Abstract
Measuring brain activity in developmental populations remains a major challenge despite great technological advances. Among the numerous available methods, functional near-infrared spectroscopy (fNIRS), an imaging modality that probes the hemodynamic response, is a powerful tool for recording brain activity in a great variety of situations and populations. Neurocognitive studies with infants have often reported inverted hemodynamic responses, i.e. a decrease instead of an increase in regional blood oxygenation, but the exact physiological explanation and cognitive interpretation of this response remain unclear. Here, we first provide an overview of the basic principles of NIRS and its use in cognitive developmental neuroscience. We then review the infant fNIRS literature to show that the hemodynamic response is modulated by experimental design and stimulus complexity, sometimes leading to hemodynamic responses with non-canonical shapes. We also argue that this effect is further modulated by the age of participants, the cortical regions involved, and the developmental stage of the tested cognitive process. We argue that this variability needs to be taken into account when designing and interpreting developmental studies measuring the hemodynamic response.
Keywords: fNIRS, Inverted Hemodynamic Response, Experimental complexity, Infants, Development
1. Introduction
Functional Near Infrared Spectroscopy (fNIRS) is an increasingly popular brain imaging method in developmental neuroscience, as it is easy to use with and well tolerated by infants and children, and has several advantages over other imaging techniques in these developmental populations. However, NIRS is a relatively new technique. Its basic principles are thus less well-known, and the experimental designs are less well established than for most other imaging modalities. Moreover, the field evolves quickly, hence analysis techniques vary greatly from one laboratory to another. Here, we first provide a general overview of the physiological and physical principles behind fNIRS, with special attention to developmental populations. Then we discuss a methodological issue that had received increasing attention in the literature: the impact of experimental design, stimulus complexity as well as the age and developmental stage of participants on the shape of the hemodynamic response. We argue that these factors need to be taken into account when planning and interpreting NIRS studies with infants and children.
1.1. Basic principles of fNIRS
NIRS is an optical method that can be used to monitor brain activity at rest, in response to sensory stimulation, or in a cognitive task, from birth to adulthood in healthy as well as in clinical populations. NIRS takes advantage of the differential near-infrared light absorption properties of oxy- and deoxyhemoglobin (HbO and HbR, respectively) to detect the concentration changes of these two chromophores in blood related to focal brain activity, i.e. the hemodynamic response.
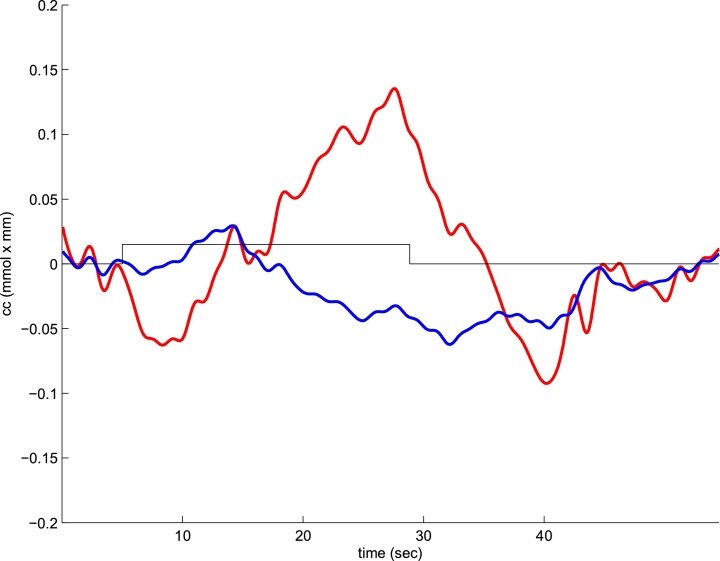
At the physiological level, NIRS relies on specific properties of the neuro-vascular coupling in the brain. Increased neural activity triggers an increase in oxygen delivery to the active region a few seconds later. As a result, there is an initial decrease in HbO followed by a strong increase, while HbR concentration decreases (after a possible brief initial overshoot). Upon continued stimulation, HbO levels remain high and then return to baseline, sometimes after an undershoot. In parallel, HbR levels remain low and then return to baseline after a possible overshoot. This pattern of variations in HbO and HbR concentrations over time defines the Hemodynamic Response Function (HRF).1 A typical newborn infant hemodynamic response is depicted in Fig. 1. Importantly, more oxygen is delivered to the active brain regions than what is taken up by the tissues, which is why it is possible to measure regional changes in blood oxygenation.
Fig. 1.
A typical Hemodynamic Response Function as observed in a newborn participant in our laboratory. Red: HbO, blue: HbR, rectangle: stimulation.
At the physical level, NIRS takes advantage of the optical properties of biological tissues in the red and near-infrared range. In the near-infrared window, i.e. light whose wavelength is above 700 nm, head tissues are almost transparent to light, and HbO and HbR have well separated absorption coefficients. Using these optical properties, NIRS measures the difference in light intensity between a source emitting near-infrared light at several wavelengths and light detectors placed at a systematic distance. Typically two wavelengths are used, one on each side of the isosbestic point of the absorption spectra of HbR and HbO, e.g. one <780 nm and one >830 nm (Scholkmann et al., 2014). A paired source and detector form a measurement channel. In turbid media, photons travel across a broad distribution of random paths due to the effects of scattering (Fukui et al., 2003; Okada and Delpy, 2003a, Okada and Delpy, 2003b). A large proportion of the light detected by the detectors travels through a donut shaped trajectory between a source and the detectors coupled with it. Light that travels in other directions does not reach the detectors, it is scattered. The measured light intensities can be converted into concentrations of HbO and HbR using the Beer-Lambert law, modified to take into account specificities of light travelling through a biological, thus optically non-ideal, non-homogeneous medium, such as the scatter and the non-linear, random path of photons. As most commercially available NIRS systems used in cognitive developmental research are unable to quantify the scatter and the actual paths of photons, these systems can only measure relative concentrations of HbO and HbR, i.e. concentration change relative to a previous time point. Absolute concentrations of HbO and HbR can be measured with NIRS systems able to quantify the scatter and the pathlength (for a review of such commercially available systems, see Wolf et al., 2007). Absolute oxygenation levels are highly informative for clinical purposes, while relative concentrations are sufficient in most experimental settings, where differences between two conditions or a condition and baseline are of interest.
The ideal source-detector separation is variable as a function of the brain region tested, the age of participants and other factors. There is a trade-off between spatial resolution and penetration depth: the farther the optodes, the deeper the penetration, but the lower the spatial resolution and the higher the noise. In adults, the penetration into the cortex is about 0.5 cm with a source-detector distance of 3 cm (Strangman et al., 2002). In infants, several source-detector distances have been tested (between about 2–5 cm), providing a penetration depth into the cortex greater than in adults, up to several centimeters in preterm newborns. Multi-distance channel set-ups, with short-distance channels of less than 2 centimeters, can also be used to record HbO and HbR concentration changes in non-brain tissues. This signal can then be subtracted from the signal obtained from regular channels to improve the signal-to-noise ratio (Emberson et al., 2016). Like functional Magnetic Resonance Imaging (fMRI), fNIRS records the hemodynamic response as a proxy for neural activity in a localized brain region. But unlike fMRI, which detects only HbR, fNIRS records both HbO and HbR, providing more information about the hemodynamic response (Steinbrink et al., 2006).
The hemodynamic response in infants is often reported to be slower to peak, delayed and/or slower to go back to baseline or different in shape than the adult hemodynamic response, although no systematic longitudinal investigation of the infant and child hemodynamic response has been undertaken to date. The current paper aims to address this gap by reviewing the existing literature in order to describe the variations in the shape and latency of the hemodynamic response found in studies with infants and young children.
1.2. Why use fNIRS in infant research?
The ease of use of NIRS is particularly relevant for developmental research. Sources and detectors are mounted on a flexible cap that can be easily placed on the participant’s head. In addition, NIRS does not require a shielded room. Furthermore, NIRS is relatively motion-tolerant compared to fMRI, allowing a behavioral paradigm to be used simultaneously. NIRS requires no tracer substance or gel. It is completely silent and uses no magnetic field or radio pulses. It is thus perfectly safe and non-invasive. It is also possible to take the set-up outside the laboratory to reach specific populations and study cognitive development under ecological conditions, such as under-nourishment or recurrent infections in rural Africa (Lloyd-Fox et al., 2016) (Fig. 2A).
Fig. 2.
Pictures of different fNIRS headgears and their respective approximative channel locations. A: The UCL system (adapted from Lloyd-Fox et al., 2018). B: The Hitachi ETG-4000 system (adapted from May et al., 2011). C: The NIRx NIRScout system as used in our laboratory.
fNIRS can be used with developmental populations2 for whom fMRI is complicated or impossible (Fig. 2B and C). Newborns can be tested directly in their bassinet, rendering experiments much easier (Benavides-Varela et al., 2012; Issard and Gervain, 2017; Pena et al., 2003; Rossi et al., 2012). The relatively high motion-tolerance of fNIRS is relevant for testing infants and children, as well as clinical populations likely to produce involuntary movements such as epileptic patients. NIRS also allows longer, even continuous monitoring. The NIRS cap can be left on patients’ heads for several hours to capture seizures or other activity whose onset is unpredictable (Wallois et al., 2010). Another clinical population for whom fNIRS is of particular interest is patients with cochlear implant. Cochlear implant users cannot be placed in an MRI because of the metallic parts of the implant. They cannot be tested with EEG either as the implant interferes with the EEG signal. fNIRS has proven useful to monitor activity in the auditory cortex with children following cochlear implantation (Sevy et al., 2010). fNIRS is thus a useful alternative to fMRI when the age or the pathology of the tested developmental population is incompatible with MRI or with EEG.
