Highlights
-
•
Hippocampal memory related activation changes throughout childhood and adolescence.
-
•
Pubertal development contributes to brain activations in addition to age.
-
•
Timing of pubertal development moderates brain activation patterns.
-
•
Hippocampal and prefrontal activation show non-linear developmental trajectories.
Keywords: Episodic memory, Puberty, Hippocampus, DLPFC, Testosterone, Longitudinal
Abstract
The current study investigated longitudinal change in hippocampal and prefrontal contribution to episodic retrieval. Functional neuroimaging data were collected during an item-context association memory task for children between the ages of 8 and 14 with individuals scanned 1–3 times over the course of 0.75–3.7 years (Timepoint 1 N = 90; Timepoint 2 N = 83, Timepoint 3 N = 75). We investigated developmental changes in functional activation associated with episodic retrieval (correct item-context > incorrect item-context contrast) and asked whether pubertal changes contributed to developmental changes in pattern of activation. Non-linear developmental trajectories were observed. In the hippocampus, activation decreased with age during childhood and then increased into early adolescence. In the dorsolateral prefrontal cortex, activation was largely absent initially, but quickly accelerated over time. Independent of age, changes in pubertal status additionally predicted increases in item-context activation in initially older children, and decreases in initially younger children across both regions and two indicators of puberty: the Pubertal Development Scale and salivary testosterone. These findings suggest that changes in both age and pubertal status uniquely contribute to memory-related activation, and the timing of pubertal onset may play an important role in the neural mechanisms supporting memory retrieval.
1. Introduction
Episodic memory, or the ability to remember specific information regarding experienced events, develops from early childhood into adolescence (Cycowicz et al., 2001; Ghetti and Lee, 2010; Raj and Bell, 2010). However, the neural mechanisms underlying these developmental improvements are still largely unknown. The current study used longitudinal fMRI methods to begin to fill this gap. We examined developmental trajectories of brain activation supporting memory retrieval and distinguished the role of change in age and pubertal status in this process.
1.1. Development of episodic retrieval and changes in hippocampal and prefrontal activations
The hippocampus supports episodic memory in adults (Diana et al., 2007; Konkel and Cohen, 2009) and a growing literature based on cross-sectional methods shows developmental increases in memory related hippocampal activation in children (DeMaster et al., 2016; Ghetti et al., 2010; Sastre et al., 2016). In some cases, the pattern of results hinted to a non-linear developmental trajectory, such that memory-related activation observed in younger children seemed to be attenuated in older children and re-emerged more strongly later in development (Ghetti et al., 2010). However, cross-sectional sampling may give rise to the appearance of non-linear trajectories. For this reason, longitudinal designs can help determine whether this non-linearity holds when within-individual changes over time are assessed. Furthermore, there is initial cross-sectional evidence of functional differences along the anterior-posterior hippocampal axis such that the hippocampal head may be particularly sensitive to developmental differences in episodic memory (DeMaster and Ghetti, 2013; Schlichting et al., 2017). However, the longitudinal development of hippocampal sub-region function has not been previously examined.
Nevertheless, some studies failed to show age-related differences in hippocampal activation and reported cortical differences primarily in lateral prefrontal regions (Güler and Thomas, 2013; Ofen et al., 2007; Tang et al., 2017). Prefrontal regions play an important role in memory-related attentional processes (Simons and Spiers, 2003), and there is little question that regions such as the dorsolateral prefrontal cortex (DLPFC) are recruited more strongly in adults compared to children (DeMaster and Ghetti, 2013; Ofen et al., 2012; Tang et al., 2017). Although we are primarily interested in hippocampal activation, we examined developmental change in the DLPFC to highlight similarities and differences in developmental trajectories.
Overall, previous studies demonstrate an inconsistent picture about the development of hippocampal function, which may depend on the use of different stimuli, paradigms, and reliance on cross-sectional design. In the current study, we used an accelerated longitudinal design to investigate individual trajectories, including non-linear patterns, and differentiated within person change vs. between person differences. Critically, we examined these age-related developmental changes in the context of pubertal development.
1.2. Pubertal development and changes in hippocampal and prefrontal activation
The examination of age-related changes alone may not fully capture neurodevelopmental processes at the transition into adolescence (Dahl et al., 2018). Based on extensive work on sex hormones in animal models (see Juraska et al., 2013 for a review), recent research in humans has highlighted the important link between puberty and neurodevelopmental outcomes (Herting and Sowell, 2017; Peper et al., 2011). Furthermore, pubertal development occurs later in males compared to females (Shirtcliff et al., 2009), suggesting that sex and timing of pubertal onset may be important factors to consider. Taken together, this research suggests that age-related differences in patterns of activation may be, at least in part, accounted for by pubertal changes.
The hippocampus may be particularly sensitive to pubertal changes given the density of sex steroid hormone receptors observed in this structure in animal models (Sarkey et al., 2008). Research in humans has yielded mixed results. More advanced pubertal development has been associated with smaller hippocampal volumes in both sexes (Neufang et al., 2009), males only (Hu et al., 2013; Satterthwaite et al., 2014), or females only (Bramen et al., 2011). However, the opposite association has also been reported in both sexes (Goddings et al., 2013), males only (Bramen et al., 2011), or females only (Hu et al., 2013).
Some of these discrepancies may be due to differences in the age ranges and measures of puberty examined. Although hormonal, physical, and report measures of puberty are correlated (Shirtcliff et al., 2009), differences are also observed (e.g., Koolschijn et al., 2014). For example, testosterone increases more in adolescent males (Biro et al., 1995) compared to females (Legro, 2000), leading to potentially different brain-testosterone relations in males vs. females. Overall, this research highlights the importance of examining multiple indicators of pubertal development.
These previous studies have mainly focused on volumetric changes and the functional significance of these findings remains largely unknown. Some recent work has examined the association between puberty and hippocampal activation mostly in the context of emotional face processing (Moore et al., 2012; Pagliaccio et al., 2015): pubertal change predicted greater hippocampal activation during early adolescence but not middle childhood, consistent with the idea that timing of pubertal changes may be important (Moore et al., 2012). However, the role of puberty on memory-related hippocampal function is unknown. If pubertal change brings about more precise differentiation of function, then puberty may predict increased memory-related activation in the hippocampus. However, rodent models have shown that pubertal hormones inhibit hippocampal synaptic plasticity and memory performance (Hebbard et al., 2003; Shen et al., 2010), leading to the possibility that pubertal development may lead to functional declines.
Pubertal development has also been associated with decreases in prefrontal volume (Peper et al., 2009) and cortical thickness (Nguyen et al., 2013), and changes in prefrontal function during social (Pfeifer et al., 2013) or emotional processes (Moore et al., 2012). Therefore, we examined whether longitudinal changes and pubertal effects would also extend to in the DLPFC, which is a region sensitive to developmental improvements in memory function (Ofen et al., 2007).
2. Methods
2.1. Design overview
We investigated longitudinal changes in functional activations associated with memory retrieval during childhood and adolescence and its relation to pubertal development. Children ages 8- to 14-years-olds were assessed at up to 3 separate time points. Functional scans were collected while children performed an episodic memory task in which they recalled which of three contexts had been associated previously with target items. Pubertal change was measured using parental report of pubertal status and a salivary assay of testosterone level. We elected to focus on testosterone given its associations with hippocampal structure in humans (Bramen et al., 2011; Neufang et al., 2009) and hippocampal synaptic plasticity in animals (Hebbard et al., 2003). By including both age and pubertal change, we characterized their unique contribution to the functional development of hippocampal sub-regions and prefrontal cortex.
2.2. Participants
Children were recruited from the Sacramento, California region through flyers and were given monetary compensation for their participation. Participants completed informed consent and the study was approved by the University of California, Davis Institutional review board.
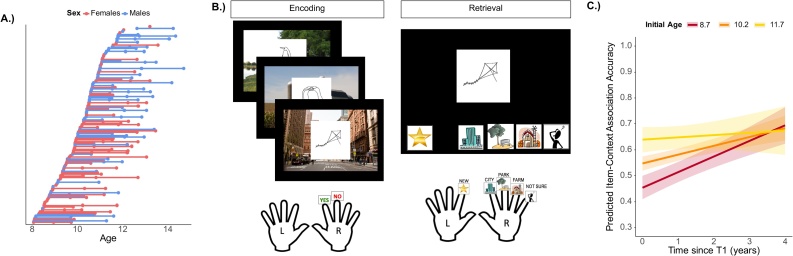
Initial time-point (T1) cross-sectional analyses examining age-related differences are reported in Sastre et al. (2016). T1 included data from 90 children (50 females, range = 8.05–12.0 years, M = 9.92 years, SD = 1.05 years). The second time-point (T2) occurred on average 1.33 years later (range = .76–3.08 years, SD = .49 years) and included 83 children (46 females, range = 8.85–13.0 years, M = 10.9 years, SD = 1.14 years). The third time-point (T3) occurred on average 1.17 years later (range = .49–1.88 years, SD = .40 years) and included 75 children (33 females, range = 9.42–14.7 years, M = 12.0 years, SD = 1.16 years). In total, 248 longitudinal scans were analyzed across 127 unique participants contributing data for one (N = 43), two (N = 47), or three (N = 37) observations occurring over the course of 0.75 to 3.7 years (See Fig. 1a).
