Abstract
To better characterize the neural correlates of the full spectrum of reading ability, this fMRI study examined how variations in reading ability correlate with task-based brain activity during reading among a large community sample of adolescents (N = 234). In addition, complimentary approaches taking advantage of empirical as well as independent meta-analytic information were employed to isolate neural substrates of domain-general executive processes that are predictive of reading ability. Age-related differences in brain activity were also examined. Better reading was associated with increased activation in left anterior and inferior temporal regions and parts of orbitofrontal cortex, along with reduced activation in the thalamus and left frontal eye field (FEF). Converging evidence suggests that FEF activity corresponds to executive processes during reading. In contrast, activity in temporal regions is likely to reflect cognitive processes specific to reading. Older adolescents also demonstrated increased activation in an orbitofrontal region that overlaps with the aforementioned age-independent, reading-related regions, along with reduced activity in parietal and occipital regions. These results suggest that comparedto poor readers, proficient readers benefit from efficient reading-specific processes and require less executive effort, implemented via the FEF, during a reading comprehension task.
Keywords: Executive Function, Reading, Frontal eye field, fMRI, Individual differences
1. Introduction
There is wide variability among individuals in their ability to comprehend written language. Understanding the mechanisms of such individual differences is of considerable theoretical and practical importance. For example, low reading ability is associated with a host of negative outcomes including the likelihood of academic and occupational underachievement or the development of mental disorders (e.g., Goldston et al., 2007). Although substantial progress has been made in understanding the general cognitive and neural mechanisms of reading comprehension (for reviews, see Landi et al., 2013; Price, 2012), much less is known regarding the neural substrates that underpin individual differences in reading ability. In this study, we therefore examined how neural activity changes as a function of individuals’ reading ability, as well as the roles of domain-general executive processes vs. reading-specific processes in influencing these individual differences.
Reading comprehension is generally supported by a typically left lateralized language network, the core of which includes the inferior frontal gyrus (IFG) and the middle and superior temporal gyri (Ferstl et al., 2008a,b; Friederici and Gierhan, 2013; Martin et al., 2015). After lower-level visual features are processed by occipital visual areas, prelexical word form recognition is accomplished primarily by the visual word form area (Dehaene and Cohen, 2011). Multiple regions of the core language network, which spread across posterior and anterior regions of the left hemisphere, are involved in higher-level reading processes like accessing sematic information and syntactic analysis (Dronkers et al., 2004; Friederici, 2011; Price, 2012). To achieve comprehension, domain-general processes like working memory and executive functions are also essential (more details below; e.g., Fedorenko and Thompson-Schill, 2014).
As for determining neural activity associated with differences amongst individuals in reading ability, most neuroimaging studies so far have adopted a group-differences approach, examining variations in the pattern of brain activity between two (or more) groups of participants that differ in their level of reading skill. This comparison is most often between dyslexic (or poor) readers and a control group of average readers. People with dyslexia exhibit reduced activation in portions of the left language network mentioned above, including inferior temporal regions, fusiform gyrus, and temporo-parietal regions (Maisog et al., 2008; Norton et al., 2015; Paulesu et al., 2014). Such reduced activation is thought to reflect deficits in phonological and orthographic processing associated with dyslexia. Furthermore, a meta-analysis suggested that individuals affected by dyslexia also showed reduced activation in the fronto-parietal network (FPN), suggesting potential deficits in the executive system as well (Paulesu et al., 2014).
Very few studies have examined brain activity as a function of levels of reading ability in a continuous manner among members of a non-clinical population during performance of a reading-related task. Moreover, the information obtained so far is not consistent. For example, in two studies of this nature, Prat and colleagues showed in one study that adults with smaller vocabulary size had increased activity in right paracentral lobule during sentence reading (Prat and Just, 2011), and in another that such individuals had increased activity in right hemisphere homologues of left hemisphere language areas while reading passages that require causal inference (Prat et al., 2011). Welcome and Joanisse (2012) have also reported that adults with higher reading ability show less activity in the right subgenual anterior cingulate cortex and the left middle temporal gyrus during word recognition.
Even fewer studies have examined this issue of the neural substrates underlying individual differences in reading ability specifically among adolescents. In the only study we know to have addressed this issue, Ryherd et al. (2018) examined multivariate brain patterns during a reading task and observed that reading ability was positively related to activity in the left language network, but negatively related to activity in the executive network.
In the present study, we intentionally focus on adolescent readers as we wished to determine how individual variation in patterns of brain activation during adolescence is related to reading proficiency. Previous neuroimaging studies of this nature have predominantly focused on either earlier ages (<13 years old), when children are developing basic reading skills, or adulthood, when reading is likely quite proficient (>20 years old). Adolescence, however, is an important developmental period during which key aspects of an individual’s reading skills continue to develop. For instance, adolescents’ vocabulary and word-level skills grow steadily (Nippold, 2007; Perfetti et al., 2005) while the fluent word recognition functions of the visual word form area become increasingly refined (Schlaggar and McCandliss, 2007). Adolescents also begin to master higher-level reading skills like complex syntax and figurative language, as well as the abilities to appreciate texts’ social context and synthesize multiple viewpoints. To better understand these developmental changes during adolescence, a sub-goal of this study was also to determine the degree to which age affects patterns of reading-related brain activation during this developmental period.
Another goal of the study was to examine the degree to which neural activation associated with domain-general executive processes (EP) might influence individual differences in reading ability. Domain-general executive processes have been argued to be a major contributor to differences in individuals’ reading abilities (Cartwright, 2012; Gernsbacher, 1997; Wagner and Sternberg, 1987). These domain-general abilities are supported by the frontoparietal network (i.e., dorsal lateral prefrontal cortex, DLPFC, anterior cingulate cortex, and inferior parietal lobule; Banich, 2009; Botvinick et al., 2001) and the dorsal attention network (i.e., frontal eye field, FEF, and regions along the intraparietal sulcus; Corbetta and Shulman, 2002; Fox et al., 2006). Multiple studies have shown that individuals with better executive abilities tend to be stronger readers as well (Columbus et al., 2015; Kieffer et al., 2013; Protopapas et al., 2007; van der Sluis et al., 2007). Deficits in executive processes may also be an important etiological factor among individuals with specific-reading comprehension disorder, who have a selective deficit in reading comprehension with intact word recognition abilities (for a review, see Hudson et al., 2016).
Executive processes may also interact with processes that are specific to the reading domain. For instance, the verbal efficiency theory proposes that the amount of domain-general executive resources available for higher-level comprehension processes while reading is determined by individual’s word identification efficiency (Perfetti, 1985, 1988). According to this theory, one would expect reversed activation patterns in executive brain regions and reading-specific regions. As such, an important sub-goal of this study was to examine the neural mechanisms through which executive and reading-specific processes contribute to variations in reading ability.
