Abstract
Inhibitory control deficits are a hallmark in ADHD. Yet, inhibitory control includes a multitude of entities (e.g. ‘inhibition of interferences’ and ‘action inhibition’). Examining the interplay between these kinds of inhibitory control provides insights into the architecture of inhibitory control in ADHD. Combining a Simon task and a Go/Nogo task, we assessed the interplay of ‘inhibition of interferences’ and ‘action inhibition’. This was combined with EEG recordings, EEG data decomposition and source localization. Simon interference effects in Go trials were larger in ADHD. At the neurophysiological level, this insufficient inhibition of interferences in ADHD related to the superior parietal cortex. Simon interference effects were absent in action inhibition (Nogo) trials in ADHD, compared to controls. This was supported by bayesian statistics. The power of effects was higher than 95%. The differential effects between the groups were associated with modulations of neurophysiological response selection processes in the superior frontal gyrus. ADHD is not only associated with deficits in inhibitory control. Rather, the organization and architecture of the inhibitory control system is different in ADHD. Distinguishable inhibitory control processes operate on a hierarchical ‘first come, first serve’ basis and are not integrated in ADHD. This is a new facet of ADHD.
Keywords: ADHD, Inhibitory control, EEG, Parietal cortex
1. Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a prevalent childhood onset disorder (Faraone et al., 2015) associated with deficits in cognitive control (Greenhill et al., 2008; Kieling and Rohde, 2012; Thomas et al., 2015). One instance of cognitive control is the ability to inhibit responses (Diamond, 2013). Deficits in ‘inhibitory control’ are frequently reported and of high clinical relevance in ADHD (Bari and Robbins, 2013; Chmielewski et al., 2018a; Fallgatter et al., 2005, 2004; Paul-Jordanov et al., 2010; Pliszka et al., 2007; Seifert et al., 2003). However, ‘inhibitory control’ actually refers to a multitude of aspects, like the ‘inhibition of interferences’ and ‘action inhibition’ (Sebastian et al., 2012): The ‘inhibition of interferences’ can, for example, be examined using Simon tasks where subjects choose between a left- and a right-hand key press according to the identity of a stimulus presented either to the left or right on the screen. Although stimulus position is irrelevant for the task, performance is better when the required response spatially corresponds to the stimulus location (congruent condition) than when it does not correspond (incongruent condition) (Keye et al., 2013; Ridderinkhof, 2002). In incongruent trials, irrelevant information about the stimulus location needs to be inhibited/controlled to allow correct responding (De Jong et al., 1994; Keye et al., 2013; Kornblum et al., 1990; Mückschel et al., 2016). Several studies consistently show deficits in the inhibition of interferences in ADHD; i.e. patients show an increased interference/congruency effect compared to healthy controls (Mullane et al., 2009; Rubia et al., 2011). Processes related to ‘action inhibition’ can, for example, be examined using Go/Nogo tasks. There, subjects have to respond as quickly as possible to a Go-stimulus and to withhold a response when a (rare) Nogo stimulus is presented. Here, ADHD patients have also been shown to exhibit deficits inhibiting an incorrect response on NoGo trials (Albrecht et al., 2013; Coghill et al., 2014a,b).
Critically, deficits in the ‘inhibition of interferences’ and ‘action inhibition’ have thus far been considered independently in ADHD research, which may leave the impression of distinct dysfunctional entities of inhibitory control. Yet, ‘inhibition of interferences’ and ‘action inhibition’ are not isolated entities of inhibitory control. Rather, they mutually affect each other (Chmielewski et al., 2018, 2018b; Chmielewski and Beste, 2017). This interplay of different kinds of inhibitory control is elusive in ADHD, but provides insights into the architecture of inhibitory control that goes beyond a mere assessment of the degree of inhibitory control deficits in ADHD:
Evidence for an interplay of the ‘inhibition of interferences’ and ‘action inhibition’ comes from experiments combining the above-mentioned “Simon Tasks” and “Go/Nogo tasks”. In fact, exerting inhibitory control to resolve interference affects how well actions can be inhibited (Chmielewski et al., 2018; Chmielewski and Beste, 2017). On the one hand‚ ‘inhibition of interference’ complicates response execution (leading to the Simon effect). On the other hand, it facilitates response inhibition (Chmielewski et al., 2018; Chmielewski and Beste, 2017). The reason is that inhibitory control is exerted to overcome automated responding in incongruent trials. When being integrated with action inhibition processes, this reduces the automaticity of inappropriate response tendencies in Nogo trials and improves action inhibition (Chmielewski et al., 2018; Chmielewski and Beste, 2017). Owing to the ADHD-inherent deficits in the ‘inhibition of interference’ and in ‘action inhibition’, a possible hypothesis is that the effect of congruent and incongruent trials during the inhibition of actions is stronger in ADHD patients than controls. Yet, it needs to be stressed that inhibitory control processes resolving Simon-interference are initiated after processes triggering the incorrect action impulse evoked by the onset of the stimulus (Ridderinkhof, 2002). The stimulus onset (i.e. the stimulus identity) also triggers action inhibition processes. In case of incongruent Simon-Nogo trials it is possible that a first inhibitory control process is triggered by the Nogo stimulus’ identity and that a second process is then triggered to control the further impact of the irrelevant stimulus location (i.e. Simon interference). Crucially, processes inhibiting the Simon interference are weaker in ADHD (Mullane et al., 2009). Moreover, ADHD patients have problems integrating cognitive operations that are only slightly separated in time (Bluschke et al., 2018b; Marusich and Gilden, 2014). Therefore, it is more reasonable to hypothesize that the congruency effect during the inhibition of actions is weaker in ADHD patients compared to controls; i.e. action inhibition processes cannot be facilitated by processes related to the inhibition of interference. If this is the case, this will suggest that there are not only inhibitory control deficits in ADHD. Rather, this will show that ADHD is also associated with a qualitatively different architecture of inhibitory control: Two entities of inhibitory processes are likely to be abnormally isolated in ADHD, and ADHD patients are not able to integrate different kinds of inhibitory control.
To examine the interplay of different inhibitory control processes, we apply a neurophysiological approach combining EEG recordings with source localization and temporal signal decomposition methods. The latter are used because standard event-related potential (ERP)-components are composed of various amounts of signals from different sources (Huster et al., 2015; Stock et al., 2017). Moreover, ERPs can only yield accurate insights into neurophysiological processes when there is little intra-individual variability (Ouyang et al., 2011a, 2015a). Importantly, intra-individual variability increases with longer RT (Wagenmakers and Brown, 2007), which is especially an issue for incongruent trials. Moreover, in ADHD, intra-individual variability on the neurophysiological level is considerably high (Alba et al., 2016; Bluschke et al., 2018b, 2017; Gonen-Yaacovi et al., 2016). Using residue iteration decomposition (RIDE) it is possible to account for intra-individual variability and to distinguish between different inhibitory control subprocesses that are otherwise intermingled in ERPs (Mückschel et al., 2017a,c; Ouyang et al., 2015a). RIDE decomposes the data in different clusters: the S-cluster indicates early stimulus-related (gating) processes, the R-cluster reflects response-related processes (i.e., motor execution) and the C-cluster reflects intermediate processes between S and R (i.e., response selection) (Mückschel et al., 2017a; Ouyang et al., 2017; Verleger et al., 2017). RIDE cluster are somewhat different to classical ERP-components in that a RIDE cluster usually comprises different ERP-component. For example, the S-cluster the P1 and N1 ERP-components are shown, while the C-cluster contains information that is usually capture by the P3 ERP-component. The N2-ERP component can be seen in all three clusters (Mückschel et al., 2017a, 2017c), because this ERP-component is known to reflect a mixture of stimulus and response-related processes (Folstein et al., 2008). The R-cluster has already been shown to be modulated by interfering information (Mückschel et al., 2017a). Therefore, we hypothesize that congruency effects in Go trials and differences between ADHD patients and controls will be reflected by modulations in the R-cluster. The interplay of the ‘inhibition of interferences’ and ‘action inhibition’ (i.e., NoGo trials) has been shown to modulate response selection processes reflected by the C-cluster in healthy adult participants (Chmielewski et al., 2018; Chmielewski and Beste, 2017). There, the C-cluster amplitude revealed an interaction “Go/Nogo x congruency” in that there was no difference in the C-cluster amplitude on Go trials, while there was a difference on Nogo trials. While such results from healthy adult subjects cannot be directly seen as hint for the processes that are likely to occur in adolescence, they still suggest that response selection processes may be of particular importance. Therefore, we hypothesize that above-mentioned differential effects between congruent and incongruent NoGo trials in the control and the ADHD group are reflected by the C-cluster. Therefore, we hypothesize that there is an interaction “Go/Nogo x congruency x group” that is mainly driven by differential group effects in Nogo trials than Go trials. That is, differences in the congruency effects between ADHD patients and controls are supposed to be evident for Nogo trials, but not for Go trials. On a neurophysiological level, alterations in fronto-parietal processes have been suggested to relate to deficits in inhibition of interference processes in ADHD (Rubia et al., 2011). Moreover, the interplay between ‘action inhibition’ and ‘inhibition of interferences’ modulates activity in fronto-parietal regions (Chmielewski et al., 2018). Therefore, we hypothesize that these regions are associated with modulations in the R-cluster and the C-cluster.
