Significance
Injecting small electrical currents into the somatosensory cortex evokes vivid tactile sensations that can be used to convey sensory feedback from brain-controlled bionic hands. To evoke intuitive touch percepts requires that we understand how these are shaped by stimulation parameters. Here, we characterize the ability of monkeys to discriminate microstimulation frequency over a wide range (from 10 to 400 Hz) and show that animals can discern changes in frequency up to about 200 Hz. On some electrodes, behavioral performance is mediated by frequency-dependent changes in sensory quality that are largely independent of sensation magnitude. On others, animals cannot distinguish changes in frequency from changes in amplitude. We discuss the implications of our findings for neural coding and artificial touch.
Keywords: sensory feedback, artificial touch, temporal coding, bionic hands, neuroprosthetics
Abstract
Intracortical microstimulation (ICMS) of the somatosensory cortex evokes vivid tactile sensations and can be used to convey sensory feedback from brain-controlled bionic hands. Changes in ICMS frequency lead to changes in the resulting sensation, but the discriminability of frequency has only been investigated over a narrow range of low frequencies. Furthermore, the sensory correlates of changes in ICMS frequency remain poorly understood. Specifically, it remains to be elucidated whether changes in frequency only modulate sensation magnitude—as do changes in amplitude—or whether they also modulate the quality of the sensation. To fill these gaps, we trained monkeys to discriminate the frequency of ICMS pulse trains over a wide range of frequencies (from 10 to 400 Hz). ICMS amplitude also varied across stimuli to dissociate sensation magnitude from ICMS frequency and ensure that animals could not make frequency judgments based on magnitude. We found that animals could consistently discriminate ICMS frequency up to ∼200 Hz but that the sensory correlates of frequency were highly electrode dependent: On some electrodes, changes in frequency were perceptually distinguishable from changes in amplitude—seemingly giving rise to a change in sensory quality; on others, they were not. We discuss the implications of our findings for neural coding and for brain-controlled bionic hands.
Intracortical microstimulation (ICMS) delivered to the somatosensory cortex has been shown to evoke tactile percepts, the location and magnitude of which can be systematically manipulated (1–6), a phenomenon that can be exploited to convey sensory feedback from sensorized bionic hands. The output of sensors on the prosthetic fingers can drive stimulation through electrodes located in the appropriate region of the somatosensory homunculus, thereby intuitively conveying information about contact location on the hand; the strength of stimulation can be modulated to produce sensations whose magnitude depends on the output of the sensor, thereby intuitively conveying information about contact pressure (6). Ideally, the electrically induced neuronal activity would mimic its mechanically induced counterpart in able-bodied individuals, which would lead to completely natural sensation (7, 8). However, limitations inherent to electrical stimulation, in the number of stimulating channels, and in our understanding of cortical circuitry severely restrict our ability to produce naturalistic neuronal activity.
Despite its unnaturalness, however, ICMS leads to sensations that are reported by human subjects as being natural or nearly so (2, 9), and ICMS-based feedback leads to improved functionality for brain-controlled bionic hands (10). In light of this, we seek to refine our understanding of how the parameters of ICMS shape the percept, in the hopes of achieving increasingly intuitive and useful artificial touch. As alluded to above, the effects of ICMS amplitude on the evoked sensations have been extensively studied as has the effect of stimulation location on the cortical sheet (i.e., through different electrodes) (1, 2, 5, 6, 9). Changes in ICMS frequency have been shown to evoke discriminable percepts in studies with nonhuman primates (11, 12). However, the range of frequencies tested only spanned a small fraction of that relevant for neuroprosthetics (from 10 to 36 Hz).
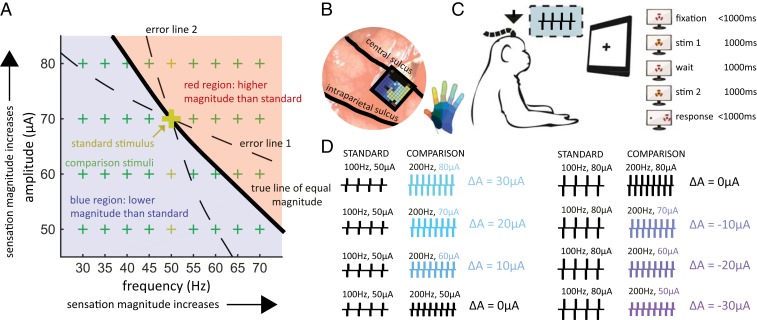
Furthermore, the question remains how changes in frequency affect the evoked percept. Indeed, sensitivity to ICMS increases with frequency (5) so the ability to discriminate frequency may rely on frequency-dependent changes in perceived magnitude. Alternatively, differences in the quality of the percept, which have been reported by human subjects to accompany changes in ICMS frequency (9, 13), may serve as the basis for discriminating frequency. Of course, these 2 possibilities are not mutually exclusive as subjects could use sensation magnitude, quality, or a combination of both to determine which of 2 stimuli (of equal amplitude) is higher in frequency. In theory, we could empirically distinguish one strategy from the other by equalizing the sensation magnitude across frequencies: the higher the frequency, the lower the amplitude (solid line in Fig. 1A). In practice, this approach would require a precise, electrode-by-electrode characterization of the relative contribution of frequency and amplitude to perceptual magnitude. Any miscalculation in the trade-off between frequency and amplitude would allow the subject to use differences in magnitude to make frequency judgments (dashed lines in Fig. 1A). An alternative approach is to vary ICMS amplitude independently of frequency: If the lower-frequency stimulus is sufficiently higher in amplitude than the higher-frequency stimulus to overcome the frequency-dependent difference in sensory magnitude, a strategy based on magnitude will lead to an incorrect frequency judgment. If stimulus amplitude varies unpredictably from stimulus to stimulus (green crosses in Fig. 1A), reliance on magnitude thus leads to poor overall performance.
Fig. 1.
Experimental design. (A) Frequency/amplitude trade-off in perceived magnitude, illustrated with the stimuli used with a 50-Hz standard frequency. Each point on the surface represents a pair of stimulation parameters. Sensation magnitude increases with both frequency and amplitude. The thick black line describes stimuli whose sensory magnitude is equal to that of a 50-Hz, 70-µA stimulus (large gold +). In theory, the standard stimulus could then be paired with other comparison stimuli on the line and the animals could not discriminate frequency based on differences in magnitude. However, if the estimate of this line is incorrect (dashed lines), then the animals can still make frequency judgments based on differences in magnitude: Following error line 1, every comparison frequency greater than the standard frequency will feel more intense and every comparison frequency lower than the standard will feel less intense. The inverse is true for error line 2. As the relative contributions of frequency and amplitude to intensity vary across electrodes, characterizing the isointensity contour is challenging. The alternative approach is to present stimuli that tile the frequency and amplitude space (green +s), so that the magnitude of the higher-frequency stimulus is sometimes higher and sometimes lower than that of the lower-frequency stimulus. Reliance on magnitude will lead to poor overall performance and lower rewards. The separation between the blue and red regions will shift if the standard frequency is presented at other amplitudes (small gold +s). (B) A Utah electrode array (UEA) was implanted in the hand representation of area 1 (the implant of monkey C is shown). (C) The animal faced a monitor that signaled the trial sequence (shown on the right, red markers denote the gaze). The animal maintained fixation on a central target while 2 ICMS pulse trains were sequentially delivered and then reported its frequency judgment by making a saccade to one of 2 targets. The animal was rewarded if it selected the pulse train with the higher frequency (regardless of stimulus amplitude). (D) Example of standard-comparison frequency pair (not all amplitude combinations shown). Colors denote the difference in amplitude between the comparison and the standard stimulus. The largest amplitude difference was ±30 µA.
