Abstract
Objective: Preeclampsia (PE) is a multi-systemic complication of pregnancy often characterised with the onset of hypertension and proteinuria after 20 weeks of gestation. Today, PE is the leading cause of maternal and perinatal morbidity and mortality worldwide. An early detection of PE would allow a chance to plan the appropriate monitoring and for clinical management to be immediately done following early detection thus making prophylactic strategies much more effective.
Materials and methods: This systematic review aims to evaluate the potential of the various serum biomarkers and diagnostic modalities (uterine artery Doppler, MAP, and maternal history) available for early prediction of PE with articles included and obtained through MEDLINE Full Text, Pubmed, Science Direct, ProQuest, SAGE, Taylor and Francis Online, Google Scholar, HighWire and Elsevier ClinicalKey.
Results: Ninety-five articles were found that fulfilled all of our inclusion criteria. Placental growth factor (PlGF), pregnancy associated plasma protein A (PAPP-A), soluble fms-like tyrosine kinase (sFLT) and placental protein 13 (PP-13) were the most commonly studied biomarkers. Whereas uterine Doppler scanning and Mean Arterial Pressure (MAP) were the most commonly studied out of other modalities.
Conclusion: Current evidence shows serum biomarkers such as PIGF, PP-13 and sFlt yielded the best results for a single biomarker with others having conflicting results. However, a combination model with other diagnostic modalities performed better than a single biomarker. In the future, new techniques will hopefully provide sets of multiple markers, which will lead to a screening program with clinically relevant performance. However further studies are required to improve current methods.
Key Words: Preeclampsia, Early Diagnosis, Biomarkers, Doppler Ultrasonography, Diagnostic Model
Introduction
Preeclampsia (PE) is a multi-systemic complication of pregnancy often characterised with the onset of hypertension and proteinuria after 20 weeks of gestation, in a previously normotensive women (1, 2). On a global scale, PE is responsible for 14% of all maternal deaths and over 500,000 fetal deaths per annum making it the leading cause of maternal and perinatal morbidity and mortality (3, 4). Although its presentation is predominantly late term with a mild clinical course, severe maternal complications do occur most often resulting in marked elevations of blood pressure and end-organ dysfunction(5).
Severe PE can cause renal failure; hemolysis, elevated liver enzymes, and low platelet (HELLP) syndrome; liver haemorrhage and rupture; eclampsia; cerebral haemorrhage; and maternal death. Women suffering the condition has been found to carry a higher risk of complications and death resulting from their pregnancy with 10-25% of all PE cases resulting in maternal death and 15-20% of them resulting in preterm births (6-8). In addition to this, women with a history of PE have an elevated risk of cardiovascular diseases later in life (9, 10).
The impact of PE far extends from its mother to also its children. In foetuses, approximately 12-25% of fetal growth restriction (FGR) are attributable to PE (6). Newborns diagnosed with FGR at birth have a two to eight fold increased risk for hypertension, cardiovascular disease, diabetes mellitus or renal disease as adults (11, 12). They are also at risk of hypoxic induced neurologic injury and preterm delivery (2, 13). One quarter of still births and neonatal deaths are associated with PE and are especially prevalent in developing countries due to the lack of neonatal care facilities (6, 8).
Considering the importance of PE, the development of an efficient management system for the disorder is crucial to tackle the problem of PE. However, currently the diagnosis, screening and management of PE remains controversial and no single standard has so far been agreed upon (1, 14). The existence of a screening process would be essential to improve both pregnancy outcome and optimise the utilisation of resources in antenatal care (5). An early detection of PE would allow a chance to plan the appropriate monitoring and for clinical management to be immediately done following early detection of the disease thus making prophylactic strategies much more effective (13, 15). This systematic review aims to evaluate the potential of the various serum biomarkers and diagnostic modalities available for early prediction of PE.
Materials and methods
The databases searched to obtain the articles included MEDLINE Full Text, Pubmed, Science Direct, Pro Quest, SAGE, Taylor and Francis Online, Google Scholar, High Wire and Elsevier Clinical Key. The search strategies used included availability of full text written in English from 1 January 2005 till 1 March 2018.
Keywords used were “early detection or synonyms” AND “first trimester or synonyms” AND “preeclampsia”. When multiple articles for a single study was found, the most recent publication was used. Relevance of studies was assessed by using an approach based on title, abstract and full text. Studies were included if they were original studies and if they have a Randomized Controlled Trial (RCT) or Cohort study design.
Results
Our initial search resulted in 4,518 papers found, of which 4,280 were excluded on the basis of title and abstract. Of the remaining 238, 143 were excluded because they did not meet the inclusion criteria or were duplicated publications. Finally 95 articles were found that fulfilled all of our inclusion criteria.
Placental growth factor (PlGF), pregnancy associated plasma protein A (PAPP-A), soluble fms-like tyrosine kinase (sFLT) and placental protein 13 (PP-13) were the most commonly studied biomarkers. Whereas uterine Doppler scanning and Mean Arterial Pressure (MAP) were the most commonly studied out of other modalities. Outcomes of interest were the sensitivity, specificity and predictive value of modalities on predicting PE occurrence, reports on its advantages and drawbacks and its overall effect on mortality as well as morbidity of participants. It must be noted that studies included were based on published reports and take into account that studies reporting positive effects are more readily accepted to be published as compared to studies reporting negative effects.
Discussion
Preeclampsia can be classified into early and late onset and is widely accepted as different forms of the disease. Early onset PE (EO-PE), requiring delivery 34 weeks before gestation, is commonly associated with adverse maternal and neonatal outcomes (6, 13). In contrast, late-onset PE (LO-PE), with delivery at or after 34 weeks, is mostly associated with mild maternal disease and a low rate of fetal involvement. The perinatal outcomes of late-onset PE are usually favourable (16, 17).
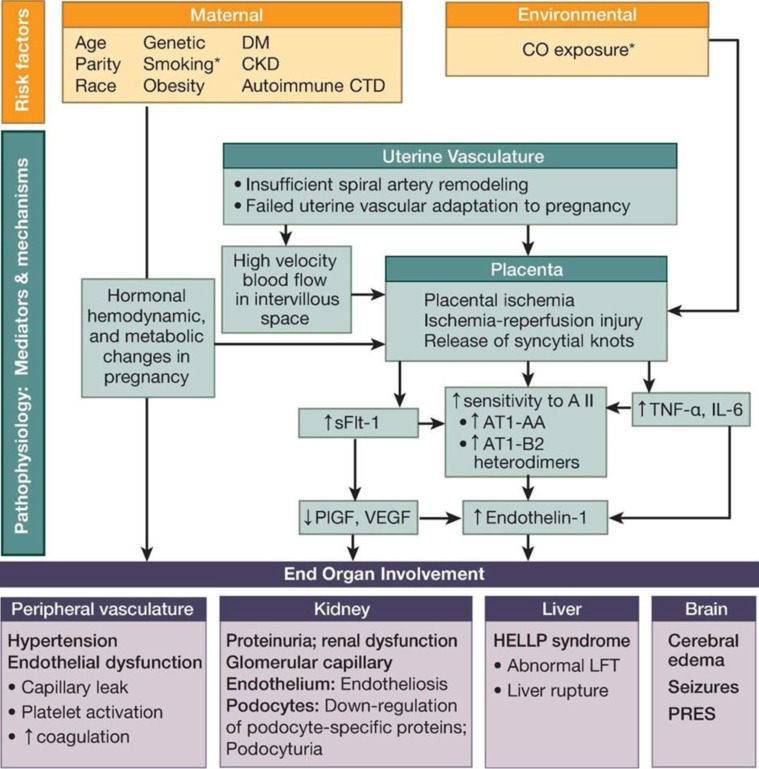
PE is considered to be a complex interaction between placental factors, maternal constitutional factors and immunological as well as vascular changes due to pregnancy resulting in the characteristic hypertension and proteinuria. Several pathophysiological mechanisms have been implicated in the pathogenesis of PE, however currently the mechanisms involved behind this disease still remains incompletely elucidated which complicates the prediction and treatment of PE (18-2) (Figure 1).
