Highlights
-
•
The human brain has a protracted developmental trajectory and is inherently adaptive.
-
•
A dynamic, developmental aspect is largely missing from neurobiological models of psychopathology.
-
•
Longitudinal data are key to progress in our understanding of social anxiety disorder.
-
•
Analytical recommendations for longitudinal imaging studies are made.
Keywords: Social anxiety, Development, Longitudinal imaging, Adolescence, fMRI, Psychopathology
Abstract
Longitudinal studies offer a unique window into developmental change. Yet, most of what we know about the pathophysiology of psychiatric disorders is based on cross-sectional work. Here, we highlight the importance of adopting a longitudinal approach in order to make progress towards identifying the neurobiological mechanisms of social anxiety disorder (SAD). Using examples, we illustrate how longitudinal data can uniquely inform SAD etiology and timing of interventions. The brain’s inherently adaptive quality requires that we model risk correlates of disorders as dynamic in their expression. Developmental theories regarding timing of environmental events, cascading effects and (mal)adaptations of the developing brain will be crucial components of comprehensive, integrative models of SAD. We close by discussing analytical considerations when working with longitudinal, developmental data.
1. Introduction
The wider availability of magnetic resonance imaging (MRI) facilities, together with increasingly sophisticated and streamlined analysis tools, has led to a dramatic increase in studies detailing changes in neural achitectures with development. These advances in paediatric neuroimaging have deepened our appreciation of the prolonged post-natal brain maturation that occurs during the first decades of human life. As the primary organ within which interactions between genetic and environmental factors play out, the brain’s inherently adaptive quality requires that we model risk correlates as similarly dynamic in their expression. That is, a developmental perspective will be key to making significant progress towards understanding etiological pathways to, and identifying biomarkers for, psychiatric conditions. Yet, as will be evident in the review below, this dynamic aspect is still largely missing from neurobiological models of psychopathology.
From the earliest beginnings of in-vivo imaging, especially functional MRI (fMRI), clinicians and scientists alike envisoned that brain-based data would quickly impact and inform psychiatric diagnoses (e.g., David et al., 1994). In particular, there were expectations that we would be able to determine biomarkers of atypical functioning, which would also provide insights into the optimal type and timing of theraputic efforts. While a few examples of these do exist, neuroimaging data has neither revolutionized nosology of mental health disorders, nor provided solid grounds to make recommendations about therapeutics for specific psychiatric disorders (e.g., Paulus, 2017). In this review, we illustrate how charting developmental trajectories (focusing on within-subject designs) is central to our understanding of psychiatric etiology. Longitudinal designs have long been considered preferable in developmental work using MRI – not least because adding multiple time points per participant significantly increases power (Steen et al., 2007). This is also reflected in recent consortium efforts such as the ABCD study,2 which follows a large cohort of youth over the second decade of life, mapping functional and structural brain development in relation to a wide array of environmental and individual difference variables. However, with few notable exceptions in the field of psychopathology (e.g., Cicchetti and Rogosch, 2002), there is little work using these designs to determine how trait-like individual differences in youth (measured as cross-sectional correlates of psychiatric illness) may also be, or interact with, maturational differences.
Here, we highlight how a comprehensive longitudinal approach is best suited to study risk correlates, and specifically how these data allow us to ‘connect the dots’ on some of the early indicators we have for sensitive periods and diverging trajectories, specifically in SAD. Sensitive periods are defined as time periods where environmental input (or lack thereof) plays an increased role in shaping a neural system. We focus on the juncture of adolescence (spanning the years from late childhood to early adulthood) and review what is known about SAD risk expression as the adolescent years unfold. Lastly, we provide some practical recommendations on the implementation of longitudinal modeling with multiple cognitive or environmental factors alongside brain-based indices of change in youth populations.
2. Mapping change in individual trajectories
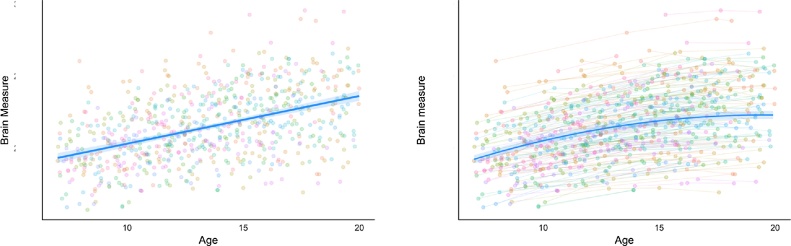
Thus far, paediatric imaging studies of pathophysiology have mainly focused on case control comparisons with youth who fulfill certain diagnostic criteria in order to identify disorder-related differences in activation or connectivity. Results of these studies paint a complex picture and are often inconsistent with respect to the directionality of group mean activation and connectivity differences in relevant brain regions in group comparisons between control groups and pediatric clinical populations. In part, these inconsistencies can be explained by the cross-sectional research approach. (see Fig. 1 for an illustration). A cross-sectional approach, even if adequately powered (which many neuroimaging studies are not, given effect sizes), can obscure developmental trends and the many different mechanisms at play that map onto different symptom profiles. Inconsistent findings may therefore reflect both heterogeneity within symptom-based diagnostic categories (i.e., sub-categories of brain-circuit dysfunction) and accentuated individual differences in development (i.e., differences in timing of developmental expression).
Fig. 1.
Hypothetical data to illustrate how longitudinal designs can reveal developmental trends that may remain obscured in cross-sectional data. The same data treated i) cross-sectional (left) ii) longitudinal (right). The model with the best fit for the data treated cross-sectionally is linear whereas the optimal fit model for the same data treated longitudinally is quadratic.
Developmental timing of symptom onset may suggest that different pathophysiological mechanisms are at play. In the context of a hierarchical, region/circuit-specific patterns of brain maturation, it is plausible that both primary pathophysiology and secondary effects on developmental trajectories present differently. For instance, parietal and lateral frontal network regions may be more heavily relied on to carry out cognitive control tasks earlier in development, with less reliance on medial and ventral frontal neural regions (e.g., Durston et al., 2006; Ordaz et al., 2013). Hence, as the networks supporting cognitive control functions shift during development (alongside changes in cognitive stategies or performance), it is likely that clinically-relevant symptoms emerging along this developmental process have different neurobiological manifestations.
Developmental timing will also give rise to secondary effects on maturational trajectories. Therefore, an understanding of circuit development will likely precede a comprehensive understanding of symptom-specific dysfunctions and the identification of early biomarkers for psychiatric conditions. Within-subject designs uniquely parse out the degree to which individual-differences are really maturational differences or are time-invariant trait differences. Most likely, they will be a combination of both. In order to make room for the role of developmental timing, dynamic conditional effects of risk factors, and cascading effects on outcomes, it is critical that we move beyond cross-sectional designs and focus our efforts on mapping change in individual trajectories over time.
3. Mapping brain development
Although longitudinal designs, including multiple brain scans of the same individual, are a significant investment of time and resources, the numerous theoretical gains warrant the costs. Placing the identification of psychiatric biomarkers in the context of development may seem impossibly complex because it means examining sophisticated interactions between age/age of onset, symptoms and cognitive/emotional processes, and exploring age beyond linear trends. Paradoxically, we may find that it is easier to identify mechanisms of pathophysiology alongside discovering mechanistic principles of neural organization that cut across development and disorder. For instance, development can help us understand how cognitive functions are supported when specific regions or networks are less available or efficient, or the effects of hyper/hypo-activation of particular regions or networks on distributed neural activity within the network. These may be similar to processes relevant for disorder. But even beyond understanding principles of organization, there are many gains to be made.
