Highlights
-
•
Children aged 4.5–5.5 years completed a go/no-go task while recording EEG.
-
•
P3b amplitudes were assessed, indexing inhibition and attention processes.
-
•
Higher household income related to larger P3b amplitudes on go and no-go trials.
-
•
Results show that SES has implications for children’s neural processing.
Keywords: Socioeconomic status, Income, Executive function, P3b, ERP, Preschool
Abstract
While it is well established that lower socioeconomic status (SES) is associated with poorer executive functioning (EF), how SES relates to the neural processing of EF in childhood remains largely unexplored. We examined how household income and parent education related to amplitudes of the P3b, an event-related potential component, during one EF task. We assessed the P3b, indexing inhibition and attention allocation processes, given the importance of these skills for academic success. Children aged 4.5–5.5 years completed a go/no-task, which assesses inhibitory control and attention, while recording EEG. The P3b was assessed for both go trials (indexing sustained attention) and no-go trials (indexing inhibition processes). Higher household income was related to larger P3b amplitudes on both go and no-go trials. This was a highly educated sample, thus results indicate that P3b amplitudes are sensitive to household income even within the context of high parental education. Findings build on the behavioral literature and demonstrate that SES also has implications for the neural mechanisms underlying inhibition and attention processing in early childhood.
1. Introduction
Socioeconomic status (SES) has broad implications for development, with high SES parents able to invest time and money in their children’s development and children from lower SES families at risk for adverse outcomes including poorer health, psychological well-being, and academic achievement (see for review; Bradley and Corwyn, 2002; Hackman et al., 2010; Reardon, 2011). In particular, the SES gap in executive functioning (EF), encompassing higher-order cognitive thinking, is evident by kindergarten (Farah et al., 2006; Raver et al., 2013). Despite implications of EF for long-term academic and socioemotional outcomes (de Wilde et al., 2015; Morgan et al., 2018; Riggs et al., 2006), the mechanisms underlying SES-EF associations are not fully understood.
One promising approach is to assess how SES relates to neural mechanisms of EF at school entry, when EF is rapidly developing (Carlson, 2005; Farah, 2017). Given that many processes underlie behavioral performance, neural measures help tease apart specific aspects of processing while children perform EF tasks. Indeed, neural processing has been proposed to be a factor underlying socioeconomic gaps in cognitive development (Pavlakis et al., 2015). Thus, the brain could be the intermediary in explaining how SES shapes life outcomes (Farah, 2017) and could serve as an underlying mechanism in understanding how SES shapes EF. Additionally, the practical implication of assessing neural processes is their potential to serve as biomarkers with predictive power for later outcomes (Farah, 2017; Gabrieli et al., 2015; Raizada and Kishiyama, 2010). EF demands increase throughout school and EF becomes increasingly essential for academic success as children get older (McClelland and Cameron, 2012). Therefore, neural measures could aid in predicting EF and academic performance (Greenberg, 2006; Harms et al., 2014; Raizada and Kishiyama, 2010).
1.1. Event-related potentials and the P3b
Event-related potentials (ERPs) are a feasible method for understanding how children neurally process EF tasks (Grammer et al., 2014; Willner et al., 2015). One widely studied ERP component is the P3b, indexing inhibition processes and sustained attention (Davis et al., 2003; Eimer, 1993). It is the third positive peak in the ERP waveform and is typically assessed in parietal regions (see for review, Polich, 2007). The P3b has been assessed in children during flanker (Rueda et al., 2004) and go/no-go tasks (Willner et al., 2015). The go/no-go taps multifaceted inhibitory control processes, as children must control their attention and focus on the task but also must inhibit motor responses to the target stimulus.
The P3b is a probable candidate that may vary by SES. In one kindergarten study, P3b amplitudes were reported for the go condition only during a go/no-go where children won and lost points; and larger P3b amplitudes predicted better academic performance in first grade (Willner et al., 2015). This demonstrates the practical role of ERPs in predicting outcomes. Further, given that inhibition and attention vary by SES (e.g., Lawson et al., 2017) and these processes are critical for learning and academic success (Allan et al., 2014; Diamond, 2013), an ERP component that indexes these processes is a probable candidate to vary by SES. Moreover, research suggests the P3b increases in amplitude through adolescence (Downes et al., 2017). Taken together, it is possible that higher SES would relate to larger P3b amplitudes, perhaps indicating more developed neural processing.
1.2. Income, parent education, and neural processing
When assessing socioeconomic context, it is important to consider how to conceptualize and operationalize SES. SES is a complex construct that reflects financial resources and capital (Hackman and Farah, 2009). The most common indicators are household income, parental education, and parental occupation (see for review, Ursache and Noble, 2016). There is debate whether to combine these measures into a composite or assess them separately (Ursache and Noble, 2016). While using composite SES measures is common, others argue that SES constructs (e.g., income and parent education) have different implications for development and are conceptually distinct (Duncan and Magnuson, 2003, 2012). For instance, household income has been more related to academic success while parent education has been associated with both academic and behavioral outcomes (Duncan and Magnuson, 2003).
Additionally, these aspects of SES differentially relate to structural brain development as income was associated with cortical thickness while parent education related to hippocampal volume (Noble et al., 2015). Moreover, other studies have assessed how only one aspect of SES (such as parent education) relates to the brain in childhood (Stevens et al., 2009). It is possible that income and parent education could relate to brain development and adaptive outcomes via different mechanisms. Parent education may be more related to parenting style while income could enable access to learning materials and higher quality child-care. Therefore, assessing how income and parent education separately relate to neural EF processes would provide a more comprehensive and specific assessment of the role of the socioeconomic context.
1.3. Current study
Given that low SES children are at risk for poor EF by kindergarten entry, it is critical to understand the neural mechanisms that contribute to this disparity. The goal of this study was to take a first step and examine how indices of SES relate to the P3b in a go/no-go task in 4.5–5.5 year olds. This task taps two aspects of EF including inhibition and attention processes. Based on previous literature, we expected the P3b to be larger on no-go trials compared to go trials (Abdul Rahman et al., 2017; Davis et al., 2003). We explored the separate contributions of household income and parent education to provide specificity in how socioeconomic context may relate to neural processing. We assessed the P3b on go and no-go trials to examine whether income and parent education mattered for the P3b on sustained attention (go) or inhibition (no-go) trials, or whether there were global effects. We expected higher income and higher levels of parent education would be associated with larger P3b amplitudes.
