Abstract
Interest in the ontogeny of memory blossomed in the twentieth century following the initial observations that memories from infancy and early childhood are rapidly forgotten. The intense exploration of infantile amnesia in subsequent years has led to a thorough characterization of its psychological determinants, although the neurobiology of memory persistence has long remained elusive. By contrast, other phenomena in the ontogeny of memory like infantile generalization have received relatively less attention. Despite strong evidence for reduced memory specificity during ontogeny, infantile generalization is poorly understood from psychological and neurobiological perspectives. In this review, we examine the ontogeny of memory persistence and specificity in humans and nonhuman animals at the levels of behavior and the brain. To this end, we first describe the behavioral phenotypes associated with each phenomenon. Looking into the brain, we then discuss neurobiological mechanisms in the hippocampus that contribute to the ontogeny of memory. Hippocampal neurogenesis and critical period mechanisms have recently been discovered to underlie amnesia during early development, and at the same time, we speculate that similar processes may contribute to the early bias towards memory generalization.
Keywords: Ontogeny, Infantile amnesia, Infantile generalization, Hippocampus, Neurogenesis, Critical period
1. Introduction
Experiences during early life, including infancy, can have profound effects on neuropsychological development in adolescence and even adulthood. Remarkably, while the impact of these experiences can endure throughout the lifespan, their memory fades with age. This paradox, known as infantile amnesia (IA), has dominated psychological research on the ontogeny of memory since the publication of Campbell and Spear’s formative review on the subject nearly a half-century ago (Campbell and Spear, 1972). In recent years, interest in the neural correlates of IA has been reignited, owing to developments in modern neuroscience techniques that have significantly advanced our understanding of the neurobiology of memory (Josselyn and Frankland, 2012; Josselyn et al., 2015; Luo et al., 2018; Madsen and Kim, 2016; Seo et al., 2018). Indeed, recent studies using some of these techniques have identified circuit and molecular mechanisms in the brain that are responsible for infantile forgetting (Akers et al., 2014; Travaglia et al., 2016a; Guskjolen et al., 2018). Thus, with the help of contemporary approaches, developmental neuroscience is now shedding light on decades-old questions about the infant mind and brain.
Capitalizing on these advances, we suggest that now is the time for researchers examining the neurobiology of infant and child memory to expand beyond IA. While IA may be the most well-studied and robust phenomenon in the ontogeny of memory, others such as infantile generalization (IG)—the bias towards memory generalization during early development—have also been described across species (Anderson and Riccio, 2005; Keresztes et al., 2018; Rudy and Pugh, 1996). Importantly, the neurobiology of IG and other aspects of developing memory (e.g., enhanced extinction and reversal learning (Gogolla et al., 2009; Guskjolen et al., 2017; Johnson and Wilbrecht, 2011), temporary suppression of fear memories (Bath et al., 2016; Pattwell et al., 2011), and others) are relatively unexplored, and neurobiological research on these phenomena may yield insights relevant to neurodevelopmental disorders (Casey et al., 2015).
In this review, we examine the ontogeny of memory persistence (IA) and specificity (IG) from both psychological and neurobiological perspectives. We first provide an overview of historical behavioral findings on IA, before moving onto neurobiological mechanisms contributing to IA that have recently been identified in the rodent hippocampus. Moving beyond IA, we then consider a different well-documented phenomenon in the ontogeny of memory, IG. To this end, we describe IG at the behavioral level and discuss previous psychological theories related to memory generalization during development. Finally, we offer several hypotheses on how the developing brain may produce generalized memories. Importantly, in our discussions of both IA and IG, we review human and nonhuman animal research together, with the goal of demonstrating that these phenomena in the ontogeny of memory—their behavioral phenotypes and their neural underpinnings—are conserved across species.
2. Psychological foundations of infantile amnesia
It is perhaps not surprising that IA has dominated research on the ontogeny of memory, as the phenomenon has intrigued scientists dating back to the late-nineteenth century (Henri and Henri, 1895; Miles, 1893; Potwin, 1901; Freud, 1914). The earliest published accounts of IA heavily relied on retrospective surveys, and sometimes anecdotal evidence, to determine the earliest memories adults and older children could recall. Despite the methodological issues associated with these types of self-reports, these initial studies consistently found that the earliest experiences that could be recalled were for events that occurred around the third year of life, leading to Freud’s definition of IA as “the failure of memory for the first years of our lives” (Freud, 1914). These initial findings have since been supported by numerous empirical studies published across the following century, all converging on the idea that the memories from our first years are absent, or at best limited (Josselyn and Frankland, 2012; Rubin and Schulkind, 1997; Pillemer and White, 1989; Peterson, 2002). The key findings from this body of literature will be briefly summarized here, but a more complete account of these studies can be found in reviews by Rovee-Collier and Cuevas (2009a), Rovee-Collier and Cuevas (2009b), Bauer (2015), and Josselyn and Frankland (2012).
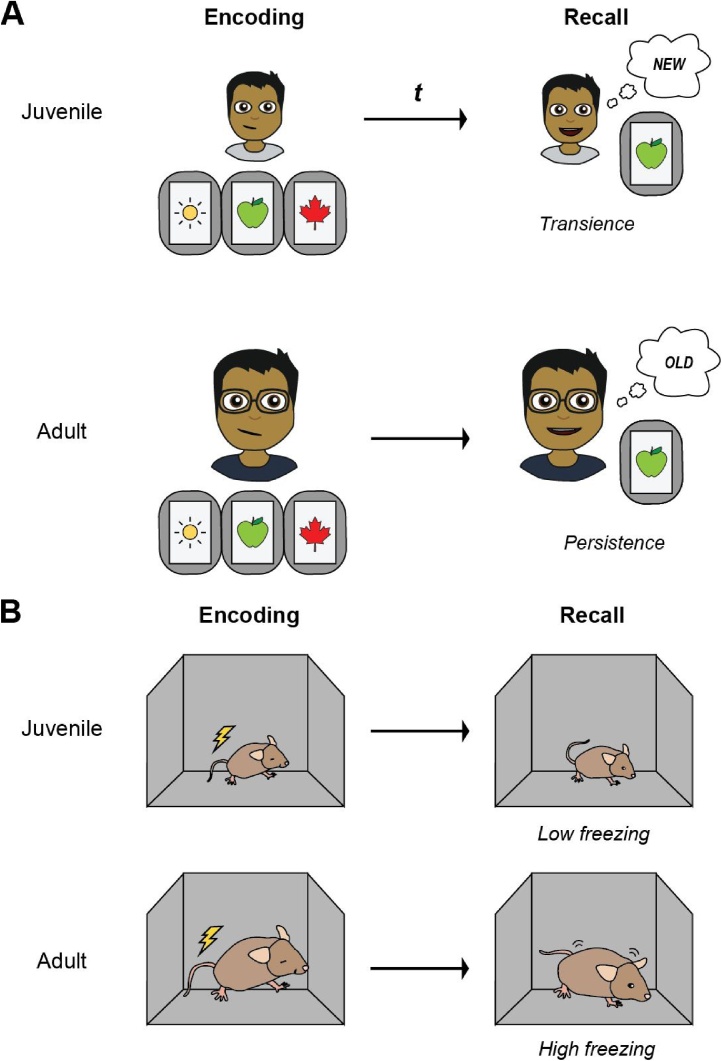
It is now generally accepted that IA refers to the absence of episodic memories from the first 2–3 years of life and an additional period, sometimes referred to as childhood amnesia, from approximately 3–7 years of age from which our memories are low in number and quality (Fig. 1A) (Josselyn and Frankland, 2012; Rubin and Schulkind, 1997; Rubin, 2000). Interestingly, poorer memory for these early years of life seems to be distinct from normal forgetting in adulthood. For example, while a child in their early teens may find it difficult to remember events occurring 10 years ago, this is usually not an issue for younger and older adults, with the latter being able to recall memories from many decades prior. This suggests that the passage of time is not responsible for IA (Wetzler and Sweeney, 1986), but instead that IA represents a period of accelerated forgetting.
Fig. 1.
Infantile amnesia in humans and rodents. (A) Memories for episodic events are increasingly forgotten over time in young children, while episodic memories in adults are more persistent. In the mnemonic similarity task, retention of previously learned objects increases with age. (B) In rodents, infantile amnesia of episodic-like memories can be modelled using contextual fear conditioning tasks. Juvenile rodents show rapid forgetting of the shock-paired environment (low freezing at recall), while adult mice exhibit fear (high freezing) over long delays.
Why then are early memories forgotten so quickly? IA typically refers to the loss of early declarative memories—memories for facts and events that can be consciously recalled (Squire, 1994, 2004), including episodic memories. One possibility is that infants may not be able to form episodic memories, or memories for specific past events (Tulving, 1972). However, research on children as young as 24 months (an age that allows infants to verbally retell experiences) has shown that infants are able to form memories for specific episodes and recount these memories in detail days and sometimes even weeks later (Bauer et al., 2000; Hudson et al., 1992; Nelson, 1993; Bauer, 2007a). Therefore, episodic memories can be formed during early childhood, just not remembered over longer periods of time.
Infantile forgetting also cannot be accounted for by differences in memory encoding across development. In a series of experiments, Rovee-Collier and colleagues trained infants ranging from 2- to 18-months in operant tasks to meet the same response criteria (kicking in a mobile task or lever presses in a train task), which allowed the authors to ask whether infants rapidly forget because they initially form weaker memories. These studies showed that older infants still displayed better memory retention over longer intervals despite younger infants showing equivalent encoding (Rovee-Collier and Cuevas, 2009a; Hartshorn and Rovee-Collier, 1997; Hartshorn et al., 1998a). Thus, these studies demonstrated that weaker memory formation at younger ages is not responsible for IA. Rather, given equivalent encoding, memory retention appears to increase with age linearly over the first 18 months of life.
If memories during infancy can be remembered shortly after formation, but not later in life, what happens to them — are these memories gone, or just inaccessible? In other words, does amnesia for early memories represent a failure of memory storage or memory retrieval? Experimental evidence from both human and animal models of IA suggests the latter; that infantile memories once forgotten can be reinstated (at least, partially) under the right conditions. For example, reminder treatments such as presenting a cue from learning can partially reinstate forgotten memories or extend the natural retention of memories in human infants (Hildreth and Rovee-Collier, 1999; Rovee-Collier et al., 1980; Davis and Rovee-Collier, 1983). Similar work has been conducted using rodent models of IA, which have been especially useful for determining the fate of infantile memories over long stretches of time.
Studies examining infant memory in rodents also support that forgetting in IA is due to a retrieval deficit. Like humans, rodents rapidly forget episodic-like memories—memories for fearful and other novel events that occurred within a distinct context—that are formed during infancy (Fig. 1B) (Guskjolen et al., 2017; Campbell and Campbell, 1962; Ramsaran et al., 2016; Robinson-Drummer and Stanton, 2015; Westbrook et al., 2014). However, these memories encoded during infancy can be recovered later in life using reminders. Brief environmental reminders presented before retrieval can alleviate forgetting in fear, spatial navigation, and novelty-based memory tasks (Travaglia et al., 2016a; Guskjolen et al., 2017; Richardson et al., 1986; Travaglia et al., 2018).
Recovering infantile memories with reminders in rodents requires presentation of cues that are very specific to the initial learning experience. For example, to reinstate infant contextual fear memories, rodents must be re-exposed to a shock stimulus or shock and training context presented on separate occasions (Travaglia et al., 2016a; Smith and Spear, 1984; Spear and Smith, 1978). Likewise, after infant mice have forgotten the location of the escape platform in a watermaze (an episodic-like navigation task), mice must be placed in the exact platform location, but not other nearby locations, to recover the spatial memory (Guskjolen et al., 2017). Researchers have also shown that forgotten infant memories can be recovered using reminder cues up to one month after encoding, when the rodent subjects were approaching adulthood (Guskjolen et al., 2017). Thus, these experiments indicate that highly specific cues are required to reinstate forgotten memories from infancy, but the correct cues can be effective much later in life.
Despite these results, for humans, the likelihood of reinstating childhood memories for real-world events is low. The specificity of cues required to reinstate forgotten memories in empirical rodent studies poses an issue, since the configuration of cues that would be required for recovering human memories outside of experimental contexts may be impossible to know, especially following delays of many years. Further, these reminder experiments in rodents use tasks that form novel, salient memories in infants, and whether reminders would be effective in recovering everyday, mundane memories in rodents or humans is still unknown. It is possible that IA represents a storage or retrieval failure, depending on the salience or importance of the original infantile memory. Nonetheless, the apparent “reactivation” of infant memories with reminder cues confirms that some memories that appear lost to IA are not completely gone, just harder to access. Importantly, this suggests that some memories formed in the infant brain endure in some form into adulthood, and that neurodevelopment possibly contributes to the difficulty in retrieving these memories later in life.
3. Emerging neurobiological mechanisms of infantile amnesia
Current psychological theories of IA have emphasized the emergence of adult-like memory persistence with the development of other psychological or neurobiological processes. For example, some theories of IA have posited that infant memories quickly fade because infants lack language (Nelson, 1993), sense of self (Howe and Courage, 1993), or theory of mind (Perner and Ruffman, 1995), which may be crucial for maintaining memories of an autobiographical nature. These hypotheses are unsatisfactory in fully explaining IA however, as they fail to explain why non-human animals also exhibit amnesia for early life events (Akers et al., 2014; Travaglia et al., 2016a; Guskjolen et al., 2017; Campbell and Campbell, 1962; Robinson-Drummer and Stanton, 2015; Akers et al., 2012). Therefore IA, a phenomenon conserved across species, must also be rooted in a neurobiological process that is conserved across species.