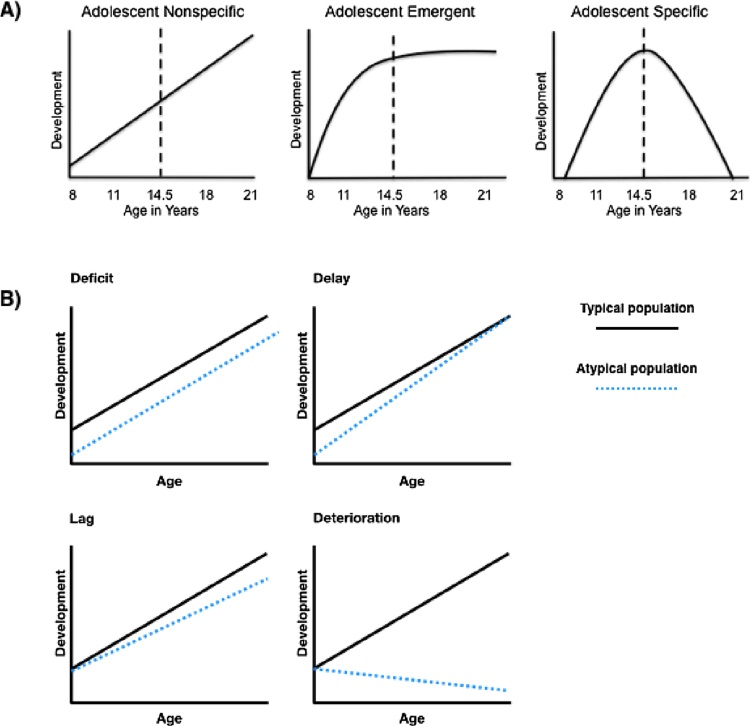
First, a key benefit of mapping developmental trajectories is that it would allow us to understand what constitutes typical variations and what represents risk or a risk trajectory in a given context. Building comprehensive, continuous trajectories of cognitive and brain development in well characterized samples will allow us to differentiate the type of developmental differences (i.e., developmental delay, lag, deterioration or overall deficit; see Fig. 2B), diverging trajectories and changing risk correlates. Mechanistic insights crucial to therapeutic recommendations are almost impossible to gain with cross-sectional approaches, even with finely binned cross-sectional data. For instance, when studying a phenotype compared to healthy controls, it would be important to distinguish a “delay in maturation” from a “deficit” explanation (as detailed in Fig. 2B). As illustrated in Fig. 1 (which treats the same set of data points as cross-sectional and longitudinal), the best model fit differs between the two designs. Hence, while the quadratic trend revealed in the longitudinal treatment of the data would support a delayed maturation explanation for the phenotype, the cross-sectional trend would support a deficit explanation. Longitudinal data is therefore pertinent in differentiating these alternatives, especially in regard to the distinction between maturational and stable deficit.
Fig. 2.
A) Hypothesized patterns of cognitive and brain development during adolescence. Of particular concern for the current paper are the adolescent-specific changes, which are likely to indicate a period of heightened plasticity for development (Adapted with permission from Casey, 2013). B) Hypothesized developmental trajectories of functioning (Adapted with permission from Reichenberg et al., 2010).
Secondly, mapping individual trajectories of change in developing populations will allow us to understand whether particular networks undergo periods of significant and rapid maturation. Rapid maturational change of individual structures or brain function does not necessarily mean that these structures or functions are amenable to external influence (in terms of both environmental insult and intervention) at the time of change. However, it is plausible that early emerging functions will result in earlier brain specialization, thus leaving the cognitive/brain function in question less amenable to interventions at later time points (Johnson et al., 2015). Hence, differentiating changes that are unique to a developmental period from those that are non-specific on-going changes, or emerging changes that will mature at the later stage (Fig. 2A) may be an important piece of the puzzle. When mapping change in relation to external factors, we can move closer to understanding whether there are sensitive periods for the development of certain functions and impact of particular (therapeutic) experiences.
Each type of developmental difference and pathway comes with its own implications for therapeutic approaches. For example, we could either develop programs that target specific points of divergence from typical trajectories, or help to compensate for delay early on. No matter how these trajectories play out, getting a better handle on timing information (i.e. understanding when and how risk factors are expressed in development) will be crucial if we are to devise interventions that target both brain function and behavior effectively (Cohen Kadosh et al., 2013a,b; Johnson et al., 2015). Choice and timing of therapeutics for at-risk youth is particularly tricky. Whilst “the earlier the better” may appear to be the most intuitive approach, intervening early for those at risk is a more delicate decision.
In the following section we illustrate the benefits of this approach, using examples from the developmental or clinical neuroscience literature to show how key findings in the literature need to be extended with longitudinal data. Social anxiety disorder (SAD) lends itself well as an illustrative clinical example, with a body of work on a reliably identified early risk profile (behavioral inhibition). It is noteworthy that the processes discussed below are affected across several psychiatric illnesses; from a functional standpoint, key processes are often those that track with several symptom dimensions (e.g., reward and threat processing, attention and cognitive control, working memory etc.).
4. Adolescence as a time of risk expression: the case of social anxiety disorder
Adolescence is a transitional period marked by changes on many levels: developments in brain structure and function, genetic and hormonal changes with puberty, as well as new social-environmental demands (e.g., Dahl, 2004). As early as prenatal development, risk factors such as adverse environments and factors intrinsic to the brain such as the quality of neural processing (i.e., the sampling of the early environment) significantly impact cognitive and emotional variables (e.g., Andersen, 2003). It may be that that these early risk factors only find their full expression during the second decade of life. The timing of adolescence-associated social, hormonal, and neural changes may compound risk for mental health disorders in this developmental period (Haller et al., 2014, 2015; Keshavan et al., 2014; Paus et al., 2008). In the psychiatric literature, adolescence has long been recognized as a period of great vulnerability: age-of-onset data suggest that initial impairing symptoms of many psychiatric disorders, including anxiety disorders, often emerge at the adolescent juncture (Kessler et al., 2005, 2007; Wittchen et al., 1999).
SAD is one such example. There is a pronounced increase of SAD symptomatology at the juncture of adolescence persisting to explain a significant proportion of adult SAD (Gregory et al., 2007; Kessler et al., 2005; Wittchen et al., 1999). The hallmark of SAD is a disabling and persistent fear and consequential avoidance of social situations. Individuals with SAD fear negative evaluation and social rejection, with concerns usually centering on thoughts of humiliation and embarrassment (American Psychiatric Association. APA, 1994; Clark and Wells, 1995; Foa et al., 1996). The course of SAD is often chronic, and is associated with co-morbid depressive symptoms and low self-esteem (Cox et al., 2004). Since peer interactions carry important learning experiences for adolescents, avoidance of social exchanges is particularly impairing and disruptive during adolescence. Additionally, as poor social interactions in school environments also impact academic success, intervening early is all the more important. What do we know about the processing characteristics of SAD, and the way in which the adaptive and dynamic brain adjusts to biased input?
