ABSTRACT
Introduction
Gallbladder polyps (GBPs) are generally harmless, but the planning of diagnosis and treatment of the GBP is of clinical importance due to the high mortality risk of delays in the diagnosis of gallbladder carcinomas that show polypoid development.
Materials and methods
GBPs are usually incidentally detected during ultrasonographic (USG) examinations of the abdomen. The risk of carcinoma development from polypoid lesions in the literature is reported as 0-27%. There is no consensus about the management of the GBPs. Herein, we reviewed the contemporary data to update our knowledge about diagnosis and treatment of gallbladder polyps.
Results
Polyps can be identified in five different groups, primarily as neoplastic and non-neoplastic. Cholesterol polyps account for 60% of all cases. The most common (25%) benign polypoid lesions after cholesterol polyps are adenomyomas.
Conclusion
Ultrasonography and endoscopic ultrasonography seems to be the most important tool in differential diagnosis and treatment. Ultrasonography should be repeated in every 3-12 months in cases that are thought to be risky. Nowadays, the most common treatment approach is to perform cholecystectomy in patients with polyps larger than 10 mm in diameter. Radical cholecystectomy and/or segmental liver resections should be planned in cases of malignancy.
How to cite this article
Dilek ON, Karsu S, et al. Diagnosis and Treatment of Gallbladder Polyps: Current Perspectives. Euroasian J Hepatogastroenterol 2019;9(1):40-48.
Keywords: Diagnosis, Gallbladder polyps, Pathology, Treatment, Ultrasonography.
INTRODUCTION
Gallbladder polyps (GBPs) are benign lesions originating from the mucosa. Polyps are usually harmless, but cases with a diameter of more than 1 cm and adenomatous features are of clinical importance due to the possibility of developing cancer. In the literature, the rate of cancer development from polyps has been reported as 0-27%.1-4 The likelihood of diagnostic confusion increases the clinical significance of the polypoid lesions of the gallbladder because of the polypoid appearance of gallbladder cancers at first. The fact that the algorithms for diagnosis and treatment have not been developed yet can cause stress on the doctors and anxiety in the patients.
Clinically, there are differences in the diagnosis, follow-up and treatment approaches of benign lesions. Herein, controversial issues in the diagnosis and treatment of GBP are discussed in light of current developments.
GALLBLADDER POLYPS
Demographic Findings
The widespread and effective use of ultrasonography (USG) has caused an increase in the diagnosis of the GBPs.5 It is one of the most common diseases seen in the biliary system. GBP is detected in around 3-7% of healthy individuals in the community.1,6-8 GBPs prevalence is increasing among people who have increased age, male gender, hypertension, diabetes, hepatitis C infection, impaired fasting glycemia and obese.9
The incidence is either equal or close in both genders.4,5 Although polypoid lesions are seen in all ages, they are more common in individuals that are over 40 years old. In their study that included 3600 Danish people, Jorgensen and Jensen found that the incidence of polypoid lesions was 4.6% in males and 4.3% in females and 5.9% and 5.8%, respectively over the age of 70 years.5,7,8 Segawa et al. reported that the polyp detection rate was 6.3% for men and 3.5% for women in their study including 21,771 people.10
In cholecystectomy specimens, the rate of polyps changed in between 0.5% and 13.8%.4,5,11 Ozmen et al.11 detected this rate as 1.3% in the series of 1718 cholecystectomy cases, Koga et al. found it as 9.7% in 411 cases, and Sun et al.4 detected 194 (4.9%) polyps in a case series of 3955 patients.
In the performed studies, there was no correlation found between polyp formation and factors known to play a role in stone formation such as age, gender, weight, gestational status, and hormone use. During childhood, they can be seen more in patients with Peutz-Jeghers syndrome, leukodystrophy, and pancreatobiliary malunion.11,12
Pathology
Description of GBPs changes according to their structure. Most of the polypoid lesions are a condition in which triglycerides, cholesterol esters, and their precursors are deposited in the lamina propria of the gallbladder. The fact remains that some GBPs arise from the layer structures of the gallbladder wall are real polyps. Polypoid lesions originating from gallbladder epithelium that is determined to be malignant are known as gallbladder cancer. Patients with pedunculated malignant polyps are often papillary, while those without peduncles are nodular type polyps. Histopathologically, they are usually adenocarcinomas. According to WHO classification, a 3-stage grading system (well, moderately and poorly differentiated) has been proposed for gallbladder carcinomas, which consider the architectural and cytological changes.13-15 It is noted that well-differentiated carcinomas are papillary carcinomas and may be difficult to distinguish from GBPs.
On the contrary, Stringer et al. suggest that GBPs may be classified primary (adenoma, hyperplasia, heterotopia) and secondary (Peutz-Jegher syndrome, leukodystrophy, pancreato-biliary malunion) in children.12 However, there is no consensus on the grading of GBPs. GBPs are initially divided into two categories as benign and malignant (Table 1). Benign GBP lesions can be divided into two groups: neoplastic (true polyp) and non-neoplastic.
Table 1.
Polypoid lesions of the gallbladder
|
|
||
|
|
||
| Non-neoplastic (Pseudotumor) |
|
|
|
| Benign |
|
|
|
|
|
||
| Neoplastic (Tumor) | Other* (Mesenchymatous tumors) |
|
|
| Malign |
|
||
Neoplastic Polyps
Neoplastic lesions mainly include adenomas and mesenchymatous tumors. They are true polyps. Although the incidence of adenomatous polyps is not completely known, it accounts for 4% of polypoid lesions.16 Neoplastic lesions tend to be single while non-neoplastic lesions tend to be multiple. They are considered as precancerous lesions. Less common (5-10%) neoplastic types of benign polyps have the potential to be malignant.4,17
The malignant formation is believed to be originated from the flat and dysplastic epithelium. They can occur anywhere on the gallbladder wall. Lesions that originate from the gallbladder mucosa may be single or multiple (Fig. 1). Multiple ones are also called papillomatosis (adenomatosis) (Fig. 2). There are two types, papillary and non-papillary (tubulary). Their diameters may range from 2 to 20 mm. They may be accompanied by gallstones. Smok et al. reported the adenoma incidence rate as 0.09% in their series with 12.153 cholecystectomy cases.18
Fig. 1.
