Highlights
-
•
We investigated brain networks underlying working memory maintenance in children.
-
•
Higher alpha phase synchrony was found for correct compared to incorrect responses.
-
•
Working memory maintenance was associated with dominant fronto-temporal connections.
-
•
Maintenance-related network included the left dorsolateral prefrontal cortex.
-
•
Our results implicate sustained alpha phase synchrony with successful performance.
Keywords: Working memory, MEG, Children, Alpha oscillations, Functional connectivity
Abstract
Working Memory (WM) supports a wide range of cognitive functions, and is positively associated with academic achievement. Although fMRI studies have revealed WM networks in adults, little is known about how these networks develop to support successful WM performance in children. Using magnetoencephalography, we examined the networks underlying the maintenance of visual information in 6-year-old children. We observed an increase in mean whole-brain connectivity that was specific to the alpha frequency band during the retention interval associated with correct compared to incorrect responses. Additionally, our network analysis revealed elevated alpha synchronization during WM maintenance in a distributed network of frontal, parietal and temporal regions. Central hubs in the network were lateralized to the left hemisphere with dominant fronto-temporal connections, including the dorsolateral prefrontal cortex, middle temporal and superior temporal gyri, as well as other canonical language areas. Local changes in power were also analysed for seeds of interest, including the left inferior parietal lobe, which revealed an increase in alpha power after stimulus onset that was sustained throughout the retention period of WM. Our results therefore implicate sustained fronto-temporal alpha synchrony during the retention interval with subsequent successful WM responses in children, which may be aided by subvocal rehearsal strategies.
1. Introduction
Working memory (WM), the ability to maintain and manipulate information held in mind, plays a crucial role in the development of many higher-order cognitive abilities. Although the core network of brain areas recruited for WM processes are well established in adults, less is known about when and how these networks are activated to support WM in children. One popular paradigm to delineate the different stages of WM is the delayed-match-to-sample task. In this task, a brief stimulus is presented (encoding), followed by a delay (retention), and then a test stimulus is presented requiring participants to respond if the test stimulus matches the sample (recognition). In adults, a large body of functional MRI (fMRI) studies has established a core network of regions underlying WM, including fronto-parietal regions such as the dorsolateral prefrontal cortex (dlPFC) and the superior and inferior parietal cortex, that mediate maintenance processes (Curtis and D’Esposito, 2003; Jonides et al., 1998; Eriksson et al., 2015; Goldman-Rakic, 1995; Pessoa et al., 2002).
Compared to electrophysiological measures, fMRI does not provide the temporal resolution required to delineate the precise timing of neural processes that support maintenance processes. Early electrophysiological studies in primates revealed sustained increases in a subset of dlPFC neurons during the retention period, which was interpreted to reflect storage of information in WM (Funahashi et al., 1989; Fuster and Alexander, 1971). However, recent studies suggest that persistent dlPFC activity is not related to the direct storage of information, but rather reflects directed attention towards relevant representations maintained in sensory cortices (for a review, see Sreenivasan et al., 2014). Persistent fMRI BOLD activity during the retention interval has also been found in parietal areas (D’Esposito et al., 1998; Rowe et al., 2000), with other regions recruited with respect to stimulus type (i.e. spatial versus object) and task design.
In children, the neural basis of WM has yielded less consistent results, with some studies reporting reduced prefrontal activation compared to adults (Casey et al., 1995; Geier et al., 2009; Olesen et al., 2007; Scherf et al., 2006; Taylor et al., 2012), while other studies report no frontal recruitment in children (Ciesielski et al., 2006). In one fMRI study, age-related differences were found to be most pronounced during long retention periods, with children recruiting a more distributed network of regions during longer delays, suggesting a more immature neural system when task demands are increased (Geier et al., 2009). Given that efficient WM processes rely on intact fronto-parietal circuits (Crone et al., 2006; Klingberg et al., 2002), it is likely that functional connectivity within WM networks increases with age to support maturing performance. One way to delineate the functional networks that support working memory is to investigate the network of regions recruited during successful compared to unsuccessful outcomes. One such study, Pessoa et al. (2002) used fMRI to analyze activity during different stages of a WM task, including the encoding, retention and recognition periods. Importantly, they found BOLD activity in a network of fronto-parietal regions (e.g. left dlPFC, intraparietal sulcus, and right frontal eye field) during the delay period, rather than encoding, predicted successful performance on a trial-by-trial basis.
More recently neurophysiological studies using magnetoencephalography (MEG) have provided frequency-specific information on the neural underpinnings of WM (Jensen et al., 2002; Palva et al., 2010; Roux and Uhlhaas, 2014). Alpha oscillations (8–14 Hz) have been shown to be modulated by task performance, with one study revealing that enhanced phase synchrony in the alpha band was associated with successfully remembered items in a Sternberg-like WM paradigm (Freunberger et al., 2009). Conversely, items that were cued ‘not to be remembered’ were associated with decreased alpha phase synchrony (Freunberger et al., 2009). Similarly, increased alpha phase-locking was shown to be associated with better performance (i.e. correct categorization) on a perceptual discrimination task (Hanslmayr et al., 2005). These and related MEG findings emphasize the important role of alpha oscillations in enabling effective stimulus encoding and maintenance of information in WM (Jensen et al., 2002; Jokisch and Jensen, 2007; Sauseng et al., 2005).
Palva et al. (2010) also found enhanced inter-regional synchrony in MEG with increasing memory-load among fronto-parietal regions in alpha, beta and gamma frequency bands; they found alpha synchrony to be most strongly associated with increasing memory load, showing alpha rhythms to be closely linked with task demands (Palva et al., 2010). This is consistent with prior studies that link long-range alpha synchrony with WM and attentional processes (Anderson et al., 2014; Hsieh et al., 2011; Jensen et al., 2002; Schack and Klimesch, 2002; Stipacek et al., 2003). These studies, however, were conducted in adults.
Thus, in the present study, MEG was used to examine the spatio-temporal dynamics underlying visual WM in 6-year-old children. MEG is uniquely suited to capture the rhythmical oscillatory activity of neuronal populations, and allow for the investigation of large-scale functional networks involved in WM maintenance. We investigated whole-brain connectivity time-courses during the retention interval associated with both correct and incorrect responses to determine the frequency band of interest for our analysis. We expected that the frequency band of interest important for successful WM outcomes would show significant differences between correct and incorrect trials during the retention period. Specifically, we hypothesized that alpha-band connectivity would be related to WM performance, showing increased connectivity for successful compared to unsuccessful responses. Additionally, we expected sustained alpha synchrony throughout the retention interval to support stable maintenance processes (Palva et al., 2010; Pessoa et al., 2002). In our network analysis (performed on correct trials only, i.e., correct hits), we expected enhanced alpha synchronization compared to baseline among a network of frontal, parietal, and temporal regions during WM maintenance. Given that prefrontal regions and their connections are undergoing continued maturation at this age, we expected limited dlPFC involvement, due to its protracted maturation within prefrontal regions, and possibly increased recruitment from other prefrontal regions in support of WM.
