Abstract
The aim of the current study was to develop an fMRI task capable of characterizing individual differences in reading and attentional domains. Forty-nine students with a range of reading and attentional control abilities completed an event-related fMRI oddball task consisting of printed word and false font stimuli. Reading network activation was assessed by contrasting printed words with false font stimuli. Left inferior frontal gyrus and superior/middle temporal gyrus showed a main effect of stimulus type. The magnitude of the difference in activation between words and false font was correlated with word reading for both regions and reading fluency for superior/middle temporal gyrus. Regions including bilateral middle cingulate, insula and right inferior frontal gyrus showed a main effect of trial type. The difference in activation between oddball and standard trials in the right superior/middle temporal gyrus and left cerebellum was correlated with attentional control measures. Results indicate the task tapped both reading and attentional control resources. Understanding the contribution of the neural networks supporting each of these domains may provide insight into the shared neural deficits underlying the co-morbidity between developmental dyslexia and attention deficit hyperactivity disorder.
Keywords: Reading, Attentional control, Developmental dyslexia, ADHD, fMRI, Oddball
1. Introduction
Developmental dyslexia (DD) is a brain – based reading disorder characterized by impaired decoding and encoding at the single word level (Snowling, 2000), with current definitions focused on dysfluent word recognition as well as poor spelling and decoding abilities (Lyon et al., 2003). The prevalence of comorbid inattentive behaviors is significantly higher than would be expected by chance, with 25%-40% of individuals with DD also meeting criteria for attention deficit–hyperactivity disorder (ADHD; Willcutt and Pennington, 2000). These disorders are typically studied as two distinct deficits although comorbidity is high and occurs more often than chance in both clinic–referred and community-based samples. This suggests that comorbidity is not the result of a selection artifact (Willcutt and Pennington, 2000) and should be considered in studies of either disorder. Although cognitively and behaviorally dissociable, DD and ADHD may have similar or shared underlying neural correlates (Boada et al., 2012). The involvement of overlapping neural networks may help to explain the high co-occurrence of these disorders, although there are few available neuroimaging paradigms which can concurrently evaluate their shared neural attributes.
1.1. The reading network
Research has identified a complex neural “reading network,” consisting of a predominantly left–hemisphere network of inferior frontal, temporoparietal, and occipitotemporal cortical regions (Martin et al., 2015). Three distinct yet complementary neural pathways, or systems, have been shown to be involved in reading. The reading network’s dorsal system is comprised of left temporoparietal areas including the angular gyrus, supramarginal gyrus (SMG), and posterior superior temporal gyrus (STG), which are thought to play a role in mapping orthographic information to phonological and semantic properties of written words (Xu et al., 2001). The ventral system is associated with left ventral occipitotemporal cortex (VOT) extending into the middle and inferior temporal gyrus, which serve to link the automatic processing of the orthographic features of written language necessary for automatic word recognition (Cohen et al., 2000, 2002). The anterior system is focused in the left inferior frontal gyrus (IFG) and is important for a number of processes such as phonological recoding and semantic integration (Poldrack et al., 1999; Zhu et al., 2012, 2013).
1.2. Attentional control network
Neuroimaging research connected to attentional control has identified a cingulo-fronto-parietal attentional control network, associated with fronto-striatal and fronto-parietal pathways thought to be the primary substrate for attention and executive functioning (Bush, 2011). This network consists of connections between the lateral frontal pole, anterior cingulate cortex, dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, inferior parietal lobe, and various subcortical regions (Bush, 2010). The attentional control network is thought to facilitate goal-directed processes and provides for the ability to respond to changing task demands. Functional neuroimaging research has revealed that individuals with attentional deficits, such as those associated with ADHD, show decreased functioning of this cingulo-fronto-parietal network (Castellanos and Proal, 2012). These differences in the attentional control network have been observed during tasks designed to tap attentional control, such as response inhibition and go/no-go tasks (Dickstein et al., 2006; Rubia, 2011), as well as the oddball task (Kiehl et al., 2001, 2005).
1.3. Overlapping neural networks
Few studies have investigated the shared components of the neural networks associated with DD and ADHD (see Germanò et al., 2010). Those studies which have investigated shared neural profiles between these disorders have suggested structural abnormalities associated with the IFG pars triangularis (Kibby et al., 2009), which plays a role in phonological processing (Eckert et al., 2003) and attentional control (Depue et al., 2010); and striatal dysfunctions associated with difficulties in selective attention (Shafritz et al., 2004). Research suggests that the pulvinar-cortical pathway might mediate interactions between visual language and attention in those with DD (Pugh et al., 2013) and has been shown to be underutilized in those with ADHD (Xi et al., 2012). Additional structural abnormalities such as lower cerebellar volume (Castellanos et al., 2002; Eckert et al., 2003; Rubia, 2007; Stoodley, 2014) and differences in hemispheric asymmetry (Hynd et al., 1993; Pueyo et al., 2000) have been observed in both disorders. Comparing across the separate ADHD and DD literatures also suggests similar functional deficits in temporal regions (Hoeft et al., 2007; Rubia, 2007; Shaywitz et al., 2002; Temple et al., 2001). However, it is difficult to quantify the extent of overlap between reading and attentional control networks because each domain has been assessed using different functional imaging tasks targeting the specific network of interest.
1.4. Benefits of a shared task paradigm
The reading network is typically assessed using fMRI tasks designed to tap into component processes such as lexical decision-making, semantic judgment, or real versus nonsense word-reading (Germanò et al., 2010; Ramus et al., 2003; Snowling, 2000). Conversely, the attentional control network is typically tapped via orienting, sustained attention, and response inhibition tasks. The lack of overlapping or shared fMRI paradigms across these domains limits the ability to study the interface between their neural networks. Development of a single fMRI paradigm with both reading and attentional components would allow for the assessment of independent and overlapping neural networks, while also accounting for shared task demands.
Addtionally, obtaining high quality fMRI data in special child populations, such as those with DD and/or ADHD, can be challenging. Administering separate reading and attentional tasks in a single scan session can be time consuming and cognitively taxing. For example, the Pugh et al. (1996) paradigm is a well validated fMRI reading task but is limited in its utility with special populations because in its full implementation, it can take approximately 45–60 minutes to complete. Conversely, the standard event-related fMRI oddball task typically only takes between 6 and 8 min per run (e.g. Stevens et al., 2007) but with multiple runs collected, it can be extremely taxing on attentional resources.
