Abstract
Background
Clostridioides difficile infection (CDI) is the leading cause of antibiotic-associated and health care–associated diarrhea in humans. Recurrent CDI (R-CDI) occurs in ~20%–30% of patients with CDI and results in increased morbidity, mortality, and hospital costs. Genomic analyses have shown overlap of C. difficile isolates from animals and people, suggesting that a zoonotic reservoir may contribute to recurrence. The objective of this study was to determine whether pet ownership is a risk factor for recurrence of CDI.
Methods
We conducted a case–control study among patients with recurrent CDI (cases; n = 86) and patients with nonrecurrent CDI (controls; n = 146). Multivariable logistic regression modeling was used to determine the association between recurrence of CDI and pet ownership while accounting for patient-level risk factors.
Results
Pet ownership was not significantly associated with recurrence of CDI (odds ratio [OR], 1.02; 95% confidence interval [CI], 0.38–2.72; P = 0.965) among all patients (n = 232). However, among the subset of patients with community-associated or community-onset health care facility–acquired CDI (n = 127), increasing contact with pets was increasingly protective against recurrence: for every point increase in a pet contact score (out of 7 possible points), the odds of recurrence decreased by 14% (OR, 0.86; 95% CI, 0.74–1.00; P = 0.051).
Conclusions
Close interactions with pets appear protective against the recurrence of community-acquired CDI. A potential mechanism may involve beneficial contributions to the microbiota of pet owners afflicted with CDI, as has been observed for other conditions such as atopy, obesity, and food allergies. However, more research is needed to understand the interactions between pets, owners, and their microbiota.
Keywords: Clostridioides difficile, microbiome, pets, recurrence, zoonosis
The recurrence of Clostridioides difficile infection, the leading cause of health-care associated diarrhea, results in increased morbidity, mortality, and hospital costs. Close contact with pets appears to be protective against recurrence in patients with community-acquired or -onset C. difficile infection.
Clostridioides difficile is the leading cause of antibiotic- and health care–associated diarrhea in humans [1]. In recent decades, there has been a marked increase in the incidence and severity of CDI worldwide [2], and community-acquired CDIs are now more common than hospital-acquired infections [3, 4]. Recurrent CDI (R-CDI), defined by the Infectious Diseases Society of America (IDSA) as the presence of diarrhea and a positive C. difficile stool assay within 2–8 weeks of the initial episode, occurs in ~20%–30% of patients with CDI [5, 6]. R-CDI is often poorly responsive to treatment and results in the need for additional medications, longer courses of therapy, and increased medical costs, morbidity, and mortality [7].
Antibiotics are generally considered the primary risk factor for the development and recurrence of CDI. However, patients can develop CDI outside of a health care facility without the prior use of antibiotics, and patients who have experienced CDI can experience recurrence without additional exposure to antibiotics. In 1 study, up to 83% of people who experienced R-CDI were infected with an identical strain during recurrence [8], suggesting that inadequate clearance of the index infection, re-infection from the original source, or re-infection from a related source could be occurring.
Carriage of C. difficile has been documented in many species of domesticated animals, and companion animals were posited as a potential reservoir species as early as 1983 [9]. Overlap between strains of C. difficile isolated from people and animals [10–12] and evidence of potential interspecies clonal transmission events of C. difficile [10, 13] have led to speculation that CDI could be a zoonotic disease. The goal of this study was to investigate whether pet ownership is a risk factor for recurrence of CDI.
METHODS
Subject Enrollment and Outcome Adjudication
All patients seen at the University of Pennsylvania Health System (UPHS) between January 1, 2014, and March 1, 2019, who experienced diarrhea with a positive C. difficile toxin assay by either polymerase chain reaction (PCR; BD Max Cdiff, Becton Dickinson, Cockeysville, MD, USA) before September 26, 2017, Xpert C. difficile (Cepheid, Sunnyvale, CA, USA) thereafter, or immunoassay (Cdiff Quik Chek Complete, TechLab, Blacksburg, VA, USA) were identified as potentially eligible study subjects. The study was approved by the Institutional Review Board of the University of Pennsylvania (protocol 829159).
