography-related ventral pathway is important in Chinese reading. Thus, in the present study, we investigated both dorsal and ventral pathways in Chinese children with dyslexia.
What are the cognitive deficits underlain by the disrupted fibertracts? Reading is built on spoken language (Perfetti and Sandak, 2000) and consists of a series of complex cognitive processes, from decoding visual (orthographic) to auditory (phonological) information and to accessing conceptual (semantic) representations (e.g. Fiez and Petersen, 1998; McCandliss and Noble, 2003; Ramus, 2004). According to the dual route theory of reading, word reading can be achieved through two discrete routes: the grapho-phonological or indirect route for regular or novel words that transforms visual words into their auditory counterparts via grapheme-to-phoneme correspondences; the lexicosemantic or direct route for exception or frequent words that corresponds to a direct association between the visual form of the word and its meaning (Coltheart et al., 2001). Accordingly, researchers proposed that reading recruits two distinct neural routes in the left hemisphere: a dorsal phonological route and a ventral orthographic route (Jobard et al., 2003; Schlaggar and McCandliss, 2007). DTI studies have suggested that this functional dissociation is instantiated by the dorsal and ventral white matter pathways. The left AF in the dorsal route was found to be associated with phonological processing (Saygin et al., 2013; Vandermosten et al., 2012a; Yeatman et al., 2011), while the left IFOF and ILF in the ventral pathway have been found to be important for orthographic processing (Epelbaum et al., 2008; Vandermosten et al., 2012a; Zhao et al., 2016). Alternatively, other studies have suggested a semantic (Duffau et al., 2005; Han et al., 2013) and phonological (Vandermosten et al., 2015; Welcome and Joanisse, 2014) involvement of the left IFOF. Therefore, it remains unclear whether the ventral pathway is a purely orthographic processing route or whether it also participates in other cognitive processes.
All these findings were obtained in alphabetic languages. It remains a highly debated issue to what extent the neural basis of dyslexia differs across different languages and cultures (Perfetti et al., 2006; Pugh, 2006; Siok et al., 2004; Ziegler, 2006). As is well established, Chinese is a logographic language that differs from alphabetic languages in visual-orthographic and semantic properties (Shu et al., 2003). Firstly, Chinese writing is reputed for its visual complexity (constituted by strokes with different shapes and directions) and orthographic complexity (position and composition rules for different radicals) (Chen and Kao, 2002; Shu et al., 2003). Visual-orthographic skills have been found to be particularly important for reading development and dyslexia in Chinese children (Ho et al., 2004; Li et al., 2012). Secondly, morphology in Chinese differs from many other languages (Packard, 2000). Morphemes are the smallest unit of meaning. In Mandarin Chinese, there are about 7,000 morphemes, but only 1,300 syllables (Chao, 1976). Thus, more than five morphemes or characters share the same syllable, which results in a large number of homophones and homographs (Packard, 2000). A reader must be able to distinguish between the homophones and homographs that share the same syllable, but with different morphemes/meanings. Morphological awareness becomes increasingly important in reading development as children grow older (Shu et al., 2006). As mentioned above, previous studies on the IFOF and ILF in the ventral pathway have emphasized their role in orthographic processing and in semantic processing, but the evidence remains limited and ambiguous. No direct evidence is available on whether the integrity of the left ventral pathway is associated with orthographic and semantic processing skills in children with dyslexia.
In summary, although white matter disruptions in dyslexia have been found in alphabetic writing systems, it remains unclear whether Chinese children with dyslexia would show a similar disruption pattern. In the current study, by using state-of-the-art white matter tractography methods in each child’s native space, we examined for the first time white matter microstructure in a group of Chinese children with dyslexia and their age-matched controls. The first aim of this study was to systematically examine the differences between Chinese children with and without dyslexia in the key white matter pathways, specifically in the dorsal (AF) and ventral (IFOF and ILF) pathways. Furthermore, we aimed to investigate the associations between the disrupted tracts and the reading-related cognitive skills, including phonological, orthographic and morphological processing skills. Based on previous studies in alphabetic languages (e.g. Vandermosten et al., 2012a) and the complex properties of Chinese language (e.g. Shu et al., 2003), we expected that Chinese children with dyslexia might show disruptions in both the left AF in the dorsal pathway (similar with alphabetic languages), and the left IFOF/ILF in the ventral pathway (more specific to Chinese). Furthermore, we hoped to clarify the respective functional roles of the dorsal and ventral pathways: FA values along the dorsal pathway might be particularly correlated with phonological processing skills (similar with alphabetic languages), while FA values along the ventral pathway might be more correlated with orthographic and morphological processing skills (more specific to Chinese).
2. Method
2.1. Participants
40 Chinese children participated in this study. There were 18 children with dyslexia (11 boys and 7 girls) and 22 control children (11 boys and 11 girls) with a mean age of 11.1 years old. Table 1 shows that the two groups were well matched in sex (χ2(1) = 0.482, p = 0.537), age, and nonverbal IQ (ps>0.05). All the children were recruited from primary schools in Beijing. Their nonverbal IQ was in the normal range (C-WISC overall performance IQ score ≥ 80; Gong and Cai, 1993). All participants were native Mandarin speakers and had normal or corrected-to-normal visual acuity. Informed written consent was obtained from both the parents and their children. Ethical approval for the present study was obtained from the Institutional Review Board of Beijing Normal University Imaging Center for Brain Research.
Table 1.
Descriptive statistics for control children and children with dyslexia.
| Control(n = 22) | Dyslexia(n = 18) | t | |||
|---|---|---|---|---|---|
| (11F, 11M) |
(7F, 11M) |
||||
| Mean | S.D. | Mean | S.D. | ||
| Age | 133.66 | 10.89 | 133.29 | 11.42 | 0.107 |
| Grade | 4.95 | 0.95 | 4.83 | 0.92 | 0.406 |
| Performance-IQ | 104.95 | 10.01 | 100.06 | 7.40 | 1.712 |
| Verbal-IQ | 105.62 | 9.48 | 97.72 | 9.23 | 2.625* |
| Character recognition | 118.91 | 18.91 | 92.50 | 12.40 | 5.090*** |
| Word list reading | 92.48 | 20.08 | 65.81 | 12.68 | 4.888*** |
| Phoneme deletion | 19.64 | 5.29 | 14.00 | 6.64 | 2.990** |
| Rapid automatized naming | 16.80 | 3.90 | 20.59 | 5.26 | −2.615* |
| Digit recall | 17.76 | 2.53 | 16.22 | 1.99 | 2.088* |
| Lexical decision | 0.90 | 0.06 | 0.84 | 0.07 | 2.805** |
| Morphological production | 23.77 | 4.13 | 18.67 | 3.22 | 4.286*** |
Note: *p < 0.05, **p < 0.01, ***p < 0.001. Rapid automatized naming was calculated in time.
