Highlights
-
•
Using fNIRS, we examined how children recruit PFC while engaging cognitive control.
-
•
Activation increased with cognitive control demands more in left than right PFC.
-
•
It was higher in left PFC in competitive than cooperative contexts, and in right PFC in cooperative and neutral compared to competitive contexts.
-
•
Older children showed greater variations in PFC activation than younger children.
-
•
Cognitive control is supported by more differentiated PFC recruitment with age.
Keywords: Prefrontal cortex, Cognitive control, Cooperation, Competition, Children, Functional near-infrared spectroscopy (fNIRS)
Abstract
Emerging cognitive control during childhood is largely supported by the development of distributed neural networks in which the prefrontal cortex (PFC) is central. The present study used fNIRS to examine how PFC is recruited to support cognitive control in 5–6 and 8-9-year-old children, by (a) progressively increasing cognitive control demands within the same task, and (b) manipulating the social context in which the task was performed (neutral, cooperative, or competitive), a factor that has been shown to influence cognitive control. Activation increased more in left than right PFC with cognitive control demands, a pattern which was more pronounced in older than younger children. In addition, activation was higher in left PFC in competitive than cooperative contexts, and higher in right PFC in cooperative and neutral than competitive contexts. These findings suggest that increasingly efficient cognitive control during childhood is supported by more differentiated recruitment of PFC as a function of cognitive control demands with age.
1. Introduction
Cognitive control development, which enables goal-directed regulation of thoughts and actions, supports growing autonomy and adaptive behaviors during childhood. As cognitive control is achieved through prefrontally-mediated goals biasing information processing in posterior regions, its protracted development is intertwined with the development of the prefrontal cortex (PFC) (Crone and Steinbeis, 2017; Luna et al., 2010; Moriguchi and Hiraki, 2013). Yet, little is known about how PFC recruitment changes from early to middle childhood, a developmental window characterized by profound transformations in cognitive control engagement. Furthermore, although cognitive control is often implemented in social contexts in which children interact with other individuals and/or actions are socially relevant, the influence of such social contexts on PFC recruitment has been largely overlooked in children. Thus, the present study addresses potential differences from early to middle childhood in PFC recruitment as a function of variations in both cognitive control demands and social contexts.
Despite the widespread idea that cognitive control development reflects exclusively engagement of more control with age, it may also (and perhaps foremost) reflect better and more flexible engagement of control to meet specific task demands (Chevalier, 2015). For instance, studies using confirmatory factor analysis, which examines whether variance across tasks tapping control is explained by one or multiple latent factors (i.e., control components), showed cognitive control is unitary at age 3 (e.g., Wiebe et al., 2011; Willoughby et al., 2010) and progressively differentiates into two and three partially separable components through middle childhood and adolescence, respectively (e.g., Huizinga et al., 2006; Lee et al., 2013). In other words, with age children engage control in an increasingly differentiated manner as a function of specific demands to inhibit responses, update information in working memory, or switch between tasks. This progressive differentiation is accompanied by a diversification of control strategies with age, including proactive strategies, verbalization, and active information maintenance (Camos and Barrouillet, 2011; Chatham et al., 2009; Chevalier et al., 2015; Fatzer and Roebers, 2012). Thus, school-age children engage control in a less rigid fashion than preschoolers, more flexibly tailoring control to changing task demands, which may include both better control mobilization when demands increase and greater control release when demands decrease (Chevalier et al., 2013).
Increasingly efficient cognitive control with age may be supported by more differentiated recruitment of PFC, as reflected by two major trends. First, PFC activation may increase in prefrontal regions that show progressive specialization (i.e., which are increasingly critical in supporting control) with age (e.g., Buss et al., 2014; Moriguchi and Hiraki, 2009; Perlman et al., 2014) and decrease in other, less critical prefrontal regions (Adleman et al., 2002; Bunge et al., 2002; Casey et al., 1997; Morton et al., 2009), leading to a progressive shift from diffuse to focal pattern of prefrontal activation (Durston et al., 2006; Marsh et al., 2006; Tamm et al., 2002; Tsujii et al., 2009). Second, differentiation may also result in greater modulation of activation in critical prefrontal regions. That is, not only may older children recruit PFC more than younger children when control demands increase, but they may also release control more when demands are low (see Durston et al., 2002).
Furthermore, PFC recruitment during childhood may vary as a function of the immediate social context, as cooperation and competition can enhance children’s behavioural performance on tasks tapping cognitive control (Butler and Walton, 2013; Fischer et al., 2018; Qu, 2011). Competition may increase enjoyment, motivation, and task engagement (Cagiltay et al., 2015; Conti et al., 2001; Plass et al., 2013), which in turn may result in better cognitive control engagement. In cooperative contexts, socially shared goals may be especially salient and thus more easily maintained in working memory (Decety et al., 2004; Qu, 2011). If these social contexts influence cognitive control through different processes, they may be associated with different patterns of prefrontal activation, yet this has never been studied in children. In adults, although both cooperation and competition lead to greater activation in superior frontal gyrus relative to neutral contexts, they are associated with distinct patterns of activation in medial prefrontal cortex in a task that required the participant and other player to take turns to reproduce complex visuospatial patterns (Decety et al., 2004). Furthermore, cooperation in adults is associated with greater activation in right inferior gyrus (Liu et al., 2015), greater signal coherence across partners in right superior frontal gyrus (Cui et al., 2012), but lower activation in left superior frontal gyrus (Decety et al., 2004) than competition. Therefore, these diverging patterns of prefrontal activation suggest cooperation and competition may enhance cognitive control through distinct processes in adults.
The present study used fNIRS, an especially appropriate neuroimaging technique for younger children (Lloyd-Fox et al., 2010; Moriguchi and Hiraki, 2013), to investigate how cognitive control may be supported by changes in PFC recruitment from early to middle childhood, by manipulating two factors that influence cognitive control engagement: control demands and the social context in which cognitive control is engaged. Specifically, 5–6- and 8–9-year-olds completed a task in which inhibition and switching demands were progressively introduced, in three social contexts: neutral, cooperative, and competitive. Both preschool and school-age children were targeted because of important gains in flexible cognitive control engagement between these two periods of development, making it especially likely to reveal different patterns of PFC recruitment with age. We expected performance to increase with age, decrease with increasing cognitive control demands, and benefit from cooperative and competitive contexts. Importantly, more differentiated PFC recruitment with age should result in greater variation of prefrontal activation as a function of control demands in older children than in younger children. Although more exploratory, we expected this pattern to be more pronounced in cooperative and competitive than neutral contexts, as these contexts enhance cognitive control in children. However, as they may affect cognitive control through distinct factors (goal salience and motivation, respectively) and are associated with distinct patterns of prefrontal activation in adults, we explored whether they would be associated with different patterns of prefrontal activation. In contrast, if PFC recruitment simply increases with age in an undifferentiated manner, older children should show greater prefrontal activation than younger children across the board and even more so as control demands increase.