4.1. Threat interpretation
In many ways, SAD represents an exaggerated presentation of a typical developmental phenomenon in adolescence: increased salience and reactivity to social cues, and sensitivity to social exclusion. Central to cognitive models of (social) anxiety are systematic biases in the way social information is processed (e.g., Clark and Wells, 1995). Biases are thought to give rise to preferential processing of threatening information, maintaining social fears by shaping maladaptive patterns of social avoidance. For instance, skewed interpretations of everyday ambiguous social experiences (e.g., a frown of an audience member during your presentation or a laugh behind you in the hallway – note that social interactions are often ambiguous as one must always infer mental states indirectly via verbal/non-verbal cues) have generally shown robust associations with social anxiety (Stopa and Clark, 2000; Amin et al., 1998; Amir et al., 2012; Constans et al., 1999; Huppert et al., 2003). Developmental differences in interpretational style and its links to (social) anxiety have been found in cross-sectional work, with more robust associations between social anxiety and interpretations of social stimuli across studies in adolescent samples compared to younger populations (e.g., Creswell et al., 2014; Miers et al., 2008). It is possible that interpretational style is more malleable to interventions before adolescence, with the growing importance of peers and socio-cultural norms increasingly guiding behavior, it is likely that social cue interpretation fine-tunes during puberty. With increasing age, youth have a greater quantity of social experiences to inform their thinking, and cognitive styles may also become more stable and global (e.g., Nolen-Hoeksema et al., 1992; Nolen-Hoeksema, 1996). Hence, interpretational style may only become sufficiently stable and function as a maintenance factor in adolescence, not earlier in development.
Increased affective responding to negative social cues in youth with SAD, or youth selected for increased social worries, have been linked to increased amygdala sensitivity as well as differential responses in several frontal regions such as the anterior cingulate cortex (ACC), medial PFC (mPFC), ventro/dorsal-lateral PFC (vl/dlPFC), and insula (e.g., Battaglia et al., 2012; Jarcho et al., 2016; Killgore and Yurgelun-Todd, 2005). These data have been replicated and extended with tasks that engage the participant in more dynamic social exchanges online (Jarcho et al., 2013b and 2016; Guyer et al., 2009). Brain networks implicated in perturbed processing in SAD are the same as those that have been documented to undergo prolonged structural and functional change across adolescence (Mills et al., 2014; Goddings et al., 2014). Cross-sectional data on typically developing youth suggest that sensitivities in the neural responses of subcortical affect- and reward-processing regions (such as the amygdala and striatum) to simple threatening or rewarding stimuli peak around adolescence (Chein et al., 2011; Ernst et al., 2005; Passarotti et al., 2009; Somerville et al., 2011; Van Leijenhorst et al., 2010). There is some contention as to whether these peak sensitivities occur before, at the start, or in mid-adolescence (Hare et al., 2008; Somerville et al., 2011, 2013; Gee et al., 2013a,b). Functional developmental trajectories of frontal areas in response to socio-affective stimuli are equally complex, with reports of adolescent-emergent or -unique trends in tasks that require automatic or effortful emotion regulation (e.g., McRae et al., 2012; Somerville et al., 2013). Inconsistencies in the directionality of functional developmental differences in child/adolescent/adult groups across studies have made it difficult to draw firm conclusions about developmental change (Crone and Dahl, 2012). It is plausible that age and/or pubertal status may significantly affect how social stimuli are processed, possibly obscuring more subtle SAD-related differences. Overall, it is clear that we need to integrate findings on SAD-linked functional responses of key emotion generation and regulation regions with the emerging corpus of work on typical developmental trajectories of peak sensitivities of regions in these networks to understand when and how neural trajectories diverge.
4.2. Emotion regulation
As well as studying mean activation differences in individual regions, recent studies have explored typical and anxiety-linked developmental changes in functional connectivity between regions of social-affective and social-cognitive brain networks. Task-based functional connectivity is an indicator of co-activation between different areas during engagement in different task conditions (Friston, 2011), and may represent an approach that more closely aligns with the notion that computations are carried out in concert by distributed networks. However, changes in coupling (as opposed to a valence ‘switch’ in connectivity) can be difficult to interpret, as we cannot know what drives differences (i.e., it could be either node, both nodes, the connectivity between nodes, or a third region influencing both regions). With this in mind, different functional connectivity patterns and age-related changes of these patterns in response to emotionally evocative stimuli in youth with increased levels of (social) anxiety have been reported in a handful of studies (e.g., Spielberg et al., 2014a,b; Gold et al., 2016; Hardee et al., 2013). Findings are inconsistent and difficult to compare across studies due to the different task-related processes and specific contrasts employed across studies. Some studies find increased negative coupling in youth with anxiety in fronto-limbic circuits, both in task conditions where attention is explicitly directed at threat features of stimuli (e.g., Gold et al., 2016), or where attention capture by threat is bottom-up (Hardee et al., 2013). Other studies have found no difference (e.g., McClure et al., 2007), or significantly less negative coupling (e.g., Monk et al., 2008; Hare et al., 2008), in anxious youth as compared to typically developing peers. Two studies have additionally examined age as a factor. Gold et al. (2016) reported that anxious adolescents exhibited the inverse task-related connectivity compared to anxious adults, with increased negative coupling of amygdala-PFC circuit in anxious adolescents and increased positive coupling in anxious adults during threat appraisal. Kujawa et al. (2016) reported a positive association between amygdala-ACC connectivity and age in anxious youth during a face-emotion processing task, with the reverse age trend observed in the typically developing controls.
These results are particularly interesting in the context of two lines of work. Firstly, recent cross-sectional studies on typical developmental changes in functional connectivity have documented a normative developmental ‘switch’ from positive to negative connectivity in the amygdala-medial PFC network when viewing emotional faces compared to a baseline condition (i.e., non-threat specific) (Gee et al., 2013b; Wu et al., 2016). The authors reported that children aged 4–9 years showed positive connectivity, whilst from early adolescence (10–13 years) to adulthood, youth exhibited negative connectivity (Gee et al., 2013b). Additionally, the authors found that the switch in coupling is related to individual differences in declining separation anxiety.
Secondly, work examining the effects of early adverse experiences on functional connectivity of limbic-prefrontal circuits in youth samples consistently suggests that childhood social experiences result in neural adaptions in these circuits (although we note that it is often difficult to disentangle heritable vulnerability from the quantified environmental factors) (e.g., Herringa et al., 2016; Gee et al., 2013a,b; Silvers et al., 2016). Specifically, Gee et al. (2013a) found that early adversity is associated with a shift in timing of the normative amygdala-PFC connectivity pattern. The authors found that youth who had experienced early caregiving adversity exhibited negative amygdala-mPFC connectivity (the more “mature” phenotype) earlier in development. There is also evidence that these adaptions are linked to internalizing outcomes. Results from studies by Silvers et al. (2016) and Herringa et al. (2016) suggest that increased fronto-limbic connectivity to negative stimuli may represent an adaptive augmentation in this population, a source of resilience against internalizing symptoms in adolescence. Childhood adversity predicted increased connectivity in this circuit only in adolescents with low levels of internalizing symptoms (Herringa et al., 2016). While both lines of work speak to this circuit being a potential developmental target, the latter highlights that we need to be cautious and contextualize what we determine to be adaptive. Together, this body of work highlights the importance of interpreting pathophysiological findings in youth in the light of age-related change; risk and resilience trajectories and pathophysiological underpinnings of subgroups need to be discerned with longitudinal data.