The inclusion criteria for children with dyslexia was to be more than 1.5 SDs below grade level on a standardized test of Chinese character reading, or more than 1.5 SDs below grade level on a standardized test of word list reading and more than 1 SDs below grade level on Chinese character reading. This criterion was previously established in studies in mainland China (Shu et al., 2006; Zhang et al., 2012). The inclusion criterion for control children was to be above -0.5 SD of the norm on both Chinese character reading and word list reading tasks.
2.2. Behavioral measures
2.2.1. General reading ability
2.2.1.1. Character recognition
This task was based on one hundred and fifty Chinese single characters. Characters were arranged in decreasing frequency and increasing difficulty (Li et al., 2012). The experimenter required the children to name the characters as accurately as possible with no time limit. The measure was terminated when the participants failed on 15 successive characters. This measure of character recognition ability is widely used to represent Chinese children’s reading accuracy (Li et al., 2012; McBride-Chang and Kail, 2002; Pan et al., 2011).
2.2.1.2. Word list reading
The word list reading task consisted of one hundred and eighty 2-character words. All of the words were printed on a sheet of A4 paper in a 9 (column) x 20 (row) matrix (Zhang et al., 2012). The children’s task was to name the words as quickly and accurately as possible. The final score of the task was the number of words read correctly per minute. The word list reading task is viewed as a valid task of Chinese children’s reading fluency (Zhang et al., 2012).
2.2.2. Phonological processing skill
Following a long line of research since Wagner and Torgesen (1987), we chose to measure phonological processing through its three classic subcomponents: phonological awareness, verbal short-term memory and rapid automatized naming, using tasks similar to those used in alphabetic languages.
2.2.2.1. Phoneme deletion
In the phoneme deletion test, participants were required to delete a target phoneme from a monosyllabic character (e.g. ‘Say /gua1/ without the phoneme /g/’). The target phoneme was the first, middle, or final phoneme of the character. There were twenty-six items in total and the final score was the number of correct items (Xue et al., 2012).
2.2.2.2. Rapid automatized naming
In the rapid automatized naming task (RAN), the experimenter presented the participants with an A4 paper consisting of 5 (column) x 5 (row) digits. The child was asked to name the digits twice as quickly and accurately as possible (Shu et al., 2006). The final score was the mean time of the two tests. This task has been widely used in previous Chinese studies (Lei et al., 2011; Pan et al., 2011).
2.2.2.3. Digit recall
In this digit recall task, the experimenter orally presented a series of digit sequences to the children. Participants were required to repeat the digits after the presentation of the experimenter. The test was terminated if the child failed to repeat five consecutive digit sequences. The final score was the correct number of the sequences. This task is used to measure children’s verbal short-term memory (Xia et al., 2016).
2.2.3. Orthographic processing skill
2.2.3.1. Lexical decision
In this task, the experimenter presented the children with two hundred characters (forty real characters, forty non-characters with ill-formed radicals, forty non-characters with real radicals in illegal positions, forty stroke combinations and forty filler items). The children’s task was to judge whether the stimulus presented to them was a real character or not (Su et al., 2015). The accuracy was recorded as the final score of this task. This task has been used to measure children’s orthographic processing skill in previous studies (Su et al., 2015; Zhou et al., 2015).
2.2.4. Morphological processing skill
2.2.4.1. Morphological production
In this task, children were orally presented with a target morpheme in a 2-morpheme word (e.g. the target morpheme /cao3/ meaning grass from /cao3 di4/ meaning grass land) (Shu et al., 2006). Children’s task was to produce 2 new words including the target morpheme. It was required for the target morpheme to have, in one word, the same meaning as in the initial word (e.g. /ye3 cao3/ meaning wild grass, /cao3/ meaning grass), and in the other word, a different meaning from that in the initial word (e.g. /liao2 cao3/ meaning hasty and careless, /cao3/ meaning careless). This morphological production task has been successfully used in previous studies (Shu et al., 2006; Song et al., 2015).
2.3. Diffusion weighted imaging, acquisition and analysis
A 3 T MRI scanner (Siemens Trio, Germany) with a 12-channel head coil was used to acquire the diffusion-weighted images (DWI) of all the participants at Beijing Normal University. A single-shot spin-echo echo-planar imaging sequence was applied to collect the DWI data (coverage of the whole brain; TR = 8000 ms; TE = 89 ms; acquisition matrix = 128 × 128; field of view = 282 × 282 mm2; slice thickness = 2.2 mm with no gap). The diffusion sensitizing gradients were applied along 30 non-collinear directions with a b-value of 1000 s/mm2 and one image with a b-value of 0 s/mm2. The resolution was 2.2 × 2.2 × 2.2 mm3. In order to improve signal-to-noise ratio, we repeated the DWI sequence twice.
The processing of the raw diffusion-weighted data was performed using the software ExploreDTI (http://www.exploredti.com), including registration of the raw DWI images and correction for participant motion and geometrical distortions (Leemans and Jones, 2009). Then the Levenberg–Marquardt nonlinear regression was applied to fit the tensor model to the data (Marquardt, 1963). Based on the eigenvalues of the diffusion tensor, fractional anisotropy (FA), a scalar value representing the degree of diffusion anisotropy, was computed (Basser and Pierpaoli, 1996). After that, an interpolated streamline algorithm was used to perform whole-brain tractography (step length = 0.5 mm, maximum angle threshold = 35°). Voxels with an FA below the threshold value of 0.2 were excluded from the tractography. This initial analysis yields whole-brain tractography independently from regions-of-interest. Finally, the diffusion tensor maps and tractography data were imported into the TrackVis software tool (http://www.trackvis.org; Wedeen et al., 2008).
Tract dissections were performed in each child’s native space using a region-of-interest (ROI) approach. Fig. 1 displays our tracts of interest. The protocol for defining the ROIs for each fiber tract has been described in detail in previous tractography studies (Catani et al., 2007, 2005; Catani and Thiebaut de Schotten, 2008): the three segments of AF (AF-anterior, AF-posterior, AF-direct) were dissected following Catani et al. (2005), the IFOF following Catani and Thiebaut de Schotten (2008), and the ILF following Catani and Thiebaut de Schotten (2008). Like in previous studies (Rojkova et al., 2016; Zhao et al., 2016), we automated some steps of tract dissection in order to minimize the subjective variability associated with manual dissection. We defined ROIs on the MNI152 template (provided by FSL, http://www.fmrib.ox.ac.uk/fsl/). The FA map of each child was registered to the MNI152 template using Advanced Normalization Tools combining affine with diffeomorphic deformations (ANTs, http://www.picsl.upenn.edu/ANTS/; Avants et al., 2008; Klein et al., 2009). Then the inverse deformation was used to bring the ROIs defined on the MNI152 template to the native space of each participant. Each dissected tract was visually inspected to determine whether it had been successfully reconstructed. In that case, it was then further corrected in the native space of each child. All visual inspections and corrections were carried out by two anatomists (MS and AC) under the supervision of expert anatomist MTS.