1.5. Current study
The aim of the current study was to develop and provide initial validation of a single fMRI task capable of quickly and reliabily localizing both reading and attentional control networks within the same child, as well as characterizing individual differences in each domain. The fast localizer reading – attention paradigm (FastLoc-R/A), a variant of the fast localizer task (Malins et al., 2016), involves a passive reading element integrated with an oddball component requiring an active response to a predetermined target that is presented only a portion of the time. This novel task was designed to localize brain regions associated with both reading and attentional control networks in children with a range of reading abilities and inattentive behaviors. Additionally, the task was validated by identifying activated brain regions which showed relations to standardized reading and attentional control measures.
2. Materials and methods
2.1. Participants
Participants were recruited from elementary schools in the greater Atlanta area as part of a longitudinal study of reading intervention approved by the Georgia State University/Georgia Tech Institutional Review Board. Before participation in the study all parents/students provided informed consent/assent. DD readers were recruited from a longitudinal reading intervention study after being identified by their teachers as struggling readers and then meeting study-based low achievement criteria for DD diagnosis, defined as scoring one standard deviation below age-norm expectations (SS ≤ 85) on standardized reading assessments. Typically developing readers (n = 14) were also included to provide for a full range of reading abilities. All participants were also screened for attentional impairments. Participants had a verbal and/or performance intelligence standard score at or above 80 on one of the subtests of the Wechsler Abbreviated Scale of Intelligence - II (WASI-II; Wechsler, 2011) in order to rule out intellectual disabilities. Children with serious emotional/psychiatric disturbances or other chronic neurological conditions were excluded. Participants in the current study included those students who had completed all relevant behavioral measures and received a baseline MRI scan (including functional sequences) as part of participation in the longitudinal reading intervention study.
All participants were scanned using the same MRI scanner and using the same fMRI sequences (detailed in Section 2.3.1). Seventy-one participants qualified for inclusion in the current study and were included in the analysis of in-scanner behavioral data. Imaging data was assessed for quality; data were excluded if 40% or more of collected volumes exceeded the thresholds of 0.3 mm point-to-point movement and/or 10% outliers. In all, 49 participants were included in the final imaging analysis, with the other 22 excluded for excessive motion (see Table 1).
Table 1.
Descriptive Statistics for Behavioral Only and Imaging Analysis Groups.
| Variable | Behavioral (n = 71) M(SD) |
Imaging (n = 49) M(SD) |
|---|---|---|
| Age | 9.40 (0.66) | 9.43 (0.65) |
| WASI – 2 Matrix Reasoning | 46.80 (9.35) | 47.43 (10.08) |
| DD Diagnosis (%) | 80.28 | 73.47 |
| ADHD Diagnosis (%) | 31.01 | 34.69 |
| Medicated for ADHD symptoms (%) | 21.43 | 18.37 |
| WJ3 Basic Reading Composite | 91.03 (13.41) | 92.55 (15.09) |
| WJ3 Reading Fluency | 91.44 (14.32) | 92.80 (16.11) |
| BRIEF Initiate | 52.89 (10.52) | 52.49 (11.10) |
| BRIEF Monitor | 52.67 (10.99) | 52.76 (11.27) |
| BRIEF Plan/Organize | 53.89 (13.22) | 54.53 (12.04) |
| BRIEF Shift | 51.64 (11.99) | 51.73 (11.63) |
Note: WASI-2 = Wechsler Abbreviated Scale of Intelligence, Second Edition. WJ3 = Woodcock-Johnson III Tests of Achievement. BRIEF = Behavior Rating Inventory of Executive Function.
2.2. Behavioral measures
2.2.1. Reading measures
Subtests of the Woodcock - Johnson III Tests of Achievement were used to index reading skill (Woodcock et al., 2001). The Basic Reading Composite standard score (WJ3-Basic) was used to assess word reading accuracy. The standard score on the Reading Fluency (WJ3-RF) subtest was used to assess reading fluency. Both of these measures have been shown to have high reliability/validity and have been normed for use with school - aged populations.
2.2.2. Attentional control measures
To measure inattentive behaviors, the Behavior Rating Inventory of Executive Functioning (BRIEF; Gioia et al., 2000) was administered. The BRIEF is an individualized, norm-referenced measure of executive function behaviors designed for school-aged students. The Shift, Initiate, Plan/Organize, and Monitor subscales of the BRIEF questionnaire were completed by a parent or guardian who rated their child on behavioral regulation and metacognitive functions associated with attentional control. Higher scores on each of these subscales are associated with higher levels of inattentive behavior/poor attentional control.
2.3. Magnetic resonance imaging
2.3.1. MRI data acquisition
Images were acquired using a 3 T Siemens Trio scanner with a 12-channel head coil located at the GSU/GaTech Center for Advanced Brain Imaging in Atlanta, Georgia. T2*-weighted images were acquired in an axial-oblique orientation parallel to the intercommissural line (32 slices; 4 mm slice thickness; no gap) using single-shot echo planar imaging (matrix size = 64 × 64; voxel size = 3.438 × 3.438 × 4 mm; FoV = 220 mm; TR =2000 ms; TE =30 ms; flip angle = 80°). To allow for stabilization of the magnetic field, the first six volumes within each run were discarded. Anatomical scans were collected in the same orientation as the functional volumes (MPRAGE; matrix size = 256 × 256; voxel size = 1 × 1 × 1 mm; FoV = 256 mm; TR =2530 ms; TE =2.77 ms; flip angle = 7°); these were acquired either following or between the functional runs. Across all trials in the experiment, the time between trial onsets was jittered between 4 and 13 s. Because the TR was 2 s in length, trials started either at the beginning or the middle of a TR, with the likelihood for this equally balanced across all stimulus conditions (Belin et al., 1999). Each child completed between two and four runs of the functional task, each of which was 5:22 (158 volumes) in duration. For all participants, we attempted to collect all four runs of the experiment; however, there were some cases in which only two or three runs were collected due to issues of timing and/or participant discomfort/fatigue (of the 49 participants, six participants completed three runs, and one participant completed two runs).
