Abstract
Purpose
To compare the efficacy and toxicity of hypofractionated radiotherapy versus conventional fractionated radiotherapy in postmastectomy breast cancer using meta-analysis.
Methods
The PubMed, EMbase, Cochrane Library, Google Scholar, Wan Fang and CNKI databases were searched to identify controlled clinical trials comparing hypofractionated radiotherapy versus conventional fractionated radiotherapy in postmastectomy breast cancer. Overall survival (OS) was the primary endpoint, and disease-free survival (DFS), locoregional recurrence (LRR), distant metastasis (DM), acute skin toxicity, acute lung toxicity, late skin toxicity, lymphedema,, shoulder restriction, and late cardiac related toxicity were the secondary endpoints.
Results
Twenty-five controlled clinical trials involving 3871 postmastectomy breast cancer patients were included in this meta-analysis according to the selection criteria. The meta-analysis revealed that there were no significant differences in OS (OR = 1.08, 95% CI = 0.87~1.33, P = 0.49), DFS (OR = 1.13, 95% CI = 0.91~1.40, P = 0.28), LRR (OR = 1.01, 95% CI = 0.76~1.33, P = 0.96), DM (OR = 1.16, 95% CI = 0.85~1.58, P = 0.34), acute skin toxicity (OR = 0.94, 95% CI = 0.67~1.32, P = 0.72), acute lung toxicity (OR = 0.94, 95% CI = 0.74~1.20, P = 0.62), late skin toxicity (OR = 0.98, 95% CI = 0.75~1.27, P = 0.88), lymphedema (OR = 0.99, 95% CI = 0.77~1.28, P = 0.94), shoulder restriction (OR = 0.75, 95% CI = 0.43~1.31, P = 0.31), or late cardiac related toxicity (OR = 1.17, 95% CI = 0.82~1.65, P = 0.39) between the two groups.
Conclusions
The results of this study show that compared to conventional fractionated radiotherapy, hypofractionated radiotherapy is not significantly different with respect to efficacy or toxicity in postmastectomy breast cancer. Additional large randomized clinical trials are needed to further confirm this conclusion.
Keywords: Breast cancer, Hypofractionated radiotherapy, Conventional radiotherapy, Postmastectomy
Introduction
Breast cancer has the highest incidence rate and causes the second highest number of deaths among cancers in women according to cancer statistics from 2019 in the United States [1]. It is well accepted that postmastectomy radiotherapy (PMRT) improves long-term outcomes by reducing local recurrence and cancer mortality in breast cancer after mastectomy [2, 3]. The most recent National Comprehensive Cancer Network (NCCN) guidelines recommend the conventional fractionated radiotherapy (CFRT) schedule for PMRT, which consists of a total dose (TD) of 45.0 Gy to 50.4 Gy given in 25 to 28 fractions over 5 weeks or more and delivered to the chest wall and regional lymph nodes. However, with advances in radiotherapy technology, methods to reduce toxicity, overall treatment time and cost have gradually attracted the attention of researchers.
Previous reports indicate that breast cancer has a low ratio of α/β over the range of 2.0~4.0 Gy, and this low α/β ratio suggests that the efficacy of hypofractionated radiotherapy (HFRT) regimens are equivalent to CFRT in breast cancer [4]. Data from randomized controlled trials from the United Kingdom and Canada confirm this conclusion to a certain extent [5–11]. However, most of the patients in these trials were early breast cancer patients who underwent breast-conserving surgery. Adjuvant treatment of breast cancer after mastectomy remains controversial, and there are few relevant prospective randomized controlled clinical trials (RCTs) that address adjuvant treatment internationally. In Lancet Oncology, 2019, Wang et al. [12] report 5-year outcomes of a randomized, non-inferiority, open-label, phase 3 trial in China that compared postmastectomy HFRT with CFRT directed to the chest wall and the supraclavicular and level III axillary nodal regions in 820 patients with locally advanced breast cancer (at least four positive axillary lymph nodes). There were no significant differences in the 5-year cumulative incidence of locoregional recurrence, 5-year overall survival or 5-year disease-free survival between groups. Furthermore, acute and late toxicities were similar in both groups. This finding suggests that hypofractionated postmastectomy radiotherapy (HF PMRT) is safe and effective for patients with a high risk for breast cancer, exhibiting low toxicity and high local control rates. In addition, 15% (336/2236) and 8% (177/2215) of patients with postmastectomy HFRT were included in the START A and START B trials, respectively, and there was no significant difference in local recurrence or late toxicities between the two groups over a long-term follow-up of 10 years [5–8].
Clinically, HFRT could reduce the cost of cancer treatment, provide more convenient treatment and allow providers to treat more patients. There is growing interest in using the HF PMRT scheme, although the number of patients receiving HFRT after mastectomy in the United States is currently small (1.1%) [13]. In recent years, HFRT use in patients who underwent breast-conserving surgery has been written into NCCN and other treatment guidelines and has been gradually applied in the clinic, but using HFRT in patients after mastectomy remains controversial. Therefore, we aimed to use evidence-based medicine to compare the efficacy and toxicity of HFRT and CFRT after mastectomy.
Methods
Study protocol
A search of PubMed, EMBASE, Cochrane Library, Google Scholar, Wan Fang, and CNKI was conducted up through February 25, 2019. MeSH or Emtree terms combined free terms were used: “breast cancer”, “mastectomy”, “radiotherapy”, “hypofractionated” and “conventional fractionated”.
Selection criteria
Inclusion Criteria: (1) Surgical mastectomy in patients diagnosed with breast cancer by pathology. (2) Controlled trials comparing HFRT to CFRT after mastectomy. (3) Inclusion of study sample size > 20 cases. (4) Complete information is provided in the literature. Exclusion Criteria: (1) Review articles, case reports, meeting abstracts, and lectures. (2) No clear diagnosis was made in enrolled patients. (3) Inclusion of study sample size < 20 cases. (4) Incorrect data, incomplete data or unable to extract required data. (5) Duplicate publications.
Data extraction
Data were independently screened and extracted by two reviewers, including patient eligibility, study design, baseline characteristics, and number of events for all outcomes and interventions. Overall survival (OS) was the primary endpoint, and disease-free survival (DFS), locoregional recurrence (LRR), distant metastasis (DM), acute skin toxicity, acute lung toxicity, late skin toxicity, lymphedema, shoulder restriction, and late cardiac related toxicity were secondary endpoints. Any disagreement was resolved by consensus.
Quality assessment
Quality assessment of included studies was independently performed by two authors, and disagreements were resolved by consensus. Study quality was evaluated using the Newcastle-Ottawa scale (NOS). Primary evaluations included measurement of exposure factors, comparability between groups, and patient selection. Each study with NOS scores ≥6 was considered a high-quality study, whereas studies with NOS scores<6 were considered low-quality studies. Quality assessment results of included studies are summarized in Table 1.