The objective of the present study, then, was to characterize the ability of nonhuman primates (Rhesus macaques) to discriminate the frequency of ICMS applied to the somatosensory cortex over a wide range of frequencies (from 10 to 400 Hz) independently of amplitude and to assess the degree to which changes in frequency affect the magnitude and quality of the percept. Results from the present study provide a quantitative basis for the design of sensory feedback algorithms that modulate ICMS frequency and amplitude to shape artificial touch and complement previous and future subjective reports from humans.
Results
Three monkeys, implanted with electrode arrays (Utah electrode arrays [UEAs]) in Brodmann’s area 1 of the somatosensory cortex, judged which of 2 1-s-long ICMS pulse trains was higher in pulse frequency (Fig. 1 B and C). On each experimental block, consisting of several hundred trials, a standard stimulus (at 20, 50, 100, or 200 Hz) was paired with several comparison stimuli whose frequencies varied around the standard frequency. The amplitudes of the standard and comparison stimuli also varied from trial to trial (50, 60, 70, or 80 µA presented in every possible combination in random order, all above typical detection thresholds) (Fig. 1D; for all stimulus parameters, see SI Appendix, Table S1, Frequencydiscrimination stimulus set 1). The animal was rewarded when it correctly reported which of the 2 stimuli was higher in frequency. As discussed above, the large, behaviorally irrelevant variations in amplitude were intended to reduce or abolish the informativeness of perceived magnitude, which is modulated by changes in both frequency and amplitude (Fig. 1A).
Frequency Discrimination with Equal Amplitudes.
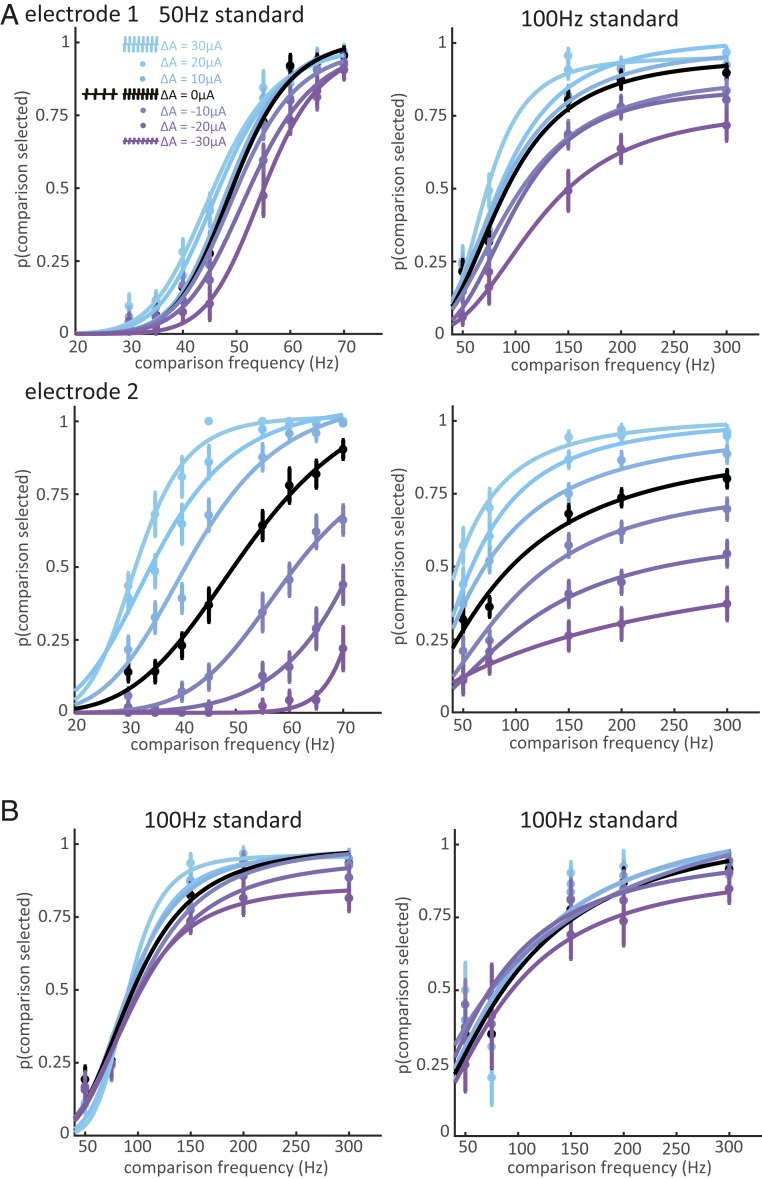
On all electrodes tested, the animals were able to reliably discriminate the frequency of ICMS pulse trains when the amplitudes of the standard and comparison were equal, except over the highest frequency range (from 200 to 400 Hz) (Fig. 2A). Indeed, the animals reached near perfect performance with the 20-, 50-, and 100-Hz standards and, with the 200-Hz standard, for frequencies below 200 Hz. However, when both frequencies were 200 Hz and above, performance leveled off, often below 75%, suggesting that further increases in frequency had no impact on the evoked sensation. To gauge the animals’ sensitivity to changes in frequency, we computed the just noticeable difference (JND), which denotes the frequency increment or decrement required to achieve 75% discrimination performance. We found that JNDs increased with standard frequency from around 3 Hz for a 20-Hz standard to 95 Hz for the 200-Hz standard (Fig. 2B). Weber fractions—the ratio of the JND to the standard frequency—increased dramatically, from 0.15 to 0.5, between 50 and 100 Hz, indicating a much higher sensitivity to changes in frequency in the low range (Fig. 2C). Frequency discrimination performance was independent of stimulus amplitude at the low frequencies but improved somewhat with increasing amplitude at the high frequencies (Fig. 2A and SI Appendix, Fig. S1). Note that, while only trials with equal-amplitude pairs were included in this analysis, these trials were interleaved with many more trials on which the standard and comparison amplitudes differed. The monkeys therefore had to develop a strategy geared toward selecting the higher frequency in the face of task-irrelevant changes in magnitude. If the animals had been trained with only equal-amplitude pairs, sensation magnitude would be a reliable cue, and the animals may have performed better by developing a strategy that exploits this cue.
Fig. 2.
Frequency discrimination with equal amplitudes. (A) Performance on the frequency discrimination task when stimulus amplitudes were equal, averaged across all electrodes (n = 1 each from monkeys A and B for the 20-Hz standard; n = 5 from monkeys A and B for both the 50-Hz and 200-Hz standard; n = 8 from monkeys A, B, and C for the 100-Hz standard). Different colors denote different stimulus amplitudes. Error bars show the SEM across electrodes. The animals achieved high performance for frequencies below 200 Hz. (B) Just noticeable difference (JND) as a function of standard frequency. JNDs at each amplitude were averaged for each electrode. (C) Weber fractions as a function of standard frequency. Error bars in B and C denote the SEM across all electrodes tested at each standard.
Frequency Discrimination with Unequal Amplitudes.
