Abstract
Individual differences in temperament have been theorized to be supported by differential recruitment of key neural regions, resulting in the distinct patterns of behavior observed throughout life. Although a compelling model, its rigorous and systematic testing is lacking, particularly within the heightened neuroplasticity of early childhood. The current study tested a model of the link between temperament, the brain, and behavior for cognitive flexibility in a sample of 4-5-year-old children (N = 123) using functional near-infrared spectroscopy (fNIRS) to assess prefrontal cortex (PFC) activation. Structural Equation Modeling (SEM) was used to explore the link between survey reports of temperamental effortful control, and both performance-based and neuroimaging measures of cognitive flexibility. Results indicated that greater parent-reported temperamental effortful control was associated with better performance on a cognitive flexibility task, and less activation of the DLPFC in preschoolers. These findings support the theorized model of the interrelatedness between temperamental tendencies, behavior, and brain activation and suggest that better temperamentally regulated children use the DLPFC more efficiently for cognitive flexibility.
Keywords: Cognitive flexibility, Functional near-infrared spectroscopy (fNIRS), Effortful control, PFC, Preschool, Executive function
1. Introduction
Decades of research on early temperamental traits have supported a model in which individual differences in behavioral responses are supported by differential recruitment of key neural regions and networks, which in turn are linked to patterns of healthy functioning across the lifespan (Fox et al., 2005; Hayden et al., 2007; Kagan et al., 1987; Posner and Rothbart, 2007). For example, behavioral inhibition, a temperamental tendency to experience fear in unfamiliar situations, has been associated with increased activation of the amygdala (Pérez-Edgar et al., 2007), as well as with hyperattention to threat-related stimuli and a tendency to withdraw from social situations (Chronis-Tuscano et al., 2009; Pérez-Edgar et al., 2010). Even though this is a compelling model for the relation between brain and behavior within temperamental domains, research in early childhood often does not consider all parts of this model within the same investigation, and the research that does, often focuses on behavioral inhibition and related temperamental traits. During the preschool years, children dramatically improve their ability to control their attention and behaviors (i.e., effortful control) (Kochanska and Knaack, 2003), and this seems to have significant consequences for healthy developmental (Eisenberg et al., 2009). A better understanding of the interrelatedness of constitutionally-based temperamental traits, laboratory-observed behaviors, and brain activity in this age range could help clarify which children are more likely to have persistent problems with effortful control, resulting in maladaptive patterns of functioning later in life. Thus, in the current study, we tested this integrative model within the domain of effortful control, which has been hypothesized to define variability in executive function through prefrontal cortex (PFC) activation to advance research on this temperamental trait.
Executive function behaviors, defined as the cognitive processes necessary for goal-directed behaviors, including working memory, inhibitory control, and cognitive flexibility (Miyake et al., 2000), have long been a focus within developmental psychology and cognitive neuroscience (Diamond, 2006; Nigg, 2017) and are a transdiagnostic behavioral and neural focus for the development of mental health problems (Shanmugan et al., 2016). We focused our investigation on the construct of cognitive flexibility, the ability to shift behaviors and cognitions based on contextual demands (Armbruster et al., 2012; Berg, 1948) as this ability becomes increasingly necessary throughout childhood when children shift from behavioral strategies of self-regulation (e.g. self-soothing behaviors) to cognitive approaches (e.g. reappraisal; Perlman and Pelphrey, 2010; Zelazo and Cunningham, 2007). Moreover, cognitive flexibility has emerged as an important correlate of healthy development both concurrently and longitudinally, making it an important ability to study in early childhood (Bock et al., 2015; Duncombe et al., 2013; Hawes et al., 2016).
Cognitive flexibility matures along a steep slope during early childhood (ages 3–6; Diamond, 2006; Garon et al., 2008) and this is thought to be driven by neurodevelopmental changes in the PFC (Diamond, 2002; Ezekiel et al., 2013). Specifically, the structural and functional changes that the PFC undergoes during the preschool years (Giedd et al., 1999; Tsujimoto, 2008), allow for the substantial increases in cognitive development observed during this period (Diamond, 2006). Specific to cognitive flexibility, several studies have consistently pointed to the lateral region of the PFC, the dorsolateral prefrontal cortex (DLPFC), as supporting this ability in adults (Armbruster et al., 2012; Niendam et al., 2012). Similar patterns of DLPFC activation have also been found in studies with younger samples (Crone, 2009; Morton et al., 2009; Schroeter et al., 2004). For example, one study in 3-5-year-olds found that children showed similar increases in activation in the DLPFC to adults on a modified Dimensional Change Card Sort task (DCCS; Moriguchi and Hiraki, 2009); a classic test of cognitive flexibility. In a follow-up longitudinal study, children improved their ability to recruit this region in a one-year span, suggesting that maturation in behavioral performance is supported by neural development of this region (Moriguchi and Hiraki, 2011). Another study, using a preschool appropriate version of the classic Stroop task (Stroop, 1935), found that cognitive flexibility was associated with increased activation in the left DLPFC in children 3-5-year-olds (Li et al., 2017). Thus, there is ample support for the role of the DLPFC for underlying the behaviors inherent in flexible cognition. Although there is substantial research on cognitive flexibility, it is important to highlight the ways in which these tasks differ across procedures. For example, although the traditional Stroop capitalizes on automatic processing by creating a mismatch with the voluntary processing of stimuli, the most traditional cognitive flexibility task in childhood, the DCCS, uses rule-shifting as its primary way of eliciting cognitive flexibility. Moreover, the modified Stroop task used in the Li et al. (2017) study, the same one used in this study, takes a slightly different approach from the traditional Stroop task by reducing the amount of perceptual conflict across Stroop and NonStroop blocks (by reducing the number of features that need to be attended) making it a viable task for very young children who often fail to pass the DCCS task. Thus, while these tasks are considered to measure cognitive flexibility, we acknowledge that they all differ on the demands placed on cognitive flexibility and that this needs to be considered more explicitly when interpreting previous findings.