Table 1.
Baseline characteristics and dose fraction of 3871 patients in 25 studies with breast cancer after mastectomy
| Study (year published) | Sample size | Age (years) | Clinical stage | Interventions (Gy/fractions) | Outcomes | NOS | The type of RT technique | RT area and prescriptive method used | Bolus | Breast reconstruction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HFRT | CFRT | HFRT | CFRT | |||||||||
| Wang (2019) [12] | 401 | 409 | 24~74 | II~III | 43.5/15 | 50/25 | OS, DFS, LRR, Toxicities | 9 | 2DRT, 3DCRT, or IMRT |
CW: a single appositional field with 6–9 MeV electron beam, depending on CW thickness SPC: anteroposterior field using a 6 MV photon, combined 6 MV photon, and 9–15 MeV electron or a 10~12 MeV electron beam |
5 mm bolus to CW | None |
| Abhilash (2016) [14] | 30 | 30 | 25~67 | II~III | 39/13 | 50/25 | LRR, Toxicities | 6 | 2DRT |
CW: bilateral tangent pair fields SPC: a direct anterior field Cobalt 60 teletherapy machine with gamma energy 1.25 MeV |
NR | None |
| Bi (2011) [15] | 51 | 49 | 28~64 | II~III | 43.5/15 | 50/25 | OS, LRR, Toxicities | 6 | 2DRT |
CW: a 6~12 MeV electron beam SPC: 12 MeV electron and 6 MV X-ray beams mixed |
5 mm bolus to CW | None |
| Bedi (2018) [16] | 30 | 30 | 39~61 | II~III | 42.5/16 | 50/25 | Toxicities | 5 | 3DCRT |
CW: two tangential fields using 6 MV X-ray beam SPC: a direct anterior field with 6 MV photon beam |
Universal bolus to CW | None |
| Das (2018) [17] | 55 | 53 | 48/50 a | I~III | 42.56/16 | 50/25 | DFS, LRR, DM, Toxicities | 6 | 2DRT |
CW: medial and lateral tangential fields SPC: anteroposterior field Cobalt 60 teletherapy machine |
NR | None |
| Eldeeb (2012) [18] | 66 | 41 | 25~67 | I~III | 45/17、40/15 | 50/25 | LRR, Toxicities | 7 | 2DRT |
CW: two tangential fields SPC and Axillary: an anterior field 6 MV photon linear accelerator or Cobalt 60 machine |
NR | None |
| El-Sayed (2012) [19] | 159 | 84 | 30~69 | II~III | 42.5/16、39/13 | 50/25 | OS, DFS, LRR, Toxicities | 7 | 2DRT |
CW: medial and lateral tangential fields using 6 MV photon beam SPC: anteroposterior field using 6 MV photon beam |
NR | None |
| Elsayed (2014) [20] | 25 | 22 | 33~70 | II~III | 42.72/16 | 50/25 | OS, DFS, Toxicities | 8 | 2DRT |
CW and Axillary: two tangential fields using 6 MV photon beam SPC: a direct anterior field using 6 MV photon beam |
NR | None |
| Fatma (2018) [21] | 50 | 50 | 31~68 | II~III | 40/15 | 50/25 | DFS, LRR, Toxicities | 7 | 3DCRT |
CW: two tangential fields using 6 MV or 15 MV photon beam SPC: an anterior field using 6 MV or 15 MV photon beam |
NR | None |
| He (2016) [22] | 48 | 63 | 25~57 | I~II | 40/15 | 50/25 | Toxicities | 6 | 3DCRT | CW and SPC: 6 MV photon beam | NR | None |
| Huang (2013) [23] | 70 | 70 | 25~65 | II~III | 43.5/15 | 48.5/22 | LRR | 6 | NR | RT area: CW and SPC | NR | None |
| Jin (2013) [24] | 164 | 136 | 23~75 | NR | 45/15 | 50/25 | OS, DM, LRR, Toxicities | 6 | NR | RT area: CW, SPC, Axillary, Axillary dome or internal mammary lymph node region if necessary | NR | None |
| Kalita (2018) [25] | 25 | 25 | 27~70 | II~III | 40/15 | 50/25 | Toxicities | 5 | 2DRT, |
CW: two parallel opposed tangential fields SPC and Axillary: a separate direct anterior field All fields using 6 MV photon beam |
NR | None |
| Kouloulias (2016) [26] | 87 | 30 | 33~78 | II~III | 48.3/21、42.56/16 | 50/25 | Toxicities | 7 | 3DCRT | CW and SPC using 6 MV photon beam | NR | None |
| Kumbhaj (2013) [27] | 46 | 45 | 31~70 | I~III | 40/17 | 50/25 | DFS, LRR, DM, Toxicities | 6 | 2DRT, |
CW: tangent pair technique by using Cobalt 60 machine; SPC: NR |
NR | None |
| Pinitpatcharalert (2011) [28] | 148 | 67 | 44~56 | I~III | 42.72/16 | 50/25 | OS, DFS, Toxicities | 8 | 2DRT |
CW: medial and lateral tangential chest wall fields SPC: anteroposterior ipsilateral supraclavicular field Axillary boost: clinical N2 disease, inadequate node excision (less than 10 nodes), or perinodal invasion CW and SPC field were treated with cobalt 60 |
5 mm bolus to CW | None |
| Purohit (2016) [29] | 25 | 25 | 18~65 | II~III | 40/15 | 50/25 | Toxicities | 6 | 2DRT |
CW: medial tangential and lateral tangential fields SPC: anteroposterior field two field technique by using Cobalt 60 energy source |
NR | None |
| Rastogi (2018) [30] | 50 | 50 | 21~79 | II~III | 42.72/16 | 50/25 | DFS, LRR, DM | 7 | 3DCRT | CW and SPC: single beam energy, or a combination of both 6 and 15 MV photon beam was used depending on patients’ anatomy | None | |
| Wang (2010) [31] | 31 | 30 | 32~71 | NR | 41.6/13 | 50/25 | OS, Toxicities | 5 | 2DRT | CW and SPC: 12 MeV electron and 6 MV X-ray beams mixed, two parallel opposed tangential fields | NR | None |
| Wu (2003) [32] | 177 | 149 | 26~74 | I~III | 45/15 | 50/25 | OS, LRR | 8 | 2DRT, |
SPC, Axillary and Axillary dome: 8 MV X-ray, and the 20 Gy SPC was added with 1.5 cm bolus Internal mammary: 14 MeV electron beam or mixed with 8 MV X-ray; CW: tangent to 8 MV X-ray or 6–8 MeV electron beam |
