The family of peptides controlling metabolism and reproduction in vertebrate and invertebrate animals has deep evolutionary roots. In vertebrates, the primary peptide controlling sexual differentiation and reproduction is gonadotropin-releasing hormone (GnRH), a decapeptide with “relatives” extending to the alpha factor, a tridecapeptide mating pheromone of yeast (Saccharomyces cerevisiae) (1). The yeast pheromone is able to reproduce the biological actions of GnRH in the mammalian pituitary gland, thus suggesting that structural and functional properties of the GnRH-related superfamily (2) of ligands and peptides have been highly conserved during evolution. Invertebrate peptides evolved extensively within the bilaterian lineage and appear to have retained the ancestral role of mobilizing metabolic resources in response to stress, including the necessities of reproduction. In PNAS, Andreatta et al. (3) build on previous work showing that the marine bristleworm Platynereis dumerilii has a circalunar (monthly) clock that interacts in an independent manner with the circadian clock (4). In this most recent study (3), the authors use gene-editing techniques to generate animals mutant for the gene encoding corazonin1, a peptide belonging to the GnRH superfamily, and demonstrate that the animals are normal with respect to development and reproduction, yet show metabolic problems with energy homeostasis.
Cells releasing endocrine signals are likely to predate the appearance of a nervous system, since they can be found in extant metazoans (sponges and cnidarians; Fig. 1) lacking nerve cells (5), animals that predate the split giving rise to protostomes (Arthropoda and annelids; Fig. 1), and deuterostomes (echinoderms and chordates; Fig. 1). A subject of intense discussion in the literature is the naming of the peptides related to GnRH / GnRH receptors (6), where evolutionary analysis of ligands is closely coupled with their receptors because they usually coevolve. One member of the GnRH superfamily, corazonin (first named because it stimulated the heart of the American cockroach), is now considered a descendent of an “ancient stress-related peptide” that restores metabolic homeostasis by altering stress physiology to coordinate increased food intake and diminished energy stores, as shown in Drosophila (7). Belonging to the same GnRH superfamily, adipokinetic hormones (AKHs: peptide family with over 60 members) also play a major role in mobilizing stored energy metabolites during energetic demand in insects (8).
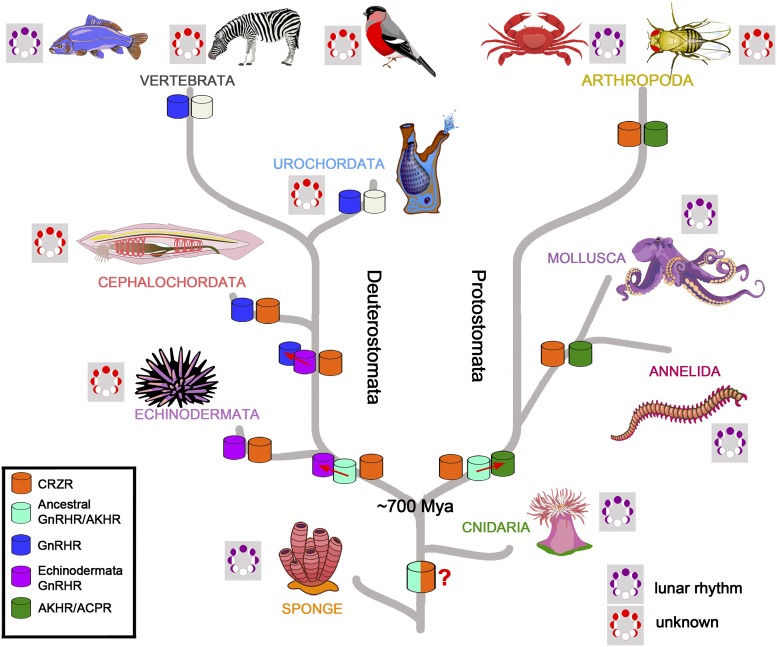
Fig. 1.
Evolution of peptide signaling pathways and lunar rhythms. Reduced evolutionary tree representing the 2 principal lineages of animal evolution, protostomata and deuterostomata, arising ∼700 MYa from basal groups of sponges and cnidarians. Evolutionary changes in AKH/corazonin/ACP/GnRH receptor super family evolution data from ref. 9. Empty symbols (vertebrates and urochordates) represent loss of the receptor type. Symbols of moon phases indicate whether animals in groups show activity based on lunar rhythms (purple): Arthropoda, Christmas Island crabs; Mollusca, bobtail squid; although it should be noted that invertebrate spawning is more tightly coupled to the circadian cycle than to the circalunar cycle (21). Annelida group contains polychaete worm P. dumerilii discussed in the paper.