Cases were patients with recurrent CDI, defined as a patient who had documentation of a subsequent positive C. difficile toxin assay within 56 days of the index CDI diagnosis or index discharge with interim resolution of symptoms [14]. Controls were patients who did not experience a subsequent positive C. difficile toxin assay within 8 weeks of the index CDI diagnosis or index discharge. Only patients who continued to receive care within the UPHS for the year after initial CDI diagnosis (ie, did not die or did not transfer to another health system) were considered eligible. A history of CDI before enrollment was not an exclusion criterion. CDI was defined as health care facility onset (HFO) if the patient developed diarrhea and had a positive C. difficile assay >3 days after admission [15]. Community-onset health care facility–associated (COHFA) CDI was defined as occurring within 28 days of discharge from a health care facility [15]. All other patients were considered to have community-acquired (CA) CDI. Patients were also classified as having either PCR + or enzyme immunoassay (EIA) + CDI, as evidence suggests that the latter group is more likely to be truly infected and the former colonized [16].
Surveys to Ascertain Primary Exposure
A survey instrument was adapted from a previously developed instrument [17] to ascertain the number and species of pets in the household at the time the patient was first diagnosed with CDI, the duration of the pets’ residence in the household, and the degree of contact between patients and pets. A cumulative contact score was derived as follows: The patient is the primary care provider for their pet(s) (feeding, grooming, bathing, medicating, exercising): yes = 2 points, no = 0 [2]; the pet(s) sleep(s) in/on the human participant’s bed: yes = 3 points, no = 0 [3]; the participant allows their pet(s) to lick their face or hands: yes = 4 points; no = 0. People without pets were assigned a contact score of 0.
Potential study subjects were sent a mailing containing an introduction letter with a link to the survey, a paper copy of the survey, a printed visual aid with pictures of antibiotics to enhance recall of previously administered courses of drugs [18], and a postage-paid return envelope. Subjects who did not return the paper survey or complete the online survey within 2 weeks were called a maximum of 2 times to participate over the phone. The control status of the subject was confirmed with a question asking the patient if he/she had been subsequently diagnosed with CDI within the established time frame at a hospital facility not in the UPHS and by verifying the medical record.
Secondary Exposure Ascertainment
The following data related to the index diagnosis of CDI were collected from the medical records: age, sex, race, ethnicity, episodes of CDI 5 years before the index diagnosis, duration of hospitalization of inpatients, comorbidities used to calculate the Charlson comorbidity score or associated with CDI (eg, inflammatory bowel disease), gastric acid suppression or immunosuppression at the time of the index diagnosis, whether the patient was hospitalized within a month before admission or after discharge, and antibiotics prescribed before and during/after the index diagnosis. A Charlson comorbidity score was calculated for each patient.
Statistical Analysis
Descriptive analyses included computation of means with 95% confidence intervals (95% CIs), standard deviations, medians, interquartile ranges (IQRs) of continuous variables, and tabulation of categorical variables. Categorical variables were compared between cases and controls using the chi-square test, and continuous variables were compared using the Student t or Wilcoxon rank-sum test as appropriate. Bivariable analysis was conducted to determine the unadjusted association between potential risk factors (pet ownership, number of pets, and pet contact score) and R-CDI. Variables that represented putative risk factors for recurrence, variables trending to be associated with R-CDI on bivariable analysis (P < 0.15), and variables involved in confounding the association between pet ownership and recurrence of CDI (ie, their inclusion in the model resulted in a >15% change in the effect size of the primary association of interest) were added to the model in a step-wise fashion. Stratified analyses were performed post hoc by infection type (eg, health care facility–onset vs community-acquired/community-onset CDI) and diagnostic assay type (PCR+ vs EIA+). Statistical interactions between key variables were assessed by performing stratified analyses. Model fits were examined using Aikaike Information Criteria. All analyses were conducted with Stata 15 (StataCorp, State College, TX, USA), with 2-sided tests of hypotheses and a P value <0.05 as the criterion for statistical significance.