2. Materials and methods
2.1. Participants
Twenty-seven healthy, typically-developing children were recruited to the study at 6 years of age. Children were recruited via hospital flyers, public schools, and word of mouth in the Toronto area. Three children were excluded due to excessive movement in the MRI and/or MEG scanner, and four children were excluded due to poor task performance (<60% accuracy). The final sample consisted of 20 children (mean age: 6.65 ± 0.34 years; 10 females). Exclusion criteria included a history of neurological disorder, uncorrected visual deficits, colour blindness, and a diagnosis of significant learning or neurodevelopmental disorders such as autism spectrum disorder. Additionally, all children were screened and approved for MEG and MRI compatibility (i.e. without any metal implants/devices). Informed consent and verbal assent were obtained from the parents and children, respectively. The study protocol was approved by the Hospital for Sick Children (SickKids) research ethics board and is in accordance with the declaration of Helsinki. All testing was conducted at SickKids.
To ensure that all children included in the study analyses were without any significant cognitive delay, all children were also administered the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II) (Wechsler, 2013) to provide an estimate of full-scale IQ. Children also completed two sub-tests of the Working Memory Test Battery for Children (WMTB-C) (Gathercole and Pickering, 2000) to assess working memory ability.
2.2. Stimuli
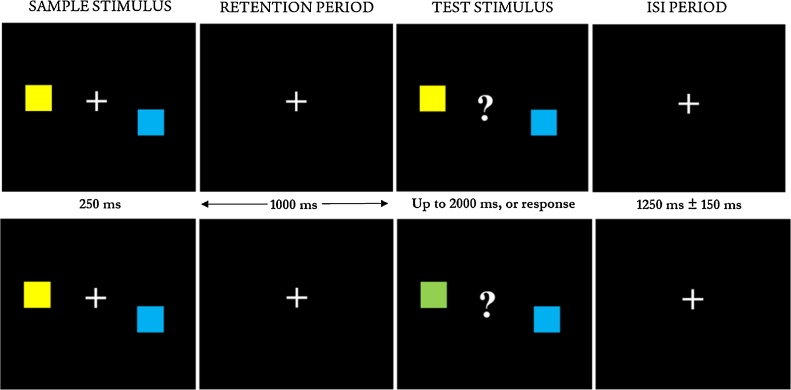
The WM paradigm was adapted from a delayed match-to-sample task employed by Palva et al. (2010). Children were first presented with two coloured squares (sample stimulus), followed by a retention period (fixation cross) during which they were instructed to remember the colour of the squares. Following the retention period (1000 ms), two coloured squares were then presented (test stimulus) and participants were requested to indicate, via button-pad, whether the sample and test stimuli were the same or different (Fig. 1). The central fixation cross presented during the sample stimulus, was replaced by a question mark during the test stimulus, prompting children to respond. Children responded by pressing either a left or right button on a MEG-compatible button-pad with their thumbs, corresponding to either same or different responses, which was randomized across subjects. The test stimulus was presented until a response was given, or up to 2000 ms. A fixation cross appeared after the presentation of the test stimulus, and remained for the duration of the inter-stimulus interval (ISI; between the presentation of the test stimulus and the next trial’s sample stimulus) and was jittered between 1100 and 1400 ms. Half of the test stimuli comprised “different” trials, in which the colour of one square changed between the sample and test stimulus (Fig. 1 – lower panel), and the other half “same” trials (Fig. 1 – upper panel). The task was run for two periods of 7 min each, with a short break in between. All children received training prior to MEG testing to ensure full understanding of the task and instructions. The task also included a higher memory-load condition with four coloured squares, interleaved with the 2-square condition; however, due to poor task performance, this condition was not included in the current analyses. The task was designed to aim for 100 correct trials in each load condition (2- or 4-square condition), or discontinue when the time limit was reached.
Fig. 1.
Stimuli for the delayed match-to-sample task. Children were presented with the sample stimulus for 250 ms, followed by a 1000 ms retention interval during which they are instructed to remember the initial stimulus. A second, test stimulus was then presented for up to 2000 ms, or until the child responded. The inter-stimulus interval (ISI) period varied between 1100 and 1400 ms. The upper panel shows an example of the "same" condition, as the colour of the squares in the sample and test stimulus match. The lower panel shows the "different" condition, as the colour of one square has changed (from yellow to green). Task adapted from Palva et al. (2010).
2.3. MEG data acquisition
MEG data were recorded using a 151-channel CTF MEG system (MISL, Canada) housed in a magnetically shielded room (MSR) at SickKids. Children lay supine with their head inside the MEG helmet. Stimulus timing was controlled using Presentation software (Neurobehavioral Systems Inc., Albany, CA) running on a dedicated stimulus computer. Stimuli were projected into the MSR via a system of mirrors, a back projection screen, and a digital projector located outside of the MSR. The stimuli were projected on the screen at a viewing distance of 79 cm and visual angle of 6.9°. Three localization coils were placed on the right and left pre-auricular points and the nasion. The localization coils were energised enabling continuous tracking of head position within the helmet. Data were recorded continuously at 600 Hz, and a third-order spatial gradient was applied to attenuate distant environmental noise and optimize brain sources.
2.4. MRI data acquisition
Anatomical 3 T MRI images were acquired with a 12-channel head coil (MAGNETOM Tim Trio, Siemens AG, Erlangen, Germany) at SickKids. Reference fiducial coil placements were placed in the same location as the MEG localization coils for co-registration to the MEG data. A T1-weighted MRI image of the brain was obtained using a 3D SAG MRPAGE sequence: GRAPPA = 2, FA = 9°, TR/TE = 2300/2.96 ms, FOV = 28.8 x 19.2 cm, 240 × 256 matrix, 192 slices, slice thickness = 1.0 mm, scan time = 5:03 min.
2.5. MEG preprocessing and source reconstruction
MEG analyses were performed on the 1 s retention period (between the sample and test stimulus) using MATLAB R2015a software (Mathworks Inc., Natick, MA) and the FieldTrip toolbox (git commit 4c12371; Oostenveld et al., 2011). Retention periods associated with subsequently correctly and incorrectly recognized trials were separated in the analysis. For incorrect trials, only false alarm trials in which a response was provided (i.e. button press) were included in the analyses (miss trials were excluded). Epochs for each trial were created from -1 s pre-stimulus to 2 s post-stimulus onset, to prevent boundary effects at the beginning and end of these data segments. Epochs where head motion exceeded 10 mm from the initial head position were excluded from analysis, consistent with motion thresholds for MEG studies in children (Doesburg et al., 2013; Pang, 2011; Taylor et al., 2011). MEG data were bandpass filtered from 1 to 150 Hz with a 4th order two-pass Butterworth filter and a 60 Hz notch. Independent component analysis (ICA) was then performed for each participant and ocular and cardiac components were identified by visual inspection and manually excluded from the MEG recording (Oostenveld et al., 2011). Additionally, epochs containing muscle artefacts were excluded from analysis, as were epochs with MEG sensor signals exceeding a threshold of 2500 fT.
