Significance
Understanding social influences on how apes acquire tool behaviors can help us model the evolution of culture and technology in humans. Humans scaffold novice tool skills with diverse strategies, including the transfer of tools between individuals. Chimpanzees transfer tools, and this behavior meets criteria for teaching. However, it is unclear how task complexity relates to this form of helping. Here, we find differences between 2 wild chimpanzee populations in rate, probability, and types of tool transfer during termite gathering. Chimpanzees showed greater helping in the population where termite gathering is a more complex tool task. In wild chimpanzees, as in humans, regular and active provisioning of learning opportunities may be essential to the cultural transmission of complex skills.
Keywords: tool use, cumulative culture, social learning, chimpanzee, prosociality
Abstract
Cumulative culture is a transformative force in human evolution, but the social underpinnings of this capacity are debated. Identifying social influences on how chimpanzees acquire tool tasks of differing complexity may help illuminate the evolutionary origins of technology in our own lineage. Humans routinely transfer tools to novices to scaffold their skill development. While tool transfers occur in wild chimpanzees and fulfill criteria for teaching, it is unknown whether this form of helping varies between populations and across tasks. Applying standardized methods, we compared tool transfers during termite gathering by chimpanzees in the Goualougo Triangle, Republic of Congo, and in Gombe, Tanzania. At Goualougo, chimpanzees use multiple, different tool types sequentially, choose specific raw materials, and perform modifications that improve tool efficiency, which could make it challenging for novices to manufacture suitable tools. Termite gathering at Gombe involves a single tool type, fishing probes, which can be manufactured from various materials. Multiple measures indicated population differences in tool-transfer behavior. The rate of transfers and probability of transfer upon request were significantly higher at Goualougo, while resistance to transfers was significantly higher at Gombe. Active transfers of tools in which possessors moved to facilitate possession change upon request occurred only at Goualougo, where they were the most common transfer type. At Gombe, tool requests were typically refused. We suggest that these population differences in tool-transfer behavior may relate to task complexity and that active helping plays an enhanced role in the cultural transmission of complex technology in wild apes.
The emergence of cumulative technology is a defining aspect of human evolution. Identifying the factors that facilitate the transfer of complex skills in humans and other animals is essential for understanding the pedagogical settings that may have accompanied the inception of hominin tool technologies (1, 2). More broadly, this endeavor is necessary for understanding what underpins cultural behavior (3, 4). Teaching and imitation, deemed “high-fidelity social learning mechanisms,” are hypothesized to play a critical role in the emergence of cumulative culture, as these mechanisms may ensure the faithful replication of complex behaviors between individuals and support the retention of progressive innovations (5). Studies of chimpanzee tool use can help us better model the tool behavior of our last common ancestor, as well as the social influences that may support the accumulation of technological complexity. All studied chimpanzee populations use tools, and populations vary in the complexity of tool-assisted foraging behaviors (6–8). This provides an opportunity to examine whether there is an enhanced role for social learning, including deployment of high-fidelity social learning mechanisms, in populations where tool behaviors are more complex.
Social learning of tool use and other behaviors in nonhuman primates largely comprise processes by which a novice learns from a conspecific who does not actively facilitate the novice’s learning (9). For example, stimulus and local enhancement may occur when novices learn independently after their attention is drawn to a conspecific’s tool or tool site. Reuse of others’ tools may further aid learning, and, developmentally, tool reuse precedes the independent manufacture of tools for immature chimpanzees, capuchins, and New Caledonian crows (10). Emulation and imitation learning may occur when novices have the opportunity to observe skilled tool-using models (9). Among chimpanzees, close association between mothers and offspring over many years facilitates “education by master-apprenticeship” (11), involving repeated opportunity for observation in close proximity. At Gombe, Tanzania, the amount of time spent watching mothers termite fish is a significant predictor of the age at which infants learn this tool skill. Females watch more, acquire the skill earlier, and imitate their mothers’ technique (12). At Bossou, Guinea, offspring with more opportunity to observe ant dipping acquire this skill at younger ages (13).
Skilled tool users may sometimes more actively scaffold (sensu ref. 14) the development of novices’ tool skills. At Taï Forest, Côte d’Ivoire, mothers were observed actively intervening to assist offspring during nut-cracking, a complex task for which full mastery is not reached until adulthood (15, 16). Offspring are also allowed to use their mothers’ hammers, which positively impacts immatures’ nut-cracking performance (14). In the Goualougo Triangle, Republic of Congo, transfers of termite-fishing probes from skilled to less-competent conspecifics satisfy functional criteria for teaching (17): transfers occur in the presence of a learner; they impose a cost on tool donors, in the form of reduced tool use and feeding; and they provide a benefit to tool recipients, who show increased tool use and feeding after receiving a tool. Further, mothers deploy strategies that mitigate the cost of transfers, indicating they are sensitive to and may even anticipate offspring need for a tool (18). This type of active, costly facilitation is predicted to occur when it would otherwise be difficult for a learner to acquire information or skills (19). In humans, the provisioning of tools is a common way of scaffolding the development of technological skills in novices, who may spend years learning to independently manufacture complex tools (20).
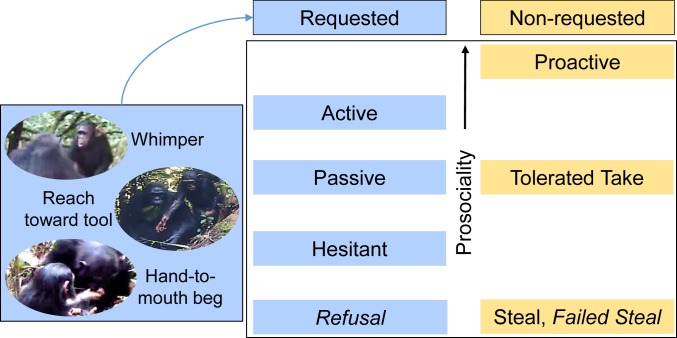
The transfer of objects, including tools, is a principal means for investigating instrumental helping, a form of prosociality by which one individual helps another achieve an action goal (21). Prosocial behaviors are those performed by one individual to benefit another, while costly behaviors that occur between nonkin may further be considered altruistic (22). Object transfer can involve varying degrees of prosociality (Fig. 1). Chimpanzees and capuchins transfer objects upon request in experiments (21, 23), and chimpanzees will even transfer the specific tool a conspecific requires, indicating that they can understand others’ goals (24, 25). Such requested transfers (termed “reactive” in ref. 26) are a more precise index of prosocial response compared to nonrequested transfers in which an object possessor simply tolerates another’s action (26, 27). The exception is proactive object transfers, which are the most prosocial in that they are initiated by the possessor rather than the recipient. These are rare outside of humans but have been observed in captive chimpanzees (e.g., ref. 24). Greater prosociality is also inferred when helping occurs after shorter latencies. For example, among chimpanzees, individuals who help more also help more quickly (28). Prosociality is not inferred when an object is stolen or a possessor refuses the transfer of an object (Fig. 1).
Fig. 1.
