Abstract
The use of movie-watching as an acquisition state for functional connectivity (FC) MRI has recently enabled multiple groups to obtain rich data sets in younger children with both substantial sample sizes and scan durations. Using naturalistic paradigms such as movies has also provided analytic flexibility for these developmental studies that extends beyond conventional resting state approaches. This review highlights the advantages and challenges of using movies for developmental neuroimaging and explores some of the methodological issues involved in designing pediatric studies with movies. Emerging themes from movie-watching studies are discussed, including an emphasis on intersubject correlations, developmental changes in network interactions under complex naturalistic conditions, and dynamic age-related changes in both sensory and higher-order network FC even in narrow age ranges. Converging evidence suggests an enhanced ability to identify brain-behavior correlations in children when using movie-watching data relative to both resting state and conventional tasks. Future directions and cautionary notes highlight the potential and the limitations of using movies to study FC in pediatric populations.
Keywords: Naturalistic viewing, Movies, Resting state, Intersubject correlations, Head motion, Children
1. Introduction
The term “naturalistic paradigms” traces its roots to a shift in the field of vision research from using highly constrained artificial stimuli such as bars of light to also studying neural responses to complex natural images such as landscape photographs (for reviews, see (Felsen and Dan, 2005; Hasson et al., 2010). Early functional magnetic resonance imaging (fMRI) studies with movies used the term natural vision (Hasson et al., 2004) or natural viewing conditions (Bartels and Zeki, 2004a, 2005). In some cases, the specifier “free viewing” was added to emphasize the absence of a fixation cross or instructions to the participant to pay attention to a certain location on the screen (Bartels and Zeki, 2004b). The un-natural characteristics of, for example, commercial movies, are many, including multiple camera angles, zooms, pans, scene-cuts, the inclusion of music and exaggerated sound effects, the ability to skip time, etc. In 2009, Hasson et al. used the term “naturalistic,” and we adopt it here to describe stimuli that are complex, dynamic and rich (Hasson et al., 2009). A key feature of this class of stimuli is their dynamics: the stimuli themselves are composed of multiple elements, all of which have highly complex time courses, and are consequently distinct from conventional paradigms. As Bottenhorn et al. noted, these are stimuli that require continuous, real-time integration of dynamic streams of information (Bottenhorn et al., 2018). In this way, naturalistic paradigms aim to evoke more naturalistic patterns of neural responses; they do not necessarily aim to mimic the natural world.
The scope of naturalistic paradigms in neuroscience is broad, including the use of lullabies to study infants (Wild et al., 2017), so-called “hyperscanning” to scan two participants interacting in real time via a live video-feed between two MRI machines (Redcay et al., 2010), listening to stories or music (Finn et al., 2018; Regev et al., 2013) and using virtual reality goggles to simulate social scenarios (Pelphrey et al., 2004). Such stimuli are being used across modalities, including electroencephalography (Dmochowski et al., 2012; Tie et al., 2015), functional near infrared spectroscopy (fNIRS) (Hirsch et al., 2017) and eye-tracking (Klin et al., 2002), as well as across species (Mantini et al., 2012; Xu et al., 2018). The complexity of naturalistic paradigms introduces both theoretical and practical challenges for neuroscientists. Felsen and Dan discussed the difficulties of analyzing the stimulus-response relationship when the stimulus itself is dynamic and complex. Almost by definition, a movie violates the tenet of the scientific method of altering one variable at a time. In a critique of natural viewing paradigms, Rust and Movshon stated “The main—and in our view, crippling—challenge is that the statistics of natural images are complex and poorly understood” (Rust and Movshon, 2005). In other words, when using naturalistic paradigms, the stimulus properties (e.g., tracking the presence or absence of faces, local motion changes, or certain auditory features) are too complicated to isolate and model with any confidence. Movies are also technically difficult to work with: sound and video editing are entire specialties on their own, and movies are notoriously difficult to display consistently in scientific settings. Specifically, because computers dynamically alter the allocation of processing resources when handling digital data streams, it is challenging to maintain precise timing of a movie over repeated viewings. This is more of a problem for the temporal precision needed for electroencephalography than for fMRI, but attaining successful, consistent in-scanner movie presentation remains a struggle.
Despite these challenges, a major reason for the growing use of naturalistic paradigms in developmental fMRI has been the effect of movie-watching on head motion during scanning. With conventional task-based fMRI, scanning young awake children was possible because child-friendly tasks maintained interest and helped minimize head movement (Rajagopal et al., 2014). With the advent of functional connectivity (FC) MRI (fcMRI), parameters changed significantly. In fcMRI, researchers identify spatially separate brain regions or voxels that have correlated BOLD-signal time courses (see also Box 1). When doing this type of correlation-based connectivity analysis, even small head movements that were tolerable in task-based fMRI can cause spurious signal artifact (Power et al., 2012; Van Dijk et al., 2012). Further, fcMRI is usually conducted using “task-free” or resting state conditions wherein participants lay quietly in the MRI machine with no external stimulation. Because children naturally move more than adults, and because they experience the instructions to “lay still and relax” as a particularly demanding task, getting enough usable FC data from awake children under the age of 7 years has remained a formidable challenge.1 Even now, more than 20 years after the advent of resting state, only a few studies have used task-free conditions with substantial numbers of awake children under the age of 6 years (e.g., de Bie et al., 2012; Langeslag et al., 2013; Wee et al., 2018; Xiao et al., 2016), while others include only a few subjects under the age of 6 (Fareri et al., 2015; Gabard-Durnam et al., 2014; Muetzel et al., 2016). The major studies delineating developmental trajectories of resting state FC in awake children all begin at age 7 or 8 years (Alexander-Bloch et al., 2013; Dosenbach et al., 2010; Fair et al., 2008; Thomason et al., 2011; Yang et al., 2014). The problem is becoming even more formidable as a growing literature suggests that substantially more data are required to achieve reliable FC measures (Birn et al., 2013; Laumann et al., 2017; Noble et al., 2017; O’Connor et al., 2017; Pannunzi et al., 2017; Zuo et al., 2013).
Box 1. Terms and Acronyms (in order of appearance).
BOLD = Blood oxygen level dependent; describes the signal of interest in functional MRI, reflects the ratio of oxygenated and de-oxygenated hemoglobin in a given region of the brain.
FC = functional connectivity; FC is inferred when two or more spatially separate brain regions have correlated BOLD-signal time courses.
Resting state = the name given to the acquisition state used for studies that identify functional connectivity without an active task. Is further qualified as “eyes open” or “eyes closed” as these conditions can alter the patterns of results.
Task-free rest = a more specific way of talking about resting state imaging, sometimes just called “rest.” Acknowledges that remaining extremely still in a magnet is not particularly restful.
Movie = a series of images presented at a frame rate (usually greater than 24 frames/second) in which continuity across the images is used to portray movement and progression over time such that the viewer can not identify individual frames from within the stream of visual information.
Naturalistic stimuli = a class of stimuli that aim to evoke more naturalistic patterns of neural responses than traditional controlled artificial stimuli. Naturalistic paradigms are typically complex and dynamic, and longer in duration than many conventional stimuli. This is most often a relative term, for example a point-light-display of a man dancing would be naturalistic relative to still images of the same, but would be conventional and controlled relative to the biological motion shown in a film clip of a child playing soccer.
Framewise displacement (FD) = a complex measure of in-scanner head motion that calculates the position of the brain from one data point (frame) to the next, can be calculated using a variety of parameters, e.g., in 6 or 24 dimensions, etc.
Independent component analysis (ICA) = analysis that sorts a data set into components such that the fewest number of independent components explain the most variance; considered a “data-driven” approach.
Intersubject correlations (ISCs) = analysis pioneered by Hasson et al. in which the similarity of BOLD-signal time-courses from the same voxels across different subjects are studied. This approach isolates stimulus-induced responses because only responses that are shared (i.e., time-locked) across subjects reach significance. Sometimes called “intersubject synchrony” (Hasson et al., 2004, Nummenmaa et al. 2018).
Intersubject functional connectivity (ISFC) = an approach that combines ISCs and FC by identifying a seed region in one subject’s brain, and calculating the correlations with that seed region throughout another subject’s brain. This approach isolates functional connectivity patterns that are shared across subjects, and therefore are stimulus-evoked. Also called intersubject functional correlation (Simony et al., 2016).
Alt-text: Box 1
The idea of using movies to improve compliance is not new; labs and hospitals have been showing cartoons during structural MRI sequences to improve image quality and avoid sedation during pediatric scans for years (Raschle et al., 2012, 2009). In 2013, Cantlon and Li published an elegant study using clips from Sesame Street to study math-related processes in children ages 4–11 years (Cantlon and Li, 2013). They noted that head motion during the movie clips was significantly lower than during an age-appropriate task. In 2015, Vanderwal et al. published results from a small sample of young children, ages 4–7 years, studied in an MRI simulator, showing that movies could be used to attain relatively long scanning blocks with both lower mean head movement and fewer head movement spikes relative to rest. As the idea that functional connectivity can be studied using a variety of acquisition states has gained traction in the field, groups have increasingly used movies to investigate functional brain development in children (see Table 1), providing new insights about brain development, particularly in children under the age of 7 years.
Table 1.