Individual differences in cognitive flexibility have been theorized to be part of a broader effortful control temperamental construct. Effortful control, including attentional focusing, inhibitory control, perceptual sensitivity, and low intensity pleasure (Rothbart et al., 2001), is often defined as the constitutionally based ability to voluntarily suppress or change attention and behavior (Rothbart et al., 2001). Effortful control early in life is thought to offer the foundations over which more complex forms of cognitive control develop during childhood, resulting in more efficient and intricate forms of cognitive control later in life (Kochanska and Knaack, 2003). Individual differences in temperamental effortful control have been found to strongly predict better outcomes throughout life, such as better school functioning (Sánchez-Pérez et al., 2018) and decreased emotional and behavioral problems (Eisenberg et al., 2009). Studies probing associations between temperamental effortful control and cognitive flexibility-related behaviors within the laboratory have also supported this link. For example, toddlers who performed better on a conflict resolution task were reported by the parent as being high in all subdomains of effortful control (Gerardi-Caulton, 2000). Collectively, these studies suggest coherence between a reliable set of behaviors (cognitive flexibility/executive functions), its underlying neural processes (DLPFC activation), and its classification as a temperamental domain (effortful control), yet existing studies have examined only disparate links rather than focusing on the individual differences model as a whole.
The present study aimed to test a model of the relationship between parent-reported variability in temperamental effortful control, cognitive flexibility-related task behavior and cognitive flexibility-related DLPFC activation, to offer insight into how these aspects of cognitive control are integrated in early childhood. We used Functional Near-Infrared Spectroscopy (fNIRS) to assess DLPFC activation, as it allows for the non-invasive measurement of hemoglobin change in the cortex while tolerating more movement than other neuroimaging tools, resulting in greater compliance and more reliable signal (Aslin and Mehler, 2005). We then capitalized on the use of structural equation modeling (SEM) to assess the associations amongst these constructs. We did this by assessing the subdomains of temperamental effortful control (attentional focusing, inhibitory control, perceptual sensitivity, and low intensity pleasure) to clarify how temperamental tendencies towards greater effortful control are associated with neural activation during a cognitive flexibility task. We hypothesized that parent-reported effortful control would be associated with both behavioral and brain measures of cognitive-flexibility, such that children rated by parents as being higher in temperamental effortful control would perform better on the task, suporting the hypothesized model. Finally, to confirm the specificity of our model for effortful control, we explored two alternative models with the temperamental domains of surgency and negative affectivity. We hypothesized that only parent-reported temperamental effortful control would predict behavioral responses and DLPFC activation during a cognitive flexibility task.
2. Method
2.1. Participants
One hundred and fifty-one preschool-aged children (M = 57.91 months., SD = 7.24; 46–71 months-old; 70 girls) were recruited for a larger study on the neural underpinning of emotional development. Children were reported by their parent as being 68% White, 23% Black/African-American, 6% Biracial, 2% Asian American, and 1% Native American or Pacific Islander. Children were identified as being 95% Non-Hispanic. Family annual income ranged substantially, 70 families (47%) reported an income of less than $60,000, 55 (36%) reported an income of $61,000-$120,000, and 26 (17%) reported an income higher than $121,000 a year.
Exclusion criteria included the child having any current or past psychiatric diagnosis or neurological disorders, as well as a history of loss of consciousness or sensory impairments. Because this task was part of a series of tasks children had to complete wearing the cap, 10 children either declined to play or were too tired to do the task. Of the 141 who completed the cognitive flexibility task, 18 were removed due to computer errors during fNIRS data collection leaving a final sample of 123 children for further analyses. Children with usable fNIRS data did not differ from children without usable data on demographic variables or any of the four effortful control subscales (all ts < 1.693, ps > .093).
2.2. Cognitive flexibility task
Children completed a child-friendly Stroop task that has been successfully employed to assess cognitive flexibility (Li et al., 2017). Children sat at a child-sized desk where they were asked to complete the Pet Store Stroop task (Fig. 1) as part of a battery of cognitive tasks. This task is based on the traditional Stroop (Stroop, 1935), but was modified to be engaging for the preschool age group. Children were told that all the animals in a pet shop had escaped from their cages and were asked to put each animal back in the correct cage. Children were told that they would see an animal and hear a sound (either a dog, a cat, a bird, or a frog; sound lasted for 2 s) and to place each animal in the appropriate cage located on the corners of the screen (e.g., a dog house; 3 additional seconds). A single animal was presented on the center of the screen on each trial. The animal and the cages on the corners of the screen were presented for a total of 5 s. Children were told that sometimes the animals would try to trick them by dressing up as other animals and to pay attention to the sound the animal made to choose the right cage. For the NonStroop control condition, animals made the sound of their species (e.g., a cat made a “Meow” sound). In the Stroop condition, animals made the sound of a different animal (e.g., a cat made the dog sound “Woof”). In this condition, children had to ignore what the animal looked like to place them on the cage based on the sound they made. Children completed a practice session consisting of one NonStroop and one Stroop block before the task began. The task consisted of three NonStroop and three Stroop blocks presented in alternating order, starting with a NonStroop block. Each block consisted of six NonStroop or Stroop trials. There was a 1 s inter-stimulus interval between the trials and a 15 s inter-block interval rest period between blocks. The tasked lasted roughly 5 min. Accuracy and reaction times were recorded for later analysis. Reaction time was calculated from the onset of each trial.
