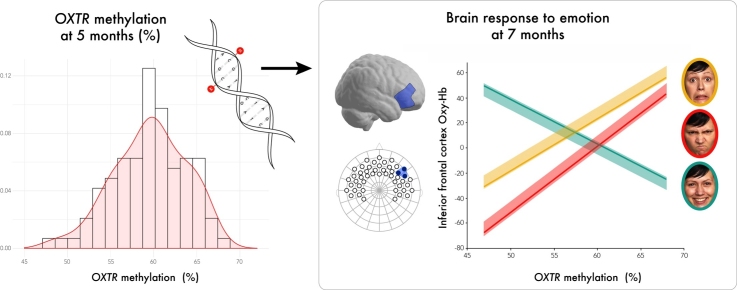
Graphical abstract
Keywords: Oxytocin, Infancy, fNIRS, Inferior frontal cortex, Emotion, Epigenetics, DNA methylation
Highlights
-
•
First developmental neuroimaging epigenetics study with human infants.
-
•
Oxytocin receptor gene methylation (OXTRm) assessed in a large sample of infants.
-
•
OXTRm predicts inferior frontal brain responses to emotional faces using fNIRS.
-
•
Higher OXTRm linked to enhanced brain responses to angry and fearful faces.
-
•
OXTRm contributes to variability in social brain function early in ontogeny.
Abstract
The neural capacity to discriminate between emotions emerges early in development, though little is known about specific factors that contribute to variability in this vital skill during infancy. In adults, DNA methylation of the oxytocin receptor gene (OXTRm) is an epigenetic modification that is variable, predictive of gene expression, and has been linked to autism spectrum disorder and the neural response to social cues. It is unknown whether OXTRm is variable in infants, and whether it is predictive of early social function. Implementing a developmental neuroimaging epigenetics approach in a large sample of infants (N = 98), we examined whether OXTRm is associated with neural responses to emotional expressions. OXTRm was assessed at 5 months of age. At 7 months of age, infants viewed happy, angry, and fearful faces while functional near-infrared spectroscopy was recorded. We observed that OXTRm shows considerable variability among infants. Critically, infants with higher OXTRm show enhanced responses to anger and fear and attenuated responses to happiness in right inferior frontal cortex, a region implicated in emotion processing through action-perception coupling. Findings support models emphasizing oxytocin’s role in modulating neural response to emotion and identify OXTRm as an epigenetic mark contributing to early brain function.
1. Introduction
The ability to detect and discriminate between emotional expressions is a vital social skill that emerges early in human development. Behavioral and neuroscience work consistently demonstrates that by seven months of age, infants distinguish between emotional expressions conveyed through faces, voices, and bodies (Grossmann, 2015). At the same time, infants have also been shown to vary in their responses to emotional displays (Krol et al., 2015a; Rajhans et al., 2015). Identifying factors that contribute to variability in emotion processing in infancy is important to achieve a more mechanistic understanding of healthy and atypical socio-emotional development. In prior work, individual differences in emotion processing have been linked to overt social behaviors in neurotypical adults and infants (Marsh et al., 2007; Grossmann et al., 2018), and have in more extreme forms been associated with a host of mental health disorders in adolescents and adults (Gur et al., 2017; Lee et al., 2016). One factor that may contribute to variability in emotion processing is the endogenous oxytocin system.
The neurohormone oxytocin influences a range of social and emotional brain processes (Kanat et al., 2014). Intranasal administration of oxytocin has been shown to enhance prosocial behaviors, facilitate the formation of social bonds, and modulate the perception of emotional information (Leppanen et al., 2017; Kosfeld et al., 2005; Gamer et al., 2010; Marsh et al., 2010). Although exogenous administration studies are informative, the exploration of individual differences within the endogenous system allows us to further understand the role of oxytocin in vivo, shedding light on individual differences in socio-developmental trajectories. One mechanism that has the potential to drive observed variability is epigenetic modification of genes, which impacts transcription without changing underlying genetic structure (Jaenisch and Bird, 2003). DNA methylation is an epigenetic modification that leads to the addition of a methyl group to a cytosine residue, commonly in the context of a 5′-Cytosine-phosphate-Guanine-3′ dinucleotide pair (CpG site) (Bird, 2011).
The human oxytocin receptor gene (OXTR) contains a specific DNA methylation-dependent regulatory region in its promoter (termed MT2). Increased methylation of this region was found to be associated with reduced expression of OXTR, suggesting a regulatory role on gene transcription (Kusui et al., 2001). Later work by Gregory, Connelly, and colleagues (Gregory et al., 2009) profiled the methylation status of every CpG site in MT2 in a sample of post-mortem human neurotypical and autistic brain tissue, identifying six CpG sites at which methylation differed depending on autism diagnosis. Remarkably, several of these sites are conserved in the prairie vole, indicating that the animal model can provide insights into the human system. Indeed, recent work in prairie voles by Perkeybile and colleagues (Perkeybile et al., 2018) has found that both brain- and blood-derived OXTRm levels at these sites are negatively associated with gene expression in the brain and highly correlated with each other. This work highlights (1) the regulatory potential of DNA methylation at specific sites in OXTR on expression of oxytocin receptor, and (2) the ability to use DNA methylation levels derived from peripheral tissues, such as blood and perhaps saliva, as viable markers of the endogenous oxytocinergic system in the brain. The use of saliva is particularly important for studies that require non-invasive techniques, such as research with human infants. Finally, in human adults, blood-derived variability at these CpG sites within the promoter has been linked to susceptibility to disorders associated with social deficits as well as individual differences in the brain’s response to social information. For example, increased OXTRm has been associated with callous unemotional traits, anorexia nervosa, postpartum depression, and autism spectrum disorder (ASD) (Gregory et al., 2009; Dadds et al., 2014; Bell et al., 2015; Kim et al., 2014). Further, increased OXTRm has been associated with a heightened amygdala response to angry and fearful faces in neurotypical adults (Puglia et al., 2015). Taken together, OXTRm may be a useful epigenetic mark reflective of individual differences in adult social function, including emotion perception. OXTRm has not been investigated in human infants, and it remains unknown whether it may contribute to variability in emotion perception early in human ontogeny.
