Highlights
-
•
Neurodevelopment is malleable with Cogmed in neonatal critical illness survivors.
-
•
Global and specific changes in white matter microstructure immediately post-Cogmed.
-
•
Increased FA in the SLF was associated with better verbal working-memory post-Cogmed.
Keywords: Neurodevelopmental disorders, Neuropsychology, Cognitive remediation, Critical care outcomes, Neuroimaging, Diffusion tensor imaging, Memory
Abstract
In a nationwide randomized controlled trial, white matter microstructure was assessed before and immediately after Cogmed Working-Memory Training (CWMT) in school-age neonatal critical illness survivors. Eligible participants were survivors (8–12 years) with an IQ ≥ 80 and a z-score of ≤ −1.5 on (working)memory test at first assessment. Diffusion Tensor Imaging was used to assess white matter microstructure. Associations between any training-induced changes and improved neuropsychological outcome immediately and one year post-CWMT were evaluated as well. The trial was conducted between October 2014–June 2017 at Erasmus MC-Sophia, Rotterdam, Netherlands. Researchers involved were blinded to group allocation. Participants were randomized to CWMT(n = 14) or no-intervention(n = 20). All children completed the CWMT. Global fractional anisotropy(FA) increased significantly post-CWMT compared to no-intervention(estimated-coefficient = .007, p = .015). Increased FA(estimated coefficient = .009, p = .033) and decreased mean diffusivity(estimated-coefficient = −.010, p = .018) were found in the left superior longitudinal fasciculus(SFL) post-CWMT compared no-intervention. Children after CWMT who improved with >1SD on verbal working-memory had significantly higher FA in the left SLF post-CWMT(n = 6; improvement = .408 ± .01) than children without this improvement post-CWMT(n = 6; no-improvement = .384 ± .02), F(1,12) = 6.22, p = .041, = .47. No other structure-function relationships were found post-CWMT. Our findings demonstrate that white matter microstructure and associated cognitive outcomes are malleable by CWMT in survivors of neonatal critical illness.
1. Introduction
The number of critically ill neonates surviving to discharge after neonatal intensive care admission is increasing worldwide (Harrison and Goodman, 2015; Zeitlin et al., 2013). A clearly delimited group of survivors of neonatal critical illness are children treated with neonatal extracorporeal membrane oxygenation (ECMO) due to severe respiratory failure and children with congenital diaphragmatic hernia (CDH) treated without ECMO. Since the first neonatal ECMO treatment applied in 1975, over 40,000 neonates have been treated with ECMO worldwide (Extracorporeal, Life, Support, Organization, 2018). The most frequent underlying diagnoses for neonatal ECMO are meconium aspiration syndrome (MAS) and congenital diaphragmatic hernia (CDH). The survival rate following MAS is over 90%. CDH is a rare congenital anatomical malformation associated with significant mortality and morbidity due to pulmonary hypoplasia and pulmonary hypertension. In the most severe cases of CDH necessitating treatment with ECMO, mortality rates are 49% (Extracorporeal, Life, Support, Organization, 2018). Over the past decade, standardized treatment protocols for CDH patients have led to lower mortality (Snoek et al., 2016). ECMO is a rescue therapy for neonates with severe but reversible (cardio) respiratory failure that is used when maximal conservative treatment fails to maintain adequate oxygenation. In ECMO, the right common carotid artery and/or right internal jugular vein are cannulated for drainage and return of blood and usually permanently ligated. Oxygenation of blood takes place in an artificial lung as part of an extracorporeal circuit (Lequier, 2004; Rais-Bahrami and Short, 2000). Normally, autoregulation maintains cerebral blood flow over a wide range of cerebral perfusion pressures. In critically ill neonates, cerebral autoregulation can be disturbed due to prolonged hypoxia before ECMO or due to ECMO itself, potentially having long-lasting effects on neurodevelopment (Greisen, 2005; Kontos et al., 1978; Panerai et al., 1995; Boylan et al., 2000). The assessment of long-term outcome, and in particular neuropsychological outcomes, in these children is therefore increasingly important.
The brain is rapidly developing during the first months of life and therefore particularly vulnerable in both children treated with ECMO as well as CDH children not treated with ECMO, subjecting them to ‘growing into deficit’. The concept of ‘growing into deficit’ is a highly important concept within the field of developmental cognitive neuroscience. Children treated with neonatal ECMO and/or CDH are a perfect of example of this phenomenon. Survivors of ECMO and/or CDH are at increased risk of specific attention and memory deficits that start to emerge in the school-age years and have been found to persist into adolescence (Leeuwen et al., 2017; Madderom et al., 2016). These deficits have been found to be specifically associated with global white matter microstructure alterations and with specific alterations in limbic regions of the brain, namely the hippocampus, cingulum and parahippocampal part of the cingulum (Schiller et al., 2017a; Cooper et al., 2015; Schiller et al., 2017b). These brain abnormalities are thought to be due to conditions these children are at high risk of during the period of neonatal critical illness, such as hypoxia-ischemia, inflammation, exposure to anesthetics and stress (Schiller et al., 2018a).
Working-memory, one of the fundamental building blocks for higher cognitive functioning, is highly associated with academic performance (Gathercole et al., 2008) and at risk of impairment following neonatal ECMO (Madderom et al., 2016; Cooper et al., 2015). The few training programs to improve cognitive functioning that exist have received increasingly more attention over the years, and are based on the idea that repetitive mental exercise of one cognitive task results in improved functioning that may generalize to other tasks with similar underlying skills. The effectiveness of these working-memory training programs remains a topic of debate (for a review please refer to (Melby-Lervag and Hulme (2013a))). The most well-known program and widely evaluated for children with working-memory problems is Cogmed Working Memory Training(CWMT) (Klingberg et al., 2002). CWMT consists of a specific set of working-memory tasks that are performed on a computer at home. The difficulty level of the working-memory tasks is continuously adjusted to child’s ability (Klingberg et al., 2002). Studies have demonstrated near-transfer effects immediately after CWMT, i.e. improvements on trained and untrained working-memory tasks (Melby-Lervag et al., 2016; Melby-Lervag and Hulme, 2013b). Using a single-blind randomized controlled trial, we recently showed near-transfer effects on untrained working-memory tasks in school-age survivors of neonatal ECMO and/or CDH immediately post-CWMT compared to untrained controls (Schiller et al., 2018b). However, this improvement was not maintained at the one year post-intervention assessment. Interestingly, far transfer effects were found on visuospatial memory delayed recall, an untrained test, which persisted one year post-intervention compared to controls (Schiller et al., 2018b). Given the fact that more than 50% of these survivors have visuospatial memory deficits later in life (Leeuwen et al., 2017) and considering the importance of memory for both academic performance and participation in society, this is a promising finding.
Diffusion Tensor Imaging (DTI) is an imaging technique that can quantify microstructural characteristics of white matter. Using this technique, several studies in adults have shown plasticity in white matter microstructure immediately following working-memory training, indicating DTI to be a reliable technique to detect training-induced change in white matter microstructure (Caeyenberghs et al., 2016; Takeuchi et al., 2010). Of specific interest in the context of working-memory and working-memory training is the superior longitudinal fasciculus (SLF), as it is the main connection between the parietofrontal network, known for its importance in working-memory performance, and has been found to change following working-memory training in various studies in adults (Takeuchi et al., 2010; Metzler-Baddeley et al., 2017; Olesen et al., 2004). However, one study in adults did not find any training-induced changes in white matter microstructure after CWMT, but no cognitive improvements were found either (Nyberg et al., 2018). In addition to the SLF, changes in white matter microstructure may be more widespread following CWMT in children as global alterations in white matter microstructure, especially in the limbic system, have been found in survivors of neonatal ECMO and/or CDH compared to healthy controls (Schiller et al., 2017b).Furthermore, compared to adults, there is less specificity of brain function in children, which may lead to increased neuroplasticity (Johnson, 2011; Jolles and Crone, 2012). However, this remains highly speculative as neuroplasticity likely depends on a combination of factors, including age (Kolb et al., 2013).