Invertebrate peptides in the AKH/corazonin / AKH-corazonin receptor−related peptide (ACP) / GnRH superfamily share a common origin with vertebrate GnRHs. Yet these ligands differ in the number of amino acids: The GnRH peptides (in vertebrates and urochordates) are composed of 10 amino acids, whereas protostome, echinoderm, and amphioxus GnRH-like peptides are an 11- or 12-residue peptide that contains 2 amino acids after an N-terminal pyro-Glu. The protostome peptides, corazonin and adipokinetic hormones, are thought to be orthologous to the GnRH family, due to the presence of several amino acids typical of GnRHs and sequence similarity among their receptors and GnRH receptors, but the evolutionary relationship remains controversial (9, 10). The naming of the GnRH-like peptides used in the paper of Andreatta (ref. 3, tables S1−S3) is subject to discussion, as the use of “GnRH” to describe invertebrate peptides may imply a closer evolutionary relationship than exists [see Tsai (11) for a discussion on naming of invertebrate peptides].
In vertebrates, the regulation of many endocrine processes originates in the hypothalamus of the brain; this includes control of appetite, energy balance, and puberty. While a coordinated link between energy balance and reproduction is essential to ensure successful propagation of the species, vertebrates and invertebrates have evolved distinct mechanisms. Strikingly, only in the lineage leading to vertebrates (Fig.1), has GnRH ultimately been coopted into an endocrine function specialized for the stimulation of gonadotropin release from the anterior pituitary (12). This function does not appear to exist in the lineage of protostomes (Fig. 1); thus it is not surprising that Andreatta et al. (3) do not find a defect in reproductive function in the crz1−/− mutant animals. Yet the elucidation of downstream target genes of CRZ1 signaling involved with glycoprotein and carbohydrate metabolism harkens back to the ancestral necessity of guaranteeing sufficient resources (metabolic) to ensure successful reproduction. This is made more crucial in the marine worm, a uniparous animal whose “reproduce and die” strategy of survival places great pressure on the single reproductive event.
Recently, my laboratory (13) and others have shown that the zebrafish, Danio rerio, lacks the gnrh1 gene and appears not to require GnRH as a reproductive peptide, leaving the mystery of what peptide has been coopted to control reproduction. A potential candidate, the gonadotropin-inhibitory hormone (GnIH), may also be a candidate for circalunar reproductive pathway in Platynereis (14, 15). In the grass puffer, a fish with a semilunar spawning cycle, genes encoding GnIH and its receptor (as well as Kiss2) showed distinct seasonal, daily, and circadian variations in the brain, suggesting common regulatory mechanisms involving melatonin, circadian clock, and water temperature (16).
The marine bristleworm is a nonstandard model system that represents a large group of animals, the polychaetes, with more than 10,000 described species encompassing a wide variety of reproductive strategies. Studies in the marine bristleworm will allow us to better understand the evolution and cooption of peptide-receptor signaling pathways that regulate environmental control of reproduction. The advent of gene-editing techniques allowed for the generation of the crz1−/− mutant, and bioinformatic techniques underlie the data mining necessary to elucidate metabolic pathways that appear to “cue” from the lunar cycle, thus allowing these widely dispersed marine animals to coordinate their only spawning event. What is lacking are data collected
from the field that potentially could be extremely revealing, as “domestication” (maintaining animals in captivity) and social context strongly affect hormonal states and thus behavior. This is evidenced in attempts to explain individual variation in circulating
In PNAS, Andreatta et al. build on previous work showing that the marine bristleworm Platynereis dumerilii has a circalunar (monthly) clock that interacts in an independent manner with the circadian clock (4).
hormone levels above breeding baselines in vertebrates (17) where cellular components of target tissues such as receptors, metabolizing enzymes, and other factors clearly modulate effects of peptide hormones. Ideally, samples of circulating CRZ1 peptide in premetamorphosis and postmetamorphosis bristleworms coordinated with the natural lunar cycle would confirm the results obtained in the laboratory, although authors (3) do acknowledge both the necessity and difficulty of obtaining such data. In the face of the climate crisis, the marine bristleworm may prove to be an indicator species we can use to assess hormonal and metabolic variations in response to environmental stress already evidenced in our oceans, with the potential of avoiding future catastrophes in the natural world (18).
The role of the moon in the coordination of reproduction is most obvious in aquatic species where it is important to coordinate broadcast spawning over large areas, as has been observed with spawning of coral reefs, some marine fishes, and crabs (19) (20). In the modern world illuminated by artificial lighting at all hours of the circadian clock, we often forget that the moon exerts a huge effect on the planet’s ecosystems: The moon pulls the oceans, creating the tides (many animals respond to the tides as natural cues coordinating reproduction); the moon stabilizes Earth’s climate by reducing variability in the axial tilt; and, finally, important for this paper, the moon can act as an environmental cue (zeitgeber) setting the genetically encoded biological clock that most organisms possess. The polychaete Platynereis has been shown previously to express circadian rhythm genes that can be modulated either directly or indirectly by the circalunar clock, yet the circalunar clock is independent of these gene oscillations (4). A striking finding in this paper (3) is that the CRZ1-like peptide appears to act as a link coordinating the sensation of the lunar cycle with the metabolic resources necessary for spawning. Future research will hopefully elucidate the mechanisms by which lunar cycle is transduced to a communal sensory signal coordinating the brain–reproductive axis in diverse populations of animals.