Episodic and episodic-like memories in humans and nonhuman animals, respectively, require the hippocampus for their encoding and initial retrieval (Squire, 2004; Tulving and Markowitsch, 1998). In humans, the hippocampus is activated during the formation and recall of episodic memories (Dolan and Fletcher, 1997; Eldridge et al., 2000; Moscovitch et al., 2016), and individuals with hippocampal damage suffer from episodic memory deficits (Penfield and Milner, 1958; Scoville and Milner, 1957; Vargha-Khadem et al., 1997). Research conducted in rodent models parallels these results from human studies. Neuronal activity in rodent hippocampi contains complex codes of discrete experiences (Leutgeb et al., 2005; McKenzie et al., 2014), and inactivation or activation of hippocampal subfields using different approaches can enhance or impair memory encoding (Kheirbek et al., 2013; Stefanelli et al., 2016) or block or induce memory recall (Kim and Fanselow, 1992; Liu et al., 2012; Park et al., 2016; Vetere et al., 2017). Clearly, the hippocampus has a critical role in mediating episodic memory, and this function is conserved across species. Accordingly, hippocampal development across early life likely has strong implications for the ontogeny of memory in humans and nonhuman animals.
A predominant and long-standing neurobiological theory of IA held by memory researchers studying humans and nonhuman animals alike is that the emergence of hippocampal function underlies the development of memory persistence (Bauer, 2006; Newcombe et al., 2007; Rudy and Morledge, 1994; Nadel and Zola-Morgan, 1984; Mullally and Maguire, 2014). Indeed, the hippocampus undergoes tremendous anatomical development during the earliest stages of life (Mullally and Maguire, 2014; Donato et al., 2017; Gogtay et al., 2006; Lavenex and Banta Lavenex, 2013; Li et al., 2009; Schwartzkroin et al., 1981), which may underlie the emergence of hippocampal memory functions such as spatial cognition at very young ages (Langston et al., 2010; Wills et al., 2010). At a finer level of analysis, hippocampal neurobiology also undergoes key changes at the cellular and molecular levels during infancy. Alterations in gene and receptor expression profiles (Travaglia et al., 2016a; Eastwood et al., 2006; Law et al., 2003a,b; Robinson-Drummer et al., 2018; Travaglia et al., 2016b), synaptic transmission (e.g., an age-dependent increase in neurotransmitter release (Dumas and Foster, 1995) and downscaling of silent synapses (Liao et al., 1999; Petralia et al., 1999), and other mechanisms during infancy have been proposed to trigger the onset of hippocampal function, thereby permitting memory stability (Dumas and Rudy, 2010). However, the finding that forgotten infantile memories encoded before the supposed onset of hippocampal function can be later recovered using specific reminders challenges the idea that IA reflects an incompetent hippocampus. Instead, this raises the possibility that the immature hippocampus can form memories but is unable retrieve them later on. If infantile memories in the hippocampus are successfully formed, why are they forgotten?
In 1972, Campbell and Spear speculated that the rapidly changing neural landscape of the developing brain may be responsible for IA (Campbell and Spear, 1972). Specifically, they thought that either the ongoing maturation of the brain after memory formation or the immaturity of the brain at the time of memory formation could contribute to forgetting of infant memories. Recent studies on the neurobiology of IA suggest that both of these scenarios could be true. Here we will focus on two neurobiological mechanisms of IA discovered in the rodent hippocampus, although it should be noted that other mechanisms have been identified in the basolateral amygdala (BLA) (Madsen and Kim, 2016).
First, our lab has shown that the ongoing high levels of neurogenesis in the hippocampus produces rapid forgetting of memories previously formed by infants (Akers et al., 2014), indicating a role of ongoing hippocampal maturation in forgetting. At the same time, work by Cristina Alberini’s group suggests that infant forgetting is due to immaturity of the hippocampus at the time of learning, and that critical period mechanisms triggered by early experience mature the hippocampus so that it can later retain and express long-term memories (Travaglia et al., 2016a, 2018). Importantly, these two developmental changes in hippocampal neurobiology are conserved across species (Law et al., 2003a,b; Knoth et al., 2010) and therefore could contribute to IA in human infants.
3.1. Infantile amnesia by ongoing hippocampal neurogenesis
Campbell and Spear were the first to consider that the ongoing maturation of the immature brain might affect the ability to recall previously encoded memories (Campbell and Spear, 1972). They describe a process by which “the increase in number of synaptic junctions following learning somehow impedes access to the previously learned response.” Interestingly, new synapses in the hippocampus are continuously generated through the process of new neuron addition, or neurogenesis, which occurs in the dentate gyrus (DG) of mammals throughout life (Amrein et al., 2011; Christian et al., 2014; Frankland et al., 2013). Neurogenesis is strongly regulated by age such that infants have significantly high numbers of proliferating and immature neurons in the DG, but this sharply declines during childhood (Josselyn and Frankland, 2012; Knoth et al., 2010; Boldrini et al., 2018; Seki and Arai, 1995; Sorrells et al., 2018; Zhao et al., 2008). This means that the pattern of neuronal connectivity in memory circuits is under constant remodeling in the infant hippocampus. If stable synaptic connectivity is required for memory persistence (Hayashi-Takagi et al., 2015; Roy et al., 2016), ongoing neurogenesis in the developing DG likely would disrupt memories over time. Based on this idea, our lab developed the neurogenic hypothesis of IA, which posited that the extensive creation of new neurons in the DG during infancy would re-wire the hippocampus, making it difficult to retrieve memories formed during infancy (Josselyn and Frankland, 2012; Frankland et al., 2013). The observations that memory retention increases with age (Rubin and Schulkind, 1997; Rovee-Collier and Cuevas, 2009a; Campbell and Campbell, 1962) as hippocampal neurogenesis declines (Akers et al., 2014; Dolan and Fletcher, 1997; Knoth et al., 2010; Seki and Arai, 1995; Restivo et al., 2015) provided support for our hypothesis. Now, a series of studies from our lab has tested this hypothesis and shown that hippocampal neurogenesis is a mechanism of forgetting in the developing (and mature) brain (Akers et al., 2014; Epp et al., 2016; Gao et al., 2018; Ishikawa et al., 2016).
Although neuroscientists had long been interested in the role neurogenesis plays in memory processes (Christian et al., 2014; Deng et al., 2010; Shors et al., 2001), surprisingly no work had examined the effect of ongoing neurogenesis on previously acquired memories. Key to our neurogenic hypothesis of IA was that hippocampal neurogenesis after memory formation would cause forgetting. Following this logic, we predicted that increasing or decreasing neurogenesis after memory formation should shorten and lengthen retention, respectively (Frankland et al., 2013). We tested this empirically in adult mice by first asking whether increasing neurogenesis after learning would produce infant-like forgetting. Indeed, increasing neurogenesis using environmental (running wheels for voluntary exercise), pharmacological (memantine or the antidepressant fluoxetine), or genetic (deletion of the tumor-suppressing gene p53 from neural progenitors) manipulations after learning caused forgetting in a variety of hippocampus-dependent memory tasks (Akers et al., 2014; Epp et al., 2016; Gao et al., 2018; Ishikawa et al., 2016). Furthermore, if we reduced neurogenesis by genetically ablating neural progenitor cells, we could ameliorate natural forgetting in adult mice, thereby extending the normal retention of hippocampal memories (Epp et al., 2016).
Our experiments in adult mice demonstrated that we could bidirectionally alter memory retention by speeding up or slowing down the process of hippocampal neurogenesis. We next asked whether reducing postnatal neurogenesis in the DG of infant mice would prevent IA, allowing infant mice to express memories after longer delays. As expected, infant mice that had high levels of hippocampal neurogenesis could retain contextual and spatial memories for one day but not one month, consistent with the IA phenotype (Akers et al., 2014; Guskjolen et al., 2017; Akers et al., 2012). However, by reducing hippocampal neurogenesis with temozolamide (a DNA alkylating agent) or the same transgenic approach used by Epp et al. (2016) to ablate dividing cells in the DG, we could prevent the rapid forgetting in these mice (Akers et al., 2014). These experiments offered the first causal evidence that high levels of postnatal neurogenesis contribute to amnesia in infants.
While hippocampal neurogenesis is conserved across mammals, high levels of postnatal neurogenesis are absent in precocial species (Guidi et al., 2005). Precocial rodents such as guinea pigs have longer gestation periods (approximately 9 weeks) compared to altricial rats and mice (3 weeks), allowing much of their neurological maturation to occur pre- rather than postnatally (Altman and Das, 1967). Consequently, precocial rodents are born more neurologically mature than their altricial counterparts and can perform functions that are developmentally delayed in mice and rats (e.g., walking and vision) immediately at birth. In alignment with our neurogenic hypothesis of IA, Campbell and colleagues previously reported that 5-day-old guinea pigs have comparable memory retention to adults (Campbell et al., 1974). In other words, precocial guinea pigs that have low levels of postnatal neurogenesis do not display IA. Based on these previous findings, we tested whether the unusually long retention of infant memories in precocial rodents was causally linked to their low levels of postnatal neurogenesis. Compared to mice, in precocial guinea pigs and degus there was only a modest decrease in dividing and immature cells in the DG between infancy and adulthood, and IA was not observed in infants of either species, even after a month delay (Akers et al., 2014). We reasoned that if the lack of IA in guinea pigs and degus was related to their low levels of neurogenesis, pro-neurogenic manipulations should cause forgetting in these infant rodents as they do in adult mice. Indeed, enhancing neurogenesis in infant guinea pigs and degus made them exhibit the alitricial-like phenotype of accelerated forgetting in infancy (Akers et al., 2014). The significance of these findings is twofold. First, they show that precocial animals lacking postnatal neurogenesis are an exception to IA. Second and more importantly, they imply that the link between neurogenesis and forgetting may generalize to other mammals, including altricial humans in which postnatal and adult hippocampal neurogenesis occurs (Josselyn and Frankland, 2012; Knoth et al., 2010; Boldrini et al., 2018; Sorrells et al., 2018; Kempermann et al., 2018).
One question that remained unanswered from our initial studies is how new neurons impede recall of previously formed memories. The finding that forgotten memories can be reinstated with reminders suggests that some infantile memories, although largely inaccessible, must persist in the brain over time. Numerous studies examining the physical representation of memories in the brain, or engrams, converge on the idea that memory engrams involved at the time of learning must be sufficiently reactivated to recall a memory (Josselyn et al., 2015; Liu et al., 2012; Park et al., 2016; Tanaka et al., 2014). The stability of neuronal circuits is crucial for this reactivation process since artificial (Hayashi-Takagi et al., 2015; Abdou et al., 2018; Ryan et al., 2015) or pathological (Roy et al., 2016) disruption of synaptic connectivity impairs both engram reactivation and behavioral expression of memories. We hypothesized that post-encoding neurogenesis might produce forgetting in the same way. By adding and possibly removing synapses in the hippocampus through neurogenesis (Frankland et al., 2013; Lopez et al., 2012; Yasuda et al., 2011), new neurons may prevent the reactivation of engrams in the hippocampus and other brain regions necessary for memory recall.
Motivated by this idea, we predicted that optogenetic excitation of the engram (Liu et al., 2012) should be able to reawaken infant memories later in life similar to reminder treatments. We recently tested this using transgenic mice in which we could indelibly “tag” engrams with channelrhodopsin (ChR2) during infancy and reactivate them later in life using blue-light stimulation (Guskjolen et al., 2018; Guenthner et al., 2013). Activating engrams in the DG “tagged” during infancy led to partial recovery of contextual fear memories encoded up to 3-months prior, demonstrating that some memories formed in the infant hippocampus indeed persist there into adulthood. Perhaps more interestingly, we found that optogenetic recovery of infantile memories was associated with better reinstatement of encoding activity patterns (i.e., reactivation of the engram) in the hippocampus and other downstream brain regions. IA can therefore be viewed as a brain-wide phenomenon since the addition of new neurons in the DG disrupts reactivation of the engram not only within hippocampus, but also across the brain.
3.2. Infantile amnesia as a developmental critical period for memory
While our data on neurogenesis-mediated forgetting support the idea that continuous maturation of the hippocampus after learning contributes to IA, it is also possible that immaturity of the hippocampus at the time of learning plays a role in rapid forgetting (Campbell and Spear, 1972). Consistent with the ongoing maturation hypothesis of IA, this view proposes that the infant hippocampus is competent to form episodic-like memories but diverges by stating that infant memories are stored in a distinct form that precludes their retrieval once the hippocampus matures (Alberini and Travaglia, 2017). Critically, this hypothesis also asserts that learning during infancy actively matures the hippocampus, which consequently makes infant memories inaccessible. Thus, early learning experiences trigger hippocampal maturation and consequently IA. This model of experience-dependent maturation of the hippocampus parallels that of critical periods in sensory systems during which early sensory experience promotes the emergence of adult-like brain functions (Hensch, 2005; Lee et al., 2017; Nabel and Morishita, 2013). This may mean that IA reflects a critical period for hippocampal memory.
Recent work by Travaglia et al. supports the notion that the hippocampus undergoes a critical period during which it gains the capacity to retain long-term memories (Travaglia et al., 2016a, 2018). Using hippocampus-dependent passive avoidance (PA) and object location (OL) tasks (Bambah-Mukku et al., 2014; Mumby et al., 2002; Quevedo et al., 1999), the authors first established that infant rats forget some hippocampal memories within hours of learning, but these memories can be reinstated with reminder treatments later in life (Travaglia et al., 2016a, 2018). Given that reminder treatments could reactivate the seemingly lost memories similar to previous studies (Guskjolen et al., 2017; Richardson et al., 1986), the authors asked what changes in the developing hippocampus might occlude retrieval of the infant memories in the absence of reminders.