Fig. 1.
Illustration of the tracts of interest.
Note: This figure was produced from a representative participant (sagittal view in the left hemisphere). Different tracts are displayed in different colors: green = arcuate fasciculus-anterior (AF-anterior), yellow = arcuate fasciculus-posterior (AF-posterior), red = arcuate fasciculus-direct (AF-direct), blue = inferior frontal occipital fasciculus (IFOF), orange = inferior longitudinal fasciculus (ILF).
Finally, mean fractional anisotropy (FA), perpendicular (radial diffusivity, RD), and parallel diffusivities (axial diffusivity, AD), which are indirect measures of white matter microstructural properties, were extracted along each tract.
2.4. Statistical analysis
Statistical analysis was performed in SPSS Statistics v.20 (IBM Corporation, Somers, New York). Firstly, demographic and behavioral differences between groups were tested through independent-samples t-tests. Secondly, in order to test group differences in the FA of each tract between children with and without dyslexia, ANCOVAs were performed with FA as dependent variable, diagnosis and sex as between-subject factors, and age, whole brain mean FA and head motion parameter as covariates. The head motion parameter was calculated by averaging the absolute values of z-scores of the six head motion parameters (3 translation and 3 rotation directions) (Zhao et al., 2016). The statistically significant results were then interpreted by analyzing the other two diffusivity measures (AD, RD) with the same ANCOVAs. Thirdly, partial correlation analyses were performed between the FA of the tracts showing group differences and each child’s reading performance, controlling for age, sex, whole brain mean FA and head motion parameter. Finally, hierarchical linear regression models were used with FA of each tract as dependent variable, and cognitive skills as independent variables, controlling for age, sex, whole brain mean FA and head motion parameter in the first step, and diagnosis in the second step. As there were three measures of phonological skill (phoneme deletion, RAN and digit recall), we made a composite variable for phonological skill by averaging the z-scores of the three tasks. Results were corrected for multiple comparisons of tracts using the False Discovery Rate (FDR) correction (Benjamini and Hochberg, 1995). In the Results section, we report uncorrected p-values and then compare them to the FDR-corrected alpha threshold q value.
3. Results
3.1. Demographic and behavioral results
Demographic and behavioral characteristics are reported in Table 1. K-S test for normality showed that all the variables were normally distributed (ps>0.05). No differences were found in sex, age, grade and nonverbal IQ between children with and without dyslexia (ps > 0.05). Regarding behavioral measures, children with dyslexia performed significantly worse than their age-matched peers on all of the reading and reading-related cognitive skills (ps < 0.05). Finally, no significant difference was found in the head motion parameter between children with and without dyslexia (p>0.05)
3.2. Group differences
Tractography success rates were higher than 95% on each segment of the arcuate fasciculus in both hemispheres, except for the right AF-direct, which could not be reconstructed in 5 participants, consistently with previous studies (Catani et al., 2007; Yeatman, et al., 2011). The tractography success rate of the ventral tracts reached 100% in both hemispheres. Fig. 1 shows an example of fiber tracking in one participant.
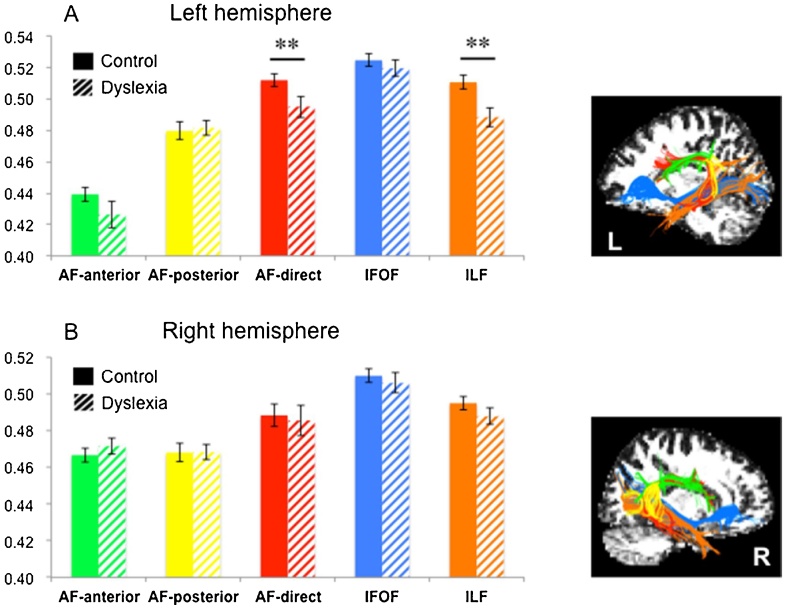
In the ANCOVAs with diagnosis and sex as between-subject factors and age, whole brain mean FA and head motion parameter as covariates, significant diagnosis effects were found in two left-hemisphere tracts (Table 2). One was the left arcuate fasciculus direct segment (AF-direct) (F(133) = 10.190, p = 0.003, ηp2 = 0.236), the other one was the left inferior longitudinal fasciculus (ILF) (F(133) = 10.760, p = 0.002, ηp2 = 0.246). Significant lower fractional anisotropy was found in children with dyslexia relative to control children in these two tracts (Fig. 2). Those results survived the FDR correction (ps < q = 0.010). In addition, neither sex effects nor sex by diagnosis interactions were observed (Table 2).
Table 2.
FA comparisons between control children and children with dyslexia on bilateral ventral and dorsal tracts.
| Tractsa | Control | Dyslexia | Diagnosis effect | Sex effect | Interaction effect |
|---|---|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | F (p value)b | F (p value) | F (p value) | |
| Left hemisphere | |||||
| AF-anterior | 0.439(0.021) | 0.426(0.036) | 2.583(0.118) | 0.475(0.496) | 2.243(0.144) |
| AF-posterior | 0.480(0.025) | 0.482(0.019) | 0.251(0.620) | 0.174(0.680) | 0.013(0.909) |
| AF-direct | 0.512(0.020) | 0.495(0.028) | 10.190**(0.003) | 0.280(0.600) | 0.234(0.632) |
| IFOF | 0.525(0.018) | 0.519(0.021) | 1.673(0.205) | 0.024(0.879) | 0.709(0.406) |
| ILF | 0.510(0.020) | 0.488(0.026) | 10.760**(0.002) | 0.013(0.909) | 2.468(0.126) |
| Right hemisphere | |||||
| AF-anterior | 0.467(0.018) | 0.472(0.019) | 0.948(0.337) | 0.225(0.638) | 1.765(0.193) |
| AF-posterior | 0.468(0.023) | 0.468(0.018) | 0.085(0.772) | 0.208(0.652) | 3.519(0.070) |
| AF-direct | 0.488(0.027) | 0.485(0.032) | 0.004(0.949) | 3.944(0.057) | 0.941(0.340) |
| IFOF | 0.510(0.017) | 0.506(0.023) | 1.369(0.250) | 0.713(0.404) | 0.029(0.865) |
| ILF | 0.495(0.017) | 0.488(0.020) | 1.916(0.176) | 0.730(0.399) | 0.241(0.627) |
Note: Age, whole brain mean FA and head motion parameter as covariates.
