Abstract
Due to heightened level of neuroplasticity, there is a sensitive period (2–4 years after birth) that exists for optimal central auditory development. Using diffusion tensor imaging combined with resting-state functional connectivity (rsFC) analysis, this study directly investigates the structural connectivity alterations of the whole brain white matter (WM) and the functional reorganization of the auditory network in infants with sensorineural hearing loss (SNHL) during the early sensitive period. 46 bilateral profound SNHL infants prior to cochlear implantation (mean age, 17.59 months) and 33 healthy controls (mean age, 18.55 months) were included in the analysis. Compared with controls, SNHL infants showed widespread WM alterations, including bilateral superior longitudinal fasciculus, inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, right corticospinal tract, posterior thalamic radiation and left uncinate fasciculus. Moreover, SNHL infants demonstrated increased rsFC between left/right primary auditory cortex seeds and right insula and superior temporal gyrus. In conclusion, this study suggests that SNHL in the early sensitive period is associated with diffuse WM alterations that mainly affect the auditory and language pathways. Furthermore, increased rsFC in areas mainly associated with auditory and language networks may potentially reflect reorganization and compensatory activation in response to auditory deprivation during the early sensitive period.
Keywords: Sensorineural hearing loss, Resting-state functional magnetic resonance imaging, Functional connectivity, Sensitive period, Diffusion tensor imaging
1. Introduction
With the establishment of newborn hearing screening programs, the number of infants identified with congenital sensorineural hearing loss (SNHL) is on the rise, as it is estimated to affect 1–2 of every 1000 newborns in Western countries (Kral and O’Donoghue, 2010). Hearing loss caused by SNHL is due to alterations in the vestibulocochlear nerve, inner ear, or central processing pathways of the brain. Although there are many hypothesized pathologies, including various infections, ototoxic agents, inner ear malformations and immunologic diseases, genetic causes account for at least 50% of congenital SNHL (YA and M, 2006). Individuals with congenital bilateral profound SNHL typically have no hearing experiences after birth, which can dramatically alter white matter (WM) and cortical development, and lead to negative affects not only on language but also on cognitive and social functions (Huang et al., 2015; Lin et al., 2008; Liu et al., 2015; Tarabichi et al., 2018). Cochlear implantation (CI) has become a routine surgical procedure worldwide for the management of profound SNHL. Specifically, age at implantation is one of the key factors for improved language outcomes (Richter et al., 2002; Svirsky et al., 2004; Tomblin et al., 2005).
During early development, the brain undergoes crucial changes. Researchers studying the auditory cortex have reported that peak synaptic density is attained at 2–4 years of age in children with typical hearing (Kral and O’Donoghue, 2010). These data also correspond to electroencephalographic studies on implanted children, which have also established the existence of, and the time limits for, a sensitive period for CI within the first 3.5–4.0 years after birth (best before the 2nd year of life) (Kral and Sharma, 2012; Sharma and Campbell, 2011). Taken together, the maturation of the auditory cortex has a sensitive period during the first 2–4 years of life, during which the central auditory system remains maximally plastic, and CI surgery is more likely to be successful. More recently, one study stated that the development of auditory, language and vision processing function might be affected by congenital severe SNHL before 4 years of age (Xia et al., 2017). However, there has been little work investigating how the structural and functional connectivity changes in bilateral profound SNHL infants during this sensitive period.
Advanced non-invasive MR techniques, including diffusion tensor imaging (DTI) and functional MRI (fMRI), have been applied to investigate microstructural and functional changes in patients with SNHL. DTI represents a neuroimaging modality, which measures the displacement of water molecules within WM tracts, providing insight into changes in WM microstructure and thus serving as a biomarker of tissue integrity (Tarabichi et al., 2018). The most commonly used quantitative parameter is fractional anisotropy (FA), which provides information about fiber integrity (Steele et al., 2013). Studies using DTI have reported altered microstructure of extensive WM in SNHL patients, including auditory pathways, such as the lateral lemniscus, inferior colliculus, auditory cortex and auditory radiation (Karns et al., 2016; Lin et al., 2008), as well as some non-auditory areas (Rachakonda et al., 2014). These findings suggested axonal loss or lack of myelination caused by the auditory deprivation in SNHL patients. Additionally, in children with a mean age of 4–5 years, some DTI studies found that those who had a good outcome with CI showed better neural integrity in brain areas associated with language and auditory pathways, which suggested that the conservation of the microstructural integrity of these brain areas is important, potentially providing clinically useful information for guidance of CI (Chang et al., 2012; Huang et al., 2015). Therefore, DTI provides an effective approach for examining structural connectivity affected by SNHL.
Furthermore, fMRI has been used to evaluate the effect of hearing loss on cerebral activity in SNHL patients. Resting-state functional connectivity (rs-FC) MRI depends on the spontaneous neural activity of brain and is usually used to evaluate the functional network organization that occurs when a subject is not performing a specific task (Biswal et al., 1995; Fair et al., 2007; Fox et al., 2005). Recent rs-FC studies have found abnormal brain FC induced by unilateral or bilateral hearing loss (Li et al., 2015; Liu et al., 2015; Schmithorst et al., 2014; Shi et al., 2016; Tobyne et al., 2017; Wang et al., 2014; Xu et al., 2016; Zhang et al., 2015, 2016). For example, in children with severe congenital SNHL who do not have cochlear implants (aged 5–14 years), the primary auditory cortex (A1) was found to be less connected with the motor cortex, whereas the visual cortex showed strengthened connectivity with motor and speech cortices (Shi et al., 2016). Liu and colleagues evaluated whole-brain FC changes related to the auditory cortex in adult patients with left-sided SNHL, and found that the SNHL group showed significant FC changes in the auditory system, recognition network, visual cortex and language network (Liu et al., 2015). These findings suggest that the functional reorganizations occurred as a response to auditory deficits. However, most of these studies focused on subjects who had a relatively long history of hearing deprivation and so had matured beyond the sensitive period of sensory development and plasticity.
Using DTI in conjunction with rs-FC analysis, the present study directly investigates the structural connectivity alterations of the whole brain WM tracts as well as functional reorganization of the auditory network in infants with bilateral profound SNHL who are under 3 years of age. This study aimed to obtain a better understanding of the brain connectivity characteristics associated with congenital SNHL, especially in infants prior to cochlear implantation during their early sensitive period.