Biochemical assays of hippocampal tissue revealed that during infancy, but not later developmental stages, learning triggered a change in the subunit composition of N-methyl-D-aspartate (NMDA) receptors (Travaglia et al., 2016a). NMDA receptors in the hippocampus are essential for most types of synaptic plasticity and learning (Paoletti et al., 2013; Shimizu et al., 2000; Tsien et al., 1996), and undergo developmental changes in subunit composition in experimentally naïve animals (Paoletti et al., 2013). Specifically, at birth NMDA receptors throughout the brain are predominately composed of GluN2B subunits which negatively regulate AMPA receptor expression and immature synapse formation (Gray et al., 2011; Kelsch et al., 2014; Wang et al., 2011). However, during the second postnatal week GluN2A subunits are progressively added to NMDA receptors, establishing “mature” NMDA receptor function in part by lowering the threshold for the induction of synaptic plasticity (Yashiro and Philpot, 2008). Travaglia, Alberini, and colleagues observed that the hippocampi of infant mice that learned in the PA or OL tasks had a higher GluN2 A/GluN2B ratio (i.e., they more abundantly expressed the GluN2 A subunit compared to GluN2B) compared to naïve infants (Travaglia et al., 2016a, 2018). This molecular phenotype was similar to more mature hippocampi from juveniles and adults that could retain long-term memories (Travaglia et al., 2016a), suggesting that learning during infancy actively matured the hippocampus. A similar increase in GluN2A/GluN2B ratio is observed across human development between infancy and early adolescence (Law et al., 2003a,b), suggesting that early learning might engage a similar subunit exchange in humans.
If early learning experiences transition the hippocampus from an immature (primarily expressing GluN2B) to mature (primarily expressing GluN2A) state, might this represent a critical period for memory? Critical periods are stages of early development characterized by heightened plasticity in brain circuitry and sensitivity of developmental trajectories (behavioral and neural) to ongoing experience (Hensch, 2005). Importantly, the emergence of adult-like behavioral and neural functions depends on exposure to the appropriate experiences during the critical period. For example, balanced visual inputs during early life are essential for the proper development of ocular dominance patterning in the primary visual cortex (V1) (Hensch, 2005; Lee et al., 2017; Nabel and Morishita, 2013; Pizzorusso et al., 2002). Visual stimuli during this critical period triggers a host of molecular changes in V1 including the switch in the composition of NMDA receptors. In rodents, heightened expression of GluN2A is observed shortly after eye-opening and precedes the end of ocular dominance plasticity. Moreover, sensory deprivation around the time of eye-opening delays GluN2A expression, thereby prolonging the critical period in V1 (Matta et al., 2011; Philpot et al., 2001), indicating that the incorporation of GluN2A into NMDA receptors is necessary for maturation of the visual system.
These findings in V1 parallel the discovery that infant learning promotes GluN2A expression in the hippocampus towards adult levels (Travaglia et al., 2016a, 2018), suggesting that early learning may mature the hippocampus in the same way that early visual stimulation develops the visual cortex. In line with this predication, manipulations that cause early expression of GluN2 A in V1 and precociously close the visual critical period, such as engaging brain-derived neurotrophic factor (BDNF) or activating metabotropic glutamate receptor 5 (mGluR5) (Matta et al., 2011; Hanover et al., 1999), should accelerate hippocampal development and lead to an early decline in IA. In line with this prediction, infusions of BDNF or mGluR agonist DHPG into the hippocampus of infant rats led to an early switch in NMDA receptor subunits and allowed the infants to form more persistent PA and OL memories (Travaglia et al., 2016a, 2018). In other words, acute interventions of BDNF and DHPG matured the hippocampus, allowing it to remember experiences from infancy for longer durations. Numerous critical period mechanisms have been identified in V1 including the switch in NMDA receptor subunits (Philpot et al., 2001), maturation of perineuronal nets (Pizzorusso et al., 2002), and expression of endogenous prototoxin Lynx1 (Morishita et al., 2010) or myelin-signalling Nogo receptor (McGee et al., 2005). Whether these critical period mechanisms also contribute to IA remains to be determined, and perhaps more interestingly, whether they regulate other phenomena in the ontogeny of memory is a promising avenue for future research.
4. Psychological foundations of infantile generalization
Ontogenetic memory research has historically focused on memory persistence, while overlooking other aspects of memory that develop similarly across the first years of life. For example, memory specificity also undergoes a protracted course of development that begins at birth (Keresztes et al., 2018; Barr and Brito, 2013). Specificity is a key aspect of memory that controls how information is used to guide behavior. On one end of the continuum, memories can contain rich details about the place and timing of an event, as well as the experienced sights, smells, and sounds. Remembering the particulars of these features permits one to distinguish between similar episodes of the same event. In this way, learned information can be applied only in the appropriate contexts.
While absolute specificity may seem optimal for mnemonic function, incidents of the same event are never experienced identically. For example, the same event can take place in different locations or at different times of day. Therefore, a degree of memory generalization is often necessary to recall memories when the circumstances of an experience (i.e., the context or associated cues) are altered in some way. In other words, generalization allows information to be used flexibly in situations that differ from the original learning event. Memory generalization is regulated by numerous factors including the initial encoding experience (e.g., stimulus intensity or amount of learning trials), memory age (e.g., recent versus remote memory), and stress. The latter of these is associated with maladaptive memory generalization and is hallmark of neuropsychiatric diseases. However, some generalization can be adaptive, for instance in dynamic environments or in novel situations for which there are no corresponding memories to guide behavior (Barr and Brito, 2013; Richards and Frankland, 2017). The latter of these scenarios may be especially common during early life when many life experiences are novel. Accordingly, in this section, we will focus on age, which also is known to regulate memory generalization, but is an understudied area in memory research.
The balance between memory specificity and generalization undergoes a complex trajectory across early development. The ability to generalize memories across cues and contexts emerges over the first 18 months (Barr and Brito, 2013). From this point, memories in toddlers and younger children (approximately 18 months - 10 years) lack detail and are recalled with cues that differ from encoding (i.e., skewed towards generalization). This bias towards memory generalization gradually declines into adulthood when detailed memories are favored, but general knowledge can be utilized depending on the situation (i.e., skewed towards specificity). Here, we will focus on the middle stage of this developmental course which we will refer to as the infantile generalization (IG) period. Although IG has been observed in developing humans and nonhuman animals, this phenomenon is poorly characterized relative to IA. In this section, we will synthesize the current literature related to IG by first reviewing empirical evidence supporting IG as an early stage of memory development. We further discuss previous psychological theories of memory generalization in infants and children. In the following section, we propose neurobiological mechanisms that may be responsible for IG.
The value and utility of memory generalization is underscored by its early emergence in ontogeny. The ability to retrieve memories in novel situations or contexts emerges within the first 1–2 years of life, as illustrated by a series of influential studies by Harlene Hayne and colleagues using the deferred imitation task. In the deferred imitation task, infants are shown a series of actions with a set of props (usually a puppet) during a demonstration phase and then given the opportunity to reproduce the actions following a delay (Meltzoff, 1985). Notably, deferred imitation is thought to assess episodic memory in nonverbal infants (Richmond and Nelson, 2007), since performance is vulnerable to medial temporal lobe damage in older individuals (Adlam et al., 2005; McDonough et al., 1995). Infants as young as 6-months old can imitate actions the day after the demonstration (Barr et al., 1996; Hayne et al., 1997), but their memory is highly specific in that they fail to imitate when features of the puppet (e.g., color or shape) or the location in which the task is performed is changed (Barnat et al., 1996; Hayne et al., 2000; Learmonth et al., 2004). Conversely, generalization in deferred imitation is first observed at 12 months when infants can retrieve action sequences using puppets that differ in color from the demonstration puppet, and by 18-months, infants can generalize their memory across puppets that differ in color and shape and across novel environments (e.g., imitation in the laboratory when demonstrated at home) (Hayne et al., 1997, 2000; Learmonth et al., 2004). A similar course of development is observed using visual paired-comparison task, in which 18-month old but not 6- and 12-month old infants exhibit a novelty preference when the stimulus background color is changed between encoding and retrieval (Robinson and Pascalis, 2004). While the emergence of memory generalization varies by task—in the operant train task, generalization across train sets (i.e., cues) and rooms in the child’s house (i.e., contexts) is observed as early as 9 months (Hartshorn et al., 1998b)—memory generalization across stimuli is consistently observed at 18 months of age. These studies indicate that memory generalization emerges early during infancy, providing the foundation for IG throughout early development.
Central to our conceptualization of IG is the idea that not only is generalized memory utilized during early development, but also that generalization may be prioritized over specificity. In line with this view, retrieval in younger children would favor less-detailed, more gist-like memories (Fig. 2A). Indeed, when asked to recall specific instances of familiar events (e.g., trips to the grocery store, recent meals, playground activities), 3-5-year old children focus on the routine, rather than distinctive elements of the events, describing what usually happens instead of what did happen (i.e., “you do X” instead of “I did X”) (Hudson et al., 1992; Bauer, 2007b; Nelson and Gruendel, 1981; Todd and Perlmutter, 1980; Fivush and Hammond, 1990). At the same time, these children more easily answer questions that probe general, semantic memory content rather than specific, episodic details. For example, 3-5-year-old children more easily answer questions like “what happens at dinner,” that ask for the typical event structure, compared to “what happened at dinner,” which requires recollection of a specific experience from the past (Fivush, 1984; Fivush et al., 1984). Interestingly, children under the age of 5 disproportionately recall general event information despite being able to remember events in great detail (Bauer et al., 2000; Nelson, 1993; Bauer, 2007a), suggesting that the specifics are not lost, but rather overshadowed by generalities. By comparison, beginning around 6 years of age, children’s verbal recall of events becomes more elaborate, and continues to increase in complexity and specificity over the next few years (Price and Goodman, 1990). Over this time, children recall more component activities within events as well as variations in the structure of repeated events (Hudson et al., 1992; Farrar and Goodman, 1992; Slackman et al., 1986), indicating that they can and readily do discriminate episodes of similar events.
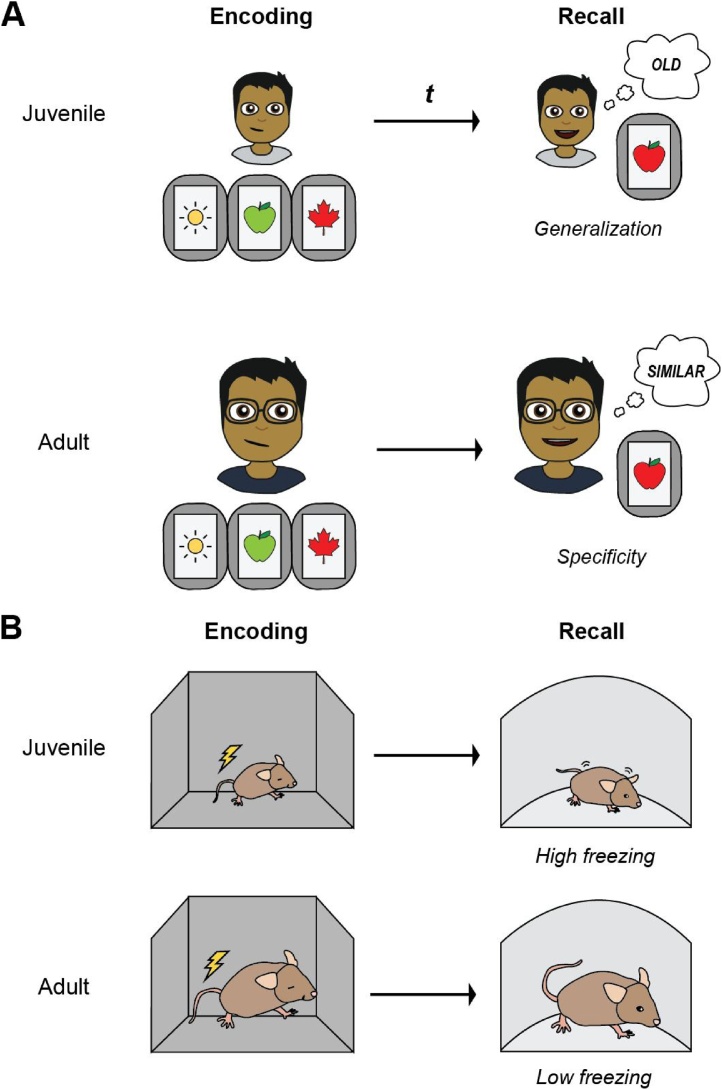
Fig. 2.
Infantile generalization in humans and rodents. (A) Memories for episodic events are recalled generally in young children, while memories in adults are recalled more precisely. In the mnemonic similarity task, discrimination of similar lure stimuli from previously learned objects increases with age. (B) In rodents, infantile generalization of episodic-like memories can be modelled using contextual fear conditioning tasks. Juvenile rodents generalize learned fear across similar environments (high freezing at recall in a novel context), while adult mice recall fear memories specifically (low freezing in a novel context).
Consistent with the emphasis on memory generalities during verbal recall, IG is characterized by generalized memory retrieval—that is, memory retrieval in the presence of cues that differ from initial learning (Fig. 2B). This key feature of IG is supported by empirical studies of memory in both young children and rodents. These studies converge to show that the shape of generalization gradients differs between children and adults. Namely, in contrast to adults that display steep generalization gradients at memory retrieval (i.e., peak responding to conditioned stimuli and low responding to others) (Dunsmoor et al., 2017, 2009; Dunsmoor and Paz, 2015; Ghirlanda and Enquist, 2003; Greenberg et al., 2013; Lissek et al., 2008; Lissek and Grillon, 2012; Schiele et al., 2016), young children respond to a wider range of stimuli after learning, thereby producing broader gradients (Schiele et al., 2016; Block et al., 1970; Mednick and Lehtinen, 1957; Meyer and Orgel, 1969). In fact, children below the age of 5 years produce flat generalization gradients. Children conditioned to respond to the presentation of particular lines, shapes, or auditory tones do so equally even when the stimuli are modified at testing (e.g., rotated, enlarged, etc.) (Block et al., 1970; Landau, 1969, 1968a; Landau, 1968b). Similarly, generalization gradients in adult and infant (approximately 17-day old) rodents mirror those observed in humans. Whereas adult rodents discriminate between conditioned and unconditioned tones and contexts after learning (Armony et al., 1997; Grosso et al., 2018; Han et al., 2008; Rajbhandari et al., 2016), infant rats exhibit flat generalization gradients across auditory (Rudy and Pugh, 1996; Rudy and Hyson, 1984), context (Anderson and Riccio, 2005), and gustatory stimuli (Chotro and Alonso, 2003) after conditioning (but see (Rohrbaugh and Riccio, 1968) for example of better context discrimination in infant compared to adult rats).