2. Materials and methods
2.1. Participants
Participants included 30 five to six-year-old children (M = 5.9 years, SD = 0.6, age range = 5.1–6.9, 16 girls) and 30 eight to nine-year-old children (M = 8.8 years, SD = 0.6, age range = 8.1–10.0, 6 girls). They were mostly Caucasian (56 children) and from middle to high socioeconomic backgrounds (49 children had at least one parent with a university degree). The data of one additional younger child were dropped as she failed to complete the session. Families were recruited from the community through adverts on social media. Informed written consent was obtained from the accompanying caregivers. All children provided verbal and written assent to participate. Parents received £10 compensation for their time and travel costs. Each child received age-appropriate prizes and a ‘young scientist’ certificate. The study received approval from the local university’s research ethics committee.
2.2. Experimental design
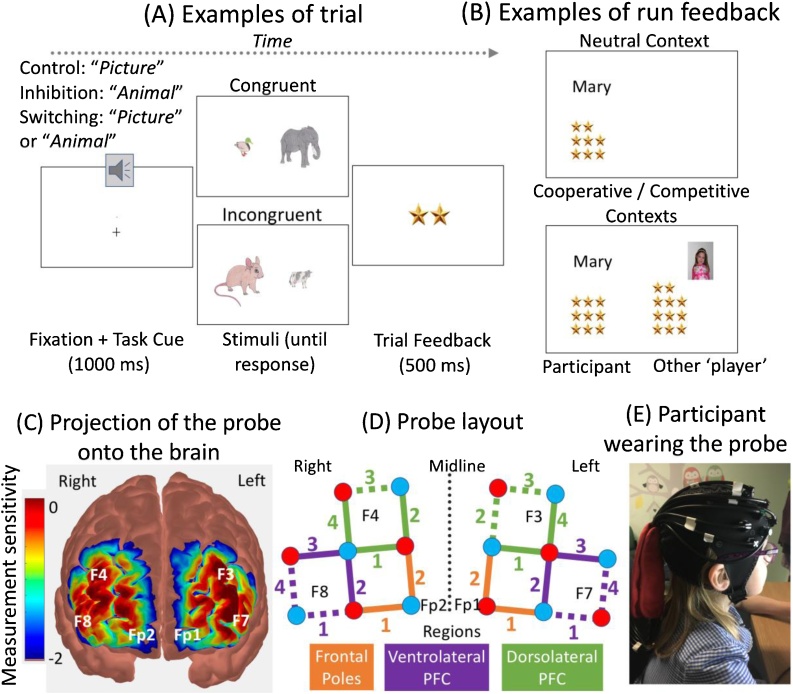
Children were tested individually in a child-friendly laboratory setting. Each child completed a modified version of the Real Animal Size Test (RAST; Catale and Meulemans, 2009). On each trial, children were presented with a pair of cartoon animals and instructed to identify the animal that was either visually bigger (Picture Game) or bigger in real life (Animal Game), by pressing on the gamepad button located on the same side as the bigger animal on the screen, as quickly and accurately as they could. The stimuli were drawn from four small animals in real life (ant, mouse, duck, cat) and four large animals in real life (cow, bear, elephant, whale). These animals were selected because (a) they are familiar to children and (b) they can be unambiguously classified as big or small, as confirmed by children successfully naming and categorizing them as big or small when presented on a sheet before the task started. Each time, one of the two animals presented side by side was visually bigger than the other (side counterbalanced across trials; Fig. 1). The animal visually bigger on the screen was also bigger in real life in congruent trials (e.g., a visually big elephant with a visually small duck), whereas it was smaller in real life in incongruent trials (e.g., a visually big mouse with a visually small cow). Congruent and incongruent trials were equally frequent in all cognitive control demand phases and social contexts. Each trial started with a 1000-ms fixation cross accompanied by an auditory task cue (i.e., either the word ‘picture’ or the word ‘animal’) that was then replaced with a pair of animals (stimuli) until a response was entered. The subsequent 500-ms trial feedback consisted of two stars if the response was correct and fast (i.e., faster than the prior response), one star if it was correct but slow (i.e., slower than the prior response), or a red cross if it was incorrect. After each run of 6 trials, children were shown how many stars they accumulated during the run (feedback).
Fig. 1.
Real Animal Size Test and fNIRS probe. (A) After the fixation cross and auditory task cue, children had to press on the side of the animal that was either bigger on the screen (Picture Game) or in real life (Animal Game). (B) After each trial run, participants were shown either just their own score beside their first name (Neutral condition) or their own score, and that of the other ‘player’ beside the other player’s picture (Cooperative and Competitive conditions). (C) Projection of the probe onto a standard brain atlas with 10–20 system landmarks. (D) Probe layout. Sources are indicated in red and detectors in blue. Digits in color indicate the channel number within each region. Brain regions are shown in orange, purple, and green. Channels marked with dotted lines showed no difference between HbO and HbR (see Table 1) and were not included in statistical analyses. (E) Example of a participant wearing the fNIRS probe (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
2.2.1. Cognitive control demands
The task started with a Control phase in which children were instructed to press the gamepad button on the same side as the animal that was visually bigger (Picture Game). The word ‘picture’ was orally presented with the fixation cross on all trials. As the animals’ visual size is especially salient, cognitive control demands were relatively low in this phase. Children then completed the Inhibition phase in which they had to press the button on the same side as the animal that was bigger in real life (Animal Game), as cued by the word ‘animal’ orally presented alongside the fixation cross on all trials. This phase required inhibiting visual size to attend to real-life size instead. Finally, in the Switching phase, children unpredictably switched back and forth between the Picture and Animal games, as a function of the auditory cue (either ‘picture’ or ‘animal’) presented with the fixation cross. As this phase required both inhibition and task-switching, it was especially taxing in terms of cognitive control. Each phase started with initial practice trials that were followed by 5 runs of 6 test trials each (2 congruent and 4 incongruent trials). Trial runs were separated by at least 10 s to allow the hemodynamic response function to return to baseline before the start of the following run. The stars were used to ensure children stayed motivated throughout the session and for the social context manipulation (see below). Children were told that the more stars they accumulated in total throughout the session, the nicer the prizes they would get at the very end. In reality, all children received the same prizes regardless of their performance.
2.2.2. Social context
Each participant completed all three phases of the RAST in three social contexts: cooperative, competitive, or neutral (order counterbalanced across participants). At the start of the session, the experimenters pretended that another child (Amy or Ben) would also play the same game but in a different room. To make it more convincing, they showed the participant a picture of the other ‘player’ (matched on the participant’s age and gender) and pretended to take a picture of the participant that would be similarly shown to the other ‘player’. In the Cooperative context, the participant was told that they played with the other ‘player’ as a team, and each player would receive half of the stars that the team accumulated by the end of the game (to be later traded for prizes). In the Competitive context, the participant was told they played against the other ‘player’ and the player who accumulated the most stars would receive their own stars at the end of the game, while the other one would not receive any stars for this round (condition). In the Neutral context, children played on their own and were told they would receive the stars they themselves accumulated, regardless of the other player’s performance.