2. Patients and methods
2.1. Patients
This study is comprised of 86 subjects: 50 patients with congenital bilateral profound SNHL and 36 healthy controls (HC). All SNHL participants failed the newborn hearing screening examinations. As a confirmatory test, the auditory brainstem response (ABR) was measured at 42 days. All the patients with ABR results greater than 90 dB were documented as having bilateral profound hearing loss. Their parents were then referred by the Department of Otolaryngology for MRI scans as a presurgical evaluation for CI and consented to participate in our fMRI protocol. SNHL infants who had any malformation or abnormality found in the high-resolution computed tomography scan (HRCT) of the temporal bone or MRI of the brain and inner ear scans were not included in the study. Moreover, all deaf infant participants did not wear hearing aids, and had no history of infections, ototoxic drugs, cytomegalovirus, trauma or any other neural diseases. The control group was well matched to the patient group in terms of age and sex. Participants in the control group received clinical MRI scans with sedation for non-hearing related indications, and their parents agreed to additional sequence scans and hearing tests. Inclusion criteria included: gestational age of at least 36 weeks, no single frequency greater than 25 dB and normal neuroanatomy as determined by a pediatric neuroradiologist. Exclusion criteria included: a variety of central nervous system diseases, such as white matter hypoplasia, abnormal neuronal migration, trauma, tumor, infection, epilepsy and so on. Signed informed consent was obtained from every subject’s parents prior to entering the study. All examinations were approved by the hospital ethics committee.
2.2. MRI acquisition
MR images were acquired using a Siemens Verio Tim 3.0 T MR scanner (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil. All the infants were sedated with 50–60 mg/kg of 10% chloral hydrate orally, 15 min before MR imaging. Hearing protection was provided with earplugs and headphones. During MRI, infants were continuously monitored by a pulse oximeter and closely observed by a pediatrician. All infants underwent anatomical MRI, DTI and fMRI acquisitions using the protocol detailed below.
2.2.1. Anatomical data acquisition
Anatomical MRI consisted of axial and sagittal T1-weighted images (T1WI), as well as axial and coronal T2-weighted images (T2WI) to check for brain lesions and/or abnormalities. Parameters were as follows: T1WI: TR/TE = 300/2.5 ms, slice thickness = 4 mm, interlayer spacing = 1.2 mm, matrix = 320 × 320, FOV = 220 × 220 mm2, flip angle = 70°, slices covering the whole brain. T2WI: TR/TE = 6000/93 ms, slice thickness = 4 mm, the interlayer spacing = 1.2 mm, matrix = 320 × 320, FOV = 220 × 220 mm2, flip angle = 120°, slices covering the whole brain.
2.2.2. DTI data acquisition
Diffusion tensor images were acquired using an SE diffusion echo-planar imaging sequence with the following parameters: TR/TE = 4345/95 ms, slice thickness = 4 mm, matrix = 128 × 128, FOV = 220 × 220 mm2, flip angle = 90°, voxel size = 1.7 × 1.7 × 4 mm3, 21 directions of diffusion gradients, b = 800 s/mm2, and 30 slices acquired by covering the whole brain.
2.2.3. fMRI data acquisition
Resting-state fMRI data were acquired using the echo-planar imaging sequence, with the following parameters: TR/TE = 2000/30 ms, slice thickness = 4 mm, matrix = 70 × 70, FOV = 220 × 220 mm2, flip angle = 90°, voxel size = 3.1 × 3.1 × 4 mm3, 30 slices acquired covering the whole brain and total volumes = 190. T1-weighted anatomical images were also obtained using a 3D MPRAGE sequence with the following parameters: TR/TE = 2400/3.16 ms, inversion time (TI) = 900 ms, slice thickness = 1 mm, matrix = 224 × 256, FOV = 220 × 220 mm2, flip angle = 9°, voxel size = 1.0 × 1.0 × 1.0 mm3 and 128 sagittal slices covering the whole brain.
2.3. Data processing
2.3.1. DTI data processing: whole brain tract-based spatial statistics
DTI data was preprocessed using FMRIB Software Library (FSL) software library tools (www.fmrib.ox.ac.uk/fsl/) (Smith et al., 2004). Firstly, DTI images underwent eddy current correction and linear registration to the non-diffusion weighted image in order to correct for head motion. Images were then brain-extracted using Brain Extraction Tool (BET) and entered into FDT toolbox (FMRIB’s Diffusion Toolbox) for generating FA maps. Following this, tract-based spatial statistics (TBSS) (Smith et al., 2006) were applied to the data. Instead of aligning onto a standard FMRIB58 template, a new target image was selected; that is the one with the minimum mean displacement score from all other subjects in the group using TBSS option (tbss_2_reg -n) (Ball et al., 2010). FA images from each participant were co-registered to the target image using non-linear registration in FNIRT (FMRIB’s Non-linear Registration Tool), and then affine-aligned into Montreal Neurological Institute 152 standard space. After that, a mean FA image was created, which was then thinned to generate a mean FA skeleton of white matter tracts. A threshold of 0.2 was selected to define the border of major fiber bundles.
For voxel-wise analyses of FA measures, differences between the SNHL group and healthy controls were tested in a general linear model (GLM) framework with t-test using nonparametric permutation testing (number of permutations = 5000), FSL’s randomize and controlling for age and sex. Family wise error (FWE) corrected p value of <0.05 was identified as significant, according to the threshold-free cluster enhancement method.
2.3.2. Functional data processing: seed-based resting-state functional connectivity analysis
Resting-state fMRI data was preprocessed using the DPARSF toolbox and REST toolbox version 1.8 (www.restfmri.net) in SPM8 (http://www.fil.ion.ucl.ac.uk). The first 10 functional volumes were discarded for signal equilibrium and the subjects’ adaptation to scanning noise. Slice-timing and realignment for head motion correction were then performed. All subjects with a head motion greater than 2.0 mm translation or 2.0°rotation in any direction were excluded. After that, functional images were co-registered to a corresponding T1-weighted high-resolution image. T1-weighted images were segmented using University of North Carolina 2-year-old tissue probability maps (Feng et al., 2011). Following that, images were normalized to the 2-year-old brain template, resampled to 3 mm isotropic voxels, and smoothed with a full width at half maximum (FWHM) Gaussian Smoothing kernel of 6 mm. Additional preprocessing steps were also conducted to minimize the effect of physiological artifacts on the resting-state signal. These steps including: temporal band-pass filtering (0.01−0.08 Hz), regression of rigid body head motion parameters in 6 directions, regression of the whole-brain averaged signal, regression of cerebrospinal fluid (CSF) signal averaged from a ventricular region mask, regression of white matter signal averaged from a white matter mask.
Functional connectivity was then performed using the seed-voxel correlation approach, in which the time-course signal in a seed region is correlated to all voxels in the whole brain. For a particular A1 seed, a mask of the left and right Heschl’s gyrus (HG) were defined as regions of interest (ROIs) based upon the 2-year-old Automated Anatomical Labeling (AAL) (Feng et al., 2011). Correlation coefficients were then transformed to z values using the Fisher r-to-z transformation to improve normality.
All analyses at the group level were conducted using SPM8 software. For within-group FC analysis, a one-sample t-test was performed based on the individual z values to determine the brain regions that showed positive FC to each ROI in each group (false discovery rate (FDR) corrected threshold of p < 0.05 was set). For between-group comparisons, two-sample t-tests were performed to identify brain regions that showed significant FC differences to each ROI (FDR corrected threshold of p < 0.05 was set). Analyses were performed after regressing out nuisance covariates including age, sex, and head motion. Results were visualized with REST Slice Viewer and BrainNet Viewer.
