Abstract
Background
Insects harbor a myriad of microorganisms, many of which can affect the sex ratio and manipulate the reproduction of the host. Leptocybe invasa is an invasive pest that causes serious damage to eucalyptus plantations, and the thelytokous parthenogenesis, low temperature resistance, protection in galls, generation overlap and small body of L. invasa contribute to its rapid invasion and population growth. However, the endosymbiotic bacterial composition, abundance and sex differences of L. invasa remain unclear. Therefore, this research aimed to identify the bacterial communities in L. invasa adults and compare them between the sexes of L. invasa lineage B.
Results
The Illumina MiSeq platform was used to compare bacterial community composition between females and males of L. invasa by sequencing the V3–V4 region of the 16S ribosomal RNA gene. A total of 1,320 operational taxonomic units (OTUs) were obtained. These OTUs were subdivided into 24 phyla, 71 classes, 130 orders, 245 families and 501 genera. At the genus level, the dominant bacteria in females and males were Rickettsia and Rhizobium, respectively.
Conclusion
The endosymbiotic bacteria of L. invasa females and males were highly diverse. There were differences in the bacterial community of L. invasa between sexes, and the bacterial diversity in male specimens was greater than that in female specimens. This study presents a comprehensive comparison of bacterial communities in L. invasa and these data will provide an overall view of the bacterial community in both sexes of L. invasa with special attention on sex-related bacteria.
Keywords: Leptocybe invasa, 16S rRNA, High throughput sequencing, Bacteria, Diversity
Introduction
There are numerous microorganisms living in insects, including bacteria, fungi, yeast and viruses, that play a vital role in the growth and reproduction of host insects (Dillon & Dillon, 2004; Doğanlar, 2005; Crotti et al., 2012; Frago, Dicke & Godfray, 2012; Engel & Moran, 2013; Hammer & Bowers, 2015). Over the course of long-term coevolution, microorganisms develop a close relationship with host insects, which may have an effect on the reproduction, survival, community interactions, and the ability to resist predators and vectors of the hosts (Oliver et al., 2003; Oliver et al., 2010; Moran, 2007; Clark et al., 2008; Moran, McCutcheon & Nakabachi, 2008; Moya et al., 2008). In light of the significant functions of the microorganisms, they have received much attention from the international academic community. In some insects, the diversity and function of endosymbiotic bacteria have been well studied. For instance, the bacteria in termites are mainly Bacteroidetes, Firmicutes and Actinobacteria and can assist their hosts in breaking down lignocellulose and promoting the nitrogen cycle (Warnecke et al., 2007; Brune, 2014). The bacteria in Aphis gossypii improve its resistance and adaptation (Łukasik et al., 2013a; Łukasik et al., 2013b). In recent years, manipulating endosymbionts for pest control has raised wide concern, and its theory and methods have been applied successfully to some extent. Introduction of antimalarial endosymbionts into the mid gut of host pests could inhibit the breeding of plasmodia and in turn reduce the efficiency of mosquito transmission of malaria (Wang et al., 2012). Mixed application of antibiotics and insecticides effectively reduced the quantity of endosymbionts in Nilaparvata lugens while improving the control effect of insecticides (Shentu et al., 2016). Based on research on the related incompatible insect technique (IIT), researchers used the maternally inherited endosymbiotic bacterium Wolbachia for sterilization, which had good effects on eliminating the fecundity of mosquitoes (Zheng et al., 2019). Obviously, it is necessary to clarify the bacterial composition and diversity in insects, which are the bases of manipulating endosymbionts for pest control. In addition, previous investigations have shown that sex is an important factor affecting bacterial diversity. For example, due to different attack behaviors, the overall diversity and richness of bacterial communities associated with female Dendroctonus valens are higher than those associated with males of this beetle species (Xu et al., 2016). The bacterial composition of mosquitoes was also affected by sex (Minard, Mavingui & Moro, 2013; Zouache et al., 2011). Different anatomies and life histories between male and female flies could provide differential opportunities for bacterial colonization (Tang et al., 2012).
The blue gum chalcid Leptocybe invasa Fisher & LaSalle (Hymenoptera: Eulophidae: Tetrastichinae) is a cosmopolitan pest that damages many Eucalyptus species (Mendel et al., 2004; Le et al., 2018). L. invasa, originating in Australia, was first recorded in 2000 and has since been discovered in 45 countries of Asia, Europe, Africa, Oceania and America (Le et al., 2018; Zheng et al., 2014a). A new study demonstrated that an increasing number of areas will become suitable for L. invasa due to climate warming (Huang et al., 2019). Every delicate twig, vein and petiole of eucalyptus trees may provide a spawning ground for this pest, and galls ultimately lead to stunted growth of the trees, causing great losses in local eucalyptus plantations (Mendel et al., 2004; Zheng et al., 2014a; Huang et al., 2018). DNA barcode data indicated that L. invasa includes two genetically separate lineages (lineages A and B). Researchers considered the Italian, Argentinean and Tunisian populations to belong to lineage A and the Chinese population to belong to lineage B (Le et al., 2018; Dittrich-Schröder et al., 2018). The absence of natural enemies, presence of large amounts of suitable host plants, small size, protection in galls, strong resistance to low temperature and thelytokous parthenogenesis of L. invasa caused its rapid invasion and growth of in China (Zheng et al., 2014a). As a result, it has become one of the most difficult pests to control (Zheng et al., 2014a; Huang et al., 2018; Le et al., 2018). It is important in tems of theory and application to study the endosymbiotic bacterial diversity of L. invasa and then control the wasps by using these endosymbiotic bacteria.
To date, few studies have reported on the overall endosymbiotic bacteria of L. invasa, which is an invasive gall-inducing insect. Only a few studies have comprehensively examined the endosymbiotic bacteria in this species. Wang et al. (2018) cultured 11 strains from female adults of L. invasa in winter using traditional methods and classified them into three phyla (Firmicutes, Actinobacteria, and Proteobacteria), three classes (Bacilli, Actinobacteria, and Gammaproteobacteria) and four orders (Bacillales, Micrococcales, Lactobacillales, and Enterobacterales) that were related to growth, development, nutrition metabolism and immunity. Nugnes et al. (2015) researched the bacteria living in adults among different populationsvia denaturing gradient gel electrophoresis (DGGE) analysis and found that Rickettsia occurred in the reproductive tissues of female L. invasa, suggesting a relationship with its thelytokous parthenogenesis. L. invasa harbors a myriad of bacteria, and bacterial differences between sexes have strong effects on insects, such as effects on reproductive regulation (Wang et al., 2018; Nugnes et al., 2015). Therefore, the overall endosymbiotic bacterial composition and abundance of L. invasa and the differences between sexes are important to study.
In this study, the endosymbiotic bacteria in female and male adults of L. invasa were indentified by 16S rRNA sequencing of the V3–V4 region to shed light on their internal bacterial compositions. The females and males were also compared to address sexual differences in the endosymbionts. These results will provide a valuable bacterial pool for L. invasa and will further contribute to understanding its reproductive strategies and invasion mechanisms.
