Abstract
E3 ubiquitin ligases are the most expanded components of the ubiquitin proteasome system (UPS). They mediate the recognition of substrates and later transfer the ubiquitin (Ub) of the system. Really Interesting New Gene (RING) finger proteins characterized by the RING domain, which contains 40–60 residues, are thought to be E3 ubiquitin ligase. RING-finger proteins play significant roles in plant growth, stress resistance, and signal transduction. In this study, we mainly describe the structural characteristics, classifications, and subcellular localizations of RING-finger proteins, as well the physiological processes of RING-finger proteins in plant growth and development. We also summarize the functions of plant RING-finger proteins in plant stress resistance. Finally, further research on plant RING-finger proteins is suggested, thereby establishing a strong foundation for the future study of plant RING-finger proteins.
Keywords: RING-finger proteins, adversity stress, plant development
1. Introduction
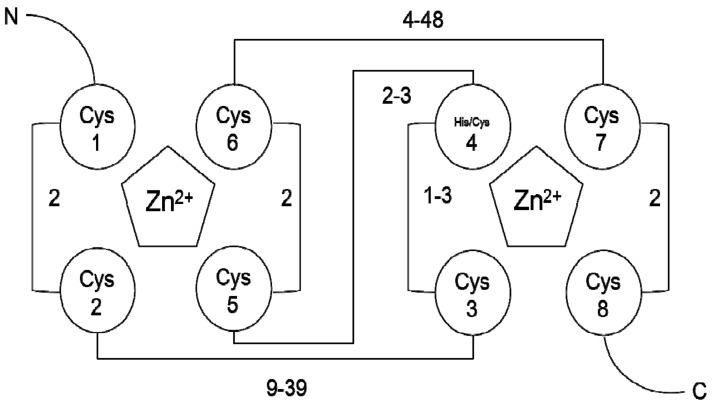
Proteins diversify their functions needed for different modifications. Ubiquitin (Ub) is a protein consisting of 76 amino acids that is known as a post-translational protein modifier in all eukaryotes that affect the fate of the protein. Ub targets cellular proteins via three distinct enzymes: the Ub-activating enzyme E1, the Ub-conjugating enzyme, enzyme E2, and Ub-ligase enzyme E3 in UPS (the ubiquitin proteasome system) [1]. Ub-dependent targets are degraded specifically by the 26S proteasome based on the E3 presence, which specifically recognizes their substrates and catalyzes the isopeptide bond between substrates with Ub. Really Interesting New Gene (RING)-finger proteins, as a large family of E3 types, exist widely in eukaryotes. Previous studies have shown that RING-finger proteins are widely involved in the regulation of various physiological and biochemical processes, including plant growth and development, stress resistance, and hormone signaling responses [2,3,4,5,6]. However, compared with known DNA-binding zinc finger domains, the RING-finger domain acts as a protein–protein interaction domain [2,7] and is necessary to catalyze the E3 ligase activity of RING-finger proteins [8]. RING-finger proteins contain a conserved cysteine-rich finger domain (RING-finger Domain) consisting of 40–60 residues arranged as Cys-X2-Cys-X(9-39)-Cys-X(1-3)-His-X(2-3)-Cys/His-X2-Cys-X(4-48)-Cys-X2-Cys (Figure 1) [9]. It forms eight spatially conserved Cys and His residues as metal ligands (ML) to chelate two zinc atoms and define a cross-brace secondary structure that serves as a platform for binding E2s. X represents any amino acid, but the choice of these amino acids is preferred, which determines the structural and functional diversity of the family members [10]. However, in all of these loop variants, the substituted amino acids can participate in the Zn2+ connection, so the global three-dimensional structure of the domain is conserved [11]. These characteristics facilitate the classification of RING-finger proteins based on their domain architectures [8,9].
Figure 1.