Figure 1.
Etiology and Pathophysiologic Mechanisms of PE21
AT1-AA – autoantibodies to angiotensin II type 1 receptor; AT1-AA-B2 – heterodimers, angiotensin II type 1 receptor-bradykinin type 2 receptor heterodimers; CO – carbonmonoxide; CKD – chronic renal disease; CTD – connective tissue disease; DM – diabetes mellitus; HELLP – hemolysis, elevated liver enzymes, low platelet count; IL-6 – interleukin 6; LFT – liver function tests; PIGF- placental growth factor; PRES – posterior reversible encephalopathy syndrome; sFlt-1 – soluble fms-like tyrosine kinase 1; TNFα – tumor necrosis factor α; VEGF – vascular endothelial growth factor.
*Reduced risk of pre-eclampsia
Currently, the diagnosis of PE is made on the basis of hypertension (arterial pressure exceeding 140/90 mmHg on at least 2 occasions, > 4 hours apart) and proteinuria (> 300 mg/dL/24h) found over 20 weeks of pregnancy (22, 23).
In contrast, it is recommended by the World Health Organization and supported by recent meta-analyses that the prevalence of PE can potentially be halved by the initiation of low-dose aspirin at 16 weeks or earlier (1, 24-26).
No screening procedure to predict PE is recommended by the American College of Obstetricians and Gynecologists (ACOG) beyond obtaining an appropriate medical history to evaluate for risk factors which makes the early diagnosis of more than half of PE cases which occurs among healthy nulliparous women to be difficult (27, 28).
Extensive research in the last several years on these mediators and signalling pathways as a result of the need for early detection in the first trimester to reduce maternal mortality and morbidity has however identified a series of early biophysical and biochemical markers of PE (29, 30) (Table 1).
Table 1.
| A disintegrin and metalloprotease 12 (ADAM12) | L-Arginine |
| Activin-A | L-Homoarginine |
| Adiponectin | Leptin |
| Adrcnomedullin | Magnesium |
| Alpha fetoprotein | Matrix mctalloproteinase-9 Microalbuminuria |
| Alpha-l-microglobulin | Microtransferrinuria |
| Ang-2 angiopoietin-2 | N-Acetyl-/J-glucosaminidase |
| Antiphospholipid antibodies | Neurokinin B |
| Antithrombin III | Neuropeptide Y |
| Atrial natriuretic peptide | Neutrophil gelatinase-associated lipocalin |
| Beta2-microglobulin | P-Selectin |
| C-reactive protein | Pentraxin 3 |
| Calcium | Placenta growth factor |
| Cellular adhesion molecules | Placental protein 13 |
| Circulating trophoblast | Plasminogen activator inhibitor-2 |
| Corticotropin release hormone | Platelet activation |
| Cytokines | Platelet count |
| Dimethylarginine (ADMA) | Pregnancy associated plasma protein-A |
| Endothelin | Prostacyclin |
| Estriol | Relaxin |
| Ferritin | Resistin |
| Fetal DNA | Serum lipids |
| Fetal RNA | Soluble cndoglin |
| Free fetalhemoglobin | Soluble fms-like tyrosine kinase |
| Fibronectin | Thromboxane |
| Genetic markers | Thyroid function |
| Haptoglobin | Total proteins |
| Hematocrit | Transferrin |
| Homocysteine | Tumor necrosis factor receptor-1 Uric acid |
| Human chorionic gonadotropin | Urinary calcium to creatinine ratio |
| Human placental growth hormone | Urinary kallikrein |
| Inhibin A | Vascular endothelial growth factor |
| Insulin-like growth factor | Visfatin |
| Insulin-like growth factor binding protein Insulin | Vitamin D |
| resistance | |
| Isoprostanes |
AT1-AA – autoantibodies to angiotensin II type 1 receptor; AT1-AA-B2 – heterodimers, angiotensin II type 1 receptor-bradykinin type 2 receptor heterodimers; CO – carbonmonoxide; CKD – chronic renal disease; CTD – connective tissue disease; DM – diabetes mellitus; HELLP – hemolysis, elevated liver enzymes, low platelet count; IL-6 – interleukin 6; LFT – liver function tests; PIGF- placental growth factor; PRES – posterior reversible encephalopathy syndrome; sFlt-1 – soluble fms-like tyrosine kinase 1; TNFα – tumor necrosis factor α; VEGF – vascular endothelial growth factor.
*Reduced risk of pre-eclampsia
Serum Biomarkers
Due to the extensive number of biomarkers as shown in the table above, biomarkers were only evaluated if they have at least 10 journal articles discussing their diagnostic use.
Vascular Endothelial Growth Factor and Placental Growth Factor (PlGF): PIGF, a dimeric glycoprotein, is a member of the VEGF Family synthesized in villous and extravillouscytotrophoblast and has both angiogenicand pro-inflammatory properties (46, 47). PE occurs when impaired placentation followed by ischemia results in increasing circulation of anti-angiogenic factors (sFlt-1 and sEng) which later antagonize a number of pro-angiogenic factors such as PIGF (48, 49). As a consequence, the concentration of important angiogenic and PIGFs in maternal circulation is reduced evidently during the first and second trimester leading to impaired endothelial function and subsequently EO-PE (5, 50).
Maternal serum levels of PIGF in pregnancies destined to develop PE, especially EO-PE, increases less or remains low throughout pregnancy.(51-53) Significant differences can be seen as early as 9 weeks, where PIGF can first be measured.(54) The detection rate (DR) of PIGF during the first trimester was found to be between 41% to 59% for EO-PE and 33% for LO-PE (51-56). This can be improved by the addition of serum PAPP-A and maternal history from 51% to 74% with a false positive rate (FPR) of 10% foe EO-PE and 33% to 43% with a false positive rate of 5% for LO-PE (55, 57).
PAPP-A (Pregnancy Associated Plasma Protein A): PAPP-A is a syncytiotrophoblast derived insulin-like growth factor binding protein protease and has long been used in risk calculation for chromosomal abnormalities such as Down’s syndrome. It regulates the bioavailability of free IGF at the placental-decidual interface during human implantation. Low concentrations of PAPP-A in the first trimester of pregnancy are highly associated with chromosomal aneuplodies. However, in chromosomally normal pregnancies, there is evidence that low maternal serum PAPP-A is linked with increased risk for subsequent development of PE (58).