AF = arcuate fasciculus, IFOF = Inferior fronto-occipital fasciculus, ILF = Inferior longitudinal fasciculus. b. p values were uncorrected, *p < 0.05, **p < 0.01, p values surviving FDR correction (p < 0.010) were in bold.
Fig. 2.
FA values of the white matter tracts between children with and without dyslexia.
Group comparisons of mean fractional anisotropy (FA) of each tract in the left (A) and right (B) hemisphere. Error bars represent ± 1 standard error of the mean, *p < 0.05, **p < 0.01.
To help interpret the diagnosis effects on the left AF-direct and the left ILF, we calculated the other two diffusivity measures, axial diffusivity (AD) and radial diffusivity (RD), and compared them between children with and without dyslexia (Tables S1 and S2).Significant lower AD was found in children with dyslexia relative to typical readers in the left AF-direct (F(133) = 5.845, p = 0.021, ηp2 = 0.150) and the left ILF (F(133) = 7.733, p = 0.009, ηp2 = 0.190). Interestingly, a significant diagnosis by sex interaction was found in the AD of the left ILF (F(133) = 6.129, p = 0.019, ηp2 = 0.157). Further analysis showed that there was no difference between children with and without dyslexia in boys (F(117) = 0.192, p = 0.666), while the difference was significant in girls (F(113) = 9.254, p = 0.009), with girls with dyslexia having lower AD than normal-reading ones. This interaction pattern is shown in Fig. S1. Significantly higher RD was found in children with dyslexia relative to typical readers in the left AF-direct (F(133) = 6.293, p = 0.017, ηp2 = 0.160) and the left ILF (F(133) = 10.523, p = 0.003, ηp2 = 0.242).
3.3. Correlations between neuroanatomical and behavioral measures
In order to clarify the relationship between the disrupted tracts and reading-related behavioral measures, partial correlation analyses were run between the FA of the two target tracts and behavioral measures, controlling for age, sex, whole brain mean FA and head motion parameters. Table 3 shows that better character recognition was associated with an increased FA in both the left AF-direct and left ILF. At the underlying cognitive level, the FA of the left AF-direct was positively correlated with phoneme deletion, RAN and digit recall (ps<0.05). Regarding the left ILF, significant correlations were found with morphological production (r = 0.513, p = 0.001) and digit recall (r = 0.420, p = 0.012). After FDR correction for multiple tests, all correlations remained significant (ps < q = 0.029). No other significant correlation was found (ps>0.05). We then computed correlations within groups: similar trends of a stronger relationship between AF-direct and phonological skills, and between ILF and morphological skills were found in children with dyslexia, albeit non-significant in this reduced dataset (Table S3). No correlation approached significance within the control group.
Table 3.
Partial correlations between the FA of left AF-direct, left ILF and behavioral measures controlling for age, sex, whole brain mean FA and head motion parameter.
| CR | PD | RAN | Digit | LD | MP | ||
|---|---|---|---|---|---|---|---|
| AF-direct | r | 0.407* | 0.392* | −0.435** | 0.462** | 0.237 | 0.194 |
| p | 0.014 | 0.018 | 0.008 | 0.005 | 0.178 | 0.256 | |
| ILF | r | 0.465** | 0.097 | −0.127 | 0.420* | −0.084 | 0.513** |
| p | 0.004 | 0.575 | 0.460 | 0.012 | 0.637 | 0.001 |
Note: AF-direct=arcuate fasciculus-direct; ILF = inferior longitudinal fasciculus; CR = character recognition; PD = phoneme deletion; RAN = rapid automatized naming; Digit = digit recall; LD = lexical decision; MP = morphological production. p-values are uncorrected, *p < 0.05, **p < 0.01, p values surviving FDR correction (p < 0.029) are in bold.
In order to clarify the role of the left AF-direct and the left ILF in phonological vs. morphological processing, we carried out hierarchical linear regression analyses (Table 4) of the FA of the two tracts with age, sex, whole brain mean FA and head motion parameter included in the first step. To exclude the control the contribution of group differences on the dependent variables, we entered diagnosis in the second step. Finally, phonological processing skill (composite standard score of phoneme deletion, RAN and digit recall) and morphological skill (standard score of morphological production) were entered in the third step. A factor analysis yielding a single component and explaining 64.73% of the variance confirmed that a composite phonological score was legitimate.
Table 4.
Hierarchical regression models using control variables and cognitive skills to predict the FA of the left arcuate fasciculus-direct and the left inferior longitudinal fasciculus.
| Left AF-direct |
Left ILF |
||||
|---|---|---|---|---|---|
| Step | ΔR2 | Beta | ΔR2 | Beta | |
| 1 | Control variables | 0.154 | 0.133 | ||
| Age | −0.084 | −0.210 | |||
| Sex | 0.013 | −0.026 | |||
| Whole brain mean FA | 0.125 | 0.356* | |||
| Head motion parameter | 0.463** | −0.036 | |||
| 2 | Group variable | 0.180** | 0.189** | ||
| Diagnosis | −0.408* | −0.267 | |||
| 3 | Cognitive variables | 0.150* | 0.083 | ||
| Phonological processing | 0.563** | −0.130 | |||
| Morphological processing | −0.360 | 0.421* | |||
Note: *p < 0.05, **p < 0.01. ΔR2 is the R2 change at each hierarchical step; Beta is the standardized regression coefficient.
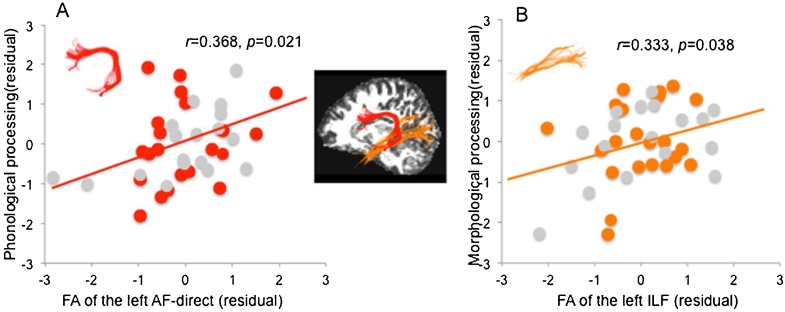
It was found that when control and group variables were statistically controlled, phonological skill remained a significant predictor of the FA of left AF-direct (β = 0.563, p = 0.006), while morphological skill remained significantly associated with the FA of left ILF (β = 0.421, p = 0.048). Fig. 3 illustrates this dissociation. The scatterplots show that phonological scores are positively correlated with the FA of the left AF-direct (r = 0.368, p = 0.021), while morphological scores are positively correlated with the FA of the left ILF (r = 0.333, p = 0.038).