RESULTS
Survey Response
A total of 750 survey invitations were sent to prospective participants. Of those, 56 were returned unopened to the sender for having an incorrect address. One hundred eleven participants completed the paper or online survey, whereas 120 participated over the phone, for a total of 231 completed surveys (response rate of 31%). The remaining participants either declined to participate (n = 35), died (n = 14), or could not be reached by phone (n = 414). Characteristics of responders and nonresponders are presented in Supplementary Table 1.
Subject Characteristics
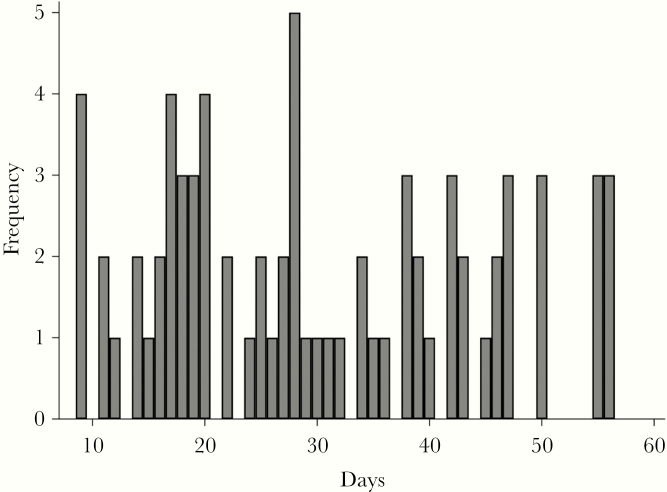
Of the 232 completed surveys, 86 were from cases (ie, people who had experienced recurrent CDI within 56 days) and 146 were from controls (ie, people who did not experience recurrence of CDI). Health care facility–onset cases accounted for 104 cases (45.0%), community-onset health care facility–acquired cases accounted for 27 cases (11.7%), and community-associated accounted for 101 cases (43.3%). The duration between the index diagnosis and recurrence for cases is shown in Figure 1.
Figure 1.
Distribution of the duration between index diagnosis and recurrence for 86 patients who experienced recurrent Clostridioides difficile infection at the University of Pennsylvania Health System (Philadelphia, PA, USA).
Demographic information is shown for all patients and patients with community-onset or community-acquired CDI in Table 1. There were no significant differences in any demographic parameters between cases and controls. Most (80%) of the patients had been administered antimicrobials before developing the index episode of CDI, and almost all (98%) of the patients who experienced CDI were treated with antimicrobials. The only parameters that were significantly associated with being a case for all patients were prior episodes of CDI (P = 0.003) and subsequent additional hospitalization either a month before (P = 0.010) or after (P = 0.001) the index visit. Among patients with community-acquired or community-onset CDI, only the Charlson comorbidity index (P = 0.040) and pet ownership (P = 0.083) were significantly and borderline significantly associated with being a case, respectively.
Table 1.