In the current study, we applied a developmental neuroimaging epigenetics approach to examine how OXTRm impacts emotional face processing during early postnatal development. Previous work shows that infants’ facial emotion discrimination abilities emerge between five and seven months (Leppanen and Nelson, 2009; Peltola et al., 2009), we therefore focused our study on this age range. At five months, OXTRm levels were measured from saliva, a tissue we show is highly correlated with blood methylation levels, and allows for non-invasive, infant-friendly collection. Others have shown saliva useful in identifying epigenetic marks predictive of social behavior in healthy and clinical populations (Haas et al., 2016; Smith et al., 2015). At seven months, brain responses to happy, angry, and fearful facial expressions were measured from inferior frontal cortex (IFC) using functional near-infrared spectroscopy (fNIRS). We focused on IFC due to its involvement in facial emotion processing and its atypical function and structure in disorders characterized by social impairment, such as ASD (Dapretto et al., 2006). We hypothesized that variability in OXTRm modulates infant brain responses to facial emotions, representing an early detectable endophenotype. Specifically, as increased blood- and brain-derived OXTRm has been associated with decreased oxytocin receptor expression (Gregory et al., 2009; Perkeybile et al., 2018), we predicted that higher OXTRm would be associated with a heightened neural response to negative (fearful and angry) facial expressions in IFC. In contrast, we predicted that greater OXTRm would be linked to a reduced brain response to positive (happy) facial expressions. This hypothesis is based on intranasal studies in adults as well as infant work demonstrating that experiential and genetic variability linked to higher oxytocin increases sensitivity to positive expressions (Krol et al., 2015a; Marsh et al., 2010; Krol et al., 2015b). To complement our infant findings, we examined the possibility of a similar relation between OXTRm and IFC function during emotion processing in adults by analyzing previously collected fMRI data.
Prior work exists suggesting that both infant temperament and maternal traits can impact how infants respond to others’ emotional expressions. For example, infants’ responses to emotional displays in others are influenced by their own fearful temperament (Rajhans et al., 2015; de Haan et al., 2004). Moreover, reduced positive maternal engagement has been linked to heightened neural responsiveness to fear in seven-month-old infants (Grossmann et al., 2018) and maternal anxiety was found to be linked to how mothers respond to infants’ emotional cues (Emery et al., 2014) and to their infants’ temperament (Austin et al., 2005). We therefore decided to assess whether and how infant fearful temperament links to brain response to emotional faces, which allowed us to determine whether behavioral signs of fearfulness are associated with infant IFC response to fear in others. In addition, we explored the possibility that maternal trait anxiety might modulate infant fearful temperament, brain response, and OXTRm.
2. Material and methods
2.1. Participants
Ninety-eight infants (49 females) of European descent participated in this study at five months (Mage = 147.97 days, SD = 14.44) and seven months of age (Mage = 214.07 days, SD = 7.25). Infants who did not provide at least three artifact-free fNIRS trials per emotion were excluded in the reported seven-month analyses. Fourteen out of 98 infants were excluded on the basis of this criterion, resulting in a final fNIRS sample of N = 84. Infants were born at standard gestational age (>38 weeks) and normal birthweight (>2,500 g). Sixty-eight of the infants had vaginal births and 16 were delivered through caesarean section. There were no medical issues regarding development at any time of testing, and no known mental health history in any of the parents or older siblings of the infants. All infants had mothers on maternity leave at both testing points. Parents provided written informed consent for participation and were compensated with travel money, a toy for the infant, and a photograph of the infant at each visit. All procedures were approved by the Leipzig University Medical School Ethics Committee and were conducted in accordance with the Declaration of Helsinki.
2.1.1. Infant DNA isolation and epigenetic procedures
Passive drool was collected from infants at five months using collection kits from DNA Genotek (CS-2 sponges stored in OG-250 kits) (Ottawa, Canada) and was stored at room temperature until DNA isolation. Saliva collection kits were incubated at 50 °C for 1 h followed by centrifugation for 10 min at 1000 RPM to release all liquid from sponges. 500 μL of saliva was used to isolate DNA using the manual purification protocol from DNA Genotek. DNA was stored in Hydration Solution from Qiagen (Hilden, Germany) (10 mM Tris, 1 mM EDTA, pH 7–8) and quantitated using nanodrop. 200 ng of DNA was subject to bisulfite treatment (MECOV50 Kit, Thermo Fisher Scientific, Waltham, MA). Sample locations within 96-well plates were randomized.
The subsequent procedures were processed in triplicate (for every one sample, three replicates were generated): 40 ng of bisulfite-converted DNA was amplified using polymerase chain reaction (PCR) with a PyroMark PCR Kit (Qiagen) using 0.2 μM primers (Table 1). This amplified a 116-base-pair region on the coding strand of OXTR containing CpG-924 (hg38, chr3:8,769,044−8,769,160). Note that analysis of this CpG site was hypothesis-driven due to previous work indicating its association with reduced gene expression and hypermethylation in ASD (Gregory et al., 2009). In addition, this CpG site is not on the commercially-available Illumina 450 K or Epic (850 K) arrays (San Diego, CA). Each PCR plate contained methylation standards (0, 50, and 100% methylated) and negative controls from bisulfite conversion and PCR. Successful PCR amplification of a single 116-base-pair fragment was confirmed using agarose gel electrophoresis. Non-converted cytosines were quantified using pyrosequencing (PyroMark Q24, Qiagen) (see Table 1). Replicate variability averaged 2.08%. Reported DNA methylation levels are the average of the three technical replicates.
Table 1.
Listed are the steps taken in the polymerase chain reaction (PCR) protocol followed by the primers used during PCR (Forward and Reverse) and subsequent pyrosequencing (Sequencing) in order to generate targeted analysis of OXTR CpG site -924.