The primary aim of this study was to investigate the neurobiological plasticity immediately following CWMT using diffusion tensor imaging(DTI) in school-age survivors of neonatal ECMO and/or CDH that were part of a nationwide single-blind randomized controlled trial. Our secondary aim was to evaluate whether, if there were changes in white matter microstructure, these were associated with the cognitive improvements observed following CWMT in verbal and visuospatial working-memory and in visuospatial memory delayed recall (Schiller et al., 2018b). We hypothesized that white matter microstructure, more specifically an increase in FA/lower MD in the SLF, would change immediately following CWMT. Furthermore, we hypothesized that increased FA/lower MD in the SLF would be associated with immediate improvements in working-memory (Metzler-Baddeley et al., 2017) and that increased FA/lower MD in the parahippocampal part of the cingulum would be associated with the long-term improvements in visuospatial memory delayed recall (Schiller et al., 2017a).
2. Method
2.1. Design and population
This nationwide randomized controlled trial, conducted between October 2014 and June 2017, compared CWMT to no training in school-age neonatal ECMO and/or CDH survivors(NTR4571). Inclusion criteria for the trial were: school-age children (8–12 years) treated with ECMO or treated for CDH without ECMO in the first weeks of life at either of the two neonatal ECMO centers in the Netherlands (Erasmus MC-Sophia Children’s Hospital in Rotterdam or the Radboud University Medical Center in Nijmegen), IQ ≥ 80 and memory impairment (z-score ≤ −1.5 on one or more memory tests (Lezak et al., 2004)). All children were born at term. Children had not previously undergone working-memory training. Exclusion criteria were: usage of psychopharmaceutic drugs (e.g. methylphenidate) and/or genetic syndromes that are known to affect neuropsychological functioning. Eligible children were randomized into either the CWMT group or the non-training group by an independent researcher not involved with the assessment of the children (Fig. S1). Randomization was performed by drawing from sealed, opaque envelopes containing a paper with either ‘intervention’ or ‘no intervention’. The MRI exam and neuropsychological assessments were performed by researchers blinded to group allocation.
Ethical approval was granted by the Erasmus MC Medical Ethical Review Board(MEC-2014-001). All families received an application package with written informed consent for the parents and children 12 years of age that was discussed with the family and filled out before participating in the trial. The complete trial methods as well as the sample size calculation based on the primary outcome measure are described elsewhere (Schiller et al., 2018b).
2.2. Intervention
Children in the CWMT group completed the CWMTRM version for children from the ages 7 to 17 years. Children trained at home for 45 min a day, five days a week, for five consecutive weeks, as recommended in the manufacturer’s instructions (Klingberg et al., 2002). The level of the tasks adapted automatically to ensure that the child was continuously performing at the maximum of his or her ability. As part of the program, each child was supervised by a certified CWMT coach, who provided support to the family and feedback on the training results once a week over the phone and by e-mail. The CWMT coach was able to closely monitor the child’s performance via online access. The minimum number of training sessions recommended by the CWMT program is 20 sessions, which we implemented.
Children in the non-training group did not receive any training.
2.3. Outcome measures
After the standardized, neuropsychological assessment at baseline to determine eligibility, eligible participants underwent an MRI exam (baseline or T0). After six weeks, the MRI exam was repeated in all participants (post-treatment or T1). The neuropsychological assessment was repeated, and again after one year following the baseline measurement (T2, neuropsychological assessment only). The neuropsychological outcomes included tests of attention, verbal and visuospatial working-memory, verbal and visuospatial short- and long-term memory, executive functioning and visuospatial processing. For this study, we only used those cognitive outcomes that improved following CWMT, i.e. verbal working-memory (Digit Span of the WISC-III-NL (Kort and Compaan, 1999)), visuospatial working-memory (Spatial Span of the Wechsler Nonverbal Scale of Ability (Wechsler and Naglieri, 2006)) and visuospatial memory delayed recall (the Rey Complex Figure Test (RCFT) (Watanabe et al., 2005)). Please refer File A.1 for a full description of the tests used.
Prior to the MRI procedure, all children underwent a mock scanning session to become familiarized with the MRI-scanner environment (White et al., 2013). All MRI data were acquired on the same 3 T GE MR-750 system using an 8-channel head coil (General Electric, Milwaukee, WI). A full description of the sequences and scanning protocol is provided in File A.2. After DTI image preprocessing, voxel-wise scalar maps of fractional anisotropy(FA) and mean diffusivity(MD) were computed. FA provides a rotationally invariant measure of diffusion, with 0 being completely isotropic (equal in all directions) and 1 being completely anisotropic (diffusion along only one axis). A higher FA generally represents a greater coherence of white matter fibers. MD is the rate of diffusion of water(hydrogen) averaged in all directions. Lower MD is suggestive of increased integrity in axonal membranes, packing, or myelin. The FSL plugin ‘AutoPtx’ for fully automated probabilistic fiber tractography was used to create subject-specific, probabilistic representations of multiple white matter bundles (de Groot et al., 2015). Automated (Muetzel et al., 2015) and visual inspection of the neuroimaging data resulted in 61 DTI datasets (87%) with usable image quality. All scans were reviewed by a certified neuroradiologist (M.S.), blinded for medical history and outcome.
2.4. Statistical analysis
Clinical and demographic characteristics and neuropsychological outcome at baseline were compared between groups using independent samples t-tests and ANOVA(normally distributed variables), Mann-Whitney U or Fisher’s exact tests(non-normally distributed continuous or categorical variables, respectively).
First, to evaluate our primary aim, we assessed whether white matter microstructure changed immediately after CWMT. We were particularly interested in changes in the SLF as it is involved in working-memory and has previously been shown to be affected by CWMT (Metzler-Baddeley et al., 2017). Linear mixed models were estimated to assess whether white matter microstructure changed in the CWMT group compared to the non-training group at immediately post-CWMT. This method accounts for within-subject correlations and allows for missing values in the dependent variable. Outcome at baseline (T0) and post-treatment (T1) were both included in the model as time-points, and mean outcome at baseline was constrained to be equal (Liu et al., 2009). This was done to account for the fact that participants were randomized to either the CWMT or non-training group and that there were no differences in treatment between the groups at baseline (Liu et al., 2009). The brain diffusion measures at both time-points were the dependent variables, and group, time-point, age and gender as well as the group by time-point interaction term were included as independent variables. Results of the linear mixed models were summarized using the estimated marginal means (the predicted values of the dependent variable adjusted for the effects of the independent variables) of the group by time-point interaction.
We also analyzed changes in global FA and global MD immediately post-CWMT using linear mixed models. Described by our group in more detail elsewhere (Schiller et al., 2017b), global white matter microstructure was calculated using a weighted(by tract volume) average score of FA/MD of the association and limbic system white matter tracts(uncinate, inferior fronto-occipital fasciculus, SLF, inferior longitudinal fasciculus, cingulum bundle and parahippocampal part of cingulum) (Equation 1), known to be involved with cognitive functioning in children (Muetzel et al., 2015; Schmithorst et al., 2005):
| (1) |
where i denotes the tract, Vol denotes the volume of the tract, and n is the number of tracts. The same formula was used for global MD, replacing FA for each tract with the MD measure.
If global FA or global MD changed significantly following CWMT, additional analyses were performed with the individual white matter tracts. The same setup of linear mixed models were used to now assess whether white matter microstructure in the individual tracts (independent variables) changed in the CWMT group compared to the non-training group at T1. Brain structures were analyzed in the left and right hemispheres separately as laterality differences have been shown in the organization of working-memory and specific cognitive functions (Ocklenburg et al., 2014). In all linear mixed models, we adjusted for age at T1 and gender (Lebel et al., 2008). For the additional analyses on group differences in the individual white matter tracts, the False Discovery Rate (FDR) (Benjamini and Hochberg, 1995) correction was applied to account for multiple testing. These results were considered statistically significant at the FDR-corrected p < .05.
Our previous findings showed that children trained with CWMT improved significantly on verbal working-memory and visuospatial working-memory immediately post-CWMT and visuospatial memory delayed recall one year after CWMT compared to non-trained children (Schiller et al., 2018b). For our second aim, we therefore assessed whether (if any) immediate training-induced changes in white matter microstructures were associated with the cognitive improvements found either immediately or one year post-CWMT using univariate linear regression models. The dependent variable was the brain parameter immediately post-CWMT in the CWMT group and the independent variable was the cognitive outcome measure either immediately or one year post-CWMT, dichotomized to: improved (>1 SD improvement from T0 to T1 or T2) versus not improved (<1 SD improvement from T0 to T1 or T2). In these structure-function analyses, we adjusted for FA/MD at baseline, age at T1 or T2 (as appropriate) and gender. Neuropsychological test scores were converted to z-scores (individual score minus the population mean divided by the population SD). Assumptions of normality were checked using normal probability plots. Scores were inverted where appropriate so that a higher score always equated with better performance.
Statistical analyses were performed using SPSS 21.0 (IBM Corporation, Armonk, NY) and the Stats, lme4 and lmerTest packages in R Statistical Software version 3.1.3 (R Core Team, 2014). The estimated marginal means are reported by group and time-point for the linear mixed model analyses. Effect sizes were calculated in the linear regression models using partial eta squared() and interpreted according to Cohen’s guidelines(0.01=small, 0.06=medium, 0.14=large) (Cohen, 1988).
3. Results
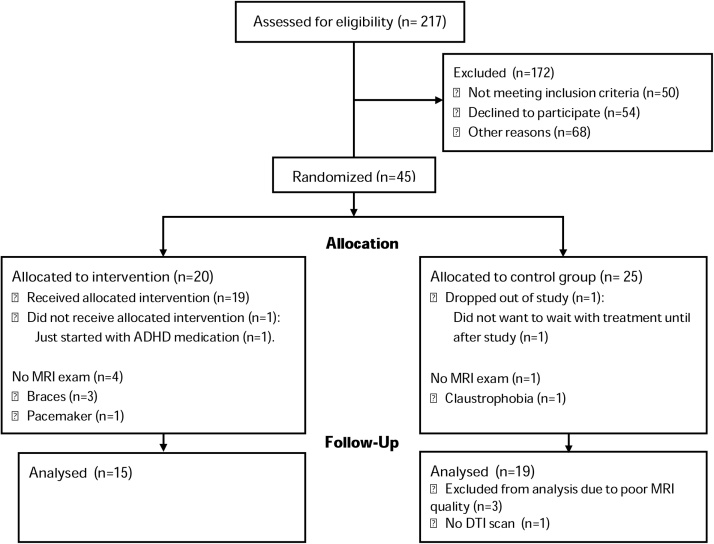
Of 34 eligible children with useable DTI data, 15 were in the CWMT group (13 with an MRI at both time-points) and 19 in the non-training group (18 with an MRI at both time-points) (Fig. 1). Children included in this study were generally representative of the wider trial cohort, except that children included in this study had higher IQ’s than the children who were excluded (excluded mean IQ ± SD: 92 ± 11; included: 101 ± 12, p = .038).All children in the CWMT group completed the 25 sessions, except one who completed 20 sessions. Sensitivity analyses on cognitive outcome were performed without this child’s data and showed no difference, therefore the child was not excluded from the analyses (Schiller et al., 2018b). Since nearly all children received the same training protocol, dose-dependent relationships with CWMT could not be evaluated. Demographic and clinical characteristics did not differ between the CWMT group and the non-training group (Table 1). There were no differences in global white matter microstructure or white matter microstructure on any of the tracts between the two groups at baseline (Table 2).
Fig. 1.
CONSORT flow diagram.
Abbreviations: ADHD, Attention Deficit Hyperactivity Disorder.
Table 1.
Study population characteristics.
| Characteristics | All (n = 34) |
Control (n = 19) |
CWMT (n = 15) |
P-value |
|---|---|---|---|---|
| a) Demographic | ||||
| Age (years) | 10 ± 2 | 10 ± 2 | 10 ± 1 | .821 |
| Gender | .728 | |||
| Male | 21 (62%) | 10 (67%) | 11 (62%) | |
| Handedness | ||||
| Right | 28 (82%) | 16 (84%) | 12 (80%) | .749 |
| Ethnicity | .355 | |||
| Dutch | 29 (85%) | 15 (79%) | 14 (93%) | |
| Maternal education level† | .242 | |||
| Low | 5 (15%) | 2 (11%) | 3 (20%) | |
| Moderate | 11 (32%) | 5 (26%) | 6 (40%) | |
| High | 18 (53%) | 12 (63%) | 6 (40%) | |
| Type of education child | .228 | |||
| Regular | 23 (68%) | 13 (68%) | 10 (67%) | |
| Regular with help | 9 (26%) | 6 (32%) | 3 (20%) | |
| Special education | 2 (6%) | 0 (0%) | 2 (13%) | |
| IQ | 101 ± 12 | 100 ± 12 | 102 ± 12 | .576 |
| b) Clinical | ||||
| Birthweight (kilograms) | 3.5 (2.8–3.2) | 3.5 (3.3–3.8) | 3.4 (3.0–4.0) | .650 |
| Gestational age (weeks) | 40 (39–41) | 40 (40–41) | 40 (40–42) | .483 |
| ICU stay (days) | 16 (10–20) | 16 (10–21) | 16 (9–18) | .918 |
| Mechanical vent. (days) | 10 (8–16) | 11 (8–16) | 10 (9–16) | .762 |
| Chronic lung disease‡ | 3 (9%) | 2 (11%) | 1 (8%) | .787 |
| Abnormal CUS§ | 3 (9%) | 2 (12%) | 1 (9%) | .823 |
| CDH-non-ECMO | 11 (32%) | 5 (26%) | 6 (40%) | .646 |
| ECMO treatment¶ | 23 (68%) | 14 (74%) | 9 (60%) | .475 |
| Type of ECMO | .176 | |||
| VA | 15 (65%) | 8 (57%) | 7 (78%) | |
| VV | 7 (30%) | 6 (43%) | 1 (11%) | |
| VV conversion to VA | 1 (4%) | 0 (0%) | 1 (22%) | |
| Age start ECMO (days) | 1 (1–2) | 2 (1–4) | 1 (1–2) | .557 |
| Hours on ECMO | 109 (85–180) | 114 (84–185) | 104 (84–161) | .831 |
N (%), mean ± SD or median (interquartile range) is reported where appropriate. Dutch refers to children with two native Dutch parents. †Based on the highest level of education completed by the mother (Centraal, Bureau, voor, de, Statistiek, (Statistics, Netherlands), 2006). ‡Chronic lung disease defined as oxygen dependency at 28 days of life (Jobe and Bancalari, 2001). §Abnormal CUS: hemorrhagic infarct posterior cerebral artery (n = 1), focal densities thalami (n = 2). ¶Diagnoses underlying ECMO treatment in the neonatal period were congenital diaphragmatic hernia (n = 2), meconium aspiration syndrome (n = 17), persistent pulmonary hypertension of the newborn (n = 2), infant respiratory distress syndrome (n = 1), and cardiac anomaly (n = 1).Abbreviations: CWMT, Cogmed Working Memory Training; IQ, Intelligence Quotient; CUS, cranial ultrasound; CDH, congenital diaphragmatic hernia; ECMO, extracorporeal membrane oxygenation; VA, venoarterial; VV, venovenous.
Table 2.