Demographic and Clinical Characteristics of 232 Patients who Experienced Recurrent Clostridioides difficile Infection (Cases) and Patients who Did Not Experience Recurrent Clostridioides difficile Infection (Controls)
| All Patients (n = 232) | Patients With Community-Acquired or Community-Onset C. difficile Infection (n = 131) | |||||
|---|---|---|---|---|---|---|
| Controls (n = 146) | Cases (n = 86) | P Value | Controls (n = 88) | Cases (n = 43) | P Value | |
| Sex, No. (%) | ||||||
| Female | 93 (63.7) | 47 (54.7) | 0.174 | 63 (71.6) | 26 (67.9) | 0.200 |
| Male | 53 (36.6) | 39 (45.4) | 25 (28.4) | 17 (39.5) | ||
| Mean (SD) age, y | 56.1 (16.1) | 57.8 (16.8) | 0.463 | 53.7 (16.8) | 58.2 (17.6) | 0.164 |
| Race, No. (%) | ||||||
| White | 107 (75.4) | 67 (77.9) | 0.832 | 66 (77.7) | 32 (74.4) | 0.196 |
| Black | 30 (21.1) | 17 (19.8) | 16 (18.8) | 9 (20.9) | ||
| Asian | 5 (3.6) | 2 (2.3) | 3 (3.5) | 2 (4.7) | ||
| Ethnicity, No. (%) | ||||||
| Non-Hispanic | 138 (97.2) | 82 (95.4) | 0.466 | 82 (96.5) | 41 (95.4) | 0.757 |
| Hispanic | 4 (2.8) | 4 (3.5) | 3 (3.5) | 2 (4.7) | ||
| Median (IQR) length of stay among inpatients, d | 9 (3–20) | 8 (4–18) | 0.943 | 3 (1–5) | 4 (3–7) | 0.198 |
| Mean (SD) Charlson comorbidity index score | 4.12 (2.71) | 4.41 (2.74) | 0.414 | 3.3 (2.6) | 4.3 (3.0) | 0.040 |
| Experienced prior episodes of CDI within previous 5 y, No. (%) | 9 (6.2) | 15 (17.4) | 0.003 | 7 (8.0) | 7 (16.3) | 0.148 |
| Inflammatory bowel disease, No. (%) | 22 (15.2) | 15 (17.4) | 0.649 | 20 (22.7) | 11 (25.6) | 0.718 |
| Received antibiotics before CDI diagnosis, No. (%) | 110 (78.0) | 71 (82.6) | 0.409 | 66 (76.7) | 35 (81.4) | 0.546 |
| Gastric acid suppression, No. (%) | 83 (57.2) | 42 (48.8) | 0.215 | 44 (50.0) | 18 (41.9) | 0.381 |
| Immunosuppression, No. (%) | 54 (37.2) | 31 (36.1) | 0.856 | 16 (29.1) | 11 (28.2) | 0.925 |
| Additional hospitalization before index diagnosis/hospitalization, No. (%) | 34 (23.5) | 34 (39.5) | 0.010 | 16 (18.2) | 11 (25.6) | 0.326 |
| Additional hospitalization after index diagnosis/hospitalization, No. (%) | 24 (17.0) | 33 (38.4) | 0.001 | 14 (15.9) | 9 (20.9) | 0.478 |
| Pet ownership, No. (%) | 73 (50.4) | 39 (45.4) | 0.4363 | 51 (58.0) | 18 (41.9) | 0.083 |
Abbreviations: CDI, Clostridioides difficile infection; IQR, interquartile range.
Relationship Between Pet Ownership and CDI Recurrence Risk
A total of 112 (48.3%) respondents were pet owners, which is slightly lower than the national average of 57% [19]. Among the 112 pet owners, 56 (50.0%) owned dogs only, 26 (23.2%) owned cats only, 23 (20.5%) owned dogs and cats, and 6 (2.3%) owned a combination of cats, dogs, and other species (including birds, rabbits, and hamsters). The mean (SD) contact score among pet owners was 4.3 (2.2) points, out of a possible total of 7 (non–pet owners had a score of 0).
Univariable analysis showed no significant association between pet ownership and recurrence of CDI (odds ratio [OR], 0.83; 95% CI, 0.49–1.42) (Table 2), regardless of whether patients had EIA+ or PCR+ toxin tests (OR, 0.70; 95% CI, 0.30–1.62; vs OR, 0.92; 95% CI, 0.45–1.86) (Supplementary Table 1). A modest but nonsignificant dose–response association between degree of contact between pets and protection against recurrence of CDI was observed: For every point increase in the contact score, the odds of experiencing recurrence decreased by 3.5% (OR, 0.96; 95% CI, 0.87–1.07). The total number of pets within a household was not associated with recurrence (OR, 1.03; 95% CI, 0.90–1.18).
Table 2.