Categorization of transfer types according to the level of prosociality. Transfer types are arranged vertically from most (Top) to least (Bottom) prosocial. Transfers are grouped into 2 categories: requested (blue) in which the potential recipient first requests the tool by whimpering and/or reaching toward the tool, or by making hand-to-mouth gestures; and nonrequested (yellow) in which the recipient receives, takes, or attempts to take the tool without first requesting it. While requested and nonrequested transfer types are presented together, note that Active, Passive, and Hesitant requested transfers may more clearly index prosocial behavior. Requests make the potential recipient’s goal more salient, and they inherently involve a possessor physically relinquishing a tool, while nonrequested transfers are more ambiguous (23). The exception is Proactive transfers, which are the most prosocial because they are initiated by the possessor rather than the recipient. Refusals, and Steal/Failed Steal transfers, are ranked comparably because for each of these, the possessor does not, or does not willingly, relinquish a tool; thus, these are not considered prosocial. Italics indicate that no possession change occurs.
In an experiment with human children, success at solving tasks of increasing difficulty varied with the number of prosocial acts received, suggesting that prosocial helping could facilitate social transmission of complex tasks (2). Chimpanzees have been observed transferring tools in numerous contexts in the wild (11, 15, 14, 18, 29–31). However, no standardized comparisons have been conducted to evaluate whether and how this form of scaffolding varies between populations and across tasks of differing complexity. Such a comparison could help illuminate to what extent the accumulation of technological complexity is linked to variation in cultural transmission and prosocial helping.
Termite gathering is an ideal task for such a comparison because it occurs in multiple chimpanzee populations, varying in tool techniques and characteristics as well as task complexity (32–34). This variation is exemplified by chimpanzee populations in the Goualougo Triangle, Republic of Congo, and in Gombe, Tanzania. Both populations exhibit a minimum of 22 different types of tool use, some of the largest tool repertoires of any nonhuman tool user (8). Tool use and manufacture are more complex in the termite-gathering context at Goualougo compared to Gombe. At Goualougo, as observed elsewhere in the Congo Basin, chimpanzees gather invertebrate resources with tool sets (32), which involve the sequential use of 2 or more different tools (35). In contrast, single tools are used by chimpanzees at Gombe, as has been observed in other populations in East and West Africa (32). There are also regional differences in tool selection and manufacture. In the Goualougo Triangle, chimpanzees manufacture fishing probes and puncturing sticks from selected raw plant materials (8). They also intentionally modify herb probes to fashion brush tips, a design feature that is more efficient than an unmodified probe for gathering insects (36). At Gombe, individuals use one tool type, fishing probes, to acquire termites, and probes are manufactured from various materials, such as twigs, bark, grass, or vine (34, 37). Further, there are differences in the timing and sequence of skill acquisition by immature chimpanzees. At Gombe, all individuals have acquired the termite-gathering skill by an age of 5.5 y, and chimpanzees learn to make tools before or around the same time they learn to fish (12). At Goualougo, the acquisition of tool skills extends into subadulthood, and youngsters learn to make tools only after they have already become adept termite fishers.
We compared tool-transfer behavior during termite gathering between Goualougo and Gombe chimpanzees. In selecting these 2 populations, we held constant several factors that are deemed important proximate regulators of helping behavior (23, 26): intrinsic motivation and physical capabilities (same species), social distance between individuals (at both sites, transfers occurred principally between mothers and offspring), proximity to food (the tool task involves extraction of embedded Macrotermes termites), and opportunity for a potential recipient to signal their need by making a direct request (both tasks occur in terrestrial contexts, and chimpanzees in both populations can make gestural and vocal requests in close proximity). A key difference between populations was the complexity of the termite-gathering task. Tool use among chimpanzees in the Congo Basin comprises some of the best evidence for cumulative technology in the animal kingdom (8, 36, 38). Specifically, given the requirements of tool manufacture at Goualougo, we hypothesized that there would be greater need and benefit associated with transferring tools to youngsters during termite gathering in this population relative to termite fishing at Gombe.
To test this hypothesis, we first compared the rate of tool transfers, predicting that there would be a higher overall rate of tool transfer at Goualougo compared to Gombe. In addition, we compared the degree to which tool transfers reflected a prosocial response. We predicted that at Goualougo compared to Gombe, requests or attempts to take tools would more often result in a change of possession. We expected that this effect would be strongest for transfers involving a request and that such requested transfers would happen with shorter latencies at Goualougo. Finally, we predicted that rates of resistance by tool possessors would be lower at Goualougo compared to Gombe. To facilitate comparison, we also categorized transfers into types (Fig. 1) according to presence of request, the possessor’s reaction, and whether the tool changed possession (Table 1 and Movies S1–S9).
Table 1.
Definition of transfer types as well as counts and percentages of fishing-probe transfer types for each population
| Transfer type* | Definition | Goualougo (n = 110) | Gombe (n = 106) | |||
| n | % | n | % | |||
| Preceded by request | ||||||
| Active | Possessor moves to facilitate transfer or divides tool so recipient can take a portion† (U, P) | 22 | 20 | 0 | 0 | |
| Passive | Possessor allows recipient to take tool without showing either facilitation or hesitation‡ (U, P) | 10 | 9.1 | 2 | 1.9 | |
| Hesitant | Recipient begs, then grasps tool; possessor transfers tool only after delaying or resisting the transfer (U, P, S§) | 12 | 10.9 | 5 | 4.7 | |
| Refusal | Possessor does not transfer tool despite begging; possessor may actively resist transfer (e.g., pull away) (U, P) | 14 | 12.7 | 40 | 37.7 | |
| Possession change | Tool changes possession after a beg, but possessor’s specific reaction is not visible (U, P) | 4 | 3.6 | 0 | 0 | |
| Unknown possession change | Possession change cannot be discerned, and possessor’s specific reaction is not visible (U, P) | 1 | 0.9 | 0 | 0 | |
| Total number of requests | 63 | 57.3 | 47 | 44.3 | ||
| Not preceded by request | ||||||
| Proactive | Possessor initiates transfer; tool changes possession (U, P) | 0 | 0 | 0 | 0 | |
| Tolerated take | Possessor allows recipient to take tool; possessor shows neither facilitation nor hesitation¶ (U, P, S) | 15 | 13.6 | 26 | 24.5 | |
| Steal | Recipient takes tool from possessor, who reacts negatively (e.g., attempts to keep tool or threatens stealer#,||) (U, P, S) | 8 | 7.3 | 8 | 7.5 | |
| Failed steal | Recipient tries unsuccessfully to take possessor’s tool; possessor exhibits a negative reaction, as in “steal” (U, P, S) | 16** | 14.5 | 15 | 14.2 | |
| Failed attempt | Recipient tries unsuccessfully to take possessor’s tool; possessor does not react (U, P, S) | 8 | 7.3 | 10 | 9.4 | |
| Total number of take attempts | 47 | 42.7 | 59 | 55.7 | ||
n, number of transfers.
Transfer types were categorized according to whether or not they were preceded by a request, whether a possession change occurred, whether the tool possessor protested the transfer, and whether at the time of transfer the tool was in use (U), physical possession (P), or spatial possession (S). The table excludes 2 transfers for which it could not be discerned whether or not there was a request.
Sensu “active-passive” and “active” transfer (29).
Sensu “passive” transfer (29).
Transfers could be classified as a Hesitant transfer if a tool was in the possessor’s spatial possession at the time of possession change only if the tool was initially in use or physical possession. For example, a Hesitant transfer was coded if there was a request after which the possessor dropped the tool on the ground, and the recipient took possession.
If the tool was in use or in physical possession, this is equivalent to “passive” if there is no begging; if the tool was in spatial possession, this is equivalent to “recovery” (29).
Adapted from ref. 46.
Includes one transfer that occurred in a play context.
