Abstract
Humans generate internal models of their environment to predict events in the world. As the environments change, our brains adjust to these changes by updating their internal models. Here, we investigated whether and how 9-month-old infants differentially update their models to represent a dynamic environment. Infants observed a predictable sequence of stimuli, which were interrupted by two types of cues. Following the update cue, the pattern was altered, thus, infants were expected to update their predictions for the upcoming stimuli. Because the pattern remained the same after the no-update cue, no subsequent updating was required. Infants showed an amplified negative central (Nc) response when the predictable sequence was interrupted. Late components such as the PSW were also evoked in response to unexpected stimuli; however, we found no evidence for a differential response to the informational value of surprising cues at later stages of processing. Infants rather learned that surprising cues always signal a change in the environment that requires updating. Interestingly, infants responded with an amplified neural response to the absence of an expected change, suggesting a top-down modulation of early sensory processing in infants. Our findings corroborate emerging evidence showing that infants build predictive models early in life.
Keywords: Internal models, Predictive models, Predictive processing, Development, Event-Related potentials
1. Introduction
Uncertainty is fundamental to everyday life. When deciding on which pizza to order to which shares to buy in the stock market, we try to handle the unknown. To deal with the uncertainty embedded in life, we form internal models of how the world works. It has been suggested that our brain constantly predicts its sensory input using predictive models that are organized through a hierarchy of increasingly complex hypotheses about the states of the world. The parts of the input that cannot be predicted by one’s current model, namely the prediction errors, are propagated back to the upper levels in the hierarchy to update the internal models further (Friston, 2010).
Little is known about how internal models are generated and updated in the early years of life – a period during which infants go through rapid physical and mental developmental changes. In this study, we investigated whether 9-month-old infants spontaneously form new internal models that would allow them to make predictions in a dynamic environment. Moreover, we examined whether and how infants update their models of an experimental environment when confronted with changes.
1.1. Updating of predictive models in adults
Sudden changes in the environment elicit discrepancies between what has been predicted and what is observed that require the adjustment of internal models (Barto et al., 2013; Friston, 2010). In an fMRI study, O’Reilly (2013a, 2013b) investigated how adults update their internal models in response to changes in the environment. Their findings suggest a distinct role of parietal and anterior cingulate cortex in surprise and model updating. Using a saccadic planning paradigm, they presented adults with target stimuli that changed location every few trials. After the target had appeared several times in the same location (i.e. expected trials), two different surprising trials could follow in which the target appeared at a new unexpected location. One of them was the update trial, after which targets continued to appear around the same, new area for several trials. The other one was the one-off trial, in which the target appeared at a new location only for once and afterwards the targets continued to appear around the location prior to the one-off trial. Because the targets continued to appear at the new location only after the update trials, participants were required to update their models in update but not in one-off trials. Data revealed activation in the parietal cortex when an immediate motor response was programmed as the location of the targets unexpectedly changed in both trial types. When participants had to update their internal models to accommodate the change of target locations, anterior cingulate cortex was specifically active. These findings suggest that adults process stimuli differentially based on their information value and adjust their internal models when required to represent the statistics of the outside world accurately.
1.2. Predictive internal models in infants
Although there is ample evidence in the adult literature on how predictive internal models are optimized (Egner et al., 2010; van Pelt et al., 2016; Wacongne et al., 2011), our knowledge on how the infant brain forms and updates its predictive models is limited. In a functional near infrared spectroscopy (fNIRS) study using an omission paradigm, Emberson et al. (2015) provided the first evidence that the predictive architecture of the brain is already present as early as 6 months. Emberson et al. (2015) demonstrated that after a learning period, when images were unexpectedly omitted, infants showed activation in the occipital cortex, as if an image was presented, suggesting that they generated predictions about the visual input. Importantly, this activation was not observed, if omission was expected to happen. Measuring EEG during a cross-modal cueing paradigm, Kouider et al. (2015) provided further evidence that infants form predictions as a result of learning associations between auditory cues and visual categories and their neural responses differ dependent on the prior knowledge they acquired. Whereas early components were amplified for predicted events as compared to unexpected ones, late components, such as the positive slow wave (PSW), were enhanced by unexpected events. These studies together suggest that the infant brain is already capable of forming predictions based on prior information acquired via a brief exposure of stimuli, and that sensory activity is modulated by the violations of these predictions. Although these studies provide initial evidence on the formation of predictive models in the infant brain, it remains unknown whether infants, like adults, update their internal models in response to changes in the environment.
1.3. Neurocognitive markers of violation of predictions in infants and adults
An adult’s brain responds to the violations of predictions as early as 100 ms after onset of the violation with activation mostly observed at the frontal central regions of the brain. In the EEG, this response is reflected, for example, in the N1, which is an early event-related potential (ERP) (Hsu et al., 2014; Stefanics et al., 2014). Direct evidence on the early precursors of ERP components (such as the N1 in adults) for infants is scarce (de Haan, 2013; Marinović et al., 2014), because using the same experimental paradigm to test adults and infants is not often feasible. Still, it has been suggested that one of the most prominent infant ERP components, namely the negative central (Nc), might be considered a neural marker of violations of predicted regularities (Jeste et al., 2015). The Nc is a mid-latency component that is largely observed in young children around frontal central regions of the brain (Hoehl et al., 2008; Reynolds and Richards, 2005; Striano et al., 2006; Webb et al., 2005). In general, the infant Nc is assumed to capture how much attention infants allocate when observing stimuli (Reynolds et al., 2014; Richards, 2003). Although the precise functional significance of the Nc component is still under debate, there is considerable evidence suggesting that the Nc is amplified when stimuli are unexpected (Ackles and Cook, 2007; Jeste et al., 2015; Kaduk et al., 2013; Snyder et al., 2010). This is because unexpected events bring about involuntary capture of attention to evaluate the significance of the events before taking actions accordingly (Friedman et al., 2001). Based on the cortical source analysis of infant ERP data, Reynolds and Richards (2005) suggested that areas in the prefrontal cortex including the anterior cingulate cortex likely are the generators of the infant Nc component. Accordingly, here, we used the Nc to examine whether infants responded with surprise to unexpected changes in a predictable sequence of events.