MEG data were co-registered to each individual’s MRI image using the reference fiducial coil placements, and single shell head models were constructed based on fiducial positions (Lalancette et al., 2011; Nolte, 2003). Ninety cortical and sub-cortical seed locations from the Automated Anatomical Labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002) were unwarped from standard Montreal Neurological Institute (MNI) space into each individual’s brain space using SPM8′s normalization functions. The AAL atlas is a commonly used anatomical atlas in functional neuroimaging studies, and provides widespread coverage of cortical and sub-cortical brain areas. The linearly constrained minimum variance (LCMV; Van Veen et al., 1997) beamformer was used to estimate broadband source activity from a single point at the centroid of each of the 90 AAL parcels for each subject. Beamforming is a spatial filtering approach that estimates activity at each source in the brain while maximally suppressing background noise (Van Veen et al., 1997). In this manner, source activity from regions of interest can be isolated from other influences, including artefacts generated by ocular movements or cardiac and muscle activity. The LCMV beamformer was run with 5% regularization and covariance matrices calculated on the selected epochs between 1–150 Hz. The source time series were normalized by the estimate of noise at each source location to obtain the Neural Activity Index (Van Veen et al., 1997) time series.
2.6. Functional connectivity analysis
After source reconstruction, the time series from each seed region were filtered into theta (4–7 Hz), alpha (8–14 Hz), beta (15–30 Hz), and low gamma (30–55 Hz) frequency bands. The Hilbert transform was applied to the filtered time series to extract the instantaneous phase values for each time sample for each frequency. Inter-regional phase synchronization was calculated over the retention period and averaged over trials using the weighted phase lag index (wPLI) (Vinck et al., 2011). wPLI is a measure of functional connectivity, which is believed to reflect the efficiency of communication between brain regions (Fries, 2005). wPLI provides values ranging from 0 (no phase locking, random phase difference) to 1 (maximum phase locking, constant phase difference). Specifically, wPLI measures the non-zero phase leads and lags between sources by weighting the magnitude of the imaging component cross-spectrum (Vinck et al., 2011). wPLI gives optimal weighting to 90-degree phase differences, and is thus able to suppress signals associated with artificial synchrony. In this manner, wPLI is able to delineate phase activity associated with true brain interactions from that of artefacts or external noise. wPLI assumes that true phase synchrony between two sources will possess a consistent, non-zero phase difference, making it less susceptible to artefactual coherence and inflated / spurious correlations due to volume conduction or beamformer leakage. Phase synchrony was calculated at each frequency band, across all source pairs for the entire 1 s retention period, and then averaged across trials. This resulted in a 90 × 90 connectivity matrix of wPLI values for all pairwise combinations of reconstructed sources in the brain at each sample, for each child.
To determine whether phase synchrony was sustained throughout the retention period, and to identify frequency bands important in maintaining these networks, the time course for mean whole-brain connectivity was computed for each frequency band. wPLI values were averaged over all node pairs (90 × 90 nodes) to obtain a single timecourse of mean whole-brain connectivity. The values were z-scored to the baseline (subtracting the mean between -500 to 0 ms from the entire timeseries then dividing by baseline variance), then averaged across subjects, resulting in a single timeseries for each condition. The time course in connectivity was compared between correct and incorrect trials to examine how whole-brain networks engaged in the maintenance of visual stimuli were differentially activated. To identify significant differences between the connectivity time series while controlling the false positive rate due to multiple comparisons on the 600 time points, we employed a permutation test. On each of the 1000 permutations, the condition labels of each timeseries were randomly permuted, the t-statistic computed between the randomly labelled timeseries, then thresholded at t >1.7. The length of the largest contiguous supra-threshold window was recorded into the null distribution. Windows of contiguously supra-threshold differences in the unpermuted timeseries were therefore statistically significant and corrected for multiple-comparisons if supra-threshold windows were larger than 95% of supra-threshold windows from the null distribution.
As we were interested in the associations between brain and behaviour, we averaged alpha connectivity (wPLI) values over the retention interval of correct hits (normalized to baseline) and tested associations with WM behavioural accuracy (%). Pearson’s correlations between mean whole brain alpha connectivity (normalized to baseline) over the retention interval associated with correct responses, and behavioural accuracy (%) were computed. Two participants were excluded from this portion of the analysis due to a low number of error trials (i.e. they had high accuracy).
2.7. Maintenance-related network analysis
Group averaged adjacency matrices for correct trials (i.e., correct hits) were then compiled and submitted to a Network-Based Statistic (NBS) analysis (Zalesky et al., 2010) in order to identify regions recruited during successful WM outcomes. Statistical contrasts between conditions of interest (retention period vs. baseline) were computed. NBS identifies network components (i.e. contiguous set of inter-regional connections) that significantly differ between conditions. In this study, the retention period (active window) and the pre-stimulus ISI period (baseline) were compared to identify networks involved in WM maintenance. NBS controls for the Family-Wise Error Rate (FWER) when performing mass univariate testing on a graph. NBS first applies a univariate threshold to every element in the connectivity matrix, resulting in each edge having a single test statistic value (t-value). Then a test-statistic threshold is applied such that only contiguously connected nodes (i.e. components) exceeding the desired significance level are included in the network analysis. In this manner, NBS assigns statistical significance at the network level, rather than at the level of individual connections. The graph components are ascribed a corrected p-value by the FWER using permutation testing, with 5000 permutations.
The NBS univariate threshold was adapted to the data distribution in each frequency band (Zalesky et al., 2010, 2012). To target strong network differences between conditions, a conservative univariate t-threshold of t ≥ 2.0 was applied. Node degree was used to determine the degree of connectedness—the number of edges connected to a particular node. Nodes with high degree are considered network hubs, and therefore crucial to efficient network communication. Brain networks were then visualized using the BrainNet Viewer Connectivity Toolbox (Xia et al., 2013).
2.8. Time-frequency analyses
Additionally, we looked at local power changes in seeds of interest, based on the literature of regions involved in WM maintenance in adults (Gazzaley et al., 2004; Palva et al., 2010; Pessoa et al., 2002), as well as seeds that were identified as network hubs in our network analysis. Analyses were computed for correct trials only. Time-frequency representations (TFRs) reflecting the proportion change in power relative to baseline (-0.5 to 0 s), were computed for the 1 s retention interval (0.25–1.25 s). Spectral power estimations, averaged across trials, were computed for each selected seed’s time-series using a Morlet wave transformation, as implemented in Fieldtrip (Oostenveld et al., 2011). This was computed for the frequency window between 8–20 Hz (at 0.5 Hz bins) and at each 5-ms time point between -1 s pre-stimulus to 3 s post-stimulus onset. To test for statistical differences between conditions of interest, proportion change in power (relative to baseline) values were averaged over the entire retention interval, and over the frequency band of interest (i.e. 8–14 Hz), for each selected seed. A dependent t-test was used to compute significant differences between the mean values of the retention interval compared to the baseline period. A False Discovery Rate (FDR) correction applied using the Benjamini-Hochberg procedure was applied to correct for multiple comparisons (q<0.05) (Benjamini and Hochberg, 1995). Additionally, to test whether relative alpha power was correlated with alpha synchrony in our maintenance network, we extracted the average alpha power per subject in all the nodes found to be significant in our network analysis (48 nodes). The proportion change in power averaged over the entire retention interval (relative to baseline) was calculated for each of the 48 nodes and the values were averaged for each child. These values were then correlated with alpha network strength for each child. Network strength was calculated by taking the sum of the wPLI value (z-scored to the baseline) for all significant connections in our network analysis (54 connections), which indexes the recruitment of this network in each child.