Fig. 3.
Partial correlations between the FA of the left dorsal and ventral tracts and phonological and morphological processing skills, controlling for age, sex, whole brain mean FA, head motion parameter and group. (A) Correlation between the FA of AF-direct and phonological processing skill; (B) Correlation between the FA of ILF and morphological processing skill. Red or orange dots represent control participants, grey dots represent children with dyslexia (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Note: The brain figure in the middle was produced from a representative participant (sagittal view in the left hemisphere). Different tracts are displayed with different colors: red = arcuate fasciculus-direct (AF-direct), orange = inferior longitudinal fasciculus (ILF).
4. Discussion
By applying state-of-the-art white matter tractography analysis in each individual’s native space, the present study investigated the integrity of 5 reading-related tracts in the dorsal and ventral pathways of each hemisphere in a sample of Chinese children with and without dyslexia. Children with dyslexia showed significantly reduced fractional anisotropy in the left dorsal pathway, namely the arcuate fasciculus-direct (AF-direct), and in the left ventral pathway, namely the inferior longitudinal fasciculus (ILF). Further correlation analyses revealed a functional dissociation between left AF-direct and ILF: the AF-direct was associated with phonological processing skills (phoneme deletion, rapid automatized naming, digit recall), while the ILF was associated with morphological processing skill. Finally, a diagnosis by sex interaction was observed in the axial diffusivity of the left inferior longitudinal fasciculus, due to differences in girls only.
4.1. Fiber tract disruptions in Chinese children with dyslexia
The finding of the left AF and left ILF may support a double-pathway deficit hypothesis in Chinese dyslexia. On the one hand, the differences observed in the left AF are consistent with a series of previous DTI studies using either VBA analysis (Carter et al., 2009; Deutsch et al., 2005; Klingberg et al., 2000; Rimrodt et al., 2010; Steinbrink et al., 2008) or tractography analysis (Vandermosten et al., 2012a). The finding of a disrupted left arcuate fasciculus in Chinese children with dyslexia suggests that this dorsal pathway plays a similar role in reading acquisition and in dyslexia in Chinese as in alphabetic languages.
On the other hand, the finding of a reduced fractional anisotropy in the left ILF for children with dyslexia is more novel. Some studies have suggested a role for the left ILF in reading alphabetic languages (Epelbaum et al., 2008; Lebel et al., 2013; Nikki et al., 2017; Rollins et al., 2009; Yeatman et al., 2012; Zemmoura et al., 2015). These studies were either in brain-lesioned patients (Epelbaum et al., 2008; Zemmoura et al., 2015) or in participants with a wide range of reading skills (Lebel et al., 2013; Yeatman et al., 2012). Only two studies were specifically in children with dyslexia: Rollins et al.'s (2009) study was in children with dyslexia (n = 18) with a wide age range (6–16 years) and they found that FA values of the ILF in the dyslexia group were higher than those in the control group, up to around 11 years of age. In Nikki et al. (2017) study, they reported a positive correlation between ILF FA and reading comprehension in poor readers, while word reading was unrelated to white matter integrity in either group. Thus, the observation of a reduced fractional anisotropy in the left ILF in children with dyslexia in the present study does not seem to be the same result as in previous studies in alphabetical languages.
To our knowledge, there have only been two previous studies exploring developmental aspects of white matter microstructure in China, in a population of normally developing children (Qiu et al., 2008, 2011). Thus it is of great value to further explore to what extent white matter pathways may differ between Chinese children with and without dyslexia. In the present study, we found white matter disruptions in the left dorsal pathway (AF-direct) and in the left ventral pathway (ILF), with reduced FA in children with dyslexia. Similar group differences observed in axial diffusivity (AD) and radial diffusivity (RD) suggested that AD was reduced and RD was increased. Unfortunately, this does not allow us to unambiguously attribute the reduced FA in children with dyslexia to a single specific factor, whether reduced myelination, axonal density or diameter, etc., but rather suggests a multifaceted disruption of white matter.
4.2. Functional dissociation between AF and ILF
Consistent with our hypothesis, we found a functional dissociation between the left dorsal (AF-direct) and ventral (ILF) pathways. FA values along the left AF-direct were correlated with phonological processing skills, whose role is thought to hold in all languages. In contrast, FA values along the left ILF were correlated with the more Chinese-specific morphological processing skill. The dissociation remained even after inclusion of diagnosis and other control variables in the regression (Table 4, Fig. 3). This suggests that this functional dissociation is not simply a product of group differences.
The location of the AF-direct overlapped with the dorsal phonological route proposed in previous fMRI studies (Schlaggar and McCandliss, 2007). This is also consistent with a number of previous DTI studies showing associations between the arcuate fasciculus and phonological processing skills (Rimrodt et al., 2010; Steinbrink et al., 2008; Vandermosten et al., 2012a; Yeatman et al., 2011). This suggests that Chinese phonological processing has at least partly the same brain basis (the left arcuate fasciculus) as in alphabetic languages.
In contrast, the FA of the left ILF was positively correlated with morphological processing skill after controlling for age, sex and whole-brain mean FA. A study on pure alexic patients suggested that the left ILF might serve as a route to transfer primary visual input to the Visual Word Form Area (VWFA), and that it may be correlated with orthographical processing skills (Epelbaum et al., 2008). The correlation between the left ILF and morphological processing skill can be understood considering the nature of the task and of Chinese reading. In the morphological production task, children needed to recognize the target morpheme (e.g. 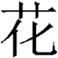 hua1/ meaning “flower”) orally presented in a compound word (e.g.
hua1/ meaning “flower”) orally presented in a compound word (e.g.  hua1 cao3/ meaning “flowers and plants”, with /hua1/ meaning “flower”), then produce 2 new words with the same target morpheme (e.g.
hua1 cao3/ meaning “flowers and plants”, with /hua1/ meaning “flower”), then produce 2 new words with the same target morpheme (e.g.  /hua1 huan2/ meaning “floral hoop”) and with a different morpheme (e.g.
/hua1 huan2/ meaning “floral hoop”) and with a different morpheme (e.g.  /hua1 fei4/ meaning “spending money”, with /hua1/ meaning “spend”). Like phonological awareness tasks, the Chinese morphological production task can be performed purely orally, but performance can be greatly enhanced if one can access the orthography of the corresponding characters, thus accessing the visual word form area through the ILF. The task then requires doing further semantic manipulations, possibly involving more anterior parts of the temporal lobe, which have been found to be associated with language comprehension (Ge et al., 2015), further along the ILF.
/hua1 fei4/ meaning “spending money”, with /hua1/ meaning “spend”). Like phonological awareness tasks, the Chinese morphological production task can be performed purely orally, but performance can be greatly enhanced if one can access the orthography of the corresponding characters, thus accessing the visual word form area through the ILF. The task then requires doing further semantic manipulations, possibly involving more anterior parts of the temporal lobe, which have been found to be associated with language comprehension (Ge et al., 2015), further along the ILF.