Importantly, after each run of trials in the cooperative and competitive contexts, children were shown both their run score (i.e., the number of stars they accumulated during the run) presented besides their first name on the left-hand side of the screen, and the other player’s run score, which was shown besides the other player’s picture on the right-hand side of the screen. The participant’s run score was always accurate, whereas that of the other ‘player’ was calculated by adding or subtracting 0, 1, or 2 stars from the participant’s score (counterbalanced across runs). In contrast, children only saw their own run score in the neutral context to minimize the risk that they may believe they were (still) playing with or against the other ‘player.’ Finally, at the end of each round, children were asked to recall the context in which they just played by choosing among three pictures showing either a single cartoon character (neutral), two characters shaking hands (cooperative), or two characters on a start line (competitive). Testament to the success of the social context manipulation, the vast majority of children answered all questions correctly; only 3 younger children provided correct answers for just 2 of the 3 conditions.
In each social context, children completed five runs of six trials for each of the three cognitive control phases. As children were tested in all three social contexts, they completed 270 trials in total.
2.2.3. fNIRS recording
Concentrations of oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR) were measured while participants completed the RAST, using an NTS Diffuse Optical Topography System (University College London/Gowerlabs, London, UK), operating at wavelengths of 780 and 850 nm and with a 10-Hz sampling rate (Everdell et al., 2005). The NIRS probes contained 16 optodes, including 8 sources and 8 detectors, mounted on a customized nylon cap fitting the participant’s head circumference and arranged in a layout of 20 channels covering the left and right frontal poles (Fp1, Fp2), ventrolateral (F7, F8) and dorsolateral (F4, F3) prefrontal cortices, as assessed using AtlasViewer (Aasted et al., 2015) (Fig. 2). Channel distance was proportional to cap size (Wijeakumar et al., 2015) based on a 58-cap with 30 mm channels: 25 mm for the 48 cm cap (one younger child), 26 mm for the 50 cm cap (5 younger and 3 older children), 27 mm for the 52 cm cap (15 younger and 9 older children), 28 mm for the 54 cm cap (9 younger and 16 older children), and 29 mm for the 56 cm cap (2 older children). The optodes were placed on the cap using plastic adapters permanently affixed to the cap relative to the 10–20 landmarks (directly shown on the cap). After measuring the participant’s head circumference and fitting the corresponding cap size, correct cap placement was checked by ensuring that the Cz landmark on the cap sat exactly halfway between the nasion and inion and halfway between the two ears, as indicated by tape measurement. Pictures of the cap on the participants’ head were taken so that placement could be checked again a posteriori.
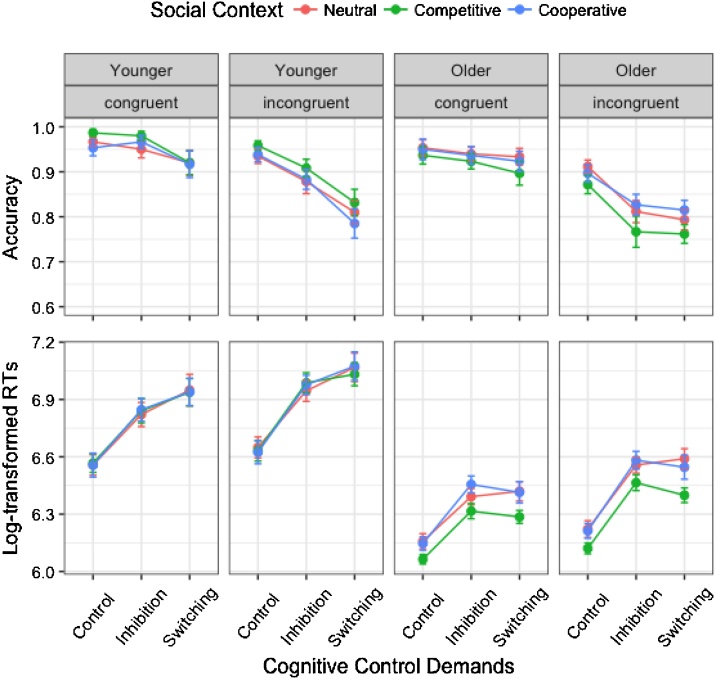
Fig. 2.
Accuracy (top panel) and log-transformed response times (RTs; bottom panel). Error bars indicate standard errors. In both age groups, performance decreased with increasing cognitive control demands, and was especially affected by competition.
2.3. Statistical analysis
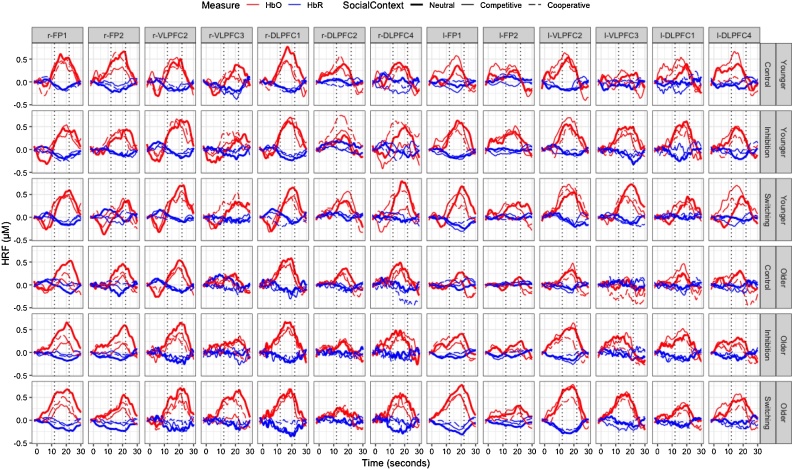
Response times were log-transformed (to correct for skewness and minimize baseline differences between age groups) and analyzed after removing trial outliers lower than 200 ms or greater than 3 standard deviations above the mean or 10,000 ms (1.9% of trials). fNIRS data for each run of test trials were processed using HomER2 (Huppert et al., 2009). The raw data were first converted from intensity to optical density measurements. Motion artefacts were detected by channel (using a standard deviation threshold of 40 absorbance units and amplitude threshold of 0.20 over .5 s intervals) and corrected using Wavelet filtering (iqr = 1.50) (Brigadoi et al., 2014; Cooper et al., 2012). Data were subsequently band-pass filtered (.01–.5 Hz). They were converted from optical density to concentration with differential pathlength factor values derived from Scholkmann and Wolf (2013) using mean age for each age group: 5.5 for 780 nm and 4.5 for 850 nm for younger children, and 5.6 and 4.6 (respectively) for older children. The data were then segmented from 2 s pre run onset to 30 s post run onset (with the initial 2 s serving as baseline), and averaged across the 5 runs for each phase and condition. The window when the hemodynamic response function (HRF) was maximal (12–22 sec after run onset) was determined by visual inspection. This 10-sec window was used to calculate oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR) changes, as this window adequately captured peak HRF in both age groups across all cognitive control demand phases, social contexts and channels (see Fig. 3), despite variability in mean run durations (Supplemental Materials)1 . Paired t-tests showed mean HbO was significantly greater than mean HbR in 13 channels after Bonferonni correction (see Table 1); the other channels were dropped from subsequent analyses as they did not show specific task-related activity (e.g., Buss and Spencer, 2017). The remaining 13 channels (Fig. 1, Fig. 3) were entered in the subsequent analysis, which focused on HbO, as it is considered a more sensitive parameter of blood flow (e.g., Cui et al., 2011) and has been used in most developmental studies (see Moriguchi and Hiraki, 2013). However, the analysis of HbR is provided in Supplemental Materials.