Fig. 1.
Sample description, task design, and behavioral results. A.) Participants’ age is plotted at each visit. Individual participants are connected by a horizontal line. B.) During encoding participants viewed a series of black-and-white drawings on one of three background scenes (city, park, farm). During retrieval, participants indicated whether drawings were previously seen by using their right hand and further indicated if the item was presented with a city (pointer finger), park (middle finger), farm (ring finger), or they did not know the associated scene (pinky finger). Participants indicated if a drawing was not previously presented using their left hand (pointer finger) represented by a star. C.) The initial age X time since T1 interaction for the longitudinal model of item-context association accuracy. Results demonstrate that younger children at T1 increase their accuracy more over time than older children at T1. Initial age lines represent median centered scores of -1.5 (8.7 years), 0 (10.2 years), and 1.5 (11.7 years). Bands represent 95% confidence intervals.
All reported participants contributed data from at least 2 of 3 retrieval runs per time-point. Data from an additional 36 observations at T1, 28 observations at T2, and 8 observations at T3 were collected and excluded due to below chance behavioral recognition accuracy (T1 N = 7; T2 N = 4, T3 N = 1), only one retrieval run of the task completed (T1 N = 12; T2 N = 3, T3 N = 1), or excessive head motion leading to less than 2 usable retrieval runs (T1 N = 17; T2 N = 21, T3 N = 6). Excessive head motion was defined as more than 25% interpolated volumes per run (See fMRI Data Analysis).
2.3. Task procedure
Data were collected as part of a larger project investigating the development of hippocampal contribution on episodic memory. MRI data were collected at the UC Davis Imaging Research Center in Sacramento, California. Participants first completed a short training protocol using a mock scanner followed by 3 scanned study/test cycles of an item-context association memory task (See Fig. 1b).
During each encoding run, participants were shown 48 item-scene pairs composed of a black and white line drawing of an object or animal (Cycowicz et al., 1997) superimposed on one of three scenes (i.e., city, park, or farm). Participants were instructed to remember the item-context associations for an upcoming memory test. To facilitate encoding children indicated whether the drawing belonged to the corresponding scene.
During each retrieval run, participants completed an item-context association task on 48 studied items and 16 new items presented on a black background. Participants responded with their right hand to indicate that the drawing had been previously seen and via specific fingers to indicate which scene the drawing had been seen with previously (i.e., city, park, or farm using pointer, middle, index finger respectively) or if they recognized the item but did not know which scene it went with (indicated by an uncertain cartoon character using the pinky finger). When participants did not recognize the item, they responded with their left hand and pressed the “new” button (indicated by a star). Each drawing was presented for 4000 ms followed by a jitter fixation period ranging from (500–8000 ms). Only neuroimaging data during retrieval scans were considered for the purposes of this study.
2.4. Behavioral analysis
We calculated item-context associations as the number of studied items with the correctly identified background divided by the total number of correctly identified studied items, as commonly done in memory research (e.g., Cycowicz et al., 2001; Demaster et al., 2013).
2.5. fMRI data acquisition
Data were collected with a Siemens 3 T MRI scanner using a 32-channel head coil. Functional data were acquired using a gradient EPI sequence with repetition time (TR) = 2000 ms, echo time (TE) = 23 ms, no interslice gap, flip angle = 90◦, field of view (FOV) = 204 mm, 37 slices per volume, and voxel size = 3 mm isotropic. For coregistration of functional images, a T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) was acquired (TR = 2500 ms, TE = 3.24 ms, FOV = 224 mm, voxel size = 0.7 mm isotropic).
2.6. fMRI data analysis
Data were analyzed using SPM8 (www.fil.ion.ucl.ac.uk/spm). Preprocessing included slice timing correction, rigid body motion correction aligned to the first volume with sinc interpolation, coregistration to the MPRAGE, spatial normalization using T1 template in SPM, and spatial smoothing using a 6-mm full-width half-maximum isotropic Gaussian kernel. Subject motion was corrected such that volumes with >1 mm motion or >2% signal change were interpolated with values using ArtRepair (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html). Runs with more than 25% of volumes replaced were excluded. We excluded participants who contributed fewer than 2 runs.
General-linear-modeling was used to estimate activations during correct item-context (i.e., item correctly identified with context background) and incorrect item-context (i.e., item correctly identified as previously seen, but chose incorrect context background or “Not Sure” background option) conditions. Incorrect responses and correct identifications of new items were also modeled, but not examined in the current analyses. Events were convolved using a canonical hemodynamic response function and modeled using the duration from trial onset to participants’ response. The model also included a session effect, general linear trend, and motion parameters as covariates of non-interest. The contrast of interest was the item-context association defined as correct item-context > incorrect item-context. The average number of correct item-context trials submitted to analysis was M(SD) = 54(25) at T1, M(SD) = 68(25) at T2, and M(SD) = 75(22) at T3. The average number of incorrect item-context trials was M(SD) = 48(19) at T1, M(SD) = 44(15) at T2, and M(SD) = 44(15) at T3.
Regions of interest (ROI) analyses were conducted using Marsbar (http://marsbar.sourceforge.net/). A-priori ROIs included AAL maps of left and right hippocampus, further divided into head, body, and tail using previously defined cutoffs in MNI space (DeMaster and Ghetti, 2013; Sastre et al., 2016) such that the head was separated from the body using a plane at y=-20 for the left side and y=-18 for the right side, and the body was separated from tail using a plane at y=-36 for the left side and y=-34 for the right side.
We additionally selected sub-regions in the DPLFC. The DLPFC ROIs were selected to be similar in size to the hippocampal regions and defined as 8 mm spheres around the center of AAL maps for the middle frontal gyrus (Right: 37, 32, 33; Left: -34, 32, 34). Mean parameter estimates were determined by averaging signal across voxels within each ROI for the target contrast (i.e., correct item-context > incorrect item-context). Parameter estimates more than 3.29 standard deviation units from the mean (i.e., p < .001 two-tailed) for each region of interest were excluded from the reported analyses (<1.7% of observations). The overall pattern of results was similar when including these outliers.
2.7. Puberty and hormone data acquisition
Pubertal status was measured with two indicators including parent report of physical markers using the Puberty Developmental Scale (PDS) (Petersen et al., 1988) and testosterone levels collected via saliva samples. Saliva samples were collected through passive drool at home or in the lab, depending on the time of day the fMRI session was scheduled. Children were instructed to chew gum for 30 s in order to increase saliva production before filling the test vial. Saliva samples were collected during the morning (M = 8.52 h, SD=1.41 h) and/or afternoon (M = 15.5 h, SD=1.97 h), with 182 observations including both samples (T1 = 65, T2 = 60, T3 = 57), 11 observations including only a morning sample (T1 = 4, T2 = 1, T3 = 6), and 40 observations including only an afternoon sample (T1 = 14, T2 = 14, T3 = 12). Morning samples were instructed to be taken immediately after waking and before eating or drinking.
Prior to assay, samples were centrifuged at 3000 rpm for 20 min to separate the aqueous component from mucins and other suspended particles. Salivary concentrations of testosterone were estimated in duplicate using the Expanded Range Salivary Testosterone EIA Kit (Salimetrics LLC, State College, PA). Intra- and inter-assay coefficients of variation are 4.97% and 5.71%, respectively and assay sensitivity is 1.0 pg/mL. Testosterone levels were correlated across morning and afternoon samples (Testosterone: T1 r = .61, T2 r = .74, T3 r = .57, ps<.001) and were therefore averaged when two samples were available. Estradiol and DHEAS were also extracted from salivary samples, but we focus on testosterone for the reasons described in the Introduction.
2.8. Statistical analyses
We first investigated age-related changes in behavioral item-context accuracy. We next examined pubertal development separately for our two indicators of puberty including PDS and testosterone. Finally, age and pubertal related changes were assessed for item-context accuracy and item-context activation (i.e., correct > incorrect item-context associations) for both hippocampus and DLPFC separately.
Longitudinal data analyses were conducted with multilevel modeling (MLM) using R and package nlme (Pinheiro et al., 2018). MLM is robust to missing cases and can account for different intervals between time points (Shaw et al., 2008; Verbeke, 1997). Longitudinal models were specified in a similar manner to previous research using this sample (Fandakova et al., 2017). The outcome of interest, , was modeled with following equations:
Level 1:
Level 2:
The level 1 model included a random intercept, (predicted score at initial time point for individual i), random slope, (predicted rate of change for individual i), (time in years since initial assessment for individual i), and time-dependent residual, . The random slope was included when it significantly increased fit via the log-likelihood ratio test relative to a random intercept only model. The level 2 model included , which was centered around the median age of the sample at the initial time point (10.2 years) in order to increase the interpretation of (predicted score for a median aged child at the initial time point). Regression coefficient accounts for cross-sectional differences in the intercept. While we refer to the initial observation as T1, note that due to exclusions (See Participants), for some participants the initial observation in the regression model may have occurred during their second (N = 27) or third (N = 10) visit.
