Abstract
Left temporal-parietal white matter structure is consistently associated with reading abilities in children. A small number of longitudinal studies show that development of this area over time is altered in children with impaired reading. However, it remains unclear how brain developmental patterns relate to specific reading skills such as fluency, which is a critical part of reading comprehension. Here, we examined white matter development trajectories in children with dysfluent reading (20 dysfluent and inaccurate readers, 36 dysfluent and accurate readers) compared to non-impaired readers (n = 14) over 18 months. We found typical age-related increases of fractional anisotropy (FA) in bilateral temporal-parietal areas in non-impaired readers, but a lack of similar changes in dysfluent readers. We also found steeper decreases of mean diffusivity (MD) in the right corona radiata and left uncinate fasciculus in dysfluent inaccurate readers compared to dysfluent accurate readers. Changes in diffusion parameters were correlated with changes in reading scores over time. These results suggest delayed white matter development in dysfluent readers, and show maturational differences between children with different types of reading impairment. Overall, these results highlight the importance of considering developmental trajectories, and demonstrate that the window of plasticity may be different for different children.
Keywords: Dyslexia, Reading, Fluency, Accuracy, Diffusion tensor imaging, White matter
1. Introduction
Reading is a complex task requiring efficient communication between multiple brain areas. While most children learn to read relatively easily, 17–21% struggle to read despite adequate intelligence, motivation, and opportunity (Ferrer et al., 2015). Reading impairment can lead to reduced academic achievement and diminished future career prospects, and is a risk factor for mental health problems (Goldston et al., 2007; Morris and Turnbull, 2007; Wilson et al., 2009). Specific reading impairment, or dyslexia, is characterized by difficulties reading accurately and/or fluently (Lyon et al., 2003), and has a neurobiological basis.
Diffusion tensor imaging (DTI), a neuroimaging technique sensitive to white matter microstructure, has provided evidence of widespread disrupted structural brain connectivity underlying specific reading impairments. White matter features related to reading ability have been identified in classical dorsal and ventral reading pathways (e.g., the superior longitudinal, arcuate, inferior fronto-occipital, uncinate, and inferior longitudinal fasciculi), as well as the splenium of the corpus callosum (Dougherty et al., 2007; Frye et al., 2008; Odegard et al., 2009; Travis et al., 2016; Vandermosten et al., 2012). A meta-analysis of DTI reading studies identified the most consistent findings in an area of left temporal-parietal white matter intersected by the left superior longitudinal fasciculus and corona radiata (Vandermosten et al., 2012). Furthermore, gains in reading skills have been associated with changes in white matter structure in this left temporal-parietal region following intensive reading remediation for children (Huber et al., 2018; Keller and Just, 2009).
There is growing interest in understanding the neural underpinnings of the components of the reading process, such as fluency. Reading fluency, which refers to the combination of reading accuracy and speed, is a key component of reading comprehension (Fuchs et al., 2001). Oral reading fluency at baseline, and gains in fluency over time, are predictive of reading comprehension (Kim et al., 2010). Dysfluency, or the inability to read accurately and quickly, is a widespread deficit in poor readers (Shaywitz and Shaywitz, 2005), and one that may persist longer than inaccurate decoding. However, relatively little is known about how structural brain connectivity is related to the different components of reading. One study in children found that white matter structure in ventral connections (uncinate, inferior fronto-occipital, and inferior longitudinal fasciculi) was correlated with fluency and comprehension, but not sight word reading (Arrington et al., 2017). Another study in adolescents and young adults found that fluency, decoding, and sight word reading were each related to widespread white matter structure, but that sight word reading was uniquely related to several frontal white matter regions (Lebel et al., 2013). Both accuracy and processing speed are related to white matter microstructure in the arcuate fasciculus in children (Skeide et al., 2016).
Longitudinal studies are able to investigate brain development trajectories, which are often more sensitive measures of developmental disorders than analyses at a single time point (Giedd et al., 2008). Furthermore, understanding trajectories of brain development as they relate to specific reading difficulties can highlight periods of maximal brain plasticity that might be ideal targets for intervention. Component processes in reading, including phonological processing and fluency, are related to development of gray matter volume and cortical thickness in inferior frontal, inferior parietal, and superior temporal areas in children (Clark et al., 2014; Houston et al., 2014; Linkersdorfer et al., 2015; Lu et al., 2007; Xia et al., 2016). Two DTI studies showed that word reading abilities in children predicted white matter development in dorsal and ventral white matter tracts (Wang et al., 2017; Yeatman et al., 2012), and others have observed relationships between changes in reading skills and white matter structure, including the superior and inferior longitudinal fasciculi and the arcuate fasciculus in children with prenatal alcohol exposure (Treit et al., 2013) or with a family history of dyslexia (Wang et al., 2017).
