Significance
Phosphate is crucial for the origin of life because it is ubiquitous in key biomolecules. A major issue is that prebiotic syntheses use concentrated phosphate to incorporate phosphate into biomolecules, whereas natural waters are generally phosphate-poor because phosphate reacts with calcium to form low-solubility apatite minerals. Here we show that carbonate-rich lakes can concentrate phosphate to >1 molal levels by locking up calcium in carbonate minerals, which prevents phosphate removal by apatite precipitation. Phosphate-rich lakes may have preferentially formed on the prebiotic Earth because of carbonic acid weathering under CO2-rich atmospheres and the absence of microbial phosphate consumption. This specifically points to an origin of life in carbonate-rich lakes, and so defines aqueous conditions that prebiotic chemists should consider.
Keywords: phosphate, origin of life, early Earth, carbonate-rich lakes
Abstract
Phosphate is central to the origin of life because it is a key component of nucleotides in genetic molecules, phospholipid cell membranes, and energy transfer molecules such as adenosine triphosphate. To incorporate phosphate into biomolecules, prebiotic experiments commonly use molar phosphate concentrations to overcome phosphate’s poor reactivity with organics in water. However, phosphate is generally limited to micromolar levels in the environment because it precipitates with calcium as low-solubility apatite minerals. This disparity between laboratory conditions and environmental constraints is an enigma known as “the phosphate problem.” Here we show that carbonate-rich lakes are a marked exception to phosphate-poor natural waters. In principle, modern carbonate-rich lakes could accumulate up to ∼0.1 molal phosphate under steady-state conditions of evaporation and stream inflow because calcium is sequestered into carbonate minerals. This prevents the loss of dissolved phosphate to apatite precipitation. Even higher phosphate concentrations (>1 molal) can form during evaporation in the absence of inflows. On the prebiotic Earth, carbonate-rich lakes were likely abundant and phosphate-rich relative to the present day because of the lack of microbial phosphate sinks and enhanced chemical weathering of phosphate minerals under relatively CO2-rich atmospheres. Furthermore, the prevailing CO2 conditions would have buffered phosphate-rich brines to moderate pH (pH 6.5 to 9). The accumulation of phosphate and other prebiotic reagents at concentration and pH levels relevant to experimental prebiotic syntheses of key biomolecules is a compelling reason to consider carbonate-rich lakes as plausible settings for the origin of life.
Phosphate is central to the origin of life (1–4) because it is a key component of nucleotides, phospholipids, and metabolites such as adenosine triphosphate used in cellular replication, compartmentalization, and energy transfer, respectively (5). A major issue for prebiotic chemistry is that phosphate combines with Ca2+ down to micromolar levels to form apatite-group minerals [e.g., Ca5(PO4)3(OH,F,Cl)] or with Fe3+ and Al3+ in acidic solutions to form Fe/Al phosphates (6). However, laboratory experiments seeking to understand prebiotic phosphorylation (i.e., adding a phosphoryl group to an organic molecule) commonly use ∼1 molal phosphate to overcome phosphate’s poor reactivity with organics in water (2–4, 7, 8). Concentrated phosphate also catalyzes acid/base and nucleophilic reactions and acts as a pH and chemical buffer (8). The geochemical difficulty of forming concentrated phosphate, combined with its central role in prebiotic syntheses, is a long-standing enigma known as “the phosphate problem” (1, 4).
Difficulties with using aqueous phosphate have motivated research on more reactive phosphorylating agents such as struvite (MgNH4PO4·6H2O), polyphosphates, and amidophosphates (9–11), and alternative solvents such as formamide and urea (12, 13). These approaches are generally problematic because the proposed reagents would have been scarce in plausible early Earth environments (10, 14, 15). Reduced phosphorus compounds, such as phosphite , may also form when water reacts with meteoritic material, and are more reactive and soluble than orthophosphate (1, 16); however, phosphorylation with phosphite primarily yields phosphonates, that is, compounds with P–C bonds vs. P–O–C bonds in organophosphates. These issues have motivated recent proposals for an origin of life in extremely acidic hot spring environments (17), which can accumulate aqueous phosphate as species, but measured phosphate concentrations in acid hot springs are limited to <1 mM (18).
Here we to show that carbonate-rich lakes are an exception to the rule of low phosphate concentrations found in most natural waters. Using environmental data, laboratory experiments, and geochemical models, we find that >1 molal phosphate concentrations could have occurred on the early Earth in carbonate-rich lake environments. This provides a plausible geochemical source of concentrated, soluble phosphate needed in prebiotic syntheses, and points to a specific environment for the origin of life in carbonate-rich lakes.
High Phosphate Concentrations in Present-Day, Carbonate-Rich Lakes
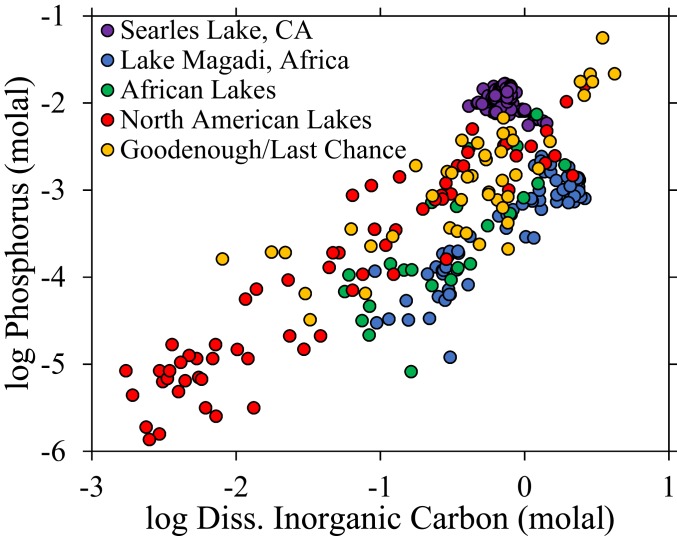
Phosphate concentrations are remarkably high in closed-basin, carbonate-rich lakes and generally increase with increasing carbonate alkalinity (Fig. 1) (see SI Appendix, Appendix A for other dissolved species). Searles Lake, located within the Mojave Desert in California, is an economically important carbonate-, boron-, and sulfate-rich groundwater brine within a dry lake basin that contains 5 to 17 mM phosphate (19). Even higher total phosphorus concentrations are found in Goodenough and Last Chance Lakes in British Columbia, which can exceed 50 mM during periods of desiccation in the summer and autumn (20, 21). Other carbonate-rich lakes, such as Lake Magadi in Africa (22, 23) and Mono Lake in California (24), contain ∼1 mM phosphate.
