Significance
Even though preterm brains are immature and will undergo a myriad of developmental processes, they already display great interindividual differences in their functional connectivity (FC) architecture. Interestingly, functionally less variable brain regions are morphologically more mature around term birth than functionally more complex and variable brain regions. Despite the overall resemblance of variability patterns in preterm infants and adults, some brain regions, especially in higher cognitive networks, show a developmental shift toward greater FC variability in adults, related to postnatal cortical expansion. Our result of robust variability distribution in the preterm brain indicates the need to develop individualized FC measures that may lead to predictors of nervous function in this high-risk population.
Keywords: functional connectivity, cortical development, preterm infants, individual differences
Abstract
Functional connectivity (FC) is known to be individually unique and to reflect cognitive variability. Although FC can serve as a valuable correlate and potential predictor of (patho-) physiological nervous function in high-risk constellations, such as preterm birth, templates for individualized FC analysis are lacking, and knowledge about the capacity of the premature brain to develop FC variability is limited. In a cohort of prospectively recruited, preterm-born infants undergoing magnetic resonance imaging close to term-equivalent age, we show that the overall pattern could be reliably detected with a broad range of interindividual FC variability in regions of higher-order cognitive functions (e.g., association cortices) and less interindividual variability in unimodal regions (e.g., visual and motor cortices). However, when comparing the preterm and adult brains, some brain regions showed a marked shift in variability toward adulthood. This shift toward greater variability was strongest in cognitive networks like the attention and frontoparietal networks and could be partially predicted by developmental cortical expansion. Furthermore, FC variability was reflected by brain tissue characteristics indicating cortical maturation. Brain regions with high functional variability (e.g., the inferior frontal gyrus and temporoparietal junction) displayed lower cortical maturation at birth compared with somatosensory cortices. In conclusion, the overall pattern of interindividual variability in FC is already present preterm; however, some brain regions show increased variability toward adulthood, identifying characteristic patterns, such as in cognitive networks. These changes are related to postnatal cortical expansion and maturation, allowing for environmental and developmental factors to translate into marked individual differences in FC.
The intrinsic functional connectivity (FC) architecture of the human brain is individually unique (1) and reflects cognitive variability in healthy individuals (2, 3). In previous work investigating interindividual variability of FC in adults, we showed that highest interindividual variability exists in higher-order association cortices, while unimodal, sensory, and motor cortices show lower interindividual variability (4).
Individual differences in structural and functional brain architecture are significantly shaped during prenatal life and can already be detected as early as the third trimester (5), in neonates (6), and in young infants (7). It is a neuroscientific and philosophical truism that not all individual differences in cognition and behavior observed in adults are determined by the architecture of the neonatal brain; genetic and environmental factors impact the developing brain after the neonatal period (7), resulting in variable changes over time, with increasing variance in some brain regions but decreasing variance in others (7). However, several research questions have not yet been fully elucidated, with a potentially significant impact on functional MRI (fMRI) application in scientific and clinical settings and subsequent challenges for analysis. First, understanding the characteristic pattern of FC variance in the immature brain undergoing profound environmental stimulation in the earliest stages of development will help understand how neonatal variance translates into individual differences in connectivity architecture in later developmental stages. Second, the high risk of developmental abnormalities in the cohort of preterm infants is an outstanding example demonstrating the clinical need for quantifiable, individualized imaging markers that can indicate or even predict neurodevelopmental abnormalities in one of the largest high-risk pediatric patient populations.
Although FC has been assessed in the neonatal brain preterm (8, 9) and at full term (10, 11), the assessment of interindividual FC variability in preterm infants and neonates remains challenging. The variance observed across individuals cannot be fully attributed to interindividual differences unless intrasubject variance is accounted for. Therefore, we have suggested to estimate intrasubject variance, which may arise from technical as well as biological sources, from repeated scans of each subject. We used this estimate to model “pure” intersubject variability of functional connections (4). Repeated measurements in newborns are uncommon, however. An alternate route to achieve this goal is a “split-session” approach (12) in which one resting state session is divided into a “test” and a “retest” part to estimate the spatial distribution of intrasubject variability of FC.
We aimed to define the characteristic pattern of FC variance detectable close to term-equivalent age and test its robustness in 2 independent cohorts of preterm infants that underwent postnatal environmental challenges imposed in an early stage of brain maturation. We then compared this pattern to well-established FC characteristics in healthy adults. We further aimed to identify brain regions that undergo developmental changes in variability and to explore how the changes in functional variability may relate to structural development, specifically cortical expansion and maturation. Our results will inform research in the field of diagnosis and prediction of nervous function in high-risk constellations, as well as the developmental and environmental impacts on individual differences in FC.