The goal of the current study was to extend this previous work by investigating the relationship between trajectories of white matter development and reading fluency. To this end we studied two types of dysfluent readers, dysfluent accurate and dysfluent inaccurate readers, and compared these to non-impaired readers. We aimed to examine group differences and changes with age in white matter tracts that have previously been shown to be altered in poor readers. We hypothesized that children who were fluent readers would have higher fractional anisotropy and lower mean diffusivity than dysfluent readers, and that the fluent readers would have the largest developmental changes (increases of fractional anisotropy and decreases of mean diffusivity) over time in white matter connections related to reading.
2. Methods
2.1. Participants
Participants were 70 healthy right-handed children (44 male/26 female). All children received a reading assessment and an MRI scan at baseline (age: 9.5 ± 1.3 years), and again approximately 18 months later (age: 11.0 ± 1.3 years; 1.5 ± 0.1 years after the first scan). Participants were originally recruited from a number of sources, including referrals from health care providers and community advertisements. Children where the cause of the reading problem was likely attributable to emotional disturbance, clinically apparent neurogenetic disorders, brain injury, sensory disorders, or social, cultural, or economic disadvantage were excluded from the study. Children were also excluded for contraindications to MRI. The Institutional Review Board at Yale University approved this study and written informed consent was obtained from parents or guardians.
2.2. Reading assessments
Within two months of their MRI scan, children were assessed using the Gray Oral Reading Test-IV (GORT) Fluency; the Woodcock Johnson (WJ) Letter-Word Identification and Word Attack subtests (which together provide a Basic Reading score); and the Test of Word Reading Efficiency (TOWRE) Sight Words and Phonological Decoding. All children were also assessed at baseline using the Wechsler Abbreviated Scale of Intelligence (WASI) to provide full scale IQ scores. Participants were divided into three groups, based on their baseline reading assessments. Children were classified as dysfluent readers if their GORT Fluency, TOWRE Sight Words, or TOWRE Phonological Decoding scores were below the 25th percentile, or if any of their reading scores were 1.5 standard deviations or more below their Full Scale IQ score. Both methods validly identify children as dyslexic readers, and there is little evidence of differences between subgroups of children formed with one criterion versus the other (Ferrer et al., 2010; Shaywitz et al., 1992, 2003). The dysfluent group was further subdivided into dysfluent readers with inaccurate decoding skills (WJ Basic Reading or WJ Word Attack scores below the 25th percentile) or dysfluent readers with accurate decoding skills (Basic Reading and Word Attack scores above the 25th percentile). Control subjects had scores on all reading assessments above the 40th percentile. The three groups of participants were: control subjects (fluent readers with accurate decoding; n = 14), dysfluent readers with accurate decoding (n = 36), and dysfluent readers with inaccurate decoding (n = 20). Table 1 provides participant information for each group.
Table 1.
Subject demographics. Demographic information is given for 70 subjects at baseline, including age, gender, IQ, and reading assessments (WJ, GORT, TOWRE).
| Sample mean ± standard deviation |
|||
|---|---|---|---|
| Dysfluent, inaccurate | Dysfluent, accurate | Non-impaired | |
| N | 20 | 36 | 14 |
| Age at baseline MRI | 10.0 ± 1.2 | 9.4 ± 1.3 | 9.2 ± 1.2 |
| Gender | 15 m/5f | 23 m/13f | 6m/8f |
| WASI FSIQ | 102 ± 14* | 105 ± 10* | 125 ± 20 |
| Reading Scores | |||
| GORT Fluency | 3 ± 2*◊ | 5 ± 2* | 13 ± 2 |
| WJ Letter-Word ID | 82 ± 7*◊ | 98 ± 6* | 120 ± 10 |
| WJ Word Attack | 87 ± 5*◊ | 98 ± 7* | 112 ± 10 |
| WJ Basic Reading | 84 ± 6*◊ | 98 ± 6* | 118 ± 10 |
| TOWRE Sight Words | 79 ± 10*◊ | 91 ± 8* | 116 ± 12 |
| TOWRE Phonological Decoding | 80 ± 6*◊ | 90 ± 9* | 112 ± 11 |
Group differences: *significantly different from controls, ◊ significantly different from dysfluent accurate group; p < 0.05.
2.3. Image acquisition and processing
All subjects underwent MRI scanning on a 1.5T Siemens Sonata scanner at Yale University. DTI images were acquired using spin echo echo-planar imaging with twenty-eight 5 mm slices (no gap), image matrix of 64 × 64, field of view = 240 × 240 mm2, TE = 85 ms, TR = 9000 ms, b = 1000 s/mm2, six diffusion-encoding directions, and six averages. Total DTI acquisition time was 7:24 min.