Studies using movies as an acquisition state to study aspects of functional connectivity and/or intersubject correlations in pediatric neuroimaging, ordered by participant age.
| Movie | First author(s), year | Age range | Sample size | Run duration | Measures of interest |
|---|---|---|---|---|---|
| Child's choice | Long et al. (2017) | 2-6y | 44 | 8m 20s | ReHo, ALFF, eigenvector centrality (whole brain), |
| of movie | (4.0 ± 0.72) | (77 scans) | modeling age-related changes, some longitudinal data | ||
| Partly Cloudy | Richardson et al. (2018) | 3.5-12y | 122 | 5m 36s | social and pain network FC and ISCs, related to ToM |
| (silent version) | (6.7 ± 2.3) | behavioral measures, also reverse correlation GLM | |||
| Toy Story | Moraczewski et al. (2018) | 4y and 6y | 23 and 23 | 6m | ISCs in children vs. adults, relationship to |
| (4.4, 6.6) | attention-based measures | ||||
| Elmo's World | Rohr et al. (2017) | girls 4-7y | 44 | 18m | FC using DAN seeds, correlated with age and selective |
| (5.3 ± 0.8) | attention measures while controlling for age | ||||
| Rohr et al. (2018) | girls 4-7y | 60 | 18m | FC of ICA-derived networks correlated with three | |
| (5.5 ± 0.8) | attentional measures while controlling for age | ||||
| lava lamp | Xiao et al. (2016) | age 5, then 6 | 46 | 3m 33s | graph theory (degree centrality) and seed-based FC |
| screensaver | (5.5, 6.5) | related to age and language scores | |||
| Child's choice | Emerson et al. (2015) | 6y | 33 | 5m | ICA and seed-based comparison of inter- and |
| of movie | (6.1 ± 0.11) | (approx) | intra-network FC, rest vs. movie, focus on FP network | ||
| screensaver | Riggins et al. (2016) | 4y and 6y | 21 and 19 | 6m | hippocampal FC related to episodic memory abilities |
| (sparse visuals) | (4.5, 6.5) | ||||
| Blankenship et al. (2017) | 4-10y | 97 | 6m | hippocampal FC by age (cross-sectional) | |
| (6.7 ± 1.42) | |||||
| Sesame Street (compiled clips) |
Emerson and Cantlon (2012) | 4–12y | 24 | 20 m 20s | ROIs defined via number-matching task, then used |
| (8.24 ± 2.26) | calculate FC during movie, related to math scores | ||||
| Cantlon and Li, 2013 | 4.3-10.8y | 27 | 20 m 20s | ISCs (child-child, child-adult) in math-related ROIs | |
| (7.1 ± 1.6) | |||||
| Finding Nemo | Greene et al. (2018) | 5-15y (11.1) | 24 | 6m 50s | head motion, and whole-brain FC matrices (n = 17) |
| Big Hero 6 | |||||
| Despicable Me | |||||
| The Present | Alexander et al. (2017) | 5-21y | 900+ | 3m 30s | demographics, phenotypic outcomes, head motion, |
| Despicable Me | 10m | quality control assessments (Healthy Brain Network) | |||
| Bugs Bunny | Anderson et al. (2013) | 7-30y | 26 | 50m | ISCs, FC and graph theory measures in individuals |
| (20.3 ± 6.3) | with Down Syndrome and healthy participants | ||||
ReHo = regional homogeneity, ALFF = amplitude of low frequency fluctuations, FC = functional connectivity, ISCs = intersubject correlations, ToM = Theory of Mind, GLM = general linear model, DAN = dorsal attention network, ICA = independent component analysis, FP = frontoparietal network, ROIs = regions of interest, s=second, m = minute, y = year.
This review focuses specifically on the use of movies (which we define as moving images presented at more than 24 frames/second (Read et al., 2000)), in developmental fMRI. Many labs have used movies in fMRI for more conventional task-based designs, such as those showing clips of actors changing facial expressions (Schultz and Pilz, 2009), point-light-displays to evoke biological motion processing (Sifre et al., 2018), or movie clips that feature various classes of objects (Deen et al., 2017). Here, however, we are interested in the use of longer movies that are used as an acquisition state for recently developed fMRI analyses, for example, conducting resting state analyses on movie-watching data. We aim to highlight the advantages and limitations of using complex movie paradigms as an acquisition state for developmental neuroimaging, to explore some of the methodological issues involved in designing developmental studies using such movies, and finally, to summarize emerging themes regarding functional brain development under naturalistic movie-watching conditions
2. Head motion and age
When comparing head motion outcomes across pediatric studies, numerous factors must be taken into account, including the length of the run(s) in question, total scan duration, whether task-free rest or frustrating or boring tasks were included, the type and extent of behavioral training, the nature of the movie used, whether sound was included or not, any clinical symptomatology, and of course, the age range and age distribution of participants. To date, only small sample sizes have been available for head motion studies, which is especially limiting as different ages demonstrate different levels of compliance. A recent examination of head motion in a sample of 24 healthy children (mean age 11.1 years) found that movies conferred a significant advantage in children ages 5–10, but not for children older than age 10 (Greene et al., 2018).
Here we report head motion during a movie and task-free rest in a larger sample of 5–21 year olds (N = 356, mean age 9.9 years) from the Healthy Brain Network (HBN) data (henceforth referred to as HBN-356 for convenience, see Fig. 1a). At the group level, movie-watching conferred a significant advantage with regard to head motion. However, when examined by age, the null finding during adolescence was replicated. In HBN-356, after age 12 years, a major improvement in head motion compliance during both rest and movies occurred, negating the head motion advantage of movies relative to rest. Both studies used cartoons (e.g., Finding Nemo, Despicable Me), and testing adolescents with more age-specific stimuli might alter these outcomes (see also Box 2). Fig. 1e shows the best-fit line for each participants’ framewise displacement by their age. The steep slope for task-free rest demonstrates how developmentally skewed this condition is across a broad age range. The slope for movies is less steep, indicating that despite not being as helpful for adolescent head motion, using movies in a sample that includes both younger children and adolescents helps to minimize the effect of motion as an inherent age-based confound. A third finding is that, despite movement functioning as a trait in children as it does in adults (Engelhardt et al., 2017), the participants with the worst head movement during rest were not usually the worst during movie-watching (Fig. 1c). In other words, movies appear to be particularly helpful for the children with the most head motion during task-free resting state conditions. Overall, for scanning in children under the age of 10 years, the data converge, showing highly significant improvements in head motion.
Fig. 1.
Pediatric head motion in movies and rest (N = 356, mean age 10.42 years ± 3.4), from a help-seeking community sample. Two 5-minute rest runs were concatenated to match the duration of the movie run (Despicable Me (DM)). A) Sample age distribution shows skew towards younger subjects. B) For the group as a whole, the movie significantly improved head motion relative to rest (p < 0.0001). C) The 50 subjects with the highest mean framewise displacement (FD) during rest are labeled in red. Most of the red dots are below the diagonal, indicating that the 50 worst subjects during rest were not also the worst during the movie. D) When binned by age, mean head motion advantage was greatest for children under age 10 years. E) The best-fit lines show the striking effect of age on head motion for rest, which was somewhat diminished during the movie. All data are from the publicly available Healthy Brain Network biobank (PI: Michael Milham, Alexander et al., 2017). Methods for these analyses are in Supplementary materials. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Box 2. Comment on cartoons.
Cartoons are cinematically unique in many ways. For example, cartoons have significantly more scene cuts than most live action movies, and scene cuts are potent directors of visual attention and engagement (Smith, 2013). Cartoons also feature extraordinarily tight and exaggerated audiovisual congruency, which also relates strongly to attention and arousal, particularly in children (Rajagopal et al., 2014). Cartoons are also often highly imaginative and playful, altering the physical laws of the universe in ways that appeal to children. Due to these features, some researchers have concerns that cartoons are not as “ecologically valid” as other movies. In adults, for example, recruitment of dorsal medial prefrontal cortex (DMPFC) has been shown to differ when participants view artificial intelligence characters relative to human faces (Han et al., 2005). However, a study of 10-year old children reported the same recruitment of dorsal medial prefrontal cortex (DMPFC) to both cartoon and real-life figures (Han et al., 2007), and cartoons have long been used effectively to study social cognition and mentalizing in adults (e.g., Gallagher et al., 2000). At this point, the engagement-based advantages of cartoons outweigh the potential disadvantages, and our current recommendation is that researchers are fortunate to harness the work of an entire industry that is developmentally targeted, and designed to keep children engaged and entertained.
Alt-text: Box 2
These data also show that movies are not a panacea for in-scanner head movement; the mean framewise displacement (FD) for the HBN subsample used here was 0.84 mm ± 1.2 for rest, and 0.60 mm ± 0.85 for the movie. The HBN recruits help-seeking children from the community, so it is selective for children with a broad range of neurodevelopmental and psychiatric symptoms. Careful behavioral training in all young child groups remains essential, and combining movies with real-time motion monitoring may also be useful for some studies (Dosenbach et al., 2017; Thesen et al., 2000). Appropriate approaches to reporting and balancing motion across ages and conditions are still crucial, as is the use of rigorous approaches to mitigating motion artifact (Grayson and Fair, 2017; Power et al., 2015; Yan et al., 2013).