Fig. 1.
Example of Pet Store Stroop ‘NonStroop’ and ‘Stroop’ trials. Children put the animals on the cage corresponding to the sound the animal made.
2.3. fNIRS data acquisition and preprocessing
Non-invasive optical imaging was collected throughout the task with a continuous-wave NIRScout fNIRS system (NIRx Medical Technologies LLC, Glen Head, NY). A total of 8 LED light sources emitted light at 760 nm and 850 nm and were measured by four photodiode light detectors, resulting in a total of 10 measurement channels per wavelength. Signals were collected at 15.625 Hz. Sensors were mounted on a child-friendly elastic neoprene cap. Probes were positioned using the 10–20 coordinate system. The dorsomedial sources were placed on AF3/AF4, and the ventromedial sources were placed on Fp1/Fp2. Hair was parted by a trained research assistant to improve signal detection. The setup took approximately 5 min.
Preprocessing and activation analyses were carried out using the NIRS Brain AnalyzIR toolbox (Santosa et al., 2018). Raw signals were converted to changes in optical density, then corrected for motion artifacts via the Temporal Derivative Distribution Repair (TDDR) method, which uses a robust regression approach to reduce the magnitude of extreme fluctuations in the signal (Fishburn et al., 2018). This is done by calculating the temporal derivatives of the signal and iteratively reweighting the values using Tukey’s bisquare function until the weights of the observations are stabilized. To account for signal drift, data were then detrended by regressing out a discrete cosine transform regressor matrix with a maximum frequency of 1/128 Hz. After this, data were transformed to oxygenated hemoglobin concentration using the modified Beer-Lambert law (Delpy and Cope, 1997) using a differential path length factor of 6 and a partial volume correction of 60 for both wavelengths.
2.4. fNIRS analyses
Activation during the task was quantified by convolving the boxcar function for each block (for both ‘NonStroop’ and ‘Stroop’ conditions) with the canonical hemodynamic response function (HRF) and submitting to a general linear model. To account for variability in the HRF, the temporal and dispersion derivatives were estimated and discarded. An autoregressive iteratively-reweighted least squares approach (Barker et al., 2013) was used to estimate the coefficients to account for the presence of serial correlations in the data. Following this, a weighted mixed effects model was used to model condition as a fixed effect and subject as a random effect. To assess the activation associated with cognitive flexibility, a t-contrast of ‘Stroop’ versus ‘NonStroop’ was carried out, and the false discovery rate (FDR) correction was used to control for multiple comparisons associated with having multiple channels (Benjamini and Hochberg, 1995). The results from this analysis were used to assess the channels where there was a significant difference in activation between the ‘Stroop’ and ‘NonStroop’ condition (Section 3.2 of the results). After this, subject-level activation betas were extracted from the channel with the greatest activation at the group-level for the ‘Stroop’ vs. ‘NonStroop’ contrast. This single beta value per subject quantifies the amount of activation associated with cognitive flexibility. The global activation beta value for each participant was extracted and exported to SPSS 24 (IBM Corp., Armonk, NY) for inclusion in the SEM model and follow-up analyses (Sections 3.3–3.5 of the results).
2.5. Temperamental effortful control
The Child Behavioral Questionnaire short form (CBQ; Rothbart et al., 2001) is a widely used and validated measure of child temperament. It consists of 94 items that assess three broad aspects of temperament: effortful control, negative affectivity, and surgency.
Factor analytic reports have consistently found four subscales to load onto a latent factor of temperamental effortful control (low intensity pleasure, inhibitory control, attentional focusing, and perceptual sensitivity; Simonds et al., 2007). Thus, we used all four subscales for our analyses. One child had an attentional focusing value that was more than 3 SD below the mean, their score was winsorized to the next smallest value in the dataset to improve the normality of distribution for this variable (Wilcox, 2011). Reliability for all subscales ranged from acceptable to good (Effortful control subscales: α = .63–.77; Negative Affectivity: α = .53–.81; Surgency: α = .53–.85; these alphas are similar to those reported on Rothbart et al., 2001).
2.6. Analytic plan
We used SEM to test our hypotheses. First, because effortful control has been hypothesized to be composed of four different subscales, we confirmed the loading of the four subscales onto an effortful control latent factor. We then tested the direct path from effortful control to activation, and from effortful control to behavior while also considering children’s age. SEM analyses were conducted using IBM AMOS version 25 (Arbuckle, 2017). When there was missing data (e.g., the child had fNIRS but not accuracy data due to a light touch on the touchscreen computer), parameter estimates were conducted using Full Information Maximum Likelihood (FIML; Enders and Bandalos, 2001). Indices used to estimate model fit included a chi-square test, the comparative fit index (CFI), the Tucker-Lewis fit index (TLI), the incremental fit index (IFI), and the root mean square error of approximation (RMSEA). Standard guidelines were used to estimate good model fit, such as CFI, TLI, and IFI values higher than .90, and RMSEA value smaller than .06 (Hoyle, 1995; Schreiber, 2008). To assess the specificity of the effortful control–PFC activation relation, we carried out follow-up analyses with two alternative models using the negative affectivity and surgency factors in place of the effortful control factor. Finally, partial correlations were used to explore which aspects of effortful control related most strongly to activation when controlling for age and accuracy.
3. Results
3.1. Behavioral performance on the cognitive flexibility task
Accuracy was good for both ‘NonStroop’ (M = 76.49%; SD = 18.57%;) and ‘Stroop’ conditions (M = 66.26%; SD = 25.80%). Children were more accurate on ‘NonStroop’ compared to ‘Stroop’ trials, t(107) = 5.021, p < .001. Reaction time also varied based on condition (t(106) = 6.283, p < .001). Children were faster on the ‘NonStroop’ (M = 2740.30 ms; SD = 495.94 ms) compared to the ‘Stroop’ (M = 2985.35 ms; SD = 495.11 ms) condition.
3.2. PFC activation
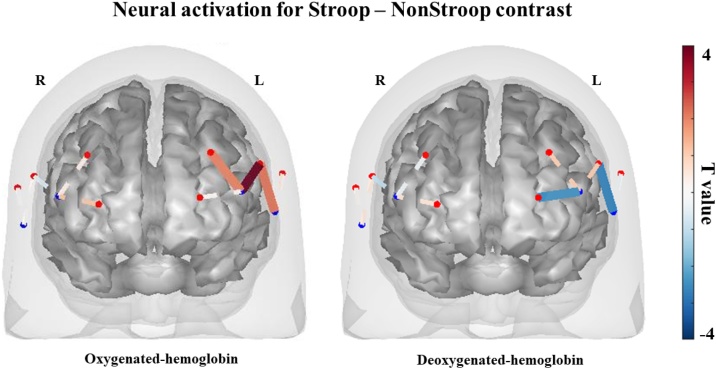
There was a significant increase in oxy-hemoglobin concentrations between Stroop and NonStroop conditions in three channels of the left DLPFC (Fig. 2; the time-course of the fNIRS signal for a representative participant can be found on Supplemental Fig. 1). Thus, the Stroop condition elicited significantly more activation than the NonStroop condition in this region (p < .05; FDR corrected).
Fig. 2.
Cognitive flexibility fNIRS activation shown for the Stroop-NonStroop contrast for oxygenated- and deoxygenated-hemoglobin thresholded at p < .05, FDR corrected for the number of channels.
3.3. Associations between temperamental effortful control, cognitive flexibility-related behavior, and DLPFC activation
Means, standard deviations, and correlations can be found in Table 1. Initial analyses indicated that reaction time during both ‘NonStroop’ and ‘Stroop’ conditions and the difference in reaction time between conditions were not associated with any of our variables of interest and are not considered further. The SEM model resulted in an excellent fit, X2(11) = 7.099, p = .791, CFI = 1.00, TLI = 1.00, IFI = 1.00, RMSEA = .00. For clarity, all estimates presented are standardized estimates. As expected, attentional focusing (β = .73, p < .001), inhibitory control (β = .70, p < .001), perceptual sensitivity (β = .52, p < .001), and low intensity pleasure (β = .55, p < .001), all loaded onto our effortful control latent variable (Fig. 3). Thus, consistent with previous research, all subscales were part of an effortful control latent factor (Rothbart et al., 2001; Simonds et al., 2007).
Table 1.
Descriptive statistics and correlations among predictors.
| Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
|
4.94 | 0.92 | – | |||||
|
4.71 | 0.92 | .526 | – | ||||
|
6.08 | 0.78 | .402 | .347 | – | |||
|
5.37 | 0.88 | .335 | .391 | .327 | – | ||
|
66.26 | 25.80 | .249 | .209 | .221 | .113 | – | |
|
58.09 | 7.39 | .160 | .031 | -.026 | -.010 | .400 | – |
Note. Bold = p < .05; *Age = age in months.
Fig. 3.
SEM model of effortful control predicting PFC activation during the cognitive flexibility task. Estimates are standardized. Errors not included in the figure for clarity. Bold = significant estimate; dashed line = nonsignificant path.
Effortful control predicted activation on the Stroop-NonStroop contrast (β = -.26, SE = 10.15, p = .019), such that greater effortful control predicted less DLPFC activation during cognitive flexibility. Activation did not predict accuracy (accuracy for ‘Stroop’ trials only; β = .01, SE = .06, p = .912). The path from effortful control to accuracy was significant (β = .27, SE = 6.82, p = .017), suggesting that effortful control was associated with increased behavioral performance. Lastly, age predicted accuracy (β = .36, SE = .30, p < .001), such that older children were more accurate in the task1 .
3.4. Follow-up analyses of specific aspects of temperamental effortful control and cognitive flexibility-related DLPFC activation
To decompose associations between cognitive flexibility related activation and the four aspects of effortful control (attentional focusing, inhibitory control, perceptual sensitivity, and low intensity pleasure), partial correlations were conducted between activation and each of these subscales while controlling for children’s accuracy and age. Attentional focusing was the only aspect of effortful control associated with activation when controlling for age and accuracy (r(105) = -.208, p = .032; Fig. 4), such that being better able to focus attention was associated with less activation during the task.
Fig. 4.
Correlation between DLPFC activation during cognitive flexibility and attentional focusing controlling for accuracy and age. Blue lines represent 95% confidence interval of the prediction line (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.5. Specificity of temperamental effortful control associations with DLPFC activation
To assess the specificity of the relation between effortful control and cognitive flexibility-related DLPFC activation and behavior, two follow-up models were conducted to test this model in relation to other core domains of temperament, using the negative affectivity and the surgency temperamental factors. Based on previous work, the negative affectivity model included a latent factor composed of the sadness, fear, anger, discomfort, and soothability subscales (Rothbart et al., 2001) (see Supplemental Table 1 for mean, standard deviation and correlations among negative affectivity domains). This model had good fit X2(17) = 18.68, p = .347, CFI = .98, TLI = .95, IFI = .98, RMSEA = .028. The negative affectivity latent factor did not predict activation (β = .09, SE = 6.07, p = .449) or accuracy (β = -.16, SE = 3.96, p = .154).
The second model included the surgency latent factor composed of the subscales high intensity pleasure, impulsivity, activity level, positive anticipation, and shyness, which loads negatively (the smiling/laughter subscale was originally included in the model, but inclusion of this subscale resulted in an unidentified model, and was thus removed from the final model (see Supplemental Table 2 for mean, standard deviation and correlations among surgency domains). The model showed excellent fit X2(17) = 15.79, p = .539, CFI = 1.00, TLI = 1.00, IFI = 1.00, RMSEA = .000. As expected, the surgency latent factor did not predict activation (β = -.02, SE = 6.82, p = .804) or accuracy (β = -.12, SE = 4.38, p = .205).
4. Discussion
The goal of the current study was to test a model of individual differences linking variability in temperamental effortful control to cognitive flexibility-related behaviors and DLPFC activation in preschoolers. As hypothesized, we found significant associations between temperamental effortful control and both behavioral and brain measures of cognitive flexibility, serving as strong evidence for the coherence of these measures during the preschool years. Our findings, thus, support an integrative model of individual differences in which temperamental tendencies towards greater control of behavior are tightly linked to the recruitment of one of the neural regions that make flexible cognition possible. Additionally, it extends theory on the domain of effortful control by showing evidence of the links between parent-reported temperamental effortful control and cognitive flexibility at both the behavioral and neural level in preschoolers during a laboratory task. Specifically, we found that this pattern can be explained via links between the attentional focusing sub-domain of effortful control, such that children who were higher in their attentional focusing showed less activation in the left DLPFC, suggesting that children who are temperamentally better at modulating their attention required less DLPFC recruitment to efficiently perform on a cognitive flexibility task. Although we focused on effortful control, our integrative model shows the advantages of taking a more comprehensive approach to exploring temperamental traits and can be used to understand temperament more broadly. This model can also be easily expanded to consider how various temperamental dimensions (e.g., fearfulness and effortful control) interact to predict socioemotional profiles.
Our finding that the left DLPFC showed greater activation for trials requiring cognitive flexibility adds to a growing body of research on the vital role of this region for cognitive flexibility (Li et al., 2017; Schroeter et al., 2004). When examining task performance, we found that older children performed better, as would be expected from previous studies (Zelazo et al., 1996). We failed, however, to find any age effects on DLPFC recruitment. It is possible that this lack of age effects was due to the task being simple enough that even the youngest children in our sample could engage in some form of cognitive flexibility, and thus, were still able to recruit the DLPFC to a similar degree as the older children. Our behavioral findings are consistent with previous studies linking greater parent-rated temperamental effortful control with better performance on executive function tasks (e.g., Gerardi-Caulton, 2000). Additionally, we were able to extend knowledge of these links to the neural level by demonstrating that effortful control relates to the underlying neural regions that support these behaviors, namely the DLPFC. Moreover, the results of our follow-up analyses indicate specificity of this DLPFC activation model to the dimension of effortful control, rather than temperament more broadly. These results serve as evidence of the importance of considering an integrative model of individual differences when trying to understand children’s variability in behavior and address recent calls to use more complex models and latent variable approaches for modeling neuroimaging data to study individual differences (Cooper et al., 2019).
Our finding that greater effortful control was linked to less activation of the DLPFC offers some evidence for how early temperamental tendencies might influence developmental trajectories. The fact that better effortful control was associated with less DLPFC activation suggests that children with greater effortful control might have neural systems that perform more efficiently from a younger age or that the DLPFC is better integrated with a neural circuit that supports executive function in children who show better cognitive flexibility (e.g. fronto-parietal network; Dosenbach et al., 2008; Zanto and Gazzaley, 2013). Increased integration of this network might, in turn, place children on more adaptive developmental trajectories. Thus, results from our model suggest that parent-reported variability in temperamental effortful control is linked to more complex forms of cognitive control by allowing for a greater integration of neural networks. The fact that we found this link in preschoolers is particularly meaningful as the neural systems underlying cognitive control are not fully developed at this age (Diamond, 2002), highlighting that these behavioral tendencies are malleable and more susceptible to intervention. Moreover, future research exploring how early in life these links become evident, will help further clarify when intervention efforts would be most useful.