A central objective of the present study was to assess the extent to which changes in frequency shape the evoked percept beyond modulating its magnitude. Indeed, one might expect higher microstimulation frequencies to evoke stronger sensations, given the increased sensitivity at higher frequencies as reflected in lower detection thresholds (5). However, increases in microstimulation amplitude (charge per pulse) also evoke stronger sensations (2, 6). To the extent that frequency discrimination judgments were based on intensive differences, then, we expected the psychometric functions to shift to lower or higher frequencies (i.e., left- or rightward) depending on the amplitude difference between the standard and comparison stimulus. As expected, animals exhibited a systematic bias toward selecting the higher-amplitude stimulus (Fig. 3A), as evidenced by a left- or rightward shift in the psychometric functions when the standard stimulus was lower or higher in amplitude than the comparison stimulus, respectively. The direction of the bias was consistent across standard frequencies and electrodes, and the magnitude of the bias increased monotonically as the difference between the comparison and standard amplitude increased. The magnitude of the bias also varied widely across electrodes: On some electrodes, amplitude differences only slightly contaminated frequency judgments (Fig. 3 A, Top); on others, amplitude differences dominated the animal’s choices (Fig. 3 A, Bottom). All 3 monkeys were able to perform the task with a weak amplitude bias on some electrodes (Fig. 3 A and B).
Fig. 3.
Frequency discrimination with unequal amplitudes. (A, Top) Behavioral performance at one electrode from monkey A for standard frequencies of 50 and 100 Hz. Colors indicate amplitude differences between the comparison and standard frequencies (blue indicates the comparison stimulus amplitude was higher and purple indicates it was lower). The animal’s choices were slightly biased toward the higher amplitude. (A, Bottom) The same monkey’s performance for a different electrode. The monkey could perform frequency discrimination with equal amplitudes at both electrodes (black), but amplitude exerted a powerful influence on its frequency judgments when stimulation was delivered through this electrode. Error bars show the SEM across training blocks. (B) Behavioral performance on one low-bias electrode in monkey B (Left) and one in monkey C (Right).
To maximize reward, the monkeys had to distinguish changes in frequency independently of amplitude. Implementation of this strategy would have yielded psychometric functions that overlapped completely regardless of the difference in amplitude between standard and comparison stimuli, and the small amplitude-related biases on many electrodes demonstrate that the monkeys were capable of learning this strategy. The persistence of large amplitude-dependent biases on some electrodes, even after extensive training on those electrodes, indicates that changes in frequency could not be distinguished from changes in amplitude. We conclude that the sensory correlate of frequency changes on those electrodes was a change in sensory magnitude.
Differences Across Electrodes.
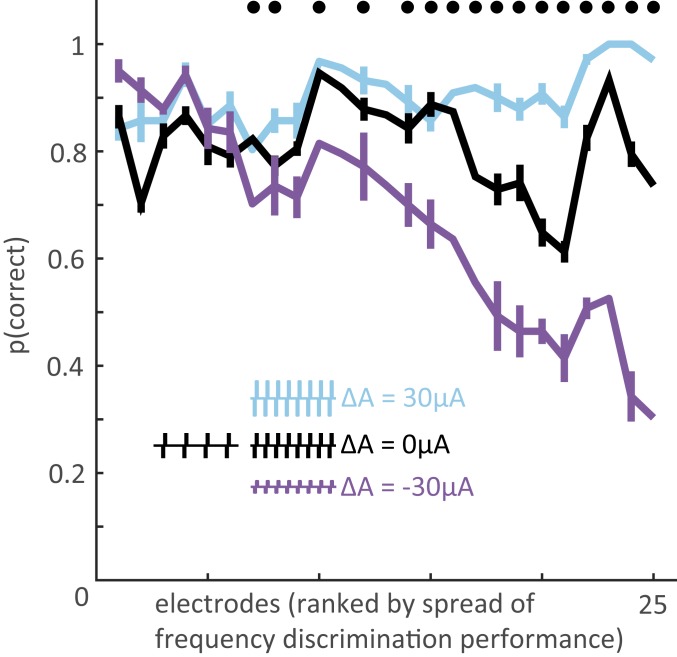
After extensively testing a few electrodes as described above, we had monkey B perform the frequency discrimination task using a more restricted set of stimuli to sample electrodes more widely across the arrays. In this set, the frequency difference between the 2 paired stimuli was always 100 Hz, with the low frequency stimulus spanning the range from 70 to 170 Hz; the amplitude combinations were the same as those tested in the full set (SI Appendix, Table S1, Frequencydiscrimination stimulus set 2). The 25 electrodes tested (8 with full psychometric curves, 17 with this reduced stimulus set) yielded a wide range of amplitude biases (Fig. 4 and SI Appendix, Fig. S2) [2-way ANOVA of performance with amplitude difference and stimulation electrode as factors; interaction term: F(48,12,660) = 12.2, P < 0.001]: Performance was consistently high when the higher-frequency stimulus was also higher in amplitude (blue in Fig. 4) whereas performance in the converse conditions—when the higher-frequency stimulus was lower in amplitude—varied widely (purple in Fig. 4). Poor performance when the high-frequency stimulus was lower in amplitude indicated a reliance on intensity differences to perform the frequency discrimination task.
Fig. 4.
Magnitude of the amplitude bias across electrodes. For the 25 electrodes tested (5 from monkey A, 17 from monkey B, 3 from monkey C), asymptotic performance on the frequency discrimination task with a frequency difference of 100 Hz (with base frequency ranging from 70 to 170 Hz) and amplitude differences of −30, 0, and 30 µA. Electrodes are ranked by spread, computed as the difference in performance between the 2 amplitude extremes (cyan and purple). Error bars represent the SEM performance at each base frequency. Data points without error bars represent the 100-Hz vs. 200-Hz performance for the 8 electrodes that were extensively tested (full psychometric curves were obtained). Black dots indicate electrodes at which differences in amplitude had a significant effect on performance (χ2 test, P < 0.01). The effect of amplitude on performance differed significantly across electrodes (2-way ANOVA of performance with amplitude difference and stimulation electrode as factors, interaction term P < 0.001).
Next, we investigated the possible causes for differences in frequency sensitivity across electrodes. ICMS frequency discrimination has been previously hypothesized to be dependent on the response properties of the stimulated population: In area 3b, electrodes that impinged upon cortical neurons with rapidly adapting responses (RA-like) (which exhibit responses to stimulus transients but not to sustained skin indentations) yielded better performance on an ICMS frequency discrimination task than did electrodes that impinged upon neurons with slowly adapting responses (SA-like) (which exhibit sustained responses to static indentations) (11, 12). Note that the distinction between RA- and SA-like responses has been called into question as most cortical neurons exhibit intermediate responses, even in area 3b (14, 15). Nonetheless, we tested this hypothesis by examining the responses to mechanical indentations delivered to the receptive field of the neurons surrounding each electrode tested. From these responses, we computed an adaptation index to gauge how “RA-like” the response was at each electrode (14, 16) (a higher index value indicates more “RA-like” response properties) (SI Appendix, Adaptation index). We found no consistent relationship between adaptation index and the susceptibility to the amplitude confound (SI Appendix, Fig. S3). Note, however, that the relationship between adaptation properties and frequency discrimination performance was observed for ICMS applied to area 3b, not area 1 as in the present study, which may explain the discrepancy with previous findings.
Another possibility is that differences in discrimination performance reflect differences in sensitivity to ICMS. That is, the monkey may have had difficulties perceiving the pulse trains on some electrodes, thereby leading to poor discrimination performance. To test this hypothesis, we measured the detection thresholds on a subset of 12 electrodes used in the frequency discrimination experiment (measured at 100 Hz) (Methods and SI Appendix, Table S1, Detection task) and found no relationship between frequency discrimination performance and detection threshold (SI Appendix, Fig. S4). In other words, the poor performance on frequency discrimination on some electrodes cannot be attributed to an inability to feel the stimulation.
Disentangling Frequency and Amplitude Effects.