2.3.2. “Fast” localizer reading - attention task (Fastloc-R/A)
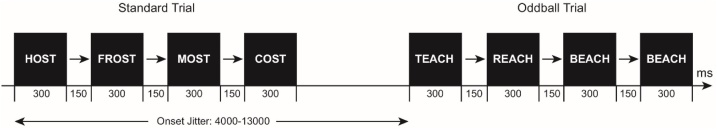
Participants were asked to complete a Fastloc-R/A task similar to the “fast” localizer task (Malins et al., 2016). On each trial of this task, four items in either the visual and auditory modality were presented to participants in a rapid, sequential fashion (described in detail in Appendix A.1.). Participants were asked to make a button press response only when the third and fourth items of the set were identical (i.e. oddball trials). Oddball trials occurred in one third of the trials in each condition. Standard trials were defined as trials in which all four stimuli in the set differed, and therefore a participant response was not required (Fig. 1). Items were consistent with those used in Malins et al. (2016), with minor modifications such that all stimuli were deemed familiar and appropriate for children. Each of the four runs of the task consisted of 48 trials that were evenly distributed across the eight stimulus conditions (six visual and two auditory; only false font and the four types of visual words were analyzed in the current study), for a total of 24 trials in each condition across the whole experiment. Within each condition, the ratio of standard to oddball trials was 2:1. Participants were instructed to respond as quickly and as accurately as possible via button press with their right thumb every time the target stimulus was presented, and were instructed not to respond to the standard trials.
Fig. 1.
Illustration of a visual word standard trial followed by a visual word oddball trial in the fast localizer reading – attention task.
Prior to the scanning session, participants were familiarized with the task in a mock-scanner using a shortened version of the task with different items. A commercially available MRI compatible response device was used to acquire behavioral responses. Stimulus events and behavioral responses were time-locked to scanner data acquisition timings using software run on a separate computer. Performance on oddball trials was used to assess attentional control resources and validate the FastLoc-R/A task, with measures consistent with previous fMRI oddball paradigms (Kiehl et al., 2001, 2005), and with expected reaction times expanded to account for younger participants. Reaction times were computed on oddball trials in which the participant responded correctly between 250 and 2250 ms post-stimulus onset. Omission errors included any missed responses or any response with a latency of greater than 2250 ms following onset of the target stimulus on oddball trials. Errors of commission were defined as responses to standard trials or responses with a latency of less than 250 ms following the onset of the target stimulus on oddball trials. Task performance for word only trials was correlated with reading measures whereas task performance for false font only trials was correlated with attentional control measures. False font only trials were used to index domain-general aspects of attentional control believed to be more closely linked to behavioral measures, and to reduce confounds associated with overlapping reading behaviors.
2.3.3. Analysis of MRI data
After standard preprocessing (see details in Appendix A.2.), single-subject statistical maps were entered into a groupwise analysis (3dMVM in AFNI) that tested for main effects of stimulus type (false font or visual words of the following four types: unrelated, shared orthography and shared phonology, shared orthography but different phonology, or semantically related) and trial type (standard, oddball). This analysis also included subject age and performance IQ as covariates of non-interest. We expected reading-related areas to show main effects of stimulus type, whereas we expected attention-related areas to show main effects of trial type.
Resultant maps were thresholded at a voxelwise threshold of p = 0.0001, cluster corrected at p = 0.05. Cluster correction was performed using the latest recommendations for 3dClustSim in AFNI (Eklund et al., 2016). Spatial autocorrelation function parameters were estimated with respect to each subject’s error time series (3dFWHMx), and Monte Carlo simulations (10,000 iterations) were performed using a whole brain mask with input values consisting of the average parameter estimates across subjects. The cluster threshold for a corrected alpha level of p = 0.05 was four voxels.
There were several resulting clusters that were larger than 1000 voxels in size and spanned multiple anatomic regions. To more closely examine network-specific sub-regions within these larger clusters, we identified local peaks using the program 3dExtrema (minimum separation distance of ten voxels, or 30 mm). Regions of interest were then created by centering spheres with a radius of 6 mm on local peak coordinates. Bonferroni-corrected pairwise t-tests were performed within the smaller clusters identified from the main effects analysis as well as the spherical ROIs within the larger clusters. From the set of clusters that showed a main effect of stimulus type, we defined reading-related brain areas as regions that showed greater activation for all words compared to false font. From the set of clusters that showed a main effect of trial type, we defined attention-related brain areas as regions that showed greater activation for oddball compared to standard trials. In order to reduce confounds associated with the reading-based demands of the attentional task, we selected a priori ROIs within the previously defined attentional control network that have been shown to be involved in monitoring and planning. Both of these skills are required for the oddball task (Bush, 2011; Kiehl et al., 2001, 2005) but are not typically associated with the left-lateralized reading network. Once we defined reading and attention-related brain regions, we then calculated effects of interest for each subject by subtracting beta weights between conditions (i.e., words minus false font; oddball minus standard trials). Individual differences in neural responses were then assessed via correlations with out-of-scanner behavioral measures of reading and attentional control.
3. Results
3.1. Behavioral analysis
3.1.1. Preliminary analysis
Performance on reading and attentional control measures fell within expected ranges (see Table 1). WJ3-Basic and WJ3-RF were significantly, positively correlated with each other (r = 0.84, p < 0.001). Behavioral measures of reading and attentional control were negatively correlated (r = −0.32 to −0.48, p < 0.001), with increased reading performance associated with lower levels of reported behavioral inattention. Those excluded from the imaging group analysis for exceeding the motion threshold did not differ on reading or attentional control measures from those included in the analysis, suggesting that the excluded subgroup was at least not systematically different based on these performance measures.
Participants completed the in-scanner task with near perfect accuracy, with shorter reaction times on word only oddball trials compared to false font. Mean reaction time was 859.10 (SD 172.38) and 915.46 (SD 223.77) for word only and false font trials, respectively. Errors of commission (9%, SD 0.14) and omission (8%, SD 0.13) were relatively rare for word only trials. Errors of commission (9%, SD 0.15) and omission (14%, SD 0.15) were only slightly higher for false font trials.
3.1.2. Behavioral correlations
After FDR correction for multiple comparisons, behavioral performance on the FastLoc-R/A task was significantly, negatively correlated with behavioral measures of reading, with increased reading scores associated with decreased errors in FastLoc-R/A task performance. WJ3-Basic was negatively correlated with omission errors for word only trials (r = −0.32, p < 0.01). WJ3-RF was negatively correlated with errors of omission (r = −0.33, p < 0.01) and commission (r = −0.37, p < 0.01) for word only trials.