Results
Tool Transfers.
Transfer rate.
We detected a significant difference between populations in the rate of tool transfers (transfers/hour: Mann–Whitney U test: U = 27, P = 0.021). The probability that the tool-transfer rate for a recipient at Goualougo would be larger than for a recipient at Gombe was 0.79 (95% confidence interval = 0.55 to 0.95). The transfer rate for immature chimpanzees was an average of 3.4 transfers/hour at Goualougo (14 individuals, n = 45 transfers) and 1.1 transfers/hour at Gombe (9 individuals, n = 33 transfers). Individual transfer rates at Goualougo ranged from 0 to 7.4/h and at Gombe from 0 to 5.2/h. At both sites, there were several immatures who experienced multiple transfers on the same day (Goualougo, 6/14 individuals; Gombe, 3/9 individuals).
Possession change of fishing probes.
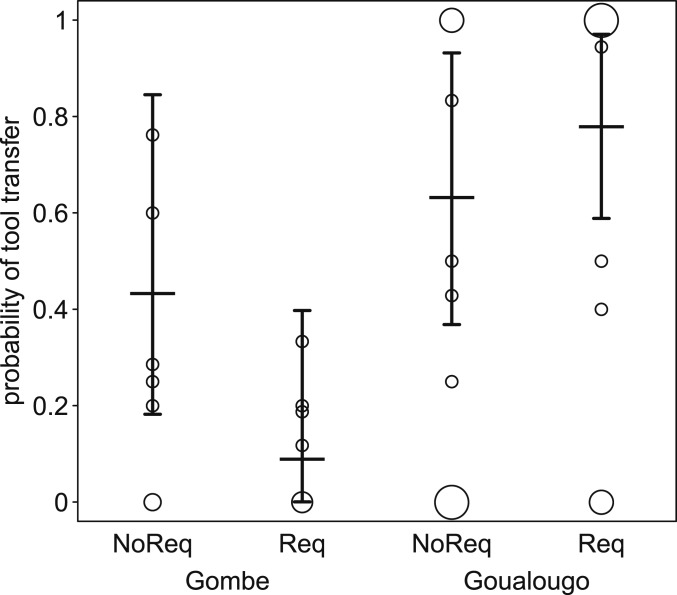
Tool-transfer probability clearly differed between the 2 populations (full-null model comparison: χ2 = 16.195, degrees of freedom [df] = 2, P < 0.001), whereby we found a significant interaction between population and request status (χ2 = 9.687, df = 1, P = 0.002). In fact, while the probability of a transfer was similar at Gombe and Goualougo when the tool was not requested, the probability of a transfer after a request was considerably higher at Goualougo as compared to Gombe (SI Appendix, Table S1 and Fig. 2). We also detected significant effects of the 2 control predictors: recipient age (1.915 ± 1.144, χ2 = 7.260, df = 2, P = 0.027) and sex (−1.489 ± 0.746, χ2 = 4.064, df = 1, P = 0.044), whereby the probability of a transfer was higher in the 5- to 10-y age class relative to the 0- to 5-y age class, and also higher for females. Within both populations, the majority (71% at Gombe and 82% at Goualougo) of requests or attempts to take tools involved mothers and offspring.
Fig. 2.
Tool-transfer probability and how it depended on tool request status and population. Indicated are the fitted model and its confidence limits (horizontal lines with error bars) and the observed transfer probabilities per possessor. The area of the symbols depicts the number of possessors per population and request status with the same transfer probability, such that larger symbols correspond to a greater number of possessors at that value (range: 1 to 8). NoReq, no request; Req, request.
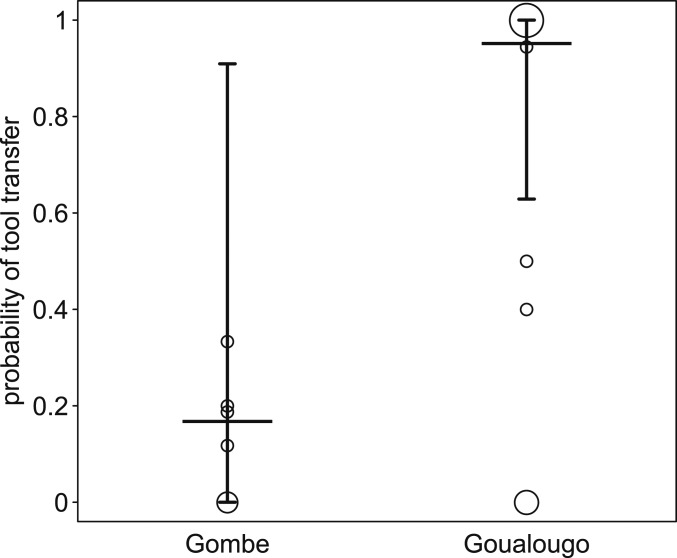
The requested tool-transfer model, including only the subset of transfer events preceded by request, also revealed a clear difference between populations, with a higher probability of transfer following a request at Goualougo compared to Gombe (full-null model comparison: χ2 = 7.400, df = 1, P = 0.007; SI Appendix, Table S2 and Fig. 3).
Fig. 3.
Probability of requested tool transfer and how it differed between populations. Indicated are the fitted model and its confidence limits (horizontal lines with error bars) and the observed transfer probabilities per possessor. The area of the symbols depicts the number of possessors per population with the same transfer probability, such that larger symbols correspond to a greater number of possessors at that value (range: 1 to 8).
Fishing-probe transfer-event types.
With respect to types of tool-transfer events, 63/110 (57.3%) at Goualougo and 47/106 (44.3%) at Gombe were preceded by a request. At Goualougo, 48/63 of these requests (76.2%) resulted in a change of tool possession, compared to 7/47 requests (14.9%) at Gombe. The most common type of requested transfer at Goualougo was Active (n = 22, 19.6%), and this was also the most frequently observed transfer type at Goualougo overall (Table 1). No Active transfer events occurred at Gombe. In contrast, the most numerous type was Refusal (n = 40, 85%), consisting of a request followed by the possessor’s refusal to transfer the tool. We did not observe any Proactive transfers.
In both populations, immature chimpanzees also attempted to take tools without first requesting them. At Goualougo, novices were sometimes permitted to take tools without a reaction (Tolerated Take, n = 15); novices also stole (Steal, n = 8) or attempted to steal (Failed Steal, n = 16) tools. At Gombe, chimpanzees were also permitted to take tools without a reaction (Tolerated Take, n = 26), and, as at Goualougo, novices occasionally also stole (Steal, n = 8) or attempted to steal (Failed Steal, n = 15) tools.
Request Behavior and Latency to Transfer.
Request behavior could be assessed in detail for 31 transfers at Goualougo and 42 at Gombe. Also at Goualougo, requesting behavior most often involved a combination of reaching and whimpering together (n = 17 transfers), followed by just reaching (n = 10 transfers), or occasionally just whimpering (n = 5 transfers). At Gombe, reaching (n = 22 transfers) and reaching and whimpering (n = 17) were observed, while only whimpering was not. At Gombe but not Goualougo, hand-to-mouth gestures were observed (n = 3 transfers), twice together with reaches toward the tool and once in conjunction with whimpering.
At Goualougo, the mean latency in seconds between an immature chimpanzee requesting a tool and a possessor relinquishing it was 11 s (SD = 7, n = 38 transfers). At Gombe, the mean latency to tool transfer was 15.8 s (SD = 18.3, n = 7 transfers).