Results
We included a total of 116 preterm infants who were scanned near term as part of the Attention to Infants at Respiratory Risk study conducted by the German Center for Lung Research in cooperation with the Departments of Neonatology and Radiology at Ludwig Maximilian University of Munich and Justus-Liebig University Giessen. All subjects were scanned using the same type of scanner and the same scanning protocol. After initial recruitment of 116 preterm infants who completed the fMRI protocol, 29 subjects were excluded based on neuroradiologic and clinical criteria, 24 subjects were excluded based on head motion criteria, and 13 subjects were excluded for low signal-to-noise ratio of the functional images (SI Appendix, Fig. S1 provides details of the exclusion criteria.) A total of 50 infant imaging studies passed all quality control criteria and underwent further analyses. Twenty-five neonates were included from the Munich site (15 females; mean gestational age at birth, 27.6 wk ± 17.8 d; mean gestational age at scan, 36.3 wk ± 16.1 d; all scanned in spontaneous sleep), and 25 neonates were included from the Giessen site (18 females; mean gestational age at birth, 28.1 wk ± 14.6 d; mean gestational age at scan, 37.1 wk ± 10.2 d; 22 scanned while sedated, 3 scanned in spontaneous sleep). Further details on the clinical course are provided in SI Appendix, Table S1.
Interindividual Variability of FC Architecture Can Be Reliably Detected around Term Birth.
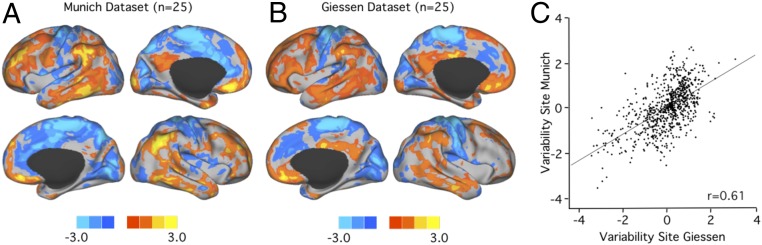
Intersubject FC variability was quantified at each of the 2,420 evenly distributed cortical regions of interest (ROIs) of the neonatal brains after correction for intrasubject variance and technical noise (details in SI Appendix, Methods and Fig. S2 for details). Robustness of the spatial distribution of FC variability maps is an important prerequisite for their potential use in developing individualized FC parameters in preterm neonates (13). To test whether FC variability shows a robust pattern that can be reliably detected in independent samples of preterm neonates around term, we calculated and mapped FC variability in the Munich and Giessen samples separately and quantified the similarity of the results. We found that the spatial distribution was highly reproducible across 2 independent samples of preterm neonates. The spatial distribution of variability observed in the Munich cohort (n = 25; Fig. 1A) was well replicated in the independent Giessen cohort (n = 25; Fig. 1B), with a Pearson correlation between the 2 maps of r = 0.61 (PSPIN < 0.001). The PSPIN value was generated by spin-based permutation testing that accounted for spatial autocorrelation of the data (Fig. 1C).
Fig. 1.
Interindividual variability of FC architecture can be reliably estimated around term birth in preterm infants. Maps of interindividual variability estimated in 25 preterm infants scanned around term at Ludwig Maximilian University of Munich (A) and 25 preterm infants scanned around term at Justus-Liebig University Giessen (B) showed very similar spatial distributions. Variability above the global mean is shown in warm colors, and variability below the global mean is shown in cool colors. (C) Accordingly, intersite reliability, expressed as the spatial Pearson correlation coefficient between 2 maps, is high (r = 0.61, PSPIN < 0.001).
To assess the impact of gestational age at birth on FC variability, we compared 2 groups of preterm infants chosen from 2 ends of the gestational age range of the study cohort, that is, 15 infants with a mean gestational age of 24.9 wk ± 5.6 d and 15 infants with a mean gestational age of 30.3 wk ± 4.9 d. We found a very similar overall pattern of variability distribution in these 2 groups, as indicated by a significant correlation between variability maps (r = 0.66, PSPIN < 0.001; SI Appendix, Fig. S3), suggesting that the effect of prematurity was not dominating the variability pattern observed (Fig. 1).