Materials & Methods
Insect sampling
Branches of DH 201-2 (Eucalyptus grandis × E. tereticornis) (Myrtales: Myrtaceae) harboring galls of L. invasa were removed from the Teaching and Experiment Base of Forestry College, Guangxi University (108°17′E, 22°51′N), Nanning city, Guangxi Zhuang Autonomous Region from July to August 2018. The branches were placed in a plastic bottle filled with water to retain freshness and transferred into a sealed net cage (40 cm × 40 cm × 80 cm) at room temperature to keep the adults from escaping. The water in the plastic bottle was renewed daily until the emergence of L. invasa adults. Sexes were identified by morphological observation (Zheng et al., 2014b).
DNA extraction
Fifty adults of each sex of L. invasa newly emerged within 12 h were fasted for 6 h. Then, both samples were sterilized externally with 75% ethanol for 2–5 min and rinsed 3 times with sterilized water to remove microbes on the surface. The total bacterial DNA of each sample was extracted using a Power Soil DNA Isolation Kit (MO BIO Laboratories) according to the manufacturer’s instructions. The quality and quantity of DNA were assessed by the ratios of 260 nm/280 nm and 260 nm/230 nm. Then, the qualified DNA was stored at −80 °C for further processing. The DNA of each individual was extracted by using a Chelex-100 and proteinase K-based method (Gebiola et al., 2009).
PCR amplification and cloning of the bacterial 16S rRNA gene
Amplification of the V3–V4 hypervariable region of the bacterial 16S rRNA gene was performed by using the universal bacterial primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GACTACHVGGGTWTCTAAT-3′). PCRs were carried out in 50 µL solutions containing 10 µL of 10 × buffer, 0.2 µL of Q5 High-Fidelity DNA Polymerase, 10 µL of High GC Enhancer, 1 µL of dNTPs, 10 µM each forward and reverse primer, 60 ng of genomic DNA and enough ddH2O to reach 50 µL. The amplifications were performed in a ABI Applied Biosystems 9902 thermal cycler with an initial denaturation step at 95 °C for 5 min, followed by 35 cycles of annealing and extension (each cycle consisted of 95 °C for 1 min, 50 °C for 1 min and extension at 72 °C for 1 min) and a final extension at 72 °C for 7 min. The PCR products were checked by electrophoresis on an agarose gel (1.8% agarose, 1 × TBE), stained with ethidium bromide and visualized under ultraviolet light. The products from the first round of PCR were purified with VAHTS™ M DNA Clean Beads. The second round of PCR was then performed in a 40 µL reaction containing 20 µL of 2 × Phµsion HF MM, 8 µL of ddH2O, 10 µM each forward and reverse primer and 10 µL of PCR product produced in the first round. The second round of PCR was run under the following conditions: initial denaturation at 98 °C for 30 s, followed by 10 cycles at 98 °C for 10 s, 65 °C for 30 s and 72 °C for 30 s and a final extension at 72 °C for 5 min. Finally, all PCR products were quantified and pooled by Quant-iT™ dsDNA HS Reagent. High-throughput sequencing analysis of bacterial rRNA genes was performed on the purified, pooled sample by using the Illumina HiSeq 2500 platform at Biomarker Technologies Co., Ltd., Beijing, China.
Bioinformatics and statistical analysis
After sequencing, PE reads obtained with HiSeq sequencing were merged by overlapping to obtain raw tags. To obtain clean tags, the raw tags were denoised, sorted and separated by using Trimmomatic (version 0.33). The remaining sequences were filtered for redundancy, and all unique sequences in each sample were clustered into operational taxonomic units (OTUs) on the basis of 97% similarity. Low-abundance OTUs were identified and eliminated by using UCHIME v4.2. Taxonomic assignment of the OTUs was conducted with the Silva reference database. Species abundance tables were generated by QIIME, and community structures in every taxon category was plotted by R software. The relative abundances of the bacteria were determined by percentages.
Alpha diversity based on Chao1 richness and ACE richness estimators, as well as the Simpson and Shannon diversity indices, was evaluated by using the mothur v.1.11.0 program. Among these measure, Chao1 and ACE reflected species richness in the samples, the Shannon index reflected community diversity, the Simpson index reflected the dominance of species in the community, and the coverage index reflected the degree to which the sequencing results represented the actual composition of the microorganisms in the samples.
Molecular characterization and phylogenetic analyses
COI was amplified by using the forward primer LCO1490 and reverse primer HCO2198 (Nugnes et al., 2015). The 16S rRNA gene of Rickettsia was amplified by using the primers listed in Table S1. The PCR program for both genes (COI and 16S rRNA) was as follows: 3 min of initial denaturation at 94 °C, 30 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min and a final extension of 5 min at 72 °C. PCR products were observed by using 1.0% agarose gel electrophoresis, and the amplified fragments were directly sequenced by TsingKe Biological Technology Co., Ltd, Beijing, China. Representative sequences of other regions were downloaded from GenBank, and sequence alignment was completed by using Clustal X. The neighbour-joining method was used to construct a consensus phylogenetic tree with MEGA7 software. To evaluate the branch support of the phylogenetic tree, bootstrap analysis of 1,000 replicates was performed.
Accession numbers
Data is available at NCBI SRA, accession numbers: SRR9591039, SRR9591038. The COI and 16S rRNA sequences determined in this study have been deposited in the GenBank database with Accession number MN524231 and MN524230, respectively.
Results
Sex of L. invasa specimens in this study
All female and male specimens were identified on the basis of morphology. In this study, a total of 656 females and 51 males were collected (Table S2). The materials were deposited at the Forest Conservation Laboratory, College of Forestry, Guangxi University, Nanning 530004, China.
Sequencing and classification
A total of 533,266 raw tags (370,680 from males and 162,586 from females) were obtained for L. invasa, and 476,235 clean tags (328,833 from males and 147,402 from females) were generated (Table S3), which were classified into different OTUs based on 97% similarity. Among the 476,235 clean tags, a total of 1,320 OTUs were obtained; of these 1,320 OTUs, 154 were common to both sexes, and 38 and 1128 were specific to female and male adults, respectively (Fig. 1).
Figure 1. Venn diagram of OTU distribution in Leptocybe invasa female and male adults.
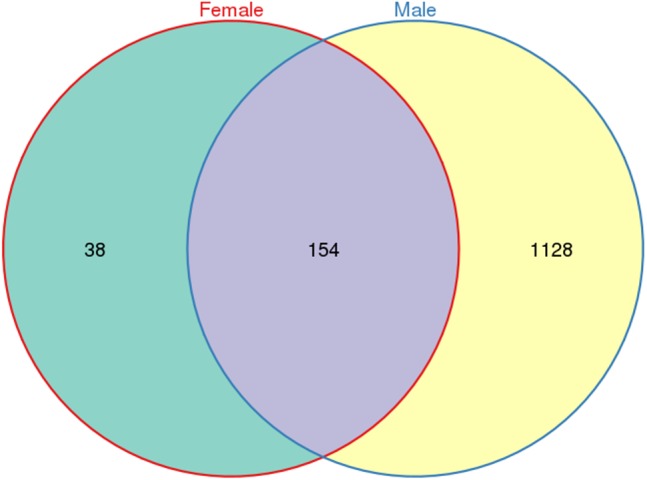
Numbers within compartments indicate OTU counts of according to mathematical sets.