The cross structure between Really Interesting New Gene (RING)-finger protein sequences [9]. The circle represents the cysteine (Cys) and histidine (His) residue; the pentagonal form represents the bound Zn2+; the connecting line represents the minimum and maximum range of the number of linked amino acids; N represents the N-terminus, C represents the C-terminus, Cys1 represents ML, and Cys1 and Cys2 together with Cys5 and Cys6 bind the first Zn2+, whereas Cys3 and Cys4 together with Cys7 and Cys8 bind the second Zn2+.
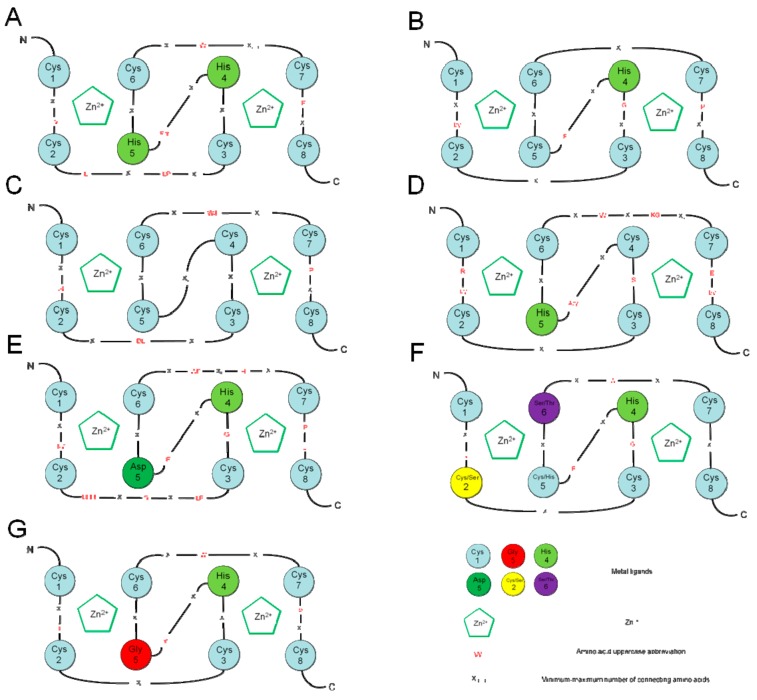
With more extensive research, many RING-finger proteins have been identified in plants. There are 477 RING domains detected from 469 predicted proteins in the whole Arabidopsis thaliana proteome. These members are mainly divided into seven subtypes according to structural differences: RING-H2(241), RING-HC(186), RING-v(25), RING-C2(10), RING-D(10), RING-S/T(4), and RING-G(1). RNIG-H2 and RING-HC are two canonical subclasses of RING-finger domains, accounting for 50% and 39% of RING-finger domains, respectively [9]. However, the latest study suggests that there are 508 RING domains predicted in Arabidopsis thaliana due to the improved annotation of the A. thaliana genome. These are also divided into seven subtypes: RING-H2(258), RING-HC(191), RING-v(26), RING-C2(16), RING-D(7), RING- S/T(3), and RING-G(1) [12]. According to the type of the fifth conserved ML, the ML containing histidine is called RING-H2, and the one containing cysteine is called RING-HC. Other RING-finger types differ mainly in the spacing between the ML or the position of one or more metal ligands (Figure 2). The majority of these RING-finger proteins have been proven to possess E3 activity by ubiquitination essays in vitro, even the RING-finger proteins with substituted zinc binding acid residues or with slightly altered spacing [9,13,14]. A total of 425 RING-finger proteins were identified in whole rice (Oryza sativa) proteome and are divided into four types: RNIG-H2 (281), RING-HC (119), RING-v (23), and RING-C2 (2) [15]. Eighty percent of rice RING-finger proteins have been shown to possess E3 ligase activity [16,17]. At the same time, 731 RING-finger domains in 715 predicted proteins were divided into eight types: RING-H2 (371), RING-HCa (215), RING-HCb (47),RING-v (44), RING-C2 (38), RING-D (10), RING-S/T (5), and RING-G (1) [18]. A total of 756 RING domains in 734 predicted proteins were identified in the whole Brassica oleracea proteome. These domains can be further classified into eight RING types: RING-H2 (355), RING-HCa (215), RING-HCa (47),RING-v (49), RING-C2 (86), RING-D (11), RING-S/T (4), and RING-G (1) [19]. Moreover, 688 RING domains were identified from 663 predicted proteins in the whole apple (Malus domestica) proteome which were further divided into NINE RING types: RING-H2 (367), RING-HC (208), RING-v (35), RING-S/T (11),RING-C2(10), RING-D (1), RING-G (2), RING-mHC (44), and RING-mH2 (10) [20]. A total of 474 RING domains were identified from 469 potential proteins encoded in the Solanum lycopersicum genome, which are further divided into 7 RING types: RING-H2 (248), RING-HCa (142), RING-HCb (21), RING-v (40), RING-C2 (20), RING-S/T (2), and RING-G (1) [21]. In Ostreococcus tauri, only 65 RING domains were identified from 65 predicted proteins and further divided into 8 RING types: RING-H2 (25), RING-HC (28), RING-v (7), RING-C2 (1), C3HCHC2 (1),C2HC5 (1), C3GC3S (1), and C2SHC4 (1) [22].
Figure 2.
The structure diagram of different RING-finger domains. (A) RING-H2; (B), RING-HC; (C) RING-C2; (D), RING-v; (E) RING-D; (F) RING-S/T; (G) RING-G; N represents the N-terminus, C represents the C-terminus; 1–8 represent the ML, the blue circle represents the Cys amino acid residue, the dark green circle represents the Asp amino acid residue, the red circle represents the Gly amino acid residue, the light green circle represents the His amino acid residue, the purple circle represents the Ser/Thr amino acid residue, and and the yellow circle represents the Cys/Ser. The shape pentagons represent the Zn+; the red letter represents the conserved amino acid residue, and X stands for any amino acid.
2. RING-Finger Protein Subcellular Localization
Most of the plant RING-finger proteins are found in the nucleus, cytoplasm, and cell membrane. For example, Arabidopsis RFI2 is located in the nucleus [23], and rice OsCOIN is located in the nucleus and cytoplasm [24]. Meanwhile, maize ZmRFP1 is located on the cell membrane [25] (Table 1). There are also a few proteins located in the endoplasmic reticulum or other parts of the cell. RmaIH1 is located in the endoplasmic reticulum [26], and OsHCI1 is mainly distributed in the vicinity of the cytoskeleton in rice [27] (Table 1). According to recent research, the localization of RING-finger proteins is related to their function to a great extent. RING-finger proteins located in the nucleus are mainly involved in the degradation of transcription factors or other nuclear expression proteins [28,29].
Table 1.
The subcellular localizations of RING-finger proteins.
| Gene Name | Plant Species | Type | Subcellular Localization | References |
|---|---|---|---|---|
| AtAIRP1 | A. thaliana | RING-HC | Cell membrane | Ryu M.Y. et al., 2010 [30] |
| AtAIRP4 | A. thaliana | RING-HC | Cytoplasm | Yang L. et al., 2016 [31] |
| AtATRF1 | A. thaliana | RING-HC | Nucleus | Qin X. et al., 2017 [32] |
| ATL9 | A. thaliana | RING-HC | Endoplasmic network | Beerocal M. et al., 2010 [33] |
| BIG BROTHER | A. thaliana | RING-H2 | Dish S. et al., 2006 [34] | |
| BRH1 | A. thaliana | RING-H2 | Wang X. et al., 2018 [35] | |
| CIP8 | A. thaliana | RING-H2 | Hardtke C.S. et al., 2002 [36] | |
| COP1 | A. thaliana | RING-HC | Nucleus | Deng X.W. et al., 1991, Von Arnim A.G. et al., 1993 [37,38] |
| DRIP1 | A. thaliana | RING-HC | Nucleus | