Abstract
The ability to selectively direct attention to a certain location or modality is a key neurocognitive skill. One important facet of selective attention is anticipation, a foundational biological construct that bridges basic perceptual processes and higher-order cognition. The current study focuses on the neural correlates of bodily anticipation in 6- to 8-year-old children using a task involving tactile stimulation. Electroencephalographic (EEG) activity over sensorimotor cortex was measured after a visual cue directed children to monitor their right or left hand in anticipation of tactile stimulation. Prior to delivery of the tactile stimulus, a regionally-specific desynchronization of the alpha-range mu rhythm occurred over central electrode sites (C3/C4) contralateral to the cue direction. The magnitude of anticipatory mu rhythm desynchronization was associated with children’s performance on two executive function tasks (Flanker and Card Sort). We suggest that anticipatory mu desynchronization has utility as a specific neural marker of attention focusing in young children, which in turn may be implicated in the development of executive function.
Keywords: Child electroencephalogram, Mu rhythm, Tactile, Somatosensory, Executive function, Anticipation, Attention
1. Introduction
Selective attention, the deployment of focused attention to task-relevant features of the environment, is fundamental to human cognition. It facilitates sensory processing (Awh et al., 2000), perception (Anderson and Ding, 2011; Posner and Driver, 1992) and is crucial to performance on cognitively-demanding tasks (Gazzaley and Nobre, 2012; Posner and Rothbart, 2007). In children, electroencephalographic (EEG) activity during auditory selective attention has been associated with variation in non-verbal IQ (Isbell et al., 2016) and academic achievement (Stevens and Bavelier, 2012). Investigations of selective attention in childhood typically focus on EEG or behavioral responses following presentation of the target stimulus (Isbell et al., 2016; Markant and Amso, 2016; Ruberry et al., 2017), with event-related responses influenced by the presentation of simultaneous distractors that compete for attention allocation.
One key facet of selective attention is anticipation. Anticipation refers to the preparatory actions and neural activation associated with expectation of an upcoming stimulus. Selective attention tasks often invoke anticipation by incorporating a preparatory cue that conveys relevant information, for example spatial location or modality, relevant to an impending target stimulus (Jensen and Mazaheri, 2010; Posner, 1980).
There is growing evidence that neural indices of visual anticipatory attention are related to broader cognitive skills both in children (Shimi et al., 2015) and adults (Scheeringa et al., 2009; Zanto and Gazzaley, 2009). However, the role of anticipatory attention in the development of self-regulatory abilities has not been closely examined, and is the focus of the current work. Our interest in this connection is driven by the premise that the ability to prospectively adjust to impending events is a fundamental property of self-regulating systems. As such, the ability to anticipate facilitates the maintenance or adaptation of an organism’s state in the face of environmental perturbations (Marshall, 2016; Sokol et al., 2010; Vernon, 2014).
Much of the extant research on anticipatory attention in adults and children involves behavioral and neural responses related to the expectation of visual stimulation. Alongside this work, there is also growing interest in aspects of anticipation related to somatosensory stimulation (Ferri et al., 2017). Interest in developmental aspects of somatosensory anticipation partly stems from work on the role of touch in early caregiving interactions (Gliga et al., 2018; Reddy et al., 2013) and aspects of bodily connections between self and other (Marshall and Meltzoff, 2015). Early in life (and indeed, throughout the lifespan) the flow and regulation of social interaction and joint action with others is implicitly facilitated by attention to (and control of) one’s own body, which in turn is connected to the perception of others’ bodies and actions (Marshall, 2018; Marshall and Meltzoff, 2014). Examining developmental aspects of attention to one’s own body (Gliga and Dehaene-Lambertz, 2005; Meltzoff et al., 2018; Somogyi et al., 2017) may therefore provide a window into an aspect of attention that is closely connected to the growth of social interaction and self-regulatory abilities.
In the current study, we take the novel approach of investigating children’s ability to deploy anticipatory attention to their bodies (indexed by neural activity during anticipation of tactile stimulation), as a potential correlate or predictor of self-regulatory abilities (as measured by executive function tasks) and other cognitive capacities. This interest aligns with recent work that has emphasized the role of sensorimotor influences on emerging executive function skills (Gottwald et al., 2016).
1.1. Development of anticipatory attention
The early development of anticipatory abilities may provide a window into emerging cognitive skills (Rothbart et al., 2006). Classic behavioral work examined aspects of visual anticipation in infancy (Colombo, 2001; Haith et al., 1988; Johnson et al., 1991). Behavioral indicators of infant anticipatory attention have been used to predict the development of self-regulation, motor control, and temperament traits related to executive function (Papageorgiou et al., 2014; Reddy et al., 2013; Sheese et al., 2008). Executive function is the ability to plan, organize, and monitor the execution of goal-directed actions (Kochanska et al., 2001; Zelazo et al., 2013). This construct encompasses various domains such as working memory, cognitive flexibility, and inhibition, which support the voluntary control of attention and behavior (Blair and Raver, 2015; Diamond, 2013; Miyake et al., 2000).
One suggestion arising from work linking selective attention and executive function is that ‘low-level’ indicators of attentional processing reciprocally influence the development of ‘higher-order’ executive function abilities (Gazzaley and Nobre, 2012; Raver et al., 2012; Tarantino et al., 2017). Consistency of selective attention deployment in early childhood is identified as a precursor to emergence of complex executive function abilities (Garon et al., 2008; Hendry et al., 2016; Isbell et al., 2018; Veer et al., 2017). Individual differences in target stimulus response time as well as target stimulus detection accuracy are common behavioral measures of performance on selective attention and executive function tasks (Willoughby et al., 2018). We speculate that variation in these measures may be partially attributed to individual differences in the ability to adaptively prepare for a stimulus as a function of trial-by-trial task demands. As such, we propose anticipation as a key component process in determining the regularity with which participants filter and focus their attention to an upcoming stimulus. Across individuals, anticipatory abilities may index how dynamic prediction (the strength of prior experience) informs perception of upcoming, cue-directed sensory events (Haith et al., 1988; Holmboe et al., 2018). However, there is limited work characterizing individual differences in behavioral and neural indices of anticipation in childhood (beyond infancy) or its potential relations with executive function and selective attention.
1.2. Neural indicators of anticipatory attention
There is continued interest in oscillatory activity in the alpha frequency band (8–13 Hz) in the EEG in relation to various aspects of adult cognitive functioning (Klimesch, 2012; Sadaghiani and Kleinschmidt, 2016; Zanto and Gazzaley, 2009). Studies using event-related spectral perturbation (ERSP) have demonstrated the modulation of alpha oscillations during anticipation of visual (Worden et al., 2000), auditory (Weisz et al., 2011), and somatosensory target stimuli (Haegens et al., 2012; Jones et al., 2010; Shen et al., 2017). When attention is directed by a spatially informative cue to monitor one visual hemifield, one ear, or one hand in anticipation of stimulation of that location, there is typically an event-related desynchronization (ERD; a reduction in band power) in the alpha band over contralateral sensory cortex (Banerjee et al., 2011; Katus et al., 2015; Weisz et al., 2011). In children (Vollebregt et al., 2015) and adults (Jensen and Mazaheri, 2010), alpha ERD is observed over the contralateral occipital region following a directional cue that precedes a visual target presented to the left or right visual field. Greater anticipatory alpha desynchronization reflects a bias in sensory processing in the context of impending action (Engel et al., 2013), and is further associated with heightened perceptual salience of target stimuli (Foxe and Snyder, 2011; Thut, 2006).
Contemporary accounts of alpha-range activity are often grounded in the inhibition-timing hypothesis (Klimesch et al., 2007), positing that increases in the amplitude of alpha-range oscillations arise from synchronized timing of cortical firing in an underlying neural population, which functionally gates the processing of unnecessary features in the immediate environment (Jensen and Mazaheri, 2010; Scheeringa et al., 2009; Thut, 2006). From this perspective, decreases in alpha power are thought to reflect the ‘release from inhibition,’ facilitating the deployment of attention to specific features of the environment and subsequent selective sensory processing (Klimesch, 2012). Regionally-specific alpha ERD contributes to the coordination of larger-scale brain networks that enable dynamic control of perception and working memory (Klimesch, 1999; Sadaghiani and Kleinschmidt, 2016).
Although much of the extant work on alpha power fluctuations has focused on the visual alpha rhythm at posterior sites, another prominent alpha-range rhythm is the sensorimotor mu rhythm that is prominent at central electrode sites (Jones et al., 2010; Kuhlman, 1978; Pfurtscheller, 1989). The mu rhythm is present in infancy and childhood and is functionally distinct from the occipital alpha rhythm (Marshall et al., 2002; Stroganova et al., 1999). Although developmental work on the mu rhythm has emphasized motor aspects of this oscillation (Liao et al., 2015; Marshall et al., 2011), there is increasing interest in somatosensory aspects of the mu rhythm (Marshall and Meltzoff, 2014, Marshall and Meltzoff, 2015), in part driven by findings from adults that mu oscillations may be primarily generated in somatosensory cortex (Ritter et al., 2009).