Fig. 1.
Phosphorus concentrations in carbonate-rich lakes, including Searles Lake (19, 69), Lake Magadi (22, 23), Goodenough and Last Chance Lakes (20, 36), various other North American lakes (36, 69–71), and various other African lakes (72–75). Inorganic phosphate was determined in most analyses; however, the Goodenough and Last Chance Lake analyses are for total phosphorus (inorganic + organic).
Closed-basin lakes accumulate high salt concentrations, including phosphates, from chemical weathering within their hydrologic basins, followed by the inflow of weathering products via streams to evaporating lake brines (25). Carbonate-rich lakes, in particular, develop when atmospheric or volcanogenic CO2 gas dissolves in water to form carbonic acid , which chemically weathers rocks to form dissolved cations (Na+, K+, Ca2+, and Mg2+) and carbonate alkalinity ( and ). Upon evaporation of these fluids, Ca2+ and Mg2+ ions precipitate as carbonates and silicates, and the residual carbonate alkalinity concentrates as Na–HCO3–CO3-rich brine (26).
Phosphate is thought to accumulate in carbonate-rich lakes because the carbonate alkalinity strongly precipitates Ca2+ from solution as low-solubility Ca2+ carbonates, which allows phosphate to reach much higher concentrations before saturating with respect to apatite (27, 28). However, the specific minerals that control Ca2+ and phosphate concentrations, and the maximum phosphate concentrations possible, are unknown because apatites and carbonates form poorly defined and/or metastable phases in carbonate-rich brines. Na+, Mg2+, , , and F− ions typically found at high concentrations in carbonate-rich lakes extensively substitute in apatites (26, 29, 30), which greatly influences their solubility, nucleation, and crystallization rate (30). Furthermore, even low phosphate concentrations inhibit the nucleation and growth of both calcite (CaCO3) and aragonite (a low-temperature CaCO3 polymorph), so other carbonate phases likely control Ca2+ concentrations in carbonate-rich lakes (31). A final issue is that biology cycles organic and inorganic phosphorus in carbonate-rich lakes, which makes it difficult to separate biotic vs. abiotic influences on phosphate concentrations.
Experiments on Phosphate Accumulation in Carbonate-Rich Brines
The preceding considerations motivated a series of experiments to determine how much phosphate can accumulate by abiotic processes in carbonate-rich lakes. We start by noting that carbonate-rich lakes can only accumulate phosphate if Ca2+ precipitates in carbonates instead of apatites; otherwise, phosphate will be removed from solution by much higher Ca2+ influxes to lake basins. Consequently, how much phosphate can accumulate in carbonate-rich lakes is determined by what phases Ca2+ preferentially precipitates in. If Ca2+ precipitates as apatite, it will limit phosphate concentrations in the brine. On the other hand, if Ca2+ carbonates form, then phosphate can accumulate at higher concentrations.
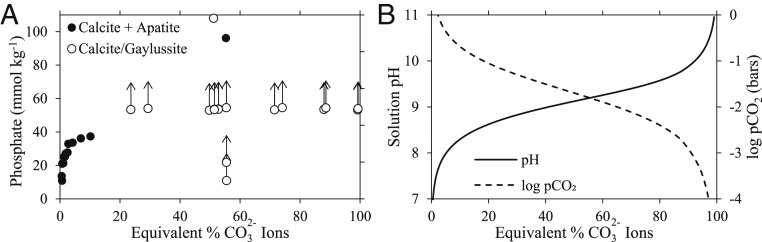
To investigate Ca2+ precipitation in phosphate- and carbonate-rich brines, we slowly added Ca2+ using gypsum (CaSO4·2H2O) and phosphate using Na2HPO4 to various saturated Na2CO3/NaHCO3 brines in the laboratory at room temperature (∼23 °C). We then analyzed the salts that precipitated for mineralogy and chemistry (Methods). Results indicate that the formation of apatite and Ca2+ carbonate salts depends primarily on the relative concentrations of carbonate vs. bicarbonate ions in solution (Fig. 2A). High ion concentrations strongly precipitate Ca2+ from solution as gaylussite (Na2CO3·CaCO3·5H2O) and/or calcite (open circles in Fig. 2A), which allows phosphate to accumulate up to ∼100 mmol⋅kg−1. Gaylussite is commonly found in carbonate-rich lakes, such as in Searles Lake and nearby Mono Lake (32); however, the formation of calcite is surprising because even trace phosphate levels inhibit calcite nucleation and growth (31). We infer that calcite probably formed indirectly through incongruent dissolution of primary gaylussite based on the slow conversion of gaylussite to calcite we observed over 4 wk (SI Appendix, Appendix B, Fig. S4), which is supported by observations of gaylussite converting into CaCO3 in Mono Lake (33, 34).
Fig. 2.
Results from experiments on phosphate- and carbonate-rich brines. (A) The concentration of phosphate in saturated Na–HCO3–CO3 brines after 2 wk of equilibration with 50 to 100 mmol⋅kg−1 Ca2+ in the form of gypsum (CaSO4·2H2O) and initial phosphate concentrations from 10 to 100 mmol⋅kg−1. Solid circles indicate solutions saturated with respect to both apatite and calcite (determined via XRD; see Methods). Open circles indicate solutions that are saturated with respect to calcite and/or gaylussite (Na2CO3·CaCO3·5H2O); hence, phosphate must be more concentrated than these points for apatite to precipitate (indicated by the upwards arrows). The x axis gives the equivalent percentage of ions relative to the total carbonate alkalinity, that is, . (B) The modeled pCO2 and pH of the experimental saturated Na–HCO3–CO3 brines at 25 °C, which correspond to the solutions in A.