Data was visually inspected for motion artifacts (including signal drop out, venetian blind artifact, and mechanical vibration artifact). Volumes with substantial motion corruption (i.e., more than 10%) were removed from analysis before processing. Participants with more than 8 scan volumes removed (out of a total of 36) were excluded from analysis. Data was processed using FSL’s diffusion pipeline, including eddy current correction, motion correction, brain extraction (BET, 0.2 threshold), and fitting of the diffusion tensor model at each voxel. From the tensor, maps of fractional anisotropy (FA), mean diffusivity (MD), and axial and radial diffusivity (AD, RD) were calculated for each subject. After fitting to the tensor model, non-linear registration was performed on each subject’s FA maps to the MNI152 1 mm standard brain. FSL’s Tract Based Spatial Statistics (TBSS) (Smith et al., 2006) was used to calculate a mean white matter skeleton using an FA threshold value of 0.20. Each subjects’ FA data was then projected onto the skeleton. Similarly, MD, AD, and RD were projected onto the skeleton to create white matter skeleton maps for each parameter. Brain areas were selected for analysis based on previous DTI studies of reading fluency (Arrington et al., 2017; Lebel et al., 2013), and dyslexia (Vandermosten et al., 2012). Using the JHU ICBM-DTI-81 atlas, mean FA, MD, AD, and RD were calculated for each subject from the following regions of interest (ROIs): the splenium of the corpus callosum (sCC); anterior limb of the internal capsule (ALIC); anterior and posterior corona radiata (aCR, pCR); sagittal stratum (includes the inferior longitudinal and fronto-occipital fasciculi); uncinate fasciculus; and superior longitudinal fasciculus. For all regions except the corpus callosum, left and right were calculated separately, giving a total of 13 ROIs. We also calculated the average FA and MD across the entire skeleton to provide reference values. Parameter values were calculated at each time point for each subject.
2.4. Statistical analysis
Group differences in reading scores at baseline were tested using a one-way ANOVA with p < 0.05. Changes in reading scores over time were tested using paired samples t-tests across the whole group, and within each group separately.
Linear mixed effects models were used to test for group differences, age-related changes, and group-by-age interactions for each tract. Gender was included as a covariate. Significance was set to p < 0.05. A supplementary analysis was conducted controlling for IQ to ensure IQ differences were not driving results. Because regions were selected based on a priori hypotheses about white matter regions related to reading, multiple comparison corrections were not performed for these models.
Relationships between changes in diffusion parameters and changes in reading scores over time were tested using Pearson correlations, controlling for gender. As age-related changes were linear (see results), we did not correct for age.
FA and MD were analyzed as primary outcomes of interest. RD and AD were analyzed in any areas where FA and/or MD showed significant results, in order to investigate which white matter factors may be influencing any observed group differences.
3. Results
3.1. Demographics
Participants had normal to above-average scores on the WASI (Wechsler, 1999) (108 ± 16), although both dysfluent groups had significantly lower IQ scores than controls (p < 0.05; Table 1). All three groups differed on reading scores, with the dysfluent inaccurate group scoring lowest, then the dysfluent accurate group, and the non-impaired group scoring highest; see Table 1. There were no significant group differences in age or gender.
3.2. Age-related changes and gender differences in diffusion parameters
Ten of 13 regions had significant age-related increases of FA (p < 0.05); only the right anterior corona radiata and the bilateral uncinate fasciculus did not. All regions showed significant age-related decreases of MD (p < 0.05). The left anterior corona radiata was the only region with gender differences in FA values; males had higher FA (p = 0.008). The left and right ALIC, left and right anterior CR, and left SLF all had higher MD values in females (p = 0.007, 0.007, 0.028, 0.009, and 0.048, respectively); the left and right sagittal stratum had higher MD in males (p = 0.003, p = 0.013, respectively).
Across the whole skeleton, MD had significant age-related decreases (p = 0.032), but FA did not change significantly. Neither had significant differences between males and females.
3.3. Group differences in diffusion parameters
Group differences were significant for FA in the left and right posterior CR (p = 0.01, p = 0.02, respectively), and for MD in the right anterior and posterior corona radiata (p = 0.006, p = 0.035, respectively) and left uncinate fasciculus (p = 0.039). However, these regions also had significant group-age interactions, so findings are best interpreted in that context.