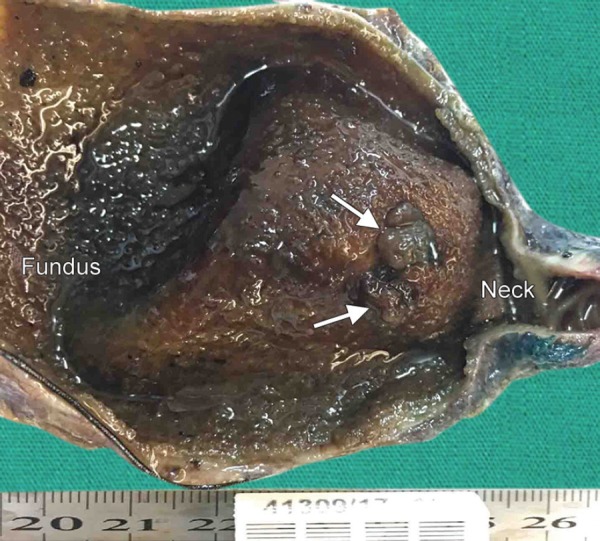
Sagittal section of gallbladder specimen shows true neoplastic polyps on the neck (arrows)
Figs 2A and B.
Adenomatous polyps of gallbladder (adenomatosis); (A) fresh and (B) fixed
In the systematic analysis of Elmasry et al., 64 (0.60%) of 5482 cases were found to be adenomas or malignancies.19 The risk of cancer is high when the diameter is greater than 1 cm. Approximately 1/4 of adenomas become cancerous (6-36%) and all adenomas over 12 mm in diameter are considered to contain cancer cells.6,20,21 In other studies, the risk of developing cancer in adenomatous polyps greater than 1 cm in diameter is reported to be 25-75%.21-23 In Japan, Kozuka et al. reported that in their 1600 cholecystectomy series, there were 18 adenomas (1.1°%) and 7 (39%) of them had cancer foci and the diameter of all these cases was greater than 12 mm. In the same series, it is also reported that adenomatous tissue remains were detected in 15 out of 17 patients with gallbladder carcinoma.21
Non-neoplastic Polyps
The majority of benign lesions are considered as non-neoplastic lesions. The most common non-neoplastic polyps include cholesterol deposits and hyperplasia of inflammatory, granulomatous, ectopic, and heterotopic tissues. In the series by Roa et al. with 219 polyps, 85% of the cases were detected as non-neoplastic polyps, and 15% were adenomas. Seventy-five percent of the non-neoplastic cases were reported to be located in the proximal half and 88% of the adenomas were located in the distal half. It was also reported that 95% of non-neoplastic cases had a diameter of less than 10 mm.24
Cholesterol polyps are the most common clinically encountered lesions polypoid lesions and have no neoplastic features. It is accepted that cholesterol polyps are formed as a result of phagocytosis of cholesterol esters and other lipids (triglyceride and esterified sterols) by macrophages found in the lamina propria and coverage of the columnar epithelium by cholesterol-containing foamy histiocytes. Cholesterol polyps attached to the wall by a stalk (“ball on the wall”). Lipids can similarly be stored in epithelial and stromal layers in lesser amounts. It is believed that the accumulation develops as a result of a disorder in cholesterol metabolism.24-26 These lesions are considered more to be pseudopolyps. They constitute 60-70% of all polypoid lesions.3,4 They are more common in multiparous women aged 40-50 years.
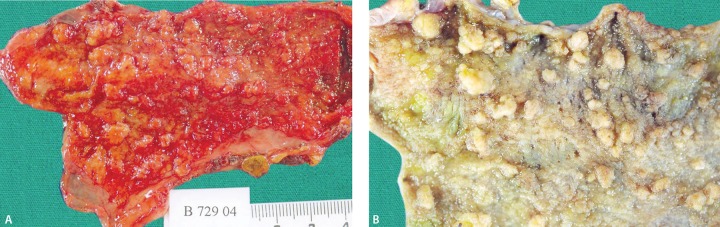
Cholesterol polyps can often be multiple (64.7%), single or broad. Their diameters are usually less than 10 mm (2-10 mm) and they may be pedunculated.17,24,26,27 Broad lesions are referred to as cholesterolosis. In cholesterolosis, the mucosa gains a carpet pattern with yellow-green colored papillary structures with a diameter of less than 1 mm. This appearance is also called strawberry gallbladder (Fig. 3). Interestingly, there is no relation between cholesterolosis and gallstone formation. There was also no correlation between cholesterol polyps and serum cholesterol levels.
Fig. 3.
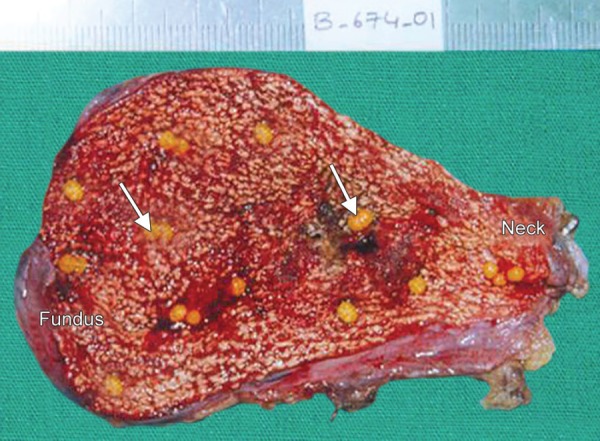
The opened gallbladder specimen shows round, yellow, pure cholesterol gallstones (arrows) and a geographic yellow mucosal surface caused by cholesterolosis (strawberry gallbladder)
Adenomyomas are hyperplastic non-neoplastic lesions developing from the gallbladder wall. Adenomyomas are the most common benign polypoid lesions after cholesterol polyps (25%). It is reported that it is mostly seen in women over 50 years old and with a frequency of 2.5-5%.17,24,27-29 These lesions are generally considered to develop during the cholecystitis episodes as a result of mucosal hyperplasia or thickening of the muscular tissue that forms the bile duct wall without an inflammatory response. It is believed that hyperplasia also contributes to the development of adenomyosis with the branching and dilatation of the Rokitansky-Aschoff sinuses in the muscular layer of the gallbladder. It is believed that compartmentalization and increased neuromuscular activity may be responsible for the excessive increase of intraluminal pressure in the biliary tree.29 They are usually located in the fundus (Fig. 4). They may be developed in generalized (adenomyomatosis), annular, segmentary, and localized forms.6,29 It is reported that segmental adenomyomatous lesions on the gallbladder wall may be confused with cancer by causing concentric narrowing (hourglass gallbladder).28,29 Although it is generally accepted that there is no risk of cancer, there are also studies claiming it to be precancerous.30 The NCCN guidelines accept that adenomyomatosis of the gallbladder has a potential risk of developing gallbladder cancer.
Fig. 4.
