Highlights
-
•
Quantified variability in evoked neural responses of children with and without dyslexia.
-
•
A subset of children with dyslexia had significantly higher variability in cortex.
-
•
Higher variability observed in auditory and visual domains in multiple reading network nodes.
-
•
Risk alleles in KIAA0319 were related to degree of variability in auditory cortex.
-
•
Results support unstable neural responses as a mechanism for some cases of dyslexia.
Keywords: KIAA0319, gene, reading, neural variability, subgroups, mechanisms
Abstract
Individuals with dyslexia exhibit increased brainstem variability in response to sound. It is unknown as to whether increased variability extends to neocortical regions associated with audition and reading, extends to visual stimuli, and whether increased variability characterizes all children with dyslexia or, instead, a specific subset of children. We evaluated the consistency of stimulus-evoked neural responses in children with (N = 20) or without dyslexia (N = 12) as measured by magnetoencephalography (MEG). Approximately half of the children with dyslexia had significantly higher levels of variability in cortical responses to both auditory and visual stimuli in multiple nodes of the reading network. There was a significant and positive relationship between the number of risk alleles at rs6935076 in the dyslexia-susceptibility gene KIAA0319 and the degree of neural variability in primary auditory cortex across all participants. This gene has been linked with neural variability in rodents and in typical readers. These findings indicate that unstable representations of auditory and visual stimuli in auditory and other reading-related neocortical regions are present in a subset of children with dyslexia and support the link between the gene KIAA0319 and the auditory neural variability across children with or without dyslexia.
1. Introduction
Dyslexia is a heritable, neurobiological disorder that is diagnosed by difficulty in acquiring reading as compared to typically-developing (TD) peers despite average nonverbal IQ and adequate schooling (Lyon et al., 1995; Peterson and Pennington, 2012; Schulte-Körne, 2010; Shaywitz, 1998). The precise neural and genetic bases for dyslexia are unknown. Given the observation that there is no causal gene for dyslexia (Galaburda et al., 2006; Müller et al., 2016; Scerri and Schulte-Körne, 2010) and even some controversy about the neural (Boets et al., 2013; Ramus and Szenkovits, 2008) and behavioral deficits present (Lorusso et al., 2014; Morris et al., 1998; Vaessen et al., 2009; Ziegler et al., 2008), it is likely that there is no unitary basis for dyslexia. Instead, it is likely that there are several subgroups within this broad diagnostic category. One such subgroup may be marked by inconsistent neural responses to speech sounds, which may in turn interfere with efficient letter-to-sound mapping during reading acquisition. Here, we tested the hypotheses that some, but not all, children with dyslexia have a specifically unstable neocortical response to visual and auditory stimuli and that this instability is associated with a specific risk gene for dyslexia (KIAA0319).
Although dyslexia is often conceptualized as a specific language-based disorder, there is also evidence suggesting a broader perceptual basis. Individuals with dyslexia often have difficulty with phoneme awareness that both developmentally precedes learning to read and accompanies difficulty in reading (Harm and Seidenberg, 1999; Rey et al., 2002; Schulte-Körne et al., 1999). There is evidence, however, that this deficit in phoneme awareness may be caused by abnormal neural responses to auditory stimuli (Hornickel and Kraus, 2013; Kovelman et al., 2012; Lehongre et al., 2011; Neef et al., 2017, 2016 but see Boets et al., 2013; Ramus and Szenkovits, 2008). These abnormal responses include delayed latency, reduced amplitude, and diminished mismatch negativity (Kujala, 2007; Näätänen et al., 1978) and are present in response to verbal (speech sounds) and non-verbal (tones) auditory stimuli (Erez and Pratt, 1992; Mcanally and Stein, 1996; Schulte-Körne and Deimel, 1999). In addition, struggling readers with no formal diagnosis of dyslexia exhibit increased neural variability in response to synthetic consonant sounds in the auditory brainstem compared to their typically reading peers (Hornickel and Kraus, 2013; Neef et al., 2017, 2016).
Increased variability in brain responses to perceptual inputs may be linked with a specific gene that is associated with some, but not all, instances of dyslexia. There is a strong genetic component to dyslexia, with approximately 68% concordance in monozygotic twins (DeFries et al., 1987) and a number of genes have been identified as susceptibility genes (Galaburda et al., 2006; Lind et al., 2010; Scerri et al., 2011). One of these genes, KIAA0319, is located on chromosome 6p22.3 and is involved in neuronal migration (Galaburda et al., 2006) and inhibition of axonal growth (Franquinho et al., 2017). Reduced expression of KIAA0319 in rats causes increased variability in neural responses to tones, to short bursts of broadband noise, and to speech sounds (Centanni et al., 2014a). A genetic composite in KIAA0319, including the single nucleotide polymorphisms (SNPs) rs6935076 and rs761100, was related to the degree of speech sound response variability observed in the auditory brainstem of typically developing children as well as performance on spelling measures (Neef et al., 2017). These SNPs have been consistently named as risk SNPs for dyslexia, with the minor allele conferring increased risk (Cope et al., 2005; Müller et al., 2016; Paracchini et al., 2008). Taken together, these results suggest that the gene KIAA0319 plays a role in the consistency of neural responses in typical readers. It is unknown whether this gene is associated with neural inconsistency in children with dyslexia.
The current study was designed to test four specific hypotheses. First, we hypothesized that increased variability for sound would occur not only in the brainstem as previously found (Hornickel and Kraus, 2013), but also in neocortical regions associated with audition. We therefore examined whether variability in stimulus-driven neural responses to consonant-vowel-consonant speech sounds was significantly higher in the primary auditory cortex (found on Heschl’s gyrus in humans) in children with dyslexia compared to their typically reading peers. Second, we hypothesized that only a subset of children with dyslexia would show increased neural variability. This hypothesis is consistent with others who propose that not all children with dyslexia are characterized by altered basic perception (Boets et al., 2013; Paul et al., 2006). If only a subset of children with dyslexia demonstrate significantly increased variability, then it is possible either that globally increased variance is a marker of this subgroup or that such variance is specific to the auditory domain. Third, we hypothesized that increased variability is not limited to speech (verbal stimuli) and extends to other kinds of stimuli and to reading network regions beyond auditory cortex. To test this hypothesis, we examined variability of response to nonverbal auditory (tones), verbal visual (printed letters), and nonverbal visual (unnamable shapes) stimuli in several reading network regions. Fourth, we hypothesized that either or both of two specific variants in the dyslexia-susceptibility gene KIAA0319 (rs6935076 or rs761100) are related to the level of neural variability observed. This hypothesis was motivated by evidence that this gene is associated with greater variability of brain responses in rats (Centanni et al., 2014a, 2014b). We focused on these specific SNPs because they have been linked to dyslexia in humans (Cope et al., 2005; Müller et al., 2016; Neef et al., 2017; Paracchini et al., 2008).
2. Methods and Materials
2.1. Participants
We recruited 48 children from the greater Boston area (7-14 y/o). From this sample, a subset of 32 participants with dyslexia (DYS, N = 20, 6 females) or without dyslexia (typically developing/TD, N = 12, 4 females) were selected so that participants across groups were age-matched (10.10 ± 2.05 y/o in TD vs. 11.18 ± 2.01 y/o in DYS; unpaired t-test, t (29) = 1.43, p = 0.16). All children were required to have been exposed to English from birth with no history of neuropsychological conditions, including ADHD and autism. All children scored in the average range (greater than a standard score of 85) on measures of nonverbal IQ (Reynolds Intelligence Assessment Scales; RIAS; Reynolds and Kamphaus, 2003) and oral language (Clinical Evaluation of Language Fundamentals; CELF-4; Semel et al., 1987). Children were placed in the dyslexia group if they had a previous dyslexia diagnosis and if they scored below 90 on at least two of the following four subtests: Sight Word Efficiency (SWE; TOWRE; Torgensen et al., 1999), Phonemic Decoding Efficiency (PDE; TOWRE), Word Identification (WID; Woodcock Reading Mastery Test/WRMT-3; Woodcock et al., 2001), and Word Attack (WA; WRMT-3). This approach takes into account both performance-based models of dyslexia (including the 25th percentile approach; Catts et al., 2005; Meyer et al., 1998) as well as IQ-cutoff definitions (above -1 standard deviation on nonverbal IQ and < -1 standard deviation on 2 of the 4 reading measures; (Catts et al., 2005). Of the 16 children who were excluded from the original 48 participants, 3 were excluded for low oral language scores, 1 was excluded due to age exceeding the target range, 1 was excluded from the TD group for having a sibling with a diagnosed reading impairment, 4 were excluded for having a prior dyslexia diagnosis with reading scores above our threshold for dyslexia, 1 was excluded from the dyslexia group for neural variability greater than 3 standard deviations above the group mean, and 6 were excluded for poor neural imaging quality. Characteristics of the finalized groups are summarized in Table 1. All parents provided written consent and children provided written and verbal assent to participate in the study.
Table 1.