In summary, we sought to identify the neural substrates activated during a reading comprehension task that are associated with adolescents’ reading ability, as determined via a comprehensive behavioral battery among a large sample of adolescents (N = 234 after quality assurance). A potential explanation for the inconsistent results across prior studies of the neural substrates of individual differences in reading is that these studies were consistently underpowered (N<33). For a correlational analysis of this nature, however, a sufficient sample size is essential to obtain robust and reliable effects (Yarkoni, 2009). We also examined the degree to which reading-related neural activity changes over the course of adolescence and how the neural substrates of domain-general executive processes correlate with individual differences in reading ability (see Analytic Strategy in Method).
We predicted that a lower-level of reading ability would be associated with hypoactivation within a subset of left language network regions, mainly involving middle and inferior temporal gyrus, primarily based upon results from studies of poor readers compared to control participants. We also predicted that lower reading ability would be associated with an increased need to engage executive resources in order to adequately comprehend text, which would be associated with increased activation within the frontoparietal network and the dorsal attention network. We also expected that such activation might decrease within the adolescent time period, as youth become more fluent readers.
2. Method
2.1. Analytic strategy
The primary goal of the study was to determine task-based neural correlates of individual differences in reading ability among a large sample of adolescents. To this end, we used a covariate analysis to determine the degree to which brain activity during a reading comprehension task (contrast: passage reading vs. symbol reading; see supplemental material, SM 1) was associated with individual differences in reading ability. As an adjunct, to identify brain correlates of individual differences in executive ability, activity during an N-back task (Contrast: 2-back vs. 0-back; see SM 1) was also examined for its associations with executive functioning behavior. While these analyses controlled for age differences among participants to find patterns of association independent of age, additional analyses were also performed to determine age-related patterns of activation for each task.
Another goal of the study was to examine whether the neural underpinnings of domain-general executive processes are associated with individual differences in reading ability. We did so via three complimentary approaches. In the first approach, we classified regions identified in the reading covariate analysis (i.e., whose activity is related to individual differences in reading ability) into domain-general executive regions and reading-specific regions. This was achieved by using the meta-analytic tool of Neurosynth (Yarkoni et al., 2011) to generate reading-specific and executive maps, which then were used to mask the reading covariate effects. In the second approach, the conjunction map of the reading covariate effect and the N-back covariate effect was obtained, thereby identifying regions whose task-state activity is predictive of both reading and executive abilities. We inferred that these regions are likely supporting the domain-general executive processes of interest. In the third approach, we examined the covariate effect of executive ability on activation during the reading task. This result provides information on the how adolescents with different executive abilities utilize various brain regions in the context of a reading task. If the results of these three approaches converged on one or more regions, then it would provide strong evidence supporting the existence of domain-general executive regions that are predictive of individuals’ reading ability.
2.2. Participants
Participants for the study were drawn from the Learning Disabilities Innovation Hub Sample, which is based upon a collaboration between The Ohio State University, Case Western Reserve University, the University of Colorado Boulder and Vanderbilt University. The sample for the current image analyses consisted of 324 healthy individuals (mean age = 16.92, SD = 1.56; age range, 13.33–23; male = 146; 79 MZ twin pairs, 73 same-sex DZ twin pairs and 20 singletons). After the quality assurance procedures (see procedure details in supplementary material, SM, 3.2), the final sample size was 234 for the reading task (107 male, mean age = 17.13, SD = 1.56, 51 MZ twin pairs, 36 same-sex DZ twin pairs, and 60 singletons) and 249 for the N-back task (113 male, mean age = 17.06, SD = 1.57, 53 MZ twin pairs, 45 same-sex DZ twin pairs, and 53 singletons). All participants had normal or corrected-to-normal vision and had no history of neurological disorders. Informed consent was obtained from each family prior to participation, and all study procedures were approved by the Institutional Review Board at The Ohio State University, Case Western Reserve University Social/Behavioral Institutional Review Board, and the University of Colorado Boulder Institutional Review Board.
2.3. Behavioral assessments
Participants completed the behavioral tests described below in a counter-balanced fashion in which each twin completed the behavioral measures or imaging session first and then switched with his or her co-twin.
2.3.1. Reading assessments
Reading achievement was measured using four assessments. The Woodcock Reading Mastery Test - Revised (WRMT-R) Word Identification and Word Attack subtests (Woodcock, 1998) measures decoding of real and pseudo-words, respectively. The Passage Comprehension subtest of the WRMT-R is a cloze form task in which participants read a sentence or short passage and provide a semantically-appropriate word. WRMT-R tasks have reported reliabilities of 0.84 – 0.98 for the Word Identification and Word Attack subtests in the age groups included in the present study and 0.68 – 0.92 for the Passage Comprehension subtest. In addition to the Word Comprehension subtest (not administered), these three measures make up the WRMT-R Total Reading score, which demonstrates high concurrent reliability with Peabody Individual Achievement Test (PIAT) Reading composite (0.87), Woodcock-Johnson Reading Achievement score (0.88 – 0.90), and the Woodcock Reading Achievement Test (WRAT) Reading composite (0.90 – 0.92). A timed measure of reading was also obtained with the Test of Word Reading Efficiency, Second Edition (Torgesen et al., 2012). In the Phonemic Decoding Efficiency subtest, participants had 45 s to read as many pseudo-words as they could from a list. Average reported reliability for the TOWRE-2 subtest ranges from 0.90 – 0.93. Test performance was broadly normative among the sample of 234 individuals whose reading-related imaging data passed quality control, with narrower standard deviations due to the non-independence of twins; standard score M (SD) [Range] were as follows: Word Identification: 96.9 (7.2) [71–114]; Word Attack: 95.0 (10.0) [68–135]; Passage Comprehension: 105.5 (8.4) [82–133]; and Phonemic Decoding Efficiency: 95.4 (9.5) [64–119].
A latent reading factor score was made from the four reading measures’ normative scores. Correlations between these measures ranged from r = 0.40 to 0.66 (M = 0.54; SD = 0.12) within the sample of 234 individuals whose reading comprehension imaging data survived quality control. The reading factor displayed a close fit to the data (X2(1) = 1.6, p = .21; CFI = 0.99; RMSEA = .04), with standardized loadings of 0.95, 0.66, 0.65, and 0.75 for the measures listed in the order above.
2.3.2. Executive function assessments
Factor scores were calculated for parent-based executive functioning ratings on the Behavior Rating Inventory of Executive Function (BRIEF) Initiate, Working Memory, Plan/Organize, and Monitor subscales. Of note, these subscales comprise four of the five measures that constitute the BRIEF metacognition index. Correlations between these subscales ranged from r = 0.62 to 0.82 (M = 0.71; SD = 0.07) within the sample of 249 individuals whose N-back imaging data survived quality control. The latent executive functioning factor displayed a close fit to the data (X2(2) = 4.6, p = 0.10; CFI = 0.99; RMSEA = 0.07), with standardized loadings of 0.78, 0.87, 0.95, and 0.77 for the subscales listed in the order above. With regards to the BRIEF’s reliability, the normative samples’ inter-rater and test-retest reliabilities (average 5 weeks) are .57 and 0.87, respectively. Internal consistency (Cronbach's alpha) are: Metacognitive Index, a = 0.96; Initiate subscale; a = .82, Working Memory subscale: a = .92; Plan/Organize subscale: a = .91; Monitor subscale: a = .85. With the exception of a slightly decreased mean and variance on the Monitor index of the BRIEF, parent ratings of EF were also broadly normative among the sample of 249 individuals whose N-back imaging data passed quality control, with narrower standard deviations due to the non-independence of twins; T-score M (SD) [Range] for each of the indices, reverse scaled so that, like the reading measures, higher scores represented better executive functioning behavior, were as follows: Initiate: 51.0 (9.3) [17–64]; Working Memory: 50.9 (9.3) [23–60]; Plan/Organize: 52.4 (7.4) [35–62]; and Monitor: 44.2 (5.1) [29–52].