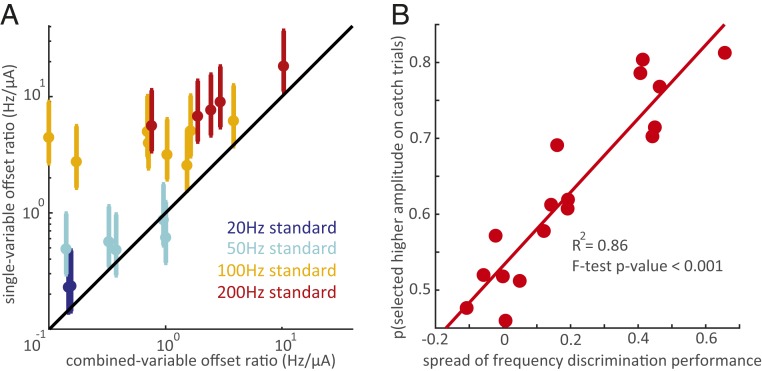
One possibility is that ICMS frequency and amplitude exert the same effect on the evoked percept, namely modulating its magnitude. If this were the case, any increase in frequency could be completely reversed by a concomitant decrease in amplitude (and vice versa). Under this assumption, we can estimate the relative impact of frequency and amplitude on sensory magnitude from the animals’ behavioral performance (Gauging the relative contribution of amplitude and frequency to discrimination judgments). Discrimination performance for a given pair of stimuli is then determined by a weighted sum of the frequency and amplitude differences, and the ratio of the weights—which we will call the offset ratio—can be used as an index of the relative impact of frequency and amplitude on sensory magnitude. For an electrode with a very strong amplitude bias, the offset ratio was 0.96 Hz/µA (Fig. 3A, electrode 2 with the 50-Hz standard). That is, an increase of 0.96 Hz (from 50 Hz) was equivalent to an increase of 1 µA (from the standard amplitude) on this electrode. For an electrode with a weak amplitude bias, the offset ratio was 0.14 Hz/µA (Fig. 3A, electrode 1 with the 50-Hz standard). We could then compare these offset ratios, obtained from frequency discrimination performance when both frequency and amplitude varied, to offset ratios obtained from discrimination performance when one parameter varied while the other was held constant. Specifically, from amplitude discrimination experiments with frequency held constant (performed in an earlier study and published in ref. 5) (SI Appendix, Table S1, Amplitudediscrimination task), we estimated sensitivity to changes in amplitude, and, from frequency discrimination experiments (with amplitude held constant), we estimated sensitivity to changes in frequency (SI Appendix, Fig. S6 and Generating equivalent frequency-amplitude trade-offs using single-variable discrimination). We could then recalculate the offset ratio based on these measurements and assess the degree to which the relative sensitivities to frequency and amplitude derived from the single-parameter experiments matched those derived from the combined parameter experiments. If frequency and amplitude exert the same influence on perception (both only affecting sensation magnitude), the offset ratios computed for the single- and variable-parameter experiments should match. To the extent that frequency has a different impact on sensation than does amplitude, the offset ratio should be systematically lower for the combined-variable experiments than for the single-variable ones. If frequency impacts sensation quality, the single-variable experiments should lead to an underestimate of the discriminability of frequency in the presence of amplitude confounds because changes in quality are relatively robust to changes in sensory magnitude.
Consistent with the quality-modulation hypothesis, the offset ratios derived from combined-variable experiments were consistently and significantly lower (in Hz/µA) than were those derived from the single-variable discrimination experiments (Fig. 5A) (Mann–Whitney test, P < 0.01). That is, the effect of frequency relative to that of amplitude was systematically and strongly underestimated from single-variable experiments, especially for electrodes with a weak amplitude bias (compare isoperformance contours in SI Appendix, Fig. S6). These results are thus inconsistent with the hypothesis that amplitude and frequency influence the percept in the same way and support the conclusion that changes in microstimulation frequency affect the evoked sensation beyond simply modulating its magnitude. However, the extent to which this is the case is highly electrode dependent. On some electrodes (those overlapping the unity line in Fig. 5A), changes in frequency and changes in amplitude seem to be completely interchangeable.
Fig. 5.
Disentangling the effects of ICMS frequency and amplitude. (A) Offset ratios (the equivalent trade-off between frequency and amplitude changes) computed from single-variable discrimination experiments versus the offset ratios computed from combined-variable discrimination experiments. Each data point represents 1 electrode at 1 standard frequency (2 points for the 20-Hz standard, 5 for the 50- and 200-Hz standards, and 8 for the 100-Hz standard). Different colors denote different standard frequencies. The y axis error bars show the range of offset rates obtained by pairing each electrode’s same-amplitude frequency discrimination performance with the amplitude discrimination performance from all electrodes tested in the single-variable amplitude discrimination task (Data Analysis), and the marker shows the mean of these estimates. All but 2 electrodes at the 50-Hz standard are above the unity line, indicating that the relative effect of frequency is consistently greater in the combined-variable experiment. The single-variable offset ratios were significantly different from the combined-variable offset ratios (Mann–Whitney test, P < 0.01). (B) Proportion of catch trials in which the animal selected the higher amplitude stimulus versus spread, the difference in performance between the 2 amplitude extremes (+30 µA to −30 µA). Each data point represents 1 electrode from Monkey B. Probability of selecting the higher amplitude increased significantly with performance spread (linear regression R2 = 0.86, P < 0.001). The animal had negligible or no preference for the higher amplitude stimulus on catch trials at low-spread electrodes, confirming that the animal was not using sensation intensity to select higher frequencies. Catch trials represented ∼5% of trials at each electrode. The number of catch trials performed at each electrode ranged from 49 to 161.
To further test the hypothesis that frequency changes have nonintensive effects on perception, we introduced catch trials on which the 2 stimuli in the pair had the same frequency but different amplitudes (Catch trials and SI Appendix, Table S1). To the extent that the animal relied on intensive cues to make its judgment, it would select the higher-amplitude stimulus. To the extent that it judged the stimuli along a frequency-specific continuum and ignored differences in intensity (as it was rewarded to do), it would be equally likely to select either stimulus, having no basis to choose one or the other. We found that, for electrodes with a weak amplitude bias (those on the left in Fig. 4), the animal was equally likely to pick either stimulus on catch trials (Fig. 5B). It could reliably select the higher frequency in all amplitude conditions at these electrodes yet showed no preference for higher-intensity stimuli when both frequencies were equal, indicating that it did not rely on intensity to make frequency judgments. For electrodes with a strong amplitude bias, on which we hypothesized the animal was performing an intensity discrimination task, the animal was highly likely to pick the higher amplitude stimulus on catch trials.
In fact, further examination of the catch trials revealed that the animal switched behavioral strategy in a context-dependent manner (SI Appendix, Fig. S7 and Validation of catch trials). When the stimulus set was such that the task could be performed using sensation magnitude (when only same-amplitude pairs were presented, or when the higher-frequency stimulus always was much lower in amplitude than was the lower-frequency stimulus), the animal relied more heavily on intensive cues to perform the task on all electrodes. Indeed, on same-amplitude pairs, the animal exhibited a strong tendency to pick the stronger stimulus on catch trials (SI Appendix, Fig. S7). On pairs where the high-frequency stimulus was much lower in amplitude, the tendency was reversed. When amplitude variations precluded a reliance on intensive cues, the animal switched to a strategy that was less dependent on intensive cues, as evidenced by a lack of preference for one stimulus over the other on catch trials, but this ambivalence was only observed on electrodes with weak amplitude biases.
Dependence of the Sensory Correlates of ICMS Frequency on the Spatial Pattern of Recruitment.