The majority of studies found a significant association between PAPP-A and EO-PE with detection rates from 22% to 43%. However, it is not an effective standalone screening tool for PE because only 8-23% of affected cases have serum levels below the 5th percentile with the reported odds ratio varying between 1.5 and 4.6 (51, 55, 56, 59).
Placental Protein 13 (PP-13): PP-13 is a 32-kDa dimer protein belonging to the galectin super-family, a family of carbohydrate binding proteins called b-galactoside-specific lectins highly expressed in the placenta, specifically by the syncytiotrophoblast (60). PP-13 is suggested to be involved in the remodelling of the common fetomaternal blood-spaces through binding to proteins between the placenta and the endometrium. In normal pregnancies, serum PP-13 gradually increases to double or triple their values before delivery (61, 62). Several studies have demonstrated that low concentrations of PP-13 as early as 5-7 weeks predicts the onset of PE (41, 63). A number of studies on PP-13 found DR varying from 36% to 80% while Chafetz et al reported a quite high sensitivity (79%) and specificity (90%) ( 39, 54, 64, 65). These results can be further improved by combining PP-13 with maternal history and MAP as demonstrated by Gonen et al. with DR reaching 92% at a 12% FPR (41).
Serum Soluble Flt-1: sFlt-1 is a truncated splice variant of the membrane bound Flt-1. This splice variant circulates freely in the serum, where it binds and neutralizes VEGF and PIGF, preventing interaction with their biologically active transmembrane receptor (66). Several studies have reported sFlt-1 levels to rise as early as 5 weeks before the onset of PE. sFlt-1 levels are correlated directly with severity of disease and inversely related with the onset of clinical symptoms mainly hypertension and proteinuria (13,17, 67). sFlt-1 use however, would be improved if used in conjunction with other serum biomarkers such as PIGF instead (sFlt/PIGF ratio) as sFlt has a drawback of low sensitivity despite its high specificity (68). A study by Boucoiran et al, found sFlt to have a 90% specificity and 25% sensitivity (69). These findings are further supported by Myatt et al, with an 80% specificity and 21% sensitivity as well as various other studies (17, 35, 37, 70, 71).
β-humanChorionic Gonadotropin (β-hCG): β-hCG is a promoter of cell growth and differentiation in the embryo secreted by syncytiotrophoblastic cells of the placenta with the primary function of maintaining the vascular supply of placenta during pregnancy (72,73). In normal pregnancies, its levels increase until 9 to 10 weeks, decreasing afterwards. However, studies have shown that in those who develop PE, β-hCG levels continue to be elevated well beyond to the second trimester (5). Unfortunately, studies reviewed showed that it has a low predictive value for PE (34, 74-78).
However, a large scale retrospective analysis study by Cohen et al. involving almost 2,200 women showed that a combination of first and second trimester serum biomarkers, pregnancy-associated plasma protein A (PAPP-A), free βhCG, and maternal serum alpha-fetoprotein (msAFP) has a predictive probability of 91% of PE (73).
Inhibin-A and Activin-A: Inhibin-A and Activin-A are glycoprotein hormones produced by the fetoplacental unit which has been suggested to be involved in the feedback loop regulating hCG levels during pregnancy (5). An early study by Muttukrishna et al. showed that those suffering from PE has serum concentrations of both markers 10-times greater compared to controls in their third trimester (79).
Later on, several studies has exhibited that both inhibin-A and activin-A are increased in the maternal blood of first trimester patients who later develop PE compared to pregnant women with normal pregnancies (74, 78, 80-82). However, a study by Spencer et al. reported that when used alone, DR of inhibin-A and activin-A for PE to be 35% and 20% respectively. Better results were obtained when used with a combination of other markers (82).
Soluble Endoglin: Soluble endoglin is an auxiliary co-receptor of transforming growth factor β2 (TGF-β2). This co-receptor interferes with binding of TGF-β1 to its receptor, and thus was reported to play a role in the alterations in vasculogenesis and angiogenesis which occurs during PE, including effects on the production of nitric oxide, vasodilation and, capillary formation by endothelial cells as well as hypoxia and oxidative stress (83, 84).
Animal models have shown that sEng, interacts with another anti-angiogenic protein, sFlt-1 inducing severe PE-like disease (85). Various clinical studies has also reported that high levels of soluble endoglin was found in the plasma of women with PE.(85, 93) This was in contrast to 2 studies by Myers et al (35). And Myatt et al (37). Which reported no change in sEng levels.
However, a large scale prospective cohort study by Kusanovic et al. involving over 1,600 pregnant women studying sEng levels at early and mid-trimester concluded that it is a promising biomarker in predicting PE, although best diagnostic performances were obtained when combined with other biomarkers (42).
A Disintegrin and Metalloprotease 12 (ADAM-12): ADAM12 is a placenta-derived member of the ADAM protein family, hypothesised to take part in placental growth and development.(5) Furthermore, Gack et al. demonstrated that ADAM12 was the most upregulated transcription factor in placental tissues of women with PE (94). Several studies have examined ADAM12 serum levels between 8 to 14 weeks of pregnancy with conflicting results (74, 95-98). Reduced ADAM12 levels was reported by Laigaard et al. and Spencer et al. in pregnancies complicated by PE, both studies concluded the potential for PE as an early biomarker (96, 97). In contrast, studies by Poon et al, Audibert et al and Wortelboer et al. reported no change in ADAM12 levels in those suffering from PE (74, 95, 98). Further studies would thus be required to confirm these findings.
Other proposed modalities
Maternal History: Maternal history including ethnic origin, parity, body mass index (BMI) and personal or family history (PE, anti-phospolipid syndrome, chronic kidney disease, insulin-dependent diabetes, multiple pregnancies, pre-existing hypertension, and nulliparity) are well-known risk factors of PE (107, 108). Among women considered as high-risk, approximately 25% will develop PE compared with 5% in the general population (27) (Table 2).
Table 2.
| Parameter | Classified by category | Classified by risk profile |
|---|---|---|
| Maternal age | Personal history | Personal risk profile |
| Maternal ethnicity | ||
| Tobacco use in pregnancy | ||
| Parity | ||
| Education level | ||
| Conception method | ||
| Family history of preeclampsia | ||
| Prior preeclampsia | ||
| Prior preterm birth | ||
| Renal disease | Past medical conditions | Cardiovascular risk profile |
| Hypertension | ||
| Systolic blood pressure | Physical examination | |
| Diastolic blood pressure | ||
| Mean arterial blood pressure | ||
| Prior gestational diabetes | History | Metabolic risk profile |
| Pre-existing diabetes mellitus | Past medical conditions | |
| Maternal height | Physical examination | |
| Maternal weight | ||
| Maternal body mass index | ||
| Thrombophilia | Past medical conditions | Prothrombotic risk profile |
| Uterine artery Doppler index | Placental blood flow | |
| Uterine artery notching |
However screening strategies using maternal history alone for detection of PE perform moderately well at best. The National Institute of Clinical Excellence (NICE) found that a screening strategy based on maternal history and risk factors categorizes more than 60% of pregnant women as high risk and predicts fewer than 30% of those to develop PE with a false positive rate of 10%b (15, 109). As the pathophysiology of PE is thought to include abnormal placentation, the evaluation of uterine artery blood flow resistance may be able to support the results of risk factors (13). An assessment of risk by Poon et al. based on maternal history, blood pressure and uterine artery Doppler has found the detection rate of PE to be higher than based on maternal history alone (107, 109).