We used a model building procedure that separately tested effects of interest in a hierarchical fashion. For behavioral and puberty data analyses, the first model included main effects of initial age, time since T1 in years, and sex. The second model included the quadratic effect of time to test for non-linear relationships using orthogonal polynomials, which help stabilize estimates by using uncorrelated regressors (Little et al., 2006). The remaining models included the following interactions: 1) initial age by time since T1, to determine whether the change over time depended on initial age, 2) sex by initial age, and 3) sex by time since T1, to determine if there were any sex differences. Each model was tested sequentially using log-likelihood ratio tests, and interactions were only included if they significantly increased the fit relative to the previously best fit model.
For fMRI data analysis, our first model included main effects of initial age, time since T1 in years, sex, hemisphere, initial puberty, and change in puberty since T1. Modeling of hippocampal data included an additional main effect of sub-region (head, body, vs. tail). The second model included the quadratic effect of time using orthogonal polynomials to test for non-linear developmental changes, as suggested by previous cross-sectional research (Ghetti et al., 2010). In order to limit the number of interactions tested, our remaining models only tested the following interactions starting with 1) initial age by time since T1 to determine whether activation changes over time depended on initial age, 2.) sex by initial puberty, and 3) sex by change in puberty to determine if the effects of pubertal change on activation depended on sex, 4) initial age by change in puberty, which reflects the timing of changes in puberty and determines whether the relation between activation and longitudinal changes in puberty depended on initial age, and 5) initial age by change in puberty by sex (and related lower order interaction) to further determine if longitudinal changes in puberty by initial age depended on sex.
Each model was tested sequentially using log-likelihood ratio tests, and interactions were only included if they significantly increased fit relative to the previously best fit model. For each region, two separate models were tested, where either PDS score or testosterone was used as an indicator of puberty to assess whether similar patterns would emerge across different measures. The best fit models were estimated for the hippocampus and these models were then applied to DLPFC in order to assess whether similar effects would be observed prefrontal regions. Tables and figures demonstrate the final models and include interactions terms that significantly increased fit.
3. Results
3.1. Developmental change in memory for item-context associations
Longitudinal analysis of item-context association accuracy revealed a significant effect of initial age b = .06, p < .001 and time, b=.03, p < .001, indicating that older children were more accurate than younger children at T1, as reported in Sastre et al. (2016), and improvements were also observed over time. However, the interaction between initial age and time was also significant, b=-.02, p = .009, indicating that younger children at T1 showed greater improvements over time than older children who remained relatively stable in performance (Fig. 1c; Table 1). These results confirm typical age-related improvements in memory retrieval.
Table 1.
Longitudinal Model Results for Item-Context Associations, PDS, and Testosterone.
| Item-Context Associations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI |
|||||||||
| SD | b | SE | df | T | p | Lower | Upper | ||
| Random Effect | |||||||||
| Intercept | 0.14 | 0.12 | 0.17 | ||||||
| Time | 0.04 | 0.03 | 0.06 | ||||||
| Fixed Effect | |||||||||
| Intercept | 0.55 | 0.02 | 124 | 30.09 | <0.001 | *** | 0.51 | 0.59 | |
| Initial Age | 0.06 | 0.01 | 124 | 4.76 | <0.001 | *** | 0.04 | 0.09 | |
| Time | 0.03 | 0.01 | 119 | 5.00 | <0.001 | *** | 0.02 | 0.05 | |
| Gender | −0.01 | 0.02 | 124 | −0.36 | 0.72 | −0.06 | 0.04 | ||
| Initial Age*Time | −0.02 | 0.01 | 119 | −2.64 | 0.01 | ** | −0.03 | 0.00 | |
| PDS | |||||||||
| SD | b | SE | df | T | p | Lower | Upper | ||
| Random Effect | |||||||||
| Intercept | 0.13 | 0.26 | 0.40 | ||||||
| Time | 0.26 | 0.07 | 0.22 | ||||||
| Fixed Effect | |||||||||
| Intercept | 1.63 | 0.05 | 122 | 31.58 | <0.001 | *** | 1.53 | 1.73 | |
| Initial Age | 0.12 | 0.03 | 122 | 3.45 | <0.001 | *** | 0.05 | 0.19 | |
| Time | 0.50 | 0.04 | 115 | 13.33 | <0.001 | *** | 0.42 | 0.57 | |
| Gender | −0.41 | 0.07 | 122 | −5.56 | <0.001 | *** | −0.56 | −0.26 | |
| Initial Age*Time | 0.11 | 0.03 | 115 | 4.38 | <0.001 | *** | 0.06 | 0.16 | |
| Gender*Time | −0.21 | 0.05 | 115 | −4.17 | <0.001 | *** | −0.32 | −0.11 | |
| Testosterone | |||||||||
| SD | b | SE | df | T | p | Lower | Upper | ||
| Random Effect | |||||||||
| Intercept | 9.40 | 6.50 | 13.58 | ||||||
| Fixed Effect | |||||||||
| Intercept | 34.50 | 2.30 | 122 | 14.96 | <0.001 | *** | 29.92 | 39.05 | |
| Initial Age | 4.72 | 1.54 | 122 | 3.07 | 0.003 | ** | 1.68 | 7.76 | |
| Time | 6.55 | 1.65 | 105 | 3.97 | <0.001 | *** | 3.28 | 9.84 | |
| Gender | −4.62 | 3.36 | 122 | −1.38 | 0.17 | −11.27 | 2.02 | ||
| Initial Age*Time | 3.85 | 1.10 | 105 | 3.51 | <0.001 | *** | 1.67 | 6.03 | |
| Gender*Time | 7.03 | 2.23 | 105 | 3.15 | 0.002 | ** | 2.61 | 11.45 | |
3.2. Pubertal development
Cross-sectional assessments across our two assessments of pubertal development showed convergence. Each time point showed positive correlations between PDS and testosterone for both males (T1 r = .40, p = .02; T2 r = .55, p = .001; T3 r = .61, p < .001) and females (T1 r = .45, p = .002; T2 r = .39, p = .01; T3 r = .28, p = .11). Correlations were often only moderately strong, further justifying our decision to assess these measures separately (see Supplementary Results for additional cross-sectional correlations).
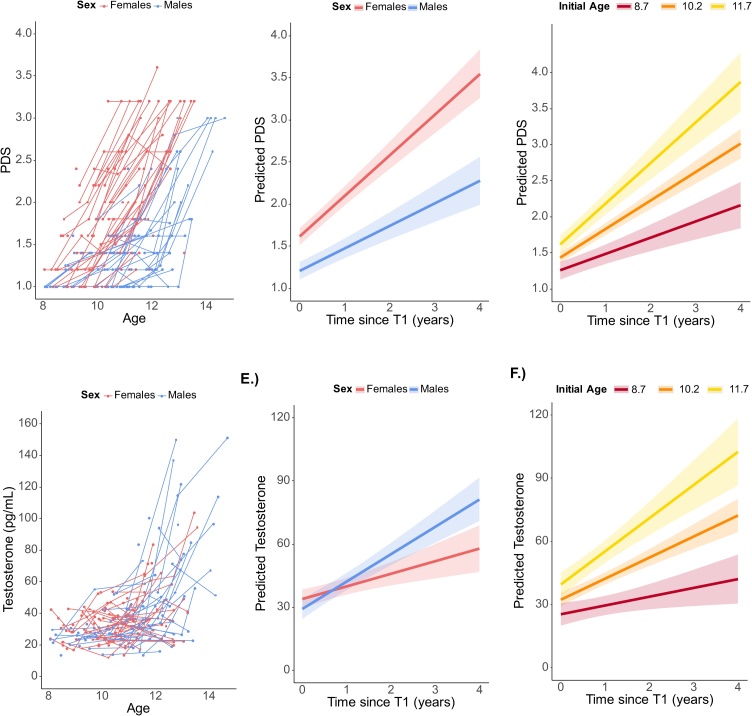
Longitudinal pubertal change was tested separately for PDS scores and testosterone (Table 1). PDS scores showed a significant interaction between initial age and time, b = .11, p < .001, indicating that those children who were older at T1 showed the greatest increase in PDS scores over time (Fig. 2b). Additionally, the time X sex interaction was significant, b=-.21, p < .001, such that longitudinal increases in PDS were greater in females than males (Fig. 2c).
Fig. 2.
Longitudinal change in PDS (top panels) and testosterone (bottom panels) A.) PDS scores plotted for each subject as a function of age for each time point. B.) Longitudinal model results of the sex X time since T1 interaction showing that females PDS scores increased more over time than males. C.) Longitudinal model results of the initial age X time since T1 interaction showing that PDS scores increased more for children who were older at T1. D.) Testosterone plotted for each subject as a function of age for each time point. E.) Longitudinal model results of the sex X time since T1 interaction showing that testosterone increased more over time for males than females. F.) Longitudinal model results of the initial age X time since T1 interaction showing that testosterone increased more for children who were older at T1. Initial age lines represent median centered scores of -1.5 (8.7 years), 0 (10.2 years), and 1.5 (11.7 years). Bands represent 95% confidence intervals.