Expectation of tactile stimulation in adults elicits changes in the mu rhythm that exhibit a contralateral, somatotopic pattern of organization, in accord with the organization of the homuncular strip (Penfield and Boldrey, 1937). In one study with adults, Haegens et al. (2012) presented an arrow (pointing left or right) that directed the participant to attend to which of their hands would subsequently receive tactile stimulation. As has been found in other adult studies involving expectation of touch, anticipatory ERD of the mu rhythm occurred over central sites contralateral to the direction of the spatial cue (Haegens et al., 2012; Jones et al., 2010; Shen et al., 2017; Zhang and Ding, 2010). Although this contralateral mu ERD in anticipation of touch has been well established in adults, to our knowledge there is no prior published study of this effect in children.
1.3. Current study
The dual objectives of the present study are: (i) first to characterize changes in mu rhythm activity during anticipation of tactile stimulation in children aged 6–8 years, and (ii) then to examine whether individual differences in measures of bodily attention are related to children’s performance on executive function tasks. We employed a task in which a visual cue directed children to focus their attention on a specific bodily location (the left or right hand) in anticipation of tactile stimulation to that location. The logic of presenting a preparatory cue in a different modality from the target stimulus allows temporal and spatial differentiation of anticipatory activity (over sensory cortex relevant to the target) from neural responses elicited by the cue (Foxe and Snyder, 2011; Mazaheri et al., 2014).
There are also several strengths of employing somatosensory rather than visual targets: (i) Compared with the visual modality, tactile attention is not complicated by factors such as ocular shifts or visual preferences (Kennett et al., 2007; Papageorgiou et al., 2014); (ii) Neural indices of anticipation of touch are readily measurable through EEG recordings from electrodes overlying somatosensory cortex (Anderson and Ding, 2011; Haegens et al., 2011; Jones et al., 2010); (iii) The ability to focus attention to a body part in expectation of touch may be amenable to change and enhancement via specific interventions (Black and Fernando, 2014; Kerr et al., 2013; Tang et al., 2012); and (iv) cross-modal anticipation is of special relevance to theories in developmental science.
Drawing upon previous studies of childhood EEG during selective attention (Coch et al., 2005) and given our focus on executive function – which develops rapidly prior to school entry (Blair and Raver, 2015; Bull et al., 2008) and by age 6 years can serve as a psychometrically-consistent, reliable predictor of children’s later academic performance (Wiebe et al., 2011) – we investigated the links between bodily attention and cognitive skills in a sample of children aged 6–8 years, measuring neural responses in expectation and in response to tactile stimulation. We speculate that the early emergence of bodily awareness (Marshall and Meltzoff, 2015; Bremner, 2016) may provide a developmental explanation for why neural indicators in expectation of touch are involved in building self-regulation, because infants’ prediction of impending tactile sensations that directly contact the body may support goal-directed actions as well as predictive aspects of social interaction. The ability to anticipate tactile sensations may cascade into habitual individual differences in the prediction of events across modalities (Kiverstein and Rietveld, 2018), such that anticipatory neural activity captures variance in self-regulatory capacity that is foundational to multiple domains of cognitive development (Nigg, 2017). This also fits with the idea that the planning and regulating of bodily actions are central to executive function (Dick and Overton, 2010; Gottwald et al., 2016; Hendry et al., 2016; Pezzulo, 2012; Zelazo et al., 2013).
We had several specific aims and hypotheses. Consistent with the anticipatory mu rhythm response evident in adults, we predicted that children would exhibit mu rhythm desynchronization over contralateral central electrodes during the interval between the cue and the target stimuli (tactile stimulation of the left or right hand). Given the conceptual links between anticipatory attention, the ability to focus attention, and the development of higher-order executive abilities, we further hypothesized that the extent of mu desynchronization would be related to children’s performance on executive function tasks. Drawing upon previous EEG investigations of post-stimulus auditory selective attention and visual anticipatory attention in children (Isbell et al., 2016; Shimi et al., 2015), we expected neural indicators of heightened attention (i.e., greater mu desynchronization or ERD) to relate to higher-order cognitive abilities (i.e., the executive function measures), but not necessarily to detection of target stimuli (Jones et al., 2010; Murphy et al., 2016).
2. Methods
2.1. Participants
Families were recruited from a diverse urban environment through community outreach, commercially available mailing lists, and online advertisements. Families were not invited to participate if their child had any medical or psychological diagnoses, was left-handed, or was on long-term medication. One hundred families with 6 to 8-year-old children (M = 7.2 years, SD = 0.6; 47 male) visited the investigators’ laboratory for one visit. Twenty children were excluded from analyses due to technical issues (n = 8), because the child did not meet eligibility criteria (n = 4), did not tolerate cap preparation (n = 2), or had an insufficient number of artifact-free trials (minimum of 30 trials per condition; n = 6). Caregivers provided demographic information upon arrival to the lab.1 Compared with the analyzed sample (N = 80), the 20 excluded children did not differ in race, family income, age, maternal education, gender and NIH Cognitive Toolbox task scores. Our sample (85% Non-Hispanic) consisted of 28 children identified as African-American, 25 children identified as Caucasian, 24 children identified as mixed race or multiple races, and 2 children identified by their caregiver as “other race”. Prior to data collection, children were read an assent form in the presence of their caregiver that outlined the protocol. Children were then fitted with an EEG cap and tactile stimulators, seated at a table facing a computer screen, and instructed to rest their hands on their lap, under the table and out of sight.
2.2. Somatosensory selective attention task
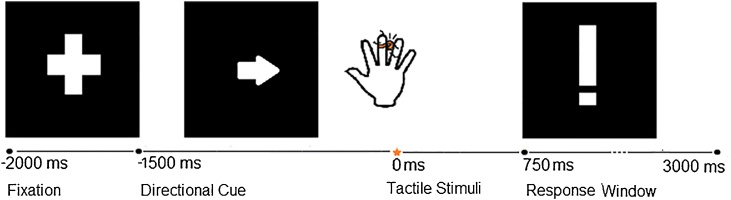
Research assistants introduced the experimental task as a game in which an arrow on a monitor screen would tell the child which hand (left or right) to pay attention to. Children were told to prepare for tactile stimulation to the middle finger hand indicated by the direction of the arrow and instructed to respond to each tactile stimulus on the indicated hand by pressing a foot pedal once if they felt one tap or twice if they felt two taps. The specific sequence of visual stimuli in each trial comprised a fixation cross for 500 ms, followed by the arrow cue for 2250 ms, followed by a response screen that read “Copy with Your Foot!” (Fig. 1). The direction of the arrow was randomized, with an equal number (60) of left and right trials. Two tactile stimuli were delivered in rapid succession (“double stimuli”) on 20 out of the 120 trials. Prior to the experimental trials, 5 practice trials were presented to ensure that children distinguished between the single and double tactile stimuli. The tactile discrimination task served to enhance anticipation of the tactile stimuli and as an incentive to keep children engaged. The foot that children used to respond to stimuli was counterbalanced (right or left) between participants. Children were offered breaks every 20 trials, with the task completed in 15–20 min.
Fig. 1.
Trial structure: A fixation point was displayed for 500 ms, followed by an arrow (directional cue) displayed continuously for 2250 ms, with the delivery of the tactile stimulus occurring 1500 ms later (at 0 ms, as indicated by the star). The response prompt was displayed at 750 ms after tactile stimulus onset.
The percentage of correct behavioral responses to the single and double stimuli was computed for each participant. Incorrect responses could be false alarms (single tactile stimuli were responded to as double) or misses (double stimuli were responded to as single). Trials involving double tactile stimuli were excluded from further EEG analysis, since the electrophysiological response to the double stimuli targets was different than to the single stimuli.
Tactile stimuli were delivered to the distal tip of the left and right middle fingers using an inflatable membrane (10 mm diameter; MEG Services International, Coquitlam) mounted in a plastic casing and secured with a finger clip. The membrane was inflated by a short burst of compressed air delivered via flexible polyurethane tubing (3 m length, 3.2 mm outer diameter). The compressed air delivery was controlled by STIM stimulus presentation software in combination with a pneumatic stimulator unit (both from James Long Company, Caroga Lake) and an adjustable regulator that restricted the airflow to 60 psi. To generate each tactile stimulus, the STIM software delivered a 10 ms trigger that served to open and close a solenoid in the pneumatic stimulator. Expansion of the membrane started 15 ms after trigger onset and peaked 35 ms later, with a total duration of membrane movement of around 100 ms. These latencies were taken into account such that the zero point for analyses is the onset of tactile stimulation at the participant’s finger.