Further contextualizing these findings and offering meaningful specificity to our model, attentional focusing emerged as a particularly important correlate of cognitive flexibility-related DLPFC activation. Conceptually, attentional focusing is the temperamental domain within our effortful control measure that is closest to cognitive flexibility, likely acting as one the foundational process upon which more complex forms of cognitive flexibility such as rule-set shifting, and other executive functions, develop throughout childhood. Although there is still limited research on the link between temperamental attentional control and its neural correlates in childhood, the findings from our model suggest that the underlying brain mechanisms involved in cognitive control support temperamental tendencies in protecting against the emergence of behavioral problems by making cognitive control of behavior more efficient, effective, and less taxing. Findings from studies on disorders marked by cognitive rigidity or inflexibility, such as behavioral problems, offer some support for this idea. For example, a study on 8-12-year-olds with behavioral problems found that children who showed improvements in symptoms after a 14-week treatment program showed decreases in activation of ventrolateral PFC (VLPFC) from pre- to post-treatment, suggesting that the neural underpinnings of cognitive control processes changed for children who responded to treatment to be more like their typically developing peers which in turn resulted in decreases in their behavioral problems (Lewis et al., 2008). Research on irritability in younger children also offer some support for this. Findings from irritability research suggest that increased DLPFC activation could be a compensatory mechanism for the regulation of frustration in children high on irritability (Fishburn et al., 2019a, 2019b; Li et al., 2017; Perlman et al. (2014)). Children who have greater temperamental effortful control might tax their regulatory resources less by requiring less activation to achieve a similar level of behavioral performance as their peers compared to children who engage in these compensatory mechanisms. A recent paper on 3-7-year-olds LPFC activation and irritability seems to support this (Grabell et al., 2018), showing that at the normative range of irritability, less activation was associated with less irritability. It is important to point out, however, that some developmental dual process models suggest that temperamental traits like behavioral inhibition interact with effortful control in more complex ways, such that in some contexts, high effortful control might in fact potentiate risk for later psychopathology in children high in behavioral inhibition (Henderson et al., 2015). Thus, an important extension of our work will be to consider associations between temperament and brain function in children who are experiencing symptoms of disorders where cognitive inflexibility is common and to more thoughtfully consider how other temperamental traits might qualify the links between effortful control and adaptive functioning. Taken together, those studies, combined with our findings, will help elucidate variations in developmental trajectories toward (or away) psychopathology and could help improve intervention efforts by clarifying who is most likely to improve.
4.1. Limitations and future directions
This study represents an important contribution to our understanding of the integration of individual differences in temperament, behavior, and its neural underpinnings in early childhood, but some limitations should be noted. First, our study did not include longitudinal data, limiting our interpretation of how these associations might change across the critical early childhood period, including our inability to establish the earliest age at which these linkages can be detected. Additionally, contrary to our expectations, we failed to find a significant association between children’s behavior and brain activation during the cognitive flexibility task. Although unexpected, correlations between neuroimaging measures and behavioral responses are often lacking in neuroimaging research (e.g., Davis et al., 2003). Moreover, given the age range of our sample, it is likely that our Pet Store Stroop cognitive flexibility task also required children to engage in inhibitory control and working memory. Future studies should aim to better parse out the role of these three dimensions of executive function on prefrontal activation to assess dimension specific links between brain activity and temperamental traits. Additionally, although our decision to use a simplified version of a Stroop task ensured that even the youngest children in the sample could complete the task, this reduced the cognitive demands placed on the participants. In future studies, this task could be modified to force children to change modalities across blocks. For example, by having blocks that can only be correctly responded to by using visual cues and other blocks that require auditory cues. Lastly, our task only measured one form of cognitive flexibility and it is possible that tasks that measure other aspects of cognitive flexibility would show a different pattern of results. Also, we focused on the prefrontal cortex given its importance for executive functions, but this model could apply to the relation among many neural networks and behaviors. Lastly, although our results highlighted the link between the left DLPFC and effortful control, this aspect of temperament is likely linked with the recruitment of the PFC more broadly, and not just this specific region. Future studies should expand on this model and its implications for developmental trajectories by more thoroughly examining these associations.
4.2. Concluding remarks
While the link between behavioral measures and individual differences in temperament has been established in previous studies, few have considered neural activation in conjunction with these measures as part of an integrative model for understanding individual differences in very young children. The current study offers novel support for this model of individual differences, serving as a step towards better understanding how these levels become increasingly integrated across the childhood years, laying the foundation for longitudinal work exploring their integration across childhood.
Declaration of conflict of interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by the National Institute of Mental Health grant R01 MH107540, PI: Perlman. Laura E. Quiñones-Camacho was supported by the National Institute of Mental Health training grant (NIMH T32 MH018951; PI: Brent). M. Catalina Camacho was supported by the National Science Foundation Graduate Research Fellowship under grant No. 174745. We thank Dr. Theodore J. Huppert for his guidance in collecting and analyzing the fNIRS data. We also thank Lisa M. Bemis, Christina O. Hlutkowsky, and the undergraduate research assistants of the Laboratory for Child Brain Development for their help in data collection and management. Lastly, we thank the children and families who participated in the study.
Footnotes
Because 11 children had a Stroop accuracy of below 25% (i.e., below chance for this task), we conducted the SEM model without these children to assess if the patterns reported on the results section changed when only children performing above chance were included in the analyses. This model resulted in similar fit, X2(11) = 11.476, p = .404, CFI = .995, TLI = .987, IFI = .996, RMSEA = .020. The results of the model remained the same when only children performing above chance were included. Effortful control still predicted activation (β = -.26, SE = 10.54, p = .022) and accuracy (β = .30, SE = 5.76, p = .016), and activation was still not predictive of accuracy (β = .04, SE = .05, p = .661).