Fig. 3.
Group average of the hemodynamic response function (HRF) for each channel. The time window used for statistical analysis (12–22 s) is denoted by the dotted vertical lines. HbO = oxygenated hemoglobin. HbR = deoxygenated hemoglobin. FP = frontal pole. DLPFC = dorsolateral prefrontal cortex. VLPFC = ventrolateral prefrontal cortex.
Table 1.
HbO-HbR comparisons for all channels, and significant pairwise comparisons for Channel × Cognitive Control Demands and Channel × Social Context effects on HbO for each channel showing significantly greater HbO than HbR.
| Channel | HbO vs. HbR | HbO: Cognitive Control Demands | HbO: Social Context |
|---|---|---|---|
| r-FP1 | t(59) = 4.81, p<.001, d = .62* | -- | Cm (.28 μM) < N (.47 μM), p = .033 |
| r-FP2 | t(59) = 5.24, p<.001, d = .68* | -- | -- |
| r-VLPFC1 | t(59) = .74, p = .462, d = .10 | ||
| r-VLPFC2 | t(59) = 5.43, p<.001, d = .70* | -- | -- |
| r-VLPFC3 | t(57) = 3.49, p = .001, d = .46* | C (.12 μM) < S (.30 μM), p = .026 | -- |
| r-VLPFC4 | t(59) = .69, p = .491, d = .09 | ||
| r-DLPFC1 | t(57) = 6.63, p<.001, d = .87* | -- | -- |
| r-DLPFC2 | t(57) = 4.21, p<.001, d = .55* | -- | -- |
| r-DLPFC3 | t(57) = 1.52, p = .134, d = .20 | ||
| r-DLPFC4 | t(57) = 5.29, p<.001, d = .69* | C (.22 μM) < S (.42 μM), p = .025 | Cm (.18 μM) < N (.40 μM), p = .022 Cm (.18 μM) < Co (.39 μM), p = .028 |
| l-FP1 | t(59) = 5.67 p<.001, d = .73* | C (.18 μM) < I (.34 μM), p = .012 C (.18 μM) < S (.44 μM), p<.001 |
-- |
| l-FP2 | t(59) = 3.23, p = .0020, d = .42* | -- | -- |
| l-VLPFC1 | t(59) = 2.19, p = .033, d = .28 | ||
| l-VLPFC2 | t(59) = 7.04 p<.001, d = .91* | C (.32 μM) < S (.58 μM), p<.001 | Co (.33 μM) < Cm (.55 μM), p = .011 |
| l-VLPFC3 | t(59) = 3.88, p<.001, d = .50* | C (.02 μM) < I (.27 μM), p = .001 I (.27 μM) < S (.48 μM), p = .008 |
-- |
| l-VLPFC4 | t(58)=-2.17, p = .034, d = .28 | ||
| l-DLPFC1 | t(59) = 4.63, p<.001, d = .60* | C (.26 μM) < I (.38 μM), p = .046 S (.24 μM) < I (.38 μM), p = .027 |
-- |
| l-DLPFC2 | t(58) = 1.61, p = .112, d = .21 | ||
| l-DLPFC3 | t(58) = .59, p = .560, d = .08 | ||
| l-DLPFC4 | t(59) = 4.98, p<.001, d = .64* | C (.18 μM) < S (.44 μM), p = .010 I (.23 μM) < S (.44 μM), p = .036 |
Co (.08 μM) < Cm (.39 μM), p = .006 |
r = right; l = left; FP = frontal pole; VLPFC = ventrolateral prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; HbO = oxygenated hemoglobin; HbR = deoxygenated hemoglobin; C = control; I = inhibition; S = switching; Co = cooperative; Cm = competitive; N = neutral; * = significant after Bonferroni correction (p < .0025); -- no significant pairwise comparisons.
Linear mixed models were run on accuracy and log-transformed response times (RTs) to examine the fixed effects of age group (younger, older), control demands (control, inhibition, switching), social context (neutral, cooperative, competitive), congruency (congruent, incongruent), and gender (boys, girls). In addition to random intercepts for subjects, random slopes were included for congruency, control demands, and social contexts as they provided the best fit to the data. The model on HbO included channel (r-FP1, r-FP2, r-VLPFC2, r-VLPFC3, r-DLPFC1, r-DLPFC2, r-DLPFC4, l- FP1, l-FP2, l-VLPFC2, l-VLPFC3, l-DLPFC1, l-DL-PFC4), control demands (control, inhibition, switching), social context (neutral, cooperative, competitive), age group (younger, older), and gender (boys, girls). Mean run duration was also entered to control for its potential effects given the variability in mean run durations (Supplemental Materials). Congruency could not be included as it was manipulated within rather than between runs of trials. In addition to random intercepts for subjects, random slopes for control demands and social contexts were included as they provided the best fit. Given that gender distribution differed between age groups, χ2(1, N = 60) = 7.18, p = .007, gender was entered in all models to control for its effect. Models were run using lme4 package in R (R Development Core Team, 2012). Satterthwaite approximations for degrees of freedom are reported. Only significant effects are reported.
3. Results
3.1. Behavior
Response accuracy decreased as control demands increased, F(2, 57) = 32.08, p < .001, η2p = .53, and from congruent to incongruent trials, F(1, 58) = 96.03, p = < .001, η2p = .62 (Fig. 2). In addition, control demands interacted with congruency, F(2, 696) = 21.85, p < .001, η2p = .06, due to a significant drop in performance between the control and inhibition phases on incongruent trials, p < .001, but not on congruent trials, p = .485. Importantly, age group, which did not have a significant main effect, showed an interaction with control demands, F(2, 57) = 3.65, p = .032, η2p = .11. Younger children’s performance decreased significantly between inhibition and switching (.93 vs. .86, p < .001), whereas older children’s dropped mostly from control to inhibition (.92 vs. .87, p < .001). Social context, whose main effect was not significant, showed an interaction with age group, F(2, 57) = 5.17, p = .008, η2p = .15. Older children responded more accurately in the cooperative (.89) and neutral (.89) contexts than in the competitive context (.86), p = .014 and p = .012, respectively, whereas there were no differences in younger children.