Testosterone also showed a significant interaction between initial age and time, b = 3.85, p < .001, such that those children who were older at T1 showed greater increases in salivary testosterone over time (Fig. 2e). Furthermore, the time X sex interaction was significant, b = 7.03, p = .002, such that longitudinal increases in testosterone were greater in males than females, as expected (Fig. 2f).
Longitudinal models showed typical sex differences, such that physical characteristics of puberty were higher and increased more steeply over time in females, while testosterone levels were higher and increased more steeply over time in males.
3.3. Relation between pubertal development and memory for item-context associations
We investigated whether pubertal change was associated with behavioral memory performance above and beyond age-related differences. We added the following predictors to the final item-context association model in a hierarchical fashion separately for PDS and testosterone: 1) initial puberty and change in puberty to assess the main effects of puberty and puberty change, 2) initial puberty by sex and change in puberty by sex interactions to further test if the relations between puberty and behavior differed based on sex, and 3) initial age by change in puberty interaction to test if the effects of puberty change on behavior depended on initial age.
Neither initial PDS or change in PDS were significant predictors, ps>.22. Adding the PDS by sex interactions, p = .21, or the initial age by change in PDS interaction did not significantly improve model fit, p = .52. Similar results were observed with testosterone: neither initial testosterone or change in testosterone significantly predicted behavior, ps>.46. Adding the testosterone by sex interactions, p = .84, or the initial age by change in testosterone interaction did not significantly increase fit, p = .54. Thus, pubertal development was not associated with behavioral changes in memory performance beyond age.
3.4. fMRI results
3.4.1. Developmental change in hippocampal activation
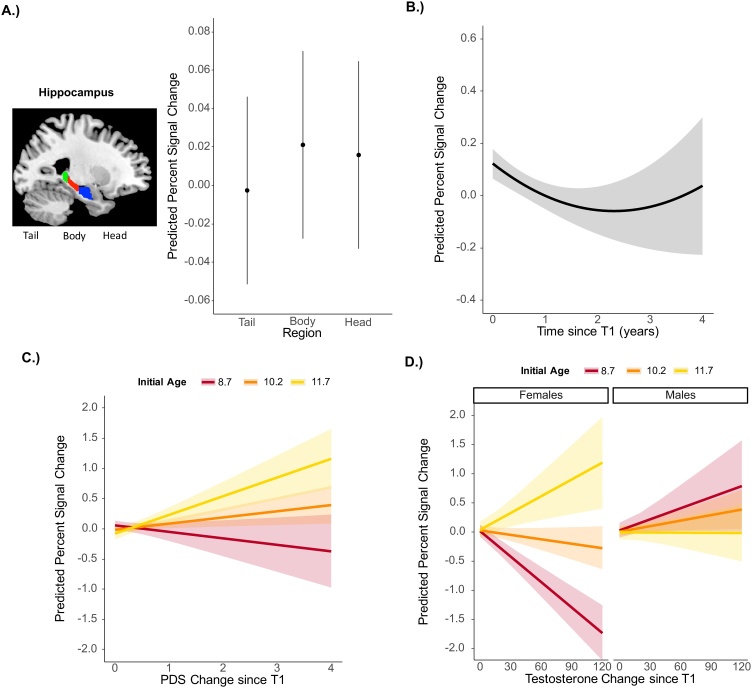
Our main interest was to examine how longitudinal changes in age and puberty predict hippocampal function during episodic retrieval (i.e., correct > incorrect item-context associations). We examined longitudinal models based on the model building procedure described in the Materials and Methods section. The final models are reported in Table 2 and Fig. 3. We found both linear, b=-2.72, p = .03, and quadratic, b=1.24, p < .001, effects of time such that hippocampal activation followed a U-shape pattern with a small initial decrease followed by increases in activation over time (Fig. 3b). Critically, changes in puberty uniquely predicted hippocampal activation as demonstrated by an initial age X change in PDS interaction, b = .14, p < .001; children who were older at T1 showed a positive relation between change in PDS over time and hippocampal activation whereas children younger at T1 showed a weak negative relation (Fig. 3c; Supplementary Fig. S1a). We also included item-context association accuracy at T1 and change in item-context association accuracy over time in order to investigate whether changes in performance influence activation levels or our pattern of results more generally. Change in item-context accuracy was significant, b = .15, p < .001, suggesting that activation was positively related to task performance improvements over time. T1 item-context accuracy was not significant, p = .32. Critically, all our effects of interest held when including these additional predictors (linear time, b=-3.75, p=.003; quadratic time, b=1.37, p<.001; initial age X change in PDS, b=.18, p<.001). Thus, changes since T1 in age and PDS both uniquely contributed to hippocampal activation and this pattern persisted even when we accounted for improvements in behavioral accuracy. Finally, there was a main effect of region, such that the hippocampal tail showed significantly lower activation than the body, b=-.024, p = .04 across time points; the tail, b=-.018, p=.12, or body, b=-.005, p = .66 did not significantly differ from the head (Fig. 3a).
Table 2.
Longitudinal Model Results for Hippocampal Item-Context Association Activation.
| Hippocampus | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI |
|||||||||
| SD | B | SE | df | t | p | Lower | Upper | ||
| PDS | |||||||||
| Random Effect | |||||||||
| Intercept | 0.281 | 0.245 | 0.322 | ||||||
| Time | 0.255 | 0.213 | 0.304 | ||||||
| Fixed Effect | |||||||||
| Intercept | −0.005 | 0.038 | 1294 | −0.13 | 0.90 | −0.080 | 0.070 | ||
| Initial Age | −0.047 | 0.023 | 120 | −2.06 | 0.04 | * | −0.091 | −0.002 | |
| region:Body | 0.024 | 0.012 | 1294 | 2.01 | 0.04 | * | 0.001 | 0.047 | |
| region:Head | 0.018 | 0.012 | 1294 | 1.56 | 0.12 | −0.005 | 0.041 | ||
| Time | −2.719 | 1.225 | 1294 | −2.22 | 0.03 | * | −5.123 | −0.315 | |
| Time2 | 1.241 | 0.345 | 1294 | 3.59 | <0.001 | *** | 0.564 | 1.919 | |
| Gender | 0.011 | 0.049 | 120 | 0.22 | 0.83 | −0.087 | 0.108 | ||
| Hemisphere | 0.001 | 0.010 | 1294 | 0.13 | 0.89 | −0.018 | 0.020 | ||
| Initial PDS | 0.018 | 0.056 | 120 | 0.33 | 0.74 | −0.092 | 0.128 | ||
| Change in PDS | 0.102 | 0.041 | 1294 | 2.48 | 0.01 | * | 0.021 | 0.184 | |
| Initial Age*Change in PDS | 0.139 | 0.040 | 1294 | 3.47 | <0.001 | *** | 0.060 | 0.218 | |
| Testosterone | |||||||||
| Random Effect | |||||||||
| Intercept | 0.289 | 0.252 | 0.332 | ||||||
| Time | 0.287 | 0.240 | 0.344 | ||||||
| Fixed Effect | |||||||||
| Intercept | 0.029 | 0.038 | 1190 | 0.76 | 0.45 | −0.045 | 0.103 | ||
| Initial Age | 0.005 | 0.033 | 114 | 0.15 | 0.88 | −0.061 | 0.071 | ||
| region:Body | 0.017 | 0.011 | 1190 | 1.54 | 0.12 | −0.005 | 0.040 | ||
| region:Head | 0.014 | 0.011 | 1190 | 1.22 | 0.22 | −0.008 | 0.036 | ||
| Time | −1.899 | 1.243 | 1190 | −1.53 | 0.13 | −4.339 | 0.541 | ||
| Time2 | 0.703 | 0.334 | 1190 | 2.11 | 0.04 | * | 0.048 | 1.357 | |
| Gender | −0.018 | 0.049 | 114 | −0.38 | 0.71 | −0.114 | 0.078 | ||
| Hemisphere | 0.004 | 0.009 | 1190 | 0.47 | 0.64 | −0.014 | 0.022 | ||
| Initial Testosterone | 0.000 | 0.002 | 114 | −0.20 | 0.85 | −0.004 | 0.003 | ||
| Change in Testosterone | −0.003 | 0.002 | 1190 | −1.61 | 0.11 | −0.006 | 0.001 | ||
| Gender * Change in Testosterone | 0.006 | 0.002 | 1190 | 2.65 | 0.01 | ** | 0.001 | 0.010 | |
| Initial Age*Change in Testosterone | 0.008 | 0.001 | 1190 | 5.39 | <0.001 | *** | 0.005 | 0.011 | |
| Initial Age * Gender | −0.019 | 0.044 | 114 | −0.42 | 0.67 | −0.107 | 0.069 | ||
| Initial Age* Gender * Change in Testosterone | −0.010 | 0.002 | 1190 | −4.60 | <0.001 | *** | −0.015 | −0.006 | |
Fig. 3.
