Abstract
Introduction
The incidence of tongue squamous cell carcinoma (TSCC) has increased in recent decades. However, the function of long non-coding RNA (lncRNA) CASC9 in the occurrence and progression of TSCC is unclear. In this work, we attempted to clarify the role of lncRNA CASC9 in determining the phenotype of TSCC cells, and to clarify the underlying mechanisms.
Methods
We used qRT-PCR analysis to identify the level of CASC9 mRNA expression in TSCC clinical samples and cell lines. We investigated cell proliferation, and cell migration and invasion of TSCC cells transfected with siCASC9 or siNC using CCK-8 and transwell assays. Bioinformatics analysis and a luciferase reporter assay were employed to predict and verify the target microRNA (miRNA).
Results
CASC9 was up-regulated in the TSCC tissues and cells, and predicted a poor prognosis. CASC9 silencing significantly inhibited cell proliferation, migration, and invasion of the TSCC cells compared with the non-targeting control small interfering RNA (siCtrl) treatment. miR-423-5p was predicted as the targeting miRNA of CASC9; this was verified by a luciferase reporter assay. CASC9 expression showed a negative correlation with miR-423-5p expression and a positive correlation with SOX12 expression. The miR-423-5p inhibitor can rescue the carcinogenesis effect of CASC9 on TSCC cells.
Conclusion
Our work indicates that CASC9 plays a role in TSCC tumorigenesis; this novel information will improve TSCC molecular targeting therapy.
Keywords: CASC9, tongue squamous cell carcinoma, miR-423-5p, proliferation, invasion, migration
Introduction
Tongue squamous cell carcinoma (TSCC) is considered as the most common and malignant form of head and neck squamous cell carcinoma.1,2 A third of the tongue margin and the front of the tongue are the areas in which TSCC is most likely to occur.3 TSCC is most common in old men, and is related to smoking, drinking, and other factors.3 Certain oral inflammatory changes—such as leukoplakia, erythema, and lichen planus—are also associated with TSCC. The tongue is abundantly supplied with lymph and blood vessels, and frequent tongue body activity facilitates metastasis and local recurrence; moreover, mortality due to TSCC has increased in recent decades.4 Surgery, chemotherapy, radiotherapy, and other methods have been used to treat TSCC.5 Although medical technology has improved considerably, the prognosis for TSCC patients remains unfavorable. The 5-year survival rate of TSCC is only approximately 50%.5 Local or regional recurrence and cervical lymph node metastasis remain important challenges for clinical treatment. To effectively prevent the recurrence of TSCC, and ultimately improve the rate of patient survival,6 a better understanding the molecular mechanism regulating TSCC occurrence and development is required.
Long non-coding RNA (lncRNA) is a newly identified class of RNA; as the name suggests, lncRNAs typically comprise more than 200 nucleotides and lack protein-coding capability. There is a lot of evidence that lncRNAs play an important part in a lot of cellular physiological processes by regulating gene expression.7,8 There are reports that CASC9 lncRNA is overexpressed in esophageal squamous cell cancer, lung adenocarcinoma, and hepatocellular carcinoma.9–11 Ma et al investigated the CASC9 expression was upregulated in oral carcinoma tissues and associated with patients’ survival time.12 Moreover, other reports suggest that CASC9 is closely correlated with the Tumor-Node-Metastasis (TNM) stage of tumor metastasis.10 However, the biological function of CASC9 in TSCC remains unknown.
In this work, we attempted to identify the biological function of CASC9 lncRNA in the progression of TSCC. Therefore, we investigated the expression and regulatory effect of CASC9 in TSCC. We also investigated the underlying molecular mechanism by which CASC9 regulates malignant tumor phenotype. Finally, our study demonstrated that CASC9 interacted with miR-423-5p to enhance the expression of SOX12, and promotes TSCC development and progression.
Materials and Methods
Patients and Tissue Specimens
We examined 94 TSCC samples from The Second Hospital of Nanjing (Nanjing, China) taken between 2001 and 2013. We obtained TSCC tumor samples and matched adjacent normal tissues from the Oncology Department at The Second Hospital of Nanjing. All the TSCC and normal samples were collected with the written informed consent of the patients. According to the TNM classification for TSCC (Union for International Cancer Control, UICC 2017, 8th edition),13 the patients were divided into 4 stages: Stage I (n=18), stage II (n=28), stage III (n=26) and stage IV (n=22). The tissues were immediately frozen in liquid nitrogen and stored at −80°C. Frozen tissue sections were investigated by two pathologists. The whole study was performed in accordance with the Declaration of Helsinki, and it was also approved by the Ethics Committee of The Second Hospital of Nanjing.
Cell Culture and Transfection
We purchased human oral keratinocytes (HOKs) and human TSCC cell lines (TCA8113, SCC25, and CAL27) from the Chinese Academy of Sciences. For the cultures, we used DMEM (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% FBS (Invitrogen, Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin (Gibco, Thermo Fisher Scientific, Inc.) in a humidified atmosphere containing 5% CO2 at 37°C. We replaced the culture medium every 2 days or following the thawing of the cells. We passaged the cells when they reached ~80–90% confluence. Cells growing in the logarithmic stage were seeded in a 6-well plate at a density of 5 × 103.
For cell transfection, we obtained miR-423-5p mimics, miR-423-5p inhibitors, small interfering RNA (siRNA)-targeted CASC9 (siCASC9-1: 5ʹ-GCCUGUGAUAGCAGAACAAUU-3ʹ, siCASC9-2: 5ʹ-GGAAGAAUUUCCAGAGUUUUU-3ʹ), and relative controls (siCtrl: 5ʹ-UUCUCCGAACGUGUCACGUUU-3ʹ) from GenePharma Co., Ltd. (Shanghai, China). We performed cell transfection by using a Lipofectamine® 2000 (Invitrogen, Thermo Fisher Scientific, Inc.) in 5 × 106 cells at a 50 nM concentration. The following experiments were carried out 48 h after transfection. After transfection for 24 h, we determined the level of CASC9 expression by qRT-PCR.