Analysis of alpha diversity
Alpha diversity was estimated by five indices: Chao1, the Shannon index, the Simpson index, the ACE and coverage. The results in Table 1 show that the bacteria in L. invasa adults were diverse in both sexes. The Chao1 (229.50 vs 1282.00) and ACE (212.84 vs 1282.28) values were lower in the females than in the males. Good agreement was also observed between the Simpson and Shannon indices. The Shannon index (0.59 vs 6.13) was lower in the females than in the males, while the Simpson index (0.85 vs 0.01) was higher in the female wasps than in the male wasps, indicating that the diversity of the bacterial community in males was higher than that in females. The coverage was near 100% for both males and females, illustrating a higher probability of bacteria being detected than of bacteria being undetected.
Table 1. Statistics of alpha diversity indices of the bacteria in female and male adults of Leptocybe invasa.
| Sample | ACE | Chao1 | Simpson | Shannon | Coverage |
|---|---|---|---|---|---|
| Female | 212.84 | 229.50 | 0.85 | 0.59 | 1.00 |
| Male | 1282.28 | 1282.00 | 0.01 | 6.13 | 1.00 |
The analysis of community composition and species abundance
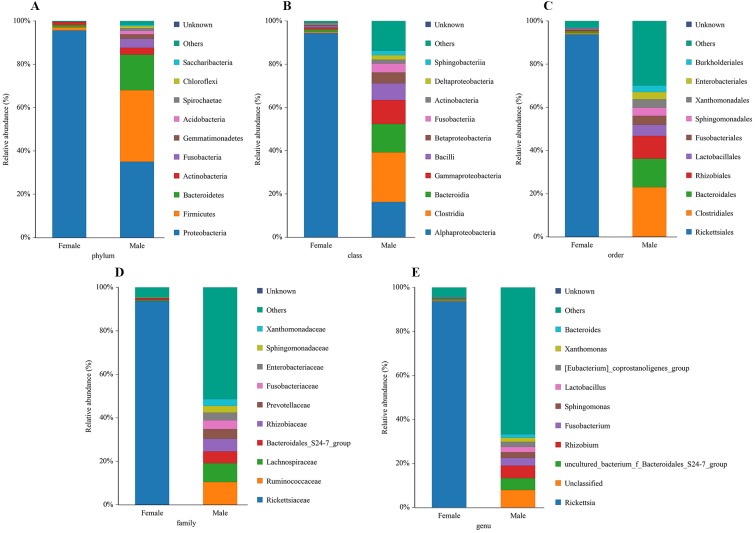
The bacterial community composition and species abundance in both sexes of L. invasa were analyzed (abundances greater than 0.1%) based on the results of the OTUs (Table 2, Fig. 2). A total of 24 phyla were detected and classified in the samples. Proteobacteria was the dominant bacterial phylum annotated in females and males, accounting for 95.63% and 34.99% of bacteria, respectively. At the genus level, Rickettsia (with an abundance of 93.67%) and Rhizobium (with an abundance of 5.73%) were the dominant bacteria in females and males, respectively. In addition, it was noteworthy that the abundance of Rickettsia was less than 1% in males (Table 3).
Table 2. Basic composition of the bacterial colonies in female and male adults of Leptocybe invasa.
| Sample | Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|
| Female | 10 | 26 | 44 | 76 | 122 |
| Male | 24 | 69 | 127 | 238 | 487 |
| Female-specific | 0 | 2 | 3 | 7 | 14 |
| Male-specific | 14 | 45 | 86 | 169 | 379 |
| Sex-in common | 10 | 24 | 41 | 69 | 108 |
| Total | 24 | 71 | 130 | 245 | 501 |
Figure 2. Relative abundance of top 10 bacteria at the levels of phylum (A), class (B), order (C), family (D) and genu (E) in females and males of Leptocybe invasa.
Table 3. Relative abundance of dominate bacteria at the levels of genus in female and male adults of Leptocybe invasa.
| Genus | Female (%) | Male (%) |
|---|---|---|
| Rickettsia | 93.67 | 0.04 |
| Uncultured_bacterium_f_Bacteroidales _S24-7_group | 0.71 | 5.37 |
| Lactobacillus | 0.31 | 2.38 |
| Sphingomonas | 0.25 | 2.62 |
| Bacteroides | 0.11 | 1.65 |
| Fusobacterium | 0.04 | 3.49 |
| [Eubacterium]_coprostanoligenes _group | 0 | 2.34 |
| Rhizobium | 0 | 5.73 |
| Unknown | 0 | 0.01 |
| Xanthomonas | 0 | 1.83 |
| Others | 4.48 | 66.68 |
| Unclassified | 0.44 | 7.86 |
Molecular characterization and phylogenetic analyses
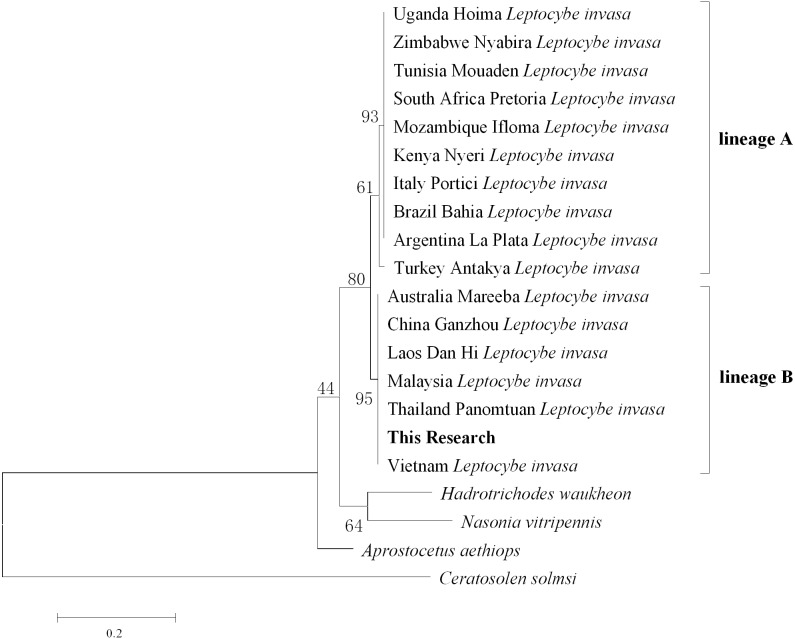
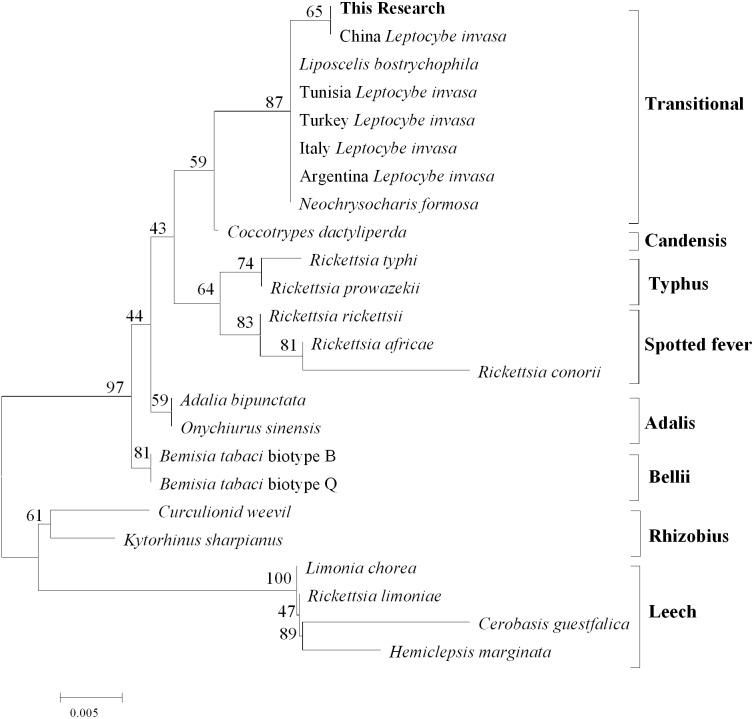
After comparison with Genebank, the identification of L.invasa in this research was lineage B and the phylogenetic tree of COI also indicated that the population of this research belonged to the lineage (Fig. 3). The phylogenetic analysis of 16S rRNA genes revealed that the Rickettsia of L. invasa symbionts belonged to the Rickettsia transitional group (Fig. 4).