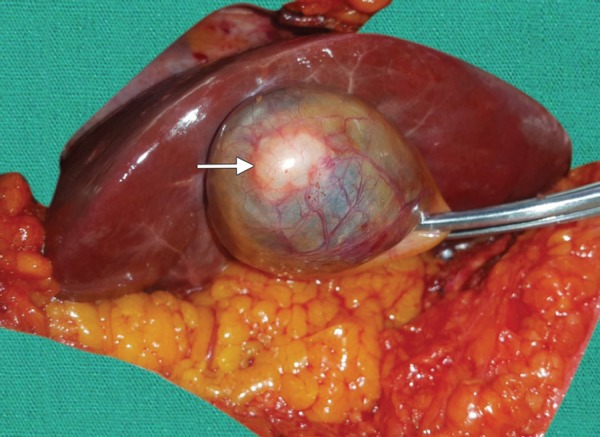
Gallbladder adenomyomatosis with focal wall thickening (arrow) involving the fundal region
Hyperplastic polyps are lesions that are characterized by papillary hyperplasia. They may be primary and secondary. Unlike the secondary forms, there is no association between primary papillary hyperplasia and gallstones, cholecystitis, or other inflammatory processes.16,26
Inflammatory type polyps are frequently associated with gallbladders that are chronically inflamed with stones. They constitute 10% of benign polypoid lesions. It is accepted that they develop as a result of the local inflammatory response in the mucosa. They contain cells responsible for chronic inflammation (lymphocytes and plasma cells), granulation, and fibrous tissue elements. The lesion is a polypoid structure extending toward the lumen, with a peduncle and its vasculature. They are usually solitary and 5-10 mm in diameter. It is accepted that malignancy does not develop due to chronic inflammatory process.16,18,24
Lymphoid polyps coexist with lymphoid hyperplasia and often chronic cholecystitis, as in other gastrointestinal organs. They are smaller than cholesterol polyps. Lymphoid polyps are lesions covered with mucosa with/without a peduncle. They can be localized in all the layers of the wall of the gallbladder.16 Salmonella typhi may be detected in some cases.31,32 Fibrous polyps can be found in association with acute and chronic calculous cholecystitis.
Granulomatous polyps or granulomas are the lesions growing towards the lumen. Acute or chronic inflammatory processes may be encountered. Their diameter is usually less than 10 mm. However, they are longer than fibrous or lymphoid polyps. Interestingly, their histological appearance resembles the fibroadenomas of the breast.16
In addition, polypoid melanomas or metastatic lesions can be detected rarely.12,17,24 Other polyps that can be defined as stomach heterotopic tissue, carcinoid tumor, leiomyoma, fibroma, and neurofibromas are very rare.16
Clinical Features
The uncertainty about the early diagnosis and differential diagnosis of GBP and the choice of treatment approach are among the important issues in the clinic. While there is no clinical symptom in 1/3 of the patients, in half of the cases, patients may resort to polyclinic with dyspeptic complaints manifested by abdominal pain, nausea, and vomiting.
Cholesterol polyps generally have no clinical signs. If any symptom occurs, most of them resemble cholecystitis. They may occasionally cause abdominal pain and even rarely pancreatitis, such as gallbladder stones. Pedunculated polyps rarely rupture, forming colic-like pain, jaundice, and cholangitis. In the literature, cases that caused hemobilia and mechanical cholestasis have also been reported.1,5
Diagnosis
Because most of the GBP cases do not show any clinical symptoms, they are incidentally detected by ultrasonography performed without a prediagnosis of polyps during routine examinations for other causes. The differential diagnosis of focal wall thickening of gallbladder should be made with polyps, adenomyomatosis, carcinoma, xanthogranulomatous cholecystitis, metastasis, chronic cholecystitis and tumefactive sludge (sludge balls).19,22
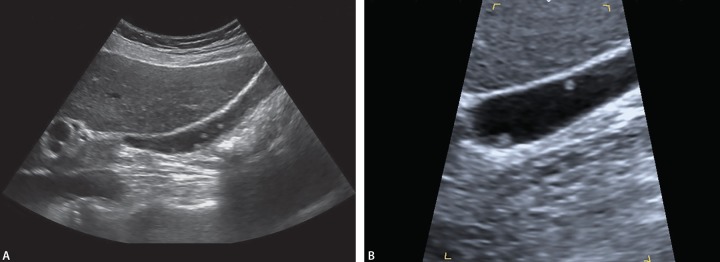
In USG, hyperechoic lesions that are soft tissue protrusions extending into the lumen of the pouch or adjacent to the wall that show no acoustic shadowing, do not change localization with the position are characteristic for polyps (Figs 5 and 6). Malignant or benign lesions may be single or multiple, pedunculated or sessile and have different echogenicity (hypo, hyper, iso), different surface (smooth or nodular) and may be superficial or flat in appearance.22,33,34
Fig. 5.
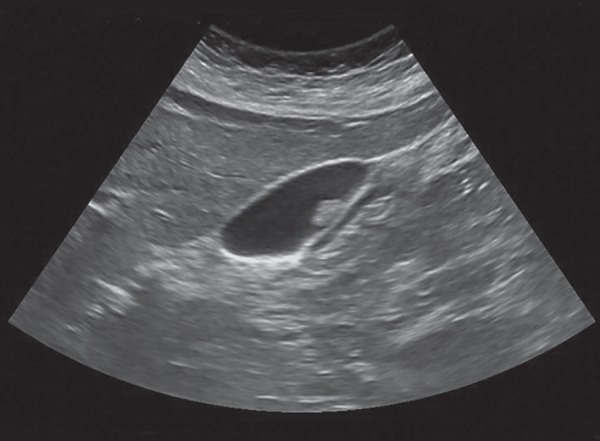
Transabdominal USG shows that a sesile gallbladder polyp were defined as immobile and lack an acoustic shadow
Fig. 6A and B.