1.4. Neurocognitive markers of updating of predictions in infants and adults
Beyond detecting change, efficient systems should use prediction errors to adjust their internal models of the world. For example, the P3b component in adults, observed 300–400 ms after stimulus onset around parietal-central channels, has been assumed to represent updating of memory representations (Polich, 2007). Interestingly, it has been shown that the P3b response is evoked also in the absence of stimuli, when a predicted input is deliberately omitted (Wacongne et al., 2011). These findings indicate that the P3b is elicited when one’s predictions are violated by the current input, which calls for an updating of the internal models (Marzecová et al., 2017; Kolossa et al., 2015). In developing populations, there is still little evidence on which electrophysiological signals potentially mark the updating of internal models (Kouider et al., 2015). Yet, a promising candidate, namely the positive slow wave (PSW), is a late infant ERP component elicited approximately 700–1000 ms after stimulus onset. The PSW has traditionally been considered to represent memory updating especially in response to only partially encoded infrequent stimuli (de Haan, 2013; Elsner et al., 2013). Moreover, the infant PSW is assumed to be an early precursor of the P3 in adults (de Haan, 2013; Marinović et al., 2014). Here, we used the PSW to examine whether updating occurred if that was required given the changes in the environment but not if no updating was needed.
Our previous research has shown that infants at 14 months of age can generate internal models that represent the statistics of a dynamic environment and adjust their internal models only when necessary (Kayhan et al., 2019). Using eye-tracking, we identified differences in saccadic latencies indicating that infants at 14 month of age can differentiate surprising information in the extent to which it is relevant to updating predictions. However, how infants’ brains form generative models that allow them to make predictions and how they respond to the violations of these predictions remain unanswered.
To have an accurate representation of their environment, infants not only need to detect changes that result in violations of their predictions but also use this information to update their internal models accordingly. However, given the vast of amount of information in the outside world, it is important to update the models selectively, that is, only if updating is beneficial for future predictions. We set out to investigate whether and how infants’ brains process unexpected information differentially and update its models only when the change is relevant for the future.
1.5. The current study
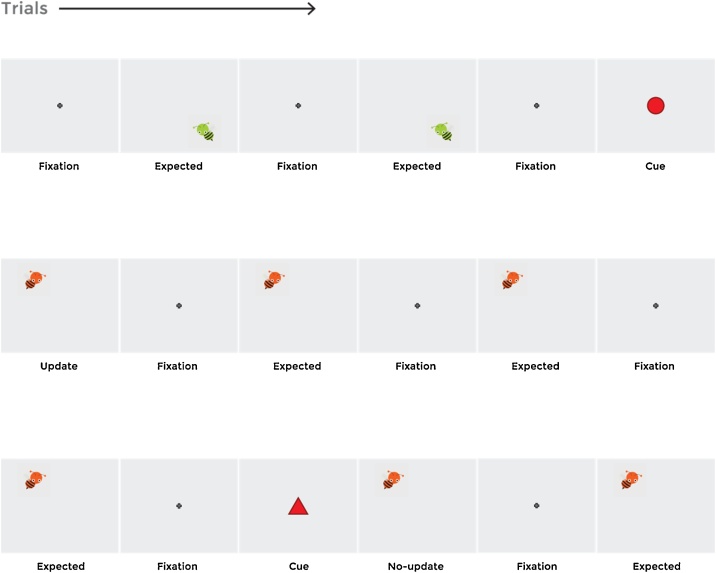
In this study, we investigated whether and how 9-month-old infants dynamically and selectively update their internal models of the world when confronted with changes. In an audio-visual EEG paradigm, we presented infants with a continuous sequence of stimuli which followed a predictable pattern (i.e. the same stimuli were repeated for several trials in a row; expected trials). Two surprising stimuli were interspersed among the expected trials that served as cues of how the sequence would continue. Following the update cue, the pattern was altered whereas the same sequence continued after the no-update cue. We hypothesized that if participants formed predictions based on the repeated observations of the predictable stimuli, they would show a prediction error response when their predictions were violated by the unexpected appearance of the cues. Moreover, if they learned the differential predictive value of the cues, they would expect the following stimuli to be different from the immediately preceding sequence after observing the update cue, whereas they would not expect any change in the sequence following the no-update cue (see Fig. 1).
Fig. 1.
Illustration of the different stimulus types within a continuous sequence of presentation. Each cue was accompanied by a sound, which was counterbalanced. Note that the bee images following the cues were analyzed separately and were not included in the expected trials. In this figure, only two colors are used for illustration purposes. In the experiment, the bees could appear in four different colors.
We defined the ERP components of interest for this study based on the literature. First, we examined the Nc in response to the presentation of the cues. We predicted that infants would show larger Nc amplitudes in response to the unexpected cues – both the update and no-update one – as compared to the expected stimuli (i.e. repetition of the same stimuli). As the sudden appearance of the cues should be equally unexpected for the two trial types, we predicted no difference in the Nc amplitude between the two types of unexpected cues at this early stage of processing.
To determine whether infants updated based on the information content of the cues at later stages of processing, we examined the PSW response. We reasoned that if infants learned the information value of the cues, the update-related activity should only be triggered in response to the update cue but not the no-update cue. Therefore, we predicted that infants would show a larger PSW in response to the update cue as compared to the no-update cue and expected stimuli. Finally, to explore infants’ internal models and their effect on subsequent sensory processing; we calculated the Nc and PSW for the stimuli following the cues. This way, we aimed at examining infants’ reactions to an observed change or absence of a change in the environment, which provides additional information on the models they generated during the experiment.
2. Method
2.1. Participants
We recruited 60 9-month-old infants (M = 272.58 days, SD = 8.86 days, range: 251–289 days, 32 girls). In total, 37 infants were excluded from the analyses. One infant was unwilling to wear the EEG cap and the other 36 infants were excluded during data processing due to a lack of sufficient artifact-free trials for the analyses (see EEG data processing). This high attrition rate is expected in infant EEG studies (Stets et al., 2012). The final sample consisted of 23 infants (M = 272.27 days, SD = 9.31 days, range: 251–288 days, 11 girls). Participants were recruited from a database of volunteer families, and parents gave written informed consent for the study. Families received baby books or a monetary reward for their participation. The local ethics review board approved the study (CMO 2012/012-NL39352.091.12).
2.2. Stimuli and design
Infants observed a continuous sequence of stimuli presented on a computer screen. The cue stimuli were circles and triangles, and the target stimuli consisted of differently colored cartoon-bees that alternated between four colors. The bees appeared on one of eight spots on an imaginary circle around the center of the screen, where the fixation image and the bees appeared, to make sure that the targets were always as far in the periphery. There were 90 expected trials, 25 update, and 25 no-update trials in the experiment.
Each trial started with a gray screen. After 500 ms, a fixation image (i.e. a cross) appeared in the center of the screen lasting for 1000 ms (see Fig. 1). In expected trials, the fixation image was followed by a bee image that had the same color and appeared at the same location (with the same sound) as in the previous trial (1500 ms). In total, an expected trial lasted for 3000 ms. In update and no-update trials, either a red triangle or a red circle respectively (1500 ms) appeared in between the fixation image and the bee image in order to provide participants with a cue that was informative about the upcoming trial type. The cues indicated whether the bee would change its location, color and sound (i.e. update trial) or would appear at the same location and in the same color as it was in the trial before (i.e. no-update trial). Together with the cues, update and no-update trials lasted for 4500 ms in total. The entire stimuli presentation took approximately 9 min.
To keep infants’ attention on the task as long as possible, we used auditory stimuli that accompanied the images. The fixation cross was accompanied by a brief beep sound to draw infants’ attention to the beginning of the trial. When the bee appeared a brief jumping sound was played that differed in pitch for the eight possible locations. The appearance of the cues (i.e. the circle and the triangle) was also accompanied by sounds. For the cues, two sounds were created by using a unique sound which was then played backwards to create the other sound to minimize the differences between the auditory information.
The colors of the bees, the shape of the cue, and all associated sounds were counterbalanced. The positions of the bees and the presentation order were randomized across participants. The visual stimuli were adjusted using open source software GIMP (version 2.8.16) and the auditory stimuli were created with the software Audacity (version 2.0.5). The experiment was implemented in Presentation Software (Neurobehavioral Systems, Inc., Albany, CA).
2.3. Procedure
Each session took approximately 60 min including a warm-up phase during which the families were informed about the EEG procedure. While EEG was recorded, infants sat on their parent’s lap in an electrically shielded testing room. Parents were instructed to keep the interaction with their child minimal during the measurement. The session was recorded via cameras, which also allowed us to continuously monitor the parent and the child during the experiment in the control room. If the infant started to get fussy, brief breaks were introduced. The experiment ended when all trials were completed or when the infant disengaged. Families were debriefed about the exact content of the experiment after the measurement.
2.4. EEG recordings
We collected EEG data using 32 Ag/AgCl active electrodes arranged in the 10–20 layout, which were placed in an infant-sized actiCap (Brain Products GmbH, Munich, Germany). The signal was amplified using a BrainAmp DC EEG amplifier, band-pass filtered with a low cut-off at 0.1 Hz and high cut-off at 125 Hz, and digitized at 500 Hz. We strived to keep all impedances below 60 kΩ. All electrodes were referenced to the left mastoid online with AFz as the ground. To record the EEG data, we used Brain Vision Recorder software (Brain Products GmbH, Germany).
2.5. EEG data processing
We processed the data in MATLAB (version R2013b, Mathworks, Inc.) using the FieldTrip toolbox (Oostenveld et al., 2011, http://www.fieldtriptoolbox.org/). First, the EEG data were time-locked to the expected, update, and no-update stimuli and segmented into epochs including 500 ms of pre-stimulus period during which the fixation cross was visible and 2000 ms of post-stimulus phase showing either the cues or the bee image.
To eliminate EEG artifacts such as eye movements, we first visually inspected the data (while being blind to conditions). We then padded the data for filtering (5 s windows), applied a high-pass filter and performed a baseline correction on the entire window. We excluded extremely noisy trials and channels before running an independent component analyses (ICA) to correct for eye movement artifacts. To ensure that eye movement artifacts were identified correctly, we visually inspected the components using topographic and time course plots to extract eye-movement components. Then, noisy channels (max. 3 channels) were interpolated using the nearest channels. Subsequently, we visually inspected the data again, excluded trials with a kurtosis value greater than 6 and rejected any remaining trials with artifacts before re-referencing the electrodes to the linked mastoids. Participants who failed to provide more than two trials per condition were excluded from the final sample. Due to the nature of the experimental paradigm and the strict preprocessing procedure to ensure inclusion of high quality data only, the amount of trials per condition was limited. Mean number of trials and standard deviations (SD) for all trial types for the final sample are shown in Table 1.
Table 1.
Mean number of trials and standard deviations (SD) for all trial types for the infants in the final sample.
| Mean number of trials and standard deviations (SD) | ||
|---|---|---|
| Trial type | Infants (N = 23) | |
| Mean | SD | |
| Update | 6.74 | 3.19 |
| No-update | 5.52 | 3.38 |
| Expected | 21.57 | 12.59 |
| Bee After Update | 7.43 | 3.91 |
| Bee After No-update | 5.96 | 3.40 |
For ERPs, we baseline-corrected the preprocessed data using a 200 ms pre-stimulus period and applied a .1–30 Hz band-pass filter. We then calculated ERP averages per participant per trial type. In line with previous studies testing infants of similar age ranges, we defined a time window between 350 to 550 ms for the Nc1 (Striano et al., 2006), and calculated the mean amplitude during this window around the frontal-central channels (i.e. Fz, FCz, Cz, FC1, FC2, C3, F3, C4, F4). Based on the literature, we investigated the PSW 700 to 1000 ms after stimulus onset (Grossmann and Johnson, 2007; Kopp and Lindenberger, 2011) around central-parietal regions (i.e. P3, Pz, P4, CP1, CP2, CP5, CP6), because ERP research in adults showed that these regions are involved in updating of generative internal models (Conroy and Polich, 2007). Outliers beyond two standard deviations were excluded from the overall trial average. For additional transparency, we provide the ERP time-courses in Fig. 2, Fig. 3.
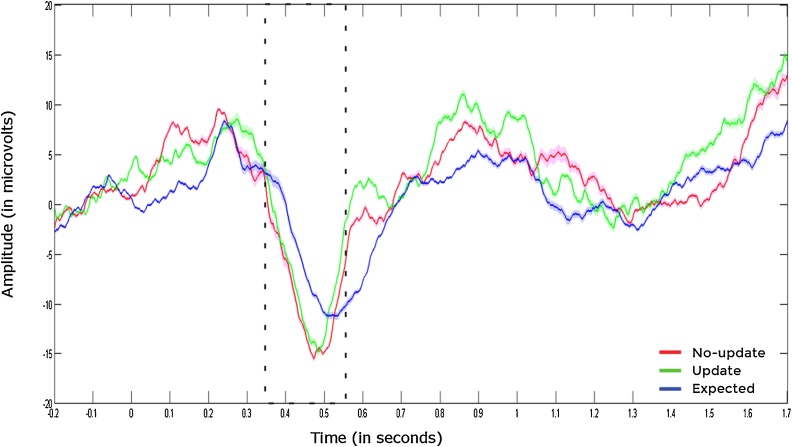
Fig. 2.
The highlighted area indicates the Nc component averaged across frontal-central electrodes (i.e. Fz, FCz, Cz, FC1, FC2, C3, F3, C4, F4) for each trial type. The blue line represents expected trials, whereas the green and red lines represent update and no-update trials, respectively. Shaded areas around the lines represent the standard error of the mean.
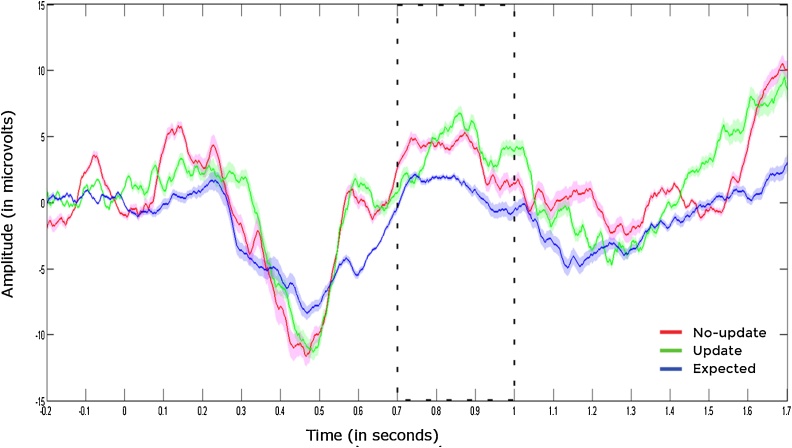
Fig. 3.
The highlighted area indicates the PSW component averaged across central-parietal electrodes (i.e. P3, Pz, P4, CP1, CP2, CP5, CP6) for each trial type. Blue line represents expected trials whereas green and red lines represent update and no-update trials, respectively. Shaded areas around the lines represent standard error of the mean.
3. Results
3.1. Negative central component (Nc)
We predicted that the cues would elicit an amplified Nc response as compared to the standard stimulus (i.e. the repeated bee images), if infants indeed perceive them as unexpected. In order to test our hypothesis statistically, we first ran a repeated measures ANOVA with trial type (expected, update, no-update) as within-subjects factor. Because Mauchly's Test of Sphericity indicated that the assumption of sphericity had been violated (p < .05), we used Greenhouse-Geisser corrections. This analysis revealed a significant main effect of trial type, F (1.46, 27.70) = 4.94, p = .023, 2 = 0.21, suggesting that infants showed differential central negativity response across the different trial types (see Fig. 2).
We ran follow-up tests using the Least Significant Differences method for pairwise comparisons of the estimated marginal means to investigate further whether the differences in amplitudes across different trials were in line with our predictions. As hypothesized, data revealed that both in the update (md = -3.89, SE = 1.17, p = 0.003) and the no-update trials (md = -5.68, SE = 2.18, p = 0.017), infants showed significantly stronger negativity in comparison to the expected trials. There was no significant difference in response between update and no-update trials (md = 1.79, SE = 2.03, p = 0.390).
3.2. Positive slow wave (PSW)
We examined whether infants showed differential responses in later components such as the PSW (see Fig. 3). Because Mauchly's Test of Sphericity indicated that the assumption of sphericity had been violated (p < .05), we used Greenhouse-Geisser corrections. A repeated measures ANOVA with trial type (expected, update, no-update) as within-subjects factor revealed a significant main effect of trial type, F (1.37, 27.45) = 4.01, p = .044, η2 = 0.17, indicating that infants’ responses in the later components differed between trials.
Based on our hypothesis on updating, we examined whether infants dissociated between the unexpected cues and showed larger positivity in update trials as compared to the other trial types. Follow-up tests using the Least Significant Differences method for pairwise comparisons of estimated marginal means revealed that participants showed more positivity in response to both update (md = 2.82, SE = 0.88, p = 0.004) and the no-update trials (md = 4.18, SE = 1.65, p = 0.020) as compared to the expected trials. This finding suggests that the unexpected information modulated infants’ responses in later stages of processing as well. However, while infants encoded the unexpected appearance of the cues differently than the predicted events, there was no indication that they dissociated between the two types of cues (md update vs. no-update = -1.37, SE = 1.82, p = 0.461).
3.3. Event related potentials in response to the stimuli following the cues
The data suggest that infants generated a model on the basis of the exposure to the experimental environment. This allowed them to form predictions about upcoming stimuli which, when violated, elicited an enhanced neural response. To explore the nature of the internal models infants might have built throughout the experiment further, we investigated their neural response to the stimuli following the update and no-update cues. These analyses enabled us to examine infants’ neural responses with respect to the change or the absence of a change in the environment signaled by the different cues, which provides further information about the content of their models. More specifically, the stimuli after the update cue were different from those in the previous expected trials (i.e. color, location and sound changed), whereas infants observed the same stimuli as in the previous expected trials following the no-update cue (see Fig. 1). Given that there were no physical differences between the stimuli following the no-update cue and those preceding expected trials, any differences in ERPs in responses to these two stimuli would inform us about how infants’ predictions were modulated by the cues.
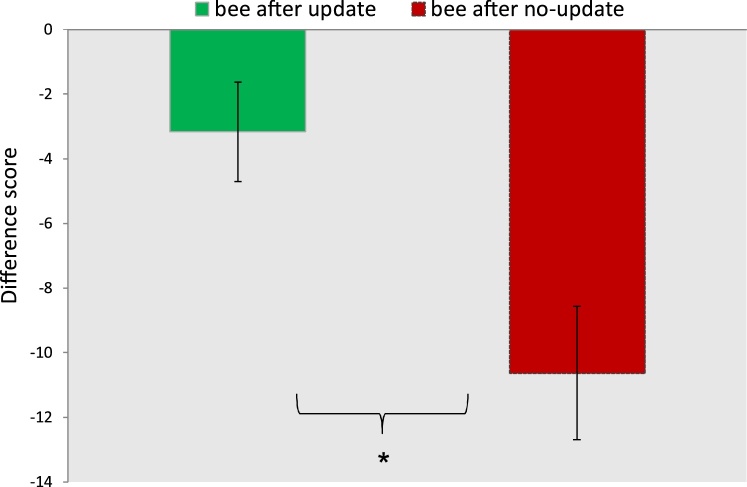
In order to directly compare the response amplitudes between the stimuli following the two types of cues, we calculated two separate difference score measures (DS) as used in the literature (Jeste et al., 2015). To obtain the first difference score, we subtracted the Nc amplitude in response to the stimuli following the update cues from the Nc amplitude in the expected trials. We followed the same procedure to calculate a difference score for the no-update trials. With a paired sample t-test, we then compared the difference scores for these two new conditions (Fig. 4). This analysis revealed a significant difference between conditions (t (22) = 3.51, p = 0.002). The difference score in the image after no-update trials (M= -10.63, SD = 9.88) was significantly larger than the difference score for the image after update trials (M= -3.16, SD = 7.37).
Fig. 4.
Difference scores for the Nc in bee after update and bee after no-update conditions. The error bars represent the standard error of the mean. *p < 0.01.
The significant difference in DS between the image after no-update and the image after update trials is especially remarkable since the stimuli before and after the no-update cues were identical. This finding implies that infants expected a change in the environment following any cue and they reacted with an enhanced neural response to the omission of this change suggesting a top-down modulation of early sensory processing in infants.
We also calculated a difference score (DS) on the PSW amplitude in response to the image following the cues separately, which we tested statistically with a paired sample t-test. This analysis revealed no significant difference between conditions (t (22) = 0.03, p = 0.976). Together, these findings suggest that infants’ observation of a change or absence of a change significantly modulated early processing stages only.
4. Discussion
In this study, we investigated whether 9-month-old infants build predictions based on a brief exposure to a sequence of audio-visual events and show error responses when these predictions are violated. Moreover, we examined whether infants use the informative value of the violations, here the different unexpected events, to adjust their internal models accordingly. Infants showed an amplified Nc when a predictable sequence was unexpectedly interrupted suggesting that they formed a model about the structure of events and responded to the violations of these predictions. The unexpected appearance of the cues modulated later components as well, namely the positive slow wave (PSW). However, we found no evidence for a differential response to the two types of unexpected cues at later stages of processing suggesting that infants’ predictive models were not modulated differentially based on whether the unexpected information required model updating or not. We further examined infants’ responses to the change (or absence of a change) in the target stimuli following the cues to understand the models infants generated during the experiment. Infants showed an enhanced response to the absence of a change. In other words, instead of predicting an upcoming continuation of the previous pattern as indicated by the no-update cue, infants rather generated one model: surprising cues always signal a change in the environment that requires updating.
Why did infants form expectations based on the update cue whereas they did not seem to learn what the no-update cue signaled? First, even though the two cues had a different shape and were accompanied by a different sound, it might simply be that the cues were perceptually too similar. Therefore, infants possibly formed only a single model, namely that unexpected stimuli were followed by a change in the sequence. It could also be that the task in the current study was too demanding for 9-month-old infants, as they had to associate the unexpected cues with a future change in the predicted sequence. In other words, infants had to form associations between two temporally distinct stimuli for the update and no-update trials separately, and keep these associations in memory to perform the task. Thus, it might be that 9-month-old infants might lack the required working memory skills to perform the current task as expected.
An alternative explanation might be that limited cognitive resources might have led infants to process a stimulus that did not signal any change in the environment (i.e. distractor) to a lesser extent than a stimulus that was relevant for the future (Wills et al., 2007). Although infants learned that a change would occur in the predicted sequence following the update cue, they did not have any information about how exactly the stimulus would change (i.e. the new position, tone and color remained unpredictable). However, following the no-update cue, it was certain that the image would be the same as the one in the previous sequence (i.e. the same position, tone and color). Because the update cue represented more uncertainty, hence more information gain, than the no-update cue, infants might have used this cue to form a model about the experimental environment in order to use their limited resources maximally (Gottlieb, 2012).
We showed that both early and late sensory responses were modulated by predictions generated by the infants’ internal models. When infants observed a sudden interruption in the predicted sequence of events, they showed a prominent Nc response. The unexpected appearance of the cues modulated a late component (i.e. the PSW) as well, observed in the posterior regions of the brain. Our results show similarities with the recent findings in the infant literature indicating that late components such as slow waves are modulated by unexpected events, potentially representing further consolidation of internal models (Kouider et al., 2015). In this study, as infants likely associated all cues with future changes in the sequence, it is reasonable to assume that the PSW responses to both types of cues indicated the updating of a single model (i.e. all unexpected cues signal change thus require updating). These findings are also in line with adult studies showing that the P3b observed in posterior channels represents adjustments in generative internal models (Marzecová et al., 2017; Kolossa et al., 2015). The current study provides further support to the recent theoretical arguments proposing that although internal models advance given maturation and increased experience during life span, a basic form of a model generation system might be functional early in life (Emberson et al., 2015).
One might argue that infants’ neural responses to the cues reflect simple adaptation processes rather than the modulation of a predictive internal model (Garrido et al., 2009). According to this view, the repetition of the same input stimulates the same pathways resulting in the adaptation of the synapses, thus, reducing the activation in response to the expected stimuli. However, the rare stimuli activate pathways that were not exploited by the repetitive observation of the same stimuli, which results in distinct neural responses to unexpected stimuli (Wacongne et al., 2011). Relatedly, it could be that infants responded to the unexpected stimuli in a bottom-up manner and did not generate a predictive model. This interpretation seems to be unlikely because if infants’ responses were not modulated by top-down predictions, we would have observed no difference in activation patterns following the cues in the early components, in particular, after the no-update cue. Infants’ amplified Nc responses to the bees following the no-update cue as compared to the update cue suggest that infants have likely built one general model. That is, surprising events (i.e. cues) always signal changes in the sequence. In turn, when their predictions were violated in the absence of a change in stimuli, infants responded with an increased Nc. Thus, our data show that infants used unexpected information to generate a model of the experimental environment to make predictions and these predictions modulated their neural responses. These findings are in line with emerging evidence showing that infants form predictions based on prior experience and these top-down predictions shape their sensory processing (Emberson et al., 2018, 2015; Kouider et al., 2015).
In a recent eye-tracking study, we investigated whether and how infants and adults construct and adjust their internal models in a changing environment (Kayhan et al., 2019). Using a saccadic planning paradigm, we showed participants – 14-month-olds and adults – differently colored stimuli that appeared at an unexpected location every few trials. The colors indicated whether the subsequent stimulus would appear at a new location (i.e. update trials) or at the same location as previously (i.e. no-update trials). Results showed that 14-month-old infants did dissociate between different types of unexpected events in the extent to which they were relevant to modulating their internal models, unlike the 9-month-old infants in the current study.
What might explain the seemingly different results of these two studies? In addition to age difference between the participants, there might also have been differences in task demands. Whereas in the eye-tracking study, infants were required to differentiate between the two types of unexpected events to initiate an eye-movement to correct target locations so that they could follow the sequence, there was no behavioral cost to generating only one model in the current paradigm. Here, we chose to present the cues in the center of the screen because we aimed to minimize saccade-related artifacts in the EEG response during the update and no-update trials, which might have influenced infants’ performance in the task. In other words, infants did not have to dissociate between different types of unexpected events to prepare eye-movement responses to locations in the periphery during the critical trials (i.e. update and no-update trials). Therefore, they might have allocated their resources to form the update model only, as it was functionally more relevant as compared to the no-update model (i.e. update cue signaled a change in the environment).
Besides minor methodological differences to previous studies, developmental differences in cognitive functioning might play a role in explaining our findings. It could be that the ability to disentangle surprise from updating only develops around the end of the first year in life. Successful dissociation of surprise and update requires accurate representation of the statistical regularities in the changing environment. It has been widely reported that infants advance in their statistical learning skills by the end of their first year of life (Saffran and Kirkham, 2018, for a review). Therefore, it might be that with increasing experience in detecting statistical regularities throughout the first year of life, infants’ ability to distinguish surprise from updating develops.
As Piaget already pointed out in the past century, children likely build internal models of their environment or “schemas” based on their interactions with the world (Piaget, 1952). They use assimilation and accommodation mechanisms to integrate new information into their existing schemas to improve them further such that they represent the outside world more accurately. Although Piaget’s theoretical work has set the stage to interesting research questions, recent advances in theoretical and brain sciences provided us with cutting-edge tools that greatly extend our knowledge. Using an audio-visual EEG paradigm, here we show that infants generate predictive models of the experimental environment based on the repeated observations of a sequence of stimuli and respond to the violations of their predictions when the sequence is unexpectedly interrupted. Moreover, our findings reveal that when expected environmental changes do not occur, infants show an amplified neural response in the early stages of processing, suggesting a top-down modulation of early sensory processing in 9-month-old infants. These findings are important as they corroborate the emerging evidence suggesting that the basic machinery to build generative models might be functional early on in development.
As children get older, they might advance in generating sophisticated internal models of their environment. In Piaget’s terms, development reflects the increases in the number and complexity of the schemas that a person learned. Relatedly, although a basic system to build predictive internal models might be functional already in infancy (Emberson et al., 2015; Kouider et al., 2015), the capacity to form complex models might increase with maturation and experience. Once the models advance, more precise and detailed predictions generated by these models can be formed (Kwisthout et al., 2017). An interesting avenue for future work would be to investigate whether and how the generative models evolve during the first years of life.
To summarize, the current study shows that 9-month-old infants form and update their internal models to represent the changes in a dynamic environment. Infants formed predictions based on the statistical information and responded to the violation of these predictions with an amplified negative central (Nc). Late components such as the PSW were evoked in response to unexpected events as well; however, in contrast to our hypotheses, we found no evidence for a differential response to the informational value of surprising cues at later stages of processing. Instead, infants generated an overall update model. That is, infants associated all unexpected cues with future changes in the sequence. Remarkably, when a predicted change was omitted, infants responded with an amplified neural response suggesting a top-down modulation of early sensory processing in infants. These findings contribute to the emerging literature suggesting that the basic machinery to build predictive models might be functional early on in life.
Footnotes
It should be noted that the time window of 350 to 550 ms for the Nc analysis is after the cue onset in “update” and “no-update” trials and after bee image onset in “expected” trials. Same procedure was used for the PSW component examined 700 to 1000 ms after stimulus onset.
References
- Ackles P.K., Cook K.G. Attention or memory? Effects of familiarity and novelty on the Nc component of event-related brain potentials in six-month-old infants. Int. J. Neurosci. 2007;117(6):837–867. doi: 10.1080/00207450600909970. [DOI] [PubMed] [Google Scholar]
- Barto A., Mirolli M., Baldassarre G. Novelty or surprise? Front. Psychol. 2013;4:907. doi: 10.3389/fpsyg.2013.00907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy M.A., Polich J. Normative variation of P3a and P3b from a large sample: gender, topography, and response time. J. Psychophysiol. 2007;21(1):22–32. [Google Scholar]
- de Haan M. Psychology Press; London: 2013. Infant EEG and Event-related Potentials. [Google Scholar]
- Egner T., Monti J.M., Summerfield C. Expectation and surprise determine neural population responses in the ventral visual stream. J. Neurosci. 2010;30(49):16601–16608. doi: 10.1523/JNEUROSCI.2770-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner B., Jeschonek S., Pauen S. Event-related potentials for 7-month-olds’ processing of animals and furniture items. Dev. Cogn. Neurosci. 2013;3:53–60. doi: 10.1016/j.dcn.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Boldin A.M., Robertson C.E., Cannon G., Aslin R.N. Expectation affects neural repetition suppression in infancy. Dev. Cogn. Neurosci. 2018:100597. doi: 10.1016/j.dcn.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emberson L.L., Richards J.E., Aslin R.N. Top-down modulation in the infant brain: learning-induced expectations rapidly affect the sensory cortex at 6 months. Proc. Natl. Acad. Sci. 2015;112(31):9585–9590. doi: 10.1073/pnas.1510343112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D., Cycowicz Y.M., Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev. 2001;25(4):355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friston K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010;11(2):127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- Garrido M.I., Kilner J.M., Stephan K.E., Friston K.J. The mismatch negativity: a review of underlying mechanisms. Clin. Neurophysiol. 2009;120(3):453–463. doi: 10.1016/j.clinph.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J. Attention, learning, and the value of information. Neuron. 