3. Results
3.1. Behavioural and outcome results
Children included in the study performed with a mean accuracy of 80.15%. Reaction times (RTs) did not differ between correct and incorrect trials (t(19)= -0.36, p = 0.73): correct responses had a mean RT of 1409.97.2ms (± 119.85ms) and incorrect responses had a mean RT of 1426.83ms (± 264.53ms). All children fell within the average to high-average range for the digit (mean score: 113.20 ± 11.92) and block recall (107.50 ± 13.67) sub-tests of the WMTB-C. Full-scale IQ scores showed all children were within the average to superior range (113 ± 14.16).
3.2. Whole-brain connectivity time-courses associated with correct and incorrect responses
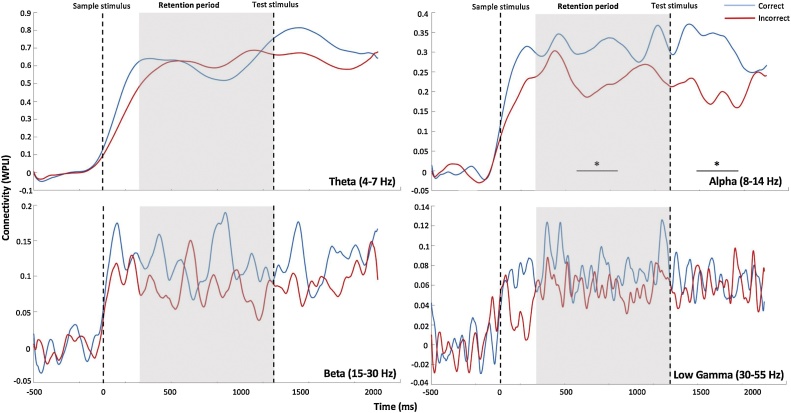
Whole-brain connectivity results revealed significant differences between correct and incorrect trials only for the alpha-band (pcorr<0.05, Fig. 2). As illustrated in Fig. 2 (upper right panel), whole-brain connectivity in the alpha-band (8–14 Hz) peaked soon after stimulus onset, and remained stable across the retention interval and through to the presentation of the test stimulus. This effect was observed for both correct (blue line) and incorrect trials (red line); however, there was significant reduction in connectivity for incorrect trials during the course of the retention period (pcorr = 0.011). This maintenance effect was frequency-specific, as only the alpha frequency band showed significant differences in connectivity between correct and incorrect trials (see Fig. 2). We therefore focused our primary analyses on the alpha-band. We also found greater connectivity compared to baseline for correct responses during the recognition phase (Fig. 2).
Fig. 2.
Mean whole brain connectivity time series are depicted for correct (blue line) and incorrect (red line) responses for each frequency band. The onset of the sample stimulus is represented by the first dotted line, and the retention period is represented by the shaded grey area. Significant differences between correct and incorrect responses (pcorr = 0.011; upper right panel) were observed in the alpha frequency band (8–14 Hz) only during the retention interval and the recognition period (i.e. presentation of the test stimulus).
3.3. Alpha band WM maintenance network
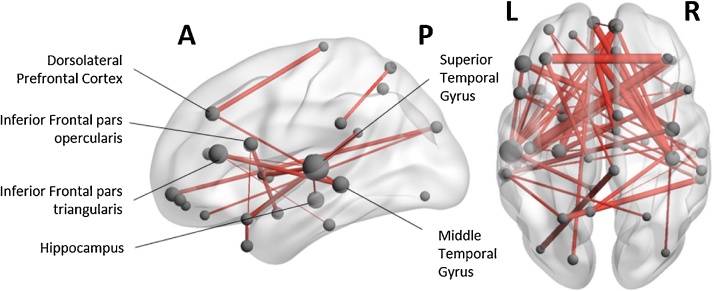
Functional connectivity results revealed children also had elevated synchronization in the alpha frequency band during WM retention compared to the baseline ISI period (pcorr<0.001, Fig. 3). The alpha-network encompassed significant ventrolateral (bilateral inferior frontal gyri), dorsolateral (bilateral middle frontal gyri) and medial (right superior medial frontal gyrus, bilateral medial orbital gyri) prefrontal areas, as well as temporal (left hippocampus, bilateral middle temporal and superior temporal gyri, bilateral middle and superior temporal poles), and parietal (bilateral superior parietal lobules, paracentral lobules, supramarginal gyri, left precuneus) nodes; and areas in occipital (left superior occipital gyrus, right middle occipital gyrus) cortices (see supplementary table 1 for a complete list of network nodes). We used node degree to identify network hubs (regions that are central to the network and information flow). The left inferior frontal triangularis, left hippocampus, and the left middle temporal gyrus (MTG), and the left superior temporal gyrus (STG) were identified as the most connected seeds in the network (i.e. high node degree). In addition, the left dlPFC showed high degree within the network. Alpha synchronization was strong between hemispheres, with dominant fronto-temporal connections (Fig. 3).
Fig. 3.
Functional connectivity network during working memory maintenance in the alpha frequency band. Children showed elevated alpha synchronization during the retention period compared to baseline, in a distributed network of frontal, parietal, and temporal regions (pcorr<0.001). Nodes were scaled by degree (the number of edges connected to a particular node), such that larger node size reflects nodes with higher degree (i.e. a greater number of connections to other nodes in the network).
3.4. Brain-behaviour associations
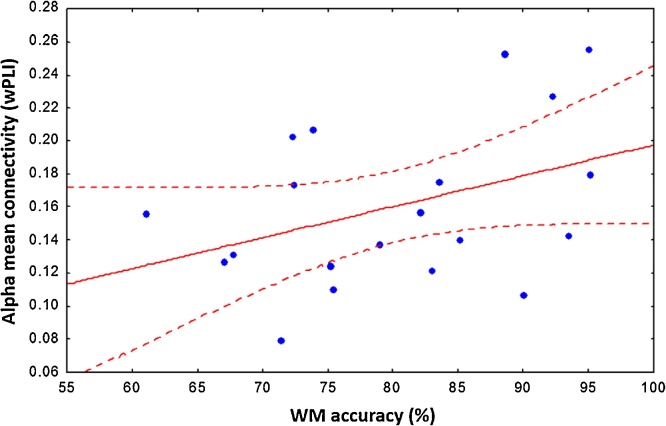
To investigate brain-behaviour associations, we computed Pearson’s correlation between whole-brain alpha connectivity averaged over the retention interval (normalized to baseline) and behavioural WM accuracy (%). This correlation showed a trend towards significance (r = 0.39, p = 0.087, see Fig. 4). We also performed correlations between specific nodes of interest (e.g. major hubs in our network analysis) and task performance, but found that none of the associations were significantly associated with WM accuracy (p>0.05).