In situ Hybridization (ISH)
ISH assay was performed as previously described.14 In brief, samples were fixed and embedded in paraffin. Then, sample sections were incubated in graded alcohols and incubated in 3% hydrogen peroxide (H2O2) for 30 min. Biotin-conjugated probes and streptavidin-horseradish peroxidase conjugate were used for ISH. The samples were finally stained with hematoxylin (Solarbio Technology Co., LTD, Beijing, China) and observed with a light microscope (Nikon Corporation, Tokyo, Japan).
Immunofluorescence Assay
Cells were seeded in 6-well plates at a density of 1×106 cells/well. At the bottom of each well, a sterile slide was pre-placed. DMEM medium (10% FBS) with a volume of 1 mL was contained in each well. After 24 h incubation at 37°C, 5% CO2, all slides were removed from the well plates and cells on the slides were fixed using a 4% paraformaldehyde solution for 15 min. Triton-100 (0.1%, Beijing solarbio science﹠technology, China) was used to treat cells for 10 min. Cells were then rinsed with PBS and incubated with 5% bovine serum albumin for 30 min at 37°C. Subsequently, CASC9 probe (Shanghai WSHT Biotechnology Inc., China) were used to incubate cells overnight at 4°C. Cells were then subjected to incubation with FITC-labeled secondary antibody for 1 h at room temperature. The DAPI solution was used to stain the nucleus for 10 min at 37°C. After PBS rinsing, the slides were sealed with neutral resin and observed under a fluorescence microscope.
qRT-PCR
We extracted total RNA from TSCC tissues and normal tissues using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. For microRNA (miRNA) analysis, we carried out qRT-PCR using a TaqMan MicroRNA Reverse Transcription kit and a TaqMan Universal PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. We used the following universal miRNA qRT-PCR primers: U6 (forward, 5ʹ-AACGAGACGACGACAGAC-3ʹ and reverse 5ʹ-GCAAATTCGTGAAGCGTTCCATA-3ʹ); and miR-423a-5p (forward 5ʹ-GGGACAGGTGAGGTTCTTG-3ʹ and reverse 5ʹ-GAGTTGGAGGTTGCGTTAGA-3ʹ). For mRNA analysis, we carried out qRT-PCR using a TaqMan High-Capacity complementary DNA (cDNA) Reverse Transcription Kit and a TaqMan Fast PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific, Inc.) according to the manufacturer’s instruction. We used the GAPDH as an internal control. The qRT-PCR protocol included initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 20 s, and extension at 72°C for 10 s. The expression level was measured using the 2−ΔΔCq method. The primer sequences were: CASC9 (forward, 5ʹ-AGCAGCAAATGTGTCCATC-3ʹ and reverse, 5ʹ-CAGACAGCAGCAAAGCAAT-3ʹ); SOX12 (forward, 5ʹ-GAGGAAACGGGGTCAGG-3ʹ and reverse, 5ʹ-CAAACAGGGAGGGAGAGG-3ʹ), and GAPDH (forward, 5ʹ-AAGGTCGGAGTCAACGGA-3ʹ and reverse, 5ʹ-TTAAAAGCAGCCCTGGTGA-3ʹ).
Cell Proliferation Assay
We determined cell viability using a cell counting kit-8 (CCK-8) assay. We seeded cells into 96-well plates at 1 × 104 cells/well and cultured them overnight. After transfection for 48 h, we added 10 μL of CCK-8 reagent (Dojindo Molecular Technologies, Dojindo, Japan) to the wells, and cultured the cells continuously for 2 h at 37°C. We measured the absorbance at 450 nm on an automated microplate reader (BioTek Instruments, Winooski, VT, USA).
Cell Migration and Invasion Assay
We assayed cell migration using Transwell method. We grew the cells to approximately 50% confluence and transfected them with the corresponding siRNA. After 24 h, we incubated the cells in serum-free medium for 24 h. We trypsinized the cells, then introduced 5 × 104 cells in serum-free medium to the upper chamber. We added a medium containing 10% FBS in the inferior cavity. We incubated the cells for 24 h at 37°C, and removed all non-migrating cells. We then fixed the cells that had migrated to the bottom of the membrane in 4% paraformaldehyde, and stained them with 0.5% crystal violet. Finally, we examined the stained cells under a microscope, assessed them in five random fields, and calculated the average number. The invasion assay was then performed, and the upper chamber was precoated with 1 mg/mL Matrigel (BD Biosciences). The remaining steps are taken as migration analysis.
Bioinformatics Analysis
We predicted the CASC9 target miRNAs using the online software programs starBase (http://starbase.sysu.edu.cn) and miRanda (http://www.microrna.org). miR-423-5p was ranked first and was predicted as a target of CASC9. To identify the targeting genes of miR-423-5p, we used the online software programs TargetScan (http://www.targetscan.org/) and miRanda (http://www.microrna.org). The 3ʹ-untranslated region (3ʹ-UTR) of the SOX12 was analyzed to contain miR-423-5p binding site.
Luciferase Activity Assay
We individually inserted wild-type CASC9 and mutant CASC9 into pmirGLO reporter vectors (Promega, Madison, WI, USA). We co-transfected human dermal microendothelial cells (HDECs) with miR-423a-5p mimics and wild-type (WT) CASC9 or mutant (MUT) CASC9 using Lipofectamine 2000 (Invitrogen). We measured the relative luciferase activity 48 h after transfection on the dual- luciferase report analysis system (Promega). The data were expressed as the ratio of Renilla luciferase activity to firefly luciferase activity. Luciferase report analysis was performed to verify the direct binding of miR-423a-5p to SOX12 3ʹ-UTR as described above.