2.4. Imaging analysis
After acquisition (SM 2), MRI images were preprocessed (SM 3.1) with FSL version 5.0.8 (analysis group, FMRIB, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl/), quality controlled (SM 3.2) and went through lower-level processing (SM 3.3).
2.4.1. Higher-level GLM analyses
Covariate effects reflecting individual differences in reading ability (characterized by the reading factor score) and executive abilities (characterized by the executive functioning factor score) were obtained via a higher-level GLM. For statistical inference, we adopted a multi-level block permutation method (Winkler et al., 2015) implemented in FSL's PALM function (Winkler et al., 2014). Due to the gender differences reported in the literature, data from males and females were treated as different variance groups, allowing separate estimation of variance for each group (via the “vg” argument of PALM). In the design matrix, EVs (explanatory variables or regressors) modeling average activation and covariate of mental ability (EP factor scores for N-back task and reading factor scores for reading task) were included for each of the male and female groups separately. Standardized (Z-transformed) age was included as a confounding EV for the covariate analyses. As noted above, we also examined the effect of age in another analysis that did not take individual differences in levels of abilities into account.
To account for non-independence due to the family structure of this sample, nested exchangeability blocks were defined that restricted permutations to the same family type. Specifically, during the permutation process, a twin pair as a whole can only switch places with another twin pair of the same type (MZ or DZ). In addition, the two co-twins within a twin pair can also switch places. In this manner, family structure was accounted for without directly modeling these complicated repeated-measures factors. 5000 permutations were performed. Covariate effects averaged across males and females are reported. Results of group average activations are reported in another paper (Wang et al., 2019). This paper focuses on the reading- and executive-related covariate effects.
We considered three methods for correction of voxel-wise multiple comparisons across all voxels in the brain as well as multiple comparisons across multiple contrasts using the same data. In all cases p values were determined through permutation testing at the voxel level using the PALM tool in FSL, taking into account the twin structure in the data via exchangeability blocks as described above. The three multiple comparisons approaches were: family-wise error rate (FWER) correction at p < 0.05, false discovery rate error (FDR) correction at p < 0.05, and an uncorrected p < 0.005 voxel-wise threshold along with a minimal cluster extent threshold of 10 voxels. None of the results survived the FWER correction, which can be quite conservative and of low power (Logan and Rowe, 2004). The latter two methods yielded substantial significant results. The FDR threshold results are reported throughout the manuscript, as it is a well-recognized method (Poldrack et al., 2008).
2.5. Reliability analysis
Split-half reliability of the main and covariate effects were also examined. For both the N-back and reading tasks, the samples were divided into two sub-samples matched for reading ability, executive ability, age and gender. Each co-twin was assigned to a separate sub-sample so that there is no family structure within each group. Using the same permutation procedure utilized for the main analyses, reliability was defined as the voxel-wise correlation of the raw probability maps between the two sub-samples (calculated via FSL command “fslcc”).
2.6. Examining the role of executive processes in individual reading ability
Three complimentary approaches were employed to examine the neural correlates of domain-general executive processes that are predictive of individuals’ reading ability. Each method is explained in more detail below.
2.6.1. Masking reading covariate effects with Neurosynth maps
First, we utilized the meta-analytic tool of Neurosynth (http://www.neurosynth.org/; Yarkoni et al., 2011), which integrates information from hundreds of studies and covers a broad range of operational definitions of specific concepts, to separate the executive- vs. reading-specific components of reading ability-related brain activation. Here we used reverse (rather than forward) inference maps from Neurosynth in order to provide a selective map of activation related to a specific mental operation. In contrast, forward inference maps show all brain regions activated by a mental operation of interest, even if that brain region (e.g., visual cortex) is activated by a myriad of operations. We then used these two masks to determine which portions of the reading covariate map are likely driven by reading-specific versus executive processes.
We utilized the 200-word topic sets extracted with a standard topic modeling approach from the abstracts of all articles in the Neurosynth database as of July 2015 (11,406 articles). We utilized topic 196 for reading (N = 314 studies) and topic 022 for executive aspects of working memory (N = 485 studies). Reverse inference maps for each of these two topics were downloaded from the Neurosynth website. We then determined which regions in the reading Neurosynth map (minus those for the Neurosynth EF map) were activated in the reading covariate map. Regions identified by this process should be those regions in the reading covariate map that are likely driven by reading-specific processes. Similarly, the overlapping regions between the executive topic Neurosynth map and the reading covariate results are likely driven by executive processes.
2.6.2. Conjunction of covariate effects
The assumption for this analysis was that domain-general executive regions should be important in predicting both reading ability and executive ability. The appropriate method to conduct a conjunction analysis has been a topic of considerable debate. Nichols et al. (2005) argued that all the comparisons in the conjunction should be individually significant, so that a proper null hypothesis of logical “AND” is tested. However, Friston et al. (2005) suggested that such an approach results in “a very conservative procedure, particularly in the context of multiple comparisons” and is “generally unnecessary”. We therefore reported results under both standards. When applying the later standard to identify such regions, we identified those regions that were significant both in the map of brain activation during the reading task that co-varied with reading ability and in the map of brain activation during the N-back task that covaried with executive ability. Each map was individually thresholded at α < 0.0707 (square root of 0.005, so that the conjunction effect has a significance level of 0.005; Friston et al., 2005), binarized, and multiplied via FSL command “fslmaths” to obtain common areas between the two maps. Local peaks of averaged T values are reported for these common regions.
2.6.3. Covariate effect of executive ability in reading task
In the third approach, we examined the extent to which individuals with different levels of executive ability demonstrate varying brain activity during the reading task. Individuals’ executive ability, as assessed by parent-rated executive functioning behavior, was entered into the higher-level covariate GLM model of the reading task, which also contained explanatory variables of age and the group average. The same group-level analytical procedures from the other covariate analyses were applied.
3. Results
3.1. Behavioral results
Participants achieved a high degree of comprehension, as was reflected by an average accuracy rate of 85.5% (S.D. = 16.6%) for the picture-judging trials in the reading task. Moreover, reading ability, as assessed by the reading factor score, was not related to accuracy on the picture-judging trials, r(232) = 0.06, p = 0.40, suggesting that readers with different levels of reading ability all exhibited similar levels of comprehension on our neuroimaging reading task. The average hit rate for repeated stimuli was 92.4% (S.D. = 9.7%) and average false alarm rate is 1.1% (S.D. = 3.7%).