Children continue to demonstrate enhanced memory generalization compared to adults past the age of 5 years. However, around this time they begin to show some discrimination between learned and novel stimuli. Interestingly, this stage of development corresponds to the same time when episodic details first become regularly incorporated into verbal recall (see above), which may signal the first step towards the development of adult-like memory specificity. For example, in a large-scale study comparing fear generalization in children (8–10 years) and adults (18–50 years), children were more fearful of non-conditioned face stimuli (i.e., CS- and generalization stimuli; GSs) than adults, despite having poorer memory for the scream-paired face (i.e., CS+) (Schiele et al., 2016). Even though children generalized more than adults, they still displayed more fear to the CS + compared to other stimuli, indicating that they could discriminate. While this study involved a fear conditioning paradigm, broader generalization gradients are also observed in children between the ages of 5–10 years after learning positive (El-Bar et al., 2017) and neutral associations (Mednick and Lehtinen, 1957; Landau, 1969). Thus, like IA, IG seems to be agnostic to memory valence (Josselyn and Frankland, 2012). Furthermore, enhanced generalization has been reported across visual (Schiele et al., 2016; Landau, 1969, 1968a; Landau, 1968b; Glenn et al., 2012; Lau et al., 2011; Michalska et al., 2016), auditory (Block et al., 1970; Gao et al., 2010), spatial (Mednick and Lehtinen, 1957; Tempone, 1966), temporal (Droit-Volet et al., 2001; Droit-Volet and Wearden, 2001), and even semantic (Meyer and Orgel, 1969) dimensions of stimuli in human children up to the age of 12. The observations of generalization across tasks that vary in valence, stimulus modality, and performance demands indicate that IG is a robust phenomenon in childhood memory.
When does the bias towards memory generalization end during development? The answer to this question remains unclear, as there is a lack of systematic experiments on IG. Few studies have directly compared memory generalization in children and adults and even fewer have assessed memory generalization across different stages of childhood (Schiele et al., 2016; Ngo et al., 2018; Rollins and Cloude, 2018). In a study using contextual fear conditioning in rats ranging in age from infancy to adulthood, the transition to adult-like discrimination occurred between P18 and P25 (Anderson and Riccio, 2005), corresponding to a shift during pre-adolescence in human development (Madsen and Kim, 2016). This data tracks with some human studies on developmental memory generalization—a notable increase in fear and spatial discrimination occurs around the age of 10 years in humans (Mednick and Lehtinen, 1957; Glenn et al., 2012; Tempone, 1966)—but other data supports that generalization at recall emerges more gradually. For instance, memory discrimination first improves around the age of 5 (Block et al., 1970; Landau, 1969, 1968a; Landau, 1968b; Droit-Volet et al., 2001; Ngo et al., 2018), but also continues to improve between pre-adolescence and adolescence in humans (9–12 and 13–18 years, respectively) (El-Bar et al., 2017) and rodents (approximately 5 weeks) (Ito et al., 2009). Therefore, it is possible that IG may contain multiple stages (similar to infantile and childhood portions of IA), or that later improvements in mnemonic discrimination represent the development of other, higher-order cognitive faculties. To fully understand the developmental course of IG, more research is needed in humans and animal models that assesses memory generalization across many stages of development using a range of comparable behavioral tasks. In this way, true cross-species comparisons can be made to determine the boundaries of IG.
In addition to further research characterizing IG at the behavioral level, more work is needed to determine why generalized memory is favored during childhood. Multiple theoretical perspectives have been put forth to explain the prominence of memory generalization during early development, but little empirical evidence exists to support these hypotheses. First, in line with ecological models of memory development (Rovee-Collier and Cuevas, 2009a; Spear, 1984), some have proposed that infants and young children may be biased towards recalling general or semantic forms of memory due to their lack of life experiences. In doing so, young children may be able to better predict subsequent occurrences of an event after a single, novel experience (Hudson et al., 1992; Fivush et al., 1984). Indeed, in the context of word referent learning, infants as young as 12 months can rapidly evaluate statistical task information to learn ambiguous word-object pairings (Altmann, 2017; Smith and Yu, 2008) (although they also show a tendency to generalize learned word names from exemplars to similar, novel objects (e.g., similar shapes) (Colunga and Smith, 2005; Smith et al., 2014), lending support to the notion that infants quickly identify commonalities between events to guide behavior. This is in stark contrast to semanticized memory in adults, which is typically thought to emerge gradually over time or as one accumulates similar experiences of an event (Richards and Frankland, 2017; Desiderato et al., 1966; Moscovitch et al., 2006; Richards et al., 2014; Tompary and Davachi, 2017; Winocur et al., 2010). While a shortage of experiences likely contributes to memory generalization in children (Hudson et al., 1992), it alone cannot account for IG since increasing experience with an event (e.g., number of experiences or training trials) in young children does not result in comparable memory specificity to older children and adults (Price and Goodman, 1990; Farrar and Goodman, 1992; Landau, 1968b) and sometimes even widens young children’s generalization gradients (Tempone, 1966). Therefore, other age-related factors must also contribute to IG.
Other explanations of memory generalization during development have focused on the distinction between perceptual confusion and learned equivalence. In other words, does memory generalization during development occur because children are unable to perceive differences between stimuli or because children learn to treat discriminable stimuli as functionally equivalent? Both of these scenarios may contribute to IG. On one hand, perceptual confusion is likely to occur if previously learned stimulus features are forgotten (Barr and Brito, 2013). Following this line of thinking, memory generalization should be absent shortly after memory formation and increase with time (Desiderato et al., 1966), consistent with the co-occurrence of IA during development. Some studies of memory generalization in infant humans (Borovsky and Rovee-Collier, 1990) and rats (Anderson and Riccio, 2005) have shown that memory is retrieved specifically after shorter delays (days and hours in adults and infants, respectively) but generally after longer delays (weeks and days, respectively), which may suggest that forgetting memory details over time produces generalization. However, other researchers have shown that infants and children exhibit generalization immediately after memory encoding (Mednick and Lehtinen, 1957; Landau, 1969, 1968b; Droit-Volet et al., 2001; Bauer and Dow, 1994). Even more interesting is the finding that infants recall generalized memories involving stimuli they can perceptually discriminate (Landau, 1969; Rudy and Hyson, 1984). In one striking example, 14-day old rats were shown to discriminate between a 2 kHz tone and tones that were ± 0.2 kHz using a habituation procedure, but when 17-day old rats were trained to associate the 2 kHz tone with the taste of sucrose, licking behavior was observed in response to tones that were ± 1 kHz in frequency (Rudy and Hyson, 1984). Thus, associative memory formation may modify how cues are processed during childhood in a way that elicits memory retrieval more generally.
Lastly, and perhaps not surprisingly, some theories related to IG have emphasized the role of IA in the early bias toward memory generalization. Different theories such as the fuzzy-trace theory (Brainerd and Reyna, 2004), rooted in human cognitive psychology, and forgetting of stimulus attributes (Brainerd and Reyna, 2004; Riccio et al., 1994, 1984), developed primarily from work done in rodents, share this view. Similar to the idea of perceptual confusion, these theories put forth that memories are encoded simultaneously as detailed (also referred to as verbatim or episodic) and general (also gist or semantic) representations, that are differentially affected by forgetting. Namely, episodic information is vulnerable to accelerated forgetting during childhood while semantic information is not (Nelson and Ross, 1980). Consequently, the forgetting of detailed information over time in children may promote reliance on generalized memory representations. Indeed, the ontogenetic profiles of early life amnesia and generalization overlap significantly, with shifts in mnemonic discrimination following shortly after increases in memory retention (see above). Experiments in humans and rodents have also found that reminders prior to testing alleviate forgetting as well as increase memory specificity in infants (Anderson and Riccio, 2005; Borovsky and Rovee-Collier, 1990), which suggests that IA and IG during development may be functionally linked. If so, an exciting area of future research is to determine whether these phenomena share similar underlying mechanisms. Our increased understanding of the neurobiology of IA might provide a foundation for the beginnings of neurobiological research on IG.
5. Potential neurobiological mechanisms of infantile generalization
Contemporary neuroscience research is only now uncovering the roots of IA at the levels of circuits, cells, and molecules in the brain (Akers et al., 2014; Travaglia et al., 2016a). In contrast, neurobiological research on other developmental memory phenomena has lagged behind, with little research being aimed at identifying the brain mechanisms responsible for IG. Previous research aimed at understanding the neural correlates of specific versus generalized memories in adults has implicated the hippocampus in balancing memory precision (Moscovitch et al., 2016; Ruediger et al., 2011; Guo et al., 2018). Therefore, like the ontogeny of memory persistence, the ontogeny of memory specificity may also be regulated by hippocampal development.
Here, we adopt the view that maturation of the hippocampus may drive the transition to adult-like memory specificity (Keresztes et al., 2018). Given its role in encoding and recalling richly detailed memories, we propose that the poor specificity of childhood memory may reflect the immaturity of the hippocampus at many levels. In this section, we highlight two candidate mechanisms in the developing hippocampus that may account for memory generalization during early life. The aspects of hippocampal maturation discussed here are restricted to the scale of circuits and molecules; however, at a larger scale, asymmetrical anatomical development of hippocampal subfields may also contribute to IG (reviewed by Keresztes et al., 2018; Gomez and Edgin, 2016). The mechanisms we discuss—hippocampal neurogenesis and perineuronal nets—may similarly regulate the allocation of memory representations to similar or distinct neuronal populations. It is our view that this general mechanism may underlie memory generalization during multiple stages of development, including childhood (Armony et al., 1997; Grosso et al., 2018; Ghosh and Chattarji, 2015; Schlichting and Preston, 2015; Schlichting et al., 2014).
5.1. Hippocampal neurogenesis, pattern separation, and pattern integration
As discussed above, hippocampal neurogenesis is elevated during infancy and early childhood in altricial species, and our group discovered that these high rates of postnatal neurogenesis are causally linked to amnesia for early hippocampal memories (Akers et al., 2014). Yet, neuroscience research spanning over a decade supports a broader role for newly generated neurons in memory (reviewed in Deng et al., 2010; Abrous and Wojtowicz, 2015). As a prime example, hippocampal neurogenesis is widely accepted to be critical for pattern separation—the process of transforming similar neural representations into distinct output representations—by the DG (Bernier et al., 2017; Treves and Rolls, 1994). At the neuronal population level, this entails coding of memory engrams for similar experiences in orthogonal groups of cells (Santoro, 2013). Indeed, in adult mice, rates of hippocampal neurogenesis are tied to engram overlap in the DG and CA3. Studies have shown that elevating neurogenesis promotes greater separation of cell assemblies representing different experiences (McAvoy et al., 2016), whereas reducing neurogenesis has the opposite effect (Niibori et al., 2012). At the behavioral level, these neural phenotypes in the hippocampus manifest as enhancements or impairments in behavioral discriminations, respectively (McAvoy et al., 2016; Niibori et al., 2012; Clelland et al., 2009; Sahay et al., 2011). These findings extend to adult humans, in which exercise and stress—factors known to modulate rates of hippocampal neurogenesis—have similar effects on mnemonic discrimination (Dery et al., 2013; Kheirbek et al., 2012).
Hippocampal neurogenesis may contribute to pattern separation at the behavioral and neuronal population levels by engaging inhibitory circuits in the hippocampus. First, granule cells 4–6 weeks after cell division are more excitable than their mature counterparts (Christian et al., 2014; Marin-Burgin et al., 2012; Mongiat et al., 2009), allowing these specific neurons to be preferentially allocated to memory engrams (Park et al., 2016; Gu et al., 2012; Josselyn and Frankland, 2018; Kee et al., 2007; Yiu et al., 2014). Activation of these immature neurons during memory encoding and retrieval thus likely recruits feed-forward inhibition in the DG and CA3 (Meltzer et al., 2005). Immature neurons in the DG form contacts with inhibitory interneurons in the hilus and CA3 (Restivo et al., 2015; Wilke et al., 2013), and in doing so, indirectly promote circuit inhibition and sparse activation of mature principal neurons in the DG and downstream structures (Niibori et al., 2012; Drew et al., 2016; Ikrar et al., 2013). Therefore, by driving inhibitory interneuron activity, these immature neurons influence the uniqueness of hippocampal engrams (i.e., engram size and separation) (Stefanelli et al., 2016; Guo et al., 2018), thereby conferring specificity to memories.
If greater hippocampal neurogenesis improves pattern separation, why then do children with relatively high levels of neurogenesis discriminate memories so poorly? Based on the experiments described above, it should follow that the immature DG is an excellent pattern separator. One possibility is that the neural circuitry for pattern separation is not yet fully developed in children. If true, increasing levels of neurogenesis would have minimal effects on pattern separation abilities. Some human cognitive neuroscientists subscribe to this view, based on the findings that young children perform poorly on mnemonic similarity tasks in which they must discriminate novel lure stimuli from previously encountered exemplars (performance in these tasks is thought to require hippocampal pattern separation) (Keresztes et al., 2018; Ngo et al., 2018; Rollins and Cloude, 2018) and that the DG and CA3 in humans and nonhuman primates undergo slower anatomical development compared to other subfields (Lavenex and Banta Lavenex, 2013; Gomez and Edgin, 2016; Keresztes et al., 2017; Lee et al., 2014). Because of the more protracted development of the DG, early memories may be encoded less precisely downstream in CA1, which has been identified as a locus for memory integration (Schlichting et al., 2014). However, functional imaging data in children performing pattern separation behaviors is needed to support this claim.