3. FC modulation: movies vs. rest
A researcher might adopt the use of movies for practical reasons such as decreasing head motion. The next question quickly becomes, “What are movies doing to FC patterns in the brain?” Just as with conventional tasks, two perspectives on FC modulation during movie-watching have been argued: the first is that movies are potent tasks, evoking highly specific patterns of FC; the second is that the brain has an underlying level of functional organization that is captured well during naturalistic conditions. We contend that both are valid perspectives, and that either perspective can be leveraged depending on the analytic approach being implemented.
Seed-based analyses (which identify networks by placing a region-of-interest (“seed”) in the brain and then looking throughout the brain to see what other regions are functionally correlated with the seed) conducted in children (n = 17) during movie-watching showed that FC network structure was highly similar during movie and task-free rest for all network seeds tested (Greene et al., 2018). Independent component analysis (ICA) of movie-watching data in girls ages 4–7 years (n = 60), and in a separate group of 6-year old children (n = 33) yielded components that were easily identified as intrinsic connectivity networks (Emerson et al., 2015; Rohr et al., 2018). When using canonical resting-state network masks, a consensus is emerging in the adult literature that movie-watching modulates mean FC in visual and higher order networks, especially the frontoparietal network, and also the default and dorsal attention networks (Betti et al., 2013; Gao and Lin, 2012; Vanderwal et al., 2015). This has also been shown in children (Emerson et al., 2015; Greene et al., 2018). The underlying assumption is that as with any acquisition state, these FC measures capture a combination of invariant and task-evoked dynamic properties (Buckner et al., 2013; Ren et al., 2017; Simony et al., 2016), and as discussed below, such measures are reliable and can be meaningfully related to age and behavioral correlates.
Task specific networks that emerge during movie-watching are also being studied (Kim et al., 2017). These networks become more pronounced when researchers first use intersubject approaches to essentially filter out intrinsic connectivity relationships that are by nature not correlated across subjects (see intersubject functional connectivity (ISFC) in Fig. 4, and in the Box 1) (Simony et al., 2016). This “pure” approach to movie-watching FC demonstrates a modular network structure, with the canonical FC networks split up into different modules. For example, dorsal attention network regions were split into two clusters. Moreover, the authors point out that the general external/internal categorization of networks was not maintained, meaning that parts of task-positive networks were integrated with regions from default and other networks to form combined modules during movie-watching (Kim et al., 2017). This intriguing approach therefore reveals network interactions that are likely crucial to everyday life and may have important developmental trajectories, but work of this sort has not yet been conducted in pediatric samples.
Fig. 4.
Spectrum of analytic approaches to movie-watching data, with examples. Studies to the left highlight the combined use of multiple types of analyses on the same data set, and asterisks denote pediatric studies. Intersubject approaches comprise a unique hybrid insofar as they are model-free, but depend on time-locked signal changes.
It is important to note that the task-evoked properties of movie-watching data make it difficult to leverage large resting state datasets for comparison, replication, machine learning or normative statistics. Some large datasets are starting to include movie-watching conditions, for example in children ages 5–21 years (Alexander et al., 2017) and adults ages 18–87 years (Taylor et al., 2017) which helps, but also introduces questions about cross-movie comparisons.
4. FC modulation: movie vs. movie
Questions about cross-movie comparisons also arise as some groups have allowed each child participant to select their own movie, averaging across different movies when calculating FC measures (Emerson et al., 2015; Long et al., 2017). Strengths of this approach are that it works well—the scan durations in these studies are the longest for their respective age ranges to date. It also likely diminishes some confounds by balancing each child’s engagement with the movie, an approach that might be particularly helpful with a sample that includes both preschool children and adolescents. Increasing the number of different movies used within a sample also increases generalizability of findings (Westfall et al., 2016). The major concern with collapsing across multiple movies is that each movie varies in innumerable ways, confounding the interpretation of results.
Depending on the research question, however, data so far suggest that the variance introduced across different movies is less than that which occurs either across separate scanning sessions or across subjects. Using a unique data set in which 10 subjects were scanned on 12 separate days with a mix of 10-minute movie clips, task-free rest, Inscapes (see below) and a Flanker task (Stroop-like inhibition task), O’Connor et al. examined the reliability of whole-brain FC measures using a hierarchical linear mixed model (O’Connor et al., 2017). Intra-class correlation coefficients revealed an “impressively high degree of between-condition reliability for most connections” whereas between-session reliability was significantly lower. Along these lines, an unsupervised test-retest matching algorithm has also been shown to exhibit high accuracies for matching an individual subject’s FC matrix from one scanning session to their own FC matrix from another scanning session. This test-retest matching was successful across two movies (Inscapes and Ocean’s Eleven) and across a movie and rest (Vanderwal et al., 2017). Finally, the first meta-analysis to be conducted across naturalistic paradigms found 6 patterns of convergent activation common to a broad range of naturalistic stimuli, including movies, virtual reality paradigms, and listening to music or speech (Bottenhorn et al., 2018).
To date, studies directly comparing FC patterns across movies (rather than movie vs. rest) are scarce. Here we use data from 10 adults (Healthy Brain Network Serial Scanning Initiative) to calculate the mean FC for canonical networks during four distinct movies as well as rest and a Flanker task (Fig. 2). The movies were selected to be distinct from each other. They include a mainly nonverbal animation (Wall-E opening scene), a highly suspenseful science fiction clip with multiple settings, characters and special effects (The Matrix, scene with two pills), a dialogue-driven, highly social scene shot in a single environment (A Few Good Men, courtroom scene) and an animation of abstract shapes and music called Inscapes. Qualitatively, spring-embedded graph visualizations look similar for Wall-E, The Matrix, Inscapes and Rest, but A Few Good Men and Flanker have more distinctive graphs. In line with previous work, the frontoparietal network appeared to change inter-network relationships the most across conditions, becoming more integrated with other networks during movie-watching (Emerson et al., 2015; Gao and Lin, 2012). Despite clear topological shifts, the mean FC for both the frontoparietal and dorsal attention networks did not differ significantly across conditions in this small sample. Differences in mean FC of the default network were found only for Flanker relative to Wall-E and A Few Good Men.
Fig. 2.
FC organization across four movies, task and rest (n = 10, healthy adults) using canonical networks. 2a) When the top 10 percent of edges were visualized using spring-embedded graph tools, the frontoparietal and default networks appeared to be the most variable across conditions. During the Flanker task (a basic memory and response inhibition task), the frontoparietal network was more discrete, and was positioned between the default and remaining networks. Graphs appeared to be similar across Rest, Inscapes, Wall-E and The Matrix, whereas the graph for A Few Good Men appeared more spherical, with more inter-network integration. This may have been due to the highly social nature of the scene, indicating that the brain was most integrated during the sustained and intense high-level social processing. 2b) Mean functional connectivity (FC) by network was calculated using dual regression. Repeated measures ANOVA was nonsignificant for both the frontoparietal and dorsal attention networks. Despite topographical changes in these networks across conditions as depicted in the spring graphs, mean FC was not significantly different in this small sample. Follow-up t-tests for default network showed significant differences in FC between Flanker and Wall-E, and between Flanker and A Few Good Men (p < .05).
5. Inscapes
Given that different movies can modulate FC in different ways as discussed above, some groups have used particular movie types to try to decrease the amount of task-driven modulation. For example, Xiao et al. used a movie of a slowly changing lava lamp, and Riggins et al. used a screensaver with only occasional, sparse visual shapes and colors (Riggins et al., 2016; Xiao et al., 2016).
Vanderwal et al. attempted to optimize and standardize this type of approach by making a movie specifically for acquiring functional connectivity data in young children that would be somewhat more engaging than the lava lamp or screensaver approach, and could be shared across labs. In 2015, they introduced a 7-minute movie called Inscapes. Professional artists created the original animation, musical score and soundscape, with consultation from developmental specialists. The movie features a series of slowly moving abstract shapes with a soothing, consistent piano score that was meant to blend with the scanner sounds. It has no scene cuts, and there are no sudden pans or zooms. Inscapes is freely available (www.headspacestudios.org), and is currently being used by multiple research groups in North America, Europe and Asia in both pediatric and adult samples. Preliminary head motion results coming from large samples of both young children and children with disorders such as minimally verbal autism spectrum disorder are promising, but it remains to be seen whether Inscapes results in better head motion outcomes relative to other movies for children as it did in adults (Vanderwal et al., 2017). Overall, the film minimizes some important developmental confounds such as language and social processing, and may be useful to researchers because of these unique features. Neurally, the movie evokes strong connectivity in the default network, and frontoparietal connectivity patterns are more similar to rest than during conventional movies (Vanderwal et al., 2015). The paradigm thus offers a standardized, developmentally informed option for researchers wanting a non-social movie to use during scanning that improves head motion and arousal levels, and still preserves individual differences in FC patterns (Vanderwal et al., 2017) (See Box 3).
Box 3. To include rest?
One frequently asked question is whether to include a block of task-free rest in a pediatric study that uses movies. Recent key studies in children elected not to include task-free rest (Long et al., 2017; Morazcewski et al., 2018; Richardson et al., 2018), and this may be an important consideration in why they were successful in these young age ranges. If task-free rest is essential to the study question, we follow the recommendations of the organizers of the Healthy Brain Network, who noted higher head motion in the second 3 min of a 6-minute rest block. Splitting up the rest block into two shorter runs has been beneficial. However, particularly with children younger than 10 years of age, the data indicate that omitting task-free rest is a defensible position whereas including rest that has significant motion artifact is not.