4.3. Behavioral inhibition
There have been several studies looking at behavioral inhibition (BI) as a risk profile to understand pathways to SAD (Goldsmith and Gottesman, 1981; Matheny, 1989). BI is a reliably and early identified temperamental factor associated with heightened sensitivity to novel situations and people, and avoidance of unfamiliar stimuli in general (Kagan et al., 1987). Evidence from both longitudinal and high-risk family studies has demonstrated that BI is a developmental and familial risk factor for anxiety disorders, specifically SAD (Biederman et al., 2014; Clauss and Blackford, 2012; Hayward et al., 1998; Rosenbaum et al., 2000; Schwartz et al., 1999). Interestingly, although BI is often used as an early proxy for SAD, there is large variability in outcomes, with many children developing neither clinical nor sub-clinical levels of social anxiety – BI represents only one possible pathway to clinical anxiety. What are the mechanisms fueling the BI to SAD risk trajectory, what are the mechanisms of continuity and change?
Numerous studies have found evidence for biased attention orienting (i.e., preferential orienting and/or maintenance of attention to social threat such as angry faces) in adults and youth with SAD or with high trait levels of social anxiety relative to their non-socially anxious controls (e.g., Roy et al., 2008; Stirling et al., 2006). Some studies suggest more complex attentional patterns of hyper vigilance-avoidance depending on length of threat exposure (e.g., Gamble and Rapee, 2009; Kircanski et al., 2015). Similarly, there is some evidence suggesting that children with BI also show atypical attentional patterns (Pérez-Edgar et al., 2010; Pine et al., 2009), which act as moderators between early temperament and later anxiety symptoms by biasing the information processed (Pérez-Edgar et al., 2010; 2011). Specifically, a stable hyper-vigilant attention bias pattern is thought to increase risk for SAD onset in early adolescence for BI children (Fox, 2010) – although the small number of studies and inconsistent results make it difficult to disentangle the timing and emergence of the (likely reciprocal) relations between attention orienting patterns, BI and risk for SAD. While some fMRI work has compared youth with a history with BI to either healthy volunteers or youth with anxiety disorders on relevant tasks (e.g., Hardee et al., 2013), very little work has examined BI by social anxiety by age symptom interactions to directly assess the BI to SAD link.
Another body of work examining the role of attention in the BI pathway to SAD has focused on response monitoring (i.e., regulating behavior, based on attending to and subsequently adjusting one’s output) and inhibition. These processes that are more effortful in their execution are thought to have a more prolonged maturational trajectory than the more automatic attention orienting described above. Both cross-sectional and longitudinal studies have found evidence that networks underpinning attentional control, including the dorso-lateral and medial (dl/m) PFC, anterior cingulate cortex (ACC) and parietal regions, continue to develop throughout adolescence, where performance gains are related to increases in activation in regions such as the dorsal ACC (Henderson et al., 2015; Munakata et al., 2012; Ordaz et al., 2013; Rothbart and Rueda, 2005). Relations among effortful control, BI, and risk for SAD are complex, such that effortful control may moderate risk (i.e. it is plausible that increased inhibitory control promotes inflexibility of attentional deployment in social contexts) thereby inflating developmental risk for SAD specifically in youth with a history of BI (i.e., only conferring risk in this subgroup), while linking to positive outcomes in non-BI youth. The body of work on the neural underpinnings of attention control using fMRI in this population is small but growing. An early study (Jarcho et al., 2013a) demonstrated that adults with a history of BI performed similarly on an emotion-based attention control task but showed increased neural responses in the dmPFC during trials requiring effortful control. It is unclear when this presumably compensatory activation pattern first emerges, whether it represents a trait-like correlate of BI, or whether it emerges along the developmental path as cognitive control and regulation networks mature. It is also unclear how this activation pattern links to SAD across development. Given that there are many different pathways to SAD, it may be that, for instance, despite being phenotypically identical, neural manifestations of SAD in individuals with and without BI are different, both with regards to maturational timing and overall pathophysiological architecture. Further, longitudinal data are needed to understand the mechanisms of change in the BI to SAD trajectory, especially in the key period of adolescence where youth often move from at-risk status to experiencing clinically significant and impairing SAD. A better understanding of trajectories in at-risk groups would greatly impact therapeutics for SAD in these subgroups.
BI risk unfolds as attention control systems mature. Experimental interventions targeting attention biases/control often require sustaining attention to complete a repetitive task to train attention control or change a processing bias; hence we may see intervention effects increase linearly or possibly in a quadratic fashion (reaching a plateau or declining towards adulthood) from childhood to adolescence – at least in anxious youth. When thinking about targeted early intervention for at-risk youth, understanding trajectories is particularly important. BI at-risk status will not, in the majority of cases, result in clinically impairing levels of anxiety. Training attention control in a BI child could potentially have adverse effects resulting in a lack of attentional flexibility (maybe via increasing compensatory activation in the ACC). Our understanding of pathways to the SAD phenotype, risk and protective mechanisms and how these play out across development is still very limited. Therapeutic experiences ought to have more impact during periods of increased plasticity due to maturing neural circuitry. Hence, therapeutics in pediatric at-risk populations requires weighing considerations of vulnerability and opportunity.
4.4. Remaining questions
In order to move our understanding of developmental paths to anxiety forward, it is crucial to assess whether the transition to adolescence represents an inflection point (i.e., a time of significant change in typical development) or/and a central point of divergence for anxiety-relevant functional connectivity indices between key emotion regulation regions. Are differences in functional connectivity already present before puberty? If so, do differences become more pronounced? Is an early or delayed valence change of coupling related to, or predictive of, a rise of anxiety in adolescence? How much ‘normative’ variability is there at the juncture to adolescence, and how does this relate to indices of pubertal status and significant social experiences (e.g., parenting, positive and negative peer interactions)? How do youth at risk (e.g., youth with a history of BI) compare – does this juncture represent a time when risk groups diverge rapidly? What about compensatory adaptions and how do these inform therapeutics in at-risk youth? Should preventions target at-risk youth before or during adolescence? Only when we go beyond cross-sectional approaches to chart individual trajectories and links with anxiety can we answer these questions comprehensively. Once we have determined when critical maturational time courses occur, we can move to determine whether, for instance, changes in coupling also represent windows at which external influences (i.e., therapeutic efforts) have increased impact, whether training abilities can preemptively shape trajectories towards more adaptive outcomes, and whether these functions remain plastic and malleable into adulthood.
5. Interim conclusion
Together, these examples illustrate why it is important to go beyond extrapolating predictions from adult models to understand: i) developmental changes in risk correlates and cognitive phenotypes and ii) timing of symptom onset (or prediction of transition to disorder) by assessing how potentially unique cognitive and emotional trajectories in normative development may shape and bring out risks at certain developmental periods. It is plausible that typical developmental processes in adolescence exaggerate individual differences and perceived functional impairment, as maturational trajectories change neural dynamics alongside increasingly sophisticated cognitive skills and newfound autonomy. It is only when we chart developmental trajectories that we can test these mechanistic hypotheses directly.
It is enticing to think that progress in developmental cognitive neuroscience and biological psychiatry will be made by singular big discoveries and paradigm shifts. Given the complexity of research questions and the limitations of current imaging tools, it is more likely that progress in this field will be gradual, driven by collaborative efforts. In line with this, we need to support work on the reliability of fMRI measures, and encourage data sharing to establish larger datasets. Large data can capture the heterogeneity of adolescent samples and disorders (e.g., gender specific trajectories and outcomes) to ultimately establish standardized scores of variability in function and structure across development. Thinking about new ways of phenotyping participants, assessing risk, balancing a trans-diagnostic framework with examining specificity of pathophysiology and outcomes are among some of the newer challenges when designing a longitudinal study. In the next section, we will discuss some of the basic analytical considerations when working with longitudinal, developmental imaging data.
