Abstract
Sleep deprivation in youth has garnered international attention in recent years, as correlational studies have demonstrated significant relationships between lack of sleep and detrimental behavioral and academic outcomes. However, no study to date has systematically examined the neurophysiological consequences of a single night of sleep restriction (i.e., 4 h) in adolescents using ultra-high field functional neuroimaging. Much of what we know regarding the neural consequences of sleep deprivation has come from the adult literature, and among those studies, the majority use region of interest (ROI) approaches, thus disregarding the dynamic mechanisms that may subserve the behavioral effects of sleep restriction. Leveraging a crossover within-subjects design, we demonstrate that pivotal brain regions involved in the default mode and limbic regulatory centers have disrupted functioning following a night of restricted sleep compared to a night of “normal sleep”. Specifically, a normal night (i.e., 8 h) of sleep led to increased global and local efficiency of bilateral amygdala, and less efficiency in the posterior cingulate, as measured by graph theory, compared to a night of sleep restriction. Furthermore, aberrant functional connectivity patterns were identified in key fronto-limbic circuitry, suggesting a potential pathophysiological mechanism underlying the widespread effects of sleep deprivation in youth.
Keywords: Actigraphy, Adolescence, Resting-state fMRI, Sleep restriction, Default-mode network
1. Introduction
Sleep deprivation in youth is a significant national and international problem (Mindell and Owens, 2010). As children transition into adolescence, they go to sleep later, obtain less sleep, and experience more sleepiness and poorer quality sleep (Carskadon et al., 2004; Smaldone et al., 2007), with up to 25% obtaining less than 6 h (National Sleep Foundation, 2006; Bartel et al., 2015; Becker et al., 2015). Meanwhile, rates of sleep-related behavioral and emotional disorders continue to rise during adolescence (Costello et al., 2011), as does the potential for negative consequences of sleep deprivation. In adolescence, hormonal effects on brain development create windows of vulnerability (Dahl and Lewin, 2002; Andersen and Teicher, 2004), and escalating social and academic demands may increase the need for the restorative and regulating functions of sleep (Dahl and Spear, 2004; Becker et al., 2015).
In studies conducted predominantly with adults, both prolonged sleep disturbances and short-term sleep restriction have been associated with distinct changes in the brain along both anatomical and functional dimensions, even in otherwise healthy populations. Furthermore, behavioral data suggest powerful effects in cognitive and emotional domains, both following acute and chronic sleep deprivation and restriction. As a result, it is not surprising that disrupted limbic and cortical regions have been implicated as plausible pathophysiological mechanisms underlying the widespread cognitive and mental health disturbances associated with sleep disturbances.
Despite the documented effects of sleep deprivation, our understanding of the neurophysiological underpinnings of even a single night of sleep restriction in youth is limited. Sleep disturbances are thought to undermine control of regulatory centers in the brain and prime emotion or reward circuits (van der Helm and Walker, 2012; Mullin et al., 2013). Adolescents who reported relatively poor sleep over a 30-day period exhibited less recruitment of the dorsolateral prefrontal cortex (dlPFC) during cognitive control demands and greater insula activation during reward processing (Beebe et al., 2009; Telzer et al., 2013). Research conducted mostly with adults also suggests that sleep disturbances and mental health problems both may be related to “decoupling” of regulatory and reactive subsystems of the brain, such that strong emotions and impulses are under insufficient inhibitory control. The PFC has a reciprocal relationship with the amygdala, such that it can serve as a ‘braking system’ to the emotional brain (Delgado et al., 2008), exerting cognitive control over emotions in adaptive regulation of emotion and impulsivity. At least one study with adolescents (Telzer et al., 2013), and several studies with adults, have indicated that sleep disruptions may contribute to such a decoupling (Van der Helm and Walker, 2010; Killgore, 2013).
Affective dysregulation and reward sensitivity are not the only neurophysiological disruptions that manifest from poor, or lack of, sleep. Behavioral and psychiatric problems, neurobehavioral impairments, academic underachievement, autonomic dysregulation, and obesity have also been associated with sleep disturbances (El-Sheikh and Erath, 2011; Baum et al., 2014; Shochat et al., 2014; Rodríguez-Colón et al., 2015). Persistent sleep problems, even if only minor, can cause permanent neuronal cellular stress over time (Jan et al., 2010). Such effects may be more severe among youth, given the level of plasticity and rapid rate of development in young brains (Spruyt et al., 2005; Jan et al., 2010). This highlights the importance of examining neurophysiological disruptions associated with sleep restriction in youth.
Despite the widespread effects of sleep disturbances, most studies have focused solely on a priori regions of interest inclusive of primarily the PFC and the amygdala. Enhancing the novelty of this study, we leveraged the advantages of ultra-high field functional neuroimaging (i.e., higher signal-to-noise ratio, higher spatial resolution, and increased sensitivity to the blood-oxygen-level-dependent signal), to examine neurophysiological changes associated with sleep restriction (compared to a ‘normal’ night of sleep) across the entire brain. Specifically, we hypothesized that differences would emerge in critical networks that modulate global processing (i.e., the default mode network) in the brain, and that involve regions that have been previously implicated in sleep disturbances. Furthermore, rather than focusing on activation patterns or specific levels of activation signal which limit the conclusions that can be drawn about the functional architecture of specific regions/networks, we focused on examining connectivity patterns. To this end, we expected to find differences not only within prefrontal and limbic regions, but also in regions that are commonly associated with a broader cognitive-affective network. Employing a within-subjects design, participants were scanned within two hours of waking up. Resting-state functional connectivity analyses and graph theory metrics were conducted to determine differential patterns of brain connectivity and function.
2. Methods
2.1. Participants
Participants were 18 healthy adolescents (9 female) aged 13–15 years old (M age = 14.40 years ± 1.94 months) who were recruited from public schools and partook in a larger study of sleep and child development (Auburn University Sleep Study). Thirteen participants were European American and 5 were African American. Sixteen were right-handed.1 A researcher contacted the participants’ parents via phone to determine eligibility for the study. Exclusion criteria included braces/metal in the body, medication use, ADHD, asthma, diagnosed sleep disorders based on mothers’ report (e.g., sleep apnea, restless leg syndrome), learning disabilities, concussion, seizures, claustrophobia, and chronic illnesses.
2.2. Procedures
All procedures were approved by the Institutional Review Board at Auburn University. Written assent and consent were obtained from adolescents and their parents. All participants visited the Auburn University MRI Research Center (AUMRIRC) prior to giving written informed consent and, if interested in the study, were placed in the scanner to acclimate them to the study procedures and gauge their tolerability to the scanning environment. To reduce potential confounds, participation occurred during the regular school year, excluding holidays. Actigraphs were delivered to the participants.