2. Materials and methods
2.1. Participants, sample size estimation and power analysis
Previous data showed that interactive effects in this task had an effect size of ηp2 ˜ .17 (Chmielewski et al., 2018; Chmielewski and Beste, 2017). Using this effect size as the basis of an estimate for the current study, the power calculation revealed a total required sample size of N = 20 (i.e. 10 ADHD patients and 10 controls) (power = 95%). The enrolled sample size was more than twice as large. The obtained effect size of the important interactions ‘congruency x group’ were in the range between ηp2 ˜ .10 and ηp2 ˜ .17 (see results section for details). Accordingly, the post-hoc power analysis (using the actually sample size and achieved effect sizes) revealed a power above 98%. Therefore, the effects reported are highly reliable.
Only unmedicated patients with confirmed ADHD, diagnosed according to established clinical guidelines (incl. family and school interviews and questionnaires, IQ and attention testing, exclusion of possible somatic differential diagnoses via blood analyses, EEG, audiometry and vision testing) by a team of experienced child and adolescent psychiatrists and psychologists were enrolled in the study. All patients fulfilled criteria for ADHD according to ICD-10 (F90.0, F90.1 or F98.8). Patients with additional severe or acute psychiatric comorbidities (e.g. autism, tics, depressive episode etc.) were excluded. N = 23 patients (16 male, 11.9 ± 0.3 years, median age = 11.6; age range = 10–14.2 years; IQ: 106.8 ± 13.5) were included in the study. In the ADHD Symptom Checklist (Döpfner et al., 2008) parents rated (0: no problems, 3: severe problems) their children in regards to inattention (1.82 ± 0.20), hyperactivity (1.64 ± 0.18) and impulsivity (2.04 ± 0.16). N = 27 children without ADHD were included in the control group (18 male, 14.4 ± 0.36 years, median age = 14.6; age range = 11.1–15.9 years; IQ: 109 ± 14.2). None of them were taking medication and none had a psychiatric diagnosis as confirmed by clinical interview. The factor “age” was used a covariate in the statistical analyses. All subjects and their parents or legal guardians provided informed written consent and the study was approved by the local ethics committee of the Medical Faculty of the TU Dresden.
2.2. Task
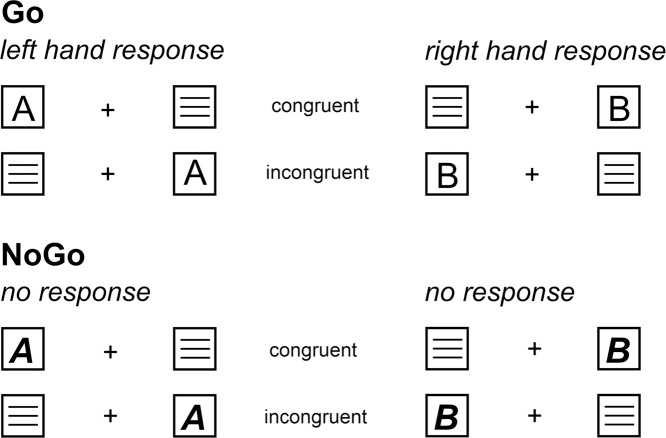
To examine the interplay of the ‘inhibition of interferences’ and ‘action inhibition’ we apply a combined Simon-Go/Nogo task, which is shown in Fig. 1 (Chmielewski et al., 2018).
Fig. 1.
Upper panel: In the Go condition (70% of all trials), which was indicated by regular letter stimuli (either letter “A” = left button or “B” = right button). Trials which required a response on the side where the target was presented were categorized as “congruent”, the others as “incongruent”. Lower panel: The Nogo condition (30% of all trials) was indicated by bold italic target stimuli.
The experiment consisted of 720 trials [70% Go and 30% Nogo trials] and was divided into six equally-sized blocks with short breaks in between. In each block, the same ratio of Go and Nogo trials was presented. Participants were seated in front of a black screen presenting a fixation cross in the middle and white boxes to the left and right of the fixation cross (distance of 1.1° visual angle). The inter-trial interval (ITI) was jittered between 1100 and 1600 ms. Each trial began with the presentation of a letter (for 200 ms) in one of the boxes, which was either in normal font (i.e. ‘A’, ‘B’), or in bold-italics (i.e. ‘A’ or ‘B’). Letters in a normal font represented Go trials, letters in combined bold and italic font represented Nogo trials. Whenever an ‘A’ was displayed, a left-hand response was required on Go trials. Whenever a ‘B’ was displayed a right-hand response was required on Go trials. The responses were carried out using a standard Cherry QWERTZ-keyboard. These responses were required regardless of the spatial position of the stimuli in the left or right box. This creates a Simon-conflict (incongruent Go trials) whenever stimuli were presented on the side opposite of the hand carrying out the response. For Nogo trials, left side ‘A’s and right side ‘B’s represented congruent Nogo trials, whereas left side ‘B’s and right side ‘A’s represented incongruent Nogo trials. Fifty percent of Go and Nogo trials were incongruent or congruent. It was ensured that all congruent and incongruent Go/Nogo conditions were equally distributed across the blocks. In Go trials, subjects were asked to respond within 250–1000 ms after stimulus presentation. An incorrect response in that time-window was coded as error and if no response was obtained, trials were coded as misses. For Nogo trials, any response within 250–1000 ms after stimulus presentation represented a false alarm (i.e. a failure to inhibit the response). If no response was given on Go trials in a time window of 500 ms, a speed up sign (‘Schneller!’) was presented above the fixation cross. Each trial ended after 1700 ms.
The behavioral data (RTs, accuracy on Go and Nogo trials) were analyzed separately for Go and Nogo conditions using repeated measures ANOVA including the factor’ congruency’ (congruent vs. incongruent) as within-subject factor and ‘group’ (ADHD vs. HC) as between-subject factor. Greenhouse-Geisser correction was applied and all post-hoc tests were Bonferroni-corrected.