2. Methods
2.1. Participants
Participants were 69 children (40 females) aged 4.5–5.5 years and their primary caregivers. Children spoke and understood English. Children were full-term singletons with no known hearing, visual, neurological, or developmental disorders. The primary goal of this study was to assess how income and parent education related to the P3b on go and no-go trials. Therefore to be included in analyses, (1) children had to have useable ERP data for both trial types and (2) children needed to understand the task. This ensured that ERPs included in analyses were only from children engaging in the task. Therefore while 125 children participated in the study, 56 were excluded leaving a final sample of 69. Children were excluded for the following reasons: did not understand the task (N = 3), which was defined as go accuracy was less than 70% and no-go accuracy was higher than their go accuracy; declined to complete the task (N = 6); did not have usable ERPs for both trial types (go and no-go; N = 29); had EEG technical difficulties (N = 5); had braided hair, which prevented electrodes from sufficiently contacting the scalp (N = 4); and declined to wear the EEG cap (N = 9). There were no differences in child age, child gender, income, or parent education, between the group of children included in the final sample and those excluded.
Of the final sample, 26 children attended preschool, 15 children were in kindergarten, and 28 children did not attend preschool or kindergarten. See Table 1 for demographic information of the final sample.
Table 1.
Demographics.
| Maternal age (years) | Minimum | Maximum | |
|---|---|---|---|
| M (SD) | 36.24 (5.76) | 22.00 | 48.00 |
| Paternal age (years) | |||
| M (SD) | 39.09 (7.50) | 23.00 | 56.00 |
| Child age (years) | |||
| M (SD) | 5.04 (0.27) | 4.55 | 5.52 |
| Child ethnicity | |||
| White | 46.40 % | ||
| Black | 7.20 % | ||
| Hispanic/Latino | 7.20 % | ||
| Asian | 14.50 % | ||
| Multiracial | 24.60 % | ||
| Income-to-needs ratio; M (SD) | 4.88 (3.72) | 0.42 | 18.42 |
| Parent Education Average; M (SD) | 4.03 (1.03) | 1.50 | 5.00 |
Effort was made to recruit a sample across the SES spectrum. Thus 38.20% of the sample was at or below an income-to-needs (ITN) ratio of 3.0, meaning they had an income less than three times the federal poverty line, given their household size. Given the high cost of living in the city where this study was conducted, an income below the threshold of 3 times the federal poverty line is considered financially strained (Ames, Lowe, Dowd, Liberman, & Youngblood, 2013). See Table 2 for a breakdown of the income and parent education distribution in the sample.
Table 2.
ITN and Parent Education Information.
| ITN grouping | Frequency | Percent | Cumulative Percent |
|---|---|---|---|
| 0.00–1.00 | 5 | 7.40 % | 7.40 % |
| 1.00–2.00 | 10 | 14.70 % | 22.10 % |
| 2.00–3.00 | 11 | 15.90 % | 38.20 % |
| 3.00–4.00 | 9 | 13.20 % | 51.50 % |
| 4.00–5.00 | 5 | 7.40 % | 58.80 % |
| 5.00–6.00 | 7 | 10.30 % | 69.10 % |
| 6.00–7.00 | 6 | 8.80 % | 77.90 % |
| 7.00–8.00 | 4 | 5.90 % | 83.80 % |
| 8.00–9.00 | 4 | 5.90 % | 89.70 % |
| 9.00–10.00 | 1 | 1.50 % | 91.20 % |
| >10.00 | 6 | 8.80 % | 100 % |
| Maternal Education | |||
| Some middle school or some high school | 3 | 4.30 % | 4.30 % |
| High school graduate or GED | 3 | 4.30 % | 8.70 % |
| Some college | 8 | 11.60 % | 20.30 % |
| 4-year college degree | 20 | 29.00 % | 49.30 % |
| Graduate degree | 35 | 50.70 % | 100.00 % |
| Paternal Education | |||
| Some middle school or some high school | 2 | 2.90 % | 2.90 % |
| High school graduate or GED | 12 | 17.60 % | 20.60 % |
| Some college | 8 | 11.80 % | 32.40 % |
| 4-year college degree | 14 | 20.60 % | 52.90 % |
| Graduate degree | 32 | 47.10 % | 100.00 % |
Note: ITN refers to the level of a household’s income relative to the federal poverty line. Thus an ITN of 1.00 means that a family has an income at the federal poverty line. An ITN of 2.00 means that family has an income 2x that of the federal poverty line, etc.
2.2. General procedure
Participants were recruited from a department-maintained database of families interested in research; from publicly available state birth records; from online advertising; and through face-to-face-recruitment events at Head Starts, diaper banks, community play groups, and a community health center. This study was approved by the university Institutional Review Board. Parent-child dyads visited the laboratory for one session lasting 1.5–2.0 h. Following informed consent, the experimenter presented the child with a sticker card with the child’s name on it and explained that by working hard at the game, they could earn stickers. Next the experimenter placed the EEG cap on the child’s head. While EEG was recording, the child completed a computerized go/no-go task in an electrically shielded booth to prevent interference with the EEG signal. The task was administered via E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA, USA). Next the child completed assessments of receptive language and nonverbal IQ. The parent completed a demographics questionnaire.