To assess the effect of prematurity vs. term birth on FC variability, we replicated the variability map in publicly available data of healthy term-born infants (n = 25) from the Developmental Human Connectome Project (http://www.developingconnectome.org). Demographic and scan parameters are provided in SI Appendix. We found that the overall pattern of variability distribution at term was similar in preterm- and term-born infants (r = 0.71, PSPIN < 0.001; SI Appendix, Fig. S4), again suggesting that the effect of prematurity was not dominating the variability pattern observed in neonates.
The Overall Characteristic Pattern of FC Variability Is Already Present around Term Birth.
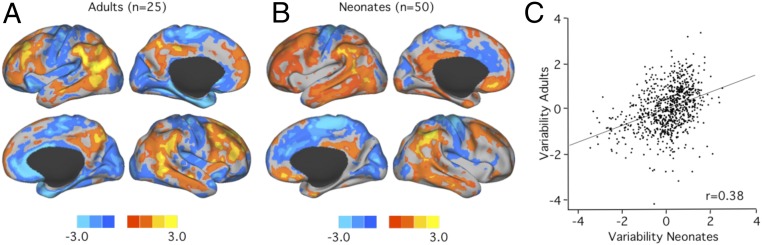
We next aimed to investigate whether the pattern of FC variability observed in neonates already shows similarity to the characteristic distribution in adults. To this end, FC variability was quantified in 25 neurologically healthy adults who had been scanned using the same scanner and sequence protocol as for the neonatal data at the Munich site (SI Appendix). Intersubject variability in adults demonstrated the characteristic nonuniform distribution across brain regions (Fig. 2A), as previously described by our group (4). FC variability was greatest in the heteromodal association cortex and minimal in unimodal sensory and motor cortices (Fig. 2A). This overall pattern could already be detected in preterm-born infants (n = 50) scanned around term-equivalent age (Fig. 2B). The similarity of the 2 maps yielded a correlation coefficient of r = 0.38 (PSPIN < 0.001; Fig. 2C). To further establish the overall similarity, we summarized functional variability in the association cortex and in the sensorimotor cortex (SI Appendix, Fig. S5A and Methods) and found that mean FC variability was clearly above the global mean in the association cortex and below the global mean in the sensorimotor cortex in both neonates and adults (SI Appendix, Fig. S5B).
Fig. 2.
The overall characteristic pattern of high FC variability in higher-order association cortices and low variability in unimodal sensorimotor cortices is already present around term birth. (A and B) The overall pattern of interindividual variability in healthy adult subjects (A) grossly resembles the pattern of interindividual variability in preterm infants (B). (C) Accordingly, the spatial correlation coefficient shows moderate similarity between the 2 maps (r = 0.38, PSPIN < 0.001).
Toward Adulthood, Some Brain Regions and Networks Show a Marked Shift in Variability, Which Can Be Partially Predicted by Developmental Cortical Expansion.
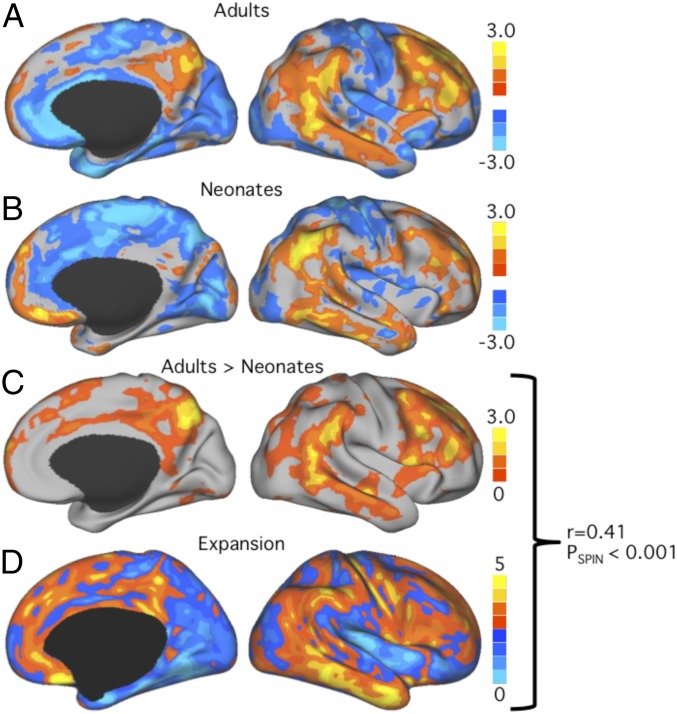
Although the overall distribution of interindividual variability was similar in adults (Fig. 3A) and neonates (Fig. 3B), some brain regions appeared to differ between these 2 groups. To specify which regions display a shift toward higher variability in adults, and to identify factors that might drive this shift, neonatal and adult FC variability maps were compared via regression analysis. Specifically, the developmental variability shift was defined as the residuals of the regression analysis of adult and neonate FC variability maps. Brain regions that displayed a positive developmental variability shift (i.e., a relative gain in variability in adults compared with the neonatal brain) included frontal, temporal, and parietal association cortex areas (Fig. 3C and SI Appendix, Fig. S7; details in Materials and Methods and SI Appendix, Methods). These ontogenetically late-developing regions (14) are essential to complex cognitive functions like reasoning and language (15). This may indicate that developmental changes in these cortical areas give rise to higher variability in FC.