USG shows multiple polyps in the same position at different angles (A and B) without acoustic shadow
There is a relationship between the diameter of the polypoid lesion and the risk of malignancy and the diameter must definitely be determined. During USG examination, gallstones smaller than 5 mm, in particular, do not leave acoustic shadows and with the presence of biliary sludge, it is difficult to distinguish polypoid lesions from gallstones and repeat USGs are recommended.25,35 In their series with 111 cases, Csendes et al. found that the majority of the GBP cases (80%) had a diameter of less than 5 mm and a single polyp.3 In the literature, it is reported that only 36-90% of GPL cases can be detected by USG. Martin et al. recently reported a metaanalysis evaluating 1816 articles. They detected a high false-positive rate (85.1%) for the diagnosis of GBPs with transabdominal ultrasonography. They also advise using alternative imaging modalities to determine the management guidelines of GBPs.36 As the diameter increases, the sensitivity, and specificity of USG increases. This ratio reaches up to 99% in cases without stones. It has been reported that stones block the appearance of polyps and cause false negativity. It was noted that GBPs can be a mask with the presence of gallstones and also small polyps cannot be differentiated from the thickened wall of the gallbladder by USG. Mucosal folds, small stones, and sludges may be impacted into the gallbladder wall, and this leads to misinterpreted images as a polyp.1 The sensitivity of USG increases to 100% and specificity increases to 86% when polyp diameter exceeds 10 mm.1,25 Also, higher ultrasound frequencies (5-12 MHz) yield better results.23
Differential diagnosis can be made with the data obtained about the localization, appearance, number, size of the polyps and whether or not they are pedunculated. Differential diagnosis and exclusion of gallbladder cancer may be most problematic in segmental and focal adenomyomas. Adenomyomatosis may be shown as “comet-tail” reverberation artifacts within the thickened gallbladder wall. However, no clear distinction can be made as to whether the lesion contains cancer. In this regard, it is reported that cholesterol polyps, adenomas, and adenocarcinomas can be differentiated with doppler USG, which can show that there is more mural blood flow in cancerous lesions. There are also studies reporting that with contrast USG (CEUS) the distinction between adenoma and adenocarcinoma can be made more easily.34 Kubota et al. reported that polypoid lesions with the same echogenicity as liver parenchyma are at risk for malignancy.37 Choi et al. suggest a scoring system that is calculated by the characteristics of the lesion structure, echo pattern, edge of the polyp, number of polyps and presence of peduncle (whether or not pedunculated). According to this, with the cutoff score numbered through 1-6, the risk of malignancy can be predicted in the rates ranging from 4.6 to 84.6%.38
On the other hand, the false positive rate in the USG examination is reported to be 6-43% in different series. This difference is thought to be due to mucosal folds, biliary sludge, or small stones impacted on the gallbladder wall.1 Also, it should not be forgotten that many polypoid lesions may be lost while holding with the forceps, crushing during removal from the trocar and during the cleaning of the bile for the purpose of preparation for the macroscopic examination.1,3
Endoscopic ultrasonographic (EUS) examinations are also recommended when the differential diagnosis is needed. With EUS, the echogenicity, structure, and diameter of the lesion can be demonstrated in more detail. Scoring systems developed by evaluating properties of the polyp with EUS may also be helpful in the differential diagnosis.39,40 Better results have been reported with contrast-enhanced EUS. Kimura et al. in their EUS study suggest that lesions with large diameter, flat or nodular surface, solid and internal echo should be evaluated as malignant whereas lesions with a hyperechogenic appearance and heterogeneous structure should be evaluated as cholesterol polyps with hyperplastic structure.41 Similar criteria were used by Sugahara et al. In their clinical screening study performed with EUS and conventional USG.42 Sugiyama et al. were able to identify the polypoid lesions with 97% accuracy with EUS and 76% with normal USG.43 USG guided percutaneous transhepatic fine needle aspiration biopsy may be recommended in cases that a definitive differential diagnosis is needed.44 However, transabdominal USG guided biopsy should be performed in selected patients because of the biopsy related complications including, tumor dissemination, bleeding, infection, and bile leakage.45,46 Besides, fine needle aspiration biopsy using EUS (EUS-FNA) is a more useful tool for differential diagnosis of the gallbladder pathologies.47
Ultrasonography-based elastography is a relatively new imaging technology that creates images of tissue stiffness for almost every tissue in the body. There are studies that suggest elastography can help in differentiating malignant and benign.48,49 With this method, inflammatory tissue can be differentiated from the masses of epithelial tissue. Teber et al. evaluated the feasibility of elastography in a preliminary report. They reported that the benign lesions have a high-strain elastographic pattern and malign lesions had low elasticity properties.49 Kapoor et al. reported that the likelihood of malignancy is high with a mean shear wave velocity of 2.7 m/s or greater with elastography (sensitivity 100%, specificity 91%).48 Magnetic resonance elastography may be another alternative diagnostic tool for validation of the size and location of lesions.50 However, there have been very few studies with elastography and controversies about its clinical usage. More clinical studies need to be carried out to understand the efficacy of elastography.
CT shows and distinguishes the marked gallbladder wall thickening, intramural nodules that are hypoechoic at the sonography and representing abscess or foci of xanthogranulomatous inflammation. In the diagnosis of lesions with low density, computerized tomography is inadequate and its sensitivity (44-77%) is low.27 In recent years, clinical studies have been published that contribute to differential diagnosis with thin-section contrast enhanced computed tomography (CECT) and positron emission tomography (PET). Sensitivity and specificity of CECT in the differential diagnosis of benign and malign lesions on the gallbladder wall were reported as 82.5% and 75.9%, respectively.23,27 Furukawa et al. reported that lesions greater than 5 mm in diameter, in particular, can be diagnosed correctly with the rate of 100% with CECT in the means of benign-malignant differentiation.51
Magnetic resonance imaging (MRI) and its combination with MRCP can be a very valuable tool in evaluating gallbladder lesions. Neoplastic lesions of the gallbladder can be evaluated using MRI on T2-weighted fat-suppressed images. Also, MRCP shows the serial contrast-enhanced images and their obstructive effect on images. MRI may be useful to differentiate gallbladder carcinoma from adenomyomatosis or emphysematous cholecystitis by depicting Rokitansky-Aschoff sinuses. MRI and MRCP would be able to detect mural thickening, focal sessile mass and hourglass configuration and also “pearl necklace sign” represents to Rokitansky-Aschoff sinuses in which has the fluid filled intramural mucosal diverticula. This sign is highly specific (92%), but only present in 70% of cases.52,53
In recent years, a large number of nuclear scanning studies have begun to be published to differentiate between benign and malignant polyps. In their studies with 50 cases of performed PET/ CT with 18F-FDG, Lee et al. reported that the presence of 18F-FDG uptake in polyps is a strong risk factor for cancer and that the ratio of liver/polyp SUVgp may be an important predictor.54 In another study, it is reported that the retention index calculated from early and delayed 18F-FDG PET/CT uptake values was higher in delayed uptakes and the sensitivity and specificity of the method were reported to be 100% and 80%, respectively.55 However, the rate of false positives is high in cases with cholecystitis. In infected cases, the results should be assessed with findings of inflammation (CRP).
There are studies suggesting that in the protein electrophoresis of the gallbladder walls of cholecystectomized patients, unlike cases with cholelithiasis, at least two additional protein bands are detected in cancerous tissues and that this can be used in the differential diagnosis.56 The method called fourier transform infrared (FTIR) spectroscopy has been reported to be able to detect molecular compositions of premalignant tissues of the gallbladder wall (lipid increase in the plasma membrane) and that it can be used in the differential diagnosis.57 However, there has not yet been the clinical use of these two methods.