In vivo Xenograft Experiments
We obtained 12 Male BALB/c nude mice (6 weeks-old) divided for two groups (n=6/group) purchased from HFK Bioscience Co. Ltd. (Beijing, China). The animal experiment was performed in accordance with relevant guidelines of the Animal Care and Use Committees at the The Second Hospital of Nanjing, and was also approved by the Committee of The Second Hospital of Nanjing. CAL27 cells transfected with shCASC9 (5ʹ-CCGGAAACUCUGGAAAUUCUUCCCUCGAGGGAAGAAUUUCCAGAGUUUUUUUUG-3ʹ) or shNC (5ʹ-CCGGACGUGACACGUUCGGAGAUUCUCGAGUUCUCCGAACGUGUCACGUUUUUUG-3ʹ) were subcutaneously injected into the mice. Tumor volume was evaluated by a formula: volume = πab2/6 (a: tumor length; b: tumor width) every 7 days. The tumor weight was weighed at day 42 post-injection.
Western Blot Analysis
We extracted proteins from cells by using a radioimmunoprecipitation assay buffer (Sigma-Aldrich), and quantified protein concentrations using a BCA Protein Assay Kit (BJBALB, Beijing, China). On 10% of the twelve alkyl sulfate polyacrylamide gel, the same amount of protein (50 μg) was isolated from each sample and transferred to PVDF membrane (Millipore, Boston, USA). The membrane was blocked with 5% skimmed milk at 37°C for 1 hr. The target protein was detected by primary anti-cyclin D1 (ab134175, 1:1000), anti-E-cadherin (ab15148, 1:800), anti-Twist (ab49254, 1:1000), anti-MTA1 (ab71153, 1:1200), and anti-GAPDH (ab9485, 1:2500) antibodies, and incubated at 4°C overnight. Afterwards, we cultured the second antibody (1:2000; Beyotime Biotechnology) of the membrane with horseradish peroxidase at 25°C for 1 hr. We visualized the immunoreactive protein bands using an enhanced chemiluminescence protein imprinting Kit (Thermo Fisher Scientific), and quantified protein band intensity by Image-Pro Plus 6.0 software. We measured the relative level of protein expression by standardizing with GAPDH.
Statistical Analysis
Data from at least three independent experiments were expressed as the mean ± standard deviation (SD). We carried out statistical analysis using IBM SPSS 19.0 statistical software. Statistical analysis was performed using Student’s t-test or ANOVA. We used Pearson’s χ2 tests to determine the correlation between clinicopathological parameters and CASC9 lncRNA expression in TSCC samples. We used Spearman correlation analysis to analyze the relationships between the levels of CASC9 and miR-423-5p, or CASC9 and SOX12 in the TSCC tissues. The Kaplan-Meier test was used to analyze the survival rates of the TSCC patients. We took P < 0.05 to indicate a statistical significance.
Results
The CASC9 Expression Was Increased in TSCC Tissues and Cells
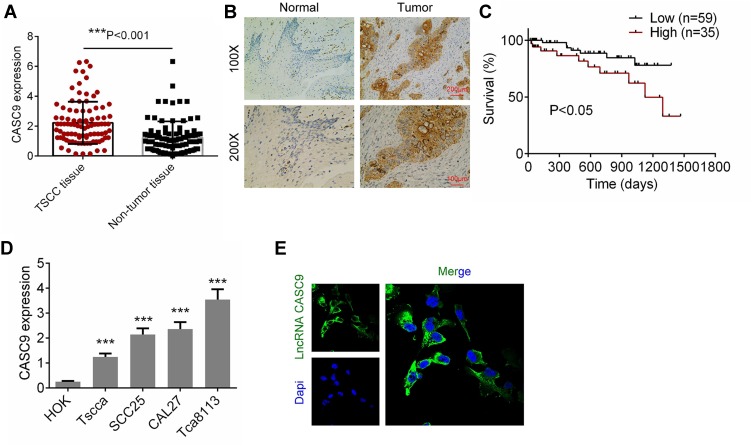
We firstly determined the levels of CASC9 expression in TSCC tissues and matched non-TSCC tissues by qRT-PCR and ISH. Figure 1A and B shows that the level of CASC9 expression increased obviously in the TSCC tissues as compared to that in the normal lingual mucous membrane (P < 0.001). Patients with high CASC9 expression exhibited a low survival rate (P<0.05, Figure 1C). We also determined the levels of expression of CASC9 in the TSCC cell lines (CAL27, TCA8113, TSCCA, and SCC25) and in the HOKs using qRT-PCR. The level of expression of CASC9 was significantly greater in the TSCC cell lines than in the HOKs (P < 0.001, Figure 1D). Subsequently, by immunofluorescence assay, we found that CASC9 was most expressed in cytoplasm (Figure 1E). To further identify the clinicopathological role of CASC9 expression in TSCC patients, we divided 94 patients into low CASC9 expression group (n=59) and high CASC9 expression group (n=35).15 As presented in Table 1, CASC9 level in TSCC tissues was positively correlated with tumor diameter, TNM stage, and lymph node metastasis. The results indicated that the abnormal expression of lncRNA CASC9 played a crucial role in the progression of TSCC.
Figure 1.
The expression of CASC9 in TSCC tissues and cell lines. (A) The mRNA expression of CASC9 in 41 paired TSCC tissues and matched adjacent non-TSCC tissues was detected by RT-qPCR. (B) The expression of CASC9 in TSCC tissues and matched adjacent non-TSCC tissues was detected by ISH. (C) Survival rates of patients with TSCC with high and low CASC9 by Kaplan-Meier survival analysis. (D) The expression of CASC9 in TSCC cell lines (CAL27, Tca8113, Tscca and SCC25) and human oral keratinocytes (HOK) were determined by RT-qPCR. (E) The cellular sublocalization of CASC9 was evaluated by immunofluorescence assay. Data is from three independent experiments and expressed as mean ± SD. ***P<0.001.
Table 1.