Fig. 3.
Toward adulthood, some brain regions show a marked shift in variability, which can be partially predicted by developmental cortical expansion. (A and B) Maps of interindividual variability of FC in 25 healthy adults (A) and 25 preterm infants (B) (Munich dataset). (C) Regression analysis revealing cortical areas that shift toward relatively greater functional variability in adults. Values above the global mean (i.e., that display a relative gain in variability toward adulthood) are shown in warm colors. (D) Map of developmental cortical expansion between a term-born infant and the average human adult PALS-B12 atlas (16). The spatial correlation coefficient shows a moderate similarity between the maps of developmental variability shift and developmental cortical expansion (r = 0.41, PSPIN < 0.001), indicating that the extent of FC variability is partially related to developmental cortical expansion.
To test this hypothesis, we compared the map of developmental variability shift (Fig. 3C) with a map of developmental cortical expansion (Fig. 3D) provided by Hill et al. (16). On a whole-surface level, developmental cortical expansion and developmental variability shift were significantly correlated (r = 0.41, PSPIN < 0.001), indicating that the increase in FC variability is partially related to developmental cortical expansion.
It also has been suggested that developmental reorganization of FC is characterized by a shift of FC hubs from the sensory-motor cortex toward frontoparietal network areas (17). To test whether these network areas are also characterized by a developmental gain in variability (Fig. 4A), variability shift was assessed within 7 specific brain networks (18) (Fig. 4B). Frontoparietal control and attentional networks showed the highest developmental gain in FC variability (Fig. 4C). Results for the right hemisphere are shown in SI Appendix, Fig. S6.
Fig. 4.
The frontoparietal control network and the attentional networks show the highest developmental shift in FC. (A) Map of developmental variability shift. (B) Boundaries of 7 cortical networks overlain in black. (C) Bar plot of the mean residuals summarized in each network.
While image acquisition in preterm infants was achieved in quiet sleep, adult imaging was performed in awake individuals. To assess the impact of the state of consciousness on FC variability pattern and its potential bias on estimating developmental changes in FC variability, we performed additional analyses comparing adults scanned while asleep (n = 17) with adults scanned while awake with eyes open (n = 17). Data from subjects scanned while asleep were provided by Zhou and colleagues and have been described in detail previously (19). Data from subjects scanned while awake were collected as part of the Brain Genomics Superstruct Project of Harvard University and the Massachusetts General Hospital (20). These 2 cohorts were matched according to age, sex, and years of education. Demographic and scanning parameters from both cohorts are provided in SI Appendix. When comparing the FC variability map of the asleep and awake samples, we found that the overall pattern of variability distribution was highly similar (SI Appendix, Fig. S8), as indicated by a significant correlation between the variability maps (r = 0.64, PSPIN < 0.001), suggesting that the effect of sleep does not significantly change the spatial distribution of interindividual variability.
Functionally Less Variable Brain Regions Are Morphologically More Mature around Term Birth than Functionally More Complex and Variable Brain Regions.