Alt-text: Box 3
6. Reliability of FC measures acquired during movie-watching
It next becomes important to understand how reliable FC measures are under movie-watching conditions. One of the first motivating factors for using movies during fMRI was that the results of natural vision studies had been found to be significantly more reliable than results from artificial stimuli (Hasson et al., 2010). In adults, movies have been shown to have higher within-subject FC correlations at the whole brain level relative to rest (Vanderwal et al., 2017), as well as higher intra-class correlations (ICCs) (Wang et al., 2017a). Graph theory measures such as degree centrality have also been shown to have significantly higher ICCs relative to rest (Wang et al., 2017b). Data regarding reliability using naturalistic paradigms in pediatric populations are not yet available. An important consideration for developmental studies attempting to identify age-related changes or longitudinal change over time is the test-retest reliability of the measure of interest (Herting et al., 2017; Telzer et al., 2018).
7. Options for evaluating task performance
One challenge inherent to task-free rest is that there is no straightforward way to measure task performance or engagement, particularly when working with young children who find it difficult to describe what their minds are doing (Tusche et al., 2014; Ye et al., 2014), or when a sample has a broad range of attentional capacities. With movies, even with passive viewing of long movie clips, the presence of a time-locked stimulus enables some experimental control for these parameters. Where developmentally appropriate, post-scan recall questions can index a participant’s comprehension and memory for the movie. Measures such as eye-tracking or skin conductance can be used and correlated across participants to identify outliers (Hasson et al., 2009). Voxel- or cluster-wise BOLD-signal time courses from particular regions can also be used in similar ways. For example, the BOLD-signal time-courses in the frontal eye fields have been used as a proxy for attention (Moraczewski et al., 2018). Movies are also excellent candidates for approaches that utilize BOLD-signal in ocular muscles to provide volume-by-volume estimates of fixation (Alexander et al., 2017; LaConte et al., 2006). These approaches, or their combinations, can equip developmental neuroimagers to assess and control for task performance during movie-watching even in young children, providing an advantage over task-free rest.
8. Analytic flexibility
Up to this point, we have been discussing the use of movies to study FC, but movies are uniquely situated to facilitate a broad array of analytic approaches that go beyond “resting state” analyses. The BOLD-signal time-courses that are evoked during movie-watching are long, contain both task-induced and task-independent sources of variability, and involve a majority of voxels. Fig. 3 provides an example of such signal variability under naturalistic conditions. The raw BOLD-signal time courses from a single low-motion subject are shown for seed regions of the frontoparietal and visual networks during a movie, rest and the Flanker task. At the group level, we computed whole-brain multi-scale entropy to capture aspects of the signal structure that go beyond variability or standard deviation that might be included in these signals (Grandy et al., 2016, Smith et al., 2014). This was motivated by Cantlon and Li’s suggestion that the variability of the BOLD-signal time-course during movie-watching contains developmentally relevant information not included in conventional task BOLD-signal responses (Cantlon and Li, 2013): Fig. 3 depicts that idea.
Fig. 3.
Signal complexity under naturalistic conditions. Spherical regions-of-interest (ROIs) were selected within frontoparietal and visual networks. Time courses from a single, low motion subject are shown. Power spectral density (PSD) of each signal shows that the variability of signal during movie (blue line) differentiates from Flanker task (red line) and rest (yellow line) in both ROIs. At the whole-brain level (n = 10), multi-scale entropy (MSE) was calculated to estimate the complexity of the BOLD-signal structure. Here, both unconstrained states (movie and rest) demonstrate significantly greater MSE than the Flanker task. These exploratory analyses are meant to visually depict the idea that signal complexity under naturalistic conditions enables multiple analytic approaches, and may be related to an enhanced ability to identify brain-behavior correlations and individual differences in FC. See supplementary materials for detailed methods. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
In addition to evoking signal variability throughout the brain, movies provide time-locked stimuli to use during data analysis, meaning that researchers have the ability to model the task in their analyses, or not. Fig. 4 schematizes the continuum of analytic approaches that are possible when using movie-watching data. Spiers and Maguire (2007) and Hasson et al. (2009) provide excellent reviews of time-locked analyses of naturalistic paradigms.
The most common movie-based approach in developmental studies to date is intersubject correlations (ISCs), a method pioneered by Hasson et al. in which the BOLD-signal time course in voxel A of subject 1 is correlated with the time course in voxel A of subject 2, and so on (see Nummenmaa et al., 2018 for a review of ISCs in adults) (Hasson et al., 2004). ISCs are unique insofar as they rely on stimulus-evoked (i.e., time-locked) signal changes, but are also model-free and whole-brain. ISCs can be examined in groups of different developmental stages (see Fig. 5), or across clinical groups such as individuals with autism spectrum disorder (Bolton et al., 2018; Hasson et al., 2009) or Down Syndrome and healthy participants (Anderson et al., 2013). Group maps of ISCs provide information about how systematic BOLD-signal responses are in a cohort, and what regions demonstrate those concerted neural responses.
Fig. 5.
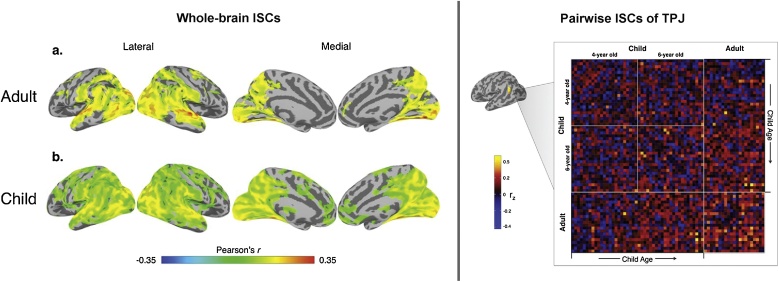
Intersubject correlations (ISCs) in adults, and children ages 4 and 6 years of age during Toy Story. Overall, ISCs are stronger in adults, but cover more of the cortex in children. When controlling for attention, the left temporoparietal junction (TPJ) exhibited age-based correlations. Panel B shows all pairwise ISCs for 4 year olds, 6 year olds, and adults. Figure reproduced and adapted from Moraczewski et al. (2018), by permission.
Both behavioral and neural variability have long been of interest as sensitive developmental measures (Campbell et al., 2015; McIntosh et al., 2008; Petroni et al., 2018; Vakorin et al., 2011), and ISCs provide a unique whole-brain way of assessing both intra-, but especially inter-subject variability, of complex neural processes in children. Three papers to date have used a developmental cross-group approach to ISCs by defining an average adult time course for each voxel or cluster, and then calculating the correlation between each child’s time course and that
adult time course at each voxel (Cantlon and Li, 2013; Moraczewski et al., 2018; Richardson et al., 2018). The advantage of generating an averaged adult time course is that it provides a model for what mature neural responses in dynamic, complex conditions might look like at each voxel. Each child’s correlation (child-adult) can then be averaged across the group of children, and brain regions that are more or less correlated with the adult response can be identified. Importantly, ISCs appear to have significant behavioral relevance, and have been shown to correlate strongly with developmental outcomes of interest such as math skills (Cantlon and Li, 2013), social cognitive scores (Richardson et al., 2018), age, and measures of attention (Moraczewski et al., 2018). One complication of using ISCs in children is that analyses depend on an intact time-course, complicating the use of volume censoring to mitigate motion artifact by requiring interpolation (Carp et al., 2013; Richardson et al., 2018). Recent approaches to denoising high-motion data sets that preserve the time course may better fit ISC analyses (Kaufmann et al., 2017) and have been shown to work well with even young children (Rohr et al., 2018).
Every movie contains numerous visual and auditory features such as luminance change and the zero-crossing rate of the soundtrack, the complexity of which refers back to a common concern regarding naturalistic paradigms as mentioned in the Introduction. Each of these features can be used as a regressor in a more task-based approach to probe evoked responses within different neurofunctional systems (Bartels et al., 2008; Lahnakoski et al., 2012; Mante et al., 2005; Whittingstall et al., 2010). Features within movies can be annotated using a number of methods, and annotated epochs can be collapsed to form an event-related design (Botzung et al., 2010; Lahnakoski et al., 2012). Reverse-correlation approaches, where changes in signal at a group or individual level are used to identify relevant features from within the movie, have also been used productively (Hasson et al., 2004; Jaaskelainen et al., 2008; Nishimoto et al., 2011). Though most of this type of stimulus-modeled work has so far been conducted with adults, Richardson et al. recently showed that a reverse correlation approach is feasible even with 3-year old participants. As can be seen in Fig. 4, this study creatively applied three different analyses to a movie-watching data set, spanning both model-driven and model-free approaches, highlighting the analytic flexibility inherent to movie-watching data. All of these approaches are complicated and time-consuming, and the makers of NeuroSynth (a popular platform for synthesizing fMRI findings by region (Yarkoni et al., 2011 #159)) are working on a project called NeuroScout that aims to provide automated multimodal feature extraction for movies that is generalizable and reproducible, and may be of use to developmental neuroimagers interested in more task-based investigations of movie-watching data (McNamara et al., 2017 #160).