Longitudinal Changes in Hippocampal Activation. Predicted hippocampal activation from the longitudinal model using PDS as an indicator of puberty showing A.) main effect of region: there was lower activation in the tail subregion relative to head subregion. Points represent average predicted activation and horizontal lines represent 95% confidence intervals B.) quadratic effect of time: activation initially decreased followed by an upward trajectory over time C.) initial age X change in PDS interaction: for initially older children, activation increased as PDS increased over time, while for initially younger children, activation decreased as PDS increased over time. D.) Predicted hippocampal activation from the longitudinal model using testosterone as an indicator of puberty showing the initial age X sex X change in testosterone interaction: for initially older females, activation increased as testosterone increased over time, while for initially younger females, activation decreased as testosterone increased over time. For males, the relation between activation and testosterone over time did not depend on initial age. Initial age lines represent median centered scores of -1.5 (8.7 years), 0 (10.2 years), and 1.5 (11.7 years). Bands represent 95% confidence intervals.
Given the significant main effect of region, we further investigated whether hippocampal region interacted with time and pubertal change since T1. However, adding the region by linear and quadratic time interaction did not significantly increase fit, p = .72, and neither did adding the region by change in PDS interaction, p = .61. Thus, we did not find any evidence of regional differences in age and pubertal change over time.
In addition, we tested whether a similar pattern of puberty-related findings would emerge if we used testosterone as an indicator of puberty instead of PDS scores using the same model building procedure for interactions as described in the methods section. We found a significant 3-way interaction between change in testosterone, initial age, and sex, b=-.010, p < .001. Similar to the PDS findings, older children at T1 showed a positive relation between change in testosterone over time and hippocampal activation while younger children at T1 showed a negative relation; however, this effect was only present in females (Fig. 3d; Supplementary Fig. S1b). This finding was confirmed when testing males and females separately, such that the interaction between initial age and change in testosterone was significant in females, b = .008, p < .001, but not males, b=-.002, p = .18. The 3-way interaction remained significant including item-context association accuracy at initial assessment and change in accuracy since T1, b=-.008, p < .001. Additionally, our findings remained unvaried when we included motion (i.e., number of repaired volumes), answering style (i.e., rate of not sure responses), and total number of trials as covariates in both PDS and testosterone models (see Supplementary Results).
Thus, although PDS scores and testosterone gave largely similar results, they were not identical. To confirm that these two indicators of puberty explained unique variance, we added both indicators of puberty in the same model. We confirmed that the 2-way interaction between change in PDS and initial age, b = .17, p < .001 and the 3-way interaction between change in testosterone, initial age, and sex, b=-.013, p < .001, remained significant, suggesting that both indicators of puberty uniquely contribute to hippocampal activation.
Overall, changes in puberty predicted increases in hippocampal activation when measured both through PDS scores as well as testosterone in females. Critically, the pattern observed with puberty depended on initial age, such that increasing puberty over time was associated with increases in activation for older children and slight declines for younger children. In addition, changes in age contributed to the development of hippocampal function. Longitudinal changes in hippocampal activation followed a non-linear trajectory with declines in activation during middle childhood and an upward trajectory beginning to emerge between late childhood and early adolescence.
3.4.2. Developmental change in DLPFC activation
We examined whether the developmental trajectory of activation during episodic retrieval (i.e., correct > incorrect item-context associations) and the contribution of pubertal development would extend to the DLPFC. We therefore applied the final model reported for the hippocampus to the DLPFC, except that we did not include region as a factor (i.e., head, body, tail). Results are reported in Table 3 and Fig. 4.
Table 3.
Longitudinal Model Results for DLPFC Item-Context Association Activation.
| DLPFC | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI |
|||||||||
| SD | b | SE | df | t | P | Lower | Upper | ||
| PDS | |||||||||
| Random Effect | |||||||||
| Intercept | 0.338 | 0.290 | 0.394 | ||||||
| Time | 0.234 | 0.188 | 0.292 | ||||||
| Fixed Effect | |||||||||
| Intercept | −0.014 | 0.054 | 337 | −0.26 | 0.80 | −0.120 | 0.092 | ||
| Initial Age | −0.013 | 0.031 | 120 | −0.43 | 0.67 | −0.074 | 0.048 | ||
| Time | −0.515 | 0.869 | 337 | −0.59 | 0.55 | −2.225 | 1.195 | ||
| Time2 | 2.057 | 0.362 | 337 | 5.68 | <0.001 | *** | 1.345 | 2.769 | |
| Gender | −0.115 | 0.068 | 120 | −1.70 | 0.09 | −0.249 | 0.019 | ||
| Hemisphere | −0.005 | 0.019 | 337 | −0.25 | 0.80 | −0.043 | 0.033 | ||
| Initial PDS | −0.100 | 0.076 | 120 | −1.31 | 0.19 | −0.250 | 0.051 | ||
| Change in PDS | 0.108 | 0.068 | 337 | 1.59 | 0.11 | −0.026 | 0.243 | ||
| Initial Age*Change in PDS | 0.061 | 0.055 | 337 | 1.09 | 0.28 | −0.049 | 0.170 | ||
| Testosterone | |||||||||
| Random Effect | |||||||||
| Intercept | 0.345 | 0.296 | 0.402 | ||||||
| Time | 0.210 | 0.164 | 0.268 | ||||||
| Fixed Effect | |||||||||
| Intercept | 0.017 | 0.047 | 307 | 0.36 | 0.72 | −0.075 | 0.109 | ||
| Initial Age | −0.002 | 0.043 | 114 | −0.05 | 0.96 | −0.087 | 0.083 | ||
| Time | −0.388 | 0.654 | 307 | −0.59 | 0.55 | −1.675 | 0.899 | ||
| Time2 | 1.485 | 0.351 | 307 | 4.23 | <0.001 | *** | 0.795 | 2.176 | |
| Gender | −0.135 | 0.063 | 114 | −2.14 | 0.03 | * | −0.261 | −0.010 | |
| Hemisphere | 0.001 | 0.020 | 307 | 0.06 | 0.95 | −0.037 | 0.040 | ||
| Initial Testosterone | −0.003 | 0.002 | 114 | −1.29 | 0.20 | −0.008 | 0.002 | ||
| Change in Testosterone | −0.003 | 0.003 | 307 | −1.12 | 0.26 | −0.008 | 0.002 | ||
| Gender * Change in Testosterone | 0.009 | 0.003 | 307 | 2.63 | 0.01 | ** | 0.002 | 0.015 | |
| Initial Age*Change in Testosterone | 0.005 | 0.003 | 307 | 1.89 | 0.06 | . | 0.000 | 0.010 | |
| Initial Age * Gender | −0.004 | 0.057 | 114 | −0.08 | 0.94 | −0.118 | 0.109 | ||
| Initial Age* Gender * Change in Testosterone | −0.008 | 0.004 | 307 | −2.19 | 0.03 | * | −0.015 | −0.001 | |
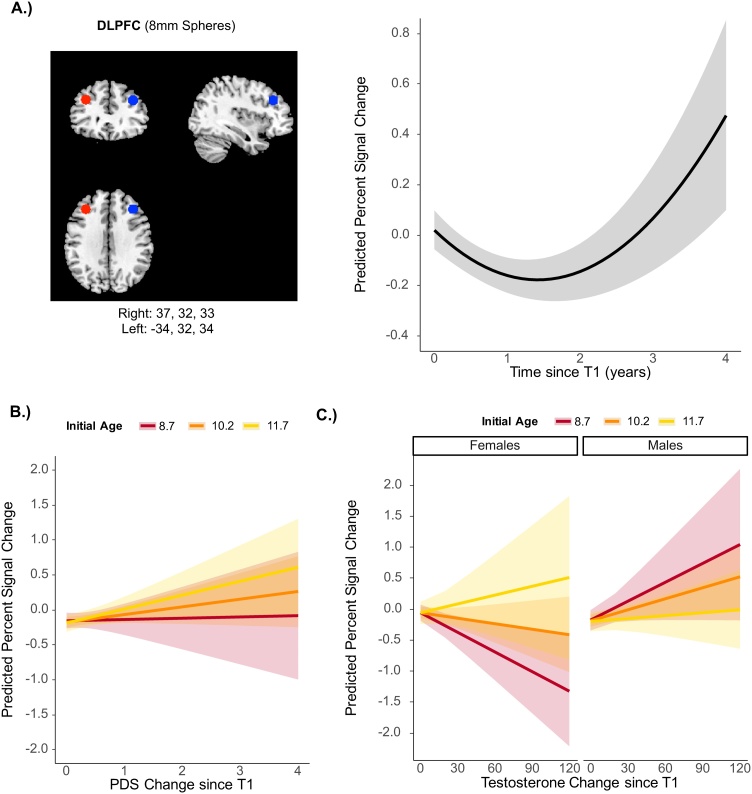
Fig. 4.