1.5 cm bolus to SPC | None |
| Wu (2014) [33] | 53 | 53 | 31~68 | II~IV b | 42/15 | 50/25 | NR | 6 | 3DCRT | RT area: CW and SPC | NR | None |
| Yu (2011) [34] | 175 | 171 | 21~76 | II~III | 45/15 | 50/25 | NR | 7 | 2DRT |
SPC and Axillary: 4 MV X-ray by single anterior field, and the 20 Gy of total dose was added with 5 mm bolus CW and internal mammary: 8 MV X-ray two parallel opposed tangential fields all beams supplemented by 100–200 KV X-ray |
5 mm bolus to SPC and Axillary | NR |
| Zhang (2015) [35] | 36 | 30 | 24~68 | II~III | 43.5/15 | 50/25 | Toxicities | 5 | 3DCRT | Irradiation target area: CW and SPC | NR | None |
| Zhao (2014) [36] | 41 | 44 | 18~70 | II~III | 42.56/16 | 50/25 | LRR, DM, Toxicities | 6 | 3DCRT | CW and SPC: two parallel opposed tangential fields by using 6 MV X-ray beam | NR | None |
| Zhao (2016) [37] | 37 | 35 | 37~59 | II~III | 42.56/16 | 50/25 | OS, LRR, DM, Toxicities | 7 | 3DCRT |
RT area: CW, SPC and Axillary, excluding the Internal mammary 6 MV X-ray beam |
5 mm bolus to CW | None |
OS overall survival, LRR locoregional recurrence, DFS disease-free survival, DM distant metastasis, HFRT hypofractionated radiotherapy, CFRT conventional fractionated radiotherapy, RT radiotherapy, NOS Newcastle-Ottawa Scale, 2DRT two-dimensional radiotherapy, 3DCRT three-dimensional conformal radiotherapy, IMRT intensity-modulated radiotherapy, NR not reported, CW chest wall, SPC supraclavicular
aMean age in HFRT group and CFRT group
bOnly one stage IV case in the HFRT group
Statistical analysis
RevMan 5.3 analysis software (Cochrane Collaboration, Copenhagen, Denmark) and STATA 14.0 (Stata Corporation, College Station, TX, USA) were used for statistical analysis. Odds ratio (OR) and 95% confidence interval (95% CI) were used for count data. Cochran’s Q test and Ι2 statistics were used to assess heterogeneity between studies. If heterogeneity was not present (P > 0.1, I2 < 50%), the fixed-effect model was adopted for analysis. Otherwise, a random-effect model was employed. The results are represented as forest maps, and potential heterogeneity was identified by sensitivity analysis. We assessed publication bias using the Egger test and funnel plots. P < 0.05 was considered statistically significant.
Results
Study selection
Two hundred twelve articles were initially retrieved, and after screening according to inclusion and exclusion criteria, 25 articles were entered into the systematic review [12, 14–37] (Fig. 1). Only 1 study was an RCT [12], and the rest were retrospective studies [14–37].
Fig. 1.
Flow chart of the study selection process
Study characteristics
The characteristics of the trials are summarized in Table 1. Studies were published in 2003–2019, with a total of 3871 patients with breast cancer, including 2080 in the HFRT group and 1791 in the CFRT group. All patients underwent mastectomy with potential differences in quality between study surgeries, and none receive breast reconstruction (except the study by Yu [34] not reported). Radiation treatment area generally included the ipsilateral side chest wall and or the ipsilateral side supraclavicular area [12, 14–37]. Eight studies [18, 20, 24, 25, 28, 32, 34, 37] proposed to add the axillary fossa, axillary dome or internal mammary lymph node region if necessary. 6 MV ~ 8 MV X-ray and 6 MeV ~ 15 MeV electron beams were generally used for radiation treatment, and fourteen trials [12, 14, 15, 17–20, 25, 27–29, 31, 32, 34] used two-dimensional radiotherapy technology, with cobalt 60 being used in some patients [14, 17, 18, 27, 29]. The median age ranged from 18 to 78 years, and the TD of radiotherapy in the HFRT group ranged from 39.0 to 48.3 Gy, with a single dose of 2.3 to 3.2 Gy given over 13 to 17 fractions. The TD in the CFRT group was 50.0 Gy, with a single dose of 2.0 Gy over 25 treatments (only 1 trial [23] 48. 5 Gy in 22 fractions). Clinical characteristics between the two groups of patients included in the study, such as age, tumor stage, pathological type, estrogen and progesterone levels, HER2 status, chemotherapy regimen, etc., were not significantly different, so results were highly reliable. Patient information, including age, tumor stage, dose fraction, radiation therapy area, prescriptive method used, etc. is listed in Table 1. NOS scores are shown in Table 1.
Meta-analysis outcomes
In the combined overall survival rate, disease-free survival rate and distant metastasis rate, there were 2 studies [19, 28], 1 study [14] and 1 study [14], respectively, that could not be submitted to meta-analysis due to no events and were not included the corresponding study outcomes for analysis.
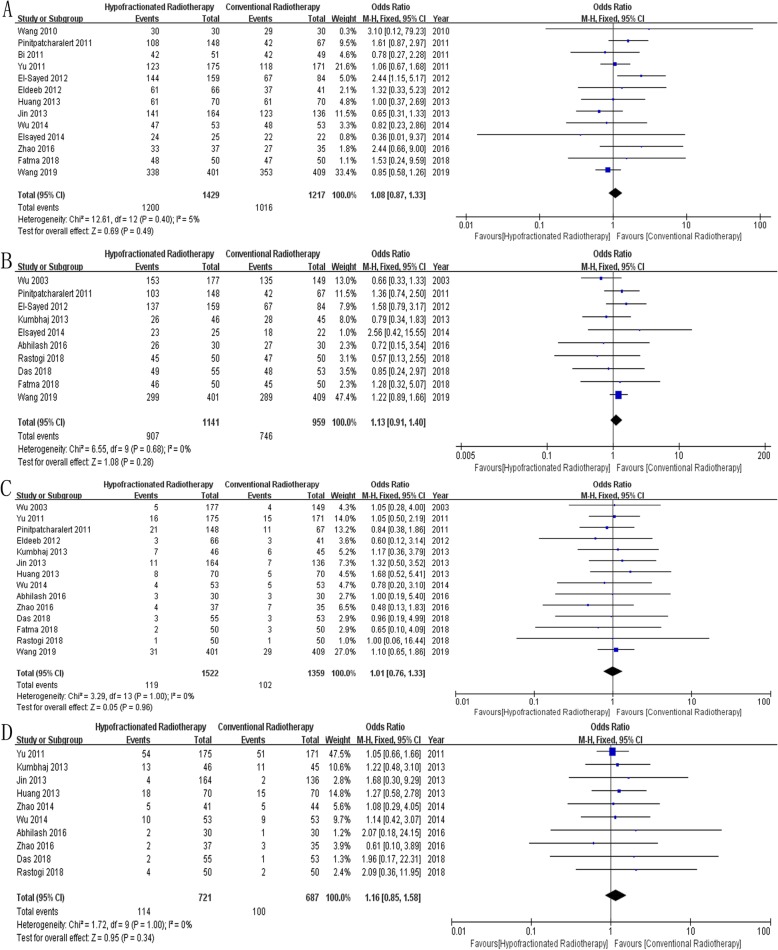
Overall Survival: Thirteen studies [12, 15, 18–21, 23, 24, 28, 31, 33, 34, 37] reported overall survival in 2646 patients, and results showed no significant difference between the two groups (OR = 1.08, 95% CI = 0.87~1.33, P = 0.49, Fig. 2a).