Fig. 4.
Pearson correlations between mean alpha connectivity (wPLI) and WM behavioural accuracy revealed a positive trend (r = 0.39, p = 0.08).
3.5. Time-frequency analysis
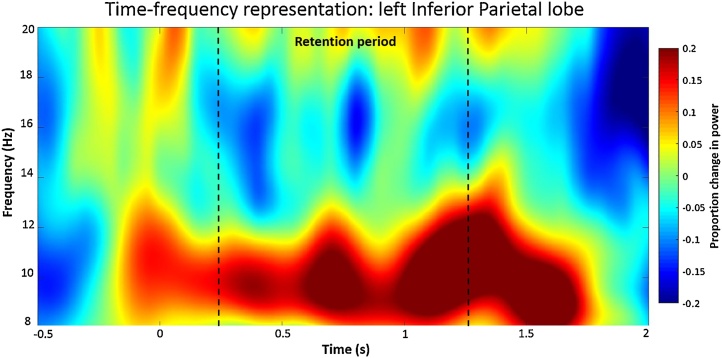
The WM literature has highlighted the role of fronto-parietal connections as important for sustaining WM processes (Eriksson et al., 2015; Jensen et al., 2002; Jokisch and Jensen, 2007; Palva et al., 2010; Pessoa et al., 2002). As such, we chose bilateral dlPFC and inferior parietal lobe (IPL) as our seeds of interest, as well as the left STG, given that it was a central hub in our network analysis. Spectral power was averaged over the alpha frequency band (8–14 Hz) and the retention interval (0.25–1.25 s), for each seed region, and a dependent t-test was computed between active versus baseline values. Only the left IPL revealed a significant difference between active versus baseline values (Fig. 5, t(19)= -2.42, p = 0.025).
Fig. 5.
Group-averaged Time-Frequency Representation during WM for the left Inferior Parietal lobule. Time (in s) is denoted on the x-axis, and frequency (in Hz) on the y-axis. The color legend reflects the proportion change in power relative to baseline (−0.5 to 0 s). The zero time-point reflects the onset of the sample stimulus, and 0.25 s the onset of the retention interval (until 1.25 s).
Time-frequency representation for the left IPL revealed an enhancement in alpha-power relative to baseline, beginning at the onset of the retention interval, and returned to baseline levels shortly after the presentation of the test stimulus (Fig. 5). The right IPL, however, did not show a strong pattern of alpha enhancement during the retention interval (see supplemental figure, S1). Instead we observed an increase in alpha-power at the onset of both the sample stimulus and soon after the presentation of the test stimulus. The TFR for the right dlPFC showed a robust increase in alpha power towards the end of the retention interval, and remained stable through to the presentation of the test stimulus (Fig. S2). We also observed sustained suppression in the high-alpha and low-beta-bands, beginning after the onset of the retention interval. There were no marked changes in enhancement/suppression observed in the left dlPFC during retention (Fig. S3). For the left STG, however, TFRs revealed alpha desynchronization towards the middle of the retention interval (Fig. S4).
3.6. Association between relative alpha power and network strength
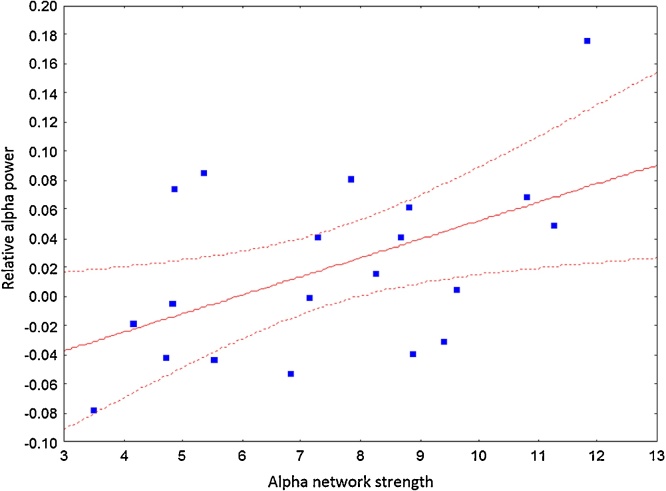
To test whether alpha power modulations were associated with alpha synchronization, we performed a Pearson’s correlation between relative alpha power (proportion change in power relative to baseline) and alpha network strength, which revealed a positive association (Fig. 6, r=0.51, p=0.02). However, to test whether increases in alpha connectivity were not solely attributed to random increases in power, we generated a set of 1000 surrogate networks with connections involving nodes from the maintenance network, with equivalent network density (n = 54 connections, i.e., edges between nodes). A surrogate distribution of r-values was then computed by regressing the network strength of these surrogate networks with alpha power (see supplemental figure, S5). The r-value from our original data (r=0.51) was significantly larger than the bulk of the surrogate distribution (p=0.036).
Fig. 6.
Pearson correlations between relative alpha power and alpha network strength revealed a significant, positive association (r = 0.51, p=0.02).
4. Discussion
4.1. Summary of findings
This study elucidates the oscillatory mechanisms underlying WM maintenance in young children. Whole brain connectivity analyses in the alpha band (8–14 Hz) revealed significant differences between correct and incorrect trials during the retention period. Alpha connectivity was found to be higher and more stable during the retention period associated with correct compared to incorrect responses. In our network analysis, children showed elevated alpha connectivity during the retention interval compared to the baseline period. This network comprised significant frontal, parietal and temporal connections that have been widely reported in WM processing in adults. The major hubs in this network included the left hippocampus, middle temporal and superior temporal gyri, as well as the left dlPFC. Additionally, time-frequency analyses showed distinct patterns of synchronization and desynchronization in brain areas linked to WM.