At low ion concentrations, Na-rich carbonated apatites and calcite phases occur together, and phosphate concentrations in equilibrium with these minerals decrease with decreasing (black circles near the left of the vertical axis in Fig. 2A). These results are consistent with Ca2+ from carbonate salts controlling the solubility of apatite. A decrease in ion concentration increases the solubility of calcite/gaylussite, which increases the concentration of Ca2+ in solution and causes phosphate to precipitate as apatite.
The relative proportion of vs. ions in solution also controls the pCO2 and pH, which we model for the experimental saturated Na–HCO3–CO3 brines in Fig. 2B (SI Appendix, Appendix B, Table S1 and Methods). By comparing modeled pCO2 values to recent estimates ranging from 0.01 to 1 bar CO2 on the early Earth (35), we find that corresponding phosphate concentrations in our experiments are between 20 and 100 mmol⋅kg−1, respectively. Furthermore, the pH ranges from circumneutral at 1 bar pCO2 to pH 9 at 0.01 bar pCO2 in saturated Na–HCO3–CO3 brines because the relatively high pCO2 acidifies the solutions. This suggests that elevated phosphate concentrations could have occurred in CO2-rich atmospheres on the early Earth.
Evaporative Concentration of Phosphate-Rich Brines
Our experiments indicate that when Ca2+ is added to phosphate- and carbonate-rich brines, apatite minerals only precipitate above 10 to 100 mmol⋅kg−1 phosphate. Applied to a closed-basin lake setting, this means that phosphate delivered via streams can accumulate up to ∼100 mmol⋅kg−1; however, at higher phosphate concentrations, Ca2+ delivered by stream inflows will precipitate phosphate from solution as apatite. Given that streams have much more Ca2+ than phosphate (36), closed-basin lakes in hydrologic balance (i.e., stream inflows balance water loss via evaporation) are constrained to <100 mmol⋅kg−1 phosphate. However, once a phosphate-rich lake forms, the brine contains much more phosphate than Ca2+ because Ca2+ is sequestered into carbonate minerals (see SI Appendix, Appendix A for lake data). Consequently, in the absence of stream inflows, evaporating lake brines can reach much higher phosphate concentrations. Such evaporation may occur seasonally because of wet and dry cycles. Alternatively, lake brines may desiccate in shoreline environments by evaporation on rocks wetted by wave action or evaporation in local pools.
During lake evaporation (in the absence of Ca-rich stream inflows), phosphate will accumulate in the residual brine until it precipitates with a more concentrated cation as a salt. This phosphate-limiting salt cannot be a Ca2+ or Mg2+ phase because these ions are minor in carbonate-rich brines. We can also exclude K+ because it is minor relative to Na+ in carbonate-rich lakes and only precipitates phosphate salts at extremely high concentrations (37). The remaining major cation is Na+, which implies that phosphate concentrations will increase in evaporating lake brines until the brine reaches saturation with respect to sodium phosphate salts. In addition, high phosphate concentrations would only form after sodium carbonate/bicarbonate and chloride salts had precipitated because phosphate is minor relative to both carbonate alkalinity and chloride.
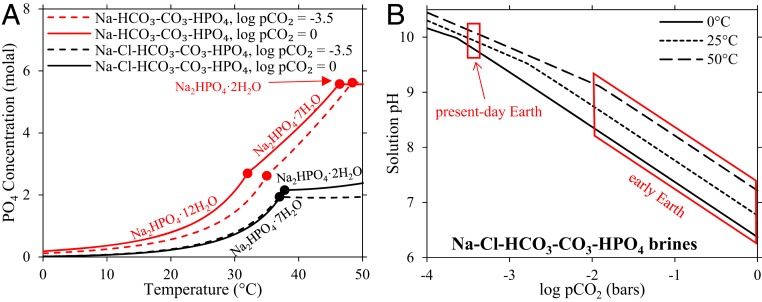
To determine the maximum phosphate concentrations possible in such brines, we model solutions saturated with respect to sodium phosphate, carbonate, and chloride salts at variable temperature (0 to 50 °C) and CO2 gas pressure (log pCO2 −3.5 to 0 bars). We find that up to 3 molal phosphate occurs in equilibrium with either Na2HPO4·7H2O or Na2HPO4·2H2O salts (Fig. 3A). Relative to pure sodium phosphate solutions (Fig. 3A, red lines), phosphate concentrations in saturated mixed brines (Fig. 3A, black lines) decrease by about one-third because the additional Na+ associated with chlorides and carbonates forces Na2HPO4 to precipitate from solution. The carbonate alkalinity precipitates as either nahcolite (NaHCO3), natron (Na2CO3·10H2O), or trona (Na2CO3·NaHCO3·2H2O) depending on the pCO2, and chloride ions precipitate as halite (NaCl).
Fig. 3.
Phosphate concentrations and pH modeled in saturated, carbonate-rich lake brines. (A) The concentration of phosphate in solutions saturated with respect to sodium phosphate. The red lines indicate the solubility of pure Na2HPO4·XH2O, where XH2O refers to the hydration state (X = 2, 7, and 12). The black lines indicate the solubility of sodium phosphate in solutions saturated with respect to sodium phosphate, chloride, and carbonate salts. Solid lines are for 1 bar atmospheric pCO2, and dashed lines are for log pCO2 = −3.5 bar (the pCO2 of present-day Earth). (B) The pH of solutions saturated with respect to sodium phosphate, chloride, and carbonate salts at variable temperature and pCO2. The range of pH and temperature of these solutions encompasses that of the black lines in A.
The modeled pH of saturated phosphate brines depends on the temperature and atmospheric pCO2 (Fig. 3B). At present-day pCO2 levels, solutions are highly alkaline (pH ∼ 10), consistent with high pHs measured in modern soda lakes (38); however, in CO2-rich atmospheres on the early Earth (log pCO2 = −2 to 0) brines range from moderately alkaline (pH 9) to slightly acidic (pH 6.5) because of acidification by CO2. These are maximum pH values because the solutions are saturated with respect to carbonate alkalinity; hence, for undersaturated solutions the pH will be lower. Temperature also affects the pH because CO2 is more soluble in solutions at lower temperatures. These results provide a plausible environmental scenario for molar phosphate concentrations at moderate pH levels used in prebiotic syntheses (8).