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100651.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Armbruster D.J., Ueltzhöffer K., Basten U., Fiebach C.J. Prefrontal cortical mechanisms underlying individual differences in cognitive flexibility and stability. J. Cogn. Neurosci. 2012;24(12):2385–2399. doi: 10.1162/jocn_a_00286. [DOI] [PubMed] [Google Scholar]
- Aslin R.N., Mehler J. Near-infrared spectroscopy for functional studies of brain activity in human infants: promise, prospects, and challenges. J. Biomed. Opt. 2005;10(1) doi: 10.1117/1.1854672. [DOI] [PubMed] [Google Scholar]
- Barker J.W., Aarabi A., Huppert T.J. Autoregressive model based algorithm for correcting motion and serially correlated errors in fNIRS. Biomed. Opt. Express. 2013;4(8):1366–1379. doi: 10.1364/BOE.4.001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57(1):289–300. [Google Scholar]
- Berg E.A. A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 1948;39(1):15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Bock A.M., Gallaway K.C., Hund A.M. Specifying links between executive functioning and theory of mind during middle childhood: cognitive flexibility predicts social understanding. J. Cogn. Dev. 2015;16(3):509–521. [Google Scholar]
- Chronis-Tuscano A., Degnan K.A., Pine D.S., Perez-Edgar K., Henderson H.A., Diaz Y. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A. Executive functions in adolescence: inferences from brain and behavior. Dev. Sci. 2009;12(6):825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Cooper S.R., Jackson J.J., Barch D.M., Braver T.S. Neuroimaging of individual differences: a latent variable modeling perspective. Neurosci. Biobehav. Rev. 2019;98:29–46. doi: 10.1016/j.neubiorev.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy D.T., Cope M. Quantification in tissue near–infrared spectroscopy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1997;352(1354):649–659. [Google Scholar]
- Davis E.P., Bruce J., Snyder K., Nelson C.A. The X-trials: neural correlates of an inhibitory control task in children and adults. J. Cogn. Neurosci. 2003;15(3):432–443. doi: 10.1162/089892903321593144. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: cognitive functions, anatomy, and biochemistry. In: Stuss D., Knight R., editors. Principles of Frontal Lobe Function. Oxford University Press; New York, NY: 2002. pp. 466–503. [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystock E., Craik F.I.M., editors. The Early Development of Executive Functions. Lifespan Cognition: Mechanisms of Change. Oxford University Press; Oxford, England: 2006. pp. 70–95. [Google Scholar]
- Dosenbach N.U., Fair D.A., Cohen A.L., Schlaggar B.L., Petersen S.E. A dual- networks architecture of top-down control. Trends Cogn. Sci. (Regul. Ed.) 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe M., Havighurst S.S., Holland K.A., Frankling E.J. Relations of emotional competence and effortful control to child disruptive behavior problems. Early Educ. Dev. 2013;24(5):599–615. [Google Scholar]
- Eisenberg N., Valiente C., Spinrad T.L., Cumberland A., Liew J., Reiser M. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Dev. Psychol. 2009;45(4):988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C.K., Bandalos D.L. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct. Equ. Model. 2001;8(3):430–457. [Google Scholar]
- Ezekiel F., Bosma R., Morton J.B. Dimensional change card sort performance associated with age-related differences in functional connectivity of lateral prefrontal cortex. Dev. Cogn. Neurosci. 2013;5:40–50. doi: 10.1016/j.dcn.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn F.A., Hlutkowsky C.O., Bemis L.M., Huppert T.J., Wakschlag L.S., Perlman S.B. Irritability uniquely predicts prefrontal cortex activation during preschool inhibitory control among all temperament domains: a LASSO approach. Neuroimage. 2019;184:68–77. doi: 10.1016/j.neuroimage.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishburn F.A., Ludlum R.S., Vaidya C.J., Medvedev A.V. Temporal Derivative Distribution Repair (TDDR): a motion correction method for fNIRS. Neuroimage. 2019;184:171–179. doi: 10.1016/j.neuroimage.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.A., Henderson H.A., Marshall P.J., Nichols K.E., Ghera M.M. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu. Rev. Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. https://doi.org/10.1146.annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Garon N., Bryson S.E., Smith I.M. Executive function in preschoolers: a review using an integrative framework. Psychol. Bull. 2008;134(1):31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Gerardi-Caulton G. Sensitivity to spatial conflict and the development of self-regulation in children 24-36 months of age. Dev. Sci. 2000;3(4):397–404. [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grabell A.S., Li Y., Barker J.W., Wakschlag L.S., Huppert T.J., Perlman S.B. Evidence of non-linear associations between frustration-related prefrontal cortex activation and the normal: abnormal spectrum of irritability in young children. J. Abnorm. Child Psychol. 2018;46(1):137–147. doi: 10.1007/s10802-017-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawes S.W., Perlman S.B., Byrd A.L., Raine A., Loeber R., Pardini D.A. Chronic anger as a precursor to adult antisocial personality features: the moderating influence of cognitive control. J. Abnorm. Psychol. 2016;125(1):64–74. doi: 10.1037/abn0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden E.P., Dougherty L.R., Maloney B., Durbin C.E., Olino T.M., Nurnberger J.I., Jr Temperamental fearfulness in childhood and the serotonin transporter promoter region polymorphism: a multimethod association study. Psychiatr. Genet. 2007;17(3):135–142. doi: 10.1097/YPG.0b013e3280147847. [DOI] [PubMed] [Google Scholar]