2.4. Magnetic resonance imaging quality control
DTI data were initially preprocessed using DTIPrep software (Liu et al., 2014) to automatically identify and correct motion and scanner-induced artifacts. None were detected. Further to this, DTI data were visually inspected by the first author on a slice-by-slice basis during analysis to identify any intensity artifacts including banding and poor signal-to-noise ratio. Again, no artifacts were detected.
For fMRI data, 4 subjects with head motion of more than 2.0 mm (2 SNHL participants and 2 controls) were excluded from further analysis. In addition, the first author visually inspected the coregistration and normalization in fMRI data processing in all datasets. As a result, 2 SNHL participants failed to coregister structural and functional images, while one control failed to normalize to the 2-year-old brain template. Thus, these 7 subjects were excluded from further analyses. In total, 79 subjects (46 SNHL infants and 33 healthy controls) without neuroanatomical anomalies were included in the study.
Given recent evidence that DTI and FC analysis are particularly susceptible to head motion (Mayer et al., 2007; Yendiki et al., 2014), Mann-Whitney U-tests were performed to ensure groups did not differ on average volume-by-volume translation or rotation parameters in either DTI data or fMRI data. For the DTI data, using b = 0 image for coregistration, there was no group difference in translation (mm) (mean SNHL = 0.98 (SD = 0.50) vs. mean HC = 1.00 (SD = 0.59), p = 0.87) or rotation (degree) (mean SNHL = 0.32 (SD = 0.20) vs. mean HC = 0.31 (SD = 0.22), p = 0.73). For the fMRI data, there was no group difference in translation (mm) (mean SNHL = 0.92 (SD = 0.42) vs. mean HC = 1.10 (SD = 0.69), p = 0.33) or rotation (degree) (mean SNHL=0.41 (SD = 0.29) vs. mean HC=0.39 (SD = 0.28), p = 0.67). In addition, previous work has shown that frame-to-frame motion has great influence on connectivity results (Power et al., 2012). Frame-wise displacement (FD) was also calculated for assessing the amount of head movement between volumes. We used a volume censoring approach, removing volumes associated with greater than 0.5 mm FD (and one volume before and two volumes after to account for temporal blurring). After that, subjects with less than 114 volumes (60% of the total volumes) were excluded. As a result, no additional subjects were excluded. For the remaining subjects (46 SNHL infants and 33 healthy controls), no significant difference was found in terms of the remaining FD (mm) (mean SNHL = 0.19 (SD = 0.06) vs. mean HC = 0.18 (SD = 0.06), p = 0.57) and the remaining number of volumes after volume removal (mean SNHL = 160.96 (SD = 16.45) vs. mean HC = 154.91 (SD = 15.91), p = 0.11) between groups using the Mann-Whitney U test.
3. Results
3.1. Demographic and clinical characteristics
Demographic and clinical data for the subjects are shown in Table 1. No significant differences were found in age and sex between two groups. The ABR of the left and right ear were significantly different between two groups.
Table 1.
Demographic and clinical data of all subjects.
| SNHL group | HC group | P value | |
|---|---|---|---|
| Number (n) | 46 | 33 | – |
| Age (months)a(mean ± SD) | 17.59 ± 6.89 | 18.55 ± 7.38 | 0.56 |
| Age range (months) | 9–36 | 9–36 | – |
| Gender (male/female)b | 21/25 | 16/17 | 0.78 |
| ABR of left ear (dB HL) (mean ± SD) | >90 | 21.55 ± 2.35 | – |
| ABR of right ear (dB HL) (mean ± SD) | >90 | 21.86 ± 2.56 | – |
Note: Statistical analyses for comparisons between groups were carried out witht-testsa or χ2 testsb.
SNHL = sensoryneural hearing loss; HC = health control; ABR = auditory brainstem response; SD = standard deviation.
3.2. Structural connectivity analysis
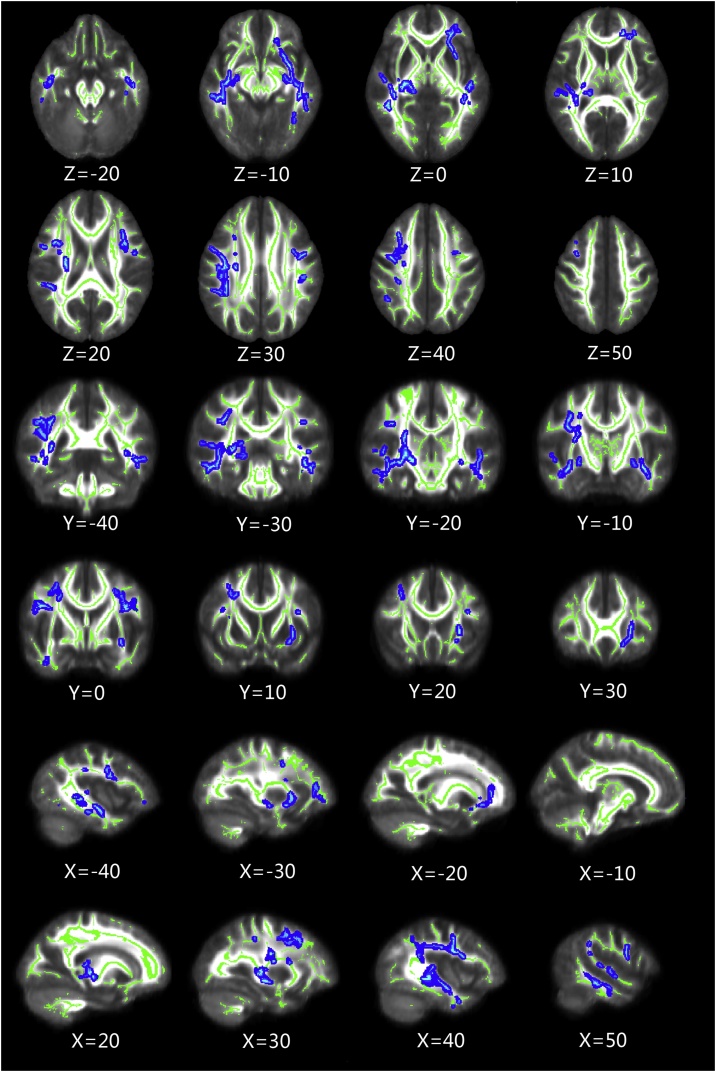
Whole-brain group-comparison results for the FA values obtained by TBSS analysis are shown in Fig. 1 and Table 2. Compared with HC, SNHL infants showed significant FA reductions of multiple WM clusters. These regions were bilateral superior longitudinal fasciculus (SLF), inferior fronto-occipital fasciculus (IFOF), inferior longitudinal fasciculus (ILF), right corticospinal tract (CST), posterior thalamic radiation (PTR) and left uncinate fasciculus (UF). There was no brain region in which the HC showed lower FA compared with the SNHL infants.
Fig. 1.