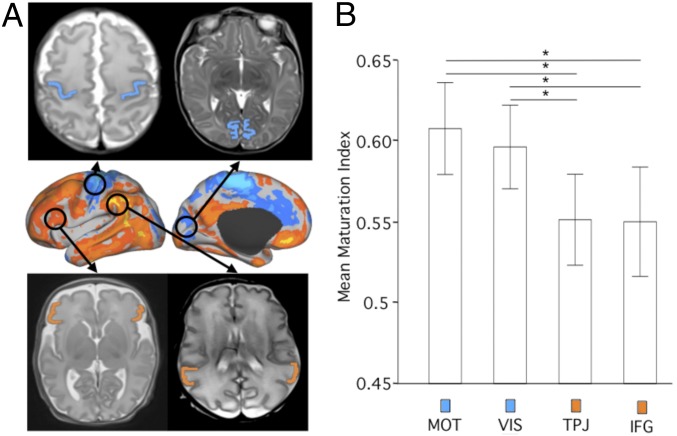
As brain regions and networks differ in their extent of interindividual FC variability in preterm neonates, we investigated whether this heterogeneity correlates with brain tissue characteristics. To do so, we computed a modified cortical maturation index (MI) (21) in brain regions of high and low FC variability in 25 preterm neonates (Munich cohort). The cortical MI, based on the T2-weighted MRI signal intensity (SI) in brain tissues normalized by the SI of cerebrospinal fluid (MI = 1 − SIcortex/SICSF; Materials and Methods), indicated significantly lower cortical maturation at term in regions of high functional variability, like the inferior frontal gyrus (IFG) and the temporoparietal junction (TPJ), compared with low-variability regions like the visual and motor cortices (P < 0.01 for each of the 4 comparisons; Fig. 5).
Fig. 5.
Functionally less variable brain regions are morphologically more mature around term birth than functionally more complex and variable brain regions. (A) Cortical maturation was evaluated in cortex areas of low and high FC variability (shown in cool and warm colors, respectively). Cortical maturation indices were estimated in the visual and motor cortices (indicated in blue; Top), and in the IFG and TPJ, indicated in orange; Bottom). (B) Bar plots with error bars indicating mean ± 2 SDs of the cortical maturation indices of each structure showing higher maturation indices in the visual (VIS) and motor (MOT) cortices compared with the IFG and TPJ. Asterisks indicate P values < 0.01 resulting from ANOVA comparing the 4 groups.
Discussion
We demonstrated that functional variability distribution can be reliably detected in the neonatal preterm brain reaching term-equivalent age. The overall pattern was similar to the distribution observed in neurologically healthy adults. However, when comparing the patterns of FC variability in neonates with the adult brain, some brain regions showed a marked shift in variability toward adulthood. This shift toward higher variability was strongest in cognitive networks like the attention and frontoparietal networks and could be partially predicted by developmental cortical expansion. Furthermore, the pattern of FC variability was reflected by brain tissue characteristics of cortical maturation. Functionally highly variable brain regions like the IFG and the TPJ displayed lower cortical maturation around term-equivalent age compared with somatosensory cortices.
Relation to Structural Brain Development.
We found that brain regions that experience the most pronounced variability gain during development are also characterized by the most pronounced cortical expansion, suggesting that developmental changes in functional variability are at least partially rooted in structural brain development. Cortical expansion is closely related to cortical folding and the degree of gyrification, which is highest in association cortex and lowest in the occipital and motor cortices (22), resulting in the highest sulcal depth variability and positional variability in association cortex (6). Our results indicate that the functional variability gain in association cortex areas during development is related to postnatal cortical expansion and folding.
Furthermore, regions displaying low functional variability near term were structurally more mature than regions with high FC variability. Specifically, the primary sensorimotor cortices showed the highest MI, while association cortex areas (e.g., IFG and TPJ) displayed significantly lower cortical maturation. This pattern of differential cortical maturation also has been shown by estimating cortical myelination across the early months of infancy (21), and cortical density across childhood (14). A similar developmental time course can be found in the dynamics of peak cortical thickness (23) and cortical lamination (24), indicating that cortical maturation processes in primary cortical areas precede those in association cortices. On a neuronal level, synaptogenesis begins earlier in primary motor regions and later in the prefrontal cortex (25). Taken together, our findings indicate that protracted structural and synaptic maturation may contribute to the developmental shift toward greater FC variability of multimodal association cortices in adults, as reported in this study.
Relation to FC Maturation.
We identified cortical areas and networks with a marked shift toward greater variability in healthy adults compared with neonates. These cortical areas were mainly located in the association cortex that included the attention and frontoparietal networks.
Again, the relation of the observation to the developmental time course is striking. Prolonged FC maturation of higher-order cortical regions has been described during the third trimester (8, 26), and during the first 2 y of life (27, 28). Accordingly, the maturation time course differs among brain networks, with sensorimotor networks maturing more rapidly than the attention and frontoparietal networks (17).