4.3. Interaction between sex and dyslexia
The few studies on dyslexia that have paid attention to the sex factor have often found that the neural basis of dyslexia might differ between males and females (Altarelli et al., 2013, 2014;Evans et al., 2014; Humphreys et al., 1990; Ramus et al., 2017). Using a subject-by-subject functionally guided approach, Altarelli et al. (2013) found a similar sex by diagnosis interaction on the cortical thickness of the visual word form area, whereby girls with dyslexia differed from control girls, whereas no such difference was observed in boys. Until now, little is known about these sex effects on the reading related tracts. The present study found a similar diagnosis by sex interaction on the axial diffusivity (AD) of the left inferior longitudinal fasciculus. And the location of the left ILF may overlap with the occipitotemporal regions observed in Altarelli et al. (2013) study. Thus the possibility that the neural basis of dyslexia may partly differ between boys and girls seems supported to some extent by our results. Nevertheless, the fact that we observed this interaction on axial diffusivity, but not on fractional anisotropy makes the interpretation of this result difficult, and a replication would be warranted before drawing conclusions.
4.4. Limitations
The work presented here of course had some limitations. In particular, the sample size of the present study remains limited (22 controls and 18 children with dyslexia respectively), especially when dividing the sample into boys and girls. The small sample size may therefore impede the generalization of the results and reduce the power of statistical tests. Another limitation was the use of the DTI model, which was imposed by the sequence parameters. The DTI model has particular problems resolving fiber orientation in fiber-crossing regions, which particularly concerns the temporoparietal regions including the arcuate fasciculus. More advanced fiber reconstruction techniques (such as spherical deconvolution), requiring more stringent sequence parameters (b > 1400 s/mm2), have shown that the DTI model may engender confusions between the AF and the superior longitudinal fasciculus (Zhao et al., 2016) or the corpus callosum (Vanderauwera et al., 2015). Therefore, it will be important to replicate our observations using such advanced tractography methods to unambiguously confirm the involvement of the arcuate fasciculus.
4.5. Universality and specificity of dyslexia in Chinese
In conclusion, the present study on Chinese children revealed both what seems to be emerging as a universal characteristic of dyslexia, namely a disrupted integrity of the left direct segment of the arcuate fasciculus, and what may be a result more specific to Chinese, namely a disrupted integrity of the left inferior longitudinal fasciculus. As discussed by Ziegler (2006), such results do not necessarily challenge a unitary theory of dyslexia, they may rather reflect that there are both language-universal causes of dyslexia: the phonological deficit reflected in the disruption of the left AF (Vandermosten et al., 2012a); and more language-dependent causes of dyslexia, depending on the specific cognitive demands of each reading system. This may be the case for morphological deficits, reflected by the disruption in the left ILF, which may play a greater role in reading disability in a morphographic language such as Chinese than in alphabetic languages.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
We thank all the children and their families for their collaboration in this study. We also thank Allyson Covello for her help in the visual correction of the tract dissection. This study was supported by the National Key Basic Research Program of China (2014CB846103), the Key Project of Philosophical and Social Science Foundation, ministry of education (11JZD041), the National Natural Science Foundation of China (31271082, 31500886, 31671126), the Fundamental Research Funds for the Central Universities (2017XTCX04), the Interdiscipline Research Funds of Beijing Normal University, the Agence Nationale de la Recherche (ANR–11–BSV4–014–01, ANR–10–LABX–0087 IEC and ANR–10–IDEX–0001–02 PSL*), the China Scholarship Council, the China Postdoctoral Science Foundation Funded Project (2016M591098), and the NSFC–CNRS Joint Research Project Grant (31611130107).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.04.002.
Contributor Information
Franck Ramus, Email: franck.ramus@ens.fr.
Hua Shu, Email: shuh@bnu.edu.cn.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Altarelli I., Leroy F., Monzalvo K., Fluss J., Billard C., Dehaene-Lambertz G., Ramus F. Planum temporale asymmetry in developmental dyslexia: revisiting an old question. Hum. Brain. Mapp. 2014;35:5717–5735. doi: 10.1002/hbm.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarelli I., Monzalvo K., Iannuzzi S., Fluss J., Billard C., Ramus F., Dehaene-Lambertz G. A functionally guided approach to the morphometry of occipitotemporal regions in developmental dyslexia: evidence for differential effects in boys and girls. J. Neurosci. 2013;33(27):11296–11301. doi: 10.1523/JNEUROSCI.5854-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross- correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural features measured using diffusion tensor imaging. J. Magn. Reson. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Plewes C., Paulson L.A., Roy D., Snook L., Concha L., Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25(4):1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false Discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995;57(57):289–300. [Google Scholar]
- Ben-Shachar M., Dougherty R.F., Wandell B.A. White matter pathways in reading. Curr. Opin. Neurobiol. 2007;17(2):258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Carter J.C., Lanham D.C., Cutting L.E., Clements-Stephens A.M., Chen X., Hadzipasic M., Kaufmann W.E. A dual DTI approach to analyzing white matter in children with dyslexia. Psychiatry Res.: Neuroimaging. 2009;172(3):215–219. doi: 10.1016/j.pscychresns.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Allin M.P., Husain M., Pugliese L., Mesulam M.M., Murray R.M., Jones D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. 2007;104(43):17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chao Y.R. vol. 9. Stanford University Press; Palo Alto, CA: 1976. (Aspects of Chinese Sociolinguistics: Essays). [Google Scholar]
- Chen X., Kao H.S.R. Visual-spatial properties and orthographic processing of Chinese characters. Cognit. Neurosci. Stud. Chin. Lang. 2002:175–194. [Google Scholar]
- Coltheart M., Rastle K., Perry C., Langdon R., Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychol. Rev. 2001;108(1):204. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Duffau H., Gatignol P., Mandonnet E., Peruzzi P., Tzourio-Mazoyer N., Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128(Pt 4):797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Deutsch G.K., Dougherty R.F., Bammer R., Siok W.T., Gabrieli J.D.E., Wandell B. Children's reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41(3):354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- Epelbaum S., Pinel P., Gaillard R., Delmaire C., Perrin M., Dupont S., Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44(8):962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Evans T.M., Flowers D.L., Napoliello E.M., Eden G.F. Sex-specific gray matter volume differences in females with developmental dyslexia. Brain Struct. Funct. 2014;219(3):1041–1054. doi: 10.1007/s00429-013-0552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez J.A., Petersen S.E. Neuroimaging studies of word reading. Proc. Natl. Acad. Sci. 1998;95(3):914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J., Peng G., Lyu B., Wang Y., Zhuo Y., Niu Z. Cross-language differences in the brain network subserving intelligible speech. Proc. Natl. Acad. Sci. 2015;112(10):2972. doi: 10.1073/pnas.1416000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B.T., Powell D.K., Xuan L., Jiang Y., Hardy P.A. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Cai T. Map Press Hunan; China: 1993. Wechsler Intelligence Scale for Children, Chinese Revision (C-WISC) [Google Scholar]