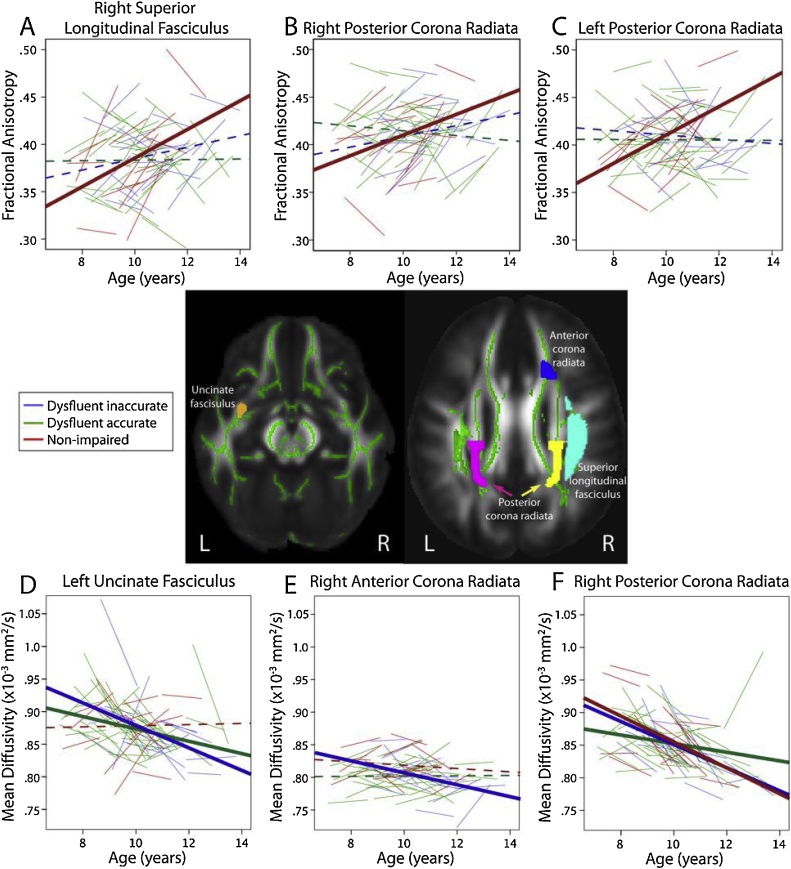
The left and right posterior CR and the right SLF had significant group-by-age interactions for FA (p = 0.008, p = 0.024, p = 0.021, respectively). In all three areas, age-related increases of FA were significant in the non-impaired group, but no significant changes of FA occurred in either of the dysfluent groups (Fig. 1). For MD, significant interactions were present in the right anterior and posterior CR (p = 0.008, p = 0.037, respectively) and the left uncincate fasciculus (p = 0.031). In the anterior CR, significant decreases of MD occurred in the dysfluent inaccurate group only, while no changes were evident in either the dysfluent accurate group or the non-impaired controls. In the right posterior CR, all groups had significantly decreasing MD, but changes in the dysfluent accurate group were slower than the other two groups. In the left uncinate fasciculus, both dysfluent groups had decreasing MD (the dysfluent inaccurate more steeply than dysfluent accurate), and the non-impaired group had no change. These results would not survive correction for multiple comparisons.
Fig. 1.
Trajectories of white matter development differed in dysfluent inaccurate, dysfluent accurate, and non-impaired readers in several brain regions (A–F). Solid lines represent significant relationships, while dotted lines represent non-significant fits; each group is shown in a different colour. The figures in the centre show the average fractional anisotropy map with the tract-based spatial statistics (TBSS) skeleton overlaid in green. Regions of interest that had significant group differences in trajectories are shown in colours.
There were no significant group differences or group-by-age interactions for whole-skeleton averaged FA or MD.
A post-hoc analysis of group-by-age interactions for axial and radial diffusivity (AD, RD) revealed no significant interactions for AD. The right posterior corona radiata had significant interactions for RD (p = 0.012), w while the right anterior corona radiata, the left posterior corona radiata, and the right SLF had trend-level interactions for RD (p = 0.054, 0.071, 0.052, respectively).
3.4. IQ as a covariate
Follow-up analysis was conducted with IQ as a covariate to understand its impact on any group differences. When IQ was included in the model, all age-by-group interactions remained significant: FA in the left and right posterior corona radiata (p = 0.001, 0.011, respectively), FA in the right SLF (p = 0.028), MD in the right anterior and posterior corona radiata (p = 0.01, 0.05, respectively), and MD in the left uncinate fasciculus (p = 0.033).
3.5. Changes in reading scores
Across all subjects, there were no significant changes in reading scores over time. Within the dysfluent accurate and non-impaired groups, however, some scores decreased significantly over time. In the dysfluent accurate group, WJ Word Attack (Δ = −2.6, p = 0.013), WJ Basic Reading (Δ = −1.8, p = 0.049), and TOWRE Phonological Decoding (Δ = −2.1, p = 0.043) scores decreased. In the non-impaired group, WJ Word Attack (Δ = −3.1, p = 0.041) and TOWRE Sight Words (Δ = −5.7, p = 0.031) scores decreased. No changes over time were significant in the dysfluent inaccurate group.
Across all subjects, changes in TOWRE Sight Words were positively correlated with changes of FA in the left SLF (r = 0.25, p = 0.040), changes in TOWRE Phonological Decoding were negatively correlated with changes of MD in the right posterior corona radiata (r = −0.30, p = 0.014), and changes of GORT Fluency were positively correlated with changes of MD in the left posterior corona radiata (r = 0.28, p = 0.019) and left SLF (r = 0.28, p = 0.02). Changes in both MD and FA averaged across the skeleton were correlated with changes in TOWRE Phonological Decoding scores (r = 0.27, p = 0.027; r = −0.28, p = 0.019, respectively). See Fig. 2. There were no significant group differences in the relationships between changes in reading scores and changes in diffusion parameters. These results would not survive correction for multiple comparisons.
Fig. 2.
Significant relationships were observed between changes in reading scores and changes in DTI parameters for the three regions shown above, as well as the average values across the skeleton (A–F). Relationships did not differ significantly by group. The brain image in the centre shows the average fractional anisotropy map with the tract-based spatial statistics (TBSS) skeleton overlaid in green. Regions of interest that had significant correlations are shown in colours.