Figure 3. Phylogenetic tree of different Leptocybe invasa populations based on COI sequences.
Figure 4. Phylogenetic analysis of different Rickettsia groups based on their 16S rRNA sequences.
Discussion
Differences in bacteria between female and male adults
This research revealed that the bacteria harbored in L. invasa had high diversity, and many of the endosymbiotic bacteria were annotated in this species for the first time. Based on alpha diversity analysis, the diversity of the endosymbiotic bacteria in males was higher than that in females (Table 1). The variation in bacterial communities between males and females may be partly explained by differences in physiology structure and between the two sexes of L. invasa; specifically, the female wasps have ovaries, which harbor an abundance of Rickettsia, allowing the genus to occupy different bacterial niches than in males (Nugnes et al., 2015). Another possibility is that insects launch innate and systematic immune responses to cope with microbe colonization (Leulier & Royet, 2009) and females have stronger immune systems than males (Kurtz et al., 2000).
Comparison of the bacteria with those in other insects
Bacterial community analysis at the phylum level demonstrated that Proteobacteria was the dominant group in female and male wasps, and Firmicutes, Bacteroidetes, Actinobacteria and Fusobacteria were also annotated. Previous studies revealed that Proteobacteria were dominant in other Hymenoptera, such as Apis cerana and leaf-cutter ants (Ahn et al., 2012; Zhukova et al., 2017). In contrast, Firmicutes and Bacteroidetes were the major bacterial phyla detected in the guts of termites (Miyata et al., 2007; Xiang et al., 2012) and bees (Mohr & Tebbe, 2006). Firmicutes and Actinobacteria were the dominant bacteria in A. mellifera and bumblebees (Ahn et al., 2012; Praet et al., 2018).
Putative functions of dominant endosymbiotic bacteria in L. invasa
Several of the bacteria detected in this study are commonly described in insects at the genus level, and some have been found in Hymenoptera, such as honeybees (Mohr & Tebbe, 2006) and termites (Xiang et al., 2012). Intriguingly, two genera, Staphylococcus and Escherichia, are known to contain cultivable species (Wang et al., 2018). Gloverin and lysozyme gene expression was upregulated when silkworm larvae were fed Escherichia and Staphylococcus, indicating that the two bacteria were closely related to the immune signaling pathway of the silkworm (Douglas, 2015). We hypothesized that Escherichia and Staphylococcus may also be involved in the immunoreaction of L. invasa. Functions have been suggested for some of the other bacterial genera detected in this study. The Enterobacteriaceae that are associated with insects help with digestion, the detoxification of toxic substances, and resistance to pathogens and enhance the adaptability of the host (Anand et al., 2010). Adding Enterobacter to feed extended the life span of Mediterranean flies (Behar, Yuval & Jurkevitch, 2005; Behar, Yuval & Jurkevitch, 2008). Similarly, Enterobacteriaceae (Hongoh & Ishikawa, 2000) and Acinetobacter (Broderick et al., 2004) facilitated carbon-nitrogen metabolism and accelerated the growth and development of host insects; e.g., the Acinetobacter belonging to termites have a nitrogen-transforming function according to Warnecke et al.’s (2007) research. Some bacteria associated with immunization were also discovered in L. invasa, such as Lactobacillus. Lactobacillus had some positive effects on insect resistance (Xia et al., 2013). In addition, Bacillales were also detected in this study and may be insect pathogens, such as Bacillus thuringiensis and Bacillus cereus (Broderick et al., 2004; Raymond et al., 2010; Song et al., 2014). In contrast, some Bacillus in termites might be involved in the degradation of cellulose and hemicellulose (Konig, 2006). In this study, Bacillales were detected in both sexes, and their specific functions require further study. Nevertheless, Acinetobacter was detected in L. invasa, and previous research showed that Acinetobacter produces an antiviral compound that inhibits tobacco mosaic virus (Lee et al., 2009). Moreover, members of Bacteroidetes specialize in the degradation of complex organic matter, including lignocellulosic compounds (Yuki et al., 2015). Bacteroidetes are also involved in the decomposition and metabolism of polysaccharides (Xu et al., 2003; Sonnenburg et al., 2010), which are beneficial for host absorption and digestion (Liu et al., 2011). In addition, Bacteroidetes also include some Azotobacter, such as Azobacteroides pseudotrichonympha, which can provide the host with amino acids for nutrition (Noda et al., 2009; Desai & Brune, 2012). Bacteroidetes involved in the degradation and fermentation of phytomass could influence the nutrient absorption of L. invasa, but further studies are needed. Many other groups of bacteria with undefined functions were detected in L. invasa for the first time in this study. Better knowledge of the bacteria associated with L. invasa will allow researchers to investigate their role in host biology.
A sequence similarity search revealed that Rhizobium was the dominant bacterium in male adults (Fig. 2, Table 3). Rhizobium produces a variety of enzymes with cellulose- and pectin-hydrolyzing activities that can hydrolyze the glycoside skeleton of the plant cell wall and play a very important role in the symbiosis between Rhizobium and leguminous plants (Robledo et al., 2008; Huang et al., 2018). Rhizobium is an endosymbiont detected in the gut of some phytophagous insects and can help the host synthesize nitrogen-containing substances that are lacking in food (Russell et al., 2009).