Association Between lncRNA CASC9 Expression and Clinicopathological Features of Patients with Tongue Squamous Cell Carcinoma
| Parameters | High CASC9 Expression (≥ Mean, n=35) | Low CASC9 Expression (< Mean, n=59) | P-Value |
|---|---|---|---|
| Sex | |||
| Male | 16 | 31 | 0.306 |
| Female | 19 | 28 | |
| Age | |||
| ≥55 | 21 | 32 | 0.372 |
| <55 | 14 | 27 | |
| Tumor diameter | |||
| ≥2 cm | 22 | 23 | 0.033* |
| <2 cm | 13 | 36 | |
| Differentiation | |||
| Moderately/poorly | 15 | 36 | 0.133 |
| Well | 20 | 23 | |
| TNM stage | 0.049* | ||
| I | 3 | 15 | |
| II | 8 | 20 | |
| III | 14 | 12 | |
| III-IV | 10 | 12 | |
| Lymph node metastasis | |||
| With | 22 | 19 | 0.01* |
| Without | 13 | 40 | |
| Distant metastasis | |||
| Yes | 22 | 25 | 0.044* |
| No | 13 | 34 | |
Note: The mean expression level was used as the threshold. For analysis of association between CASC9 levels and clinical features, Pearson’s χ2 tests were used *P<0.05.
Abbreviation: TNM, tumor node metastasis stage.
Down-Regulation of CASC9 Suppressed Cell Proliferation, Migration, and Invasion of TSCC Cells
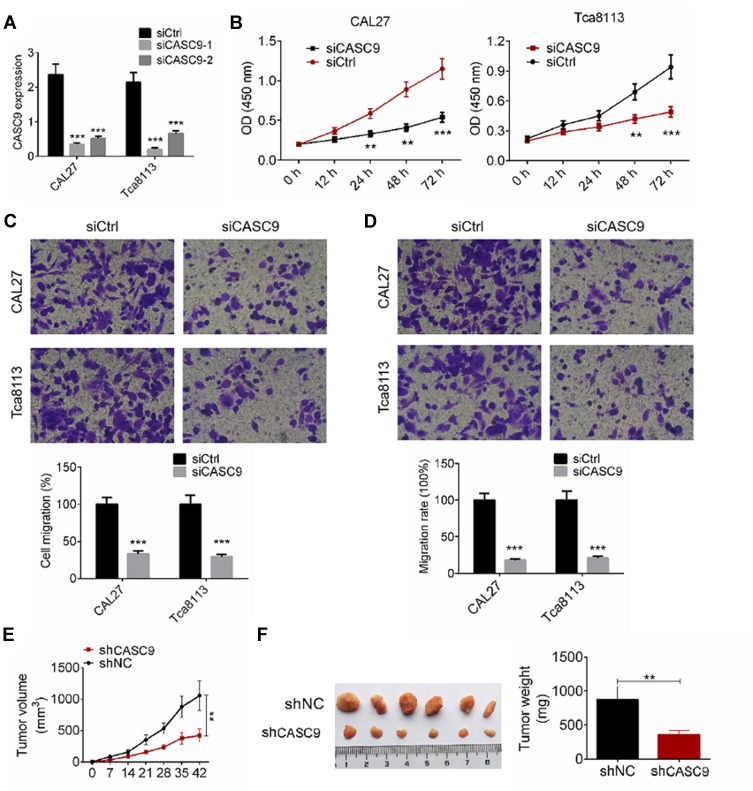
We investigated the effect of CASC9 knockdown on cell proliferation, migration, and invasion of TSCC cells. We transfected CAL27 and TCA8113 cells with siCASC9-1, or siCASC9-2 or non-targeting control (siCtrl) plasmids, and determined CASC9 expression by qRT-PCR analysis (Figure 2A). The siCASC9-1 showed a better effect on CASC9 knockdown, and it was used for further studies. We determined TSCC cell proliferation by a CCK-8 assay. As shown in Figure 2B, CASC9 knockdown significantly inhibited cell proliferation at 24, 48, and 72 h compared to the cells transfected with siCtrl (P < 0.01). We evaluated cell migration and invasion capabilities by a transwell assay. TSCC cell migration was significantly reduced by siCASC9 treatment compared to siCtrl treatment (P < 0.001, Figure 2C). Following CASC9 downregulation, the cell invasion rates of the CAL27 and TCA8113 cells decreased by 18.6 ± 2.47% and 22.3 ± 3.14%, respectively (P < 0.001, Figure 2D). The effects of CASC9 on TSCC development were measured based on a xenograft mouse model. CASC9 knockdown effectively inhibited tumor growth in vivo (P < 0.01, Figure 2E). The knockdown of CASC9 dramatically reduced the tumor size and weight (P < 0.01, Figure 2F). These results indicate that CASC9 overexpression promotes the cell proliferation, migration, and invasion of TSCC cells.
Figure 2.
Down-regulation of CASC9 inhibited cell proliferation, migration and invasion of TSCC cells. (A) CAL27 and Tca8113 cells were transfected with siCASC9-1 or siCASC9-2, or siCtrl plasmids, the mRNA expression of CASC9 was examined by RT-qPCR analysis. (B) Cell proliferation of CAL27 and Tca8113 cells transfected with siCASC9 or siCtrl was determined by CCK8 assay. (C and D) Cell migration and invasion of CAL27 and Tca8113 cells transfected with siCASC9 or siCtrl were detected by transwell assay. (E) Tumor growth curves were established by measuring tumor volume every 7 for 42 days after injection. (F) Tumor weights isolated from nude mice in each treatment group were determined on day 42 after injection. Data is from three independent experiments and expressed as mean ± SD. **P<0.01, ***P<0.001 compared with siCtrl.