9. Stimulus-independent analytic approaches
Many groups have used naturalistic paradigms as an acquisition state to study FC in adults, applying the same analyses as would be used with data acquired during task-free rest. Categorizing these approaches as “stimulus independent” specifically means that the analysis itself is not informed by, or dependent on, the time-course of the movie (i.e., it does not mean that the signal changes being captured are not informed by the time-course of the movie). FC studies of children using movie-watching data have used an array of FC measures including whole-brain (Long et al., 2017), canonical or pre-defined networks (Richardson et al., 2018; Rohr et al., 2018), seed-based networks (Emerson et al., 2015; Greene et al., 2018; Rohr et al., 2017), ROIs (Blankenship et al., 2017; Emerson and Cantlon, 2012; Riggins et al., 2016), and ICA-based measures (Emerson et al., 2015; Rohr et al., 2018).
Much has been learned about the development of FC organization using task-free rest, sleep studies, and conventional tasks (for review, see Grayson and Fair, 2017). To date, studies of FC during movie-watching are making unique contributions to the developmental literature in three main ways: first, by providing quality data in awake children in an understudied and developmentally dynamic age range; second, by providing information about how network interactions during complex, naturalistic processing develop; and third, by enabling researchers to identify robust brain-behavior relationships in developmental domains of interest.
10. FC and age in preschool children
In the first study of its kind in preschool children, Long et al. investigated the relationship between basic FC measures and age in this pivotal developmental period. Using movies as an acquisition state enabled them to collect both cross-sectional and longitudinal data from 44 children, ages 2–6 years. FC measures were found to correlate strongly with age even within this narrow age range, with r-values ranging from 0.35 to 0.65 and from −0.4 to −0.55. The strongest correlations were in precuneus and right superior temporal gyrus. Local and global FC were found to both increase and decrease with age, with different regions exhibiting different patterns: Regions exhibited increased local FC with age (frontoparietal network), increased local and global FC (default network) and mixed patterns including both local-to-global (superior temporal gyrus) and global-to-local shifts (e.g., fusiform gyrus) (Long et al., 2017).
As did the frontoparietal and default network regions in the Long et al. study, other data also show an overall pattern of FC increasing with age at a dynamic pace during early childhood. This has been demonstrated for pain and Theory of Mind networks from ages 3–12 years (Richardson et al., 2018), and with nodes of the dorsal attention network in girls ages 4–7 years (Rohr et al., 2017). A follow-up study in a larger cohort that used ICA to identify networks showed that FC increased with age in all but one of these networks (ventral attention) in girls ages 4–7—including primary sensory networks—providing an important counterpoint to the prevailing idea that brain development proceeds hierarchically from sensory to higher-order regions (Rohr et al., 2018). These cross-sectional data support the general picture that sensory FC networks seem to develop linearly throughout early childhood, while cognitive networks appear to exhibit nonlinear, network specific trajectories.
Exceptions to the pattern of increasing FC with age have also been found. Blankenship et al. examined age-related changes in hippocampal FC in children ages 4–10 years of age (Blankenship et al., 2017). They showed that most major patterns of hippocampal connectivity are already stable at this developmental stage. Exceptions were connectivity between the hippocampus and lateral temporal lobes and anterior cingulate. The authors suggest that the hippocampus becomes more connected to the default network during this period, which is theoretically consistent with the observed increase in global connectivity of the default network made by Long et al. (2017). Conversely, Rohr et al. showed that FC between dorsal attention and default network regions was negatively associated with age, meaning that negative FC was greater in the older children (Rohr et al., 2017). These regional patterns underscore the importance of delineating developmental trajectories of both intra- and inter-network FC.
11. FC organization during dynamic processing
Movie-watching entails dynamic processing that is different than occurs with most discrete, conventional tasks and with task-free rest (Bottenhorn et al., 2018 #91). Studying FC organization in this state in children has started to yield some important results about network development. In an independent component analysis of 6 year old children, frontal control and default networks became more connected during movies relative to rest, while the frontal control and dorsal attention networks showed down-regulated connectivity during movies relative to rest (Emerson et al., 2015). This rest-movie pattern is the same as has been observed in adults (Gao and Lin, 2012), but children only show marginally significant differences between rest and movie, meaning that the network interactions would be expected to strengthen with age. Relatedly, Richardson et al. used a movie that sometimes required viewers to make interpretations about a character’s inner thoughts or intentions (i.e., Theory of Mind), and sometimes required viewers to make interpretations about a character’s physical experience (i.e., pain). Their data show that as a network matures and becomes more integrated, it also exhibits more anti-correlated interactions with other networks (Richardson et al., 2018) (see Fig. 6). In their sample, network interactions were the measure that changed most dynamically, going from uncorrelated to anti-correlated with age, and the anti-correlation of two networks strongly predicted the within-network correlations, or integration, of the network. Network anti-correlation during movie-watching has also proven to be a meaningful outcome in studies of developmental disorders. In 15 children and adults with Down syndrome, Anderson et al. showed that individuals with Down syndrome demonstrated less anti-correlation relative to healthy participants. Further, increased inter-network correlation during movie-watching was associated with lower IQ scores. These data echo findings from resting state studies. For example, longitudinal resting state data from children ages 10–13 years of age shows that within-network connectivity strength increases and networks become increasingly anticorrelated (Sherman et al., 2014), and in adults during rest, the degree of anti-correlation between two networks has been shown to predict response times during inhibition tasks (Kelly et al., 2008). More broadly, network anti-correlation during rest has been related to psychiatric symptoms (Anticevic et al., 2012) and aging (Spreng et al., 2016). The data reviewed here suggest that network anti-correlation during both task-free rest and during naturalistic processing is an important component of healthy functional brain development.
Fig. 6.
Inter-region correlation analysis using Theory of Mind (ToM) and Pain networks in children and adults. As intra-network correlations increased, anti-correlations between networks also increased. The degree of integration within a network was also found to predict the degree of anti-correlation between networks. The broader developmental hypothesis is that network integration facilitates a network’s ability to interact effectively with other networks. Reproduced from Richardson et al. (2018), by permission (CC BY 4.0).
12. Brain-behavior correlations during naturalistic viewing
Multiple studies have shown that neural patterns during naturalistic viewing correlate strongly with developmentally relevant behavioral measures, including math aptitude, episodic memory abilities, social cognition scores and attention measures. Emerson and Cantlon defined ROIs using a number-based task, and then calculated FC between those ROIs under naturalistic viewing of math-based movie clips. FC values correlated with task-based reaction time (r2 = 0.27), Test of Early Mathematics Ability (TEMA) raw score (r2 = 0.32) and age (r2 = 0.17). They also showed that though FC during movies differed with age, BOLD-signal amplitude during task did not (Emerson and Cantlon, 2012). Riggins et al. found that FC involving a hippocampal network and episodic memory scores demonstrated significant interactions (Riggins et al., 2016). Their data showed an intriguing pattern whereby 4-year olds and 6-year olds often had opposite brain-behavior relationships (e.g., 4-year olds showed a negative correlation between FC of a given pair of ROIs and episodic memory scores whereas 6-year olds showed a positive correlation). The authors related these findings to developmental shifts in network segregation and integration, pointing to the possibility of fine-grained developmental trajectories of particular regions. Rohr et al. showed that in girls age 4–7, FC strength between intraparietal sulcus and frontal eye field regions (seeds of the dorsal attention network) correlated with selective attention scores (r = 0.4-0.53) (Rohr et al., 2017). In a follow-up study, using a dual regression approach to ICA-derived networks, they showed that when controlled for age, the dorsal attention network and default network, as well as primary visual and auditory networks, correlated with different attention-based outcomes (see Fig. 7) (Rohr et al., 2018).
Fig. 7.
Relationships between age and behavioral measures in primary sensory networks. Higher order networks also showed significant relationships, but these data emphasize that FC in sensory networks is developmentally dynamic during the ages of 4–7 years. Regions in red were identified via whole-brain correlation analyses, and are shown with network map underlays for context. This paper also emphasized the multi-network aspect of FC development that supports attentional maturation. From Rohr et al. (2018), by permission. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
The underlying theme is that FC relationships are, to date, extraordinarily promising in the way that they relate to cognitive and behavioral development, and that in particular, FC patterns during naturalistic conditions may reveal developmentally relevant information better than conventional tasks or task-free rest.
13. Future directions
13.1. FC network development measured longitudinally
Only one paper to date contains longitudinal FC data for preschool children (Long et al., 2017). As has been the case in developmental neuroimaging regarding both white and grey matter studies, longitudinal data are needed to definitively delineate patterns of change in the normal development of FC architecture. Given the degree of change in FC patterns identified in the work reviewed here, further investigation of early childhood FC development in particular is needed.