6. Data-analytic recommendations
“Change is the only constant” could describe both the rapid evolution of imaging methods and best practices in data analysis and reporting. Despite constant change, there are certain basic considerations for modeling changes in the brain during development that are distinct from those used for cross-sectional data analysis (for further reading see Mills and Tamnes, 2014; King et al., this issue). Given their complexity, longitudinal designs require access to better computational resources and training. Arguably, longitudinal studies also require more up-front work to settle on the best design and analysis plan to test the change processes of interest. Hence, there are several decisions that must be handled carefully when undertaking a project that models brain developmental trajectories. A few of these considerations are outlined below.
6.1. Measure of development
When modeling developmental change, it is essential to first define the measure of development. In the developmental neuroimaging literature, this measure is almost always chronological age, but other studies have attempted to model brain development along other developmental measures such as puberty (e.g., Goddings et al., 2014), or other measures of body growth (e.g., height or weight). The developmental measure used will in part determine the kind of models that can be used to describe the data. Given that body growth and puberty follow non-linear trajectories, different kinds of models might need to be tested from those tested against linearly developing measures (e.g., age).
It is worth considering how the measures of development could relate to different underlying mechanisms driving brain changes. For example, if the research focus is on cortical brain changes in the transition into adolescence, modeling these changes against pubertal development might yield different results than modeling against age. Further, modeling change against pubertal measures would be beneficial for samples with short age ranges, but larger pubertal variation.
Ultimately, the measure of development will constrain the interpretation of the results. While age is an easily quantifiable measure of development, relating brain changes to this measure cannot tell us about the potential impact of biological processes such as puberty or body growth.
6.2. Initial data processing
Many choices about parameters are made in the pre-processing stages of imaging analysis – with what could be considered a problematic amount of analytic flexibility (Carp, 2012). Unfortunately, for longitudinal scanning studies in particular, it is easy to add bias by inadvertently applying a process that is particular to only one time-point (most commonly being with regard to time one). Any particular analysis of a longitudinal dataset should strive for consistency in data processing for all participant and sessions. Variation in operating system and software version has been shown to affect brain measures that are used to quantify brain change (Gronenschild et al., 2012). The different treatment of baseline images, wherein only follow-up images are re-sampled, must also be avoided (Reuter and Fischl, 2011). Interpolation asymmetry resulting from within-subject registration has been shown to introduce artifacts that lead to bias in subsequent session data, where the difference between baseline and the first interval is disproportionately higher than changes across the overall trajectory (see Fox et al., 2011 for an illustrative example). Algorithms have been developed wherein inverse consistency is achieved, such that each time point is treated identically and registration is thus fully symmetric (e.g., Reuter et al., 2012; Wachinger et al., 2015). 3
6.3. Multilevel modeling
Longitudinal data are not suitable for simple regression analysis, as data obtained from the same individual cannot be considered independent. There are multiple analytic strategies that can handle the continuous dependent (within-participant) and independent (between-participant) variables present in longitudinal data. Which analytic strategy to use will depend on the kind of data as well as the research question.
Typical linear regression models can estimate the overall group-level trends, or fixed effects. These models are typically employed in cross-sectional studies characterizing age-related changes in brain measures of interest. Multilevel models, also known as mixed-effects models, can estimate the fixed effects of a chosen variable on a measure of interest while also taking into account the dependence of observations obtained from the same individual. This can be done by setting a random intercept for each participant in addition modeling group means (fixed effects). By adding a random intercept to the model, each individual’s developmental pattern is modeled with a regression line parallel to the overall regression line (group trend), which can account for overall differences in values for individuals. Practically, in developmental cognitive neuroscience, setting a random intercept can allow the model to account for the variability of individual brain anatomy and function.
Setting a random slope can allow further flexibility in the model by removing the assumption that individuals are going to change in the same way. This can be helpful for investigations of how individual differences or psychopathology affect the pace of brain development. For example, if it is hypothesized that some individuals will show slower cortical development than others (e.g. a different rate of change), a random slope might improve the fit of the model. Examining the random slope for each participant, it is possible to identify individuals showing aberrant growth patterns from the group average. Further, by examining the relationship between an individual’s random intercept and slope, it is possible to examine patterns between an individual’s overall measure and rate of change in the measure. For example, in a study examining how brain structure changes over age (starting from the earliest age point in the sample), correlating the random intercepts to the random slopes can illustrate if individuals with larger/smaller brain measures also exhibit slower/faster developmental trajectories. However, it is necessary to have at least three data points before a random slope can be useful. Further, these models also allow for data to be collected at uneven intervals, which makes this technique particularly attractive to longitudinal datasets with an accelerated design.
How the level 1 predictor is coded in a model has implications for the interpretation of the predicted model (Biesanz et al., 2004). For example, if age is used as the developmental measure of interest (the level 1 predictor), it can be centered with the grand mean (e.g. by subtracting the mean age of the sample from each individual’s age). When this particular centering procedure is applied prior to modeling the data, the estimates provided must be interpreted from the grand mean of the sample. But time can also be centered to different points along the timeframe of interest if the study’s aims are to understand developmental changes from a specific starting or ending point. For example, if an investigator is interested in understanding how developmental trajectories might diverge as the sample get older, centering to earliest age of the sample will allow the model estimates to be interpreted from this age forward.
Drawing from the literature discussed in the example section, applying multilevel modeling to investigations of behavioral inhibition (BI) and anxiety disorders could increase our ability to identify risk correlates related to developmental maturity. For example, if it is hypothesized that children with BI who develop biased attention patterns before adolescence will have a higher risk for developing anxiety disorders in adolescence, a longitudinal study assessing these measures across childhood and adolescence is necessary to identify if one of these periods represents a time of increased risk. Further, a longitudinal design will not only establish group norms for the trajectory of attention processes during the developmental period of interest, but also allow for individual patterns of development to be identified and compared. Some individuals might show a slower pace or reach different levels at different points in development; these two factors can be assessed by including a random intercept and a random slope in a multilevel model. Including measures of brain function and anatomy into this design can allow for hypotheses regarding how neurocognitive strategies or brain maturity further distinguish individuals at risk from those following divergent patterns that do not necessarily relate to maladaptive outcomes.
Practically, several neuroimaging software packages are now able to implement multilevel analyses, and new methods are being developed to overcome computational limitations inherent in massively univariate analysis (for a discussion see Madhyastha et al., this issue). For example, linear mixed-effects (LME) models implemented in Freesurfer’s longitudinal pipeline (Reuter et al., 2012) apply iterative algorithms such that convergence in model fitting may not be consistently achieved, which can lead to invalid results for some voxels. To overcome this issue, Guillaume et al. (2014) developed an alternative, non-iterative Sandwich Estimator.4 Here, a simple ordinary least squares model is specified for the marginal model to create parameter estimates of interest. A Sandwich Estimator is used for the standard error of these estimates, to account for the repeated measurements. This approach has the advantage of reduced model complexity as random effects are not specified, although the use of unstructured error covariance permits all random effects to be accounted for. Moreover, because it is non-iterative, this implementation benefits from significantly reduced computational time.
