Highlights
-
•
Orienting to unexpected novel events is reflected in the novlety P3 in all age groups.
-
•
Repeated familiar stimuli elicited reduced novlety P3 amplitude in all age groups.
-
•
Repeated unfamiliar stimuli elicited reduced novelty P3 amplitude only in children.
-
•
Recognition Old/New ERP effect for unfamilar stimuli is seen only in children.
-
•
Children may rely more on visual perception to process unfamiliar stimuli.
Keywords: Orienting, ERP, Development, Oddball, Novel, Memory
Abstract
For children, new experiences occur very often, and learning to differentiate between old and new events is a fundamental process necessary for appropriate reactions to stimuli. Thus the present study is concerned with maturation of brain responses to repeated novel events. We examined the effect of repetition of familiar (meaningful) and unfamiliar (meaningless) symbols on the event-related-potentials (ERPs) recorded during novelty oddball and recognition memory tasks from children, adolescents and young adults. During the novelty oddball task, repetition of the familiar symbols elicited a reduction in the novelty P3 in the ERPs of all age groups, while repetition of the unfamiliar symbols elicited a reduction in novelty P3 amplitude only in children. As expected, recognition memory performance improved with age and was better for familiar than unfamiliar symbols. For all age groups, ERPs to correctly recognized familiar old symbols elicited a larger positivity than ERPs to correctly identified new symbols, indicating a reliable memory effect. However, ERPs to unfamiliar old and new symbols did not differ in adults and adolescents but did differ in children. The data suggest that children process familiar visual symbols in a similar fashion to that of adults, and that children process unfamiliar symbols differently from adults.
1. Introduction
Orienting to a novel event is a rapid shift in attention to a change in one’s surroundings that appears to be a fundamental biological mechanism for survival and essentially functions as a "what is it" detector. Orienting appears to play a central role in human learning and development, as it facilitates adaptation to an ever-changing environment (Sokolov, 1963). Infants’ mental growth depends on their tendency to orient to unfamiliar stimuli, such as a sudden onset of a female voice (Kagan, 1994). While orienting responses measured in infants is sensitive to different variables than those observed in children and adults, it has been suggested that orienting, which is part of the attention system, may promote cognitive functioning throughout life. In this respect, orienting can be viewed as an allocational mechanism in which attention sifts through the complex multi-sensory world and selects relevant stimuli for further processing. The selection of stimuli for further processing has implications for what will be encoded into memories and how strong those memory traces will be. The ability to differentiate between relevant and irrelevant input, to inhibit the processing of irrelevant stimuli, and to sustain attention requires control, self-regulatory and inhibitory processes that improve with age (Kopp, 2002). Therefore, studying orienting behavior in children has implications for our understanding of the overall development of the attention mechanisms that are essential in all cognitive operations. Despite a large body of knowledge regarding the orienting response in infants (e.g., Clarkson et al., 1989; Marshall et al., 2009; Moffitt, 1973), orienting during childhood has not yet been extensively explored. Thus, the current study was designed to investigate changes with age in the orienting response and the formation of memories to unexpected events.
1.1. Orienting to novel events
Orienting elicits physiological and behavioral changes, but not all sensory stimuli elicit orienting to the same extent (Sokolov, 1990). For example, the orienting response diminishes with stimulus repetition, indicating that some form of memory exists for events that shape the response to repeated incidences. It has been proposed (Sokolov, 1969) that novel stimuli elicit processes that enable the construction of representations for these novel events. As stimulus exposure continues and a representation is formed, the constructed representation is then continuously compared to incoming information. Habituation occurs as the representation increasingly matches the external stimulus.
The orienting response can be measured physiologically by measuring scalp-recorded event related brain potentials (ERPs). Measuring ERPs is well suited to investigate the processing of unexpected novel events because the neurophysiological responses that correspond to these events can be recorded in the absence of overt responses. Indeed, ERPs have been used to examine orienting in infants (Kushnerenko et al., 2007; Wiebe et al., 2006), children (Cycowicz and Friedman, 1997; Cycowicz et al., 1996; Maatta et al., 2005a, Maatta et al., 2005b) and the elderly (Daffner et al., 2006; Kazmerski and Friedman, 1995; Richardson et al., 2011).
The orienting response is often investigated using ERP recordings by employing a novelty oddball paradigm. In this task, frequently occurring stimuli are randomly presented with two infrequently occurring stimulus types: target (oddball) and novel. Participants are informed about the occurrences of the frequent stimuli, and are instructed only to respond to the infrequently occurring targets. Participants are not informed prior to the experiment of the occurrence of infrequent novel events. Both target and novel stimuli elicit a positive deflection in the ERP waveforms at around 300 ms post stimulus onset. These responses are known as the P3, and in adults, differ in their scalp distribution, with the target P3 usually being characterized by a parietally-focused amplitude maximum (see for example, Johnson, 1993), whereas the novelty P3 scalp topography is frontally oriented (Courchesne et al., 1975). It has been suggested that the parietally-oriented P3 elicited by targets is associated with stimulus evaluation processes (e.g., Courchesne et al., 1975; Grillon et al., 1990), while the frontally-oriented P3, elicited by the novel stimuli, reflects an involuntary shift of attention, i.e., orienting (Friedman et al., 2001).
Habituation of orienting responses with repetition or recurrence of both visual (Courchesne, 1978a, Courchesne, 1978b) and auditory (e.g., Friedman et al., 1993a, Friedman et al., 1993b; Friedman and Simpson, 1994) novel events has been demonstrated in ERP studies by a reduction in novelty P3 amplitude and a change in its scalp topography to a more posterior focus. The change in scalp topography of the novelty P3 with repetition indicates that successive presentations of novel events alter the processing of those events. Initially uncategorized novel stimuli form a distinct class of rare events, thus eliciting brain activity with a parietal topography resembling that of target events.
Previous investigation of the orienting response in children using ERPs yielded inconsistent results. Courchesne (Courchesne, 1978a, Courchesne, 1978b) who used visual stimuli reported that the infrequent novel events elicited waveforms with different morphology and scalp distribution in children compared to adults. Courchesne proposed that this age-related difference in the ERPs implies that children and adults process infrequent novel information differently, and suggested that the neurocognitive system that is engaged in processing novel events is less mature in children. However, in a similar paradigm with repeated environmental sounds as novel events, Cycowicz et al. (1996) found only quantitative age differences (e.g., latency) and suggested that overall children process novel information in a manner similar to adults. Age-related difference was also reported by Gumenyuk et al. (2004) in a more complex novelty paradigm, where novel sounds served as distracted stimuli during a visual task, and suggested that the changes in the development of the orienting system relates to the development of the attention switching functions in children. Another variable that could explain the difference findings is the stimulus familiarity. For example, the visual novels used by Courchesne, 1978a, Courchesne, 1978b were meaningless and therefore difficult to name; in contrast the complex environmental sounds used by Cycowicz et al varied in their degree of familiarity. Thus, it seems that task demands, stimulus modality and type of stimuli all may affect processing of novel information (Oja et al., 2016).
The idea that stimulus familiarity affects the orienting response, that can be measured in the ERPs, comes from an investigation of the orienting response in adults (Cycowicz and Friedman, 1998). In that study, following the novelty oddball task where each novel event was repeated, each participant was asked to listen carefully to each of the sounds and to name them. Sounds that were named were labeled familiar, whereas those that were not named were labeled unfamiliar, and the ERPs were averaged based on each subject naming responses. Cycowicz and Friedman reported that the effect of sound familiarity emerged only after repetition, as there was no difference in novelty P3 amplitude between familiar and unfamiliar sounds on first presentation. This result implies that on first presentation both familiar and unfamiliar sounds elicited an orienting response with similar activation of frontal lobe generators. However, repetition of the familiar novel sounds elicited a significant reduction of novelty P3 amplitude over fronto-central scalp sites, suggesting that stimuli with pre-existing representations required less processing on second presentation. By contrast, repetition of unfamiliar sounds induced an increase in amplitude, primarily at posterior scalp locations, reflecting a greater processing load due to the relative unfamiliarity of the eliciting sounds.
Therefore, one goal of the current study was to investigate the effect of stimulus familiarity on the orienting response and its changes with age. To this end we used two distinct types of visual symbols. One type consisted of meaningful symbols that can be named, and the second type consisted of meaningless symbols that cannot be named. The meaningful symbols were known to most adult participants from previous exposure and therefore presumably familiar, whereas the meaningless symbols were unknown to most adults and therefore labeled unfamiliar. Both types of symbols were unexpected within the context of the experiments, and thus both were considered to be novel. We hypothesized that the visual novelty P3 in adults would show reduced amplitude for the familiar but not for the unfamiliar symbols. However, since children have less experience both with the familiar and the unfamiliar symbols, they would not show novelty P3 amplitude reduction for either stimulus type.
1.2. Memory of novel events
The detection of novel events is strongly related to memory, as it requires a comparison between incoming events and memory representations. If the incoming event matches a memory trace the stimulus is considered recognizable, otherwise, it is identified as new. Unfamiliar events are presumed to trigger learning and memory processes via attentional resources (Malcuit et al., 1996). Thus, research measuring learning and memory in infants often includes a comparison of the orienting response to the novel/new stimuli to that of familiar/old stimuli (Bahrick and Pickens, 1995; Rovee-Collier and Cuevas, 2009). In adults, unexpected events within the context of an experiment tend to be encoded in memory more effectively than non-distinct events (for review see (Ranganath and Rainer, 2003). For example, P3 amplitude elicited by highly distinctive items correlated with subsequent memory performance for those items (Fabiani and Donchin, 1995), and larger P3 amplitude for subsequently recalled words were reported for 11-year old children (Fabiani et al., 1990). The relationship between unexpected events and memory for these events can also be inferred from a lesion study suggesting that interactions between prefrontal and medial temporal regions are crucial for the generation of enhanced memory for contextually novel events (Parker et al., 1998). Additional support for these findings are provided by hemodynamic data demonstrating a relation between activation of prefrontal regions that are critical for evaluation of novel events and medial temporal cortical regions that are implicated in memory (Harper et al., 2017; Kirchhoff et al., 2000).