Carbonate-Rich Lakes on the Early Earth
Carbonate-rich lakes may have been relatively common on early Earth due to strong chemical weathering of abundant, fresh volcanic rocks (39) under early Earth’s CO2-rich atmosphere (40). Weathering would release phosphate from apatites and carbonate alkalinity from other minerals, which would accumulate in closed basins (41, 42). A potential analog for such carbonate-rich lakes on the early Earth is Lonar Lake in central India, a NaHCO3-rich lake formed from an impact crater in basalt host rock (43). Lonar Lake also precipitates gaylussite and calcite salts (44), but phosphate concentrations are relatively low (∼5 μM) because algal blooms consume the phosphate (45).
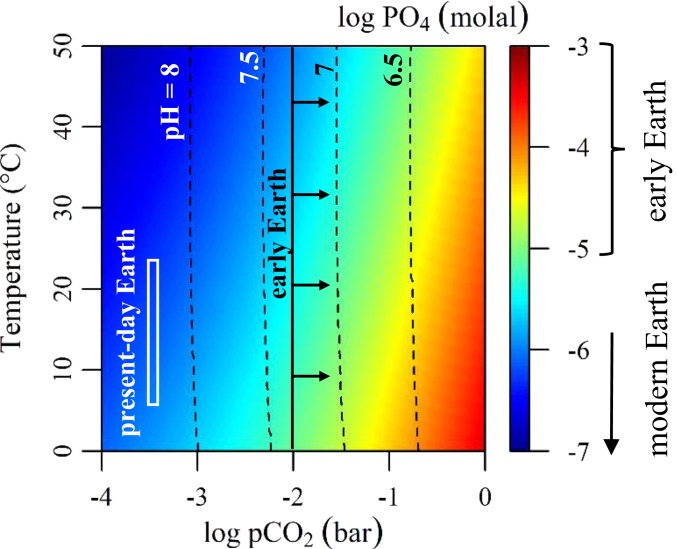
A consequence of early Earth’s CO2-rich atmosphere is that it would have enhanced the weathering of hydroxyl- and fluorapatites in mafic rocks by lowering the pH of surface waters (46, 47). Apatites are more soluble at lower pH and weather more rapidly in CO2-acidified stream and rainwater, resulting in potentially high phosphate fluxes to carbonate-rich lakes on the early Earth. By modeling the equilibrium solubility of fluorapatite and hydroxyapatite in the presence of calcite buffer as a function of temperature and CO2 pressure (Fig. 4 and Methods), we find that relatively low CO2 pressures on present-day Earth (log pCO2 = −3.5) limit phosphate to ≤1 μM, which is consistent with phosphate concentrations found in most present-day rivers and surface waters. However, in CO2-rich atmospheres relevant to the early Earth (pCO2 = 0.01 to 1 bar) (35, 40), modeled phosphate concentrations are 10 to 100 times higher, which implies a much higher phosphate weathering flux on the early Earth from streams into lakes.
Fig. 4.
Modeled phosphate concentrations (log PO4) in equilibrium with fluorapatite, hydroxylapatite, and calcite at variable pCO2 and temperature relevant to the release of phosphate into streams that supply lakes. The pH is given by dashed contours at 0.5-pH intervals.
Phosphate concentrations were likely higher in prebiotic, carbonate-rich lakes because microbial phosphate sinks were absent. Modern carbonate-rich lake environments are some of the most productive ecosystems on Earth in terms of biomass turnover because of high nutrient levels (38). As a result, phosphate measured in modern carbonate-rich lakes is probably a minimum because microbes consume the phosphate and eventually deposit it in organic matter. This may explain why Lake Magadi in Africa, a surface lake with high biomass turnover, has over 10 times lower phosphate concentrations than Searles Lake (which cannot support photosynthetic microbes because it is a groundwater brine), despite the much higher carbonate alkalinity found in Lake Magadi (Fig. 1). Consequently, in the absence of microbial consumption, carbonate-rich lakes on the prebiotic Earth may have hosted higher phosphate concentrations than typically occur today.
A potential sink for soluble phosphate on the early Earth is reduced soluble iron (Fe2+), which precipitates with phosphate as the low-solubility mineral vivianite [Fe3(PO4)2·8H2O]. However, Fe2+ also precipitates as siderite (FeCO3) in carbonate-rich brines, which limits Fe2+ to low levels and increases the solubility of phosphate from vivianite, similar to solubility relations in the CaCO3–apatite system shown in Fig. 2. Furthermore, siderite is less soluble than calcite, and unlike calcite, the nucleation and growth of siderite is not inhibited by phosphate. Although siderite crystallizes relatively slowly from solution at low temperatures (48), near-equilibrium conditions occur on a timescale of hours to days, which is fast relative to inflow and evaporation processes in closed-basin lakes. This implies that Fe2+ concentrations in anoxic, phosphate- and carbonate-rich brines will be lower than Ca2+, which suggests that Ca2+, not Fe2+, would have controlled phosphate concentrations in carbonate-rich lakes on the early Earth.
Implications for Prebiotic Chemistry
Our experiments and model results show that carbonate-rich lakes on the early Earth would have accumulated phosphate at concentration and pH levels relevant to laboratory prebiotic syntheses. Molar phosphate concentrations can occur during evaporation in lake marginal pools, on rocks wetted by wave action, and in shore sediments. Drying promotes condensation reactions such as phosphorylation by removing water (49), as well as more generally facilitating polymerization reactions (50). Condensation reactions may also have been promoted by deliquescence of highly soluble evaporites (51). Furthermore, carbonate-rich lakes are rich in clay, zeolite, and silica mineral assemblages that catalyze phosphorylation reactions because of their high reactive surface areas (52). Once phosphate reacts, changing lake levels or wave action could reintroduce the reaction products to the lake, or reaction products could undergo successive wet–dry cycles.