Using a repeated measures design, adolescents participated in two experimental conditions (restricted sleep and “normal” sleep) one week apart, each followed by an fMRI session. The order of the experimental conditions was counterbalanced across participants. Sleep manipulations were on Friday nights (to reduce the effects of weekday-weekend sleep schedule differences and sleep restriction during the school week), and fMRI assessments were on Saturday mornings during the academic year. To maintain consistency in the sleep environment, and given the young age of youth, all sleep assessments occurred in the adolescents’ homes.
To gauge participants’ regular sleep schedules and examine compliance, sleep was monitored with actigraphs for 6 nights total across two weeks (Wednesday-Friday prior to each scan). During the Wednesdays and Thursdays of these two weeks, participants were told to go to bed and to wake up at their usual times. For sleep restriction, youth were instructed to be in bed for 4 h on the Friday before the scan. Scheduled sleep times were either 2:00 a.m.–6:00 a.m. (for those scanned at 8:00 a.m.) or 3:00 a.m.–7:00 a.m. (for those scanned at 9:00 a.m.) depending on scheduled MRI time the following morning. To facilitate compliance, adolescents were called by researchers every 30 min from 10:00pm until 15 min prior to instructed bedtime to make sure they were awake. Researchers inquired about ongoing activities, fatigue, and provided suggestions for staying awake (e.g., get up and walk). In the morning, the researcher called the participants at the scheduled wake time to ensure compliance with the study protocol. For the “normal” sleep condition, participants were told to remain in bed for at least eight hours: 10:00 p.m.–6:00 a.m. for those scanned at 8:00 a.m., or 11:00 p.m.–7:00 a.m. for those scanned at 9:00 a.m. Participants were called in the evening and instructed to go to bed at the scheduled time and were called in the morning at the scheduled wake time to make sure they did not oversleep. For both conditions, participants were instructed to avoid naps and caffeine throughout the day and to arrive at the AUMRIRC within 2 h of waking. Further, for all nights during which actigraphy data were collected, a research assistant called the participants every night to obtain sleep diary information from the previous night to facilitate the coding of actigraphy data and to remind the adolescents to wear the actigraph.
2.3. Actigraphy
Actigraphs were used to estimate sleep (Octagonal Basic Motionlogger, Ambulatory Monitoring, Inc., Ardsley, NY). Actigraphy has been validated with children and adolescents for the assessment of multiple sleep parameters and has good psychometric properties when compared to polysomnography (Sadeh et al., 1994; Sadeh et al., 1995). The actigraph recorded activity in 1-min epochs between bedtime and wake time. Data were downloaded using ACTme software and were scored using the Sadeh algorithm (Sadeh et al., 1994; Sadeh et al., 1995) and the Action W-2 analysis software (ActionW User’s Guide version 2.4, 2002). Established procedures for determining sleep/wake times were used (Acebo and Carskadon, 2001). Youth reported their bedtimes and four actigraphy-based variables were derived: (a) Sleep Onset Time; (b) Awakening Time; (c) Sleep Period (bedtime to awakening time and latency); and (d) Total Actual Sleep Minutes (from sleep onset to wake time excluding wake minutes after sleep onset).
2.4. fMRI scanning & data analysis
We performed ultra-high field, high-resolution resting state functional magnetic resonance imaging (rs-fMRI) to characterize differences in resting state networks between conditions. Participants were scanned using an EPI sequence, optimized in-house to reduce susceptibility artifacts (37 slices acquired parallel to the AC-PC line, 0.85 mm × 0.85 mm × 1.5 mm voxels, TR/TE: 3000/28 ms, 70° flip angle, base/phase resolution 234/100, A > P phase encode direction, iPAT GRAPPA acceleration factor = 3, interleaved acquisition, 100 time points, total acquisition time 5:00). Participants were asked to rest with their eyes closed for the duration of the scan. Data were acquired on the AUMRIRC Siemens 7 T MAGNETOM outfitted with a 32-channel head coil by Nova Medical (Wilmington, MA). A whole-brain high-resolution 3D MPRAGE image (256 slices, 0.70 m m3 voxels, TR/TE: 2200/2.89 ms, 7° flip angle, base/phase resolution 256/100, A > P phase encode direction, iPAT GRAPPA acceleration factor = 2, total acquisition time 5:18) was also acquired for registration purposes. It is important to note that scanning utilized an ultra-high resolution sequence (i.e., submillimeter in-plane acquisition), which allows for greater sensitivity and delineation of anatomical features. This leads to improved pre-processing and registration to anatomical images, in addition to more accurate functional brain maps.
Data were analyzed using two techniques: 1) graph theory metrics and 2) independent component analysis. For graph theory analyses, we employed the ‘conn’ toolbox(Whitfield-Gabrieli and Nieto-Castanon, 2012) (https://www.nitrc.org/projects/conn/) for MATLAB to calculate nodal measures such as local efficiency, clustering coefficient, and degreeness as well as global measures such as global efficiency, average path length, and betweenness centrality. In graph theory, a brain region is considered a node, and a connection between brain regions is considered an edge. These measures are indicative of how brain regions are interacting and integrating with other brain regions. Global and local efficiency measure the ability of a region to transmit information at the whole brain (i.e., global) or regional (i.e., local) level (Latora and Marchiori, 2001), while path length is a measure of integration as it accounts for the average shortest path length between all brain region pairs. Degreeness is an indication of the number of edges, or connections, emanating from a single brain region, while a clustering coefficient indicates how many of a regions neighbors are connected to one another. Graph theory metrics are used to characterize brain networks, and are a robust alternative to activation patterns as they provide more information about the nature of the network (i.e., how functionally integrated or segregated it may be) (Rubinov and Sporns, 2010). For all graph theory metrics, the adjacency matrix (composed of 85 atlas-based regions of interest) was formed by selecting a fixed percentile (15%) of the edges in each network (i.e., those with the highest correlation coefficient values, separately for each subject), using a two-sided threshold. Paired t-tests (between conditions) were conducted. All analyses surpassing a two-sided, FDR-corrected p<0.05 are reported. We also employed FSL’s Multivariate Exploratory Linear Decomposition into Independent Components (MELODIC), a spatial ICA-based approach to resting state data. Prior to analyses, standard resting state fMRI pre-processing steps (i.e., brain extraction, slice timing correction, Gaussian smoothing (3 m m FWHM), band-pass filtering (0.008–0.09), regression of motion and physiological artifacts (using CompCor), registration to anatomical space, and normalization to MNI standard space) were performed.