FC maturation is characterized by the formation of long-range connections and the selective weakening of short-range connections between networks. We have previously shown that variability is related to the degree of long-range connections (i.e., high functional variability may be induced by the emergence of distant connectivity) (4) and the formation of hubs of distant connections in multimodal association cortices (29). Thus, the developmental variability gain in the frontoparietal and attention networks observed in this study is likely to reflect the development of the more complex connectivity architecture of these networks that comprise many long-range connections. These long-range connections are needed to support complex processes, such as sustained attention, cognitive control, decision making, and the integration of information from memory and attention systems (30). These functions are known to have a protracted course of development that might extend into adolescence (31) and to display great interindividual variability that is at least partially rooted in individual differences in brain connectivity (2, 3). From studies in adult subjects, it has been reported that the association cortex displays especially high interindividual differences in the size, location, and connection strength of functional regions (13, 32), all of which are likely to contribute to the greater functional variability observed here.
When comparing preterm infants with adults, we found a relative loss of variability in the default, visual, and motor networks. This is consistent with results published by Gao and colleagues (7) showing that the default mode network and the lateral visual network move down in the variability ranking of networks during the first 2 y of life, and with results reported by Xu and colleagues (5) showing that functional variability of the sensorimotor network significantly decreases at gestational weeks 31 to 42 in preterm infants. Previous studies have consistently documented that primary networks develop early and are already largely established around term birth (8, 33). Consequently, primary networks might have almost reached their maximal intersubject variability at around term birth and will experience much less postnatal variability increase compared with multimodal association networks.
Relation to Genetic and Environmental Factors.
We found the most pronounced shift toward higher variability in adults in the frontal and parietal association cortex areas. Both genetic and environmental factors may contribute to the relative developmental gain in FC variability observed in these regions.
Individual variability is likely influenced by complex gene–environment interactions (7, 34); however, the extent to which environmental and or genetic factors impact development seems to differ between brain regions. While significant heritability of structural variability has been reported for early-maturing brain regions such as the occipital lobes (35), greater environmental influences have been reported for the frontal and prefrontal association cortex areas (35, 36). Accordingly, a twin study in neonates showed the lowest heritability estimates for volumetric MRI measures in the prefrontal lobe (37), potentially enabling a more variable impact of postnatal environmental factors that may lead to higher intersubject variability beyond genetic determination (38). However, other studies have reported strong genetic influences on some frontal and temporal regions (39) and language-related brain regions (40). Compared with brain structure, brain function shows much lower heritability estimates (∼40%, compared with 60 to 80% for structural measures) (41). Genetic effects on FC variability show complex region- and age-dependent patterns in which genetically induced lower variability can be found in both association and primary cortices (7).
Clinical Relevance.
From a clinical perspective, advanced MRI methods in preterm infants aim to achieve improved prediction of long-term neurologic outcome to guide risk stratification and thus inform therapeutic strategies and monitoring concepts and aid parent counseling. Currently, there is no single clinical assessment tool recognized as standard of care in infants in the early months of life to predict neurologic outcome at 24 mo (42), especially with regard to neuronal function. Therefore, objective functional imaging markers are needed to supplement today’s solely structure-based assessment. Preterm birth has both proximate and long-lasting impacts on FC architecture (43). Resulting abnormalities in FC have been observed in preterm children, adolescents, and adults and have been related to performance in different cognitive and language domains (43). Although several studies have investigated FC in preterm infants scanned before or around term, associations with later cognitive outcomes remain to be established.
Importantly, before embarking on the exploration of FC changes that may ultimately carry prognostic information, robust FC markers in individuals need to be established. Determining the distribution of interindividual functional variability is a crucial prerequisite for mapping functional networks in individuals (13, 32). Here we show that the distribution of interindividual variability of FC can be reliably estimated in preterm neonates scanned at term. This finding will set the stage for the exploration of individual network boundaries and functional regions in preterm infants, enabling the development of connectivity markers that can be accurately quantified in individuals. These individualized connectivity parameters could be used to define individual risk scores and to predict long-term neurologic outcome.
Limitations.
The results of this study might not be fully transferrable to term-born infants. To date, it remains a matter of ongoing investigation if and to what extent prematurity affects resting-state connectivity at term. While some studies report connectivity differences between preterm-born infants at term compared with term-born neonates (33, 44), other studies have found no significant differences (8) and have described a relatively well-preserved functional network topography (9). The extent of FC alteration is certainly influenced by the presence and degree of structural brain damage (i.e., hemorrhage, hydrocephalus, and white matter injury) as a consequence of prematurity. For example, studies have shown connectivity differences between preterm neonates with and without “punctate white matter lesions” (44). Therefore, in this study we carefully selected a cohort of preterm infants without overt structural brain damage on MRI and without severe comorbidities (SI Appendix, Fig. S1). We subsequently excluded 25% of all cases, significantly exceeding the rate in other fMRI studies (e.g., approximately 13% of the subjects were excluded the study reported in ref. 33). Despite this effort, prematurity is still likely to influence variability in FC. However, when comparing our variability map of preterm infants with a variability map derived from healthy term-born infants from the Developmental Human Connectome Project, we found a remarkable similarity of the 2 maps (r = 0.71, PSPIN < 0.001), indicating that prematurity is not dominating the spatial distribution of FC variability.