Whole-brain group-comparison results for FA values obtained by TBSS analysis (p < 0.05, FWE corrected). The background image is the mean FA and the FA skeleton (green). Blue voxels represent regions in which FA was significantly decreased in the SNHL group relative to the HC group.
FA = fractional anisotropy, TBSS = tract–based spatial statistics, FWE = Family Wise Error, SNHL = sensorineural hearing loss, HC = healthy control (All images are in radiologic orientation).
Table 2.
Skeleton clusters of TBSS analysis showing significantly decreased FA at p < 0.05 (FWE corrected).
| Cluster index | Voxel size in total | Anatomic Region | Hemisphere L = left, R = right |
|---|---|---|---|
| A | 32,845 | Superior longitudinal fasciculus | R |
| Inferior fronto-occipital fasciculus | |||
| Inferior longitudinal fasciculus. | |||
| Corticospinal tract | |||
| Posterior thalamic radiation | |||
| B | 14,769 | Inferior fronto-occipital fasciculus | L |
| Inferior longitudinal fasciculus | |||
| Uncinate fasciculus | |||
| C | 3402 | Superior longitudinal fasciculus | L |
| D | 896 | Inferior longitudinal fasciculus. | L |
3.3. Functional connectivity analysis
3.3.1. Intra-group comparison
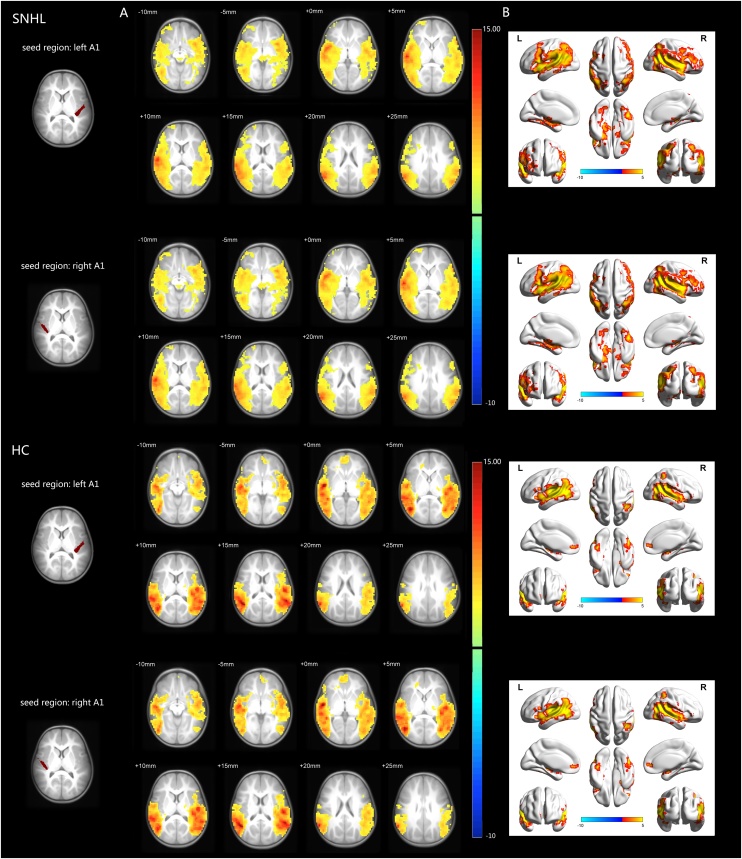
Intra-group comparison showed extensive positive FC with left/right A1 in both groups (Fig. 2). These areas are bilateral superior temporal gyrus, middle temporal gyrus, insula, inferior frontal gyrus, angular, inferior parietal lobule and Rolandic operculum, most of which are considered to be part of the auditory network, salience network and default mode network (DMN).
Fig. 2.
Intra-group functional connectivity maps in the SNHL group and HC group. A: Axial visualization, B: Surface visualization. In both groups, the left/right A1 showed similar positive functional connectivity to brain regions (color coded) of bilateral superior temporal gyrus, middle temporal gyrus, insula, inferior frontal gyrus, angular, inferior parietal lobule and Rolandic operculum, most of which are considered to be part of the auditory network, salience network and default mode network. All images were shown with FDR correction of p < 0.05. The colored bar indicates the corresponding t values.
SNHL = sensorineural hearing loss, HC = healthy control, A1 = primary auditory cortex, FDR = false discovery rate. (All images are in radiologic orientation)
3.3.2. Inter-group comparison
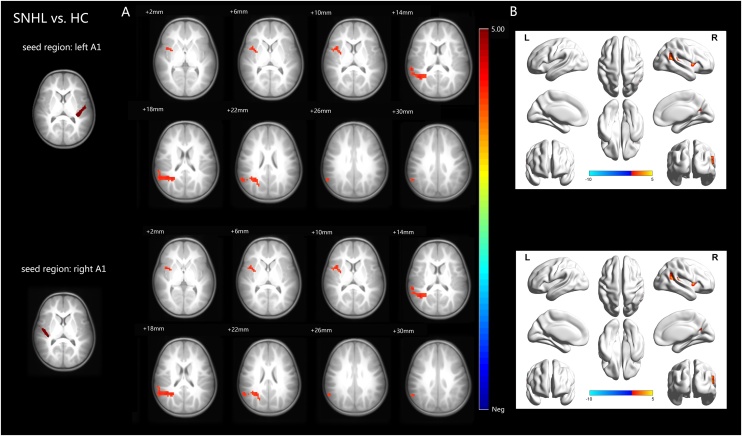
Inter-group FC analysis revealed differences between the two groups with the left/right A1 as the seeds (Fig. 3 and Table 3). Compared with HC, SNHL group showed significant increased FC in right insula and superior temporal gyrus (STG) when the left A1 was used as the seed region. Additionally, increased FC was found in the right insula and STG when the right A1 was used as the seed region. No region of decreased FC was found in SNHL group compared with HC group.
Fig. 3.
Inter-group functional connectivity analyses results. A: Axial visualization; B: Surface visualization. Compared with HC group, SNHL group showed significant increased functional connectivity in the right insula and STG when the left A1 was used as the seed region. In addition, increased functional connectivity was found in the right insula and STG when the right A1 was used as the seed region. No region of decreased functional connectivity was found in SNHL group compared with HC group. All images were shown with FDR correction of p < 0.05. The colored bar indicates the corresponding t values.
SNHL = sensorineural hearing loss, HC = healthy control, A1 = primary auditory cortex, STG = superior temporal gyrus, FDR = false discovery rate. (All images are in radiologic orientation).
Table 3.