More broadly, closed-basin lakes are ideal sites for “one-pot” organic syntheses because they accumulate and concentrate reagents via evaporation across hydrologic basins (41, 49, 53). As a result, closed-basin lakes may have accumulated organic precursors for phosphorylation, as well as condensation agents that promote phosphorylation by forming reactive intermediate species (54) and polyphosphates (14). Boron also accumulates at high concentrations in closed-basin lakes (e.g., Searles Lake contains ∼0.5 molal boron; SI Appendix, Appendix A), which promotes the regioselective phosphorylation of ribose and stabilizes the furanose form needed to synthesize nucleic acids (55).
A difficulty for an origin of life in saline lakes is that high salt concentrations tend to destabilize lipid vesicles (56) and inhibit RNA oligimerization (57), but these issues are potentially resolvable. For example, lipid vesicles can stabilize in high-salt environments by binding to nucleotides and/or amino acids (58, 59). Given that concentrated prebiotic reagents and wet/dry cycles often used in the laboratory imply saline environments, more research is needed on prebiotic chemistry in salty solutions.
Recent attempts at “one-pot” syntheses have proposed closed-basin lakes as sites for the formation of amino acids, nucleotides, and lipid precursors in reaction networks involving ultraviolet light, phosphate, sulfur, and cyanide (53, 60). Our results indicate that high phosphate concentrations used in these reaction networks (8) plausibly occurred in carbonate-rich lake environments. Furthermore, the key cyanide component needed for this chemistry also preferentially accumulates in carbonate-rich lakes to make sodium ferrocyanide evaporites, which thermally decompose to sodium cyanide above 700 °C (possibly via magmatic activity or flash heating from asteroid impacts) (42, 53, 61). Concentrated sulfur needed for prebiotic syntheses may also have accumulated as sulfite ions (62). Consequently, carbonate-rich lakes accumulate a number of key reagents at high concentrations relevant to experimental prebiotic syntheses.
Conclusions
The results presented here show that carbonate-rich lakes accumulate phosphate because Ca2+ preferentially precipitates in gaylussite and/or calcite, which increases the solubility of apatites. In carbonate-rich lakes influenced by stream inflows, phosphate levels are limited to <0.1 molal because excess Ca2+ in stream water precipitates apatite above this phosphate concentration threshold. However, in the absence of stream inflows, phosphate can enrich to >1 molal levels during evaporation because phosphate concentrations exceed Ca2+ in carbonate-rich lakes. Such evaporation could occur continuously along lake margins, or during dry seasons.
Carbonate-rich lake environments were likely common on the early Earth due to chemical weathering of mafic rocks in early Earth’s CO2-rich atmosphere, and would have been relatively phosphate-rich compared to the present day because of efficient weathering of apatite minerals and the lack of microbial phosphate sinks. Furthermore, phosphate-rich brines on the early Earth would have had slightly acidic to moderately basic pH (pH 6.5 to 9) because of elevated atmospheric CO2 levels. Thus, carbonate-rich lakes are highly plausible environments for accumulating phosphate at concentration and pH levels relevant to laboratory syntheses of prebiotic organophosphate compounds. Given the central importance of phosphate in cellular processes, and that phosphate occurs at micromolar concentrations elsewhere, our results specifically point to carbonate-rich lakes as sites for prebiotic chemistry and the origin of life.
Methods
Experimental Ca2+ and Phosphate Precipitation in Carbonate-Rich Brines.
The goal of these experiments was to determine which salt phases precipitate Ca2+ and phosphate ions in phosphate- and carbonate-rich brines. To achieve this, we added gypsum (CaSO4·2H2O) to phosphate- and carbonate-rich brines prepared in the laboratory, and allowed the mixtures to react over 1 to 4 wk. The rationale behind this methodology is that the gypsum will slowly dissolve because it is sparingly soluble, gradually adding Ca2+ to solution, which approximates the slow addition of Ca2+ via inflow waters to closed-basin lakes. A potential complication to using gypsum as the Ca2+ source is that gypsum will also add ions to solution; however, the added is <0.1 molal does not precipitate as a solid phase, and is not expected to interfere with the precipitation of carbonate or phosphate salts. After equilibration of the solution, we analyzed the resulting solid phases using inductively coupled optical emission spectroscopy (ICP-OES) and X-ray diffraction (XRD). The detailed results of these experiments are given in SI Appendix, Appendix B.
To prepare phosphate- and carbonate-rich brines, we gravimetrically added NaHCO3, Na2CO3, Na2HPO4, and NaF reagents to 150 mL of deionized water in 250-mL polyethylene bottles. We added the NaHCO3 and Na2CO3 reagents in varying proportions ranging from pure NaHCO3 to pure Na2CO3 to make nearly saturated solutions (i.e., the concentration just below the point at which salt precipitates). We determined the saturation point for each Na–HCO3–CO3 mixture at 25 °C using the THEREDA Pitzer model described in Geochemical Models. We added the Na2HPO4 reagent at concentrations ranging from 10 to 500 mmol⋅kg−1, and the NaF reagent at 0 and 10 mmol⋅kg−1. Finally, we added Ca2+ in the form of gypsum, capped the solutions, and agitated them with an orbital shaker for 1 to 4 wk.
We performed 4 series of experiments using the brine mixtures described above:
-
1)
We varied the initial NaHCO3:Na2CO3 ratio at constant equilibration time (2 wk) and initial phosphate concentration (50 mmol⋅kg−1, the highest concentration measured in carbonate-rich lakes).
-
2)
We varied the equilibration time at constant initial NaHCO3:Na2CO3 ratio (0.6:0.4) and initial phosphate concentration (50 mmol⋅kg−1).
-
3)
We varied the initial phosphate concentration at constant equilibration time (2 wk) and initial NaHCO3:Na2CO3 ratio (0.6:0.4).
-
4)
We repeated experiments 1 and 3 described above with the addition of 10 mmol⋅kg−1 F−. These experiments are motivated by relatively high F− concentration commonly found in carbonate-rich lakes (SI Appendix, Appendix B), and because F− strongly precipitates with Ca2+ and phosphate in natural systems to form fluorapatites.