Biophysical: Biophysical modalities reviewed include blood pressure, mean arterial pressure (MAP) monitoring and uterine artery doppler scan (UtAD).
Blood Pressure & Mean Arterial Pressure (MAP)
Women who subsequently developed PE are found to have a higher systolic blood pressure and MAP readings before the onset of the disease – with MAP (the sum of systolic and twice the diastolic pressure divided by three) having a higher predictive value for PE among low risk women in the first and second trimester than either systolic or diastolic readings alone (13, 110, 111). Kumar et al discovered that a combination of mean BMI, MAP, uterine artery Doppler and pulsatility index, have a distinct correlation with the onset of hypertension with a sensitivity and specificity of 76% and 80% respectively (112).
First-trimester MAP has been shown to be affected by maternal weight, height, age, racial origin, cigarette smoking, family and prior history of PE, and history of chronic hypertension. Consequently, combining these predictors may be beneficial in selecting individuals for close monitoring and early intervention during pregnancy (29). In a study by Poon et al, a combination of MAP with maternal risk factors resulted in a detection rate of 76% (113). A study by Gallo et al. performed MAP-1 (during 11-13 weeks) and MAP-2 (20-24 weeks) screening, which showed detection rates of 74.3% and 84.3% for EO-PE respectively thus proving that MAP would be best taken during these weeks (114, 115).
Maternal MAP is an easy, cost effective, and non-invasive test that can be performed to all women during their first antenatal visit. MAP can also be combined with uterine artery Doppler and biomarkers where a study reported this combined screening, with a false positive rate of 5% identified approximately 80% of PE patients before 34 weeks gestation and another study reported that 90% of PE developing pregnancies were identified in the subsequent 4 weeks (46, 55, and 57).
Uterine Artery Doppler (UtAD)
The impedance to blood flow in the uterine arteries steadily declines in the progression of a normal pregnancy where this transformation is necessary to ensure a dramatic increase of blood supply to intervillous space (46, 63, 116). However due to defective differentiation of trophoblast and impaired invasion of spiral arteries, the uteroplacental circulation remains in a state of high resistance which can be measured noninvasively by UtAD.(113, 117)UtAD uses color flow mapping to identify vessels before a pulsed wave Doppler is used where the three similar consecutive waveforms are used to obtain the Pulsatility Index (PI) and calculate the mean PI of the left and right arteries (118).
A feature of UtAD is that the detection rates and sensitivity are better suited for second trimester than first trimester screening and for pre-term/early form PE than for severe or mild PE (99, 115). A meta-analysis by Velauthar et al involving 11 studies (43,122 pregnancies) to predict PE in the first trimester with UtAD reported that abnormal UtAD has a high specificity (91%) and low sensitivity (26%) in the first trimester (119). Nevertheless, it must be kept in mind that first trimester UtAD are shown to be affected by maternal history and risk factors and adjustment must be taken (29). Considering this, a combination of maternal factors and UtAD has yielded a better detection rate (from 51% to 75%, with a false positive rate of 10%) for PE requiring delivery before 37 weeks gestation (46).
The use of UtAD remains controversial with its conflicting results however despite it, the evaluation of UtAD suggests it as an important predictor as it will allow clinicians to determine women at risk of especially EO-PE, which causes the highest perinatal morbidity and mortality, and IUGD, enabling preventive methods to be taken to minimise adverse outcomes (13, 120).
Combination Studies
sFlt/PIGF Ratio: The PIGF/sFlt-1 ratio has been brought forward as a possible predictive marker not only for preeclampsia butalso other placenta related disorders such as IUGR or stillbirth (2, 121). In a multicentre clinical study by Verlohren et al, it was reported that the PGF/sFlt-1 ratio has a DR of 82% with a 5% FPR and is especially high for EO-PE (89% detection rate). Other than these findings, it was especially important to know that PE patients with a high PIGF/sFlt-1 ratio were found to have a significantly increased risk for delivery within 7 days as the early identification and timely referral to a perinatal care unit is hypothesised to be able to reduce perinatal morbidity and mortality by 20% (122). Benton et al also performed a case control study of 44 patients to compare two commercially available sFlt/PLGF assay with both having greater than 95% sensitivity and a greater tendency for detection in the diagnosis of EO-P (123).
PlGF, sEng, and soluble fms-like tyrosine kinase 1 (sFlt1) Ratio: A large scale prospective cohort study by Kusanovic et al. involving over 1600 pregnant women studying sEng levels at early and mid-trimester concluded that best diagnostic performances were obtained by ratios in the mid-trimester and the slopes of plasma concentrations of PlGF, sEng, and soluble fms-like tyrosine kinase 1 (sFlt1) between the first and second trimester with a sensitivity of 100% for all tests and a specificity between 98-99% (42).
Maternal Characteristics, Biophysical and Biochemical Markers: A recent large scale study involving over 50,000 singleton mothers by Akolekar et al. developed a new model for PE screening wherein the combined use of maternal characteristics (race, method of conception, smoking, history of diabetes and hypertension, history of autoimmune disorders, family history of PE and obstetric history) with biophysical characters namely uterine artery pulsatility index and MAP together with serum biomarkers PAPP-A and PLGF was able to detect 96% of cases of early onset PE cases and 54% of all PE cases at a fixed false-positive rate of 10% (124).
Many other models have also been develop involving the combined use of various different maternal characteristics, biophysical characteristics and serum biomarkers with differing sensitivity and specificity (28, 31, 34, 35, 38, 41, 73, 74, 90, 101, 106, 107).
Further large scale studies comparing their uses with each other in diverse populations need to be done before these models can be implemented at large.
Limitations of the Review
This review article suffers limitations in only being able to synthesize journal articles and trials previously published which are accessible to the authors.
Concerns and Practical Implications
Although extensive research has been done focusing on the identification of women at high risk of developing PE, various studies have reported that PE cannot be predicted by previous obstetric history and risk factors alone (68, 106). PP-13 was found to be the best at predicting EO-PE as a single biomarker. However, no single marker was able to perform better compared to a combination model taking into account the various facets of PE.
As several large scale studies have shown, serum markers when combined with maternal characteristics such as age, weight, height, nulliparity, smoking status, first-trimester MAPand, uterine artery Doppler are powerful tools, able to predict PE in the first trimester.
From a public health perspective, a cost effective solution can be found through the use of clinical, uterine artery Doppler and MAP assessments, negating the use of the costly laboratory based biomarker(s) prediction strategy. As the study by Kuc et al. has shown, maternal characteristics when combined with first-trimester MAP and UtAD was able to have a high detection rate of 89.2%, in line with other studies as well (32).This may seem plausible as maternal characteristics determine the susceptibility of a mother and MAP relates to maternal vascular adaptation. Whereas biomarkers relate to placentation.
No matter the modality chosen, early detection for PE especially EO-PE is one that we must strive for, to reduce both maternal and neonatal morbidity as well as mortality. Early prediction would allow for greater risk assessments and control, more intensive monitoring of this high risk group as well as targeted prophylactic intervention, timely diagnosis and treatment.