FA and MD group differences in white matter tracts immediately after CWMT.
| Tract | Hemisphere | Mean FA T0 Non-training | Mean FA T1 Non-training | Mean FA T0 CWMT | Mean FA T1 CWMT | p | pFDR | Mean MD T0 Non-training | Mean MD T1 Non-training | Mean MD T0 CWMT | Mean MD T1 CWMT | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLF | Left | .392 (.01) | .390 (.01) | .389 (.01) | .396 (.01) | .025 | .779 (.01) | .781 (.01) | .779 (.01) | .770 (.01) | .015 | |
| Right | .388 (.01) | .387 (.01) | .388 (.01) | .392 (.01) | .287 | .779 (.01) | .783 (.01) | .766 (.01) | .762 (.01) | .208 | ||
| Global | – | .404 (.01) | .401 (.01) | .401 (.01) | .406 (.01) | .014 | .808 (.01) | .811 (.01) | .804 (.01) | .800 (.01) | .048 | |
| UNC | Left | .371 (.01) | .368 (.01) | .372 (.01) | .381 (.01) | .071 | .178 | |||||
| Right | .379 (.01) | .378 (.01) | .382 (.01) | .392 (.01) | .025 | .130 | ||||||
| IFO | Left | .445 (.01) | .442 (.01) | .449 (.01) | .451 (.01) | .265 | .320 | |||||
| Right | .446 (.01) | .438 (.01) | .442 (.01) | .447 (.01) | .048 | .160 | ||||||
| ILF | Left | .425 (.01) | .423 (.01) | .418 (.01) | .424 (.01) | .177 | .307 | |||||
| Right | .435 (.01) | .427 (.01) | .430 (.01) | .429 (.01) | .184 | .307 | ||||||
| CB | Left | .396 (.01) | .382 (.01) | .390 (.01) | .396 (.01) | .026 | .130 | |||||
| Right | .358 (.01) | .351 (.01) | .343 (.01) | .347 (.01) | .288 | .320 | ||||||
| PHC | Left | .277 (.01) | .276 (.01) | .282 (.01) | .286 (.01) | .243 | .320 | |||||
| Right | .303 (.01) | .295 (.01) | .302 (.01) | .293 (.01) | .984 | .984 |
Group*time-point interaction terms of the linear mixed model analyses showing differences in white matter microstructure between children in the CWMT group (n = 14) and non-training group (n = 20) directly after the intervention, adjusted for age and gender. T0 is the baseline assessment; T1 is the post-treatment assessment six weeks after T0. Mean weighted average FA (SD) is given for each tract per group. Results are significant at p-value < .05. The FDR-correction (Benjamini and Hochberg, 1995) was applied once for the set of additional analyses on the individual white matter tracts (i.e. once for 10 tests). PFDR < .05 was considered statistically significant. Abbreviations: FA, fractional anisotropy; MD, mean diffusivity; UNC, uncinate fasciculus; ILF, inferior longitudinal fasciculus; IFO, inferior fronto-occipital fasciculus; SLF, superior longitudinal fasciculus; CB, cingulum bundle; PHC, parahippocampal cingulum bundle.
3.1. White matter microstructure following CWMT
Using a linear mixed model analysis, we found significant group by time interactions with FA in the left SLF significantly higher in the CWMT group compared to the non-training group post-treatment (estimated coefficient = .010, p = .025), whereas MD in the left SLF was lower in the CWMT group compared to the non-training group post-treatment (estimated coefficient = -0.011, p = .015) (Tables 2, C2).
There was a significant group by time interaction with higher global FA in the CWMT group compared to the non-training group post-treatment (estimated coefficient = .008, p = .014). Additional linear mixed model analyses in the individual tracts showed a significant group by time interaction with higher FA in the right uncinate fasciculus in the CWMT group compared to the non-training group at T1 (estimated coefficient = 0.013, p = .025). However, this finding did not survive the correction for multiple testing (pFDR = .130). Furthermore, a significant group by time interaction was found with higher FA in the left cingulum bundle in the CWMT group compared to the non-training group at T1 (estimated coefficient = .020, p = .026, but this finding did not remain significant after correcting for multiple testing either (pFDR = .130)(Table 2).
Global MD did not significantly change following CWMT (estimated coefficient = -0.008, p = .048) (Tables 2, C2).
Sensitivity analyses were performed adjusted for IQ. As the results did not change, analyses adjusted only for age and gender are shown in the manuscript.
3.2. White matter microstructure and neuropsychological improvement following CWMT
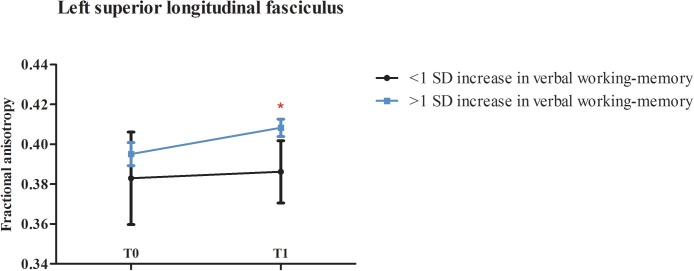
The assumption of normality was met for the neuropsychological data. Children in the CWMT who improved with more than 1 SD on verbal working-memory from baseline to post-treatment had significantly higher FA in the left SLF post-treatment (n = 6; FA left SLF (mean ± SD) at T1 = .408 ± .01) compared to children that did not show this improvement after CWMT (n = 6; FA left SLF at T1 = .384 ± .02), F(1,12) = 6.22, p = .041, = .47 (Fig. 2). This association was not found in the control group where two children improved with at least 1 SD from baseline to the post-treatment assessment (p = .175), indicating that the association between the increase in FA in the left SLF and improvement in verbal working-memory is related to the CWMT. Sensitivity analysis with improvement in verbal working-memory in the CWMT group as a continuous variable did not show a significant linear relationship with training-induced changes in the left SLF (p = .634).
Fig. 2.
Associations between cognitive improvement and changes in white matter microstructure following CWMT.
Children who improved with more than 1 SD on verbal working-memory (‘improved verbal working-memory’, n = 6) after CWMT had significantly higher FA in the left SLF at T1 than children without this improvement (‘non-improved verbal working-memory’, n = 6). *Indicates a significant association at p < .05. Error bars showing 95% confidence intervals are shown. Abbreviations: CWMT, Cogmed Working Memory Training.
Sensitivity analyses were performed adjusted for total brain volume. As the results did not change, analyses adjusted only for age and gender are shown in the manuscript. Comparing the CWMT patients who significantly improved on verbal working-memory to those who did not, we did not find any differences in handedness (p = .153) or the type of education (p = .845) between the groups.
Improvements in visuospatial working-memory immediately after CWMT or visuospatial memory delayed recall immediately or one year after CWMT were not associated with the training-induced changes in global white matter microstructure or in the individual white matter tracts.
4. Discussion
In this single-blind randomized controlled trial, we found significant white matter microstructural changes in school-age survivors of neonatal ECMO and/or CDH who were trained with CWMT compared to non-trained survivors. We found both global and specific changes in white matter microstructure immediately post-intervention in the CWMT group compared to the non-training group. Specific changes in FA in the SLF were associated with better verbal working-memory after CWMT. These findings demonstrate that neurobiological plasticity exists in survivors of neonatal critical illness despite significant alterations found in white matter microstructure in these children compared to healthy controls (Schiller et al., 2017a; Schiller et al., 2017b).