2.3. EEG collection
EEG was recorded using a stretch cap (ANT Neuro, Berlin) with electrodes placed at Fp1, Fpz, Fp2, F3, Fz, F4, F7, F8, C3, Cz, C4, CP1, CP2, T7, T8, P3, Pz, P4, P7, P8, O1, Oz, O2, and the left and right mastoids. Conducting gel was used and scalp electrode impedances were kept under 25 kΩ (values were typically lower). EEG channels were collected referenced to the vertex (Cz) and were re-referenced offline to an average mastoids reference prior to further analysis. The signal from each site was amplified using optically isolated, high input impedance (> 1 GΩ) custom bioamplifiers (SA Instrumentation, San Diego) and digitized using a 16-bit A/D converter (+/- 2.5 V input range). Bioamplifier gain was 4000 and the hardware filter (12 dB/octave rolloff) settings were .1 Hz (high-pass) and 100 Hz (low-pass).
2.3.1. EEG processing
Initial processing of the data utilized the EEG Analysis System (James Long Company, Caroga Lake) followed by analysis using the EEGLAB toolbox (Delorme and Makeig, 2004) version 13.5.4b implemented in MATLAB. Independent component analysis was used to clear the EEG data of ocular and muscle artifact (Hoffmann and Falkenstein, 2008). Visual inspection of the EEG signal was then used to reject epochs containing excessive artifact. There was no significant difference in the number of usable trials between the left and right cued conditions (p = 0.68). Out of 50 trials, the mean number of artifact-free trials per condition was 41 (SD = 5.71).
The frequency range of the mu rhythm moves from around 6–9 Hz in infancy to a higher frequency range in early childhood (Berchicci et al., 2011; Marshall et al., 2002). By the age of the current sample (6–8 years) the mu frequency band is close to the adult range of 8–13 Hz (Berchicci et al., 2011) so this frequency range was used in the present analyses. For each single-pulse trial with a correct behavioral response, an epoch of 2300 ms was extracted (beginning 2000 ms prior to onset of the tactile stimulus and extending 300 ms after tactile stimulus onset). Spectral power over this epoch was estimated using Gaussian-tapered Morlet wavelets. Changes in power were computed as event-related spectral perturbation (ERSP) following visual cue presentation (i.e. -1500 to 300 ms following tactile stimulus presentation) relative to a 500 ms baseline preceding the visual cue (i.e., -2000 to -1500 ms prior to tactile stimulation onset). For statistical analyses, a key variable was mean mu ERSP for the period from -1000 ms to 0 ms, with this time window selected to prevent contamination of anticipatory responses by changes evoked by the response to the visual cue evident from -1500 to -1000 ms.2 We additionally analyzed post-stimulus mu ERSP by extracting the mean mu ERSP for the period from the onset of the tactile stimulation at 0 ms to the following 300 ms.
2.4. Behavioral measures
Following the tactile task and removal of the EEG cap, four tasks from the NIH Cognition Toolbox (for details, see Zelazo et al., 2013) were administered to children: the Flanker task and the Card Sort task measured aspects of executive function, a picture vocabulary test measured Receptive Language by presenting children with an audio recording of a word and four images, requiring selection of the picture that matched the meaning of the word, and Processing Speed was measured by a task that required children to rapidly identify if a set of images were identical.
The dimensional change Card Sort task indexes task-switching and working memory abilities in childhood (Beck et al., 2011; Zelazo, 2006). Children were directed to select one of two test stimuli which matched the shape (truck or ball) or color (red or blue) of the target stimuli, as instructed by a verbal prompt which varied randomly between trials. In the Flanker task (Rueda et al., 2004a), children were required to indicate the direction of an central arrow that was presented between distractor or ‘flanker’ arrows. The direction of arrows was randomized by trial, such that the flanking arrows were alternatively congruent or incongruent with the target central arrow. The Flanker task indexes response inhibition, selective attention and conflict monitoring (Rueda et al., 2004a; Zelazo et al., 2013). Children’s scores on the Card Sort and Flanker tasks were calculated to reflect both accuracy and reaction time for participants who correctly identified targets on 80% of trials; accuracy alone was considered for participants who did not meet this threshold. For all four measures, we used t-standardized test scores (on a scale from 0 to 100, with a mean of 50) provided by the NIH Cognitive Toolbox, adjusted for children’s age and gender.
2.5. Plan of analysis
Statistical analyses were conducted in R (Howell, 2016) and focused on the mean mu ERSP during anticipation of (anticipatory) and in response to a tactile stimulus (post-stimulus) the left and right central electrodes (C3 and C4) overlying hand areas of sensorimotor cortex. Mean mu ERSP values for three participants were identified as outliers (Hadi, 1992; defined as having a Mahalanobi’s distance of greater than 2.5); these data were imputed using the MICE package (Vollebregt et al., 2015). Assumption checks determined that the dependent variable in the analyses, mu ERSP, was normally distributed. Table 1 shows descriptives for all relevant neural and behavioral variables. Repeated measures ANOVAs were conducted to examine differences in mu ERSP as a function of electrode and task condition (i.e., “Cue Direction”). Follow-up analyses utilized pairwise comparisons with FDR correction.
Table 1.
Descriptive Statistics for Mu ERSP and Cognitive Task Scores.
| Anticipatory Contralateral Mu ERSP |
Anticipatory Ipsilateral Mu ERSP |
Post-Stimulus Contralateral Mu ERSP |
Post-Stimulus Ipsilateral Mu ERSP |
Flanker | Card Sort | Language | Speed Processing | |
|---|---|---|---|---|---|---|---|---|
| N | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 |
| Mean | −0.581 | 0.064 | 0.110 | 0.447 | 52.08 | 51.74 | 54.94 | 44.20 |
| SE | 0.068 | 0.071 | 0.130 | 0.132 | 1.099 | 1.150 | 1.373 | 1.496 |
| SD | 0.606 | 0.639 | 1.167 | 1.181 | 9.826 | 10.29 | 12.28 | 13.38 |
| Skew | 0.226 | −0.178 | −0.501 | −0.931 | 0.101 | 0.530 | 0.199 | −0.176 |
| Kurtosis | −0.425 | 0.684 | 0.519 | 0.347 | −0.442 | 1.164 | 0.888 | 0.199 |
3. Results
3.1. Behavioral responses to single vs. double tactile pulses
Aggregated across the sample (N = 80), participants responded correctly to the single or double stimuli on 86% of trials. The rate of correct responding was not significantly related to mu ERSP or executive function scores. Only single-stimulus trials with a correct behavioral response were included in further analyses of the EEG data.
3.2. Characterizing Mu ERSP in children
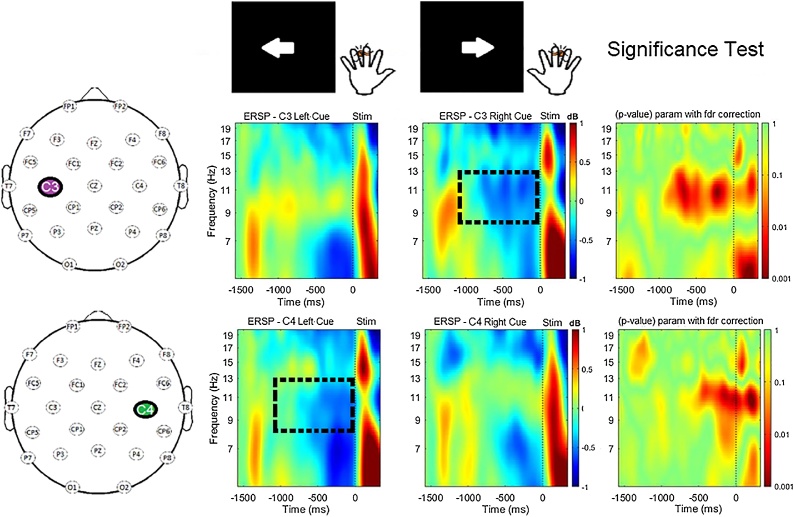
Time-frequency plots (Fig. 2) show a clear mu rhythm (8–13 Hz) desynchronization at the central electrode site (C3 or C4) contralateral to cue direction. In contrast, there is minimal change in mu power at the central electrode ipsilateral to the cue direction.
Fig. 2.
Time-frequency plots showing ERSP (event-related spectral perturbation) at left and right central sites (C3/C4) across a frequency range of 5–20 Hz for the time period from 1500 ms before the tactile stimulus to 300 ms after. The dashed boxes highlight anticipatory mu desynchronization (8–13 Hz) at electrode sites contralateral to cue direction. The response elicited by the delivery of the tactile stimulus occurs after 0 ms, which was the onset of finger stimulation. The significance test panels show statistical comparisons of ERSP within each electrode site in response to left vs. right cue.
Significant differences between contralateral and ipsilateral central sites (Fig. 2) are driven by mu rhythm desynchronization during anticipation of tactile stimuli (-1000 ms to 0 ms) at the site contralateral to the cue direction. At the left central electrode site (C3), mu desynchronization was apparent when children attended to their right hand. At the right central electrode site (C4), desynchronization of the mu rhythm was present when children attended to their left hand.