Inter-group functional connectivity analyses demonstrating significant increased functional connectivity in SNHL group compared with HC group using bilateral A1 as seeds.
| Seed regions | Brain region SNHL > HC (2-year-old AAL template) | Hemisphere L = left, R = right | Peak MNI |
Max t-value | Cluster voxels | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Left A1 | Cluster 1 | Superior temporal gyrus | R | 54 | −33 | 15 | 4.1163 | 148 | |
| Cluster 2 | Insula | R | 30 | 9 | 9 | 3.9385 | 51 | ||
| Right A1 | Cluster 1 | Superior temporal gyrus | R | 54 | −33 | 15 | 4.1229 | 141 | |
| Cluster 2 | Insula | R | 30 | 9 | 9 | 3.9271 | 50 | ||
Note: Thresholds were set at p < 0.05 FDR corrected. Two sample t-tests with gender and age as covariates were performed to test the difference between the two groups on the left/right A1 functional connectivity.
SNHL = sensorineural hearing loss, HC = healthy control, A1 = primary auditory cortex, MNI = Montreal Neurological Institute, AAL = Automated Anatomical Labeling.
4. Discussion
During development, periods of maximal neuroplasticity - known as the “sensitive period” - have been well described (Knudsen, 2004; Kral and O’Donoghue, 2010). Deprivation of auditory input during the early sensitive period has major and long-lasting impacts on intrinsic brain organization. In this multimodal MRI study, we found that the infants under 3 years of age with profound congenital bilateral SNHL demonstrated widespread alterations of WM microstructures as well as increased rs-FC between the left/right A1 seeds and the areas mainly associated with auditory and language networks. To our knowledge, this is the first study combined structural and functional connectivity analysis to explore the brain connectivity characteristics in SNHL infants during this early sensitive period.
4.1. White matter alterations
The development of synapses is regulated by neuronal activity. When activity is absent (for example, the lack of auditory input for those with bilateral profound SNHL), synaptic development is delayed and takes place later (Kral and Sharma, 2012; Kral et al., 2005), regulated by other principles which are not related to sensory input. Using a semiautomatic method for demarcating ROIs, Karns and colleagues (Karns et al., 2016) found that congenitally deaf adults had reduced FA in WM underlying bilateral HG, anterior superior temporal gyrus (STG), posterior STG and posterior corpus callosum. In addition, WM changes have also been explored using whole-brain DTI analyses in deaf adults and adolescents (Kim et al., 2009; Li et al., 2012; Miao et al., 2013). For example, Kim et al. (Kim et al., 2009) found that for deaf adults who did not have a history of wearing a hearing aid, FA values were reduced in the internal capsule, the WM tract lying close to the STG, the SLF, and the IFOF. Li et al. (Li et al., 2012) compared the WM of congenitally deaf adults to those with acquired deafness and controls, found that deaf individuals (who did not wear hearing aids before 6 years old, and no individuals used a hearing aid consistently in the past 3 years) exhibited significantly reduced FA values of the right STG, the left HG and the splenium of the corpus callosum (SCC) compared to hearing controls. Miao et al. (Miao et al., 2013) studied deaf adolescents (who had been using hearing aids for at least 5 years, and were trained in Chinese Sign Language for at least 4 years), and found that the FA values of the STG and the HG were lower than normal. Partially consistent with the above studies with adults or adolescents, our study found WM microstructure alterations detected by decreased FA in SNHL infants’ group, including association fibres (SLF, IFOF, ILF and UF) and projection fibres (CST, PTR). This indicates that bilateral profound SNHL in infants may prevent development of typical brain structural connections, which are perhaps best attributed to less myelination, axonal loss and/or fewer fibres projection caused by auditory deprivation during an early sensitive period. In addition, our results were consistent with another recent study which found a distributed pattern of FA reduction (in HG, IFOF, UF, SLF and the forceps major) in a deaf group below 4 years of age when compared with a hearing group (Park et al., 2018). However, the difference was not significant in older subjects (aged 4–8 years) (Park et al., 2018). Along with Park’s findings, WM alterations detected in our SNHL group may suggest that the development of WM is delayed in congenital deaf children within the early sensitive period. While in older children, adolescents or adults, WM alterations may be affected by other factors including the use of sign language, visual experiences and intelligence.
The SLF, which consists of three components (I, II, III), projects from the fronto-temporal and fronto-parietal regions in a bidirectional manner. The ILF interconnects temporal with ipsilateral occipital lobes (Catani et al., 2002). The IFOF connects the ipsilateral frontal and occipital lobes, ipsilateral frontal and posterior parietal and temporal lobes and mixes with the UF (Catani et al., 2002; Potapov et al., 2014). These specific association fasciculi, which mainly connect regions of fronto-parieto-temporal network, can be considered part of the speech processing stream, described by Specht as the Dual Stream Model (Hickok and Poeppel, 2007). This indicates that there are microstructural alterations, possibly reflecting delayed development, in pathways responsible for the perceptual integration of auditory and language signals in the SNHL group.
The CST contains projection fibres mainly associated with motor function (Kunimatsu et al., 2003). The microstructure alteration of this fibre tract may suggested that early hearing deprivation also affects the development of motor fibers, which may be linked to the reduced motor behavior found in infants with SNHL. Thalamic radiata contain projection fibres which lead to and from the cortex connecting with the thalamus. The medial geniculate nucleus of the thalamus is an obligatory relay for all ascending auditory information destined to reach the primary auditory cortex. MR imaging studies have shown that the thalamus, particularly the right PTR, plays an important role in auditory signal processing (Baldoli et al., 2015; Meredith and Allman, 2012). In this study, a significantly decreased FA found in the right PTR indicated microstructure alterations in the posterior thalamic projection pathways, which may be due to a lack of auditory input in SNHL infants (Kral and Pallas, 2011).
4.2. Alterations of functional connectivity
Measurements of rs-FC MRI in subjects with SNHL have shown altered FC not only in regions directly associated with auditory processing, but also in the higher-order structures involved in the functional organization of higher-level networks, including the DMN network, executive control network and language network (Dewey and Hartley, 2015; Liu et al., 2015; Tibbetts et al., 2011; Zhang et al., 2016). A1 is located in the HG, believed to be the first region of the auditory pathway. Although functional neuroplasticity in A1 has been controversial (Bavelier et al., 2006; Kral et al., 2003), recent studies have reported the functional and structural reorganization of A1 in deaf cats (Wong et al., 2014), congenitally and profoundly deaf adults (Karns et al., 2016; Scott et al., 2014), unilateral SNHL children (Propst and Greinwald, 2010) and congenital bilateral severe SNHL infants (below 4 years old) (Xia et al., 2017). In this study, we used left/right A1 as seeds to explore FC changes, and found increased FC in insula and STG in the SNHL group. These changes suggest that hearing loss affects functional adaptive reorganization in SNHL infants within the early sensitive period. The insular cortex and STG are auditory, language-related regions which are responsible for auditory conscious experience, such as allocating auditory attention (Bamiou et al., 2003), and language comprehension (Dewitt and Rauschecker, 2013; Yeatman et al., 2010). Thus, consistent with previous reports (Tibbetts et al., 2011; Xia et al., 2017), the increased FC within the auditory and language networks was also found in our study with SNHL infants. This may be explained as a compensatory mechanism of the brain for balancing the WM alteration or delayed development in these brain regions due to no sound stimulated in the early sensitive period.