Rickettsia (with an abundance of 93.67%) was the dominant bacterial genus present in female adults (Fig. 2, Table 3). Rickettsia is a maternally inherited intracellular bacterium in a wide range of arthropods and is capable of controlling populations by reproductive manipulation, such as parthenogenesis inducing (PI) (Hagimori et al., 2006; Adachi-Hagimori, Miura & Stouthamer, 2008; Giorgini et al., 2010) and male killing (MK) (Lawson et al., 2001; Von der Schulenburg et al., 2001; Majerus & Majerus, 2010). During female gamete formation in Rickettsia-carrying Neochrysocharis formosa, meiotic cells underwent only one equatorial division, and meiotic recombination was absent, which demonstrated that Rickettsia could induce parthenogenesis by changing the meiosis of wasps (Adachi-Hagimori, Miura & Stouthamer, 2008). Rickettsia also induced male embryo death in Adalia bipunctata and A. decempunctata (Hurst, Majerus & Walker, 1993; Hurst, Walker & Majerus, 1996; Werren et al., 1994). Moreover, Rickettsia affects the fitness of the host and protects it against adverse environmental conditions (Oliver et al., 2003; Sakurai et al., 2005; Chiel et al., 2009; Himler et al., 2011; Brumin, Kontsedalov & Ghanim, 2011). For instance, preadult development of the Bemisia tabaci B-biotype was faster with Rickettsia infection than without (Chiel et al., 2009). Himler et al. (2011) found that Rickettsia-carrying whiteflies produced more offspring, developed faster, had a higher rate of survival to adulthood, and produced a larger proportion of daughters than did uninfected whiteflies. Males have never been recorded in Italy, Tunisia and Argentina, and rarely in Turkey (sex ratio 0–0.5%) (Nugnes et al., 2015). These results show that L. invasa reproduces by thelytokous parthenogenesis. In contrast, males appeared more frequently in China, India and Thailand. In this study, the sex ratio was 7.2%. In addition, Nugnes et al. (2015) found that Rickettsia was located in reproductive tissues in females and passed to the next generation via vertical transmission, representing a possible reason for thelytokous parthenogenesis in L. invasa. Female L. invasa play an important role in invasion and colonization (Zheng et al., 2014a). The results of the current investigation could explain why the sex ratio in wasps is female-biased and support the hypothesis that Rickettsia can induce thelytokous parthenogenesis in L. invasa. However, both explanations require further testing. In addition, a low abundance of Rickettsia was present in males in this research. For Hymenoptera, the dominant reproductive mode is arrhenotoky; that is, diploid females develop from fertilized eggs, and haploid males develop from unfertilized eggs (Van Wilgenburg, Driessen & Beukeboom, 2006). A previous investigation suggested that Rickettsia could be passed to the offspring by vertical transmission (Nugnes et al., 2015), and a threshold density of Rickettsia bacteria in eggs is required to trigger the development of female embryos Giorgini, 2001; Giorgini et al., 2010. Removing Rickettsia by feeding antibiotics could lead to the production of more male offspring. Giorgini et al. (2010) found that Rickettsia-infected Pnigalio soemius generated only female progeny, and after 24 h, when the Rickettsia was removed by 20 mg/mL rifampin, adults produced almost all male offspring. Hagimori et al. (2006) declared that Rickettsia was related to the thelytokous parthenogenesis of N. formosa, a dominant parasite of leaf miners, and after removing Rickettsia from the adults by feeding the adults tetracycline, female offspring without Rickettsia were present. Therefore, future studies should clarify whether Rickettsia is involved in the reproductive manipulation of L. invasa accomplished via feeding with antibiotics. Furthermore, environmental factors could also influence the density of the bacteria, and endosymbiont densities and functions may change with space, time and season (Bordenstein & Bordenstein, 2011; Nugnes et al., 2015). A previous study indicated that the sex ratio of the Chinese population could change with temperature, presumably because the relationship between Rickettsia strain and Chinese population is weaker than that of Western population, which could be more susceptible to temperature (Zhu et al., 2015; Nugnes et al., 2015). In addition, another plausible explanation may be the use of different host plants (the host of lineage A is E. camaldulensis but in this research, it was DH201-2), which has been demonstrated in other systems (Ferrari, Scarborough & Godfray, 2007; Biere & Tack, 2013). Therefore, it is also essential to compare the differences in bacteria between L. invasa that parasitize different hosts.
Conclusions
The results of this study obtained by high-throughput revealed the bacterial diversity and differences between sexes in L. invasa, suggesting an abundant endosymbiotic bacterial community, and some bacteria were reported in L. invasa for the first time. Moreover, the males harbored a more diverse bacterial community than did the females. The next research should focus on the bacteria found in this study to identify their specific ecological functions and the specific sex-based regulatory mechanism of Rickettsia occurrence in L. invasa.
Supplemental Information
Acknowledgments
The authors thank Prof. Yongqiang He for sharing his knowledge of bacteria, the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources, and the members of the Guangxi Key Laboratory of Forest Ecology and Conservation.
Funding Statement
The research was financially supported by the National Natural Science Foundation of China (grant number 31560212, 31971664 and 31870634) and Guangxi Natural Science Foundation (grant number No. 2018GXNSFAA294008, 2018GXNSFDA281004 and 2018GXNSFAA138099). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Chunhui Guo conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Xin Peng performed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Xialin Zheng performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Xiaoyun Wang analyzed the data, authored or reviewed drafts of the paper, and approved the final draft.
Ruirui Wang conceived and designed the experiments, performed the experiments, prepared figures and/or tables, and approved the final draft.
Zongyou Huang conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Zhende Yang conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
Data is available at NCBI SRA: SRR9591039, SRR9591038.
The COI and 16S rDNA sequences are available at GenBank: MN524231 and MN524230.
Additional data is also available at Figshare: Guo, Chunhui (2019): mic.zip. figshare. Dataset. DOI: 10.6084/m9.figshare.8323616.v1.
References
- Adachi-Hagimori, Miura & Stouthamer (2008).Adachi-Hagimori T, Miura K, Stouthamer R. A new cytogenetic mechanism for bacterial endosymbiont-induced parthenogenesis. Proceedings of the Royal Society B-Biological Sciences. 2008;275(1652):2667–2673. doi: 10.1098/rspb.2008.0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn et al. (2012).Ahn JH, Hong IP, Bok JI, Kim BY, Song J, Weon HY. Pyrosequencing analysis of the bacterial communities in the guts of Honey Bees Apis cerana and Apis mellifera in Korea. Journal of Microbiology. 2012;50(5):735–745. doi: 10.1007/s12275-012-2188-0. [DOI] [PubMed] [Google Scholar]