13.2. Intersubject correlations
ISCs could also be studied longitudinally to delineate trajectories of functional brain development of complex processing. It remains unclear how intersubject correlations during movie-watching change with development. Some data indicate that ISCs become stronger with age, perhaps following the same “diffuse to focal” pattern that has been observed longitudinally using conventional task-based fMRI (see Fig. 5) (Cantlon et al., 2011; Durston et al., 2006). Relatedly, individuals with Down syndrome have been shown to have lower ISCs than typically developing participants (Anderson et al., 2013). So far, regions in the temporal lobe have shown developmental correlations with ISCs. For example, children were found to have stronger ISCs in BA 22 (temporal pole) than adults (Cantlon and Li, 2013), and child-adult ISCs in TPJ can be predicted by age (Moraczewski et al., 2018). It may be that developmental patterns in temporal regions are especially well-captured by ISC analyses, and/or that the social and cognitive aptitudes improving during early childhood rely on functional maturation of these regions. ISCs could conceivably play a role in helping us understand what regions emerge together with regards to complex processing in normal development, thereby also providing ways to identify individuals who are not following that pattern. They might also provide a way to group subjects into more accurate cohorts of neural maturity as opposed to biological age, which might be especially helpful around maximally dynamic periods of development such as pre-adolescence.
13.3. Individually distinct patterns of FC
Recent work has investigated individually distinct patterns of FC by employing an unsupervised test-retest matching algorithm to identify individual subjects from within a group based solely on the correlation strength between FC matrices (Finn et al., 2015). A recent study used the same matching algorithm in 797 children and adults, ages 8–23 years (Kaufmann et al., 2017). The authors showed that matching accuracies increased with age, and that females have higher matching accuracies earlier than males. These data suggest that FC becomes more individually distinct with maturation. Further, participants with increased psychiatric symptoms in their sample became “individually distinct” later than their peers. In adults, movies have been shown to preserve and/or enhance the accuracies of these matching algorithms (Vanderwal et al., 2017) and researchers postulate that the more complex the task, the more distinct individual differences in FC become (Finn et al., 2017). Movie-watching has not yet been used to study individual differences in children, but would enable this approach to span preschool years, potentially yielding new information about the development of individually distinct FC patterns.
Additionally, recent approaches using FC-based predictive modeling to truly predict (as opposed to correlating) a behavioral score of interest in individual subjects seem to work best when more volumes of data are used, and when more varied acquisition states are used (Rosenberg et al., 2016; Shen et al., 2017). These connectome-based predictive modeling approaches have been shown to work using resting-state data in children with autism spectrum disorder, and also in children with attention-deficit hyperactivity disorder (Lake et al., 2018), but have not yet leveraged the duration and variability of movie-watching data. Such studies in pediatric populations, particularly across a broad developmental range, could make a significant contribution to the field.
13.4. Hyperalignment
A significant challenge when working with developmental fMRI is to align accurately across participants. Typically, this is accomplished using structural features to align each subject to a pediatric atlas. Multiple groups have worked on ways to align data using functional signal instead. This “hyperalignment” can be accomplished using evoked BOLD-signal time courses during movie-watching, using FC relationships, or a combination of the two (Conroy et al., 2013; Feilong et al., 2018; Guntupalli et al., 2018; Sabuncu et al., 2010). Hyperalignment has been shown to boost classification accuracies of both individual subjects and of condition, both of which could help to optimize pediatric studies. The degree of the transformation needed to align each participant in representational space could itself be a developmental outcome of interest, though this is a purely conceptual hypothesis currently. Much work would be needed to decrease the duration of scan time needed to accomplish hyperalignment to make it feasible for children, but it might be possible to use the same runs of movie-watching for both alignment and analyses.
13.5. Paradigms to evoke specific symptom domains
To date, researchers have selected movies based on availability, personal preference, the intention to elicit strong emotional responses, or to be more neutral. As an example of the latter intent, Inscapes was created to evoke a specific type of processing in children. Vanderwal and colleagues have also created another publicly available animation inspired by the work of Heider and Simmel in the 1940s (Heider and Simmel, 1944). This film, called When Heider Met Simmel, features simple black and white geometric shapes. The shapes tell a fairly complex, extended social story about a child who has bad dreams, and the movie is 7 min long. The movie is being used by different groups investigating social processing and narrative interpretation (Nguyen et al., 2018 #163). From a psychiatric perspective, movies could be created to evoke symptom domains that might be more powerful and elicit important signal changes relative to ordinary tasks. The expense, time and expertise required to produce a quality animation or live-action film is generally much greater than for a conventional task, making open sharing of stimuli even more important when it comes to movies. Researchers moving forward will still need to identify what types of FC patterns are evoked by particular movies, but should aim to use analytic approaches that enable inferences beyond descriptive localization of psychological and cognitive processes (Poldrack and Farah, 2015). Eventually, it may be most productive for the field to generate a pediatric-specific database of films with well-studied behavioral and neural effects rather than testing an endless stream of different movies, similar to what has been partially accomplished with emotional films for adults (Schaefer et al., 2010; Uhrig et al., 2016).
14. Cautionary notes
14.1. Movie = social
Most movies are potent social paradigms, and our field has years of research showing that brain development during childhood and adolescence is strongly driven by social cognitive development (Blakemore, 2008; Johnson et al., 2005; Saxe et al., 2009). Particularly when discussing FC-based findings from movies with other developmental findings from resting state studies, we need to be careful not to equate distinct acquisition states. For example, Rohr et al. found increasing FC in the default network with age in a movie-watching study (Rohr et al., 2018), whereas Muetzel et al. show decreasing FC in the default network in children ages 6–10 years during task-free rest (Muetzel et al., 2016). These findings also cover somewhat different age ranges (4–7 years vs. 6–10 years), and it may be that the developmental trajectories of default network FC during early childhood are complex and will require longitudinal data to be properly understood. It is also possible that default network FC in this age range exhibits both patterns: increases with age during movie-watching and decreases with age during rest. Either way, the primacy of the social brain during childhood and the inherent social nature of most movies serve as the backdrop for most movie-watching studies in pediatric populations.
14.2. Head motion
Movement remains a paramount issue for any developmental neuroimaging study. Excellent methodological suggestions for pediatric FC studies have been provided by Grayson and Fair (2017).
14.3. Age distribution
The rate of change in FC patterns appears to be faster than previously observed trajectories (e.g., grey matter thinning, white matter tractification, BOLD-signal responses to specific discrete tasks), and where possible, it seems even more important than it has been in the past to structure developmental cohorts appropriately and with tighter age ranges, e.g., not collapsing across children and adolescents. Fully reporting the age distribution of even small age ranges in children is necessary to fully assess the data.
14.4. Describing movies
Careful descriptions of movies are as methodologically necessary as careful descriptions of tasks. Thorough reporting of movie clips can be complicated, but is important to enable replication efforts. See Supplementary Table 1 for recommended parameters to report.
14.5. Cross-condition comparisons
As discussed throughout, movie-watching data is different than resting state data, and making comparisons of findings across acquisition states should be done carefully and explicitly. At this point in time, it remains a limitation that researchers using movies can not use the large publicly available resting state datasets to replicate findings or to enlarge sample sizes, etc.
15. Main conclusions
-
1
In children younger than 10 years of age, movies provide a significant improvement in head motion during fMRI scanning relative to task-free rest, and in some cases, also relative to conventional tasks. The use of movies has recently enabled the collection of FC data in awake children under the age of 7 with substantial sample sizes and scan durations.
-
2
Movie-watching data has been shown to capture canonical intrinsic functional connectivity relationships both via ICA and using network masks that are reliable and have been shown to relate to age and behavior.
-
3
Movie-watching data has also been shown to reveal novel network topologies evoked by the complex, dynamic processing required during naturalistic viewing. Little is known about the development of these complex movie-driven modules or their interactions.
-
4
FC measures vary with age even in samples with small age ranges, indicating a steep slope of change. FC measures also demonstrate clear brain-behavior relationships with outcomes that change dynamically during early childhood. These changes and correlations relate to both higher order networks and primary sensory networks.
-
5
Intersubject correlations in particular have been used to study the development of complex processing, revealing robust brain-behavior relationships with theory of mind outcomes, math aptitude, and attention-based measures. Temporal regions frequently emerge in these whole-brain ISC maps as being related to age or behavioral measures in children.
-
6
Anti-correlation between networks appears to be a developmentally important network property that emerges for some networks after age 3 years.
-
7
Despite the use of movies, head motion in young children remains a paramount concern in developmental fMRI, and ongoing efforts to prevent and remove motion artifact in addition to using movies are needed.
Conflict of Interest
None.
Acknowledgements
The authors thank Cameron Craddock (University of Texas at Austin) for helpful comments and input during manuscript preparation. Dr. Vanderwal acknowledges the Klingenstein Third Generation Foundation for funding support. Dr. Castellanos acknowledges funding received from the National Institute of Mental Health (R61MH113663). Inscapes is copyright of Yale University, 2013, and can be downloaded at http://www.headspacestudios.org.
Footnotes
The specifier “awake” is used to differentiate from excellent and challenging work done studying FC during sleep, for example in infants under age 2 (for review, Gao et al., 2017) or with preschool aged children with autism spectrum disorder (e.g., Shen et al., 2016).