Finally, the flexibility of optode arrangement and the compatibility of the two signals makes co-recording with EEG possible (Mahmoudzadeh et al., 2013; Telkemeyer et al., 2009). The two techniques are complementary, NIRS allows spatial localization, while EEG has high temporal resolution. Furthermore, while the hemodynamic response is slow, NIRS technology itself has a higher temporal resolution than fMRI, typically allowing a sampling frequency above 10 Hz for small and medium channel numbers, whereas fMRI typically has a sampling frequency of about 0.5–1.5 Hz. Although the major determiner of temporal resolution using hemodynamic imaging is the temporal dynamics of the physiological signal itself, which is slow, the higher sampling rate is of particular interest for the identification of the temporal dynamics of cortical networks. These investigations are based on the analysis of the peak latency and phase delay of the response. This may reveal the order in which regions are activated, showing the temporal map of activation in the involved network (Mahmoudzadeh et al., 2013). NIRS can also establish functional connectivity between regions based on the synchrony of their respective activation, e.g. by computing correlations or phase synchronization between channels (Benavides-Varela et al., 2017; Homae et al., 2011; Molavi et al., 2014). Because NIRS has a higher sampling rate than fMRI, it leads to more precise temporal measures of connectivity.
1.3. Limitations of the fNIRS method
There are several constraints that need to be taken into account when using NIRS. First, the hemodynamic response is slow, operating in the order of seconds, unlike the electrophysiological response, which works at the millisecond range. Both block designs and “event-related” designs can be used, but stimulation periods need to last at least a few seconds. In block designs, a series of stimuli is presented to create long stimulation periods (typically between 5 and 30 s), separated by long baseline periods to let the hemodynamic response return to baseline. The length of the baseline periods between blocks depends on several factors such as the age of participants, the amount of activation produced and the type of design. With younger participants, the hemodynamic response may take longer to appear and to return to baseline (Arichi et al., 2012), so block designs with sufficiently long baseline periods are appropriate. In “event-related” designs, stimulation as short as 3 s have been used, separated by baseline periods as short as 4 s (Emberson et al., 2015; Homae et al., 2006; Taga and Asakawa, 2007). In this type of designs, the end of the hemodynamic response to a stimulus typically overlaps with the response to the next stimulus. This issue can be managed by using General Linear Model (GLM) for data analysis.
Another main limitation of fNIRS is its limited access to deep brain structures and its low spatial resolution. The penetration of NIR light into the head is relatively shallow at a standard 3 cm source-detector separation (with a penetration into the cortex of up to 0.5 cm in adults, 1.5 cm in infants and several centimeters in preterm newborns). Multiple source-detector distances and overlapping channels can be used in high density set-ups to create three-dimensional, i.e. tomographic, images in order to identify the source of the response with better precision and probe deeper areas (Liao et al., 2010), but these technologies are currently not yet sufficiently developed to be routinely used in developmental research. If fiducial measures of probe placement exist, i.e. the position of the probes with respect to external landmarks (ear, nasion etc.) or a co-ordinate system, then the measurement points can be mapped onto the participant’s anatomical MRI, if available, or to an age-appropriate template (Lloyd-Fox et al., 2014; Matsui et al., 2014). The cortical area activated can thus be identified with greater precision.
2. Variability of the hemodynamic response reported in the infant literature
The hemodynamic responses reported in infant fNIRS literature show important variation within and across studies. The main goal of this review is to discuss some of the factors underlying this variability. Since NIRS provides an indirect, metabolic measure of brain activity, these factors are crucial when designing fNIRS experiments and interpreting their results. The factors we will discuss below include (i) developmental changes of the hemodynamic response, (ii) the development of physiological and cognitive processes, (iii) stimulus complexity and (iv) experimental design.
Despite their importance, these factors are rarely discussed explicitly when interpreting results, and have not yet been systematically investigated across development. While we are not offering a systematic empirical investigation either, reviewing the existing developmental NIRS literature nevertheless allows us to draw certain conclusions about how these factors impact the hemodynamic response. Importantly, however, the factors are inherently linked in any single study, it is thus impossible to identify and discuss the contribution of any factor in isolation. For the same reason, we are not providing an exhaustive review of the infant NIRS literature (for more general reviews and meta-analyses of the infant NIRS literature, we refer the reader to Cristia et al. (2013) and Lloyd-Fox et al., 2010). Rather, we are focusing on studies that are comparable or matched along at least some relevant dimensions (e.g. testing the same task at different ages or testing the same age using stimuli that systematically vary), such that it becomes more apparent how factors in which they differ impact the hemodynamic response. In the sections below, we will first discuss the variability of the shape of the hemodynamic response observed at different ages in the different sensory modalities and cognitive functions. We will then describe how stimulus complexity, physiological and cognitive development and the experimental design might impact the hemodynamic response.
2.1. Variation in the shape of the hemodynamic response reported in the developmental literature between cortical regions
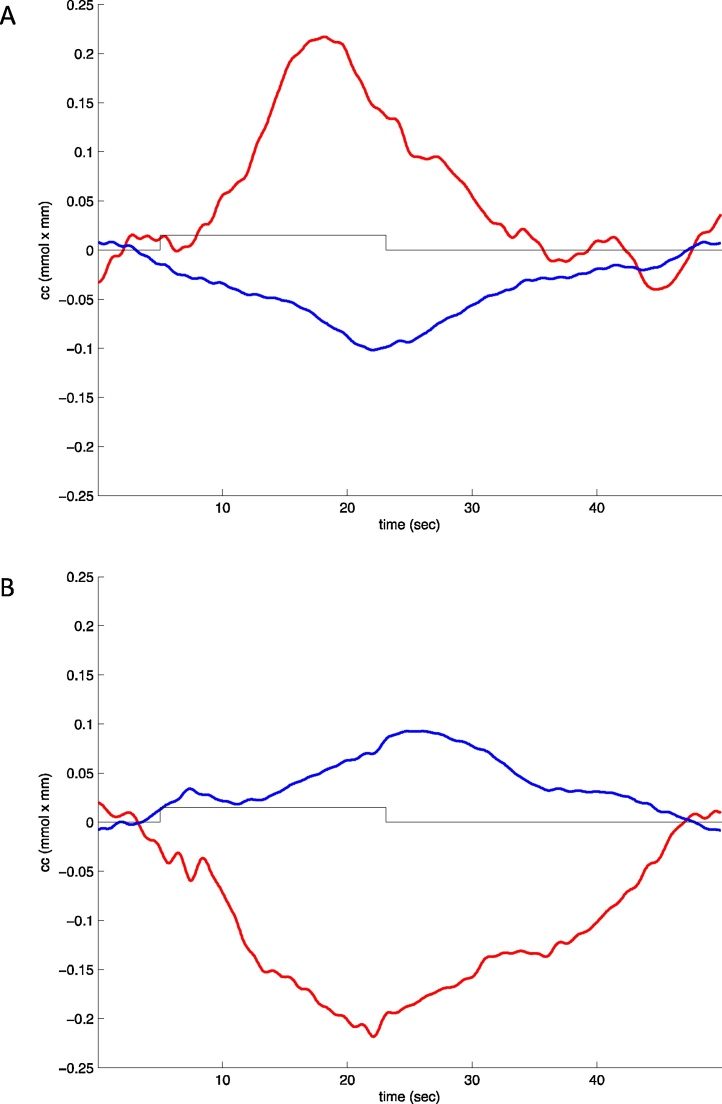
The infant fNIRS and fMRI literature shows that the shape of the hemodynamic response changes with age in different brain areas (and perceptual or cognitive tasks). Canonical (i.e. statistically significant3 increase in HbO and decrease in HbR as compared to baseline, as depicted in Fig. 3A) and inverted (statistically significant decrease in HbO and increase in HbR as compared to baseline, Fig. 3B) reponses have both been reported in the literature. Some studies also report statistically significant HbO and HbR changes in the same direction.
Fig. 3.
Canonical (A) and inverted (B) responses as observed in newborn infants in our laboratory. Red: HbO, blue: HbR.
Non-canonical responses, especially the inverted response, are not straightforward to interpret, because when absolute measures are taken, a decrease in oxygenation beyond a critical point is a signature of stroke, ischemia or other vascular problems. Most systems commonly used for research purposes only measure relative concentrations, i.e. concentration change. It is, therefore, difficult to understand the physiological and functional meaning of a relative decrease in oxygenation. Such a response pattern arises as a result of decreasing activation compared to a previously higher level of brain activity. This might occur for several possible reasons, e.g. when an inappropriately chosen baseline triggers more activation than the actual experimental stimulus, when habituation occurs, when a region is inhibited (Mullinger et al., 2014; Shmuel et al., 2002), due to reduced compensatory vascular mechanisms or indeed trauma. Do these factors also play a role in atypical hemodynamic responses observed in infants?