Knowledge of maturational changes in brain areas that might underlie developmental stages associated with orienting and memory processes has expanded in recent years (e.g., Benes, 1989, 1994; Walhovd et al., 2017), and include increase in myelination, synaptic pruning, and increase subcortical gray matter and areas of the limbic system volume (including the hippocampus and amygdala) (Huttenlocher, 1990; Jernigan and Sowell, 1997). However, the longest developmental trajectory is seen in the frontal cortex, which develops gradually throughout childhood and adolescence (Caballero et al., 2016; Case, 1992; Stuss, 1992). Because the relationship between detection of contextual novelty and memory for novel events depends on brain structures that mature throughout childhood, the current investigation included measures of children’s memory for these events. While all novel events are distinct within the context of the task, the current study assessed whether familiar and unfamiliar novel events are incidentally remembered to the same extent, and how their memorability compares across age groups. For this, ERPs were recorded while participants performed an unexpected recognition memory task for the novel events they viewed during the novelty oddball task.
In a variety of recognition memory tasks in which participants are explicitly asked to differentiate old stimuli from new stimuli, more positive-going amplitudes for old compared to new items have been demonstrated beginning at about 300 ms and often continuing to the end of the recording epoch. Collectively, these old/new effects have been labeled the “episodic memory” or EM effect (Curran et al., 2003; Friedman and Johnson, 2000). The exact number and functional significance of these EM effects is still under debate, but some EM effects do appear consistently with distinct temporal and topographic patterns in many memory studies. Relevant to the current study is the parietal EM effect that is assumed to reflect recollection processes. There is evidence that recollection from long term memory elicits parietal EM effects in children similar to those recorded in adults (Cycowicz, 2000; Cycowicz et al., 2003; Czernochowski et al., 2005).
The majority of studies demonstrating a parietal EM effect have used familiar and meaningful stimuli such as words and pictures, and only a handful of studies have demonstrated it for unfamiliar stimuli (e.g., Van Petten and Senkfor, 1996). Cycowicz and Friedman (1999) recorded ERPs in adults during their performance on a recognition memory task for auditory novel events initially presented during the novelty oddball task. Although memory for all novel sounds was poor, previously experienced sounds elicited faster reaction times than new sounds, and a robust parietal EM effect. The poor memory for the novel sounds was probably due to their brief nature and the fact that they were difficult to name. Nevertheless, these data demonstrated the formation of long-term memories of initially novel sounds. Based on these findings familiar novel symbols which are meaningful to subjects should be more likely to be remembered and should elicit the parietal EM effect, whereas unfamiliar symbols should be less likely to be remembered and should not be associated with the parietal EM effect.
The fact that children may not be as well acquainted with the familiar symbols as adults brings into question to what extent children’s memory of the familiar symbols will resemble that of adults. Based on the assumption that children need to learn many new facts about their environment in a relatively short time, they may be expected to have a system in place that differentiates between the new and the experienced even for stimuli for which they lack semantic knowledge. It is possible that while adults’ memory is based on meaning, children will remember the familiar symbols perceptually. In this scenario, children’ ERPs during the recognition memory task should be similar to that of the adults. Alternatively, if children perceived the familiar symbols as meaningless because they cannot attach meaning to them, then similar to the difficulty with the unfamiliar symbols, they will not be able to distinguish between old and new familiar symbols. Hence, their ERPs for both familiar and unfamiliar symbols will fail to show the parietal EM effect.
1.3. Aims of current study
To summarize, the goals of the current investigation were twofold. First, we examined the orienting response, as reflected by the novelty P3, to repeated familiar and unfamiliar novel visual symbols in adults, adolescents, and children. To understand the brain mechanisms involved in the encoding and subsequent remembering of those unexpected events, brain responses to the first and second presentations of visual symbols were contrasted for familiar and unfamiliar stimuli as a function of age. Second, an unexpected symbol recognition memory test was administrated following the novelty oddball task in order to examine any evidence for a parietal EM effect in all age groups, which would provide evidence of recollection of familiar and unfamiliar symbols.
2. Method
2.1. Subjects
Thirty-two young adults (age range: 20–28; mean age = 23.15; 22 women), 27 adolescents (age range: 12–14; mean age 12.95; 8 girls) and 28 children (age range: 9–11; mean age 10.09; 8 girls) participated in the current study. Half of the subjects within each age group were assigned to view either familiar or unfamiliar symbols as described below. There were no demographic differences between subjects assigned to the two experimental conditions in the three age groups. Four adolescents (1 female) and one child (female) did not complete the experiment, resulting in an uneven number of subjects who were assigned to each experimental condition. Volunteers were native English speakers, with no history of head trauma, neurological disorders, learning disabilities or hyperactivity. The study was reviewed and approved by the New York State Psychiatric Institute Institutional Review Board. Informed consent was obtained from adult participants and parents of all children. Additionally, children and adolescents signed assent forms.
2.2. Stimuli
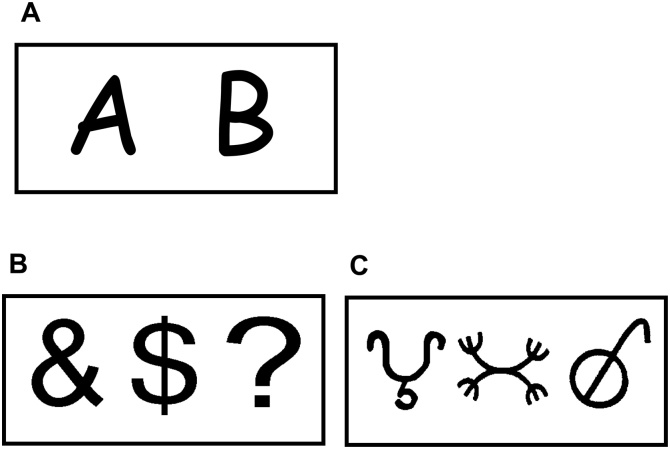
The letters A and B each served as a frequent and/or target stimuli equally often as they were counterbalanced across subjects. The novel stimuli included 72 familiar and 72 unfamiliar (total of 144) symbols collected from a Dictionary of Symbols (Liungman, 1994); for examples see Fig. 1. For the complete list of the familiar and the unfamiliar stimuli, as well as the list selection criteria, see Supplemental Material and Cycowicz and Friedman, 2007.
Fig. 1.
Examples of: A. frequent and target stimuli (letters assignment rotated across subjects); B. example of familiar and meaningful symbols; C. example of unfamiliar and meaningless symbols employed in the tasks.
2.3. Procedure
The experimental design, which includes several tasks performed by participants in all age groups, is presented in Table 1. The experiment included two stimulus conditions, familiar and unfamiliar novel symbols, that were a between subject factor. Because the familiar symbols are assumed to exist in the subject’s long-term semantic memory, their novelty is limited to the experimental context. In contrast, the unfamiliar symbols are assumed not to exist in long-term, semantic memory, and therefore their novelty is within the context of the experiment as well as the subject’s general experience. Participants performed all tasks in a quiet room (see Supplemental material for more detail), and were instructed to respond as quickly as possible. Stimulus and hand of response were counterbalanced across participants within each age group.
Table 1.
Summary of the Experimental Design.
| Age Groups (N) |
Stimulus Conditions | Tasks | Stimulus | Type | ||
|---|---|---|---|---|---|---|
| Adult | Teen | Child | ||||
| 16 | 10 | 13 | Familiar | Visual Oddball | Frequent, Target | Letters A, B |
| Novelty Visual Oddball | Frequent, Target | Letters A, B | ||||
| Novel 1, Novel 2 | Familiar symbols | |||||
| Recognition Memory | Old and New Novels | Familiar symbols | ||||
| Symbol Naming | Novels | Familiar symbols | ||||
| 16 | 13 | 14 | Unfamiliar | Visual Oddball | Frequent, Target | Letters A, B |
| Novelty Visual Oddball | Frequent, Target | Letters A, B | ||||
| Novel 1, Novel 2 | Unfamiliar symbols | |||||
| Recognition Memory | Old and New Novels | Unfamiliar symbols | ||||
2.3.1. Visual oddball task
Subjects were presented with two blocks with 100 trials each and were asked to press a button when they detected a rare letter (targets; P = 0.12) embedded in a series of frequent letters (frequents; P = 0.88). Stimulus duration was 300 ms with inter-stimulus-intervals (ISI) of 1200 ms.
2.3.2. Novelty visual oddball task
Following the visual oddball test subjects were presented with seven blocks of 100 trials each. As in the visual oddball task, subjects were instructed to press a button when they detect the targets (the same rare letter as in the visual oddball task) among the frequent events (the same frequent letter as in the visual oddball task). They were not informed nor instructed about the occurrence of the novel events. The novel stimuli (P = 0.12), which were 42 visual symbols, were randomly intermixed with frequent (P = 0.76) and target (P = 0.12) letters (see more details in the supplemental material). Repetition of the novel stimuli occurred two blocks after their initial presentation, such that the novel stimuli initially presented in the first block, for example, were repeated in the third block. Stimuli were randomized separately for each subject, with the restrictions that a target or a novel could not occur as the first or last stimulus and that two targets or novels could not be presented sequentially. Stimulus duration was 300 ms with inter-stimulus-intervals (ISI) of 1200 ms.