Association Between Pet Ownership, Pet Contact, Patient Factors, and Recurrence of Clostridioides difficile Infection (n = 232)
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value |
| Pet ownership | 0.81 | 0.47–1.38 | 0.432 | 1.02 | 0.38–2.72 | 0.965 |
| Pet contact score (0–7 points) | 0.96 | 0.87–1.06 | 0.435 | 0.98 | 0.81–1.18 | 0.802 |
| Prior CDI | 3.22 | 1.34–7.71 | 0.009 | 2.97 | 1.20–7.37 | 0.019 |
| Additional hospitalization 1 mo before index diagnosis | 2.15 | 1.21–3.84 | 0.009 | 2.03 | 1.02–3.48 | 0.042 |
| Additional hospitalization 1 mo after index diagnosis/discharge | 2.62 | 1.44–4.78 | 0.002 | 2.30 | 1.23–4.31 | 0.009 |
Abbreviations: CDI, Clostridioides difficile infection; CI, confidence interval.
Multivariable analysis controlling for previous CDI and additional hospitalization (1 month before or after the index visit) showed no significant association between pet ownership and recurrence risk (OR, 0.93; P = 0.53–1.63) (Table 2).
Stratification by Type of Infection
We then conducted stratified analyses post hoc based on whether the CDI was HFA or CA/CO-HFA. In contrast to the hypothesized relationship, we found that pet ownership and the pet contact score were borderline significantly and significantly protective, respectively, against recurrence among patients with CA or CO-HFA CDI (OR for pet ownership, 0.54; 95% CI, 0.23–1.12; and OR for contact score, 0.84; 95% CI, 0.72–0.97) (Table 3). These relationships persisted in analyses restricted to patients with either an EIA+ or PCR+ diagnosis of CDI (Supplementary Table 2). When adjusting for the Charlson comorbidity index (Table 3), pet ownership remained protective against recurrence, though the effect was no longer statistically significant (OR, 0.60; 95% CI, 0.27–1.24), and the contact score was borderline significantly protective against recurrence (OR, 0.86; 95% CI, 0.74–1.00). The distribution of cases, controls, and pet ownership among patients with or without prior CDI is shown in Supplementary Table 3.
Table 3.
Association Between Pet Ownership, Pet Contact, Patient Factors, and Recurrence of Clostridioides difficile Infection Among Patients With Community-Onset or Community-Acquired C. difficile Infection (n = 131)
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value |
| Pet ownership | 0.52 | 0.25–1.09 | 0.085 | 0.58 | 0.27–1.24 | 0.163 |
| Pet contact score (0–7 points) | 0.84 | 0.72–0.97 | 0.022 | 0.86 | 0.74–1.00 | 0.051 |
| Charlson comorbidity index | 1.15 | 1.00–1.32 | 0.040 | 1.12 | 0.976–1.29 | 0.105 |
Abbreviation: CI, confidence interval.
DISCUSSION
Consistent with other studies, we found that prior episodes of CDI [20], hospitalization both before or after the index diagnosis [21], and increasing Charlson score [22] were risk factors for recurrence. Somewhat unexpectedly, though, we found that pet ownership and increasing contact with pets were not associated with recurrence of CDI, but instead were protective against recurrence among patients with CA or CO-HFA CDI.
Pets are recognized reservoirs of bacterial pathogens that can be transferred to owners via the fecal–oral pathway [23], and healthy dogs and cats have been shown to shed C. difficile at rates of 3.4%–5.5% and 2.5%–3.4%, respectively [24, 25]. Pet owners frequently engage in activities that could facilitate such a transfer (eg, close contact, allowing pet to lick hands and face, picking up fecal matter, sleeping with pet), whereas pets engage in behavior that could allow them to become colonized with pathogens (eg, coprophagia, drinking from contaminated places such as toilets, close contact with the ground, licking paws, etc.). Although no studies have demonstrated the direct transmission of C. difficile between pets and their owners, 1 study found that 2/9 (22%) cats and 2/5 (40%) dogs belonging to owners who had experienced CDI carried C. difficile with identical PFGE profiles as their owners [26], and other studies have presented varying degrees of evidence suggesting that dogs could represent a reservoir of C. difficile [27, 28]. Less work has been done in cats, but cats have also been found to be colonized with C. difficile. We therefore originally hypothesized that a patient who had experienced CDI could transmit C. difficile to a pet, which could then act as a reservoir for recurrence of C. difficile in the index patient or colonization of people who did not carry the pathogen. As with other diseases such as methicillin-resistant Staphylococcus aureus [17], we supposed that asymptomatic pet carriers could represent a major source of infection and/or cyclical re-infection. The opposite finding—that pet ownership was protective against recurrence—was unexpected. Interestingly, the protective effect of pet ownership was stronger among EIA+ patients, who are more likely to be truly infected [16], than among patients with PCR+ assays, who are more likely to be colonized. This suggests that the protective effects of pet ownership and pet contact are real and not due to confounding by colonization (and therefore less severe illness).