6.4. Model selection
Several considerations factor into choosing the kind of model to fit to your data. The rule of thumb for modeling is to go with the simplest model that best describes the data. This heuristic of parsimony means finding a model that explains the most amount of variance using the least number of parameters. While it might be tempting to include all the measures you have for a particular dataset into your model, it is likely that a model with so many parameters will not translate to a new sample (called overfitting). Therefore, much of the literature on brain developmental trajectories has favored parametric models (Raznahan et al., 2011; Vijayakumar et al., 2016). However, nonparametric modeling, such as spline modeling, is also a potential avenue for mapping more precise developmental trajectories across larger developmental periods (e.g., Tamnes et al., 2013; Walhovd et al., 2016).
6.5. Parametric modeling
When applying parametric models to data, it is essential to choose models that are physiologically plausible. This will depend on the sample’s developmental period as well as the brain measure of interest. One study might hypothesize distinct developmental trends for a sample spanning childhood, adolescence, and adulthood, and the parametric model will need to account for this. For example, as cortical thickness is highest in the first decade, decreases across the second decade, and stabilizes in the third decade (Tamnes et al., 2017), a study spanning these ages might choose to fit a cubic (polynomial) model to the data to capture the change in slope at the beginning and end of the examined age range. However, a study investigating the second decade alone might be best suited examining the linear trajectories of the cortical thickness. Further, it is also plausible to fit a logarithmic, exponential, or other growth curve model to certain age periods, depending the data and underlying physiology.
Visualizing the raw data can assist in model selection, but it is also important to compare models with their simpler counterparts. Second and third-order polynomial models are attractive because they are able to characterize the nonlinear trends present across brain development. While a cubic polynomial model might provide a significant fit to the data, the quadratic or even linear model might be considered the better fit. This can be determined by comparing values representing the goodness of fit of a model and likelihood ratio tests. While likelihood ratio tests can only be performed between nested models, goodness of fit measures like the Akaike Information Criteria (AIC) or Bayesian Information Criteria (BIC) can be used to compare models that are not nested because they are standardized measures that take into account the goodness of fit of a chosen model, penalizing for complexity. Indeed, one of the advantages of using parametric models when examining brain developmental trajectories is that they allow for easy comparison of factors that could be influencing the development of the brain measure of interest. Comparing models with added fixed effect variables, such as group affiliation or an individual trait, allows for researchers to quantify the effect size of a particular variable on the fitted developmental trajectory.
6.6. Nonparametric models
Nonparametric models such as spline models have the advantage of mapping more precise developmental trajectories, and are becoming more common developmental neuroimaging analyses. There are several spline fitting procedures that can be used, including Generalized Additive Mixed Modeling (Walhovd et al., 2016), Generalized Estimating Equations (Chen et al., 2014), and Penalized Spline Mixed-Effect Models (Alexander-Bloch et al., 2014). Rather than specifying the precise structure expected to fit the data, spline modeling procedures attempt to identify the points of inflection in a developmental trajectory. Similar to parametric models, it is possible to specify a developmental model for a reference population using nonparametric models, and then characterize how this model might differ in another sample (e.g., different overall size of measure, different rate of change). New spline modeling techniques are being developed that take into account not only the time-varying correlation structure between different developmental segments, but also the expected weaker correlation between data points acquired from the same participant across longer intervals (Chen et al., 2014).
6.7. Differential equation models
It is also possible to specify differential equation models that take into account the within-participant dependence of observations (just like multilevel models). These multilevel differential equation models might be useful for studies investigating the additive effects of reaching certain developmental milestones off-tempo from the “neurotypical”, expected trajectory. Differential equation models have just started to be implemented in developmental cognitive neuroscience (Ziegler et al., 2017). It is important to keep in mind that these equations assume continuous change in a given process and require several time points to generate a stable model. As longitudinal developmental neuroimaging datasets continue to grow and incorporate more time points (e.g., ABCD), we will begin to realize the applicability of more sophisticated modeling approaches to our investigations of brain-behavior relationships across development.
7. Conclusion
Thus far, neuroimaging data have yet to make significant contributions to nosology or treatment of psychiatric disorders. What would it take for neuroimaging data to be useful? Following well-phenotyped at-risk groups as development unfolds allows us to understand how the adaptive, dynamic brain adjusts to biased input and processing. Additionally, using longitudinal designs to study emergent function will contribute to a more comprehensive understanding of how processes and computations are carried out in different (functional) networks. Placing the identification of biomarkers of psychiatric conditions in the context of development may seem like an impossible level of complexity to tackle. Here, we have argued that a developmental perspective may, in fact, provide a way to wrestle with the complexity of pathophysiology and help to extrapolate system-level neurocognitive mechanisms of disorder. Developmental data will be a significant step towards deriving integrative, dynamic models of psychiatric conditions. Developmental ideas of timing of environmental events, cascading effects and (mal)adaptations of the developing brain need to be key components of these models.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
SH was supported by a Medical Research Council studentship (Reference: 1242237) and a Scatcherd European Scholarship. CH was supported by an award from the European Research Council (‘Learning & Achievement’, Reference: 338065). KM was funded by a NIH extramural grant (Reference: R01MH107418). ASD is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Footnotes
Such as is implemented in FreeSurfer’s longitudinal pipeline (http://surfer.nmr.mgh.harvard.edu).
Contributor Information
Simone P.W. Haller, Email: simona.haller@gmail.com.
Kathrin Cohen Kadosh, Email: kathrin.cohenkadosh@psy.ox.ac.uk.