Disease-Free Survival: Ten studies [12, 14, 17, 19–21, 27, 28, 30, 32] reported disease-free survival in 2100 patients, and results showed no significant difference between the two groups (OR = 1.13, 95% CI = 0.91~1.40, P = 0.28, Fig. 2b).
Locoregional Recurrence: Fourteen studies [12, 14, 17, 18, 21, 23, 24, 27, 28, 30, 32–34, 37] reported locoregional recurrence in 2881 patients, and results showed no significant difference between the two groups (OR = 1.01, 95% CI = 0.76~1.33, P = 0.96, Fig. 2c).
Distant Metastasis: Ten studies [14, 17, 23, 24, 27, 30, 33, 34, 36, 37] reported distant metastasis in 1408 patients, and results showed no significant difference between the two groups (OR = 1.16, 95% CI = 0.85~1.58, P = 0.34, Fig. 2d).
Acute Skin Toxicity: Twenty-three studies [12, 14–18, 20–36] reported acute skin toxicity in 3456 patients, and results showed no significant difference between the two groups (OR = 0.94, 95% CI = 0.67~1.32, P = 0.72, Fig. 3a).
Acute Lung Toxicity: Ten studies [12, 16, 17, 20–22, 30, 34–36] reported acute lung toxicity in 1853 patients, and results showed no significant difference between the two groups (OR = 0.94, 95% CI = 0.74~1.20, P = 0.62, Fig. 3b).
Late Skin Toxicity: Seven studies [12, 14, 17, 18, 26, 30, 31] reported late skin toxicity in 1363 patients, and results showed no significant difference between the two groups (OR = 0.98, 95% CI = 0.75~1.27, P = 0.88, Fig. 3c).
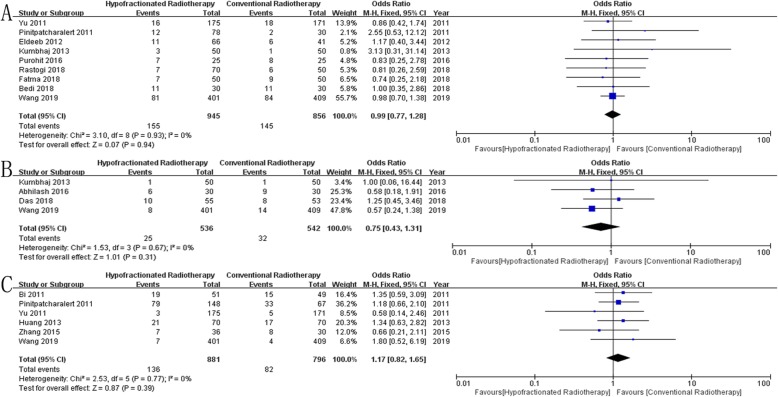
Lymphedema: Nine studies [12, 16, 18, 21, 27–30, 34] reported lymphedema in 1801 patients, and results showed no significant difference between the two groups (OR = 0.99, 95% CI = 0.77~1.28, P = 0.94, Fig. 4a).
Shoulder Restriction: Four studies [12, 14, 17, 27] reported shoulder restriction in 1078 patients, and results showed no significant difference between the two groups (OR = 0.75, 95% CI = 0.43~1.31, P = 0.31, Fig. 4b).
Late Cardiac Related Toxicity: Six studies [12, 15, 23, 28, 34, 35] reported late cardiac related toxicity in 1677 patients, and results showed no significant difference between the two groups (OR = 1.17, 95% CI = 0.82~1.65, P = 0.39, Fig. 4c).
Fig. 2.
Forest plot comparing the efficacy of HFRT with that of CFRT after mastectomy in breast cancer. a Overall Survival, b Disease-Free Survival, c Locoregional Recurrence, d Distant Metastasis
Fig. 3.
Forest plot comparing the toxicity of HFRT with that of CFRT after mastectomy in breast cancer. a Acute Skin Toxicity, b Acute Lung Toxicity, c Late Skin Toxicity
Fig. 4.
Forest plot comparing the toxicity of HFRT with that of CFRT after mastectomy in breast cancer. a Lymphedema, b Shoulder Restriction, c Late Cardiac Related Toxicity
Heterogeneity analysis and publication Bias
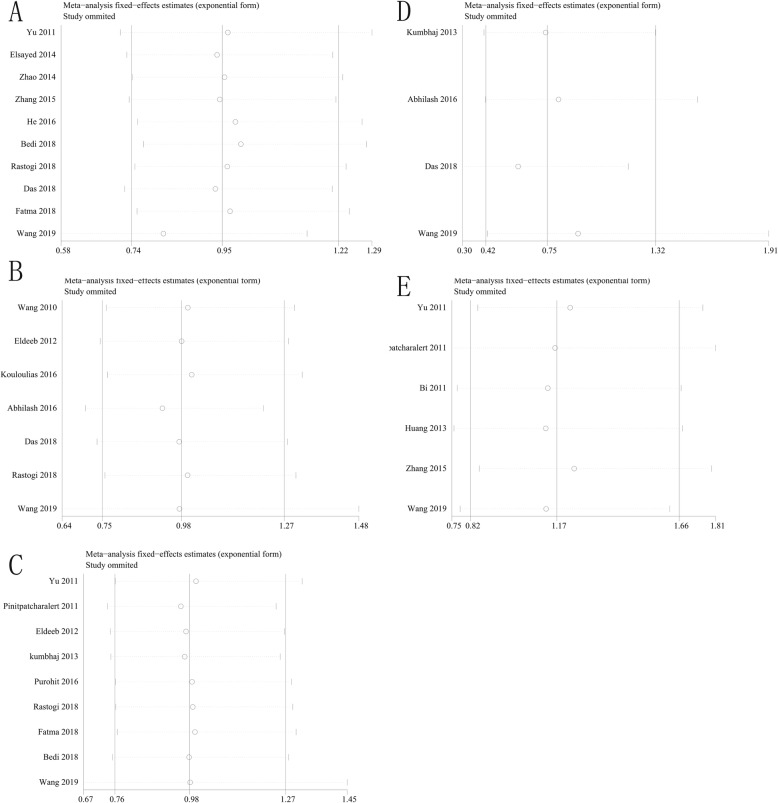
Study outcomes included overall survival, disease-free survival, locoregional recurrence, distant metastasis, acute lung toxicity, late skin toxicity, lymphedema, shoulder restriction and late cardiac related toxicity not present heterogeneity (P > 0.1, I2 < 50%), and a fixed-effect model was adopted for analysis. The study outcome acute skin toxicity presented heterogeneity (P < 0.1, I2 > 50%), and a random-effect model was employed. Meanwhile, sensitivity analysis of this study outcome did not find any abnormal studies, indicating that our research results are more stable. Further analysis of other study outcomes using sensitivity analysis did not observe significant heterogeneity (Fig. 5, Fig. 6). Publication bias results suggest that study outcome of acute skin toxicity has a published bias (P < 0.05), but there was no significant bias in the remaining study outcomes (P > 0.05) (Table 2, Fig. 7, Fig. 8).