Participant characteristics, including all children with either usable genetics data, usable imaging data, or both. Data are reported as mean ± standard deviation.
| TD (N = 12) | DYS (N = 20) | p value | |
|---|---|---|---|
| Number of females | 4 | 6 | |
| Age (Years) | 10.10 ± 2.05 | 11.18 ± 2.01 | 0.16 |
| IQ(RIAS) | 115.75 ± 10.09 | 108.05 ± 8.15 | 0.03 |
| Oral Language (CELF-4) | 113.75 ± 9.61 | 99.89 ± 10.72 | 0.001 |
| Timed sight word reading(TOWRE-SWE) | 111.50 ± 10.94 | 86.20 ± 10.92 | <0.0001 |
| Timed phonemic decoding(TOWRE-PDE) | 107.83 ± 10.82 | 80.05 ± 9.58 | <0.0001 |
| Untimed sight word reading(WRMT-WID) | 112.00 ± 14.17 | 84.44 ± 9.08 | <0.0001 |
| Untimed pseudoword reading(WRMT-WA) | 102.60 ± 19.62 | 82.13 ± 10.58 | 0.002 |
2.2. Stimuli
We presented verbal and nonverbal stimuli in the auditory or visual domain. Auditory verbal stimuli consisted of four consonant-vowel-consonant (CVC) speech sounds which were spoken by a female, native English speaker and recorded in a double-walled soundproof booth (/bad/, /dad/, /gad/, /tad/) (Centanni et al., 2013; Engineer et al., 2008). Visual verbal stimuli (printed letters) consisted of the lower-case first letter of each of these CVC sounds presented in black, Arial font in the center of a gray screen. The chosen sounds were initial consonants that are commonly difficult to discriminate by children with dyslexia (Marshall et al., 2001) and have been used in previous studies (Centanni et al., 2014a, 2014b, 2013; Engineer et al., 2008). Auditory nonverbal stimuli consisted of 500 ms tones at four frequencies (200, 500, 1500, and 2000 Hz) and visual nonverbal stimuli were four unnamable shapes (based on polygons reported previously; Alt et al., 2016) outlined in black and presented in the center of the screen. All trials were approximately 500 ms in length. To avoid habituation due to predictable stimulus onset times (Budd et al., 1998; Butler, 1968; Polich, 1990), the interstimulus interval was randomly selected between 750-1750 ms.
2.3. Imaging task
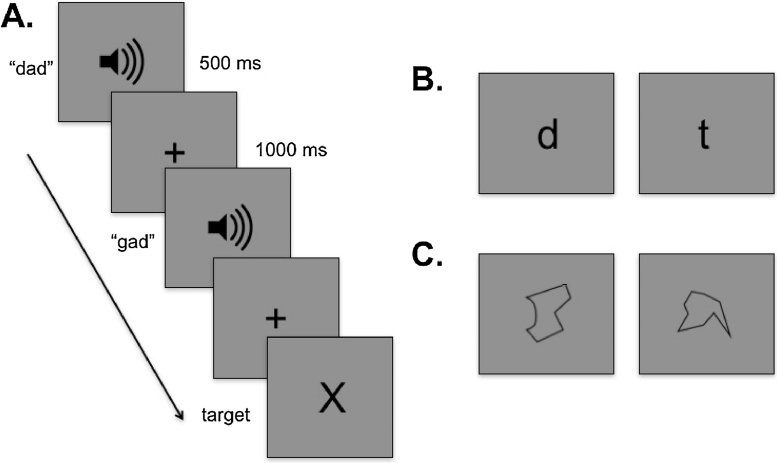
Participants were passively exposed to auditory-only and visual-only stimuli (speech sounds, tones, letters, and unnamable shapes; Fig. 1). To ensure attention to the task, participants were instructed to press a button when a visual-only ‘X’ (vigilance target) appeared on the screen, which accounted for approximately 10% of trials. The imaging session included a total of 8 blocks of 100 stimuli each (2 blocks of each of the 4 stimulus types). Both stimulus and block order were randomized to eliminate effects of imaging bias and neural fatigue across participants. The data collection portion of the imaging session lasted approximately 20 minutes in addition to customized short breaks every 3 minutes to minimize movement and ensure attention in this young population. No session lasted longer than 30 minutes. Custom Matlab programs were used to control stimulus presentation and timing (Mathworks, Natick, MA). Visual stimuli were presented using Psychtoolbox (www.psychtoolbox.org). All behavioral assessment and neural imaging procedures were approved by the Institutional Review Boards of MGH Institute of Health Professions and Massachusetts Institute of Technology.
Fig. 1.
Task design. A. Stimuli were presented in blocks of a single stimulus type with stimulus and block order randomized by participant. Stimuli were presented for 500 ms with an interstimulus interval of 1000 ms. The target stimulus (an ‘X’) was presented approximately 10% of the time to ensure participant attention. B. Examples of letter stimuli C. Examples of unnamable shape stimuli.
2.4. Magnetoencephalography data acquisition and processing
Brain activation measurements were obtained using an Elekta Neuromag Triux system equipped with a whole brain sensor array comprising 102 magnetometers and 204 planar gradiometers (306 total magnetic sensors). Magnetoencephalography (MEG) recordings were obtained at a sampling rate of 1,000 Hz and filtered between 0.03 and 330 Hz. The position of the head was measured continuously during the recordings using a set of 5 head position indicator coils placed on the head.
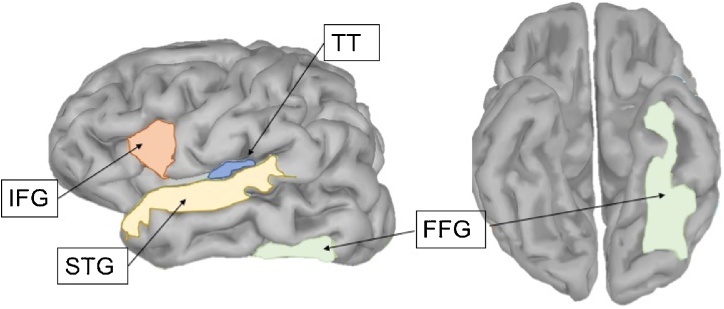
Raw MEG data were preprocessed using the Maxfilter software (Elekta Neuromag, Stockholm) to compensate for head movements and perform noise reduction with spatiotemporal filters (Taulu et al., 2004; Taulu and Simola, 2006). We used default parameters (harmonic expansion origin in head frame = [0 0 40] mm; expansion limit for internal multipole base = 8; expansion limit for external multipole base = 3; bad channels automatically excluded from harmonic expansions = 7 s.d. above average; temporal correlation limit = 0.98; buffer length = 10 s). MEG data were then analyzed using the Brainstorm software (Tadel et al., 2011). Heartbeat and eye blink artifacts were identified by an experienced observer and projected out of the signal. We then extracted trials with 200 ms baseline and 500 ms post-stimulus recordings, and every trial was baseline-corrected to remove the mean (-200 ms to 0 ms) from each channel. Trials with excessive movement (peak-to-peak value greater than 10,000 fT) were labeled and removed from the database. Children with fewer than 25 good trials per stimulus type were excluded (6 children out of the original 48). The time series were then temporally smoothed with a 40 Hz low pass filter. For each trial, the MEG data were mapped on the cortical mantle derived from Freesurfer automatic segmentation (Fischl et al., 2004). This was accomplished by first calculating a head model using an overlapping spheres model (Huang et al., 1999). Next, an inverse model was computed using a dynamic statistical parametric mapping approach (dSPM) (Dale et al., 2000). Finally, we extracted the time-series from multiple cortical regions of interest (ROIs), including auditory, visual, and language cortical areas, derived from the Destrieux-Killiany atlas (Desikan et al., 2006). These ROIs comprised left-hemisphere brain areas involved in reading and language perception: Heschl’s gyrus (transverse temporal/primary auditory cortex) as well as superior temporal gyrus, inferior frontal gyrus, and fusiform gyrus (Destrieux et al., 2010; Hickok and Poeppel, 2007) (Fig. 2).
Fig. 2.
Location of all regions of interest in left hemisphere. TT = transverse temporal/primary auditory cortex, IFG = inferior frontal gyrus, FFG = fusiform gyrus, STG = superior temporal gyrus.
2.5. Calculation of neural variability and statistical methods
Neural activation responses in each ROI were analyzed for variability across trials within every participant using variance as the metric. Two time windows were analyzed corresponding to different components of neural processing of the speech signal. First, a window including 50-100 milliseconds (ms) post stimulus onset was chosen to correspond with basic sensory processing of both the tone and the initial consonant portion (phoneme-level) of the consonant-vowel-consonant stimulus (CVC) in transverse temporal (Travis et al., 2013). Second, a window including 150-300 ms post stimulus onset was chosen to capture syllable-level processing in the remaining regions of interest (Poeppel, 2003). We first calculated the mean response within each of these windows, and then calculated variance within these means across trials of the same stimulus.