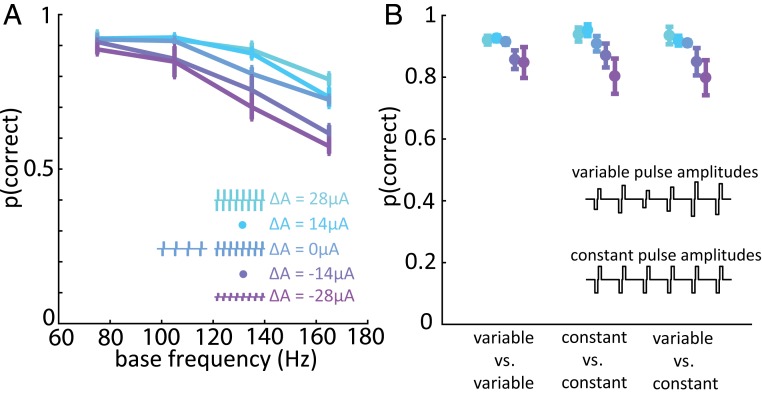
Computational modeling suggests that the perceived magnitude of a stimulus can be predicted from the population spike count (17, 18). To the extent that animals were not solely relying on differences in sensory magnitude to discriminate frequency, however, this neural code is unlikely to exclusively mediate their behavioral performance. One possibility is that the stimulus can be decoded from the spatial layout of the cortical response. For example, frequency and amplitude may each shape in a systematic way the falloff in the response with distance from the electrode tip. The individual contributions of these 2 stimulation parameters could then be untangled by sampling total evoked activity at several distances from the electrode tip. To investigate this possibility, we delivered pulse trains in which the amplitude varied from pulse to pulse over a range but was on average equal to the amplitude of corresponding constant-amplitude pulse trains (SI Appendix, Table S1, Frequency discrimination stimulus set 3). The spatial extent of the response—the pattern of recruitment—thus varied from pulse to pulse, blurring the formerly sharp separation between distinct spatial patterns of recruitment, and so reliance on a spatial pattern of activation would lead to poor frequency discrimination performance. In individual experimental blocks, we randomly interleaved trials on which 1) both pulse trains had constant amplitudes as in previous experiments, 2) both pulse trains had variable amplitudes, and 3) one pulse train was constant in amplitude and the other was variable. We found that the pulse-by-pulse variability in amplitude had a negligible effect on performance (Fig. 6 and SI Appendix, Fig. S8), suggesting that frequency discrimination does not rely on differences in spatial patterns of electrically evoked neural activation.
Fig. 6.
Varying individual pulse amplitude has a negligible effect on frequency discrimination performance. (A) Monkey B’s performance vs. base frequency in the variable-amplitude experiment for a group of 4 electrodes with weak amplitude bias. The frequency difference was always 90 Hz for the variable-amplitude experiment due to hardware constraints (Methods). Error bars in A and B show the SEM across electrodes. (B) Performance when stimulus pulse trains were both variable-amplitude were split or were both constant-amplitude, for the same 4 electrodes with weak amplitude bias. The Inset illustrates variable-amplitude and constant-amplitude pulse trains. Changing spatial distribution of the ICMS-induced activity on a pulse-by-pulse basis had little to no effect on the animal’s ability to discriminate frequency.
Discussion
In summary, we find that 1) animals can discriminate the frequency of ICMS up to ∼200 Hz, 2) changes in frequency affect both the magnitude and quality of the evoked sensation, 3) the degree to which frequency shapes sensory quality varies across electrodes, and 4) ICMS frequency discrimination does not depend on the spatial pattern of neural activation.
Microstimulation Frequency Can Be Discriminated up to ∼200 Hz.
When stimuli differing in frequency but matched in amplitude were paired, animals were able to discriminate frequency up to ∼200 Hz, at which point performance declined considerably (Fig. 2 and SI Appendix, Fig. S1), suggesting that increases in frequency beyond 200 Hz have a negligible impact on the evoked percept. This frequency cutoff coincides with the point at which detection thresholds for ICMS level off (5), beyond which intensive cues for frequency are likely no longer available. The steep decline in performance suggests that the neural code that mediates microstimulation frequency discrimination independent of amplitude also deteriorates (see below for discussion of neural codes).
Increased ICMS Frequency Leads to Increased Perceived Magnitude.
Three lines of evidence suggest that higher ICMS frequencies give rise to more intense percepts. First, increasing frequency lowers detection thresholds, indicating increased sensitivity at higher frequencies (5). Second, animals exhibit a consistent bias to select the higher-amplitude stimulus as being higher in frequency even though this leads to less reward (Figs. 3 and 4). This systematic bias implies a systematic relationship between frequency and perceived magnitude. Third, on experimental blocks comprising primarily equal-amplitude stimuli, catch trials—on which both stimuli had the same frequency but different amplitudes—resulted in the systematic selection of the higher-amplitude stimulus, consistent with the hypothesis that the animal relied in part on intensity to make these discrimination judgments (SI Appendix, Fig. S7). Note that systematic selection biases on catch trials disappeared to the extent that animals judged frequency independently of amplitude (Fig. 5B and SI Appendix, Fig. S7 and Validation of catch trials). Increased perceived magnitude at higher frequencies has also been reported for peripheral nerve stimulation (19–21) and is consistent with the hypothesis that intensity is determined by the spike rate evoked in the neuronal population (17, 18).
Changes in ICMS Frequency Can Lead to Changes in the Quality of the Evoked Percept.
On a number of electrodes, animals could select the higher frequency stimulus even when 1) it was much lower in amplitude and perceived as less intense than the stimulus with which it was paired (points in the blue region of Fig. 1A at which the frequency is higher than the standard) and 2) it was interleaved with other pairs in which the higher frequency stimulus was perceived as more intense (points in the red region of Fig. 1A). In these cases, when an animal was presented with catch trials—in which both stimuli were of equal frequency but different amplitude—it did not exhibit a bias to select the higher-amplitude stimulus (Fig. 5B and SI Appendix, Fig. S7). The animal thus demonstrated that it was largely ignoring differences in magnitude in making its frequency judgments. Furthermore, on those electrodes, the animal’s behavior was inconsistent with the hypothesis that frequency and amplitude affect only a common sensory continuum—magnitude—as evidenced by the significantly lower frequency/amplitude offset ratios derived from the combined-variable experiment compared to those derived from the single-variable experiments (Fig. 5A and SI Appendix, Fig. S6). Indeed, the amplitude bias was weaker than would be predicted based on single-parameter discrimination performance (amplitude or frequency).
Together, these observations are consistent with the hypothesis that changes in frequency also affect the quality of the evoked percept on some electrodes in a highly electrode-dependent way reflected in a wide range of frequency/amplitude offset ratios. That the animals were often able to transfer performance from one electrode to the next suggests that the effect of frequency on sensory quality was consistent across electrodes. Indeed, had the perceptual effect been very different from electrode to electrode, the animal would have had to discover the relevant sensory continuum on an electrode-by-electrode basis. The inability to distinguish frequency independently of amplitude on other electrodes indicates that the effect of frequency on those electrodes is indistinguishable from that of amplitude, is subtle and drowned out by the fluctuations in amplitude, or does not lie on a discernible continuum.
Neural Codes.
Any proposed neural code to explain the discrimination behavior must account for the animals’ ability to distinguish increases in ICMS frequency from increases in ICMS amplitude. As both stimulation parameters affect the firing rate of neural populations near the electrode tip, a pure population spike rate code cannot account for the behavior. While higher amplitudes lead to the recruitment of a larger volume of neurons (22, 23) and to changes in the spatial distribution of neuronal activity (24), the precise shape of recruitment may also be frequency dependent (25). In principle, then, the spatial pattern of neuronal activation may have shaped the resulting percept and mediated the animals’ frequency discrimination behavior. We ruled out this possibility—and others positing that frequency discrimination relies on a spatial pattern of activation—first by showing that frequency can be reliably discriminated despite large differences in average stimulation amplitude, and further by showing that performance is largely unhindered by random changes in amplitude from pulse to pulse, which in turn lead to spatial patterns of activation that vary from pulse to pulse (Fig. 6 and SI Appendix, Fig. S8).