4. Discussion
Here we show different developmental trajectories between non-impaired and dysfluent readers in superior white matter known to be associated with reading. In general, non-impaired readers showed the expected age-related increases of FA, while dysfluent readers did not. Dysfluent inaccurate readers showed more substantial decreases of MD than the other groups, perhaps suggesting compensation. Gains in sight word reading and fluency were related to changes of diffusion parameters in the superior longitudinal fasciculus and corona radiata, suggesting that strengthening of these connections underlies improvements in key reading skills. On the other hand, improvements in phonological decoding were related to diffusion changes in the right corona radiata and across the entire skeleton, suggesting a relationship with global brain structure. These results support the idea that impaired readers have different developmental trajectories than typical readers and provide evidence that white matter maturation of certain tracts varies based on the specific type of reading impairment (i.e., dysfluency alone vs dysfluency plus impaired decoding).
Non-impaired readers had significant age-related increases of FA in the right superior longitudinal fasciculus and bilateral posterior corona radiata while dysfluent readers did not show significant changes. The trajectories in non-impaired readers are expected based on white matter development patterns in typically-developing children, which show increasing FA throughout childhood in both the superior longitudinal fasciculus (Lebel and Beaulieu, 2011) and the corona radiata (Tamnes et al., 2010). The lack of significant age-related increases in dysfluent readers may reflect delayed development in this group, which has also been suggested by functional imaging studies. For example, one study showed more substantial alterations of functional connectivity in the left temporal-parietal region of children with dyslexia compared to fewer differences in adults, suggesting that individuals with dyslexia may at least partly “catch up” during development (Finn et al., 2014). A previous study found FA in the left arcuate and inferior longitudinal fasciculi increases from 7 to 11 years in children with above-average basic reading scores, but decreases in children with below-average scores (Yeatman et al., 2012). In a different study, age-related increases of FA in the left arcuate fasciculus were observed in both good and poor readers (aged 5–12 years), though the good readers had faster increases than the poor readers (Wang et al., 2017). In general, our results are consistent with these previous studies in that all observe faster FA increases in good readers than in poor readers. However, the specific location of differences and the nature of developmental changes in the poor readers varies across studies.
We found relationships bilaterally in the corona radiata and in the right superior longitudinal fasciculus, while previous relationships were found in left hemisphere regions. Previous research has suggested that the left hemisphere, particularly the intersection of the arcuate fasciculus and corona radiata, is where relationships between reading abilities and white matter microstructure are most commonly found (Vandermosten et al., 2012), although numerous studies have also observed relationships between reading skills and brain structure in right hemisphere areas, including the superior longitudinal fasciculus (Lebel et al., 2013; Richards et al., 2008; Walton et al., 2018). Indeed, here, we found significant age-group interactions in the right superior longitudinal fasciculus for FA. Interestingly, results in the left superior longitudinal fasciculus revealed a similar pattern of larger FA increases in the non-impaired group compared to the dysfluent groups, though age-group interactions were not significant (p = 0.14). One of the previous studies also examined the corona radiata, and found no group differences for FA development (Yeatman et al., 2012). However, they examined the entire corona radiata (superior cortex to subcortical gray matter), while we examined only the superior portion, which may indicate that different relationships exist along the length of the tract.
Our results also showed that dysfluent inaccurate readers had faster age-related decreases of MD in the right anterior and posterior corona radiata, as well as the left uncinate fasciculus compared to dysfluent accurate readers. MD was not investigated by the previous longitudinal studies, but it does show slightly different developmental trajectories compared to FA (Lebel et al., 2017). Decreases of MD typically occur throughout childhood in the corona radiata and uncinate fasciculus, with these areas showing continued development into the twenties (Lebel et al., 2008; Tamnes et al., 2010, 2017). The differences between dysfluent accurate and dysfluent inaccurate readers suggest that children with different component skills in reading exhibit different maturation trajectories. In general, faster decreases of MD are interpreted as faster development, which could potentially represent a catch-up, or alternatively may indicate compensation. It is possible that steeper decreases of MD suggest compensatory maturation in right-hemisphere (i.e., the corona radiata) and left ventral (i.e., uncinate) language tracts in dysfluent inaccurate readers that is not apparent in dysfluent accurate readers. Previous studies have shown increased activation in the right inferior frontal area, suggesting that it plays a compensatory role in dyslexia (Hoeft et al., 2011; Shaywitz et al., 2002), and even that this role may increase over time (Shaywitz et al., 2002). Our findings in the right anterior corona radiata and the left uncinate fasciculus are consistent with this interpretation, suggesting that structural connectivity may also underlie compensation in the right hemisphere of children with dysfluent reading.