For the N-back task, accuracy in the 0-back condition (mean = 93.5%, S.D. = 7.4%) was significantly higher than that in the 2-back condition (mean = 91.0%, S.D. = 8.2%), t(601) = 4.21, p < 0.001.
3.2. Main effects
The effects for the contrast of interest from each task are reported in Wang et al. (2019), and the resulting maps are reproduced in the SM. Patterns of main effects from the two tasks were robust and consistent with patterns observed in the literature (SM 4). The typical language network was activated in the reading task (SM Fig. 1), and the fronto-parietal network was activated in the N-back task (SM Fig. 2). Split-half reliabilities between the two subgroups for the reading task and the N-back task main effects were 0.92 and 0.95, respectively.
3.3. Covariate effects
As noted above, no results survived FWE correction (Winkler et al., 2014), which can be overly conservative and of low power (Logan and Rowe, 2004). Substantial amounts of activation were observed under FDR correction at p < 0.05 (Fig. 1) and using uncorrected threshold of p < 0.005 with a cluster extent threshold of 10 voxels. Here, we primarily focus on the FDR corrected results.
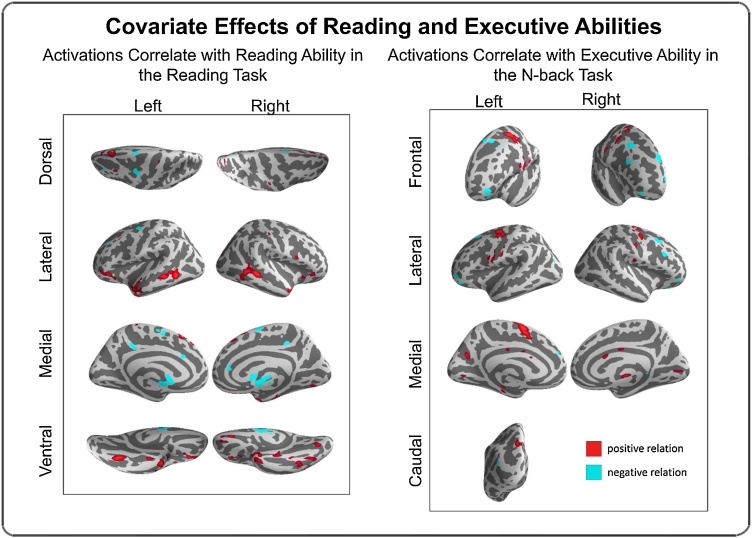
Fig. 1.
Covariate effects reflecting brain activation correlates of reading ability (left) and executive control ability (right). Regions in red exhibited a positive relation with ability (i.e., greater activation, greater ability), whereas regions in cyanshowed a negative relation with ability (i.e., less activation, greater ability). All depicted effects passed voxel-wise FDR correction (p < 0.05) for multiple comparisons (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.3.1. Reading covariate effects
Activation in large areas of the bilateral ventral frontal and temporal lobes exhibited a positive correlation with reading ability (i.e., greater reading ability was associated with greater activation). These regions included the middle temporal gyrus, temporal pole, fusiform, and orbital frontal gyrus (Fig. 1 left; SM Table 1). Negative relationships between brain activation and reading ability (i.e., lower activation related to better reading ability) were found for dorsal portions of left FEF, bilateral supplementary motor area, and the thalamus (Fig. 1 left; SM Table 1). See SM Table 1 for a complete list of results. Split-half reliability for the reading task covariate effect was 0.71.
3.3.2. N-back covariate effects
Areas where greater brain activation was correlated with better executive functioning behavior included bilateral FEF, the left supplemental motor area, and a variety of other regions distributed across the cortex (Fig. 1 right; SM Table 2). Negative covariate effects, in which better executive functioning behavior was correlated with lower level of activity, were observed in bilateral OFC, bilateral superior frontal regions, and the cerebellum (Fig. 1 right; SM Table 2). Split-half reliability for the N-back task covariate effect was 0.77.
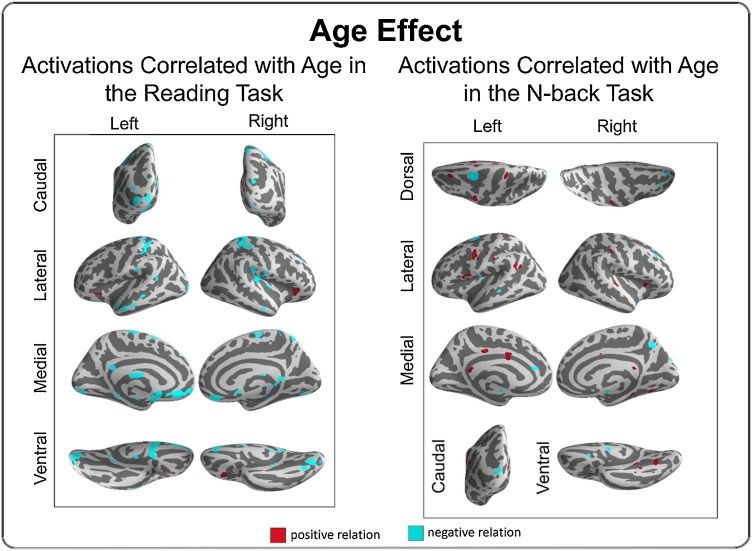
3.3.3. Age effects
In the reading task, older adolescents showed greater activation in bilateral OFC, as well as decreased activation in postcentral gyrus, superior parietal lobe, and the insular and occipital cortex (Fig. 2 and SM table 3). In the N-back task, older adolescents demonstrated greater activation in the left postcentral and precentral gyrus, right fusiform gyrus, and portions of middle and posterior cingulate cortex, as well as decreased activity in left FEF, cerebellum, and precuneus (Fig. 3 and SM table 4). Of note, this age-related left FEF region overlapped with the executive regions associated with reading ability (see below).
Fig. 2.
Covariate effects reflecting brain activation correlates of age in the reading (left) and N-back tasks (right). Regions in red have a positive relation with age (greater activation, older age), whereas regions in cyan have a negative relation with age (less activation, older age). All effects depicted passed voxel-wise FDR correction (p < 0.05) for multiple comparisons (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
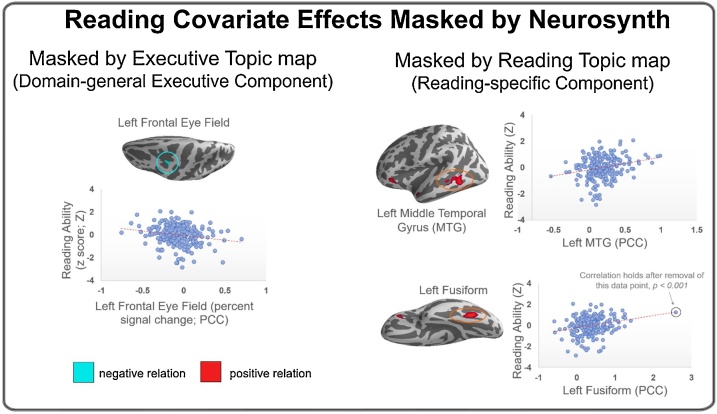
Fig. 3.