When reporting neural response variance, we describe mean and standard error of the mean (sem) across participants. To evaluate whether the amount of trial-by-trial variability was related to cognitive factors (such as IQ), phoneme awareness and/or reading ability, we compared the neural variance with the corresponding behavioral measures using Pearson’s r. Bonferroni correction was used to control for multiple comparisons. All t-tests were paired or unpaired, as appropriate, and post hoc tests were one-sided.
2.6. Sample acquisition and testing for KIAA0319 SNPs
As an optional component of the study, children were asked to provide a saliva sample for genetic testing of dyslexia-susceptibility genes. As such, children and their parents provided written consent for this component of the study, separate from the assessment and neural imaging tasks. In the subset of participants who consented to genetic testing, DNA was extracted from 2.5 mL of saliva collected in 2.5 mL of DNA stabilization buffer at Nationwide Children’s Hospital (Columbus, OH; Bruse et al., 2008). In another subset, saliva was collected using Oragene-DNA self-collection kit (OG-500; DNA Genotek Inc, Ottawa, Ontario, Canada). DNA was extracted using prepIT-L2P (DNA Genotek Inc, Ottawa, Ontario, Canada) at the Yale Center for Genome Analysis (Orange, CT). Genotyping for rs6935076 and rs761100 was conducted using allele-specific PCR-based KASP assay at LGC (Beverly, MA), with initial genotyping quality control and SNP genotype calls conducted using LGC’s in-house Kraken software and standardized protocols. rs6935076 and rs761100 had a call rate of 1 and 0.97, respectively, and satisfied Hardy-Weinberg equilibrium (p > 0.05). Minor allele frequencies for rs6935076 and rs761100 in the sample were 0.34 and 0.38, respectively. MEG and genotyping data from thirty-three participants (N = 10 typically developing readers and N = 23 children with dyslexia) were examined further. Saliva collection and analysis procedures were approved by the Institutional Review Boards of Massachusetts General Hospital, Massachusetts Institute of Technology, Nationwide Children’s Hospital, and Yale University.
3. Results
3.1. Auditory cortex responses to speech sounds are more variable in children with dyslexia
We first used the transverse temporal (TT) ROI, which is a region that includes Heschl’s gyrus (Destrieux et al., 2010), to extract activation in left primary auditory cortex (Fig. 2) and calculated variability across multiple repetitions of each stimulus within a time window of 50-100 ms after stimulus onset. This time window was chosen to correspond with basic sensory processing of the initial consonant portion (phoneme-level) of the consonant-vowel-consonant stimuli (CVC) (Travis et al., 2013).
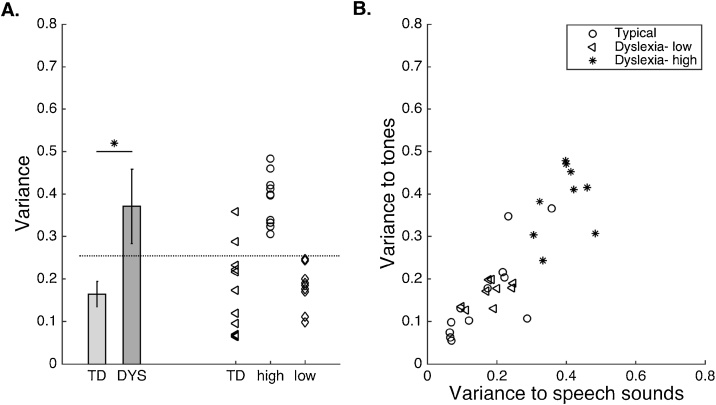
We first evaluated the hypothesis that there would be increased variability in response to speech sounds in the transverse temporal ROI (TT) of children with dyslexia (DYS group) compared to their typical peers (TD group). The DYS group exhibited a significantly higher variability of 0.37 ± 0.09 compared to 0.16 ± 0.03 in the TD group (unpaired, one-tailed t-test, t (30) = 1.82, p = 0.039; Fig. 3A).
Fig. 3.
Neural variability in left primary auditory cortex is present in a subset of children with dyslexia.A. As a group, the TT region in DYS brains responded to auditory speech sound stimuli with higher variability than TD children. Error bars are standard error of the mean. The effect was significant across groups (* p <0.05). Within the group of children with dyslexia, 50% (10/20) had variability levels that would be considered outliers compared to the TD children (high group), with the remainder of the children in the dyslexia group not differing from the TD group (low group). Dashed horizontal line indicates mean + 3 SD of typical children and the threshold for high variability. Individual symbols to the right of the bar plots represent individual children in each group. B. High variability was not specific to speech sounds. Across groups, there was a significant, positive relation between variability to speech sounds and variability to tones (r = 0.95, p <0.00001).
There was no relationship between neural variability and age (r = -0.13, p = 0.48) and no difference in neural variability between males and females overall (t (30) = 0.59, p = 0.56) or within the group of children with dyslexia (t (18) = 0.30, p = 0.76). There were also no relationships between neural variability and IQ scores overall (r = -0.04, p = 0.82) or in either group (TD: r = 0.36, p = 0.25 and DYS: r = 0.02, p = 0.94). Finally, there were no relationships between neural variability and oral language scores (across groups: r = -0.21, p = 0.25,TD: r =-0.28, p = 0.37, and DYS: r =-0.05, p = 0.83), suggesting no influence of these two cognitive-linguistic traits on neural variability.
3.2. Increased variability in primary auditory cortex was found in only a subset of children with dyslexia
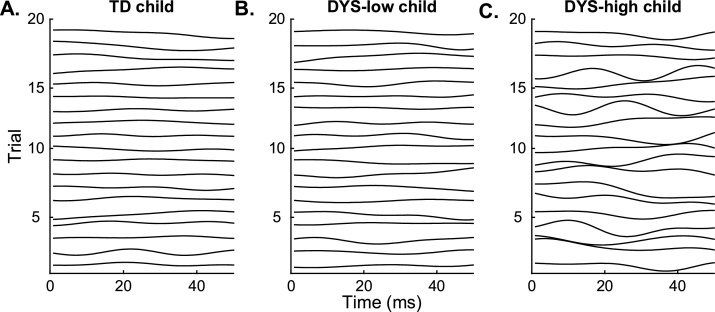
Within the DYS group, there was a notable distinction in the degree of neural variability observed: 10 out of 20 participants (50%) exhibited variability greater than that expected based on the control group (threshold was defined as TD mean + 3 standard deviations; dashed horizontal line in Fig. 3A). The group of 10 children with high variability (DYS-high) averaged 0.53 ± 0.15 compared to 0.18 ± 0.02 in children with low variability (DYS-low; Fig. 3A). Representative examples of trial-by-trial variability in each group are shown in Fig. 4. There were no significant differences in behavioral performance on oral language (t (17) = 0.94, p = 0.82) or any of the reading measures between the DYS-high and DYS-low groups (Table 2), demonstrating that the subgroup with higher variability was not due to a general language impairment.
Fig. 4.
Example responses over twenty representative presentations of the auditory stimulus /dad/. Each row represents the response in TT over a 50 ms time window for a different trial presentation of /dad/. Each panel displays a single representative child’s data from the TD group (A.), the DYS-low group (B.), and the DYS-high group (C.). The TD and DYS-low children showed consistency in the response across trials, while increased variability was observed in the DYS-high group.
Table 2.
Participant characteristics for all children with dyslexia, separated by high vs. low variability. Data are reported as mean ± standard deviation.
| DYS-high (N = 10) |
DYS-low (N = 10) |
p value | |
|---|---|---|---|
| Number of females | 3 | 2 | |
| Age (Years) | 10.91 ± 2.12 | 11.47 ± 1.96 | 0.56 |
| IQ(RIAS) | 108.00 ± 8.72 | 108.11 ± 7.93 | 0.98 |
| Oral Language (CELF-4) | 97.70 ± 10.55 | 102.33 ± 10.98 | 0.36 |
| Timed sight word reading(TOWRE-SWE) | 85.00 ± 11.42 | 87.67 ± 10.77 | 0.60 |
| Timed phonemic decoding(TOWRE-PDE) | 78.55 ± 9.83 | 81.89 ± 9.49 | 0.45 |
| Untimed sight word reading(WRMT-WID) | 81.22 ± 8.17 | 88.57 ± 9.03 | 0.11 |
| Untimed pseudoword reading(WRMT-WA) | 79.89 ± 11.61 | 85.00 ± 9.11 | 0.36 |
If exhibiting high or low variability is a trait of a child with dyslexia, then such variability ought to be reliably exhibited by a child. To examine such reliability in each child, we calculated the relationships between even-numbered trials versus odd- numbered trials (as in Hornickel and Kraus, 2013). There were significant positive correlations between variability on even- numbered versus odd-numbered trials in both the TD group (r = 0.89, p = 0.0001) and the DYS group (r = 0.85, p <0.0001). Within the DYS group, there were significant and positive correlations in both the DYS-high children (r = 0.84, p = 0.0003), and in the DYS-low children (r = 0.85, p = 0.01). These consistent relationships indicate that these measures of variability were stable within each child.