Fig. 5.
Results of sensitivity analysis. a Overall Survival, b Disease-Free Survival, c Locoregional Recurrence, d Distant Metastasis, e Acute Skin Toxicity
Fig. 6.
Results of sensitivity analysis. a Acute Lung Toxicity, b Late Skin Toxicity, c Lymphedema, d Shoulder Restriction, e Late Cardiac Related Toxicity
Table 2.
Results of publication bias
| Study Outcome | Coefficient | Standard Error | t | P > |t| | 95% Confidence Interval |
|---|---|---|---|---|---|
| Overall Survival | 0.42 | 0.56 | 0.74 | 0.47 | −0.82~1.66 |
| Disease-Free Survival | −0.43 | 0.53 | −0.82 | 0.44 | −1.64~0.78 |
| Locoregional Recurrence | −0.39 | 0.33 | −1.19 | 0.26 | −1.11~0.33 |
| Distant Metastasis | 0.42 | 0.25 | 1.67 | 0.13 | −0.16~1.00 |
| Acute Skin Toxicity | 1.57 | 0.64 | 2.45 | 0.02 | 0.24~2.89 |
| Acute Lung Toxicity | −0.50 | 0.49 | −1.01 | 0.34 | −1.63~0.64 |
| Late Skin Toxicity | −0.15 | 0.67 | −0.23 | 0.83 | −1.86~1.55 |
| Lymphedema | 0.41 | 0.38 | 1.07 | 0.32 | −0.50~1.32 |
| Shoulder Restriction | 0.39 | 1.30 | 0.30 | 0.79 | −5.19~5.98 |
| Late Cardiac Related Toxicity | −0.89 | 0.97 | −0.92 | 0.41 | −3.59~1.80 |
Fig. 7.
Publication biased funnel plot. a Overall Survival, b Disease-Free Survival, c Locoregional Recurrence, d Distant Metastasis, e Acute Skin Toxicity
Fig. 8.
Publication biased funnel plot. a Acute Lung Toxicity, b Late Skin Toxicity, c Lymphedema, d Shoulder Restriction, e Late Cardiac Related Toxicity
Discussion
For patients who underwent breast-conserving surgery and received whole-breast radiotherapy, long-term results from large randomized trials confirmed equivalent efficacy and safety of HFRT and CFRT. However, for patients who underwent surgical mastectomy the scarcity of high-level evidence has resulted in only a few patients having received HFRT [13], and only one randomized study has compared HFRT and CFRT in breast cancer patients who underwent mastectomy [12]. Other evidence for the clinical application of postmastectomy HFRT schedule has until now only been available from case series, retrospective studies, or subgroup analyses from the START randomized trials. Thus, we performed this meta-analysis to determine the efficacy and safety of postmastectomy HFRT schedule on outcomes in women with breast cancer. This meta-analysis indicated that HFRT and CFRT were equally effective with respect to overall survival (OS), disease-free survival (DFS), locoregional recurrence (LRR), and distant metastasis (DM) after breast mastectomy.
In recent small retrospective cohort studies, HF PMRT was shown to be effective with acceptable toxicity [38–41]. In a recent phase 2 trial [42], 67 women with clinical stage II to IIIa breast cancer who received a HF PMRT regimen of 36.6 Gy over 11 fractions to the chest wall and the draining regional lymph nodes with a scar boost of 4 fractions of 3.33 Gy revealed that after a median follow-up of 32 months, patients with isolated ipsilateral chest wall tumor recurrences were 3.0%, the 3-year estimated overall survival was 92.0% (95% CI, 78.9~97.1), the 3-year estimated local recurrence-free survival was 89.2% (95% CI, 74.8~95.6), the 3-year estimated distant recurrence-free survival was 90.3% (95% CI, 79.7~95.6), and low toxicity was reported. In Lancet Oncology, 2019, Shu-Lian Wang [12] reports 5-year outcomes of a randomized, non-inferiority, open-label, phase 3 trial comparing postmastectomy HFRT (43.5 Gy over 15 fractions in 3 weeks) and CFRT (50 Gy over 25 fractions in 5 weeks) directed to the chest wall and the supraclavicular and level III axillary nodal region in 820 patients with locally advanced breast cancer (at least four positive axillary lymph nodes or T3–4 tumors). All patients underwent chemotherapy, 76.5% used hormonal therapy, and the primary endpoint was 5-year locoregional recurrence. After a median follow-up of 58.5 months, there were no significant differences in the 5-year cumulative incidence of locoregional relapse (8·3% [90% CI 5·8~10·7] VS 8·1% [5·4~10·6]), 5-year overall survival (84%[90% CI 80~88] VS 86% [82~89]) and 5-year disease-free survival (74% [95% CI 70~79] VS 70% [65~76]) between the HFRT and CFRT groups. Furthermore, there was no significant difference between the two groups in the incidence of acute or late toxicities, including symptomatic radiation pneumonitis, lymphedema, ischemic heart disease, late skin toxicity, lung fibrosis or shoulder dysfunction; however, fewer patients experienced grade 3 acute skin toxicity in the HFRT group than in the CFRT group (14 [3%] of 401 patients VS 32 [8%] of 409 patients, p < 0.0001). No brachial plexopathy or rib fractures were observed, and frequencies of lymphedema and shoulder dysfunction were also reassuringly low, at less than 1% of grade 2 toxicity for both events. These results suggest that the HF PMRT regimen is safe and effective for patients with high-risk breast cancer, with low toxicity and high local control rate [12].