Reading covariate effects masked by Neurosynth topic maps of executive processes (left) and reading comprehension (right). Regions in red showed a positive relation with ability (i.e., greater activation, greater ability), whereas regions in cyan had a negative relation (i.e., less activation, greater ability). Scatter plots show reading ability versus percent signal change (averaged over a 10 mm sphere centered at the local peak) of the reading comprehension contrast in the reading task for the three regions with relatively large cluster sizes (highlighted in blue and red circles) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.4. Examining the role of executive processes in individuals’ reading ability
3.4.1. Masking reading covariate with Neurosynth maps
Masks generated with the Neurosynth reverse inference topic maps were applied to the reading covariate effect map to isolate domain-general executive regions and reading-specific regions. The only region that was identified when masked by the Neurosynth map for executive function was a portion of the FEF, for which higher activation was associated with reduced reading ability (Fig. 3 left; SM Table 5). The regions identified in the reading covariate map identified when masked by the Neurosynth map for reading included in the left middle temporal gyrus, orbital frontal gyrus, fusiform, and temporal pole, in which greater activation was associated with better reading ability (Fig. 3 right; SM Table 5).
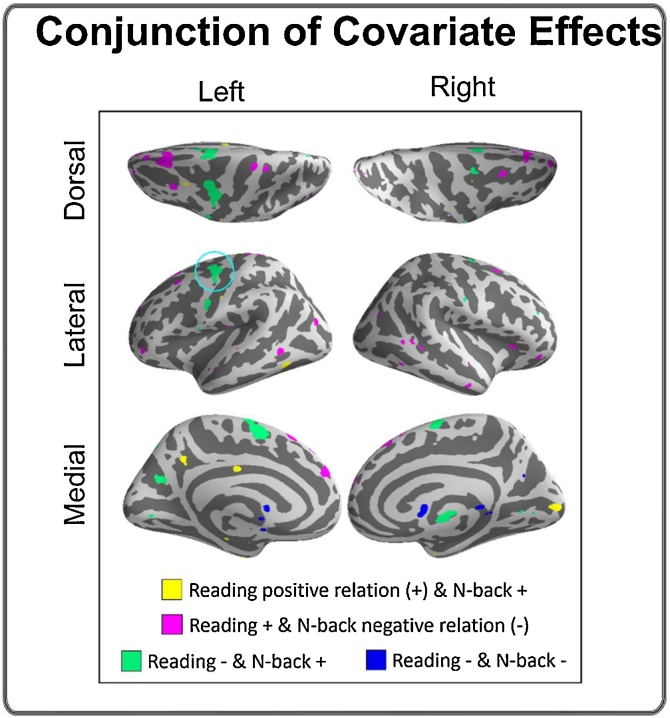
3.4.2. Conjunction of covariate effects
Significant results were observed only under the threshold proposed by Friston et al. (2005). The conjunction of the reading covariate effect (of reading ability) and the N-back covariate effect (of executive ability) revealed regions whose activity is predictive of both reading and executive behavior and may therefore be domain-general in nature. Regions revealed by the conjunction method are displayed in Fig. 4 and SM Table 6. Most of these regions demonstrated less activation with better reading ability and more activation with better executive ability. They included the left FEF, bilateral supplementary motor area, and right thalamus. Other notable conjunctions were observed for regions that showed greater activation with better reading ability and less activation with better executive ability. These included bilateral supplementary motor area, left middle temporal gyrus, and left cerebellum.
Fig. 4.
Conjunction of the reading and N-back covariate effects. “+” = positive covariate effect. “-” = negative covariate effect. Thus, “Read+ & N-back+” shows the common regions between the positive reading covariate effect and the positive N-back covariate effect.
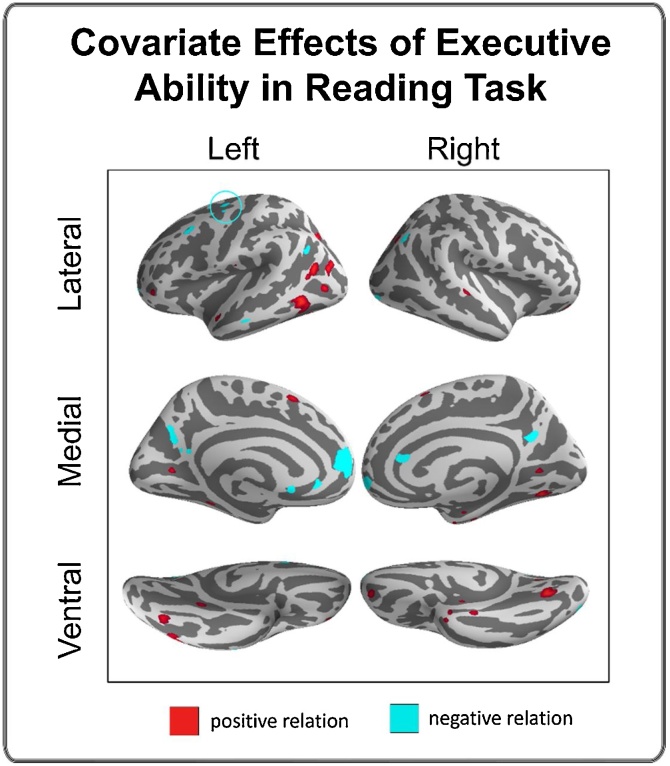
3.4.3. Covariate effect of executive ability in reading task
This covariate analysis captures regions that were differentially employed by adolescents with different executive abilities in the context of the reading task. Adolescents with higher executive ability showed lower levels of activation in FEF, DLPFC and medial aspects of orbital frontal gyrus (Fig. 5 and SM Table 7) during reading. Higher executive ability was also associated with greater activation in ventral and posterior aspects of the inferior temporal gyrus, including the visual word form area, as well as lateral and medial aspects of the occipital lobe during reading.
Fig. 5.
Covariate effects reflecting brain activation correlated with executive ability in the reading task. Regions in red have a positive relation with ability (i.e., greater activation, greater ability), and regions in cyan have a negative relation with ability (i.e., less activation, greater ability). All depicted effects passed voxel-wise FDR correction (p < 0.05) for multiple comparisons (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.5. Correlational analysis of behavioral performance and FEF activity
We subsequently performed a correlational analysis of the relationships between behavioral performance and left FEF activity, which our results convergently indicated to reflect executive processes associated with reading ability. Results showed that overall reading and executive skills, as reflected by factor scores, are both correlated with one another and related to FEF activity, thereby corroborating the above imaging analyses (Table 1). Overall, both factor scores were correlated with in-scanner performance. Performance on the two in-scanner tasks were also correlated with one another. Age was also related to the speed of response to repeated stimuli in the reading task.