The inverted response is often observed in young infants and newborns. Peak latency (i.e. time to reach the maximum of the hemodynamic response) has also been reported to decrease through infancy (Lloyd-Fox et al., in press). The hemodynamic response also varies with the measured cortical region, and thus the tested sensory modality or cognitive function, and the arousal state of the participants (i.e. asleep vs awake). We provide examples of this variability across development and sensory modalities in Appendix A in Supplementary materials. For each age and sensory modality/brain region, we report the shape of the hemodynamic response measured along with the stimuli and experimental design used, focusing on studies with unsedated infants, as sedation may impact neurovascular coupling. In the section below, we discuss in detail the potential role of these factors on the variability of the hemodynamic response, in sensory processing as well as in higher-level cognitive functions, such as language processing and social cognition.
We argue that with the maturation of the brain, the hemodynamic response increasingly often takes on a canonical shape. The hemodynamic response relies on a complex interaction between the vascular system, neurons and glial cells, all of which undergo considerable maturation throughout infancy and childhood. However, because brain maturation is not homogenous between cortical regions, the hemodynamic response may vary from one brain area to another. Below (and in Appendix A in Supplementary materilas), we thus review hemodynamic response variability separately for each sensory/cognitive function and its corresponding cortical areas.
In the temporal cortex, infant studies investigating auditory perception have demonstrated both inverted and canonical responses (Telkemeyer et al., 2009). At birth, both canonical and inverted responses were observed in the temporal cortex in response to speech and non-speech sounds between participants within the same condition (Sakatani et al., 1999), and within participants between conditions (Abboub et al., 2016; Issard and Gervain, 2017; Telkemeyer et al., 2009). Later in infancy, from 3 months onwards, infants’ temporal cortex increasingly often shows a canonical response, even in infants born preterm (Emberson et al., 2017a, Emberson et al., 2017b), although inverted responses, with increasing HbR have also been reported as late as 7–9 months in response to complex speech stimuli (Wagner et al., 2011). It is possible to obtain a canonical hemodynamic response in the temporal cortex to various auditory stimuli (tones, vocal and non-vocal sounds) in different states of alertness, including sleep, as canonical responses have been reported in sleeping newborns as well as sleeping (non-sedated) 3-month-old infants (Taga et al., 2011).
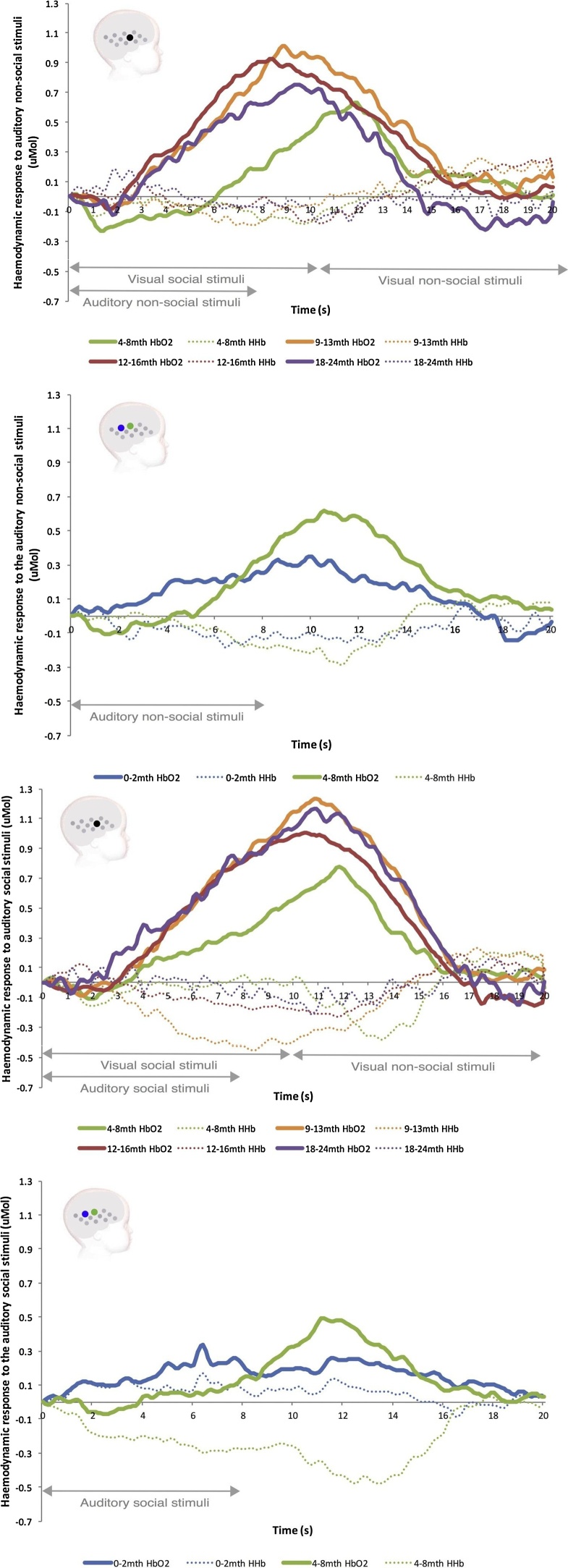
In the posterior temporo-parietal region, the hemodynamic response to social stimuli (i.e faces and ostensive, infant-directed speech) has also been intensively investigated across development. Response to the same stimuli can vary with age. At 4 months of age, infants display a delayed but canonical response, and this response becomes faster during the first two years of life, as depicted in Fig. 4 (Lloyd-Fox et al., 2018 Lloyd-Fox et al., in press). But inverted responses have also been observed at similar ages in similar ROIs. At 5–6 and 7–8 months, a series of different faces presented from different points of view evoked a canonical response, whereas a series of the same face presented from different points of view evoked an inverted response (Kobayashi et al., 2011) (Fig. 5).
Fig. 4.
Hemodyamic response in the temporo-parietal junction as a function of age. Adapted from Lloyd-Fox et al. (2018).
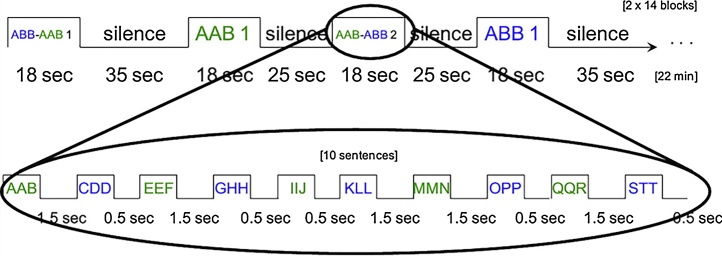
Fig. 5.
Alternating/non-alternating design with numerous variable stimuli within conditions. Adapted from Gervain et al. (2012).
Inverted hemodynamic responses have also been reported in the occipital lobe at specific time points in development. In sleeping newborns, a flickering light (projected through the eyelids) evoked a canonical response. Between 0–3 months, a checkerboard reversal evoked an increase both in HbO and HbR in awake infants (Meek et al., 1998). Later in development, between 2 and 4 months, awake infants showed a canonical response to a checkerboard reversal, but an inverted response to a face-like pattern with blinking eyes (Taga et al., 2003a, Taga et al., 2003b). Inverted responses in the occipital cortex were also observed in 4-month-old infants with pictures of faces compared to unstructured images, but in this study unstructured images also evoked inverted responses (Csibra et al., 2004). At 6 months, checkerboard pattern reversals evoked a canonical response, but unpatterned visual stimuli evoked an inverted response (Watanabe et al., 2012). The developmental trajectory of the hemodynamic response in the occipital cortex thus seems to be non-linear, varying with stimulus category.
Finally, the shape of the hemodynamic response in the frontal lobe depends on the sensory modality of the stimuli, the role the frontal lobe plays in their processing and the age of participants. For speech stimuli, the frontal lobe is typically recruited as it is part of the speech/language network (e.g. Broca’s area), whereas for visual perception it often plays a more general role related to attention. At birth, canonical responses to structured speech sounds were observed in left and right frontal cortices (Ferry et al., 2016; Gervain et al., 2008). At 3 months, both awake and sleeping infants showed a canonical hemodynamic response to speech sounds in bilateral frontal cortices (Homae et al., 2006; Homae et al., 2014). These results were replicated at 10 months (Homae et al., 2007). In the visual domain, a canonical response was measured to dynamic faces with mutual or adverted gaze at 4 months of age (Grossmann et al., 2008). In 5–7-month-old infants, a canonical response was observed to static faces, but only when the same face was repeatedly displayed during trials (Emberson et al., 2017a, Emberson et al., 2017b). By contrast, images of fruits didn’t elicit significant responses in the same frontal area. Finally, an increase in HbO was observed in the orbito-frontal cortex in response to the smell of colostrum and vanilla in newborns (Bartocci et al., 2000).