2.3. EEG recording and analysis
The EEG was recorded with a BrainAmp amplifier (Brain Products, Inc.) with an equidistant electrode setup from 60 Ag/AgCl electrodes with a sampling rate of 500 Hz (reference at Fpz, ground electrode at θ = 58, ф = 78, electrode impedances < 5 kΩ). During off-line data processing using the Brain Vision Analyzer 2 software package, a band-pass filter was applied (0.5–20 Hz, slope: 48 db/oct)1 and technical artefacts (“offsets in the data) were removed during the manual inspection of the raw data. Then, an independent component analysis was used to detect and remove pulse artefacts and horizontal and vertical eye movements. The EEG data were then segmented to the onset of the Go and Nogo stimuli (in a time window of −250 ms to 1000 ms). Only trials with correct responses on Go and trials without responses on Nogo trials were segmented and analysed further. For the segmented data, an automatic artefact rejection procedure was applied with an amplitude criterion (maximal amplitude: +200μV, minimal amplitude: −200μV) and a maximal value difference of 200μV in a 200 ms interval as well as an activity below 0.5μV in a 100 ms period as rejection criteria. After that, a current source density transformation was performed to allow a reference-free evaluation of the EEG data (Nunez and Pilgreen, 1991). It is important to note that the spatial filter properties of the CSD-transformation (Kayser and Tenke, 2015) do not violate assumptions of RIDE since the decomposition is conducted separately for each single electrode channel (Ouyang et al., 2015b). After CSD transformation, data were baseline corrected to a time interval from −200 ms to 0 ms and segments were averaged for each condition.
2.4. Residue iteration decomposition and data quantification
Full mathematical details of the residue iteration decomposition (RIDE) can be found elsewhere (Ouyang et al., 2011b, 2015c). The RIDE toolbox and manual are available at http://cns.hkbu.edu.hk/RIDE.htm. Briefly, RIDE decomposes the EEG single-trial data into three clusters. The S-cluster is correlated to the stimulus onset, the R-cluster to the response. The third C-cluster has a variable latency, which is estimated by the algorithm and iteratively improved. Since the R-cluster cannot reliably be estimated in Nogo trials due to a low frequency of responding in these trials (Ouyang et al., 2013), only the S-cluster and the C-cluster were calculated (Chmielewski et al., 2018). To estimate the C-cluster latency, RIDE uses a nested iteration scheme. During this procedure, the initial latency of the C-cluster is estimated using a time window function. Then, the S-cluster is iteratively removed, and the latency of the C-cluster is re-estimated in every iteration step using a template matching approach. The time window is assumed to cover the range within which each component is supposed to occur (Ouyang et al., 2015b). During processing, the initial time window for the estimation of the C-cluster was set to 200–700 ms after stimulus onset. The time window for the S-cluster was set to -200 to 400 ms around stimulus onset. These time windows were also applied in a previous study using the same experimental paradigm (Chmielewski et al., 2018). In a data-driven approach, single-subject RIDE cluster amplitudes were quantified as the mean amplitude in a defined time interval. The choice of electrodes and time windows was validated using a statistical procedure described in Mückschel et al. (2014). This validation procedure revealed the same electrodes and time windows as identified by visual inspection. The electrodes and time windows used for the extraction of mean activity for RIDE-Clusters are shown in the Supplemental table 1. We also analyzed standard ERP components. Details on electrode and time window selection for the ERP analysis can also be found in the Supplemental table 1.
2.5. Source localization
As in previous studies using this experimental paradigm, source localization was carried out applying sLORETA on the RIDE data (Chmielewski et al., 2018). As shown in the results section (see below), especially the C-cluster revealed differential effects between ADHD patients and healthy controls. sLORETA provides a single linear solution to the inverse problem without a localization bias (Marco-Pallarés et al., 2005; Pascual-Marqui, 2002; Sekihara et al., 2005). The reliability of sLORETA sources has been corroborated by EEG/fMRI and EEG/TMS studies (Dippel and Beste, 2015; Sekihara et al., 2005). For sLORETA, the intracerebral volume is partitioned into 6239 voxels at 5 mm spatial resolution. The standardized current density at each voxel is calculated in a realistic head model using the MNI152 template. Comparisons were based on statistical non-parametric mapping (SnPM) using the sLORETA-built-in voxel-wise randomization tests with 2500 permutations (Nichols and Holmes, 2002). Voxels with significant differences (p < .05, corrected for multiple comparisons) between contrasted groups were located in the MNI-brain www.unizh.ch/keyinst/NewLORETA/sLORETA/sLORETA.htm. It has been shown that source localization results based on ERPs and RIDE decomposed data are highly similar (Chmielewski et al., 2018).
2.6. Statistics
The behavioral data were analyzed separately for Go and Nogo conditions using mixed effects ANOVAs. These included the factor’ congruency’ (congruent vs. incongruent) as within-subject factor and ‘group’ (ADHD vs. HC) as between-subject factor. The neurophysiological data were analyzed using mixed effects ANOVAs including the factor ‘congruency’ (congruent vs. incongruent) as within-subject factors and ‘group’ (ADHD vs. HC) as between-subject factor. For P1, N1, S- and R-cluster activation the additional within-subject factor electrode was included in the mixed effects ANOVAs. Greenhouse-Geisser correction was applied and all post-hoc tests were Bonferroni-corrected All variables were normal distributed as indicated by Kolmogorov-Smirnov Tests (all z < 0.74; p > .4). The factor age was controlled using this parameter as a covariate in the statistical models.
3. Results
3.1. Behavioral data
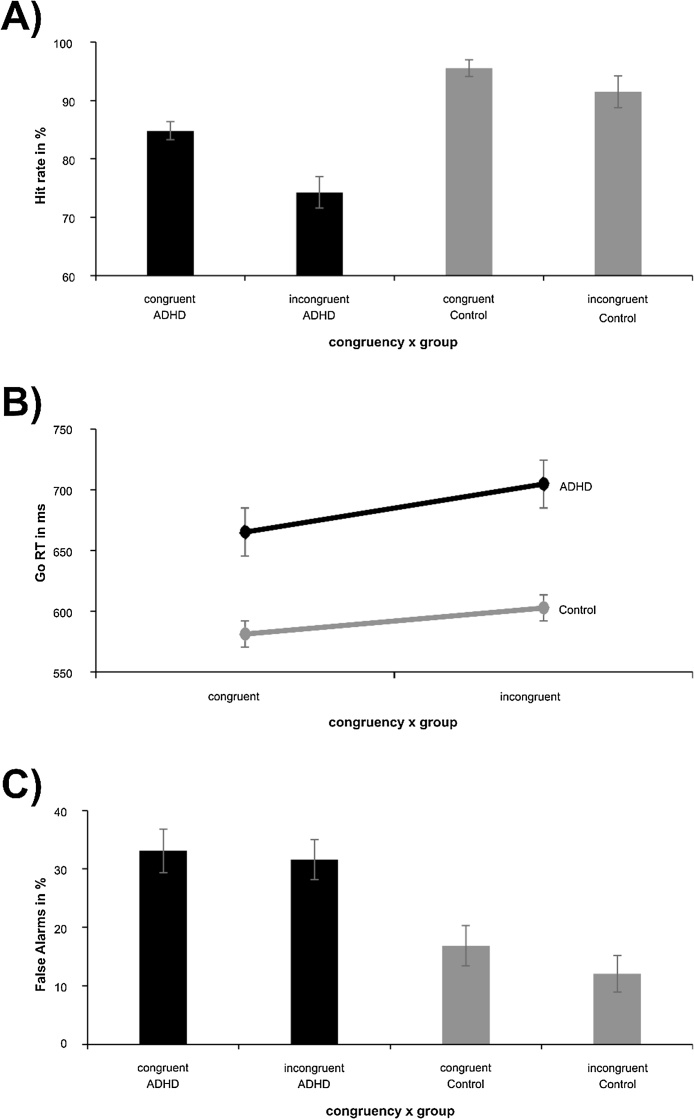
The behavioral data are shown in Fig. 2.
Fig. 2.
(A) Hit rate in percent and (B) Reaction times in ms for congruent and incongruent Go trials; (C) False alarm rate in percent for congruent and incongruent NoGo trials. The mean and standard error of the mean are given. Controls are indicated by grey color, ADHD is indicated by black color.