Table 1.
Correlations of performance across behavioral tasks, FEF activity, and age.
| EF_fs | Read_fs | L_FEF | age | 0-back_acc | 2-back_acc | Read_acc | Read_rpt | |
|---|---|---|---|---|---|---|---|---|
| EF_fs | ||||||||
| Read_fs | 0.36** | |||||||
| L_FEF | −0.16* | −0.19** | ||||||
| age | 0.01 | −0.11 | −0.05 | |||||
| 0-back_acc | 0.02 | 0.11 | 0.02 | 0.12 | ||||
| 2-back_acc | 0.17** | 0.30** | −0.07 | 0.09 | 0.70** | |||
| Read_acc | 0.11 | 0.22** | −0.08 | −0.01 | 0.14* | 0.16* | ||
| Read_rpt | 0.30** | 0.22** | −0.06 | 0.16* | 0.17* | 0.36** | 0.25** |
Note. EF_fs, factor score of executive ability; Read_fs, factor score of reading ability; L_FEF, percent signal change of FEF (10 mm sphere centered on [-36, -6, 50]) from the reading task; 0-back_acc, accuracy of 0-back trial; 2-back_acc, accuracy of 2-back trial; Read_acc, accuracy of picture judging trials in the reading task; Read_rpt, hit rate of repeated trials in the reading task.
p < 0.05.
p < 0.01.
4. Discussion
To better our understanding of neural mechanisms of individual differences in reading during adolescence, we identified brain regions whose activity is associated with individual differences in reading ability. In addition, we then isolated the regions whose level of activity are predictive of individual’s reading ability that are likely supported by domain-general executive processing as compared to domain-specific reading processing. We observed that proficient readers, as compared to poor readers, tended to show less activation in the left FEF, left SMA, and left thalamus. At the same time, proficient readers tended to show greater activation in the anterior, ventral, and middle parts of the left temporal lobe, as well as orbital frontal regions. Converging evidence from the three approaches used in the current study suggested that activation in the FEF is reflective of executive processes, whereas temporal and orbital frontal activation tended to reflect reading-specific processes.
Given that participants across the spectrum of reading ability reached similar levels of understanding during the fMRI reading comprehension task, we interpret their differing patterns of brain activation as likely reflecting differences in their cognitive processing during the reading of simple passages. More extensive inferior temporal lobe activation, including the VWFA, was observed for higher-level readers. Since these regions are known to be specialized in word-level processing, these results suggest that adolescents with higher reading ability rely more on these reading-specific regions while reading, perhaps reflecting increased efficiency and/or modularity within these regions. Meanwhile, proficient readers’ activation in executive regions (i.e., FEF, across three approaches, and DLPFC [Fig. 5]) was reduced, which may be indicative of less reliance upon higher-level executive resources.
In contrast, poorer readers may be less efficient at engaging reading-specific processes and instead rely upon executive processes to achieve adequate comprehension. Interestingly, activation of that same left FEF region that was linked to level of reading ability appears to also undergo developmental changes during adolescence, as the age effect on the N-back task revealed reduced FEF utilization among older individuals. Overall, these patterns of activation support the hypothesis that brain regions involved in both executive and reading-specific processes are important for reading comprehension. However, they also indicate that the degree of involvement of regions involved in executive processing may be related to the efficiency of reading-specific processes, as reflected by an individual’s reading ability.
This pattern echoes findings from a recent attempt to correlate reading ability with multivariate brain patterns during a reading task. Ryherd et al. (2018) found that reading ability was related to a brain pattern that loaded positively on activity in regions supporting passage comprehension, including the left fusiform, angular gyrus, and left anterior and inferior temporal gyrus, but loaded negatively on activity in traditionally executive regions, such as DLPFC and anterior cingulate cortex. Our study further suggests the possibility that poorer readers may utilize executive processes in order to compensate for their comparatively inefficient reading-specific processes. However, there seems to be a limited extent to which executive processes can facilitate reading, as no such pattern is observed across studies examining brain activation in dyslexic readers (Norton et al., 2015; Richlan et al., 2009).
A separate age-effect analysis revealed changes in reading- and executive-related brain activity that were at least partially attributable to maturation. During reading, older participants showed less activity in parietal, occipital, and insular regions, which are typically involved in sensory processing and integration. Older adolescents also demonstrated increased activity in orbital frontal regions that have been associated with memory processes, specifically generalizing information across distinct events (Zeithamova et al., 2012). Greater activation of this region may allow information processed during reading tasks to be linked more efficiently with previously acquired information, thereby aiding current comprehension.
Among the reading-specific regions identified by the Neurosynth approaches, the roles of the left fusiform and anterior temporal lobe are relatively well-established. The former is related to word form decoding (Dehaene and Cohen, 2011; McCandliss et al., 2003) and the latter is involved in access to semantic memory (Patterson et al., 2007; Pobric et al., 2007). Though often considered as parts of the language network (Ferstl et al., 2008a,b; Friederici and Gierhan, 2013), the specific roles of the left middle temporal gyrus and pars orbitalis of the left inferior frontal gyrus are less clear. Several recent studies have shown that these regions are critical for tasks that require making semantic decisions at the single-word level (Davey et al., 2016; Noonan et al., 2013; Whitney et al., 2011). Activation in the middle temporal gyrus may therefore be related to semantic access as well, much like the anterior temporal lobe, but at a lower (i.e., single-word) level. Lastly, pars orbitalis of the left inferior frontal cortex is considered to be important during higher-level language comprehension (Price, 2012; Saur et al., 2008).
Another noteworthy aspect of our results is that brain regions supporting a particular aspect of cognition, such as executive function, may not be the same regions that are sensitive to individual differences in levels of that cognitive ability. In a previous study, we used this same data set to examine brain regions underlying domain-general executive processes via a conjunction analysis of group main effects across the same reading comprehension and N-back tasks, as well as a number estimation task (Wang et al., 2019). The inferior frontal junction, VLPFC, and an inferior portion of the precentral gyrus were shown to be important for domain-general executive processes across these three tasks. In contrast, as shown in Fig. 2 (right-hand side), the executive regions identified in the current study are different than those identified in our previous analysis of this data. This finding suggests that brain mechanisms underlying individual differences in a cognitive domain may differ from the mechanisms that are engaged consistently across all individuals (Yarkoni and Braver, 2010).