The effects of certain risk factors for CDI and RCDI are likely mediated by their disruptive effect on the gut microbiota [29]. Disruption of the gut microbiota results in reduced microbial diversity, development of opportunistic species, and loss of resistance to colonization [29, 30]. Pets have been shown to significantly alter the microbial communities of household environments [31, 32], which, in turn, can alter the gut microbiota of people living in the household [33]. For example, exposure to pets can alter the gut microbiota of infants in ways that are beneficial in preventing childhood atopy, obesity, asthma, and food allergies [34–36]. Dog ownership can also significantly increase the shared skin microbiota in cohabiting adults [37], suggesting that pets transfer microbiota to their owners. As such, pets may provide microbiota that can increase resistance against the recurrence of CDI. In support, increasing contact between patients and pets, as quantified by the pet contact score, was significantly associated with protection against recurrence. However, more research is needed to verify this hypothesis.
It is unclear why the protective effect of pet ownership was only significant among patients with CA or CO-HFA CDI. These patients were less likely to have experienced additional hospitalizations before or after their index diagnosis and therefore may have had increased exposure to their pets following their index diagnosis. Additionally, because patients with community-acquired CDI may be more prone to recurrence than patients with health care facility–onset CDI [38], it is possible that protective factors such as pet ownership have a more pronounced effect among these patients than among patients with health care facility–onset CDI.
This study had several limitations. First, because this was a retrospective study and because CDI was diagnosed using molecular methods rather than culture, we were unable to identify the strain of C. difficile. Certain strains, most notably Ribotype 027, are associated with recurrence [39], and it is possible that the strain with which patients were infected could better explain the risk of recurrence than pet ownership. Second, because some of the index diagnoses occurred several years ago, patients may have had limited recall of the circumstances surrounding their diagnosis. However, we do not believe that pet ownership is something that is easily forgotten, even if it occurred several years ago. Third, patients may have had varying durations of antibiotic therapy following diagnosis of CDI, which could have impacted the likelihood of recurrence. Although no difference in the rate of recurrence has been found for patients treated with metronidazole vs vancomycin [40], little is known about the effect of therapy duration on recurrence [41], and guidelines for the treatment of CDI propose ranges of durations for antibiotic therapy [42]. Fourth, the results of the study may not be generalizable because of the relatively small study size, the low response rate (31%), and the fact that it was conducted at a single institution. Moreover, because nonresponders were significantly different from responders in several respects (Supplementary Table 2), the results may be subject to bias. Specifically, because responders were significantly more likely to have experienced prior episodes of CDI, recurrence of CDI, and gastric acid suppression, they may have been more invested in contributing to research aimed at better understanding the epidemiology of their condition. Finally, cases with short durations between the index diagnosis and the subsequent positive test may have been experiencing relapse rather than recurrence [8], which are 2 different biological phenomena and could have different contributing risk factors. However, to mitigate this possibility, the UPHS only allows the submission of repeat C. difficile assays after 9 days have elapsed since the first assay. Future studies should aim to examine the relationship between pet ownership and recurrence of CDI prospectively and more closely assess the gut microbial ecology of pet owners with and without pets.