3.2.1. Anticipatory Mu modulation compared to baseline
Following the methodological recommendations of Cuevas et al. (2014), we initially calculated absolute (without baseline-corrected) power values to confirm that contralateral mu power during the period of anticipation (-1000 ms to 0 ms) differed from power in the same frequency band during the baseline period (-2000 to -1500 ms). Pairwise comparisons indicated that at the left central electrode site (C3), mu power during anticipation of stimulation was significantly lower than during the baseline epoch (p < .001). At the right central electrode site (C4), mu power during anticipation of stimulation to the left hand was significantly less than the baseline epoch (p < .01). As expected, these differences were observed only for the contralateral hemisphere; no significant differences in mu power between baseline and the anticipatory epoch were apparent at central electrode sites ipsilateral to the cue direction. Given the confirmation of significant contralateral decreases in absolute mu power during anticipation (relative to baseline), the analyses described below uses baseline-corrected ERSP values.
3.2.2. Anticipatory Mu ERSP
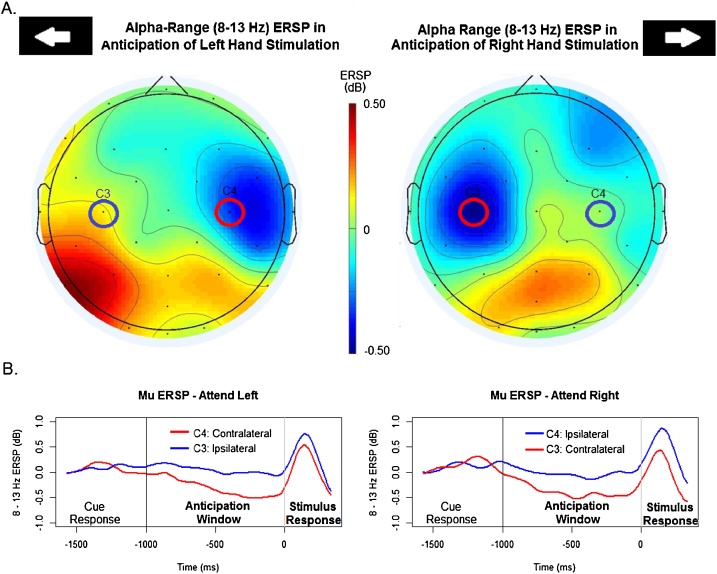
Repeated-measures ANOVA was conducted comparing mean 8–13 Hz ERSP in the -1000 to 0 ms window by electrode (C3/C4) and cue direction (left/right). No main effects were observed. There was a significant interaction between cue direction and electrode, F (1, 79) = 43.985, p < .001, η2p = 0.358. As suggested by the ERSP waveforms (Fig. 3), this interaction was driven by greater mu desynchronization at the contralateral site than at the ipsilateral site. Following a cue to expect stimulation of the right hand, significantly greater mu ERD was observed at C3 (M = -0.627, SD = 0.746) than at C4 (M = 0.053, SD = 0.746, t = -4.996, p < .001). When stimulation was expected to the left hand, significantly greater mu ERD was observed at C4 (M = -0.534, SD = 0.753) than at C3 (M = 0.074, SD = 0.841, t = -5.564, p < .001)
Fig. 3.
(A) Scalp maps showing mean ERSP for the anticipatory period (-1000 to 0 ms) at each of the 30 analyzed electrodes. The central electrodes C3 and C4 are indicated. (B) Continuous ERSP waveforms for the mu (8–13 Hz) ERSP at C3 (blue line) or C4 (red line) plotted from 1500 ms prior to tactile stimulus presentation (0 ms) and out to the following 300 ms. During the entire period plotted, the visual cue (directional arrow) is displayed. Mu rhythm desynchronization is indicated as negative ERSP values (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.2.3. Post-stimulus Mu ERSP
Repeated-measures ANOVA was conducted comparing mean 8–13 Hz ERSP in the 0–300 ms window by electrode (C3/C4) and cue direction (left/right). No significant main effects were observed. As expected, there was a significant interaction between cue direction and electrode, F (1, 79) = 13.975, p < .001, η2p = 0.152. As evident in Fig. 3, this effect was driven by differences after tactile stimulation of the right hand; following stimulation of the right hand, mu ERSP was significantly greater at the ipsilateral site C4 (M = 0.505, SD = 1.348) than at the contralateral site C3 (M = 0.054, SD = 1.416, t = -3.506, p < .001). Following stimulation to the left hand, mu ERSP was not significantly different at the contralateral site C4 (M = 0.388, SD = 1.586) compared to the ipsilateral site C3 (M = 0.167, SD = 1.455, t = 1.599, p = .114).
3.3. Cognitive abilities and anticipatory Mu ERSP
To examine the relations between task scores and mu ERSP, the conditions used in the previous ANOVA were collapsed into contralateral (mu ERSP at C3 for the right hand cue and at C4 for the left hand cue) and ipsilateral (mu ERSP at C3 for the left hand cue and at C4 for the right hand cue) mean mu ERSP values. Correlations were computed among ipsilateral and contralateral mu ERSP in anticipation of (anticipatory) and in response to (post-stimulus) a tactile stimulus, as well as measures from the NIH Cognitive Toolbox (Table 2). We note there was a strong correlation between the magnitude of contralateral anticipatory mu ERSP and post-stimulus mu ERSP at both contralateral and ipsilateral sites. Amplitude of contralateral anticipatory mu ERSP was inversely associated with the Flanker and Card Sort scores. There was not a significant correlation between Language or EF abilities and post-stimulus ipsilateral or contralateral mu ERSP, but variation in ipsilateral post-stimulus mu ERSP was associated with our measure of Processing Speed. This was confirmed by a follow-up regression analysis which found a significant relation between Processing Speed and ipsilateral post-stimulus mu ERSP, t (79) = 2.917, β = 0.444, p = 0.005, but not contralateral post-stimulus mu ERSP, t (79) = -0.174, β = -1.141, p = 0.258.
Table 2.
Correlations between Anticipatory and Post-Stimulus Mu ERSP, Cognitive Task Scores and Demographic Variables.
| Contralateral Anticipatory Mu ERSP |
Ipsilateral Anticipatory Mu ERSP |
Contralateral Post-Stimulus Mu ERSP |
Ipsilateral Post-Stimulus Mu ERSP |
Flanker (EF) |
Card Sort (EF) |
|
|---|---|---|---|---|---|---|
|
Contralateral Anticipatory Mu ERSP |
— | .017 | .707*** | .491*** | — | — |
|
Ipsilateral Anticipatory Mu ERSP |
— | — | .069 | .260** | — | — |
| Flanker | −.310** | .139 | −.003 | −.091 | — | — |
| Card Sort | −.231* | .027 | −.138 | −.161 | .599*** | — |
| Language | −.168 | −.093 | −.040 | −.076 | .322*** | .364*** |
| Processing Speed | .026 | .210 | .140 | .269** | .359*** | .272** |
| Age | −.110 | −.143 | .243* | .063 | −.064 | .029 |
| Gender | −.093 | −.127 | .038 | .050 | −.051 | −.143 |
| Family Incomea | −.310*** | −.032 | .021 | −.043 | .144 | .213 |
See Supplement for measurement details and further analyses concerning the role of family (household) income as a moderator in the relations between EF and contralateral anticipatory mu ERD.
p < .05.
p < .01.
p < .001.
To address our hypotheses on the relations between cognitive skills and neural indicators of anticipation, multiple regressions were conducted predicting scores on the Flanker, Card Sort, Receptive Language, and Processing Speed tasks from contralateral and ipsilateral mu ERSP. For both Flanker and Card Sort tasks, greater contralateral mu ERD was associated with better EF task performance. Flanker performance was related to contralateral mu ERSP, t (79) = -2.934, β = -0.314, p = 0.004, but not with ipsilateral mu ERSP. Card Sort performance was also related with contralateral mu ERSP, t (79) = -2.307, β= -0.254, p = 0.024, but not with ipsilateral mu ERSP. Processing Speed and Receptive Language scores were not related to anticipatory mu ERSP (Table 3). Further, variance accounted for in Flanker and Card Sort by contralateral ERD remained significant controlling for Processing Speed and Language as covariates (Table 4).
Table 3.
Cognitive Task Scores by Anticipatory Ipsilateral and Contralateral Mu ERSP.
| Outcome | Anticipatory Mu ERSP | b | SE | β | T | P | η2p |
|---|---|---|---|---|---|---|---|
| Flanker (EF) | (Intercept) | 48.972 | 1.458 | 33.593 | <.001* | 0.198 | |
| Contralateral | −5.095 | 1.736 | −0.314 | −2.934 | 0.004* | ||
| Ipsilateral | 2.270 | 1.647 | 0.148 | 1.379 | 0.172 | ||
| Card Sort (EF) | (Intercept) | 49.196 | 1.572 | 31.305 | <.001* | 0.156 | |
| Contralateral | −4.317 | 1.872 | −0.254 | −2.307 | 0.024* | ||
| Ipsilateral | 0.544 | 1.775 | 0.034 | 0.307 | 0.760 | ||
| Language | (Intercept) | 53.094 | 1.905 | 27.869 | <.001* | 0.036 | |
| Contralateral | 2.269 | 2.269 | −0.166 | −1.481 | 0.143 | ||
| Ipsilateral | −1.705 | 2.152 | −0.089 | −0.793 | 0.430 | ||
| Processing Speed | (Intercept) | 44.171 | 2.065 | 21.392 | <.001* | 0.047 | |
| Contralateral | 0.441 | 2.459 | 0.020 | 0.179 | 0.858 | ||
| Ipsilateral | 4.486 | 2.332 | 0.214 | 1.924 | 0.058 | ||
p < .05.