2.3. Measures
2.3.1. Go/No-go
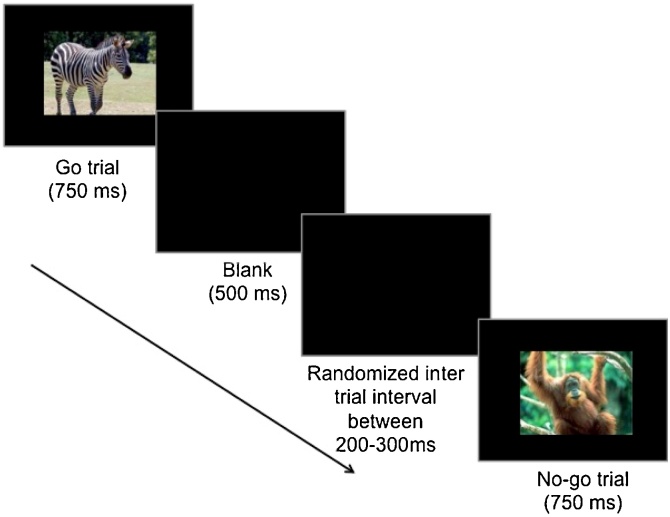
Children were told that all of the animals had escaped from their cages at the zoo and the zookeeper needed their help to catch them (He et al., 2010; Lamm et al., 2014), but that the friendly orangutans are helping them catch the animals. Therefore children were told to press a button to catch the animals each time they saw an animal (go trial) but not to press the button when they saw an orangutan (no-go trial). Thus children had to inhibit their dominant responses on no-go trials. Children completed 18 practice trials and the rules were repeated halfway through the task. Each trial consisted of an animal stimulus presented for 750 ms and then a blank screen presented for 500 ms. Each trial was followed by a blank screen with a randomized inter-trial interval between 200–300 ms. Children could respond during the presentation of the stimulus or on the blank screen. Therefore, the trial ended either at the end of the 500 ms blank screen or when the child pushed the button, whichever came first (Fig. 1).
Fig. 1.
Visual depiction of the go/no-go task. Children could respond during the stimulus presentation or during the 500 ms blank screen.
Children completed a total of 280 trials of which 75% were go trials and 25% were no-trials. The trials were broken up into four blocks. Children were shown a map of the zoo at the beginning of the task and after each block so they could track their progress and they received two stickers at the end of each block. Accuracy was computed for each trial type (go and no-go) and block. The go trials index sustained or selective attention while the no-go trials index actual inhibition processes (Lewis et al., 2017; Willner et al., 2015). Reaction time was calculated as the mean reaction time on correct go trials only. Trials with reaction times < 150 ms were excluded prior to computing the mean reaction time. The task took around 12 min to complete. See Table 3 for task descriptive statistics.
Table 3.
Task Descriptive Statistics.
| M (SD) | Min | Max | N | |
|---|---|---|---|---|
| Mean P3b amplitude for go trials | 16.68 (9.47) | −4.17 | 43.04 | 69 |
| Mean P3b amplitude for no-go trials | 19.83 (11.86) | −6.71 | 47.52 | 69 |
| Accuracy in go trials | .92 (.06) | .74 | 1.00 | 69 |
| Accuracy in no-go trials | .71 (.17) | .33 | .99 | 69 |
| Reaction time in accurate go trials (ms) | 658.87 (66.60) | 504.18 | 816.84 | 69 |
| Language | 105.91 (13.33) | 78.75 | 135.37 | 67 |
| Nonverbal IQ | 102.00 (10.97) | 83.00 | 141.00 | 67 |
2.4. Household income
Parents reported on household income and household composition. An ITN ratio based on the federal poverty level was computed with income and household composition.
2.4.1. Parent education
The parent reported highest maternal and paternal educational level. They were coded from 1 (some middle school or high school) to 5 (graduate degree). Scores were averaged to yield a parent education composite.
2.4.2. Tested covariates
2.4.2.1. Language
Children completed the picture vocabulary test (normed for ages 3–85) from the National Institutes of Health Toolbox Cognition Battery (Gershon et al., 2014). This measures receptive vocabulary and uses a computerized adaptive format based on performance. The child hears a word and sees four photographs on the screen and is asked to select the picture that most closely matches the meaning of the word. Age-adjusted scores were used. Two children did not have scores due to technical difficulties.
2.4.2.2. Nonverbal IQ
The matrices sub-scale of the Kaufman Brief Intelligence Test, Second Edition, was used. The assessment is multiple choice and involves the child pointing to pictures that reflect an understanding of both meaningful and abstract relationships. The task takes 5–10 min to complete. Age-adjusted scores were used. This assessment was not administered to two children.
2.5. Electrophysiological recording and analysis
EEG was recorded to a vertex reference using NetStation acquisition software and a Net Amp 300 amplifier (Electrical Geodesics, Inc.: Eugene, OR) connected to a Geodesic Sensor Net with 128 electrodes spaced ∼1 cm apart over the scalp. Prior to use, the 128 lead high-density net was soaked for 10 min in an electrolyte solution (6cc potassium chloride/liter distilled water) to facilitate electrical contact between the scalp and electrodes. Prior to recording, impedances were lowered by administering small amounts of the electrolyte solution to electrodes with poor contact. Data were sampled from all channels at 500 Hz.
Offline data was processed using NetStation. A bandpass filter of .3–30 Hz was applied. Continuous EEG data was then was segmented time-locked to trial onset from -100 prior to the trial to 1000 ms after the trial. As with the behavioral data, only trials in which the child responded correctly were included and additionally for go trials, when reaction time was >150 ms. Each segment was baseline corrected, using the mean voltage in the 100 ms prior to stimulus onset. Next, an automatic artifact rejection paradigm identified channels with excessive artifact (> 150 μV). In addition, segments with eye blinks (>140 μV differential average) or eye movements (>100 μV differential average) were excluded. Next bad channels were replaced via interpolation and segments for each child were averaged within each trial type (go and no-go) and re-referenced to the average reference. Each child had to have at least 10 useable trials for each trial type to be included in analyses. For the final sample, this resulted in an average 63.45 (SD = 32.45) go trials and 24.59 (SD = 12.11) no-go trials.