CONCLUSIONS
Although more research is needed to understand the transmission dynamics of C. difficile within a household and between pets and owners, it appears that pet ownership and close contact with pets are not associated with recurrence of CDI and may instead be protective against recurrence of CDI in patients with community-acquired or community-onset CDI.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. L.E.R.: study design, data collection, formal analysis, investigation, manuscript preparation, and editing. B.J.K.: study design, formal analysis, manuscript preparation, and editing. D.S.: formal analysis. J.K.L.: study design, data collection. P.T.: study design, data collection. L.C.: data collection. E.G.: data collection. P.M.: data collection. E.B.: study design, formal analysis, manuscript preparation and editing.
Availability of data. Data are available upon request.
Financial support. This work was supported by funding from the Veterinary School at the University of Pennsylvania. B.J.K. is supported by NIH K23 AI121485.
Potential conflicts of interest. None of the authors have any conflicts of interest to report. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:2369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuijper EJ, Coignard B, Tüll P; ESCMID Study Group for Clostridium difficile; EU Member States; European Centre for Disease Prevention and Control Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect 2006; 12(Suppl 6):2–18. [DOI] [PubMed] [Google Scholar]
- 3. Khanna S, Baddour LM, Huskins WC, et al. The epidemiology of Clostridium difficile infection in children: a population-based study. Clin Infect Dis 2013; 56:1401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gould CV, File TM Jr, McDonald LC. Causes, burden, and prevention of Clostridium difficile infection. Infect Dis Clin Pract 2015; 23:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maroo S, Lamont JT. Recurrent Clostridium difficile. Gastroenterology 2006; 130:1311–6. [DOI] [PubMed] [Google Scholar]
- 6. Khanna S, Pardi DS, Aronson SL, et al. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. Am J Gastroenterol 2012; 107:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olsen MA, Yan Y, Reske KA, et al. Recurrent Clostridium difficile infection is associated with increased mortality. Clin Microbiol Infect 2015; 21:164–70. [DOI] [PubMed] [Google Scholar]
- 8. Figueroa I, Johnson S, Sambol SP, et al. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Infect Dis 2012; 55(Suppl 2):S104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borriello SP, Honour P, Turner T, Barclay F. Household pets as a potential reservoir for Clostridium difficile infection. J Clin Pathol 1983; 36:84–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knetsch CW, Connor TR, Mutreja A, et al. Whole genome sequencing reveals potential spread of Clostridium difficile between humans and farm animals in the Netherlands, 2002 to 2011. Euro Surveill 2014; 19:20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keessen EC, Harmanus C, Dohmen W, et al. Clostridium difficile infection associated with pig farms. Emerg Infect Dis 2013; 19:1032–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakker D, Corver J, Harmanus C, et al. Relatedness of human and animal Clostridium difficile PCR ribotype 078 isolates determined on the basis of multilocus variable-number tandem-repeat analysis and tetracycline resistance. J Clin Microbiol 2010; 48:3744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knight DR, Kullin B, Androga GO, et al. Evolutionary and genomic insights into Clostridioides difficile sequence type 11: a diverse zoonotic and antimicrobial-resistant lineage of global one health importance. mBio 2019; 10(2):e00446-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDonald LC, Coignard B, Dubberke E, et al. ; Ad Hoc Clostridium difficile Surveillance Working Group Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol 2007; 28:140–5. [DOI] [PubMed] [Google Scholar]
- 15. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morris DO, Lautenbach E, Zaoutis T, et al. Potential for pet animals to harbour methicillin-resistant Staphylococcus aureus when residing with human MRSA patients. Zoonoses Public Health 2012; 59:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kimmel SE, Lewis JD, Jaskowiak J, et al. Enhancement of medication recall using medication pictures and lists in telephone interviews. Pharmacoepidemiol Drug Saf 2003; 12:1–8. [DOI] [PubMed] [Google Scholar]