Finally, regional homogeneity (ReHo) analyses were performed on the data. ReHo evaluates the brain activity at the voxel level by measuring the similarity or synchronization between the time course of a given voxel and its nearest neighbors (Zang et al., 2004). The rationale and advantages of using ReHo are as follows: a) intrinsic brain activity is manifested in clusters of voxels rather than in a single voxel, and hence ReHo may be a more accurate measure; b) measuring local connectivity is important as it provides an index of specialization of areas; c) local connectivity can induce alterations in remote functional connectivity (Deco et al., 2014); d) local connectivity can also determine the boundaries between functionally heterogeneous regions; and e) regional homogeneity can inform structure-function relationship in understanding the brain (Jiang and Zuo, 2016). Regional homogeneity implements Kendall’s coefficient of concordance (KCC), which relies on rank correlations of time series to assess the homogeneity of a given center voxel and its neighboring voxels.
KCC within a given cluster of voxels is equal to the parameter W (ranging from 0 to 1), where Ri is the sum rank of the ith time point; R is the mean of the Ris; K is the number of time series within a selected cluster (7, 19, or 27 voxels), and n is the number of ranks, as determined by the number of time points. For this analysis, we used a cluster size of 27 voxels. To generate connectivity maps and be subjected to statistical analyses, ReHo maps were smoothed and then converted to z-scores. Contrasts were thresholded at p = 0.00001, cluster = 50 voxels, estimated through Monte Carlo simulations, which roughly corresponds to a familywise error (FWE) corrected threshold of p < 0.05.
2.5. Behavioral tasks
Participants completed two common neurocognitive tasks, known to elicit activation throughout the fronto-limbic network (Cazzell et al., 2012; Congdon et al., 2013; Robles et al., 2014; Rasmussen et al., 2016; Schmüser et al., 2016), and that have been used in sleep deprivation studies (Killgore et al., 2011). First, participants completed the balloon analogue risk task (BART) which is a computerized measure of risk-taking behavior. Adolescents were presented with a balloon that they could “blow up”. The balloon could “pop” at any time, and the more the balloon inflated, the more points they would earn. They could choose to stop inflating the balloon, and collect the points earned for that trial at any time. If the balloon popped, the points earned from that trial were lost. Youth were not informed about the balloon’s breakpoints, which were random (Lejuez et al., 2002). The BART is sensitive in determining neural network differences in populations with varying levels of risk-sensitivity (Karen et al., 2014). For the BART, we examined the total amount of points earned over the 6-minutes they engaged in the task, the number of popped balloons, the average number of pumps per trial, the number of total trials and the number of successful trials, as well as reaction time. The second task was a Go/No-Go task, in which stimuli were presented in a continuous stream and youth performed a binary decision on each stimulus (e.g., is the stimulus in an alternating pattern with the previous stimulus?). Youth pressed a button if the stimulus met the criteria (go trial), or withheld their responses if it did not (no-go trial). There are more ‘go’ than ‘no-go’ trials, enticing the youth into a pattern of response, making the ‘no-go’ trials more difficult in people with poor impulse control (Roberts et al., 1994; Shibata et al., 1997; Braver et al., 2001; Rubia et al., 2001; Rubia et al., 2003). In this task, we examined the number of lure errors (i.e., how many times they responded when they shouldn’t have), errors of omission (i.e., how many times they did not respond when they should have), total accuracy, and reaction time during correct trials.
3. Results
3.1. Actigraphy measures
Indicative of similarity in sleep schedule on the two nights (Wednesdays and Thursdays) preceding the normal and restricted sleep conditions, adolescents went to bed, fell asleep, and woke up at similar times during the two nights preceding each sleep manipulation (Table 1). Further, adolescents woke up at similar times on the Fridays of the two sleep conditions. The sleep manipulations were effective (see Table 1), and both bedtimes and sleep onset times were later during the sleep restriction in comparison to the normal sleep condition. The sleep period (overall time in bed) and actual sleep minutes were shorter during the night of sleep restriction in comparison to that of normal sleep (Table 1). On average, participants had a difference of 3.5 h of actual sleep minutes between conditions. Descriptive information regarding sleep schedule during the normal and restriction conditions is compiled in Table 1.
Table 1.
Means and standard errors for sleep schedule and period prior to and during the normal and restriction conditions.
| Sleep Schedule and Period | Sleep | Condition | t-value |
|---|---|---|---|
| Normal | Restriction | ||
| Wednesday & Thursday | |||
| Reported Bedtime | 10:00 p.m. ± 13.04 | 10:08 p.m. ± 9.59 | −0.70 |
| Sleep Onset Time | 10:39 p.m. ± 12.73 | 10:47 p.m. ± 13.27 | −0.75 |
| Total Sleep Minutes | 413.75 ± 14.18 | 380.31 ± 13.69 | 3.26** |
| Wake Minutes | 31.72 ± 5.02 | 40.83 ± 6.80 | −2.39* |
| Awakening Time | 6:04 a.m. ± 11.62 | 5:51 a.m. ± 15.13 | 1.54 |
| Sleep Period | 455.03 ± 13.86 | 439.67 ± 17.53 | 0.12 |
| Friday – Manipulation | |||
| Awakening Time (Fri) | 6:09 a.m. ± 13.29 | 6:06 a.m. ± 14.80 | 1.46 |
| Reported Bedtime | |||
| 10:00 pm / 2:00 am | 9:55 p.m. ± 7.14 | 1:54 a.m. ± 7.88 | −61.43*** |
| 11:00 pm / 3:00 am | 11:13 p.m. ± 8.81 | 3:13 a.m. ± 8.81 | −19.24*** |
| Sleep Onset Time | |||
| 10:00 pm / 2:00 am | 10:31 p.m. ± 11.35 | 1:59 a.m. ± 7.47 | −15.28*** |
| 11:00 pm / 3:00 am | 11:38 p.m. ± 14.86 | 3:13 a.m. ± 9.85 | −19.33*** |
| Total Sleep Minutes | 401.67 ± 12.32 | 209.39 ± 8.44 | 20.06*** |
| Wake Minutes | 38.61 ± 5.46 | 16.94 ± 5.28 | 3.58** |
| Awakening Time (Sat) | |||
| 6:00 am | 5:48 a.m. ± 8.08 | 5:52 a.m. ± 7.25 | −0.75 |
| 7:00 am | 7:01 a.m. ± 12.42 | 6:55 a.m. ± 11.42 | 0.45 |
| Sleep Period | 449.83 ± 8.41 | 239.78 ± 5.43 | 26.67*** |
Note: Sleep period refers to time in bed (bedtime to awakening time). Total sleep minutes refer to actual sleep between sleep onset and wake time excluding minutes awake after sleep onset. df = 17. * p < 0.05, ** p < 0.01, two-tailed. *** p < 0.001, two-tailed.