3.3. Increased neural variability extended to nonverbal stimuli in the subset of children with dyslexia
To determine whether the increased variability in TT was specific to verbal stimuli or a general response to sound, we evaluated variability to nonverbal stimuli (e.g. tones). There was a significant and positive correlation between variability to tones and variability to speech sounds across groups (r = 0.95, p <0.0001; Fig. 3B) such that individuals with high variability to speech sounds also exhibited high variability to tones. The strong correlation between variability to tones compared to speech sounds was present in both the DYS group (r = 0.95, p <0.0001) and in the TD group (r = 0.79, p = 0.002).
Because the increased neural variability was consistently present in some children with dyslexia and consistently absent in other children with dyslexia, we separated these two groups in subsequent independent analyses involving different stimuli and different brain regions.
3.4. Increased cortical variability was not present in the left-FFG in response to either auditory or visual stimuli
To determine whether cortical variability extends beyond primary auditory cortex in dyslexia, we calculated variability to speech sounds (at the syllable-level), tones, letters, and shapes in three main components of the left-hemisphere reading network (Hickok and Poeppel, 2007): fusiform gyrus (FFG), inferior frontal gyrus (IFG), and superior temporal gyrus (STG); Fig. 2). In these regions, we analyzed variability at the syllable level: 150-300 ms after stimulus onset to account for the delay in processing time between primary sensory cortices and these association areas. We chose these regions for their specific contributions to reading- visual processing of letters within FFG (Centanni et al., 2017; Cohen et al., 2002), integration of phonemes and graphemes in the IFG (Fiez and Petersen, 1998), and phonological awareness in the STG (Blomert, 2011; McCandliss and Noble, 2003).
We ran two 3 × 2 repeated measures ANOVAs in each region of interest. One evaluated variability to auditory stimuli (group: TD, DYS-high, DYS-low vs. stimulus: speech sounds vs. tones) and the other evaluated variability to visual stimuli (group: TD DYS-high, DYS-low vs. stimulus: letters vs. shapes). In response to auditory stimuli, there was no main effect of stimulus type (F (1, 29) = 1.01, p = 0.32) or group (F (2, 29) = 2.68, p = 0.09) in the left FFG region. There were also no main effects of stimulus type (F (1, 29) = 0.74, p = 0.40) or group (F (2, 29) = 2.25, p = 0.12) in response to visual stimuli. The lack of significant effects of group or stimulus in FFG demonstrates that increased variability was not present at the level of the FFG in dyslexia in response to any of the stimulus types tested.
3.5. Increased cortical variability was present in the left-IFG to auditory and visual stimuli
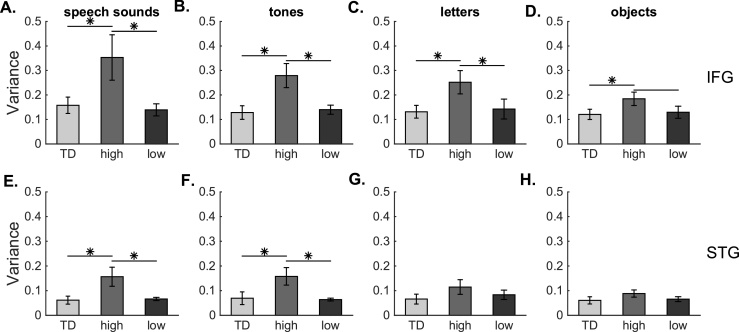
In left IFG in response to auditory stimuli, there was no significant main effect of stimulus type (F (1, 29) = 3.43, p = 0.07) but there was a significant main effect of group (F (2, 29) = 6.11, p = 0.006). There was no interaction between stimulus type and group (F (2, 29) = 1.21, p = 0.31). Post hoc t-tests revealed significantly higher variability in the DYS-high variability group compared to the TD group in response to speech sounds (DYS-high: 0.39 ± 0.11 vs. TD: 0.16 ± 0.03; unpaired, one-tailed t-test, t (21) = 2.27, p = 0.017; Fig. 5A), as well as in response to tones (DYS-high: 0.31 ± 0.05 vs. TD: 0.14 ± 0.02; unpaired, one-tailed t-test, t (21) = 3.14, p = 0.003; Fig. 5B). The DYS-high group also had significantly higher variance compared to the DYS-low group in response to speech sounds (DYS-low: 0.14 ± 0.02; unpaired, one-tailed t-test, t (18) = 2.21, p = 0.02) and tones (DYS-low: 0.14 ± 0.02; unpaired, one-tailed t-test, t (18) = 2.84, p = 0.006; Fig. 5B).
Fig. 5.
Significantly increased variability to speech sounds, tones, letters, and shapes in left hemisphere outside primary auditory cortex. Error bars are standard error of the mean. * unpaired one-tailed t-tests at p < 0.05 after Bonferroni correction. Horizontal lines without * indicate nominally significant comparisons that did not survive multiple-comparisons correction. A-D. Variability to auditory (A-B) and visual (C-D) stimuli in left inferior frontal gyrus (IFG). E-H. Variability to auditory (E-F) and visual (G-H) stimuli in left superior temporal gyrus (STG).
In response to visually presented stimuli, there was no significant main effect of stimulus type (F (1, 29) = 1.82, p = 0.19) but there was a significant main effect of group (F (2, 29) = 7.79, p = 0.002. There was no interaction between stimulus type and group (F (2, 29) = 0.84, p = 0.44). DYS-high children exhibited significantly higher variability (0.28 ± 0.05) compared to the TD group (0.13 ± 0.03) in response to letters (unpaired, one-tailed t-test, t (21) = 2.60, p = 0.008; Fig. 5C). DYS-high children also exhibited higher variability (0.20 ± 0.03) than the TD group in response to shapes (0.12 ± 0.02), (unpaired, one-tailed t-test, t (21) = 2.11, p = 0.024; Fig. 5D). There was also a significant difference between letter-evoked variability in the DYS-high vs DYS-low groups (DYS-low: 0.14 ± 0.03; unpaired, one-tailed t-test, t (18) = 2.19, p = 0.021; Fig. 5C). There was a nominally significant difference in shape-evoked variability between the DYS-high and DYS-low groups (t (18) = 1.80, p = 0.04; Fig. 5D), but this comparison did not survive Bonferroni correction. The findings that DYS-high children exhibit higher variability in response to letters and shapes supports the hypotheses that increased variability is not limited to auditory stimuli nor is it limited to primary auditory cortex.
3.6. Increased cortical variability was present in the left-STG to auditory stimuli only
The comparison of the responses to the auditory stimuli revealed no significant main effect of stimulus type in left STG (F (1, 29) = 0.05, p = 0.82) but there was a significant main effect of group (F (2,29) = 6.31, p = 0.005). There was no interaction between stimulus type and group (F (2, 29) = 0.09, p = 0.91). Post hoc t-tests in STG revealed significantly higher variability in DYS-high children compared to the TD group in response to speech sounds (DYS-high: 0.17 ± 0.04 vs. TD: 0.06 ± 0.02; unpaired, one-tailed t-test, t (21) = 2.52, p = 0.01; Fig. 5E) and tones (DYS-high: 0.18 ± 0.04 vs. TD: 0.07 ± 0.03; unpaired, one-tailed t-test, t (21) = 2.42, p = 0.012; Fig. 5F). DYS-high children also exhibited significantly higher variability compared to DYS-low children in response to speech sounds (DYS-low: 0.07 ± 0.01; t (18) = 2.19, p = 0.021) and tones (DYS-low: 0.06 ± 0.005; unpaired, one-tailed t-test, t (18) = 2.72, p = 0.007).
In response to visual stimuli, there was no significant main effect of stimulus type (F (1, 29) = 1.16, p = 0.29) but a trend in the main effect of group (F (2, 29) = 3.18, p = 0.06) in left STG (Fig. 5G-H). These findings, along with those observed in IFG, support the hypothesis that increased variability propagates through the reading network, but that increased variability to visual stimuli is only present at a later node (IFG) associated with letter-to-sound integration.
3.7. Risk alleles in KIAA0319 are related to the degree of variability in primary auditory cortex
We collected saliva samples from 33 children. Because allele frequencies vary across racial and ethnic backgrounds that could confound statistical genetic analysis, we considered only samples from non-Hispanic white children as per parent report (N = 7 typical readers and N = 16 children with dyslexia). We then quantified the number of minor/risk alleles in two well-established dyslexia-associated SNPs in the gene KIAA0319 which were previously associated with both dyslexia and neural consistency (rs6935076 and rs761100; Cope et al., 2005; Lim et al., 2014; Müller et al., 2016). At rs6935076, 2 out of 7 TD children had at least one minor allele compared to 5 children in the DYS-high group and 5 children in the DYS-low group. At rs761100, 5 out of 7 TD children had at least one minor allele compared to 3 in the DYS-high group and 3 in the DYS-low group. Only one child with dyslexia displayed minor alleles at both locations. There were no group differences (DYS vs. TD) in the genotypes in either of the SNPs (chi-squared tests, ps >0.51), so we combined the two reading-level groups (TD and DYS) for further analyses.