- 19. American Veterinary Medical Association. AVMA Pet Ownership and Demographics Sourcebook: 2017–2018 Edition. Schaumburg, IL: American Veterinary Medical Association; 2018. [Google Scholar]
- 20. McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 2002; 97:1769–75. [DOI] [PubMed] [Google Scholar]
- 21. Pépin J, Routhier S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis 2006; 42:758–64. [DOI] [PubMed] [Google Scholar]
- 22. Hardt C, Berns T, Treder W, Dumoulin FL. Univariate and multivariate analysis of risk factors for severe Clostridium difficile-associated diarrhoea: importance of co-morbidity and serum C-reactive protein. World J Gastroenterol 2008; 14:4338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Damborg P, Broens EM, Chomel BB, et al. Bacterial zoonoses transmitted by household pets: state-of-the-art and future perspectives for targeted research and policy actions. J Comp Pathol 2016; 155:S27–40. [DOI] [PubMed] [Google Scholar]
- 24. Schneeberg A, Rupnik M, Neubauer H, Seyboldt C. Prevalence and distribution of Clostridium difficile PCR ribotypes in cats and dogs from animal shelters in Thuringia, Germany. Anaerobe 2012; 18:484–8. [DOI] [PubMed] [Google Scholar]
- 25. Rabold D, Espelage W, Abu Sin M, et al. The zoonotic potential of Clostridium difficile from small companion animals and their owners. PLoS One 2018; 13:e0193411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loo VG, Brassard P, Miller MA. Household transmission of Clostridium difficile to family members and domestic pets. Infect Control Hosp Epidemiol 2016; 37:1342–8. [DOI] [PubMed] [Google Scholar]
- 27. Janezic S, Mlakar S, Rupnik M. Dissemination of Clostridium difficile spores between environment and households: dog paws and shoes. Zoonoses Public Health 2018; 65:669–74. [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez C, Taminiau B, Bouchafa L, et al. Clostridium difficile beyond stools: dog nasal discharge as a possible new vector of bacterial transmission. Heliyon 2019; 5:e01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zanella Terrier MC, Simonet ML, Bichard P, Frossard JL. Recurrent Clostridium difficile infections: the importance of the intestinal microbiota. World J Gastroenterol 2014; 20:7416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–87. [DOI] [PubMed] [Google Scholar]
- 31. Maier RM, Palmer MW, Andersen GL, et al. Environmental determinants of and impact on childhood asthma by the bacterial community in household dust. Appl Environ Microbiol 2010; 76:2663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fujimura KE, Johnson CC, Ownby DR, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol 2010; 126:410–2, 412.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Konya T, Koster B, Maughan H, et al. ; CHILD Study Investigators. Associations between bacterial communities of house dust and infant gut. Environ Res 2014; 131:25–30. [DOI] [PubMed] [Google Scholar]
- 34. Tun HM, Konya T, Takaro TK, et al. ; CHILD Study Investigators. Exposure to household furry pets influences the gut microbiota of infant at 3-4 months following various birth scenarios. Microbiome 2017; 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marrs T, Logan K, Craven J, et al. ; EAT Study Team. Dog ownership at three months of age is associated with protection against food allergy. Allergy 2019; 74:2212–9. [DOI] [PubMed] [Google Scholar]
- 36. Nermes M, Niinivirta K, Nylund L, et al. Perinatal pet exposure, faecal microbiota, and wheezy bronchitis: is there a connection? ISRN Allergy 2013; 2013:827934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife 2013; 2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001-2010. Am J Infect Control 2014; 42:1028–32. [DOI] [PubMed] [Google Scholar]
- 39. Jazmati N, Hain O, Hellmich M, et al. PCR based detection of tcdCΔ117 in Clostridium difficile infection identifies patients at risk for recurrence - a hospital-based prospective observational study. Anaerobe 2019; 57:39–44. [DOI] [PubMed] [Google Scholar]
- 40. Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative effectiveness of vancomycin and metronidazole for the prevention of recurrence and death in patients with Clostridium difficile infection. JAMA Intern Med 2017; 177:546–53. [DOI] [PubMed] [Google Scholar]
- 41. Petrosillo N. Tackling the recurrence of Clostridium difficile infection. Med Mal Infect 2018; 48:18–22. [DOI] [PubMed] [Google Scholar]
- 42. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.