2.3.3. Symbol recognition memory task
At the end of the visual novelty oddball task subjects were asked to perform an old/new recognition memory task. A total of 60 symbols were presented, of which 30 had been seen twice during the visual novelty oddball test, and 30 were "new." Each subject saw one symbol at a time and had to press one button if s/he thought the symbol was "old," and the another button if s/he thought the symbol was "new." Each subject received a different random ordering of the stimuli. Instructions emphasized speed and accuracy equally. The hands assigned to "old" and "new" responses were counterbalanced across subjects. Each stimulus was presented for 300 ms, and the ISI was 1800 ms.
2.3.4. Familiar symbol naming task
The familiar symbols were defined as familiar based on previous adults’ ratings (Cycowicz and Friedman, 2007). To assess children’s and adolescents’ familiarity with these familiar symbols, subjects in all age groups who viewed the familiar symbols received an additional symbol naming task. After subjects completed the symbol recognition memory task they were presented with a different random ordering of the familiar symbols one at a time. Subjects were instructed to name each symbol as accurately as possible, and to state ‘I do not know’ if they were unable to identify a symbol. The experimenter transcribed verbatim each response before initiating the presentation of the next symbol. Several of the adolescents (3) and the children (2) did not complete this task.
2.4. EEG recordings
EEG (5s time constant; 50 Hz upper cutoff; 200 Hz digitization rate) was recorded continuously (Sensorium amplifiers; from extended 10–20 system placements; e.g., (Nuwer et al., 1994) using an Electrocap (Electrocap International) from 62 scalp sites, including left and right mastoids, all referred to nosetip, with electrode impedance < 10 kΩ. Vertical EOG was recorded bipolarly from electrodes placed on the supraorbital and infraorbital ridges of the right eye, and horizontal EOG was recorded bipolarly from electrodes placed on the outer canthi of the two eyes. Trials were epoched off-line with 100 ms pre and 1100 ms post-stimulus periods for the oddball tasks and 100 ms pre- and 1700 ms post-stimulus periods for the recognition task. Trials containing eye movement artifact were corrected off-line using the procedure developed by Gratton (Gratton et al., 1983). In addition, single trials were visually inspected, and trials containing muscular or other artifacts were marked and excluded from further analysis.
2.5. Data analyses
ERP data were averaged for frequent, target and novel stimuli in both oddball tasks, and to correctly recognized old and correctly rejected new symbols during the recognition test. Averaged voltages were computed separately for the first (novel 1) and second (i.e., repeated, novel 2) presentation of the novel symbols. Averaged voltages for the ERP components (novelty P3 and Old/New EM effect) were calculated within time intervals defined on the basis of visual inspection of each group’s data. In all ERP analyses the Electrode factor included the midline electrodes (FPz, Fz, FCz, Cz, CPz, Pz, POz, Oz) which represent well the differences in activation over the anterior-posterior plane.
ANOVA were performed using the SPSS Program (V.11). The Greenhouse-Geisser epsilon correction (Jennings & Wood, 1976) was used where appropriate. Uncorrected degrees of freedom are reported below along with the epsilon value (ε); the P values reflect the epsilon correction. Where appropriate, significant main effects and interactions were followed-up with simple effects procedures and/or post-hoc analyses using the Tukey Honestly Significant Difference (HSD) test.
3. Results
3.1. Visual oddball and novelty visual oddball tasks
3.1.1. Behavioral performance
Table 2 shows that subjects in both familiar and unfamiliar stimulus conditions and age groups were very accurate in detecting the targets (% Hit) for both the visual oddball and novelty visual oddball tasks, and very occasionally made false alarms to frequents (% FA-F) and novels (% FA-N). Mean reaction times (RTs) for targets were submitted to a mixed design ANOVA with Age Group (Children, Adolescents, Adults) and Stimulus Condition (Familiar, Unfamiliar) as between-subject factors and Task (Visual, Novelty) as a within-subject factor. The main effect of Age Group (F2,78 = 11.68, p < 0.01) indicated, as assessed by post-hoc tests, that children were slower than adults and adolescents (ps<0.05), whereas the RTs of adolescents and adults did not differ. The main effect of Task (F1,78 = 69.19, p < 0.01) showed that RTs to targets were significantly longer in the novelty than in the visual oddball task. ANOVAs with Age group and Stimulus Condition as between-subject factors were computed separately for false alarms (FA) to: a) frequents during the visual oddball task, and to b) frequents during the novelty oddball task, and to c) novels during the novelty oddball task. Significant Age Group effects were found for FA to frequents during the visual oddball task (F2,78 = 5.46, p < 0.01) and to frequents during the novelty oddball task (F2,78 = 5.06, p < 0.01). Tukey HSD tests revealed that children made more FAs than adults during both tasks, whereas adolescents and adults did not differ. None of the ANOVAs indicated any performance difference between subjects assigned to the familiar and unfamiliar stimulus conditions.
Table 2.
Performance in the Visual Oddball and the Novelty Visual Oddball tasks: Mean (±SE) Reaction Time (RT) for Targets, Percent Correct Target Detection (Hit), Percent False Alarms for Frequents (FA-F), and for Novels (FA-N), for each age group.
| Performance |
||||||
|---|---|---|---|---|---|---|
| Group | Task | Stimulus Condition | RT (ms) | Hit (%) | FA-F (%) | FA-N (%) |
| Adults | Visual Oddball | Familiar | 421 (17) | 100 | 0.11 | |
| Unfamiliar | 395 (12) | 100 | 0.08 | |||
| Novelty Visual Oddball | Familiar | 442 (17) | 100 | 0.08 | 0.30 | |
| Unfamiliar | 413 (12) | 100 | 0.06 | 0.60 | ||
| Adolescents | Visual Oddball | Familiar | 438 (12) | 100 | 0.29 | |
| Unfamiliar | 426 (16) | 100 | 0.41 | |||
| Novelty Visual Oddball | Familiar | 474 (17) | 100 | 0.16 | 0.96 | |
| Unfamiliar | 455 (18) | 100 | 0.21 | 1.03 | ||
| Children | Visual Oddball | Familiar | 468 (20) | 100 | 0.61 | |
| Unfamiliar | 484 (16) | 100 | 0.86 | |||
| Novelty Visual Oddball | Familiar | 516 (23) | 100 | 0.32 | 1.29 | |
| Unfamiliar | 512 (21) | 100 | 0.19 | 1.03 | ||
3.1.2. ERP responses
Since the visual oddball task does not include novels and in that respect does not contribute to the aims of this paper, the data which demonstrated typical oddball ERP findings during the visual oddball task will not be presented.
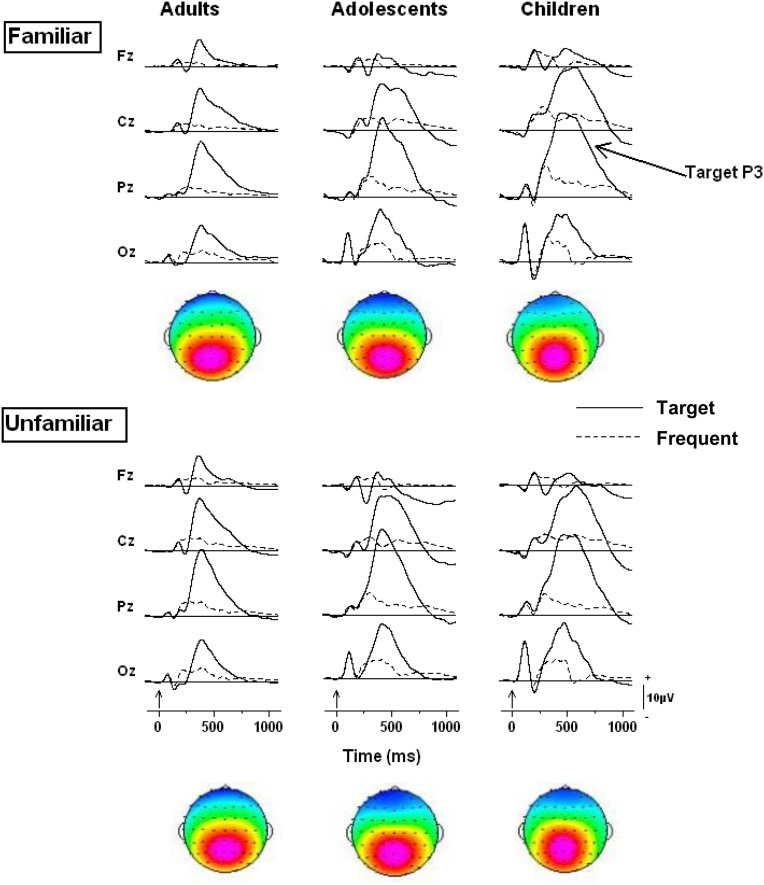
Grand mean waveforms elicited by targets and frequents during the novelty oddball task for subjects assigned to the familiar and the unfamiliar stimulus conditions in the three age groups are presented in Fig. 2. Parietally-maximal target P3s are clearly seen for all age groups in both the familiar and unfamiliar stimulus conditions. These target P3s appear highly similar for subjects assigned to the two experimental conditions. This indicates that target detection processing was similar for subjects assigned to familiar and unfamiliar conditions. Aside from the typical amplitude differences among age groups (Cycowicz, 2000), the target P3s appear similar to those already reported in previous developmental studies and, therefore, no further analysis will be performed on these data.