- Henderson H.A., Pine D.S., Fox N.A. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40(1):207–224. doi: 10.1038/npp.2014.189. https://dx.doi.org/10.1038%2Fnpp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle R.H. Sage Publications; Thousand Oaks, CA: 1995. Structural Equation Modeling: Concepts, Issues, and Applications. [Google Scholar]
- Kagan J., Reznick J.S., Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58(6):1459–1473. [PubMed] [Google Scholar]
- Kochanska G., Knaack A. Effortful control as a personality characteristic of young children: antecedents, correlates, and consequences. J. Pers. 2003;71(6):1087–1112. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Granic I., Lamm C., Zelazo P.D., Stieben J., Todd R.M. Changes in the neural bases of emotion regulation associated with clinical improvement in children with behavior problems. Dev. Psychopathol. 2008;20(3):913–939. doi: 10.1017/S0954579408000448. [DOI] [PubMed] [Google Scholar]
- Li Y., Grabell A.S., Wakschlag L.S., Huppert T.J., Perlman S.B. The neural substrates of cognitive flexibility are related to individual differences in preschool irritability: a fNIRS investigation. Dev. Cogn. Neurosci. 2017;25:138–144. doi: 10.1016/j.dcn.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Neural origin of cognitive shifting in young children. Proc. Natl. Acad. Sci. U. S. A. 2009;106(14):6017–6021. doi: 10.1073/pnas.0809747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi Y., Hiraki K. Longitudinal development of prefrontal function during early childhood. Dev. Cogn. Neurosci. 2011;1(2):153–162. doi: 10.1016/j.dcn.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton J.B., Bosma R., Ansari D. Age-related changes in brain activation associated with dimensional shifts of attention: an fMRI study. Neuroimage. 2009;46(1):249–256. doi: 10.1016/j.neuroimage.2009.01.037. [DOI] [PubMed] [Google Scholar]
- Niendam T.A., Laird A.R., Ray K.L., Dean Y.M., Glahn D.C., Carter C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg J.T. Annual research review: on the relations among self‐regulation, self‐ control, executive functioning, effortful control, cognitive control, impulsivity, risk‐ taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry. 2017;58(4):361–383. doi: 10.1111/jcpp.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Bar-Haim Y., McDermott J.M., Chronis-Tuscano A., Pine D.S., Fox N.A. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010;10(3):349. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K., Roberson-Nay R., Hardin M.G., Poeth K., Guyer A.E., Nelson E.E. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Luna B., Hein T.C., Huppert T.J. fNIRS evidence of prefrontal regulation of frustration in early childhood. Neuroimage. 2014;85:326–334. doi: 10.1016/j.neuroimage.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. Regulatory brain development: balancing emotion and cognition. Soc. Neurosci. 2010;5(5-6):533–542. doi: 10.1080/17470911003683219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Rothbart M.K., Ahadi S.A., Hershey K.L., Fisher P. Investigations of temperament at three to seven years: the children's behavior questionnaire. Child Dev. 2001;72(5):1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pérez N., Fuentes L.J., Eisenberg N., González-Salinas C. Effortful control is associated with children's school functioning via learning-related behaviors. Learn. Individ. Differ. 2018;63:78–88. [Google Scholar]
- Santosa H., Zhai X., Fishburn F., Huppert T. The NIRS brain AnalyzIR toolbox. Algorithms. 2018;11(5):73. doi: 10.3390/a11050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J.B. Core reporting practices in structural equation modeling. Res. Soc. Adm. Pharm. 2008;4(2):83–97. doi: 10.1016/j.sapharm.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Zysset S., Wahl M., von Cramon D.Y. Prefrontal activation due to Stroop interference increases during development—an event-related fNIRS study. Neuroimage. 2004;23(4):1317–1325. doi: 10.1016/j.neuroimage.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Shanmugan S., Wolf D.H., Calkins M.E., Moore T.M., Ruparel K., Hopson R.D. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am. J. Psychiatry. 2016;173(5):517–526. doi: 10.1176/appi.ajp.2015.15060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds J., Kieras J.E., Rueda M.R., Rothbart M.K. Effortful control, executive attention, and emotional regulation in 7-10-year-old children. Cogn. Dev. 2007;22:474–488. [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18(6):643–662. [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. Neuroscientist. 2008;14(4):345–358. doi: 10.1177/1073858408316002. https://doi.org/10.1177%2F1073858408316002. [DOI] [PubMed] [Google Scholar]
- Wilcox R.R. Academic pres.; Waltham, MA: 2011. Introduction to Robust Estimation and Hypothesis Testing. [Google Scholar]
- Zanto T.P., Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn. Sci. (Regul. Ed.) 2013;17(12):602–603. doi: 10.1016/j.tics.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P.D., Frye D., Rapus T. An age-related dissociation between knowing rules and using them. Cogn. Dev. 1996;11(1):37–63. [Google Scholar]
- Zelazo P.D., Cunningham W.A. Executive Function: Mechanisms Underlying Emotion Regulation. In: Gross J.J., editor. Handbook of emotion regulation. The Guilford Press; New York, NY, US: 2007. pp. 135–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.