Log-transformed response times were affected by social context, F(2, 58) = 4.13, p = .02, η2p = .124, control demands, F(2, 58) = 195.99, p < .001, η2p = .871, congruency, F(1, 58) = 208.96, p < .001, η2p = .78, and age group, F(1, 59.63) = 46.39, p < .001, η2p = .44 (Fig. 2). Moreover, age group interacted with control demands, F(2, 58) = 7.58, p = .001, η2p = .21, and social context, F(2, 58) = 3.89, p = .025, η2p = .12, and control demands also interacted with congruency, F(2, 696) = 9.71, p < .001, η2p = .03. Response times slowed down from the control to the inhibition phases in both younger (6.59 vs. 6.9 log ms, p < .001) and older (6.15 vs. 6.46 log ms, p < .001) children, with additional slowing from inhibition to switching in younger (6.99 log ms, p = .001), but not older children (6.44 log ms), p = .337. Older children responded faster in the competitive (6.27 log ms) than the cooperative (6.38 log ms, p = .002) and neutral (6.39 log ms, p = .002) contexts, whereas younger children’s RTs did not differ across social contexts.
3.2. fNIRS
At the brain level, the effect of control demands on HbO, F(2, 61.5) = 3.55, p = .034, η2p = .10, interacted with age group (whose main effect was not significant), F(2, 56.1) = 3.29, p = .044, η2p = .11 (Figs. 3 and 4). In older children, HbO significantly increased across all three phases (.15 μM, .26 μM, .37 μM), ps < .028, whereas in younger children, HbO was relatively high across all phases (.33 μM, .39 μM, .38 μM) but did not vary significantly across phases, all ps > .373.
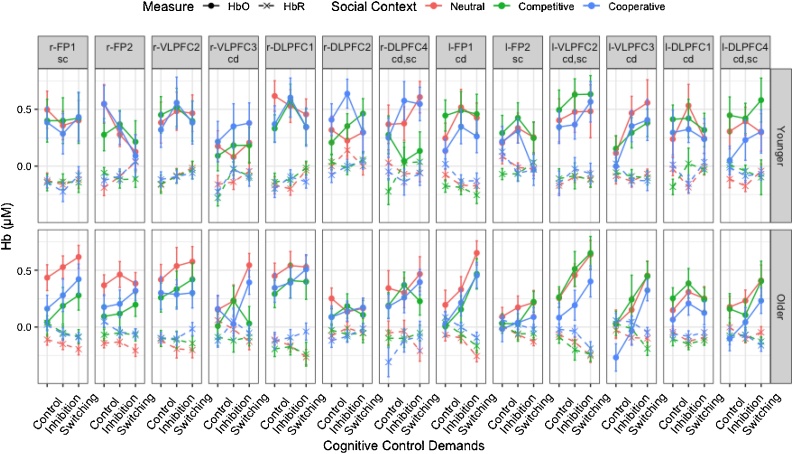
Fig. 4.
Mean changes in oxygenated (HbO) and deoxygenated (HbR) hemoglobin for each channel as a function of age group, control demands, and social context. Error bars indicate standard errors. ‘cd’= channels in which HbO significantly increased with cognitive control demands. ‘sc’ = channels in which HbO significantly varied across social contexts. HbO increased with cognitive control demands, mostly over the left PFC channels and in older children. HbO was lower in the competitive context in two right PFC channels and in the cooperative contexts in two left PFC channels.
Control demands also interacted with channel, F(24, 6383.1) = 2.67, p < .001, η2p = .01. HbO increased with control demands in all channels over the left PFC but one (l-FP2), ps < .047, whereas only two of the seven channels showed an HbO increase with control demands over the right PFC (r-VLPFC3 and r-DLPFC), ps < .027. Significant pairwise comparisons for each channel are provided in Table 1. Therefore, cognitive control demands mostly affected activation in the left PFC. One may wonder whether the less pronounced effect of cognitive control demands over the right PFC was due to overall little activation in these channels or consistently high activation across all phases. To answer this question, HbO was averaged across all seven right PFC channels and compared to the average of all six left PFC channels. HbO in the right PFC was higher than in left PFC in the control phase (.29 μM vs. .18 μM), p = .005, and did not differ from the left PFC in the inhibition (.35μM vs. .31μM) and switching phases (.35μM vs. .40μM), ps > .335. Thus, right prefrontal activation was already high in the (least demanding) control phase, while left prefrontal activation was low, and remained high in the other phases where it matched left prefrontal activation, which generally rose with control demands.
Furthermore, social context did not have significant main effect, but there was an interaction with channel, F(24, 6382.4) = 2.23, p < .001, η2p = .01. HbO was lower in the competitive than the neutral and/or cooperative contexts in two channels over the right PFC (r-FP1 and r-DLPFC4), ps < .034, whereas the opposite pattern was observed in two channels over the left PFC (l-VLPFC2 and l-DLPFC4), with lower HbO in the cooperative than the competitive contexts, ps < .012 (see Table 1 for significant pairwise comparisons).
Finally, we also checked whether HbO was significantly higher than HbR even in cognitive control demand phases and social contexts where it was lowest. In each age group, HbO was higher than HbR in all three phases, all ps < .020. In all channels showing an effect of control demands, HbO was significantly higher than HbR in all phases (ps < .027), except in the control phase for l-VLPFC3 (p = .708). Similarly, HbO was significantly higher than HbR in all social contexts (ps < .009), except for l-DLPFC4 in the cooperative context (p = .107).
3.3. Correlations between HbO and behavior
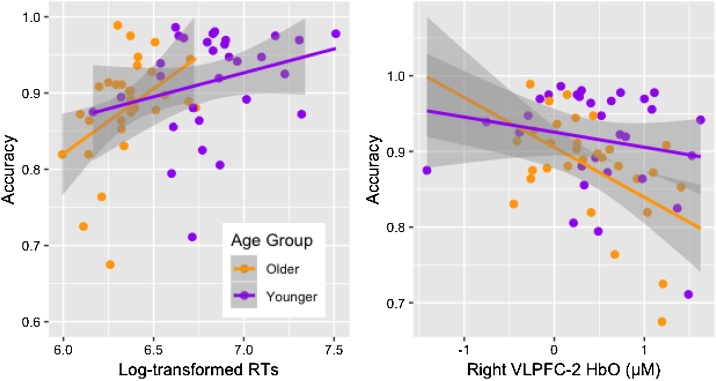
We ran Pearson’s correlations to explore whether prefrontal activation related to behavioral performance in both age groups focusing on HbO, as it varied more than HbR across channels. Given the lack of interaction between control demands and social contexts for accuracy, RTs, and HbO, correlations were run after collapsing measures across control demands and social contexts. No significant correlations were found in younger children. In older children, however, higher accuracy was associated with lower HbO in right PFC (r-VLPFC2: r = −.515, p = .004; Fig. 5), which held after False Discovery Rate (FDR) correction (Benjamini and Hochberg, 1995). Additional negative correlations, which did not pass FDR correction, were observed in right lateral PFC, further suggesting that lower HbO was associated with greater accuracy (r-VLPFC3: r = −.399, p = .032; r-DLPFC1: r = −.416, p = .025) and slower response times (r-VLPFC2: r = −.408, p = .025; r-DLPFC1: r = −.380, p = .042). Lower HbO in one left PFC channel (l-DLPFC1) was also associated with greater accuracy (r = −.415, p = .023), but this correlation did not pass FDR correction. Accuracy was positively correlated with RTs in older children (r = .406, p = .026). These results suggest lower HbO in these channels reflected more mature performance at that age.