Having excluded the population spike rate and spatial hypotheses, we hypothesize that frequency discrimination relies on the temporal structure of the ICMS-evoked activity. Indeed, each ICMS pulse synchronously activates a large population of neurons around the electrode tip (22) so periodic stimulation results in synchronized periodic responses across a large swath of the somatosensory cortex. The animals’ ability to discriminate ICMS frequency relies on the ability to detect differences in the temporal patterning in the population response, which in turn result in differences in sensation quality along a continuum that is distinct from magnitude. According to this hypothesis, the drop-off in performance for frequencies above 200 Hz is caused by an inability of neuronal populations to phase lock at these frequencies. Note that a small population of neurons in the somatosensory cortex have been shown to phase lock to vibratory stimuli up to 800 Hz (26), but it is unlikely that hundreds or thousands of neurons—confined to a restricted volume—could do so. According to this hypothesis, differences across electrodes would be due to differences in the ability of local circuits to phase lock to stimulation, a hypothesis that can be tested by recording the electrically induced neuronal activity.
Implications for Neuroprosthetics.
Regardless of the relevant perceptual continuum and neural mechanisms, ICMS frequency exerts a robust influence on the evoked percept. Indeed, we estimate that the perceptually relevant range of frequencies (from 10 to 200 Hz) accommodates 10 to 20 nonoverlapping JNDs. That is, changes in frequency can lead to tens of mutually discriminable percepts. In contrast, amplitude JNDs—which range from 14 to 30 µA (2, 5)—provide at best 5 to 7 mutually discriminable percepts, from detection threshold (around 20 to 30 µA at 300 Hz) to the maximum amplitude used for human experiments (100 µA) (2, 5, 6). ICMS frequency manipulation may therefore enable more finely graded sensory feedback than does amplitude manipulation.
To the extent that ICMS frequency and amplitude have different sensory correlates, the perceptual space that can be achieved by changing these 2 stimulation parameters is vast (∼100 to 150 discriminable percepts if we assume that the effects of frequency and amplitude are completely orthogonal). Note, however, that frequency and amplitude can never be completely dissociated, even after extensive training on the “best” electrodes. On many electrodes, frequency and amplitude cannot be dissociated at all. An ideal sensory encoding algorithm would take into consideration these electrode-specific trade-offs between frequency and amplitude, which we gauge with the offset ratio. One approach to estimate the trade-offs would be to conduct extensive psychophysical testing on each human participant to assess frequency and amplitude sensitivity on an electrode-by-electrode basis, but this is unlikely given the time and tedium this strategy entails. A more viable approach would be to discover response properties of neural tissue around electrodes that would be readily identified and diagnostic of that electrode’s frequency and amplitude sensitivity. Regardless, the challenge will be to harness stimulation frequency and amplitude, as well as other simulation parameters, taking into consideration idiosyncratic differences across electrodes, to evoke meaningful tactile percepts.
Methods
Animals.
Three male Rhesus macaques (Macaca mulatta), ranging in age from 7 to 9 years old and weighing between 9 and 10 kg, participated in this study. Animal care and handling procedures were approved by the University of Chicago Institutional Animal Care and Use Committee.
Implants.
Each animal was implanted with 1 Utah electrode array (UEA) (Blackrock Microsystems, Inc., Salt Lake City, UT) in the hand representation of area 1 (Fig. 1B). Each UEA consists of 96 1.5-mm-long electrodes with tips coated in iridium oxide, spaced 400 µm apart, and spanning 4 mm × 4 mm of the cortical surface. The hand representation in area 1 was targeted based on anatomical landmarks. Given that the arrays were continuous to the central sulcus and area 1 spans ∼3 to 5 mm of cortical surface from the sulcus (27), few if any electrodes were located in area 2. Given the length of the electrodes, their tips likely terminated in the infragranular layers of the somatosensory cortex if embedded to their base, as we have previously shown in postmortem histological analysis with other animals instrumented with identical arrays (28). We mapped the receptive field of each electrode by identifying which areas of skin evoked significant z-scored multiunit activity (16). The age of the implanted arrays used in these studies ranged from 2 mo to 4 y. The stability of sensitivity to ICMS in area 1 over multiple years has been documented (29).
Stimuli.
Intracortical stimulation (ICMS) consisted of cathodal phase-leading symmetrical biphasic pulses delivered through a 96-channel neurostimulator (CereStim R96; Blackrock Microsystems Inc., Salt Lake City, UT). Across all tested stimulation regimes, pulse train frequencies ranged from 17 to 400 Hz, pulse amplitudes ranged from 44 to 100 µA, and phase durations equaled 200 or 400 µs. The interval between phases was always 53 µs. All biphasic pulses within the same stimulus were separated by the same time interval (pulse trains were periodic). In all experiments, pulse train duration was always 1 s. In some experiments, all of the pulses in a train had the same amplitude; in others, amplitude varied from pulse to pulse (see below).
Behavioral Task.
The animals were seated at the experimental table facing a monitor, which signaled the trial progression (Fig. 1C). Eye movements were tracked with an optical eye-tracking system (MR PC60; Arrington Research, Scottsdale, AZ). The animals initiated trials by directing their gaze to a cross in the center of the monitor. A trial was aborted if the animal failed to maintain its gaze on the center until the appearance of response targets. Each trial comprised 2 successive stimulus intervals, each indicated by a circle on the video monitor, lasting 1 s, and separated by a 1-s interstimulus interval during which the circle disappeared, followed by a response interval during which 2 response targets appeared on either side of the gaze fixation point (Fig. 1C). The animals’ task was to judge which of the 2 pulse trains was higher in frequency. The animals responded by making a saccadic eye movement toward the left (selecting the first stimulus) or right (second stimulus) target. Correct responses were rewarded with juice. Psychophysical performance was calculated as the proportion of trials on which the higher frequency stimulus was selected.
Experimental Design.
Stimulus set for detailed psychometric curves.
We first performed extensive testing on a small group of electrodes, building psychometric curves spanning a wide range of frequencies. In each test block, consisting of several hundred trials, one stimulus of each pair had the same standard frequency (20, 50, 100, or 200 Hz), and the other stimulus was drawn from a set of comparison frequencies around the standard (comparisons for the 20-Hz standard: 17, 18, 22, 23, 26, and 29 Hz; comparisons for the 50-Hz standard: 30, 35, 40, 45, 55, 60, 65, and 70 Hz; comparisons for the 100-Hz standard: 50, 75, 150, 200, 250, and 300 Hz; comparisons for the 200-Hz standard: 50, 100, 150, 250, 300, 350, and 400 Hz) (SI Appendix, Table S1). Phase duration during each pulse was 200 µs at the higher frequencies (100- and 200-Hz standards) and 400 µs at the lower ones (20- and 50-Hz standards) to ensure that the stimuli were detectable (1). Stimulus amplitudes were 50, 60, 70, or 80 µA, comfortably above expected detection threshold (5). Every possible combination of frequencies (standard vs. comparisons) and amplitudes, numbering hundreds of unique stimulus pairs, was presented in each test block, ensuring the animals had to perform frequency discrimination instead of memorizing the correct responses to individual pairs of stimuli. For each electrode/standard combination, animals were trained until they reached stable performance, a process which could take weeks or even months as we incrementally included harder stimulus pairs (those with small frequency differences and large amplitude confounds). To discourage the animals’ reliance on perceptual magnitude in making their frequency judgments, we overrepresented stimulus pairs in which the higher-frequency stimulus was lower in amplitude by 50%. That way, the higher-frequency stimulus was lower in amplitude on ∼50% of trials, to compensate for the fact that the higher-frequency stimulus will feel more intense on equal-amplitude pairs. The psychometric curves were constructed after asymptotic performance was achieved. We extensively tested 5 electrodes (4 from monkey A, 1 from monkey B) at standard frequencies of 50, 100, and 200 Hz. Only 1 electrode from each of monkeys A and B was tested at the 20-Hz standard before testing was cut short by the failure of Monkey A’s array. Three electrodes from monkey C were extensively tested at only the 100-Hz standard before testing was cut short by health issues that precluded water restriction.