Cytologic analysis of bile juice using by endoscopic transpapillary gallbladder drainage (ETGD) tube does not contribute to differential diagnosis.58 But, Itoi et al. suggest that cytology using an ETGD tube is more useful for differential diagnosis of gallbladder lesions.59 On the other hand, endoscopic or percutan ultrasound-guided fine needle aspiration cytology offer clinical benefits for differential diagnosis of gallbladder wall pathologies.47,60 Ogura et al. also suggested that EUS-FNA is more sensitive, specific and accurate than ETGD for cyto-histological diagnosis of gallbladder lesions.47
There are no specific laboratory findings refer to GBPs. Liver function tests are not corelated with GBPs. There is also no corelation between GBPs and bile juice analysis.58
Follow-up
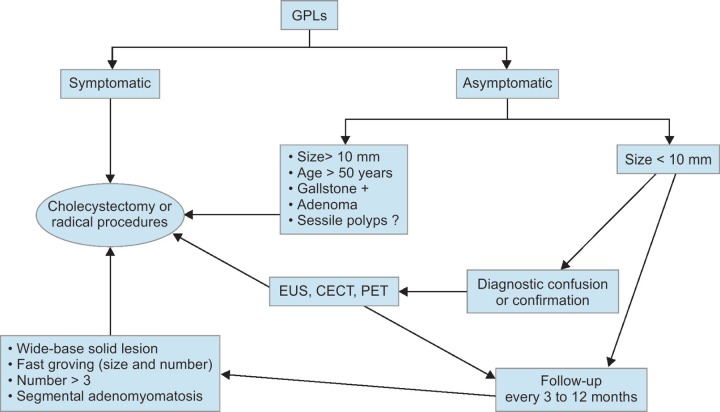
Nowadays, benign-malignant differentiation, follow-up, and treatment options are still being discussed in GBP cases. The risk of developing malignancy in GBP cases is reported as 0.57% in systematic analyzes, but it is accepted that missing gallbladder cancer will have a potentially catastrophic outcome. Several algorithms have been proposed for diagnosis, treatment, and follow-up of patients. A proposed algorithm is presented in Flowchart 1, which we have prepared in light of the available literature data.
Flowchart 1.
Proposed management algorithm for gallbladder polyps
Studies on the follow-up of polypoid lesions are very limited. In a series of 226 cases of GBPs that were followed-up by cholecystography by Eelkema et al. published in 1962, it is reported that GBP cases without stones are benign and they remain benign.61 It is known that the diagnostic value and sensitivity of cholecystography is lower than that of USG. It has been reported in a study by Moriguchi et al. that carcinoma developed in only one case in a series of 109 cases followed up for five years with USG.5 Ukai et al. reported that cholesterol polyps may show rapid growth during follow-up.62 It has been shown that stones lead to metaplastic dysplasia on polyps.63 On the other hand, an epidemiological study by Jensen et al.8 and Jorgensen et al.7 showed that polypoid lesions facilitate stone formation.
Different algorithms are proposed by radiologists for diagnosis and follow-up according to the diameter of the polypoid lesions or the thickening of the gallbladder wall.23
No clinical data are available on how long polypoid lesions with diameters less than 10 mm should be followed. It is reported in the literature that 94% of lesions that are <1 cm in diameter are benign.3,25 The observation of polypoid formations that are multiple, have a diameter less than 10 mm and remain unchanged in size after multiple measurements are considered to indicate that the lesion may be benign. However, serial USG follow-ups for every 3-6 months in the first 2 years and then every 6-12 months is recommended. In their study, Terzi et al. found malignancy in 26 out of 100 polypoid lesions. They stated that polypoid lesions accompanied by stones increase the likelihood of malignancy and cases with gall stones should go under cholecystectomy. In the same study, because of the presence of symptoms such as right upper quadrant pain, nausea, vomiting, and dyspepsia in all of the malignant cases, they suggested performing cholecystectomy in symptomatic cases as well which is different from the literature.2 Moriguchi et al. reported that in their series with 109 cases that were followed-up for 5 years, only 6% of the lesions were above 10 mm.5 In this process, they stated that lesions remained the same in 84% of the patients, grown bigger in 12% and reduced or got lost in 4%.5 In their series with 98 asymptomatic patients with polyps with a diameter of 10 mm or less which were followed-up for 6 years with serial USG, Csendes et al. found that the lesion's diameter remained the same in half of the patients, increased in 26.5% and decreased in 23.5%.3 In the same study, no malignancies or gall stones were found in the patients. Fourteen patients with enlarged diameters were operated. Among the operated patients, cholesterol polyps were detected in 70% and adenoma was detected in only one patient.3 Therefore, it is considered that laparoscopy being widely used in the last 20 years and being minimally invasive cannot be a reason to perform cholecystectomy in lesions below 10 mm.
In their studies with 1558 GBP cases, Park et al. detected neoplastic polyps in 32 cases.64 In the 8-year follow-up of these cases, the rate of neoplastic polyps was 1.7% at 1 year, 2.8% at 5 years and 4% at 8 years. It has been reported that polyps with a diameter of 10 mm or more on the same series are 24 times more likely to develop carcinoma.64 It is reported in the literature that malignancy is seen in 34-88% of polypoid lesions that are 10 mm or more in diameter.1,3,65 Being 60 years of age or older, the lesion being single and sessile, the lesion having a large peduncle and being solid, the lesion having a diameter greater than 10 mm and having a rapid increase in diameter in serial USG follow-ups and the presence of a coexisting stone may all be indicative of malignancy.1,3,4 However, it should be taken into account that lesions with peduncles and lesions that are larger than 15 mm in diameter may be cholesterol-hyperplastic polyps. Considering that cholesterol polyps constitute more than half of the polypoid lesions of the gallbladder, it is suggested that other radiological imaging methods, especially EUS examination, should be performed due to the increased risk of the tumor, even if the diameter is over 10 mm.