Behavioral performance on the FastLoc-R/A task was moderately, positively correlated with behavioral measures of inattention, with increased scores on the BRIEF subscales (indicating poorer attentional control) associated with higher error rates. BRIEF Initiate was correlated with errors of commission for false font trials (r = 0.39, p < 0.001), whereas BRIEF Monitor was correlated with errors of omission for false font trials (r = 0.28, p < 0.01).
3.2. Identification of reading and attentional control networks
The groupwise analysis of those participants who met imaging data threshold parameters (n = 49) identified a number of brain regions that showed either a main effect of stimulus type, a main effect of trial type, or an interaction between stimulus type and trial type.
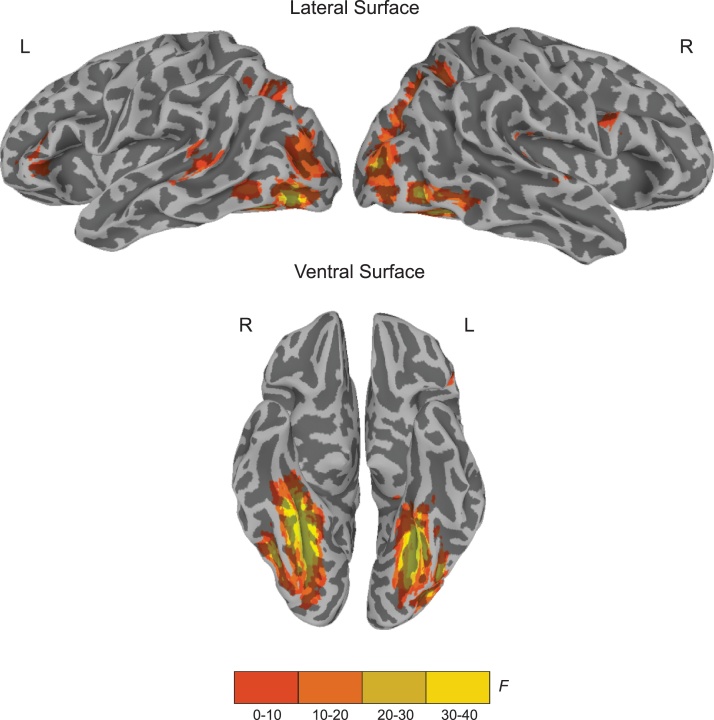
Fig. 2 presents brain regions that showed a main effect of stimulus type (listed in Table 2). This set of regions included the ventral and dorsal visual streams in both hemispheres, as well as clusters in left IFG, left MTG/STG, and several smaller clusters in the right hemisphere. From this larger set of regions, Bonferroni-corrected pairwise t-tests identified two regions that showed greater activation for words compared to false font: left IFG and left MTG/STG. These areas were identified as reading-related brain regions and used in further analyses of brain-behavior relations.
Fig. 2.
Illustration of brain regions showing a main effect of stimulus type, which represents the effect of reading in the fast localizer reading - attention task (voxelwise p = 0.0001; cluster corrected at p = 0.05).
Table 2.
Clusters Showing a Main Effect of Stimulus Typec(F = 6.24; voxelwise p = 0.0001; cluster corrected at p = 0.05).
| Region |
Talairach Coordinates of Peak |
||||
|---|---|---|---|---|---|
| L/R | Area | x | y | z | Extent (voxels)a |
| R | Ventral and dorsal visual streams | 1362 | |||
| Fusiform gyrus | 29 | −50 | −10 | ||
| Middle occipital gyrus | 32 | −83 | 6 | ||
| Superior occipital gyrus/Angular gyrus | 26 | −62 | 36 | ||
| L | Ventral and dorsal visual streams | 1050 | |||
| Fusiform gyrus | −29 | −56 | −10 | ||
| Middle occipital gyrus | −32 | −80 | 9 | ||
| Superior parietal lobule | −23 | −62 | 42 | ||
| L | Middle temporal gyrus/superior temporal gyrusb | −62 | −41 | 9 | 88 |
| L | IFG pars triangularis/ pars orbitalisb | −38 | 32 | 6 | 67 |
| R | Superior temporal gyrus | 44 | −29 | 15 | 37 |
| R | IFG pars opercularis | 47 | 5 | 27 | 25 |
| R | Fusiform gyrus/ Parahippocampal gyrus | 32 | −5 | −22 | 9 |
| R | Superior temporal gyrus | 59 | −14 | 6 | 6 |
Voxels are 3 × 3 × 3 mm, or 27 mm3, in size. Peak co-ordinates are given in LPI orientation.
Denotes clusters identified as components of the reading network for brain-behavior analysis.
Main effect of stimutblnlus type represents the effect of the reading domain.
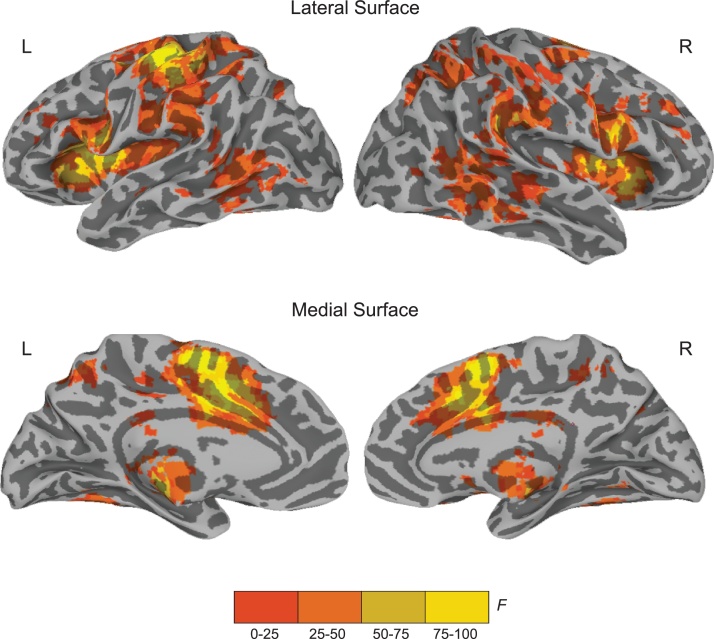
Fig. 3 presents brain regions that showed a main effect of trial type (listed in Table 3). This set of brain regions spanned multiple areas, including those previously identified as attentional control regions such as fronto-parietal regions in both hemispheres and bilateral cerebellum. All of these regions showed greater activation for oddball compared to standard trials. Eight clusters (see Table 3) previously identified as part of the attentional control network during oddball task performance (Kiehl et al., 2001; Warbrick et al., 2013) were identified as attentional control regions and used in brain-behavior analyses.