References
- Alexander-Bloch A.F., Reiss P.T., Rapoport J., McAdams H., Giedd J.N., Bullmore E.T., Gogtay N. Abnormal cortical growth in schizophrenia targets normative modules of synchronized development. Biol. Psychiatry. 2014;76(6):438–446. doi: 10.1016/j.biopsych.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. APA . 1994. Diagnostic and Statistical Manual of Mental Disorders; p. 4. [Google Scholar]
- Amin N., Foa E.B., Coles M.E. Negative interpretation bias in social phobia. Behav. Res. Ther. 1998;36(10):945–957. doi: 10.1016/s0005-7967(98)00060-6. [DOI] [PubMed] [Google Scholar]
- Amir N., Prouvost C., Kuckertz J.M. Lack of a benign interpretation bias in social anxiety disorder. Cognit. Behav. Ther. 2012;41(2):119–129. doi: 10.1080/16506073.2012.662655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27(1):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Battaglia M., Zanoni A., Taddei M., Giorda R., Bertoletti E., Lampis V. Cerebral responses to emotional expressions and the development of social anxiety disorder: a preliminary longitudinal study. Depress. Anxiety. 2012;29(1):54–61. doi: 10.1002/da.20896. [DOI] [PubMed] [Google Scholar]
- Biederman J., Hirshfeld-Becker D.R., Rosenbaum J.F., Hérot C., Friedman D., Snidman N. Further evidence of association between behavioral inhibition and social anxiety in children. Am. J. Psychiatry. 2014;158(10):1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Biesanz J.C., Deeb-Sossa N., Papadakis A.A., Bollen K.A., Curran P.J. The role of coding time in estimating and interpreting growth curve models. Psychol. Methods. 2004;9(1):30. doi: 10.1037/1082-989X.9.1.30. [DOI] [PubMed] [Google Scholar]
- Carp J. On the plurality of (methodological) worlds: estimating the analytic flexibility of fMRI experiments. Front. Neurosci. 2012;6:149. doi: 10.3389/fnins.2012.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J. The teenage brain: an overview. Curr. Dir. Psychol. Sci. 2013;22(2):80–81. doi: 10.1177/0963721413480170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., An H., Shen D., Zhu H., Lin W. Tailor the longitudinal anaysis for nih longitudinal normal brain developmental study. 2014 IEEE 11th International Symposium on Biomedical Imaging (ISBI) 2014:1206–1209. doi: 10.1109/ISBI.2014.6868092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F.A. A developmental psychopathology perspective on adolescence. J. Consult. Clin. Psychol. 2002;70(1):6. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Clark D.M., Wells A. A cognitive model of social phobia. Soc. Phobia Diagn. Assess. Treat. 1995;41(68):00022–00023. [Google Scholar]
- Clauss J.A., Blackford J.U. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J. Am. Acad. Child. Adolesc. Psychiatry. 2012;51(10):1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Johnson M.H., Dick F., Cohen Kadosh R., Blakemore S.J. Effects of age, task performance, and structural brain development on face processing. Cereb. Cortex. 2013;23(7):1630–1642. doi: 10.1093/cercor/bhs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Linden D.E.J., Lau J.Y. Plasticity during childhood and adolescence: innovative approaches to investigating neurocognitive development. Dev. Sci. 2013;16(4):574–583. doi: 10.1111/desc.12054. [DOI] [PubMed] [Google Scholar]
- Constans J.I., Penn D.L., Ihen G.H., Hope D.A. Interpretive biases for ambiguous stimuli in social anxiety. Behav. Res. Ther. 1999;37(7):643–651. doi: 10.1016/s0005-7967(98)00180-6. [DOI] [PubMed] [Google Scholar]
- Cox B.J., Fleet C., Stein M.B. Self-criticism and social phobia in the us national comorbidity survey. J. Affect. Disord. 2004;82(2):227–234. doi: 10.1016/j.jad.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Creswell C., Murray L., Cooper P. Interpretation and expectation in childhood anxiety disorders: age effects and social specificity. J. Abnorm. Child Psychol. 2014;42(3):453–465. doi: 10.1007/s10802-013-9795-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann. N.Y. Acad. Sci. 2004;1021(1):1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- David A.S., Blamire A., Breiter H. Functional magnetic resonance imaging: implications for psychology and psychiatry. Br. J. Psychiatry. 1994;164:2–7. doi: 10.1192/bjp.164.1.2. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Foa E.B., Franklin M.E., Perry K.J., Herbert J.D. Cognitive biases in generalized social phobia. J. Abnorm. Psychol. 1996;105(3):433. [PubMed] [Google Scholar]
- Fox N.A. Factors contributing to the emergence of anxiety among behaviorally inhibited children: the role of attention. New Dir. Child Adolesc. Dev. 2010;2010(127):33–49. doi: 10.1002/cd.261. [DOI] [PubMed] [Google Scholar]
- Fox N.C., Ridgway G.R., Schott J.M. Algorithms, atrophy and Alzheimer’s disease: cautionary tales for clinical trials. Neuroimage. 2011;57(1):15–18. doi: 10.1016/j.neuroimage.2011.01.077. [DOI] [PubMed] [Google Scholar]
- Friston K.J. Functional and effective connectivity: a review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- Gamble A.L., Rapee R.M. The time-course of attentional bias in anxious children and adolescents. J. Anxiety Disord. 2009;23(7):841–847. doi: 10.1016/j.janxdis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold A.L., Shechner T., Farber M.J., Spiro C.N., Leibenluft E., Pine D.S., Britton J.C. Amygdala–cortical connectivity: associations with anxiety, development, and threat. Depress. Anxiety. 2016;33(10):917–926. doi: 10.1002/da.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith H.H., Gottesman I. Origins of variation in behavioral style: a longitudinal study of temperament in young twins. Child Dev. 1981:91–103. [PubMed] [Google Scholar]
- Gregory A.M., Caspi A., Moffitt T.E., Koenen K., Eley T.C., Poulton R. Juvenile mental health histories of adults with anxiety disorders. Am. J. Psychiatry. 2007;164(2):301–308. doi: 10.1176/ajp.2007.164.2.301. [DOI] [PubMed] [Google Scholar]
- Gronenschild E.H., Habets P., Jacobs H.I., Mengelers R., Rozendaal N., Van Os J., Marcelis M. The effects of FreeSurfer version, workstation type, and Macintosh operating system version on anatomical volume and cortical thickness measurements. PLoS One. 2012;7(6):e38234. doi: 10.1371/journal.pone.0038234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume B., Hua X., Thompson P.M., Waldorp L., Nichols T.E. Alzheimer’s Disease Neuroimaging Initiative. Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. NeuroImage. 2014;94:287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., McClure‐Tone E.B., Shiffrin N.D., Pine D.S., Nelson E.E. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S.P., Cohen Kadosh K., Lau J.Y.F. A developmental angle to understanding the mechanisms of biased cognitions in social anxiety. Front. Hum. Neurosci. 2014;7:846. doi: 10.3389/fnhum.2013.00846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S.P., Kadosh K.C., Scerif G., Lau J.Y. Social anxiety disorder in adolescence: how developmental cognitive neuroscience findings may shape understanding and interventions for psychopathology. Dev. Cognit. Neurosci. 2015;13:11–20. doi: 10.1016/j.dcn.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee J.E., Benson B.E., Bar-Haim Y., Mogg K., Bradley B.P., Chen G. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol. Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward C., Killen J.D., Kraemer H.C., Taylor C.B. Linking self- reported childhood behavioral inhibition to adolescent social phobia. J. Am. Acad. Child. Adolesc. Psychiatry. 1998;37(12):1308–1316. doi: 10.1097/00004583-199812000-00015. [DOI] [PubMed] [Google Scholar]
- Henderson H.A., Pine D.S., Fox N.A. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40(1):207–224. doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Burghy C.A., Stodola D.E., Fox M.E., Davidson R.J., Essex M.J. Enhanced prefrontal-amygdala connectivity following childhood adversity as a protective mechanism against internalizing in adolescence. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging. 2016;1(4):326–334. doi: 10.1016/j.bpsc.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert J.D., Foa E.B., Furr J.M., Filip J.C., Mathews A. Interpretation bias in social anxiety: a dimensional perspective. Cognit. Ther. Res. 2003;27(5):569–577. [Google Scholar]
- Jarcho J.M., Fox N.A., Pine D.S., Etkin A., Leibenluft E., Shechner T., Ernst M. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol. Psychol. 2013;92(2):306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J.M., Leibenluft E., Walker O.L., Fox N.A., Pine D.S., Nelson E.E. Neuroimaging studies of pediatric social anxiety: paradigms, pitfalls and a new direction for investigating the neural mechanisms. Biol. Mood Anxiety Disord. 2013;3(1):1. doi: 10.1186/2045-5380-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J.M., Davis M.M., Shechner T., Degnan K.A., Henderson H.A., Stoddard J. Early-childhood social reticence predicts brain function in preadolescent youths during distinct forms of peer evaluation. Psychol. Sci. 2016;27(6):821–835. doi: 10.1177/0956797616638319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Jones E.J., Gliga T. Brain adaptation and alternative developmental trajectories. Dev. Psychopathol. 2015;27(2):425–442. doi: 10.1017/S0954579415000073. [DOI] [PubMed] [Google Scholar]