To evaluate the relationship between these SNPs and neural variability, we evaluated a total of 22 children (N = 6 TD and N = 16 DYS) whose MEG data met the minimum criterion for quality and were reported in the previous sections. We used separate ANOVAs to determine the influence of each SNP on neural variability in primary auditory cortex. Due to the high correlation between speech sound variance and tone variance in primary auditory cortex, we averaged across all auditory stimuli for these measures. Because this gene has been associated with reading skills (Paracchini et al., 2008) and auditory brainstem stability in the general population (Neef et al., 2017), we combined across groups (TD and DYS) for this analysis to increase statistical power. We first ran univariate ANOVAs to determine the effect of a minor allele (one or two) compared to no minor allele at each SNP. There was a significant main effect of minor allele at rs6945076 (F (1,20) = 5.8, p = 0.03) but not for rs761100 (F (2, 19) = 0.25, p = 0.62).
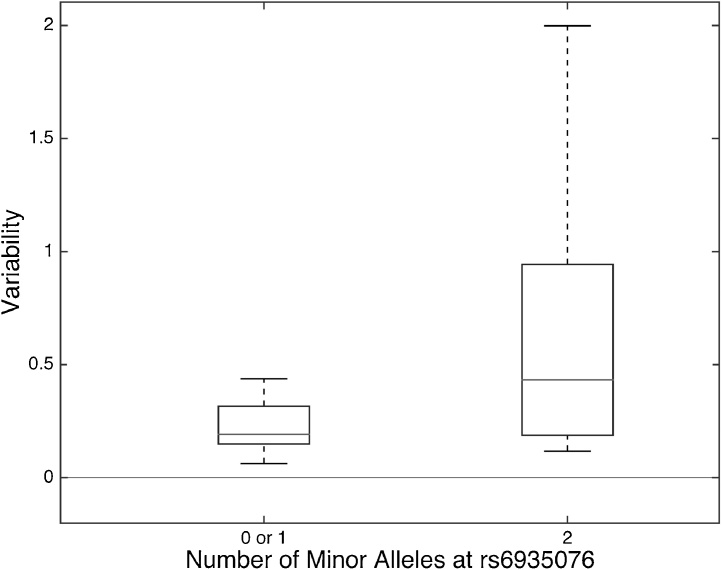
To determine whether there was an additive effect of multiple minor alleles we ran an additional ANOVA with three groups: no minor allele at rs6935076 (N = 10), one minor allele (N = 7), and two minor alleles (N = 5). There was a trend in the main effect of the minor allele at rs6935076 (F (2, 19) = 2.8, p = 0.09). A linear regression confirmed a significant positive relationship between number of minor alleles at rs6935076 and neural variability (F (2, 20) = 4.47, p = 0.04, R2 = 0.753), indicating that additional minor alleles corresponded with increased neural variability. In support of this finding, children with 2 minor alleles at rs6935076 (N = 5, all dyslexic) had significantly higher variability compared to children with 1 (N = 7, 5 dyslexic) or 0 minor alleles (N = 10, 4 dyslexic, 0.67 ± 0.38 vs. 0.23 ± 0.03; one-tailed t-test, t (20) = 2.41, p = 0.01; Fig. 6).
Fig. 6.
The presence of two minor alleles at rs6935076 is associated with high variability in children with dyslexia. Five children, all with dyslexia, had two minor alleles at a specific SNP in KIAA0319. These children exhibited significantly higher variability compared to children with 1 (N = 7, including 5 dyslexic) or 0 (N = 10, including 4 dyslexic) minor alleles at this location. * = one-tailed t-test, p = 0.01.
In response to auditory stimuli in IFG, there was no main effect for stimulus type (F (1, 19) = 0.79, p = 0.39) and a trend in the main effect of the minor allele (F (2, 19) = 2.72, p = 0.09). There was a trend in the linear model for the effect of additional minor alleles on variability to auditory stimuli in IFG (F (2, 20) = 3.92, p = 0.06). In response to visual stimuli in IFG, there were no main effects for either stimulus type (F (1, 19) = 2.53, p = 0.13) or minor allele (F (2, 19) = 0.39, p = 0.68).
In response to auditory stimuli in STG, there was no significant main effect of stimulus type (F (1, 19) = 0.17, p = 0.69) and a trend in the main effect of the minor allele (F (2, 19) = 2.94, p = 0.08). There was a trend in the linear model for the effect of additional minor alleles on variability to auditory stimuli in STG (F (2, 20) = 3.57, p = 0.07). In response to visual stimuli in STG, there were no main effects for either stimulus type (F (1, 19) = 1.36, p = 0.26) or minor allele (F (2, 19) = 1.14, p = 0.34).
4. Discussion
4.1. Summary of results
We discovered novel insights into the role of high neural variability in dyslexia. First, we confirmed the hypothesis that greater variability was reliably present in a subset of children with dyslexia, so that half of the children with dyslexia exhibited high neural variance and half of the children with dyslexia did not. Second, we confirmed the hypothesis that a subset of children with dyslexia exhibited high variability in primary auditory cortex in response to auditory stimuli, including speech sounds and tones. Third, we confirmed the hypothesis that greater variability extended beyond auditory cortex and beyond auditory stimuli. Specifically, we observed greater variability to a variety of stimulus types in IFG and STG, but only in the subgroup of children with greater neural variability as defined by primary auditory cortex responses. The current study is the first to find that inconsistent neural responses to perceptual inputs in children with dyslexia are not limited to auditory brainstem responses (Hornickel and Kraus, 2013; Neef et al., 2017), but extend to primary auditory cortex, and additional components of the reading network. Fourth, we confirmed the hypothesis that risk alleles in the dyslexia-susceptibility gene KIAA0319 (rs6935076) were significantly associated with neural variability in primary auditory cortex across groups, which confirms the relationship between risk alleles in KIAA0319 and neural variability in typically reading children and extends this finding, for the first time, into children with dyslexia.
4.2. Cortical evidence for subgroups within the dyslexia diagnosis
Our findings provide support for the hypothesis that some, but not all, children with dyslexia demonstrate stimulus-evoked variability in the brain. Half of the children with dyslexia exhibited levels of variability in primary auditory cortex that were significantly higher than their typically developing peers. The idea that inconsistent processing of auditory input is observed in some but not all children with dyslexia is consistent with often-cited evidence that around half of a previous sample of children with dyslexia exhibited deficits in auditory temporal processing (Tallal, 1980). We speculate that increased neural variability, as reported in the current study, likely interferes with reliable coding of a stimulus and therefore can lead to inaccurate perception and incorrect behavioral decisions. In support of this hypothesis, neural responses to distinct consonant sounds in rats with reduced Kiaa0319 expression were more difficult for a computer algorithm to distinguish compared to control brain responses (Centanni et al., 2014a). To test the hypothesis that inconsistent neural responses relate to abnormal temporal processing, future research should measure neural variance in humans in the high-variance dyslexia subgroup while completing the original rapid auditory temporal processing task (Tallal, 1980). If confirmed, this would provide a biological explanation for a finding that has been both well-known and fiercely debated in the field for many years.
It could have been the case that the higher variance observed in primary auditory cortex was limited to this brain region and to auditory stimuli, as no study to date has investigated variance outside of primary auditory regions: auditory brainstem in humans (Hornickel and Kraus, 2013; Neef et al., 2017) and primary auditory cortex in rodent models (Centanni et al., 2014a; Centanni et al., 2014b). We observed instead that children in the high-variability subgroup exhibited increased variance in other brain regions and to additional stimulus conditions. The DYS-high subgroup was defined by higher variability to speech sounds in primary auditory cortex, but that subgroup also exhibited higher variability to several kinds of stimuli in multiple left-hemisphere regions associated with language and reading. Children in the DYS-high group also exhibited higher variability for tones in a region encompassing primary auditory cortex, in the STG for both kinds of auditory stimuli, and in the IFG for all stimulus types tested. The STG (Blomert, 2011; McCandliss and Noble, 2003) and IFG (Boets et al., 2013; Fiez and Petersen, 1998; Grande et al., 2011; Shaywitz et al., 1998) have been implicated in speech perception and reading. These findings support the view that dyslexia is not a disorder with a unitary basis but instead, that there are several subgroups within this broad diagnostic category, including a distinct group with increased variability in brain responses to perceptual inputs.
4.3. Variability to multiple stimulus types in reading network regions
Increased variability in brain responses was found to extend to multiple regions and in response to multiple stimulus types. We discovered increased cortical variability in the DYS-high group in response to auditory stimuli in the STG and to all stimuli in the IFG. Both of these brain areas are critical components of the reading network (McCandliss and Noble, 2003). The STG appears to specialize in phonological comparisons (in a rhyming task; Paulesu et al., 1996) and complexity (Shaywitz et al., 1998). The STG exhibits increased activation in typical readers during a word pronunciation task but such activity is reduced in those with dyslexia (Rumsey et al., 1997a, 1997b). The IFG is associated with silent reading of print (Fiez and Petersen, 1998) and often exhibits reduced activation in individuals with dyslexia (Boets et al., 2013; Grande et al., 2011; Shaywitz et al., 1998). Our findings converge with previous fMRI observations that these two reading network regions are atypically activated in dyslexia and raise the possibility that one potential mechanism for this atypical activation is increased neural variability.