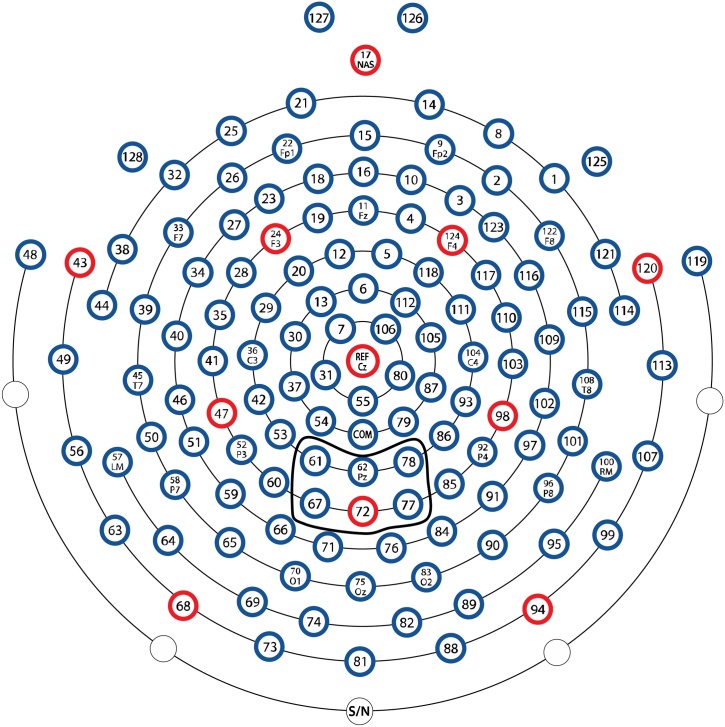
Mean P3b amplitude was computed in the time window 400–700 ms post-stimulus in the parietal region for go trials and no-go trials. This time window was selected to be generally consistent with past research (McDermott et al., 2012; Willner et al., 2015). Visual inspection of the grand-averaged waveforms confirmed that the P3b occurred in the window from 400 to 700 ms post-stimulus. Further, individual waveforms were inspected and time windows were adjusted if needed to ensure that the P3b was represented. This was the case for four children. For one child, the revised time window was 300–600 ms and for three other children, the time windows were adjusted to 350–650 ms. Visual inspection indicated that the P3b was maximal in the parietal region, consistent with past research (Davis et al., 2003; McDermott et al., 2012; Willner et al., 2015). The following electrodes with their corresponding 10–20 system sites were averaged into one parietal region: 61 (P1), 62 (Pz), 67 (PO3), 72 (POZ), 77 (PO4), 78 (P2; see Fig. 2). The mean amplitude of the go P3b was not correlated with the number of useable go segments, r (67) = .008, p = .95, and the mean amplitude of the no-go P3b was not correlated with the number of useable no-go segments, r (67) = −.06, p = .65.
Fig. 2.
The parietal electrodes used in the current study.
2.6. Analysis plan
A preliminary model was first run to check that the paradigm elicited the expected differences in P3 amplitude. We expected larger P3b amplitudes on no-go trials compared to go trials. A repeated measures analysis of variance (RM-ANOVA) was used with trial type as a within-subjects factor. Next, Pearson correlations were used to assess relations of behavioral performance to the P3b. In addition, Pearson correlations were used to assess relations of ITN and parent education to behavioral performance.
To examine possible associations of child gender, age, language, and nonverbal IQ to the P3b, each covariate was included in the repeated measures model. Gender was included as a between-subjects factor and continuous variables were included as covariates. Whenever there was an effect of the covariate in the model, the covariate was included in subsequent models. In addition, the relations between child age with income and parent education were tested using Pearson correlations.
To assess relations of ITN and parent education to the P3b, we used repeated measures analyses of covariance (RM-ANCOVAs) with the P3b as the dependent measure. Trial type (go or no-go) was included as a within-subjects factor. ITN and parent education were included as predictors in separate models. This allowed us to test for main effects of ITN and parent education as well as interactions. Statistically all continuous variables were entered as covariates (Hoffman, 2015; Sweet and Grace-Martin, 2011). We use the term “predictor” for ITN and parent education to distinguish from variables that we treated as potential covariates (e.g., age).
For all ANOVAs, post hoc analyses followed significant main effects, using Bonferroni corrections for multiple testing. In ANOVAs where the assumption of sphericity was violated, we used Greenhouse–Geisser corrections.
3. Results
3.1. Preliminary analyses
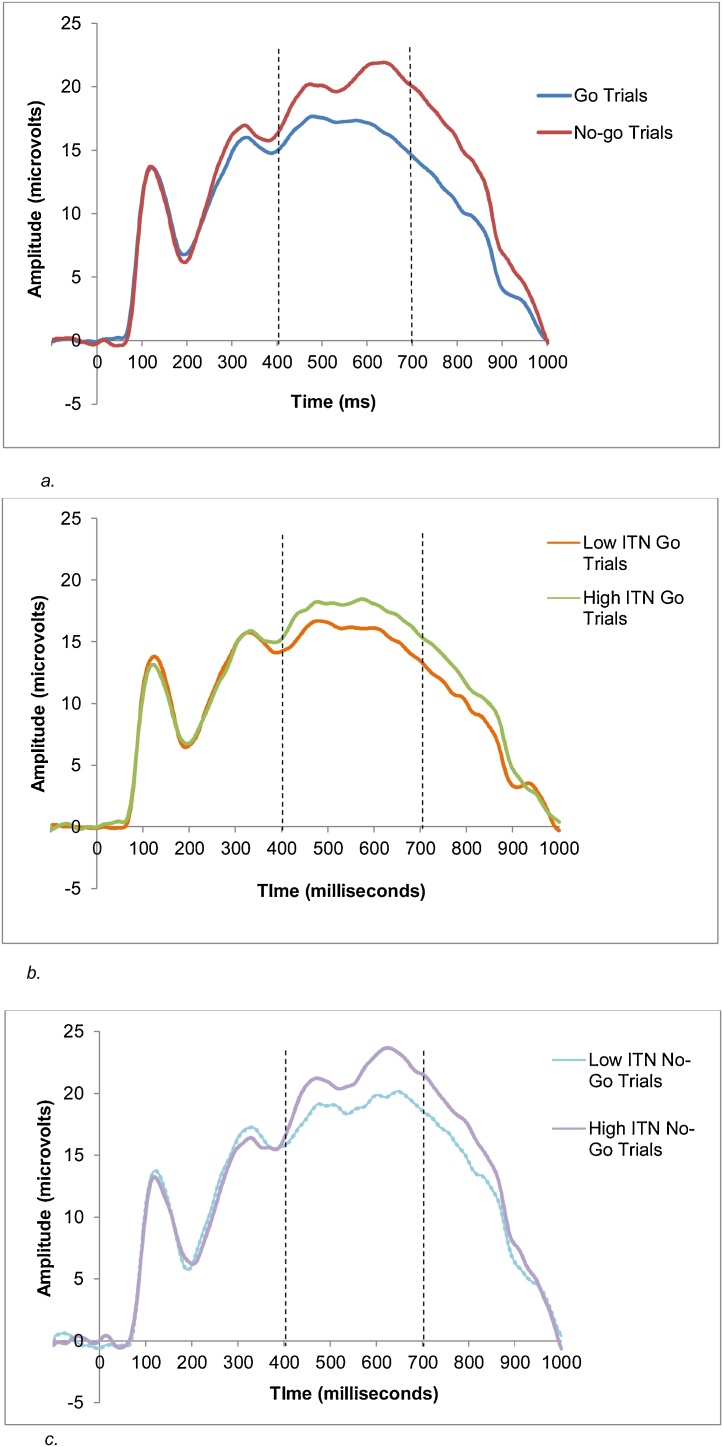
We first used a RM-ANOVA to test for an effect of trial type. As would be expected, there was a main effect of trial type, F (1, 68) = 18.10, p < .001, ηp2 = .21, such that P3b amplitudes were larger on no-go trials (M = 19.83 μV, SD = 11.86) compared to go trials (M = 16.68 μV, SD = 9.47). See Fig. 3a.
Fig. 3.
a. Grand-averaged waveform of go and no-go trials for the entire sample in the parietal region. The P3b was calculated as the mean amplitude from 400 to 700 ms (seen with the dashed lines). Four children had adjusted time windows to ensure the P3b was represented. For one child, their time window was 300–600 ms and three children had time windows of 350–650 ms. Time 0 ms indicates stimulus onset. b. Grand-averaged ERP waveforms for low and high ITN groups for go trials. The P3b was calculated as the mean amplitude from 400 to 700 ms (seen with the dashed lines). Time 0 ms indicates stimulus onset. Four children had adjusted time windows to ensure the P3b was represented. For one child, their time window was 300–600 ms and three children had time windows of 350–650 ms. Note. A median split was used to visually depict the relation between ITN and the P3b on go trials. ITN was analyzed continuously. c. Grand-averaged ERP waveforms for low and high ITN groups for no-go trials. The P3b was calculated as the mean amplitude from 400 to 700 ms (seen with the dashed lines). Time 0 ms indicates stimulus onset. Four children had adjusted time windows to ensure the P3b was represented. For one child, their time window was 300–600 ms and three children had time windows of 350–650 ms. Note. A median split was used to visually depict the relation between ITN and the P3b on no-go trials. ITN was analyzed continuously.
Accuracy on go trials was significantly correlated with the go P3b, r (67) = .27, p = .03, such that higher accuracy related to larger go P3b amplitudes. No other correlations associating behavioral performance and P3b amplitudes were significant. See Table 4 for correlations of study variables. In regards to behavioral performance and SES indices, ITN and parent education did not relate to accuracy or reaction time.
Table 4.
Correlations of Study Variables.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.ITN | – | ||||||||||
| 2.EDU | .47*** | – | |||||||||
| 3.P3b go amp | .26* | .17 | – | ||||||||
| 4.P3b no-go amp | .22† | .12 | .86*** | – | |||||||
| 5.ACC go amp | .12 | .20† | .27* | .18 | – | ||||||
| 6.ACC no-go amp | .06 | −.03 | .00 | −.13 | −.10 | – | |||||
| 7.RT go | −.01 | .07 | −.12 | -.09 | -.54*** | .48*** | – | ||||
| 8.Lang- uage |
.33** | .19 | .12 | .11 | −.01 | .09 | .09 | – | |||
| 9.Non- verbal IQ |
.19 | .04 | −.03 | −.02 | −.06 | .27* | .03 | .44*** | – | ||
| 10.Age | .02 | .02 | .11 | .07 | .41*** | −.08 | 0.24† | −.22† | −.14 | – | |
| 11.Gender | -.04 | -.03 | .15 | −.03 | −.16 | .19 | .03 | −.09 | −.18 | −.23† | – |
Note. ITN = income-to-needs ratio; EDU = parent education; amp = amplitude; ACC = accuracy; RT = reaction time.
p < 0.10.
p < 0.05.
p < 0.01.
p <0.001.
Age, language, and nonverbal IQ did not relate to P3b amplitude when included in the RM-ANCOVA, with trial type (go and no-go) as a within-subjects factor. When gender was included, there as a trial type x gender interaction, F (1, 67) = 6.31, p = .014, ηp2 = .09. While the parameter estimates did not show a significant gender difference on either trial type, the pattern suggested that females had larger P3b amplitudes on go trials (M = 17.91 μV, SD = 9.38) compared to males (M = 14.99 μV, SD = 9.51). There were no differences in no-go P3b amplitude in females (M = 19.53 μV, SD = 12.02) compared to males (M = 20.23 μV, SD = 11.84). Gender was thus included in subsequent models. Finally, child age was not significantly correlated with ITN or parent education (see Table 4).
3.2. Relations of ITN and parent education to P3b amplitudes
We ran two RM-ANCOVAs to test the roles of ITN and parent education. In the first model, trial type (go or no-go) was a within-subjects factor, gender was a between-subjects factor, and ITN was a predictor. There was a main effect of ITN, F (1, 65) = 4.21, p = .04, ηp2 = .06. Assessment of the parameter estimates indicated that higher ITN was associated with larger P3b amplitudes (see Fig. 3b and c). There was no trial type x ITN interaction.
In the next model to test for effects of parent education, we used a RM-ANCOVA with trial type (go or no-go) as a within-subjects factor, gender as a between-subjects factor, and parent education as a predictor. There was no main effect of parent education or interactions with parent education.
4. Discussion
We examined how indices of SES related to electrophysiological processing of a go/no-go task in early childhood. We focused on the P3b, an index of inhibition and attention allocation processes, given the relevance of this ERP component as a predictor of later academic outcomes. Children aged 4.5–5.5 years completed a go/no-go task and P3b amplitudes were calculated for go and no-go trials. Thus we focused on two aspects of EF that were assessed in the task: inhibitory control and attention processes. Even though the sample was highly educated, results showed that higher household income was associated with larger P3b amplitudes of both go and no-go trials. Given the SES disparities in EF and ultimately academic achievement, results highlight the potential relevance of neural processing as a mechanism to understand these behavioral differences.