Fig. 3.
Illustration of brain regions showing a main effect of trial type, which represents the effect of attentional control in the fast localizer reading – attention task (voxelwise p = 0.0001; cluster corrected at p = 0.05).
Table 3.
Clusters Showing a Main Effect of Trial Typec (F = 18.15; voxelwise p = 0.0001; cluster corrected at p = 0.05).
| Region |
Talairach Coordinates of Peak |
||||
|---|---|---|---|---|---|
| L/R | Area | x | y | z | Extent (voxels)a |
| L/R | Attentional control network | 9065 | |||
| R | Middle cingulate cortex | 11 | 14 | 39 | |
| R | Thalamusb | 11 | −17 | 3 | |
| L | Insula/Rolandic operculumb | −35 | −2 | 18 | |
| R | IFG pars opercularis/Precentral gyrusb | 56 | 5 | 21 | |
| R | Supramarginal gyrusb | 50 | −38 | 27 | |
| R | Hippocampus/White matter | 38 | −11 | −10 | |
| L | Middle temporal gyrus/Inferior temporal gyrus | −53 | −53 | −1 | |
| R | Inferior parietal lobule/ Superior parietal lobuleb | 32 | −56 | 48 | |
| L | Superior parietal lobule/Precuneus | −17 | −59 | 51 | |
| L | Brainstem | 5 | −29 | −37 | |
| L | Middle frontal gyrus/Superior frontal gyrus | −23 | 2 | 54 | |
| R | Middle frontal gyrus | 38 | 35 | 30 | |
| L | Middle frontal gyrus | −35 | 35 | 30 | |
| L/R | Cerebellum/MTG | 2873 | |||
| L | Cerebellum (VI)b | −23 | −47 | −22 | |
| R | Cerebellum (VIII/IX)b | 8 | −59 | −40 | |
| R | Cerebellum (VI) | 35 | −41 | −25 | |
| R | MTG | 50 | −50 | 3 | |
| R | MTG/STGb | 53 | −26 | −1 | 51 |
| L | Middle cingulate cortex/White matter | −5 | −29 | 27 | 21 |
| R | Superior orbital gyrus/IFG pars orbitalis | 14 | 23 | −16 | 12 |
| L | Middle occipital gyrus | −41 | −80 | −7 | 7 |
Voxels are 3 × 3 × 3 mm, or 27 mm3, in size. Peak co-ordinates are given in LPI orientation.
Denotes clusters identified as components of the attentional control network for brain-behavior analysis.
Main effect of trial type represents the effect of the attentional control domain.
Clusters showing an interaction between stimulus type and trial type are listed in Table 4. A priori hypotheses for these interactions were not generated for the current study and therefore these regions were not analyzed further.
Table 4.
Clusters Showing an Interaction between Stimulus Type and Trial Type (F = 6.24; voxelwise p = 0.0001; cluster corrected at p = 0.05).
| Region |
Talairach Coordinates of Peak |
||||
|---|---|---|---|---|---|
| L/R | Area | x | y | z | Extent (voxels)a |
| R | Posterior cingulate cortex/Middle cingulate cortex | 11 | −44 | 27 | 33 |
| L | Inferior parietal lobule/Supramarginal gyrus | −44 | −26 | 42 | 9 |
| L | Supramarginal gyrus | −44 | −29 | 33 | 7 |
| L | Superior temporal gyrus/Middle temporal gyrus | −56 | −11 | 3 | 6 |
Voxels are 3 × 3 × 3 mm, or 27 mm3, in size. Peak co-ordinates are given in LPI orientation.
3.3. Brain-behavior correlations
After FDR correction for multiple comparisons, several brain-behavior relations were identified in regions associated with reading or attentional control. The difference in activation between words and false font in the left MTG/STG was significantly correlated with WJ3-Basic (r = 0.38, p < 0.01). The difference in activation between words and false font in the left MTG/STG was also significantly correlated with WJ3-RF (r = 0.36, p < 0.01).
The difference in activation between oddball and standard trials in the right MTG/STG was negatively correlated with BRIEF Initiate (r = -0.36, p < .01), Plan/Organize (r = -0.42, p < 0.01), and Monitor scales (r = -0.32, p < 0.05). The difference in activation between oddball and standard trials in the left cerebellum (VI) was negatively correlated with BRIEF Plan/Organize (r = -0.39, p < 0.01).
4. Discussion
The primary aim of the current study was to develop and validate an fMRI paradigm capable of simultaneously localizing both reading and attentional control networks in school-aged children. To do this, we utilized the FastLoc-R/A task with a group of children who exhibited a broad range of skills in reading and attentional control, including a number of children who showed deficits in either or both domains. Analyses focused on first identifying reading and attentional control brain networks using a groupwise analysis, and then subsequently testing the sensitivity of the task to individual differences by relating neural activation within these networks to out-of-scanner measures of reading and attentional control.
A number of brain regions showed a main effect of stimulus type in the visual modality, including the dorsal and ventral visual streams, as well as left IFG and left MTG/STG. Post-hoc analyses revealed that left IFG and left MTG/STG showed greater activation for words compared to the false font condition. Additionally, the extent of activation in the left MTG/STG scaled with out-of-scanner standardized behavioral measures of single word reading and reading fluency.
The left MTG/STG has been previously implicated as a critical region in the reading network in both children and adults (Cao et al., 2006; Martin et al., 2015; Pugh et al., 1996), and has been associated with semantic processing (Price et al., 1997; Pugh et al., 1996). The left IFG is also regarded as a canonical reading-related region that is sensitive to individual differences across readers of different ages and skill levels. This region has been associated with various processes including phonological recoding (Poldrack et al., 1999), semantic integration (Zhu et al., 2013), and print-speech convergence (Preston et al., 2015). In the current study, even though the left IFG was identified as a reading-related region at the group level, we failed to observe relations between left IFG activation and individual differences in reading skills in this sample. A possible explanation could be that differences in the engagement of the left IFG may instead be reflected in other attributes of the neural signal rather than mean activity. In a similar sample of children who performed a metacognitive reading task involving matching pictures of items to visual words, individual differences in reading skill were not significantly related to mean activation in the left IFG but were instead associated with variability in activation across trials (Malins et al., 2018).