Longitudinal Changes in DLPFC Activation. Predicted DLPFC activation from the longitudinal model using PDS as an indicator of puberty showing A.) significant quadratic effect of time: activation was not present initially but followed an upward trajectory over time B.) non-significant initial age X change in PDS interaction. C.) Predicted DLPFC activation from the longitudinal model using testosterone as an indicator of puberty showing a significant initial age X sex X change in testosterone interaction: for initially older females, activation increased as testosterone increased over time, while for initially younger females, activation decreased as testosterone increased over time. For males, the relation between activation and testosterone over time did not depend on initial age. Initial age lines represent median centered scores of -1.5 (8.7 years), 0 (10.2 years), and 1.5 (11.7 years). Bands represent 95% confidence intervals.
There was a significant quadratic effect of time, b = 2.06, p < .001, such that DLPFC activation was not present initially but rapidly increased later in development (Fig. 4a). No other main effects were significant, ps>.09, and the initial age X change in PDS interaction was also not significant, b = .06, p = .28, despite a pattern that was numerically similar to that of the hippocampus (i.e., children who were older at T1 showed a more positive relation between activity and change in PDS over time) (Fig. 4b; Supplementary Fig. S2a). When we added item-context association accuracy, we found that change in item-context accuracy was significant, b = .11, p = .001, such that activation increased with improvements in accuracy across time. T1 item-context accuracy wat not significant, b=.01, p = .94. The quadratic effect of time remained significant, b=2.16, p<.001, and the initial age X change in PDS interaction approached significance, b=.11, p = .07 when we included these additional predictors.
When we assessed testosterone as an indicator of puberty, we found a significant 3-way interaction between change in testosterone, initial age, and sex, b=-0.008, p = .03. Similar to the hippocampus, older females at T1 had a positive relation between change in testosterone over time and DLPFC activation while younger females at T1 had a negative relation (Fig. 4c; Supplementary Fig. S2b). This interaction remained significant when controlling for item-context associations, b = .007, p = .05. When testing males and females separately, the interaction between initial age X change in testosterone was significant in females, b=.005, p = .05 but not males b=-.002, p = .36. Finally, our findings remained the same when we included additional covariates including motion (i.e., number of repaired volumes), answering style (i.e., rate of not sure responses), and total number of trials (See Supplementary Results).
Overall, longitudinal change in DLPFC function was also affected by changes in both age and puberty. Activation in DLFPC followed a non-linear trajectory such that item-context activation was near 0 at initial assessment but showed rapid increases during middle childhood and early adolescence. Furthermore, changes in puberty showed a similar pattern as the hippocampus in females when measured with testosterone.
4. Discussion
The ability to remember specific contextual details associated with experienced events improves throughout childhood and adolescence (Ghetti and Angelini, 2008; Ofen et al., 2007). However, little is known about functional changes in the neural substrates underlying these improvements. While the hippocampus is known to support memory retrieval in adults (Rugg et al., 2012), only a handful of cross-sectional studies have investigated hippocampal activation during memory retrieval in children (DeMaster et al., 2013, 2016; DeMaster and Ghetti, 2013; Güler and Thomas, 2013; Ofen et al., 2012; Paz-Alonso et al., 2008). Furthermore, changes during adolescence occur in the context of pubertal development, and recent work has begun to link this transition to neurodevelopmental changes in humans (Herting and Sowell, 2017). In the current study, we investigated longitudinal changes in hippocampal and prefrontal activation during memory retrieval and asked whether pubertal development could explain, at least in part, these trajectories.
4.1. The role of puberty in memory-related activation
Pubertal development predicted changes in activation above and beyond age. Pubertal investigations in humans have yielded discrepant results regarding changes in hippocampal structure (Bramen et al., 2011; Goddings et al., 2013; Koolschijn et al., 2014). Hippocampal function has rarely been explored and only in non-memory contexts (Moore et al., 2012; Pagliaccio et al., 2015); to our knowledge hippocampal memory-related function and pubertal development have not been previously examined in children. We found a positive relation between pubertal development and changes in hippocampal activation, but only for children who were older at the initial assessment; younger children at the initial assessment tended to show a negative relation, underscoring the importance of timing of pubertal change.
The timing of puberty has been hypothesized to be an important factor during adolescent development (Belsky et al., 1991) and early or non-normative pubertal development has been associated with negative behavioral and psychological outcomes (Kaltiala-Heino et al., 1982; Marceau et al., 2011). Our results show that children who exhibited more pubertal change later compared to earlier in childhood also showed an increase in memory-related activation. Larger activation of this contrast is expected in older adolescents and adults (DeMaster et al., 2013; DeMaster and Ghetti, 2013). Thus, these findings are consistent with the idea that later, instead of earlier, pubertal development leads to more adult like patterns of activation. Whether early puberty onset may be detrimental should be examined in future research. In the present research, we did not have additional observation points to assess whether and when children with an earlier pubertal onset eventually exhibit the pattern of hippocampal activation expected in adults.
In the current study, two separate measures of puberty, the Pubertal Development Scale (PDS) (Petersen et al., 1988) and testosterone, yielded somewhat different results based on sex. A similar effect was found for males and females when predicting hippocampal activation using PDS, while testosterone only affected hippocampal activation in females. Each measure of puberty also showed typical age-related increases such that children older at initial assessment experienced greater longitudinal changes in both PDS and testosterone. Females observed greater changes in PDS scores and males observed greater changes in testosterone, consistent with previous longitudinal investigation of these measures (Braams et al., 2015).
There are several potential reasons why PDS scores and testosterone showed different predictive patterns in males. PDS scores were based on parental reports and assessed physical changes including height, hair growth, skin changes, voice changes for males, and breast growth and start of menarche for females. While physical and hormonal markers of puberty are correlated (Nottelmann et al., 1987), and they were in the present study as well, PDS scores reflect more global changes while testosterone may result in more specific physical changes (Shirtcliff et al., 2009). Furthermore, females showed earlier physical indicators of puberty than males, consistent with typically observed findings (Dahl et al., 2018; Shirtcliff et al., 2009). Since females were further along in their pubertal development than males in our sample, future work should examine if a relation between testosterone and hippocampal activation would emerge if older adolescent males were assessed.
We also investigated whether a similar pattern of associations would hold in lateral prefrontal cortex. We found a partially overlapping pattern of results between the hippocampus and the DLPFC, such that longitudinal changes in testosterone were associated with increases in activation for females who were older at the initial assessment, while younger females at initial assessment showed a negative relation. This suggests that the pubertal findings associated with memory-related activity was not specific to the hippocampus. Indeed, hormonal changes in the human brain are widespread (Blakemore et al., 2010; Herting and Sowell, 2017) and testosterone has been linked to structural changes in DLPFC (Nguyen et al., 2013). However, research investigating the role of puberty on cortical structure and cognitive function in humans is highly limited. Building a comprehensive understanding of the developing brain will require research integrating pubertal influences on neural structure, function, and their relation.
While we observed activation in DLPFC and hippocampus during memory retrieval, future research should examine how hormonal influences operate in these regions. For example, it is possible pubertal hormones mainly influence the hippocampus and this in turn affects function in other memory related brain regions. Alternatively, hormonal influences may occur at both subcortical and cortical regions, but perhaps the timing of these influences may differ as suggested by the different developmental trajectories of these regions (Ghetti and Bunge, 2012). Furthermore, in the current investigation we did not find any relation between pubertal development and behavioral memory performance. We can offer the speculation that the nature of the task might determine the extent to which relations between puberty and behavior are observed (Smith et al., 2013). For example, pubertal maturation has been associated with performance in tasks involving risk and sensation-seeking, but not cognitive impulse control (Steinberg et al., 2008), suggesting that motivational aspects may influence puberty-behavioral relations. However, future research is needed to better characterize the contexts in which puberty-related changes in activation lead to changes in behavioral outcomes.
4.2. Longitudinal change in hippocampal and DLPFC activation
Age-related developmental effects were also observed beyond changes in puberty. We found non-linear development of hippocampal activation associated with successful episodic retrieval (i.e., correct > incorrect item-context associations). Hippocampal activation followed a U-shape trajectory such that initially decreasing activation was followed by increasing activation during late childhood and adolescence. Previous cross-sectional studies have suggested developmental differences in hippocampal activation (DeMaster et al., 2013; DeMaster and Ghetti, 2013), including suggestions of non-linear change (Ghetti et al., 2010). By using a longitudinal approach, we were able to reveal within-individual change during late childhood and adolescence. This finding highlights the prolonged developmental trajectory of hippocampal activation and is consistent with evidence of non-linear structural change in hippocampal volumes well into adolescence (Gogtay et al., 2006). Nevertheless, it is currently unclear how declines or increases in hippocampal volume map onto changes in activation. In a different line of developmental research, namely the development of reasoning, cortical thinning has been found to be associated with increased selectivity in the rostrolateral PFC (Wendelken et al., 2011). The extent to which developmental reductions in volume promote increased selective activation for episodic retrieval in the hippocampus and, conversely, whether declines in activation result from volumetric increases should be examined in future research.