Concerning the Go accuracy data (Fig. 2A), the mixed effects ANOVA revealed a main effect of congruency (F(1,48) = 26.96; p < .001; = .360). Hit rates (HR) were higher in the congruent (90.2 ± 1.0%) than the incongruent condition (82.9 ± 1.8%). The main effect of group revealed a lower accuracy in the ADHD (79.5 ± 1.9%), than the control group (93.5 ± 1.8%) (F(1,48) = 28.55; p < .001; = .373). Crucially, an interaction of congruency x group was detected (F(1,48) = 5.27; p = .026; = .099). Post-hoc tests showed the congruency/interference effect (CEHR = congruentHR – incongruentHR) was stronger in ADHD (10.6 ± 2.9%) than healthy controls (4.1 ± 0.8%) (t(48) = 2.30; p = .026). For the Go reaction time (RT) data (Fig. 2B), the mixed effects ANOVA showed a main effect of congruency (F(1,48) = 61.29; p < .001; = .561), and responses were faster in congruent (623 ± 17 ms) than in incongruent trials (653 ± 16 ms). A main effect of group was detected, which revealed longer RTs in ADHD (685 ± 24 ms) than controls (591 ± 22 ms) (F(1,48) = 7.68; p = .008; = .138). Again, there was an interaction of congruency x group (F(1,48) = 5.40; p = .024; = .101). The congruency/interference effect (CE) was stronger in ADHD (-39 ± 6 ms) than controls (-21 ± 4 ms) (t(48) = 2.32; p = .024).
The mixed effects ANOVA for false alarms (FA) in Nogo trials (Fig. 2C) revealed a main effect of congruency (F(1,48) = 12.32; p = .001; = .204). FAs were decreased in the congruent (22.3 ± 2.3%) compared to the incongruent condition (25.0 ± 2.5%). The main effect of group revealed higher FAs in ADHD (32.9 ± 3.5%) than controls (14.5 ± 3.3%) (F(1,48) = 14.55; p = <.001; = .233). Most importantly, there was an interaction of congruency x group (F(1,48) = 8.23; p = .006; = .146). Interestingly, in ADHD no significant FA differences between congruent (33.1 ± 4.6%) and incongruent (32.6 ± 4.3%) trials were observed (t(48) = .37; p = .718). Controls, however, revealed higher FAs in congruent (16.9 ± 2.6%) than incongruent (12.1 ± 2.2%) trials (t(48) = 5.87; p < .001), which is in line with previous findings (Chmielewski et al., 2018). Importantly, using “age” as a covariate in the analyses did not change the pattern of results (all F < 0.99; p > .456). The mean response time on erroneous Nogo trials was 256 ms ± 26 and did not differ between groups (t(48) = 0.36; p > .5). This response time is well below the deadline of the speed up sign. Therefore, the lack of the speed up sign cannot serve as an additional Nogo cue. Further analyses showing that “age” did not affect the pattern of behavioral results is shown in the Supplemental material (cf. Supplemental analysis on possible age effects).
3.2. Neurophysiological data
The analysis of the standard ERP data is presented in the Supplemental material. Briefly, the standard ERP-components did not reveal interactive effects (“condition x group”) explaining the behavioral effects. This is in line with the hypotheses. However, the RIDE decomposed data revealed differential effects in the C-cluster and the R-cluster, but not in the S-cluster. Therefore, the S-cluster is also shown in the Supplemental material.
3.3. C-cluster
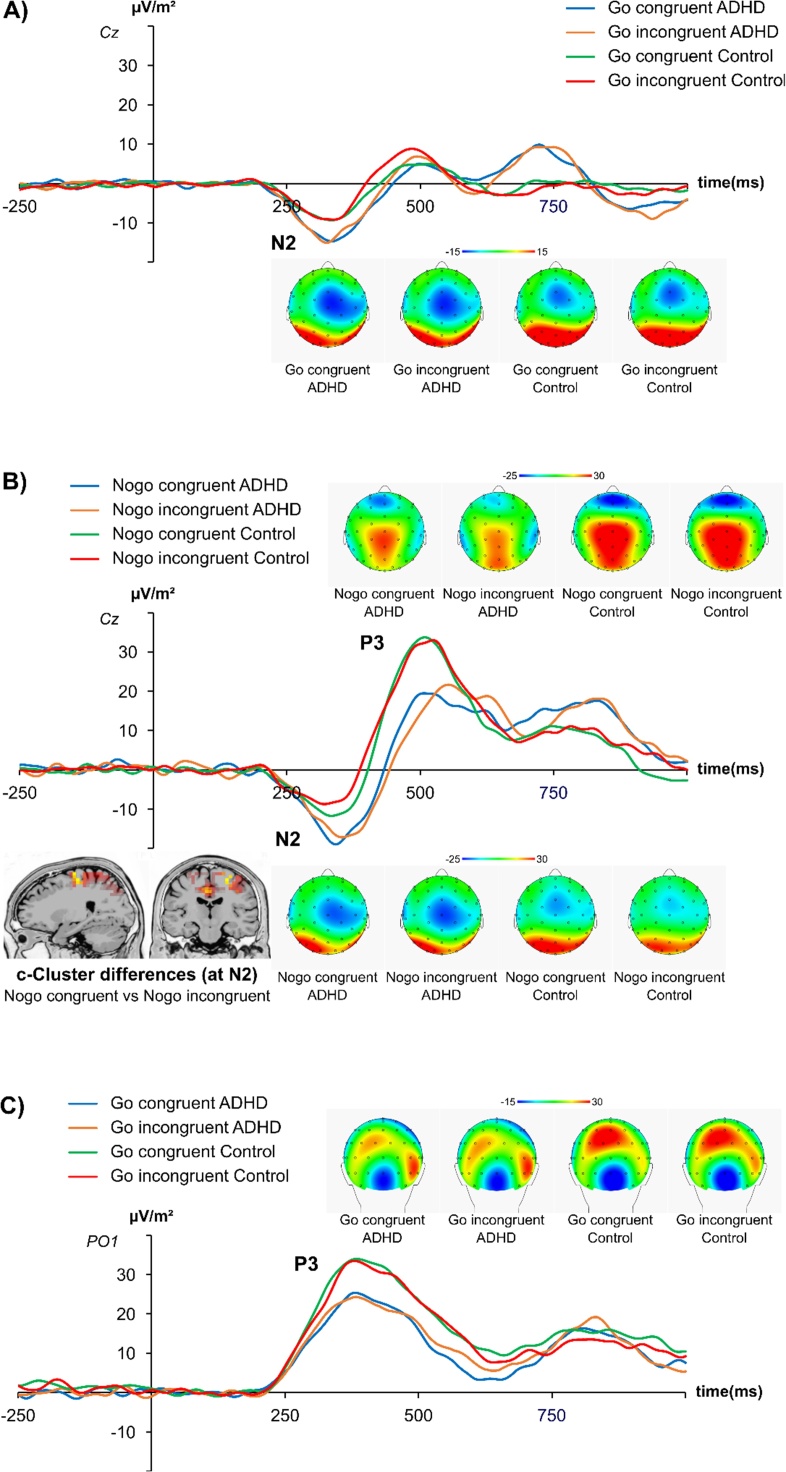
The C-cluster is shown in Fig. 3.
Fig. 3.
The C-cluster in the N2 and P3 time window and respective topography plots. The C-Cluster is shown over the whole time-range and topographic plots are shown for the N2 and P3 time-windows. (A) Go conditions at electrode Cz in the N2 time window. (B) Nogo conditions at electrode Cz in the N2 and P3 time window. The sLORETA plots show the source of the difference between the congruent and incongruent Nogo condition in the N2 time window. An area in the superior frontal gyrus (BA6) is revealed. The time windows used for data quantification are given in Supplementary Table 1. The different lines show the congruent condition in ADHD (blue), the incongruent condition in ADHD (orange), the congruent condition in controls (green) and the incongruent condition in controls (red). (C) Go conditions at electrode PO1 in the P3 time window. The analyzed time windows were 20 ms around the peak of the C-cluster in the P3 time window in each condition as outlined in Supplementary Table 1.