Our findings of specific changes in the SLF, a white matter tract connecting the frontal and parietal cortices (Metzler-Baddeley et al., 2017), following CWMT confirm previous findings of changes in connectivity in frontopatietal regions following working-memory training in healthy school-age children (8–11 years) as well as in childhood cancer survivors(12 years) (Astle et al., 2015; Conklin et al., 2015). Although most studies have focused on brain activity using fMRI and/or were performed in adults, the frontoparietal network has been consistently shown to be affected by working-memory training (Olesen et al., 2004; Klingberg, 2010; Jolles et al., 2013). Interestingly, we did not find a linear relationship between improvement in verbal working-memory and FA in the left SLF in the CWMT group. This may indicate there is a non-linear relationship between the white matter changes and cognitive improvements after CWMT. Future studies are needed to replicate these findings in larger sample sizes. Furthermore, we found that the training-induced changes in the left SLF were significantly associated with improvements in verbal working-memory. To further increase our understanding of these findings, future studies should apply tractography approaches to parse the individual components of the SLF (SLF I, II, III), as the first branch of the SLF is involved in the limbic system network (Catani et al., 2013) and may thus be particularly vulnerable in survivors of neonatal ECMO and/or CDH.
Predominantly left hemispheric alterations have been previously found following neonatal ECMO (Schiller et al., 2017b), which have been suggested to be due to right internal jugular vein cannulation in neonatal ECMO patients (Raets et al., 2013). Thus, greater alterations could equate with the opportunity for greater functional improvement in the left hemisphere, could explain the training-induced changes in the left hemisphere only. However, we have previously shown that the left-hemispheric alterations existed irrespective of ECMO treatment or type of ECMO cannulation (Schiller et al., 2017a), making this clarification unlikely. The majority of children in our cohort were right handed(80%) and right-handedness is generally associated with left hemispheric dominance for language (Szaflarski et al., 2012). This may contribute to the association between verbal working-memory improvements and the left-sided changes in white matter microstructure. This also confirms previous findings showing that working-memory functioning is lateralized, i.e. verbal working-memory corresponds with the left hemisphere and visuospatial working-memory with the right hemisphere (Ocklenburg et al., 2014; Nagel et al., 2013). However, children in the CWMT also improved significantly on visuospatial working-memory (Schiller et al., 2018b), yet we did not find any changes in the right SLF. In previous neuroimaging studies following CWMT in children as well as adults, both right-lateralized and left-lateralized changes in the frontal and parietal cortices have been demonstrated (Metzler-Baddeley et al., 2017; Conklin et al., 2015). These contrasting findings may be due to the type of image acquisition and analysis methodologies employed, such as region-of-interest versus whole-brain analyses, making it difficult to draw definitive conclusions.
Another potential explanation may be that we found training-induced increases in FA in the right uncinate fasciculus, a white matter tract that has been associated with visuospatial working-memory in children (Krogsrud et al., 2018), which disappeared after correction for multiple testing. A lack of power due to a relatively small sample size may have resulted in the lack of finding an association between improvements in visuospatial working-memory and increased FA in the right uncinate fasciculus. The increase in visuospatial working-memory is smaller than the increase in verbal working-memory and may therefore be accompanied by smaller changes in white matter microstructure that are not as easy to detect in a small group. Future studies with larger study populations are needed to further our understanding on the structure-function relationships following working-memory training.
In addition to changes in the left SLF, we found that global FA increased in the CWMT compared to the control group from baseline to post-treatment. In the majority of the white matter tracts assessed, we found that FA increased following CWMT. This global change in FA may be due to an increased capacity for plasticity in the child’s brain due to less specificity in brain function (Johnson, 2011; Jolles and Crone, 2012; Kolb et al., 2013). However, this remains highly speculative as neuroplasticity following cognitive training in children is likely to be multifactorial. The training-induced changes in global FA were not associated with any cognitive improvements following CWMT. In the same cohort, we previously showed an association between lower global FA and sustained attention deficits, a domain consistently found to be impaired following neonatal ECMO and/or CDH (Schiller et al., 2017a). However, we did not find any direct relationships between the changes in global FA and sustained attention following CMWT. Future research is needed to better understand the clinical relevance of changes in global white matter microstructure following CWMT in children.
In any case, our findings of increased FA in response to five weeks of intensive cognitive training may indicate enhanced orchestration in communication between neural circuits, which, in turn, may lead to enhanced cognitive functioning (Takeuchi et al., 2010). A potential molecular mechanism underlying these changes may be increased myelination in response to neuronal activity by the working-memory training (Takeuchi et al., 2010; Scholz et al., 2009). This increase in myelin is suggested to improve cognitive functioning because it alters conduction velocity and improves the synchronization of nervous signals (Fields, 2008). Both human and mouse models have shown that increased myelination following external manipulation can become visible within days to weeks (Scholz et al., 2009; Ishibashi et al., 2006). A similar mechanism may therefore be underlying the training-induced changes observed in our study. Specificity of training-induced changes in white matter microstructure (i.e. increase in myelin) (Dubois et al., 2014) might also explain why global MD was not as strongly affected following CWMT, although global MD did decrease following CMWT compared to the non-training group, but this finding did not reach significance. As FA and MD are summary parameters that do not give detailed histological information, this remains speculative. Future studies using more sensitive neuroimaging techniques in larger sample sizes are needed to see whether these global changes following CMWT in survivors of neonatal critical illness can be replicated to gain insight into these findings.
We did not find associations between changes in white matter microstructure and the improvements found in visuospatial memory delayed recall one year after CWMT (Schiller et al., 2018b). These cognitive improvements may be due to changes in brain activity or connectivity that were not detectable using DTI (Metzler-Baddeley et al., 2017). However, in the same cohort, we previously showed that impaired delayed visuospatial memory was specifically associated with increased MD in the parahippocampal part of the cingulum (Schiller et al., 2017a). A previous study using DTI in adults following CWMT did find training-induced changes in the parahippocampal cingulum (Metzler-Baddeley et al., 2017). In our cohort, the improvements in delayed visuospatial memory were most apparent one year post-intervention (Schiller et al., 2018b). Potential changes in the parahippocampal and temporal brain regions therefore may not have been detectable immediately following CWMT. In line with this, a recent study in a population-based cohort of school-age children (6–10 years) has shown downstream effects of behavior on the brain instead of the commonly assumed direction of brain shaping behavior (Muetzel et al., 2017). Such a downstream mechanism may explain why we see cognitive improvements immediately following CWMT without corresponding changes in the brain. Future studies conducting multimodal neuroimaging both immediately and longitudinally over the course of several years post-intervention are needed to better understand the underlying mechanism of these changes in visuospatial memory delayed recall one year post-CWMT.
In addition, previous findings by our group have demonstrated that 17-year-old survivors of neonatal ECMO experience similar visuospatial memory deficits as survivors at the age of 8–12 years of age. (Madderom et al., 2016; Leeuwen et al., 2018; Schiller et al., 2016) As such deficits may have a significant impact on societal participation, it is imperative that we explore ways to improve neuropsychological outcome in adolescent survivors as well. In other patient groups, e.g. in preterm born adolescents, positive effects of CWMT have been found in both children and adolescents (Lohaugen et al., 2011; Grunewaldt et al., 2013). As we cannot make any statements about the effectivity of CWMT in adolescent survivors of neonatal ECMO and/or CDH due to differences in underlying etiology, it is important to assess the effectiveness of a cognitive training program over the long-term as well as at later stages of development in this patient group.