In the current research, we found that the hippocampal tail showed less activation than the hippocampal body across time points, but we did not observe any significant regional differences in developmental trajectories. Several researchers have suggested differences in hippocampal function along the longitudinal axis (Poppenk et al., 2013; Ranganath and Ritchey, 2012), and age-related differences in activation profiles for hippocampal sub-regions have also been observed (DeMaster et al., 2016; DeMaster and Ghetti, 2013). For example, DeMaster and Ghetti (2013) found memory retrieval activation in anterior hippocampus for adults and posterior hippocampus in children. However, other work has shown similar overall patterns of activation across hippocampal sub-regions in children (DeMaster et al., 2013). Additionally, animal models that examine hormonal effects on the hippocampus typically focus on synaptic changes in the CA1 subfield (Hebbard et al., 2003), which is located mainly in the anterior region of the hippocampus in adult humans but also extends across the entire longitudinal axis (Duvernoy, 2005). Thus, this would suggest that perhaps developmental and pubertal effects should be more strongly observed in the hippocampal head. However, we did not find evidence for this in the current study potentially because the hippocampal head is heterogeneous and contains additional subfields (e.g., dentate gyrus and CA3) that have different developmental trajectories (Keresztes et al., 2018). Future work would benefit from determining whether specific subfields are differentially influenced by pubertal change and how these effects translate to differences across the longitudinal axis. Furthermore, in most studies reporting age-related differences in hippocampal volume, samples included children as young as 4 to 6 years of age and adults (DeMaster et al., 2014; Gogtay et al., 2006; Riggins et al., 2018; Schlichting et al., 2017), suggesting that greater developmental differences in sub-region activation may emerge if a wider age range were assessed.
We also found that DLPFC memory-related activation followed a non-linear developmental trajectory with largely absent activation early in childhood followed by an accelerated rate of increase during late childhood and adolescence. This finding is consistent with cross-sectional work that demonstrates age-related increases in memory retrieval related prefrontal activation throughout childhood and adolescence (Güler and Thomas, 2013; Ofen et al., 2007). The DLFPC showed no sensitivity to item-context association at initial assessment, when the average age was approximately 10 years. Although the current investigation focused on DLFPC because of previous attention to this region in developmental work (Ghetti and Bunge, 2012; Ofen et al., 2007), research with adults shows regional distinctions between dorsolateral, ventrolateral, and anterior prefrontal cortices during memory (Simons and Spiers, 2003), with DLPFC being particularly sensitive to post-retrieval monitoring (Dobbins et al., 2002; Rugg et al., 1999). Developmental work has begun to examine potential differences in memory related prefrontal activity (Tang et al., 2017), but more research is needed to characterize developmental differences and trajectories in sub-regions in the PFC. This future work would gain from integrating longitudinal designs with experimental manipulations of putative processes.
4.3. Limitations and conclusions
There are several potential limitations of the current study and avenues for future directions. First, the puberty scale was completed by parents as opposed to a physician assessment or by self-report. Although PDS scores are positively correlated with physician assessment, both over- and underestimations occur depending on sex and characteristic measured (Shirtcliff et al., 2009). Additionally, testosterone was assessed via saliva samples, and serum testosterone may more accurately distinguish between pubertal levels than saliva samples (Rilling et al., 1996). The typical developmental trajectories and correlation observed between the two measures in our study provides some reassurance that our measures captured changes in pubertal development. Future research should assess the role of additional hormones such as estradiol, which is better captured by serum as opposed to salivary samples (Shirtcliff et al., 2000). Research in animal models demonstrates that estradiol influences hippocampal synapse formation in females but not males (MacLusky et al., 2006), suggesting that a similar sex-dependent pattern may also emerge when examining hippocampal activation in humans. Additionally, it is not possible to fully separate age from puberty in healthy developing samples. Future research with age-matched populations who exhibit typical and atypical pubertal development can be used to further disentangle the contribution of each of these effects. Although our study focused on individual differences in puberty, several other measures including mood (Buchanan et al., 1992), sleep (Campbell et al., 2012), and SES (Foulkes and Blakemore, 2018) correlate with puberty, and might influence brain activation. Finally, our age range was limited between 8 to 14 years old and additional developmental changes are likely to continue occurring after this period.
Nevertheless, the current study is the first to investigate longitudinal changes in neural substrates underlying episodic memory retrieval while distinguishing the role age from that of puberty, highlighting the importance of accounting for both factors to characterize the neural substrates of memory development.
Conflict of interest
None.
Acknowledgements
Support for this research was provided by National Institute on Mental Health Research Grant MH091109 (to S.G. and S.A.B.). S.A.B. was additionally supported by a Jacobs Foundation Advanced Career Research Fellowship. D.S. was supported by National Institute of Child Health and Human Development Grant F32HD084313.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.10.003.
Contributor Information
Diana Selmeczy, Email: dselmeczy@ucdavis.edu.
Simona Ghetti, Email: sghetti@ucdavis.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62(4):647. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Biro F.M., Lucky A.W., Huster G.A., Morrison J.A. Pubertal staging in boys. J. Pediatr. 1995;127(1):100–102. doi: 10.1016/s0022-3476(95)70265-2. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., van Duijvenvoorde A.C.K., Peper J.S., Crone E.A. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen J.E., Hranilovich J.A., Dahl R.E., Forbes E.E., Chen J., Toga A.W. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb. Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan C.M., Eccles J.S., Becker J.B. Are Adolescents the Victims of Raging Hormones - Evidence for Activational Effects of Hormones on Moods and Behavior at Adolescence. Psychol. Bull. 1992;111(1):62–107. doi: 10.1037/0033-2909.111.1.62. [DOI] [PubMed] [Google Scholar]
- Campbell I.G., Grimm K.J., de Bie E., Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc. Natl. Acad. Sci. U.S.A. 2012;109(15):5740–5743. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D., Rothstein M., Snodgrass J.G. Picture naming by young children: norms for name agreement, familiarity, and visual complexity. J. Exp. Child Psychol. 1997;65(2):171–237. doi: 10.1006/jecp.1996.2356. [DOI] [PubMed] [Google Scholar]
- Cycowicz Y.M., Friedman D., Snodgrass J.G., Duff M. Recognition and source memory for pictures in children and adults. Neuropsychologia. 2001;39(3):255–267. doi: 10.1016/s0028-3932(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Dahl R.E., Allen N.B., Wilbrecht L., Suleiman A.B. Importance of investing in adolescence from a developmental science perspective. Nature. 2018;554(7693):441–450. doi: 10.1038/nature25770. [DOI] [PubMed] [Google Scholar]
- DeMaster D.M., Ghetti S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex. 2013;49(6):1482–1493. doi: 10.1016/j.cortex.2012.08.004. [DOI] [PubMed] [Google Scholar]
- DeMaster D.M., Coughlin C., Ghetti S. Retrieval flexibility and reinstatement in the developing hippocampus. Hippocampus. 2016;26(4):492–501. doi: 10.1002/hipo.22538. [DOI] [PubMed] [Google Scholar]
- DeMaster D.M., Pathman T., Ghetti S. Development of memory for spatial context: hippocampal and cortical contributions. Neuropsychologia. 2013 doi: 10.1016/j.neuropsychologia.2013.05.026. [DOI] [PubMed] [Google Scholar]
- DeMaster D., Pathman T., Lee J.K., Ghetti S. Structural Development of the Hippocampus and Episodic Memory: Developmental Differences Along the Anterior/Posterior Axis. Cereb. Cortex. 2014;24(11):3036–3045. doi: 10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Diana R.A., Yonelinas A.P., Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. (Regul. Ed.) 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dobbins I.G., Foley H., Schacter D.L., Wagner A.D. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35(5):989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. Springer Science & Business Media; New York: 2005. The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections With MRI. [Google Scholar]
- Fandakova Y., Selmeczy D., Leckey S., Grimm K.J., Wendelken C., Bunge S.A., Ghetti S. Changes in ventromedial prefrontal and insular cortex support the development of metamemory from childhood into adolescence. Proc. Natl. Acad. Sci. 2017;114(29):7582–7587. doi: 10.1073/pnas.1703079114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.J. Studying individual differences in human adolescent brain development. Nat. Neurosci. 2018;21(3):315–323. doi: 10.1038/s41593-018-0078-4. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Dev. 2008;79(2):339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Bunge S.A. Neural changes underlying the development of episodic memory during middle childhood. Dev. Cogn. Neurosci. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., Lee J. Children’s episodic memory. Wiley Interdiscip. Rev. Cogn. Sci. 2010;2(4):365–373. doi: 10.1002/wcs.114. [DOI] [PubMed] [Google Scholar]