IG in the presence of high rates of neurogenesis may also be reconciled by research suggesting that immature granule cells are “pattern integrators.” In other words, the broad tuning and elevated intrinsic excitability of immature granule cells (Marin-Burgin et al., 2012) may allow these cells to encode or respond to multiple different experiences. Aimone, Wiles, and Gage first proposed the pattern integration hypothesis based on their computational model of the DG in which they observed similar activation of immature granule neurons by sequentially learned events (Aimone et al., 2009). In a different line of work, researchers have shown that memory formation enhances excitability within hippocampal and amygdalar engrams for approximately 6 h, allowing a second memory to become co-allocated to the same cell assembly (Abdou et al., 2018; Cai et al., 2016; Rashid et al., 2016). While this phenomenon has not yet been demonstrated in the DG, let alone immature granule cells, these findings lend some support to the pattern integration hypothesis. That is, memory co-allocation follows the same basic principle as pattern integration: enhanced excitability allows multiple experiences to be coded by the same population of neurons.
More direct evidence for pattern integration in the DG comes from recent empirical work employing in vivo recordings to simultaneously monitor activity in immature and mature granule cells. Danielson and colleagues found that while adult mice traversed virtual environments, their immature granule cells were more active than mature cells (i.e., they were more excitable) but less spatially tuned (Danielson et al., 2016). Accordingly, immature granule cells encoded two different environments more similarly than mature cells, indicative of greater representational overlap within the immature cell population. This type of representation overlap between events is likely greater in the developing hippocampus since the pool of immature granule neurons is much larger during childhood than adulthood. Furthermore, the immature DG might be biased towards pattern integration if inhibitory circuits involved in pattern separation may still be underdeveloped. Future studies combining transgenic approaches to specifically label and manipulate immature granule cells active during learning may be able to directly test this pattern integration hypothesis and determine how it contributes to memory generalization during childhood.
5.2. Expression of perineuronal net molecules
In parallel with the protracted ontogenesis of hippocampal anatomy and circuitry, the molecular profile of hippocampus develops slowly across early life, displaying marked changes in both basal (Travaglia et al., 2016b; Mody et al., 2001) and experience-dependent (Travaglia et al., 2016a; Robinson-Drummer et al., 2018; Deal et al., 2016) transcription and translation. Therefore, much like the presence of vast numbers of young granule cells in the immature DG, the changing expression profiles of specific molecules in the developing hippocampus might modulate pattern separation within the hippocampus. More specifically, given the profound regulation of engram formation and recall by interneurons, molecules localized to inhibitory circuits may be privileged in balancing memory specificity and generalization. Here, we focus on a set of molecules that compose perineuronal nets (PNNs), structures in the brain extracellular matrix that are closely associated with inhibitory interneurons. We hypothesize that the development of PNNs around distinct classes of interneurons may establish mature inhibitory circuit function in the hippocampus, and consequently promote adult-like memory specificity.
PNNs are specialized extracellular structures found in the hippocampus and throughout the CNS (Dityatev and Schachner, 2003; Kwok et al., 2011; Sorg et al., 2016; Wang and Fawcett, 2012). A host of molecules contribute to the lattice-like structure of PNNs, with the main components including polymerized chains of hyaluronan (forming the backbone of PNNs), tenascin-R, link proteins (e.g., hyaluronan and proteoglycan link protein 1; HAPLN1), and chondroitin sulfate proteoglycans (CSPGs) (Dityatev and Schachner, 2003; Carulli et al., 2010; Costa et al., 2007; Deepa et al., 2006). Of special interest to brain development are CSPGs belonging to the lectican family (i.e., aggrecan, brevican, neurocan, and versican), which initially are expressed at low levels in rodent and human neonates but continually increase in abundance through childhood and adulthood (Favuzzi et al., 2017; Frischknecht et al., 2014; Mauney et al., 2013; Milev et al., 1998; Ueno et al., 2017). Complete PNN maturation in the rodent hippocampus measured by CSPG immunohistochemistry (i.e., staining density and/or intensity) occurs somewhere between the 2 weeks and 2 months of age (Yamada and Jinno, 2013), although adult levels of specific CSPGs like brevican may be achieved earlier (Favuzzi et al., 2017).
CSPGs in the hippocampus are not randomly dispersed in the extracellular space. Rather, throughout the adult brain they are closely associated with GABAergic cells, preferentially surrounding parvalbumin-expressing (PV+) interneurons (Favuzzi et al., 2017; Yamada and Jinno, 2013; Lensjo et al., 2017a; Yamada and Jinno, 2017; Yamada et al., 2015) (in the hippocampus, subfield CA2 is an exception Carstens et al., 2016; Lensjo et al., 2017b). Although not studied as extensively in the hippocampus, the scarcity of CSPG-containing PNNs around PV + interneurons during development in the BLA and V1 has been linked to critical periods for fear learning (Gogolla et al., 2009) and ocular dominance patterning (Pizzorusso et al., 2002; Lensjo et al., 2017a), suggesting that CSPG expression may be a brain-wide mechanism for regulating plasticity (Lee et al., 2017). Moreover, because critical periods in the BLA and V1 can be reopened in the adult brain by enzymatic digestion of CSPGs with chondroitinase, it is thought that the development of CSPG-rich, mature PNNs around PV + interneurons restricts juvenile plasticity to close critical periods (Hensch, 2005; Nabel and Morishita, 2013).
How CSGP expression specifically curbs critical period plasticity is not completely understood, but some research points to a role for CSPG accumulation in establishing adult-like inhibitory function of PV + interneurons. A recent study focusing on the CGSP brevican found that, in the mouse hippocampus, PV + interneurons wrapped by brevican received more glutamatergic inputs and fired more action potentials than interneurons lacking brevican, despite having higher firing thresholds (Favuzzi et al., 2017). In other words, PV + cells surrounded by brevican were less excitable than their naked counterparts but were more efficient once activated (see also Balmer, 2016). Going even further, these authors demonstrated that developmental deletion of brevican using knockout mice or local RNA interference prevents the normal maturation of synaptic contacts and intrinsic properties of hippocampal PV + interneurons. Consistent with these findings, others have shown that CSPG digestion in V1 of adult rats reduces firing from fast-spiking interneurons in vivo, shifting the balance between cortical excitation and inhibition (Lensjo et al., 2017a). Thus, low expression of CSPGs during development maintains heightened plasticity during critical periods by supporting weak GABAergic inhibition by PV + interneurons.
How might reduced inhibition in the developing hippocampus produce IG? The weak circuit inhibition permitted by low levels of CSPGs during development may produce overlap of different experiences at the level of cell assemblies, much like the pattern integration phenomenon described above. In adult mice with enhanced hippocampal plasticity conferred by a low PV-network state (which can be triggered by CSPG removal or housing in enriched environments), memory formation results in greater immediate-early gene (IEG) expression (Ruediger et al., 2011; Donato et al., 2013), suggesting that these memories are encoded by enlarged engrams. A similar expansion of engrams occurs when PV + interneuron activity is directly attenuated during learning using chemogenetics (Morrison et al., 2016), indicating that inhibitory activity from PV + interneurons is responsible for constraining the size of engrams. To our knowledge, hippocampal engram size has yet to be directly compared between developing and adult animals. However, basal expression of IEGs in the dorsal hippocampus is elevated in young, naïve rodents compared to naïve adults (Travaglia et al., 2016b). Additionally, the findings that fear memory retrieval and visual experience result in greater IEG induction respectively in BLA and V1 of juvenile (<P30) compared to adult (>P70) rodents support the idea that the same experiences recruit more neurons during early life compared to adulthood (Jenks et al., 2017; Syken et al., 2006; Ganella et al., 2018).
Even less is known about how two different experiences activate cell assemblies in the developing hippocampus, which has implications for memory generalization (Grosso et al., 2018). We speculate that engram expansion occurs during development, and in these scenarios, the probability of representational overlap between experiences is increased. Engram overlap in development could happen by chance since the imprecise coding of memories in large populations of neurons increases the probability of sharing cells between representations. Alternatively, weak inhibition also might allow inappropriate recall of engrams during novel experiences. In this way, recall of a previously encoded memory may guide behavior in novel contexts.
Several critical studies are needed to test the hypothesis that IG is the result of imprecise neural coding granted by immature CSPG expression. First, direct comparisons of memory representations in development (during and after IG period) and adulthood should be made using engram tagging methods similar to those used by Guskjolen and colleagues (Guskjolen et al., 2018). These experiments will identify qualitative differences in engram formation and recall between experiences (i.e., engram size and overlap) and provide direct evidence that the developing brain processes memories differently than the adult brain. These experiments should be supplemented by a complete time course of CSPG development around PV + interneurons to determine whether PNN maturation correlates with memory specificity across development, as well as memory engram size. Finally, experiments manipulating CSPGs in the hippocampus of infant and adult animals will provide causal evidence that PNN maturation in the hippocampus regulates memory specificity both at the behavioral and neural population levels.
6. Conclusions and future directions
The study of the ontogeny of memory has benefitted in recent years from rapid technological advancements in neuroscience methods. The application of advanced approaches to observe and manipulate memory molecules, cells, and circuits (Luo et al., 2018; Seo et al., 2018) in the developing brain has paved the way to a better understanding of infantile amnesia from a neurobiological perspective.
Specifically, two recent lines of research on the neural bases of IA provide support for Campbell and Spear’s original proposals that both ongoing brain maturation after learning—in the form of hippocampal neurogenesis (Akers et al., 2014)—and immaturity of the brain at the time of learning—based on a shortage of mature, GluN2 A-contraining NMDA receptors (Travaglia et al., 2016a, 2018)—contribute to amnesia for childhood memories (Campbell and Spear, 1972). Even more, research using advanced optogenetic tools in infant mice has overturned the prevailing dogma that IA reflects the infant hippocampus being unable to form declarative memories (Bauer, 2006; Newcombe et al., 2007; Nadel and Zola-Morgan, 1984; Dumas and Rudy, 2010). Instead, new work from our lab has shown that, despite being rapidly forgotten, some memories formed during infancy persist in the hippocampus into adulthood and can be reactivated by direct stimulation of hippocampal engrams (Guskjolen et al., 2018). Much is still to be learned about IA, but given the new research avenues afforded by modern neuroscience tools, interest in the psychology and neuroscience of IA will likely endure as it has now for nearly 50 years (Campbell and Spear, 1972; Madsen and Kim, 2016; Jabes and Nelson, 2015; Nelson, 1995).
Moving forward, in addition to continued research on IA, neuroscientists studying the ontogeny of memory should turn their attention to other developmental memory phenomena, such as IG. The bias towards memory generalization during infancy and early childhood is observed robustly across-species, yet a clear neurobiological mechanism for this phenomenon has yet to be identified. We speculate that developmental aspects of hippocampal maturation—high levels of postnatal neurogenesis and low levels of perineuronal net molecules—may promote similar neural coding for distinct experiences (Grosso et al., 2018; Ghosh and Chattarji, 2015; Schlichting and Preston, 2015; Schlichting et al., 2014; Schlichting and Frankland, 2017), which in turn may produce memory generalization during childhood.
As discussed above, these questions can now be answered by employing contemporary approaches that are becoming increasingly accessible in developmental research. Progress in collecting high-resolution fMRI data from human infants and children will be necessary for determining the regional contributions of the hippocampus to memory generalization and specificity (Mullally and Maguire, 2014; Prabhakar et al., 2018). In rodents, methods to tag and later observe and manipulate memory engrams in the rodent brain (Guskjolen et al., 2018) can be used localize overlapping memory representations to specific cell populations (e.g., immature granule neurons in the DG) and probe the content of generalized infant memories. Genetic mutations or viruses can also be introduced into the developing brain to knock-down or overexpress genes of interest (Favuzzi et al., 2017; Pena et al., 2017) that are predicted to influence IG (e.g., genes for CSPGs or other PNN molecules). Lastly, viral vectors carrying chemo- or optogenetic effectors can be utilized to discover how activity in defined cell types (e.g., immature granule neurons or PV + interneurons) in the hippocampus shift the balance between generalization and specificity across development (Donato et al., 2017; Wong et al., 2018; Arruda-Carvalho et al., 2017). In closing, the use of advanced methods to study memory development will accelerate our understanding of the neurobiology of IG, and potentially aid in the identification of novel therapeutic targets for treating neurodevelopmental and neuropsychiatric disorders characterized by inappropriate memory generalization.
Conflict of interest
None.
Acknowledgments
We thank Albert Park for the figure artwork. This work was supported by funding from the Natural Sciences and Engineering Council of Canada (A.I.R., M.L.S., P.W.F.), Canadian Institute of Health Research (P.W.F.), Canadian Institute for Advanced Research (P.W.F.), Canada Foundation for Innovation (M.L.S.), and Ontario Research Fund (M.L.S.).