- Anand et al. (2010).Anand AA, Vennison SJ, Sankar SG, Prabhu DIG, Vasan PT, Raghuraman T, Geoffrey CJ, Vendan SE. Isolation and characterization of bacteria from the gut of Bombyx mori that degrade cellulose, xylan, pectin and starch and their impact on digestion. Journal of Insect Science. 2010;10:1–20. doi: 10.1673/031.010.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar, Yuval & Jurkevitch (2005).Behar A, Yuval B, Jurkevitch E. Enterobacteria-mediated nitrogen fixation in natural populations of the fruit fly Ceratitis capitata. Molecular Ecology. 2005;14(9):2637–2643. doi: 10.1111/j.1365-294X.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- Behar, Yuval & Jurkevitch (2008).Behar A, Yuval B, Jurkevitch E. Community structure of the Mediterranean fruit fly microbiota: seasonal and spatial sources of variation. Israel Journal of Ecology & Evolution. 2008;54(2):181–191. doi: 10.1080/15659801.2008.10639612. [DOI] [Google Scholar]
- Biere & Tack (2013).Biere A, Tack AJM. Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Function Ecology. 2013;27:646–660. doi: 10.1111/1365-2435.12096. [DOI] [Google Scholar]
- Bordenstein & Bordenstein (2011).Bordenstein SR, Bordenstein SR. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLOS ONE. 2011;6:e29106. doi: 10.1371/journal.pone.0029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick et al. (2004).Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Applied Environmental Microbiology. 2004;70(1):293–300. doi: 10.1128/AEM.70.1.293-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumin, Kontsedalov & Ghanim (2011).Brumin M, Kontsedalov S, Ghanim M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Science. 2011;18(1):57–66. doi: 10.1111/j.1744-7917.2010.01396.x. [DOI] [Google Scholar]
- Brune (2014).Brune A. Symbiotic digestion of lignocellulose in termite guts. Nature Reviews Microbiology. 2014;12(3):168–180. doi: 10.1038/nrmicro3182. [DOI] [PubMed] [Google Scholar]
- Chiel et al. (2009).Chiel E, Inbar M, Mozes-Daube N, White JA, Hunter MS, Zchori-Fein E. Assessments of fitness effects by the facultative symbiont, Rickettsia, in the sweetpotato whitefly (Hemiptera: Aleyrodidae) Annals of the Entomological Society of America. 2009;102(3):413–418. doi: 10.1603/008.102.0309. [DOI] [Google Scholar]
- Clark et al. (2008).Clark ME, Bailey-Jourdain C, Ferree PM, England SJ, Sullivan W, Windsor DM, Werren JH. Wolbachia modification of sperm does not always require residence within developing sperm. Heredity. 2008;101(5):420–428. doi: 10.1038/hdy.2008.71. [DOI] [PubMed] [Google Scholar]
- Crotti et al. (2012).Crotti E, Balloi A, Hamdi C, Sansonno L, Marzorati M, Gonella E, Favia G, Cherif A, Bandi C, Alma A, Daffonchio D. Microbial symbionts: a resource for the management of insect-related problems. Microbial Biotechnology. 2012;5(3):307–317. doi: 10.1111/j.1751-7915.2011.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai & Brune (2012).Desai MS, Brune A. Bacteroidales ectosymbionts of gut flagellates shape the nitrogen-fixing community in dry-wood termites. ISME Journal. 2012;6(7):1302–1313. doi: 10.1038/ismej.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon & Dillon (2004).Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annual Review of Entomology. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Dittrich-Schröder et al. (2018).Dittrich-Schröder G, Hoareau TB, Hurley BP, Wingfield MJ, Lawson S, Nahrung HF, Slippers B. Population genetic analyses of complex global insect invasions in managed landscapes: a Leptocybe invasa (Hymenoptera) case study. Biological Invasions. 2018;20(9):2395–2420. doi: 10.1007/s10530-018-1709-0. [DOI] [Google Scholar]
- Doğanlar (2005).Doğanlar O. Occurrence of Leptocybe invasa Fisher & La Salle, 2004 (Hymenoptera: Chalcidoidea: Eulophidae) on Eucalyptus camaldulensis in Turkey, with description of the male sex. Zoology in the Middle East. 2005;35:112–114. doi: 10.1080/09397140.2005.10638116. [DOI] [Google Scholar]
- Douglas (2015).Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annual Review of Entomology. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel & Moran (2013).Engel P, Moran NA. The gut microbiota of insects-diversity in structure and function. FEMS Microbiology Reviews. 2013;37(5):699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- Ferrari, Scarborough & Godfray (2007).Ferrari J, Scarborough CL, Godfray HCJ. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia. 2007;153:323–329. doi: 10.1007/s00442-007-0730-2. [DOI] [PubMed] [Google Scholar]
- Frago, Dicke & Godfray (2012).Frago E, Dicke M, Godfray HCJ. Insect symbionts as hidden players in insect-plant interactions. Trends in Ecology & Evolution. 2012;27(12):705–711. doi: 10.1016/j.tree.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Gebiola et al. (2009).Gebiola M, Bernardo U, Monti MM, Navone P, Viggiani G. Pnigalio agraules (Walker) and Pnigalio mediterraneus Ferriére and Delucchi (Hymenoptera: Eulophidae): two closely related valid species. Journal of Natural History. 2009;43:2465–2480. doi: 10.1080/00222930903105088. [DOI] [Google Scholar]
- Giorgini (2001).Giorgini M. Induction of males in thelytokous populations of Encarsia meritoria and Encarsia protransvena: a systematic tool. BioControl. 2001;46(4):427–438. doi: 10.1023/A:1014181431482. [DOI] [Google Scholar]
- Giorgini et al. (2010).Giorgini M, Bernardo U, Monti MM, Nappo AG, Gebiola M. Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae) Applied and Environmental Microbiology. 2010;76(8):2589–2599. doi: 10.1128/AEM.03154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagimori et al. (2006).Hagimori T, Abe Y, Date S, Miura K. The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Current Microbiology. 2006;52(2):97–101. doi: 10.1007/s00284-005-0092-0. [DOI] [PubMed] [Google Scholar]
- Hammer & Bowers (2015).Hammer TJ, Bowers MD. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia. 2015;179(1):1–14. doi: 10.1007/s00442-015-3327-1. [DOI] [PubMed] [Google Scholar]
- Himler et al. (2011).Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE, Chiel E, Duckworth VE, Dennehy TJ, Zchori-Fein E, Hunter MS. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science. 2011;332(6026):254–256. doi: 10.1126/science.1199410. [DOI] [PubMed] [Google Scholar]
- Hongoh & Ishikawa (2000).Hongoh Y, Ishikawa H. Evolutionary studies on uricases of fungal endosymbionts of aphids and planthoppers. Journal of Molecular Evolution. 2000;51(3):265–277. doi: 10.1007/s002390010088. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2019).Huang MY, Ge XZ, Shi HL, Tong YG, Shi J. Prediction of current and future potential distributions of the eucalyptus pest Leptocybe invasa (Hymenoptera: Eulophidae) in China using the CLIMEX model. Pest Management Science. 2019;75:2958–2968. doi: 10.1002/ps.5408. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2018).Huang ZY, Li J, Lu W, Zheng XL, Yang ZD. Parasitoids of the eucalyptus gall wasp Leptocybe spp.: a global review. Environmental Science and Pollution Research. 2018;25(30):29983–29995. doi: 10.1007/s11356-018-3073-0. [DOI] [PubMed] [Google Scholar]