This is the first study to assess and demonstrate neuroplasticity following CWMT in neonatal ECMO and/or CDH survivors. Despite this, our study has some limitations. First, our small sample size limits the interpretability as well as the generalizability of our findings, in particular of the analyses focusing on associations between brain changes and cognitive improvement in the CWMT group. However, since the association between increased FA in the SLF and improved verbal working-memory had a large effect size (0.47) (Cohen, 1988), coupled with prior literature supporting this link (Metzler-Baddeley et al., 2017; Olesen et al., 2004), we regard this to be a reliable finding. Nonetheless, future studies are needed with larger sample sizes before we can draw definitive conclusions. Second, we used a non-active control group for ethical considerations against subjecting children to an intensive training without potential benefits, which limits our ability to attribute our findings to the specific characteristics of the CWMT training. However, similar cognitive gains as well as neuroplasticity have been demonstrated following working-memory training even when compared to a non-adaptive training program (Metzler-Baddeley et al., 2017; Astle et al., 2015; Klingberg et al., 2005; Dunning et al., 2013; Spencer-Smith and Klingberg, 2015). Third, we were not able to conduct an MRI exam one year post-intervention, limiting our understanding of neuroplasticity following CWMT. Fourth, children from the full sample (described in Schiller et al. 2018) who did not participate in the MRI component had a significantly lower IQ than those children who did participate in the MRI study (excluded mean IQ ± SD: 92 ± 11; included: 101 ± 12). This may limit the generalizability of our findings. Future studies that combine neuroimaging and neuropsychological assessment at multiple time points following CWMT are needed. Lastly, the indices extracted using DTI are not histologically specific and may reflect different white matter properties, and therefore the underlying neurobiology of the changes are less clear.
In the future, studies using more sensitive neuroimaging techniques, such as MRI with higher magnetic field strength(e.g. 7 T), multi-shell imaging sequences that can better measure crossing and branching fiber tracts, as well as multimodal imaging techniques will further increase our understanding of neuroplasticity following cognitive training. High-field imaging (e.g., 7 T) offers improved signal-to-noise ratio, allowing for higher resolution data to be collected, not only in the context of DTI but also structural neuroimaging. In addition, functional MRI, including resting-state and MR-spectroscopy, expand beyond structural features of the brain, which is highly relevant in neurocognition.
5. Conclusions
This is the first study to assess and demonstrate neuroplasticity with DTI following CWMT in school-age survivors of neonatal ECMO and/or CDH using a single-blind randomized controlled trial. We found both global and specific changes in white matter microstructure immediately post-intervention in the CWMT group compared to the non-training group. Importantly, specific changes in FA in the SLF immediately post-CWMT were associated with better verbal working-memory at this time. These findings demonstrate that neurobiological plasticity exists in survivors of neonatal critical illness, despite significant alterations found in white matter microstructure in these children compared to healthy controls. In future studies, more sensitive and multimodal neuroimaging techniques should be used to assess the effect of CMWT over the long-term as well as at later stages of development in this patient group.
Funding
This study was supported by the Sophia Stichting Wetenschappelijk Onderzoek (SSWO): S14-21 and Revalidatiefonds (project number: R2014006). The neuroimaging infrastructure was supported in part by the Netherlands Organization for Health Research and Development (ZonMw) TOP project number 91211021.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100678.
Appendix C
Table C1.
Neuropsychological outcome.
| Control (raw scores) |
CWMT (raw scores) |
Control (z-scores) |
CWMT (z-scores) |
|||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 | |
| Working-memory | ||||||||
| Digit Span (WISC-III-NL) | 12.74 (2.86) | 13.50 (3.65) | 12.07 (2.87) | 15.47 (3.11) | 0.30 (0.91) | 0.43 (1.00) | 0.02 (1.09) | 1.27 (0.93) |
| Spatial Span (WNV) | 14.74 (4.37) | 14.61 (4.13) | 14.47 (3.31) | 18.00 (4.60) | 0.19 (1.13) | 0.09 (1.12) | 0.14 (0.99) | 1.08 (1.22) |
| Verbal memory | ||||||||
| RAVLT Immediate recall | 32.89 (7.28) | 39.00 (11.02) | 36.00 (8.06) | 40.33 (8.71) | −1.72 (1.08) | −0.68 (1.22) | −1.41 (1.05) | −0.91 (1.43) |
| RAVLT Delayed recall | 5.26 (2.83) | 6.33 (3.82) | 5.93 (3.26) | 8.00 (3.27) | −2.08 (1.10) | −1.53 (1.34) | −1.71 (1.24) | −0.82 (1.32) |
| Visuospatial memory | ||||||||
| RCFT immediate recall | 10.84 (7.53) | 12.50 (7.94) | 10.90 (4.76) | 15.03 (8.27) | −1.54 (1.11) | −1.26 (1.24) | −1.59 (0.85) | −0.91 (1.44) |
| RCFT delayed recall | 10.63 (7.29) | 12.86 (8.60) | 10.20 (5.89) | 14.93 (8.06) | −1.58 (1.07) | −1.19 (1.39) | −1.59 (1.19) | −0.87 (1.36) |
| RCFT recognition | 18.74 (3.36) | 19.17 (2.41) | 19.93 (2.12) | 20.80 (1.66) | −0.82 (1.64) | −0.66 (1.22) | −0.31 (1.26) | 0.22 (0.79) |
| Sustained attention | ||||||||
| DCT | 18.43 (6.98) | 16.47 (5.10) | 18.69 (4.41) | 16.21 (3.27) | −1.13 (2.33) | −0.35 (1.64) | −1.18 (1.55) | −0.24 (1.17) |
| Selective attention | ||||||||
| TMT section B | 54.37 (30.42) | 48.83 (27.48) | 51.00 (28.05) | 39.80 (18.92) | −0.18 (1.05) | 0.02 (1.41) | −0.08 (1.39) | 0.31 (1.22) |
| STROOP (interference score) | −0.56 (0.92) | 0.33 (1.03) | −0.27 (1.12) | 0.00 (1.07) | −0.56 (0.92) | 0.33 (1.03) | −0.27 (1.12) | 0.00 (1.07) |
| Processing speed | ||||||||
| TMT section A | 18.53 (9.84) | 20.00 (10.12) | 21.40 (8.18) | 22.93 (17.75) | 0.50 (0.86) | 0.23 (0.99) | −0.02 (1.09) | 0.35 (0.77) |
| Executive functioning | ||||||||
| BADS-C-NL Key Search | 8.11 (4.76) | 8.28 (5.20) | 8.73 (4.54) | 5.60 (4.44) | 0.05 (1.19) | 0.52 (1.20) | 0.31 (1.10) | −0.35 (1.02) |
| BADS-C-NL Modified Six Elements | 12.13 (3.31) | 11.71 (3.74) | 11.87 (3.83) | 13.47 (2.48) | −0.13 (0.97) | −0.20 (1.11) | −0.07 (1.06) | 0.29 (1.13) |
| Visuospatial processing | ||||||||
| RCFT Copy | 26.92 (7.68) | 28.17 (7.08) | 29.67 (3.87) | 28.44 (5.45) | 0.05 (0.76) | 0.18 (0.74) | 0.37(0.62) | 0.09 (0.84) |
Cognitive outcomes pre- and post-CWMT for both study groups are reported as z-scores (mean = 0, SD = 1). There were 19 children in the Control group and 15 in the CWMT group. T0 is the baseline assessment; T1 is the assessment immediately after CWMT. For the DCT and TMT tests, reaction time scores are given. Z-scores were inverted where appropriate so that a higher score always equated with better performance.
Abbreviations: CWMT, Cogmed Working Memory Training; WISC, Wechsler Intelligence Scale for Children; WNV, Wechsler Non-verbal Intelligence test; RAVLT, Rey Auditory Verbal Learning Test; RCFT, Rey Complex Figure Test; DCT, Dot Cancellation Test; TMT, Trail Making Test; BADS-C-NL, Behavioural Assessment of the Dysexecutive Syndrome.
Table C2.
White matter microstructure after CWMT.
| Estimated coefficient | p | |
|---|---|---|
| FA left SLF | ||
| Group | .010 | .025 |
| Age | .008 | .004 |
| Gender | .007 | .284 |
| MD left SLF | ||
| Group | −.011 | .015 |
| Age | −.009 | .002 |
| Gender | .005 | .448 |
| Global FA | ||
| Group | .008 | .014 |
| Age | .005 | .029 |
| Gender | .000 | .093 |
Results of the linear mixed models summarized using the estimated coefficient (the predicted values of the dependent variable adjusted for the effects of the independent variables). Group is the intervention group (CWMT, n = 13) compared to the non-training group (n = 18). P value < .05 is considered statistically significant.