3.2. Graph theory metrics
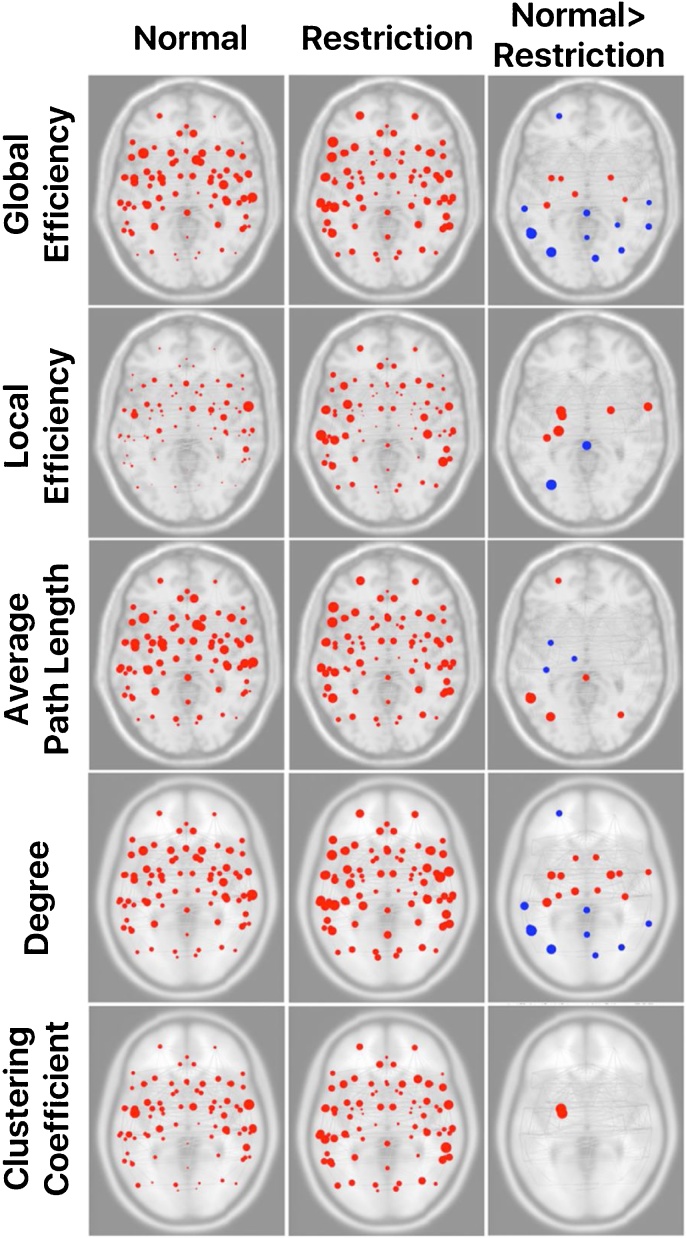
In comparison to a night of restricted sleep, a normal night of sleep was associated with greater local and global efficiency in bilateral amygdala and left posterior fusiform gyrus. Additionally, the left amygdala exhibited a significantly greater clustering coefficient along with the left anterior parahippocampus following a normal night of sleep in comparison to sleep restriction. Limbic circuitry inclusive of bilateral amygdala, fusiform, thalamus, and right nucleus accumbens had higher degreeness following normal compared to restricted sleep. In contrast, the posterior cingulate cortex (PCC) and left superior portions of the lateral occipital cortex demonstrated greater local and global efficiency, while other sensory processing/integration regions also exhibited greater global efficiency (i.e., left angular gyrus, precuneus, bilateral anterior supramarginal gyri, and right superior parietal lobule) following a night of sleep restriction. Differences in average path length were also noted such that increases in sensory processing networks were observed following a normal night of sleep, while greater path lengths were exhibited in the left thalamus and fusiform regions following a night of sleep restriction (Fig. 1, Table 2).
Fig. 1.
Significant differences across graph theory metrics between normal and restricted sleep conditions. Red dots indicate regions where normal > restriction, and blue dots indicate regions where restriction > normal. Dot size is an indication of the magnitude (t-value). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Table 2.
Regions of significant difference as a result of acute sleep restriction.
| Graph Theory Results | |||
|---|---|---|---|
| Global Efficiency Differences | |||
| Contrast | Region | t-value | p-FDR corrected |
| Normal > Restriction | Left Amygdala | 3.15 | 0.035 |
| Left Anterior Temporal Fusiform Cortex | 3.62 | 0.018 | |
| Left Posterior Temporal Fusiform Cortex | 3.80 | 0.018 | |
| Left Thalamus | 3.47 | 0.020 | |
| Right Amygdala | 3.46 | 0.020 | |
| Right Posterior Temporal Fusiform Cortex | 2.97 | 0.040 | |
| Restriction > Normal | Left Angular Gyrus | −5.92 | 0.001 |
| Left Anterior Supramarginal Gyrus | −3.64 | 0.018 | |
| Left Frontal Pole | −3.45 | 0.020 | |
| Left Inferior Temporal Gyrus (temporooccipital part) | −3.99 | 0.018 | |
| Left Superior Lateral Occipital Cortex | −5.61 | 0.001 | |
| Posterior Cingulate | −3.86 | 0.018 | |
| Precuneous | −2.98 | 0.040 | |
| Right Anterior Supramarginal Gyrus | −2.98 | 0.040 | |
| Right Cuneal Cortex | −4.06 | 0.018 | |
| Right Middle Temporal Gyrus (temporooccipital part) | −3.66 | 0.018 | |
| Right Superior Lateral Occipital Cortex | −3.67 | 0.018 | |
| Right Superior Parietal Lobule | −3.03 | 0.040 | |
| Local Efficiency | |||
| Normal > Restriction | Left Amygdala | 4.21 | 0.019 |
| Left Anterior Parahippocampal Cortex | 3.81 | 0.028 | |
| Left Hippocampus | 4.44 | 0.018 | |
| Left Posterior Temporal Fusiform Cortex | 3.31 | 0.047 | |
| Right Amygdala | 3.33 | 0.047 | |
| Right Anterior Superior Temporal Gyrus | 3.65 | 0.028 | |
| Restriction > Normal | Left Superior Lateral Occipital Cortex | −4.37 | 0.018 |