- Hurst, Majerus & Walker (1993).Hurst GDD, Majerus MEN, Walker LE. The importance of cytoplasmic male killing elements in natural populations of the two spot ladybird, Adalia bipunctata (Linnaeus) (Coleoptera: Coccinellidae) Biological Journal of the Linnean Society. 1993;49(2):195–202. doi: 10.1111/j.1095-8312.1993.tb00898.x. [DOI] [Google Scholar]
- Hurst, Walker & Majerus (1996).Hurst GDD, Walker LE, Majerus MEN. Bacterial infections of hemocytes associated with the maternally inherited male-killing trait in British populations of the two spot ladybird, Adalia bipunctata. Journal of Invertebrate Pathology. 1996;68(3):286–292. doi: 10.1006/jipa.1996.0098. [DOI] [PubMed] [Google Scholar]
- Konig (2006).Konig H. Bacillus species in the intestine of termites and other soil invertebrates. Journal of Applied Microbiology. 2006;101:620–627. doi: 10.1111/j.1365-2672.2006.02914.x. [DOI] [PubMed] [Google Scholar]
- Kurtz et al. (2000).Kurtz J, Wiesner A, Gotz P, Sauer KP. Gender differences and individual variation in the immune system of the scorpionfly Panorpa vulgaris (Insecta: Mecoptera) Development and Comparative Immunology. 2000;24(1):1–12. doi: 10.1016/S0145-305X(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Lawson et al. (2001).Lawson ET, Mousseau TA, Klaper R, Hunter MD, Werren JH. Rickettsia associated with male-killing in a buprestid beetle. Heredity. 2001;86(4):497–505. doi: 10.1046/j.1365-2540.2001.00848.x. [DOI] [PubMed] [Google Scholar]
- Le et al. (2018).Le NH, Nahrung HF, Griffiths M, Lawson SA. Invasive Leptocybe spp. and their natural enemies: global movement of an insect fauna on eucalypts. Biological Control. 2018;125:7–14. doi: 10.1016/j.biocontrol.2018.06.004. [DOI] [Google Scholar]
- Lee et al. (2009).Lee JS, Lee KC, Kim KK, Hwang IC, Jang C, Kim NG, Yeo WH, Kim BS, Yu YM, Ahn JS. Acinetobacter antiviralis sp. nov. from tobacco plant roots. Journal of Microbiology and Biotechnology. 2009;19(3):250–256. doi: 10.4014/jmb.0901.083. [DOI] [PubMed] [Google Scholar]
- Leulier & Royet (2009).Leulier F, Royet J. Maintaining immune homeostasis in fly gut. Nature Immunology. 2009;10(9):936–938. doi: 10.1038/ni0909-936. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2011).Liu N, Yan X, Zhang ML, Xie L, Wang QA, Huang YP, Zhou XG, Wang SY, Zhou ZH. Microbiome of fungus-growing termites: a new reservoir for lignocellulase genes. Applied and Environmental Microbiology. 2011;77(1):48–56. doi: 10.1128/AEM.01521-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łukasik et al. (2013a).Łukasik P, Guo H, Van Asch M, Ferrari J, Godfray HCJ. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. Journal of Evolutionary Biology. 2013a;26(12):2654–2661. doi: 10.1111/jeb.12260. [DOI] [PubMed] [Google Scholar]
- Łukasik et al. (2013b).Łukasik P, Van Asch M, Guo HF, Ferrari J, Godfray HCJ. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecology Letters. 2013b;16(2):214–218. doi: 10.1111/ele.12031. [DOI] [PubMed] [Google Scholar]
- Majerus & Majerus (2010).Majerus TMO, Majerus MEN. Discovery and identification of a male-killing agent in the Japanese ladybird Propylea japonica (Coleoptera: Coccinellidae) BMC Evolution Biology. 2010;10:37. doi: 10.1186/1471-2148-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel et al. (2004).Mendel Z, Protasov A, Fisher N, La Salle J. Taxonomy and biology of Leptocybe invasa gen. & sp. n. (Hymenoptera: Eulophidae), an invasive gall inducer on Eucalyptus. Australian Journal of Entomology. 2004;43:101–113. doi: 10.1111/j.1440-6055.2003.00393.x. [DOI] [Google Scholar]
- Minard, Mavingui & Moro (2013).Minard G, Mavingui P, Moro CV. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasites & Vectors. 2013;6:2–12. doi: 10.1186/1756-3305-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata et al. (2007).Miyata R, Noda N, Tamaki H, Kinjyo K, Aoyagi H, Uchiyama H, Tanaka H. Influence of feed components on symbiotic bacterial community structure in the gut of the wood-feeding higher termite Nasutitermes takasagoensis. Bioscience Biotechnology and Biochenmistry. 2007;71(5):1244–1251. doi: 10.1271/bbb.60672. [DOI] [PubMed] [Google Scholar]
- Mohr & Tebbe (2006).Mohr KI, Tebbe CC. Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environmental Microbiology. 2006;8(2):258–272. doi: 10.1111/j.1462-2920.2005.00893.x. [DOI] [PubMed] [Google Scholar]
- Moran (2007).Moran NA. Symbiosis as an adaptive process and source of phenotypic complexity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8627–8633. doi: 10.1073/pnas.0611659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, McCutcheon & Nakabachi (2008).Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annual Review of Genetics. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- Moya et al. (2008).Moya A, Pereto J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote–animal symbioses. Nature Review of Genetics. 2008;9(3):218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- Noda et al. (2009).Noda S, Hongoh Y, Sato T, Ohkuma M. Complex coevolutionary history of symbiotic Bacteroidades bacteria of various protist in the gut of termites. BMC Evolutionary Biology. 2009;9:1–12. doi: 10.1186/1471-2148-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugnes et al. (2015).Nugnes F, Gebiola M, Monti MM, Gualtieri L, Giorgini M, Wang JG, Bernardo U. Genetic diversity of the invasive gall wasp Leptocybe invasa (Hymenoptera: Eulophidae) and of its Rickettsia endosymbiont, and associated sex-ratio differences. PLOS ONE. 2015;10(5):e0124660. doi: 10.1371/journal.pone.0124660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver et al. (2010).Oliver KM, Degnan PH, Burke GR, Moran NA. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annual Review of Entomology. 2010;55:247–266. doi: 10.1146/annurev-ento-112408-085305. [DOI] [PubMed] [Google Scholar]
- Oliver et al. (2003).Oliver KM, Russell JA, Moran NA, Hunter MS. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1803–1807. doi: 10.1073/pnas.0335320100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praet et al. (2018).Praet J, Parmentier A, Schmid-Hempel R, Meeus I, Smagghe G, Vandamme P. Large-scale cultivation of the bumblebee gut microbiota reveals an underestimated bacterial species diversity capable of pathogen inhibition. Environmental Microbiology. 2018;20(1):214–227. doi: 10.1111/1462-2920.13973. [DOI] [PubMed] [Google Scholar]