Changes in reading parameters were correlated with changes in brain structure in several areas. Larger increases of FA in the left superior longitudinal fasciculus were related to larger increases on TOWRE Sight Words, which is well-aligned with previous studies that have observed larger gains in WJ Word ID associated with faster changes of FA (Wang et al., 2017) or larger decreases of MD (Treit et al., 2013) in the superior longitudinal fasciculus. Larger gains in TOWRE Phonological Decoding were associated with greater decreases of MD in the right posterior corona radiata, as well as across the entire white matter skeleton. This suggests that, although the right posterior corona radiata exhibited group differences in diffusion parameter trajectories, the relationship between changes in phonological decoding and changes in brain white matter may reflect a more global relationship. Interestingly, the relationships between gains in reading measures and brain maturation were similar across groups. This suggests that even though baseline differences, as well as different trajectories of development are observed across fluency groups, the fundamental relationship between the underlying brain changes and changes in reading measures is similar.
We also observed a positive correlation between fluency gains and MD changes in the left superior longitudinal fasciculus and superior corona radiata. Most subjects had decreasing MD over time, and smaller drops in MD were generally associated with bigger gains in fluency. These left temporal-parietal areas often exhibit deficits in individuals with dyslexia (Vandermosten et al., 2012). Although the relationships were opposite to those expected (i.e., larger reading fluency gains were associated with less maturation), this may be due to the fact that the dysfluent readers had smaller age-related changes in these areas and also the most potential for improvements in fluency. Nonetheless, this indicates that maturation in this reading area is related to fluency improvement. Furthermore, changes in fluency were not related to changes across the entire brain, suggesting that these relationships are specific to these left hemisphere reading areas.
The results for FA showed similar types of alterations in children with dysfluent reading, regardless of their reading accuracy (i.e., both showed a lack of the typical age-related increases of FA). The MD results, on the other hand, differentiate the dysfluent accurate readers from the dysfluent inaccurate readers. In all four regions with significant age-group interactions for MD, the dysfluent inaccurate group showed faster MD decreases than the dysfluent accurate group. These differences were in secondary reading areas (e.g., right hemisphere), and may thus reflect compensation in the dysfluent inaccurate group, where additional brain areas must support reading more than they would in the non-impaired group. It is difficult to tell whether dysfluent accurate readers did not show this compensation because they are less impaired, or because they have a different underlying neurobiological mechanism.
In this study, participant groups were defined based on reading score cut-offs. However, reading scores and brain measures exist on a continuum, and there is considerable individual variation. It is possible that the dysfluent accurate and dysfluent inaccurate groups represent the lower end of a continuum of scores, or that they have distinct neural phenotypes. Previous studies showing correlations between brain white matter measures and reading scores in typical subjects (Arrington et al., 2017; Beaulieu et al., 2005) suggest the former interpretation. Our data showed similar relationships between changes in reading scores and changes in brain diffusion properties over time, which may suggest that there is a continuum of reading scores and a continuum of brain measures and brain development.
FA and MD are sensitive to multiple white matter processes including myelination, axonal density, axon diameter, and axon coherence. FA is a measure of the directionality of diffusion, while MD reflects the average water movement. AD and RD can offer more insight into the specific processes underlying changes than FA and MD alone. RD had significant or trend-level group-by-age interactions for each of the four tracts with significant differences in FA and/or MD trajectories; AD had no significant group-by-age interactions. As RD is more specific to myelination and axonal packing (Song et al., 2003, 2005), this suggests that the differences in trajectories are likely related to increasing myelination and/or axonal packing in the non-impaired group, with a lack of similar changes in the dysfluent group. Future studies using techniques more specific to white matter processes (e.g., myelin water imaging) may help further understand the specific physiological processes driving white matter maturation in these groups (Basser and Jones, 2002; Geeraert et al., 2017).
This study has some limitations. First, the DTI data acquired used only 6 gradient encoding diffusion directions. While 6-direction DTI data is not ideal and no longer used for new protocols, as long as there is sufficiently high signal-to-noise ratio, it provides similar sensitivity to group differences as data with more diffusion directions (Lebel et al., 2012). The voxels used here were also larger than typical DTI acquisitions, which makes it difficult to localize changes to specific areas of white matter tracts, and can cause partial volume artifacts. To help reduce partial volume problems, we used tract-based spatial statistics to first skeletonize the white matter and then overlaid ROIs to extract average diffusion parameters across a region. Future studies using improved imaging techniques will be able to better localize white matter differences. We did not collect data on pubertal status of the children who participated in this study. Previous studies suggest that puberty has small effects on brain maturation, over and above effects of age and gender (Genc et al., 2017; Herting et al., 2012), though it remains unclear whether this holds throughout the brain (Lebel et al., 2017). Future studies incorporating pubertal status may be able to more cleanly isolate developmental differences between reading groups.
In sum, we observed differing maturational trajectories between children who were dysfluent compared to non-impaired readers. Both dysfluent accurate and dysfluent inaccurate readers failed to show the expected age-related increases in FA that were evident in the non-impaired group, suggesting delayed development. On the other hand, the dysfluent inaccurate group had steeper decreases of MD than the dysfluent accurate group, perhaps reflecting compensation. These changes in brain white matter diffusion parameters underlie changes in reading skills over time, as evidenced by significant correlations in left posterior reading areas. Our results highlight maturational differences between children with different types of reading impairment, and may help guide the timing of interventions to improve reading abilities, which should be targeted to a time when white matter is still developing, to take advantage of the brain’s plastic state.