| Posterior Cingulate | −3.74 | 0.028 | |
| Average Path Length | |||
| Normal > Restriction | Left Angular Gyrus | 5.88 | 0.002 |
| Left Frontal Pole | 3.47 | 0.035 | |
| Left Inferior Temporal Gyrus (temporooccipital part) | 3.64 | 0.034 | |
| Left Superior Lateral Occipital Cortex | 5.13 | 0.004 | |
| Posterior Cingulate | 3.70 | 0.034 | |
| Right Superior Lateral Occipital Cortex | 3.75 | 0.034 | |
| Restriction > Normal | Left Anterior Temporal Fusiform Cortex | −3.36 | 0.039 |
| Left Posterior Temporal Fusiform Cortex | −3.50 | 0.035 | |
| Left Thalamus | −3.21 | 0.048 | |
| Degree | |||
| Normal > Restriction | Left Posterior Temporal Fusiform Cortex | 4.33 | 0.013 |
| Left Accumbens | 2.87 | 0.041 | |
| Left Amygdala | 3.53 | 0.028 | |
| Left Anterior Temporal Fusiform Cortex | 3.94 | 0.016 | |
| Left Hippocampus | 3.02 | 0.035 | |
| Left Thalamus | 3.77 | 0.018 | |
| Right Accumbens | 3.04 | 0.035 | |
| Right Amygdala | 4.12 | 0.015 | |
| Right Anterior Middle Temporal Gyrus | 2.98 | 0.035 | |
| Right Anterior Temporal Fusiform Cortex | 3.16 | 0.035 | |
| Right Posterior Temporal Fusiform Cortex | 3.31 | 0.035 | |
| Right Thalamus | 3.05 | 0.035 | |
| Restriction > Normal | Left Angular Gyrus | −5.04 | 0.009 |
| Left Anterior Supramarginal Gyrus | −3.90 | 0.016 | |
| Left Frontal Pole | −3.06 | 0.035 | |
| Left Inferior Temporal Gyrus (temporooccipital part) | −3.45 | 0.029 | |
| Left Superior Lateral Occipital Cortex | −4.56 | 0.012 | |
| Posterior Cingulate | −3.19 | 0.035 | |
| Precuneous | −3.00 | 0.035 | |
| Right Cuneal | −3.00 | 0.035 | |
| Right Middle Temporal Gyrus (temporooccipital part) | −3.08 | 0.035 | |
| Right Superior Lateral Occipital Cortex | −2.95 | 0.036 | |
| Clustering Coefficient | |||
| Normal > Restriction | Left Amygdala | 4.80 | 0.010 |
| Left Anterior Parahippocampal Cortex | 4.69 | 0.010 | |
3.3. Independent component analysis: functional networks
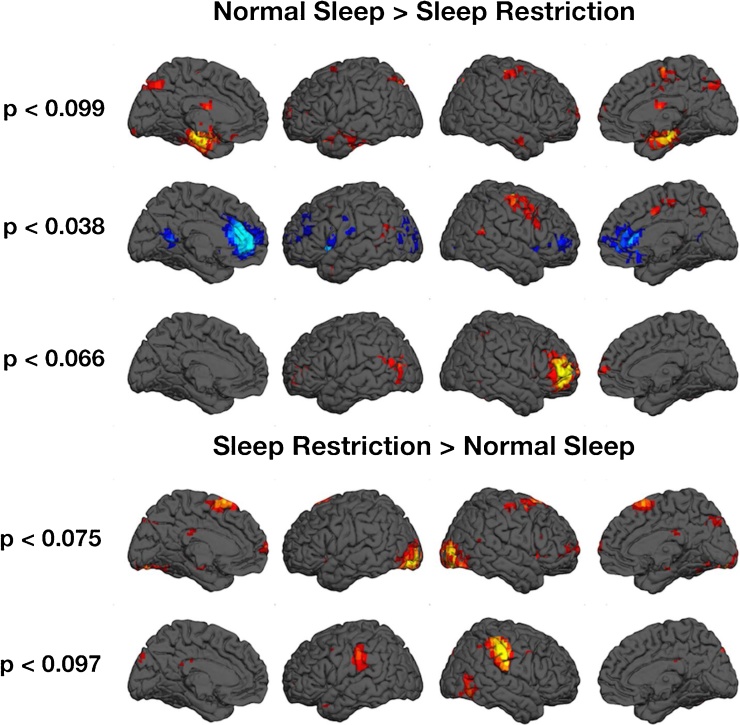
Our data demonstrate hyperactive nodes within the default mode network (i.e., the anterior cingulate cortex (ACC; BA24/32), PCC (BA30), and the inferior/middle frontal gyri (BA9/46)) associated with sleep restriction, along with hypoactive regions of the dorsolateral prefrontal cortex (dlPFC). Although not significant, it is noteworthy that differences between conditions within the limbic network approached significance, with normal sleep demonstrating greater activity (Fig. 2).
Fig. 2.
Neurophysiological differences between normal and restricted sleep as evidenced by independent component analyses of task-independent fMRI.
3.4. Regional homogeneity (ReHo)
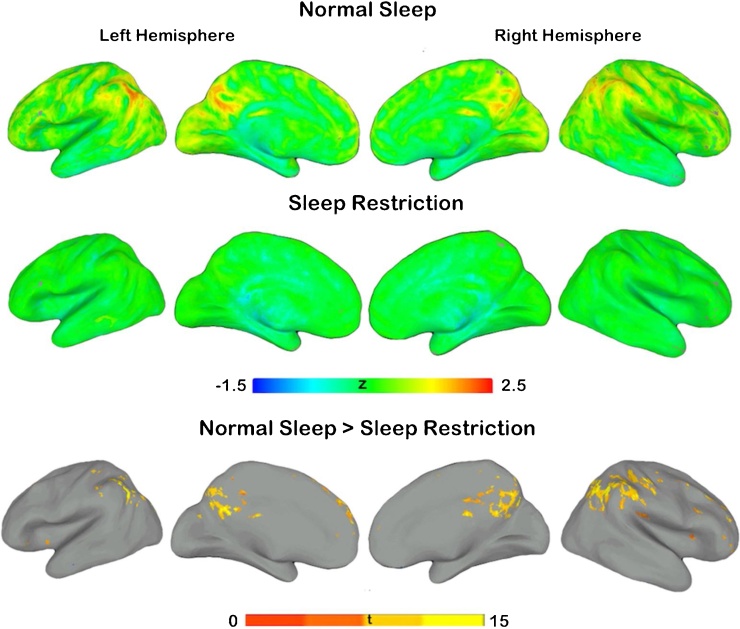
Statistically significant differences in ReHo were found with increased local connectivity in participants following normal sleep compared to restricted sleep. Local connectivity differences were identified primarily throughout the right hemisphere inclusive of the supramarginal gyrus, posterior cingulate, precuneus, medial prefrontal cortex (MPFC), and middle cingulate gyrus (Fig. 3, Table 3). Only one local connectivity difference was noted in the left hemisphere (inferior parietal lobule). No significant increases in connectivity were identified in the sleep restriction > normal sleep contrast.