Fig. 2.
A. Grand mean waveforms at four midline scalp locations for targets and frequents in the novelty oddball tasks for subjects assigned to the familiar and unfamiliar stimulus conditions. Solid lines represent targets, dashed lines represent frequents. Vertical arrows on the time-line represent stimulus onset.
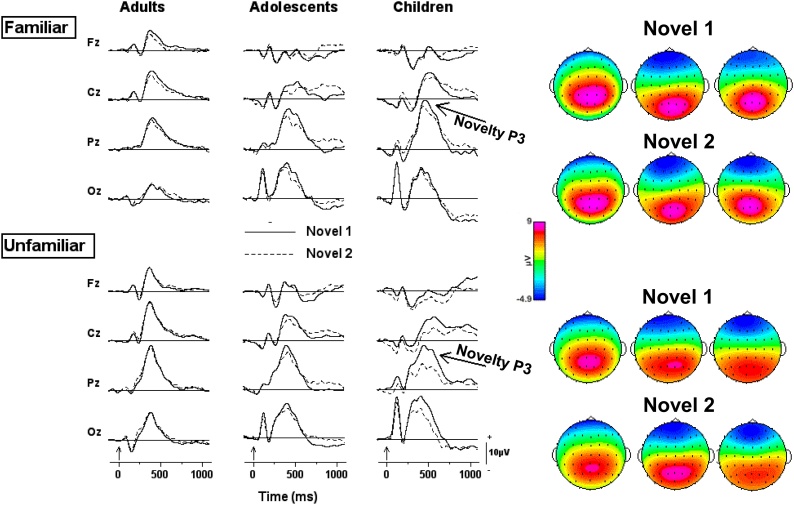
The ERPs elicited by first and second presentations of familiar and unfamiliar novel symbols are depicted in Fig. 3 for each age group. It is notable that compared with auditory novel stimuli which elicit fronto-parietal P3s scalp distribution (e.g., Cycowicz et al., 1996; Fabiani and Friedman, 1995) the novel visual stimuli elicited a more posterior scalp distribution in all age groups, with the children showing the most posterior scalp distribution. For the adults, an amplitude reduction of the novelty P3 at fronto-central sites is seen for second presentations of novel items only in the familiar stimulus condition. The waveforms elicited by first and second unfamiliar novel presentations overlap completely. The adolescents show results similar to those of the adults; there is an amplitude reduction of the novelty P3 for subjects in the familiar stimulus condition, although at somewhat more posterior locations than that of the young adults, with no difference between first and second presentations for subjects in the unfamiliar stimulus condition. By contrast, the children’s waveforms show a large amplitude reduction with novel repetition for the unfamiliar stimulus condition, and a small amount of amplitude reduction (with repetition) for the familiar stimulus condition.
Fig. 3.
Grand mean waveforms for first and second presentations of the novel symbols at four midline scalp locations during the visual novelty oddball task. The waveforms are depicted for subjects assigned to familiar and unfamiliar stimulus conditions for each age group. Vertical arrows on the time-line represent stimulus onset.
To capture the novelty P3, whose peak latency differed in the three age groups, averaged voltage data were measured between 320 and 500 ms for the adults, 370 and 630 ms for the adolescents, and 380 and 680 ms for the children. To determine if repetition influenced the ERP indices, averaged voltages were submitted to a mixed design ANOVA with Age Group (Children, Adolescents, Adults) and Stimulus Condition (Familiar, Unfamiliar) as between-subject factors, and Presentation (novel 1, novel 2) and Electrode as within-subject factors. A main effect of Presentation (F1,76 = 8.04, p < 0.01) indicated that novelty P3 amplitude was reduced with repetition. However, this effect was modulated by the interaction of Age Group, Stimulus Condition and Presentation (F2,76 = 3.92, p < 0.02). Table 3 presents the averaged voltage data for this triple interaction. To further explore this interaction, separate ANOVAs were performed for subjects assigned to each stimulus condition, with Age Group as a between-subjects factor and Presentation and Electrode as within-subject factors. For the familiar stimulus condition, a main effect of Presentation (F1,36 = 4.25, p < 0.05) again indicated that novelty P3 amplitude was reduced with repetition. There was no interaction with age group, which indicates that the reduction in novelty P3 amplitude was similar across all age groups. For the unfamiliar stimulus condition, the main effect of Presentation (F1,40 = 4.04, p < 0.05) was moderated by Age Group as seen in the significant interaction (F2,40 = 4.66, p < 0.05). Post-hoc testing revealed that reduction in novelty P3 amplitude with repetition in the unfamiliar stimulus condition was reliable for the children but not the adolescents and adults.
Table 3.
Averaged Voltages in μV (±SE) of the Novelty P3 for First (Novel 1) and Second (Novel 2) Presentations for the Two Conditions and the Three Age Groups.
| Age Groups |
||||
|---|---|---|---|---|
| Stimulus Condition | Novel | Adults | Adolescents | Children |
| Familiar | 1 | 8.78 (1.46) | 7.27 (1.85) | 7.62 (1.62) |
| 2 | 7.19 (1.45) | 5.24 (1.83) | 6.94 (1.61) | |
| Unfamiliar | 1 | 11.45 (1.46) | 7.80 (1.62) | 8.28 (1.60) |
| 2 | 11.27 (1.45) | 8.23 (1.61) | 3.20 (1.55) | |
3.2. Symbol recognition memory task
3.2.1. Behavioral performance
Table 4 presents the mean correct RTs and accuracy data during the recognition memory tasks for the three age groups in both stimulus conditions. In a mixed design ANOVA with Age Group (Children, Adolescents, and Adults) and Stimulus Condition (Familiar, Unfamiliar) as between-subject factors and Item (New, Old) as a within-subjects factor, mean RTs did not differ among the age groups or stimulus conditions. However, the main effect of Item (F1,78 = 17.07, p < 0.01) indicated faster RTs to old compared to new symbols.
Table 4.
Performance in the Symbol Recognition Memory task: Mean (±SE) Reaction Time (mRT), Percent Correct Old (Hit), Percent False Alarms for New (FA), and Discrimination (dL) and Bias (cL) measures, for each stimulus condition and group.
| Mean RT (ms) |
Performance Accuracy |
||||||
|---|---|---|---|---|---|---|---|
| Group | Stimulus Condition | New | Old | Hit | FA | dL | CL |
| Adults | Familiar | 708 (31) | 681 (33) | 0.78 | 0.16 | 3.32 | 0.32 |
| Unfamiliar | 739 (20) | 710 (22) | 0.67 | 0.25 | 1.93 | 0.22 | |
| Adolescents | Familiar | 696 (37) | 682 (35) | 0.79 | 0.21 | 3.01 | −0.05 |
| Unfamiliar | 675 (34) | 633 (40) | 0.62 | 0.35 | 1.21 | 0.04 | |
| Children | Familiar | 810 (26) | 717 (38) | 0.79 | 0.29 | 2.56 | −0.16 |
| Unfamiliar | 678 (57) | 656 (53) | 0.54 | 0.34 | 1.08 | 0.33 | |
ANOVAs with Age Group and Stimulus Condition as between-subject factors were computed separately for percentage Hits, FA, discrimination (dL), and response bias (cL) measures. These latter two indices, dL and cL, were obtained according to the procedures described by Snodgrass & Corwin (Snodgrass and Corwin, 1988). A Stimulus Condition main effect for percentage Hits (F1,78 = 35.75, p < 0.01) indicated that in all age groups familiar symbols were recognized at a higher rate than unfamiliar symbols. For the discrimination measure (dL), a main effect of Age Group was found (F2,78 = 3.22, p < 0.05). Tukey HSD testing revealed that children performed more poorly than adults, with no difference between adolescents and adults. The Stimulus Condition main effect (F1,78 = 32.46, p < 0.01) for the discrimination measure indicated that all age groups performed more poorly with unfamiliar compared to familiar stimuli. Similarly, the main effect of Stimulus Condition (F1,78 = 9.21, p < 0.01) indicated that there were more FA responses to unfamiliar than familiar stimuli. The bias measure cL did not differ among groups or stimulus conditions. The main effect of Age Group (F2,78 = 4.8, p < 0.01), followed by Tukey HSD testing, showed that children had more FA than adults, but adolescents did not differ from adults.