We also found that this increased variability is not limited to verbal stimuli, as we observed increased variability to tones in transverse temporal, IFG, and STG in a subset of children with dyslexia (the DYS-high group). Abnormal responses to tones are well-established in dyslexia, as diminished mismatch negativity, delayed latency, and reduced amplitude are often observed in EEG recordings of individuals with dyslexia (Kujala et al., 2000; Lachmann et al., 2005; Maurer et al., 2003; Schulte-Körne et al., 1998). Children with dyslexia that have specific phonological awareness deficits displayed diminished mismatch negativity to both tones and speech sounds, while children with dyslexia that performed poorly only on sight word reading did not display such deficits (Lachmann et al., 2005). Because the mismatch negativity measure requires the brain to encode a repetitive stimulus, increased neural variability may impact the brain’s ability to habituate and therefore reduce the effect of the deviant stimulus, as is seen in dyslexia (Jaffe-Dax et al., 2018; Perrachione et al., 2016). Further research is needed to evaluate whether the increased neural variability we observed is related to the reduced mismatch negativity observed in other studies using EEG and fMRI.
We also observed increased variability to shapes in IFG in the DYS-high subgroup compared to the TD group. Further research will be needed to determine whether increased variability to unnamable visual objects in the IFG is due to the influence of a multi-sensory integration region that is amplifying the variability that originates in the auditory system or due to some other mechanism.
4.4. Role of KIAA0319 in cortical variability
The current findings also support existing evidence that dyslexia genes, specifically KIAA0319, may play a role in causing variability throughout the brain and points to this SNP as a potential marker for this feature. Specific gene variants in KIAA0319 are present in children with increased neural variability in auditory brain stem responses, but are not present in children with control level neural consistency (Neef et al., 2017). Neef et al., (2017) used a single composite value derived from minor alleles in three individual SNPs (rs6935076, rs761100, and rs2179515) to reflect genetic variation in KIAA0319. One of our aims was to unpack the involvement of this gene and to determine whether specific SNPs in this gene were associated with neural variability. In the current study, we observed a significant relationship between risk alleles in rs6935076 but not rs761100 and neural variability. It is therefore possible that the significant associations between KIAA0319 SNPs, auditory brainstem variability, and spelling performance reported in Neef et al., 2017, can be accounted for by rs6935076. Although rs6935076 is a non-functional polymorphism, it could be in linkage disequilibrium with a nearby functional element mediating neural variability. Future research is needed to better understand the functional consequences of the region surrounding rs6935076 in the genome, as well as its role in other ethnicities. In the genetic analysis, one child in the TD group with self-reported non-Caucasian ancestry was excluded from the genetic analysis due to the confounding effects of differences in allele frequency of rs6935076 across different populations. However, it is important to note that this child carried a minor allele at rs6935076 and exhibited increased neural variability above the threshold for outliers. Although we cannot draw any conclusions about how this particular variant is associated with neural variability in diverse populations given a single data point, this observation supports additional studies on this gene in different world populations.
4.5. Potential implications for increased variability for reading acquisition
The presence of inconsistent speech sound and/or letter representations in neocortex (also referred to as increased neural noise) may interfere with the formation of letter-to-sound correspondences in newly forming reading networks (Hancock et al., 2017). Increased variability in the reading network could disrupt reading acquisition because reading is a multisensory process which requires the integration of auditory speech sound representations with visual representations of letters. Although variability in primary sensory brain regions may be disruptive for reading acquisition, variability associated with cognitive flexibility in the inferior frontal gyrus is positively correlated with reading scores in typically developing individuals based on data from an fMRI study (Malins et al., 2018). We observed increased variance in both IFG and in primary sensory areas, perhaps due to the greater temporal resolution of MEG compared to fMRI. It may be that the millisecond level precision detected through MEG is required to detect differences in processing speech signals, given the fine temporal structure of individual speech sounds. In any case, the precise negative or positive consequence of increased variability of brain responses for language or reading may depend on the specific mental operations and brain measures involved.
Our findings suggest that increased stimulus-evoked variability in the brain may be a biomarker of a dyslexia subtype. In support of this hypothesis, neural habituation to a variety of repetitive stimuli is reduced in individuals with dyslexia and, importantly, this effect is not specific to linguistic stimuli (Perrachione et al., 2016). If a stimulus were represented in an unstable or noisy fashion, the resultant variability would reduce habituation because a repeated stimulus would be encoded as different percepts across repetitions. There is also evidence that variability may be present even in the absence of a stimulus. Reading-related group differences in response to a variety of stimuli are present in both the Alpha (Duffy et al., 1980) and Beta (Dimitriadis et al., 2013) frequency bands of the neural response and also occur during rest conditions, suggesting these differences are reflective of baseline differences in brain function across groups. The strength of temporal correlations present during rest in these frequency bands correspond with reading scores (Dimitriadis et al., 2013), supporting the hypothesis that widespread increases in variability may have a negative impact on reading acquisition.
Additional evidence for increased baseline variability in dyslexia comes from research in animal models of impaired auditory processing relevant to dyslexia. Suppression of either Kiaa0319 or Dcdc2 in a rat model causes increased spontaneous firing within auditory cortex in the absence of an external stimulus (Centanni et al., 2016; Centanni et al., 2014a). Although it is difficult to determine whether increased spontaneous firing is the cause of the increased resting variability observed in humans (Dimitriadis et al., 2013; Duffy et al., 1980), a greater amount of spontaneous neural firing in the absence of any external stimulus is likely to increase the variability across time windows. Further studies are needed to determine whether abnormal habituation responses are driven by the same mechanisms that drive inconsistent cortical responses, or whether these represent separate processes.
4.6. Limitations of the current study
The small sample size in our study is a limitation in interpreting our results. In the current study, we evaluated neural responses to a variety of stimuli in 20 children with dyslexia and 12 age-matched, typically reading peers. This sample size was too small to determine whether the proportion of children with inconsistent neural responses generalizes to the population of children with reading disorders. The small sample size is also a limitation for our genetic finding, although research showing a link between these same SNPs and auditory brainstem consistency in a larger sample supports our results (Neef et al., 2017). Further, with regard to the genetic results, we were forced to restrict our genetic analyses to a replication on a small number of SNPs due to limited power conferred by our small sample size and were unable to fully investigate the hypothesis that this gene confers an increased risk of high cortical variability in dyslexia. Ideally, more adequately powered genome-wide association studies could identify any additional variants of small effect size that contribute to cortical variability. In addition, we included samples only from non-Hispanic white children. The relationship between KIAA0319 and neural variability should be evaluated in other populations to determine whether these findings are generalizable.
A second limitation is the low spatial resolution of the reconstructed cortical activation maps due to MEG physics. While this technique provides better spatial precision than EEG and better temporal precision than fMRI, the MEG reconstructed maps encompass overlapping activity. Therefore, activity attributed to nearby cortical regions of interest are likely blurred, which may bias some statistical tests. The regions of interest selected here should be sufficiently spatially separated to allow for confidence in these findings, but future studies should confirm these results when new techniques and/or algorithms are developed to better separate these sources. Finally, in the current study, we did not acquire structural images of each child and instead used an MRI scan from one of our enrolled children (which was acquired in a separate study) as the default anatomy for all participants. Therefore, the exact location of each region of interest may vary slightly.
4.7. A neuro-genetic trait for greater or lesser neural variability
The present findings suggest a trait-like, or stable individual difference, in neural variability that spans typical reading ability and atypical reading disability and that is associated with KIAA0319. Children with typical reading ability, children with reading disability but typical neural variability, and children with reading disability plus enhanced neural variability all exhibited consistent neural variability across odd-and-even numbered trials to auditory stimuli in primary auditory cortex. These findings indicate that degree of neural variability is a trait-like characteristic of all the children. Similarly, the correlation between neural variability and KIAA0319 spanned all the children. Further research will need to elucidate how such an apparently continuous neuro-genetic trait appears to be problematic in only half of children with dyslexia. It is noteworthy that the two groups of children with dyslexia who differed so much in the typicality or atypicality of neural variability differed so little on all language, cognition, and reading scores.
The present findings, therefore, point to potential alternative neuro-genetic pathways to similar phenotypical disabilities in reading in general, and more mechanistically toward one specific neuro-genetic pathway in particular. Increased neural variability in some children with dyslexia may decrease the strength of the association between speech sound representations in the brain and the corresponding letter. Weaker sound-to-letter associations may impede the brain’s ability to develop automaticity and therefore impede reading acquisition, leading to dyslexia in a subset of children.
Conflict of Interest
None.