Treatment
Cholecystectomy is recommended when cases with GBPs have pain and colic as clinical symptoms and follow-up with USG is recommended when they have dispeptic complaints. Today, among surgeons, because lesions larger than 10 mm in diameter are more likely to be malignant, the surgical approach is accepted as a priority in the treatment. It is reported that local recurrence is seen usually within 6 months in 30-50% of the cases that underwent laparoscopic cholecystectomy and have a diagnosis of malignancy in the pathologic examination.20,24,26 In cholecystectomized cases, the prevalence of port site metastasis rate reported in the literature ranged between 0-40%.66 The risk of the tumor increases as the grade goes up. The risk is even higher in perforated gallbladder cases. During laparoscopic cholecystectomy, perforation develops in 25-30% of cases and may lead to the intraperitoneal spread of tumors. In the case of perforation, the surgical field should be washed with abundant saline and be aspirated. In addition, whatever the cause, the cholecystectomy materials should be taken out in an endobag. The removed gallbladder should be carefully inspected and palpated. After removal of the gallbladder, the gas in the abdomen should be drained from the trocars (desufflate the pneumoperitoneum). Such protective treatments will reduce the risk of tumor seeding and port-site recurrence in cases of malignancy.67,68 In fact, Kimura et al. recommend cholecystectomy by laparotomy for cholesterol polyps with a diameter larger than 10 mm.41 Csendes et al. recommend performing cholecystectomy in lesions larger than 10 mm in diameter and open cholecystectomy in the presence of malignancy.3 Lee et al. suggest that frozen should be done in polypoid lesions with a diameter larger than 2 cm and recommend open cholecystectomy in these patients.1
The NCCN guidelines (v.3.2018) recommends that laparoscopic simple cholecystectomy is sufficient for patients with pTis and pT1a cancer in laparoscopic cholecystectomy material.69 Radical cholecystectomy is recommended for pT1b cases. Radical intervention should be preferred for pT2 and pT3 lesions. In the series of Shih et al., mean survival was 6 months for pT2 cases who underwent laparoscopic cholecystectomy and 18 months for radical (extended) resection cases.70-73 In cases with detected polypoid tumors; it is recommended to also perform liver segment 4b and 5 resections, investigation of malignancy with frozen in the cystic duct, and dissection of hepatic hilus lymph glands with classical cholecystectomy. Although the effect of the dissection of the lymph node dissection in the hepatoduodenal region to survival is controversial, there are some who recommend removal of 6 lymph nodes in terms of defining the prognosis.74 In their series with 107 cases, Shih et al.70 reported a 5-year overall survival of 15% (8 months) and 5-year survival of 33% (21 months) in patients who were incidentally diagnosed and had undergone laparoscopic cholecystectomy. In the same series, it has also been stated that 74% of cases with incidental carcinoma required reexploration.
As a result; USG is the gold standard in diagnosing GBP. There is a consensus that in cases of diagnostic difficulty EUS with high sensitivity and specificity should be performed.
Polyp size is the most important predictor for neoplastic polyps. A diameter greater than 10 mm is a risk factor alone. Because of the possibility of malignancy of polypoid formations containing risk factors, surgery should be recommended first and follow-up should be recommended in clinically nonsymptomatic polypoid formations after the differential diagnosis. Especially in non-neoplastic and polypoid lesions below 10 mm, a “watch-and-wait strategy” should be recommended every 6-12 months with USG. On the other hand; prophylactic cholecystectomy can be performed due to “social indications” such as insomnia, insurance problems, stress that affects the patient's lifestyle, and follow-up problems. The NCCN guidelines (v.3.2018) recommend that prophylactic cholecystectomy should be performed for patients who have polyps >1 cm.69
There is a consensus on performing surgery in patients with clinical complaints. Surgery is recommended in cases with over 50 years of age, polyp diameter of more than 10 mm, broad based solid lesions, and coexistence with cholelithiasis which are accepted as independent risk factors. Cholecystectomy is recommended in cases with the rapid growth of polyp size between two USGs, with sessile (broad-based) polyps and with more than three millimetric polyps. Surgery should also be planned in segmental adenomyomatosis cases, because they may be mistaken with cancer.
The procedure to be performed in the treatment of the disease is laparoscopic cholecystectomy. Prophylactic cholecystectomy is recommended when the risk of malignancy continues. Due to the risk of cancer, the specimens should be removed by being put in an endobag. Open cholecystectomy should be preferred when cancer is suspected. In cases that the tumor is localized, partial liver resections containing 1-3 cm of free margin are preferred. It is also suggested to perform lymph node dissection around the cystic duct and hilus. In cases where cancer is detected, partial liver resections (segments 4b and 5), wedge resection or hepatectomy may be performed according to the localization of the lesion and its features.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Lee KF, Wong J, et al. Polypoid lesions of the gallbladder. Am J Surg. 2004;188:186–190. doi: 10.1016/j.amjsurg.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Terzi C, Sökmen S, et al. Polypoid lesions of the gallbladder: report of 100 cases with special reference to operative indications. Surgery. 2000;127:622–627. doi: 10.1067/msy.2000.105870. [DOI] [PubMed] [Google Scholar]
- 3.Csendes A, Burgos AM, et al. Late follow-up of polypoid lesions of the gallbladder smaller than 10 mm. Am Surg. 2001;234:657–660. doi: 10.1097/00000658-200111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun XJ, Shi JS, et al. Diagnosis and treatment of polipoid lesions of the gallbladder: report of 194 cases. Hepatobiliary Pancreat Dis Int. 2004;3:591–594. [PubMed] [Google Scholar]
- 5.Moriguchi H, Tazawa J, et al. Natural history of polypoid lesions in the gallbladder. Gut. 1996;39:860–862. doi: 10.1136/gut.39.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilhartz LE. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 7th ed. W.B. Saunders Co.; Philadelphia: 2002. Polyps of the gallbladder. pp. 1125–1130. [Google Scholar]
- 7.Jorgensen T, Jensen KH. Polyps in the gallbladder. A prevalence study. Scand J Gastroenterol. 1990;25:281–286. [PubMed] [Google Scholar]