Analysis of Solids after Equilibration.
After the brine/salt mixtures equilibrated, we removed the solid phases by filtering. We then removed excess brine from the filtered solids by rinsing with ethanol and dried them in air for ∼10 min. We then powdered the solid samples and analyzed them using a Bruker D8 Discover XRD from 10 to 60° 2θ. Furthermore, we analyzed the solids for Na, Ca, S, and P using ICP-OES. To prepare the solid samples for ICP-OES analysis, we first dried the samples in a vacuum oven at 60 °C overnight, and then acidified weighed aliquots of the dried samples (∼50 mg) with 1 mL of ∼2 M nitric acid (which completely dissolved the solid). The acid-dissolved samples were then diluted as needed for ICP-OES analysis.
We calculated the content of the equilibrated solid phases (on a dry weight basis) by assuming molar charge balance via the equation
| [1] |
The total calculated weight percent of Ca2+, Na+, , , and components on a dry basis is typically within several percentage of 100% (SI Appendix, Appendix B, Table S2).
Calculation of Aqueous Species after Equilibration.
To calculate ion concentrations in the solutions after equilibration, we assumed that all of the Ca2+ had completely precipitated as solid phases; consequently, the total concentration of all other ions precipitated from solution may be determined relative to Ca2+. We also tested this assumption on a representative suite of samples and found that ∼99.9% of the Ca2+ remained in solid phases over the course of the experiments (see discussion in SI Appendix, Appendix B). For any given ion X, the concentration in solution after equilibration is given by
| [2] |
where subscripts indicate concentrations in the equilibrated solid, the initial, and the final solutions. We neglect F− concentrations because this ion is minor in the experimental solutions. Finally, to determine the pH, pCO2, and inorganic carbon speciation of the equilibrated solutions, we modeled the solution composition determined above in equilibrium with 100 mL of headspace gas, which is the headspace volume in our experiments, using the THEREDA Pitzer model at 25 °C (described in Geochemical Models).
Geochemical Models.
We use two approaches to model phosphate solubility at variable temperature and composition. To model solution chemistry at relatively low concentrations in Fig. 4, we use the phreeqc.dat database in the geochemical program PHREEQC (63). We supplemented phreeqc.dat with temperature-dependent equilibrium constants for fluorapatite [Ca5(PO4)3F] (64) and hydroxylapatite [Ca5(PO4)3OH] (65). To model concentrated solutions in the Na–Cl–P–CO2–H–OH system from 0 to 60 °C in Fig. 3, we developed a Pitzer model in PHREEQC using parameters from several existing models. The resulting PHREEQC database file, as well as a detailed description of the Pitzer model equations and the model fits to literature data, are given in SI Appendix, Appendix C.
For Pitzer parameters involving NaCl, NaHCO3, and Na2CO3 salts, we use Pitzer parameters and solubility products from CHEMCHAU (66), which is parameterized from 0 to 100 °C. We include NaCl, Na2CO3·7H2O, Na2CO3·H2O, NaHCO3, Na2CO3·10H2O, NaHCO3·Na2CO3·2H2O, and CO2 gas phases from CHEMCHAU. For phosphate Pitzer parameters, we use parameters from the THEREDA R-12 Pitzer database (37, 67, 68), and assume that Pitzer parameters involving phosphate are temperature-invariant. To model the temperature-dependent solubility of Na2HPO4·12H2O, Na2HPO4·7H2O, Na2HPO4·2H2O, Na2HPO4, NaH2PO4·2H2O, NaH2PO4·H2O, and NaH2PO4 solid phases, we fit literature data on the solubility of these salts to the temperature-dependent expression for equilibrium constants in PHREEQC, using the THEREDA solubility product at 25 °C as a basis. Finally, to model the dissociation constants of phosphoric acid (H3PO4) to , , and , we use the temperature-dependent dissociation constants in the phreeqc.dat database.
Data Availability.
Experimental data from this study are available in SI Appendix, Appendix B, Tables S1 and S2. The PHREEQC database file used to make Fig. 3 is given in SI Appendix, Appendix C.
Supplementary Material
Acknowledgments
We thank 2 anonymous reviewers for their constructive comments. This research was funded by the Simons Collaboration on the Origin of Life grant 511570 (to D.C.C.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916109117/-/DCSupplemental.