Conclusion
PE is a hypertensive pregnancy disorder with multifactorial origins. First trimester screening for PE will allow for a personalized risk estimate to the population early in their pregnancy. Serum biomarkers such as PIGF and sFlt yielded the best results for a single biomarker with others having conflicting results. However, a combination model with other diagnostic modalities including maternal history, MAP, and uterine artery Doppler performed better than a single biomarker. Further research is needed as current models have a low predictive value and guidelines need to be created to implement this method in Indonesia. In the future, new techniques will hopefully provide sets of multiple markers, which will lead to a screening program with clinically relevant performance and early prophylactic strategies could be made for high risk women.
Acknowledgments
The authors would like to thank AndrianaKumalaDewi, MD (Obstetrician and Gynecologists) for her valuable inputs provided during the preparation of this manuscript.
Conflict of Interests
Authors have no conflict of interests.
Notes:
Citation: Sunjaya AF, Sunjaya AP. Evaluation of Serum Biomarkers and Other Diagnostic Modalities for Early Diagnosis of Preeclampsia. J Fam Reprod Health 2019; 13(2): 56-69.
References
- 1.World Health Organization (WHO) WHO Recommendations for Prevention and Treatment of pre-eclampsia and eclampsia. World Health Organization (WHO) Department of Reproductive Health and Research; 2011. [PubMed] [Google Scholar]
- 2.Savaj S, Nosratolah D Vaziri. An overview of recent advances in pathogenesis and diagnosis of preeclampsia. Iranian Journal of Kidney Diseases. 2012;6:334–8. [PubMed] [Google Scholar]
- 3.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 4.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21:521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 5.Kuc S, Wortelboer EJ, van Rijn BB, Franx A, Visser GH, Schielen PC. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv . 2011;66:225–39. doi: 10.1097/OGX.0b013e3182227027. [DOI] [PubMed] [Google Scholar]
- 6.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 7.WHO; UNICEF; UNFPA; The World Bank. Maternal Mortality in 2005. Geneva: WHO, UNICEF, UNFPA and The World Bank, Department of Reproductive Health and Research; 2007. [Google Scholar]
- 8.Jeyabalan A. Epidemiology of preeclampsia: impact of obesity. Nutr Rev. 2013;71 (Suppl 1):S18–25. doi: 10.1111/nure.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noris M, Perico N, Remuzzi G. Mechanisms of disease: Pre-eclampsia. Nat Clin Pract Nephrol. 2005;1:98–114. doi: 10.1038/ncpneph0035. [DOI] [PubMed] [Google Scholar]
- 10.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Y, Zhu F, Ding Y. Serum screening in first trimester to predict pre-eclampsia, small for gestational age and preterm delivery: systematic review and meta-analysis. BMC Pregnancy Childbirth. 2015;15:191. doi: 10.1186/s12884-015-0608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa Fda S, Murthi P, Keogh R, Woodrow N. Early screening for preeclampsia. Rev Bras Ginecol Obstet. 2011;33:367–75. doi: 10.1590/s0100-72032011001100008. [DOI] [PubMed] [Google Scholar]
- 14.Pedrosa AC, Matias A. Screening for pre-eclampsia: a systematic review of tests combining uterine artery Doppler with other markers. J Perinat Med. 2011;39:619–35. doi: 10.1515/jpm.2011.077. [DOI] [PubMed] [Google Scholar]
- 15.Thilaganathan B, Wormald B, Zanardini C, Sheldon J, Ralph E, Papageorghiou AT. Early-pregnancy multiple serum markers and second-trimester uterine artery Doppler in predicting preeclampsia. Obstet Gynecol . 2010;115:1233–8. doi: 10.1097/AOG.0b013e3181dd5137. [DOI] [PubMed] [Google Scholar]
- 16.Yu CK, Khouri O, Onwudiwe N, Spiliopoulos Y, Nicolaides KH Fetal medicine foundation second-trimester screening group. Prediction of pre-eclampsia by uterine artery Doppler imaging: relationship to gestational age at delivery and small-for-gestational age. Ultrasound Obstet Gynecol. 2008;31:310–3. doi: 10.1002/uog.5252. [DOI] [PubMed] [Google Scholar]
- 17.Park HJ, Shim SS, Cha DH. Combined screening for early detection of pre-eclampsia. Int J Mol Sci. 2015;16:17952–74. doi: 10.3390/ijms160817952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 20.Visser N, van Rijn BB, Rijkers GT, Franx A, Bruinse HW. Inflammatory changesin preeclampsia: current understanding of the maternal innate and adaptive immune response. Obstet Gynecol Surv. 2007;62:191–201. doi: 10.1097/01.ogx.0000256779.06275.c4. [DOI] [PubMed] [Google Scholar]
- 21.Craici IM, Wagner SJ, Weissgerber TL, Grande JP, Garovic VD. Advances in thepathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 2014;86:275–85. doi: 10.1038/ki.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wibowo N, Irwinda R, Frisdianty E. Indonesian Society of Obstetrician and Gynecologists; Ministry of Health Republic of Indonesia; 2010. Pedoman Nasional Pelayanan Kedokteran Diagnosis dan tatalaksana pre-eklampsia. [Google Scholar]
- 23.Royal College of Obstetricians and Gynaecologists. Royal College of Obstetricians and Gynaecologists. 2011. Hypertension in pregnancy: the management of hypertensive disorders during pregnancy London. [Google Scholar]
- 24.Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–14. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 25.Roberge S, Villa P, Nicolaides K, Giguère Y, Vainio M, Bakthi A, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31:141–6. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 26.Roberge S, Guguere Y, Villa P, Nicolaides K, Vainio M, Forest JC, et al. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Fetal Diagnosis and Therapy. 2012;31:141–6. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 27.Committee Opinion No. 638: First-trimester risk assessment for early-onset preeclampsia. Obstet Gynecol. 2015;126:e25–7. doi: 10.1097/AOG.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 28.Kenny LC, Black MA, Poston L, Taylor R, Myers JE, Baker PN, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers: the Screening for Pregnancy Endpoints(SCOPE) international cohort study. Hypertension. 2014;64:644–52. doi: 10.1161/HYPERTENSIONAHA.114.03578. [DOI] [PubMed] [Google Scholar]
- 29.Wright D, Akolekar R, Syngelaki A, Poon LC, Nicolaides KH. A competing risks model in early screening for preeclampsia. Fetal Diagn Ther. 2012;32:171–8. doi: 10.1159/000338470. [DOI] [PubMed] [Google Scholar]