| Step | Process | Temperature (°C) | Duration (s) | Cycle(s) |
|---|---|---|---|---|
| 1. | Initial denaturation | 95 | 15 | 1 |
| 2. | Denaturation | 94 | 30 | 50 |
| Annealing | 56 | 30 | ||
| Elongation | 72 | 30 | ||
| 3. | Elongation | 72 | 10 | 1 |
| 4. | Hold | 4 | until analysis | 1 |
| Primer | Sequence |
|---|---|
| Forward (TSL101F) | 5′-TTGAGTTTTGGATTTAGATAATTAAGGATT-3′ |
| Reverse (TSL101R) | 5′-biotin-AATAAAATACCTCCCACTCCTTATTCCTAA-3′ |
| Sequencing (TSL101S) | 5′-AGAAGTTATTTTATAATTTTT-3′ |
2.1.2. Tissue comparison analysis
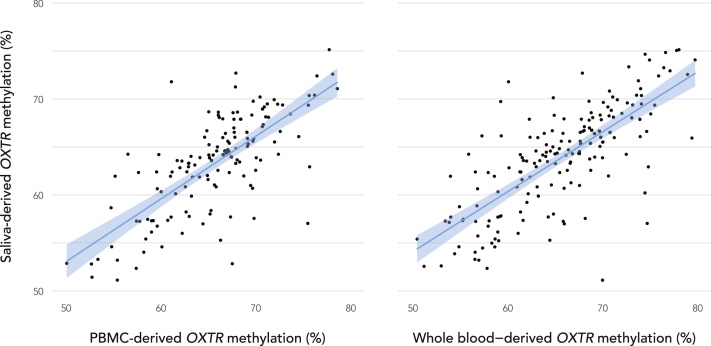
In prairie voles, peripheral blood-derived OXTRm has been shown to be highly correlated with brain-derived OXTRm, and both measures were shown to predict OXTR expression (Perkeybile et al., 2018). To determine reliability of OXTRm values derived from saliva at CpG site -924, we conducted an additional tissue comparison analysis in a sample of 206 neurotypical adults (112 females; Mage = 36.9, SD = 22.6). Participants provided both passive drool and intravenous blood for assessment of methylation derived from peripheral blood mononuclear cells (PBMCs) (N = 142), and/or assessment of whole blood methylation (N = 181). Epigenetic analyses were performed using the same procedures outlined above. Replicate variability averaged 1.34% and reported DNA methylation levels are the average of three technical replicates. Further information on adult tissue collection and DNA isolation can be found in Supplementary Information.
2.2. fNIRS procedure
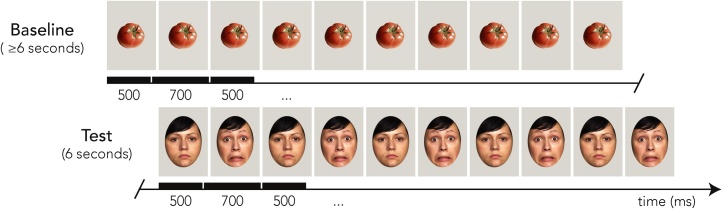
Infants underwent the same fNIRS paradigm as in (Grossmann et al., 2018). Infants were presented with photographs of five Caucasian females expressing happiness, anger, fear, and neutrality. These actresses were chosen from a validated and published stimulus set (FACES Collection) (Ebner et al., 2010) and had expressions with average recognition accuracies at or above 93.25% [see Ebner et al., 2010 for details]. Using Adobe Photoshop CS5 (San Jose, CA), faces were placed below an oval in the center of a gray background. Baseline images consisted of photographs of five inanimate objects (vegetables) provided by Otsuka and colleagues (Otsuka et al., 2007), placed centrally within the same gray background. These stimuli have been successfully used as baseline images in infant fNIRS studies concerned with face processing (Grossmann et al., 2018; Otsuka et al., 2007; Nakato et al., 2011, 2009). The visual angles of the facial and baseline stimuli were around 15.7° × 21.7° and 16.8° × 16.8°, respectively (see Fig. 1).
Fig. 1.
Infant fNIRS paradigm. Dynamically changing stimuli were presented to infants while fNIRS was recorded. Neutral faces rapidly and repeatedly changed to one of three target emotions (happiness, anger, and fear). Each emotional test trial was preceded by at least six seconds of baseline (inanimate vegetable) stimuli [from Nakato et al., 2011]. A fear trial is displayed here as an example.
Infants were seated on a parent’s lap in a dimly lit room, facing a screen (52 cm × 32 cm) at a distance of approximately 60 cm. A small plastic ring was provided for each infant to hold during the experiment to reduce arm and body movements. A camera attached to the bottom of the screen allowed for online tracking of infant behavior as well as offline coding of attention during trials. Stimuli were presented using Presentation software (Neurobehavioral Systems, Berkeley, CA).
As in (Grossmann et al., 2018), the experimental paradigm consisted of blocks of three randomized trials each of happiness, anger, and fear. Each block began with an attention-getter to orient infants to the center of the screen [a video clip of a shaking rattle accompanied by sound, see Krol et al., 2015a]. At the beginning of each trial, a brief 150-millisecond bell sound (about 600 Hz) occurred to maintain infant attention. Trial presentation was pseudo-randomized such that each infant viewed every possible actress-emotion combination, no actress expressed the same emotion consecutively, and no emotional expression was repeated more than twice in a row. Similar pseudo-randomization parameters were used for the baseline stimuli such that no image served as baseline twice in a row, and every possible baseline-emotion combination was presented.
Baseline and face stimuli were presented in the following fashion in order to create dynamically changing visual stimulation: The baseline consisted of six seconds of the same photograph changing from its original size (500 ms) to a slightly larger size (˜1° increase in visual angle) (700 ms) at least five times. The face presentation consisted of six seconds of the same actress changing from a neutral expression (500 ms) to the target emotion (700 ms) five times (please see Fig. 1 for a detailed schematic of this paradigm). This method of pseudo-dynamic presentation of facial expressions was adapted from previous infant fNIRS paradigms (Nakato et al., 2011), and ensured that infants maintained attention during the relatively long trials that fNIRS measurement requires (in comparison to EEG, for example). Infants viewed an average of 31.02 total trials (range = 18–43; SD = 5.63).