Resistance.
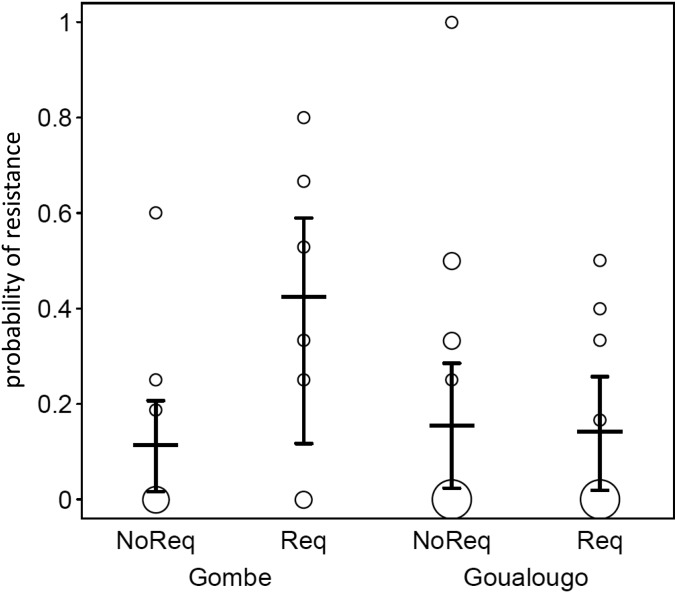
The probability of resistance differed between populations (full-null model comparison: χ2 = 7.211, df = 2, P = 0.027), and we again found a significant interaction between population and request status (χ2 = 4.688, df = 1, P = 0.030; SI Appendix, Table S3). In fact, while resistance probability was generally low at Goualougo and also at Gombe when there was no request, this probability more than doubled at Gombe following a request (Fig. 4).
Fig. 4.
Resistance probability and how it depended on tool request status and population. Indicated are the fitted model and its confidence limits (horizontal lines with error bars) and the observed transfer probabilities per possessor. The area of the symbols depicts the number of possessors per population and request status with the same transfer probability, such that larger symbols correspond to a greater number of possessors at that value (range: 1 to 11). NoReq, no request; Req, request.
Discussion
In this study, we compared the scaffolding of tool skills between 2 chimpanzee populations in which tool technologies differ in complexity. There were significant population differences in tool-transfer behavior. First, we found that tool transfers occurred approximately 3 times as often at Goualougo as at Gombe. Second, we found that there was a higher probability of tool transfer following a request at Goualougo. Request behavior makes an individual’s goals highly salient, and so the possessor’s response to a request is a strong index of the responder’s motivation to help (23). Consistent with these findings, requests were more likely to be met with resistance at Gombe than at Goualougo. Resistance behaviors provide a clear indicator that an individual is attempting to prevent tool transfer. We also found population differences with respect to transfer types. At Gombe, we did not observe any Active transfers, while at Goualougo, Active transfers were the most common response. These population differences were evident despite holding relatively constant key factors that might affect helping behavior, such as opportunity for request (23, 26).
We suggest that these population differences in scaffolding of tool use could reflect the differing complexity of the tool tasks between populations, particularly the material and design demands associated with the production of tool sets at Goualougo. Transfers of fishing probes as well as other tool types in this context provide information about tool material, dimensions, and design, and they also provide an opportunity to practice with an appropriate tool. This may be particularly critical in cases where raw material and form influence tool effectiveness (39) as is the case for brush-tipped fishing probes (36) and likely also puncturing sticks. Given that we have previously documented that tool transfers at Goualougo function as a form of teaching (18), the present results highlight the intersection of high-fidelity social learning and instrumental helping in the context of this complex task, where it could be challenging for novices to acquire tools, and thus to develop tool skills, without assistance.
The demands of tool manufacture at Goualougo may also help to explain the significance of age as a predictor of tool transfer. Tool-transfer probability was higher for individuals between the ages of 5 to 10 y relative to those aged 0 to 5 y. At Goualougo, chimpanzees do not manufacture brush-tipped probes until, on average, after 4 y of age, with some individuals not observed independently making a tool until after an age of 5 y. They may continue to refine tool manufacture skills during the juvenile period and to use tools manufactured by skilled conspecifics even after they have begun manufacturing tools independently. Mothers appear to remain willing to transfer tools even to adolescent offspring, as we observed that 94% of transfer attempts involving recipients that were 10 to 15 y old (n = 16, with 14 of these including a request) resulted in a change of possession. The age effect is principally the result of differences within the Goualougo dataset, as individuals at Gombe rarely attempt to take or request tools after age 5 y. At Gombe, infants begin making fishing probes between the ages of 1.5 to 3.5 y (12). During the juvenile period, there may be less need or incentive to take or request conspecifics’ tools because of the comparative ease of tool manufacture.
We also detected potential subtle variations in maternal responses to female versus male offspring’s attempts to take tools. Females in both populations were more successful at acquiring tools, and there was a significant effect of sex on the likelihood of tool transfer, including both requested and nonrequested transfers. At Gombe, female infants spent more time watching their mothers (12), so the observed difference could also be associated with females’ increased interest in or identification of opportunities to request or retrieve tools. At Goualougo, further research will be required to help identify whether, like at Gombe, there are sex differences in activity patterns or social-learning strategies that may help to account for this difference.
The similarity of request behavior at Goualougo and Gombe indicates that population differences did not result from differences in the requestor’s initiative, but from differences in the response of the tool possessors. At Gombe, transfers in response to requests are rare and typically unsuccessful regardless of requestor characteristics. Continued data collection will also help to illuminate how both age and sex influence success upon request within the Goualougo population. Although we did not detect significant effects of age or sex in the model based on only requested transfers, success upon request was 93% for females (26/28 requests) and 62% for males (16/26 requests). In addition, the requests of older individuals were rarely refused. Stealing or attempting to steal tools was more characteristic of young infants, and individuals may increasingly adopt the more successful strategy of requesting tools as they get older.
Patterns of scaffolding at Goualougo showed some similarities to Taï, where expert tool users select tools based on conditional assessment of multiple variables (40). Mothers modify their behavior in ways that facilitate offspring tool use, for example, by allowing offspring to use their hammers and take intact nuts. Further, use of mothers’ tools improves offspring efficiency (14), which highlights the possibility that access to tools manufactured or selected by skilled models is of particular importance in the context of complex tasks. In contrast to Goualougo, transfers upon request were rare, and mothers at Taï tended not to actively hand over hammers to offspring. This could reflect differing tool properties, as wooden and stone hammers are heavier than lightweight herb probes; if they are set down during nut-cracking, offspring could more easily pick up these tools without a request.
In addition to differences in task complexity, other factors could influence the population differences we observed between Goualougo and Gombe chimpanzees. Compared to Goualougo, where chimpanzees gather termites year-round (41), at Gombe, termite gathering is concentrated during the rainy season from October to December (37, 42). Climate at Gombe is highly seasonal, and chimpanzees show reduced body weights in the drier months preceding the rainy season (43). Adult females termite fish more than do males at Gombe, and females, compared to males, are hypothesized to be more reliant on insects as a food source (44). Termites and other insect resources provide nontrivial macronutrients as well as various micronutrients for Gombe chimpanzees (45). Mothers at Gombe, compared to Goualougo, may be less inclined to relinquish their tools if their foraging efforts in this context are limited to a short time period.