As an important caveat, the executive process of working memory focused on in this study should not be confused with working memory capacity (WMC), which refers to the amount of information that can be temporarily held on-line for processing. It has been suggested that the process of reading can benefit from larger WMC (Just and Carpenter, 1992). Ideally, measurement of WMC should be relatively pure, without additional processing requirements, such as occurs in the digit span task, in which individuals are presented with a sequence of numerical digits and asked to recall them in order. However, multiple studies have used more complex span tasks to assess WMC that involve additional operations as well. One such task, the reading span task, requires participants to read aloud a series of sentences and then recall the final word of each sentence. It had been suggested that in addition to WMC, reading skill is also a major component of performance on the reading span task (Farmer et al., 2012; MacDonald and Christiansen, 2002). This task may therefore be suboptimal for assessing the relationship between reading ability and WMC. Studies where “pure” WMC was measured, (e.g., a forward digit span task or a word span task), typically find minimal or no correlation between reading ability and WMC (Daneman and Carpenter, 1980; Daneman and Merikle, 1996). Executive processes of working memory, however, have been found to predict unique variance in reading ability after controlling for WMC (Swanson and Howell, 2001).
4.1. Limitations and future directions
When interpreting the findings of the current study, some limitations must be kept in mind. First, while significant, the covariate effects reported in this study are generally of moderate effect size and can only explain a limited amount of variance in reading ability. This may be a general limitation of the precision of imaging studies involving individual differences, potentially due to variations in the degree and extent of brain activation across individuals. Second, the parent-rated measures used to create executive covariate scores are more reflective of real-world executive behavior than task-based measures of executive functioning (Toplak et al., 2013). Given that performance-based and parent-rated measures of executive processes assess somewhat different constructs (Mahone et al., 2002; Toplak et al., 2009), future studies would benefit from adopting a more extensive battery of executive function tests to both more fully capture various aspects of executive processes and permit comparison of these measures’ utility. Third, we might have observed a different pattern of results if we had used a more taxing reading comprehension task. More specifically, the task we used may not have been challenging enough to tax good readers’ executive processes. An interesting follow-up would be to test the relationship again with a more challenging task in which readers of differing ability demonstrate different levels of comprehension. In that case, activation in executive control regions might show a positive relationship with reading ability, as opposed to the negative relationship observed in the present study. Finally, while our sample did contain some twin pairs, the current sample size was underpowered for a formal twin design analysis. Follow-up studies with larger sample sizes would better enable estimation of the heritability of the effects reported in this study. Such studies would also afford insight into which components of the observed individual difference effects are more likely to be influenced by genes as compared to the environment.
5. Conclusion
The present study provides insights into the neural and cognitive basis of individual differences in reading performance among adolescents using a variety of converging approaches. In general, our results suggest that proficient readers tend to engage neural mechanisms supporting lower-level reading-specific processing skills, such as word-form decoding (via VWFA) and semantics (via middle and anterior temporal regions), which perhaps results in more automated reading. This engagement may preclude their need to employ additional executive resources, as indicated by reduced activity in the left FEF. Conversely, poor readers appear to require more top-down executive resources to ensure successful reading comprehension. The educational and clinical implications of these results may be that while it is important to develop specific reading skills, especially at earlier ages, it may be equally or even more important to somehow train, enhance, or scaffold the necessary executive processes for later adolescent readers who do not excel in reading.
Declarations of interest
None.
Author note
Kai Wang and Marie T. Banich, Institute of Cognitive Science, University of Colorado Boulder.
Daniel R. Leopold, Marie T. Banich, and Erik G. Willcutt, Department of Psychology and Neuroscience, University of Colorado Boulder.
Daniel R. Leopold, Andrew E. Reineberg, and Erik G. Willcutt, Institute for Behavioral Genetics, University of Colorado Boulder.
Laurie E. Cutting and Stephanie N. Del Tufo, Peabody College of Education and Human Development, Vanderbilt University.
Lee A. Thompson, Department of Psychological Sciences, Case Western Reserve University.
Frank J. Kanayet, John Opfer, Zhong-Lin Lu, and Stephen A. Petrill, Department of Psychology, The Ohio State University
Acknowledgments
Research reported in this paper was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number HD075460 (Petrill, P.I.). Data entry via the Redcap system was supposed by grant UL1TR001070 from the National Center for Advancing Translational Sciences. DRL is also supported by NICHD F31HD091967. This research was also supported by NICHD P50 HD027802 (Willcutt, Center P.I., Banich, P.I., Project 3). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also thank Kathy Pearson for her computational help with data archiving, quality assurance, and pre-processing.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100647.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Banich M.T. Executive function: the search for an integrated account. Curr. Dir. Psychol. Sci. 2009;18(2):89–94. [Google Scholar]
- Botvinick M.M., Braver T.S., Barch D.M., Carter C.S., Cohen J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108 doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Cartwright K. Insights from cognitive neuroscience: the importance of executive function for early reading development and education. Early Educ. Dev. 2012;23 [Google Scholar]
- Columbus G., Sheikh N.A., Côté-Lecaldare M., Häuser K., Baum S.R., Titone D. Individual differences in executive control relate to metaphor processing: an eye movement study of sentence reading. Front. Hum. Neurosci. 2015;8(1057) doi: 10.3389/fnhum.2014.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Daneman M., Carpenter P.A. Individual differences in working memory and reading. J. Verbal Learning Verbal Behav. 1980;19(4):450–466. [Google Scholar]
- Daneman M., Merikle P.M. Working memory and language comprehension: a meta-analysis. Psychon. Bull. Rev. 1996;3(4):422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- Davey J., Thompson H.E., Hallam G., Karapanagiotidis T., Murphy C., De Caso I. Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage. 2016;137:165–177. doi: 10.1016/j.neuroimage.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. The unique role of the visual word form area in reading. Trends Cogn. Sci. (Regul. Ed.) 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Dronkers N.F., Wilkins D.P., Van Valin R.D., Redfern B.B., Jaeger J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Farmer T.A., Misyak J.B., Christiansen M.H. Individual differences in sentence processing. In: Spivey M., Joanisse M., McRae K., editors. Cambridge Handbook of Psycholinguistics. Cambridge University Press; Cambridge, UK: 2012. pp. 353–364. [Google Scholar]