- Kagan J., Reznick J.S., Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987:1459–1473. [PubMed] [Google Scholar]
- Keshavan M.S., Giedd J., Lau J.Y.F., Lewis D.A., Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Angermeyer M., Anthony J.C., Graaf R.on De, Demyttenaere K., Gasquet I. Lifetime prevalence and age-of-onset distributions of mental disordersin the World Health Organization’s World Mental Health SurveyInitiative. World Psychiatry. 2007;6(3):168–176. [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D., Yurgelun-Todd D.A. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- Kircanski K., Joormann J., Gotlib I.H. Attention to emotional information in social anxiety disorder with and without co-occurring depression. Cognit. Ther. Res. 2015;39(2):153–161. [Google Scholar]
- Kujawa A., Wu M., Klumpp H., Pine D.S., Swain J.E., Fitzgerald K.D. Altered development of amygdala-anterior cingulate cortex connectivity in anxious youth and young adults. Biol. Psychiatry Cognit. Neurosci. Neuroimaging. 2016;1(4):345–352. doi: 10.1016/j.bpsc.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny A.P. Children’s behavioral inhibition over age and across situations: genetic similarity for α trait during change. J. Pers. 1989;57(2):215–235. doi: 10.1111/j.1467-6494.1989.tb00481.x. [DOI] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., Parrish J.M., Adler A., Blair R.J.R. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch. Gen. Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miers A.C., Blöte A.W., Bögels S.M., Westenberg P.M. Interpretation bias and social anxiety in adolescents. J. Anxiety Disord. 2008;22(8):1462–1471. doi: 10.1016/j.janxdis.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Tamnes C.K. Methods and considerations for longitudinal structural brain imaging analysis across development. Dev. Cognit. Neurosci. 2014;9:172–190. doi: 10.1016/j.dcn.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cognit. Affect Neurosci. 2014;9(1):123–131. doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., Bradley B.P., Mai X., Louro H.M. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch. Gen. Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata Y., Snyder H.R., Chatham C.H. Developing cognitive control: three key transitions. Curr. Dir. Psychol. Sci. 2012;21(2):71–77. doi: 10.1177/0963721412436807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Chewing the cud and other ruminations. Ruminative Thoughts. 1996;9:135–144. [Google Scholar]
- Nolen-Hoeksema S., Girgus J.S., Seligman M.E. Predictors and consequences of childhood depressive symptoms: a 5-year longitudinal study. J. Abnorm. Psychol. 1992;101(3):405. doi: 10.1037//0021-843x.101.3.405. [DOI] [PubMed] [Google Scholar]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33(46):18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti A.M., Sweeney J.A., Pavuluri M.N. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc. Cognit. Affect. Neurosci. 2009;4:387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P. Evidence-based pragmatic psychiatry—a call to action. JAMA Psychiatry. 2017;74(12):1185–1186. doi: 10.1001/jamapsychiatry.2017.2439. [DOI] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Bar-Haim Y., McDermott J.M., Chronis-Tuscano A., Pine D.S., Fox N.A. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10:349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Reeb-Sutherland B.C., McDermott J.M., White L.K., Henderson H.A., Degnan K.A. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. J. Abnorm. Child Psychol. 2011;39(6):885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine D.S., Helfinstein S.M., Bar-Haim Y., Nelson E., Fox N.A. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology. 2009;34:213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D. How does your cortex grow? J. Neurosci. 2011;31(19):7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J.F., Biederman J., Hirshfeld-Becker D.R., Kagan J., Snidman N., Friedman D. A controlled study of behavioral inhibition in children of parents with panic disorder and depression. Am. J. Psychiatry. 2000;157(12):2002–2010. doi: 10.1176/appi.ajp.157.12.2002. [DOI] [PubMed] [Google Scholar]
- Rothbart M.K., Rueda M.R. The development of effortful control. Developing individuality in the human brain: a tribute to Michael I. Posner. 2005:167–188. [Google Scholar]
- Roy A.K., Vasa R.A., Bruck M., Mogg K., Bradley B.P., Sweeney M. Attention bias toward threat in pediatric anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(10):1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C.E., Snidman N., Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J. Am. Acad. Child. Adolesc. Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Silvers J.A., Lumian D.S., Gabard-Durnam L., Gee D.G., Goff B., Fareri D.S. Previous institutionalization is followed by broader Amygdala–Hippocampal–PFC network connectivity during aversive learning in human development. J. Neurosci. 2016;36(24):6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cognit. Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Olino T.M., Forbes E.E., Dahl R.E. Exciting fear in adolescence: does pubertal development alter threat processing? Dev. Cognit. Neurosci. 2014;8:86–95. doi: 10.1016/j.dcn.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Jarcho J.M., Dahl R.E., Pine D.S., Ernst M., Nelson E.E. Anticipation of peer evaluation in anxious adolescents: divergence in neural activation & maturation. Soc. Cognit. Affect. Neurosci. 2014:nsu165. doi: 10.1093/scan/nsu165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen R.G., Hamer R.M., Lieberman J.A. Measuring brain volume by MR imaging: impact of measurement precision and natural variation on sample size requirements. Am. J. Neuroradiol. 2007;28(6):1119–1125. doi: 10.3174/ajnr.A0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling L.J., Eley T.C., Clark D.M. Preliminary evidence for an association between social anxiety symptoms and avoidance of negative faces in school-age children. J. Clin. Child Adolesc. Psychol. 2006;35(3):431–439. doi: 10.1207/s15374424jccp3503_9. [DOI] [PubMed] [Google Scholar]
- Stopa L., Clark D.M. Social phobia and interpretation of social events. Behav. Res. Ther. 2000;38(3):273–283. doi: 10.1016/s0005-7967(99)00043-1. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Walhovd K.B., Dale A.M., Ostby Y., Grydeland H., Richardson G. Brain development and aging: overlapping and unique patterns of change. NeuroImage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes C.K., Herting M.M., Goddings A.L., Meuwese R., Blakemore S.J., Dahl R.E., Güroğlu B., Raznahan A., Sowell E.R., Crone E.A., Mills K.L. Development of the cerebral cortex across adolescence: a multisample study of interrelated longitudinal changes in cortical volume, surface area and thickness. J. Neurosci. 2017:3302–3316. doi: 10.1523/JNEUROSCI.3302-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb. Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N., Allen N.B., Youssef G., Dennison M., Yücel M., Simmons J.G., Whittle S. Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum. Brain Mapp. 2016;37(6):2027–2038. doi: 10.1002/hbm.23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachinger C., Golland P., Magnain C., Fischl B., Reuter M. Multi-modal robust inverse-consistent linear registration. Hum. Brain Mapp. 2015;36(4):1365–1380. doi: 10.1002/hbm.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Giedd J., Dale A.M., Brown T.T. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb. Cortex. 2016:bhv301. doi: 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.U., Stein M.B., Kessler R.C. Social fears and social phobia in a community sample of adolescents and young adults: prevalence, risk factors and co-morbidity. Psychol. Med. 1999;29(02):309–323. doi: 10.1017/s0033291798008174. [DOI] [PubMed] [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D. Age‐related changes in amygdala–frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 2016;37(5):1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G., Ridgway G.R., Blakemore S.J., Ashburner J., Penny W. Multivariate dynamical modelling of structural change during development. NeuroImage. 2017;147:746–762. doi: 10.1016/j.neuroimage.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]