3.2.2. ERP responses
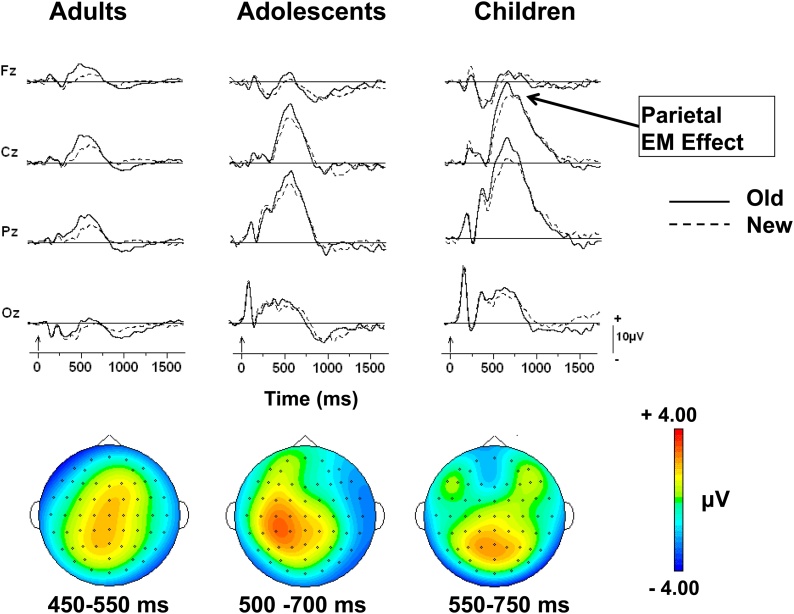
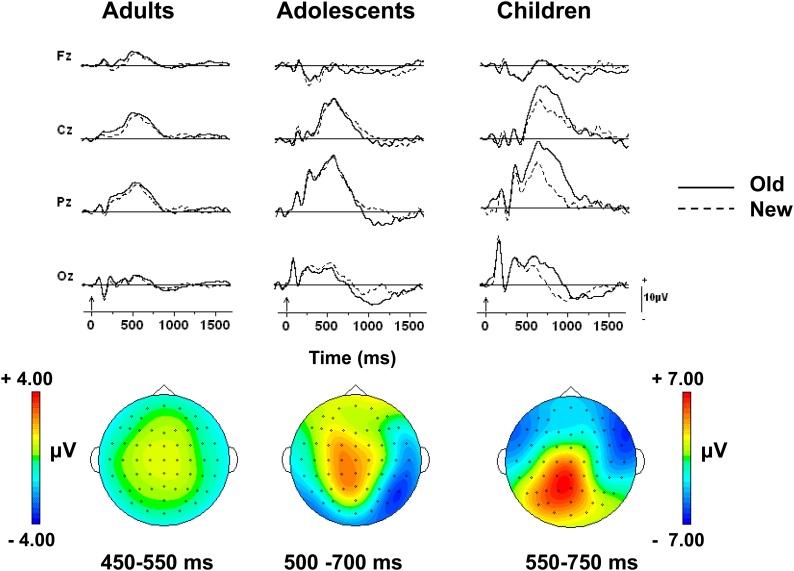
The grand mean waveforms elicited by correctly recognized old and correctly rejected new items for the three age groups are presented in Fig. 4 for the familiar symbols. It is clear that larger positive amplitudes are elicited by old compared to new items for all age groups; i.e., that the data are indicative of robust episodic memory (EM) or old/new effects. Based on the scalp topography presented in Fig. 4, it seems likely that this EM effect is synonymous with the parietal EM presumed to reflect recollection.
Fig. 4.
Grand mean waveforms for correctly recognized old and correctly rejected new familiar symbols at four midline scalp locations during the symbol recognition memory task. The waveforms are depicted for each age group. Vertical arrows on the time-line represent stimulus onset. Scalp topographies are based on the old minus new difference waveforms and are depicted for the parietal EM effects.
To determine if there were developmental differences in the parietal EM effect, averaged voltages were submitted to a mixed design ANOVA with Age Group (Children, Adolescents, Adults) as between-subjects factor, and Item (New, Old) and Electrode as within-subject factors. To capture the parietal EM effect, which differed in latency among age groups, averaged voltages were measured in three-100 ms intervals between 300 and 600 ms for adults, between 400 and 700 ms for adolescents, and 450 and 750 ms for children. Main effects of Item for the three-100 ms intervals (F1,36 = 10.24, p < 0.01, for the first bin; F1,36 = 14.19, p < 0.01, for the second bin; F1,36 = 4.99, p < 0.05, for the third bin) indicated that correctly recognized old familiar symbols elicited larger amplitudes than correctly rejected new familiar symbols. These analyses further suggest that the parietal EM effect was similar across age group since there was no interaction between Item and Age Group.
Fig. 5 presents grand mean waveforms elicited by correctly recognized old and correctly rejected new items for the unfamiliar symbols for the three age groups. Unlike the ERPs elicited by the familiar symbols, there is no amplitude difference between the old and new unfamiliar symbols for the adult and adolescent groups, whereas the children maintain a great amplitude difference between old and new. Because subjects in all age groups performed more poorly in the unfamiliar than the familiar stimulus condition, there were fewer correct trials in this condition. To reduce variability in the data due to low number of trials, only averages with a minimum of 8 trials were included in this analysis. This resulted in eliminating one participant in the 9–10 year olds group. The same ANOVA described for the familiar stimulus condition was applied for the unfamiliar stimulus condition. Main effects and interactions for the first two 100 ms bins were not significant. However, for the third 100 ms bin the three-way interaction of Age Group, Item and Electrode was significant. (F14,273 = 37.47, ε = 0.285, p < 0.05). A simple effects procedure followed to allow interpretation of this interaction. These tests revealed that for both adults and adolescents there were no amplitude differences between correctly recognized old or correctly rejected new unfamiliar symbols. For children, the interaction of Item and Electrode was significant (F7,84 = 5.04, ε = 0.265, p < 0.05). In post hoc tests it was found that correctly identified old unfamiliar symbols elicited significantly larger amplitudes than correctly rejected new symbols at Cz, CPz, Pz, POz, Oz, with the largest amplitude at the parietal electrode sites.
Fig. 5.
Grand mean waveforms for correctly recognized old and correctly rejected new unfamiliar symbols at four midline scalp locations during the symbol recognition memory task. The waveforms are depicted for each age group. Vertical arrows on the time-line represent stimulus onset. Scalp topographies are based on the old minus new difference waveforms and are depicted for the parietal EM effects.
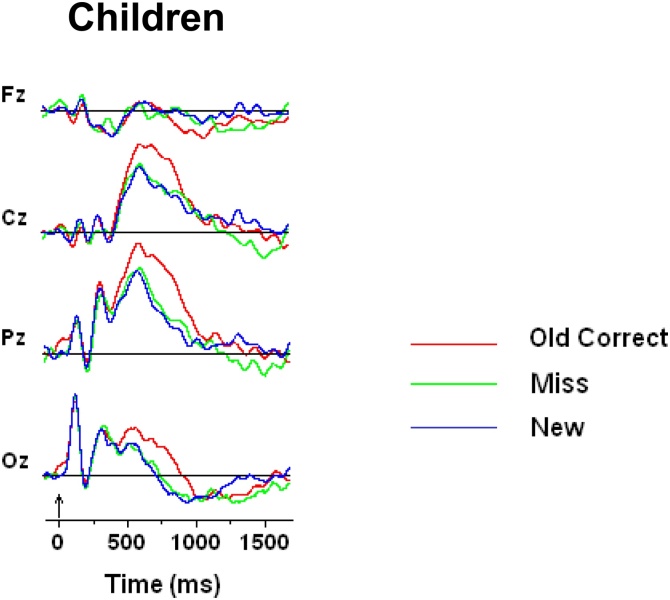
Children’s performance was just above chance, so it is of interest to look at the ERP elicited by old symbols that were incorrectly judged as new. If children’s responses were based on guessing alone, the ERPs elicited by either the correctly or incorrectly identified old items should not differ. Fig. 6 presents average waveforms for new, old correct and old incorrect for the children. The Figure demonstrates that ERP elicited by new and old incorrect symbols are identical and different from ERP elicited by correctly detected old symbols.
Fig. 6.
Grand mean waveforms for correctly recognized old, for old items that were missed, and for correctly rejected new unfamiliar symbols at four midline scalp locations during the symbol recognition memory task. Vertical arrows on the timeline represent stimulus onset.
3.3. Familiar symbol naming task
More than half of the familiar symbols viewed during the novelty oddball task were appropriately named by all the subjects. Of the 60 familiar symbols, the average number of symbols named by adults was 53 (range: 44–58), by adolescents 49 (41–54), and by children 42 (33–55). Children were more likely to say “I do not know” than the older groups. The average number of “do not know” responses for adults was 2.4 (range: 0–6), for adolescents 4.8 (0–9), and for children 8.8 (0–22).
3.4. Summary
Familiar and unfamiliar visual symbols elicited similar novelty P3s in all age groups. While repetition of familiar symbols elicited reduced novelty P3 amplitude in all the groups, novelty P3 amplitude for repeated unfamiliar symbols did not change in adults and adolescents. In all age groups familiar symbols were better remembered than unfamiliar symbols. A parietal EM effect that is presumed to reflect recollection was recorded during the recognition task of the familiar but not during the recognition task of the unfamiliar visual symbols for the adults and adolescents. In contrast, a parietal EM effect was recorded during both familiar and unfamiliar conditions for children.
4. Discussion
The present data demonstrate age-related differences in processing visual novel symbols in an unexpected way. Our working hypotheses were that children who are less acquainted with the familiar symbols will show different processing of these symbols when compared to adults. It was expected that participants in all age groups who were never exposed to the unfamiliar symbols would demonstrate similar brain processing to these unfamiliar events. The fact that no age related differences in brain activation was found during the orienting and memory tasks for the familiar symbols is not so difficult to interpret. However, it is less obvious as to why only children showed a reduction in novelty P3 amplitude to repeated unfamiliar symbols. Moreover, it is surprising that only the children who performed at about chance during the recognition task demonstrated a parietal EM effect. To address these issues, we first discuss the findings of the novelty oddball task, followed by a discussion of the findings of the symbol recognition task.
4.1. Orienting to novel events
In our study, the repetition of familiar novel events led to a reduction in novelty P3 amplitude for adults, adolescents and children. These findings are consistent with previous studies using auditory novel events, demonstrating that as subjects become more experienced with the novel environmental sounds either by repetition (Cycowicz et al., 1996; Kazmerski and Friedman, 1995) or recurrence (Cycowicz and Friedman, 1997), there is a reduction in the magnitude of the novelty P3. In those studies, the reduced amplitude was interpreted as reflecting attenuated activation of the orienting system for novel events as they became less “novel” with repetition. This explanation is viable here for repetition of visually presented familiar novel symbols. While, these findings are inconsistent with Courchesne, 1978a, Courchesne, 1978b who reported that ERPs to novel visual patterns differed in morphology between children and adults indicating that children processed novel stimuli differently than adults, others reported similar ERPs morphology between children and adults. For example, using photographs as novel events Thomas & Nelson (1996) also did not find a morphological difference in ERPs recorded from eight-year-old children compared to those of adults. Similarly, a recent study by Maatta et al. (Maatta et al., 2005a, Maatta et al., 2005b), who investigated selective attention in children, employed infrequent complex tone bursts that elicited orienting responses similar in ERP morphology and basic topographic features to those reported by us in our previous and current investigations.