On the basis of these examples, a great diversity of hemodynamic response shapes can be observed across development, showing an interaction between the age of participants and the cortical areas measured. The temporal cortex seems to present canonical responses earlier than the occipital and frontal cortices, and follows a more linear developmental trajectory than the occipital cortex. This latter shows a canonical response at birth, but an inverted response later in infancy. Finally, the frontal cortex shows more variable responses, depending on stimulus complexity and age of participants. Social stimuli, such as speech and faces, elicit canonical responses earlier than non-social stimuli (such as fruits or flashing lights).
2.2. The factors influencing the hemodynamic response
In addition to the variability over developmental time and brain areas, the hemodynamic response seems to be further influenced by the complexity of the stimuli used, the development of the cognitive processes tested, and the experimental design, even within the same age or brain area.
Using blood oxygenation as a measure of neuronal activity relies on the assumption that the amount of change in oxygenation reflects the amount of neuronal activity. In cognitive neuroscience paradigms, changes in oxygenation in response to experimental conditions are typically compared between one another or to a baseline. The usual interpretation of this difference in hemodynamic response amplitude (or localization) is that it reflects differences in processing effort. It is, therefore, relevant to compare NIRS results, at least briefly, to models of processing effort based on behavioral measures (for a more detailed and not NIRS-specific comparison of behavioral and brain imaging studies in infants, see Turk-Browne et al., 2008). In this perspective, it can readily be explained that factors such as stimulus complexity, familiarity or the infants’ developmental state all shape the hemodynamic response.
In developmental psychology, the effect of stimulus complexity/familiarity on infants’ behavior, such as looking time, has received much attention (Goldowsky and Newport, 1993; Kidd et al., 2012, Hunter and Ames 1988). Although the factors influencing infants’ looking behavior are not fully understood, one model (Kidd et al., 2012) holds that the probability for infants to look away from a stimulus is a U-shaped function of stimulus complexity. If stimuli are too simple or too complex, infants disengage (Kidd et al., 2012). While a systematic investigation of whether such a U-shaped function may also characterize NIRS responses when stimulus complexity is systematically varied is still lacking, the review of the NIRS literature we presented above suggests that parallels may exist. NIRS responses are often canonical in shape and largest in amplitude when stimuli are in the middle range of complexity, while overly complex or too simple stimuli more often evoke atypical responses.
Another model (Hunter and Ames, 1988), focusing on the impact of familiarity, predicts that when initially exposed to some stimuli, infants will show a preference for them for a period of time, as they are still in the process of exploring, encoding or learning them. Once encoding, memorization or learning is completed, infants’ interest in the familiar stimulus decreases, and they readily show interest in novel stimuli. During the transition between these two periods, it is hypothesized that infants show no preference as they are equally attracted by the familiar and the novel stimuli (Hunter and Ames, 1988). This behavioral familiarization/habituation pattern also shows some similarities with the hemodynamic response, especially when experimental designs based on repeated exposure to the same stimuli are used (neural habituation). We discuss studies based on such experimental designs below.
Another relevant question is how different degrees of complexity or familiarity may change the type of processing mechanisms triggered. One relevant developmental model is the « less is more » hypothesis (Goldowsky and Newport, 1993), which states that learners with limited memory capacity, such as infants, may use generalization, rule learning or categorization mechanisms to reduce the memory demand of highly variable or highly complex input, whereas learners with greater memory capacity tend to memorize or rote learn the input set. A change in stimulus complexity might thus trigger a qualitative rather than a quantitative change in cognitive processing, resulting in an entirely different pattern of responses in the NIRS data. The link between qualitatively different cognitive processing mechanisms and their neural signatures is not straightforward and remains unknown for most cognitive tasks, especially in development.
To the extent that the fNIRS response is indeed a measure of cognitive effort, it is important to take into account factors such as stimulus complexity, cognitive and physiological development and experimental design, when interpreting NIRS results, especially non-canonical ones. In the next sections, we discuss how these three factors modify cognitive effort and the hemodynamic response.
3. Modulation of the hemodynamic response by complexity
3.1. Variation due to stimulus complexity
The complexity and the familiarity of the stimuli are two potential factors that impact the hemodynamic response. Below, we provide examples for three different patterns of results that infant studies have found between their experimental conditions, and discuss to what extent these differences are attributable to stimulus complexity and/or familiarity.
First, some studies found canonical reponses in all conditions, with a significant difference in amplitude across conditions. Familiarity plays a clear role in such differences. For instance, in the auditory domain, monolingual and bilingual newborns were presented with tone pairs in which the members differed along an acoustic dimension (pitch, duration or intensity) that either matched or did not match the acoustic cues carrying prosodic prominence in the language(s) the infants heard in utero (Abboub et al., 2016). The newborns’ left temporo-parietal and right temporal cortices responded more to sound patterns that were inconsistent with the patterns found in the infants’ native language, i.e. were unfamiliar, as compared to patterns that were consistent with it, i.e. were familiar, suggesting that processing unfamiliar melodic patterns often requires extra effort, resulting in a larger hemodynamic response. In the olfactory modality, vanilla smell evoked a larger response than the mother’s colostrum in newborns’ frontal cortex (Bartocci et al., 2000). Actually, responses to the mother’s colostrum negatively correlated with the infants’ age (0–200 h after birth). In older infants, the frontal region responded increasingly less to colostrum, probably due to the gradual replacement of colostrum with breastmilk and the decreasing relevance/familiarity of its smell. By contrast, vanilla was a new odor, which infants didn’t encounter outside the testing sessions and to which all infants responded with a large hemodynamic response independently of age. Stimulus complexity also modulates the amplitude of the hemodynamic response. In an artificial grammar learning study with newborns, for example, trisyllabic sequences containing a repetition (e.g. “mulele”) evoked a larger hemodynamic response than sequences containing no repetition (e.g. “mulevi”) in the frontal and temporal regions (Gervain et al., 2012; Gervain et al., 2008). In this case, the presence of a repetition created an underlying abstract structure, absent from the random sequences, and triggering a larger hemodynamic response. As another example of the role of complexity, at 3 months, both natural and sine wave speech induced a greater response than complex tones in the left posterior temporal and the right temporo-parietal cortex and the left frontal cortex (Homae et al., 2014). Natural speech and its sine wave equivalents are both acoustically more complex than tones, leading to a larger hemodynamic response. Differences in stimulus complexity also produce canonical responses of different amplitudes in the visual domain. In 3-month-old infants, for example, videos of mobile objects elicited a significantly greater hemodynamic response than checkerboard pattern reversal in both frontal and occipital regions (Watanabe et al., 2008). Mobile objects were visually more complex than the checkerboard, containing more colors and a motion pattern more sophisticated than the reversal. Similarly, during live social interaction, the frontal cortex of 7-month-old infants showed larger responses to direct than to averted gazes, direct gazes being more engaging than averted gazes (Urakawa et al., 2015). When stimuli evoke canonical responses, complexity produces larger responses both in the visual and auditory domains.
Second, another pattern of results often observed in infant studies is a significant hemodynamic response in one condition, compared with a null response in some other condition. Differences in familiarity between conditions have been observed to play a role. For instance, comparing the responses to forward (FW) speech, backward (BW) speech, and silence in neonates, forward speech produced a classical canonical hemodynamic response in the left temporal cortex, but no significant response was measured during silence and backward speech (Pena et al., 2003). FW and BW speech are of the same complexity, as BW speech is the time-reversed version of FW speech, but BW speech is less familiar than FW speech, and may thus not be processed by the speech/language network of the newborn brain. Importantly, the language used in this study was the one the infants heard prenatally (Italian). Follow-up studies the contrasted responses to the native language with an unfamiliar language, both played forward and backward. Interestingly, one study found a larger response to forward speech and no significant response for silence and backward speech in the left temporo-parietal cortex of neonates, but only for the neonates’ native language, Japanese. For English, the unfamiliar language, a bilateral temporal response was found with no difference between FW and BW speech (Sato et al., 2012). Comparing responses to FW and BW English and FW and BW Spanish in English-exposed newborns, the same unfamiliar Spanish stimuli evoked diminished non-significant responses in the fronto-temporal region when contrasted with English, the native language, but a larger FW-selective response when paired with Silbo Gomero, a Spanish based whistled language, therefore a non-speech-like but linguistic sound (May et al., 2017). These results suggest that familiarity shapes the observed hemodynamic response, but also that familiarity itself may be modulated by contextual effects. The unfamiliarity of the stimuli has been observed to trigger the absence of a significant hemodynamic response at even more fine-grained levels in speech processing. Phonologically possible words, allowed by language-universal constraints on syllable structure, evoked a significant response, but no significant response was found for words violating these constraints (Gómez et al., 2014). In the social visual domain, the occipital cortex of 4-month-old infants exhibited an inverted response to pictures of faces, but no response to unstructured images constructed from the same spatial frequencies and color distribution (Csibra et al., 2004).
Third, yet other studies observed canonical responses in some conditions and inverted ones in others. Comparing the native language with a non-native language in a paradigm similar to those used in the previously mentioned studies, but using low-pass filtered, thus less complex speech stimuli, a canonical, direction-insensitive hemodynamic response to the native language, English, was observed, as well as to the unfamiliar language, Tagalog, played backwards, but an inverted hemodynamic response was observed to FW Tagalog (May et al., 2011). These results suggest that stimulus complexity interacts with familiarity when modulating the hemodynamic response. Canonical and inverted responses can also be observed within the same study when complexity alone is manipulated. At birth, for instance, canonical responses were observed to normal and moderately time-compressed speech (Issard and Gervain, 2017), but highly time-compressed speech produced an inverted response. For visual stimuli, a checkerboard reversal pattern evoked a canonical response in the occipital cortex of 3-month-olds, but a blinking face evoked an inverted response (Taga et al., 2003a, Taga et al., 2003b). Similarly, at 6 months, unpatterned visual stimuli evoked an inverted hemodynamic response, while checkerboard pattern reversals evoked a canonical hemodynamic response (Watanabe et al., 2012). For social stimuli, at 5 months faces with direct gaze and the participant’s own name evoked a canonical response in the left frontal cortex, but faces with averted gaze or another name evoked inverted hemodynamic responses (Grossmann et al., 2010a, Grossmann et al., 2010b).
Together, these results suggest that the hemodynamic response is highly modulated by the presented stimuli within the same sensory modality, cognitive ability and age group. In line with the idea that the hemodynamic response is a reflection of cognitive effort, more complex and thus more demanding stimuli often elicit a larger hemodynamic response than simpler stimuli. However, the effects of stimulus complexity and familiarity are non-linear: when stimuli become overly complex or demanding, null or inverted responses can appear.
3.2. Variation related to developmental changes
Brain maturation and the developmental stage of the tested cognitive function also modulate how the experimental stimuli are responded to.
At the neurobiological level, the immaturity of the vascular system in infants has been argued to play a role in the appearance of inverted responses. Indeed, they could be the result of insufficient cerebral blood flow (Meek et al., 1998). In young infants, the cerebral blood flow may sometimes be insufficient. In this case, HbR would not be fully eliminated, leading to an increase in HbR and a decrease in HbO. Infants’ immature vascular system may insufficiently respond to the metabolic demand of the neural population. Indeed, in the somato-sensory cortex of preterm neonates, blood flow has been shown to increase immediately after stimulus onset and to return to baseline prior to HbR and HbO. Arterial to venous transit time was found to be longer than in adults (Roche-Labarbe et al., 2014). This increased transit time is consistent with an immaturity of the brain vasculature in infancy (Norman and O’Kusky, 1986). The capillary bed increases during the first months of life followed by a process of remodeling the vascular network as a function of local neural activity (Kozberg and Hillman, 2016).
The hypothesis of an occasional decoupling between the vascular and the neural part of the neuro-vascular system is further confirmed by EEG-NIRS co-registration studies in infants. In highly premature newborns, a change in a single consonant in French syllables elicited larger electrophysiological and hemodynamic responses than no change, whereas a change of voice from male to female elicited a larger electrophysiological response, but a smaller hemodynamic response than no change (Mahmoudzadeh et al., 2013, Mahmoudzadeh et al., 2017). This confirms that the relationship between neural and vascular activity is not linear in the developing brain.
In parallel with biological maturation, perceptual, cognitive and learning skills also develop, and their developmental stage impacts how stimuli are processed. In social cognition, for instance, 4 different age groups ranging from 4 to 24 months all showed a canonical hemodynamic response to social stimuli such as peek-a-boo videos and social sounds, with the peak latency decreasing over developmental time (Lloyd-Fox et al., in press). This may reflect infants’ increasing expertise in social stimuli during the first two years of life. Similarly, when comparing activity evoked by social auditory stimuli in 0–2-month-olds and 4–8–month-olds, the same authors reported a larger and more typical response in the older group. Interestingly, it has been shown that specialization for the human voice occurs between 4 and 7 months: the left and right superior temporal cortices showed increased responses to the human voice when compared to non-vocal sounds at 7, but not at 4 months (Grossmann et al., 2010a, Grossmann et al., 2010b). This supports the hypothesis that infants present a larger response when they master the targeted cognitive skill, for instance when they specialize for the processing of the human voice as the most important social sound. Similarly in visual social stimuli, 5–6 and 7–8-month-old infants presented with pictures of the same or different faces showed a decrease in HbO in the right temporal area for different faces at 5–6 months, but an increase in HbO for the same stimuli at 7–8 months. This confirms the hypothesis that mastery of a cognitive skill leads to greater responses.
Similar developmental trends can be observed in the speech perception domain. For instance, a developmental change was found in response to the native dialect vs. another dialect: 3-month-old Parisian infants showed a similar canonical response to both Parisian and Quebecois French talking faces, while 5-month-olds showed an advantage for the Parisian stimuli in the left temporal areas (Cristia et al., 2013). Similarly, in a pitch accent discrimination task, Japanese 4-month-olds showed canonical activation bilaterally, while 10-month-olds already exhibited left-lateralized, i.e. language-specific responses to the same stimuli, i.e. a null response in the right hemisphere (Sato, Mazuka et al., 2010). In the same vein, 6–7-month-olds present a greater hemodynamic response to across-category than within-category phonemic changes, mirroring behavioral data. By contrast, the responses to the two conditions are similar at 10–11 months, as the phonological system undergoes reorganization, attuning to the native language. Then the response to across-category phonemic change becomes larger again with a left hemisphere advantage at 13–14 and 25–28 months (Minagawa-Kawai et al., 2007). Perceptual narrowing, i.e. attunement to the native language, brings about a reorganization, whereby infants switch from an auditory to a linguistic processing of speech sounds and shape their phonological space to better match the phoneme contrasts found in the native language during the second half of the first year of life. This changes the nature and efficiency of the underlying cognitive processing, and therefore its metabolic costs.
Together, these data suggest that development has a considerable impact on how the infant brain processes the same stimuli. As sensory and cognitive functions develop, the newly emerging cognitive or perceptual skills often lead to additional, higher level steps of processing, for instance because a novel, more abstract representation is formed or because a category is learned or reshaped, and might thus evoke a larger hemodynamic response. By contrast, for cognitive functions where processing becomes more automatic and/or faster with development, a smaller hemodynamic response is observed. Therefore, similarly to the U-shaped developmental curves observed in several perceptual and cognitive domains at the behavioral level, developmental change may not be linear in direction at the neural level either, with increases in hemodynamic response amplitude early in development and decreases later.
4. Modulation of the hemodynamic response by experimental design
Specific experimental designs can have an effect on the hemodynamic response, as they modify the context of stimulus presentation by adding another level, that of local and global stimulus arrangement/context. Below we discuss how this can influence the shape of the hemodynamic response.
4.1. Simple event-related or block designs
The simplest and most commonly used design in infant NIRS studies is the presentation of stimuli belonging to different experimental conditions in an interleaved, random order, where one long (in event-related designs) or several shorter (in block designs) stimuli from the same condition are presented (for a duration of several seconds to allow for the hemodynamic response to build up). Both canonical (Homae et al., 2007; Taga et al., 2003a, Taga et al., 2003b) and inverted (Telkemeyer et al., 2009; Watanabe et al., 2012) responses have been observed in such designs, as shown in many of the studies reviewed above.
In this design, the order of stimuli or blocks is randomized. Any interaction between consecutive stimuli is, therefore, random, and should not influence the hemodynamic response in a systematic way. Rather, the measured hemodynamic response is expected to be influenced only by the properties of the stimuli.
4.2. Repetition effects
Repetition effects might arise when stimuli or blocks from the different conditions are not intermixed, but rather grouped together by condition, resulting in a large number of repetitions of the same stimulus type.4 Repetitions can influence the neural and hence the hemodynamic response by producing either enhancement, or suppression. Repetition suppression can be defined as a decrease of neural activity in response to the repeated presentation of the same stimulus or stimulus category. Initially shown in non-human primates at the single cell level, this effect was later demonstrated at the level of the hemodynamic response in humans, first in adults (Buckner and Koutstaal, 1998) and more recently in infants (Nakano et al., 2009). Repetition enhancement can be defined as an increase of neural activity in response to repeated presentation of the same stimulus or stimulus category. Both repetition enhancement and suppression effects have been found in adults depending on experimental parameters, especially stimulus complexity and quality. Suppression is more common. Repetition enhancement is mainly assumed to occurs when stimuli are degraded, masked or when participants do not have a stable memory representation of the stimuli (Henson and Rugg, 2003; Henson et al., 2002; Henson et al., 2000; Naccache and Dehaene, 2001; Segaert et al., 2013).
Similarly to adults, both repetition suppression and enhancement have been found in developmental populations. Indeed, several studies have found an increase of the hemodynamic response when a condition or a stimulus was repeated. Bouchon et al. (2015) presented newborns with trisyllabic sequences following either an ABB (e.g. “mulele”) or an ABC pattern (e.g. “mulevi”), following-up on Gervain et al. (2008). In contrast to Gervain et al. (2008), however, who presented blocks in an interleaved, random order, Bouchon et al. (2015) grouped together all blocks of the same condition. Gervain et al. (2008) found an increasingly greater response over the course of the experiment for the ABB patterns as compared to the ABC ones, whereas Bouchon et al. (2015) showed a repetition enhancement effect during the time course of the ABC condition. The grouped vs. interleaved presentation modes may have contributed to these differences, although it needs to be noted that the two studies also differed in stimulus complexity, as Gervain et al. (2008) used 280 unique trisyllabic sequences, each of which occurred only once, whereas Bouchon et al. (2015) used 24 different sequences, each repeated 6 times.
Repetition suppression effects were also found in infants. At 3–4 months, the frontal cortex showed reduced hemodynamic response over time to the repetition of the same syllable 15 times (Nakano et al., 2009). Similarly, 5–7-month-olds showed a reduced hemodynamic response in the frontal cortex when hearing the same word 8 times in a block as compared to blocks containing 8 different words (Emberson et al., 2017a, Emberson et al., 2017b).
What factors determine whether enhancement or suppression is observed? The two studies that showed a repetition suppression effect both repeated the exact same physical stimuli within each condition (Emberson et al., 2016; Nakano et al., 2009), whereas in the studies that found enhancement, repetition occurred at a more abstract, structural level (e.g. in Gervain et al. (2008) and Bouchon et al. (2015) different trisyllabic sequences were presented, but they all shared a common ABB pattern). Repetition enhancement might thus reflect the ongoing effort to build a representation of the stimulus category, whereas repetition suppression may typically occur when the category is encoded in a more stable way (see Nordt et al., 2016 for a discussion).
The repetition suppression effect serves as a basis to create powerful experimental designs for functional studies of the cortex, namely the habituation/dishabituation design, and it has been suggested that the neural mechanisms might be analogous to the cognitive mechanisms underpinning behavioral habituation paradigms (Turk-Browne et al., 2008). In these experiments, a stimulus is repeated several times to produce neural suppression, followed by the presentation of a test stimulus, different from the repeated stimulus along the tested dimension (Grill-Spector and Malach, 2001). This design provides a powerful tool to show differential encoding of two stimulus categories. Specifically, a rebound of activity is observed when a novel test stimulus is presented, as the suppression effect subsides. If the two types of stimuli were presented in a simple randomized fashion, activity could be similar for both. This design is widely used in adult fMRI as well as in adult and infant EEG studies. In infant EEG studies, the habituation effect is revealed by a response suppression when the test stimulus is preceded by stimuli from the same category as compared to stimuli preceded by a different-category item (e.g. Friedrich and Friederici, 2004; Gliga and Dehaene-Lambertz, 2007; Jeschonek et al., 2010; Torkildsen et al., 2009).
Habituation designs have recently been adapted for infant NIRS studies to reveal fine-grained discrimination abilities. For instance, newborns were familiarized with repeated words and the hemodynamic response to a change in consonant or vowel was measured in the test phase. A rebound of the hemodynamic response was observed in the right frontal cortex in response to a consonant change (Benavides-Varela et al., 2012). Here, the suppression effect reveals a subtle discrimination ability, namely that of the consonant/vowel distinction.
4.3. Alternating presentation
Alternating/non-alternating designs are often used in developmental NIRS studies to show subtle discrimination. This design was originally developed for behavioral paradigms to “offer a more sensitive measure than the between-trial comparisons” (Best and Jones, 1998). Two types of blocks are presented: homogeneous or non-alternating blocks composed of stimuli from the same experimental condition, and alternating blocks composed of stimuli from two different conditions in alternation.
One of the first NIRS studies using this design presented 4- and 10-month-old infants with disyllabic words containing either a high-low or a low-high pitch accent, typical of Japanese, or pure tone pairs corresponding to the pitch contours of the words. In each of these two conditions, half of the blocks contained only one of the two contours, the other half contained an alternation between the two. The alternating blocks elicited a larger response in the word condition than in the pure tone condition in the 10-month-old infants (Sato et al., 2010). Another example for the use of alternating/non-alternating designs comes from the field of number perception. 6-month-old infants were presented with blocks of 10 different images containing either 8 or 16 dots varying in size, color and spatial position. Half of the blocks contained images of 8 dots only, the other half contained alternating images of 8 and 16 dots. Alternating blocks elicited a larger response than non-alternating blocks in the right parietal region (Edwards et al., 2016). In newborns, experiments that used an alternating/non-alternating design have sometimes found a greater response to non-alternating than to alternating blocks (Gervain et al., 2012; Gervain et al., 2016; Issard and Gervain, 2017). In newborns and infants, auditory short-term memory is less developed than in adults or children, estimated between 800 and 1500 ms for newborns (Cheour et al., 2002). This estimation is much shorter than the interblock interval required for the hemodynamic response to decline, but is similar to the delay typically used between items within blocks. If the two conditions are presented side by side as is the case within alternating blocks, then it may be easier for newborns to remember the previous stimulus, compare it to the current stimulus, and potentially discriminate between the two.
5. Conclusions: the hemodynamic response as a reflection of cognitive effort
In this paper, we reviewed a wide variety of studies in which fNIRS is used with typical and clinical developmental populations of various ages in different perceptual and cognitive tasks. Experimental results suggest that in addition to the variability over developmental time and brain areas, the hemodynamic response seems to be further influenced by the complexity of the stimuli used, the development of the cognitive processes tested, and the experimental design, even within the same age or brain area. Each of these factors has a different impact on the hemodynamic response, and they interact with one another.
Stimulus complexity seems to influence the hemodynamic response in a non-linear fashion. Based on the existing literature, we have argued that when stimuli are too simple, they evoke smaller responses than when stimuli are of middle-range complexity. Overly complex stimuli lead to null or inverted responses.
Cognitive development can modulate the hemodynamic response within the same cortical area. When a cognitive function is more developed, a canonical response is more likely to be observed. In experiments testing a less developed function (i.e. younger participants), a larger response may be interpreted as a signature of more efforts to process the stimuli. When the stimuli are particularly difficult for the tested developmental stage, inverted responses can more often be observed.
Experimental design can also influence the hemodynamic response: a repeated presentation of identical or similar stimuli might lead to a reduction of the hemodynamic response (i.e. habituation), while designs in which conditions alternate might evoke different hemodynamic response amplitudes between alternating and non-alternating blocks.
The influence of these modifying factors may be best understood in a common framework combining behavioral and neural mechanisms in which the hemodynamic response is interpreted as processing effort. For an a priori prediction of expected effects and an appropriate interpretation of NIRS results, it is essential to have an operational model, behavioral and/or neural, of the processing mechanisms involved, and how the different factors manipulated in a given study might modulate processing effort.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This research was supported by a HFSP Young Investigator Grant nr. RGY 0073/2014, an ANR grant nr. ANR-15-CE37-0009-01 and a French Investissements d’Avenir - Labex EFL program grant (ANR-10-LABX-0083) to JG. We thank Katie Von Holzen and the anonymous reviewers for their insightful comments and suggestions on previous versions of the manuscript.
Footnotes
In the text, we distinguish between the Hemodynamic Response Function (HRF), i.e. the canonical, idealized physiological response defined here, and the actual NIRS signal measured in any specific participant, which might approximate the canonical HRF (to different degrees) or not.
In adults, other niches for fNIRS also exist, e.g. measuring brain activation during intensive motion such as sports or heavy cognitive tasks, for instance pilots flying planes in flight simulators etc., all of which are incompatible with MRI.
In the infant literature, it is sometimes the case that only one species of Hb shows a significant response, typically HbO, and the other fails to reach significance, although it shows change. As is common practice in infant work, we therefore adopt this looser definition of the canonical and inverted response shapes in our review, since it is often simply a matter of statistical analysis/power that one Hb species doesn’t reach significance.
Strictly speaking, simple block designs also take advantage of repetition effects as they present several stimuli from the same condition to elicit a larger response than event related designs. However they don’t measure the effect of the repetition on the response to individual stimuli within a block, but rather the overall response to the whole block. For this reason, we discuss the simple block design together with event-related designs, and separately from designs with a large number of block repetitions, the aim of which is to specifically probe the repetition effect.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.01.009.
Contributor Information
Cécile Issard, Email: cecile.issard@etu.parisdescartes.fr.
Judit Gervain, Email: judit.gervain@parisdescartes.fr.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Abboub N., Nazzi T., Gervain J.1. Prosodic grouping at birth. Brain Lang. 2016;162:46–59. doi: 10.1016/j.bandl.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Arichi T., Fagiolo G., Varela M., Melendez-Calderon A., Allievi A., Merchant N., Edwards A.D. Development of BOLD signal hemodynamic responses in the human brain. Neuroimage. 2012;63(2):663–673. doi: 10.1016/j.neuroimage.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci M., Winberg J., Ruggiero C., Bergqvist L.L., Serra G., Lagercrantz H. Activation of olfactory cortex in newborn infants after odor stimulation: a functional near-infrared spectroscopy study. Pediatr. Res. 2000;48(1):18–23. doi: 10.1203/00006450-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Benavides-Varela S., Hochmann J.-R., Macagno F., Nespor M., Mehler J. Newborn’s brain activity signals the origin of word memories. Proc. Natl. Acad. Sci. U. S. A. 2012;109(44):17908–17913. doi: 10.1073/pnas.1205413109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Varela S., Siugzdaite R., Gómez D.M., Macagno F., Cattarossi L., Mehler J. Brain regions and functional interactions supporting early word recognition in the face of input variability. Proc. Natl. Acad. Sci. U. S. A. 2017;114(29):7588–7593. doi: 10.1073/pnas.1617589114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best C., Jones C. Stimulus-alternation preference procedure to test infant speech discrimination. Infant Behav. Dev. 1998;21:295. [Google Scholar]
- Bouchon C., Nazzi T., Gervain Judit. Hemispheric asymmetries in repetition enhancement and suppression effects in the newborn brain. PLOS ONE. 2015;10(10):e0140160. doi: 10.1371/journal.pone.0140160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Koutstaal W. Functional neuroimaging studies of encoding, priming, and explicit memory retrieval. Proc. Natl. Acad. Sci. 1998;95(3):891–898. doi: 10.1073/pnas.95.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheour M., Čėponiené R., Leppänen P., Alho K., Kujala T., Renlund M., Näätänen R. The auditory sensory memory trace decays rapidlyin newborns. Scand. J. Psychol. 2002;43(1):33–39. doi: 10.1111/1467-9450.00266. [DOI] [PubMed] [Google Scholar]
- Cristia A., Dupoux E., Hakuno Y., Lloyd-Fox S., Schuetze M., Kivits J., Minagawa-Kawai Y. An online database of infant functional near infraRed spectroscopy studies: a community-augmented systematic review. PLOS ONE. 2013;8(3):e58906. doi: 10.1371/journal.pone.0058906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csibra G., Henty J., Volein Á., Elwell C., Tucker L., Meek J., Johnson M.H. Near infrared spectroscopy reveals neural activation during face perception in infants and adults. J. Pediatr. Neurol. 2004;02(02):085–089. [Google Scholar]
- Edwards L.A., Wagner J.B., Simon C.E., Hyde D.C. Functional brain organization for number processing in pre-verbal infants. Dev. Sci. 2016;19:757–769. doi: 10.1111/desc.12333. [DOI] [PubMed] [Google Scholar]
- Emberson L.L., Richards J.E., Aslin R.N. Top-down modulation in the infant brain: learning-induced expectations rapidly affect the sensory cortex at 6 months. Proc. Natl. Acad. Sci. 2015;112(31):9585–9590. doi: 10.1073/pnas.1510343112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Crosswhite S.L., Goodwin J.R., Berger A.J., Aslin R.N. Isolating the effects of surface vasculature in infant neuroimaging using short-distance optical channels: a combination of local and global effects. Neurophotonics. 2016;3(3):031406. doi: 10.1117/1.NPh.3.3.031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Boldin A.M., Riccio J.E., Guillet R., Aslin R.N. Deficits in top-down sensory prediction in infants at risk due to premature birth. Curr. Biol. 2017;27(3):431–436. doi: 10.1016/j.cub.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Cannon G., Palmeri H., Richards J.E., Aslin R.N. Using fNIRS to examine occipital and temporal responses to stimulus repetition in young infants: evidence of selective frontal cortex involvement. Dev. Cogn. Neurosci. 2017;23:26–38. doi: 10.1016/j.dcn.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry A.L., Fló A., Brusini P., Cattarossi L., Macagno F., Nespor M., Mehler J. On the edge of language acquisition: inherent constraints on encoding multisyllabic sequences in the neonate brain. Dev. Sci. 2016;19:488–503. doi: 10.1111/desc.12323. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Friederici A.D. N400-like semantic incongruity effect in 19-month-olds: processing known words in picture contexts. J. Cogn. Neurosci. 2004;16(8):1465–1477. doi: 10.1162/0898929042304705. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Ajichi Y., Okada E. Monte Carlo prediction of near-infrared light propagation in realistic adult and neonatal head models. Appl. Opt. 2003;42(16):2881–2887. doi: 10.1364/ao.42.002881. [DOI] [PubMed] [Google Scholar]
- Gómez D.M., Berent I., Benavides-Varela S., Bion R.A.H., Cattarossi L., Nespor M., Mehler J. Language universals at birth. Proc. Natl. Acad. Sci. 2014;111(16):5837–5841. doi: 10.1073/pnas.1318261111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J., Macagno F., Cogoi S., Peña M., Mehler J. The neonate brain detects speech structure. Proc. Natl. Acad. Sci. 2008;105(37):14222–14227. doi: 10.1073/pnas.0806530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J., Berent I., Werker J.F. Binding at birth: the newborn brain detects identity relations and sequential position in speech. J. Cogn. Neurosci. 2012;24(3):564–574. doi: 10.1162/jocn_a_00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervain J., Werker J.F., Black A., Geffen M.N. The neural correlates of processing scale-invariant environmental sounds at birth. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Gliga T., Dehaene-Lambertz G. Development of a view-invariant representation of the human head. Cognition. 2007;102(2):261–288. doi: 10.1016/j.cognition.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Goldowsky B.N., Newport E.L. Modeling the effects of processing limitations on the acquisition of morphology: the less is more hypothesis. The Proceedings of the 24th Annual Child Language Research Forum; Center for the Study of Language (CSLI); 1993. [Google Scholar]
- Grill-Spector K., Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol. 2001;107(1–3):293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grossmann T., Johnson M.H., Lloyd-Fox S., Blasi A., Deligianni F., Elwell C., Csibra G. Early cortical specialization for face-to-face communication in human infants. Proc. R. Soc. Lond. B: Biol. Sci. 2008;275(1653):2803–2811. doi: 10.1098/rspb.2008.0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Oberecker R., Koch S.P., Friederici A.D. The developmental origins of voice processing in the human brain. Neuron. 2010;65(6):852–858. doi: 10.1016/j.neuron.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Parise E., Friederici A.D. The detection of communicative signals directed at the self in infant prefrontal cortex. Front. Hum. Neurosci. 2010:4. doi: 10.3389/fnhum.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R.N.A., Rugg M.D. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41(3):263–270. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- Henson R., Shallice T., Dolan R. Neuroimaging evidence for dissociable forms of repetition priming. Science. 2000;287(5456):1269–1272. doi: 10.1126/science.287.5456.1269. [DOI] [PubMed] [Google Scholar]
- Henson R.N.A., Shallice T., Gorno-Tempini M.L., Dolan R.J. Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cereb. Cortex. 2002;12(2):178–186. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Asakawa K., Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neurosci. Res. 2006;54(4):276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Taga G. Prosodic processing in the developing brain. Neurosci. Res. 2007;59(1):29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Nakano T., Taga G. Large-scale brain networks underlying language acquisition in early infancy. Front. Psychol. 2011:2. doi: 10.3389/fpsyg.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homae F., Watanabe H., Taga G. The neural substrates of infant speech perception. Lang. Learn. 2014;64(s2):6–26. [Google Scholar]
- Hunter M.A., Ames E.W. A multifactor model of infant preferences for novel and familiar stimuli. Adv. Infancy Res. 1988;5:69–95. [Google Scholar]
- Issard C., Gervain J. Adult-like processing of time-compressed speech by newborns: a NIRS study. Dev. Cogn. Neurosci. 2017;25:176–184. doi: 10.1016/j.dcn.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschonek S., Marinovic V., Hoehl S., Elsner B., Pauen S. Do animals and furniture items elicit different brain responses in human infants? Brain Dev. 2010;32(10):863–871. doi: 10.1016/j.braindev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kidd C., Piantadosi S.T., Aslin R.N. The goldilocks effect: human infants allocate attention to visual sequences that are neither too simple nor too complex. PLOS ONE. 2012;7(5):e36399. doi: 10.1371/journal.pone.0036399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Otsuka Y., Nakato E., Kanazawa S., Yamaguchi M.K., Kakigi R. Do infants represent the face in a viewpoint-invariant manner? Neural adaptation study as measured by near-infrared spectroscopy. Front. Hum. Neurosci. 2011;5:153. doi: 10.3389/fnhum.2011.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S.M., Gregg N.M., White B.R., Zeff B.W., Bjerkaas K.A., Inder T.E., Culver J.P. Neonatal hemodynamic response to visual cortex activity: high-density near-infrared spectroscopy study. J. Biomed. Opt. 2010;15(2) doi: 10.1117/1.3369809. 026010-026010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Elwell C.E. Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010;34(3):269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Richards J.E., Blasi A., Murphy D.G.M., Elwell C.E., Johnson M.H. Coregistering functional near-infrared spectroscopy with underlying cortical areas in infants. Neurophotonics. 2014;1(2):025006. doi: 10.1117/1.NPh.1.2.025006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Fox S., Moore S., Darboe M., Prentice A., Papademetriou M., Blasi A., Elwell C.E. fNIRS in Africa & Asia: an objective measure of cognitive development for global health settings. FASEB J. 2016;30(1 Supplement):18–1149. (1149) [Google Scholar]
- Lloyd-Fox S., Begus K., Halliday D., Pirazzoli L., Blasi A., Papademetriou M., Elwell C.E. Cortical specialisation to social stimuli from the first days to the second year of life: a rural Gambian cohort. Dev. Cogn. Neurosci. 2018 doi: 10.1016/j.dcn.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudzadeh M., Dehaene-Lambertz G., Fournier M., Kongolo G., Goudjil S., Dubois J., Wallois F. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proc. Natl. Acad. Sci. 2013 doi: 10.1073/pnas.1212220110. 201212220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudzadeh M., Wallois F., Kongolo G., Goudjil S., Dehaene-Lambertz G. Functional maps at the onset of auditory inputs in very early preterm human neonates. Cereb. Cortex. 2017;27(4):2500–2512. doi: 10.1093/cercor/bhw103. [DOI] [PubMed] [Google Scholar]
- Matsui M., Homae F., Tsuzuki D., Watanabe H., Katagiri M., Uda S., Taga G. Referential framework for transcranial anatomical correspondence for fNIRS based on manually traced sulci and gyri of an infant brain. Neurosci. Res. 2014;80:55–68. doi: 10.1016/j.neures.2014.01.003. [DOI] [PubMed] [Google Scholar]
- May L., Byers-Heinlein K., Gervain J., Werker J.F. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front. Psychol. 2011;2:222. doi: 10.3389/fpsyg.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L., Gervain J., Carreiras M., Werker J.F. The specificity of the neural response to speech at birth. Dev. Sci. 2017 doi: 10.1111/desc.12564. 00:e12564. [DOI] [PubMed] [Google Scholar]
- Meek J.H., Firbank M., Elwell C.E., Atkinson J., Braddick O., Wyatt J.S. Regional hemodynamic responses to visual stimulation in awake infants. Pediatr. Res. 1998;43(6):840–843. doi: 10.1203/00006450-199806000-00019. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y., Mori K., Naoi N., Kojima S. Neural attunement processes in infants during the acquisition of a language-specific phonemic contrast. J. Neurosci. 2007;27(2):315–321. doi: 10.1523/JNEUROSCI.1984-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molavi B., May L., Gervain J., Carreiras M., Werker J.F., Dumont G.A. Analyzing the resting state functional connectivity in the human language system using near infrared spectroscopy. Front. Hum. Neurosci. 2014:7. doi: 10.3389/fnhum.2013.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullinger K.J., Mayhew S.D., Bagshaw A.P., Bowtell R., Francis S.T. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG–BOLD–CBF study in humans. Neuroimage. 2014;94(Supplement C):263–274. doi: 10.1016/j.neuroimage.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Naccache L., Dehaene S. The priming method: imaging unconscious repetition priming reveals an abstract representation of number in the parietal lobes. Cereb. Cortex. 2001;11(10):966–974. doi: 10.1093/cercor/11.10.966. [DOI] [PubMed] [Google Scholar]
- Nakano T., Watanabe H., Homae F., Taga G. Prefrontal cortical involvement in young infants’ analysis of novelty. Cereb. Cortex. 2009;19(2):455–463. doi: 10.1093/cercor/bhn096. [DOI] [PubMed] [Google Scholar]
- Nordt M., Hoehl S., Weigelt S. The use of repetition suppression paradigms in developmental cognitive neuroscience. Cortex. 2016 doi: 10.1016/j.cortex.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Okada E., Delpy D.T. Near-infrared light propagation in an adult head model. I. Modeling of low-level scattering in the cerebrospinal fluid layer. Appl. Opt. 2003;42(16):2906–2914. doi: 10.1364/ao.42.002906. [DOI] [PubMed] [Google Scholar]
- Okada E., Delpy D.T. Near-infrared light propagation in an adult head model. II. Effect of superficial tissue thickness on the sensitivity of the near-infrared spectroscopy signal. Appl. Opt. 2003;42(16):2915–2922. doi: 10.1364/ao.42.002915. [DOI] [PubMed] [Google Scholar]
- Pena M., Maki A., Kovacic D., Dehaene-Lambertz G., Koizumi H., Bouquet F., Mehler J. Sounds and silence: an optical topography study of language recognition at birth. Proc. Natl. Acad. Sci. U. S. A. 2003;100(20):11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche-Labarbe N., Fenoglio A., Radakrishnan H., Kocienski-Filip M., Carp S.A., Dubb J., Franceschini M.A. Somatosensory evoked changes in cerebral oxygen consumption measured non-invasively in premature neonates. NeuroImage. 2014;85(0 1) doi: 10.1016/j.neuroimage.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S., Telkemeyer S., Wartenburger I., Obrig H. Shedding light on words and sentences: near-infrared spectroscopy in language research. Brain Lang. 2012;121(2):152–163. doi: 10.1016/j.bandl.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Sakatani K., Chen S., Lichty W., Zuo H., Wang Y.P. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum. Dev. 1999;55(3):229–236. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Sato Y., Sogabe Y., Mazuka R. Development of hemispheric specialization for lexical pitch-accent in Japanese infants. J. Cogn. Neurosci. 2010;22(11):2503–2513. doi: 10.1162/jocn.2009.21377. [DOI] [PubMed] [Google Scholar]
- Sato H., Hirabayashi Y., Tsubokura H., Kanai M., Ashida T., Konishi I., Maki A. Cerebral hemodynamics in newborn infants exposed to speech sounds: a whole-head optical topography study. Hum. Brain Mapp. 2012;33(9):2092–2103. doi: 10.1002/hbm.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholkmann F., Kleiser S., Metz A.J., Zimmermann R., Mata Pavia J., Wolf U., Wolf M. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage. 2014;85:6–27. doi: 10.1016/j.neuroimage.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Segaert K., Weber K., de Lange F.P., Petersson K.M., Hagoort P. The suppression of repetition enhancement: a review of fMRI studies. Neuropsychologia. 2013;51(1):59–66. doi: 10.1016/j.neuropsychologia.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Sevy A.B.G., Bortfeld H., Huppert T.J., Beauchamp M.S., Tonini R.E., Oghalai J.S. Neuroimaging with near-infrared spectroscopy demonstrates speech-evoked activity in the auditory cortex of deaf children following cochlear implantation. Hear. Res. 2010;270(1–2):39–47. doi: 10.1016/j.heares.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A., Yacoub E., Pfeuffer J., Van de Moortele P.-F., Adriany G., Hu X., Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36(6):1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Steinbrink J., Villringer A., Kempf F., Haux D., Boden S., Obrig H. Illuminating the BOLD signal: combined fMRI–fNIRS studies. Magn. Reson. Imaging. 2006;24(4):495–505. doi: 10.1016/j.mri.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Strangman G., Boas D.A., Sutton J.P. Non-invasive neuroimaging using near-infrared light. Biol. Psychiatry. 2002;52(7):679–693. doi: 10.1016/s0006-3223(02)01550-0. [DOI] [PubMed] [Google Scholar]
- Taga G., Asakawa K., Hirasawa K., Konishi Y. Hemodynamic responses to visual stimulation in occipital and frontal cortex of newborn infants: a near-infrared optical topography study. Early Hum. Dev. 2003;75:203–210. doi: 10.1016/j.earlhumdev.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Taga G., Asakawa K., Maki A., Konishi Y., Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proc. Natl. Acad. Sci. 2003;100(19):10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga G., Watanabe H., Homae F. Spatiotemporal properties of cortical haemodynamic response to auditory stimuli in sleeping infants revealed by multi-channel near-infrared spectroscopy. Phil. Trans. R. Soc. A. 2011;369(1955):4495–4511. doi: 10.1098/rsta.2011.0238. [DOI] [PubMed] [Google Scholar]
- Telkemeyer S., Rossi S., Koch S.P., Nierhaus T., Steinbrink J., Poeppel D., Wartenburger I. Sensitivity of newborn auditory cortex to the temporal structure of sounds. J. Neurosci. 2009;29(47):14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkildsen J., von K., Friis Hansen H., Svangstu J.M., Smith L., Simonsen H.G., Moen I., Lindgren M. Brain dynamics of word familiarization in 20-month-olds: effects of productive vocabulary size. Brain Lang. 2009;108(2):73–88. doi: 10.1016/j.bandl.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Turk-Browne N.B., Scholl B.J., Chun M.M., Turk-Browne N.B., Scholl B.J., Chun M.M. Babies and brains: habituation in infant cognition and functional neuroimaging. Front. Hum. Neurosci. 2008;2:16. doi: 10.3389/neuro.09.016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa S., Takamoto K., Ishikawa A., Ono T., Nishijo H. Selective medial prefrontal cortex responses during live mutual gaze interactions in human infants: an fNIRS study. Brain Topogr. 2015;28(5):691–701. doi: 10.1007/s10548-014-0414-2. [DOI] [PubMed] [Google Scholar]
- Wagner J.B., Fox S.E., Tager-Flusberg H., Nelson C.A. Neural processing of repetition and non-repetition grammars in 7- and 9-month-old infants. Front. Psychol. 2011:2. doi: 10.3389/fpsyg.2011.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallois F., Patil A., Héberlé C., Grebe R. EEG-NIRS in epilepsy in children and neonates. Neurophysiol. Clin.e/Clin. Neurophysiol. 2010;40(5–6):281–292. doi: 10.1016/j.neucli.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Homae F., Nakano T., Taga G. Functional activation in diverse regions of the developing brain of human infants. Neuroimage. 2008;43(2):346–357. doi: 10.1016/j.neuroimage.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Homae F., Taga G. Activation and deactivation in response to visual stimulation in the occipital cortex of 6-month-old human infants. Dev. Psychobiol. 2012;54(1):1–15. doi: 10.1002/dev.20569. [DOI] [PubMed] [Google Scholar]
- Wolf M., Ferrari M., Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J. Biomed. Opt. 2007;12(6):062104. doi: 10.1117/1.2804899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.