CASC9 Regulated the Expression Levels of Oncogenesis Proteins and Tumor Suppressors
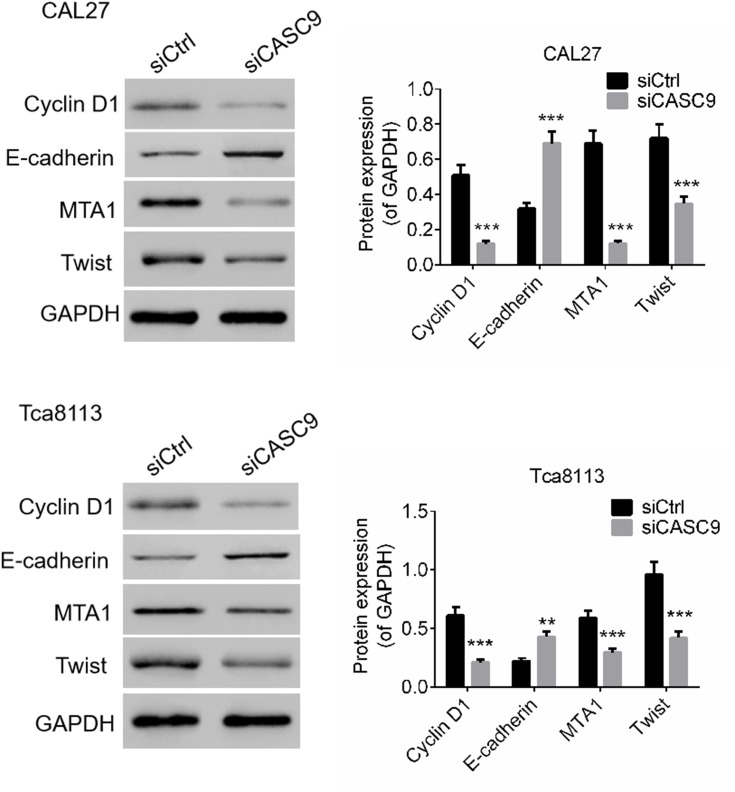
We determined the effects of CASC9 silencing on proliferation-, migration-, and invasion-related proteins by Western blot. As presented in Figure 3, compared to siCtrl treatment, the levels of E-cadherin expression in the CAL27 and TCA8113 cells were significantly promoted by siCASC9 treatment (P < 0.001). Moreover, the expression levels of cyclin D1, MTA1, and Twist in the TSCC cells were notably inhibited by siCASC9 transfection compared to those in the siCtrl-treated cells (P < 0.001). The results imply that CASC9 regulates the expression of E-cadherin, cyclin D1, MTA1, and Twist, which affect the cell activities of TSCC cells.
Figure 3.
CASC9 knockdown regulated protein expressions of E-cadherin, Cyclin D1, MTA1 and Twist of TSCC cells. The protein levels of E-cadherin, Cyclin D1, MTA1 and Twist of CAL27 and Tca8113 cells transfected with siCASC9 or siCtrl was determined by Western blot. Data is from three independent experiments and expressed as mean ± SD. **P<0.01, ***P<0.001 compared with siCtrl.
CASC9 Interacted with miR-423-5p/SOX12
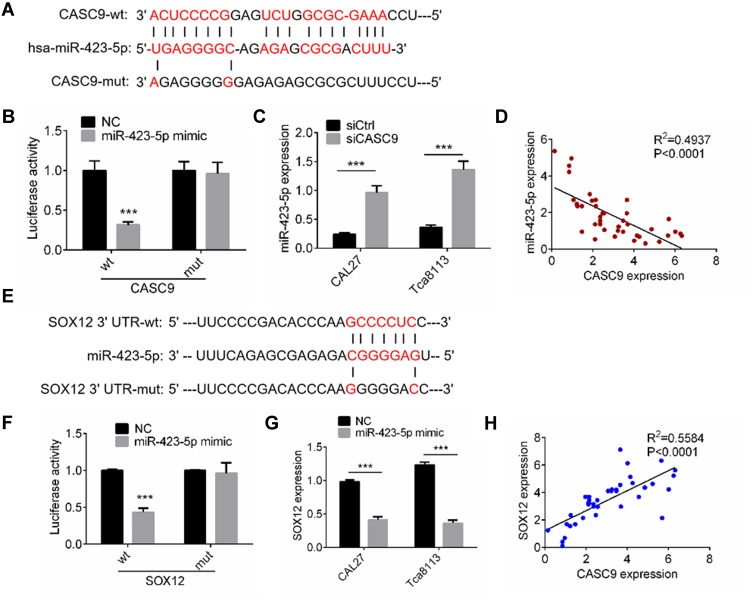
To investigate the underlying mechanism by which CASC9 regulates the cell activities of TSCC cells, we predicted the target miRNAs of CASC9 using an online tool (http://mirdb.org). miR-423-5p was the highest-ranking potential target. The binding sites are shown in Figure 4A. We constructed wild-type CASC9 (CASC9-wt) and mutant CASC9 (CASC9-mut) luciferase reporter plasmids. As shown in Figure 4B, the luciferase activity in 293T cells transfected with CASC9-wt was reduced by miR-423-5p mimic treatment, whereas the miR-423-5p mimic had no significant effect on luciferase activity in the 293T cells transfected with CASC9-mut. We also used qRT-PCR to investigate miR-423-5p expression in TSCC cells transfected with siCASC9 or siCtrl, and found that it increased significantly in siCASC9 cells compared to siCtrl cells (P < 0.001, Figure 4C). We also determined the level of miR-423-5p expression in the TSCC clinical samples, and Spearman correlation analysis revealed that CASC9 expression was negatively correlated with miR-423-5p expression (P < 0.001, Figure 4D).
Figure 4.