Acknowledgments
The authors thank Chris Bartlett and Mike Goode for assistance with genetic sample processing and testing. We also thank Kelly Halverson, Leila Denna, Mona Anchan, Grace Lee, Natalie Ross, Rachel Romeo, and Katy Cabbage for assistance with participant recruitment, data collection, and assessment scoring. Finally, we thank the families who participated in this research. Funding for this study was provided by the MGH Institute of Health Professions (PI: Hogan), the Halis Foundation for Dyslexia Research at MIT (PI: Gabrieli), and a generous donation from Noam and Lisa Bardin (to Centanni/Gabrieli).
References
- Alt M., Hogan T., Green S., Gray S., Cabbage K., Cowan N. Word learning deficits in children with dyslexia. Journal of Speech, Language, Hearing Research. 2016;60:1012–1028. doi: 10.1044/2016_JSLHR-L-16-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomert L. The neural signature of orthographic-phonological binding in successful and failing reading development. Neuroimage. 2011;57:695–703. doi: 10.1016/j.neuroimage.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Boets B., de Beeck H., Van dermosten M., Scott S.K., Gillebert C.R., Mantini D., Bulthe J., Sunaert S., Wouters J., Ghesquiere P. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342:1251–1254. doi: 10.1126/science.1244333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd T.W., Barry R.J., Gordon E., Rennie C., Michie P.T. Decrement of the N1 auditory event-related potential with stimulus repetition: Habituation vs. refractoriness. Int. J. Psychophysiol. 1998;31:51–68. doi: 10.1016/s0167-8760(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Butler R.A. Effect of changes in stimulus frequency and intensity on habituation of the human vertex potential. J. Acoust. Soc. Am. 1968;44:945–950. doi: 10.1121/1.1911233. [DOI] [PubMed] [Google Scholar]
- Catts H., Adlof S., Hogan T., Weismer S. Are specific language impairment and dyslexia distinct disorders? Journal of Speech, Language, Hearing Research. 2005;48:1378–1396. doi: 10.1044/1092-4388(2005/096). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T., Booker A., Chen F., Sloan A., Carraway R., Rennaker R., LoTurco J., Kilgard M. Knockdown of Dyslexia-Gene Dcdc2 Interferes with Speech Sound Discrimination in Continuous Streams. Journal of Neuroscience. 2016;36:4895–4906. doi: 10.1523/JNEUROSCI.4202-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T., Booker A., Sloan A., Chen F., Maher B.J., Carraway R.S., Khodaparast N., Rennaker R., Loturco J.J., Kilgard M.P. Knockdown of the Dyslexia-Associated Gene Kiaa0319 Impairs Temporal Responses to Speech Stimuli in Rat Primary Auditory Cortex. Cereb. Cortex. 2014;24:1753–1766. doi: 10.1093/cercor/bht028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T., Chen F., Booker A., Engineer C., Sloan A., Rennaker R., LoTurco J., Kilgard M. Speech sound processing deficits and training-induced neural plasticity in rats with dyslexia gene knockdown. PLoS One. 2014;9:e98439. doi: 10.1371/journal.pone.0098439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni T., King L., Eddy M., Whitfield-Gabrieli S., Gabrieli J. Development of sensitivity versus specificity for print in the visual word form area. Brain Lang. 2017;170:62–70. doi: 10.1016/j.bandl.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Centanni T.M., Engineer C.T., Kilgard M.P. Cortical speech-evoked response patterns in multiple auditory fields are correlated with behavioral discrimination ability. J. Neurophysiol. 2013;110:177–189. doi: 10.1152/jn.00092.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L., Lehéricy S., Chochon F., Lemer C., Rivaud S., Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125:1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cope N., Harold D., Hill G., Moskvina V., Stevenson J., Holmans P., Owen M.J., O’Donovan M.C., Williams J. Strong Evidence That KIAA0319 on Chromosome 6p Is a Susceptibility Gene for Developmental Dyslexia. Am. J. Hum. Genet. 2005;76:581–591. doi: 10.1086/429131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Liu A.K., Fischl B.R., Buckner R.L., Belliveau J.W., Lewine J.D., Halgren E. Dynamis statistical parametric mapping: combining fMRI and MEG for high resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- DeFries J., Fulker D., LaBuda M. 1987. Evidence for a genetic aetiology in reading disability of twins. [DOI] [PubMed] [Google Scholar]
- Desikan R., Ségonne F., Fischl B. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C., Fischl B., Dale A., Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis S.I., Laskaris N.A., Simos P.G., Micheloyannis S., Fletcher J.M., Rezaie R., Papanicolaou A.C. Altered temporal correlations in resting-state connectivity fluctuations in children with reading difficulties detected via MEG. Neuroimage. 2013;83:307–317. doi: 10.1016/j.neuroimage.2013.06.036. [DOI] [PubMed] [Google Scholar]
- Duffy F., Denckla M., Bartels P.G.S. Dyslexia: Regional differences in brain electrical activity by topographic mapping. Ann. Dyslexia. 1980;7:412–420. doi: 10.1002/ana.410070505. [DOI] [PubMed] [Google Scholar]
- Engineer C.T., Perez C.A., Chen Y.T.H., Carraway R.S., Reed A.C., Shetake J.A., Jakkamsetti V., Chang K.Q., Kilgard M.P. Cortical activity patterns predict speech discrimination ability. Nat. Neurosci. 2008;11:603–608. doi: 10.1038/nn.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez A., Pratt H. Auditory event-related potentials among dyslexic and normal-reading children: 3CLT and midline comparisons. Int. J. Neurosci. 1992;63:247–264. doi: 10.3109/00207459208987200. [DOI] [PubMed] [Google Scholar]
- Fiez J.A., Petersen S.E. Neuroimaging studies of word reading. Proc. Natl. Acad. Sci. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J.W., Makris N., Ségonne F., Quinn B.T., Dale A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Franquinho F., Nogueira-Rodrigues J., Duarte J.M., Esteves S.S., Carter-Su C., Monaco A.P., Molnár Z., Velayos-Baeza A., Brites P., Sousa M.M. The Dyslexia-susceptibility Protein KIAA0319 Inhibits Axon Growth Through Smad2 Signaling. Cereb. Cortex. 2017;27:1732–1747. doi: 10.1093/cercor/bhx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda A.M., LoTurco J., Ramus F., Fitch R.H., Rosen G.D. From genes to behavior in developmental dyslexia. Nat. Neurosci. 2006;9:1213–1217. doi: 10.1038/nn1772. [DOI] [PubMed] [Google Scholar]
- Grande M., Meffert E., Huber W., Amunts K., Heim S. Word frequency effects in the left IFG in dyslexic and normally reading children during picture naming and reading. Neuroimage. 2011;57:1212–1220. doi: 10.1016/j.neuroimage.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Hancock R., Pugh K.R., Hoeft F. Neural Noise Hypothesis of Developmental Dyslexia. Trends Cogn. Sci. 2017;21:434–448. doi: 10.1016/j.tics.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm M.W., Seidenberg M.S. Phonology, reading acquisition, and dyslexia: insights from connectionist models. Psychol. Rev. 1999;106:491–528. doi: 10.1037/0033-295x.106.3.491. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hornickel J., Kraus N. Unstable Representation of Sound: A Biological Marker of Dyslexia. Journal of Neuroscience. 2013;33:3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.X., Mosher J.C., Leahy R.M. A sensor-weighted overlapping sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 1999;44:423. doi: 10.1088/0031-9155/44/2/010. [DOI] [PubMed] [Google Scholar]
- Jaffe-Dax S., Kimel E., Ahissar M. Shorter cortical adaptation in dyslexia is broadly distributed in the superior temporal lobe and includes the primary auditory cortex. eLife. 2018 doi: 10.7554/eLife.30018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman I., Norton E.S., Christodoulou J.A., Gaab N., Lieberman D.A., Triantafyllou C., Wolf M., Whitfield-Gabrieli S., Gabrieli J.D.E. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cereb. Cortex. 2012;22:754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala T. The role of early auditory discrimination deficits in language disorders. Journal of Psychophysiology. 2007;21(3-4):239–250. [Google Scholar]
- Kujala T., Myllyviita K., Tervaniemi M., Alho K., Kallio J., Näätänen R. Basic auditory dysfunction in dyslexia as demonstrated by brain activity measurements. Psychophysiology. 2000;37:262–266. [PubMed] [Google Scholar]