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.10.004.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alexander, L.M., et al., The healthy brain network biobank: an open resource for transdiagnostic research in pediatric mental health and learning disorders. bioRxiv, 2017. [DOI] [PMC free article] [PubMed]
- Alexander-Bloch A. The convergence of maturational change and structural covariance in human cortical networks. J. Neurosci. 2013;33(7):2889–2899. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.S. Abnormal brain synchrony in Down syndrome. Neuroimage Clin. 2013;2:703–715. doi: 10.1016/j.nicl.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A., Zeki S., Logothetis N.K. Natural vision reveals regional specialization to local motion and to contrast-invariant, global flow in the human brain. Cereb. Cortex. 2008;18(3):705–717. doi: 10.1093/cercor/bhm107. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. The chronoarchitecture of the human brain—natural viewing conditions reveal a time-based anatomy of the brain. Neuroimage. 2004;22(1):419–433. doi: 10.1016/j.neuroimage.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bartels A., Zeki S. Functional brain mapping during free viewing of natural scenes. Hum. Brain Mapp. 2004;21(2):75–85. doi: 10.1002/hbm.10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A., Zeki S. Brain dynamics during natural viewing conditions—a new guide for mapping connectivity in vivo. Neuroimage. 2005;24(2):339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Betti V. Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron. 2013;79(4):782–797. doi: 10.1016/j.neuron.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.J. Development of the social brain during adolescence. Q. J. Exp. Psychol. (Hove) 2008;61(1):40–49. doi: 10.1080/17470210701508715. [DOI] [PubMed] [Google Scholar]
- Blankenship S.L. Development of hippocampal functional connectivity during childhood. Hum. Brain Mapp. 2017;38(1):182–201. doi: 10.1002/hbm.23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T.A.W. Brain dynamics in ASD during movie-watching show idiosyncratic functional integration and segregation. Hum. Brain Mapp. 2018;39(6):2391–2404. doi: 10.1002/hbm.24009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenhorn, K.L., et al., Cooperating yet distinct brain networks engaged during naturalistic paradigms: a meta-analysis of functional MRI results. bioRxiv, 2018. [DOI] [PMC free article] [PubMed]
- Botzung A. Component neural systems for the creation of emotional memories during free viewing of a complex, real-world event. Front. Hum. Neurosci. 2010;4:34. doi: 10.3389/fnhum.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Yeo B.T. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Campbell K.L. Idiosyncratic responding during movie-watching predicted by age differences in attentional control. Neurobiol. Aging. 2015;36(11):3045–3055. doi: 10.1016/j.neurobiolaging.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon J.F., Li R. Neural activity during natural viewing of sesame street statistically predicts test scores in early childhood. PLoS Biol. 2013;11(1):e1001462. doi: 10.1371/journal.pbio.1001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlon J.F. Cortical representations of symbols, objects, and faces are pruned back during early childhood. Cereb. Cortex. 2011;21(1):191–199. doi: 10.1093/cercor/bhq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: comment on power. Neuroimage. 2013;76:436–438. doi: 10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Conroy B.R. Inter-subject alignment of human cortical anatomy using functional connectivity. Neuroimage. 2013;81:400–411. doi: 10.1016/j.neuroimage.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bie H.M. Resting-state networks in awake five- to eight-year old children. Hum. Brain Mapp. 2012;33(5):1189–1201. doi: 10.1002/hbm.21280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B. Organization of high-level visual cortex in human infants. Nat. Commun. 2017;8:13995. doi: 10.1038/ncomms13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmochowski J.P. Correlated components of ongoing EEG point to emotionally laden attention—a possible marker of engagement? Front. Hum. Neurosci. 2012;6:112. doi: 10.3389/fnhum.2012.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F. Real-time motion analytics during brain MRI improve data quality and reduce costs. Neuroimage. 2017;161:80–93. doi: 10.1016/j.neuroimage.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Emerson R.W., Cantlon J.F. Early math achievement and functional connectivity in the fronto-parietal network. Dev. Cogn. Neurosci. 2012;2(Suppl 1):S139–S151. doi: 10.1016/j.dcn.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson R.W. Network-level connectivity dynamics of movie watching in 6-year-old children. Front. Hum. Neurosci. 2015;9:631. doi: 10.3389/fnhum.2015.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt L.E. Children’s head motion during fMRI tasks is heritable and stable over time. Dev. Cogn. Neurosci. 2017;25:58–68. doi: 10.1016/j.dcn.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A. The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. U. S. A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S. Normative development of ventral striatal resting state connectivity in humans. Neuroimage. 2015;118:422–437. doi: 10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feilong, M., et al., Reliable individual differences in fine-grained cortical functional architecture. bioRxiv, 2018. [DOI] [PMC free article] [PubMed]
- Felsen G., Dan Y. A natural approach to studying vision. Nat. Neurosci. 2005;8(12):1643–1646. doi: 10.1038/nn1608. [DOI] [PubMed] [Google Scholar]
- Finn E.S. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18(11):1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S. Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage. 2017;160:140–151. doi: 10.1016/j.neuroimage.2017.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S. Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative. Nat. Commun. 2018:9. doi: 10.1038/s41467-018-04387-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gao W., Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum. Brain Mapp. 2012;33(1):192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Functional connectivity of the infant human brain: plastic and modifiable. Neuroscientist. 2017;23(2):169–184. doi: 10.1177/1073858416635986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy T.H. On the estimation of brain signal entropy from sparse neuroimaging data. Sci. Rep. 2016;6:23073. doi: 10.1038/srep23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. Neuroimage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D.J. Behavioral interventions for reducing head motion during MRI scans in children. Neuroimage. 2018;171:234–245. doi: 10.1016/j.neuroimage.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntupalli J.S., Feilong M., Haxby J.V. A computational model of shared fine-scale structure in the human connectome. PLoS Comput. Biol. 2018;14(4):e1006120. doi: 10.1371/journal.pcbi.1006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. Distinct neural substrates for the perception of real and virtual visual worlds. Neuroimage. 2005;24(3):928–935. doi: 10.1016/j.neuroimage.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Han S.H., Jiang Y., Humphreys G.W. Watching cartoons activates the medial prefrontal cortex in children. Chin. Sci. Bull. 2007;52(24):3371–3375. [Google Scholar]
- Hasson U. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hasson U. Shared and idiosyncratic cortical activation patterns in autism revealed under continuous real-life viewing conditions. Autism Res. 2009;2(4):220–231. doi: 10.1002/aur.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Malach R., Heeger D.J. Reliability of cortical activity during natural stimulation. Trends Cogn. Sci. 2010;14(1):40–48. doi: 10.1016/j.tics.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider F., Simmel M. An experimental study of apparent behavior. Am. J. Psychol. 1944;57:243–259. [Google Scholar]
- Herting M.M. Test-retest reliability of longitudinal task-based fMRI: implications for developmental studies. Dev. Cogn. Neurosci. 2017;33:17–26. doi: 10.1016/j.dcn.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. Frontal temporal and parietal systems synchronize within and across brains during live eye-to-eye contact. Neuroimage. 2017;157:314–330. doi: 10.1016/j.neuroimage.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaskelainen I.P. Inter-subject synchronization of prefrontal cortex hemodynamic activity during natural viewing. Open Neuroimaging J. 2008;2:14–19. doi: 10.2174/1874440000802010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H. The emergence of the social brain network: evidence from typical and atypical development. Dev. Psychopathol. 2005;17(3):599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat. Neurosci. 2017;20(4):513–515. doi: 10.1038/nn.4511. [DOI] [PubMed] [Google Scholar]
- Kelly A.M. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim D. A new modular brain organization of the BOLD signal during natural vision. Cereb. Cortex. 2017:1–17. doi: 10.1093/cercor/bhx175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- LaConte S.M., P.S, Heberlein K.A., Hu X.P. Predictive eye estimation regression (PEER) for simultaneous eye tracking and fMRI. Proceedings 14th Scientific Meeting, International Society for Magnetic Resonance in Medicine. 2006 [Google Scholar]
- Lahnakoski J.M. Stimulus-related independent component and voxel-wise analysis of human brain activity during free viewing of a feature film. PLoS One. 2012;7(4):e35215. doi: 10.1371/journal.pone.0035215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake, E.M.R., et al., The functional brain organization of an individual predicts measures of social abilities in autism spectrum disorder. bioRxiv, 2018.