- 30.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 31.Odibo AO, Zhong Y, Goetzinger KR, Odibo L, Bick JL, Bower CR, et al. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta. 2011;32:598–602. doi: 10.1016/j.placenta.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuc S, Koster MP, Franx A, Schielen PC, Visser GH. Maternal characteristics,mean arterial pressure and serum markers in early prediction of preeclampsia. PLoS One. 2013;8:e63546. doi: 10.1371/journal.pone.0063546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Lorenzo G, Ceccarello M, Cecotti V, Ronfani L, Monasta L, Vecchi Brumatti L, et al. First trimester maternal serum PIGF, free β-hCG, PAPP-A, PP-13, uterine artery Doppler and maternal history for the prediction of preeclampsia. Placenta. 2012;33:495–501. doi: 10.1016/j.placenta.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Goetzinger KR, Singla A, Gerkowicz S, Dicke JM, Gray DL, Odibo AO. Predicting the risk of pre-eclampsia between 11 and 13 weeks' gestation by combining maternal characteristics and serum analytes, PAPP-A and free β-hCG. Prenat Diagn. 2010;30:1138–42. doi: 10.1002/pd.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, et al. SCOPE consortium. Angiogenic factors combined with clinical risk factors to predict preterm pre-eclampsia in nulliparous women: a predictive test accuracy study. BJOG. 2013;120:1215–23. doi: 10.1111/1471-0528.12195. [DOI] [PubMed] [Google Scholar]
- 36.D'Antonio F, Rijo C, Thilaganathan B, Akolekar R, Khalil A, Papageourgiou A, et al. Association between first-trimester maternal serum pregnancy-associated plasma protein-A and obstetric complications. Prenat Diagn. 2013;33:839–47. doi: 10.1002/pd.4141. [DOI] [PubMed] [Google Scholar]
- 37.Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. First-trimester prediction of preeclampsia in nulliparous women at low risk. Obstet Gynecol. 2012;119:1234–42. doi: 10.1097/AOG.0b013e3182571669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youssef A, Righetti F, Morano D, Rizzo N, Farina A. Uterine artery Doppler and biochemical markers (PAPP-A, PIGF, sFlt-1, P-selectin, NGAL) at 11 + 0 to 13 + 6 weeks in the prediction of late (> 34 weeks) pre-eclampsia. Prenat Diagn. 2011;31:1141–6. doi: 10.1002/pd.2848. [DOI] [PubMed] [Google Scholar]
- 39.Chafetz I, Kuhnreich I, Sammar M, Tal Y, Gibor Y, Meiri H, et al. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197:35. doi: 10.1016/j.ajog.2007.02.025. e1-7. [DOI] [PubMed] [Google Scholar]
- 40.Vandenberghe G, Mensink I, Twisk JW, Blankenstein MA, Heijboer AC, van Vugt JM. First trimester screening for intra-uterine growth restriction and early-onset pre-eclampsia. Prenat Diagn. 2011;31:955–61. doi: 10.1002/pd.2807. [DOI] [PubMed] [Google Scholar]
- 41.Gonen R, Shahar R, Grimpel YI, Chefetz I, Sammar M, Meiri H, et al. Placental protein 13 as an early marker for pre-eclampsia: a prospective longitudinal study. BJOG. 2008;115:1465–72. doi: 10.1111/j.1471-0528.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 42.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poon LC, Stratieva V, Piras S, Piri S, Nicolaides KH. Hypertensive disorders in pregnancy: combined screening by uterine artery Doppler, blood pressure and serum PAPP-A at 11-13 weeks. Prenat Diagn. 2010;30:216–23. doi: 10.1002/pd.2440. [DOI] [PubMed] [Google Scholar]
- 44.Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–8. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 45.Ranta JK, Raatikainen K, Romppanen J, Pulkki K, Heinonen S. Decreased PAPP-A is associated with preeclampsia, premature delivery and small for gestational age infants but not with placental abruption. Eur J Obstet Gynecol Reprod Biol. 2011;157:48–52. doi: 10.1016/j.ejogrb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Poon LC, Nicolaides KH. Early prediction of preeclampsia. Obstet Gynecol Int. 2014;2014:297397. doi: 10.1155/2014/297397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010;202:161. doi: 10.1016/j.ajog.2009.09.016. e1-161.e11. [DOI] [PubMed] [Google Scholar]
- 48.Lockwood CJ, Krikun G, Caze R, Rahman M, Buchwalder LF, Schatz F. Decidual cell-expressed tissue factor in human pregnancy and its involvement in hemostasis and preeclampsia-related angiogenesis. Ann N Y Acad Sci. 2008;1127:67–72. doi: 10.1196/annals.1434.013. [DOI] [PubMed] [Google Scholar]
- 49.Milkat B, Gellhaus A, Wagner N, Birdir C, Kimmig R, Koninger A. Early detection of maternal risk for preeclampsia. ISRN Obstet Gynecol. 2012;2012:172808. doi: 10.5402/2012/172808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crispi F, Llurba E, Domínguez C, Martín-Gallán P, Cabero L, Gratacós E. Predictive value of angiogenic factors and uterine artery Doppler for early-versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:303–9. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 51.Foidart JM, Munaut C, Chantraine F, Akolekar R, Nicolaides KH. Maternal plasmasoluble endoglin at 11-13 weeks' gestation in pre-eclampsia. Ultrasound Obstet Gynecol. 2010;35:680–7. doi: 10.1002/uog.7621. [DOI] [PubMed] [Google Scholar]
- 52.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti angiogenic factors inmaternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes incirculating level of angiogenic factors from the first to second trimester aspredictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239. doi: 10.1016/j.ajog.2006.10.909. e1-6. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) andanti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction ofearly, intermediate and late pre-eclampsia from maternal factors, biophysical andbiochemical markers at 11-13 weeks. Prenat Diagn. 2011;31:66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 56.Wortelboer EJ, Koster MP, Cuckle HS, Stoutenbeek PH, Schielen PC, Visser GH. First-trimester placental protein 13 and placental growth factor: markers for identification of women destined to develop early-onset pre-eclampsia. BJOG. 2010;117:1384–9. doi: 10.1111/j.1471-0528.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- 57.Tayyar A, Garcia-Tizon Larroca S, Poon LC, Wright D, Nicolaides KH. Competing risk model in screening for pre-eclampsia by mean arterial pressure and uterine artery pulsatility index at 30–33 weeks’gestation. Fetal Diagn Ther. 2014;36:18–27. doi: 10.1159/000360792. [DOI] [PubMed] [Google Scholar]
- 58.Mikat B, Zeller A, Scherag A, Drommelschmidt K, Kimmig R, Schmidt M. βhCG and PAPP-A in first trimester: predictive factors for preeclampsia. Hypertens Pregnancy. 2012;31:261–7. doi: 10.3109/10641955.2011.638956. [DOI] [PubMed] [Google Scholar]
- 59.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11-13 weeks. Prenat Diagn. 2011;31:66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 60.Sekizawa A, Purwosunu Y, Yoshimura S, Nakamura M, Shimizu H, Okai T, et al. PP13 mRNA expression in trophoblasts from preeclamptic placentas. Reproductive Sciences. 2009;16:408–13. doi: 10.1177/1933719108328615. [DOI] [PubMed] [Google Scholar]