Materials and Methods
We evaluated a total of 116 preterm infants who were scanned around term at Ludwig Maximilian University of Munich and Justus-Liebig University Giessen. Parents of all scanned neonates provided written informed consent. The study was approved by the Ludwig Maximilian University of Munich and Justus-Liebig University Giessen Institutional Review Boards. Resting-state fMRI and anatomic data were collected on 3-T Skyra scanners (Siemens Medical Solutions). Subjects with structural brain damage on MRI, severe comorbidities, or insufficient image quality were excluded from further analyses (SI Appendix, Methods and Fig. S1). Twenty-five healthy adult subjects (mean age, 27.0 ± 3 0.8 y; 13 females) were recruited at the Munich site as an adult comparison sample, using the same scanner and protocol as used for the neonatal data. Interindividual variability was estimated analogous to our previous work on functional variability in adults (4) and summarized within atlas-based functional networks (18) and within anatomically defined association and primary cortex regions. The similarity of the variability maps derived from the 2 neonatal samples and the similarity of neonatal and adult variability maps were assessed using Pearson correlation. Developmental changes in variability were assessed using a generalized linear model approach that was applied to regress out the neonatal functional variability map (derived from the Munich site; n = 25) from the adult functional variability map. The map of developmental variability gain was related to a map of developmental cortical expansion (16) using Pearson correlation. The potential impact of spatial dependence of vertices on the correlation analyses was assessed using spin-based permutation testing (45–47) (SI Appendix, Methods). Cortical maturation of primary and association cortex areas was assessed based on the normalized T2 signal of manually segmented regions of interest. Further information on the imaging protocols, subject characteristics, MRI data preprocessing, and variability analyses is provided in SI Appendix, Methods.
Code and Data Availability.
The MATLAB code for the calculation of interindividual variability in FC is available through our website (http://nmr.mgh.harvard.edu/bid/DownLoad.html). Data are available on request from A.H. or S. Stoecklein.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Helmholtz Zentrum Muenchen (to A.H. and S.S.) and a Physician Scientist grant from the Translational Program of the German Lung Research Center (to K.F.). H.L. is supported by National Key Research and Development Program of China Grant 2016YFC1306303; NIH Grants 1R01 NS091604, 1R01 DC017991, and 1R21 MH121831; Beijing Municipal Science & Technology Commission Grant Z161100002616009, and National Natural Science Foundation of China Grants 81790650 and 81790652. D.W. is supported by National Institutes of Mental Health Grant K01MH111802 and a National Alliance for Research on Schizophrenia and Depression Young Investigator Grant.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907892117/-/DCSupplemental.
References
- 1.Finn E. S., et al. , Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18, 1664–1671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koyama M. S., et al. , Resting-state functional connectivity indexes reading competence in children and adults. J. Neurosci. 31, 8617–8624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Heuvel M. P., Stam C. J., Kahn R. S., Hulshoff Pol H. E., Efficiency of functional brain networks and intellectual performance. J. Neurosci. 29, 7619–7624 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller S., et al. , Individual variability in functional connectivity architecture of the human brain. Neuron 77, 586–595 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y., et al. , Development and emergence of individual variability in the functional connectivity architecture of the preterm human brain. Cereb. Cortex 29, 4208–4222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill J., et al. , A surface-based analysis of hemispheric asymmetries and folding of cerebral cortex in term-born human infants. J. Neurosci. 30, 2268–2276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao W., et al. , Intersubject variability of and genetic effects on the brain’s functional connectivity during infancy. J. Neurosci. 34, 11288–11296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doria V., et al. , Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. U.S.A. 107, 20015–20020 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyser C. D., et al. , Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb. Cortex 26, 322–333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fransson P., et al. , Early development of spatial patterns of power-law frequency scaling in FMRI resting-state and EEG data in the newborn brain. Cereb. Cortex 23, 638–646 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Gao W., et al. , Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One 6, e25278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller S., et al. , Reliability correction for functional connectivity: Theory and implementation. Hum. Brain Mapp. 36, 4664–4680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., et al. , Parcellating cortical functional networks in individuals. Nat. Neurosci. 18, 1853–1860 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogtay N., et al. , Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101, 8174–8179 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman-Rakic P. S., Topography of cognition: Parallel distributed networks in primate association cortex. Annu. Rev. Neurosci. 11, 137–156 (1988). [DOI] [PubMed] [Google Scholar]