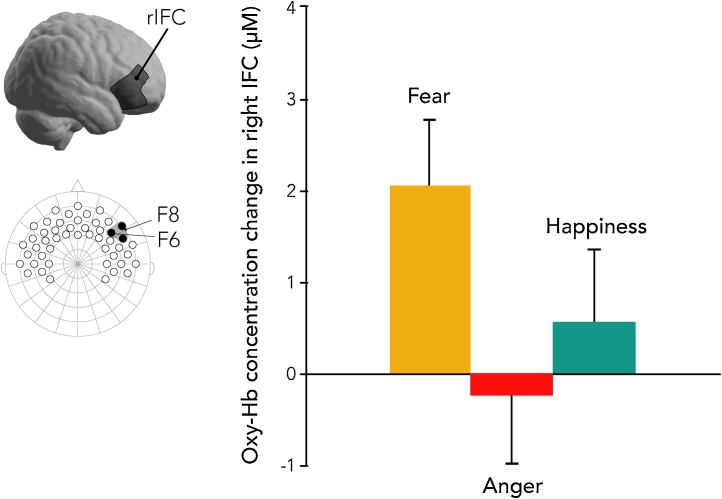
Infant fNIRS data were recorded using a NIRScout system and NIRStar acquisition software (NIRx, Berlin, Germany). A custom-built elastic cap (EasyCap, Woerthsee-Etterschlag, Germany) contained 32 optodes (16 sources, 16 detectors) placed at an approximately 2.5 cm distance. This arrangement comprised a total of 49 channels (source-detector pairs) placed over frontal and temporal cortices of both hemispheres (see Fig. 3). Data were recorded at a sampling rate of 6.25 Hz. Near-infrared light was emitted at two wavelengths (760 nm and 850 nm) with a power of 20 mW/wavelength. The system automatically adjusted light intensity in order to provide optimal gain. Regions of interest (ROIs) were selected to analyze activation within IFC of each hemisphere. Three-channel groupings were chosen using Kabdebon and colleagues’ anatomical correlations of the infant 10–20 system (Kabdebon et al., 2014) and through the use of nirsLAB software (NIRx), which estimates the projection of designated channels onto MNI space (see Fig. 3).
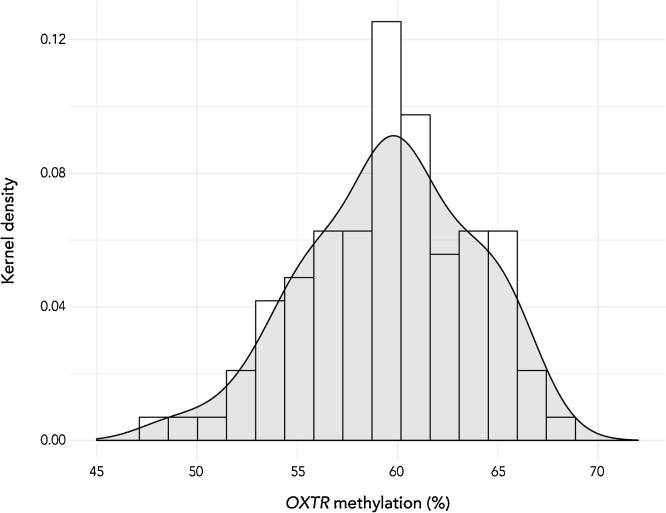
Fig. 3.
Graph displays distribution of salivary-derived methylation levels at OXTR CpG site -924 (histogram with overlaid density plot). Methylation levels ranged from 47.91% to 67.98% (M = 59.57, SD = 4.22).
2.3. fNIRS data analysis
Videos from each session were coded for infant attention to each trial. Trials were only included in the fNIRS analyses if infants had attended to the screen at least four of the six seconds for which both baseline and face stimuli were presented. Additionally, the fNIRS data were visually inspected for motion artifacts. Trials with motion artifacts were removed from further analyses. The remaining data was analyzed using the Matlab-based software Nilab2 (NIRx). Data were filtered with a 0.2-Hz low-pass filter and a 12-s high-pass filter in order to remove changes too slow to be related to experimental stimuli (i.e., fluctuations due to drift). Using six-second time windows after face onset (equaling the stimulus presentation duration of each emotional face), measurements were converted into oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) using the Beer-Lambert law. The average concentration changes of oxy-Hb and deoxy-Hb in response to each emotional expression were extracted per channel, per infant. For ease of understanding and to avoid working with small decimals, all hemoglobin concentrations were re-scaled (*10,000).
2.4. Questionnaires
The Interpersonal Reactivity Index [IRI; Davis, 1983] was administered to mothers to assess dispositional empathic distress. The Personal Distress subscale assesses the extent to which one reports “self-oriented” feelings of anxiety and unease in tense interpersonal situations, and is considered a measure of vicarious anxiety. The Edinburgh Postnatal Depression Scale was also administered to detect potential signs of postpartum depression [EPDS; Cox et al., 1987], particularly due to its previous link to OXTRm in mothers (Bell et al., 2015). To assess infant fearful temperament, parents completed the Revised Infant Behavior Questionnaire [IBQ-R; Gartstein and Rothbart, 2003] at the seven-month visit. The fear subscale assesses the extent to which an infant displays startle and distress to sudden changes in simulation, as well as inhibited approach to novel objects and social stimuli. Additional demographic information from the mother was documented using an in-house questionnaire during both visits (i.e., breastfeeding, parity, education, delivery method) (Krol et al., 2014).
2.5. Adult fMRI analysis
To determine whether relationships between OXTRm and neural response to emotional faces are similar in adulthood, we analyzed data from a previously published adult fMRI study (Puglia et al., 2015). Ninety-six healthy adults aged 18–30 years (M = 22.79, SD = 3.38) were included in the adult sample. Only self-identified Caucasians of European descent were included to avoid population stratification effects. Intravenous blood was collected in BD Vacutainer Cell Preparation Tubes with sodium citrate (BD Biosciences, Franklin Lanes, NJ), and DNA was isolated from PBMCs using reagents supplied in the Gentra Puregene Blood Kit (Qiagen, Hilden, Germany). Methylation analysis then proceeded exactly as described above in the infant dataset. Adults completed an emotional face matching task in which they were presented with triads of faces depicting fearful and angry emotions, and asked to determine which of two probe faces on the bottom of the screen matched the emotion of a target face depicted at the top of the screen via button press while undergoing fMRI [see Puglia et al., 2015 for detailed information]. Participants matched the orientation of ovals as a sensorimotor control.
At the subject level, we modeled each emotion target separately and computed contrasts for Faces > Ovals, Angry Faces > Ovals, and Fearful Faces > Ovals in FSL (Smith et al., 2004). The contrast of parameter estimates (COPE) from these analyses for each individual was carried forward to group-level analyses testing for linear relationships between OXTRm and neural response within IFC ROIs. Left and right IFC ROIs were created using the pars triangularis of inferior frontal gyrus provided in FSL’s Harvard–Oxford Cortical Structural Atlas (Desikan et al., 2006) at a probabilistic threshold of 20, which maximally overlapped with the infant-defined ROI. Z-statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of p < 0.05. We tested for outliers and data points with undue influence by ensuring that the absolute value of the standardized residuals < 3 and Cook’s distance (D) < 1 (Cook and Weisberg, 1982) for each data point. To illustrate significant effects, clusters that survived correction were registered to subject space, and mean Z-statistic values were extracted for each participant from these clusters and plotted against their OXTRm value.