Children showed a reduction in novelty P3 to repeated familiar symbols even though the events were defined as familiar based on a normative study of young adults. It is possible that many of the visual symbols presented were not as familiar to the children. Indeed, at the end of ERP data collection each participant was presented with the familiar symbols one at a time and was asked to name them. While the adults could correctly name the majority of the symbols the children were less accurate and more often stated “do not know.” Given that the children’s data included trials of novel events that were not named, the question remains why in the familiar condition children showed the same habituation (reduced amplitude with repetition) as the adults. One possibility is that while fewer symbols were named, there were enough trials with strong signals to show the habituation in novelty P3 amplitude with repetition. Alternatively, it is possible that symbols which children could not name were still familiar by other measures. For example, the children may have had some perceptual familiarity with the symbols that they may have encountered in their daily life even if they did not know what the symbols represented. Based on this suggestion, habituation of novel events can occur for stimuli in which familiarity is not based on semantic knowledge. It is possible that pre-exposure to visual stimuli induced perceptual memory sufficient to permit habituation of the orienting response.
In contrast to the familiar symbols, we found that repetition of unfamiliar symbols did not elicit an amplitude reduction of the novelty P3 for the young adults or the adolescents, whereas a reliable reduction was seen for the children. The adult and adolescent data are consistent with a previous study using unfamiliar environmental sounds (Cycowicz and Friedman, 1998) in which adults did not show a reduction in novelty P3 amplitude. Given that the orienting response has been shown to direct attention to new and unfamiliar events, the results for the children are surprising because one would expect that as long as the event is unfamiliar it will elicit orienting in order for the individual to fully assess and further process the unfamiliar events. While this seems to be the case for the adults and adolescents, children appeared to process the unexpected unfamiliar novel symbols completely differently from the adults.
There are two contrasting ways in which reduction in novelty P3 amplitude could occur. According to one possibility children did not process the unfamiliar symbols as much as adults, while the second explanation suggests that children had greater perceptual processing for the unfamiliar symbols than adults. According to the first possibility, children may have evaluated the symbols within the context of the task in which they were instructed to attend to target events. Regardless of stimulus familiarity, they may have quickly dismissed the symbols as irrelevant, making no attempt to further process them. Based on this view the reduction of novelty P3 amplitude showed reduced activation of the orienting system, signifying lack of further processing. The possibility that ignoring novel events could result in reduced novelty P3 amplitude is supported by findings in young adults who were presented with an auditory novelty oddball task under ignore and attend conditions (Friedman et al., 1998). In that study subjects were told to read a book during the ignore condition. For both ignore and attend conditions novel events elicited a novelty P3 amplitude that was reduced with repetition. The current study, however, is different in that the children overall did not ignore the stimuli because they were very accurate in detecting the targets. Nevertheless, they may have concentrated on detecting targets while selectively reducing their attention to the novel symbols.
The second explanation suggests that children processed the unfamiliar symbols more than adults and relies on theories of repetition priming. Repetition priming has been demonstrated as facilitation in processing of repeated stimuli as measured in faster RTs and greater accuracy for repeated events (Tulving and Schacter, 1990). A priming effect in the form of a larger amplitude ERP is often reported for a repeated stimulus relative to its first presentation (e.g., Bentin et al., 1985; Friedman et al., 1993a, Friedman et al., 1993b; Paller and Gross, 1998). However, a priming effect can also be seen as a reduction in ERP amplitude (Ferrari et al., 2017) or in fMRI activity (Soldan et al., 2010) to the repeated stimuli. These findings suggest that a neuronal model (template) is formed during the first presentation of the stimuli, and those stimuli no longer evoke the same magnitude of brain activation upon repetition.
The majority of the ERP repetition priming studies have employed stimuli such as words or pictures that have preexisting representations in semantic memory. However, it has been shown that other types of stimulus material also elicit the ERP priming effect. For example, Rugg and Nagy (1987) demonstrated robust priming for orthographically illegal nonwords that contain no semantic information. Similarly, several studies report an ERP priming effect for two-dimensional representations of unfamiliar and unnameable objects (e.g., Nessler et al., 2001; Rugg et al., 1995). Based on these studies, perceptual fluency between first and second presentations may result in a different magnitude of activity of the neuronal system that is involved in processing these stimuli. This explanation brings into question why in the current investigation this effect was seen only in children for the unfamiliar symbols. Previous studies employing stimuli with no preexisting representations have reported repetition priming in adults (Nessler et al., 2001; Rugg, 1987; Rugg and Nagy, 1987; Rugg et al., 1995). Several differences in experimental paradigm such as differences in task instructions and lag between repetitions may have resulted in a disparity between the current and previous priming studies. However, if task parameters reduced priming in adults how is it that priming was seen in the children?
There is a possibility that children have the ability to perceptually process new and unfamiliar visual stimuli to a greater extent than adolescents and adults. Evidence that children spontaneously attend to perceptual details comes from a study in which children and adults were instructed to perform an induction task (a type of task in which participants are expected to generalize and categorize items based on given constrains) followed by an incidental recognition memory test (Sloutsky and Fisher, 2004). Contrary to other recognition memory tests in children, in this task children’s memory performance surpassed that of adults. Sloutsky and Fisher (2004) suggested that the superior performance by children was due to differences in cognitive operations during the study phase. During the study phase the adults employed category-based induction using their conceptual knowledge. This resulted in reduced discrimination among members of the same category during the recognition test. In contrast, the authors suggested that children used similarity-based induction and therefore perceptually encoded the stimuli so their ability to discriminate between exemplars was better than that of adults. It seems that lack of semantic knowledge left children with greater ability to perceptually process the stimuli. Based on this hypothesis, we suggest that unfamiliar symbols without semantic representations are more difficult for adults to process, and therefore formation of representations for them is less likely to occur with repetition, leading to no change in novelty P3 amplitude. However, reduction in novelty P3 amplitude for the unfamiliar symbols in children could be due to children’s spontaneous tendency to perceptually process the symbols, thus creating perceptual representations that can be compared to incoming stimuli. For children this capability is particularly beneficial because they need to learn large amounts of new information and to distinguish between the truly novel and the experienced. Moreover, for children meaningless symbols may seem to be figures that they have yet to learn. Therefore, children may be more attentive to the unfamiliar symbols because, being novel, those symbols are potentially new learning materials. Although more research is needed to investigate this possibility, such a processing stance may explain the reduction in amplitude of the orienting response for the unfamiliar symbols in the children only.
4.2. Memory of novel events
Subsequent to the orienting response, it has been suggested that attentional resources are involved in further processing of the events to create memory traces for the novel events, so further discrimination of truly novel events from those that have already been experienced can occur (Cycowicz et al., 1996). To determine the existence of such memory traces, all subjects were asked to perform an unexpected recognition memory task following the novelty oddball task. In spite of the fact that all participants were neither warned about the occurrence of the symbols nor instructed to memorize them, their memory performance was above chance in all age groups for the familiar symbols. For the unfamiliar symbols, accuracy was above chance for the adults and adolescents only. For both familiar and unfamiliar symbols RTs were faster to old than to new, and memory performance improved with age.
Children demonstrated that they further processed and memorized the familiar symbols in a similar fashion to that of adolescents and adults. This was not the case in a previous report of an unexpected sound recognition task following an auditory novelty oddball task (Cycowicz, 2000). In that study subjects of all age groups heard a list of new and old novel sounds (from the previous novelty oddball task) and were asked to make old/new judgments. Subjects in all age groups performed poorly on this task, but differences in ERPs to old and new stimuli were observed for the adults and adolescents but not for the children. However, in that study the sounds included both familiar and unfamiliar stimuli. It is thus possible that for children who performed more poorly, a larger proportion of unfamiliar sounds reduced the memory effect in the ERP data. Once there were enough meaningful stimuli, as in the familiar condition, the parietal EM effect was observed in children.
Dual-process theories of recognition memory (Jacoby & Dallas, 1981; Mandler, 1980) posit both a familiarity process that is relatively fast and automatic and might not be under conscious control and a recollection process that requires effort and conscious deliberation. Accumulating evidence suggests that an EM effect peaking between 500 and 800 ms with a parietal scalp distribution reflects recollection (Curran et al., 2003; Mark & Rugg, 1998; Trott et al., 1999). The recognition results reported here for the familiar symbols support the notion that during the unexpected recognition memory task, conscious recollection of the familiar symbols occurred in all age groups.