For long-term survival of breast cancer patients, the late treatment toxicities are also important. The results of this study found that there were no differences in acute skin toxicity, acute lung toxicity, late skin toxicity, lymphedema, shoulder restriction, or late cardiac related toxicity between the two groups. And we also found that no grade 2/3/4 late lung toxicity patients were observed, and the incidence of grade 1 was very low in the included studies [12, 14, 15, 23, 28, 32, 35]. Further, the randomized trial result showed that late lung toxicity may be increased in patients after HFRT (P = 0.08), but it was not statistically significant, which is worthy of further follow-up and research. Many studies have shown that the incidence of grade 2–4 acute skin toxicity after HF PMRT is between 10 and 25% [12, 28, 38, 39, 42], and this meta-analysis showed that the incidence of grade 2–4 acute skin toxicity in 3456 patients across 23 trials was 17.3%, which in line with reported rates. Similar results were observed in the PMRT subgroup of the UK START study (12%, 513/4451), which compared to the CFRT group, there was no significant difference in lymphedema or moderate or marked breast/shoulder/arm symptoms, etc. in patients receiving HFRT [5, 6]. Another late toxicity that should be considered is cardiac-related toxicity in patients after HFRT. Previous studies have shown that the incidence of cardiovascular events in patients with breast cancer after radiotherapy is very low, and the use of HFRT was not observed to increase that risk compared to CFRT [43–45]. The 10-year follow-up results of the UK START studies revealed that fatal cardiac events in START A and START B were 1.3 and 0.5%, respectively, while the incidence of ischemic heart disease was low (0.7%), and there was no significant difference between these two groups [5]. The proportion of patients with the late cardiac related toxicity was higher (52.1%) in the Pinitpatcharalert [28] study that the meta-analysis indicated, with 3.0% (1/67) in the CFRT group and 3.0% (3/148) in the HFRT group reporting patient deaths from cardiovascular events. This proportion is higher than in previous studies, which may be due to the small number of patients included, but there was no statistically significant difference between these two groups. The meta-analysis in our study showed no significant differences in late cardiac-related toxicity between the two groups, consistent with the above findings, indicating that HFRT does not significantly increase the risk of cardiovascular related events in breast cancer patients.
A higher dose per treatment fraction might increase the risk of toxicities in the setting of regional nodal irradiation (RNI) [46], but hypofractionated RNI was not observed to increase toxicity in one randomized clinical trial [12]. Two recent studies reported that the efficacy and safety of hypofractionated RNI were acceptable [47, 48]. One was based on UK START trials, with 864/5861 patients who experienced adjuvant lymphatic radiotherapy (LNRT) (PMRT 202/864, 23.4%) assessed using the EORTC QLQ-BR23 scale, protocol-specific questions and by physicians [47]. The long-term results from START trials suggest that appropriately dosed hypofractionated LNRT is safe, according to patient and physician-assessed arm and shoulder symptoms, a conclusion consistent with the findings for > 2.0 Gy schedules delivered to the breast/chest wall [47]. Another retrospective study reviewed 257 patients with stage IIa to IIIc breast cancer receiving hypofractionated RNI, with 80.2% patients having PMRT, 99.6% undergoing chemotherapy, 81.3% having hormonal treatment, and 25.3% having anti-HER2 targeted therapy. The median follow-up time was 64 months (range, 11 to 88 months), and the 5-year OS, DFS, locoregional recurrence (LRR)-free survival, and distant metastasis (DM)-free survival was 86.6, 84.4, 93.9 and 83.1%, respectively. During study follow-up, no acute symptomatic pneumonitis, cardiac events, brachial plexopathy or rib fractures occurred, and the incidence of grade 2–4 lymphedema was 5.8% [48]. The above findings suggest that the HFRT schedule may be acceptable in breast cancer patients who require RNI. However, prospective trials are necessary to confirm these results.
Hypofractionated radiotherapy could help to contain the costs of cancer care by mitigating financial toxicity and can be performed in most radiotherapy centers, even at small-scale hospitals. Studies have reported that the cost of using hypofractionated whole breast irradiation (WBI) in the United States is 31.7% lower than that of conventional fractionated WBI [49], and one study in Asia also indicated that the total cost of treatment for hypofractionated WBI compared to conventional fractionated WBI was reduced by about one-third [50]. It should be noted that although hypofractionated PMRT is not the same as the target area irradiated by hypofractionated WBI, the treatment technique and radiotherapy fraction are similar, and it can still shorten the treatment cycle, reduce the time of patient trips to the hospital, and save medical resources, which is more cost-effective. This issue is even more important in low- and middle-income countries.
The inclusion and exclusion criteria of this study were strict, the literature search was comprehensive, and the results are highly credible, but the following limitations do exist: 1. Quality of the included studies was unequal, and the number of included studies was limited. There may be differences in the quality of surgery between studies, and the quality of surgery could not be evaluated. There are many methods for adjuvant treatment of breast cancer, and all adjuvant treatments could not be evaluated. 2. Included studies did not provide survival data for patients of different age, tumor stage, positive lymph node numbers, pathological type, estrogen/progesterone levels, and could not be further analyzed for their impact on efficacy. However, the clinical characteristics of the two groups of patients included in the study, including age, tumor stage, pathological type, estrogen and progesterone levels, HER2 status, postoperative chemotherapy, etc., were not significantly different, so the reliability of these results was still high. 3. Most of the current studies were retrospective (only one was an RCT), and the quality of research methods is unequal. There are differences in treatment methods, radiotherapy, loss of follow-up descriptions, etc. It is difficult to extract all treatment data and then evaluate them. 4. Some trials used outdated radiotherapy techniques, and the use of hypofractionation schedules is variable. 5. Limited follow-up times in these included trials, multi-center prospective clinical trials and long-term follow-up are still needed for verification.
Conclusions
The results of this study show that there is no statistically significant difference in efficacy or toxicity between hypofractionated radiotherapy and conventional fractionated radiotherapy after breast mastectomy. Hypofractionated radiotherapy is a safe and effective radiotherapy schedule, but the current study is still primarily retrospective and requires large-scale randomized clinical trials to confirm this conclusion along with long-term follow-up of patients who experience late toxicities.
Acknowledgements
Not applicable.
Abbreviations
- CFRT
Conventional fractionated radiotherapy
- DFS
Disease-free survival
- DM
Distant metastasis
- HF PMRT
Hypofractionated postmastectomy radiotherapy
- HFRT
Hypofractionated radiotherapy
- LNRT
Lymphatic radiotherapy
- LRR
Locoregional recurrence
- NCCN
National Comprehensive Cancer Network
- NOS
Newcastle-Ottawa scale
- OR
Odds ratio
- OS
Overall survival
- PMRT
Postmastectomy radiotherapy
- RCTs
Randomized controlled clinical trials
- RNI
Regional nodal irradiation
- TD
Total dose
- WBI
Whole breast irradiation
Authors’ contributions
LL, YY and QG conceived and coordinated the study; designed, performed, and analyzed the experiments; and wrote the manuscript. BR, QP, LZ and YZ collected and analyzed the data. YT revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Funding
This study was sponsored and funded by the Jiangsu Medical Innovation Team (No. CXDT-37) and Medicine Outstanding Leader of Suzhou (No. 62).