Fig. 3.
Regional homogeneity (ReHo) differences between normal sleep and sleep restriction.
Table 3.
Significant differences in regional homogeneity between normal sleep and sleep restriction conditions. Data were thresholded at p < 0.0001.
| Regional Homogeneity (Normal Sleep > Sleep Restriction) | |||||
|---|---|---|---|---|---|
| Region | Cluster size | Peak Coordinates (MNI) |
|||
| (in voxels) | x | y | z | t | |
| Right Supramarginal Gyrus | 2021 | 62 | −46 | 32 | 14.77 |
| Right Precuneus/Posterior Cingulate | 1069 | 6 | −50 | 38 | 12.49 |
| Right Superior Medial Gyrus | 467 | 2 | 56 | 10 | 11.46 |
| Left Inferior Parietal Lobule | 421 | −50 | −52 | 50 | 11.83 |
| Right Middle Cingulate Cortex | 233 | 2 | −20 | 32 | 11.42 |
| Right Middle Frontal Gyrus | 195 | 50 | 14 | 44 | 11.86 |
| Right Middle Frontal Gyrus | 141 | 24 | 52 | 26 | 11.60 |
| Right Middle Frontal Gyrus | 85 | 38 | 56 | 16 | 11.34 |
| Right Middle Frontal Gyrus | 69 | 44 | 30 | 38 | 15.19 |
| Right Rolandic Operculum | 51 | 50 | −24 | 18 | 14.14 |
3.5. Behavioral results
For the BART, one-tailed, paired t-tests revealed significant effects for the average number of pumps per trial (M±SEM: Mrestriction = 4.81 ± 0.18, Mnormal = 4.55 ± 0.22, t(17) = 1.796, p < 0.05), the number of successful trials (Mrestriction = 14.78 ± 1.18, Mnormal = 17.78 ± 1.41, t(17) = -3.956, p < 0.001), and the number of blocks (Mrestriction = 22.06 ± 0.84, Mnormal = 23.94 ± 1.07, t(17) = -2.058, p < 0.03). The number of popped balloons approached significance (Mrestriction = 7.28 ± 0.71, Mnormal = 6.17 ± 0.53, t(17) = 1.668, p = 0.057). For the Go/NoGo task, sleep restriction led to more errors of omission (Mrestriction = 15.11 ± 4.72, Mnormal = 7.00 ± 1.49, t(17) = 1.739, p < 0.05), greater reaction times (Mrestriction = 327.40 ± 13.77 ms, Mnormal = 302.14 ± 10.27 ms, t(17) = 2.072, p < 0.03), and less accuracy (Mrestriction = 206.44 ± 5.38, Mnormal = 215.44 ± 1.62, t(17) = −1.771, p < 0.05). These results suggest that across tasks, the sleep restriction condition led to greater risk-taking behavior, and less accurate responding.
4. Discussion
The extant literature has attempted to understand the neuropathophysiological mechanisms underlying sleep deprivation by focusing on the obvious targets of affective processing – namely, fronto-limbic and fronto-striatal connections – predominantly with adult samples. Using whole-brain analyses (i.e., as opposed to seed-based or ROI-based approaches), strong methodology (i.e., a within-subjects crossover design), novel analytic methods (i.e., graph theory and regional homogeneity), and a 7 T scanner, we identify additional neural components that may contribute to the system-wide effects of a single night of sleep restriction during mid-adolescence.
Our data support previous findings of disrupted striatal and limbic circuitry in adults (De Havas et al., 2012; Mullin et al., 2013), and extend them to youth. Building on the literature, we used graph theory metrics to explicate the neural environment resulting from a night of sleep restriction (4 h in bed) and a night of normal sleep (8 h in bed). Specifically, we found that following normal sleep, key limbic structures (i.e., bilateral amygdala) appear to have greater local efficiency (e.g., greater average path connecting all neighbors of a given node) and global efficiency (e.g., the inverse of the average shortest path length between all node pairs), as well as a higher degreeness (e.g., the number of edges originating from a single node). These results suggest that the functional integrity of the amygdala may break down following sleep restriction, resulting in less efficient processing. Similar patterns were observed for the accumbens and left hippocampus. Of note is that our graph theory metrics did not highlight many PFC regions, as one would expect, with the exception of the frontal pole, which had greater global efficiency and degreeness in the sleep restriction condition. We did note that the default mode network, inclusive of the dlPFC and regions critically involved in a host of affective and cognitive processes (i.e., ACC and PCC), were significantly different between conditions. Together, these data confirm and extend findings regarding the coupling of limbic and cognitive control centers (Shao et al., 2014).
Across analyses, however, we observed convergent evidence for significant aberrations in PCC function associated with sleep restriction. Specifically, we found differences in the default mode network, inclusive of the ACC and PCC, along with greater local and global efficiency in the PCC concomitant with shorter average path lengths following a night of sleep restriction. We also demonstrated regional homogeneity differences. This result is intriguing because of the vast array of processes that the PCC is involved in, including reward prediction, learning, attention, memory, emotion, nociception, and interoception (Johnson et al., 2006; Vogt et al., 2006; Pearson et al., 2011; Leech and Sharp, 2014). Furthermore, the precuneus/PCC node is considered the core of the default mode network (DMN) with vital neurofunctional roles in task positive and task negative networks (Utevsky et al., 2014). Therefore, sleep restricted participants who showed weaker local connectivity of this region may have poor flexibility in cognitive processes compared to participants who had normal sleep. We demonstrate behavioral indices suggesting that sleep restriction lead to less accuracy and increased reaction time on a task of inhibitory control and increased risky choice behavior on the BART (i.e., greater average number of balloon pumps, but less success). Given the functional connectedness and vast structural connectivity (Vogt et al., 2006), these data suggest that the PCC may be a neural hub at the center of sleep restriction or deprivation. Indeed, recent studies in adults have noted functional connectivity differences within the PCC as a result of sleep alterations (De Havas et al., 2012; Killgore et al., 2012; Shao et al., 2014). With such extensive system-wide effects resulting from a single night of sleep restriction, it is plausible that the PCC may be causing the downstream effects within fronto-limbic and fronto-striatal circuitry commonly associated with sleep disruptions.
Additionally, it should be noted that the main clusters of local connectivity differences (as determined by ReHo analyses) between the two conditions were largely throughout the parietal cortex, comprising of the inferior parietal lobule, precuneus/PCC, and supramarginal gyrus, consistent with the results of the ICA analysis. These regions are involved in several different cognitive processes, such as visual attention (Chambers et al., 2004; Stevens et al., 2005), working memory (Uncapher and Wagner, 2009), and semantic processing (Binder et al., 2009). Weaker local connectivity of these regions in sleep deprived participants in our study may reflect the impact of sleep on these cognitive functions.
Together, these complementary analytic approaches may suggest that following sleep restriction, there are concomitant network changes such that the amygdala-dlPFC relationship becomes altered via less efficient local and global processing. Furthermore, the PCC becomes more engaged and may mediate the cognitive, emotional, and interoceptive effects commonly described in sleep deprivation studies. Lastly, trends toward greater activity throughout occipital and parietal networks may suggest heightened sensory processing, or hypervigilent states. These data highlight the importance of whole-brain analyses to better understand the neurodynamics underlying sleep restriction, which may inform our understanding of sleep deprivation.
It should be noted that this study has limitations. First, while we collected two days of sleep actigraphy data prior to the manipulation, this does not account for potential habitual sleep patterns leading up to the study. As such, it is possible that participants may have already been operating under abnormal sleep patterns, which could have affected the results of the study. For example, our two groups differed in total sleep minutes for the two nights preceding the manipulation, despite going to bed, falling asleep, and waking up at similar times. We did not account for these differences in our analyses, so it may be possible that the results are indicative of differences that emerge from a more chronic, rather than acute, sleep restriction. Second, while the use of ultra-high field functional neuroimaging is novel, the field is relatively new, and it is possible that there are limitations in using this methodology (i.e., greater artifacts).
To our knowledge, these data are the first ultra-high field submillimeter functional neuroimaging data to emerge in an adolescent sample. The neurofunctional consequences of one night of sleep restriction are largely unknown in this population, and utilizing a 7 T magnet may have afforded greater sensitivity to detect subtle network disruptions. Additionally, it is possible that the added signal-to-noise ratio of the 7 T, which is primarily driving the increased BOLD sensitivity, coupled with the increased spatial resolution, provided an opportunity to identify regions that are involved in neural circuitry that would not otherwise be detected at lower field strengths. Thus, future studies should be employed to deconstruct the contributions of regions that have been largely overlooked in the sleep deprivation literature (i.e., ACC, PCC, and sensory processing regions).
Conflict of Interest
None.
Acknowledgements
This research was supported by Auburn University’s Internal Grants Program. The authors express their gratitude to Professor Avi Sadeh who was instrumental in the development of the sleep restriction component of the study design. The authors would also like to thank Bridget Wingo (research coordinator), and the many graduate students who were instrumental in the organization, recruitment, and data collection for this project including Jerry E. Murphy, M.S., Lauren A. J. Kirby, M.S., Ekjyot Saini, and Ashley Hill, and Jose Omar Maximo for his contributions to some of the analyses of the data.
Footnotes
ICA and graph theory analyses were performed with and without the left-handed individuals. Because no differences emerged between statistical maps, we included the individuals in all group analyses. Importantly, the functional neuroimaging analyses performed for this project did account for the within-subjects nature of the data.
References
- Acebo C., Carskadon M.A. Bradley Sleep Center, Brown University; Providence, RI: 2001. Scoring Actigraph Data Using ACTION-W 2. [Google Scholar]
- ActionW User’s Guide version 2.4 (2002). Ardsley, NY: Ambulatory Monitoring, Inc.
- Andersen S.L., Teicher M.H. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Bartel K.A., Gradisar M., Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med. Rev. 2015;21:72–85. doi: 10.1016/j.smrv.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Baum K.T., Desai A., Field J., Miller L.E., Rausch J., Beebe D.W. Sleep restriction worsens mood and emotion regulation in adolescents. J. Child Psychol. Psychiatry. 2014;55:180–190. doi: 10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S.P., Langberg J.M., Byars K.C. Advancing a biopsychosocial and contextual model of sleep in adolescence: a review and introduction to the special issue. J. Youth Adolesc. 2015;44:239–270. doi: 10.1007/s10964-014-0248-y. [DOI] [PubMed] [Google Scholar]
- Beebe D.W., DiFrancesco M.W., Tlustos S.J., McNally K.A., Holland S.K. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav. Brain Funct. 2009;5:1–7. doi: 10.1186/1744-9081-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver T.S., Barch D.M., Gray J.R., Molfese D.L., Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Carskadon M.A., Acebo C., Jenni O.G. Regulation of adolescent sleep: implications for behavior. Ann. N.Y. Acad. Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Cazzell M., Li L., Lin Z.-J., Patel S.J., Liu H. Comparison of neural correlates of risk decision making between genders: an exploratory fNIRS study of the balloon analogue risk task (BART) NeuroImage. 2012;62:1896–1911. doi: 10.1016/j.neuroimage.2012.05.030. [DOI] [PubMed] [Google Scholar]
- Chambers C.D., Payne J.M., Stokes M.G., Mattingley J.B. Fast and slow parietal pathways mediate spatial attention. Nat. Neurosci. 2004;7:217–218. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Congdon E., Bato A., Schonberg T., Mumford J., Karlsgodt K., Sabb F., London E., Cannon T., Bilder R., Poldrack R. Differences in neural activation as a function of risk-taking task parameters. Front. Neurosci. 2013;7(173) doi: 10.3389/fnins.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E.J., Copeland W., Angold A. Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? J. Child Psychol. Psychiatry. 2011;52:1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E., Lewin D.S. Pathways to adolescent health sleep regulation and behavior. J. Adolesc. Health. 2002;31:175–184. doi: 10.1016/s1054-139x(02)00506-2. [DOI] [PubMed] [Google Scholar]
- Dahl R.E., Spear L.P., editors. Adolescent Brain Development: Vulnerabilities and Opportunities. New York Academy of Sciences; New York, NY: 2004. [DOI] [PubMed] [Google Scholar]