In spite of the fact that for both familiar and unfamiliar symbols adults’ and adolescents’ memory performance was above chance, familiar symbols were remembered better than, and elicited brain activity distinct from, that of the unfamiliar symbols. The ERP waveforms recorded from adults and adolescents did not show any significant amplitude difference between correctly recognized old and correctly rejected new unfamiliar symbols. Thus, the parietal EM effect was observed for the familiar but not the unfamiliar symbols. In contrast, memory performance of children was at chance for the unfamiliar symbols, but the parietal EM effect was evident in the children’s ERP waveforms of both familiar and unfamiliar symbols. Thus, the ERP data of the adults and adolescents cannot provide a neuronal correlate for memory (above chance recognition) of the unfamiliar symbols in these groups. It is possible to argue that the memory traces for the unfamiliar symbols are too weak to be detected in the ERPs. Due to a larger proportion of guesses and overall low recognition rates, increases in the noise level and variability of the waveforms might have masked the EM effect. Alternatively, it is possible that the EM effect may depend on contacting a preexisting representation in lexical memory. This explanation was offered by Berman and Friedman (Berman and Friedman, 1993), who observed EM effects for words that children could read correctly but not for words that children could not read correctly. A similar explanation is viable in the present study, in which the unfamiliar and thus unnamable visual symbols would not have had preexisting representations in a “symbolic” semantic memory store.
Although children performed at chance, the ERP recordings suggest that old symbols that were correctly remembered have different memory traces than old symbols that were not correctly remembered (see Fig. 6). The data suggest that children’s chance performance does not reflect guessing because if children simply guessed correctly for some of the old symbols then there should have been no difference in the ERP activity between old correct and old incorrect (misses) symbols. In the current design, when the recognition test was unexpected and resulted in overall low performance by all age groups, it seems that a small number of stimuli were truly remembered by children and that the majority of those stimuli had strong memory traces. ERP memory effects in relatively poorly performing children have been reported by Hepworth et al., (Hepworth et al., 2001), who recorded ERPs during a continuous memory task to repeated verbal and facial stimuli. In their study, children’s performance was just above chance but old faces elicited larger ERP amplitudes when compared to new faces.
This interpretation fits well with our earlier suggestion regarding the reduction in novelty P3 amplitude with repetition of the unfamiliar symbols in the children’s group only. If it is correct that children form a stronger perceptual template for the unfamiliar symbols than adults, then these templates can be used when children retrieve item information during the recognition task. Only those symbols that were correctly recognized had a memory trace that was still available, therefore eliciting larger ERP amplitudes. However, old symbols were incorrectly rejected as new because for them memory templates were not easily available.
4.3. Conclusion
The current study demonstrates developmental differences in processing stimuli for which there is no preexisting representation. Our data show that orienting and evaluations of unexpected familiar visual events reflected in the novelty P3 are similar across age groups. In contrast, there is an age difference in evaluations and orienting of unfamiliar symbols. Adults and adolescents do not show habituation with repetition of unfamiliar symbols’ presentations, which suggests that they engage in further processing of stimuli for which they lack semantic representations. With repetition, children show a reduction in novelty P3 amplitude which suggests a reduction in processing of unexpected stimuli, probably due to the availability of recently created memory traces.
There is a quantitative difference in the encoding of familiar symbols as recognition performance improves with age, partially due to children’s lack of familiarity with some symbols. However, a similar parietal EM effect recorded in all age groups suggests the involvement of similar brain circuitry in retrieval processes. Unfamiliar symbols appear to be less memorable than familiar symbols by all age groups with children performing at chance. While RTs to old symbols are faster than RTs to new symbols, low memory performance is associated with a lack of a parietal EM effect in adults and adolescents. In contrast, children who appear to form a stronger memory trace during the novelty oddball task also show a parietal EM effect for unfamiliar symbols.
The overall data suggest that selecting stimuli from a complex environment to be further evaluated and processed is determined not only by the physical characteristics of the stimuli themselves, but also by the individual’s interests, motivation, and cognitive strategies through which the individual perceives the events. During the developmental years, when much information is acquired, those aspects are important in enabling the children to quickly learn to select those events in their environment that are worthy of further exploration.
Declarations of interest
None.
Acknowledgments
The author thanks Dr. David Friedman for his material and intellectual support during the data collection of this study. In addition, the author thanks Mr. Charles L. Brown III for computer programming and technical assistance, Mr. Martin Duff for his assistance in data collection, and all the volunteers who generously gave their time to participate in the study. This work was supported by National Institute of Child Health and Human Development (HD37193).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100615.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Bahrick L.E., Pickens J.N. Infant memory for object motion across a period of three months: implications for a four-phase attention function. J. Exp. Child Psychol. 1995;59(3):343–371. doi: 10.1006/jecp.1995.1017. [DOI] [PubMed] [Google Scholar]
- Benes F.M. Myelination of cortical-hippocampal relays during late adolescence. Schizophr. Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Benes F.M. Development of the corticolimbic system. In: Dawson G., Fisher K.W., editors. Human Behavior and the Developing Brain. Guilford Press; New York: 1994. pp. 176–206. [Google Scholar]
- Bentin S., McCarthy G., Wood C.C. Event-related potentials, lexical decision and semantic priming. Electroencephalogr. Clin. Neurophysiol. 1985;60(4):343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Berman S., Friedman D. A developmental study of ERPs during recognition memory: effects of picture familiarity, word frequency, and readability. J. Psychophysiol. 1993;7:97–114. [Google Scholar]
- Caballero A., Granberg R., Tseng K.Y. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci. Biobehav. Rev. 2016;70:4–12. doi: 10.1016/j.neubiorev.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. The role of the frontal lobes in the regulation of cognitive development. Special issue: the role of frontal lobe maturation in cognitive and social development. Brain Cogn. 1992;20(1):51–73. doi: 10.1016/0278-2626(92)90061-p. [DOI] [PubMed] [Google Scholar]
- Clarkson M.G., Clifton R.K., Swain I.U., Perris E.E. Stimulus duration and repetition rate influence newborns’ head orientation toward sound. Dev. Psychobiol. 1989;22(7):683–705. doi: 10.1002/dev.420220704. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Changes in P3 waves with event repetition: long-term effects on scalp distribution and amplitude. Electroencephalogr. Clin. Neurophysiol. 1978;45(6):754–766. doi: 10.1016/0013-4694(78)90143-8. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials form childhood to adulthood. Electroencephalogr. Clin. Neurophysiol. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Hillyard S.A., Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr. Clin. Neurophysiol. 1975;39(2):131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Curran T., Tepe K.L., Piatt C. ERP explorations of dual processes in recognition memory. In: Zimmer H., Mecklinger A., Lindenberger U., editors. Building in Human Memory: A Neurocognitive Approach. Oxford University Press; Oxford: 2003. [Google Scholar]
- Cycowicz Y.M. Memory development and event-related brain potentials in children. Biol. Psychol. 2000;54:145–174. doi: 10.1016/s0301-0511(00)00055-7. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D. A developmental study of the effect of temporal order on the ERPs elicited by novel environmental sounds. Electroencephalogr. Clin. Neurophysiol. 1997;103:304–318. doi: 10.1016/s0013-4694(97)96053-3. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D. Effect of sound familiarity on the event-related potentials elicited by novel environmental sounds. Brain Cogn. 1998;36:30–51. doi: 10.1006/brcg.1997.0955. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D. The effect of intention to learn novel, environmental sounds on the novelty P3 and old/new recognition memory. Biol. Psychol. 1999;50:35–60. doi: 10.1016/s0301-0511(99)00004-6. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D. Visual novel stimuli in an ERP novelty oddball paradigm: effects of familiarity on repetition and recognition memory. Psychophysiology. 2007;44(1):11–29. doi: 10.1111/j.1469-8986.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D., Rothstein M. An ERP developmental study of repetition priming by auditory novel stimuli. Psychophysiology. 1996;33(6):680–690. doi: 10.1111/j.1469-8986.1996.tb02364.x. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D., Duff M. Pictures and their colors: what do children remember? J. Cogn. Neurosci. 2003;15(5):759–768. doi: 10.1162/089892903322307465. [DOI] [PubMed] [Google Scholar]
- Czernochowski D., Mecklinger A., Johansson M., Brinkmann M. Age-related differences in familiarity and recollection: ERP evidence from a recognition memory study in children and young adults. Cogn. Affect. Behav. Neurosci. 2005;5(4):417–433. doi: 10.3758/cabn.5.4.417. [DOI] [PubMed] [Google Scholar]
- Daffner K.R., Ryan K.K., Williams D.M., Budson A.E., Rentz D.M., Wolk D.A., Holcomb P.J. Increased responsiveness to novelty is associated with successful cognitive aging. J. Cogn. Neurosci. 2006;18(10):1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Fabiani M., Donchin E. Encoding processes and memory organization: a model of the von Restorff effect. J. Exp. Psychol. Learn. Mem. Cogn. 1995;21(1):224–240. doi: 10.1037//0278-7393.21.1.224. [DOI] [PubMed] [Google Scholar]