CASC9 interacted with miR-423-5p/SOX12. (A) A CASC9-mut as well as a CASC9-wt luciferase reporter plasmid was cloned by mutating the predicted miR-423-5p binding site in CASC9. (B) The luciferase reporter plasmids were co-transfected into 293T cells with miR-423-5p mimic or NC. The luciferase activities were measured by dual luciferase assays. (C) The miR-423-5p expression in CAL27 and Tca8113 cells transfected with siCASC9 or siCtrl was assessed by RT-qPCR. (D) Pearson’s analysis was performed to identify the correlation between miR-423-5p expression and CASC9 expression in TSCC clinical samples. (E) A SOX12-mut as well as a SOX12-wt luciferase reporter plasmid was cloned by mutating the predicted miR-423-5p binding site in SOX12. (F) The luciferase reporter plasmids were co-transfected into 293T cells with miR-423-5p mimic or NC. The luciferase activities were measured by dual luciferase assays. (G) The SOX12 expression in CAL27 and Tca8113 cells transfected with miR-423-5p mimic or NC was assessed by RT-qPCR. (H) Pearson’s analysis was performed to identify the correlation between SOX12 expression and CASC9 expression in TSCC clinical samples. Data is from three independent experiments and expressed as mean ± SD. ***P<0.001 compared with siCtrl.
To study the miR-423-5p targets, we predicted the target genes using TargetScan and miRDB. SOX12 had the highest score among all the potential targets. Therefore, we constructed SOX12 3ʹ-UTR-wt and SOX12 3ʹ-UTR-mut luciferase reporter plasmids (Figure 4E). For the luciferase assay, we transfected the 293T cells with the miR-423-5p mimic or the control (NC). The results revealed that the miR-423-5p mimic notably reduced luciferase activity in the SOX12 3ʹ-UTR-wt 293T cells compared to the NC (P < 0.001, Figure 4F). Transfection with the miR-423-5p mimic had no effect on luciferase activity in the SOX12 3ʹ-UTR-mut 293T cells (Figure 4F). The expression of SOX12 decreased markedly in the TSCC cells transfected with the miR-423-5p mimic as compared to the NC cells (P < 0.001, Figure 4G). Our investigation of SOX12 expression in the TSCC tissues revealed a positive correlation with CASC9 expression (P < 0.0001, Figure 4H). The results described above indicated that CASC9 regulated TSCC cell activities by interacting with miR-423-5p/SOX12.
CASC9 Affected Cell Phenotype by Sponging miR-423-5p
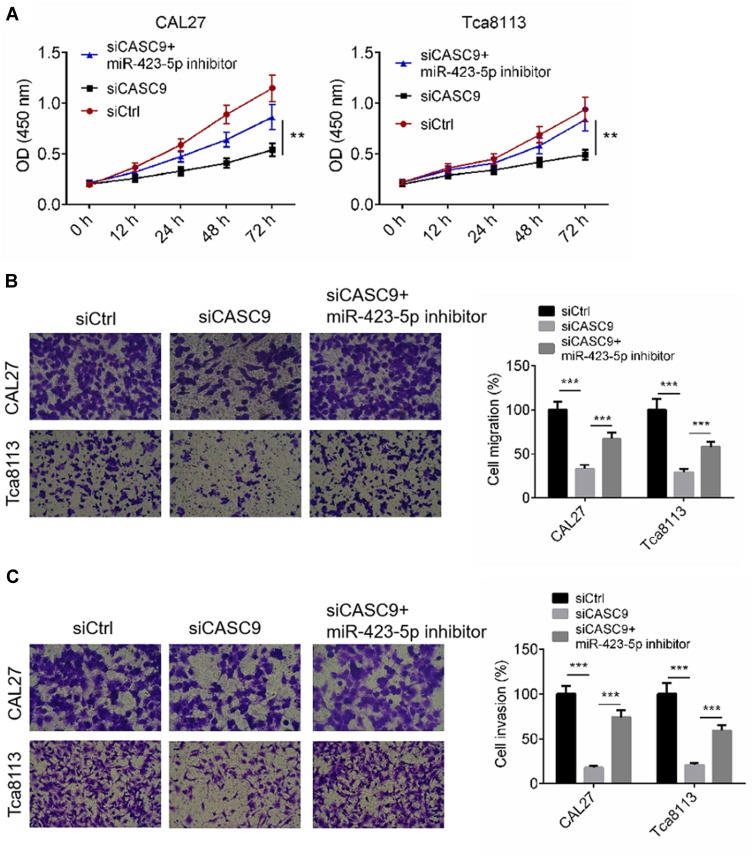
To determine whether CASC9 regulates cell activity by sponging miR-423-5p, we transfected TSCC cells with siCASC9, then treated them with an miR-423-5p inhibitor. Cell proliferation, migration and invasion of TSCC cells transfected with siCtrl, or siCASC9 or siCASC9 + miR-423-5p inhibitor were evaluated by CCK8 and transwell assay, respectively. Figure 5A illustrates that CASC9 silencing effectively suppressed the proliferation of the TSCC cells, and the miR-423-5p inhibitor rescued the anti-proliferation effect (P < 0.01). The cell migration and invasion rates of the CAL27 and TCA8113 cells were declined by siCASC9 treatment, and the miR-423-5p inhibitor reversed the decreases in the migration and invasion rates caused by siCASC9 treatment (P < 0.001, Figure 5B and C). The data indicate that CASC9 affects cell activities by sponging miR-423-5p.
Figure 5.
CASC9 mediated cell proliferation, migration and invasion through targeting miR-423-5p. (A) Cell proliferation of CAL27 and Tca8113 cells transfected with siCtrl, or siCASC9 or siCASC9 + miR-423-5p inhibitor was identified by CCK8 assay. (B and C) Cell migration and invasion of CAL27 and Tca8113 cells transfected with siCtrl, or siCASC9 or siCASC9 + miR-423-5p inhibitor was identified by transwell assay. Data is from three independent experiments and expressed as mean ± SD. **P<0.01, ***P<0.001.