References
- Abdou K. Synapse-specific representation of the identity of overlapping memory engrams. Science. 2018;360(6394):1227–1231. doi: 10.1126/science.aat3810. [DOI] [PubMed] [Google Scholar]
- Abrous D.N., Wojtowicz J.M. Interaction between neurogenesis and hippocampal memory system: new vistas. Cold Spring Harb. Perspect. Biol. 2015;7(6) doi: 10.1101/cshperspect.a018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlam A.L. Deferred imitation of action sequences in developmental amnesia. J. Cogn. Neurosci. 2005;17(2):240–248. doi: 10.1162/0898929053124901. [DOI] [PubMed] [Google Scholar]
- Aimone J.B., Wiles J., Gage F.H. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61(2):187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers K.G. Ontogeny of contextual fear memory formation, specificity, and persistence in mice. Learn. Mem. 2012;19(12):598–604. doi: 10.1101/lm.027581.112. [DOI] [PubMed] [Google Scholar]
- Akers K.G. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344(6184):598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Alberini C.M., Travaglia A. Infantile amnesia: a critical period of learning to learn and remember. J. Neurosci. 2017;37(24):5783–5795. doi: 10.1523/JNEUROSCI.0324-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Das G.D. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214(5093):1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Altmann G.T. Abstraction and generalization in statistical learning: implications for the relationship between semantic types and episodic tokens. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2017;372(1711) doi: 10.1098/rstb.2016.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I., Isler K., Lipp H.P. Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur. J. Neurosci. 2011;34(6):978–987. doi: 10.1111/j.1460-9568.2011.07804.x. [DOI] [PubMed] [Google Scholar]
- Anderson M.J., Riccio D.C. Ontogenetic forgetting of stimulus attributes. Learn. Behav. 2005;33(4):444–453. doi: 10.3758/bf03193183. [DOI] [PubMed] [Google Scholar]
- Armony J.L. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cereb. Cortex. 1997;7(2):157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Arruda-Carvalho M. Optogenetic examination of prefrontal-amygdala synaptic development. J. Neurosci. 2017;37(11):2976–2985. doi: 10.1523/JNEUROSCI.3097-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer T.S. Perineuronal nets enhance the excitability of fast-spiking neurons. eNeuro. 2016;3(4) doi: 10.1523/ENEURO.0112-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambah-Mukku D. A positive autoregulatory BDNF feedback loop via C/EBPbeta mediates hippocampal memory consolidation. J. Neurosci. 2014;34(37):12547–12559. doi: 10.1523/JNEUROSCI.0324-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnat S.B., Klein P.J., Meltzoff A.N. Deferred imitation across changes in context and object: memory and generalization in 14-month-old infants. Infant Behav. Dev. 1996;19(2):241–251. doi: 10.1016/S0163-6383(96)90023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R., Brito N. From specificity to flexibility: early developmental changes in memory generalization. In: Bauer P.J., Fivush R., editors. Wiley-Blackwell Handbook on the Development of Children’s Memory. John Wiley and Sons; Chichester: 2013. pp. 453–479. [Google Scholar]
- Barr R., Dowden A., Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behav. Dev. 1996;19(2):159–170. [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J. Constructing a past in infancy: a neuro-developmental account. Trends Cogn. Sci. 2006;10(4):175–181. doi: 10.1016/j.tics.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Bauer P.J. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. Remembering the Times of Our Lives: Memory in Infants and Beyond. [Google Scholar]
- Bauer P.J. Event memory. In: Damon W., Lerner R.M., editors. Handbook of Child Psychology. John Wiley and Sons; Hoboken, NJ: 2007. pp. 373–425. [Google Scholar]
- Bauer P.J. Development of episodic and autobiographical memory: the importance of remembering forgetting. Dev. Rev. 2015;38:146–166. doi: 10.1016/j.dr.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.J., Dow G.A. Episodic memory in 16- and 20-month-old children: specifics are generalized but not forgotten. Dev. Psychol. 1994;30(3):403–417. [Google Scholar]
- Bauer P.J. Parameters of remembering and forgetting in the transition from infancy to early childhood. Monogr. Soc. Res. Child Dev. 2000;65(4) p. i-vi, 1-204. [PubMed] [Google Scholar]
- Bernier B.E. Dentate gyrus contributes to retrieval as well as encoding: evidence from context fear conditioning, recall, and extinction. J. Neurosci. 2017;37(26):6359–6371. doi: 10.1523/JNEUROSCI.3029-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J.D., Sersen E.A., Wortis J. Cardiac classical conditioning and reversal in mongoloid, encephalopathic, and normal child. Child Dev. 1970;41(3):771–785. [Google Scholar]
- Boldrini M. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22(4):589–599 e5. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovsky D., Rovee-Collier C. Contextual constraints on memory retrieval at six months. Child Dev. 1990;61(5):1569–1583. [PubMed] [Google Scholar]
- Brainerd C.J., Reyna V.F. Fuzzy-trace theory and memory development. Dev. Rev. 2004;24(4):396–439. doi: 10.1016/j.dr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D.J. A shared neural ensemble links distinct contextual memories encoded close in time. Nature. 2016;534(7605):115–118. doi: 10.1038/nature17955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B.A., Campbell E.H. Retention and extinction of learned fear in infant and adult rats. J. Comp. Physiol. Psychol. 1962;55:1–8. doi: 10.1037/h0049182. [DOI] [PubMed] [Google Scholar]
- Campbell B.A., Spear N.E. Ontogeny of memory. Psychol. Rev. 1972;79(3):215–236. doi: 10.1037/h0032690. [DOI] [PubMed] [Google Scholar]
- Campbell B.A. Species differences in ontogeny of memory: indirect support for neural maturation as a determinant of forgetting. J. Comp. Physiol. Psychol. 1974;87:193–202. [Google Scholar]
- Carstens K.E. Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J. Neurosci. 2016;36(23):6312–6320. doi: 10.1523/JNEUROSCI.0245-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133(Pt 8):2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Glatt C.E., Lee F.S. Treating the developing versus developed brain: translating preclinical mouse and human studies. Neuron. 2015;86(6):1358–1368. doi: 10.1016/j.neuron.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotro M.G., Alonso G. Stimulus preexposure reduces generalization of conditioned taste aversions between alcohol and non-alcohol flavors in infant rats. Behav. Neurosci. 2003;117(1):113–122. [PubMed] [Google Scholar]
- Christian K.M., Song H., Ming G.L. Functions and dysfunctions of adult hippocampal neurogenesis. Annu. Rev. Neurosci. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland C.D. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colunga E., Smith L.B. From the lexicon to expectations about kinds: a role for associative learning. Psychol. Rev. 2005;112(2):347–382. doi: 10.1037/0033-295X.112.2.347. [DOI] [PubMed] [Google Scholar]
- Costa C. Mapping of aggrecan, hyaluronic acid, heparan sulphate proteoglycans and aquaporin 4 in the central nervous system of the mouse. J. Chem. Neuroanat. 2007;33(3):111–123. doi: 10.1016/j.jchemneu.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Danielson N.B. Distinct contribution of adult-born hippocampal granule cells to context encoding. Neuron. 2016;90(1):101–112. doi: 10.1016/j.neuron.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.M., Rovee-Collier C.K. Alleviated forgetting of a learned contingency in 8-week-old infants. Dev. Psychol. 1983;19(3):353–365. [Google Scholar]
- Deal A.L. Limbic system development underlies the emergence of classical fear conditioning during the third and fourth weeks of life in the rat. Behav. Neurosci. 2016;130(2):212–230. doi: 10.1037/bne0000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa S.S. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 2006;281(26):17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery N. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front. Neurosci. 2013;7:66. doi: 10.3389/fnins.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderato O., Butler B., Meyer C. Changes in fear generalization gradients as a function of delayed testing. J. Exp. Psychol. 1966;72(5):678–682. doi: 10.1037/h0023798. [DOI] [PubMed] [Google Scholar]
- Dityatev A., Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat. Rev. Neurosci. 2003;4(6):456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Dolan R.J., Fletcher P.C. Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature. 1997;388(6642):582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- Donato F., Rompani S.B., Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504(7479):272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- Donato F. Stellate cells drive maturation of the entorhinal-hippocampal circuit. Science. 2017;355:6330. doi: 10.1126/science.aai8178. [DOI] [PubMed] [Google Scholar]
- Drew L.J. Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons. Hippocampus. 2016;26(6):763–778. doi: 10.1002/hipo.22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droit-Volet S., Wearden J.H. Temporal bisection in children. J. Exp. Child Psychol. 2001;80(2):142–159. doi: 10.1006/jecp.2001.2631. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S., Clement A., Wearden J. Temporal generalization in 3- to 8-year-old children. J. Exp. Child Psychol. 2001;80(3):271–288. doi: 10.1006/jecp.2001.2629. [DOI] [PubMed] [Google Scholar]
- Dumas T.C., Foster T.C. Developmental increase in CA3-CA1 presynaptic function in the hippocampal slice. J. Neurophysiol. 1995;73(5):1821–1828. doi: 10.1152/jn.1995.73.5.1821. [DOI] [PubMed] [Google Scholar]
- Dumas T.C., Rudy J.W. Development of the hippocampal memory system: creating networks and modifiable synapses. In: Blumberg M.S., Freeman J.H. Jr, Robinson S.R., editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford University Press; New York, NY: 2010. pp. 587–606. [Google Scholar]
- Dunsmoor J.E., Paz R. Fear generalization and anxiety: behavioral and neural mechanisms. Biol. Psychiatry. 2015;78(5):336–343. doi: 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Dunsmoor J.E., Mitroff S.R., LaBar K.S. Generalization of conditioned fear along a dimension of increasing fear intensity. Learn. Mem. 2009;16(7):460–469. doi: 10.1101/lm.1431609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor J.E. Threat intensity widens fear generalization gradients. Behav. Neurosci. 2017;131(2):168–175. doi: 10.1037/bne0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood S.L. Synaptophysin protein and mRNA expression in the human hippocampal formation from birth to old age. Hippocampus. 2006;16(8):645–654. doi: 10.1002/hipo.20194. [DOI] [PubMed] [Google Scholar]
- El-Bar N. Over-generalization in youth with anxiety disorders. Soc. Neurosci. 2017;12(1):76–85. doi: 10.1080/17470919.2016.1167123. [DOI] [PubMed] [Google Scholar]
- Eldridge L.L. Remembering episodes: a selective role for the hippocampus during retrieval. Nat. Neurosci. 2000;3(11):1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Epp J.R. Neurogenesis-mediated forgetting minimizes proactive interference. Nat. Commun. 2016;7:10838. doi: 10.1038/ncomms10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar M.J., Goodman G.S. Developmental changes in event memory. Child Dev. 1992;63(1):173–187. [PubMed] [Google Scholar]
- Favuzzi E. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron. 2017;95(3):639–655. doi: 10.1016/j.neuron.2017.06.028. e10. [DOI] [PubMed] [Google Scholar]
- Fivush R. Learning about school: the development of kindergartners’ school scripts. Child Dev. 1984;55(5):1697–1709. [PubMed] [Google Scholar]
- Fivush R., Hammond N. Autobiographical memory across the preschool years: toward reconceptualising childhood amnesia. In: Fivush R., Hudson J., editors. Knowing and Remembering in Young Children. Cambridge University Press; New York: 1990. [Google Scholar]
- Fivush R., Hudson J., Nelson K. Children’s long-term memory for a novel event: an exploratory study. Merrill-Palmer Q. 1984;30(3):303316. [Google Scholar]
- Frankland P.W., Kohler S., Josselyn S.A. Hippocampal neurogenesis and forgetting. Trends Neurosci. 2013;36(9):497–503. doi: 10.1016/j.tins.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Freud S. The Macmillan Company; New York, NY: 1914. Psychopathology of Everyday Life. [Google Scholar]
- Frischknecht R. Neural ECM molecules in axonal and synaptic homeostatic plasticity. Prog. Brain Res. 2014;214:81–100. doi: 10.1016/B978-0-444-63486-3.00004-9. [DOI] [PubMed] [Google Scholar]
- Ganella D.E. Neurocircuitry of fear extinction in adult and juvenile rats. Behav. Brain Res. 2018;351:161–167. doi: 10.1016/j.bbr.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Gao Y. The development of skin conductance fear conditioning in children from ages 3 to 8 years. Dev. Sci. 2010;13(1):201–212. doi: 10.1111/j.1467-7687.2009.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao A. Elevation of hippocampal neurogenesis induces a temporally graded pattern of forgetting of contextual fear memories. J. Neurosci. 2018;38(13):3190–3198. doi: 10.1523/JNEUROSCI.3126-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlanda S., Enquist M. A century of generalization. Anim. Behav. 2003;66(1):15–36. [Google Scholar]
- Ghosh S., Chattarji S. Neuronal encoding of the switch from specific to generalized fear. Nat. Neurosci. 2015;18(1):112–120. doi: 10.1038/nn.3888. [DOI] [PubMed] [Google Scholar]
- Glenn C.R. The development of fear learning and generalization in 8–13 year-olds. Dev. Psychobiol. 2012;54(7):675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N. Perineuronal nets protect fear memories from erasure. Science. 2009;325(5945):1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gogtay N. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16(8):664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Gomez R.L., Edgin J.O. The extended trajectory of hippocampal development: implications for early memory development and disorder. Dev. Cogn. Neurosci. 2016;18:57–69. doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.A. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71(6):1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg T. Neural reactivity tracks fear generalization gradients. Biol. Psychol. 2013;92(1):2–8. doi: 10.1016/j.biopsycho.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Grosso A. A neuronal basis for fear discrimination in the lateral amygdala. Nat. Commun. 2018;9(1):1214. doi: 10.1038/s41467-018-03682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci. 2012;15(12):1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner C.J. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78(5):773–784. doi: 10.1016/j.neuron.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi S. Postnatal neurogenesis in the dentate gyrus of the guinea pig. Hippocampus. 2005;15(3):285–301. doi: 10.1002/hipo.20050. [DOI] [PubMed] [Google Scholar]