2012;76(2):281–295. doi: 10.1016/j.neuron.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T., Johnson M.H. The development of the social brain in human infancy. Eur. J. Neurosci. 2007;25(4):909–919. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- Hoehl S., Wiese L., Striano T. Young infants’ neural processing of objects is affected by eye gaze direction and emotional expression. PLoS One. 2008;3(6):e2389. doi: 10.1371/journal.pone.0002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.F., Hamalainen J., Waszak F. Both attention and prediction are necessary for adaptive neuronal tuning in sensory processing. Front. Hum. Neurosci. 2014;8:152. doi: 10.3389/fnhum.2014.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste S.S., Kirkham N., Senturk D., Hasenstab K., Sugar C., Kupelian C., Baker E., Sanders A.J., Shimizu C., Norona A., Paparella T., Freeman S.F.N., Johnson S.P. Electrophysiological evidence of heterogeneity in visual statistical learning in young children with ASD. Dev. Sci. 2015;18(1):90–105. doi: 10.1111/desc.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaduk K., Elsner B., Reid V.M. Discrimination of animate and inanimate motion in 9-month-old infants: an ERP study. Dev. Cogn. Neurosci. 2013;6:14–22. doi: 10.1016/j.dcn.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayhan E., Hunnius S., O’Reilly J.X., Bekkering H. Infants differentially update their internal models of a dynamic environment. Cognition. 2019;186:139–146. doi: 10.1016/j.cognition.2019.02.004. [DOI] [PubMed] [Google Scholar]
- Kolossa A., Kopp B., Fingscheidt T. A computational analysis of the neural bases of Bayesian inference. Neuroimage. 2015;106:222–237. doi: 10.1016/j.neuroimage.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Kopp F., Lindenberger U. Effects of joint attention on long‐term memory in 9‐month‐old infants: an event‐related potentials study. Dev. Sci. 2011;14(4):660–672. doi: 10.1111/j.1467-7687.2010.01010.x. [DOI] [PubMed] [Google Scholar]
- Kouider S., Long B., Le Stanc L., Charron S., Fievet A.C., Barbosa L.S., Gelskov S.V. Neural dynamics of prediction and surprise in infants. Nat. Commun. 2015;6 doi: 10.1038/ncomms9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwisthout J., Bekkering H., van Rooij I. To be precise, the details don’t matter: on predictive processing, precision, and level of detail of predictions. Brain Cogn. 2017;112:84–91. doi: 10.1016/j.bandc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Marinović V., Hoehl S., Pauen S. Neural correlates of human–animal distinction: an ERP-study on early categorical differentiation with 4-and 7-month-old infants and adults. Neuropsychologia. 2014;60:60–76. doi: 10.1016/j.neuropsychologia.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Marzecová A., Widmann A., SanMiguel I., Kotz S.A., Schröger E. Interrelation of attention and prediction in visual processing: effects of task-relevance and stimulus probability. Biol. Psychol. 2017;125:76–90. doi: 10.1016/j.biopsycho.2017.02.009. [DOI] [PubMed] [Google Scholar]
- O’Reilly J.X. Making predictions in a changing world-inference, uncertainty, and learning. Front. Neurosci. 2013;7(105):1–10. doi: 10.3389/fnins.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly J.X., Schüffelgen U., Cuell S.F., Behrens T.E., Mars R.B., Rushworth M.F. Dissociable effects of surprise and model update in parietal and anterior cingulate cortex. Proc. Natl. Acad. Sci. 2013;110(38):E3660–E3669. doi: 10.1073/pnas.1305373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;(1):1–9. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. International Universities Press; New York: 1952. The Origins of Intelligence in Children. [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G.D., Bahrick L.E., Lickliter R., Guy M.W. Neural correlates of intersensory processing in 5‐month‐old infants. Dev. Psychobiol. 2014;56(3):355–372. doi: 10.1002/dev.21104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G.D., Richards J.E. Familiarization, attention, and recognition memory in infancy: an event-related potential and cortical source localization study. Dev. Psychol. 2005;41(4):598–614. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.E. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Dev. Sci. 2003;6(3):312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran J.R., Kirkham N.Z. Infant statistical learning. Annu. Rev. Psychol. 2018;69:181–213. doi: 10.1146/annurev-psych-122216-011805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K.A., Garza J., Zolot L., Kresse A. Electrophysiological signals of familiarity and recency in the infant brain. Infancy. 2010;15(5):487–516. doi: 10.1111/j.1532-7078.2009.00021.x. [DOI] [PubMed] [Google Scholar]
- Stefanics G., Kremláèek J., Czigler I. Visual mismatch negativity: a predictive coding view. Front. Hum. Neurosci. 2014;8:666. doi: 10.3389/fnhum.2014.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stets M., Stahl D., Reid V.M. A meta-analysis investigating factors underlying attrition rates in infant ERP studies. Dev. Neuropsychol. 2012;37(3):226–252. doi: 10.1080/87565641.2012.654867. [DOI] [PubMed] [Google Scholar]
- Striano T., Reid V.M., Hoehl S. Neural mechanisms of joint attention in infancy. Eur. J. Neurosci. 2006;23(10):2819–2823. doi: 10.1111/j.1460-9568.2006.04822.x. [DOI] [PubMed] [Google Scholar]
- van Pelt S., Heil L., Kwisthout J., Ondobaka S., van Rooij I., Bekkering H. Beta-and gamma-band activity reflect predictive coding in the processing of causal events. Soc. Cogn. Affect. Neurosci. 2016;11(6):973–980. doi: 10.1093/scan/nsw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacongne C., Labyt E., van Wassenhove V., Bekinschtein T., Naccache L., Dehaene S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc. Natl. Acad. Sci. 2011;108(51):20754–20759. doi: 10.1073/pnas.1117807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S.J., Long J.D., Nelson C.A. A longitudinal investigation of visual event‐related potentials in the first year of life. Dev. Sci. 2005;8(6):605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Wills A.J., Lavric A., Croft G.S., Hodgson T.L. Predictive learning, prediction errors, and attention: evidence from event-related potentials and eye tracking. J. Cogn. Neurosci. 2007;19(5):843–854. doi: 10.1162/jocn.2007.19.5.843. [DOI] [PubMed] [Google Scholar]