References
- 1.Gulick A., Phosphorus as a factor in the origin of life. Am. Sci. 43, 479–489 (1955). [Google Scholar]
- 2.Pasek M. A., Gull M., Herschy B., Phosphorylation on the early Earth. Chem. Geol. 475, 149–170 (2017). [Google Scholar]
- 3.Pasek M. A., Kee T. P., “On the origin of phosphorylated biomolecules” in Origins of Life: The Primal Self-Organization, Egal R., Lankenau D.-H., Mulkidjanian A. Y., Eds. (Springer, Berlin, 2011), pp. 57–84. [Google Scholar]
- 4.Schwartz A. W., Phosphorus in prebiotic chemistry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1743–1749, discussion 1749 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westheimer F. H., Why nature chose phosphates. Science 235, 1173–1178 (1987). [DOI] [PubMed] [Google Scholar]
- 6.Lindsay W. L., Chemical Equilibria in Soils (John Wiley & Sons, New York, 1979). [Google Scholar]
- 7.Fernández-García C., Coggins A. J., Powner M. W., A chemist’s perspective on the role of phosphorus at the origins of life. Life (Basel) 7, 1–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powner M. W., Gerland B., Sutherland J. D., Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 459, 239–242 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Handschuh G. J., Orgel L. E., Struvite and prebiotic phosphorylation. Science 179, 483–484 (1973). [DOI] [PubMed] [Google Scholar]
- 10.Gull M., Pasek M. A., Is struvite a prebiotic mineral? Life (Basel) 3, 321–330 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibard C., Bhowmik S., Karki M., Kim E. K., Krishnamurthy R., Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 10, 212–217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saladino R., Crestini C., Pino S., Costanzo G., Di Mauro E., Formamide and the origin of life. Phys. Life Rev. 9, 84–104 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Burcar B., et al. , Darwin’s warm little pond: A one‐pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea‐based solvent. Angew. Chem. Int. Ed. Engl. 55, 13249–13253 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Keefe A. D., Miller S. L., Potentially prebiotic syntheses of condensed phosphates. Orig. Life Evol. Biosph. 26, 15–25 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Karki M., Gibard C., Bhowmik S., Krishnamurthy R., Nitrogenous derivatives of phosphorus and the origins of life: Plausible prebiotic phosphorylating agents in water. Life (Basel) 7, 1–28 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasek M. A., Rethinking early Earth phosphorus geochemistry. Proc. Natl. Acad. Sci. U.S.A. 105, 853–858 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deamer D. W., Assembling Life: How Can Life Begin on Earth and Other Habitable Planets? (Oxford University Press, 2018). [Google Scholar]
- 18.Mulkidjanian A. Y., Bychkov A. Y., Dibrova D. V., Galperin M. Y., Koonin E. V., Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. U.S.A. 109, E821–E830 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith G. I., Stuiver M., Subsurface stratigraphy and geochemistry of late Quaternary evaporites, Searles Lake, California. Geol. Surv. Prof. Pap. 1043, 1–130 (1979). [Google Scholar]
- 20.Hirst J. F., “Sedimentology, diagenesis and hydrochemistry of the saline, alkaline lakes on the Cariboo Plateau, Interior British Columbia, Canada,” PhD thesis, University of Saskatchewan, Saskatoon: (2013). [Google Scholar]
- 21.Renaut R. W., Long P. R., Sedimentology of the saline lakes of the Cariboo plateau, Interior British Columbia, Canada. Sediment. Geol. 64, 239–264 (1989). [Google Scholar]
- 22.Eugster H. P., Chemistry and origin of the brines of Lake Magadi, Kenya. Mineral. Soc. Am. Spec. Pap. 3, 213–235 (1970). [Google Scholar]
- 23.Jones B. F., Eugster H. P., Rettig S. L., Hydrochemistry of the lake Magadi basin, Kenya. Geochim. Cosmochim. Acta 41, 53–72 (1977). [Google Scholar]
- 24.Mono Basin Ecosystem Study Committee and National Research Council , “The Mono Basin ecosystem: Effects of changing lake level” (National Academies Press, Washington, DC, 1987), p. 272.
- 25.Hardie L. H., Eugster H. P., The evolution of closed-basin brines. Mineral. Soc. Am. Spec. Pap. 3, 273–290 (1970). [Google Scholar]
- 26.Eugster H. P., Hardie L. A., “Saline lakes” in Lakes (Springer, New York, 1978), pp. 237–293. [Google Scholar]
- 27.Gulbrandsen R. A., Physical and chemical factors in the formation of marine apatite. Econ. Geol. 64, 365–382 (1969). [Google Scholar]
- 28.Nathan Y., Sass E., Stability relations of apatites and calcium carbonates. Chem. Geol. 34, 103–111 (1981). [Google Scholar]
- 29.Eugster H. P., Jones B. F., Behavior of major solutes during closed-basin brine evolution. Am. J. Sci. 279, 609–631 (1979). [Google Scholar]
- 30.McConnell D., Apatite: Its Crystal Chemistry, Mineralogy, Utilization, and Geologic and Biologic Occurrences (Springer-Verlag, New York, 1973). [Google Scholar]
- 31.Reddy M. M., Crystallization of calcium carbonate in the presence of trace concentrations of phosphorus-containing anions: I. Inhibition by phosphate and glycerophosphate ions at pH 8.8 and 25°C. J. Cryst. Growth 41, 287–295 (1977). [Google Scholar]
- 32.Fahey J. J., Saline minerals of the Green River formation. U.S. Geol. Surv. Prof. Pap. 405, 1–50 (1962). [Google Scholar]
- 33.Bischoff J. L., Herbst D. B., Rosenbauer R. J., Gaylussite formation at Mono Lake, California. Geochim. Cosmochim. Acta 55, 1743–1747 (1991). [Google Scholar]
- 34.Bischoff J. L., Stine S., Rosenbauer R. J., Fitzpatrick J. A., Stafford T. W. Jr, Ikaite precipitation by mixing of shoreline springs and lake water, Mono Lake, California, USA. Geochim. Cosmochim. Acta 57, 3855–3865 (1993). [Google Scholar]
- 35.Krissansen-Totton J., Arney G. N., Catling D. C., Constraining the climate and ocean pH of the early Earth with a geological carbon cycle model. Proc. Natl. Acad. Sci. U.S.A. 115, 4105–4110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clarke F. W., “The composition of the river and lake waters of the United States” (US Government Printing Office, 1924), p. 135. [Google Scholar]
- 37.Scharge T., Muñoz A. G., Moog H. C., Thermodynamic modelling of high salinary phosphate solutions. I. Binary systems. J. Chem. Thermodyn. 64, 249–256 (2013). [Google Scholar]
- 38.Jones B. E., Grant W. D., Duckworth A. W., Owenson G. G., Microbial diversity of soda lakes. Extremophiles 2, 191–200 (1998). [DOI] [PubMed] [Google Scholar]
- 39.Condie K. C., Earth as an Evolving Planetary System (Academic Press, ed. 3, 2016). [Google Scholar]