- Guo N. Dentate granule cell recruitment of feedforward inhibition governs engram maintenance and remote memory generalization. Nat. Med. 2018;24(4):438–449. doi: 10.1038/nm.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskjolen A., Josselyn S.A., Frankland P.W. Age-dependent changes in spatial memory retention and flexibility in mice. Neurobiol. Learn. Mem. 2017;143:59–66. doi: 10.1016/j.nlm.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Guskjolen A., Kenney J.W., de la Parra J., Yeung B.R.A., Josselyn S.A., Frankland P.W. Recovery of “lost” infant memories in mice. Curr. Biol. 2018;28(14):2283–2290. doi: 10.1016/j.cub.2018.05.059. [DOI] [PubMed] [Google Scholar]
- Han J.H. Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn. Mem. 2008;15(6):443–453. doi: 10.1101/lm.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J.L. Brain-derived neurotrophic factor overexpression induces precocious critical period in mouse visual cortex. J. Neurosci. 1999;19(22):RC40. doi: 10.1523/JNEUROSCI.19-22-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K., Rovee-Collier C. Infant learning and long-term memory at 6 months: a confirming analysis. Dev. Psychobiol. 1997;30(1):71–85. [PubMed] [Google Scholar]
- Hartshorn K. The ontogeny of long-term memory over the first year-and-a-half of life. Dev. Psychobiol. 1998;32(2):69–89. [PubMed] [Google Scholar]
- Hartshorn K. Developmental changes in the specificity of memory over the first year of life. Dev. Psychobiol. 1998;33(1):61–78. doi: 10.1002/(sici)1098-2302(199807)33:1<61::aid-dev6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525(7569):333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayne H., MacDonald S., Barr R. Developmental changes in the specificity of memory over the second year of life. Infant Behav. Dev. 1997;20(2):233–245. [Google Scholar]
- Hayne H., Boniface J., Barr R. The development of declarative memory in human infants: age-related changes in deferred imitation. Behav. Neurosci. 2000;114(1):77–83. doi: 10.1037//0735-7044.114.1.77. [DOI] [PubMed] [Google Scholar]
- Henri V., Henri C. On our earliest recollections og childhood. Am. J. Psychol. 1895;7:303–304. [Google Scholar]
- Hensch T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hildreth K., Rovee-Collier C. Decreases in the response latency to priming over the first year of life. Dev. Psychobiol. 1999;35(4):276–289. [PubMed] [Google Scholar]
- Howe M.L., Courage M.L. On resolving the enigma of infantile amnesia. Psychol. Bull. 1993;113(2):305–326. doi: 10.1037/0033-2909.113.2.305. [DOI] [PubMed] [Google Scholar]
- Hudson J.A., Fivush R., Kuebli J. Scripts and episodes: the development of event memory. Appl. Cogn. Psychol. 1992;6(6) [Google Scholar]
- Ikrar T. Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circuits. 2013;7:204. doi: 10.3389/fncir.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R. Hippocampal neurogenesis enhancers promote forgetting of remote fear memory after hippocampal reactivation by retrieval. Elife. 2016;5 doi: 10.7554/eLife.17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito W. Enhanced generalization of auditory conditioned fear in juvenile mice. Learn. Mem. 2009;16(3):187–192. doi: 10.1101/lm.1190809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabes A., Nelson C.A. 20 years after “The ontogeny of human memory: a cognitive neuroscience perspective,” where are we? Int. J. Behav. Dev. 2015;39(4):293–303. [Google Scholar]
- Jenks K.R. Arc restores juvenile plasticity in adult mouse visual cortex. Proc. Natl. Acad. Sci. U. S. A. 2017;114(34):9182–9187. doi: 10.1073/pnas.1700866114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C., Wilbrecht L. Juvenile mice show greater flexibility in multiple choice reversal learning than adults. Dev. Cogn. Neurosci. 2011;1(4):540–551. doi: 10.1016/j.dcn.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn S.A., Frankland P.W. Infantile amnesia: a neurogenic hypothesis. Learn. Mem. 2012;19(9):423–433. doi: 10.1101/lm.021311.110. [DOI] [PubMed] [Google Scholar]
- Josselyn S.A., Frankland P.W. Memory allocation: mechanisms and function. Annu. Rev. Neurosci. 2018;41:389–413. doi: 10.1146/annurev-neuro-080317-061956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josselyn S.A., Kohler S., Frankland P.W. Finding the engram. Nat. Rev. Neurosci. 2015;16(9):521–534. doi: 10.1038/nrn4000. [DOI] [PubMed] [Google Scholar]
- Kee N. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kelsch W. GluN2B-containing NMDA receptors promote glutamate synapse development in hippocampal interneurons. J. Neurosci. 2014;34(48):16022–16030. doi: 10.1523/JNEUROSCI.1210-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A. Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proc. Natl. Acad. Sci. U. S. A. 2017;114(34):9212–9217. doi: 10.1073/pnas.1710654114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A. Hippocampal maturation drives memory from generalization to specificity. Trends Cogn. Sci. 2018;22(8):676–686. doi: 10.1016/j.tics.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek M.A. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012;15(12):1613–1620. doi: 10.1038/nn.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek M.A. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 2013;77(5):955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Knoth R. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5(1):e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok J.C. Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 2011;71(11):1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Landau J.S. Postdiscrimination generalization in human subjects of two different ages. J. Exp. Psychol. 1968;76:656–663. doi: 10.1037/h0025676. [DOI] [PubMed] [Google Scholar]
- Landau J.S. Line-orientation generalization in children and adults as a function of the number of training trials. Psychon. Sci. 1968;13:219–220. [Google Scholar]
- Landau J.S. Size generalization in children as a function of test method and age. Psychon. Sci. 1969;16(2):57–58. [Google Scholar]
- Langston R.F. Development of the spatial representation system in the rat. Science. 2010;328(5985):1576–1580. doi: 10.1126/science.1188210. [DOI] [PubMed] [Google Scholar]
- Lau J.Y. Distinct neural signatures of threat learning in adolescents and adults. Proc. Natl. Acad. Sci. U. S. A. 2011;108(11):4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P., Banta Lavenex P. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav. Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Law A.J. Expression of NMDA receptor NR1, NR2A and NR2B subunit mRNAs during development of the human hippocampal formation. Eur. J. Neurosci. 2003;18(5):1197–1205. doi: 10.1046/j.1460-9568.2003.02850.x. [DOI] [PubMed] [Google Scholar]
- Law A.J. Changes in NMDA receptor subunit mRNAs and cyclophilin mRNA during development of the human hippocampus. Ann. N. Y. Acad. Sci. 2003;1003:426–430. doi: 10.1196/annals.1300.043. [DOI] [PubMed] [Google Scholar]
- Learmonth A.E., Lamberth R., Rovee-Collier C. Generalization of deferred imitation during the first year of life. J. Exp. Child Psychol. 2004;88(4):297–318. doi: 10.1016/j.jecp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Ekstrom A.D., Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. Neuroimage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Lee H.H. Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol. Psychiatry. 2017;22:680–688. doi: 10.1038/mp.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjo K.K. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J. Neurosci. 2017;37(5):1269–1283. doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensjo K.K. Differential expression and cell-type specificity of perineuronal nets in hippocampus, medial entorhinal cortex, and visual cortex examined in the rat and mouse. eNeuro. 2017;4(3) doi: 10.1523/ENEURO.0379-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309(5734):619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Li Y., Mu Y., Gage F.H. Development of neural circuits in the adult hippocampus. Curr. Top. Dev. Biol. 2009;87:149–174. doi: 10.1016/S0070-2153(09)01205-8. [DOI] [PubMed] [Google Scholar]
- Liao D. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat. Neurosci. 1999;2(1):37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Lissek S., Grillon C. Learning models of PTSD. In: Beck J.G., Sloan D.M., editors. The Oxford Handbook of Traumatic Stress Disorders. Oxford University Press; New York: 2012. [Google Scholar]
- Lissek S. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav. Res. Ther. 2008;46(5):678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C.M. Competition from newborn granule cells does not drive axonal retraction of silenced old granule cells in the adult hippocampus. Front. Neural Circuits. 2012;6:85. doi: 10.3389/fncir.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Callaway E.M., Svoboda K. Genetic dissection of neural circuits: a decade of progress. Neuron. 2018;98(4):865. doi: 10.1016/j.neuron.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen H.B., Kim J.H. Ontogeny of memory: an update on 40 years of work on infantile amnesia. Behav. Brain Res. 2016;298(Pt A):4–14. doi: 10.1016/j.bbr.2015.07.030. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335(6073):1238–1242. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta J.A. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron. 2011;70(2):339–351. doi: 10.1016/j.neuron.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney S.A. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol. Psychiatry. 2013;74(6):427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy K.M. Modulating neuronal competition dynamics in the dentate gyrus to rejuvenate aging memory circuits. Neuron. 2016;91(6):1356–1373. doi: 10.1016/j.neuron.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough L. The deferred imitation task as a nonverbal measure of declarative memory. Proc. Natl. Acad. Sci. U. S. A. 1995;92(16):7580–7584. doi: 10.1073/pnas.92.16.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee A.W. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309(5744):2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83(1):202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S.A., Lehtinen L.E. Stimulus generalization as a function of age in children. J. Exp. Psychol. 1957;53(3):180–183. doi: 10.1037/h0047497. [DOI] [PubMed] [Google Scholar]
- Meltzer L.A., Yabaluri R., Deisseroth K. A role for circuit homeostasis in adult neurogenesis. Trends Neurosci. 2005;28(12):653–660. doi: 10.1016/j.tins.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Meltzoff A.N. Immediate and deferred imitation in fourteen- and twenty-four-month-old infants. Child Dev. 1985;56:62–72. [Google Scholar]
- Meyer W.J., Orgel A. The effects of age, stimulus intensity, and training trials on mediated stimulus generalization. J. Exp. Child Psychol. 1969;7(2):326–338. doi: 10.1016/0022-0965(69)90054-x. [DOI] [PubMed] [Google Scholar]
- Michalska K.J. A developmental analysis of threat/safety learning and extinction recall during middle childhood. J. Exp. Child Psychol. 2016;146:95–105. doi: 10.1016/j.jecp.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles C. A study of individual psychology. Am. J. Psychol. 1893;6:534–558. [Google Scholar]
- Milev P. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem. Biophys. Res. Commun. 1998;247(2):207–212. doi: 10.1006/bbrc.1998.8759. [DOI] [PubMed] [Google Scholar]
- Mody M. Genome-wide gene expression profiles of the developing mouse hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2001;98(15):8862–8867. doi: 10.1073/pnas.141244998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat L.A. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4(4):e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D.J. Parvalbumin interneurons constrain the size of the lateral amygdala engram. Neurobiol. Learn. Mem. 2016;135:91–99. doi: 10.1016/j.nlm.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr. Opin. Neurobiol. 2006;16(2):179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu. Rev. Psychol. 2016;67:105–134. doi: 10.1146/annurev-psych-113011-143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally S.L., Maguire E.A. Learning to remember: the early ontogeny of episodic memory. Dev. Cogn. Neurosci. 2014;9:12–29. doi: 10.1016/j.dcn.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby D.G. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn. Mem. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E.M., Morishita H. Regulating critical period plasticity: insight from the visual system to fear circuitry for therapeutic interventions. Front. Psychiatry. 2013;4:146. doi: 10.3389/fpsyt.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L., Zola-Morgan S. Infantile amnesia: a neurobiological perspective. In: Moscovitch M., editor. Infant Memory. Plenum Press; New York: 1984. [Google Scholar]
- Nelson K. The psychological and social origins of autobiographical memory. Psychol. Sci. 1993;4:7–14. [Google Scholar]
- Nelson C.A. The ontogeny of human memory: a cognitive neuroscience perspective. Dev. Psychol. 1995;31(5):723–738. [Google Scholar]
- Nelson K., Gruendel J. Generalized event representations: basic building blocks of cognitive development. In: Lamb M.E., Brown A.L., editors. Advances in Developmental Psychology. Lawrence Erlbaum Associates; Hillsdale, NJ: 1981. pp. 131–158. [Google Scholar]
- Nelson K., Ross G. The generalities and specifics of long‐term memory in infants and young children. New Dir. Child Adolesc. Dev. 1980;1980(10):87–101. [Google Scholar]
- Newcombe N.S., Lloyd M.E., Ratliff K.R. Development of episodic and autobiographical memory: a cognitive neuroscience perspective. Adv. Child Dev. Behav. 2007;35:37–85. doi: 10.1016/b978-0-12-009735-7.50007-4. [DOI] [PubMed] [Google Scholar]
- Ngo C.T., Newcombe N.S., Olson I.R. The ontogeny of relational memory and pattern separation. Dev. Sci. 2018;21(2) doi: 10.1111/desc.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niibori Y. Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat. Commun. 2012;3:1253. doi: 10.1038/ncomms2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Park S. Neuronal allocation to a hippocampal engram. Neuropsychopharmacology. 2016;41(13):2987–2993. doi: 10.1038/npp.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S.S. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proc. Natl. Acad. Sci. U. S. A. 2011;108(3):1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena C.J. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356(6343):1185–1188. doi: 10.1126/science.aan4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W., Milner B. Memory deficit produced by bilateral lesions in the hippocampal zone. AMA Arch. Neurol. Psychiatry. 1958;79(5):475–497. doi: 10.1001/archneurpsyc.1958.02340050003001. [DOI] [PubMed] [Google Scholar]
- Perner J., Ruffman T. Episodic memory and autonoetic consciousness: developmental evidence and a theory of childhood amnesia. J. Exp. Child Psychol. 1995;59(3):516–548. doi: 10.1006/jecp.1995.1024. [DOI] [PubMed] [Google Scholar]
- Peterson C. Children’s long-term memory for autobiographical events. Dev. Rev. 2002;22:370–402. [Google Scholar]