- 16.Hill J., et al. , Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. U.S.A. 107, 13135–13140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power J. D., Fair D. A., Schlaggar B. L., Petersen S. E., The development of human functional brain networks. Neuron 67, 735–748 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo B. T., et al. , The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou S., et al. , Dynamic functional connectivity states characterize NREM sleep and wakefulness. Hum. Brain Mapp. 40, 5256–5268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes A. J., et al. , Brain genomics superstruct project initial data release with structural, functional, and behavioral measures. Sci. Data 2, 150031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leroy F., et al. , Early maturation of the linguistic dorsal pathway in human infants. J. Neurosci. 31, 1500–1506 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zilles K., et al. , Quantitative analysis of sulci in the human cerebral cortex: Development, regional heterogeneity, gender difference, asymmetry, intersubject variability and cortical architecture. Hum. Brain Mapp. 5, 218–221 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Shaw P., et al. , Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostovic I., Rakic P., Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J. Neurocytol. 9, 219–242 (1980). [DOI] [PubMed] [Google Scholar]
- 25.Huttenlocher P. R., Dabholkar A. S., Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 387, 167–178 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Cao M., et al. , Early development of functional network segregation revealed by connectomic analysis of the preterm human brain. Cereb. Cortex 27, 1949–1963 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao W., et al. , Functional network development during the first year: Relative sequence and socioeconomic correlations. Cereb. Cortex 25, 2919–2928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao W., Alcauter S., Smith J. K., Gilmore J. H., Lin W., Development of human brain cortical network architecture during infancy. Brain Struct. Funct. 220, 1173–1186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sepulcre J., et al. , The organization of local and distant functional connectivity in the human brain. PLoS Comput. Biol. 6, e1000808 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent J. L., Kahn I., Snyder A. Z., Raichle M. E., Buckner R. L., Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 100, 3328–3342 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klenberg L., Korkman M., Lahti-Nuuttila P., Differential development of attention and executive functions in 3- to 12-year-old Finnish children. Dev. Neuropsychol. 20, 407–428 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Li M., et al. , Performing group-level functional image analyses based on homologous functional regions mapped in individuals. PLoS Biol. 17, e2007032 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smyser C. D., et al. , Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex 20, 2852–2862 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C. H., et al. , Genetic influences on cortical regionalization in the human brain. Neuron 72, 537–544 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brun C. C., et al. , Mapping the regional influence of genetics on brain structure variability—A tensor-based morphometry study. Neuroimage 48, 37–49 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon U., Perusse D., Lee J. M., Evans A. C., Genetic and environmental influences on structural variability of the brain in pediatric twin: Deformation- based morphometry. Neurosci. Lett. 493, 8–13 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Gilmore J. H., et al. , Genetic and environmental contributions to neonatal brain structure: A twin study. Hum. Brain Mapp. 31, 1174–1182 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petanjek Z., et al. , Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 108, 13281–13286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rimol L. M., et al. , Cortical thickness is influenced by regionally specific genetic factors. Biol. Psychiatry 67, 493–499 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson P. M., et al. , Genetic influences on brain structure. Nat. Neurosci. 4, 1253–1258 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Jansen A. G., Mous S. E., White T., Posthuma D., Polderman T. J. C., What twin studies tell us about the heritability of brain development, morphology, and function: A review. Neuropsychol. Rev. 25, 27–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caesar R., et al. ; PREMTiME Study Group , Early prediction of typical outcome and mild developmental delay for prioritisation of service delivery for very preterm and very low birthweight infants: A study protocol. BMJ Open 6, e010726 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon S. H., et al. , Functional magnetic resonance connectivity studies in infants born preterm: Suggestions of proximate and long-lasting changes in language organization. Dev. Med. Child Neurol. 58 (suppl. 4), 28–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cai Y., Wu X., Su Z., Shi Y., Gao J. H., Functional thalamocortical connectivity development and alterations in preterm infants during the neonatal period. Neuroscience 356, 22–34 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Alexander-Bloch A. F., et al. , On testing for spatial correspondence between maps of human brain structure and function. Neuroimage 178, 540–551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon E. M., et al. , Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reardon P. K., et al. , Normative brain size variation and brain shape diversity in humans. Science 360, 1222–1227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.