Discussion
TSCC is one of the most common carcinomas in the head and neck. The screening of molecular targets to improve our understanding of TSCC metastasis will provide new information for TSCC therapy. Several researchers have reported that long non-coding RNA exerts an important role in the occurrence and progression of certain carcinomas. CASC9 lncRNA is overexpressed in several malignant tumors such as esophagus cancer,9 lung adenocarcinoma,10 and hepatocellular carcinoma.11 CASC9 is closely related with tumor cell proliferation, migration, and invasion.16 In this study, we found that CASC9 expression was increased in TSCC tissues and cells. Liang et al indicated that CASC9.5 expression level was increased in lung cancer tissues, and was closely correlated with the TNM, tumor size, tumor metastasis, and tumor metabolism of lung cancer.10 The analysis of the clinical data from our study showed that CASC9 expression level in TSCC tissues was positively associated with tumor diameter, TNM stage, and lymph node metastasis. The results indicated that CASC9 overexpression is associated with TSCC progression.
Xu et al investigated that CASC9 was overexpressed in esophageal cancer, and promoted the cell proliferation, migration, and invasion of esophageal cancer cells.17 Previous studies have demonstrated that CASC9 can also promote lung adenocarcinoma cell proliferation and metabolism in vivo and in vitro.10 In the present study, we transfected TSCC cells with siCASC9, and investigated its effects on cell proliferation, migration, and invasion. The results suggest that the downregulation of CASC9 inhibits cell viability and reduces the ability of TSCC cells to migrate and invade. Moreover, CASC9 knockdown inhibited tumor growth of TSCC in vivo. We also determined the protein expression levels of cell proliferation- and metastasis-related biomarkers (E-cadherin, cyclin D1, MTA1, and Twist). Cyclin D1 is a regulator of cyclin-dependent kinases (CDKs), and promotes cell cycle progression from G1 to S.18,19 E-cadherin, MTA1, and Twist play important roles in TSCC tumor cell migration and invasion.20–22 Our results indicate that CASC9 knockdown effectively regulates the protein expression of E-cadherin, cyclin D1, MTA1, and Twist. The results also indicate that CASC9 promotes the cell proliferation, migration, and invasion of TSCC cells by regulating oncogenesis proteins and tumor suppressors. We further predicted the target miRNA for CASC9, and the results suggest that CASC9 interacts with miR-423-5p. The expression of miR-423-5p is downregulated in many kinds of tumors.23–25 Pan et al reported that miR-423-5p inhibited the cell proliferation and invasion of colon cancer cells.24 Yang et al indicated that miR-423-5p might serve as a diagnostic indicator and function as a tumor suppressor in ovarian carcinoma.25 In this study, the results showed that miR-423-5p was upregulated in TSCC cells that had been transfected with siCASC9. Moreover, the clinical statics showed that CASC9 expression was negatively related to miR-423-5p expression. The miR-423-5p inhibitor treatment rescued the effect of siCASC9 on the cell proliferation, migration, and invasion of TSCC cells. We used bioinformatic analysis to confirm the target mRNA for miR-423-5p; SOX12 achieved the highest score and was verified by a luciferase reporter. SOX12 is considered a biomarker for some cancers, and may stimulate the proliferation and metastasis of lung cancer cells,26 hepatocellular carcinoma cells,27 breast cancer cells,28 and renal cell carcinoma.29 We investigated SOX12 expression in TSCC tissues, and found a positive correlation with CASC9 expression. These results demonstrated that CASC9 regulates TSCC cell proliferation, migration, and invasion by interacting with miR-423-5p/SOX12.
In summary, we found that CASC9 was up-regulated in TSCC tissues and cells. High expression levels of CASC9 indicates a poor prognosis and is correlated with the clinicopathological features of TSCC, and thus CASC9 can be considered as a potential prognostic factor for survival of TSCC patients. CASC9 may promote the cell proliferation, migration, and invasion of TSCC cells by targeting miR-423-5p/SOX12 axes. However, further studies are necessary to help us clarify the molecular mechanism of CASC9 in TSCC. The present study might improve our understanding of the mechanism underlying TSCC metastasis, and contribute to the development of an effective molecular target therapy.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yang X, Tian X, Wu K, et al. Prognostic impact of perineural invasion in early stage oral tongue squamous cell carcinoma: results from a prospective randomized trial. Surg Oncol. 2018;27(2):123–128. doi: 10.1016/j.suronc.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 2.Aiempanakit K. Digital metastasis of tongue squamous cell carcinoma. JAAD Case Rep. 2018;4(2):200–202. doi: 10.1016/j.jdcr.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayaraj R, Kumarasamy C. Comment on ‘Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis’. Br J Cancer. 2018;118(5):e11. doi: 10.1038/bjc.2017.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ananthi S, Lakshmi C, Atmika P, Anbarasu K, Mahalingam S. Global quantitative proteomics reveal deregulation of cytoskeletal and apoptotic signalling proteins in oral tongue squamous cell carcinoma. Sci Rep. 2018;8(1):1567. doi: 10.1038/s41598-018-19937-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren ZH, Wu HJ, Zhang S, et al. A new surgical strategy for treatment of tongue squamous cell carcinoma based on anatomic study with preliminary clinical evaluation. J Craniomaxillofac Surg. 2015;43(8):1577–1582. doi: 10.1016/j.jcms.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 6.Hussein AA, Forouzanfar T, Bloemena E, et al. A review of the most promising biomarkers for early diagnosis and prognosis prediction of tongue squamous cell carcinoma. Br J Cancer. 2018;119:724–736. doi: 10.1038/s41416-018-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji D, Zhong X, Jiang X, et al. The role of long non-coding RNA AFAP1-AS1 in human malignant tumors. Pathol Res Pract. 2018;214:1524–1531. doi: 10.1016/j.prp.2018.08.014 [DOI] [PubMed] [Google Scholar]