4.2. Alpha network synchrony supports successful WM performance
Whole brain connectivity time series revealed a significant difference between correct and incorrect trials during the retention interval for the alpha band only; revealing stronger and more stable connectivity throughout the retention interval preceding subsequently correctly recognized trials (Fig. 2). Sustained cortical activity during the retention interval has been interpreted to underlie maintenance processes critical to sustaining visual representations in the absence of sensory input (Fuster and Alexander, 1971; Goldman-Rakic, 1995). While our current results reflect whole-brain connectivity over time, previous studies in adults have reported sustained activity during the retention interval in discrete cortical regions, including prefrontal (Cohen et al., 1997; Courtney et al., 1997; Funahashi et al., 1989), temporal (Chelazzi et al., 1998; Fuster and Jervey, 1982), and parietal (Chafee and Goldman-Rakic, 1998, 2000; Sakai et al., 2002) areas. Evidence from fMRI and EEG studies has also shown that task performance declines when neural activity was not sustained during the retention interval (Funahashi et al., 1993; Pessoa et al., 2002; Sakai et al., 2002). Our results are thus consistent with the adult literature suggesting that sustained activity during the retention period is necessary to support successful performance, extending this to now include young children. Recent studies have also implicated alpha oscillations as being critical to facilitating task performance. Freunberger et al. (2009) found alpha coherence to be stronger in adults for successfully remembered items, and that forgetting during an episodic memory task was related to reduced alpha phase coupling. This suggests that alpha plays a role in the directed attention to internal representations that is critical across age groups in maintaining relevant information in WM. Reduced alpha connectivity observed during the retention period associated with incorrect responses may therefore be a result of endogenous changes in attention. We speculate that children may not have dedicated enough attentional resources to maintain the representations in WM during incorrect trials, resulting in the decreased connectivity observed during the retention interval.
Although the current task may not seem particularly demanding, we found these young children had difficulty responding adequately in the higher load condition (which was excluded from the current analyses due to low accuracy). Mean accuracy in our sample was 80% after excluding those who performed below 60% accuracy, which allowed us, however, a sufficient number of error trials to include in our analyses. Few studies have directly compared the differential activity patterns associated with correct and incorrect responses in a WM task due to a limited number of error trials. In one fMRI study in adults, differential BOLD activity during the WM retention interval associated with correct responses revealed sustained signal amplitude in a network of fronto-parietal regions predicted successful WM performance on a trial-by-trial basis (Pessoa et al., 2002). Consistent with our results, this suggests that effective maintenance of information relies on sustained signals during the retention interval, and that reduced activity associated with incorrect trials likely reflects disrupted maintenance processes. Our results therefore critically implicate strong and sustained alpha connectivity during the retention interval with subsequent successful WM responses in young children. Most importantly, our results demonstrate for the first time that whole-brain alpha signal can be used to predict successful and unsuccessful outcomes.
Although our association between mean alpha connectivity and behavioural accuracy showed a positive trend at the whole-brain level, brain-behaviour associations were not strengthened using specific seeds of interest, such as the left hippocampus or dlPFC. These results suggest that looking at associations at the network level rather than focusing on specific seeds may yield more sensitive brain-behaviour associations. These results also suggest that other frequency bands, namely beta and gamma which were not seen in the 6-year-olds, may increasingly become involved as WM processes mature into adulthood (Palva et al., 2010).
4.3. Alpha network connectivity during WM maintenance
The alpha phase-synchronized networks during WM maintenance encompassed significant frontal, temporal, and parietal interactions (Fig. 3). These regions largely overlap with areas observed in adult fMRI studies of WM maintenance (Courtney et al., 1997; Eriksson et al., 2015; Gazzaley et al., 2004; Palva et al., 2010; Pessoa et al., 2002). Previous research in adults has also implicated large-scale alpha synchrony among key fronto-parietal regions (Palva et al., 2010), to support the attentional and central executive functions of WM (Kastner and Ungerleider, 2000; Palva and Palva, 2007). For instance, Palva et al. (2010) found that alpha synchrony among fronto-parietal structures strengthened with increases in memory load, which was interpreted to reflect the modulation of top-down attentional control that sustains object representations in visual WM (Palva et al., 2010). Our results also revealed a bilateral network, with major hubs lateralized to the left hemisphere, including the left pars opercularis and pars triangularis of the left inferior frontal gyrus. This may indicate that children used verbal rehearsal strategies (i.e. repeating colours during retention interval) to guide correct responses. Supporting this interpretation, these left-lateralized hubs are regions known to be involved in language processing and the phonological loop (see Supplementary Table 1; Cabeza and Nyberg, 2000; Rogalsky et al., 2008; Rottschy et al., 2012). Given how short the retention interval was, repeating more than two colours during the retention would not have been possible, which could have contributed to the poor performance in the higher load condition. Coupled with the notion that many classic WM regions are still maturing at this age, young children may still be developing efficient mechanisms to support the transient loading and maintenance of visual information in WM (Baddeley, 1992). As task demands increase (i.e. increasing memory load), most children fail to perform above 60% accuracy. Therefore, given the time constraints (i.e. short encoding and retention periods) and capacity limitations, this task provides appropriate cognitive demands to probe the development of WM mechanisms in children. In a similar version of this task, Palva et al. (2010) found that adults were able to complete this task with up to six coloured squares. Thus, WM maintenance processes may develop early in childhood, while the central executive functions of WM continue to mature throughout childhood to support greater attentional and task demands. In our study children showed more fronto-temporal interactions within the left hemisphere, rather than the classic fronto-parietal circuitry recruited in adults. Stronger fronto-temporal connections, may indicate that children rely more on recognition processes (e.g. matching test and sample stimulus), known to rely on temporal lobes (Bergmann et al., 2012; Urbain et al., 2016), whereas parietal areas are associated with more executive aspects of WM (Collette et al., 2005; Eriksson et al., 2015; Koenigs et al., 2009). These results support behavioural findings that demonstrate the ability to hold information in WM is evident in early childhood (Diamond and Goldman-Rakic, 1989), and that the neural underpinnings that support these processes may increasingly recruit parietal areas as executive functions of WM continue to mature.
Previous fMRI studies have also uncovered distinct regions recruited for WM in children, but have lacked the temporal resolution to delineate regions recruited for the different stages of WM. Our results identified central hubs in the network to be the left prefrontal areas, and middle and superior temporal gyri. Given the ongoing functional and structural maturation in the dlPFC in children, we were surprised by the extent of dlPFC recruitment in the alpha network. It has been shown that when distractors are presented during the retention period, or when task demands are increased (i.e. increasing memory load), dlPFC activation increases accordingly (Höller-Wallscheid et al., 2017). Our study showed that sufficient cognitive demands were placed on children, thus necessitating recruitment of the dlPFC to sustain representations or rehearsal of information in WM.
4.4. Local changes in alpha power
To investigate local changes in power, we selected five seeds of interest based on the adult literature highlighting the fronto-parietal network in WM maintenance. Results for the left IPL (Fig. 5) revealed a strong increase in alpha power relative to baseline, beginning after stimulus onset and suppressed shortly after the end of the retention interval. In line with the ‘gating by inhibition’ hypothesis (Jensen and Mazaheri, 2010; Klimesch et al., 2007), we speculate that this sustained increase in alpha may be related to suppression of the dorsal (‘where’ pathway) visual stream. Our task engaged the ventral visual stream (‘what’ pathway), requiring children to remember only the colour of the squares as the location of the squares was irrelevant. In another MEG study, Jokisch and Jensen (2007) observed a robust increase in alpha synchronization in the parieto-occipital sulcus (dorsal visual stream) during the retention interval of a delayed-match-to-sample WM task. In their task, participants were required to remember the identity or orientation of a face, engaging the ventral and dorsal visual stream, respectively (Jokisch and Jensen, 2007). In line with our results, the authors found an increase in alpha power in the dorsal stream when the ventral stream was engaged in WM maintenance. Additionally, this increase in alpha response towards the end of the retention interval may signal the ‘gating’ of incoming stimuli in order to protect ongoing maintenance processes (Heinrichs-Graham and Wilson, 2015).