To our knowledge, this is the first study to demonstrate that the P3b in early childhood varies by socioeconomic context, specifically household income. Studies have begun to assess the neural bases of EF in early childhood (e.g., Downes et al., 2017) and the association between SES and behavioral measures of EF is well-established (e.g., Lawson et al., 2017). Yet to our knowledge this is one the first studies to examine how SES also matters for neural processing of some aspects of EF during a go/no-go task. We focused on the P3b as it indexes complex attention processes and inhibition (Polich, 2007), all relevant aspects of EF that are critical for academic success (Allan et al., 2014). Results indicate the P3b is sensitive to the environment, generally consistent with one study finding that P3b amplitudes were larger on no-go trials for never institutionalized children compared to a foster care group (McDermott et al., 2012). However, P3b amplitudes did not differ on go trials and the nature of this sample should be acknowledged, such that the foster care group was comprised of children who were originally raised in institutional care and thus experienced extreme early psychosocial deprivation. In addition, this result builds on the small body of literature that has found SES differences on ERPs of auditory selective attention (D’Angiulli et al., 2008; Stevens et al., 2009) and visual target detection (Kishiyama et al., 2008). Our finding demonstrates that SES also is important for neural attention and inhibition processes that are required to perform go/no-go task.
Past research speaks to the predictive power of the P3b such that higher P3b amplitudes in kindergarten predicted better adaptive learning behaviors in first grade (Willner et al., 2015). Concurrent relations have also been found between the P3b and academic achievement (Harmon-Jones et al., 1997; Hillman et al., 2012), suggesting the importance of neural inhibition and attention processing for school success. We extend this literature by assessing how the socioeconomic context may play a role in the development of the P3b, as we show SES linked differences in the P3b by school entry. Future research is needed to longitudinally assess these constructs throughout early childhood to more comprehensively understand how the P3b may mediate relations between SES and later EF and academic outcomes.
An important question is why we see differences in P3b amplitude by household income. This is notable, given that this sample was highly educated. Thus, even within this highly educated sample, income mattered. SES co-occurs with other risks and income could thus reflect different aspects of the child’s environment. Families with lower incomes may only be able to afford to live in areas with more environmental risks and toxin exposure (see for review, Evans, 2004; Hackman and Farah, 2009) which could negatively affect brain development. Chronic stress also is a probable mechanism (Hackman and Farah, 2009). Living on a low income could be stressful for many reasons including worry about affording rent or enough food. Indeed, lower income families have more food insecurity, which affects cognitive development (Johnson and Markowitz, 2018). Lower-income parents may work multiple jobs and have less time for quality interactions with their children. Finally, parents may not be able to financially invest in cognitively stimulating learning materials and trips (Bradley and Corwyn, 2002). It may be that these aspects of SES (e.g., chronic stress) are more affected by income and less closely associated with parent education, and could in part help explain our result linking household income and child neural processing. For instance, even if parents are less educated, if they have a higher income, they likely would not experience the stressors and risks discussed above.
While a portion of our sample was economically strained, given the high cost of living where this study was conducted, the majority of parents were college educated. Thus, we may not have had the variability necessary to detect effects of parent education. Future research including a more diversely educated sample is critical to assessing the role of parent education for the P3b. It is possible with more education variability we would see differences in the P3b. Indeed, income and parent education were linked to different aspects of brain structure (Noble et al., 2015). Parent education has also related to ERPs of auditory selective attention (Stevens et al., 2009). Future research including a sample that spans income and parent education continuums, as well as assessing ERPs of different aspects of EF, is needed to better understand how these SES indices matter for EF neural processing.
A strength of our study is that we assessed SES continuously. We demonstrate that differences in neural processing are not only present when comparing extreme SES groups, but are evident across the income spectrum. This is consistent with a study showing variation in brain structure on an SES continuum (Noble et al., 2015) and also with a meta-analysis, which showed child behavioral EF varied across the SES spectrum (Lawson et al., 2017). In addition, we build on past studies which have used dichotomous groups to demonstrate SES differences in neural processing (D’Angiulli et al., 2008; Kishiyama et al., 2008; Stevens et al., 2009). However, a limitation is that we did not pre-register our hypotheses, specifically that we expected higher levels of income and education to be associated with higher P3b amplitudes.
We also assessed how SES related to behavioral performance on the go/no-go task.
ITN and parent education did not relate to any behavioral measure. Thus, within our subsample that had useable ERP data, SES differences in P3b amplitudes were more pronounced than behavioral differences. However, past research has found SES differences on behavioral performance of the go/no-go task (Noble et al., 2007, 2005). Future research including a larger sample in a longitudinal study is critical to more thoroughly examine the role of SES for both the P3b and behavioral performance.
Our data suggested that the go/no-go task did elicit the expected P3b amplitudes. As we anticipated and consistent with the literature (Abdul Rahman et al., 2017; Davis et al., 2003; Falkenstein et al., 1999), P3b amplitudes were larger on no-go trials compared to go trials. In addition, there was a link between behavioral performance and the P3b such that children who had higher accuracy on go trials had larger P3b amplitudes on go trials. This suggests that the P3b does indeed index neural processing of sustaining or maintaining attention.
While to our knowledge, no studies have assessed how indices of SES relate to P3b amplitudes on a go/no-go task, we did not expect an SES by trial type interaction as behavioral differences by SES are typically seen for both inhibitory control and attention (Dilworth-Bart et al., 2007; Mezzacappa, 2004; Noble et al., 2005). In our study, SES did not differentially relate to the neural processing of sustained attention (i.e., go trials) and to inhibition (i.e., no-go trials). Instead, we saw a global effect such that children from families with higher incomes had larger P3b amplitudes on both of these trial types. This is consistent with SES-linked behavioral differences in both inhibitory control and attention. It is possible that the variation in the P3b by income level indicates the extent to which children recruit neural systems. Research suggests that P3b amplitudes increase with age through adolescence (see for review, Downes et al., 2017). Thus it is possible that children from higher income families are showing more mature neural processing and therefore show increased P3b amplitudes on both go and no-go trials. A longitudinal study assessed ERPs in low and higher SES children at ages 4 and 5 using an auditory selective attention task (Wray et al., 2017). Results showed that low SES children at age 5 showed similar ERP patterns to the high SES children at age 4, suggesting that low SES children were delayed relative to their high SES peers. Future research exploring how income level relates to the P3b over time is needed to further tease apart this possibility.