In a previous study using a passive version of this task with skilled adult readers, the left IFG and left MTG/STG showed similar patterns of activity for words and false font (Malins et al., 2016). However, the current study failed to detect differences between words and false font in several regions that showed differences in skilled adult readers, including left VOT. This may have arisen for several reasons. First, the left VOT is thought to be progressively tuned throughout development as children become more proficient readers (Church et al., 2008; McCandliss et al., 2003; Turkeltaub et al., 2003; Wandell et al., 2012), and activation within this region scales with reading proficiency even when accounting for age (Sandak et al., 2004; Shaywitz et al., 2002), so this discrepancy may be due to differences across studies in both age and reading skill. This is particularly likely given that the current sample is oversampled for struggling readers. Second, the active nature of the current task may have promoted more extensive activation of extrastriate cortex in the visual system responsible for lower-level processing of visual information, which could have obscured subtle differences in left VOT regions.
The attentional control manipulation that was included in the FastLoc-R/A task in the current study consisted of a repetition judgment task, in which oddball trials were compared to standard trials, and a number of brain regions showed differential engagement. In particular, a network of brain regions showed greater activation for oddball compared to standard trials, including the left superior parietal lobule/precuneus, right supramarginal gyrus, right MTG/STG, right IFG pars opercularis/precentral gyrus, right superior and inferior parietal lobule, thalamus, and regions of the cerebellum. These regions have been previously implicated in attentional control responsible for sustained attention and vigilance involved in target detection (Ardekani et al., 2002; Clark et al., 2000; Kiehl et al., 2001; Stevens et al., 2000; Tamm et al., 2006; Warbrick et al., 2013; Wynn et al., 2015).
While historically used in conjunction with EEG research, the oddball task paradigm has been shown to be sensitive to individual differences in neural activity associated with attentional control in children and adults with ADHD compared to controls (Alexander et al., 2008; Barry Robert et al., 2009; Lopez et al., 2006; Marzinkzik et al., 2012). Children and adolescents with ADHD have shown reduced brain activation in attentional control regions such as the right middle frontal gyrus, bilateral cingulate cortex, inferior parietal lobule/supramarginal gyrus, insula, thalamus, and cerebellum (Orinstein and Stevens, 2014; Tegelbeckers et al., 2015). This is particularly true in the case of inattentive subtype ADHD where the predominant symptoms include the inability to focus on and attend to task relevant information. The oddball task may be most sensitive to individual differences in ADHD inattentive subtype because it requires vigilance and sustained attention. Inattention, rather than hyperactivity, is most common with ADHD in the presence of co-morbid reading impairments such as DD (Boada et al., 2012; McGrath et al., 2011). Therefore, an oddball paradigm that taps into attentional as well as reading resources, such as the FastLoc-R/A task, may serve to help us better understand the shared and distinctive neural circuitry of ADHD and DD.
One caveat associated with the oddball paradigm is that oddball trials require a motor response (i.e. button press), which is not required in the standard condition. As expected, several regions that showed a main effect of trial type in the current study were associated with this motor response; for this reason, we performed brain-behavior correlations only for the regions thought to be involved in attentional control rather than response execution. More specifically, we examined the relationship between behavioral ratings of attentional control and neural activation in eight regions associated with the attentional control network. Two of these regions, the right MTG/STG and the left cerebellum (VI) were correlated with subscales of the BRIEF thought to be associated with the ADHD inattentive subtype. Consistent with previous ADHD fMRI oddball literature (Orinstein and Stevens, 2014; Tegelbeckers et al., 2015), increased levels of behavioral inattention were associated with decreased activation in these attentional control regions. Other regions showing reduced activation in children and adolescents with ADHD, such as bilateral middle frontal gyrus and right inferior/superior parietal lobule, also showed a main effect of trial type but were not correlated with behavioral measures of inattention. However, previous studies utilizing the oddball paradigm examined group differences in neural activation in participants with ADHD versus controls, whereas the current study looked at a range of attentional control and reading abilities. Examining attentional control along a continuum allowed us to correlate activation with behavioral measures of attention and therefore validate the FastLoc-R/A task.
The current study also examined in - scanner FastLoc-R/A task performance in relation to behavioral measures of attentional control and reading. It should be noted that the FastLoc-R/A places a heavy demand on verbal working memory in addition to reading and attentional control. There are several underlying brain regions, such as the left IFG and MTG/STG, that play a role in each of these cognitive processes. In an effort to reduce the confounds associated with this overlap, attentional control regions used for behavioral analyses were selected a priori and included only those regions that are associated with the attentional control network but are not directly related to the reading network.
Overall, the oddball task was performed with high accuracy, and reaction times for oddball trials fell within the expected range for the age group. This is consistent with previous research utilizing a similar visual oddball task (Alexander et al., 2008; Barry Robert et al., 2009; Kiehl et al., 2001, 2005; Orinstein and Stevens, 2014; Tegelbeckers et al., 2015). One caveat associated with the FastLoc-R/A task is that both reading and attentional control measures do occur in the context of print (words versus false font resembling print). There was a small negative correlation between errors of omission on false font only trials and the WJ3 Basic (r = −0.28, p = 0.02), with an increased number of task errors associated with poorer word reading ability; however, error rates on the task were low enough to suggest that children of all skill levels in reading and attentional control are capable of performing this task. The FastLoc-R/A was sensitive to individual differences in both reading and attentional control in the current sample, even with near perfect accuracy, suggesting that it may be ideal for assessing individual differences in each of these domains.
4.1. Conclusions
To our knowledge, this is the first fMRI paradigm capable of characterizing behavioral and neural aspects of reading and attentional control within the same task. We were able to localize brain regions associated with each domain that are also associated with standardized measures of reading and attentional control. Defining reading and attentional control networks using a common task significantly reduces scan time and increases data quality and reliability. While this study sought to validate the FastLoc-R/A paradigm, future research could use the paradigm to characterize overlapping neural networks associated with DD and ADHD. Understanding the contributions of the networks supporting reading and attentional control may provide a better understanding of the shared neural deficits underlying the co-morbidity between DD and ADHD, and may also provide a new classification model for such children based on a range of attentional and language functioning.