For the C-cluster, the data analysis revealed an interaction “Go/Nogo x congruency x group “(F(1,48) = 4.77; p = .031; = .101). This is important, because the integration between two process is usually defined as an interaction effect between the two corresponding factors. Importantly, for Go trials (Fig. 3A), no main or interaction effects were observed in the negativity in the N2 time window (all F ≤ 1.89; p ≥ .176). However, for Nogo trials (Fig. 3B), an interaction of congruency x group was observed in the negativity amplitude in the N2 time window (F(1,48) = 5.63; p = .022; = .105). Post-hoc paired t-tests revealed that this was due to non-significant amplitude differences between congruent and incongruent Nogo trials in ADHD (congruent: -17.03 ± 3.71 μV/m2; incongruent: -18.73 ± 2.99 μV/m2; t(22) = .71; p = .486), but smaller amplitudes in incongruent (-7.45 μV/m2 ± 2.58) than congruent (-12.86 μV/m2 ± 2.75) Nogo trials (t(26) = -2.89; p = .008) in controls. There were no significant main effects (all F < 1.54; p > .221). This interaction parallels the effects in the FA data. The sLORETA analysis (Fig. 3B) shows that amplitude modulations in the N2 time window depending on group and experimental condition were due to modulations of neural activity in the superior frontal gyrus (BA6). Crucially, also when analyzing the “Go/Nogo x congruency x group “differently, i.e. examining whether there is an interaction “Go/Nogo x congruency “for each group separately, revealed that there was no interaction in the ADHD group (F(1,22) = 0.29; p > .6), but a significant interaction in the control group (F(1,26) = 4.76; p = .038; = .155). As mentioned above, the integration between two processes is usually defined as an interaction effect between the two corresponding factors. The lack of a significant interaction “Go/Nogo x congruency “in ADHD patients, opposed to healthy controls supports that there is not integration between the inhibition of interferences and action inhibition in ADHD. To substantiate the lack of “Go/Nogo x congruency “in the ADHD group, Bayesian statistics were calculated to evaluate the relative strength of evidence for the null hypothesis (Masson, 2011); the probability of the null hypothesis being true, given the obtained data (p(H0|D)). This can be done using a transformation of the sum-of-squares values generated by the ANOVA (Masson, 2011). This analysis revealed p(H0|D) > .80, which provides positive evidence for the null hypothesis.
For the positive amplitudes in the C-cluster in the P3 time window, no main or interaction effects were evident for Go trials (Fig. 3C) (all F ≤ 2.45; p ≥ .124). For Nogo trials (Fig. 3B), only a main effect of group was observed, showing that amplitudes were smaller in the ADHD (20.44 ± 4.82 μV/m2), than the control group (33.79 ± 4.45 μV/m2) (F(1,48) = 4.14; p = .047; = .079). All other main or interactions were not significant (all F ≤ .73; p ≥ .399). Using “age” as a covariate in the analyses did not change the pattern of results (all F < 1.09; p > .4). Further analyses showing that “age” did not affect the pattern of results is shown in the Supplemental material (cf. Supplemental analysis on possible age effects).
3.4. R-cluster
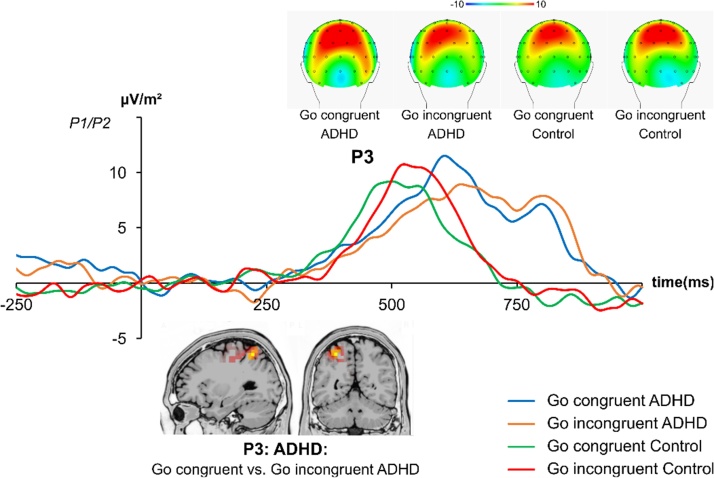
The R-cluster is shown in Fig. 4.
Fig. 4.
The R-cluster in the P3 time window at averaged electrodes P1/P2 and respective topography plots for Go trials. The R-Cluster is shown over the whole time-range and topographic plots are shown for the time-windows specified in Supplementary table 1. The sLORETA plots show the source of the effects. An area in the superior parietal cortex (BA7) is shown. The different lines show the congruent condition in ADHD (blue), the incongruent condition in ADHD (orange), the congruent condition in controls (green) and the incongruent condition in controls (red). The time windows used for data quantification are given in Supplementary Table 1.
The R-cluster can only be analyzed in Go trials, since it depends on the frequent execution of responses (Ouyang et al., 2015a). As can be seen in Fig. 4, the R-cluster was maximal at parietal electrode sites. Further details on the selection of electrode sites are provided in the Supplemental material. The mixed effects ANOVA for the parietal positivity (at electrodes P1 and P2) only revealed a significant interaction of congruency x group (F(1,48) = 9.97; p = .003; = .172). The sLORETA analysis shows that amplitude modulations depending on group and experimental condition were due modulations of neural activity in the superior parietal cortex (BA7). To follow up this interaction, congruent and incongruent trial amplitudes were compared within each group. Post-hoc paired t-test showed that the amplitudes did only differ between congruent and incongruent trials in ADHD (congruent: 11.26 ± 2.12 μV/m2; incongruent: 7.59 ± 1.69 μV/m2; t(22) = 3.11; p = .005), but not in controls (congruent: 9.29 ± 1.57 μV/m2; incongruent: 10.83 ± 1.38 μV/m2; t(26) = -1.34; p = .189). The congruency/interference effect (CE) was stronger in ADHD (3.67 ± 1.18 μV/m2) than controls (-1.55 ± 1.15 μV/m2) (t(48) = 3.16; p = .003). All other main effects and interactions did not reach significance (all F ≤ 1.65; p ≥ .205). Using “age” as a covariate in the analyses did not change the pattern of results (all F < 1.29; p > .389).
4. Discussion
Although inhibitory control deficits are a hallmark in ADHD, the precise interrelation between different entities of inhibitory control has been elusive. Yet, the examination of this interrelation provides insights into the cognitive organization and architecture of the inhibitory control system beyond the mere degree of inhibitory control deficits in ADHD. Power calculations revealed a high power of the observed effects. Since ADHD patients were unmedicated, this factor cannot have affected the effects. Though the age was different between the ADHD and the control group, which is a limitation of the study, this factor did not affect behavioral and neurophysiological data, when controlling for it in analyses of covariance. This is all the more the case since the data replicates numerous previous findings from the Simon effect. Considering the Simon effect in Go trials, this was larger in ADHD patients than controls (Mullane et al., 2009). On a neurophysiological level, these deficits in ‘inhibition of interference’ were reflected by the R-cluster, which has been suggested to denote processes of response execution and preparation (Ouyang et al., 2011a), and which has previously been shown to be modulated by interfering information/conflict effects (Mückschel et al., 2017a). In the ADHD group, the R-cluster amplitude was smaller in incongruent trials than congruent trials at parietal electrode sites. No such effects were evident in controls showing a smaller Simon effect. Crucially, it is the activation of the incorrect response motor effector by the incongruent information that elicits the Simon effect (Keye et al., 2013). It is therefore possible that the smaller R-cluster in incongruent trials in ADHD indicates an insufficient inhibition of the interfering information. This leads to the enhanced congruency effect in Go trials in the ADHD group. This assumption was supported by the sLORETA analysis showing that these modulations were associated with superior parietal areas (BA7), as dysfunctions of parietal areas are known to contribute to increased Simon effects/deficits in ADHD (Rubia et al., 2011). Moreover, this also matches other data showing that superior parietal, inhibitory mechanisms are involved in the selection of motor effectors (response channel) (Bernier et al., 2012; Cisek and Kalaska, 2002; Jaffard et al., 2008; Sulpizio et al., 2017). It may be argued that the Simon effect is usually reflected by an augmented ERP amplitude in incongruent trials compared to in congruent conditions. The findings that this is not case in the current data may therefore be counterintuitive. However, it needs to be noted classical ERPs intermingle different processing codes and signals from different sources (Huster et al., 2015; Stock et al., 2017) and are moreover affected by intra-individual variability (Ouyang et al., 2011a, 2015a). The latter increases with longer RT (Wagenmakers and Brown, 2007), which is the case for incongruent trials. Importantly, the RIDE data accounted for all of these aspects, which is the reason why the pattern of amplitude effects may diverge from classical ERP findings.