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Liu, Yongqiang Yang and Qi Guo contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019 [J] CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Mcgale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials [J] Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials[J] Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Qi X, White J, Li X. Is α/β for breast cancer really low? An analysis of large randomized clinical trials for radiation therapy of breast cancer[J] Int J Radiat Oncol Biol Phys. 2009;75(3):S146. doi: 10.1016/j.ijrobp.2009.07.345. [DOI] [Google Scholar]
- 5.Haviland J, Owen J, Dewar J, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials [J] Lancet Oncol. 2013;14(11):1086–1094. doi: 10.1016/s1470-2045(08)70077-9. [DOI] [PubMed] [Google Scholar]
- 6.Hopwood P, Haviland JS, Sumo G, et al. Comparison of patient-reported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised standardisation of breast radiotherapy (START) trials[J] Lancet Oncol. 2010;11(3):231–240. doi: 10.1016/S1470-2045(09)70382-1. [DOI] [PubMed] [Google Scholar]
- 7.Bentzen SM, Agrawal RK, Aird EG, et al. The UK standardisation of breast radiotherapy (START) trial a of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial[J] Lancet Oncol. 2008;9(4):331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentzen SM, Agrawal RK, Aird EG, et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial[J] Lancet. 2008;371(9618):1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer[J] J Natl Cancer Inst. 2002;94(15):1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 10.Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial[J] Radiother Oncol. 2005;75(1):9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial[J] Lancet Oncol. 2006;7(6):467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: a randomised, non-inferiority, open-label, phase 3 trial[J] Lancet Oncol. 2019;20(3):352–360. doi: 10.1016/S1470-2045(18)30813-1. [DOI] [PubMed] [Google Scholar]
- 13.Venigalla S, Guttmann DM, Jain V, et al. Trends and patterns of utilization of hypofractionated postmastectomy radiotherapy: a national cancer database analysis [J] Clinical Breast Cancer. 2018;18(5):e899–e908. doi: 10.1016/j.clbc.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abhilash GH, Dhull AK, Atri R, et al. Comparison of hypofractionated radiation therapy versus conventional radiation therapy in post mastectomy breast cancer[J] J Evid Based Med Healthc. 2016;3(26):1177–1181. doi: 10.18410/jebmh/2016/270. [DOI] [Google Scholar]
- 15.Bi LP, Li YJ, Gong XH, et al. Clinical study of hypofractionated radiotherapy in breast cancer after modified radical mastectomy[J] J CIin Med Practice. 2011;15(13):21–23. doi: 10.3969/j.issn.1672-2353.2011.13.007. [DOI] [Google Scholar]
- 16.Bedi N, Yadav HP, Banipal RPS, Aggarwal S, Gupta Y. Hypofractionated radiotherapy in breast cancer: Is it safe?[J] HECS Int J Comm Health Med Res. 2018;4(4):47–53. doi: 10.21276/ijchmr. [DOI] [Google Scholar]
- 17.Das P, Das TK, Jana A, et al. Comparison of result and outcome of conventional and hypofractionated radiotherapy in post-operative breast cancer patients[J] Int J Med Sci Public Health. 2018;7(6):452–456. doi: 10.5455/ijmsph.2018.0102010032018. [DOI] [Google Scholar]
- 18.Eldeeb H, Awad I, Elhanafy O. Hypofractionation in post-mastectomy breast cancer patients: seven-year follow-up[J] Med Oncol. 2012;29(4):2570–2576. doi: 10.1007/s12032-012-0192-1. [DOI] [PubMed] [Google Scholar]
- 19.El-Sayed MI, AbdelWanis ME. Comparison of hypofractionated and conventional radiotherapy protocols in breast cancer patients: a retrospective study[J] J Cancer Sci Ther. 2012;4(6):158–163. doi: 10.4172/1948-5956.1000132. [DOI] [Google Scholar]
- 20.Elsayed MA, Magdy KAA. Post-mastectomy hypofractionation radiotherapy in breast cancer patients[J] Cancer and Oncology Research. 2014;2(7):87–93. doi: 10.13189/cor.2014.020701. [DOI] [Google Scholar]
- 21.Fatma MFA, Khater A. Hypofractionated versus conventionally fractionated radiotherapy in post-mastectomy breast Cancer patients[J] Journal of Cancer Therapy. 2018;9:941–954. doi: 10.4236/jct.2018.911078. [DOI] [Google Scholar]
- 22.He HL, Liu HM, Xu SK, et al. Acute adverse reactions of hypofractionated and conventional fractionated radiotherapy in patients after radical mastectomy[J] Int Med Health Guidance News. 2016;22(5):625–628. doi: 10.3760/cma.j.issn.1007-1245.2016.05.010. [DOI] [Google Scholar]
- 23.Huang J. The effect of hypofractionated radiotherapy and conventional fraction radiotherapy in local advanced breast cancer patients after radical mastectomy [J] Yiayao Qianyan. 2013;16:158–159. doi: 10.3969/j.issn.2095-1752.2013.16.163. [DOI] [Google Scholar]
- 24.Jin XY, Duan QY. Compare the treatment effect of radiotherapy with different fractionated doses in post-mastectomy[J] Sichuan Med J. 2013;34(1):106–108. doi: 10.3969/j.issn.1004-0501.2013.01.048. [DOI] [Google Scholar]
- 25.Kalita AK, Bhattacharyya M, Jagtap VK, et al. Radiotherapy in Post Mastectomy High Risk Breast Cancer: Early results of a Prospective Study comparing Conventional versus Hypofractionated Radiotherapy[J] J Med Sci Clin Res. 2018;06(07):743–751. doi: 10.18535/jmscr/v6i7.125. [DOI] [Google Scholar]
- 26.Kouloulias V, Mosa E, Zygogianni A, et al. A retrospective analysis of toxicity and efficacy for 2 Hypofractionated irradiation schedules versus a conventional one for post-mastectomy adjuvant radiotherapy in breast Cancer[J] Breast Care. 2016;11(5):328–332. doi: 10.1159/000449433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumbhaj P, Sharma R, Saini P, et al. A study of two different dose fractionation schedules of post mastectomy Chest Wall irradiation in carcinoma breast patients[J] Int J Med Sci Public Health. 2013;2(4):1001–1005. doi: 10.5455/ijmsph.2013.040820131. [DOI] [Google Scholar]
- 28.Pinitpatcharalert A, Chitapanarux I, Euathrongchit J, et al. A retrospective study comparing hypofractionated radiotherapy and conventional radiotherapy in postmastectomy breast cancer[J] J Med Assoc Thail. 2011;94(Suppl 2):S94–102. doi: 10.1016/j.ijrop.2011.9.029. [DOI] [PubMed] [Google Scholar]