Assessing the neural correlates of inhibition and attention is important for its potential to help explain SES differences in behavioral EF and academic success. An important future direction is to assess longitudinal relations between SES, ERPs, and later outcomes to move towards understanding how neural processing may be a mechanism for understanding how SES impacts later outcomes. Further, the current study only included one task that tapped certain aspects of EF. Future research including tasks that index additional EF skills, such as working memory and cognitive flexibility, are critical for understanding the role of SES for children’s neural processing. Additionally, while this study cannot speak to causal relations between SES and neural processing, it contributes to characterizing SES differences in cognitive functioning. This can help inform the development of experimental designs to test for causal relations, with eventual implications of designing target interventions (Hackman and Farah, 2009). Moreover, our findings expand upon the broader literature on effects of SES for the developing brain by demonstrating that SES relates to the neural processing of inhibition and attention processes. Together, this body of research underscores how early in life the brain is sensitive to socioeconomic context. This has serious implications for policy efforts to address the socioeconomic gap early before SES differences are entrenched.
Given that ERPs may be a tool to identify children at risk, information on how specific indices of SES are shaping ERPs can better inform policy and intervention efforts. We demonstrate that by preschool age, children are already showing differences in neural processing, with implications for later inhibition and attention processes. It is noteworthy that income was implicated for neural processing, despite the high level of education in our sample. This fits in with a recent movement for boosting family incomes with the hopes of improving child outcomes (Duncan et al., 2014).
The nature of the cognitive processes indexed by the P3b is complex and researchers offer different interpretations of what the P3b practically means. In addition to being interpreted as an index of attention, inhibition and controlled processing (Polich, 2007), the P3b has also been proposed to index context updating and working memory processes (Donchin, 1981). In a way, working memory is inherently involved in all tasks, given that to perform a task correctly, one must hold the instructions/rules in working memory. Indeed, larger P3b amplitudes have been related to better performance on the backward digit span, an assessment of working memory (Brydges et al., 2014). Regardless of the exact interpretation of the P3b, our study is a first step in demonstrating that neural processing of inhibition and attention processes varies by socioeconomic context. Future research is needed to more precisely characterize the nature of this relation.
5. Conclusion
The relation of lower SES and poorer behavioral EF is well established. We demonstrate that by kindergarten entry, there are already SES-linked differences in the neural processing of inhibitory control and sustained attention. Specifically, higher income related to larger P3b amplitudes in 4.5–5.5 year olds, despite the highly educated nature of the sample. This study adds to the growing literature on effects of SES on the developing brain and demonstrates that household income is important for neural processes of attention and inhibition.
Declaration of Competing Interest
The authors have no conflicts of interest to report.
Funding
This research was supported by the American Psychological Association of Graduate Students Basic Psychological Research Grant and the Boston University Clara Mayo Memorial Fellowship to Ashley St. John.
Acknowledgements
We thank Basak Oztahtaci and the Brain and Early Experiences Lab for assistance in data collection. We are extremely grateful to the children and families who participated, without whom this work would not be possible.
Contributor Information
Ashley M. St. John, Email: astjohn@bu.edu.
Kayla Finch, Email: kfinch@bu.edu.
Amanda R. Tarullo, Email: Atarullo@bu.edu.
References
- Abdul Rahman A., Carroll D.J., Espy K.A., Wiebe S.A. Neural correlates of response inhibition in early childhood: evidence from a Go/No-go task. Dev. Neuropsychol. 2017;42(5):336–350. doi: 10.1080/87565641.2017.1355917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan N.P., Hume L.E., Allan D.M., Farrington A.L., Lonigan C.J. Relations between inhibitory control and the development of academic skills in preschool and kindergarten: a meta-analysis. Dev. Psychol. 2014;50(10):2368–2379. doi: 10.1037/a0037493. [DOI] [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F. Socioeconomic status and child development. Annu. Rev. Psychol. 2002;53(1):371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- Brydges C.R., Fox A.M., Reid C.L., Anderson M. Predictive validity of the N2 and P3 ERP components to executive functioning in children: a latent-variable analysis. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.M. Developmentally sensitive measures of executive function in preschool children. Dev. Neuropsychol. 2005;28(2):595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- D’Angiulli A., Herdman A., Stapells D., Hertzman C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22(3):293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- Davis E.P., Bruce J., Snyder K., Nelson C.A. The X-trials: neural correlates of an inhibitory control task in children and adults. J. Cogn. Neurosci. 2003;15(3):432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- de Wilde A., Koot H.M., van Lier P.A.C. Developmental links between children’s working memory and their social relations with teachers and peers in the early school years. J. Abnorm. Child Psychol. 2015;44(1):19–30. doi: 10.1007/s10802-015-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth-Bart J.E., Khurshid A., Vandell D.L. Do maternal stress and home environment mediate the relation between early income-to-need and 54-months attentional abilities? Infant Child Dev. 2007;16(5):525–552. [Google Scholar]
- Donchin E. Surprise!… surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Downes M., Bathelt J., de Haan M. Event-related potential measures of executive functioning from preschool to adolescence. Dev. Med. Child Neurol. 2017;59(6):581–590. doi: 10.1111/dmcn.13395. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Magnuson K.A. Off with hollingshead: socioeconomic resources, parenting, and child development. In: Bornstein M.H., editor. Socioeconomic Status, Parenting, and Child Development. Erlbaum Associates; Mahwah, NJ: 2003. pp. 83–106. [Google Scholar]