Abbreviations: FA, fractional anisotropy; SLF, superior longitudinal fasciculus; MD, mean diffusivity.
Appendix A. Supplementary data
The following are Supplementary data to this article:
Trial outline. For short descriptions of the neuropsychological tests and questionnaires used, please refer to Schiller, et al. [22]. *IQ > 80 and a z-score ≤ -1.5(20) on one or more memory tests. Abbreviations: CWMT, Cogmed Working Memory Training.
References
- Astle D.E., Barnes J.J., Baker K., Colclough G.L., Woolrich M.W. Cognitive training enhances intrinsic brain connectivity in childhood. J. Neurosci. 2015;35:6277–6283. doi: 10.1523/JNEUROSCI.4517-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Met. 1995;57:289–300. [Google Scholar]
- Boylan G.B., Young K., Panerai R.B., Rennie J.M., Evans D.H. Dynamic cerebral autoregulation in sick newborn infants. Pediatr. Res. 2000;48:12–17. doi: 10.1203/00006450-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K., Metzler-Baddeley C., Foley S., Jones D.K. Dynamics of the human structural connectome underlying working memory training. J. Neurosci. 2016;36:4056–4066. doi: 10.1523/JNEUROSCI.1973-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Dell’acqua F., Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Centraal, Bureau, voor, de, Statistiek, (Statistics, Netherlands) 2006. Standaard onderwijsindeling 2006 (the dutch standard classification of education) 2018. [Google Scholar]
- Cohen J. 2nd ed. Lawrence Earlbaum Associates; Hilsdale, NJ: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Conklin H.M., Ogg R.J., Ashford J.M., Scoggins M.A., Zou P., Clark K.N., Martin-Elbahesh K., Hardy K.K., Merchant T.E., Jeha S., Huang L., Zhang H. Computerized cognitive training for amelioration of cognitive late effects among childhood cancer survivors: a randomized controlled trial. J. Clin. Oncol. 2015;33:3894–3902. doi: 10.1200/JCO.2015.61.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J.M., Gadian D.G., Jentschke S., Goldman A., Munoz M., Pitts G., Banks T., Chong W.K., Hoskote A., Deanfield J., Baldeweg T., de Haan M., Mishkin M., Vargha-Khadem F. Neonatal hypoxia, hippocampal atrophy, and memory impairment: evidence of a causal sequence. Cereb. Cortex. 2015;25:1469–1476. doi: 10.1093/cercor/bht332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot M., Ikram M.A., Akoudad S., Krestin G.P., Hofman A., van der Lugt A., Niessen W.J., Vernooij M.W. Tract-specific white matter degeneration in aging: the Rotterdam study. Alzheimers Dement. 2015;11:321–330. doi: 10.1016/j.jalz.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Huppi P.S., Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Dunning D.L., Holmes J., Gathercole S.E. Does working memory training lead to generalized improvements in children with low working memory? A randomized controlled trial. Dev. Sci. 2013;16:915–925. doi: 10.1111/desc.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extracorporeal, Life, Support, Organization . 2018. ECLS Registry Report International Summary January, 2018. Ann Arbor, MI. [Google Scholar]
- Fields R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole S.E., Durling E., Evans M., Jeffcock S., Stone S. Working memory abilities and children’s performance in laboratory analogues of classroom activities. Appl. Cogn. Psychol. 2008:22. [Google Scholar]
- Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 2005;81:423–428. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Grunewaldt K.H., Lohaugen G.C., Austeng D., Brubakk A.M., Skranes J. Working memory training improves cognitive function in vlbw preschoolers. Pediatrics. 2013;131:e747–754. doi: 10.1542/peds.2012-1965. [DOI] [PubMed] [Google Scholar]
- Harrison W., Goodman D. Epidemiologic trends in neonatal intensive care, 2007–2012. JAMA Pediatr. 2015;169:855–862. doi: 10.1001/jamapediatrics.2015.1305. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Dakin K.A., Stevens B., Lee P.R., Kozlov S.V., Stewart C.L., Fields R.D. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe A.H., Bancalari E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Interactive specialization: a domain-general framework for human functional brain development? Dev. Cogn. Neurosci. 2011;1:7–21. doi: 10.1016/j.dcn.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D.D., Crone E.A. Training the developing brain: a neurocognitive perspective. Front. Hum. Neurosci. 2012;6:76. doi: 10.3389/fnhum.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles D.D., van Buchem M.A., Crone E.A., Rombouts S.A. Functional brain connectivity at rest changes after working memory training. Hum. Brain Mapp. 2013;34:396–406. doi: 10.1002/hbm.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn. Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Training of working memory in children with adhd. J. Clin. Exp. Neuropsychol. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Fernell E., Olesen P.J., Johnson M., Gustafsson P., Dahlstrom K., Gillberg C.G., Forssberg H., Westerberg H. Computerized training of working memory in children with adhd--a randomized, controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kolb B., Mychasiuk R., Muhammad A., Gibb R. Brain plasticity in the developing brain. Prog. Brain Res. 2013;207:35–64. doi: 10.1016/B978-0-444-63327-9.00005-9. [DOI] [PubMed] [Google Scholar]
- Kontos H.A., Wei E.P., Navari R.M., Levasseur J.E., Rosenblum W.I., Patterson J.L., Jr. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am. J. Physiol. 1978;234:H371–383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Kort W., Compaan E.L. 1999. Wisc nl iii. Handleiding. NIP Dienstencentrum. [Google Scholar]
- Krogsrud S.K., Fjell A.M., Tamnes C.K., Grydeland H., Due-Tonnessen P., Bjornerud A., Sampaio-Baptista C., Andersson J., Johansen-Berg H., Walhovd K.B. Development of white matter microstructure in relation to verbal and visuospatial working memory-a longitudinal study. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leeuwen L., Schiller R.M., Rietman A.B., van Rosmalen J., Wildschut E.D., Houmes R.J.M., Tibboel D., IJ H. Risk factors of impaired neuropsychologic outcome in school-aged survivors of neonatal critical illness. Crit. Care Med. 2017 doi: 10.1097/CCM.0000000000002869. [DOI] [PubMed] [Google Scholar]
- Leeuwen L., Schiller R.M., Rietman A.B., van Rosmalen J., Wildschut E.D., Houmes R.J.M., Tibboel D., IJ H. Risk factors of impaired neuropsychologic outcome in school-aged survivors of neonatal critical illness. Crit. Care Med. 2018;46:401–410. doi: 10.1097/CCM.0000000000002869. [DOI] [PubMed] [Google Scholar]
- Lequier L. Extracorporeal life support in pediatric and neonatal critical care: a review. J. Intensive Care Med. 2004;19:243–258. doi: 10.1177/0885066604267650. [DOI] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Loring D.W. 4th ed. Oxford University Press; Oxford: 2004. Neuropsychological Assessment. [Google Scholar]
- Liu G.F., Lu K., Mogg R., Mallick M., Mehrotra D.V. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat. Med. 2009;28:2509–2530. doi: 10.1002/sim.3639. [DOI] [PubMed] [Google Scholar]
- Lohaugen G.C., Antonsen I., Haberg A., Gramstad A., Vik T., Brubakk A.M., Skranes J. Computerized working memory training improves function in adolescents born at extremely low birth weight. J. Pediatr. 2011;158 doi: 10.1016/j.jpeds.2010.09.060. 555–561 e554. [DOI] [PubMed] [Google Scholar]