Table 4.
Executive Function Scores predicted by Ipsilateral and Contralateral Anticipatory Mu ERSP, accounting for Speed Processing and Language.
| Outcome | b | S.E. | β | T | P | η2p |
|---|---|---|---|---|---|---|
| Flanker | ||||||
| (Intercept) | 27.514 | 5.185 | 5.306 | <.001* | .262 | |
| Contralateral Mu ERSP | −4.504 | 1.591 | −0.278 | −2.830 | 0.006* | |
| Ipsilateral Mu ERSP | 1.560 | 1.532 | 0.101 | 1.018 | 0.312 | |
| Speed Processing | 0.237 | 0.073 | 0.323 | 3.243 | 0.002* | |
| Language | 0.207 | 0.079 | 0.259 | 2.612 | 0.011* | |
| Card Sort | ||||||
| (Intercept) | 26.902 | 5.677 | 4.738 | <.001* | .193 | |
| Contralateral Mu ERSP | −3.531 | 1.742 | −0.208 | −2.026 | 0.046* | |
| Ipsilateral Mu ERSP | 0.121 | 1.677 | 0.008 | 0.072 | 0.942 | |
| Speed Processing | 0.259 | 0.087 | 0.310 | 2.991 | 0.004* | |
| Language | 0.193 | 0.080 | 0.251 | 2.410 | 0.018* | |
p < .05.
4. Discussion
In the current study we characterized sensorimotor mu rhythm activity in the EEG signal at central electrode sites during anticipation of tactile stimulation in children aged 6–8 years. As predicted, a clear desynchronization of the mu rhythm was apparent at central electrode sites contralateral to the cue direction in anticipation of tactile stimulation. The extent of this regionally specific anticipatory event-related desynchronization (ERD) of the mu rhythm was related to children’s executive function skills, specifically accounting for 20% of the variance in Flanker scores and 15% of the variance in Card Sort scores. Contralateral anticipatory mu ERD remained significantly associated with these aspects of EF when controlling for other cognitive covariates, specifically Receptive Language and processing Speed. Importantly, anticipatory mu ERD was not associated with accuracy of children’s behavioral responses to tactile stimulation or to their scores on the tasks measuring Receptive Language and Processing Speed.
Our findings contribute to the developmental cognitive neuroscience literature linking electrophysiological indices of selective attention to cognitive skills in children (Isbell et al., 2016; Shimi et al., 2015). To our knowledge, this is the first investigation of children’s oscillatory brain responses during the monitoring of bodily sensations. We suggest that attention in the tactile modality is of special neuropsychological significance, particularly given the status of touch as “the first sense” (Fulkerson, 2013), its interconnection with other modalities in early development (Bremner and Spence, 2017; Meltzoff et al., 2018; Saby et al., 2015), and the potential of work on attention to one’s own body to inform interventions targeted at improving attentional and executive abilities in young children (Diamond and Ling, 2016; Isbell et al., 2018).
To systematically manipulate children’s attention on bodily sensations, we implemented a paradigm in which a visual cue directed participants to monitor their right or left middle finger in expectation of tactile stimulation. A clear desynchronization of the mu rhythm was observed over somatosensory cortex contralateral to the cued hand, consistent with findings in adults (Haegens et al., 2011; Jones et al., 2010; Shen et al., 2017). The lateralized pattern of anticipatory mu modulation is evidence that children selectively deployed attention to one of their hands, specifically monitoring the cued location for upcoming stimulation. Findings from the adult literature support the use of anticipatory mu desynchronization as an index of attention to one’s own body (Jones et al., 2010; Kerr et al., 2013).
In our sample, the extent of anticipatory mu ERD prior to tactile stimulation was associated with the magnitude of mu modulation elicited to the tactile stimulus itself in both contralateral and ipsilateral central sites. Post-stimulus brain responses (including mu modulation) in adults have previously been associated with anticipatory mu desynchronization (Shen et al., 2017) and to participant-reported detection of near-threshold tactile stimulation (Zhang and Ding, 2010). In the current study we did not expect to find a meaningful association between children’s behavioral responses to the tactile stimulation and anticipatory mu modulation, since the single versus double pulses were highly discriminable and there was little variation in performance on the task. Even so, our investigation informs models of action-oriented representation in which anticipatory shifts in neural activity reflect biases in sensory processing in the context of impending action (Engel et al., 2013).
The study of anticipatory attention, particularly in cross-modal contexts (Meltzoff, 1990), has the potential to further inform theories and mechanisms in developmental science. The ability to deploy anticipatory attention is present early in life (Johnson et al., 1991; Rueda et al., 2005; Tarantino et al., 2017; Xie et al., 2017), and we hypothesize childhood anticipation as a foundational skill situated at the interface of sensory processing and higher-order cognition (Elke and Wiebe, 2017; Silverman, 2018). By three years of age, children’s performance on visual selective attention tasks accounts for significant variation in their emerging executive function abilities (Veer et al., 2017). The association we found between executive function scores and mu rhythm desynchronization during anticipation of tactile stimulation serves as a complement to other studies linking children’s cognitive abilities with aspects of event-related potential (ERP) responses to stimuli presented during auditory (Isbell et al., 2016) and visual (Shimi et al., 2015) selective attention tasks. A separate body of work has identified relations between childhood executive function and baseline alpha-range EEG power and coherence in frontal sites (Kraybill and Bell, 2013; Whedon et al., 2016).
Our novel findings support a role for anticipatory attention in the coordination and regulation of goal-directed behavior, and further suggest that electrophysiological measures of anticipatory bodily attention are useful for exploring the mechanisms involved. The specificity of the observed effects was confirmed by the relations between EF and mu ERSP being significant only at central sites contralateral to the cue. The more general cognitive skills of Processing Speed and Receptive Language, which have been associated with alpha-range activity at rest in children (Whedon et al., 2016), were not related to the extent of anticipatory mu ERD. Processing Speed alone was associated with post-stimulus ipsilateral mu modulation, consistent with interpretation of ipsilateral increases in amplitude as an indicator of gated sensory processing (Haegens et al., 2012; Zhang and Ding, 2010).
The anticipatory modulation of the mu rhythm in our child sample appeared similar in magnitude and morphology to contralateral anticipatory responses in adults (Haegens et al., 2011; Shen et al., 2017). This is notable given that typical neural indices of selective attention (e.g. ERP responses evoked by visual and auditory stimuli) appear to have a prolonged developmental trajectory, distinct from adult responses in latency and direction (positive/negative) of evoked activity (Coch et al., 2005; Knowland et al., 2014; Rueda et al., 2004b). This finding invites work with younger children, and suggests that mu rhythm modulation during anticipatory attention tasks may potentially be a useful indicator of attention focusing across a relatively wide age range.
In terms of limitations and future directions, our cross-sectional design limits directional interpretations of the relations between selective attention and executive function. Future studies would benefit from additional measures of executive function and could follow younger children longitudinally, to test if the emergence of various aspects of executive function (inhibitory control, working memory, etc.) may be predicted by earlier attentional focusing abilities (Markant and Amso, 2016; Veer et al., 2017), particularly those specific to anticipation (Holmboe et al., 2018). Another point of note is the common role of spatial attention implicit in both the executive function measures (especially the Flanker task) and our somatosensory selective attention task (Ristic and Kingstone, 2009). Relevant work in early childhood has typically focused on post-stimulus EEG or behavioral responses following presentation of the target stimulus, and often involves the presentation of simultaneous distracting stimuli in a spatial location and/or modality other than that indicated by the cue (Markant and Amso, 2016; Veer et al., 2017; Murphy et al., 2016). Our findings imply that while post-stimulus EEG may be a useful correlate of processing speed, it is anticipatory EEG modulation (even in absence of distractors) that indexes variation in the ability to regulation of attention and action, i.e. execution function. Future researchers should consider whether their EEG baseline-correction occurs during presentation of a neutral fixation stimulus or a target-stimulus relevant cue; if the latter, researchers should be aware that variation already present in the pre-stimulus, cue-driven anticipatory period cannot easily be dissociated from activity in the post-stimulus period (Luck, 2014).
There is evidence that the ability to attend selectively to targets via attentional focusing (Heim and Keil, 2012; Veer et al., 2017) appears distinct from the slower-developing ability to inhibit attention to potential distractors (vigilance or inhibition; Holmboe et al., 2018). Since the presentation of distractors may interfere with attention deployment (Plebanek and Sloutsky, 2017), our study required children to attend and respond to tactile stimulation delivered only to the cued hand (Shen et al., 2017). As such, our task demands differ from studies of selective attention which involve presentation of simultaneous distractors, which can leverage distractors to study suppression of attention using synchronization of alpha-range EEG at ipsilateral sites (Haegens et al., 2011; Murphy et al., 2016; Zanto and Gazzaley, 2009).