In addition, many studies have shown that connectivity with the auditory-visual cortex and auditory-somatosensory cortex can be altered by hearing loss due to the adaptive and compensatory processes which originate from the cross-modality plasticity in the auditory cortex (Chen et al., 2017; Merabet, 2010; Sharma et al., 2015; Stropahl et al., 2016). However, in this study, the absence of FC changes in the visual or somatosensory associated cortices may suggest that the functional reorganization patterns in infants with SNHL within the developmental sensitive period is different from that in subjects who have relatively long-term auditory deprivation. An alternative explanation may be around the short duration of deafness in infants who have no experience in sign language or hearing aid use. Recent studies have found a strong correlation between cross-modal re-organization and poor outcomes with CI in deaf adults (Buckley and Tobey, 2011; Sandmann et al., 2015). In addition, previous studies using fMRI with auditory stimulation also found that children who perform well with CI appear to activate networks which participate in language function and attention, while children who perform poorly with CI repeatedly show functional specialization of auditory cortical areas for visual processing (Deshpande et al., 2016; Giraud and Lee, 2007). Thus, we have here provided further evidence for the existence of the sensitive period in the development of the central auditory system. In addition, this may be part of the functional basis that explains the most optimal outcomes of CI carried out within early sensitive period.
4.3. Limitations and future directions
Despite these interesting findings, firstly, certain inherent limitations due to the small sample size must be addressed. Secondly, using the TBSS and rs-FC analysis presented here, we are trying to reveal the alterations of structural and functional connectivity in congenitally deaf infants within the sensitive period. For further exploration of neuroimaging biomarkers for predicting CI outcomes, long-term follow-up of this group needs to be implemented. Additionally, more work is needed to extend the predictive value of fMRI from group level to individual level. Thirdly, the specific causes of deafness were not addressed in this study. However, SNHL infants who had any malformation or abnormality found in the conventional CT or MRI were not included in the study. Besides, all deaf infant participants had no history of infections, ototoxic drugs, cytomegalovirus, trauma or any other neural diseases. Consequently, it makes the cause of SNHL in our study mostly genetic or unknown reasons. Some studies have suggested that WM integrity (Tan et al., 2010) and cortical function (Liegeois et al., 2003) may be closely related to individual genetic composition. Thus, it will be equally important to investigate genetic contributions to WM and cortical function development in hearing loss subjects. With the enlargement of sample size and the advances in molecular biology, further studies will be able to focus on the association of genetic variation with neuroimaging endophenotypes in SNHL infants, and so may be able to provide insight into the role of genetic causes in shaping auditory system structure and function. Fourthly, lateralization is an important property for the brain functions. Fourthly, analyses directly tested the hemispheric differences confirmed that the interaction of deafness and task was more left lateralized, while the main effect of deafness was more right lateralized (Twomey et al., 2017). More recently, one study measured cerebrovascular activity during language processing in a group of deaf children and found left lateralization while producing speech, sign, sign-supported English, or a combination of languages (Payne et al., 2019). However, the infants included in our study were too young to be able to perform the handedness assessment, which would further make the assessment of lateralization inaccurate. Thus, the lateralization development after auditory deprivation and its relationship between the changes of WM integrity and FC are still in need of further exploration. Finally, our findings were from resting state conditions while the infants were sedated with chloral hydrate, which represents a limitation for comparing data obtained during natural sleeping conditions. Previous work has demonstrated the feasibility of fMRI for assessment of auditory cortical function in hearing loss infants, even with the use of sedation (Altman and Bernal, 2001; Patel et al., 2007). However, more research is required to demonstrate the utility of fMRI as effective approaches to highlight auditory cortical function in sedated infant subjects.
5. Conclusion
Our study used multimodal imaging to inspect both the structural and functional connectivity changes in bilateral profound SNHL infants before 3 years of age. In summary, we observed diffuse WM microstructural alterations extended to fibres that mainly within the auditory and language pathways, which may reflect delayed WM development in SNHL group. Additionally, FC changes were found between the left/right A1 and areas mainly involved in auditory and language networks, which may reflect compensatory functional reorganization. Combining DTI and rs-FC analysis, our findings have the potential to provide novel insights into the brain connectivity characteristics of SNHL infants during the early sensitive period.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
We thank all the children and their families for their collaboration in this study.
References
- Altman N.R., Bernal B. Brain activation in sedated children: auditory and visual functional MR imaging. Radiology. 2001;221:56–63. doi: 10.1148/radiol.2211010074. [DOI] [PubMed] [Google Scholar]
- Baldoli C., Scola E., Della Rosa P.A., Pontesilli S., Longaretti R., Poloniato A., Scotti R., Blasi V., Cirillo S., Iadanza A., Rovelli R., Barera G., Scifo P. Maturation of preterm newborn brains: a fMRI-DTI study of auditory processing of linguistic stimuli and white matter development. Brain Struct. Funct. 2015;220:3733–3751. doi: 10.1007/s00429-014-0887-5. [DOI] [PubMed] [Google Scholar]
- Ball G., Counsell S.M., Merchant N., Arichi T., Doria V., Rutherford M.A., Edwards A.D., Rueckert D., Boardman J.P. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage. 2010;53:94–102. doi: 10.1016/j.neuroimage.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Bamiou D.E., Musiek F.E., Luxon L.M. The insula (Island of reil) and its role in auditory processing. Literature review. Brain Res. Rev. 2003;42:143. doi: 10.1016/s0165-0173(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Bavelier D., Dye M.W.G., Hauser P.C. Do deaf individuals see better? Trends Cogn. Sci. 2006;10:512–518. doi: 10.1016/j.tics.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckley K.A., Tobey E.A. Cross-modal plasticity and speech perception in pre- and postlingually deaf cochlear implant users. Ear Hear. 2011;32:2–15. doi: 10.1097/AUD.0b013e3181e8534c. [DOI] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Chang Y., Lee H.R., Paik J.S., Lee K.Y., Lee S.H. Voxel-wise analysis of diffusion tensor imaging for clinical outcome of cochlear implantation: retrospective study. Clin. Exp. Otorhinolaryngol. 2012;5(Suppl (1)):S37–42. doi: 10.3342/ceo.2012.5.S1.S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.C., Puschmann S., Debener S. Increased cross-modal functional connectivity in cochlear implant users. Sci. Rep. 2017;7:10043. doi: 10.1038/s41598-017-10792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande A.K., Tan L., Lu L.J., Altaye M., Holland S.K. fMRI as a preimplant objective tool to predict postimplant Oral language outcomes in children with cochlear implants. Ear Hear. 2016;37:e263–272. doi: 10.1097/AUD.0000000000000259. [DOI] [PubMed] [Google Scholar]