3. Results
Findings from our tissue comparison analysis show that saliva-derived OXTRm levels are highly correlated with PBMC-derived methylation (r(140) = 0.74, p < 0.0001) and whole blood-derived methylation (r(179) = 0.77, p < 0.0001) (Fig. 2). This suggests that OXTRm derived from saliva may be considered a peripheral alternative for use with vulnerable populations. Infant saliva OXTRm levels ranged from 47.91% to 67.98% methylated (M = 59.57, SD = 4.22) and were normally distributed (Zskewness = 1.34, p > 0.05) (Fig. 3). There was no association of infant OXTRm with maternal postpartum depression score, maternal demographic and breastfeeding variables, or method of delivery (ps < 0.24).
Fig. 2.
To determine reliability of OXTR methylation values obtained from saliva at CpG site -924, a tissue comparison study was performed in which 206 healthy adults provided both passive drool and intravenous blood for assessment of methylation derived from peripheral blood mononuclear cells (PBMCs) (N = 142), and/or assessment of whole blood methylation (N = 181). Saliva-derived methylation levels are highly correlated with PBMC-derived methylation (r(140) = 0.74, p < 0.0001) and whole blood-derived methylation (r(179) = 0.77, p < 0.0001).
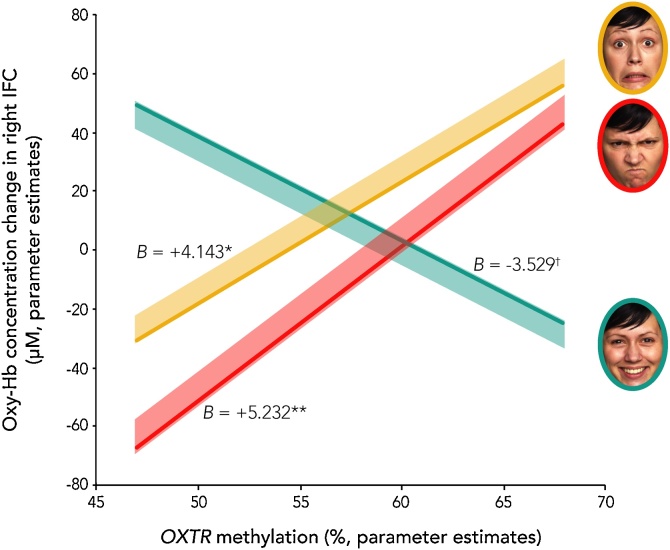
The association of infant OXTRm with the IFC response to emotional expressions was assessed using repeated-measures ANOVAs for each hemisphere (within-subject factor: emotion) with OXTRm as a continuous between-subjects factor. A main effect of emotion was present in right IFC, F(2, 160) = 6.177, p = 0.003. In general, infants displayed greater neural activation to fearful faces than to angry and happy faces (Fig. 3, for oxy-Hb distributions see Supplemental Fig. 1). An interaction between OXTRm and emotion was also present, F(2, 160) = 6.223, p = 0.002 (Fig. 4). Parameter estimates from the model indicate a negative association of OXTRm with right IFC in response to happiness (B = −3.529, p = 0.097), and positive associations of OXTRm and right IFC in response to anger (B = 5.232, p = 0.01) and fear (B = 4.143, p = 0.033) (Fig. 5). To test whether the differences between the individual slopes were significant, t-values were calculated using equations as specified by Robison and colleagues (Robison et al., 2013). The slope characterizing OXTRm and the brain response to happy faces was significantly different from the slopes for both angry faces (t[162] = −4.268, p < 0.001) and fearful faces (t[162] = −3.802, p < 0.001). Anger and fear slopes were not significantly different from each other (t[162] = 0.557, p = 0.289). This suggests that OXTRm associates with the brain response to happy faces differently (negatively) than it does for the response to anger and fear (positively). There was neither a main effect of emotion nor an emotion x OXTRm interaction within left IFC (ps > 0.47). Note that it is not uncommon in infant literature to find greater discrimination of emotion in right hemisphere (Missana et al., 2014).
Fig. 4.
The right inferior frontal cortex (IFC) discriminates between emotions at seven months of age. Oxygenated hemoglobin (Oxy-Hb) concentration change is reported in the right IFC. The ROI from our cap template is shown, as well as its projection to MNI space. Using fNIRS, we show that the right IFC discriminates between angry, happy, and fearful faces at seven months of age. Plotted are the means from our model, error bars represent ±1 SEM.
Fig. 5.
The right IFC response at seven months of age is modulated by OXTR methylation in a valence-dependent manner. Specifically, increased OXTR methylation is associated with increased oxygenated hemoglobin (Oxy-Hb) in right IFC while viewing anger and fear, and reduced Oxy-Hb to happiness. Lines plotted are the parameter estimates from our model, and shaded regions represent the upper and lower 95% confidence intervals; **p < .01; *p < 0.05, †p < 0.10.
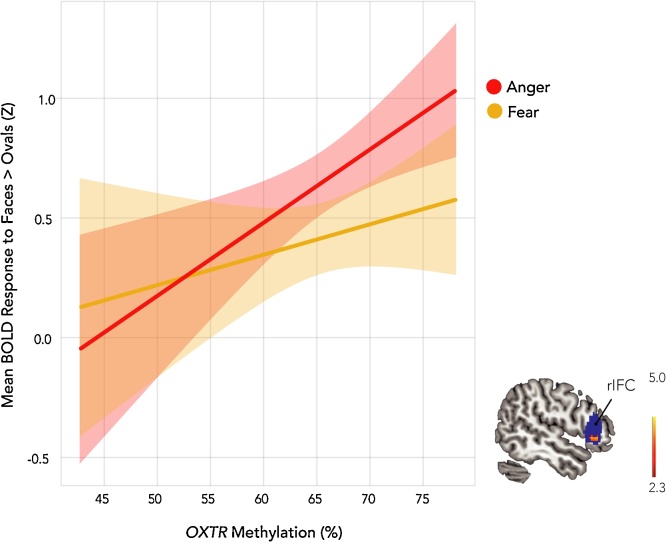
In order to explore whether our pattern of infant findings in IFC are similar in adulthood, we analyzed fMRI data from a previously collected sample of adults during the viewing of angry and fearful faces [for detailed information on this study, see Puglia et al., 2015]. Tests for emotion-specific relationships between OXTRm and neural response to anger and fear revealed a positive association between OXTRm and neural response to angry targets in right (Z = 3.33, k = 38, MNI coordinates (x,y,z) = 50, 32, −2) and left (Z = 4.44, cluster extent (k) = 107, x,y,z = −52, 30, 2) IFC, thus corroborating our infant findings and suggesting persistence into adulthood (Fig. 6). Relationships between OXTRm and neural response to fearful targets alone were not significant but showed a positive trend. A positive association between OXTRm and the neural response to both angry and fearful faces was also found in left IFC (Z = 3.82, k = 71, x,y,z = −54, 30, 2).