Fig. 5.
Correlations between accuracy, log- transformed response times (RTs), and HbO. All measures are collapsed across control demands and social contexts. In both panels, correlations are significant for older children but not for younger children. Lower HbO in r-VLPFC2 was associated with greater accuracy in older children.
4. Discussion
The present study investigated age-related changes in PFC recruitment while children engaged cognitive control in response to varying control demands and in different social contexts. Children’s performance decreased with cognitive control demands and older children responded faster, though slightly less accurately, when competing with an opponent. Prefrontal activation varied more with cognitive control demands over the left than right PFC. It was higher in two channels over the left PFC with competition relative to cooperation, and in two channels over the right PFC with cooperative or neutral contexts relative to competition. Importantly, PFC activation showed less pronounced variations with cognitive control demands in younger than older children. Finally, in older children, less activation in both right and left lateral PFC, mostly in channels unaffected by control demands, was associated with better performance.
Although younger and older children showed similar prefrontal activation overall, older children showed greater modulation of prefrontal activation as a function of control demands, which suggests more efficient tailoring of cognitive control engagement rather than simply more control engagement with age. This neural pattern aligns well with behavioral evidence for increasing flexibility in cognitive control engagement during childhood (e.g., Chatham et al., 2009), and greater differentiation in control engagement between trials with low and high demands in older than younger children (Chevalier et al., 2013). It is also consistent with fMRI evidence later in development, showing that school-age children also show generally less variable PFC recruitment as a function of cognitive control demands than adults. For instance, a prior study found that although activation in ventral PFC increased with interference in adults while inhibiting responses, primary school-age children showed maximal activation even for lower interference levels (Durston et al., 2002; see also Davis et al., 2003). Similarly, when switching between tasks, children recruited pre-SMA/SMA both when the task switched and when it repeated, whereas pre- SMA/SMA activation was observed in adults only in task switch trials (i.e., most demanding trials; Crone et al., 2006).
Furthermore, activation generally varied more with cognitive control demands over the left than right PFC (when both age groups were considered together). However, this is not because HbO over the right PFC was low across all phases, but instead because it was relatively high over the right PFC even in the control phase, as shown by greater activation than in the channels over the left PFC. In older children, lower activation in three channels over the right lateral PFC (r- VLPFC2, r-VLPFC3, r-DLPFC1) was associated with better behavioural performance, although only the correlation with r-VLPFC2 held after FDR correction. Lower activation over the right hemisphere, which showed less sensitivity to variations in control demands than left PFC, may have supported more efficient control engagement. This interpretation would be consistent with increasingly efficient cognitive control being associated with a shift from diffuse to focal activation, that is, activation decrease in most prefrontal regions but increase in prefrontal regions that show growing specialization with age and correlate with behavioral performance (Durston et al., 2006; Marsh et al., 2006; Tamm et al., 2002; Tsujii et al., 2009). Interestingly, in the present study, greater performance was not associated with greater left prefrontal activation, suggesting that more efficient control engagement may rely more on decrease in (less relevant) right PFC activation than increase in (more relevant) left PFC activation with age.
Greater differentiation of PFC recruitment as a function of control demands with age speaks against the idea that cognitive control progresses only through engagement of more cognitive control. Instead it points out more flexible and efficient engagement of cognitive control with age. In older children, who showed more differentiated PFC recruitment as a function of variations in control demands, less prefrontal activation in some channels, mostly over the right PFC, was associated with better performance. Less reliance on PFC, especially when task demands are low, may come with greater reliance on posterior regions, reflecting more automatized processing with age, and hence reduced need for cognitive control engagement. Conversely, greater PFC engagement in younger children may compensate for less posterior activity (Luna et al., 2010). Such a shifting pattern of activation could, in turn, be driven by greater structural and functional connectivity between frontal and posterior regions, leading to greater communication both within and across neural networks (e.g. Grayson and Fair, 2017; Marek et al., 2015). Consistently, frontoposterior connectivity supports cognitive control performance in young children (Buss and Spencer, 2017).
An important question is how cognitive control engagement becomes increasingly flexible and efficient with age. The dorsal anterior cingulate cortex (dACC) may play a key role here, as it has been argued to integrate information about task demands and internal states (predicting expected reward and effort associated with tasks) in order to signal to lateral PFC the need to adjust control engagement (Shenhav et al., 2013). As the cingulo- opercular network (which includes dACC) shows an increasing number of links with other networks with age (Kelly et al., 2009; Marek et al., 2015), dACC may better signal control adjustment needs to lateral PFC as children grow older. Consistently, dACC volume correlates with cognitive control performance in children (Fjell et al., 2012). Unlike adults, who strategically use variations in task demands to avoid unnecessary cognitive effort (Kool et al., 2010), younger children seem oblivious to such variations (Niebaum et al., 2019), potentially because of immature signals from dACC to lateral PFC.
Furthermore, in the present study, prefrontal activation was sensitive to social contexts. Activation in two channels over the right PFC was greater in the neutral (r-FP1, r-DLPFC4) and/or cooperative contexts (r-DLPFC4) relative to competition. Thus, competition may have contributed to a pattern of activation more focally restricted to the left PFC, perhaps through enhanced motivation (Decety et al., 2004), which in turn led to greater engagement of cognitive control. However, this pattern, which is associated with better performance in older children, was accompanied by both slightly lower accuracy and faster RTs in older children, suggesting the possibility of a speed-accuracy tradeoff. Greater motivation to beat the opponent may have resulted in less cognitive control engagement. If so, however, competition should also have been associated with lower activation in the left PFC channels, which was not observed. To further explore this possibility, we computed an speed-accuracy tradeoff index (by adding percent decreases in accuracy and RTs from the cooperative and neutral to the competitive contexts) but observed no correlation with HbO drop in the competitive context (ps > .36). Nevertheless, the exact role of competition on control engagement will need to be clarified in future research.