Reduced stimulus set.
After extensively testing a few electrodes, which took weeks or even months for each electrode and standard frequency, we developed a reduced stimulus set to test the animals’ performance at a faster pace over a wide range of electrodes. In this stimulus set, the frequency difference was always 100 Hz, a salient difference according to the psychometric curves obtained from the full set, and the base (lower) frequency was 70, 80, 90, 100, 110, 120, 130, or 170 Hz. The amplitudes were the same as in the full set (50, 60, 70, or 80 µA) and, again, parametrically combined (SI Appendix, Table S1). Here, too, we overrepresented stimulus pairs in which the higher frequency stimulus had a lower amplitude during the training phase, to reduce the animals’ reliance on intensive cues in making their frequency judgments. We tested 17 electrodes from monkey B with this stimulus set. Instead of first training to asymptotic performance while incrementally adding harder stimulus pairs, we had the animal complete several thousand trials (from 2,500 to 6,000) with the complete stimulus set at each electrode. On 4 of the 17 electrodes, the animal performed the frequency discrimination task on 2 subsets of stimuli in separate experimental blocks: one containing only pairs with equal amplitudes and one containing only pairs in which the base stimulus’s amplitude was 30 µA higher than that of the comparison stimulus (SI Appendix, Fig. S7, Table S1, and Validation of catch trials).
Catch trials.
Included in the reduced stimulus set was a small proportion of trials (∼5%) on which the 2 stimuli in the pair were at the same frequencies but differed in amplitude by 30 µA. On these catch trials, all base frequencies were used. The animal was rewarded randomly during these trials. The animal’s bias toward the higher or lower amplitude stimulus in the absence of frequency differences gauged its reliance on intensive cues (Fig. 5B and SI Appendix, Fig. S7).
Variable-amplitude pulse trains.
The mean amplitudes of the pulse train were 58, 72, and 86 µA, but the amplitudes of individual pulses spanned a range around the mean (SI Appendix, Table S1). For each stimulus, one of the following sets of individual pulse amplitudes was randomized and then repeated with the frequency-appropriate interpulse interval to complete a 1-s-long stimulus: 44 to 72, in increments of 2; 58 to 86, in increments of 2; and 72 to 100, in increments of 2. One of the stimuli in each pair was at 75, 105, 135, or 165 Hz, and the other was 90 Hz higher. Our implementation was constrained by our stimulation hardware, which required that the frequency be a multiple of the number of different pulse amplitudes used (to last exactly 1 s). The maximum number of different amplitudes was 15 so we used frequencies that were multiples of 15 for this experiment. For each pair of frequencies, every possible combination of amplitudes (variable or constant) was tested. Of the 17 electrodes of monkey B tested with the reduced stimulus set, we tested (with variable-amplitude pulse trains) 4 electrodes showing small effects of amplitude on performance and 3 electrodes showing large amplitude effects.
Detection threshold measurements.
One interval on each trial contained a 100-Hz pulse train at 10, 25, 40, 55, or 70 µA, and the other interval was empty. The animal reported which interval contained the stimulus. Twelve electrodes from monkey B covering the range of susceptibilities to amplitude were tested. The animal was trained to perform the detection task only after all data collection for the frequency discrimination experiments was complete.
Data Analysis.
Psychophysics.
We built psychometric curves by fitting performance at each comparison frequency to a cumulative normal density function. Just noticeable differences (JNDs) and Weber fractions were calculated using only trials on which both the stimuli in the pair were equal in amplitude using a criterion performance of 75% correct (Fig. 2). JNDs were calculated as the average of the frequency differences required for threshold performance above and below the standard frequency. These were nearly equal for standard frequencies of 20 and 50 Hz but tended to be asymmetric at higher frequencies (with the upper JND greater than the lower one). If performance did not reach threshold for comparison frequencies above the standard for a given electrode and standard, only the frequency difference below the standard was used. This only occurred with the 200-Hz standard frequency.
Gauging the relative contribution of amplitude and frequency to discrimination judgments.
The behavioral data show that the animals’ judgments depended on both frequency and amplitude, but the relative contribution of these 2 stimulation parameters varied from electrode to electrode. To assess the relative contributions of frequency and amplitude to discrimination judgments, we modeled the position of each stimulus along the task-relevant sensory dimension as a weighted combination of the stimulus’s frequency and amplitude. To predict performance we first subtracted the value of the standard stimulus from that of the comparison along this sensory continuum for each pair. The resulting differences then constituted the input to a sigmoid (cumulative normal density function). The resulting function comprised 3 free parameters: 2 regression weights (frequency and amplitude) and 1 sigmoid parameter (SD). For each of the 5 electrodes through which the complete stimulus set was delivered, the function was optimized to predict behavioral performance. The model provided an accurate fit of the behavioral data (R2 mean ± SEM) (SI Appendix, Fig. S5B). The regression weights gauge the relative contribution of frequency and amplitude in determining the animals’ choices (SI Appendix, Fig. S5B). For example, to the extent that the regression weight for amplitude was low, we concluded that the animal was able to discriminate frequency independent of amplitude on that electrode.
Generating equivalent frequency-amplitude trade-offs using single-variable discrimination.
We wished to test the hypothesis that frequency discrimination performance was based entirely on intensive cues. That is, changes in frequency and changes in amplitude had the same effect on the evoked percept. To test this hypothesis, we first assessed discriminability when only frequency or only amplitude changed and then assessed whether the performance in these experiments could account for performance when both parameters varied (Fig. 5 and SI Appendix, Figs. S5 and S6). To gauge sensitivity to ICMS amplitude, we used previously collected behavioral data in amplitude discrimination task, published in Kim et al. (5). In these experiments, the standard amplitude was 70 µA, and the comparisons were 40, 50, 60, 80, 90, and 100 µA. Psychometric curves were built based on amplitude discrimination performance averaged across all frequencies (50, 100, 250, and 500 Hz) because we found that frequency had a negligible effect on amplitude JNDs. To gauge sensitivity to ICMS frequency, we restricted the analysis to trials in which both stimuli had amplitudes of 70 µA.