- 40.Zahnle K., Schaefer L., Fegley B., Earth’s earliest atmospheres. Cold Spring Harb. Perspect. Biol. 2, a004895 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stüeken E. E., Buick R., Schauer A. J., Nitrogen isotope evidence for alkaline lakes on late Archean continents. Earth Planet. Sci. Lett. 411, 1–10 (2015). [Google Scholar]
- 42.Toner J. D., Catling D. C., Alkaline lake settings for concentrated prebiotic cyanide and the origin of life. Geochim. Cosmochim. Acta 260, 124–132 (2019). [Google Scholar]
- 43.Fredriksson K., Dube A., Milton D. J., Balasundaram M. S., Lonar Lake, India: An impact crater in basalt. Science 180, 862–864 (1973). [DOI] [PubMed] [Google Scholar]
- 44.Anoop A., et al. , Palaeoenvironmental implications of evaporative gaylussite crystals from Lonar Lake, central India. J. Quaternary Sci. 28, 349–359 (2013). [Google Scholar]
- 45.Tambekar D. H., Pawar A. L., Dudhane M. N., Lonar Lake water: Past and present. Nat. Environ. Pollut. Technol. 9, 217–221 (2010). [Google Scholar]
- 46.Hao J., Sverjensky D. A., Hazen R. M., Mobility of nutrients and trace metals during weathering in the late Archean. Earth Planet. Sci. Lett. 471, 148–159 (2017). [Google Scholar]
- 47.Piccoli P. M., Candela P. A., Apatite in igneous systems. Rev. Mineral. Geochem. 48, 255–292 (2002). [Google Scholar]
- 48.Jimenez-Lopez C., Romanek C. S., Precipitation kinetics and carbon isotope partitioning of inorganic siderite at 25°C and 1 atm. Geochim. Cosmochim. Acta 68, 557–571 (2004). [Google Scholar]
- 49.Stüeken E. E., et al. , Did life originate from a global chemical reactor? Geobiology 11, 101–126 (2013). [DOI] [PubMed] [Google Scholar]
- 50.Ross D. S., Deamer D., Dry/wet cycling and the thermodynamics and kinetics of prebiotic polymer synthesis. Life (Basel) 6, 1–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell T. D., et al. , Prebiotic condensation through wet-dry cycling regulated by deliquescence. Nat. Commun. 10, 4508 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hazen R. M., Sverjensky D. A., Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb. Perspect. Biol. 2, a002162 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel B. H., Percivalle C., Ritson D. J., Duffy C. D., Sutherland J. D., Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lohrmann R., Orgel L. E., Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science 171, 490–494 (1971). [DOI] [PubMed] [Google Scholar]
- 55.Ricardo A., Carrigan M. A., Olcott A. N., Benner S. A., Borate minerals stabilize ribose. Science 303, 196 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Deamer D., The role of lipid membranes in life’s origin. Life (Basel) 7, 1–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyakawa S., et al. , Studies in the mineral and salt-catalyzed formation of RNA oligomers. Orig. Life Evol. Biosph. 36, 343–361 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Black R. A., et al. , Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells. Proc. Natl. Acad. Sci. U.S.A. 110, 13272–13276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornell C. E., et al. , Prebiotic amino acids bind to and stabilize prebiotic fatty acid membranes. Proc. Natl. Acad. Sci. U.S.A. 116, 17239–17244 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritson D. J., Battilocchio C., Ley S. V., Sutherland J. D., Mimicking the surface and prebiotic chemistry of early Earth using flow chemistry. Nat. Commun. 9, 1821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burcar B., et al. , A Stark contrast to modern Earth: Phosphate mineral transformation and nucleoside phosphorylation in an iron and cyanide‐rich Early Earth scenario. Angew. Chem. Int. Ed. Engl. 58, 16981–16987 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Ranjan S., Todd Z. R., Sutherland J. D., Sasselov D. D., Sulfidic anion concentrations on early earth for surficial origins-of-life chemistry. Astrobiology 18, 1023–1040 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appelo C. A. J., Postma D., Geochemistry, Groundwater and Pollution (CRC Press, Boca Raton, FL, ed. 2, 2005). [Google Scholar]
- 64.McCann H. G., The solubility of fluorapatite and its relationship to that of calcium fluoride. Arch. Oral Biol. 13, 987–1001 (1968). [DOI] [PubMed] [Google Scholar]
- 65.McDowell H., Gregory T. M., Brown W. E., Solubility of Ca5(PO4)3OH in the system Ca(OH)2-H3PO4-H2O at 5, 15, 25, and 37°C. J. Res. Natl. Bur. Stand., A Phys. Chem. 81A, 273–281 (1977). [Google Scholar]
- 66.Marion G. M., Catling D. C., Crowley J. K., Kargel J. S., Modeling hot spring chemistries with applications to martian silica formation. Icarus 212, 629–642 (2011). [Google Scholar]
- 67.Scharge T., “Thermodynamic database for phosphate” in Thermodynamic Reference Database (THEREDA, 2017), pp. 1–24. [Google Scholar]
- 68.Scharge T., Munoz A. G., Moog H. C., Thermodynamic modeling of high salinary phosphate solutions II. Ternary and higher systems. J. Chem. Thermodyn. 80, 172–183 (2015). [Google Scholar]
- 69.Whitehead H. C., Feth J. H., Recent chemical analyses of waters from several closed-basin lakes and their tributaries in the western United States. Geol. Soc. Am. Bull. 72, 1421–1425 (1961). [Google Scholar]
- 70.Allison I. S., Mason R. S., Sodium Salts of Lake County, Oregon. (Oregon Department of Geology and Mineral Industries, 1947), vol. 17, pp. 1–12. [Google Scholar]
- 71.Newton V. C., Baggs D. W., Geologic Evaluation of the Alkali Lake Disposal Site (State of Oregon, Department of Geology and Mineral Industries, 1971). [Google Scholar]
- 72.Ashton P. J., Schoeman F. R., Limnological studies on the Pretoria Salt Pan, a hypersaline maar lake. Hydrobiologia 99, 61–73 (1983). [Google Scholar]
- 73.Imhoff J. F., Sahl H. G., Soliman G. S., Trüper H. G., The Wadi Natrun: Chemical composition and microbial mass developments in alkaline brines of eutrophic desert lakes. Geomicrobiol. J. 1, 219–234 (1979). [Google Scholar]
- 74.Talling J. F., Talling I. B., The chemical composition of African lake waters. Int. Rev. Gesamten Hydrobiol. Hydrograph. 50, 421–463 (1965). [Google Scholar]
- 75.Melack J. M., Kilham P., Photosynthetic rates of phytoplankton in East African alkaline, saline lakes. Limnol. Oceanogr. 19, 743–755 (1974). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental data from this study are available in SI Appendix, Appendix B, Tables S1 and S2. The PHREEQC database file used to make Fig. 3 is given in SI Appendix, Appendix C.