- Han Z., Ma Y., Gong G., He Y., Caramazza A., Bi Y. White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain. 2013;136(Pt 10):2952–2965. doi: 10.1093/brain/awt205. [DOI] [PubMed] [Google Scholar]
- Ho C.S.-H., Chan D.W.-O., Lee S.-H., Tsang S.-M., Luan V.H. Cognitive profiling and preliminary subtyping in Chinese developmental dyslexia. Cognition. 2004;91(1):43–75. doi: 10.1016/s0010-0277(03)00163-x. [DOI] [PubMed] [Google Scholar]
- Humphreys P., Kaufmann W.E., Galaburda A.M. Developmental dyslexia in women: neuropathological findings in three patients. Ann. Neurol. 1990;28(6):727–738. doi: 10.1002/ana.410280602. [DOI] [PubMed] [Google Scholar]
- Jobard G., Crivello F., Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20(2):693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Chitnis X.A., Job D., Khong P.L., Leung L.T., Marenco S., Symms M.R. What happens when nine different groups analyze the same DT-MRI data set using voxel-based methods. Paper Presented at the Proceedings of the 15th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Berlin; 2007. [Google Scholar]
- Keller T.A., Just M.A. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Andersson J., Ardekani B.A., Ashburner J., Avants B., Chiang M.C. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T., Hedehus M., Temple E., Salz T., Gabrieli J.D., Moseley M.E., Poldrack R.A. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25(2):493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Lebel C., Shaywitz B., Holahan J., Shaywitz S., Marchione K., Beaulieu C. Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain & Lang. 2013;125(2):215–222. doi: 10.1016/j.bandl.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The b-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61(6):1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Lei L., Pan J., Liu H., McBride-Chang C., Li H., Zhang Y., Zhang Z. Developmental trajectories of reading development and impairment from ages 3 to 8 years in Chinese children. J. Child Psychol. Psychiatry. 2011;52(2):212–220. doi: 10.1111/j.1469-7610.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- Li H., Shu H., McBride-Chang C., Liu H., Peng H. Chinese children's character recognition: visuo-orthographic, phonological processing and morphological skills. J. Res. Read. 2012;35(3):287–307. [Google Scholar]
- Marquardt D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. For. Ind. Appl. Math. 1963;11(2):431–441. [Google Scholar]
- Martin A., Kronbichler M., Richlan F. Dyslexic brain activation abnormalities in deep and shallow orthographies: a meta-analysis of 28 functional neuroimaging studies. Hum. Brain. Mapp. 2016;37:2676–2699. doi: 10.1002/hbm.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride-Chang C., Kail R.V. Cross-cultural similarities in the predictors of reading acquisition. Child Dev. 2002;73(5):1392–1407. doi: 10.1111/1467-8624.00479. [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Noble K.G. The development of reading impairment: a cognitive neuroscience model. Ment. Retard. Dev. Disabil Res. Rev. 2003;9(3):196–205. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- Moreau D., Stonyer J., McKay N., Waldie K. No evidence for systematic White matter correlates of dyslexia: an activation likelihood estimation Meta-analysis. Brain Res. 2018 doi: 10.1016/j.brainres.2018.01.014. (in press) [DOI] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cognit. Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nikki A.C., Kulesz P.A., Juranek J., Cirino P.T., Fletcher J.M. White matter microstructure integrity in relation to reading proficiency. Brain & Lang. 2017;174:103–111. doi: 10.1016/j.bandl.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., McCandliss B.D. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44(11):2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Odegard T.N., Farris E.A., Ring J., McColl R., Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47(8):1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Packard J.L. Cambridge University Press; 2000. The Morphology of Chinese: A Linguistic and Cognitive Approach. [Google Scholar]
- Pan J., McBride-Chang C., Shu H., Liu H., Zhang Y., Li H. What is in the naming? A 5-year longitudinal study of early rapid naming and phonological sensitivity in relation to subsequent reading skills in both native Chinese and English as a second language. J. Educ. Psychol. 2011;103(4):897. [Google Scholar]
- Perfetti C.A., Sandak R. Reading optimally builds on spoken language: implications for deaf readers. J. Deaf Stud. & Deaf Education. 2000;5(1):32. doi: 10.1093/deafed/5.1.32. [DOI] [PubMed] [Google Scholar]
- Perfetti C.A., Tan L.H., Siok W.T. Brain-behavior relations in reading and dyslexia: implications of Chinese results. Brain Lang. 2006;98(3):344–346. doi: 10.1016/j.bandl.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Pugh K.R. A neurocognitive overview of reading acquisition and dyslexia across languages. J. Aging Health. 2006;9(5):370–408. doi: 10.1111/j.1467-7687.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Mencl W.E., Jenner A.R., Katz L., Frost S.J., Lee J.R., Shaywitz B.A. Neurobiological studies of reading and reading disability. J. Commun. Disord. 2001;34(6):479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Qiu D., Tan L.-H., Zhou K., Khong P.-L. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. Neuroimage. 2008;41(2):223–232. doi: 10.1016/j.neuroimage.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Qiu D., Tan L.H., Siok W.T., Zhou K., Khong P.L. Lateralization of the arcuate fasciculus and its differential correlation with reading ability between young learners and experienced readers: a diffusion tensor tractography study in a chinese cohort. Hum. Brain. Mapp. 2011;32(12):2054–2063. doi: 10.1002/hbm.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F. The neural basis of reading acquisition. In: Gazzaniga M.S., editor. The Cognitive Neurosciences III. MIT Press; Cambridge, MA: 2004. p. 815824. [Google Scholar]
- Ramus F., Altarelli I., Jednoróg K., Zhao J., Scotto di Covella L. Brain asymmetries and sex differences in developmental dyslexia. In: Galaburda A.M., Gaab N., Hoeft F., McCardle P., editors. Dyslexia and Neuroscience: The Geschwind-Galaburda Hypothesis 30 Years Later. Brookes; Baltimore, MD: 2017. p. 7886. [Google Scholar]