- 29.Purohit R, Sharma N, Sharma N, et al. Comparison of acute toxicities in conventional and hypofractionated radiotherapy in post-mastectomy breast cancer[J] J Med Sci Clin Res. 2016;04(06):10721–10724. doi: 10.18535/jmscr/v4i6.07. [DOI] [Google Scholar]
- 30.Rastogi K, Jain S, Bhatnagar AR, et al. A comparative study of hypofractionated and conventional radiotherapy in postmastectomy breast cancer patients[J] Asia Pac J Oncol Nurs. 2018;5(1):107–113. doi: 10.4103/apjon.apjon_46_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Gong MY, Zeng LZ, et al. The short-term curative effects and adverse effects of hypofractionated postoperative radiotherapy for breast cancer[J] World Health Digest. 2010;07(23):109–111. doi: 10.3969/j.issn.1672-5085.2010.23.098. [DOI] [Google Scholar]
- 32.Wu JX, Hui ZG, Li YX, et al. Post-mastectomy radiotherapy with different fractionated dose schemes in early breast cancer[J] Chin J Oncol. 2003;25(3):285–288. doi: 10.3760/j.issn:0253-3766.2003.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Wu EW, Xing AM, Zhao J, et al. Comparison of the effects of hypofractionated radiotherapy and conventional radiotherapy after radical mastectomy for breast cancer[J] Prac J Med & Pharm. 2014;31(1):34–35. [Google Scholar]
- 34.Yu ZL, Zhao JG, Zhang BZ, et al. Research on the long-term efficacy of different irradiation methods for postoperative breast cancer[J] Inner Mongolia Med J. 2011;43(12):1414–1417. doi: 10.3969/j.issn.1004-0951.2011.12.003. [DOI] [Google Scholar]
- 35.Zhang W. Comparative analysis of radiotherapy toxicity of conventional fractionation and larger fractionation after breast cancer modified radical surgery[J] Chinese Community Doctors. 2015;31(29):81–83. doi: 10.3969/j.issn.1007-614x.2015.29.52. [DOI] [Google Scholar]
- 36.Zhao SH, Cao XM, Liu GQ, et al. Clinical trial of postmastectomy hypofractionation radiotherapy in breast cancer[J] J Modern Oncol. 2014;22(9):2120–2123. doi: 10.3969/j.issn.1672-4992.2014.09.36. [DOI] [Google Scholar]
- 37.Zhao XB, Ren GS. Analysis of radiotherapy optimization regimens after modified radical mastectomy[J] Eur Rev Med Pharmacol Sci. 2016;20(22):4705–4709. [PubMed] [Google Scholar]
- 38.Ko DH, Norriss A, Harrington CR, et al. Hypofractionated radiation treatment following mastectomy in early breast cancer: the Christchurch experience[J] J Med Imaging Radiat Oncol. 2015;59(2):243–247. doi: 10.1111/1754-9485.12242. [DOI] [PubMed] [Google Scholar]
- 39.Koukourakis MI, Panteliadou M, Abatzoglou IM, et al. Postmastectomy hypofractionated and accelerated radiation therapy with (and without) subcutaneous amifostine cytoprotection[J] Int J Radiat Oncol Biol Phys. 2013;85(1):e7–13. doi: 10.1016/j.ijrobp.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 40.Doré M, Cutuli B, Cellier P, et al. Hypofractionated irradiation in elderly patients with breast cancer after breast conserving surgery and mastectomy: analysis of 205 cases[J] Radiat Oncol. 2015;10(1):161. doi: 10.1186/s13014-015-0448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee S, Arunsingh M, Agrawal S, et al. Outcomes following a moderately Hypofractionated adjuvant radiation (START B type) schedule for breast Cancer in an unscreened non-Caucasian population[J] Clin Oncol. 2016;28(10):e165–e172. doi: 10.1016/j.clon.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Khan AJ, Poppe MM, Goyal S, et al. Hypofractionated postmastectomy radiation therapy is safe and effective: first results from a prospective phase II trial[J] J Clin Oncol. 2017;35(18):2037–2043. doi: 10.1200/JCO.2016.70.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan EK, Woods R, McBride ML, et al. Adjuvant Hypofractionated versus conventional whole breast radiation therapy for early-stage breast Cancer: long-term hospital-related morbidity from cardiac causes[J] Int J Radiat Oncol Biol Phys. 2014;88(4):786–792. doi: 10.1016/j.ijrobp.2013.11.243. [DOI] [PubMed] [Google Scholar]
- 44.Stokes EL, Tyldesley S, Woods R, et al. Effect of nodal irradiation and fraction size on cardiac and cerebrovascular mortality in women with breast Cancer treated with local and Locoregional radiotherapy[J] Int J Radiat Oncol Biol Phys. 2011;80(2):403–409. doi: 10.1016/j.ijrobp.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 45.Marhin W, Wai E, Tyldesley S. Impact of fraction size on cardiac mortality in women treated with tangential radiotherapy for localized breast Cancer[J] Int J Radiat Oncol Biol Phys. 2007;69(2):483–489. doi: 10.1016/j.ijrobp.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 46.Vinh-Hung V, Nguyen NP, Verschraegen C. Hypofractionated nodal irradiation for breast cancer: a case for caution[J] JAMA Oncol. 2019;5(1):13–14. doi: 10.1001/jamaoncol.2018.5061. [DOI] [PubMed] [Google Scholar]
- 47.Haviland JS, Mannino M, Griffin C, et al. Late normal tissue effects in the arm and shoulder following lymphatic radiotherapy: results from the UK START (standardisation of breast radiotherapy) trials[J] Radiother Oncol. 2018;126(1):155–162. doi: 10.1016/j.radonc.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellefqih S, Elmajjaoui S, Aarab J, et al. Hypofractionated regional nodal irradiation for women with node-positive breast Cancer[J] Int J Radiat Oncol Biol Phys. 2017;97(3):563–570. doi: 10.1016/j.ijrobp.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 49.Greenup RA, Camp MS, Taghian AG, et al. Cost comparison of radiation treatment options after lumpectomy for breast cancer[J] Ann Surg Oncol. 2012;19(10):3275–3281. doi: 10.1245/s10434-012-2546-5. [DOI] [PubMed] [Google Scholar]
- 50.Karasawa K, Kunogi H, Hirai T, et al. Comparison of hypofractionated and conventionally fractionated whole-breast irradiation for early breast cancer patients: a single-institute study of 1,098 patients[J] Breast Cancer. 2014;21(4):402–408. doi: 10.1007/s12282-012-0406-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].