- Dewey R.S., Hartley D.E. Cortical cross-modal plasticity following deafness measured using functional near-infrared spectroscopy. Hear. Res. 2015;325:55–63. doi: 10.1016/j.heares.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Dewitt I., Rauschecker J.P. Wernicke’s area revisited: parallel streams and word processing. Brain Lang. 2013;127:181–191. doi: 10.1016/j.bandl.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Dosenbach N.U., Church J.A., Cohen A.L., Brahmbhatt S., Miezin F.M., Barch D.M., Raichle M.E., Petersen S.E., Schlaggar B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. USA. 2007;104:13507–13512. [Google Scholar]
- Feng S., Yap P.T., Wu G., Jia H., Gilmore J.H., Lin W., Shen D. Infant brain atlases from neonates to 1- and 2-year-olds. Plos One. 2011;6:e18746. doi: 10.1371/journal.pone.0018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Essen D.C.V., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A.L., Lee H.J. Predicting cochlear implant outcome from brain organisation in the deaf. Restor. Neurol. Neurosci. 2007;25:381. [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Huang L., Zheng W., Wu C., Wei X., Wu X., Wang Y., Zheng H. Diffusion tensor imaging of the auditory neural pathway for clinical outcome of cochlear implantation in pediatric congenital sensorineural hearing loss patients. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns C.M., Stevens C., Dow M.W., Schorr E., Neville H.J. Atypical white-matter microstructure in congenitally deaf adults: a region of interest and tractography study using diffusion-tensor imaging. Hear. Res. 2016;343:72–82. doi: 10.1016/j.heares.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.J., Park S.Y., Kim J., Lee D.H., Park H.J. Alterations of white matter diffusion anisotropy in early deafness. Neuroreport. 2009;20:1032–1036. doi: 10.1097/WNR.0b013e32832e0cdd. [DOI] [PubMed] [Google Scholar]
- Knudsen E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kral A., O’Donoghue G.M. Profound deafness in childhood. N. Engl. J. Med. 2010;363:1438–1450. doi: 10.1056/NEJMra0911225. [DOI] [PubMed] [Google Scholar]
- Kral A., Pallas S.L. 2011. Development of the Auditory Cortex; pp. 443–463. [Google Scholar]
- Kral A., Sharma A. Developmental neuroplasticity after cochlear implantation. Trends Neurosci. 2012;35:111–122. doi: 10.1016/j.tins.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A., Schröder J.H., Klinke R., Engel A.K. Absence of cross-modal reorganization in the primary auditory cortex of congenitally deaf cats. Exp. Brain Res. 2003;153:605–613. doi: 10.1007/s00221-003-1609-z. [DOI] [PubMed] [Google Scholar]
- Kral A., Tillein J., Heid S., Hartmann R., Klinke R. Postnatal cortical development in congenital auditory deprivation. Cereb. Cortex. 2005;15:552. doi: 10.1093/cercor/bhh156. [DOI] [PubMed] [Google Scholar]
- Kunimatsu A., Aoki S., Masutani Y., Abe O., Mori H., Ohtomo K. Three-dimensional white matter tractography by diffusion tensor imaging in ischaemic stroke involving the corticospinal tract. Neuroradiology. 2003;45:532–535. doi: 10.1007/s00234-003-0974-4. [DOI] [PubMed] [Google Scholar]
- Li Y., Ding G., Booth J.R., Huang R., Lv Y., Zang Y., He Y., Peng D. Sensitive period for white-matter connectivity of superior temporal cortex in deaf people. Hum. Brain Mapp. 2012;33:349–359. doi: 10.1002/hbm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhu Q., Geng Z., Song Z., Wang L., Wang Y. Study of functional connectivity in patients with sensorineural hearing loss by using resting-state fMRI. Int. J. Clin. Exp. Med. 2015;8:569–578. [PMC free article] [PubMed] [Google Scholar]
- Liegeois F., Baldeweg T., Connelly A., Gadian D.G., Mishkin M., Vargha-Khadem F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat. Neurosci. 2003;6:1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- Lin Y., Wang J., Wu C., Wai Y., Yu J., Ng S. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: changes in radial diffusivity and diffusion anisotropy. J. Magn. Reson. Imaging. 2008;28:598–603. doi: 10.1002/jmri.21464. [DOI] [PubMed] [Google Scholar]
- Liu Z., Yi W., Gerig G., Gouttard S., Ran T., Fletcher T., Styner M. Quality control of diffusion weighted images. Front. Neuroinf. 2014;8:4. [Google Scholar]
- Liu B., Feng Y., Yang M., Chen J.Y., Li J., Huang Z.C., Zhang L.L. Functional connectivity in patients with sensorineural hearing loss using resting-state MRI. Am. J. Audiol. 2015;24:145–152. doi: 10.1044/2015_AJA-13-0068. [DOI] [PubMed] [Google Scholar]
- Mayer A.R., Franco A.R., Ling J., Canive J.M. Assessment and quantification of head motion in neuropsychiatric functional imaging research as applied to schizophrenia. J. Int. Neuropsychol. Soc. 2007;13:839–845. doi: 10.1017/S1355617707071081. [DOI] [PubMed] [Google Scholar]
- Merabet L.B. Neural reorganization following sensory loss: the opportunity of change. Nat. Rev. Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M.A., Allman B.L. Early hearing-impairment results in crossmodal reorganization of ferret core auditory cortex. Neural. Plast. 2012;2012:601591. doi: 10.1155/2012/601591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W., Li J., Tang M., Xian J., Li W., Liu Z., Liu S., Sabel B.A., Wang Z., He H. Altered white matter integrity in adolescents with prelingual deafness: a high-resolution tract-based spatial statistics imaging study. AJNR Am. J. Neuroradiol. 2013;34:1264–1270. doi: 10.3174/ajnr.A3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.H., Chung W.H., Kwon H., Lee J.M. Evaluation of cerebral White matter in prelingually deaf children using diffusion tensor imaging. Biomed Res. Int. 2018;2018:6795397. doi: 10.1155/2018/6795397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.M., Cahill L.D., Ret J., Schmithorst V., Choo D., Holland S. Functional magnetic resonance imaging of hearing-impaired children under sedation before cochlear implantation. Arch. Otolaryngol. Head Neck Surg. 2007;133:677–683. doi: 10.1001/archotol.133.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne H., Gutierrez-Sigut E., Woll B., MacSweeney M. Cerebral lateralisation during signed and spoken language production in children born deaf. Dev Cogn Neurosci. 2019;36:100619. doi: 10.1016/j.dcn.2019.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapov A.A., Goryainov S.A., V Yu Z., Pitskhelauri D.I., Kobyakov G.L., Pronin I.N., Zakharova N.E., Tanoyan A.A., Ogurtsova A.A., Buklina S.B. The long-associative pathway of the white matter: modern view from the perspective of neuroscience. Zhurnal Voprosy Nerokhirurgii Imeni N.n.burdenko. 2014;78:66–77. [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propst E.J., Greinwald J.V. Neuroanatomic differences in children with unilateral sensorineural hearing loss detected using functional magnetic resonance imaging. Arch. Otolaryngol. Head Neck Surg. 2010;136:22–26. doi: 10.1001/archoto.2009.208. [DOI] [PubMed] [Google Scholar]