Fig. 6.
The right inferior frontal cortex (IFC) response to emotional faces is modulated by OXTR methylation in adults. Using fMRI, we find a positive association between OXTR methylation and adult neural response to angry faces present in the right IFC (Z = 3.33, p < 0.001; cluster extent (k) = 38, x,y,z = 50, 32, −2) as indexed through the blood oxygenation level dependent signal (BOLD), thus corroborating infant findings and suggesting persistence into adulthood. Relationships between OXTR methylation and neural response to fearful targets were not significant but showed a positive trend. Shaded regions represent the upper and lower 95% confidence intervals. The color bar represents a Z statistical map.
In order to better understand the relationship between OXTRm and infant IFC response to emotion, we investigated the possible association of infant fearful temperament and maternal anxiety with our findings. We investigated whether right IFC response to negative emotion was associated with infant fearful temperament as assessed by the IBQ-R (Gartstein and Rothbart, 2003). The right IFC response to fear correlated positively with infant fearful temperament, r(70) = 0.242, p = 0.043, such that higher fearful temperament was associated with increased IFC response to fear. We further examined potential links of maternal interpersonal reactivity traits to methylation, brain and behavioral measures obtained from the infants. This analysis specifically focused on maternal personal distress, that is, self-oriented feelings of personal anxiety and unease in response to others’ tense experience, measured by the IRI (Davis, 1983). Our analysis revealed that maternal dispositional personal distress positively correlates with infant fearful temperament and infant OXTRm (r(68) = 0.288, p = 0.017 and r(76) = 0.286, p = 0.012, respectively).
4. Discussion
In the current study, we examined how epigenetic modification of OXTR associates with emotional face processing in human infancy using a developmental neuroimaging approach. First, we provide novel evidence that by five months of age, human infants vary extensively in the epigenetic modification of OXTR, similar to the variability found in adults (Gregory et al., 2009; Puglia et al., 2015; Jack et al., 2012). Second, our analysis revealed that OXTRm levels measured at five months of age are systematically linked to variability in right IFC response to emotional facial expressions at seven months of age, suggesting this relationship is established early in development. As predicted, greater OXTRm is associated with increased response to negative (fearful and angry) facial expressions and reduced response to positive (happy) facial expressions in right IFC. A similar association between OXTRm and IFC response to anger and fear was also shown in a sample of adults using fMRI. Our results indicate that, from early in human development, epigenetic modification of the oxytocin receptor is variable and is predictive of brain responses to emotional facial information.
The current findings are in agreement with oxytocin administration studies that report a valence-specific impact on the processing of emotions (Gamer et al., 2010; Marsh et al., 2010; Gamer and Buchel, 2012; Kirsch et al., 2005) and critically extend them by identifying similar effects early in development now using an endogenous marker of the oxytocin system. Our findings complement theoretical frameworks stipulating that oxytocin facilitates approach tendencies while simultaneously reducing the tendency to withdraw from aversive contexts (Kemp and Guastella, 2011). The analysis of adult fMRI data included in the current study indicates that the association between OXTRm and emotional face processing in right IFC is similar in adults and infants. This suggests that the relationship between epigenetic variability and right IFC function may be stable across development, pointing to OXTRm as an enduring modulator of emotional brain function.
As a general finding, our fNIRS results revealed that emotional faces differentially modulate responses in right IFC by seven months of age with fearful faces evoking greater responses than happy and angry faces. This adds to existing neural and behavioral evidence indicating that by this age, infants discriminate between these emotions and typically show a heightened attentional response to fear (Bayet et al., 2017; Jessen and Grossmann, 2016; Peltola et al., 2013). Previous fNIRS studies in infants had focused only on temporal cortex responses and not included fearful faces as stimuli (Nakato et al., 2011), thus our results extend prior findings by identifying the right IFC as an important modulator of neural attention to emotions at an early age. The IFC is implicated in understanding the intentions of others, and is activated during both the imitation and passive viewing of emotional expressions (Dapretto et al., 2006). The perception-action model places the IFC in a theoretical framework that suggests overlapping representations for performing and observing actions. In an emotional context, viewing another’s emotional expression spontaneously activates one’s own representations of the target expresser, the emotional expression, and context; thus laying the foundation for empathic responding (de Waal and Preston, 2017). Our data further show that infants’ right IFC response to fearful faces is positively associated with their behaviorally displayed fearfulness. This finding is in favor of the interpretation that the recruitment of right IFC might be linked to perception-action coupling during emotion processing. It is also important to mention that heightened fearful temperament in infancy has been linked to the development of anxiety disorders in childhood and adolescence (Goldsmith and Lemery, 2000). Notably, IFC structure and function are consistently reported to be atypical among individuals diagnosed with disorders characterized by social dysfunction such as ASD and bipolar disorder (Dapretto et al., 2006; Cho et al., 2009; Patriquin et al., 2016). From a clinical perspective, the current results point to enhanced right IFC recruitment during fearful face processing as an early detectable potential brain marker of fearfulness.
Finally, we provide evidence that maternal interpersonal traits, specifically their vicarious personal distress, link to infants’ OXTRm and fearful temperament, which are both associated with enhanced brain responses to fearful faces. Specifically, greater empathic distress reported by mothers was associated with greater fearfulness and OXTRm among the infants. This suggests that maternal anxious traits displayed during social interactions with others, including her own infant, are associated with increased OXTRm (likely indexing lower amount of oxytocin receptor) among infants. Previous research with animals has shown that maternal care critically impacts the epigenome of the offspring (Weaver et al., 2004). Moreover, there is behavioral research indicating that human mothers’ social anxiety may be transmitted to infants (de Rosnay et al., 2006), supporting the link seen between maternal personal distress and infant fearfulness. Given the correlational nature of our findings, it is not possible to draw any conclusions concerning the directionality (causality) of these effects. Critically, research is needed that directly examines how maternal behavior influences epigenetic changes in OXTR during early development and how it might predict infant outcomes.