The time-frequency representation for the right dlPFC showed a robust increase in alpha power towards the end of the retention interval that was sustained during the recognition phase (Fig. S5). The left dlPFC, however, one of the hubs in our network analysis, showed attenuated local alpha power compared to the right dlPFC (Fig. S6). In line with fMRI studies reporting sustained dlPFC activity spanning the retention interval, we also expected to find sustained alpha suppression in children, since local alpha suppression is thought to reflect cortical engagement (Pfurtscheller and Lopes Da Silva, 1999). However, we found increased alpha power in bilateral dlPFC towards the end of the retention period and recognition phase. Heinrichs-Graham and Wilson (2015) speculated that this increase in alpha power, reflecting inhibitory processes, may arise when the probability of forgetting is most likely to occur. These findings provide additional support for the alpha inhibition hypothesis, as increased alpha power in the dlPFC may reflect top-down attentional and/or inhibitory control over other brain areas, such as temporal areas, in order to maintain relevant representation in WM (Jensen et al., 2002; Klimesch et al., 2007; von Stein et al., 2000). Thus, results from our study extend those of Sauseng et al. (2005) in adults who showed an increase in alpha power over prefrontal regions, while occipital regions were suppressed. In addition to enhanced functional coupling, they found a consistent latency shift between prefrontal and occipital areas, possibly reflecting the modulation of activity in the visual cortex by prefrontal brain areas (Sauseng et al., 2005). Thus, an increase in alpha power could protect ongoing WM maintenance processes by inhibiting external or internal distractions. Finally, the left STG showed a decrease in alpha power towards the middle of the retention interval (Fig. S7). Together with findings from our whole-brain connectivity analysis, which showed strong interactions between the left STG and other prefrontal areas, local increases in alpha power in right dlPFC and suppression in the left STG, may reflect the network involved in top-down processing.
5. Conclusions
This study is the first to uncover the inter-regional oscillatory mechanisms underlying successful WM responses in young children. We found increased mean whole brain connectivity in the alpha frequency band during the retention interval associated with correct (hits) compared to incorrect (false alarm) responses. Importantly, this effect was found to be specific to the alpha frequency band, implicating alpha synchrony as an important marker of WM in development. This difference in connectivity may be attributed to fluctuations in attention that are necessary to sustain representations in WM, which is further supported by recruitment of bilateral dlPFC during WM maintenance. This interpretation is reinforced by previous findings in adults that suggest strong, sustained signals during WM maintenance as being reflective of increased attention to stored representations to enable a correct response. Further, we found strengthened alpha synchrony among fronto-temporal regions during WM maintenance, rather than the classic fronto-parietal network reported in adults, suggesting a different use of WM strategies (e.g. recognition processes) in young children. Central hubs in the network were lateralized to the left hemisphere, including the dorsolateral prefrontal cortex, middle temporal and superior temporal gyri. Our results support the role of alpha inter-regional synchrony as a mechanism for sustaining memory of visual stimuli, which is already active in young children. Together, these findings provide us with a normative framework to understand the development of functional networks that support successful WM performance in children.
Conflict of Interest
None.
Funding
This work was supported by the Canadian Institutes of Health Research; Contract grant numbers: MOP-137115; Contract grant sponsor: Hospital for Sick Children.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2018.09.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Anderson D.E., Serences J.T., Vogel E.K., Awh E. Induced alpha rhythms track the content and quality of visual working memory representations with high temporal precision. J. Neurosci. 2014;34(22):7587–7599. doi: 10.1523/JNEUROSCI.0293-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baddeley A. Working memory Alan Baddeley. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57(1):289–300. [Google Scholar]
- Bergmann H.C., Rijpkema M., Fernández G., Kessels R.P.C. Distinct neural correlates of associative working memory and long-term memory encoding in the medial temporal lobe. NeuroImage. 2012;63(2):989–997. doi: 10.1016/j.neuroimage.2012.03.047. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cohen J.D., Jezzard P., Turner R., Noll D.C., Trainor R.J., Giedd J., Kaysen D., Hertz-Pannier L., Rapoport J.L. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. NeuroImage. 1995;2(3):221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Chafee M.V., Goldman-Rakic P.S. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J. Neurophysiol. 1998;79(6):2919–2940. doi: 10.1152/jn.1998.79.6.2919. http://www.ncbi.nlm.nih.gov/pubmed/9636098 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Chafee M.V., Goldman-Rakic P.S. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J. Neurophysiol. 2000;83(3):1550–1566. doi: 10.1152/jn.2000.83.3.1550. http://www.ncbi.nlm.nih.gov/pubmed/10712479 [DOI] [PubMed] [Google Scholar]
- Chelazzi L., Duncan J., Miller E.K., Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J. Neurophysiol. 1998;80(6):2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Lesnik P.G., Savoy R.L., Grant E.P., Ahlfors S.P. Developmental neural networks in children performing a categorical N-back task. NeuroImage. 2006;33(3):980–990. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Cohen J.D., Perlstein W.M., Braver T.S., Nystrom L.E., Noll D.C., Jonides J., Smith E.E. Temporal dynamics of brain activation during a working memory task. Nature. 1997 doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Collette F., Van Der Linden M., Laureys S., Delfiore G., Degueldre C., Luxen A., Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Hum. Brain Mapp. 2005;25(4):409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney S.M., Ungerleider L.G., Keil K., Haxby J.V. Transient and sustained activity in a distributed neural system for human working memory. Nature. 1997;386(6625):608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Wendelken C., Donohue S., van Leijenhorst L., Bunge S.A. Neurocognitive development of the ability to manipulate information in working memory. Proc. Natl. Acad. Sci. 2006;103(24):9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C.E., D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Esposito M., Aguirre G.K., Zarahn E., Ballard D., Shin R.K., Lease J. Functional MRI studies of spatial and nonspatial working memory. Cogn. Brain Res. 1998;7(1):1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Diamond A., Goldman-Rakic P.S. Comparison of human infants and rhesus monkeys on Piaget’s AB task: evidence for dependence on dorsolateral prefrontal cortex. Exp. Brain Res. 1989;74(1):24–40. doi: 10.1007/BF00248277. [DOI] [PubMed] [Google Scholar]
- Doesburg S.M., Vidal J., Taylor M.J. Reduced theta connectivity during set-shifting in children with autism. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J., Vogel E.K., Lansner A., Bergström F., Nyberg L. Neurocognitive architecture of working memory. Neuron. 2015;88(1):33–46. doi: 10.1016/j.neuron.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freunberger R., Fellinger R., Sauseng P., Gruber W., Klimesch W. Dissociation between phase-locked and nonphase-locked alpha oscillations in a working memory task. Hum. Brain Mapp. 2009;30(10):3417–3425. doi: 10.1002/hbm.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Funahashi S., Bruce C.J., Goldman-Rakic P.S. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S., Bruce C.J., Goldman-Rakic P.S. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J. Neurosci. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. https://doi.org/0270-6474/93/131479-19$05.00/O [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J.M., Alexander G.E. Neuron activity related to short-term memory. Science. 1971;173(3997):652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Fuster J.M., Jervey J.P. Neuronal firing in the inferotemporal cortex of the monkey in a visual memory task. J. Neurosci. 1982;2:361–375. doi: 10.1523/JNEUROSCI.02-03-00361.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole S.E., Pickering S.J. Assessment of working memory in six- and seven-year-old children. J. Educ. Psychol. 2000;92(2):377–390. [Google Scholar]