Conflict of Interest
None.
Acknowledgments
Funding was provided by the National Institute of Child Health and Human DevelopmentP50 HD25802, R01 HD046171, and R01 HD057655. We also thank supporters of the Yale Center for Dyslexia & Creativity, and the Seedlings Foundation. Scholarship research training support was provided by the NSERC CREATE International and Industrial Imaging Training (I3T) Program (AB, BG).
References
- Arrington N.C., Kulesz P.A., Juranek J., Cirino P.T., Fletcher J.M. White matter microstructure integrity in relation to reading proficiency. Brain Lang. 2017;174:103–111. doi: 10.1016/j.bandl.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Jones D.K. Diffusion-tensor MRI: theory, experimental design and data analysis – a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Beaulieu C., Plewes C., Paulson L.A., Roy D., Snook L., Concha L., Phillips L. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Clark K.A., Helland T., Specht K., Narr K.L., Manis F.R., Toga A.W., Hugdahl K. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 2014;137:3136–3141. doi: 10.1093/brain/awu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R.F., Ben-Shachar M., Deutsch G.K., Hernandez A., Fox G.R., Wandell B.A. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E., Shaywitz B.A., Holahan J.M., Marchione K., Shaywitz S.E. Uncoupling of reading and IQ over time: empirical evidence for a definition of dyslexia. Psychol. Sci. 2010;21:93–101. doi: 10.1177/0956797609354084. [DOI] [PubMed] [Google Scholar]
- Ferrer E., Shaywitz B.A., Holahan J.M., Marchione K.E., Michaels R., Shaywitz S.E. Achievement gap in reading is present as early as first grade and persists through adolescence. J. Pediatr. 2015;167:1121–1125. doi: 10.1016/j.jpeds.2015.07.045. e1122. [DOI] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Holahan J.M., Scheinost D., Lacadie C., Papademetris X., Shaywitz S.E., Shaywitz B.A., Constable R.T. Disruption of functional networks in dyslexia: a whole-brain, data-driven analysis of connectivity. Biol. Psychiatry. 2014;76:397–404. doi: 10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R.E., Hasan K., Xue L., Strickland D., Malmberg B., Liederman J., Papanicolaou A. Splenium microstructure is related to two dimensions of reading skill. Neuroreport. 2008;19:1627–1631. doi: 10.1097/WNR.0b013e328314b8ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs L.S., Fuchs D., Hosp M., Jenkins J.R. Oral reading fluency as an indicator of reading copmetence: a theoretical, empirical, and historical analysis. Sci. Stud. Read. 2001;5:239–256. [Google Scholar]
- Geeraert B.L., Lebel R.M., Mah A.C., Deoni S.C., Alsop D.C., Varma G., Lebel C. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. Neuroimage. 2018;182:343–350. doi: 10.1016/j.neuroimage.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Genc S., Seal M.L., Dhollander T., Malpas C.B., Hazell P., Silk T.J. White matter alterations at pubertal onset. Neuroimage. 2017;156:286–292. doi: 10.1016/j.neuroimage.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Lenroot R.K., Shaw P., Lalonde F., Celano M., White S., Tossell J., Addington A., Gogtay N. vol. 289. 2008. Trajectories of anatomic brain development as a phenotype; pp. 101–112. (Novartis Foundation Symposium). discussion 112–108, 193–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldston D.B., Walsh A., Mayfield Arnold E., Reboussin B., Sergent Daniel S., Erkanli A., Nutter D., Hickman E., Palmes G., Snider E., Wood F.B. Reading problems, psychiatric disorders, and functional impairment from mid- to late adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:25–32. doi: 10.1097/01.chi.0000242241.77302.f4. [DOI] [PubMed] [Google Scholar]
- Herting M.M., Maxwell E.C., Irvine C., Nagel B.J. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., McCandliss B.D., Black J.M., Gantman A., Zakerani N., Hulme C., Lyytinen H., Whitfield-Gabrieli S., Glover G.H., Reiss A.L., Gabrieli J.D. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. U. S. A. 2011;108:361–366. doi: 10.1073/pnas.1008950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston S., Lebel C., Katzir T., Manis F.R., Kan E., Rodriguez G.R., Sowell E.R. Reading skill and structural brain development. Neuroreport. 2014;25:347–352. doi: 10.1097/WNR.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber E., Donnelly P.M., Rokem A., Yeatman J.D. Rapid and widespread white matter plasticity during an intensive reading intervention. Nat. Commun. 2018;9:2260. doi: 10.1038/s41467-018-04627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T.A., Just M.A. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-S., Petscher Y., Schatschneider C., Foorman B. Does growth rate in oral reading fluency matter in predicting reading comprehension achievement? J. Educ. Psychol. 2010;102:652–667. [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C., Benner T., Beaulieu C. Six is enough? Comparison of diffusion parameters measured using six or more diffusion-encoding gradient directions with deterministic tractography. Magn. Reson. Med. 2012;68:474–483. doi: 10.1002/mrm.23254. [DOI] [PubMed] [Google Scholar]