While the current study focused on epigenetic modification of OXTR and the neural response to emotional expressions, it should be acknowledged that DNA methylation of other genes, including the serotonin receptor and oxytocin, has been associated with neural processing of socio-emotional stimuli in adults (Haas et al., 2016; Frodl et al., 2015). Future work targeting additional, regulatory CpG sites on other genes in a hypothesis-driven manner is needed to gain a more comprehensive understanding of how epigenetic modifications relate to and may interact in accounting for variability in socio-emotional brain functions.
In addition, there is limited work on the distribution of oxytocin receptor in the primate brain, and virtually no information existing on its distribution in the developing infant brain. Limited work in the non-human primate brain reports the majority of OXTR in subcortical regions (Freeman et al., 2014a, b; Freeman and Young, 2016). Infant fNIRS is limited to measuring responses from cerebral cortex, and thus did not allow us to examine responses in subcortical brain regions. In this context, it is important to mention that while we report an association of OXTRm and IFC responding, it is unknown whether OXTR is expressed in the cerebral cortex during prenatal and early postnatal human brain development. It is possible that OXTRm primarily acts on subcortical brain processes that then affect processes in connected brain regions in the cerebral cortex.
Finally, we would like to emphasize that working with infants required us to select a non-invasive, peripheral marker of OXTRm, namely, DNA derived from saliva. Previous work from our team demonstrates the feasibility of using peripheral tissues through human post-mortem studies (Gregory et al., 2009) and animal models (Perkeybile et al., 2018). Specifically, OXTRm derived from blood is highly correlated with methylation derived from brain, the causal tissue of interest. Critically, in the current study we report data from a large sample of adults showing a high correlation between blood- and saliva-derived methylation levels. Given these data, saliva appears to be a viable tissue for the assessment of OXTRm.
5. Conclusions
In summary, we identified OXTRm as an important factor contributing to individual variability in right IFC function during emotion processing in infancy. Our data indicate that OXTRm is already variable at five months of age, and may be a particularly promising epigenetic mark to study individual differences in socio-developmental trajectories. Moreover, given the considerable literature on the role of IFC in emotion understanding and its atypical function in psychopathologies characterized by social impairment, the current study suggests that functional variability is already present in infancy and may guide potential behavioral interventions for at-risk infants. Finally, we provide preliminary evidence that maternal anxious disposition is associated with variability in infant OXTRm, adding to the existing work with animals linking maternal care to epigenetic modifications seen in the offspring. Taken together, the current findings establish the importance of understanding the contribution of the epigenome to early variability in social brain function.
Funding
This research was supported by the Max Planck Society (TG), the University of Virginia (TG and JC), an International Max Planck Research School (IMPRS NeuroCom) scholarship, an NIH NRSA T32 Research Training in Neuroendocrinology Fellowship, and a Hartwell Biomedical Research Fellowship (to KK), National Science Foundation Grant 1228522, “Examining an Epigenetic Biomarker of Social Perception” (JC and JM), and National Science Foundation Grant 1729289, “Epigenetic influences on the early development of social brain functions” (TG, JM, and JC).
Conflict of interest statement
The authors declare no conflicts of interest.
Acknowledgements
We thank Caterina Boettcher and Jenny Tippmann for their assistance with infant data collection at the Max Planck Institute for Human Cognitive and Brain Sciences, Nicole Altvater-Mackensen for her guidance during fNIRS analyses, Travis S. Lillard and Elisabeth F. Schott for their guidance during epigenetic analyses, Robert G. Moulder for his statistical support, and all families who participated. We thank Diogo Fortes, Amalia McDonald, Andrew Graves, Tyler Santander, Katie Lancaster, Morgan Lynch, and Manisha Nannapaneni for their assistance with adult data collection and preparation of biological samples.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100648.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Austin M.P. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Hum. Dev. 2005;81(2):183–190. doi: 10.1016/j.earlhumdev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Bayet L. Fearful but not happy expressions boost face detection in human infants. Proc. R. Soc. B Biol. Sci. 2017;284(1862):1–9. doi: 10.1098/rspb.2017.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A.F. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front. Genet. 2015;6(243) doi: 10.3389/fgene.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. The dinucleotide CG as a genomic signalling module. J. Mol. Biol. 2011;409(1):47–53. doi: 10.1016/j.jmb.2011.01.056. [DOI] [PubMed] [Google Scholar]
- Cho H.S. Reduced activation in the mirror neuron system during a virtual social cognition task in euthymic bipolar disorder. Biol. Psychiatry. 2009;65(8):145s. doi: 10.1016/j.pnpbp.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Cook R.D., Weisberg S. Chapman and Hall; New York: 1982. Residuals and Influence in Regression. [Google Scholar]
- Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression. Development of the 10-item edinburgh postnatal depression scale. Br. J. Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Dadds M.R. Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev. Psychopathol. 2014;26(1):33–40. doi: 10.1017/S0954579413000497. [DOI] [PubMed] [Google Scholar]
- Dapretto M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44(1):113–126. [Google Scholar]
- de Haan M. Maternal personality and infants’ neural and visual responsivity to facial expressions of emotion. J. Child Psychol. Psychiatry. 2004;45(7):1209–1218. doi: 10.1111/j.1469-7610.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- de Rosnay M. Transmission of social anxiety from mother to infant: an experimental study using a social referencing paradigm. Behav. Res. Ther. 2006;44(8):1165–1175. doi: 10.1016/j.brat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- de Waal F.B.M., Preston S.D. Mammalian empathy: behavioural manifestations and neural basis. Nat. Rev. Neurosci. 2017;18(8):498. doi: 10.1038/nrn.2017.72. [DOI] [PubMed] [Google Scholar]