- 61.Than NG, Romero R, Kim CJ, McGowen MR, Papp Z, Wildman DE. Galectins: guardians of eutherian pregnancy at the maternal-fetal interface. Trends Endocrinol Metab. 2012;23:23–31. doi: 10.1016/j.tem.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, et al. A primate subfamily of galectins expressed at thematernal-fetal interface that promote immune cell death. Proc Natl Acad Sci U SA. 2009;106:9731–6. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huppertz B, Abe E, Murthi P, Nagamatsu T, Szukiewicz D, Salafia C. Placental angiogenesis, maternal and fetal vessels--a workshop report. Placenta. 2007;28:S94–6. doi: 10.1016/j.placenta.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternalserum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility indexas markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–34. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 65.Khalil A, Cowans NJ, Spencer K, Goichman S, Meiri H, Harrington K. First trimester maternal serum placental protein 13 for the prediction of pre-eclampsia in women with a priori high risk. Prenat Diagn. 2009;29:781–9. doi: 10.1002/pd.2287. [DOI] [PubMed] [Google Scholar]
- 66.Kar M. Role of biomarkers in early detection of preeclampsia. J Clin Diagn Res. 2014;8:BE01–4. doi: 10.7860/JCDR/2014/7969.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26:236–41. doi: 10.1038/jhh.2011.29. [DOI] [PubMed] [Google Scholar]
- 68.Wu P, van den Berg C, Alfirevic Z, O'Brien S, Röthlisberger M, Baker PN, et al. Early pregnancy biomarkers in pre-eclampsia: A systematic review and meta-analysis. Int J Mol Sci. 2015;16:23035–56. doi: 10.3390/ijms160923035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boucoiran I, Thissier-Levy S, Wu Y, Wei SQ, Luo ZC, Delvin E, et al. MIROS study group. Risks for preeclampsia and small for gestational age: predictive values of placental growth factor, soluble fms-like tyrosine kinase-1, and inhibin A in singleton and multiple-gestation pregnancies. Am J Perinatol. 2013;30:607–12. doi: 10.1055/s-0032-1329691. [DOI] [PubMed] [Google Scholar]
- 70.Bills VL, Varet J, Millar A, Harper SJ, Soothill PW, Bates DO. Failure toup-regulate VEGF165b in maternal plasma is a first trimester predictive markerfor pre-eclampsia. Clin Sci (Lond) 2009;116:265–72. doi: 10.1042/CS20080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giguère Y, Massé J, Thériault S, Bujold E, Lafond J, Rousseau F, et al. Screening for pre-eclampsia early in pregnancy: performance of a multivariable model combining clinical characteristics and biochemical markers. BJOG. 2015;122:402–10. doi: 10.1111/1471-0528.13050. [DOI] [PubMed] [Google Scholar]
- 72.Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102. doi: 10.1186/1477-7827-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen JL, Smilen KE, Bianco AT, Moshier EL, Ferrara LA, Stone JL. Predictive value of combined serum biomarkers for adverse pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2014;181:89–94. doi: 10.1016/j.ejogrb.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 74.Audibert F, Boucoiran I, An N, Aleksandrov N, Delvin E, Bujold E, et al. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am J Obstet Gynecol. 2010;203:383. doi: 10.1016/j.ajog.2010.06.014. e1-8. [DOI] [PubMed] [Google Scholar]
- 75.Brameld KJ, Dickinson JE, O'Leary P, Bower C, Goldblatt J, Hewitt B, et al. First trimester predictors of adverse pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2008;48:529–35. doi: 10.1111/j.1479-828X.2008.00912.x. [DOI] [PubMed] [Google Scholar]
- 76.Spencer K, Yu CK, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;25:949–53. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 77.Canini S, Prefumo F, Pastorino D, Crocetti L, Afflitto CG, Venturini PL, et al. Association between birth weight and first-trimester free beta-human chorionic gonadotropin and pregnancy-associated plasma protein A. Fertil Steril. 2008;89:174–8. doi: 10.1016/j.fertnstert.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 78.Zwahlen M, Gerber S, Bersinger NA. First trimester markers for pre-eclampsia: placental vs. non-placental protein serum levels. Gynecol Obstet Invest. 2007;63:15–21. doi: 10.1159/000094672. [DOI] [PubMed] [Google Scholar]
- 79.Muttukrishna S, Knight PG, Groome NP, Redman CW, Ledger WL. Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet. 1997;349:1285–8. doi: 10.1016/s0140-6736(96)09264-1. [DOI] [PubMed] [Google Scholar]
- 80.Akolekar R, Minekawa R, Veduta A, Romero XC, Nicolaides KH. Maternal plasma inhibin A at 11-13 weeks of gestation in hypertensive disorders of pregnancy. Prenat Diagn. 2009;29:753–60. doi: 10.1002/pd.2279. [DOI] [PubMed] [Google Scholar]
- 81.Akolekar R, Etchegaray A, Zhou Y, Maiz N, Nicolaides KH. Maternal serum activin a at 11-13 weeks of gestation in hypertensive disorders of pregnancy. Fetal Diagn Ther. 2009;25:320–7. doi: 10.1159/000235878. [DOI] [PubMed] [Google Scholar]
- 82.Spencer K, Cowans NJ, Nicolaides KH. Maternal serum inhibin-A and activin-A levels in the first trimester of pregnancies developing pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:622–6. doi: 10.1002/uog.6212. [DOI] [PubMed] [Google Scholar]
- 83.Oujo B, Perez-Barriocanal F, Bernabeu C, Lopez-Novoa JM. Membrane and soluble forms of endoglin in preeclampsia. Curr Mol Med. 2013;13:1345–57. doi: 10.2174/15665240113139990058. [DOI] [PubMed] [Google Scholar]
- 84.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, et al. CPEP Study Group. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 86.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 87.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–42. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 88.Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol. 2007;197:174. doi: 10.1016/j.ajog.2007.03.058. e1-5. [DOI] [PubMed] [Google Scholar]
- 89.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol. 2007;197:176. doi: 10.1016/j.ajog.2007.03.036. e1-6. [DOI] [PubMed] [Google Scholar]
- 90.Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet Gynecol. 2008;111:1403–9. doi: 10.1097/AOG.0b013e3181719b7a. [DOI] [PubMed] [Google Scholar]
- 91.Stepan H, Geipel A, Schwarz F, Krämer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175. doi: 10.1016/j.ajog.2007.08.052. e1-6. [DOI] [PubMed] [Google Scholar]
- 92.De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D'anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:837–42. doi: 10.1080/00016340802253759. [DOI] [PubMed] [Google Scholar]
- 93.Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 2007;197:28. doi: 10.1016/j.ajog.2007.04.010. e1-6. [DOI] [PubMed] [Google Scholar]
- 94.Gack S, Marmé A, Marmé F, Wrobel G, Vonderstrass B, Bastert G, et al. Preeclampsia: increased expression of soluble ADAM12. J Mol Med (Berl) 2005;83:887–96. doi: 10.1007/s00109-005-0714-9. [DOI] [PubMed] [Google Scholar]
- 95.Wortelboer EJ, Koster MP, Cuckle HS, Stoutenbeek PH, Schielen PC, Visser GH. First-trimester placental protein 13 and placental growth factor: markers for identification of women destined to develop early-onset pre-eclampsia. BJOG. 2010;117:1384–9. doi: 10.1111/j.1471-0528.2010.02690.x. [DOI] [PubMed] [Google Scholar]