- Petralia R.S. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat. Neurosci. 1999;2(1):31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Philpot B.D. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29(1):157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- Pillemer D.B., White S.H. Childhood events recalled by children and adults. Adv. Child Dev. Behav. 1989;21:297–340. doi: 10.1016/s0065-2407(08)60291-8. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298(5596):1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Potwin E.B. Study of early memories. Psychol. Rev. 1901;8(6):596–601. [Google Scholar]
- Prabhakar J. Memory-related hippocampal activation in the sleeping toddler. Proc. Natl. Acad. Sci. U. S. A. 2018;115(25):6500–6505. doi: 10.1073/pnas.1805572115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D.W., Goodman G.S. Visiting the wizard: children’s memory for a recurring event. Child Dev. 1990;61(3):664–680. [PubMed] [Google Scholar]
- Quevedo J. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn. Mem. 1999;6(6):600–607. doi: 10.1101/lm.6.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajbhandari A.K. Graded fear generalization enhances the level of cfos-positive neurons specifically in the basolateral amygdala. J. Neurosci. Res. 2016;94(12):1393–1399. doi: 10.1002/jnr.23947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsaran A.I., Sanders H.R., Stanton M.E. Determinants of object-in-context and object-place-context recognition in the developing rat. Dev. Psychobiol. 2016;58(7):883–895. doi: 10.1002/dev.21432. [DOI] [PubMed] [Google Scholar]
- Rashid A.J. Competition between engrams influences fear memory formation and recall. Science. 2016;353(6297):383–387. doi: 10.1126/science.aaf0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L. Development of adult-generated cell connectivity with excitatory and inhibitory cell populations in the hippocampus. J. Neurosci. 2015;35(29):10600–10612. doi: 10.1523/JNEUROSCI.3238-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio D.C., Richardson R., Ebner D.L. Memory retrieval deficits based upon altered contextual cues: a paradox. Psychol. Bull. 1984;96(1):152–165. [PubMed] [Google Scholar]
- Riccio D.C., Rabinowitz V.C., Axelrod S. Memory. When less is more. Am. Psychol. 1994;49(11):917–926. doi: 10.1037//0003-066x.49.11.917. [DOI] [PubMed] [Google Scholar]
- Richards B.A., Frankland P.W. The persistence and transience of memory. Neuron. 2017;94(6):1071–1084. doi: 10.1016/j.neuron.2017.04.037. [DOI] [PubMed] [Google Scholar]
- Richards B.A. Patterns across multiple memories are identified over time. Nat. Neurosci. 2014;17(7):981–986. doi: 10.1038/nn.3736. [DOI] [PubMed] [Google Scholar]
- Richardson R., Riccio D.C., Axiotis R. Alleviation of infantile amnesia in rats by internal and external contextual cues. Dev. Psychobiol. 1986;19(5):453–462. doi: 10.1002/dev.420190506. [DOI] [PubMed] [Google Scholar]
- Richmond J., Nelson C.A. Accounting for change in declarative memory: a cognitive neuroscience perspective. Dev. Rev. 2007;27(3):349–373. doi: 10.1016/j.dr.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A.J., Pascalis O. Development of flexible visual recognition memory in human infants. Dev. Sci. 2004;7(5):527–533. doi: 10.1111/j.1467-7687.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Robinson-Drummer P.A., Stanton M.E. Using the context preexposure facilitation effect to study long-term context memory in preweanling, juvenile, adolescent, and adult rats. Physiol. Behav. 2015;148:22–28. doi: 10.1016/j.physbeh.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer P.A. Age and experience dependent changes in Egr-1 expression during the ontogeny of the context preexposure facilitation effect (CPFE) Neurobiol. Learn. Mem. 2018;150:1–12. doi: 10.1016/j.nlm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbaugh M., Riccio D.C. Stimulus generalization of learned fear in infant and adult rats. J. Comp. Physiol. Psychol. 1968;66(2):530–533. doi: 10.1037/h0026366. [DOI] [PubMed] [Google Scholar]
- Rollins L., Cloude E.B. Development of mnemonic discrimination during childhood. Learn. Mem. 2018;25(6):294–297. doi: 10.1101/lm.047142.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovee-Collier C., Cuevas K. Multiple memory systems are unnecessary to account for infant memory development: an ecological model. Dev. Psychol. 2009;45(1):160–174. doi: 10.1037/a0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovee-Collier C., Cuevas K. The Development of Memory in Infancy and Childhood. 2nd ed. Psychology Press; US: New York, NY: 2009. Chapter: the development of infant memory; pp. 11–41. [Google Scholar]
- Rovee-Collier C.K. Reactivation of infant memory. Science. 1980;208(4448):1159–1161. doi: 10.1126/science.7375924. [DOI] [PubMed] [Google Scholar]
- Roy D.S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature. 2016;531(7595):508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.C. The distribution of early childhood memories. Memory. 2000;8(4):265–269. doi: 10.1080/096582100406810. [DOI] [PubMed] [Google Scholar]
- Rubin D.C., Schulkind M.D. The distribution of autobiographical memories across the lifespan. Mem. Cognit. 1997;25(6):859–866. doi: 10.3758/bf03211330. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Hyson R.L. Ontogenesis of learning: III. Variation in the rat's differential reflexive and learned responses to sound frequencies. Dev. Psychobiol. 1984;17(3):285–300. doi: 10.1002/dev.420170308. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Morledge P. Ontogeny of contextual fear conditioning in rats: implications for consolidation, infantile amnesia, and hippocampal system function. Behav. Neurosci. 1994;108(2):227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Pugh C.R. A comparison of contextual and generalized auditory-cue fear conditioning: evidence for similar memory processes. Behav. Neurosci. 1996;110(6):1299–1308. doi: 10.1037//0735-7044.110.6.1299. [DOI] [PubMed] [Google Scholar]
- Ruediger S. Learning-related feedforward inhibitory connectivity growth required for memory precision. Nature. 2011;473(7348):514–518. doi: 10.1038/nature09946. [DOI] [PubMed] [Google Scholar]
- Ryan T.J. Memory. Engram cells retain memory under retrograde amnesia. Science. 2015;348(6238):1007–1013. doi: 10.1126/science.aaa5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro A. Reassessing pattern separation in the dentate gyrus. Front. Behav. Neurosci. 2013;7:96. doi: 10.3389/fnbeh.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele M.A. Developmental aspects of fear: comparing the acquisition and generalization of conditioned fear in children and adults. Dev. Psychobiol. 2016;58(4):471–481. doi: 10.1002/dev.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M.L., Frankland P.W. Memory allocation and integration in rodents and humans. Curr. Opin. Behav. Sci. 2017;17:90–98. [Google Scholar]
- Schlichting M.L., Preston A.R. Memory integration: neural mechanisms and implications for behavior. Curr. Opin. Behav. Sci. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M.L., Zeithamova D., Preston A.R. CA1 subfield contributions to memory integration and inference. Hippocampus. 2014;24(10):1248–1260. doi: 10.1002/hipo.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin P.A., Kunkel D.D., Mathers L.H. Development of rabbit hippocampus: anatomy. Brain Res. 1981;254(4):453–468. doi: 10.1016/0165-3806(81)90016-x. [DOI] [PubMed] [Google Scholar]
- Scoville W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6(18):2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Seo D.O., Motard L.E., Bruchas M.R. Contemporary strategies for dissecting the neuronal basis of neurodevelopmental disorders. Neurobiol. Learn. Mem. 2018 doi: 10.1016/j.nlm.2018.03.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290(5494):1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- Shors T.J. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Slackman E.A., Hudson J.A., Fivush R. Actions, actors, links, and goals: the structure of children’s event representations. In: Nelson K., editor. Event Knowledge: Structure and Function in Development. Erlbaum; Hillsdale, NJ: 1986. [Google Scholar]
- Smith G.J., Spear N.E. Analysis of treatments to alleviate forgetting in rats. Am. J. Psychol. 1984;97(4):475–491. [PubMed] [Google Scholar]
- Smith L., Yu C. Infants rapidly learn word-referent mappings via cross-situational statistics. Cognition. 2008;106(3):1558–1568. doi: 10.1016/j.cognition.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.B., Suanda S.H., Yu C. The unrealized promise of infant statistical word-referent learning. Trends Cogn. Sci. 2014;18(5):251–258. doi: 10.1016/j.tics.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg B.A. Casting a wide net: role of perineuronal nets in neural plasticity. J. Neurosci. 2016;36(45):11459–11468. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S.F. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555(7696):377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear N.E. The future study of learning and memory from a psychobiological perspective. In: Sarris V., Parducci A., editors. Perspectives in Psychological Experimentation. Erlbaum; Hillsdale, NJ: 1984. pp. 87–103. [Google Scholar]
- Spear N.E., Smith G.J. Alleviation of forgetting in preweanling rats. Dev. Psychobiol. 1978;11(6):513–529. doi: 10.1002/dev.420110602. [DOI] [PubMed] [Google Scholar]
- Squire L.R. Memory and forgetting: long-term and gradual changes in memory storage. Int. Rev. Neurobiol. 1994;37:243–269. discussion 285-8. [PubMed] [Google Scholar]
- Squire L.R. Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stefanelli T. Hippocampal somatostatin interneurons control the size of neuronal memory ensembles. Neuron. 2016;89(5):1074–1085. doi: 10.1016/j.neuron.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Syken J. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313(5794):1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Tanaka K.Z. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84(2):347–354. doi: 10.1016/j.neuron.2014.09.037. [DOI] [PubMed] [Google Scholar]
- Tempone V.J. Mediational processes in primary stimulus generalization. Child Dev. 1966;37(3):687–696. doi: 10.1111/j.1467-8624.1966.tb04318.x. [DOI] [PubMed] [Google Scholar]
- Todd C.M., Perlmutter M. Reality recalled by preschool children. New Dir. Child Adolesc. Dev. 1980;10:69–85. [Google Scholar]
- Tompary A., Davachi L. Consolidation promotes the emergence of representational overlap in the hippocampus and medial prefrontal cortex. Neuron. 2017;96(1):228–241. doi: 10.1016/j.neuron.2017.09.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglia A. Infantile amnesia reflects a developmental critical period for hippocampal learning. Nat. Neurosci. 2016;19(9):1225–1233. doi: 10.1038/nn.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglia A. Developmental changes in plasticity, synaptic, glia and connectivity protein levels in rat dorsal hippocampus. Neurobiol. Learn. Mem. 2016;135:125–138. doi: 10.1016/j.nlm.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglia A. Mechanisms of critical period in the hippocampus underlie object location learning and memory in infant rats. Learn. Mem. 2018;25(4):176–182. doi: 10.1101/lm.046946.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A., Rolls E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4(3):374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Tsien J.Z., Huerta P.T., Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87(7):1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E., Donaldson W., editors. Organization of Memory. Academic Press; New York, NY: 1972. pp. 381–403. [Google Scholar]
- Tulving E., Markowitsch H.J. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8(3):198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ueno H. Postnatal development of GABAergic interneurons and perineuronal nets in mouse temporal cortex subregions. Int. J. Dev. Neurosci. 2017;63:27–37. doi: 10.1016/j.ijdevneu.2017.08.003. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vetere G. Chemogenetic interrogation of a brain-wide fear memory network in mice. Neuron. 2017;94(2) doi: 10.1016/j.neuron.2017.03.037. p. 363-374 e4. [DOI] [PubMed] [Google Scholar]
- Wang D., Fawcett J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012;349(1):147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- Wang C.C. A critical role for GluN2B-containing NMDA receptors in cortical development and function. Neuron. 2011;72(5):789–805. doi: 10.1016/j.neuron.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Westbrook S.R., Brennan L.E., Stanton M.E. Ontogeny of object versus location recognition in the rat: acquisition and retention effects. Dev. Psychobiol. 2014;56(7):1492–1506. doi: 10.1002/dev.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzler S.E., Sweeney J.A. Childhood amnesia: a conceptualization in cognitive-psychological terms. J. Am. Psychoanal. Assoc. 1986;34(3):663–685. doi: 10.1177/000306518603400307. [DOI] [PubMed] [Google Scholar]
- Wilke S.A. Deconstructing complexity: serial block-face electron microscopic analysis of the hippocampal mossy fiber synapse. J. Neurosci. 2013;33(2):507–522. doi: 10.1523/JNEUROSCI.1600-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills T.J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328(5985):1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G., Moscovitch M., Bontempi B. Memory formation and long-term retention in humans and animals: convergence towards a transformation account of hippocampal-neocortical interactions. Neuropsychologia. 2010;48(8):2339–2356. doi: 10.1016/j.neuropsychologia.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Wong F.K. Pyramidal cell regulation of interneuron survival sculpts cortical networks. Nature. 2018;557(7707):668–673. doi: 10.1038/s41586-018-0139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J., Jinno S. Spatio-temporal differences in perineuronal net expression in the mouse hippocampus, with reference to parvalbumin. Neuroscience. 2013;253:368–379. doi: 10.1016/j.neuroscience.2013.08.061. [DOI] [PubMed] [Google Scholar]
- Yamada J., Jinno S. Molecular heterogeneity of aggrecan-based perineuronal nets around five subclasses of parvalbumin-expressing neurons in the mouse hippocampus. J. Comp. Neurol. 2017;525(5):1234–1249. doi: 10.1002/cne.24132. [DOI] [PubMed] [Google Scholar]
- Yamada J., Ohgomori T., Jinno S. Perineuronal nets affect parvalbumin expression in GABAergic neurons of the mouse hippocampus. Eur. J. Neurosci. 2015;41(3):368–378. doi: 10.1111/ejn.12792. [DOI] [PubMed] [Google Scholar]
- Yashiro K., Philpot B.D. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55(7):1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M. Multiple forms of activity-dependent competition refine hippocampal circuits in vivo. Neuron. 2011;70(6):1128–1142. doi: 10.1016/j.neuron.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu A.P. Neurons are recruited to a memory trace based on relative neuronal excitability immediately before training. Neuron. 2014;83(3):722–735. doi: 10.1016/j.neuron.2014.07.017. [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]