Despite the population differences we observed, our findings contribute to an increasing body of evidence that chimpanzees possess a robust and flexible capacity for prosocial behavior. The transfer of resources by chimpanzees may sometimes reflect a desire to reduce harassment, rather than a prosocial response, for example, in meat sharing among Gombe chimpanzees (46). A desire to reduce harassment does not explain the sharing of meat or other resources among Taï chimpanzees (47, 48) or helping in some captive experiments, however (25, 49, 50), nor do helping behaviors appear motivated by rewards (51). It is possible that from past instances of request behavior, chimpanzees have learned that relinquishing a tool is less costly than withstanding prolonged begging. It is not clear why, however, mothers at Gombe would not also readily relinquish tools if harassment is the primary impetus for transfer. At Gombe, begging bouts sometimes involved persistent gesturing and whimpering, and there was no indication that begging had greater potential to disrupt foraging at Goualougo.
We have also confirmed the capacity for proactive transfer in chimpanzees. For example, we observed a tool transfer in which a juvenile male approached his mother while self-scratching but without gesturing or vocalizing, at which point his mother divided her fishing probe and provided him with one of the resulting tools (Movie S1). On another occasion, the same juvenile struggled to insert his fishing probe, at which point his mother handed her tool to him. While not included in the present analyses because they were recorded after the sample of video footage systematically screened for transfers, these interactions indicate that under certain circumstances, chimpanzees can be sensitive not only to overt signals (requests) but also to subtler signs of need (26). Nonetheless, our results underscore that there is an important difference in the prevalence of proactive object transfer between humans and other apes (21, 26), particularly between nonkin.
To date, requested active or proactive tool transfers have not been reported in other nonhuman primate or nonprimate tool users. Further research is needed to see whether this is related to task characteristics, including complexity, or other ecological, social, or cognitive factors. Tolerated taking, however, may occur in a variety of species such as macaques (52), capuchins (53), New Caledonian crows (54), and possibly sea otters (55). The lack of tool transfers in orangutans may be related to their arboreality, as terrestrial settings could increase opportunity for observation and retrieval of discarded tools (56). New Caledonian crows produce tools that show hallmarks of cumulative change. While they have not been documented transferring tools to conspecifics, access to others’ discarded tools may promote template matching that supports the social transmission of tool form (57). In future studies across species, documenting specific dynamics of tool possession, such as proximity to discarded tools or responses to conspecifics who approach to procure these tools, could help to clarify the scope of tool-transfer behavior across different species. Continued investigation is also needed into what contributes to differences between chimpanzees and bonobos with respect to tool use and helping behavior. In captivity, bonobos rarely share toys or tools despite being willing to share food, even with strangers (refs. 58–62 but see refs. 63 and 64). Unlike chimpanzees, bonobos do not use tools for extractive foraging in the wild, and tool sharing may not support cultural transmission of tool use in bonobos. It is also possible that bonobos may value toys or tools differently than do chimpanzees (59).
Conclusion
In this study, we systematically compared tool-transfer behavior between Goualougo and Gombe chimpanzees and found significant population differences in this form of scaffolding. These differences could be related to the complexity of tool tasks differing between sites, suggesting an enhanced role of social learning in the transmission and maintenance of complex skills over generations, particularly when it intersects with a flexible capacity for prosocial helping. Broader comparative studies will continue to inform us about the capacity for different types of scaffolding, including tool transfers, across species, while assessing multiple tool contexts within species will further illuminate how helping varies with task demands. Differentiating specific types of helping is also essential for elucidating the potential cognitive underpinnings of these behaviors. These efforts are promising for illuminating the adaptive basis of helping behaviors and their role in the social transmission of tool behaviors across taxa. In humans, active provisioning of learning opportunities is essential to the cultural transmission of technology. The present research suggests that helping behaviors, including those that function to teach, may also play a role in supporting social learning of complex tool use among chimpanzees. We suggest that there may be a shared evolutionary origin for these capacities in humans and chimpanzees and that the elaboration of such skills could have contributed to the flourishing of cumulative culture in the human lineage.
Materials and Methods
Study Sites.
Goualougo Triangle, Republic of Congo.
The Goualougo Triangle is located in the southern section of the Nouabalé-Ndoki National Park (E 16°51′ to 16°56′; N 2°05′ to 3°03′). The study area includes 380 km2 of evergreen and semideciduous lowland forest, and altitudes range between 330 and 600 m. There is a primary rainy season from August to November and a short rainy season in May. Termite gathering occurs year-round and is not related to seasonally varying resource abundance (41).
Gombe, Tanzania.
Gombe National Park is located on the shore of Lake Tanganyika, at the western border of Tanzania. The park comprises 35 km2 of woodland, grassland, and riverine forest (65). Chimpanzees termite fish year-round, but particularly during the rainy season from October to December (37, 42).
Data Collection.
Data collection in the Goualougo Triangle was undertaken using remote cameras with passive infrared sensors to record chimpanzee tool behavior at termite nests. These data were archived on hard drives and converted to MPEG for review. We screened 224 h of video footage recorded between 2003 and 2011 and analyzed video footage using INTERACT 17 (66). At Gombe, all-day focal follows of mothers with immature (under age 11 y) offspring were conducted over the course of 4 termite-fishing seasons between 1998 and 2001. Once termite fishing commenced, 15-min, video-taped follows were conducted, during which the observer narrated information on tool use, apparent success, and social interactions at the mound (12).
Using a standardized protocol applied to videos from Goualougo and Gombe, we coded footage for all instances of immature chimpanzees requesting or attempting to take tools, type of tool-transfer event, requesting behavior, and any instance of resistance by tool possessors. Interobserver reliability was achieved on tool possession and type of transfers between observers (E.L. and L.B.-K.) scoring videos from the 2 sites. S.M. then independently reviewed all clips to identify supporting evidence of transfer type, such as presence of request as well as latency between request and transfer, as well as resistance. Final coding was also confirmed by representatives across sites to reach consensus (sensu ref. 67).
While we do rarely observe tool transfers between peers (e.g., 2 adults), in this study, we exclusively examined requests or attempts to take tools that occurred from younger to older individuals. This ensured the social relationship between individuals was as consistent as possible between populations, as this variable can impact the likelihood of helping behavior (23). We included age, sex, and identity of individuals involved in transfers in our analyses, given the potential influence of these variables in the context of tool skill acquisition among young chimpanzees (12, 16, 68).
Transfer rate.
We coded the duration of time individuals were present at a termite nest during which there was an opportunity for a tool transfer. This was defined as another individual being present and in possession of a termite-gathering tool. We calculated the rate of tool transfer for each individual by dividing the number of transfer events observed by the total duration of transfer opportunity in hours.
Fishing probe transfer type.
We classified all fishing-probe tool-transfer events according to transfer-event type. Transfer-event types were defined on the basis of several criteria: whether or not they were preceded by a request; whether or not the tool changed possession from one individual to another; and whether at the time preceding the transfer event, the possessor was in physical possession (tool held in mouth, hand, or foot) or spatial possession (the tool must be either within 1 m of the possessor or in passive contact with the possessor’s body, and the tool can be readily identified as a previous tool of the individual). Transfer-event types were further differentiated according to whether the tool possessor protested against the transfer. Video examples from both populations are provided for each transfer type in the Movies S1–S9.
Requests.