- Lebel C., Shaywitz B., Holahan J., Shaywitz S., Marchione K., Beaulieu C. Diffusion tensor imaging correlates of reading ability in dysfluent and non-impaired readers. Brain Lang. 2013;125:215–222. doi: 10.1016/j.bandl.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Lebel C., Treit S., Beaulieu C. Diffusion MRI of typical white matter development from childhood to adulthood. NMR Biomed. 2017 doi: 10.1002/nbm.3778. in press. [DOI] [PubMed] [Google Scholar]
- Linkersdorfer J., Jurcoane A., Lindberg S., Kaiser J., Hasselhorn M., Fiebach C.J., Lonnemann J. The association between gray matter volume and reading proficiency: a longitudinal study of beginning readers. J. Cogn. Neurosci. 2015;27:308–318. doi: 10.1162/jocn_a_00710. [DOI] [PubMed] [Google Scholar]
- Lu L., Leonard C., Thompson P., Kan E., Jolley J., Welcome S., Toga A., Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb. Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Lyon G.R., Shaywitz S., Shaywitz B.A. A definition of dyslexia. Ann. Dyslexia. 2003;53:1–14. [Google Scholar]
- Morris D., Turnbull P. A survey-based exploration of the impact of dyslexia on career progression of UK registered nurses. J. Nurs. Manag. 2007;15:97–106. doi: 10.1111/j.1365-2934.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- Odegard T.N., Farris E.A., Ring J., McColl R., Black J. Brain connectivity in non-reading impaired children and children diagnosed with developmental dyslexia. Neuropsychologia. 2009;47:1972–1977. doi: 10.1016/j.neuropsychologia.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Richards T., Stevenson J., Crouch J., Johnson L.C., Maravilla K., Stock P., Abbott R., Berninger V. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. AJNR Am. J. Neuroradiol. 2008;29:1134–1139. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A. Dyslexia (specific reading disability) Biol. Psychiatry. 2005;57:1301–1309. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Fletcher J.M., Holahan J.M., Shaywitz S.E. Discrepancy compared to low achievement definitions of reading disability: results from the Connecticut Longitudinal Study. J. Learn. Disabil. 1992;25:639–648. doi: 10.1177/002221949202501003. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R., Mencl W.E., Fulbright R.K., Skudlarski P., Constable R.T., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Fulbright R.K., Skudlarski P., Mencl W.E., Constable R.T., Pugh K.R., Holahan J.M., Marchione K.E., Fletcher J.M., Lyon G.R., Gore J.C. Neural systems for compensation and persistence: young adult outcome of childhood reading disability. Biol. Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Brain functional and structural predictors of language performance. Cereb. Cortex. 2016;26:2127–2139. doi: 10.1093/cercor/bhv042. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., Armstrong R.C. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Ostby Y., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb. Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Roalf D.R., Goddings A.-L., Lebel C. Diffusion MRI of white matter microstructure development in childhood and adolescence: methods, challenges, and progress. Dev. Cogn. Neurosci. 2018;33:161–175. doi: 10.1016/j.dcn.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis K.E., Ben-Shachar M., Myall N.J., Feldman H.M. Variations in the neurobiology of reading in children and adolescents born full term and preterm. Neuroimage Clin. 2016;11:555–565. doi: 10.1016/j.nicl.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Lebel C., Baugh L., Rasmussen C., Andrew G., Beaulieu C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J. Neurosci. 2013;33:10098–10109. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Wouters J., Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neurosci. Biobehav. Rev. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Walton M., Dewey D., Lebel C. Brain white matter structure and language ability in preschool-aged children. Brain Lang. 2018;176:19–25. doi: 10.1016/j.bandl.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Wang Y., Mauer M.V., Raney T., Peysakhovich B., Becker B.L.C., Sliva D.D., Gaab N. Development of tract-specific white matter pathways during early reading development in at-risk children and typical controls. Cereb. Cortex. 2017;27:2469–2485. doi: 10.1093/cercor/bhw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Wilson A.M., Deri Armstrong C., Furrie A., Walcot E. The mental health of canadians with self-reported learning disabilities. J. Learn. Disabil. 2009;42:24–40. doi: 10.1177/0022219408326216. [DOI] [PubMed] [Google Scholar]
- Xia Z., Hoeft F., Zhang L., Shu H. Neuroanatomical anomalies of dyslexia: disambiguating the effects of disorder, performance, and maturation. Neuropsychologia. 2016;81:68–78. doi: 10.1016/j.neuropsychologia.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Dougherty R.F., Ben-Shachar M., Wandell B.A. Development of white matter and reading skills. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E3045–3053. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]