- Madderom M.J., Schiller R.M., Gischler S.J., van Heijst A.F., Tibboel D., Aarsen F.K., IJ H. Growing up after critical illness: verbal, visual-spatial, and working memory problems in neonatal extracorporeal membrane oxygenation survivors. Crit. Care Med. 2016;44:1182–1190. doi: 10.1097/CCM.0000000000001626. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M., Hulme C. Is working memory training effective? A meta-analytic review. Dev. Psychol. 2013;49:270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M., Hulme C. Is working memory training effective? A meta-analytic review. Dev. Psychol. 2013;49:270–291. doi: 10.1037/a0028228. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M., Redick T.S., Hulme C. Working memory training does not improve performance on measures of intelligence or other measures of "far transfer": evidence from a meta-analytic review. Perspect. Psychol. Sci. 2016;11:512–534. doi: 10.1177/1745691616635612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C., Foley S., de Santis S., Charron C., Hampshire A., Caeyenberghs K., Jones D.K. Dynamics of white matter plasticity underlying working memory training: multimodal evidence from diffusion mri and relaxometry. J. Cogn. Neurosci. 2017;29:1509–1520. doi: 10.1162/jocn_a_01127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel R.L., Mous S.E., van der Ende J., Blanken L.M., van der Lugt A., Jaddoe V.W., Verhulst F.C., Tiemeier H., White T. White matter integrity and cognitive performance in school-age children: a population-based neuroimaging study. Neuroimage. 2015;119:119–128. doi: 10.1016/j.neuroimage.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Muetzel R.L., Blanken L.M.E., van der Ende J., El Marroun H., Shaw P., Sudre G., van der Lugt A., Jaddoe V.W.V., Verhulst F.C., Tiemeier H., White T. Tracking brain development and dimensional psychiatric symptoms in children: a longitudinal population-based neuroimaging study. Am. J. Psychiatry. 2017 doi: 10.1176/appi.ajp.2017.16070813. appiajp201716070813. [DOI] [PubMed] [Google Scholar]
- Nagel B.J., Herting M.M., Maxwell E.C., Bruno R., Fair D. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 2013;82:58–68. doi: 10.1016/j.bandc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg C.K., Nordvik J.E., Becker F., Rohani D.A., Sederevicius D., Fjell A.M., Walhovd K.B. A longitudinal study of computerized cognitive training in stroke patients – effects on cognitive function and white matter. Top. Stroke Rehabil. 2018;25:241–247. doi: 10.1080/10749357.2018.1443570. [DOI] [PubMed] [Google Scholar]
- Ocklenburg S., Hirnstein M., Beste C., Gunturkun O. Lateralization and cognitive systems. Front. Psychol. 2014;5:1143. doi: 10.3389/fpsyg.2014.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen P.J., Westerberg H., Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat. Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Panerai R.B., Kelsall A.W., Rennie J.M., Evans D.H. Cerebral autoregulation dynamics in premature newborns. Stroke. 1995;26:74–80. doi: 10.1161/01.str.26.1.74. [DOI] [PubMed] [Google Scholar]
- Raets M.M., Dudink J., Ijsselstijn H., van Heijst A.F., Lequin M.H., Houmes R.J., Wildschut E.D., Reiss I.K., Govaert P., Tibboel D. Brain injury associated with neonatal extracorporeal membrane oxygenation in The Netherlands: a nationwide evaluation spanning two decades. Pediatr. Crit. Care Med. 2013;14:884–892. doi: 10.1097/PCC.0b013e3182a555ac. [DOI] [PubMed] [Google Scholar]
- Rais-Bahrami K., Short B.L. The current status of neonatal extracorporeal membrane oxygenation. Semin. Perinatol. 2000;24:406–417. doi: 10.1053/sper.2000.20086. [DOI] [PubMed] [Google Scholar]
- Schiller R.M., Madderom M.J., JJCM Reuser, Steiner K., Gischler S.J., Tibboel D., van Heijst A.J.F., H. I Neuropsychological follow-up after neonatal ecmo. Pediatrics. 2016;138 doi: 10.1542/peds.2016-1313. [DOI] [PubMed] [Google Scholar]
- Schiller R.M., IJsselstijn H., Madderom M.J., Rietman A.B., Smits M., van Heijst A.F.J., Tibboel D., White T., Muetzel R.L. Neurobiologic correlates of attention and memory deficits following critical illness in early life. Crit. Care Med. 2017;45:1742–1750. doi: 10.1097/CCM.0000000000002553. [DOI] [PubMed] [Google Scholar]
- Schiller R.M., IJsselstijn H., Hoskote A., white T., Verhulst F., Van Heijst A., Tibboel D. Memory deficits following neonatal critical illness: a common neurodevelopmental pathway. Lancet Child Adolesc. Health. 2018;2:281–289. doi: 10.1016/S2352-4642(17)30180-3. [DOI] [PubMed] [Google Scholar]
- Schiller R.M., van den Bosch G.E., Muetzel R.L., Smits M., Dudink J., Tibboel D., Ijsselstijn H., White T. Neonatal critical illness and development: white matter and hippocampus alterations in school-age neonatal extracorporeal membrane oxygenation survivors. Dev. Med. Child Neurol. 2017;59:304–310. doi: 10.1111/dmcn.13309. [DOI] [PubMed] [Google Scholar]
- Schiller R.M., Madderom M.J., van Rosmalen J., van Heijst A.F.J., de Blaauw I., Utens E., Rietman A.B., Verhulst F., Tibboel D., White T., IJ H. Working memory training following neonatal critical illness: a randomized controlled trial. Crit. Care Med. 2018;46:1158–1166. doi: 10.1097/CCM.0000000000003151. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Wilke M., Dardzinski B.J., Holland S.K. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor mri study. Hum. Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J., Klein M.C., Behrens T.E., Johansen-Berg H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek K.G., Reiss I.K., Greenough A., Capolupo I., Urlesberger B., Wessel L., Storme L., Deprest J., Schaible T., van Heijst A., Tibboel D., Consortium CE Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH Euro Consortium Consensus – 2015 update. Neonatology. 2016;110:66–74. doi: 10.1159/000444210. [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M., Klingberg T. Benefits of a working memory training program for inattention in daily life: a systematic review and meta-analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Rajagopal A., Altaye M., Byars A.W., Jacola L., Schmithorst V.J., Schapiro M.B., Plante E., Holland S.K. Left-handedness and language lateralization in children. Brain Res. 2012;1433:85–97. doi: 10.1016/j.brainres.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H., Sekiguchi A., Taki Y., Yokoyama S., Yomogida Y., Komuro N., Yamanouchi T., Suzuki S., Kawashima R. Training of working memory impacts structural connectivity. J. Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Ogino T., Nakano K., Hattori J., Kado Y., Sanada S., Ohtsuka Y. The rey-osterrieth complex figure as a measure of executive function in childhood. Brain Dev. 2005;27:564–569. doi: 10.1016/j.braindev.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Wechsler D., Naglieri J.A. Pearson; San Antonio, TX: 2006. Wechsler Nonverbal Scale of Ability. [Google Scholar]
- White T., El Marroun H., Nijs I., Schmidt M., van der Lugt A., Wielopolki P.A., Jaddoe V.W., Hofman A., Krestin G.P., Tiemeier H., Verhulst F.C. Pediatric population-based neuroimaging and the generation r study: the intersection of developmental neuroscience and epidemiology. Eur. J. Epidemiol. 2013;28:99–111. doi: 10.1007/s10654-013-9768-0. [DOI] [PubMed] [Google Scholar]
- Zeitlin J., Mohangoo A.D., Delnord M., Cuttini M., Committee E-PS The second European perinatal health report: documenting changes over 6 years in the health of mothers and babies in Europe. J. Epidemiol. Commun. Health. 2013;67:983–985. doi: 10.1136/jech-2013-203291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial outline. For short descriptions of the neuropsychological tests and questionnaires used, please refer to Schiller, et al. [22]. *IQ > 80 and a z-score ≤ -1.5(20) on one or more memory tests. Abbreviations: CWMT, Cogmed Working Memory Training.