- 8.Li ZQ, Zou R, Ouyang KX, Ai WJ. An in vitro study of the long non-coding RNA TUG1 in tongue squamous cell carcinoma. J Oral Pathol Med. 2017;46(10):956–960. doi: 10.1111/jop.12599 [DOI] [PubMed] [Google Scholar]
- 9.Gao GD, Liu XY, Lin Y, Liu HF, Zhang GJ. LncRNA CASC9 promotes tumorigenesis by affecting EMT and predicts poor prognosis in esophageal squamous cell cancer. Eur Rev Med Pharmacol Sci. 2018;22(2):422–429. doi: 10.26355/eurrev_201801_14191 [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Xiao H, Yang X, et al. Long noncoding RNA CASC9.5 promotes the proliferation and metastasis of lung adenocarcinoma. Sci Rep. 2018;8(1):37. doi: 10.1038/s41598-017-18280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noh JH, Gorospe M. AKTions by cytoplasmic lncRNA CASC9 promote HCC survival. Hepatology. 2018. doi: 10.1002/hep.30165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Xu X, Ma H. Identification of oncogenic long noncoding RNAs CASC9 and LINC00152 in oral carcinoma through genome-wide comprehensive analysis. Anticancer Drugs. 2019;30(4):356–362. doi: 10.1097/CAD.0000000000000725 [DOI] [PubMed] [Google Scholar]
- 13.Barrett AW, Tighe JV, Gulati A, et al. Staging of squamous cell carcinoma of the tongue: extrinsic lingual muscles and the 8th editions of the American Joint Committee on Cancer/Union for International Cancer Control staging manuals. Br J Oral Maxillofac Surg. 2017;55(9):921–926. doi: 10.1016/j.bjoms.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 14.Lin J, Shi Z, Yu Z, He Z. LncRNA HIF1A-AS2 positively affects the progression and EMT formation of colorectal cancer through regulating miR-129-5p and DNMT3A. Biomed Pharmacother. 2018;98:433–439. doi: 10.1016/j.biopha.2017.12.058 [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Tian T, Li ZY, et al. FSCN1 is an effective marker of poor prognosis and a potential therapeutic target in human tongue squamous cell carcinoma. Cell Death Dis. 2019;10(5):356. doi: 10.1038/s41419-019-1574-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Hu L, Liang Y, et al. Up-regulation of lncRNA CASC9 promotes esophageal squamous cell carcinoma growth by negatively regulating PDCD4 expression through EZH2. Mol Cancer. 2017;16(1):150. doi: 10.1186/s12943-017-0715-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Z, Mao W, Bao Y, Zhang M, Su X, Xu X. The long noncoding RNA CASC9 regulates migration and invasion in esophageal cancer. Cancer Med. 2016;5(9):2442–2447. doi: 10.1002/cam4.2016.5.issue-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu SY, Lan SH, Wu SR, et al. Hepatocellular carcinoma-related cyclin D1 is selectively regulated by autophagy degradation system. Hepatology. 2018;68(1):141–154. doi: 10.1002/hep.29781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito K, Maruyama Z, Sakai A, et al. Overexpression of Cdk6 and Ccnd1 in chondrocytes inhibited chondrocyte maturation and caused p53-dependent apoptosis without enhancing proliferation. Oncogene. 2014;33(14):1862–1871. doi: 10.1038/onc.2013.130 [DOI] [PubMed] [Google Scholar]
- 20.Cheng JC, Chang HM, Xiong S, So WK, Leung PC. Sprouty2 inhibits amphiregulin-induced down-regulation of E-cadherin and cell invasion in human ovarian cancer cells. Oncotarget. 2016;7(49):81645–81660. doi: 10.18632/oncotarget.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Guo H, Yao B, Helms J. miR-15b inhibits cancer-initiating cell phenotypes and chemoresistance of cisplatin by targeting TRIM14 in oral tongue squamous cell cancer. Oncol Rep. 2017;37(5):2720–2726. doi: 10.3892/or.2017.5532 [DOI] [PubMed] [Google Scholar]
- 22.Vered M, Lehtonen M, Hotakainen L, et al. Caveolin-1 accumulation in the tongue cancer tumor microenvironment is significantly associated with poor prognosis: an in-vivo and in-vitro study. BMC Cancer. 2015;15:25. doi: 10.1186/s12885-015-1030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lian J, Lin SH, Ye Y, et al. Serum microRNAs as predictors of risk for non-muscle invasive bladder cancer. Oncotarget. 2018;9(19):14895–14908. doi: 10.18632/oncotarget.24473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia W, Yu T, An Q, Cao X, Pan H. MicroRNA-423-5p inhibits colon cancer growth by promoting caspase-dependent apoptosis. Exp Ther Med. 2018;16(2):1225–1231. doi: 10.3892/etm.2018.6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang X, Zeng X, Huang Y, et al. miR-423-5p serves as a diagnostic indicator and inhibits the proliferation and invasion of ovarian cancer. Exp Ther Med. 2018;15(6):4723–4730. doi: 10.3892/etm.2018.6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Hu F, Shen S, et al. Knockdown of SOX12 expression inhibits the proliferation and metastasis of lung cancer cells. Am J Transl Res. 2017;9(9):4003–4014. [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan P, Meng L, Wang N. SOX12 upregulation is associated with metastasis of hepatocellular carcinoma and increases CDK4 and IGF2BP1 expression. Eur Rev Med Pharmacol Sci. 2017;21(17):3821–3826. [PubMed] [Google Scholar]
- 28.Ding H, Quan H, Yan W, Han J. Silencing of SOX12 by shRNA suppresses migration, invasion and proliferation of breast cancer cells. Biosci Rep. 2016;36. doi: 10.1042/BSR20160053 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Gu W, Wang B, Wan F, et al. SOX2 and SOX12 are predictive of prognosis in patients with clear cell renal cell carcinoma. Oncol Lett. 2018;15(4):4564–4570. doi: 10.3892/ol.2018.7828 [DOI] [PMC free article] [PubMed] [Google Scholar]