We coded all request behaviors after first scoring video clips for whether or not audio was sufficient to detect vocalizations and whether visibility of the individuals involved in the transfer was sufficient to allow for coding of manual gestures. In contrast to Gilby’s (46) definition with respect to begging for meat, merely sitting and staring within 3 m of a tool possessor was not sufficient to be considered begging in this study. This approach is justified given the well-known practice of young chimpanzees to observe tool use at close proximity (31). As such, to be classified as begging, both close proximity (within 3 m) and orientation to a tool possessor had to be present and accompanied by either a whimper vocalization, a whimper face, or a manual gesture. For example, if a whimper vocalization was detected, it was not scored as begging unless the individual was also in the possessor’s proximity and oriented toward the possessor. Whimpering often occurred as an ongoing sequence and so was scored once per transfer event, while all manual gestures were coded and categorized as follows: manual gestures included reaches toward the fishing probe, where the individual extends a hand toward, or touches (but does not grasp), the tool in a slow manner indicative of a request, as well as hand-to-mouth begging gestures (69). If an individual first grasped a probe without a preceding request, this was considered an attempt to take, rather than to request, the tool, and the classification of the transfer automatically diverted to the nonrequested transfer types.
Request latency.
We determined the amount of time in seconds that elapsed between the first request for a fishing probe and a change of possession. In the case of manual gestures, the time of the request was coded at the initiation of movement.
Resistance.
We identified all occurrences of a possessor exhibiting a negative reaction in response to requests or attempts to take tools. This could include instrumental actions to prevent an individual from reaching for or getting a tool, for example extending a hand or foot to hold an individual off or push an individual’s hand away. Resistance also included actions such as threatening the individual who requested or attempted to take a tool by baring teeth, lunging, or barking.
Analyses.
We first compared the rate of transfers of termite-gathering tools between sites for 14 individuals at Goualougo and 9 at Gombe with an exact Wilcoxon–Mann–Whitney U test (70) using the wilcox.exact function in the R package exactRankTests (version 0.8-30) (71). Alpha level was set at 0.05 for all analyses. Next, we compared transfers of fishing probes to immature individuals between populations as well as resistance to transfers by tool possessors. We observed 112 fishing-probe tool-transfer events at Goualougo and 106 at Gombe. When analyzing tool transfers, we excluded Steals (n = 8 at both sites), as the negative reaction from the possessor precludes them from being prosocial. Steals were retained for analyses of resistance. We excluded any remaining transfers for which individual identity or sex could not be assigned or when it was not clear whether there was a request.
While it would be ideal to use precise ages to compare populations, these were not available for all individuals in the Goualougo Triangle study (initiated in 1999, compared to research at Gombe, which was initiated in 1960) and dramatically reduced our sample size. Therefore, we adopted the approach of Estienne et al. (72), who classified chimpanzee ages from camera trap footage into age class bins (0 to 5, 5 to 10, and 10 to 15 y).
We used generalized linear mixed models (GLMMs) (73) with binomial error structure and logit link function (74) to test our first prediction that at Goualougo, compared to Gombe, chimpanzees would be more successful gaining possession of another’s tool. The key terms with fixed effects in this model were “population” and its interaction with “request status” (i.e., whether the potential recipient requested a tool transfer). We further included fixed effects for the main effect of request status, “recipient age,” and “recipient sex.” The identity of the possessor, the recipient, and the dyad (unique possessor–recipient combination) were included as random effects. We also examined the probability of tool transfer for the requested transfers only, since these are considered a stronger indicator of prosocial motivation (23, 26). As this model included only the subset of transfers that involved a request, it lacked the effects request status and the interaction of population and request status. Finally, we tested whether the tool possessor showed signs of resisting tool transfers. This model was identical to the tool-transfer model. Sample sizes for these models were 187 observations (89 transfers) of 29 possessors and 28 recipients forming 42 dyads (tool-transfer model); 101 observations (49 transfers) of 22 possessors and 23 recipients, forming 31 dyads (requested tool-transfer model); and 201 observations (with 43 cases of resistance) of 31 possessors and 30 recipients forming 44 dyads (resistance model).
Of the requested transfers at Goualougo with known outcome, a subset of 38 both met the criteria for measuring latency and involved a change of tool possession; and of the requested transfer events at Gombe, a subset of 7 met the criteria for measuring latency and involved a change of tool possession. However, we were unable to fit a model for assessing latency to transfer tools, largely because of the small number of data available for Gombe. We did not pursue a survival analysis modeling latencies of tool transfer or requested tool transfer, as we did not have continuous footage of complete fishing sessions in order to assess the total duration of time during which a transfer could have occurred.
In order to avoid cryptic multiple testing, we first compared each full model with a respective null model lacking population and the interactions it was involved in (if there was one in the respective full model) but was otherwise identical to the full model (75). This comparison was based on a likelihood ratio test (76).
All analyses were conducted in R (version 3.4.4) (77). We fitted all GLMMs using the function glmer of the lme4 package (version 1.1-17) (78). We checked for absence of collinearity (79) among predictor variables using the function vif of the package car (80) applied to a standard linear model lacking the random effects. Collinearity was not an issue in any of the models (maximum Generalized Variance Inflation Factor [squares of the nth root of GVIF, with n being twice the degrees of freedom of the respective predictor]: tool-transfer model: 1.249; requested tool-transfer model: 1.26; resistance model: 1.228) (81).
We assessed model stability by excluding levels of the random effects one at a time, fitting the respective full model to the subsets, and comparing the estimates derived with those obtained from the model for the whole dataset. We tested the significance of the individual predictors using likelihood ratio tests comparing the full models with respective reduced models lacking the effect in question (76, 82). To obtain confidence intervals of model coefficients we used a parametric bootstrap using the function bootMer of the package lme4 (78).
Supplementary Material
Acknowledgments
We are deeply appreciative of the opportunity to work in the Nouabalé-Ndoki National Park and especially the Goualougo Triangle. This research would not have been possible without the continued support of the Ministère de l’Economie Forestière du gouvernement de la République du Congo and the Agence Congolaise de la Faune et des Aires Protégées. The Wildlife Conservation Society’s Congo Program and the Nouabalé-Ndoki Foundation are integral partners in this continuing research. Special thanks are due to J. M. Fay, P. Telfer, P. Elkan, S. Elkan, B. Curran, M. Gately, E. Stokes, T. Breuer, E. Arnhem, P. Ngouembe, D. Dos Santos, and M. Ngangoue. We would also like to recognize J. R. Onononga, C. Eyana Ayina, S. Ndolo Ebika, W. Mayoukou, M. Meguessa, D. Koni, I. Singono, S. Ndassoba Kialiema, J. Wawa, D. Ngoteni, C. Abedine, and the Goualougo tracking team for their long-term commitment to wildlife conservation in the Congo Basin. At Gombe, we thank Jane Goodall and the tireless staff of the Gombe Stream Research Centre, especially D.A. Collins, S. Kamenya, A. Alimasi, and K. John, who provided key support during data collection. We are also grateful to the Gombe National Park staff, the Government of Tanzania, Tanzania National Parks, Tanzania Commission for Science and Technology, and Tanzania Wildlife Research Institute for permission to and support for carrying out this research. Finally, we are grateful to T. Merlino, T. O’Neal, and A. Zigler for their early contributions to this project. All authors thank two anonymous reviewers and the Editor for their helpful comments. Data collection for this project was funded by the Arcus Foundation, the NSF, the L.S.B. Leakey Foundation, the Wenner-Gren Foundation, the University of Minnesota Graduate School, the Columbus Zoological Park, the Cincinnati Zoo and Botanical Garden, the Houston Zoo, the Indianapolis Zoo, the Saint Louis Zoo, the Margot Marsh Biodiversity Foundation, and the Leo S. Guthman Fund.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See Commentary on page 802.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907476116/-/DCSupplemental.