One notable strength of the current work is our focus on sensorimotor mu desynchronization preceding the onset of a target tactile stimulus as an index of anticipatory processing that is not clouded by neural responses to cue, target, or distractor stimuli. The study of anticipatory neural activity across sensory modalities can further isolate the deployment of ‘endogenous’ attention, in preparation for action or while monitoring for changes in the environment (Jones and Forster, 2015; Saby et al., 2013).
4.1. Broader implications
Reliable individual differences in selective attention can be measured in infancy (Heim and Keil, 2012; Johnson et al., 1991; Markant and Amso, 2016), so assessment of selective attention could be useful for identifying children with issues in regulating behavior and attention before school entry (Calipso Gutiérrez-Hernández et al., 2017; Felver et al., 2016). Most interventions and assessments focus on visual attention, which has shown consistent and robust responses to training (Bryck and Fisher, 2012; Posner et al., 2012;), and it is currently unknown if targeting selective attention deployment in other sensory modalities is similarly effective. Longitudinal programs of research can disentangle whether selective attention, as a multi-modal construct malleable to intervention and contextual influences (Neville et al., 2013), develops in concert with, or in preparation, for the emergence of executive function skills.
Focused attention in the somatosensory modality is connected to the construct of “mindfulness” in an interesting way. For example, mindfulness meditation specifically emphasizes bodily awareness (Davidson et al., 2003; Tang and Posner, 2014). In adults and children, brief mindfulness interventions have been found to promote aspects of executive function (Flook et al., 2010; Gallant, 2016; Zeidan et al., 2010), foster self-regulation of behavior (Tang et al., 2014) and alter neural activity related to attention (Kerr et al., 2013; Tang et al., 2012). Mindfulness practice often begins with a body scan, which involves directing attention in a focused, deliberate manner to specific body parts and monitoring for sensations (Hölzel et al., 2011). This practice has notable connections to the demands of the task used in our study (Kerr et al., 2013). Interventions using aspects of mindfulness, bodily awareness and skillful, focused deployment of attention, particularly those targeting low-income children (Black and Fernando, 2014) could potentially incorporate EEG during anticipatory attention to the body as an assessment of treatment efficacy (Raizada and Kishiyama, 2010).
In conclusion, the results described here indicate that significant variance in childhood executive function scores is accounted for by anticipatory EEG modulations during a somatosensory selective attention task. The ability to anticipate stimulation and to selectively attend to relevant spatial locations are implicit to executive function task demands (Banerjee et al., 2011; Garon et al., 2008; Veer et al., 2017). The relations between modulation of mu rhythm during bodily attention and executive function can be leveraged to study both modality-specific and amodal component processes (e.g. anticipation) of executive function and attention across different ages. Anticipation may be foundational to the shift from more reactive attentional capacities in infancy, to the more self-regulated, intentional coordination of attention and action that becomes increasingly evident in childhood. Our findings extend the understanding of the active, autonomous nature of self-regulation in children, with anticipation in the somatosensory modality serving as a demonstration of how children’s attentional state is guided dynamically by cues in the immediate environment to facilitate perception and fluid action.
Conflict of Interest
None.
Acknowledgements
The authors thank Valerie Cordero, Jebediah Taylor, and Rebecca Laconi for their help with data collection, as well as Guannan Shen and Nathan Smyk for their support. The education of SMW is supported by the NSF Graduate Research Fellowship Program. This research was supported by grants from NSF (BCS-1460889 to PJM and SMA-1540619 to ANM) and the Bezos Family Foundation. Further support for data collection was provided by a CURE grant from the Pennsylvania Department of Health. The authors declare that there is no conflict of interest regarding the publication of this article.
Footnotes
Additional information and analyses involving household income, maternal education, gender and child age are detailed in the Supplement.
The Supplement includes analysis of responses to the visual cue at posterior (occipital) electrode sites, O1 and O2.
Supplementary material related to this article can be found in the online version at https://doi.org/10.1016/j.dcn.2018.08.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Anderson K.L., Ding M. Attentional modulation of the somatosensory mu rhythm. Neuroscience. 2011;180:165–180. doi: 10.1016/j.neuroscience.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Awh E., Anllo-Vento L., Hillyard S. The role of spatial selective attention in working memory for locations: evidence from event-related potentials. J. Cogn. Neurosci. 2000;12:840–847. doi: 10.1162/089892900562444. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Snyder A.C., Molholm S., Foxe J.J. Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? J. Neurosci. 2011;31:9923–9932. doi: 10.1523/JNEUROSCI.4660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D.M., Schaefer C., Pang K., Carlson S.M. Executive function in preschool children: test–retest reliability. J. Cogn. Dev. 2011;12:169–193. doi: 10.1080/15248372.2011.563485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchicci M., Zhang T., Romero L., Peters A., Annett R., Teuscher U., Bertollo M., Okada Y., Stephen J., Comani S. Development of mu rhythm in infants and preschool children. Dev. Neurosci. 2011;33:130–143. doi: 10.1159/000329095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.S., Fernando R. Mindfulness training and classroom behavior among lower-income and ethnic minority elementary school children. J. Child Fam. Stud. 2014;23:1242–1246. doi: 10.1007/s10826-013-9784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., Raver C.C. School readiness and self-regulation: a developmental psychobiological approach. Annu. Rev. Psychol. 2015;66:711–731. doi: 10.1146/annurev-psych-010814-015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner A.J. Developing body representations in early life: combining somatosensation and vision to perceive the interface between the body and the world. Dev. Med. Child Neurol. 2016;58:12–16. doi: 10.1111/dmcn.13041. [DOI] [PubMed] [Google Scholar]
- Bremner A.J., Spence C. The development of tactile perception. Adv. Child Dev. Behav. 2017;52:227–268. doi: 10.1016/bs.acdb.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Bryck R.L., Fisher P.A. Training the brain: practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. Am. Psychol. 2012;67:87–100. doi: 10.1037/a0024657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R., Espy K.A., Wiebe S.A. Short-term memory, working memory, and executive functioning in preschoolers: longitudinal predictors of mathematical achievement at age 7 years. Dev. Neuropsychol. 2008;33:205–228. doi: 10.1080/87565640801982312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipso Gutiérrez-Hernández C., Harmony T., Nélida G., -Ramírez A.-C., Barrón-Quiroz I., Guillén-Gasca V., Tre-Jo-Bautista G., Bautista-Olvera M.M. Infant Scale of Selective Attention: a proposal to assess cognitive abilities. Rev. Eval. 2017;17:1–16. https://revistas.unc.edu.ar/index.php/revaluar/article/view/17077 [Google Scholar]
- Coch D., Sanders L.D., Neville H.J. An event-related potential study of selective auditory attention in children and adults. J. Cogn. Neurosci. 2005;17:605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annu. Rev. Psychol. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Cuevas K., Cannon E.N., Yoo K., Fox N.A. The infant EEG mu rhythm: methodological considerations and best practices. Dev. Rev. 2014;34:26–43. doi: 10.1016/j.dr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Kabat-Zinn J., Schumacher J., Rosenkranz M., Muller D., Santorelli S.F., Urbanowski F., Harrington A., Bonus K., Sheridan J.F. Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Ling D.S. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev. Cogn. Neurosci. 2016;18:34–48. doi: 10.1016/j.dcn.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Overton W.F. Executive function: description and explanation. In: Sokol B., Müller U., Carpendale J.I.M., Young A.R., Iarocci G., editors. Self-and social regulation: Exploring the relations between social interaction, social cognition, and the development of executive functions. Oxford University Press; New York: 2010. pp. 7–34. [Google Scholar]
- Elke S., Wiebe S.A. Proactive control in early and middle childhood: an ERP study. Dev. Cogn. Neurosci. 2017;26:28–38. doi: 10.1016/j.dcn.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A.K., Maye A., Kurthen M., König P. Where’s the action? The pragmatic turn in cognitive science. Trends Cogn. Sci. 2013;17:202–209. doi: 10.1016/j.tics.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Felver J.C., Celis-de Hoyos C.E., Tezanos K., Singh N.N. A systematic review of mindfulness-based interventions for youth in school settings. Mindfulness. 2016;7:34–45. [Google Scholar]
- Ferri F., Ambrosini E., Pinti P., Merla A., Costantini M. The role of expectation in multisensory body representation–neural evidence. Eur. J. Neurosci. 2017;46:1897–1905. doi: 10.1111/ejn.13629. [DOI] [PubMed] [Google Scholar]
- Flook L., Smalley S.L., Kitil M.J., Galla B.M., Kaiser-Greenland S., Locke J., Ishijima E., Kasari C. Effects of mindful awareness practices on executive functions in elementary school children. J. Appl. Sch. Psychol. 2010;26:70–95. [Google Scholar]