However, most important are the findings in Nogo trials: Generally, ADHD patients committed more false alarms than controls, which fits to the well-known action inhibition deficits in ADHD (Bari and Robbins, 2013; Bluschke et al., 2016; Chmielewski et al., 2018; Paul-Jordanov et al., 2010; Pliszka et al., 2007; Seifert et al., 2003; Albrecht et al., 2013; Coghill et al., 2014a,b). Crucially, while controls revealed the usual pattern of fewer false alarms in incongruent than congruent Nogo trials (Chmielewski et al., 2018; Chmielewski and Beste, 2017), ADHD patients revealed no differences between congruent and incongruent trials. This lack of modulation is substantiated by the bayesian analysis of the data (refer results section). The lack of modulation suggests that ‘action inhibition’ and the ‘inhibition of interferences’ are unrelated entities within the inhibitory control system in the ADHD group and shows that ADHD is not only associated with deficits in inhibitory control. Rather, the organization and architecture of the inhibitory control system is qualitatively different. The neurophysiological data details what mechanisms are affected by this altered architecture of the inhibitory control system. The lack of modulatory effects in the S-cluster indicates that stimulus-driven perceptual gating and attentional selection processes are not affected by the altered inhibitory control system’s architecture in ADHD. Crucially, interactive effects were evident for the C-cluster in the N2 time window. The source localization analysis shows that superior frontal regions (BA6) are associated with these effects. Previous results suggest that the interplay of the ‘inhibition of interferences’ and ‘action inhibition’ is reflected by the C-cluster (Chmielewski et al., 2018). In controls, in the C-cluster, amplitudes in the N2 time window were smaller for incongruent than congruent Nogo trials. Importantly, no modulations between congruent and incongruent trials were evident in the ADHD group. This parallels the behavioral data. Amplitudes in the N2 time window are well-known to be increased when information is ambiguous and inconclusive (Botvinick et al., 2001; Szmalec et al., 2008). The obtained source in the superior frontal gyrus (BA6) is well-known to be involved in processes resolving conflicts and ambiguity (Rushworth et al., 2004). The superior frontal gyrus has been implicated in a cortical network mediating inhibitory control (Bari and Robbins, 2013), but also the right inferior frontal cortex has been shown to play a role (Aron et al., 2003; Aron and Poldrack, 2006). Recent evidence suggest that also modulations of the C-cluster are associated with the right inferior frontal gyrus (Mückschel et al., 2017c). However, these and other studies frequently used a stop-signal paradigms or other paradigms involving a rare presentation of inhibitory trials. Crucially, these paradigms examine action inhibition in isolation, while in the current study action inhibition is further modulated by the inhibition of interference. The observation the BA6 (superior frontal gyrus) is modulated, may be due to that. Intriguing, the Supplementary motor cortex (including BA6) affects not just the commission but also the omission of actions (Nachev et al., 2008, 2005), especially when action contingencies are ambiguously (Nachev et al., 2008). All these is clearly the case in the current experiment because the Nogo stimulus identity (i.e. ‘A’ or ‘B’) triggers ‘action inhibition’ while the spatial location of the stimulus triggers ‘inhibition of interferences’. The generally increased Nogo trial C-cluster amplitudes in the N2 time window in ADHD suggest that this ambiguity cannot be resolved. Especially incongruent Nogo stimuli require ‘action inhibition’ and ‘inhibition of interference’ processes. Importantly, processes related to the ‘inhibition of interference’ only facilitate ‘action inhibition’ processes and reduce the ambiguity whether or not to inhibit a response when these two processes become integrated successfully (Chmielewski et al., 2018; Chmielewski and Beste, 2017). This is the case in healthy controls, showing in the better response inhibition performance and a smaller C-cluster amplitude indicating a smaller ambiguity in incongruent Nogo trials. Since this was not the case in ADHD patients, the C-cluster results show that ADHD patients are not able to integrate the ‘inhibition of interferences’ with ‘action inhibition’ processes at the response selection level. An explanation for this is that ‘inhibition of interference’ processes are known to start slightly later than inhibitory control processes evoked by the Nogo stimulus identity (i.e. bold & italics letter) (Ridderinkhof, 2002) and may therefore not become integrated. Therefore, it seems that inhibitory control processes in ADHD operate on a hierarchical ‘first come, first serve’ basis at the response selection level: Whenever ‘action inhibition’ processes have been triggered, processes related to the ‘inhibition of interferences’ cannot be integrated. This interpretation is supported by the fact that ADHD patients have problems integrating cognitive processes that are only slightly separated in time (Bluschke et al., 2018b; Marusich and Gilden, 2014). Future studies should evaluate whether pharmacological treatments in ADHD are able to change this altered functional architecture in ADHD. It is well-known that inhibitory control processes are modulated by the dopaminergic and the norepinephrine system (Bari and Robbins, 2013). Moreover, optimal DA levels play an important role in the resolution of conflicts (Botvinick, 2007; Colzato et al., 2014) and the same has been suggested for norepinephrine levels (Adelhöfer et al., 2018; Bluschke et al., 2018a; Mückschel et al., 2017b; Warren et al., 2011; Warren and Holroyd, 2012). The current first-line treatment in ADHD uses methylphenidate (MPH), a mixed dopamine/norepinephrine receptor blocker (Skirrow et al., 2015; Volkow et al., 1999). It is possible that MPH normalizes the functionally aberrant inhibitory control architecture in ADHD with the consequence the action inhibition processes become integrated with processes related to the inhibition of interferences.
In summary, there are not only inhibitory control deficits in ADHD. Rather, ADHD is associated with a qualitatively different architecture of inhibitory control. Action inhibition processes cannot be facilitated by processes related to the inhibition of interference, as it is the case in healthy controls. In ADHD, two entities of inhibitory control (‘inhibition of interferences’ and ‘action inhibition’) are functionally isolated and operate on a hierarchical ‘first come, first serve’ basis. ADHD patients are not able to integrate different aspects of inhibitory control, likely due to dysfunctions at the response selection level and fronto-parietal cortices. This altered architecture is a new facet of ADHD that needs to be focused in research and clinical practice. From a clinical point of view, these findings may provide neuroscientific support for the need to give separate instructions in small, clearly defined hierarchical steps rather than presenting affected children with detailed and complex situations requiring “if-then” decisions. Behavioural and pharmacological treatments could focus on training of such integrative processes.
Conflict of Interest
None.