- 8.Jensen KH, Jorgensen T. Incidence of gallstones in a Danish population. Gastroenterology. 1991;100:790–794. doi: 10.1016/0016-5085(91)80027-7. [DOI] [PubMed] [Google Scholar]
- 9.Lee YJ, Park KS, et al. Shifting prevalence of gallbladder polyps in Korea. J Korean Med Sci. 2014;29:1247–1252. doi: 10.3346/jkms.2014.29.9.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segawa K, Arisawa T, et al. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87:630–633. [PubMed] [Google Scholar]
- 11.Ozmen MM, Patankar RV, et al. Correspondence. Epidemiology of gallbladder polyps. Scand J Gastroenterol. 1994;29:480. doi: 10.3109/00365529409096842. [DOI] [PubMed] [Google Scholar]
- 12.Stringer MD, Ceylan H, et al. Gallbladder polyps in children classification and management. J Pediatr Surg. 2003;38:1680–1684. doi: 10.1016/s0022-3468(03)00583-9. [DOI] [PubMed] [Google Scholar]
- 13.Albores-Saavedra J, Klöppel G, et al. Carcinoma of the gallbladder and extrahepatic bile ducts. In: Bosman FT, Carneiro F, Hruban RH, et al., editors. World Health Organization Classification of Tumours of the Digestive System. 4th ed. WHO Press; Geneva: 2010. pp. 263–278. [Google Scholar]
- 14.Mizobuchi N, Munechika J, et al. Three cases of intracystic papillary neoplasm of gallbladder. Abdom Radiol (NY) 2018;43:1535–1539. doi: 10.1007/s00261-018-1595-z. [DOI] [PubMed] [Google Scholar]
- 15.Jütte H, Tannapfel A. Tumor grading of the hepatobiliary system [In German with English abstract]. Pathologe. 2016;37:299–303. doi: 10.1007/s00292-016-0176-6. [DOI] [PubMed] [Google Scholar]
- 16.Ljubicic N, Zovak M, et al. Management of gallbladder polyps: An optimal strategy proposed. Acta Clin Croat. 2001;40:57–60. [Google Scholar]
- 17.Li XY, Zheng CJ, et al. Diagnosis and treatment of polypoid lesion of the gallbladder. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:689–693. [PubMed] [Google Scholar]
- 18.Smok G, Bentjerodt R, et al. Benign polypoid lesions of the gallbladder. Their relation to gallbladder adenocarcinoma. Rev Med Chil. 1992;120:31–35. [PubMed] [Google Scholar]
- 19.Elmasry M, Lindop D, et al. The risk of malignancy in ultrasound detected gallbladder polyps: A systematic review. Int J Surg. 2016;33:28–35. doi: 10.1016/j.ijsu.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 20.Liu YL, Wang JT. Hypeplastic cholecystoses: effort should be made to recognize and treat them. HB Panc Dis Int. 2006;5:334–336. [PubMed] [Google Scholar]
- 21.Kozuka S, Tsubone M, et al. Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50:2226–2234. doi: 10.1002/1097-0142(19821115)50:10<2226::aid-cncr2820501043>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Gouma DJ. When are gallbladder polyps malignant? HPB Surg. 2000;11:428–430. doi: 10.1155/2000/34201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijayakumar A, Vijayakumar A, et al. Early diagnosis of gallbladder carcinoma: An algorithm approach. ISNR Radiology, 2013 doi: 10.5402/2013/239424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roa I, de Aretxabala X, et al. Clinicopathological features of gallbladder polyps and adenomas. Rev Med Chil. 2004;132:673–679. [PubMed] [Google Scholar]
- 25.Chattopadhyay D, Lochan R, et al. Outcome of gallbladder polypoidal lesions detected by transabdominal ultrasound scanning: A nine year experience. World J Gastroenterol. 2005;11:2171–2173. doi: 10.3748/wjg.v11.i14.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persley KM. Gallbladder polyps. Curr Treat Options Gastroenterol. 2005;8:105–108. doi: 10.1007/s11938-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 27.De Matos ASB, Baptista HN, et al. Gallbladder polps; How should they be treated and when? Res Assoc Med Bras. 2010;56:318–321. doi: 10.1590/s0104-42302010000300017. [DOI] [PubMed] [Google Scholar]
- 28.Ootani T, Shirai Y, et al. Relationship between gallbladder carcinoma and the segmental type of adenomyomatosis of the gallbladder. Cancer. 1992;69:2647–2652. doi: 10.1002/1097-0142(19920601)69:11<2647::aid-cncr2820691105>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Bonatti M, Vezzali N, et al. Gallbladder adenomyomatosis: imaging findings, tricks and pitfalls. Insights Imaging. 2017;8:243–253. doi: 10.1007/s13244-017-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghavendra BN, Subramanyam BR, et al. Sonography of denomyomatosis of the gallbladder: radiologic-pathologic correlation. Radiology. 1983;146:747–752. doi: 10.1148/radiology.146.3.6402802. [DOI] [PubMed] [Google Scholar]
- 31.Espinoza JA, Bizama C, et al. The inflammatory inception of gallbladder cancer. Biochim Biophys Acta. 2016;1865:245–254. doi: 10.1016/j.bbcan.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Kumar S, et al. Infection as a risk factor for gallbladder cancer. J Surgical Oncol. 2006;93:633–639. doi: 10.1002/jso.20530. [DOI] [PubMed] [Google Scholar]
- 33.Erden A, Songür Y, et al. The role of color Doppler ultrasonography in the differentiation of gallbladder lesions. Turkish J Gastroenterol. 1999;10:132–137. [Google Scholar]
- 34.Sun LP, Guo LH, et al. Value of contrast-enhanced ultrasound in the differential diagnosis between gallbladder adenoma and gallbladder adenoma canceration. Int J Clin Exp Med. 2015;8:1115–1121. [PMC free article] [PubMed] [Google Scholar]
- 35.Collett JA, Allan RB, et al. Gallbladder polyps: prospective study. Journal of Ultrasound in Medicine. 1998;4:207–211. doi: 10.7863/jum.1998.17.4.207. [DOI] [PubMed] [Google Scholar]
- 36.Martin E, Gill R, et al. Diagnostic accuracy of transabdominal ultrasonography for gallbladder polyps: systematic review. Can J Surg. 2018;61:200–207. doi: 10.1503/cjs.011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubota K, Bandai Y, et al. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery. 1995;117:481–487. doi: 10.1016/s0039-6060(05)80245-4. [DOI] [PubMed] [Google Scholar]
- 38.Choi WB, Lee SK, et al. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointestinal Endoscopy. 2000;52:372–379. doi: 10.1067/mge.2000.108041. [DOI] [PubMed] [Google Scholar]
- 39.Azuma T, Yoshikawa T, et al. Differential diagnosis of polypoid lesions of gallbladder by endoscopic ultrasonography. Am J Surg. 2001;181:65–70. doi: 10.1016/s0002-9610(00)00526-2. [DOI] [PubMed] [Google Scholar]
- 40.Sadamoto Y, Oda S, et al. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy. 2002;34:959–965. doi: 10.1055/s-2002-35859. [DOI] [PubMed] [Google Scholar]
- 41.Kimura K, Fujita N, et al. Differential diagnosis of large-sized pedunculated polypoid lesions of the gallbladder by endoscopic ultrasonography: a prospective study. J Gastroenterol. 2001;36:619–622. doi: 10.1007/s005350170046. [DOI] [PubMed] [Google Scholar]
- 42.Sugahara E, Nakajima M, et al. Management of polypoid lesions of the gallbladder by endoscopic ultrasonography (EUS)-- a retrospective study to establish the diagnostic criteria and a prospective study to evaluate its reliability. Nihon Shokakibyo Gakkai Zasshi. 1995;92:1846–1857. [PubMed] [Google Scholar]