- Fabiani M., Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32(6):579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M., Gratton G., Chiarenza G.A., Donchin E. A psychophysiological investigation of the von Restorff paradigm in children. J. Psychophysiol. 1990;4(1):15–24. [Google Scholar]
- Ferrari V., Codispoti M., Bradley M.M. Repetition and ERPs during emotional scene processing: a selective review. Int. J. Psychophysiol. 2017;111:170–177. doi: 10.1016/j.ijpsycho.2016.07.496. [DOI] [PubMed] [Google Scholar]
- Friedman D., Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: a selective review. Microsc. Res. Technol. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Friedman D., Simpson G.V. ERP amplitude and scalp distribution to target and novel events: effects of temporal order in young, middle-aged and older adults. Cogn. Brain Res. 1994;2(1):49–63. doi: 10.1016/0926-6410(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Friedman D., Hamberger M., Ritter W. Event-related potentials as indicators of repetition priming in young and older adults: amplitude, duration, and scalp distribution. Psychol. Aging. 1993;8(1):120–125. doi: 10.1037//0882-7974.8.1.120. [DOI] [PubMed] [Google Scholar]
- Friedman D., Simpson G., Hamberger M. Age-related changes in scalp topography to novel and target stimuli. Psychophysiology. 1993;30(4):383–396. doi: 10.1111/j.1469-8986.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Friedman D., Kazmerski V.A., Cycowicz Y.M. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35:508–520. doi: 10.1017/s0048577298970664. [DOI] [PubMed] [Google Scholar]
- Friedman D., Cycowicz Y.M., Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev. 2001;25(4):355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G.H., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grillon C., Courchesene E., Ameli R., Elmasian R., Braff D. Effect of rare non-target stimuli on brain electrophysiological activity and performance. Int. J. Psychophysiol. 1990;9:257–267. doi: 10.1016/0167-8760(90)90058-l. [DOI] [PubMed] [Google Scholar]
- Gumenyuk V., Korzyukov O., Alho K., Escera C., Naatanen R. Effects of auditory distraction on electrophysiological brain activity and performance in children aged 8-13 years. Psychophysiology. 2004;41(1):30–36. doi: 10.1111/1469-8986.00123. [DOI] [PubMed] [Google Scholar]
- Harper J., Malone S.M., Iacono W.G. Theta‐and delta‐band EEG network dynamics during a novelty oddball task. Psychophysiology. 2017;54(11):1590–1605. doi: 10.1111/psyp.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth S.L., Rovet J.F., Taylor M.J. Neurophysiological correlates of verbal and nonverbal short-term memory in children: repetition of words and faces. Psychophysiology. 2001;38(3):594–600. doi: 10.1017/s0048577201002281. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P.R. Morphometric study of human cerebral cortex development. Neuropsychology. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jennings J.R., Wood C.C. The e-adjustment procedure for repeated measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Jacoby L.L., Dallas M. On the relationship between autobiographical memory and perceptual learning. J. Exp. Psychol. Gen. 1981;110:306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jernigan T.L., Sowell E.R. Magnetic resonance imaging studies of the developing brain. In: Keshavan M.S., Murray R.M., editors. Neurodevelopment & Adult Psychopathology. Cambridge University Press; United Kingdom: 1997. pp. 63–70. [Google Scholar]
- Johnson R., Jr. On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30(1):90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Kagan J. On the nature of emotion. Monogr. Soc. Res. Child Dev. 1994;59(2-3):7–24. [PubMed] [Google Scholar]
- Kazmerski V.A., Friedman D. Repetition of novel stimuli in ERP oddball paradigm: aging effects. J. Psychophysiol. 1995;9:298–311. [Google Scholar]
- Kirchhoff B.A., Wagner A.D., Maril A., Stern C.E. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J. Neurosci. 2000;20(16):6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C.B. Commentary: The codevelopments of attention and emotion regulation. Infancy. 2002;3(2):199–208. doi: 10.1207/S15327078IN0302_5. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E., Winkler I., Horváth J., Näätänen R., Pavlov I., Fellman V., Huotilainen M. Processing acoustic change and novelty in newborn infants. Eur. J. Neurosci. 2007;26(1):265–274. doi: 10.1111/j.1460-9568.2007.05628.x. [DOI] [PubMed] [Google Scholar]
- Liungman C.G. 1994. Dictionary of Symbols: Norton. [Google Scholar]
- Mandler G. Recognizing: the judgement of previous occurrence. Psychol. Rev. 1980;87:252–271. [Google Scholar]
- Mark R.E., Rugg M.D. Age effects on brain activity associated with episodic memory retrieval: an electrophysiological study. Brain. 1998;121:861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- Maatta S., Paakkonen A., Saavalainen P., Partanen J. Selective attention event-related potential effects from auditory novel stimuli in children and adults. Clin. Neurophysiol. 2005;116(1):129–141. doi: 10.1016/j.clinph.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Maatta S., Saavalainen P., Kononen M., Paakkonen A., Muraja-Murro A., Partanen J. Processing of highly novel auditory events in children and adults: an event-related potential study. Neuro Rep. 2005;16(13):1443–1446. doi: 10.1097/01.wnr.0000177014.36979.3f. [DOI] [PubMed] [Google Scholar]
- Malcuit G., Bastien C., Pomerleau A. Habituation of the orienting response to stimuli of different functional values in 4-month old infants. J. Exp. Child Psychol. 1996;62(2):272–291. doi: 10.1006/jecp.1996.0031. [DOI] [PubMed] [Google Scholar]
- Marshall P.J., Reeb B.C., Fox N.A. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Dev. Sci. 2009;12(4):568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt A.R. Intensity discrimination and cardiac reaction in young infants. Dev. Psychol. 1973;8(3):357. [Google Scholar]
- Nessler D., Mecklinger A., Penney T.B. Event related brain potentials and illusory memories: the effects of differential encoding. Cogn. Brain Res. 2001;10(3):283–301. doi: 10.1016/s0926-6410(00)00049-5. [DOI] [PubMed] [Google Scholar]
- Nuwer M.R., Lehmann D., Lopes da Silva F., Matsuoka S., Sutherling W., Vibert J.-F. IFCN guidelines for topographic and frequency analysis of EEGs and EPs. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994;91:1–5. doi: 10.1016/0013-4694(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Oja L., Huotilainen M., Nikkanen E., Oksanen-Hennah H., Laasonen M., Voutilainen A., von Wendt L., Alho K. Behavioral and electrophysiological indicators of auditory distractibility in children with ADHD and comorbid ODD. Brain Res. 2016;1632:42–50. doi: 10.1016/j.brainres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Paller K.A., Gross M. Brain potentials associated with perceptual priming vs explicit remembering during the repetition of visual word-form. Neuropsychologia. 1998;36(6):559–571. doi: 10.1016/s0028-3932(97)00132-2. [DOI] [PubMed] [Google Scholar]
- Parker A., Wilding E., Akerman C. The Von Restorff effect in visual object recognition memory in humans and monkeys. The role of frontal/perirhinal interaction. J. Cogn. Neurosci. 1998;10(6):691–703. doi: 10.1162/089892998563103. [DOI] [PubMed] [Google Scholar]
- Ranganath C., Rainer G. Neural mechanisms for detecting and remembering novel events. Nat. Rev. Neurosci. 2003;4(3):193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Richardson C., Bucks R.S., Hogan A.M. Effects of aging on habituation to novelty: an ERP study. Int. J. Psychophysiol. 2011;79(2):97–105. doi: 10.1016/j.ijpsycho.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C., Cuevas K. The development of infant memory. In: Courage N.C.M.L., editor. The Development of Memory in Infancy and Early Childhood. 2nd ed. Psychology Press; New York, NY: 2009. pp. 11–41. [Google Scholar]
- Rugg M.D. Dissociation of semantic priming, word and non-word repetition effects by event-related potentials. Q. J. Exp. Psychol. 1987;39A:123–148. [Google Scholar]
- Rugg M.D., Nagy M.E. Lexical contribution to nonword-repetition effects: Evidence from event-related potentials. Mem. Cognit. 1987;15(6):473–481. doi: 10.3758/bf03198381. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Soardi M., Doyle M.C. Modulation of event-related potentials by the repetition of drawings of novel objects. Cogn. Brain Res. 1995;3:17–24. doi: 10.1016/0926-6410(95)00014-3. [DOI] [PubMed] [Google Scholar]
- Sloutsky V.M., Fisher A.V. Induction and categorization in young children: a similarity-based model. J. Exp. Psychol. Gen. 2004;133(2):166–188. doi: 10.1037/0096-3445.133.2.166. [DOI] [PubMed] [Google Scholar]
- Snodgrass J.G., Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J. Exp. Psychol. Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sokolov E.N. Macmillian.; New York: 1963. Perception and the Conditioned Reflex. [Google Scholar]
- Sokolov E.N. The modeling properties of the nervous system. In: Coles M., MAltzman F., editors. A Handbook of Contemorary Soviet Psychology. Basic Books; New York: 1969. pp. 671–704. [Google Scholar]
- Sokolov E.N. The orienting response, and future directions of its development. Pavlov. J. Biol. Sci. 1990;25(3):142–150. doi: 10.1007/BF02974268. [DOI] [PubMed] [Google Scholar]
- Soldan A., Habeck C., Gazes Y., Stern Y. Neural mechanisms of repetition priming of familiar and globally unfamiliar visual objects. Brain Res. 2010;1343:122–134. doi: 10.1016/j.brainres.2010.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T. Biological and psychological development of executive functions. Brain Cogn. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Tulving E., Schacter D.L. Priming and human memory system. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- Thomas K.M., Nelson C.A. Age-related changes in the electrophysiological response to visual stimulus novelty: a topographical approach. Electroencephalogr. Clin. Neurophysiol. 1996;98:294–308. doi: 10.1016/0013-4694(95)00280-4. [DOI] [PubMed] [Google Scholar]
- Trott C.T., Friedman D., Ritter W., Fabiani M., Snodgrass J.G. Episodic priming for temporal source: Event-related potentials reveal age-related differences in prefrontal functioning. Psychol. Aging. 1999;14:390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Van Petten C., Senkfor A.J. Memory for words and novel visual patterns: repetition, recognition, and encoding effects in the event-related brain potential. Psychophysiology. 1996;33:491–506. doi: 10.1111/j.1469-8986.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Giedd J., Dale A.M., Brown T.T. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb. Cortex. 2017;27(2):1472–1481. doi: 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S.A., Cheatham C.L., Lukowski A.F., Haight J.C., Muehleck A.J., Bauer P.J. Infants’ ERP responses to novel and familiar stimuli change over time: implications for novelty detection and memory. Infancy. 2006;9(1):21–44. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.