Footnotes
Importantly, the results remained the same when a broader filter band-width was used that covered the entire beta frequency band from 12 to 30Hz.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100623.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Adelhöfer N., Gohil K., Passow S., Teufert B., Roessner V., Li S.-C., Beste C. The system-neurophysiological basis for how methylphenidate modulates perceptual-attentional conflicts during auditory processing. Hum. Brain Mapp. 2018;39:5050–5061. doi: 10.1002/hbm.24344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba G., Pereda E., Mañas S., Méndez L.D., Duque M.R., González A., González J.J. The variability of EEG functional connectivity of young ADHD subjects in different resting states. Clin. Neurophysiol. 2016;127:1321–1330. doi: 10.1016/j.clinph.2015.09.134. [DOI] [PubMed] [Google Scholar]
- Albrecht B., Brandeis D., Uebel H., Valko L., Heinrich H., Drechsler R., Heise A., Müller U.C., Steinhausen H.-C., Rothenberger A., Banaschewski T. Familiality of neural preparation and response control in childhood attention deficit-hyperactivity disorder. Psychol. Med. 2013;43:1997–2011. doi: 10.1017/S003329171200270X. [DOI] [PubMed] [Google Scholar]
- Aron A.R., Poldrack R.A. Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A.R., Fletcher P.C., Bullmore E.T., Sahakian B.J., Robbins T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Bari A., Robbins T.W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bernier P.-M., Cieslak M., Grafton S.T. Effector selection precedes reach planning in the dorsal parietofrontal cortex. J. Neurophysiol. 2012;108:57–68. doi: 10.1152/jn.00011.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluschke A., Broschwitz F., Kohl S., Roessner V., Beste C. The neuronal mechanisms underlying improvement of impulsivity in ADHD by theta/beta neurofeedback. Sci. Rep. 2016;6:31178. doi: 10.1038/srep31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluschke A., Chmielewski W.X., Mückschel M., Roessner V., Beste C. Neuronal intra-individual variability masks response selection differences between ADHD subtypes - a need to change perspectives. Front. Hum. Neurosci. 2017:11. doi: 10.3389/fnhum.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluschke A., Friedrich J., Schreiter M.L., Roessner V., Beste C. A comparative study on the neurophysiological mechanisms underlying effects of methylphenidate and neurofeedback on inhibitory control in attention deficit hyperactivity disorder. Neuroimage Clin. 2018;20:1191–1203. doi: 10.1016/j.nicl.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluschke A., Gohil K., Petzold M., Roessner V., Beste C. Neural mechanisms underlying successful and deficient multi-component behavior in early adolescent ADHD. Neuroimage Clin. 2018;18:533–542. doi: 10.1016/j.nicl.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M.M. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Chmielewski W.X., Beste C. Testing interactive effects of automatic and conflict control processes during response inhibition–A system neurophysiological study. NeuroImage. 2017;146:1149–1156. doi: 10.1016/j.neuroimage.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Chmielewski W.X., Mückschel M., Beste C. Response selection codes in neurophysiological data predict conjoint effects of controlled and automatic processes during response inhibition. Human Brain Mapp. 2018;39:1839–1849. doi: 10.1002/hbm.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski W.X., Tiedt A., Bluschke A., Dippel G., Roessner V., Beste C. Effects of multisensory stimuli on inhibitory control in adolescent ADHD: It is the content of information that matters. Neuroimage Clin. 2018;19:527–537. doi: 10.1016/j.nicl.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielewski W.X., Zink N., Chmielewski K.Y., Beste C., Stock A.-K. How high-dose alcohol intoxication affects the interplay of automatic and controlled processes. Addict. Biol. 2018 doi: 10.1111/adb.12700. [DOI] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Modest gaze-related discharge modulation in monkey dorsal premotor cortex during a reaching task performed with free fixation. J. Neurophysiol. 2002;88:1064–1072. doi: 10.1152/jn.00995.2001. [DOI] [PubMed] [Google Scholar]
- Coghill D.R., Hayward D., Rhodes S.M., Grimmer C., Matthews K. A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): improvements in executive functioning do not explain clinical improvement. Psychol. Med. 2014;44:1087–1099. doi: 10.1017/S0033291713001761. [DOI] [PubMed] [Google Scholar]
- Coghill D.R., Seth S., Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol. Med. 2014;44:1989–2001. doi: 10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- Colzato L.S., Sellaro R., Hulka L.M., Quednow B.B., Hommel B. Cognitive control predicted by color vision, and vice versa. Neuropsychologia. 2014;62:55–59. doi: 10.1016/j.neuropsychologia.2014.07.010. [DOI] [PubMed] [Google Scholar]
- De Jong R., Liang C.C., Lauber E. Conditional and unconditional automaticity: a dual-process model of effects of spatial stimulus-response correspondence. J. Exp. Psychol. Hum. Percept. Perform. 1994;20:731–750. doi: 10.1037//0096-1523.20.4.731. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippel G., Beste C. A causal role of the right inferior frontal cortex in the strategies of multi-component behaviour. Nat Commun. 2015 doi: 10.1038/ncomms7587. [DOI] [PubMed] [Google Scholar]
- Döpfner M., Görtz-Dorten A., Lehmkuhl G. 2008. Diagnostik-System Für Psychische Störungen Im Kindes- Und Jugendalter Nach ICD-10 Und DSM-IV, DISYPS-II. Huber, Bern. [Google Scholar]
- Fallgatter A.J., Ehlis A.-C., Seifert J., Strik W.K., Scheuerpflug P., Zillessen K.E., Herrmann M.J., Warnke A. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin. Neurophysiol. 2004;115:973–981. doi: 10.1016/j.clinph.2003.11.036. [DOI] [PubMed] [Google Scholar]
- Fallgatter A.J., Ehlis A.-C., Rösler M., Strik W.K., Blocher D., Herrmann M.J. Diminished prefrontal brain function in adults with psychopathology in childhood related to attention deficit hyperactivity disorder. Psychiatry Res. 2005;138:157–169. doi: 10.1016/j.pscychresns.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Faraone S.V., Asherson P., Banaschewski T., Biederman J., Buitelaar J.K., Ramos-Quiroga J.A., Rohde L.A., Sonuga-Barke E.J.S., Tannock R., Franke B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- Folstein J.R., Van Petten C., Rose S.A. Novelty and conflict in the categorization of complex stimuli. Psychophysiology. 2008;45:467–479. doi: 10.1111/j.1469-8986.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen-Yaacovi G., Arazi A., Shahar N., Karmon A., Haar S., Meiran N., Dinstein I. Increased ongoing neural variability in ADHD. Cortex. 2016;81:50–63. doi: 10.1016/j.cortex.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Greenhill L.L., Posner K., Vaughan B.S., Kratochvil C.J. Attention deficit hyperactivity disorder in preschool children. Child Adolesc. Psychiatr. Clin. N. Am. 2008;17:347–366. doi: 10.1016/j.chc.2007.11.004. ix. [DOI] [PubMed] [Google Scholar]
- Huster R.J., Plis S.M., Calhoun V.D. Group-level component analyses of EEG: validation and evaluation. Front. Neurosci. 2015;9:254. doi: 10.3389/fnins.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffard M., Longcamp M., Velay J.-L., Anton J.-L., Roth M., Nazarian B., Boulinguez P. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage. 2008;42:1196–1206. doi: 10.1016/j.neuroimage.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Kayser J., Tenke C.E. On the benefits of using surface Laplacian (current source density) methodology in electrophysiology. Int. J. Psychophysiol. 2015;97:171–173. doi: 10.1016/j.ijpsycho.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keye D., Wilhelm O., Oberauer K., Stürmer B. Individual differences in response conflict adaptations. Front. Psychol. 2013;4:947. doi: 10.3389/fpsyg.2013.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieling R., Rohde L.A. ADHD in children and adults: diagnosis and prognosis. Curr. Top. Behav. Neurosci. 2012;9:1–16. doi: 10.1007/7854_2010_115. [DOI] [PubMed] [Google Scholar]
- Kornblum S., Hasbroucq T., Osman A. Dimensional overlap: cognitive basis for stimulus-response compatibility--a model and taxonomy. Psychol. Rev. 1990;97:253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Marco-Pallarés J., Grau C., Ruffini G. Combined ICA-LORETA analysis of mismatch negativity. Neuroimage. 2005;25:471–477. doi: 10.1016/j.neuroimage.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Marusich L.R., Gilden D.L. Assessing temporal integration spans in ADHD through apparent motion. Neuropsychology. 2014;28:585–593. doi: 10.1037/neu0000080. [DOI] [PubMed] [Google Scholar]