- 43.Sugiyama M, Atomi Y, et al. Endoscopic ultrasonography for differential diagnosis of polypoid gallbladder lesions: analysis in surgical and follow up series. Gut. 2000;46:250–254. doi: 10.1136/gut.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu SS, Lin KC, et al. Ultrasound-guided percutaneous transhepatic fine needle aspiration cytology study of gallbladder polypoid lesions. Am J Gastroenterol. 1996;91:1591–1594. [PubMed] [Google Scholar]
- 45.Rana C, Krishnani N, et al. Ultrasound-guided fine needle aspiration cytology of gallbladder lesions: a study of 596 cases. Cytopathology. 2016;27:398–406. doi: 10.1111/cyt.12296. [DOI] [PubMed] [Google Scholar]
- 46.Heimbach JK, Sanchez W, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–360. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogura T, Kurisu Y, et al. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for thick-walled gallbladders? Dig Dis Sci. 2014;59:1917–1924. doi: 10.1007/s10620-014-3100-z. [DOI] [PubMed] [Google Scholar]
- 48.Kapoor A, Kapoor A, et al. Differentiating malignant from benign thickening of the gallbladder wall by the use of acoustic radiation force impulse elastography. J Ultrasound Med. 2011;30:1499–1507. doi: 10.7863/jum.2011.30.11.1499. [DOI] [PubMed] [Google Scholar]
- 49.Teber MA, Tan S, et al. The use of real-time elastography in the assessment of gallbladder polyps: preliminary observations. Med Ultrason. 2014;6:304–308. doi: 10.11152/mu.201.3.2066.164.1mat. [DOI] [PubMed] [Google Scholar]
- 50.Jhaveri KS, Hosseini-Nik H, et al. The development and validation of magnetic resonance elastography for fibrosis staging in primary sclerosing cholangitis. Eur Radiol. 2018 Jul 26; doi: 10.1007/s00330-018-5619-4. [DOI] [PubMed] [Google Scholar]
- 51.Furukawa H, Takayasu K, et al. CT evaluation of small polypoid lesions of the gallbladder. Hepatogastroenterology. 1995;42:800–810. [PubMed] [Google Scholar]
- 52.Elsayes KM, Oliveira EP, et al. Magnetic resonance imaging of the gallbladder: spectrum of abnormalities. Acta Radiol. 2007;48:476–482. doi: 10.1080/02841850701324102. [DOI] [PubMed] [Google Scholar]
- 53.Bilgin M, Shaikh F, et al. Magnetic resonance imaging of gallbladder and biliary system. Top Magn Reson Imaging. 2009;20:31–42. doi: 10.1097/RMR.0b013e3181b48aa2. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Yun M, et al. Risk stratification of gallbladder polyps (1-2 cm) for surgical intervention with 18F-FDG PET/CT. J Nuclear Med. 2012;53:353–358. doi: 10.2967/jnumed.111.093948. [DOI] [PubMed] [Google Scholar]
- 55.Nishiyama Y, Yamamoto Y, et al. Dual-time point 18F-FDG PET for the evaluation of gallbladder carcinoma. J Nucl Med. 2006;47:633–638. [PubMed] [Google Scholar]
- 56.Shukla VK, Goel S, et al. Electrophoretic pattern of proteins in carcinoma of the gallbladder. Eur J Cancer Prev. 2008;17:9–12. doi: 10.1097/CEJ.0b013e3280145e1b. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Zhang J, et al. Evaluation of gallbladder lipid level during carcinogenesis by an infrared spectroscopic method. Dig Dis Sci. 2010;55:2670–2675. doi: 10.1007/s10620-009-1045-4. [DOI] [PubMed] [Google Scholar]
- 58.Itsuki H, Serikawa M, et al. Indication and usefulness of bile juice cytology for diagnosis of gallbladder cancer. Gastroenterol Res Pract. 2018;2018:5410349. doi: 10.1155/2018/5410349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Itoi T, Sofuni A, et al. Preoperative diagnosis and management of thick-walled gallbladder based on bile cytology obtained by endoscopie transpapillary gallbladder drainage tube. Gastrointest Endosc. 2006;64:512–519. doi: 10.1016/j.gie.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 60.Hammoud GM, Almashhrawi A, et al. Usefulness of endoscopic ultrasound-guided fine needle aspiration in the diagnosis of hepatic, gallbladder and biliary tract Lesions. World J Gastrointest Oncol. 2014;6:420–429. doi: 10.4251/wjgo.v6.i11.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eelkema HH, Hodgeson JR. Fifteen-year follow-up of polypoid lesions of gallbladder diagnosed by cholecystography. Gastroenterology. 1962;42:144–147. [PubMed] [Google Scholar]
- 62.Ukai K, Akita Y, et al. Cholesterol polyp of the gallbladder showing rapid growth and atypical changes-A Case Report. Hepatogastroenterology. 1992;39:371–373. [PubMed] [Google Scholar]
- 63.Yamamoto M, Nakajo S, et al. Dysplasia of the gallbladder. Its histogenesis and correlation to gallbladder adenocarcinoma. Pathol Res Pract. 1989;185:454–460. doi: 10.1016/S0344-0338(89)80062-7. [DOI] [PubMed] [Google Scholar]
- 64.Park JY, Hong SP, et al. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol. 2009;24:219–222. doi: 10.1111/j.1440-1746.2008.05689.x. [DOI] [PubMed] [Google Scholar]
- 65.Bang S. Natural course and treatment strategy of gallbladder polyps. Korean J Gastroenterol. 2009;53:336–340. doi: 10.4166/kjg.2009.53.6.336. [DOI] [PubMed] [Google Scholar]
- 66.Z'graggen K, Birrer S, et al. Incidence of port site recurrence after laparoscopic cholecystectomy for preoperatively unsuspected gallbladder carcinoma. Surgery. 1998;124:831–838. [PubMed] [Google Scholar]
- 67.Reddy YP, Sheridan WG. Port-site metastasis following laparoscopic cholecystectomy: a review of the literature and a case report. Eur J Surg Oncol. 2000;26:95–98. doi: 10.1053/ejso.1999.0750. [DOI] [PubMed] [Google Scholar]
- 68.Cavallaro A, Piccolo G, et al. Managing the incidentally detected gallbladder cancer: algorithms and controversies. Int J Surg. 2014;12:S108–S119. doi: 10.1016/j.ijsu.2014.08.367. [DOI] [PubMed] [Google Scholar]
- 69.[Guideline] National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. NCCN.; 2018. Sep 1, Available at. Version 3. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shih SP, Schulick RD, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893–901. doi: 10.1097/SLA.0b013e31806beec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mainprize KS, Gould SW, et al. Surgical management of polypoid lesions of the gallbladder. Br J Surg. 2000;87:414–417. doi: 10.1046/j.1365-2168.2000.01363.x. [DOI] [PubMed] [Google Scholar]
- 72.Xu A, Zhang Y, et al. Gallbladder polypoid-lesions: what are they and how should they be treated? A Single-center experience based on 1446 cholecystectomy patients. J Gastrointest Surg. 2017 Jul 10; doi: 10.1007/s11605-017-3476-0. [DOI] [PubMed] [Google Scholar]
- 73.Boulton RA, Adams DH. Gallbladder polyps: when to wait and when to act. Lancet. 1997;349:817. doi: 10.1016/S0140-6736(05)61744-8. [DOI] [PubMed] [Google Scholar]
- 74.Shirai Y, Sakata J, et al. Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol. 2012;10:87. doi: 10.1186/1477-7819-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]