Acknowledgements
This research was supported in part under Award P01HD070837 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100674.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Alexander D.M., Hermens D.F., Keage H.A., Clark C.R., Williams L.M., Kohn M.R., Clarke S.D., Lamb C., Gordon E. Event-related wave activity in the EEG provides new marker of ADHD. Clin. Neurophysiol. 2008;119(1):163–179. doi: 10.1016/j.clinph.2007.09.119. [DOI] [PubMed] [Google Scholar]
- Ardekani B.A., Choi S.J., Hossein-Zadeh G.A., Porjesz B., Tanabe J.L., Lim K.O., Bilder R., Helpern J.A., Begleiter H. Functional magnetic resonance imaging of brain activity in children with ADHD. J. Child Psychol. Psychiatry. 2002;41:225–231. doi: 10.1016/s0926-6410(02)00137-4. [DOI] [PubMed] [Google Scholar]
- Barry Robert J., Clarke A.R., McCarthy R., Selikowitz M., Brown C.R., Heaven P.C. Event-related potentials in adults with Attention-Deficit/Hyperactivity Disorder: an investigation using an inter-modal auditory/visual oddball task. Int. J. Psychophysiol. 2009;71(2):124–131. doi: 10.1016/j.ijpsycho.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Belin P., Zatorre R.J., Hoge R., Evans A.C., Pike B. Event-related fMRI of the auditory cortex. Neuroimage. 1999;10(4):417–429. doi: 10.1006/nimg.1999.0480. [DOI] [PubMed] [Google Scholar]
- Boada R., Willicut E.W., Pennington B.P. Understanding the comorbidity between dyslexia and attention-deficit/hyperactivity disorder. Top. Lang. Disord. 2012;32(3):264–284. [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35(1):278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69(12):1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F., Bitan T., Chou T.-L., Burman D.D., Booth J.R. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J. Child Psychol. Psychiatry. 2006;47(10):1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Lee P.P., Sharp W., Jeffries N.O., Greenstein D.K., Clasen L.S., Blumenthal J.D. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. J. Am. Med. Assoc. 2002;288(14):1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn. Sci. 2012;16(1):17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J.A., Coalson R.S., Lugar H.M., Petersen S.E., Schlaggar B.L. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb. Cortex. 2008;18(9):2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V.P., Fannon S., Lai S., Benson R., Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J. Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S., Naccache L., Lehericy S., Dehanaene-Lambertz G., Henaff M.A., Michel F. The visual word form area - spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehericy S., Chochon F., Lemer C., Rivaud S., Dehaene S. Language-specific tuning of visual cortex functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Depue B.E., Burgess G.C., Bidwell L.C., Willcutt E.G., Banich M.T. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res. Neuroimaging. 2010;182(3):231–237. doi: 10.1016/j.pscychresns.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neual correlates of attention defict hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Eckert M.A., Leonard C.M., Richards T.L., Aylward E.H., Thomson J., Berninger V.W. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126(2):482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113(8):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanò E., Gagliano A., Curatolo P. Comorbidity of ADHD and dyslexia. Dev. Neuropsychol. 2010;35(5):475–493. doi: 10.1080/87565641.2010.494748. [DOI] [PubMed] [Google Scholar]
- Gioia G.A., Isquith P.K., Guy S.C., Kenworth L. Psychological Assessment Resources, Inc.; Lutz, FL: 2000. Behavior Rating Inventory of Executive Function. [Google Scholar]
- Hynd G.W., Hern K.L., Novey E.S., Eliopulos D., Marshall R., Gonzalez J.J., Voeller K.K. Attention-deficit hyperactivity disorder and asymmetry of the caudate-nucleus. J. Child Neurol. 1993;8(4):339–347. doi: 10.1177/088307389300800409. [DOI] [PubMed] [Google Scholar]
- Kibby M.Y., Kroese J.M., Krebbs H., Hill C.E., Hynd G.W. The pars triangularis in dyslexia and ADHD: a comprehensive approach. Brain Lang. 2009;111(1):46–54. doi: 10.1016/j.bandl.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A., Laurens K.R., Duty T.L., Forster B.B., Liddle P.F. An event-related fMRI study of visual and auditory oddball tasks. J. Psychophysiol. 2001;15(4):221–240. [PubMed] [Google Scholar]
- Kiehl K.A., Stevens M.C., Laurens K.R., Pearlson G., Calhoun V.D., Liddle P.F. An adaptive processing model of neurocognitive function: supporting evidence from a large scale (n=100) MRI study of an auditory oddball task. Neuroimage. 2005;25(3):899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lopez V., Lopez-Calderon J., Ortega R., Kreither J., Carrasco X., Rothhammer P., Rothhammer F., Rosas R., Aboitiz F. Attention-deficit hyperactivity disorder involves differential cortical processing in a visual spatial attention paradigm. Clin. Neurophysiol. 2006;117(11):2540–2548. doi: 10.1016/j.clinph.2006.07.313. [DOI] [PubMed] [Google Scholar]
- Lyon R., Shaywitz S.E., Shaywitz B.A. A definition of dyslexia. Ann. Dyslexia. 2003;53(1):1–14. [Google Scholar]
- Malins J.G., Gumkowski N., Buis B., Molfese P., Rueckl J.G., Frost S.J., Pugh K.R., Morris R., Mencl W.E. Dough, tough, cough, rough: a “fast” fMRI localizer of component processes in reading. Neuropsychologia. 2016;91:394–406. doi: 10.1016/j.neuropsychologia.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malins J.G., Pugh K.R., Buis B., Frost S.J., Hoeft F., Landi N., Mencl W.E., Kurian A., Staples R., Molfese P.J., Sevicik R., Morris R. Individual differences in reading skill are related to trial-by-trial neural activation variability in the reading network. J. Neurosci. 2018;38(12):2981–2989. doi: 10.1523/JNEUROSCI.0907-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Schurz M., Kronbichler M., Richlan F. Reading in the brain of children and adults: a meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzinkzik F., Wahl M., Kruger D., Gentschow L., Colla M., Klostermann F. Abnormal distracter processing in adults with attention-deficit-hyperactivity disorder. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandliss B.D., Cohen L., Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McGrath L.M., Penninton B.F., Shanahan M.A., Santerre-Lemmon L.E., Barnard H.D., Willcutt E.G. A multiple deficit model of reading disability an attention-deficit/hyperactivity disorder: search for shared cognitive deficits. J. Child Psychol. Psychiatry. 