Acknowledgments
My research is supported by Grant Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) 1160076 and Programa Iniciativa Científica Milenio (ICM)-ECONOMIA Instituto Milenio Centro Interdisciplinario de Neurociencias de Valparaíso PO9-022-F.
Footnotes
The author declares no competing interest.
See companion article on page 1097.
References
- 1.Loumaye E., Thorner J., Catt K. J., Yeast mating pheromone activates mammalian gonadotrophs: Evolutionary conservation of a reproductive hormone? Science 218, 1323–1325 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Hauser F., Grimmelikhuijzen C. J., Evolution of the AKH/corazonin/ACP/GnRH receptor superfamily and their ligands in the Protostomia. Gen. Comp. Endocrinol. 209, 35–49 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Andreatta G., et al. , Corazonin signaling integrates energy homeostasis and lunar phase to regulate aspects of growth and sexual maturation in Platynereis. Proc. Natl. Acad. Sci. U.S.A. 117, 1097–1106. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zantke J., et al. , Circadian and circalunar clock interactions in a marine annelid. Cell Rep. 5, 99–113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartenstein V., The neuroendocrine system of invertebrates: A developmental and evolutionary perspective. J. Endocrinol. 190, 555–570 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Zandawala M., Tian S., Elphick M. R., The evolution and nomenclature of GnRH-type and corazonin-type neuropeptide signaling systems. Gen. Comp. Endocrinol. 264, 64–77 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Kubrak O. I., Lushchak O. V., Zandawala M., Nässel D. R., Systemic corazonin signalling modulates stress responses and metabolism in Drosophila. Open Biol. 6, 160152 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marco H. G., Gäde G., Five neuropeptide ligands meet one receptor: How does this tally? A structure-activity relationship study using adipokinetic bioassays with the sphingid moth, Hippotion eson. Front. Endocrinol. (Lausanne) 10, 231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai T., et al. , Invertebrate gonadotropin-releasing hormone-related peptides and their receptors: An update. Front. Endocrinol. (Lausanne) 8, 217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satake H., et al. , Neuropeptides, peptide hormones, and their receptors of a tunicate, Ciona intestinalis. Results Probl. Cell Differ. 68, 107–125 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Tsai P. S., Gonadotropin-releasing hormone by any other name would smell as sweet. Gen. Comp. Endocrinol. 264, 58–63 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Holland L. Z., Sower S. A., “Insights of early chordate genomics: Endocrinology and development in amphioxus, tunicates and lampreys”: Introduction to the symposium. Integr. Comp. Biol. 50, 17–21 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Whitlock K. E., Postlethwait J., Ewer J., Neuroendocrinology of reproduction: Is gonadotropin-releasing hormone (GnRH) dispensable? Front. Neuroendocrinol. 53, 100738 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsui K., Osugi T., Son Y. L., Ubuka T., Review: Structure, function and evolution of GnIH. Gen. Comp. Endocrinol. 264, 48–57 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Di Yorio M. P., et al. , The Gonadotropin-inhibitory hormone: What we know and what we still have to learn from fish. Front. Endocrinol. (Lausanne) 10, 78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando H., Shahjahan M., Kitahashi T., Periodic regulation of expression of genes for kisspeptin, gonadotropin-inhibitory hormone and their receptors in the grass puffer: Implications in seasonal, daily and lunar rhythms of reproduction. Gen. Comp. Endocrinol. 265, 149–153 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Wingfield J. C., Ramenofsky M., Hegner R. E., Ball G. F., Whither the challenge hypothesis? Horm. Behav. 31, 104588 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Byrne M., Foo S. A., Ross P. M., Putnam H. M., Limitations of cross and multigenerational plasticity for marine invertebrates faced with global climate change. Glob. Change Biol. 31, 14882 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Jabr F., “How moonlight sets nature’s rhythms.” Smithsonian.com (2017). https://www.smithsonianmag.com/science-nature/how-moonlight-sets-nature-rhythms-180963778/. Accessed 21 June 2017.
- 20.Kaniewska P., Alon S., Karako-Lampert S., Hoegh-Guldberg O., Levy O., Signaling cascades and the importance of moonlight in coral broadcast mass spawning. eLife 4, 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney A. M., Boch C. A., Johnsen S., Morse D. E., Twilight spectral dynamics and the coral reef invertebrate spawning response. J. Exp. Biol. 214, 770–777 (2011). [DOI] [PubMed] [Google Scholar]