- Ramus F., Altarelli I., Jednoróg K., Zhao J., Scotto di Covella L. Neuroanatomy of developmental dyslexia: pitfalls and promise. Neurosci. Biobehav. Rev. 2018;84(Supplement C):434–452. doi: 10.1016/j.neubiorev.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Richards T., Stevenson J., Crouch J., Johnson L., Maravilla K., Stock P., Berninger V. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. Am. J. Neuroradiol. 2008;29(6):1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F., Sturm D., Schurz M., Kronbichler M., Ladurner G., Wimmer H. A Common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter Reading? PLoS One. 2010;5(8):e12073. doi: 10.1371/journal.pone.0012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt S.L., Peterson D.J., Denckla M.B., Kaufmann W.E., Cutting L.E. White matter microstructural differences linked to left perisylvian language network in children with dyslexia. Cortex. 2010;46(6):739–749. doi: 10.1016/j.cortex.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkova K., Volle E., Urbanski M., Humbert F., Dell’Acqua F., Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct. Funct. 2016;221(3):1–16. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Rollins N.K., Vachha B., Srinivasan P., Chia J., Pickering J., Hughes C.W. Simple developmental dyslexia in children: alterations in diffusion-tensor metrics of white matter tracts at 3 t. Radiology. 2009;251(3):882. doi: 10.1148/radiol.2513080884. [DOI] [PubMed] [Google Scholar]
- Saygin Z.M., Norton E.S., Osher D.E., Beach S.D., Cyr A.B., Ozernov-Palchik O., Gabrieli J.D. Tracking the roots of reading ability: white matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. J. Neurosci. 2013;33(33):13251–13258. doi: 10.1523/JNEUROSCI.4383-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Shen C., Li X., Dunlop M., Shi Q.Q., Liu Z.X., Lucek E., Chen Z.Q. Magnetic field rotation analysis and the applications. J. Geophysical Res.: Space Phys. 2007;112(A6) 1978–2012. [Google Scholar]
- Shu H., Chen X., Anderson R.C., Wu N., Xuan Y. Properties of school Chinese: implications for learning to read. Child Dev. 2003;74(1):27–47. doi: 10.1111/1467-8624.00519. [DOI] [PubMed] [Google Scholar]
- Shu H., McBride-Chang C., Wu S., Liu H. Understanding chinese developmental dyslexia: morphological awareness as a core cognitive construct. J. Educ. Psychol. 2006;98(1):122–133. [Google Scholar]
- Siok W.T., Perfetti C.A., Jin Z., Tan L.H. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431(7004):71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Song S., Su M., Kang C., Liu H., Zhang Y., McBride-Chang C., Zhang Z. Tracing children's vocabulary development from preschool through the school-age years: an 8-year longitudinal study. Dev. Sci. 2015;18:119–131. doi: 10.1111/desc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink C., Vogt K., Kastrup A., Müller H.-P., Juengling F., Kassubek J., Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 t. Neuropsychologia. 2008;46(13):3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Su M., Wang J., Maurer U., Zhang Y., Li J., McBride C., Shu H. Gene–environment interaction on neural mechanisms of orthographic processing in Chinese children. J. Neurolinguistics. 2015;33:172–186. doi: 10.1016/j.jneuroling.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderauwera J., Vandermosten M., Dell’Acqua F., Wouters J., Ghesquière P. Disentangling the relation between left temporoparietal white matter and reading: a spherical deconvolution tractography study. Hum. Brain. Mapp. 2015;36:3273–3287. doi: 10.1002/hbm.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Poelmans H., Sunaert S., Wouters J., Ghesquière P. A tractography study in dyslexia: neuroanatomic correlates of orthographic, phonological and speech processing. Brain. 2012;135(3):935–948. doi: 10.1093/brain/awr363. [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Vanderauwera J., Theys C., Vos A.D., Vanvooren S., Sunaert S. A DTI tractography study in pre-readers at risk for dyslexia. Developmental Cognitive Neuroscience. 2015;43:8–15. doi: 10.1016/j.dcn.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquière P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36(6):1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vellutino F.R., Fletcher J.M., Snowling M.J., Scanlon D.M. Specific reading disability (dyslexia): what have we learned in the past four decades? J. Child Psychol. Psychiatry. 2004;45(1):2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Wagner R.K., Torgesen J.K. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol. Bull. 1987;101(2):192. [Google Scholar]
- Wedeen V.J., Wang R.P., Schmahmann J.D., Benner T., Tseng W.Y.I., Dai G., de Crespigny A.J. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41(4):1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Welcome S.E., Joanisse M.F. Individual differences in white matter anatomy predict dissociable components of reading skill in adults. Neuroimage. 2014;96:261–275. doi: 10.1016/j.neuroimage.2014.03.069. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 4th edn. World Health Organization; Geneva: 2011. ). International Statistical Classification of Diseases and Related Health Problems -10th Revision. [Google Scholar]
- Xia Z., Hoeft F., Zhang L., Shu H. Neuroanatomical anomalies of dyslexia: disambiguating the effects of disorder, performance, and maturation. Neuropsychologia. 2016;81:68–78. doi: 10.1016/j.neuropsychologia.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Shu H., Li H., Li W., Tian X. The stability of literacy-related cognitive contributions to Chinese character naming and Reading fluency. J. Psycholinguist. Res. 2012:1–18. doi: 10.1007/s10936-012-9228-0. [DOI] [PubMed] [Google Scholar]
- Yang J., Shu H., Mccandliss B.D., Zevin J.D. Orthographic influences on division of labor in learning to read Chinese and English: insights from computational modeling. Bilingualism-lang. Cognit. 2013;16(2):354–366. doi: 10.1017/S1366728912000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Ben-Shachar M., Wandell B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. 2012;109(44):E3045–E3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Rykhlevskaia E., Sherbondy A.J., Deutsch G.K., Wandell B.A., Ben-Shachar M. Anatomical properties of the arcuate fasciculus predict phonological and reading skills in children. J. Cognit. Neurosci. 2011;23(11):3304–3317. doi: 10.1162/jocn_a_00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang L., Shu H., Xi J., Wu H., Zhang Y., Li P. Universality of categorical perception deficit in developmental dyslexia: an investigation of mandarin Chinese tones. J. Child Psychol. Psychiatry. 2012;53(8):874–882. doi: 10.1111/j.1469-7610.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- Zemmoura I., Herbet G., Moritzgasser S., Duffau H. New insights into the neural network mediating reading processes provided by cortico-subcortical electrical mapping. Hum. Brain. Mapp. 2015;36(6):2215–2230. doi: 10.1002/hbm.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Thiebaut de Schotten M., Altarelli I., Dubois J., Ramus F. Altered hemispheric lateralization of white matter pathways in developmental dyslexia: evidence from spherical deconvolution tractography. Cortex. 2016;76:51–62. doi: 10.1016/j.cortex.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Zhao J., Wang X., Frost S.J., Sun W., Fang S.Y., Mencl W.E. Neural division of labor in reading is constrained by culture: a training study of reading Chinese characters. Cortex. 2014;53(1):90. doi: 10.1016/j.cortex.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Xia Z., Bi Y., Shu H. Altered connectivity of the dorsal and ventral visual regions in dyslexic children: a resting-state fMRI study. Frontiers Hum. Neuroscience. 2015;9:495. doi: 10.3389/fnhum.2015.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J.C. Do differences in brain activation challenge universal theories of dyslexia? Brain Lang. 2006;98(3):341–343. doi: 10.1016/j.bandl.2005.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.