2011;52(5):547–557. doi: 10.1111/j.1469-7610.2010.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orinstein A.J., Stevens M.C. Brain activity in predominantly-inattentive subtype attention-deficit/hyperactivity disorder during an auditory oddball attention task. Psychiatry Res. Neuroimaging. 2014;223(2):121–128. doi: 10.1016/j.pscychresns.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Wagner a D., Prull M.W., Desmond J.E., Glover G.H., Gabrieli J.D. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Preston J.L., Molfese P.J., Frost S.J., Mencl W.E., Fulbright R.K., Hoeft F., Landi N., Shankweiler D., Pugh K.R. Print-speech convergence predicts future reading outcomes in early readers. Psychol. Sci. 2015;27(1):75–84. doi: 10.1177/0956797615611921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Moore C.J., Humphreys G.W., Wise R.J.S. Segregating semantic from phonological processes during reading. J. Cogn. Neurosci. 1997;9(6):727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Pueyo R., Maneru C., Vendrell P., Mataro N., Estevez-Gonzalez A., Garcia-Sanchez C. Attention deficit hyperactivity disorder. Cerebral asymmetry observed on magnetic resonance. Rev. Neurol. 2000;30(10):920–925. [PubMed] [Google Scholar]
- Pugh K.R., Landi N., Preston J.L., Mencl W.E., Austin A.C. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang. 2013;125(2):173–183. doi: 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K.R., Shaywitz B.A., Shaywitz S.E., Constable R.T., Skudlarski P., Fulbright R.K., Bronen R.A. Cerebral organization of component processes in reading. Brain. 1996;119(4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Ramus F., Rosen S., Dakin S.C., Day B.L., Castellote J.M., White S., Frith U. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126(4):841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Rubia K. Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proc. Natl. Acad. Sci. U. S. A. 2007;104(50):19663–19664. doi: 10.1073/pnas.0710329105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. "Cool" inferior frontostriatal dysfunction in attention-deficity/hyperactivity disorder versus "hot" ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol. Psychiatry. 2011;68(12):e69–87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Sandak R., Mencl W.E., Frost S.J., Rueckl J.G., Katz L., Moore D.L., Mason S.A., Fulbright R.K., Constable R.T., Pugh K.R. The neurobiology of adaptive learning in reading: a contrast of different training conditions. Cogn. Affect. Behav. Neurosci. 2004;4(1):67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Shafritz K.M., Marchoine K.E., Gore J.C., Shaywitz S.E., Shaywitz B.A. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2004;161(11):1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R., Mencl W.E., Fulbright R.K., Skudlarski P., Constable R.T. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;55(2):101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Snowling M.J. Foundations of reading acquisition and dyslexia: implication for early intervention. Br. J. Educ. Psychol. 2000;70(2):275–276. [Google Scholar]
- Stoodley C.J. Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front. Syst. Neurosci. 2014;8(92):1–17. doi: 10.3389/fnsys.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Kiehl K.A. An fMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. Am. J. Psychiatry. 2007;164(11):1737–1749. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- Stevens A.A., Skudlarski P., Gatenby J.C., Gore J.C. Event-related fMRI of auditory and visual oddbal tasks. Magn. Reson. Imaging. 2000;18:495–502. doi: 10.1016/s0730-725x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A.L. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am. J. Psychiatry. 2006;163(6):1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- Tegelbeckers J., Bunzeck N., Duzel E., Bonath B., Flechtner H.H., Krauel K. Altered salience processing in attention deficit hyperactivity disorder. Hum. Brain Mapp. 2015;36(6):2049–2060. doi: 10.1002/hbm.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E., Poldrack R.A., Salidis J., Deutsch G.K., Tallal P., Merzenich M.M., Gabrieli J.D. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuroreport. 2001;12(2):299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Gareau L., Flowers D.L., Zeffiro T.A., Eden G.F. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Wandell B.A., Rauschecker A.M., Yeatman J.D. Learning to see words. Annu. Rev. Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick T., Reske M., Shah N.J. Do EEG paradigms work in fMRI? Varying task demands in the visual oddball paradigm: implications for task design and results interpretation. Neuroimage. 2013;77:177–185. doi: 10.1016/j.neuroimage.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Wechsler D. second edition. NCS Pearson; San Antonio, TX: 2011. Wechsler Abbreviated Scale of Intelligence. (WASI-II) [Google Scholar]
- Willcutt E.G., Pennington B.F. Comorbidity of reading disability and attention-deficit/hyperactivity disorder: differences by gender and subtype. J. Learn. Disabil. 2000;33(2):179–191. doi: 10.1177/002221940003300206. [DOI] [PubMed] [Google Scholar]
- Woodcock R.W., McGrew K.S., Mather N. Woodcock-johnson III tests of achievement. Test. 2001;2001 [Google Scholar]
- Wynn J.K., Jimenez A.M., Roach B.J., Korb A., Lee J., Horan W.P., Ford J.M., Green M.F. Impaired target detection in schizophrenia and the ventral attentional network: findings from a joint event-related potential-functional MRI analysis target stimulus ERP/fMRI analysis in schizophrenia. Neuroimage Clin. 2015;9:95–102. doi: 10.1016/j.nicl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Grafman J., Gaillard W.D., Ishlii K., Vega-Bermudez F. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb. Cortex. 2001;11(3):267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]
- Zhu Z.D., Feng G., Zhang J.X., Li G., Li H., Wang S. The role of the left prefrontal cortex in sentence-level semantic integration. NeuroImage. 2013;76:325–331. doi: 10.1016/j.neuroimage.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Zhu Z.D., Hagoort P., Zhang J.X., Feng G.Y., Chen H.C., Batiaansen M., Wang S.P. The anterior left inferior frontal gyrus contributes to semantic unification. Neuroimage. 2012;60(4):2230–2237. doi: 10.1016/j.neuroimage.2012.02.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.