- Desikan R.S. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ebner N.C., Riediger M., Lindenberger U. FACES-A database of facial expressions in young, middle-aged, and older women and men: development and validation. Behav. Res. Methods. 2010;42(1):351–362. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Emery H.T. Maternal dispositional empathy and electrodermal reactivity: interactive contributions to maternal sensitivity with toddler-aged children. J. Fam. Psychol. 2014;28(4):505–515. doi: 10.1037/a0036986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.M., Young L.J. Comparative perspectives on oxytocin and vasopressin receptor research in rodents and Primates: translational implications. J. Neuroendocrinol. 2016;28(4) doi: 10.1111/jne.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.M. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta) Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.M. Neuroanatomical distribution of oxytocin and vasopressin 1a receptors in the socially monogamous coppery titi monkey (Callicebus cupreus) Neuroscience. 2014;273:12–23. doi: 10.1016/j.neuroscience.2014.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T. DNA methylation of the serotonin transporter gene (SLC6A4) is associated with brain function involved in processing emotional stimuli. J. Psychiatry Neurosci. 2015;40(5):296–305. doi: 10.1503/jpn.140180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Buchel C. Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology. 2012;37(1):87–93. doi: 10.1016/j.psyneuen.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc. Natl. Acad. Sci. U. S. A. 2010;107(20):9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein M.A., Rothbart M.K. Studying infant temperament via the revised infant behavior questionnaire. Infant Behav. Dev. 2003;26(1):64–86. [Google Scholar]
- Goldsmith H.H., Lemery K.S. Linking temperamental fearfulness and anxiety symptoms: a behavior-genetic perspective. Biol. Psychiatry. 2000;48(12):1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Gregory S.G. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7 doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T. The development of social brain functions in infancy. Psychol. Bull. 2015;141(6):1266–1287. doi: 10.1037/bul0000002. [DOI] [PubMed] [Google Scholar]
- Grossmann T., Missana M., Krol K.M. The neurodevelopmental precursors of altruistic behavior in infancy. PLoS Biol. 2018;16(9) doi: 10.1371/journal.pbio.2005281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.E. Face processing measures of social cognition: a dimensional approach to developmental psychopathology. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2(6):502–509. doi: 10.1016/j.bpsc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Haas B.W. Epigenetic modification of OXT and human sociability. Proc. Natl. Acad. Sci. U. S. A. 2016;113(27):E3816–E3823. doi: 10.1073/pnas.1602809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack A., Connelly J.J., Morris J.P. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jessen S., Grossmann T. The developmental emergence of unconscious fear processing from eyes during infancy. J. Exp. Child Psychol. 2016;142:334–343. doi: 10.1016/j.jecp.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Kabdebon C. Anatomical correlations of the international 10-20 sensor placement system in infants. Neuroimage. 2014;99:342–356. doi: 10.1016/j.neuroimage.2014.05.046. [DOI] [PubMed] [Google Scholar]
- Kanat M., Heinrichs M., Domes G. Oxytocin and the social brain: neural mechanisms and perspectives in human research. Brain Res. 2014;1580:160–171. doi: 10.1016/j.brainres.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Kemp A.H., Guastella A.J. The role of oxytocin in human affect: a novel hypothesis. Curr. Dir. Psychol. Sci. 2011;20(4):222–231. [Google Scholar]
- Kim Y.R. Differential methylation of the oxytocin receptor gene in patients with anorexia nervosa: a pilot study. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krol K.M. Breastfeeding experience differentially impacts recognition of happiness and anger in mothers. Sci. Rep. 2014;4:7006. doi: 10.1038/srep07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol K.M. Genetic variation in CD38 and breastfeeding experience interact to impact infants' attention to social eye cues. Proc. Natl. Acad. Sci. U. S. A. 2015;112(39):E5434–E5442. doi: 10.1073/pnas.1506352112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol K.M. Duration of exclusive breastfeeding is associated with differences in infants’ brain responses to emotional body expressions. Front. Behav. Neurosci. 2015;8:459. doi: 10.3389/fnbeh.2014.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusui C. DNA methylation of the human oxytocin receptor gene promotor regulates tissue-specific gene suppression. Biochem. Biophys. Res. Commun. 2001;289(3):681–686. doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Kim C.Y., Lee S.H. Non-conscious perception of emotions in psychiatric disorders: the unsolved puzzle of psychopathology. Psychiatry Investig. 2016;13(2):165–173. doi: 10.4306/pi.2016.13.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen J.M., Nelson C.A. Tuning the developing brain to social signals of emotions. Nat. Rev. Neurosci. 2009;10(1):37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen J. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci. Biobehav. Rev. 2017;78:125–144. doi: 10.1016/j.neubiorev.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Marsh A.A., Kozak M.N., Ambady N. Accurate identification of fear facial expressions predicts prosocial behavior. Emotion. 2007;7(2):239–251. doi: 10.1037/1528-3542.7.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A.A. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl.) 2010;209(3):225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Missana M. Discrimination of fearful and happy body postures in 8-month-old infants: an event-related potential study. Front. Hum. Neurosci. 2014;8(531) doi: 10.3389/fnhum.2014.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakato E. When do infants differentiate profile face from frontal face? A near-infrared spectroscopic study. Hum. Brain Mapp. 2009;30(2):462–472. doi: 10.1002/hbm.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakato E. Distinct differences in the pattern of hemodynamic response to happy and angry facial expressions in infants - a near-infrared spectroscopic study. Neuroimage. 2011;54(2):1600–1606. doi: 10.1016/j.neuroimage.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Otsuka Y. Neural activation to upright and inverted faces in infants measured by near infrared spectroscopy. Neuroimage. 2007;34(1):399–406. doi: 10.1016/j.neuroimage.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Patriquin M.A. Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder. Hum. Brain Mapp. 2016;37(11):3957–3978. doi: 10.1002/hbm.23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola M.J. Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Soc. Cogn. Affect. Neurosci. 2009;4(2):134–142. doi: 10.1093/scan/nsn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltola M.J. The emergence and stability of the attentional bias to fearful faces in infancy. Infancy. 2013;18(6):905–926. [Google Scholar]
- Perkeybile A.M. Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology. 2018;99:128–136. doi: 10.1016/j.psyneuen.2018.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia M.H. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2015;112(11):3308–3313. doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajhans P. The association of temperament and maternal empathy with individual differences in infants’ neural responses to emotional body expressions. Dev. Psychopathol. 2015;27(4):1205–1216. doi: 10.1017/S0954579415000772. [DOI] [PubMed] [Google Scholar]
- Robison C.D., Tomek S., Schumacker R.E. Tests of moderation effects: difference in simple slopes versus the interaction term. Multiple Lin. Regress. Viewpoints. 2013;39(1):16–24. [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith A.K. DNA extracted from saliva for methylation studies of psychiatric traits: evidence for tissue specificity and relatedness to brain. Am. J. Med. Genet. Part B. 2015;168(1):36–44. doi: 10.1002/ajmg.b.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I.C.G. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.