- Langeslag S.J. Functional connectivity between parietal and frontal brain regions and intelligence in young children: the Generation R study. Hum. Brain Mapp. 2013;34(12):3299–3307. doi: 10.1002/hbm.22143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann T.O. On the stability of BOLD fMRI correlations. Cereb. Cortex. 2017;27(10):4719–4732. doi: 10.1093/cercor/bhw265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X. Age-related functional brain changes in young children. Neuroimage. 2017;155:322–330. doi: 10.1016/j.neuroimage.2017.04.059. [DOI] [PubMed] [Google Scholar]
- Mante V. Independence of luminance and contrast in natural scenes and in the early visual system. Nat. Neurosci. 2005;8(12):1690–1697. doi: 10.1038/nn1556. [DOI] [PubMed] [Google Scholar]
- Mantini D. Interspecies activity correlations reveal functional correspondence between monkey and human brain areas. Nat. Methods. 2012;9(3):277–U85. doi: 10.1038/nmeth.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A.R., Kovacevic N., Itier R.J. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 2008;4(7):e1000106. doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara Q., Vega A.D.L., Yarkoni T. Developing a comprehensive framework for multimodal feature extraction. Proceedings of the 23rd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2017:1567–1574. [Google Scholar]
- Moraczewski D., Chen G., Redcay E. Inter-subject synchrony as an index of functional specialization in early childhood. Sci. Rep. 2018;8(1):2252. doi: 10.1038/s41598-018-20600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel R.L. Resting-state networks in 6-to-10 year old children. Hum. Brain Mapp. 2016;37(12):4286–4300. doi: 10.1002/hbm.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M., Vanderwal T., Hasson U. Shared understanding of narratives is correlated with shared neural responses. NeuroImage. 2018;184:161–170. doi: 10.1016/j.neuroimage.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S. Reconstructing visual experiences from brain activity evoked by natural movies. Curr. Biol. 2011;21(19):1641–1646. doi: 10.1016/j.cub.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S. Influences on the test-retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb. Cortex. 2017;27(11):5415–5429. doi: 10.1093/cercor/bhx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L., Lahnakoski J.M., Glerean E. Sharing the social world via intersubject neural synchronisation. Curr. Opin. Psychol. 2018;24:7–14. doi: 10.1016/j.copsyc.2018.02.021. [DOI] [PubMed] [Google Scholar]
- O’Connor D. The healthy brain network serial scanning initiative: a resource for evaluating inter-individual differences and their reliabilities across scan conditions and sessions. Gigascience. 2017;6(2):1–14. doi: 10.1093/gigascience/giw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannunzi M. Resting-state fMRI correlations: from link-wise unreliability to whole brain stability. Neuroimage. 2017;157:250–262. doi: 10.1016/j.neuroimage.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Viola R.J., McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychol. Sci. 2004;15(9):598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Petroni A. The variability of neural responses to naturalistic videos change with age and sex. eNeuro. 2018;5(1) doi: 10.1523/ENEURO.0244-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Farah M.J. Progress and challenges in probing the human brain. Nature. 2015;526(7573):371–379. doi: 10.1038/nature15692. [DOI] [PubMed] [Google Scholar]
- Power J.D. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105:536–551. doi: 10.1016/j.neuroimage.2014.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal A. Success rates for functional MR imaging in children. AJNR Am. J. Neuroradiol. 2014;35(12):2319–2325. doi: 10.3174/ajnr.A4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N.M. Making MR imaging child’s play – pediatric neuroimaging protocol, guidelines and procedure. J. Vis. Exp. 2009;29 doi: 10.3791/1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read P., Meyer M.-P., Gamma Group . Butterworth-Heinemann; Oxford; Boston: 2000. Restoration of Motion Picture Film. Butterworth-Heinemann Series in Conservation and Museology. viii, 359 p. [Google Scholar]
- Redcay E. Live face-to-face interaction during fMRI: a new tool for social cognitive neuroscience. Neuroimage. 2010;50(4):1639–1647. doi: 10.1016/j.neuroimage.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev M. Selective and invariant neural responses to spoken and written narratives. J. Neurosci. 2013;33(40):15978–15988. doi: 10.1523/JNEUROSCI.1580-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y. Inter-subject functional correlation reveal a hierarchical organization of extrinsic and intrinsic systems in the brain. Sci. Rep. 2017;7(1):10876. doi: 10.1038/s41598-017-11324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H. Development of the social brain from age three to twelve years. Nat. Commun. 2018;9(1):1027. doi: 10.1038/s41467-018-03399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T. Hippocampal functional connectivity and episodic memory in early childhood. Dev. Cogn. Neurosci. 2016;19:58–69. doi: 10.1016/j.dcn.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr C.S. Functional connectivity of the dorsal attention network predicts selective attention in 4-7 year-old girls. Cereb. Cortex. 2017;27(9):4350–4360. doi: 10.1093/cercor/bhw236. [DOI] [PubMed] [Google Scholar]
- Rohr C.S. Functional network integration and attention skills in young children. Dev. Cogn. Neurosci. 2018;30:200–211. doi: 10.1016/j.dcn.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 2016;19(1):165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust N.C., Movshon J.A. In praise of artifice. Nat. Neurosci. 2005;8(12):1647–1650. doi: 10.1038/nn1606. [DOI] [PubMed] [Google Scholar]
- Sabuncu M.R. Function-based intersubject alignment of human cortical anatomy. Cereb. Cortex. 2010;20(1):130–140. doi: 10.1093/cercor/bhp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R.R. Brain regions for perceiving and reasoning about other people in school-aged children. Child Dev. 2009;80(4):1197–1209. doi: 10.1111/j.1467-8624.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Schaefer A. Assessing the effectiveness of a large database of emotion-eliciting films: a new tool for emotion researchers. Cogn. Emotion. 2010;24(7):1153–1172. [Google Scholar]
- Schultz J., Pilz K.S. Natural facial motion enhances cortical responses to faces. Exp. Brain Res. 2009;194(3):465–475. doi: 10.1007/s00221-009-1721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.D. Functional connectivity of the amygdala is disrupted in preschool-aged children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(9):817–824. doi: 10.1016/j.jaac.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 2017;12(3):506–518. doi: 10.1038/nprot.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L.E. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Dev. Cogn. Neurosci. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifre R. A longitudinal investigation of preferential attention to biological motion in 2- to 24-month-old infants. Sci. Rep. 2018;8(1):2527. doi: 10.1038/s41598-018-20808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simony E. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat. Commun. 2016;7:12141. doi: 10.1038/ncomms12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. Watching you watch movies: using eye tracking to inform cognitive film theory. In: Shimamura A., editor. Psychocinematics: Exploring Cognition at the Movies. Oxford University Press; New York: 2013. [Google Scholar]
- Smith R.X., Yan L., Wang D.J. Multiple time scale complexity analysis of resting state FMRI. Brain Imaging Behav. 2014;8(2):284–291. doi: 10.1007/s11682-013-9276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers H.J., Maguire E.A. Decoding human brain activity during real-world experiences. Trends Cogn. Sci. 2007;11(8):356–365. doi: 10.1016/j.tics.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Spreng R.N. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol. Aging. 2016;45:149–160. doi: 10.1016/j.neurobiolaging.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.R. The Cambridge Centre for ageing and neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 2017;144(Pt B):262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H. Methodological considerations for developmental longitudinal fMRI research. Dev. Cogn. Neurosci. 2018;33:149–160. doi: 10.1016/j.dcn.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesen S. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn. Reson. Med. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Thomason M.E. Resting-state fMRI can reliably map neural networks in children. Neuroimage. 2011;55(1):165–175. doi: 10.1016/j.neuroimage.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tie Y. A new paradigm for individual subject language mapping: movie-watching fMRI. J. Neuroimaging. 2015;25(5):710–720. doi: 10.1111/jon.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A. Classifying the wandering mind: revealing the affective content of thoughts during task-free rest periods. Neuroimage. 2014;97:107–116. doi: 10.1016/j.neuroimage.2014.03.076. [DOI] [PubMed] [Google Scholar]
- Uhrig M.K. Emotion elicitation: a comparison of pictures and films. Front. Psychol. 2016:7. doi: 10.3389/fpsyg.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakorin V.A., Lippe S., McIntosh A.R. Variability of brain signals processed locally transforms into higher connectivity with brain development. J. Neurosci. 2011;31(17):6405–6413. doi: 10.1523/JNEUROSCI.3153-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. 2015;122:222–232. doi: 10.1016/j.neuroimage.2015.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T. Individual differences in functional connectivity during naturalistic viewing conditions. Neuroimage. 2017;157:521–530. doi: 10.1016/j.neuroimage.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Wang J. Improving the test-retest reliability of resting state fMRI by removing the impact of sleep. Front. Neurosci. 2017;11:249. doi: 10.3389/fnins.2017.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Test-retest reliability of functional connectivity networks during naturalistic fMRI paradigms. Hum. Brain Mapp. 2017;38(4):2226–2241. doi: 10.1002/hbm.23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee C.Y. Behavioral heterogeneity in relation with brain functional networks in young children. Cereb. Cortex. 2018;28(9):3322–3331. doi: 10.1093/cercor/bhx205. [DOI] [PubMed] [Google Scholar]
- Westfall J., Nichols T.E., Yarkoni T. Fixing the stimulus-as-fixed-effect fallacy in task fMRI. Wellcome Open Res. 2016;1:23. doi: 10.12688/wellcomeopenres.10298.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingstall K. Integration of EEG source imaging and fMRI during continuous viewing of natural movies. Magn. Reson. Imaging. 2010;28(8):1135–1142. doi: 10.1016/j.mri.2010.03.042. [DOI] [PubMed] [Google Scholar]
- Wild C.J. Adult-like processing of naturalistic sounds in auditory cortex by 3- and 9-month old infants. Neuroimage. 2017;157:623–634. doi: 10.1016/j.neuroimage.2017.06.038. [DOI] [PubMed] [Google Scholar]
- Xiao Y. Longitudinal changes in resting-state fMRI from age 5 to age 6years covary with language development. Neuroimage. 2016;128:116–124. doi: 10.1016/j.neuroimage.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T. Delineating the Macroscale Areal Organization of the Macaque Cortex In Vivo. Cell Reports. 2018;23(2):429–441. doi: 10.1016/j.celrep.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Connectivity trajectory across lifespan differentiates the precuneus from the default network. Neuroimage. 2014;89:45–56. doi: 10.1016/j.neuroimage.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q. Children’s mental time travel during mind wandering. Front. Psychol. 2014;5:927. doi: 10.3389/fpsyg.2014.00927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013;65:374–386. doi: 10.1016/j.neuroimage.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.