- Lachmann T., Berti S., Kujala T., Schröger E. Diagnostic subgroups of developmental dyslexia have different deficits in neural processing of tones and phonemes. Int. J. of Psychphys. 2005;56:105–120. doi: 10.1016/j.ijpsycho.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Lehongre K., Ramus F., Villiermet N., Schwartz D., Giraud A.L. Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron. 2011;72:1080–1090. doi: 10.1016/j.neuron.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lim C.K.-P., Wong A.M.-B., Ho C.S.-H., Waye M.M.-Y. A common haplotype of KIAA0319 contributes to the phonological awareness skill in Chinese children. Behav. Brain Funct. 2014;10:23. doi: 10.1186/1744-9081-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind P.A., Luciano M., Wright M.J., Montgomery G.W., Martin N.G., Bates T.C. Dyslexia and DCDC2: normal variation in reading and spelling is associated with DCDC2 polymorphisms in an Australian population sample. Eur. J. Hum. Genet. 2010;18:668–673. doi: 10.1038/ejhg.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso M.L., Cantiani C., Molteni M. Age, dyslexia subtype and comorbidity modulate rapid auditory processing in developmental dyslexia. Front. Hum. Neurosci. 2014;8:1–16. doi: 10.3389/fnhum.2014.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon G.R., Sl B.A., Catts H., Dickman E., Eden G., Fletcher J., Gilger J., Morris R., Tomey H., Viall T. Defining Dyslexia, Comorbidity, Teachers ' Knowledge of Language and Reading A Definition of Dyslexia. Ann. Dyslexia. 1995;53:1–14. [Google Scholar]
- Malins J.G., Pugh K.R., Buis B., Frost S.J., Hoeft F., Landi N., Mencl W.E., Kurian A., Staples R., Molfese P.J., Sevcik R., Morris R. Individual differences in reading skill are related to trial-by-trial neural activation variability in the reading network. J Neurosci. 2018;38(12):2981–2989. doi: 10.1523/JNEUROSCI.0907-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C.M., Snowling M.J., Bailey P.J. Rapid auditory processing and phonological ability in normal readers and readers with dyslexia. J. Speech, Lang. Hear. Res. 2001;44:925. doi: 10.1044/1092-4388(2001/073). [DOI] [PubMed] [Google Scholar]
- Maurer U., Bucher K., Brem S., Brandeis D. Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. Neuroreport. 2003;14:2245–2250. doi: 10.1097/00001756-200312020-00022. [DOI] [PubMed] [Google Scholar]
- Mcanally K.I., Stein J.F. Auditory temporal coding in dyslexia. Proc. R. Soc. London.Series B Biol. Sci. 1996;263:961–965. doi: 10.1098/rspb.1996.0142. [DOI] [PubMed] [Google Scholar]
- McCandliss B.D., Noble K.G. The development of reading impairment: a cognitive neuroscience model. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9:196–204. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- Meyer M., Wood F., Hart L., Felton R. Longitudinal course of rapid naming in disabled and nondisabled readers. Ann. Dyslexia. 1998;48:89–114. [Google Scholar]
- Morris R., Stuebing K., Fletcher J.M., Shaywitz S.E., Lyon G.R., Shankweiler D.P., Katz L., Francis D.J., Shaywitz B.A. Subtypes of reading disability: Variability around a phonological core. J. Educational Psych. 1998;90:347–373. [Google Scholar]
- Müller B., Wilcke A., Czepezauer I., Ahnert P., Boltze J., Kirsten H., LEGASCREEN consortium Association, characterisation and meta-analysis of SNPs linked to general reading ability in a German dyslexia case-control cohort. Sci. Rep. 2016;6:27901. doi: 10.1038/srep27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R., Gaillard A.W.K., Mäntysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychologica. 1978;42(4):313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Neef N.E., Müller B., Liebig J., Schaadt G., Grigutsch M., Gunter T.C., Wilcke A., Kirsten H., Skeide M.A., Kraft I., Kraus N., Emmrich F., Brauer J., Boltze J., Friederici A.D. Dyslexia risk gene relates to representation of sound in the auditory brainstem. Dev. Cogn. Neurosci. 2017;24:63–71. doi: 10.1016/j.dcn.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neef N.E., Schaadt G., Friederici A.D. Auditory brainstem responses to stop consonants predict literacy. Clin. Neurophysiol. 2016;128:484–494. doi: 10.1016/j.clinph.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Paracchini S., Steer C., Buckingham L.-L., Morris A., Ring S., Scerri T., Stein J., Pembrey M., Ragoussis J., Golding J. Association of the KIAA0319 dyslexia susceptibility gene with reading skills in the general population. Am. J. Psychiatry. 2008;165:1576–1584. doi: 10.1176/appi.ajp.2008.07121872. [DOI] [PubMed] [Google Scholar]
- Paul I., Bott C., Heim S. Phonological but not auditory discrimination is impaired in dyslexia. Eur. Journal Neurosci. 2006;24:2945–2953. doi: 10.1111/j.1460-9568.2006.05153.x. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Frith U., Snowling M., Gallagher A., Morton J., Frackowiak R.S.J., Frith C.D. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Perrachione T.K., Tufo S. Del, Winter R., Murtagh J., Cyr A., Chang P., Halverson K., Ghosh S., Christodoulou J., Gabrieli J. Dysfunction of Rapid Neural Adaptation in Dyslexia. Neuron. 2016;92:1383–1397. doi: 10.1016/j.neuron.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R.L., Pennington B.F. Developmental dyslexia. Lancet. 2012 doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time. Speech Commun. 2003;41:245–255. [Google Scholar]
- Polich J. Probability and inter-stimulus interval effects on the P300 from auditory stimuli. Int. J. Psychophysiol. 1990;10:163–170. doi: 10.1016/0167-8760(90)90030-h. [DOI] [PubMed] [Google Scholar]
- Ramus F., Szenkovits G. What phonological deficit? Q. J. Exp. Psych. 2008;61:129–141. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- Rey V., Martino S., De, Espesser R., Habib M. Temporal processing and phonological impairment in dyslexia: Effect of phoneme lengthening on or der judgment of two consonants. Brain Lang. 2002;80:576–591. doi: 10.1006/brln.2001.2618. [DOI] [PubMed] [Google Scholar]
- Reynolds C.R., Kamphaus R.W. 2003. RIAS, Reynolds Intellectual Assessment Scales. Psychological Assessment Resources. [Google Scholar]
- Rumsey J., Nace K., Donohue B., Wise D., Maisog J., Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Arch. Neurol. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Rumsey J.M., Horwitz B., Donohue B.C., Nace K., Maisog J.M., Andreason P. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Scerri T., Schulte-Körne G. Genetics of developmental dyslexia. Eur. Child Adolesc. Psychiatry. 2010;19:179–197. doi: 10.1007/s00787-009-0081-0. [DOI] [PubMed] [Google Scholar]
- Scerri T.S., Morris A.P., Buckingham L.L., Newbury D.F., Miller L.L., Monaco A.P., Bishop D.V.M., Paracchini S. DCDC2, KIAA0319 and CMIP are associated with reading-related traits. Biol. Psychiatry. 2011;70:237–245. doi: 10.1016/j.biopsych.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Körne G. The prevention, diagnosis, and treatment of dyslexia. Dtsch. Arztebl. Int. 2010;107:718. doi: 10.3238/arztebl.2010.0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Körne G., Deimel W. Pre-attentive processing of auditory patterns in dyslexic human subjects. Neurosci. Lett. 1999;276:41–44. doi: 10.1016/s0304-3940(99)00785-5. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G., Deimel W., Bartling J., Remschmidt H. The role of phonological awareness, speech perception, and auditory temporal processing for dyslexia. Eur. Child Adolesc. Psychiatry. 1999;8:28–34. doi: 10.1007/pl00010690. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G., Deimel W., Bartling J., Remschmidt H. Auditory processing and dyslexia: evidence for a specific speech processing deficit. Neuroreport. 1998;9:337–340. doi: 10.1097/00001756-199801260-00029. [DOI] [PubMed] [Google Scholar]
- Semel E.M., Wiig E.H., Secord W. Psychological Corporation, Harcourt Brace Jovanovich; 1987. CELF-R: Clinical Evaluation of Language Fundamentals--Revised. [Google Scholar]
- Shaywitz S.E. Dyslexia. N. Engl. J. Med. 1998;338:307–312. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shaywitz S.E., Shaywitz B.A., Pugh K.R., Fulbright R.K., Constable R.T., Mencl W.E., Shankweiler D.P., Liberman A.M., Skudlarski P., Fletcher J.M. Functional disruption in the organization of the brain for reading in dyslexia. Proc. Natl. Acad. Sci. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadel F., Baillet S., Mosher J.C., Pantazis D., Leahy R.M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011;2011:8. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallal P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Taulu S., Kajola M., Simola J. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr. 2004;16:269–275. doi: 10.1023/b:brat.0000032864.93890.f9. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51:1759. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Torgensen J., Wagner R., Rashotte C. TX Pro-Ed.; Austin: 1999. Test of word reading efficiency (TOWRE) [Google Scholar]
- Travis K., Leonard M., Chan A. Independence of early speech processing from word meaning. Cereb. Cortex. 2013;23:2370–2379. doi: 10.1093/cercor/bhs228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen A., Gerretsen P., Blomert L. Naming problems do not reflect a second independent core deficit in dyslexia: Double deficits explored. J. Exp. Child Psych. 2009;103:202–221. doi: 10.1016/j.jecp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Woodcock R., McGrew K., Mather N. 2001. Woodcock-Johnson® III NU Tests of Achievement. [Google Scholar]
- Ziegler J., Castel C., Pech-Georgel C. Developmental dyslexia and the dual route model of reading: Simulating individual differences and subtypes. Cognition. 2008;107:151–178. doi: 10.1016/j.cognition.2007.09.004. [DOI] [PubMed] [Google Scholar]