- Foxe J.J., Snyder A.C. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2011;2:1–13. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant S.N. Mindfulness meditation practice and executive functioning: breaking down the benefit. Conscious. Cogn. 2016;40:116–130. doi: 10.1016/j.concog.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Garon N., Bryson S.E., Smith I.M. Executive function in preschoolers: a review using an integrative framework. Psychol. Bull. 2008;134:31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Gazzaley A., Nobre A.C. Top-down modulation: bridging selective attention and working memory. Trends Cogn. Sci. (Regul. Ed.) 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T., Dehaene-Lambertz G. Structural encoding of body and face in human infants and adults. J. Cogn. Neuro. 2005;17:1328–1340. doi: 10.1162/0898929055002481. [DOI] [PubMed] [Google Scholar]
- Gliga T., Farroni T., Cascio C.J. Social touch: a new vista for developmental cognitive neuroscience? Dev. Cogn. Neurosci. 2018;12:1–4. doi: 10.1016/j.dcn.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald J.M., Achermann S., Marciszko C., Lindskog M., Gredebäck G. An embodied account of early executive-function development. Psych. Sci. 2016;27:1600–1610. doi: 10.1177/0956797616667447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi A.S. Identifying multiple outliers in multivariate data. J. R. Stat. Soc. 1992;54:761–771. https://www.jstor.org/stable/2345856 [Google Scholar]
- Haegens S., Händel B.F., Jensen O. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J. Neurosci. 2011;31:5197–5204. doi: 10.1523/JNEUROSCI.5199-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S., Luther L., Jensen O. Somatosensory anticipatory alpha activity increases to suppress distracting input. J. Cogn. Neurosci. 2012;24:677–685. doi: 10.1162/jocn_a_00164. [DOI] [PubMed] [Google Scholar]
- Haith M.M., Hazan C., Goodman G.S. Expectation and anticipation of dynamic visual events by 3.5-month-old babies. Child Dev. 1988;59:467–480. [PubMed] [Google Scholar]
- Heim S., Keil A. Developmental trajectories of regulating attentional selection over time. Front. Psychol. 2012;3:277. doi: 10.3389/fpsyg.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry A., Jones E.J.H., Charman T. Executive function in the first three years of life: precursors, predictors and patterns. Dev. Rev. 2016;42:1–33. [Google Scholar]
- Hoffmann S., Falkenstein M. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS One. 2008;3:e3004. doi: 10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmboe K., Bonneville‐Roussy A., Csibra G., Johnson M.H. Longitudinal development of attention and inhibitory control during the first year of life. Dev. Sci. 2018:12690–13612. doi: 10.1111/desc.12690. [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Lazar S.W., Gard T., Schuman-Olivier Z., Vago D.R., Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect. Psychol. Sci. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Howell D.C. Nelson Education; 2016. Fundamental statistics for the behavioral sciences.https://www.uvm.edu/∼dhowell/fundamentals8/Student%20Manual/SMIntroduction.pdf [Google Scholar]
- Isbell E., Wray A.H., Neville H.J. Individual differences in neural mechanisms of selective auditory attention in preschoolers from lower socioeconomic status backgrounds: an event-related potentials study. Dev. Sci. 2016;19:865–880. doi: 10.1111/desc.12334. [DOI] [PubMed] [Google Scholar]
- Isbell E., Calkins S.D., Swingler M.M., Leerkes E.M. Attentional fluctuations in preschoolers: direct and indirect relations with task accuracy, academic readiness, and school performance. J. Exp. Child Psychol. 2018;167:388–403. doi: 10.1016/j.jecp.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4:186–195. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H., Posner M.I., Rothbart M.K. Components of visual orienting in early infancy: contingency learning, anticipatory looking, and disengaging. J. Cogn. Neurosci. 1991;3:335–344. doi: 10.1162/jocn.1991.3.4.335. [DOI] [PubMed] [Google Scholar]
- Jones A., Forster B. Body in mind. Front. Psychol. 2015;6:56–60. doi: 10.3389/fpsyg.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.R., Kerr C.E., Wan Q., Pritchett D.L., Hämäläinen M., Moore C.I. Cued spatial attention drives functionally relevant modulation of the mu rhythm in primary somatosensory cortex. J. Neurosci. 2010;30:13760–13765. doi: 10.1523/JNEUROSCI.2969-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katus T., Müller M.M., Eimer M. Sustained maintenance of somatotopic information in brain regions recruited by tactile working memory. J. Neurosci. 2015;35:1390–1395. doi: 10.1523/JNEUROSCI.3535-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett S., Van Velzen J., Eimer M., Driver J. Disentangling gaze shifts from preparatory ERP effects during spatial attention. Psychophysics. 2007;44:69–78. doi: 10.1111/j.1469-8986.2006.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C.E., Sacchet M.D., Lazar S.W., Moore C.I., Jones S.R. Mindfulness starts with the body: somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Front. Hum. Neurosci. 2013;7:12–30. doi: 10.3389/fnhum.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiverstein J.D., Rietveld E. Reconceiving representation-hungry cognition: an ecological-enactive proposal. Adapt. Beh. 2018;26:147–163. doi: 10.1177/1059712318772778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 1999;29:169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012;16:606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition–timing hypothesis. Brain Res. Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Knowland V.C.P., Mercure E., Karmiloff-Smith A., Dick F., Thomas M.S.C. Audio-visual speech perception: a developmental ERP investigation. Dev. Sci. 2014;17:110–124. doi: 10.1111/desc.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G., Coy K.C., Murray K.T. The development of self‐regulation in the first four years of life. Child Dev. 2001;72:1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Kraybill J.H., Bell M.A. Infancy predictors of preschool and post-kindergarten executive function. Dev. Psychobiol. 2013;55:530–538. doi: 10.1002/dev.21057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman W.N. Functional topography of the human mu rhythm. Electroencephalogr. Clin. Neurophysiol. 1978;44:83–93. doi: 10.1016/0013-4694(78)90107-4. [DOI] [PubMed] [Google Scholar]
- Liao Y., Acar Z.A., Makeig S., Deak G. EEG imaging of toddlers during dyadic turn-taking: mu-rhythm modulation while producing or observing social actions. NeuroImage. 2015;112:52–60. doi: 10.1016/j.neuroimage.2015.02.055. [DOI] [PubMed] [Google Scholar]
- Luck S.J. MIT Press; 2014. An Introduction to the Event-related Potential Technique. [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Markant J., Amso D. The development of selective attention orienting is an agent of change in learning and memory efficacy. Infancy. 2016;21:154–176. doi: 10.1111/infa.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J. Embodiment and human development. Child Dev. Perspect. 2016;10:245–250. doi: 10.1111/cdep.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J. Embodiment. In: Dick A.S., Muller U., editors. Advancing Developmental Science: Philosophy, Theory, and Method, 29–40. Psychology Press; 2018. https://www.taylorfrancis.com/books/e/9781351704564/chapters/10.4324%2F8791315174686-10 [Google Scholar]
- Marshall P.J., Meltzoff A.N. Neural mirroring mechanisms and imitation in human infants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130620. doi: 10.1098/rstb.2013.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Meltzoff A.N. Body maps in the infant brain. Trends Cogn. Sci. 2015;19:499–505. doi: 10.1016/j.tics.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall P.J., Young T., Meltzoff A.N. Neural correlates of action observation and execution in 14-month-old infants: an event-related EEG desynchronization study. Dev. Sci. 2011;14:474–480. doi: 10.1111/j.1467-7687.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A., Van Schouwenburg M.R., Dimitrijevic A., Denys D., Cools R., Jensen O. Region-specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. Neuroimage. 2014;87:356–362. doi: 10.1016/j.neuroimage.2013.10.052. [DOI] [PubMed] [Google Scholar]
- Meltzoff A.N. Towards a Developmental Cognitive Science: The implications of cross‐modal matching and imitation for the development of representation and memory in infancy. Ann. N. Y. Acad. Sci. 1990;608:1–37. doi: 10.1111/j.1749-6632.1990.tb48889.x. [DOI] [PubMed] [Google Scholar]
- Meltzoff A.N., Ramírez R., Saby J.N., Larson E., Taulu S., Marshall P.J. Infant brain responses to felt and observed touch of hands and feet: an MEG study. Dev. Sci. 2018;21:e12651. doi: 10.1111/desc.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Murphy J.W., Foxe J.J., Molholm S. Neuro-oscillatory mechanisms of intersensory selective attention and task switching in school-aged children, adolescents and young adults. Dev. Sci. 2016;19:469–487. doi: 10.1111/desc.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville H.J., Stevens C., Pakulak E., Bell T.A., Fanning J., Klein S., Isbell E. Family-based training program improves brain function, cognition, and behavior in lower socioeconomic status preschoolers. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12138–12143. doi: 10.1073/pnas.1304437110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T. Annual research review: on the relations among self‐regulation, self‐control, executive functioning, effortful control, cognitive control, impulsivity, risk‐taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry. 2017;58:361–383. doi: 10.1111/jcpp.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou K.A., Smith T.J., Wu R., Johnson M.H., Kirkham N.Z., Ronald A. Individual differences in infant fixation duration relate to attention and behavioral control in childhood. Psychol. Sci. 2014;25:1371–1379. doi: 10.1177/0956797614531295. [DOI] [PubMed] [Google Scholar]
- Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Pezzulo G. An active inference view of cognitive control. Front. Psychol. 2012;3:478–499. doi: 10.3389/fpsyg.2012.00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Functional topography during sensorimotor activation studied with event-related desynchronization mapping. J. Clin. Neurophysiol. 1989;6:75–84. doi: 10.1097/00004691-198901000-00003. https://www.ncbi.nlm.nih.gov/pubmed/2915031 [DOI] [PubMed] [Google Scholar]
- Plebanek D.J., Sloutsky V.M. Costs of selective attention: when children notice what adults miss. Psychol. Sci. 2017;28:723–732. doi: 10.1177/0956797617693005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I. Orienting of attention. Q. J. Exp. Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Driver J. The neurobiology of selective attention. Curr. Opin. Neurobiol. 1992;2:165–169. doi: 10.1016/0959-4388(92)90006-7. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K. American Psychological Association; Washington: 2007. Educating the Human Brain. [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Voelker P. Control networks and neuromodulators of early development. Dev. Psychol. 2012;48:827–835. doi: 10.1037/a0025530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada R., Kishiyama M. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to leveling the playing field. Front. Hum. Neurosci. 2010;3:1–11. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver C.C., Blair C., Willoughby M. Poverty as a predictor of 4-year-dlds’ executive function: new perspectives on models of differential susceptibility. Dev. Psychol. 2012;49:292–304. doi: 10.1037/a0028343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V., Markova G., Wallot S. Anticipatory adjustments to being picked up in infancy. PLoS One. 2013;8:65289–65295. doi: 10.1371/journal.pone.0065289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic J., Kingstone A. Rethinking attentional development: reflexive and volitional orienting in children and adults. Dev. Sci. 2009;122:289–296. doi: 10.1111/j.1467-7687.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- Ritter P., Moosmann M., Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum. Brain Mapp. 2009;30:1168–1187. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart M.K., Posner M.I., Kieras J. Temperament, attention, and the development of self-regulation. In: McCartney K., Phillips D., editors. Blackwell handbook of early childhood development. Blackwell Publishing Ltd; Oxford, UK: 2006. pp. 338–357. [Google Scholar]
- Ruberry E.J., Lengua L.J., Crocker L.H., Bruce J., Upshaw M.B., Sommerville J.A. Income, neural executive processes, and preschool children’s executive control. Dev. Psychopathol. 2017;29:143–154. doi: 10.1017/S095457941600002X. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K., Davis-Stober C.P. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neurosci. 2004;5:39–42. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K. The development of executive attention: contributions to the emergence of self-regulation. Dev. Neuropsychol. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Saby J.N., Meltzoff A.N., Marshall P.J. Infants’ somatotopic neural responses to seeing human actions: I’ve got you under my skin. PLoS One. 2013;8:e77905–e77912. doi: 10.1371/journal.pone.0077905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saby J.N., Meltzoff A.N., Marshall P.J. Neural body maps in human infants: somatotopic responses to tactile stimulation in 7-month-olds. NeuroImage. 2015;118:74–78. doi: 10.1016/j.neuroimage.2015.05.097. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., Kleinschmidt A. Brain networks and α-oscillations: structural and functional foundations of cognitive control. Trends Cogn. Sci. 2016;20:805–817. doi: 10.1016/j.tics.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Scheeringa R., Petersson K.M., Oostenveld R., Norris D.G., Hagoort P., Bastiaansen M.C.M. Trial-by-trial coupling between EEG and BOLD identifies networks related to alpha and theta EEG power increases during working memory maintenance. NeuroImage. 2009;44:1224–1238. doi: 10.1016/j.neuroimage.2008.08.041. [DOI] [PubMed] [Google Scholar]
- Sheese B.E., Rothbart M.K., Posner M.I., White L.K., Fraundorf S.H. Executive attention and self-regulation in infancy. Infant Behav. Dev. 2008;31:501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Shen G., Saby J.N., Drew A.R., Marshall P.J. Exploring potential social influences on brain potentials during anticipation of tactile stimulation. Brain Res. 2017;1659:8–18. doi: 10.1016/j.brainres.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Shimi A., Nobre A.C., Scerif G. ERP markers of target selection discriminate children with high vs. low working memory capacity. Front. Syst. Neurosci. 2015;9:153–160. doi: 10.3389/fnsys.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. Bodily skill and internal representation in sensorimotor perception. Phenomenol. Cogn. Sci. 2018;17:157–173. [Google Scholar]
- Sokol B., Muller U., Carpendale J., Young A., Iarocci G. Oxford University Press; 2010. Self-and Social-regulation: the Development of Social Interaction, Social Understanding, and Executive Functions. [Google Scholar]
- Somogyi E., Jacquey L., Heed T., Hoffmann M., Lockman J.J., Granjon L. Which limb is it? Responses to vibrotactile stimulation in early infancy. Br. J. Dev. Psychol. 2017;36:384–401. doi: 10.1111/bjdp.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Bavelier D. The role of selective attention on academic foundations: a cognitive neuroscience perspective. Dev. Cogn. Neurosci. 2012;2:S30–S48. doi: 10.1016/j.dcn.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova T.A., Orekhova E.V., Posikera I.N. EEG alpha rhythm in infants. Clin. Neurophysiol. 1999;110:997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Posner M.I. Training brain networks and states. Trends Cogn. Sci. 2014;18:345–350. doi: 10.1016/j.tics.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Tang Y.-Y., Yang L., Leve L.D., Harold G.T. Improving executive function and its neurobiological mechanisms through a mindfulness-based intervention: advances within the field of developmental neuroscience. Child Dev. Perspect. 2012;6 doi: 10.1111/j.1750-8606.2012.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.-Y., Posner M.I., Rothbart M.K. Meditation improves self-regulation over the life span. Ann. N. Y. Acad. Sci. 2014;1307:104–111. doi: 10.1111/nyas.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino V., Mazzonetto I., Formica S., Causin F., Vallesi A. The neural bases of event monitoring across domains: a simultaneous ERP-fMRI study. Front. Hum. Neurosci. 2017;11:376. doi: 10.3389/fnhum.2017.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 2006;26:9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Luyten H., Mulder H., Van Tuijl C., Sleegers P.J.C. Selective attention relates to the development of executive functions in 2,5- to 3-year-olds: a longitudinal study. Early Child. Res. Q. 2017;43:84–94. [Google Scholar]
- Vernon D. 2014. Artificial Cognitive Systems: a Primer. [Google Scholar]
- Vollebregt M.A., Zumer J.M., ter Huurne N., Castricum J., Buitelaar J.K., Jensen O. Lateralized modulation of posterior alpha oscillations in children. NeuroImage. 2015;123:245–252. doi: 10.1016/j.neuroimage.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Weisz N., Hartmann T., Müller N., Obleser J. Alpha rhythms in audition: cognitive and clinical perspectives. Front. Psychol. Behav. Sci. 2011;2:73–90. doi: 10.3389/fpsyg.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whedon M., Perry N.B., Calkins S.D., Bell M.A. Changes in frontal EEG coherence across infancy predict cognitive abilities at age 3: the mediating role of attentional control. Dev. Psychol. 2016;52:1341–1352. doi: 10.1037/dev0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S.A., Sheffield T., Nelson J.M., Clark C.A., Chevalier N., Espy K.A. The structure of executive function in 3-year-olds. J. Exp. Child Psychol. 2011;108:436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby M.T., Blair C.B., Kuhn L.J., Magnus B.E. The benefits of adding a brief measure of simple reaction time to the assessment of executive function skills in early childhood. J. Exp. Child Psychol. 2018;170:30–44. doi: 10.1016/j.jecp.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Worden M.S., Foxe J.J., Wang N., Simpson G.V. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J. Neurosci. 2000;20:RC63. doi: 10.1523/JNEUROSCI.20-06-j0002.2000. http://www.jneurosci.org/content/jneuro/20/6/RC63.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Mallin B.M., Richards J.E., Xie C.W. Development of infant sustained attention and its relation to EEG oscillations: an EEG and cortical source analysis study. Dev. Sci. 2017;21:e12562. doi: 10.1111/desc.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto T.P., Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J. Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F., Johnson S.K., Diamond B.J., David Z., Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious. Cogn. 2010;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Zelazo P.D. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nat. Protoc. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo P.D., Anderson J.E., Richler J., Wallner-Allen K., Beaumont J.L., Weintraub S. II. NIH Toolbox Cognition Battery (CB): measuring executive function and attention. Monogr. Soc. Res. Child Dev. 2013;78:16–33. doi: 10.1111/mono.12032. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ding M. Detection of a weak somatosensory stimulus: role of the prestimulus mu rhythm and its top–down modulation. J. Cogn. Neurosci. 2010;22:307–322. doi: 10.1162/jocn.2009.21247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.