- Raymond et al. (2010).Raymond B, Johnston PR, Nielsen LC, Lereclus D, Crickmore N, Lereclus D, Crickmore N. Bacillus thuringiensis: an impotent pathogen? Trends in Microbiology. 2010;18(5):189–194. doi: 10.1016/j.tim.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Robledo et al. (2008).Robledo M, Jimenez-Zurdo JI, Velazquez E, Trujillo ME, Zurdo-Pineiro JL, Ramirez-Bahena MH, Ramos B, Diaz-Minguez JM, Dazzo F, Martinez-Molina E, Mateos PF. Rhizobium cellulase CelC2 is essential for primary symbiotic infection of legume host roots. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(19):7064–7069. doi: 10.1073/pnas.0802547105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell et al. (2009).Russell JA, Moreau CS, Goldman-Huertas B, Fujiwara M, Lohman DJ, Pierce NE. Bacterial gut symbionts are tightly linked with the evolution of herbivory in ants. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21236–21241. doi: 10.1073/pnas.0907926106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai et al. (2005).Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Applied and Environmental Microbiology. 2005;71(7):4069–4075. doi: 10.1128/AEM.71.7.4069-4075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shentu et al. (2016).Shentu XP, Liu NN, Tang G, Tanaka Y, Ochi K, Xu JF, Yu XP. Effects of fungicides on the yeast-like symbiotes and their host, Nilaparvata lugens Stål (Hemiptera: Delphacidae) Pesticide Biochemistry and Physiology. 2016;128:16–21. doi: 10.1016/j.pestbp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Song et al. (2014).Song F, Peng Q, Brillard J, Lereclus D, LeRoux CN. An insect gut environment reveals the induction of a new sugar-phosphate sensor system in Bacillus cereus. Gut Microbes. 2014;5(1):58–63. doi: 10.4161/gmic.27092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg et al. (2010).Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, Sonnenburg JL. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141(7):1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang et al. (2012).Tang X, Adler PH, Vogel H, Ping LY. Gender-specific bacterial composition of black flies (Diptera: Simuliidae) FEMS Microbiology Ecology. 2012;80(3):659–670. doi: 10.1111/j.1574-6941.2012.01335.x. [DOI] [PubMed] [Google Scholar]
- Van Wilgenburg, Driessen & Beukeboom (2006).Van Wilgenburg E, Driessen G, Beukeboom LW. Single locus complementary sex determination in Hymenoptera: an unintelligent design? Frontiers in Zoology. 2006;3(1):1–15. doi: 10.1186/1742-9994-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Schulenburg et al. (2001).Von der Schulenburg JHG, Habig M, Sloggett JJ, Webberley KM, Bertrand D, Hurst GDD, Majerus MEN. Incidence of male-killing Rickettsia spp. (α-Proteobacteria) in the ten-spot ladybird beetle Adalia decempunctata L. (Coleoptera: Coccinellidae) Applied Environmental Microbiology. 2001;67(1):270–277. doi: 10.1128/AEM.67.1.270-277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang SB, Ghosh AK, Bongio N, Stebbings KA, Lampe DJ, Jacobs-Lorena M. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proceedings of the National Academy of Sciences of the United States of Ameica. 2012;109(31):12734–12739. doi: 10.1073/pnas.1204158109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang RR, Hu Y, Yang ZD, Guo CH, Zhu LH, Zheng XL, Yu SZ. Isolation, identification and diversity of culturable bacteria in female adults of Leptocybe invasa Fisher & La Salle. Journal of Southern Agriculture. 2018;49(12):2432–2439. (in Chinese with English abstract) [Google Scholar]
- Warnecke et al. (2007).Warnecke F, Luginbuhl P, Ivanova N, Ghassemian M, Richardson TH, Stege JT, Cayouette M, McHardy AC, Djordjevic G, Aboushadi N, Sorek R, Tringe SG, Podar M, Martin HG, Kunin V, Dalevi D, Madejska J, Kirton E, Platt D, Szeto E, Salamov A, Barry K, Mikhailova N, Kyrpides NC, Matson EG, Ottesen EA, Zhang X, Hernandez M, Murillo C, Acosta LG, Rigoutsos I, Tamayo G, Green BD, Chang C, Rubin EM, Mathur EJ, Robertson DE, Hugenholtz P, Leadbetter JR. Metagenomic and functional analysis of hindgut microbiota of a wood-feeding higher termite. Nature. 2007;450(7169):560–565. doi: 10.1038/nature06269. [DOI] [PubMed] [Google Scholar]
- Werren et al. (1994).Werren JH, Hurst GDD, Zhang W, Breeuwer JA, Stouthamer R, Majerus ME. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata) Journal of Bacteriology. 1994;176(2):388–394. doi: 10.1128/jb.176.2.388-394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia et al. (2013).Xia XF, Zheng DD, Zhong HZ, Qin BC, Gurr GM, Vasseur L, Lin HL, Bai JL, He WY, You MS. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLOS ONE. 2013;8(7):e68852. doi: 10.1371/journal.pone.0068852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang et al. (2012).Xiang H, Xie L, Zhang J, Long YH, Liu N, Huang YP, Wang Q. Intracolonial difference in gut bacterial community between worker and soldier castes of Coptotermes formosanus. Insect Science. 2012;19(1):86–95. doi: 10.1111/j.1744-7917.2011.01435.x. [DOI] [Google Scholar]
- Xu et al. (2003).Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science. 2003;299(5615):2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2016).Xu LT, Lu M, Xu DD, Chen L, Sun JH. Sexual variation of bacterial microbiota of Dendroctonus valens guts and frass in relation to verbenone production. Journal of Insect Physiology. 2016;95:110–117. doi: 10.1016/j.jinsphys.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Yuki et al. (2015).Yuki M, Kuwahara H, Shintani M, Izawa K, Sato T, Starns D, Hongoh Y, Ohkuma M. Dominant ectosymbiotic bacteria of cellulolytic protists in the termite gut also have the potential to digest lignocellulose. Environmental Microbiology. 2015;17(12):4942–4953. doi: 10.1111/1462-2920.12945. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2014a).Zheng XL, Li J, Yang ZD, Xian ZH, Wei JG, Lei CL, Wang XP, Lu W. A review of invasive biology, prevalence and management of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae) African Entomology. 2014a;22(1):68–79. doi: 10.4001/003.022.0133. [DOI] [Google Scholar]
- Zheng et al. (2014b).Zheng XL, Yang ZD, Li J, Xian ZH, Yang J, Liu JY, Su S, Wang XL, Lu W. Rapid identification of both sexes of Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae: Tetrastichinae): a morphological perspective. African Entomology. 2014b;22(3):643–650. doi: 10.4001/003.022.0326. [DOI] [Google Scholar]
- Zheng et al. (2019).Zheng XY, Zhang DJ, Li YJ, Yang C, Wu Y, Liang X, Liang YK, Pan XL, Hu LC, Sun Q, Wang XH, Wei YY, Zhu J, Qian W, Yan ZQ, Parker AG, Gilles JRL, Bourtzis K, Bouyer J, Tang MX, Zheng B, Yu JS, Liu JL, Zhuang JJ, Hu ZG, Zhang MC, Gong JT, Hong XY, Zhang ZB, Lin LF, Liu QY, Hu ZY, Wu ZD, Baton LA, Hoffmann AA, Xi ZY. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature. 2019;572:56–61. doi: 10.1038/s41586-019-1407-9. [DOI] [PubMed] [Google Scholar]
- Zhu et al. (2015).Zhu FL, Ren SX, Qiu BL, Wu JH. Effect of temperature on life table parameters of Leptocybe invasa (Hymenoptera: Eulophidae) Austral Entomology. 2015;54:71–78. doi: 10.1111/aen.12094. [DOI] [Google Scholar]
- Zhukova et al. (2017).Zhukova M, Sapountzis P, Schlott M, Boomsma JJ. Diversity and transmission of gut bacteria in Atta and Acromyrmex leaf-cutting ants during development. Frontiers in Microbiology. 2017;8:1942. doi: 10.3389/fmicb.2017.01942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouache et al. (2011).Zouache K, Raharimalala FN, Raquin V, Tran-Van V, Raveloson LHR, Ravelonandro P, Mavingui P. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiology Ecology. 2011;75(3):377–389. doi: 10.1111/j.1574-6941.2010.01012.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
Data is available at NCBI SRA: SRR9591039, SRR9591038.
The COI and 16S rDNA sequences are available at GenBank: MN524231 and MN524230.
Additional data is also available at Figshare: Guo, Chunhui (2019): mic.zip. figshare. Dataset. DOI: 10.6084/m9.figshare.8323616.v1.