- Rachakonda T., Shimony J.S., Coalson R.S., Lieu J.E. Diffusion tensor imaging in children with unilateral hearing loss: a pilot study. Front. Syst. Neurosci. 2014;8:87. doi: 10.3389/fnsys.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter B., Eißele S., Laszig R., Löhle E. Receptive and expressive language skills of 106 children with a minimum of 2 years’ experience in hearing with a cochlear implant. Int. J. Pediatr. Otorhinolaryngol. 2002;64:111. doi: 10.1016/s0165-5876(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Sandmann P., Plotz K., Hauthal N., Vos M.D., Schönfeld R., Debener S. Rapid bilateral improvement in auditory cortex activity in postlingually deafened adults following cochlear implantation. Clin. Neurophysiol. 2015;126:594–607. doi: 10.1016/j.clinph.2014.06.029. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Plante E., Holland S. Unilateral deafness in children affects development of multi-modal modulation and default mode networks. Front. Hum. Neurosci. 2014;8:164. doi: 10.3389/fnhum.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G.D., Karns C.M., Dow M.W., Stevens C., Neville H.J. Enhanced peripheral visual processing in congenitally deaf humans is supported by multiple brain regions, including primary auditory cortex. Front. Hum. Neurosci. 2014;8:177. doi: 10.3389/fnhum.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Campbell J. A sensitive period for cochlear implantation in deaf children. J. Matern. Fetal Neonatal Med. 2011;24(Suppl 1):151–153. doi: 10.3109/14767058.2011.607614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Campbell J., Cardon G. Developmental and cross-modal plasticity in deafness: evidence from the P1 and N1 event related potentials in cochlear implanted children. Int. J. Psychophysiol. 2015;95:135–144. doi: 10.1016/j.ijpsycho.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., Yang L.Z., Liu Y., Zhao S.L., Wang Y., Gu F., Yang Z., Zhou Y., Zhang P., Zhang X. Early-onset hearing loss reorganizes the visual and auditory network in children without cochlear implantation. Neuroreport. 2016;27:197–202. doi: 10.1097/WNR.0000000000000524. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansenberg H., Bannister P.R., De L.M., Drobnjak I., Flitney D.E. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Steele C.J., Bailey J.A., Zatorre R.J., Penhune V.B. Early musical training and white-matter plasticity in the corpus callosum: evidence for a sensitive period. J. Neurosci. 2013;33:1282. doi: 10.1523/JNEUROSCI.3578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stropahl M., Chen L.C., Debener S. Cortical reorganization in postlingually deaf cochlear implant users: intra-modal and cross-modal considerations. Hear. Res. 2016;343 doi: 10.1016/j.heares.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Svirsky M.A., Teoh S.W., Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol. Neurootol. 2004;9:224. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- Tan G.C., Doke T.J., Wood N.W., Frackowiak R.S. Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. Neuroimage. 2010;53:1030–1042. doi: 10.1016/j.neuroimage.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabichi O., Kozin E.D., Kanumuri V.V., Barber S., Ghosh S., Sitek K.R., Reinshagen K., Herrmann B., Remenschneider A.K., Lee D.J. Diffusion tensor imaging of Central auditory pathways in patients with sensorineural hearing loss: a systematic Review. Otolaryngol Head Neck Surg. 2018;158(Mar (3)):432–442. doi: 10.1177/0194599817739838. 194599817739838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts K., Ead B., Umansky A., Coalson R., Schlaggar B.L., Firszt J.B., Lieu J.E. Interregional brain interactions in children with unilateral hearing loss. Otolaryngol Head Neck Surg. 2011;144:602–611. doi: 10.1177/0194599810394954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobyne S.M., Osher D.E., Michalka S.W., Somers D.C. LFC-sensory-biased attention networks in human lateral frontal cortex revealed by intrinsic functional connectivity. Neuroimage. 2017;162:362–372. doi: 10.1016/j.neuroimage.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin J.B., Barker B.A., Spencer L.J., Zhang X., Gantz B.J. The effect of age at cochlear implant initial stimulation on expressive language growth in infants and toddlers. J. Speech Lang. Hear. Res. Jslhr. 2005;48:853. doi: 10.1044/1092-4388(2005/059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey T., Waters D., Price C.J., Evans S., MacSweeney M. How auditory experience differentially influences the function of left and right superior temporal cortices. J. Neurosci. 2017;37:9564–9573. doi: 10.1523/JNEUROSCI.0846-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fan Y., Zhao F., Wang Z., Ge J., Zhang K., Gao Z., Gao J.H., Yang Y., Fan J., Zou Q., Liu P. Altered regional and circuit resting-state activity associated with unilateral hearing loss. PLoS One. 2014;9:e96126. doi: 10.1371/journal.pone.0096126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C., Chabot N., Kok M.A., Lomber S.G. Modified areal cartography in auditory cortex following early- and late-onset deafness. Cereb. Cortex. 2014;24:1778–1792. doi: 10.1093/cercor/bht026. [DOI] [PubMed] [Google Scholar]
- Xia S., Song T., Che J., Li Q., Chai C., Zheng M., Shen W. Altered brain functional activity in infants with congenital bilateral severe sensorineural hearing loss: a resting-State functional MRI study under sedation. Neural Plas. 2017;2017:1–8. doi: 10.1155/2017/8986362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Fan W., Zhao X., Li J., Zhang W., Lei P., Liu Y., Wang H., Cheng H., Shi H. Disrupted functional brain connectome in unilateral sudden sensorineural hearing loss. Hear. Res. 2016;335:138–148. doi: 10.1016/j.heares.2016.02.016. [DOI] [PubMed] [Google Scholar]
- YA B., M Y. An overview of hereditary hearing loss. ORL. 2006;68:57–63. doi: 10.1159/000091090. [DOI] [PubMed] [Google Scholar]
- Yeatman J.D., Ben-Shachar M., Glover G.H., Feldman H.M. Individual differences in auditory sentence comprehension in children: an exploratory event-related functional magnetic resonance imaging investigation. Brain Lang. 2010;114:72. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A., Koldewyn K., Kakunoori S., Kanwisher N., Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.Y., Yang M., Liu B., Huang Z.C., Chen H., Zhang P.P., Li J., Chen J.Y., Liu L.J., Wang J., Teng G.J. Changes in the default mode networks of individuals with long-term unilateral sensorineural hearing loss. Neuroscience. 2015;285:333–342. doi: 10.1016/j.neuroscience.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Zhang G.Y., Yang M., Liu B., Huang Z.C., Li J., Chen J.Y., Chen H., Zhang P.P., Liu L.J., Wang J., Teng G.J. Changes of the directional brain networks related with brain plasticity in patients with long-term unilateral sensorineural hearing loss. Neuroscience. 2016;313:149–161. doi: 10.1016/j.neuroscience.2015.11.042. [DOI] [PubMed] [Google Scholar]