References
- 1.Boyd R., Richerson P. J., Culture and the Evolutionary Process (University of Chicago, 1985). [Google Scholar]
- 2.Dean L. G., Kendal R. L., Schapiro S. J., Thierry B., Laland K. N., Identification of the social and cognitive processes underlying human cumulative culture. Science 335, 1114–1118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laland K., Galef B. G., The Question of Animal Culture (Harvard University Press, 2009). [Google Scholar]
- 4.Nishida T., “Local traditions and cultural transmission” in Primate Societies, Smuts B., Cheney D., Seyfarth R., Wrangham R., Struhsaker T., Eds. (University of Chicago Press, 1987), pp. 462–474. [Google Scholar]
- 5.Tennie C., Call J., Tomasello M., Ratcheting up the ratchet: On the evolution of cumulative culture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 2405–2415 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrew W., Chimpanzee Material Culture: Implications for Human Evolution (Cambridge University Press, 1992). [Google Scholar]
- 7.Whiten A., et al. , Cultures in chimpanzees. Nature 399, 682–685 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Sanz C. M., Morgan D. B., Chimpanzee tool technology in the Goualougo Triangle, Republic of Congo. J. Hum. Evol. 52, 420–433 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Whiten A., van de Waal E., The pervasive role of social learning in primate lifetime development. Behav. Ecol. Sociobiol. 72, 80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fragaszy D. M., et al. , The fourth dimension of tool use: Temporally enduring artefacts aid primates learning to use tools. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzawa T., et al. , “Emergence of culture in wild chimpanzees: Education by master- apprenticeship” in Primate Origins of Human Cognition and Behavior, Matsuzawa T., Ed. (Springer, 2001), pp. 557–574. [Google Scholar]
- 12.Lonsdorf E., Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Anim. Behav. 70, 673–683 (2005). [Google Scholar]
- 13.Humle T., Snowdon C. T., Matsuzawa T., Social influences on ant-dipping acquisition in the wild chimpanzees (Pan troglodytes verus) of Bossou, Guinea, West Africa. Anim. Cogn. 12 (suppl. 1), S37–S48 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Estienne V., Cohen H., Wittig R. M., Boesch C., Maternal influence on the development of nut-cracking skills in the chimpanzees of the Taï forest, Côte d’Ivoire (Pan troglodytes verus). Am. J. Primatol. 81, e23022 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Boesch C., Teaching among wild chimpanzees. Anim. Behav. 41, 530–532 (1991). [Google Scholar]
- 16.Boesch C., Boesch-Achermann H., “Tool-use in wild chimpanzees” in The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution (Oxford University Press, 2000), pp. 191–224. [Google Scholar]
- 17.Caro T. M., Hauser M. D., Is there teaching in nonhuman animals? Q. Rev. Biol. 67, 151–174 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Musgrave S., Morgan D., Lonsdorf E., Mundry R., Sanz C., Tool transfers are a form of teaching among chimpanzees. Sci. Rep. 6, 34783 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoppitt W. J., et al. , Lessons from animal teaching. Trends Ecol. Evol. 23, 486–493 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Lew-Levy S., Reckin R., Lavi N., Cristóbal-Azkarate J., Ellis-Davies K., How do hunter-gatherer children learn subsistence skills?: A meta-ethnographic review. Hum. Nat. 28, 367–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melis A. P., Warneken F., The psychology of cooperation: Insights from chimpanzees and children. Evol. Anthropol. 25, 297–305 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Cronin K., “Cognitive aspects of prosocial behavior in nonhuman primates” in Encyclopedia of the Sciences of Learning, Seel N., Ed. (Springer, 2012), pp. 581–583. [Google Scholar]
- 23.Cronin K., Prosocial behaviour in animals: The influence of social relationships, communication and rewards. Anim. Behav. 84, 1085–1093 (2012). [Google Scholar]
- 24.Yamamoto S., Humle T., Tanaka M., Chimpanzees’ flexible targeted helping based on an understanding of conspecifics’ goals. Proc. Natl. Acad. Sci. U.S.A. 109, 3588–3592 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage-Rumbaugh E. S., Rumbaugh D., Boysen S., Linguistically mediated tool use and exchange by chimpanzees (Pan troglodytes). Behav. Brain Sci. 1, 539–554 (1978). [Google Scholar]
- 26.Jaeggi A. V., Burkart J. M., Van Schaik C. P., On the psychology of cooperation in humans and other primates: Combining the natural history and experimental evidence of prosociality. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2723–2735 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Waal F. B., Suchak M., Prosocial primates: Selfish and unselfish motivations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 2711–2722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosati A. G., DiNicola L. M., Buckholtz J. W., Chimpanzee cooperation is fast and independent from self-control. Psychol. Sci. 29, 1832–1845 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Pruetz J. D., Lindshield S., Plant-food and tool transfer among savanna chimpanzees at Fongoli, Senegal. Primates 53, 133–145 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Nishida T., Hiraiwa M., Natural history of a tool-using behavior by wild chimpanzees in feeding upon wood-boring ants. J. Hum. Evol. 11, 73–99 (1982). [Google Scholar]
- 31.Lonsdorf E. V., What is the role of mothers in the acquisition of termite-fishing behaviors in wild chimpanzees (Pan troglodytes schweinfurthii)? Anim. Cogn. 9, 36–46 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Sanz C., Morgan D., Gulick S., New insights into chimpanzees, tools, and termites from the Congo Basin. Am. Nat. 164, 567–581 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Sanz C. M., Deblauwe I., Tagg N., Morgan D. B., Insect prey characteristics affecting regional variation in chimpanzee tool use. J. Hum. Evol. 71, 28–37 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Pascual-Garrido A., Cultural variation between neighbouring communities of chimpanzees at Gombe, Tanzania. Sci. Rep. 9, 8260 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brewer S. M., McGrew W. C., Chimpanzee use of a tool-set to get honey. Folia Primatol. (Basel) 54, 100–104 (1990). [DOI] [PubMed] [Google Scholar]
- 36.Sanz C., Call J., Morgan D., Design complexity in termite-fishing tools of chimpanzees (Pan troglodytes). Biol. Lett. 5, 293–296 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGrew W., Tutin C., Baldwin P. J., Chimpanzees, tools, and termites: Cross-cultural comparisons of Senegal, Tanzania, and Rio Muni. Man (Lond.) 14, 185–214 (1979). [Google Scholar]
- 38.Sanz C., Morgan D., “The complexity of chimpanzee tool-use behaviors” in The Mind of the Chimpanzee: Ecological and Experimental Perspectives, Lonsdorf E., Ross S. R., Matsuzawa T., Eds. (University of Chicago Press, Chicago, 2010), pp. 127–140. [Google Scholar]
- 39.Sousa C., “Use of leaves for drinking water” in The Chimpanzees of Bossou and Nimba, Matsuzawa T., Humle T., Sugiyama Y., Eds. (Springer, 2011), pp. 85–96. [Google Scholar]
- 40.Sirianni G., Mundry R., Boesch C., When to choose which tool: Multidimensional and conditional selection of nut-cracking hammers in wild chimpanzees. Anim. Behav. 100, 152–165 (2015). [Google Scholar]
- 41.Sanz C. M., Morgan D. B., Ecological and social correlates of chimpanzee tool use. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodall J., “Feeding” in The Chimpanzees of Gombe: Patterns of Behavior (Belknap Press, 1986), pp. 231–266. [Google Scholar]
- 43.Pusey A. E., Oehlert G. W., Williams J. M., Goodall J., Influence of ecological and social factors on body mass of wild chimpanzees. Int. J. Primatol. 26, 3–31 (2005). [Google Scholar]
- 44.McGrew W., “Evolutionary implications of sex differences in chimpanzee predation and tool use” in The Great Apes, Hamburg D. A., McCown E. R., Eds. (Benjamin/Cummings, Menlo Park, 1979), pp. 441–463. [Google Scholar]
- 45.O’Malley R. C., Power M. L., Nutritional composition of actual and potential insect prey for the Kasekela chimpanzees of Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 149, 493–503 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Gilby I., Meat sharing among the Gombe chimpanzees: Harassment and reciprocal exchange. Anim. Behav. 71, 953–963 (2006). [Google Scholar]
- 47.Samuni L., et al. , Social bonds facilitate cooperative resource sharing in wild chimpanzees. Proc. Biol. Sci. 285, 20181643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuni L., Preis A., Deschner T., Crockford C., Wittig R. M., Reward of labor coordination and hunting success in wild chimpanzees. Commun Biol 1, 138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melis A. P., Tomasello M., Chimpanzees’ (Pan troglodytes) strategic helping in a collaborative task. Biol. Lett. 9, 20130009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto S., Humle T., Tanaka M., Chimpanzees help each other upon request. PLoS One 4, e7416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warneken F., Hare B., Melis A. P., Hanus D., Tomasello M., Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan A., “Behavioral processes and social influences on the development of stone-tool use in long-tailed macaques,” PhD dissertation, Nanyang Technological University, Singapore (2016).