- 96.Spencer K, Cowans NJ, Stamatopoulou A. ADAM12s in maternal serum as a potential marker of pre-eclampsia. Prenat Diagn. 2008;28:212–6. doi: 10.1002/pd.1957. [DOI] [PubMed] [Google Scholar]
- 97.Laigaard J, Sørensen T, Placing S, Holck P, Fröhlich C, Wøjdemann KR, et al. Reduction of the disintegrin and metalloprotease ADAM12 in preeclampsia. Obstet Gynecol. 2005;106:144–9. doi: 10.1097/01.AOG.0000165829.65319.65. [DOI] [PubMed] [Google Scholar]
- 98.Poon LC, Chelemen T, Granvillano O, Pandeva I, Nicolaides KH. First-trimester maternal serum a disintegrin and metalloprotease 12 (ADAM12) and adverse pregnancy outcome. Obstet Gynecol. 2008;112:1082–90. doi: 10.1097/AOG.0b013e318188d6f9. [DOI] [PubMed] [Google Scholar]
- 99.Cuckle HS. Screening for pre-eclampsia--lessons from aneuploidy screening. Placenta. 2011;32 Suppl:S42–8. doi: 10.1016/j.placenta.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 100.Parra-Cordero M, Rodrigo R, Barja P, Bosco C, Rencoret G, Sepúlveda-Martinez A, et al. Prediction of early and late pre-eclampsia from maternal characteristics, uterine artery Doppler and markers of vasculogenesis during first trimester of pregnancy. Ultrasound Obstet Gynecol. 2013;41:538–44. doi: 10.1002/uog.12264. [DOI] [PubMed] [Google Scholar]
- 101.Herraiz I, Arbués J, Camaño I, Gómez-Montes E, Grañeras A, Galindo A. Application of a first-trimester prediction model for pre-eclampsia based on uterine arteries and maternal history in high-risk pregnancies. Prenat Diagn. 2009;29:1123–9. doi: 10.1002/pd.2383. [DOI] [PubMed] [Google Scholar]
- 102.Scazzocchio E, Figueras F, Crispi F, Meler E, Masoller N, Mula R, et al. Performance of a first-trimester screening of preeclampsia in a routine care low-risk setting. Am J Obstet Gynecol. 2013;208:203. doi: 10.1016/j.ajog.2012.12.016. e1-203.e10. [DOI] [PubMed] [Google Scholar]
- 103.Baschat AA, Magder LS, Doyle LE, Atlas RO, Jenkins CB, Blitzer MG. Prediction of preeclampsia utilizing the first trimester screening examination. Am J Obstet Gynecol. 2014;211:514. doi: 10.1016/j.ajog.2014.04.018. e1-7. [DOI] [PubMed] [Google Scholar]
- 104.Caradeux J, Serra R, Nien JK, Pérez-Sepulveda A, Schepeler M, Guerra F, et al. First trimester prediction of early onset preeclampsia using demographic,clinical, and sonographic data: a cohort study. Prenat Diagn. 2013;33:732–6. doi: 10.1002/pd.4113. [DOI] [PubMed] [Google Scholar]
- 105.Baschat AA. First-trimester screening for pre-eclampsia: moving from personalized risk prediction to prevention. Ultrasound Obstet Gynecol. 2015;45:119–29. doi: 10.1002/uog.14770. [DOI] [PubMed] [Google Scholar]
- 106.North RA, McCowan LM, Dekker GA, Poston L, Chan EH, Stewart AW, et al. Clinical risk prediction for pre-eclampsia in nulliparous women: development of model in international prospective cohort. BMJ. 2011;342:d1875. doi: 10.1136/bmj.d1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Onwudiwe N, Yu CK, Poon LC, Spiliopoulos I, Nicolaides KH. Prediction ofpre-eclampsia by a combination of maternal history, uterine artery Doppler andmean arterial pressure. Ultrasound Obstet Gynecol. 2008;32:877–83. doi: 10.1002/uog.6124. [DOI] [PubMed] [Google Scholar]
- 108.National Collaborating Centre for Women's and Children's Health (UK) Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London: RCOG Press; 2010. [PubMed] [Google Scholar]
- 109.Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J Hum Hypertens. 2010;24:104–10. doi: 10.1038/jhh.2009.45. [DOI] [PubMed] [Google Scholar]
- 110.Harris LK, Keogh RJ, Wareing M, Baker PN, Cartwright JE, Aplin JD, et al. Invasive trophoblasts stimulate vascular smooth muscle cell apoptosis by a fasligand-dependent mechanism. Am J Pathol. 2006;169:1863–74. doi: 10.2353/ajpath.2006.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cnossen JS, Vollebregt KC, de Vrieze N, ter Riet G, Mol BW, Franx A, et al. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: systematic review and meta-analysis. BMJ. 2008;336:1117–20. doi: 10.1136/bmj.39540.522049.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar M, Gupta U, Bhattacharjee J, Singh R, Singh S, Goel M, et al. Early prediction of hypertension during pregnancy in a low-resource setting. Int J Gynaecol Obstet. 2016;132:159–64. doi: 10.1016/j.ijgo.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 113.Poon LC, Staboulidou I, Maiz N, Plasencia W, Nicolaides KH. Hypertensive disorders in pregnancy: screening by uterine artery Doppler at 11-13 weeks. Ultrasound Obstet Gynecol. 2009;34:142–8. doi: 10.1002/uog.6452. [DOI] [PubMed] [Google Scholar]
- 114.Gallo D, Poon LC, Fernandez M, Wright D, Nicolaides KH. Prediction of preeclampsia by mean arterial pressure at 11-13 and 20-24 weeks' gestation. Fetal Diagn Ther. 2014;36:28–37. doi: 10.1159/000360287. [DOI] [PubMed] [Google Scholar]
- 115.Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–34. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 116.Keogh RJ, Harris LK, Freeman A, Baker PN, Aplin JD, Whitley GS, et al. Fetal-derived trophoblast use the apoptotic cytokine tumor necrosis factor-alpha-related apoptosis-inducing ligand to induce smooth muscle cell death. Circ Res. 2007;100:834–41. doi: 10.1161/01.RES.0000261352.81736.37. [DOI] [PubMed] [Google Scholar]
- 117.Cetin I, Huppertz B, Burton G, Cuckle H, Gonen R, Lapaire O, et al. Pregenesys pre-eclampsia markers consensus meeting: What do we require frommarkers, risk assessment and model systems to tailor preventive strategies? Placenta. 2011;32 (Suppl):S4–16. doi: 10.1016/j.placenta.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 118.Plasencia W, Maiz N, Bonino S, Kaihura C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;30:742–9. doi: 10.1002/uog.5157. [DOI] [PubMed] [Google Scholar]
- 119.Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE, et al. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol. 2014;43:500–7. doi: 10.1002/uog.13275. [DOI] [PubMed] [Google Scholar]
- 120.Harrington K. Early screening for pre-eclampsia and intrauterine growthrestriction. Ultrasound Obstet Gynecol. 2011;37:623–4. doi: 10.1002/uog.9018. [DOI] [PubMed] [Google Scholar]
- 121.Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis ofpre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol. 2015;45:241–6. doi: 10.1002/uog.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio indifferent types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206:58. doi: 10.1016/j.ajog.2011.07.037. e1-8. [DOI] [PubMed] [Google Scholar]
- 123.Benton SJ, Hu Y, Xie F, Kupfer K, Lee SW, Magee LA, et al. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol. 2011;205:469. doi: 10.1016/j.ajog.2011.06.058. e1-8. [DOI] [PubMed] [Google Scholar]
- 124.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risksmodel in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther. 2013;33:8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]