Using the psychometric functions derived from the single-parameter discrimination experiments, we computed equivalent frequency-amplitude trade-offs by equating changes in frequency and amplitude that resulted in equal discrimination performance. For example, if an amplitude difference of ±10 µA and a frequency difference of ±8 Hz both resulted in a discrimination performance of 65%, ±10 µA and ±8 Hz were considered to be perceptually equivalent. The resulting predicted equal intensity curves were smoothed, and the tangent to this curve (the rate of change in Hz/µA) was computed at the point corresponding to the standard frequency and 70 µA. The frequency/amplitude trade-off implied by this slope could then be compared to the trade-off obtained with the linear model above, which was derived without the assumption that the sensory consequences of amplitude and frequency changes are indistinguishable. Because the amplitude discrimination data were collected for a separate experiment several years prior to this one (retraining animals from amplitude discrimination to frequency discrimination, or training new animals to perform frequency discrimination, was a very lengthy process and imposed a long delay before we could collect any of the frequency discrimination data presented here), it was performed on different electrodes (8 total, collectively the “amplitude electrodes”) than the frequency experiment (the “frequency electrodes”). While it would have been preferable to have both amplitude discrimination and frequency discrimination data from the same electrodes, we mitigated this weakness in the design by matching each “frequency electrode” with each of the 8 “amplitude electrodes” in turn to carry out this analysis. This yielded a distribution of possible offset rates (Hz/µA) based on each amplitude sensitivity at each frequency electrode. If the frequency-amplitude trade-off computed from the model fell outside of this distribution, results from this analysis were inconsistent with the hypothesis that frequency and amplitude affect a common intensive continuum. Note that this approach constitutes a highly conservative characterization of the possible outcomes of the null hypothesis, in that electrodes that span the entire range of amplitude discrimination performance are included in the computation of the single-variable offset ratio. Strong performance on amplitude discrimination yields a lower ratio, which is thus more liable to support the null hypothesis (that frequency and amplitude exert the same effect on performance).
Statistical test of the effect of amplitude differences on performance within electrode.
Restricting the analysis to trials on which amplitude difference between the comparison and standard stimuli was −30, 0, or 30 µA and the frequency difference was 100 Hz (Fig. 4), we tested the hypothesis that outcome was independent of amplitude difference with a χ2 test. The black dots in Fig. 4 denote electrodes for which the hypothesis was rejected with an alpha level of 0.01.
Statistical significance of differences in the effect of amplitude across electrodes.
Restricting the analysis to trials where the amplitude difference was −30, 0, or 30 µA and the frequency difference was 100 Hz (Fig. 4), we performed a 2-way ANOVA with electrode, amplitude difference, and their interaction as factors.
Statistical comparison of offset ratios from single-variable and combined-variable discrimination experiments.
We used the Wilcoxon–Mann–Whitney test to assess the likelihood that offset rates derived from the combined-variable discrimination experiment (1 value per electrode-standard frequency combination) and the single-variable experiment (8 values per electrode-standard frequency combination, 1 for each electrode tested in the amplitude discrimination experiment) were drawn from the same distribution (alpha level = 0.01).
All data presented in this article are available at https://gin.g-node.org/JohnDowney/ICMS_Frequency_Discrimination_Data (30).
Supplementary Material
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS095251.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: All data presented in this article are available at https://gin.g-node.org/JohnDowney/ICMS_Frequency_Discrimination_Data.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916453117/-/DCSupplemental.
References
- 1.Berg J. A., et al. , Behavioral demonstration of a somatosensory neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 500–507 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Flesher S. N., et al. , Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 8, 361ra141 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Flesher S., et al. , “Restoring touch through intracortical microstimulation of human somatosensory cortex” in Proceedings of 2017 New Generation of CAS (NGCAS) (IEEE, 2017), pp. 185–188. [Google Scholar]
- 4.Kim S., Callier T., Tabot G. A., Tenore F. V., Bensmaia S. J., Sensitivity to microstimulation of somatosensory cortex distributed over multiple electrodes. Front. Syst. Neurosci. 9, 47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S., et al. , Behavioral assessment of sensitivity to intracortical microstimulation of primate somatosensory cortex. Proc. Natl. Acad. Sci. U.S.A. 112, 15202–15207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tabot G. A., et al. , Restoring the sense of touch with a prosthetic hand through a brain interface. Proc. Natl. Acad. Sci. U.S.A. 110, 18279–18284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensmaia S. J., Miller L. E., Restoring sensorimotor function through intracortical interfaces: Progress and looming challenges. Nat. Rev. Neurosci. 15, 313–325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bensmaia S. J., Biological and bionic hands: Natural neural coding and artificial perception. Philos. Trans. R. Soc. B Biol. Sci. 370, 20140209 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armenta Salas M., et al. , Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. eLife 7, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flesher S., et al. , Restored tactile sensation improves neuroprosthetic arm control. bioRxiv:10.1101/653428 (31 May 2019).
- 11.Romo R., Hernández A., Zainos A., Salinas E., Somatosensory discrimination based on cortical microstimulation. Nature 392, 387–390 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Romo R., Hernandez A., Zainos A., Sensing without touching: Somatosensory discrimination based on cortical microstimulation. Neuron 26, 273–278 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Lee B., et al. , Engineering artificial somatosensation through cortical stimulation in humans. Front. Syst. Neurosci. 12, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei Y.-C., Denchev P. V., Hsiao S. S., Craig J. C., Bensmaia S. J., Convergence of submodality-specific input onto neurons in primary somatosensory cortex. J. Neurophysiol. 102, 1843–1853 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saal H. P., Bensmaia S. J., Touch is a team effort: Interplay of submodalities in cutaneous sensibility. Trends Neurosci. 37, 689–697 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Callier T., Suresh A. K., Bensmaia S. J., Neural coding of contact events in somatosensory cortex. Cereb. Cortex, 10.1093/cercor/bhy337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridman G. Y., Blair H. T., Blaisdell A. P., Judy J. W., Perceived intensity of somatosensory cortical electrical stimulation. Exp. Brain Res. 203, 499–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S., Callier T., Bensmaia S. J., A computational model that predicts behavioral sensitivity to intracortical microstimulation. J. Neural Eng. 14, 016012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Anna E., et al. , A somatotopic bidirectional hand prosthesis with transcutaneous electrical nerve stimulation based sensory feedback. Sci. Rep. 7, 10930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graczyk E. L., et al. , The neural basis of perceived intensity in natural and artificial touch. Sci. Transl. Med. 8, 362ra142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valle G., et al. , Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron 100, 37–45.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Tehovnik E. J., Electrical stimulation of neural tissue to evoke behavioral responses. J. Neurosci. Methods 65, 1–17 (1996). [DOI] [PubMed] [Google Scholar]
- 23.Tolias A. S., et al. , Mapping cortical activity elicited with electrical microstimulation using FMRI in the macaque. Neuron 48, 901–911 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Histed M. H., Bonin V., Reid R. C., Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63, 508–522 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michelson N. J., Eles J. R., Vazquez A. L., Ludwig K. A., Kozai T. D. Y., Calcium activation of cortical neurons by continuous electrical stimulation: Frequency dependence, temporal fidelity, and activation density. J. Neurosci. Res. 97, 620–638 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey M. A., Saal H. P., Dammann J. F. 3rd, Bensmaia S. J., Multiplexing stimulus information through rate and temporal codes in primate somatosensory cortex. PLoS Biol. 11, e1001558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pons T. P., Garraghty P. E., Cusick C. G., Kaas J. H., A sequential representation of the occiput, arm, forearm and hand across the rostrocaudal dimension of areas 1, 2 and 5 in macaque monkeys. Brain Res. 335, 350–353 (1985). [DOI] [PubMed] [Google Scholar]
- 28.Rajan A. T., et al. , The effects of chronic intracortical microstimulation on neural tissue and fine motor behavior. J. Neural Eng. 12, 066018 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callier T., et al. , Long-term stability of sensitivity to intracortical microstimulation of somatosensory cortex. J. Neural Eng. 12, 056010 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Callier T., Brantly N., Caravelli A., Bensmaia S. J., Data from “The frequency of cortical microstimulation shapes artificial touch.” GIN. https://gin.g-node.org/JohnDowney/ICMS_Frequency_Discrimination_Data. Deposited 27 November 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.