- Fedorenko E., Thompson-Schill S.L. Reworking the language network. Trends Cogn. Sci. 2014;18(3):120–126. doi: 10.1016/j.tics.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl E.C., Neumann J., Bogler C., Cramon D.Yv. The extended language network: a meta‐analysis of neuroimaging studies on text comprehension. Hum. Brain Mapp. 2008;29(5):581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferstl E.C., Neumann J., Bogler C., von Cramon D.Y. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Hum. Brain Mapp. 2008;29(5):581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91(4):1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Gierhan S.M.E. The language network. Curr. Opin. Neurobiol. 2013;23(2):250–254. doi: 10.1016/j.conb.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Penny W.D., Glaser D.E. Conjunction revisited. Neuroimage. 2005;25(3):661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gernsbacher M.A. Two decades of structure building. Discourse Process. 1997;23(3):265–304. doi: 10.1080/01638539709544994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldston D.B., Walsh A., Mayfield Arnold E., Reboussin B., Sergent Daniel S., Erkanli A. Reading problems, psychiatric disorders, and functional impairment from mid- to late adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(1):25–32. doi: 10.1097/01.chi.0000242241.77302.f4. [DOI] [PubMed] [Google Scholar]
- Hudson N., Scheff J., Tarsha M., Cutting L.E. Reading comprehension and executive function neurobiological findings. Perspect. Lang. Lit. 2016;42(2):23–29. [Google Scholar]
- Just M.A., Carpenter P.A. A capacity theory of comprehension: individual differences in working memory. Psychol. Rev. 1992;99(1):122. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kieffer M.J., Vukovic R.K., Berry D. Roles of attention shifting and inhibitory control in fourth‐grade reading comprehension. Read. Res. Q. 2013;48(4):333–348. [Google Scholar]
- Landi N., Frost S.J., Menc W.E., Sandak R., Pugh K.R. Neurobiological bases of reading comprehension: insights from neuroimaging studies of word level and text level processing in skilled and impaired readers. Read. Writ. Q. 2013;29(2):145–167. doi: 10.1080/10573569.2013.758566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan B.R., Rowe D.B. An evaluation of thresholding techniques in fMRI analysis. Neuroimage. 2004;22(1):95–108. doi: 10.1016/j.neuroimage.2003.12.047. [DOI] [PubMed] [Google Scholar]
- MacDonald M.C., Christiansen M.H. 2002. Reassessing Working Memory: Comment on Just and Carpenter (1992) and Waters and Caplan (1996) [DOI] [PubMed] [Google Scholar]
- Mahone E.M., Cirino P.T., Cutting L.E., Cerrone P.M., Hagelthorn K.M., Hiemenz J.R. Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Arch. Clin. Neuropsychol. 2002;17(7):643–662. [PubMed] [Google Scholar]
- Maisog J.M., Einbinder E.R., Flowers D.L., Turkeltaub P.E., Eden G.F. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci. 2008;1145(1):237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Martin A., Schurz M., Kronbichler M., Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss B.D., Cohen L., Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- Nichols T., Brett M., Andersson J., Wager T., Poline J.B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nippold M.A. Later language development: an overview. In: Nippold M.A., editor. Later Language Development: School-Age Children, Adolescents, and Young Adults. 3rd ed. PRO-ED; Austin, TX: 2007. pp. 1–23. [Google Scholar]
- Noonan K.A., Jefferies E., Visser M., Lambon Ralph M.A. Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J. Cogn. Neurosci. 2013;25(11):1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Norton E.S., Beach S.D., Gabrieli J.D. Neurobiology of dyslexia. Curr. Opin. Neurobiol. 2015;30:73–78. doi: 10.1016/j.conb.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K., Nestor P.J., Rogers T.T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007;8:976. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Danelli L., Berlingeri M. Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front. Hum. Neurosci. 2014;8(830) doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti C.A. Oxford University Press; 1985. Reading Ability. [Google Scholar]
- Perfetti C.A. 1988. Verbal Efficiency in Reading Ability. [Google Scholar]
- Perfetti C.A., Landi N., Oakhill J. 2005. The Acquisition of Reading Comprehension Skill. The Science of Reading: a Handbook; pp. 227–247. [Google Scholar]
- Pobric G., Jefferies E., Ralph M.A.L. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc. Natl. Acad. Sci. 2007;104(50):20137. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Fletcher P.C., Henson R.N., Worsley K.J., Brett M., Nichols T.E. Guidelines for reporting an fMRI study. Neuroimage. 2008;40(2):409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat C.S., Just M.A. Exploring the neural dynamics underpinning individual differences in sentence comprehension. Cereb. Cortex. 2011;21(8):1747–1760. doi: 10.1093/cercor/bhq241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat C.S., Mason R.A., Just M.A. Individual differences in the neural basis of causal inferencing. Brain Lang. 2011;116(1):1–13. doi: 10.1016/j.bandl.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopapas A., Archonti A., Skaloumbakas C. Reading ability is negatively related to Stroop interference. Cogn. Psychol. 2007;54(3):251–282. doi: 10.1016/j.cogpsych.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Richlan F., Kronbichler M., Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryherd K., Jasinska K., Van Dyke J.A., Hung Y.H., Baron E., Mencl W.E., Landi N. Cortical regions supporting reading comprehension skill for single words and discourse. Brain Lang. 2018;186:32–43. doi: 10.1016/j.bandl.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D., Kreher B.W., Schnell S., Kümmerer D., Kellmeyer P., Vry M.-S. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar B.L., McCandliss B.D. Development of neural systems for reading. Annu. Rev. Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Swanson H.L., Howell M. Working memory, short-term memory, and speech rate as predictors of children’s reading performance at different ages. J. Educ. Psychol. 2001;93(4):720. [Google Scholar]
- Torgesen J.K., Wagner R., Rashotte C. Pearson Clinical Assessment; 2012. Test of Word Reading Efficiency:(TOWRE-2) [Google Scholar]
- Toplak M.E., Bucciarelli S.M., Jain U., Tannock R. Executive functions: performance-based measures and the behavior rating inventory of executive function (BRIEF) in adolescents with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2009;15(1):53–72. doi: 10.1080/09297040802070929. [DOI] [PubMed] [Google Scholar]
- Toplak M.E., West R.F., Stanovich K.E. Practitioner Review: do performance-based measures and ratings of executive function assess the same construct? J. Child Psychol. Psychiatry. 2013;54(2):131–143. doi: 10.1111/jcpp.12001. [DOI] [PubMed] [Google Scholar]
- van der Sluis S., de Jong P.F., van der Leij A. Executive functioning in children, and its relations with reasoning, reading, and arithmetic. Intelligence. 2007;35(5):427–449. [Google Scholar]
- Wagner R.K., Sternberg R.J. 1987. Executive Control In Reading Comprehension. Executive Control Processes in Reading, 1-21. [Google Scholar]
- Wang K., Banich M.T., Reineberg A.E., Leopold D.R., Willcutt E.G., Cutting L.E. 2019. Left Posterior Prefrontal Regions Support Domain-General Executive Processes Needed for Both Reading and Math. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Welcome S.E., Joanisse M.F. Individual differences in skilled adult readers reveal dissociable patterns of neural activity associated with component processes of reading. Brain Lang. 2012;120(3):360–371. doi: 10.1016/j.bandl.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Whitney C., Kirk M., O’Sullivan J., Lambon Ralph M.A., Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb. Cortex. 2011;21(5):1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Webster M.A., Vidaurre D., Nichols T.E., Smith S.M. Multi-level block permutation. Neuroimage. 2015;123:253–268. doi: 10.1016/j.neuroimage.2015.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T. Big correlations in little studies: inflated fmri correlations reflect low statistical power—commentary on Vul et al. (2009) Perspect. Psychol. Sci. 2009;4(3):294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Braver T.S. Springer Science + Business Media; New York, NY, US: 2010. Cognitive Neuroscience Approaches to Individual Differences in Working Memory and Executive Control: Conceptual and Methodological Issues Handbook of Individual Differences in Cognition: Attention, Memory, and Executive Control; pp. 87–107. [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D., Dominick A.L., Preston A.R. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75(1):168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.