- Ghetti S., DeMaster D.M., Yonelinas A.P., Bunge S.A. Developmental differences in medial temporal lobe function during memory encoding. J. Neurosci. 2010;30(28):9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.J. The influence of puberty on subcortical brain development. NeuroImage. 2013;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Nugent T.F., Herman D.H., Ordonez A., Greenstein D., Hayashi K.M. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16(8):664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Güler O.E., Thomas K.M. Developmental differences in the neural correlates of relational encoding and recall in children: an event-related fMRI study. Dev. Cogn. Neurosci. 2013;3:106–116. doi: 10.1016/j.dcn.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbard P.C., King R.R., Malsbury C.W., Harley C.W. Two organizational effects of pubertal testosterone in male rats: transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Exp. Neurol. 2003;182(2):470–475. doi: 10.1016/s0014-4886(03)00119-5. [DOI] [PubMed] [Google Scholar]
- Herting M.M., Sowell E.R. Puberty and structural brain development in humans. Front. Neuroendocrinol. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Pruessner J.C., Coupé P., Collins D.L. Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. NeuroImage. 2013;74:276–287. doi: 10.1016/j.neuroimage.2013.02.032. [DOI] [PubMed] [Google Scholar]
- Juraska J.M., Sisk C.L., DonCarlos L.L. Sexual differentiation of the adolescent rodent brain: hormonal influences and developmental mechanisms. Horm. Behav. 2013;64(2):203–210. doi: 10.1016/j.yhbeh.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Kaltiala-Heino R., Marttunen M., Rantanen P., Rimpelä M. Early puberty is associated with mental health problems in middle adolescence. Soc. Sci. Med. 2003;57(6):1055–1064. doi: 10.1016/s0277-9536(02)00480-x. [DOI] [PubMed] [Google Scholar]
- Keresztes A., Ngo C.T., Lindenberger U., Werkle-Bergner M., Newcombe N.S. Hippocampal Maturation Drives Memory from Generalization to Specificity. Trends Cogn. Sci. (Regul. Ed.) 2018 doi: 10.1016/j.tics.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A., Cohen N.J. Relational memory and the hippocampus: representations and methods. Front. Neurosci. 2009;3(2):166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn P.C.M.P., Peper J.S., Crone E.A. The influence of sex steroids on structural brain maturation in adolescence. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0083929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legro R.S. Rapid Maturation of the Reproductive Axis during Perimenarche Independent of Body Composition. J. Clin. Endocrinol. Metab. 2000;85(3):1021–1025. doi: 10.1210/jcem.85.3.6423. [DOI] [PubMed] [Google Scholar]
- Little T.D., Bovaird J.A., Widaman K.F. On the merits of orthogonalizing powered and product terms: Implications for modeling interactions among latent variables. Struct. Eq. Model.-a Multidiscip. J. 2006;13(4):497–519. [Google Scholar]
- MacLusky N.J., Hajszan T., Prange-Kiel J., Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138(3):957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Marceau K., Ram N., Houts R.M., Grimm K.J., Susman E.J. Individual differences in boys “and girls” timing and tempo of puberty: modeling development with nonlinear growth models. Dev. Psychol. 2011;47(5):1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W.E.I., Pfeifer J.H., Masten C.L., Mazziotta J.C., Iacoboni M., Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Soc. Cogn. Affect. Neurosci. 2012;7(1):35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S., Specht K., Hausmann M. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nguyen T.V., McCracken J., Ducharme S., Botteron K.N., Mahabir M., Johnson W. Testosterone-Related Cortical Maturation Across Childhood and Adolescence. Cereb. Cortex. 2013;23(6):1424–1432. doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottelmann E.D., Susman E.J., Dorn L.D., Inoff-Germain G., Loriaux D.L., Cutler G.B., Chrousos G.P. Developmental processes in early adolescence. Relations among chronologic age, pubertal stage, height, weight, and serum levels of gonadotropins, sex steroids, and adrenal androgens. J. Adolesc. Health Care. 1987;8(3):246–260. doi: 10.1016/0197-0070(87)90428-1. [DOI] [PubMed] [Google Scholar]
- Ofen N., Chai X.J., Schuil K.D.I., Whitfield-Gabrieli S., Gabrieli J.D. The development of brain systems associated with successful memory retrieval of scenes. J. Neurosci. 2012;32(29):10012–10020. doi: 10.1523/JNEUROSCI.1082-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N., Kao Y.C., Sokol-Hessner P., Kim H., Whitfield-Gabrieli S., Gabrieli J.D. Development of the declarative memory system in the human brain. Nat. Neurosci. 2007;10(9):1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., Agrawal A., Gaffrey M.S., Belden A.C. HPA axis genetic variation, pubertal status, and sex interact to predict amygdala and hippocampus responses to negative emotional faces in school-age children. NeuroImage. 2015;109:1–11. doi: 10.1016/j.neuroimage.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso P.M., Ghetti S., Donohue S.E., Goodman G.S., Bunge S.A. Neurodevelopmental correlates of true and false recognition. Cereb. Cortex. 2008;18(9):2208–2216. doi: 10.1093/cercor/bhm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J.S., Hulshoff Pol H.E., Crone E.A., van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Schnack H.G., Brouwer R.M., Van Baal G.C.M., Pjetri E., Székely E. Heritability of regional and global brain structure at the onset of puberty: a magnetic resonance imaging study in 9-year-old twin pairs. Hum. Brain Mapp. 2009;30(7):2184–2196. doi: 10.1002/hbm.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Kahn L.E., Merchant J.S., Peake S.J., Veroude K., Masten C.L. Longitudinal change in the neural bases of adolescent social self-evaluations: effects of age and pubertal development. J. Neurosci. 2013;33(17):7415–7419. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H.R., Moscovitch M., Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. (Regul. Ed.) 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Raj V., Bell M.A. Cognitive processes supporting episodic memory formation in childhood: the role of source memory, binding, and executive functioning. Dev. Rev. 2010;30(4):384–402. [Google Scholar]
- Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012;13(10):713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Riggins T., Geng F., Botdorf M., Canada K., Cox L., Hancock G.R. Protracted hippocampal development is associated with age-related improvements in memory during early childhood. Neuro Image. 2018;174:127–137. doi: 10.1016/j.neuroimage.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., Worthman C.M., Campbell B.C., Stallings J.F., Mbizva M. Ratios of plasma and salivary testosterone throughout puberty: production versus bioavailability. Steroids. 1996;61(6):374–378. doi: 10.1016/0039-128x(96)00043-8. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Fletcher P.C., Chua P.M., Dolan R.J. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. NeuroImage. 1999;10(5):520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Rugg M.D., Vilberg K.L., Mattson J.T., Yu S.S., Johnson J.D., Suzuki M. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia. 2012;50(13):3070–3079. doi: 10.1016/j.neuropsychologia.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkey S., Azcoitia I., Garcia-Segura L.M., Garcia-Ovejero D., DonCarlos L.L. Classical androgen receptors in non-classical sites in the brain. Horm. Behav. 2008;53(5):753–764. doi: 10.1016/j.yhbeh.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M., Wendelken C., Lee J.K., Bunge S.A., Ghetti S. Age- and performance-related differences in hippocampal contributions to episodic retrieval. Dev. Cogn. Neurosci. 2016;19:42–50. doi: 10.1016/j.dcn.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Vandekar S., Wolf D.H., Ruparel K., Roalf D.R., Jackson C. Sex differences in the effect of puberty on hippocampal morphology. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(3):341–350. doi: 10.1016/j.jaac.2013.12.002. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting M.L., Guarino K.F., Schapiro A.C., Turk-Browne N.B., Preston A.R. Hippocampal structure predicts statistical learning and associative inference abilities during development. J. Cogn. Neurosci. 2017;29(1):37–51. doi: 10.1162/jocn_a_01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Sabaliauskas N., Sherpa A., Fenton A.A., Stelzer A., Aoki C., Smith S.S. A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327(5972):1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Dahl R.E., Pollak S.D. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Granger D.A., Schwartz E.B., Curran M.J., Booth A., Overman W.H. Assessing estradiol in biobehavioral studies using saliva and blood spots: simple radioimmunoassay protocols, reliability, and comparative validity. Horm. Behav. 2000;38(2):137–147. doi: 10.1006/hbeh.2000.1614. [DOI] [PubMed] [Google Scholar]
- Simons J.S., Spiers H.J. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003;4(8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Smith A.R., Chein J., Steinberg L. Impact of socio-emotional context, brain development, and pubertal maturation on adolescent risk-taking. Horm. Behav. 2013;64(2):323–332. doi: 10.1016/j.yhbeh.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Albert D., Cauffman E., Banich M., Graham S., Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev. Psychol. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Tang L., Shafer A.T., Ofen N. Prefrontal cortex contributions to the development of memory formation. Cereb. Cortex. 2017:1–14. doi: 10.1093/cercor/bhx200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelken C., O’Hare E.D., Whitaker K.J., Ferrer E., Bunge S.A. Increased Functional Selectivity over Development in Rostrolateral Prefrontal Cortex. J. Neurosci. 2011;31(47):17260–17268. doi: 10.1523/JNEUROSCI.1193-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke G. vol. 126. Springer, New York, NY; New York, NY: 1997. pp. 63–153. (Linear Mixed Models for Longitudinal Data). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.