- Duncan G.J., Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Duncan G.J., Magnuson K., Votruba-Drzal E. Boosting family income to promote child development. Future Child. 2014;24(1):99–120. doi: 10.1353/foc.2014.0008. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol. Psychol. 1993;35(2):123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Evans G.W. The environment of childhood poverty. Am. Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Hoormann J., Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999;101(2–3):267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Farah M.J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron. 2017;96(1):56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- Farah M.J., Shera D.M., Savage J.H., Betancourt L., Giannetta J.M., Brodsky N.L. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D.E., Ghosh S.S., Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. 2015;85(1):11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R.C., Cook K.F., Mungas D., Manly J.J., Slotkin J., Beaumont J.L., Weintraub S. Language measures of the NIH toolbox cognition battery. J. Int. Neuropsychol. Soc.: JINS. 2014;20(6):642–651. doi: 10.1017/S1355617714000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer J.K., Carrasco M., Gehring W.J., Morrison F.J. Age-related changes in error processing in young children: a school-based investigation. Dev. Cogn. Neurosci. 2014;9:93–105. doi: 10.1016/j.dcn.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.T. Promoting resilience in children and youth. Ann. N. Y. Acad. Sci. 2006;1094(1):139–150. doi: 10.1196/annals.1376.013. [DOI] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E., Barratt E.S., Wigg C. Impulsiveness, aggression, reading, and the P300 of the event-related potential. Pers. Individ. Dif. 1997;22(4):439–445. [Google Scholar]
- Harms M.B., Zayas V., Meltzoff A.N., Carlson S.M. Stability of executive function and predictions to adaptive behavior from middle childhood to pre-adolescence. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Degnan K.A., McDermott J.M., Henderson H.A., Hane A.A., Xu Q., Fox N.A. Anger and approach motivation in infancy: relations to early childhood inhibitory control and behavior problems. Infancy. 2010;15(3):246–269. doi: 10.1111/j.1532-7078.2009.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman C.H., Pontifex M.B., Motl R.W., O’Leary K.C., Johnson C.R., Scudder M.R. From ERPs to academics. Dev. Cogn. Neurosci. 2012;2:S90–S98. doi: 10.1016/j.dcn.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. Routledge; 2015. Longitudinal Analysis: Modeling Within-Person Fluctuation and Change. [Google Scholar]
- Johnson A.D., Markowitz A.J. Associations between household food insecurity in early childhood and children’s kindergarten skills. Child Dev. 2018;89(2):e1–e17. doi: 10.1111/cdev.12764. [DOI] [PubMed] [Google Scholar]
- Kishiyama M.M., Boyce W.T., Jimenez A.M., Perry L.M., Knight R.T. Socioeconomic disparities affect prefrontal function in children. J. Cogn. Neurosci. 2008;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Lamm C., Walker O.L., Degnan K.A., Henderson H.A., Pine D.S., McDermott J.M., Fox N.A. Cognitive control moderates early childhood temperament in predicting social behavior in seven year old children: an ERP study. Dev. Sci. 2014;17(5):667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G.M., Hook C.J., Farah M.J. A meta‐analysis of the relationship between socioeconomic status and executive function performance among children. Dev. Sci. 2017;21(2018):e12529. doi: 10.1111/desc.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis F.C., Reeve R.A., Kelly S.P., Johnson K.A. Evidence of substantial development of inhibitory control and sustained attention between 6 and 8 years of age on an unpredictable Go/No-Go task. J. Exp. Child Psychol. 2017;157:66–80. doi: 10.1016/j.jecp.2016.12.008. [DOI] [PubMed] [Google Scholar]
- McClelland M.M., Cameron C.E. Self-regulation in early childhood: improving conceptual clarity and developing ecologically valid measures. Child Dev. Perspect. 2012;6(2):136–142. [Google Scholar]
- McDermott J.M., Westerlund A., Zeanah C.H., Nelson C.A., Fox N.A. Early adversity and neural correlates of executive function: implications for academic adjustment. Dev. Cogn. Neurosci. 2012;2(Supplement 1):S59–S66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Dev. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Morgan P.L., Farkas G., Hillemeier M.M., Pun W.H., Maczuga S. Kindergarten children’s executive functions predict their second-grade academic achievement and behavior. Child Dev. 2018;0(0) doi: 10.1111/cdev.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Norman M.F., Farah M.J. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Pavlakis A.E., Noble K., Pavlakis S.G., Ali N., Frank Y. Brain imaging and electrophysiology biomarkers: is there a role in poverty and education outcome research? Pediatr. Neurol. 2015;52(4):383–388. doi: 10.1016/j.pediatrneurol.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada R.D.S., Kishiyama M.M. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front. Hum. Neurosci. 2010;4:3. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver C.C., Blair C., Willoughby M. Poverty as a predictor of 4-year-olds’ executive function: new perspectives on models of differential susceptibility. Dev. Psychol. 2013;49(2):292–304. doi: 10.1037/a0028343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon S.F. The widening academic achievement gap between the rich and the poor: new evidence and possible explanations. In: Murnane R., Duncan G., editors. Whither Opportunity? Rising Inequality and the Uncertain Life Chances of Low-Income Children. Russell Sage Foundation Press; New York, NY: 2011. pp. 91–113. [Google Scholar]
- Riggs N.R., Jahromi L.B., Razza R.P., Dillworth-Bart J.E., Mueller U. Executive function and the promotion of social–emotional competence. J. Appl. Dev. Psychol. 2006;27(4):300–309. [Google Scholar]
- Rueda M.R., Posner M.I., Rothbart M.K., Davis-Stober C.P. Development of the time course for processing conflict: an event-related potentials study with 4 year olds and adults. BMC Neurosci. 2004;5(1):39. doi: 10.1186/1471-2202-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Dev. Sci. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet S., Grace-Martin K. 4th ed. Pearson; New York, NY: 2011. Data Analysis with SPSS: A First Course in Applied Statistics. [Google Scholar]
- Ursache A., Noble K.G. Neurocognitive development in socioeconomic context: multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology. 2016;53(1):71–82. doi: 10.1111/psyp.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner C.J., Gatzke-Kopp L.M., Bierman K.L., Greenberg M.T., Segalowitz S.J. Relevance of a neurophysiological marker of attention allocation for children’s learning-related behaviors and academic performance. Dev. Psychol. 2015;51(8):1148–1162. doi: 10.1037/a0039311. [DOI] [PubMed] [Google Scholar]
- Wray A.H., Stevens C., Pakulak E., Isbell E., Bell T., Neville H. Development of selective attention in preschool-age children from lower socioeconomic status backgrounds. Dev. Cogn. Neurosci. 2017;26(Supplement C):101–111. doi: 10.1016/j.dcn.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]