- De Havas J.A., Parimal S., Soon C.S., Chee M.W.L. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage. 2012;59:1745–1751. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Deco G., Ponce-Alvarez A., Hagmann P., Romani G.L., Mantini D., Corbetta M. How local excitation-inhibition ratio impacts the whole brain dynamics. J. Neurosci. 2014;34:7886–7898. doi: 10.1523/JNEUROSCI.5068-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Nearing K.I., LeDoux J.E., Phelps E.A. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M., Erath S.A. Family conflict, autonomic nervous system functioning, and child adaptation: State of the science and future directions. Dev. Psychopathol. 2011;23:703–721. doi: 10.1017/S0954579411000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan J.E., Reiter R.J., Bax M.C.O., Ribary U., Freeman R.D., Wasdell M.B. Long-term sleep disturbances in children: a cause of neuronal loss. Eur. J. Paediatr. Neurol. 2010;14:380–390. doi: 10.1016/j.ejpn.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Jiang L., Zuo X. Regional homogeneity: a multimodal, multislice neuroimaging marker of the human connectome. Neuroscientist. 2016;22:486–505. doi: 10.1177/1073858415595004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.K., Raye C.L., Mitchell K.J., Touryan S.R., Greene E.J., Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Soc. Cogn. Affect. Neurosci. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karen L.H., Rachel E.T., Susan F.T. Adolescent marijuana users have elevated risk-taking on the balloon analog risk task. J. Psychopharmacol. 2014;28:1080–1087. doi: 10.1177/0269881114550352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W. Self-reported sleep correlates with prefrontal-amygdala functional connectivity and emotional functioning. Sleep. 2013;36:1597–1608. doi: 10.5665/sleep.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D., Schwab Z.J., Weiner M.R. Self-reported nocturnal sleep duration is associated with next-day resting state functional connectivity. NeuroReport. 2012;23(74):1–745. doi: 10.1097/WNR.0b013e3283565056. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Kamimori G.H., Balkin T.J. Caffeine protects against increased risk-taking propensity during severe sleep deprivation. J. Sleep Res. 2011;20:395–403. doi: 10.1111/j.1365-2869.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- Latora V., Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., Richards J.B., Ramsey S.E., Stuart G.L., Strong D.R., Brown R.A. Evaluation of a behavioral measure of risk taking: the balloon analogue risk task (BART) J. Exp. Psychol.: Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Mindell J.A., Owens J.A., editors. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. Lippincott Williams & Wilkins; Philadelphia, PA: 2010. [Google Scholar]
- Mullin B.C., Phillips M.L., Siegle G.J., Buysse D.J., Forbes E.E., Franzen P.L. Sleep deprivation amplifies striatal activation to monetary reward. Psychol. Med. 2013;43:2215–2225. doi: 10.1017/S0033291712002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation . 2006. Sleep in America Poll. [Google Scholar]
- Pearson J.M., Heilbronner S.R., Barack D.L., Hayden B.Y., Platt M.L. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn. Sci. 2011;15:143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J., Casey B.J., van Erp T.G.M., Tamm L., Epstein J.N., Buss C., Bjork J.M., Molina B.S.G., Velanova K., Mathalon D.H., Somerville L., Swanson J.M., Wigal T., Arnold L.E., Potkin S.G. ADHD and cannabis use in young adults examined using fMRI of a Go/NoGo task. Brain Imaging Behav. 2016;10:761–771. doi: 10.1007/s11682-015-9438-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L.E., Rau H., Lutzenberger W., Birbaumer N. Mapping P300 waves onto inhibition: Go/No-Go discrimination. Electroencephalogr. Clin. Neurophysiol. 1994;92:44–55. doi: 10.1016/0168-5597(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Robles E., Emery N.N., Vargas P.A., Moreno A., Marshall B., Grove R.C., Zhang H. Patterns of responding on a balloon analogue task reveal individual differences in overall risk-taking: choice between guaranteed and uncertain cash. J. Gen. Psychol. 2014;141:207–227. doi: 10.1080/00221309.2014.896781. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Colón S.M., He F., Bixler E.O., Fernandez-Mendoza J., Vgontzas A.N., Calhoun S., Zheng Z.-J., Liao D. Sleep variability and cardiac autonomic modulation in adolescents–Penn State Child Cohort (PSCC) study. Sleep Med. 2015;16:67–72. doi: 10.1016/j.sleep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Brammer M.J., Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., Simmons A., Williams S.C., Giampietro V., Andrew C.M. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. NeuroImage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sadeh A., Sharkey K.M., Carskadon M.A. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(20):1–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- Sadeh A., Acebo C., Seifer R., Aytur S., Carskadon M.A. Activity-based assessment of sleep-wake patterns during the 1st year of life. Infant. Behav. Dev. 1995;18:329–337. [Google Scholar]
- Schmüser L., Sebastian A., Mobascher A., Lieb K., Feige B., Tüscher O. Data-driven analysis of simultaneous EEG/fMRI reveals neurophysiological phenotypes of impulse control. Hum. Brain Mapp. 2016;37:3114–3136. doi: 10.1002/hbm.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Lei Y., Wang L., Zhai T., Jin X., Ni W., Yang Y., Tan S., Wen B., Ye E., Yang Z. Altered resting-state amygdala functional connectivity after 36 hours of total sleep deprivation. PLoS One. 2014;9:e112222. doi: 10.1371/journal.pone.0112222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Shimoyama I., Ito T., Abla D., Iwasa H., Koseki K., Yamanouchi N., Sato T., Nakajima Y. The time course of interhemispheric EEG coherence during a GO/NO-GO task in humans. Neurosci. Lett. 1997;233:117–120. doi: 10.1016/s0304-3940(97)00652-6. [DOI] [PubMed] [Google Scholar]
- Shochat T., Cohen-Zion M., Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med. Rev. 2014;18:75–87. doi: 10.1016/j.smrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Smaldone A., Honig J.C., Byrne M.W. Sleepless in America: inadequate sleep and relationships to health and well-being of our nation's children. Pediatrics. 2007;119:S29–S37. doi: 10.1542/peds.2006-2089F. [DOI] [PubMed] [Google Scholar]
- Spruyt K., O’Brien L.M., Cluydts R., Verleye G.B., Ferri R. Odds, prevalence and predictors of sleep problems in school-age normal children. J. Sleep Res. 2005;14:163–176. doi: 10.1111/j.1365-2869.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- Stevens M.C., Calhoun V.D., Kiehl K.A. Hemispheric differences in hemodynamics elicited by auditory oddball stimuli. NeuroImage. 2005;26:782–792. doi: 10.1016/j.neuroimage.2005.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. The effects of poor quality sleep on brain function and risk taking in adolescence. NeuroImage. 2013;71:275–283. doi: 10.1016/j.neuroimage.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher M.R., Wagner A.D. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol. Learn. Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky A.V., Smith D.V., Huettel S.A. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Helm E., Walker M.P. The role of sleep in emotional brain regulation. In: Kring A.M., Sloan D.M., editors. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. Guildford Press; New York, NY: 2010. pp 253–263. [Google Scholar]
- van der Helm E., Walker M.P. Sleep and affective brain regulation. Soc. Personal. Psychol. Compass. 2012;6:773–791. [Google Scholar]
- Vogt B.A., Vogt L., Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Zang Y., Jiang T., Lu Y., He Y., Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]