- Gazzaley A., Rissman J., D’Esposito M. Functional connectivity during working memory maintenance. Cogn. Affect. Behav. Neurosci. 2004;4(4):580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Geier C.F., Garver K., Terwilliger R., Luna B. Development of working memory maintenance. J. Neurophysiol. 2009;101(1):84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Cellular basis of working memory. Neuron. 1995;4(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Klimesch W., Sauseng P., Gruber W., Doppelmayr M., Freunberger R., Pecherstorfer T. Visual discrimination performance is related to decreased alpha amplitude but increased phase locking. Neurosci. Lett. 2005;375(1):64–68. doi: 10.1016/j.neulet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W. Spatiotemporal oscillatory dynamics during the encoding and maintenance phases of a visual working memory task. Cortex. 2015;69:121–130. doi: 10.1016/j.cortex.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höller-Wallscheid M.S., Thier P., Pomper J.K., Lindner A. Bilateral recruitment of prefrontal cortex in working memory is associated with task demand but not with age. Proc. Natl. Acad. Sci. 2017;114(5):E830–E839. doi: 10.1073/pnas.1601983114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L.-T., Ekstrom A.D., Ranganath C. Neural oscillations associated with item and temporal order maintenance in working memory. J. Neurosci. 2011;31(30):10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 2010;4 doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O., Gelfand J., Kounios J., Lisman J.E. Oscillations in the alpha band (9-12 Hz) increase with memory load during retention in a short-term memory task. Cereb. Cortex. 2002;12(8):877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jokisch D., Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J. Neurosci. 2007;27(12):3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J., Schumacher E.H., Smith E.E., Koeppe Ra, Awh E., Reuter-Lorenz Pa, Marshuetz C., Willis C.R. The role of parietal cortex in verbal working memory. J. Neurosci. 1998;18(13):5026–5034. doi: 10.1523/JNEUROSCI.18-13-05026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L.G. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23(1):315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Training of working memory in children with ADHD. J. Clin. Exp. Neuropsychol. 2002;24(6):781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Koenigs M., Barbey A.K., Postle B.R., Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J. Neurosci. 2009;29(47):14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette M., Quraan M., Cheyne D. Evaluation of multiple-sphere head models for MEG source localization. Phys. Med. Biol. 2011;56:5621–5635. doi: 10.1088/0031-9155/56/17/010. [DOI] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoenchephalography forward calculation in realistic volume conductors. Phys. Med. Biol. 2003;48(22):3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Macoveanu J., Tegnér J., Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb. Cortex. 2007;17(5):1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:1–9. doi: 10.1155/2011/156869. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S., Palva J.M. New vistas for α-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Palva J.M., Monto S., Kulashekhar S., Palva S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. 2010;107(16):7580–7585. doi: 10.1073/pnas.0913113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang E.W. Practical aspects of running developmental studies in the MEG. Brain Topogr. 2011;24(3–4):253–260. doi: 10.1007/s10548-011-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Gutierrez E., Bandettini P., Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35(5):975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Lopes Da Silva F.H. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110(11):1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Rogalsky C., Matchin W., Hickok G. Broca’s area, sentence comprehension, and working memory: an fMRI Study. Front. Hum. Neurosci. 2008;2(October):14. doi: 10.3389/neuro.09.014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C., Langner R., Dogan I., Reetz K., Laird A.R., Schulz J.B., Fox P.T., Eickhoff S.B. Modelling neural correlates of working memory: a coordinate-based meta-analysis. NeuroImage. 2012;60(1):830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F., Uhlhaas P.J. Working memory and neural oscillations: alpha gamma versus theta gamma codes for distinct WM information? Trends Cogn. Sci. 2014;18(1):16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Rowe J.B., Toni I., Josephs O., Frackowiak R.S., Passingham R.E. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Sakai K., Rowe J.B., Passingham R.E. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat. Neurosci. 2002;5(5):479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Sauseng P., Klimesch W., Doppelmayr M., Pecherstorfer T., Freunberger R., Hanslmayr S. EEG alpha synchronization and functional coupling during top-down processing in a working memory task. Hum. Brain Mapp. 2005;26(2):148–155. doi: 10.1002/hbm.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack B., Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci. Lett. 2002;331(2):107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Sreenivasan K.K., Curtis C.E., D’Esposito M. Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci. 2014;18(2):82–89. doi: 10.1016/j.tics.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipacek A., Grabner R.H., Neuper C., Fink A., Neubauer A.C. Sensitivity of human EEG alpha band desynchronization to different working memory components and increasing levels of memory load. Neurosci. Lett. 2003;353(3):193–196. doi: 10.1016/j.neulet.2003.09.044. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Mills T., Pang E.W. The development of face recognition; Hippocampal and frontal lobe contributions determined with MEG. Brain Topogr. 2011;24(3–4):261–270. doi: 10.1007/s10548-011-0192-z. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Donner E.J., Pang E.W. fMRI and MEG in the study of typical and atypical cognitive development. Neurophysiol. Clin. Neurophysiol. 2012;42(1–2):19–25. doi: 10.1016/j.neucli.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazover B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urbain C., Vogan V.M., Ye A.X., Pang E.W., Doesburg S.M., Taylor M.J. Desynchronization of fronto-temporal networks during working memory processing in autism. Hum. Brain Mapp. 2016;37(1):153–164. doi: 10.1002/hbm.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen B.D., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vinck M., Oostenveld R., Van Wingerden M., Battaglia F., Pennartz C.M.A. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. NeuroImage. 2011;55(4):1548–1565. doi: 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- von Stein A., Chiang C., Konig P. Top-down processing mediated by interareal synchronization. Proc. Natl. Acad. Sci. 2000;97(26):14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WASI -II: wechsler abbreviated scale of intelligence - second edition. J. Psychoeduc. Assess. 2013;31(3):337–341. [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E. On the use of correlation as a measure of network connectivity. NeuroImage. 2012;60(4):2096–2106. doi: 10.1016/j.neuroimage.2012.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.