In addition, activation in two channels over the left PFC (l-VLPFC2, l-DLPFC4) was greater with competition than cooperation. As activation in these channels, which varied with control demands, seemed critical to efficient control engagement, this pattern may explain why competition but not cooperation yielded faster responses. The distinct patterns of prefrontal activation with competition and cooperation suggest that these two social contexts differently affected cognitive control engagement. Unlike competition, which may influence cognitive control through motivation, cooperation may make socially shared goals more salient and thus easier to maintain. In adults, cooperation and competition also yield distinct patterns of activation, including lower right inferior cortex activation in competition (Liu et al., 2015) and lower left superior frontal gyrus activation in cooperation (Decety et al., 2004). However, as the present study differed from prior studies in adults in both paradigms and social context manipulation, further research is needed to examine how social contexts may differentially affect PFC recruitment and cognitive control in children and adults.
Surprisingly, neither competition nor cooperation influenced younger children’s behavioural performance. Although it further suggests that younger children engage cognitive control in a manner that is more rigid and less sensitive to contextual factors than older children, this finding is discrepant with prior studies showing that cooperation and competition can influence children’s performance in early childhood (e.g., Conti et al., 2001; Fischer et al., 2018; Qu, 2011). As prior studies involved a real (adult or child) partner/opponent, one possibility is that the virtual partner/opponent not physically present in the same room as the participant did not elicit a strong enough social context to influence younger children’s performance in the present study. This is, however, speculative at this point and should be further investigated in future research.
There are several limitations to this work. First, because of the study design, it was not possible to examine how HbO may have differed between correct and incorrect trials, and between congruent and incongruent trials. Second, as hair, skull thickness, and cerebrospinal fluid change during childhood, it may be argued that age-related differences could reflect differences in these factors rather than HbO. Yet, if anything, these factors should contribute to greater impedance in older children and thus cannot satisfactorily account for smaller HbO variations in younger than older children, and their potential impact on the findings is minimized by using distinct differential pathlength factors for each age group. Third, as activation was measured in PFC only, no conclusion can be drawn about whether increasingly differentiated PFC recruitment is accompanied by rising activation in posterior regions. Similarly, due to the inherent limitations of fNIRS, which can only probe cerebral activity up to 3 cm below the scalp, dACC activation could not be examined. Thus, its potential role in PFC recruitment differentiation during childhood remains speculative. Fourth, the unbalanced gender distribution in the older children prevented us from examining gender-related changes in PFC recruitment and could constitute a potential confound with age effects. Yet, this is unlikely as (1) gender was controlled for in the statistical analyses, and (2) the same pattern was observed when including male participants only (i.e., HbO varied with control demands in older children, p < .001, but not in younger children, p = .342). Fifth, the lack of short-separation channels prevented us from separating changes in HbO related to evoked brain activity from systematic physiological interference in the superficial layers of the head (Gagnon et al., 2011). However, the fact that task-related activation was observed in a subset of channels only and varied across phases and conditions, with all other variables (including motoric response demands) being equal, strongly speaks to the robustness of the findings.
5. Conclusions
In conclusion, progress in cognitive control during childhood is associated with more differentiated prefrontal activation as a function of cognitive demands. In other words, cognitive control development reflects more flexible and efficient engagement of control, and cannot be reduced to engagement of more control with age. These results have important implications as they suggest that, rather than attempt to train control as a muscle, efforts to support children’s cognitive control should emphasize flexible control engagement. Just like motor learning involves efficient activation of the right muscles rather than greater overall muscle activation, cognitive control development involves increasingly efficient and differentiated PFC recruitment2 .
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported in part by grants from the Carnegie Trust for the Universities of Scotland (70033) and the Economic and Social Research Council (ES/N018877/1). The authors thank Sarah Jane Harrison and Maria Quezada Garcia for their help designing the stimuli, and the participating families.
Footnotes
Similar patterns of results were observed with alternative windows (10–25 or 15–20 s).
We thank an anonymous reviewer for suggesting this parallel between cognitive control development and motor learning.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100629.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aasted C.M., Cooper R.J., Petkov M.P., Boas D.A., Aasted C.M., Yücel M.A. Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics. 2015;2(2):020801. doi: 10.1117/1.NPh.2.2.020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H., Reiss A.L. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B (Methodol.) 1995;57(1):289–300. [Google Scholar]
- Brigadoi S., Ceccherini L., Cutini S., Scarpa F., Scatturin P., Selb J. Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. NeuroImage. 2014;85:181–191. doi: 10.1016/j.neuroimage.2013.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss A.T., Spencer J.P. Changes in frontal and posterior cortical activity underlie the early emergence of executive function. Dev. Sci. 2017;(September 2016):1–14. doi: 10.1111/desc.12602. [DOI] [PubMed] [Google Scholar]
- Buss A.T., Fox N., Boas D.A., Spencer J.P. NeuroImage Probing the early development of visual working memory capacity with functional near-infrared spectroscopy. NeuroImage. 2014;85:314–325. doi: 10.1016/j.neuroimage.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler L.P., Walton G.M. The opportunity to collaborate increases preschoolers’ motivation for challenging tasks. J. Exp. Child Psychol. 2013;116(4):953–961. doi: 10.1016/j.jecp.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Cagiltay N.E., Ozcelik E., Ozcelik N.S. The effect of competition on learning in games. Comput. Educ. 2015;87:35–41. [Google Scholar]
- Camos V., Barrouillet P. Developmental change in working memory strategies: from passive maintenance to active refreshing. Dev. Psychol. 2011;47(3):898–904. doi: 10.1037/a0023193. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Trainor R.J., Orendi J.L., Schubert A.B., Nystrom L.E., Giedd J.N. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J. Cogn. Neurosci. 1997;9(6):825–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Catale C., Meulemans T. The real animal size test (rast) a new measure of inhibitory control for young children. Eur. J. Psychol. Assess. 2009;25(2):83–91. [Google Scholar]
- Chatham C.H., Frank M.J., Munakata Y. Pupillometric and behavioral markers of a developmental shift in the temporal dynamics of cognitive control. Proc. Natl. Acad. Sci. U.S.A. 2009;106(14):5529–5533. doi: 10.1073/pnas.0810002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N. The development of executive function: toward more optimal coordination of control with age. Child Dev. Perspect. 2015;9(4):239–244. [Google Scholar]