- Masson M.E.J. A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behav. Res. Methods. 2011;43:679–690. doi: 10.3758/s13428-010-0049-5. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Stock A.-K., Beste C. Psychophysiological mechanisms of interindividual differences in goal activation modes during action cascading. Cereb. Cortex. 2014;24:2120–2129. doi: 10.1093/cercor/bht066. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Stock A.-K., Dippel G., Chmielewski W., Beste C. Interacting sources of interference during sensorimotor integration processes. NeuroImage. 2016;125:342–349. doi: 10.1016/j.neuroimage.2015.09.075. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Chmielewski W., Ziemssen T., Beste C. The norepinephrine system shows information-content specific properties during cognitive control – evidence from EEG and pupillary responses. NeuroImage. 2017;149:44–52. doi: 10.1016/j.neuroimage.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Chmielewski W., Ziemssen T., Beste C. The norepinephrine system shows information-content specific properties during cognitive control – evidence from EEG and pupillary responses. NeuroImage. 2017;149:44–52. doi: 10.1016/j.neuroimage.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Mückschel M., Dippel G., Beste C. Distinguishing stimulus and response codes in theta oscillations in prefrontal areas during inhibitory control of automated responses. Hum. Brain Mapp. 2017;38:5681–5690. doi: 10.1002/hbm.23757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane J.C., Corkum P.V., Klein R.M., McLaughlin E. Interference control in children with and without ADHD: a systematic review of Flanker and Simon task performance. Child. Neuropsychol. 2009;15:321–342. doi: 10.1080/09297040802348028. [DOI] [PubMed] [Google Scholar]
- Nachev P., Rees G., Parton A., Kennard C., Husain M. Volition and conflict in human medial frontal cortex. Curr. Biol. 2005;15:122–128. doi: 10.1016/j.cub.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez P.L., Pilgreen K.L. The spline-laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J. Clin. Neurophysiol. 1991;8:397–413. [PubMed] [Google Scholar]
- Ouyang G., Herzmann G., Zhou C., Sommer W. Residue iteration decomposition (RIDE): a new method to separate ERP components on the basis of latency variability in single trials. Psychophysiology. 2011;48:1631–1647. doi: 10.1111/j.1469-8986.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Herzmann G., Zhou C., Sommer W. Residue iteration decomposition (RIDE): a new method to separate ERP components on the basis of latency variability in single trials. Psychophysiology. 2011;48:1631–1647. doi: 10.1111/j.1469-8986.2011.01269.x. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Schacht A., Zhou C., Sommer W. Overcoming limitations of the ERP method with residue iteration decomposition (RIDE): a demonstration in go/no-go experiments. Psychophysiology. 2013;50:253–265. doi: 10.1111/psyp.12004. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Sommer W., Zhou C. Updating and validating a new framework for restoring and analyzing latency-variable ERP components from single trials with residue iteration decomposition (RIDE) Psychophysiology. 2015;52:839–856. doi: 10.1111/psyp.12411. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Sommer W., Zhou C. a toolbox for residue iteration decomposition (RIDE)--a method for the decomposition, reconstruction, and single trial analysis of event related potentials. J. Neurosci. Methods. 2015;250:7–21. doi: 10.1016/j.jneumeth.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Sommer W., Zhou C. A toolbox for residue iteration decomposition (RIDE)--a method for the decomposition, reconstruction, and single trial analysis of event related potentials. J. Neurosci. Methods. 2015;250:7–21. doi: 10.1016/j.jneumeth.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Hildebrandt A., Sommer W., Zhou C. Exploiting the intra-subject latency variability from single-trial event-related potentials in the P3 time range: a review and comparative evaluation of methods. Neurosci. Biobehav. Rev. 2017;75:1–21. doi: 10.1016/j.neubiorev.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- Paul-Jordanov I., Bechtold M., Gawrilow C. Methylphenidate and if-then plans are comparable in modulating the P300 and increasing response inhibition in children with ADHD. Atten. Defic. Hyperact. Disord. 2010;2:115–126. doi: 10.1007/s12402-010-0028-9. [DOI] [PubMed] [Google Scholar]
- Pliszka S.R., Liotti M., Bailey B.Y., Perez R., 3rd, Glahn D., Semrud-Clikeman M. Electrophysiological effects of stimulant treatment on inhibitory control in children with attention-deficit/hyperactivity disorder. J. Child Adolesc. Psychopharmacol. 2007;17:356–366. doi: 10.1089/cap.2006.0081. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K.R. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol. Res. 2002;66:312–323. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Rubia K., Cubillo A., Woolley J., Brammer M.J., Smith A. Disorder-specific dysfunctions in patients with attention-deficit/hyperactivity disorder compared to patients with obsessive-compulsive disorder during interference inhibition and attention allocation. Hum. Brain Mapp. 2011;32:601–611. doi: 10.1002/hbm.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F.S., Walton M.E., Kennerley S.W., Bannerman D.M. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. (Regul. Ed.) 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sebastian A., Gerdes B., Feige B., Klöppel S., Lange T., Philipsen A., Tebartz van Elst L., Lieb K., Tüscher O. Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry Res. 2012;202:132–141. doi: 10.1016/j.pscychresns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Seifert J., Scheuerpflug P., Zillessen K.-E., Fallgatter A., Warnke A. Electrophysiological investigation of the effectiveness of methylphenidate in children with and without ADHD. J. Neural Transm. 2003;110:821–829. doi: 10.1007/s00702-003-0818-8. [DOI] [PubMed] [Google Scholar]
- Sekihara K., Sahani M., Nagarajan S.S. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. Neuroimage. 2005;25:1056–1067. doi: 10.1016/j.neuroimage.2004.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirrow C., McLoughlin G., Banaschewski T., Brandeis D., Kuntsi J., Asherson P. Normalisation of frontal theta activity following methylphenidate treatment in adult attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2015;25:85–94. doi: 10.1016/j.euroneuro.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Stock A.-K., Gohil K., Huster R.J., Beste C. On the effects of multimodal information integration in multitasking. Sci. Rep. 2017;7:4927. doi: 10.1038/s41598-017-04828-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpizio V., Lucci G., Berchicci M., Galati G., Pitzalis S., Di Russo F. Hemispheric asymmetries in the transition from action preparation to execution. Neuroimage. 2017;148:390–402. doi: 10.1016/j.neuroimage.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Szmalec A., Verbruggen F., Vandierendonck A., De Baene W., Verguts T., Notebaert W. Stimulus ambiguity elicits response conflict. Neurosci. Lett. 2008;435:158–162. doi: 10.1016/j.neulet.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Thomas R., Sanders S., Doust J., Beller E., Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135:e994–e1001. doi: 10.1542/peds.2014-3482. [DOI] [PubMed] [Google Scholar]
- Verleger R., Siller B., Ouyang G., Śmigasiewicz K. Effects on P3 of spreading targets and response prompts apart. Biol. Psychol. 2017;126:1–11. doi: 10.1016/j.biopsycho.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Fowler J.S., Gatley S.J., Logan J., Ding Y.S., Dewey S.L., Hitzemann R., Gifford A.N., Pappas N.R. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high.”. J. Pharmacol. Exp. Ther. 1999;288:14–20. [PubMed] [Google Scholar]
- Wagenmakers E.-J., Brown S. On the linear relation between the mean and the standard deviation of a response time distribution. Psychol. Rev. 2007;114:830–841. doi: 10.1037/0033-295X.114.3.830. [DOI] [PubMed] [Google Scholar]
- Warren C.M., Holroyd C.B. The impact of deliberative strategy dissociates ERP components related to conflict processing vs. reinforcement learning. Front. Neurosci. 2012;6:43. doi: 10.3389/fnins.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren C.M., Tanaka J.W., Holroyd C.B. What can topology changes in the oddball N2 reveal about underlying processes? Neuroreport. 2011;22:870–874. doi: 10.1097/WNR.0b013e32834bbe1f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.