- 53.Eshchar Y., Izar P., Visalberghi E., Resende B., Fragaszy D., When and where to practice: Social influences on the development of nut-cracking in bearded capuchins (Sapajus libidinosus). Anim. Cogn. 19, 605–618 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Holzhaider J., Gray R., Hunt G., The development of pandanus tool manufacture in wild New Caledonian crows. Behaviour 147, 553–586 (2010). [Google Scholar]
- 55.Sandegren F. E., Chu E. W., Vandevere J. E., Maternal behavior in the California sea otter. J. Mammal. 54, 668–679 (1973). [PubMed] [Google Scholar]
- 56.Meulman E. J., Sanz C. M., Visalberghi E., van Schaik C. P., The role of terrestriality in promoting primate technology. Evol. Anthropol. 21, 58–68 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Jelbert S. A., Hosking R. J., Taylor A. H., Gray R. D., Mental template matching is a potential cultural transmission mechanism for New Caledonian crow tool manufacturing traditions. Sci. Rep. 8, 8956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan J., Hare B., Bonobos share with strangers. PLoS One 8, e51922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krupenye C., Tan J., Hare B., Bonobos voluntarily hand food to others but not toys or tools. Proc. Biol. Sci. 285, 20181536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hare B., Kwetuenda S., Bonobos voluntarily share their own food with others. Curr. Biol. 20, R230–R231 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Hare B., Melis A. P., Woods V., Hastings S., Wrangham R., Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto S., Non-reciprocal but peaceful fruit sharing in wild bonobos in Wamba. Behaviour 152, 335–357 (2015). [Google Scholar]
- 63.Cronin K. A., De Groot E., Stevens J. M. G., Bonobos show limited social tolerance in a group setting: A comparison with chimpanzees and a test of the relational model. Folia Primatol. (Basel) 86, 164–177 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Jaeggi A. V., Stevens J. M., Van Schaik C. P., Tolerant food sharing and reciprocity is precluded by despotism among bonobos but not chimpanzees. Am. J. Phys. Anthropol. 143, 41–51 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Clutton-Brock T., Gillet J. B., A survey of forest composition in the Gombe National Park, Tanzania. Afr. J. Ecol. 17, 131–158 (1979). [Google Scholar]
- 66.Mangold, INTERACT User Guide. Mangold International GmBH, Ed. (2017). www.mangold-international.com. Accessed 4 December 2019.
- 67.Humle T., Matsuzawa T., Ant-dipping among the chimpanzees of Bossou, Guinea, and some comparisons with other sites. Am. J. Primatol. 58, 133–148 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Inoue-Nakamura N., Matsuzawa T., Development of stone tool use by wild chimpanzees (Pan troglodytes). J. Comp. Psychol. 111, 159–173 (1997). [DOI] [PubMed] [Google Scholar]
- 69.Goodall J., Glossary of Chimpanzee Behaviors (Jane Goodall Institute, 1989). [Google Scholar]
- 70.Siegel S., Castellan N. J. Jr, Nonparametric Statistics for the Behavioral Sciences (McGraw-Hill, 1988), pp. 128–137. [Google Scholar]
- 71.Hothorn T., Hornik K., exactRankTests: Exact Distributions for Rank and Permutation Tests, Version 0.8-30 (2019).
- 72.Estienne V., Robira B., Mundry R., Deschner T., Boesch C., Acquisition of a complex extractive technique by the immature chimpanzees of Loango National Park, Gabon. Anim. Behav. 147, 61–76 (2019). [Google Scholar]
- 73.Baayen R. H., Analyzing Linguistic Data: A Practical Introduction to Statistics Using R (Cambridge University Press, 2008), pp. 278–284. [Google Scholar]
- 74.McCullagh P., Nelder J., Generalized Linear Models (Chapman & Hall, 1989), pp. 107–110. [Google Scholar]
- 75.Forstmeier W., Schielzeth H., Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 65, 47–55 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dobson A., An Introduction to Generalized Linear Models (Chapman & Hall/CRC, 2002). [Google Scholar]
- 77.R Core Team , R: A language and environment for statistical computing, Version 3.4.4 (2018).
- 78.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 79.Field A., Discovering Statistics Using SPSS (Sage, 2005). [Google Scholar]
- 80.Fox J., Weisberg S., An {R} Companion to Applied Regression (Sage Publications, ed. 2, 2011). [Google Scholar]
- 81.Fox J., Monette G., Generalized collinearity diagnostics. J. Am. Stat. Assoc. 87, 178–183 (1992). [Google Scholar]
- 82.Barr D. J., Levy R., Scheepers C., Tily H. J., Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 68, 255–278 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boesch C., Boesch H., Hunting behavior of wild chimpanzees in the Taï National Park. Am. J. Phys. Anthropol. 78, 547–573 (1989). [DOI] [PubMed] [Google Scholar]
- 84.de Waal F., Food sharing and reciprocal obligations among chimpanzees. J. Hum. Evol. 18, 433–459 (1989). [Google Scholar]
- 85.de Waal F. B., Food transfers through mesh in brown capuchins. J. Comp. Psychol. 111, 370–378 (1997). [DOI] [PubMed] [Google Scholar]
- 86.de Waal F., The chimpanzee’s service economy: Food for grooming. Evol. Hum. Behav. 18, 375–386 (1997). [Google Scholar]
- 87.Stevens J. R., Gilby I., A conceptual framework for nonkin food sharing: Timing and currency of benefits. Anim. Behav. 67, 603–614 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.