- Chevalier N., Huber K.L., Wiebe S.A., Espy K.A. Qualitative change in executive control during childhood and adulthood. Cognition. 2013;128(1):1–12. doi: 10.1016/j.cognition.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier N., Martis S.B., Curran T., Munakata Y. Metacognitive processes in executive control development: the case of reactive and proactive control. J. Cogn. Neurosci. 2015;27(6):1125–1136. doi: 10.1162/jocn_a_00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti R., Ann M., Picariello M.L. The impact of competition on intrinsic motivation and creativity : considering gender, gender segregation and gender role orientation. Pers. Individ. Dif. 2001;30:1273–1289. [Google Scholar]
- Cooper R.J., Selb J., Gagnon L., Phillip D., Schytz H.W., Iversen H.K. A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front. Neurosci. 2012;6(October):1–10. doi: 10.3389/fnins.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Steinbeis N. Neural perspectives on cognitive control development during childhood and adolescence. Trends Cogn. Sci. 2017:1–11. doi: 10.1016/j.tics.2017.01.003. xx. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Donohue S.E., Honomichl R., Wendelken C., Bunge S.A. Brain regions mediating flexible rule use during development. J. Neurosci. 2006;26(43):11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Bray S., Bryant D.M., Glover G.H., Reiss A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. 2011;54(4):2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Bryant D.M., Reiss A.L. NIRS-based hyperscanning reveals increased interpersonal coherence in superior frontal cortex during cooperation. NeuroImage. 2012;59(3):2430–2437. doi: 10.1016/j.neuroimage.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E.P., Bruce J., Snyder K., Nelson Ca. The X-trials: neural correlates of an inhibitory control task in children and adults. J. Cogn. Neurosci. 2003;15(3):432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P.L., Sommerville Ja, Chaminade T., Meltzoff A.N. The neural bases of cooperation and competition: an fMRI investigation. NeuroImage. 2004;23(2):744–751. doi: 10.1016/j.neuroimage.2004.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S., Thomas K.M., Yang Y., Ulug A.M., Zimmerman R.D., Casey B.J. A neural basis for the development of inhibitory control. Dev. Sci. 2002;5(4):F9–F16. [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella Ja, Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Everdell N.L., Gibson A.P., Tullis I.D.C., Vaithianathan T., Hebden J.C., Delpy D.T. A frequency multiplexed near-infrared topography system for imaging functional activation in the brain. Rev. Sci. Instrum. 2005;76(9):1–6. [Google Scholar]
- Fatzer S.T., Roebers C.M. Language and executive functions: the effect of articulatory suppression on executive functioning in children. J. Cogn. Dev. 2012;13(4):454–472. [Google Scholar]
- Fischer P., Camba L., Ooi S.H., Chevalier N. Supporting cognitive control through competition and cooperation in childhood. J. Exp. Child Psychol. 2018 doi: 10.1016/j.jecp.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B., Brown T.T., Kuperman J.M., Chung Y., Hagler D.J. Multimodal imaging of the self-regulating developing brain. Proc. Natl. Acad. Sci. U. S. A. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon L., Perdue K., Greve D.N., Goldenholz D., Kaskhedikar G., Boas D.A. Improved recovery of the hemodynamic response in diffuse optical imaging using short optode separations and state-space modeling. NeuroImage. 2011;56(3):1362–1371. doi: 10.1016/j.neuroimage.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson D.S., Fair D.A. Development of large-scale functional networks from birth to adulthood: a guide to the neuroimaging literature. NeuroImage. 2017;160(February):15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M., Dolan C.V., van der Molen M.W. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Huppert T.J., Diamond S.G., Franceschini M.A., Boas D.A. HomER: a review of time-series analysis methods for near- infrared spectroscopy of the brain. Appl. Opt. 2009;48(10):D280–D298. doi: 10.1364/ao.48.00d280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M.C., Di Martino A., Uddin L.Q., Shehzad Z., Gee D.G., Reiss P.T. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb. Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kool W., McGuire J.T., Rosen Z.B., Botvinick M.M. Decision making and the avoidance of cognitive demand. J. Exp. Psychol. Gen. 2010;139(4):665–682. doi: 10.1037/a0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Bull R., Ho R.M.H. Developmental changes in executive functioning. Child Dev. 2013;84(6):1933–1953. doi: 10.1111/cdev.12096. [DOI] [PubMed] [Google Scholar]
- Liu T., Saito H., Oi M. Role of the right inferior frontal gyrus in turn-based cooperation and competition: a near-infrared spectroscopy study. Brain Cogn. 2015;99:17–23. doi: 10.1016/j.bandc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13(12):1–25. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R., Zhu H., Schultz R.T., Quackenbush G., Royal J., Skudlarski P., Peterson B.S. A developmental fMRI study of self-regulatory control. Hum. Brain Mapp. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Neural origin of cognitive shifting in young children. Proc. Natl. Acad. Sci. 2009;106(14):6017–6021. doi: 10.1073/pnas.0809747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Prefrontal cortex and executive function in young children: a review of NIRS studies. Front. Hum. Neurosci. 2013;7(December):867. doi: 10.3389/fnhum.2013.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J.B., Bosma R., Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. NeuroImage. 2009;46(1):249–256. doi: 10.1016/j.neuroimage.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Niebaum J.C., Chevalier N., Guild R.M., Munakata Y. Adaptive control and the avoidance of cognitive control demands across development. Neurospychologia. 2019;123:152–158. doi: 10.1016/j.neuropsychologia.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Luna B., Hein T.C., Huppert T.J. NeuroImage fNIRS evidence of prefrontal regulation of frustration in early childhood. NeuroImage. 2014;85:326–334. doi: 10.1016/j.neuroimage.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plass J.L., Keefe P.A.O., Homer B.D., Case J., Hayward E.O., Stein M. The impact of individual, competitive, and collaborative mathematics game play on learning, performance, and motivation. J. Educ. Psychol. 2013;105(4):1050–1066. [Google Scholar]
- Qu L. Two is better than one, but mine is better than ours: preschoolers’ executive function during co-play. J. Exp. Child Psychol. 2011;108:549–566. doi: 10.1016/j.jecp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R foundation for Statistical Computing; Vienna: 2012. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ [Google Scholar]
- Scholkmann F., Wolf M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013;18(10) doi: 10.1117/1.JBO.18.10.105004. 105004- 1–1050046. [DOI] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Maturation of brain function associated with response inhibition. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tsujii T., Yamamoto E., Masuda S., Watanabe S. Longitudinal study of spatial working memory development in young children. NeuroReport. 2009;20(8):759–763. doi: 10.1097/WNR.0b013e32832aa975. [DOI] [PubMed] [Google Scholar]
- Wiebe Sa, Sheffield T., Nelson J.M., Clark Ca C., Chevalier N., Espy K.A. The structure of executive function in 3-year-olds. J. Exp. Child Psychol. 2011;108(3):436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeakumar S., Spencer J.P., Bohache K., Boas D.A., Magnotta V.A. Validating a new methodology for optical probe design and image registration in fNIRS studies. NeuroImage. 2015;106:86–100. doi: 10.1016/j.neuroimage.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M.T., Blair C.B., Greenberg M. The measurement of executive function at age 3 years: psychometric properties and criterion validity of a new battery of tasks. Psychol. Assess. 2010;22(2):306–317. doi: 10.1037/a0018708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.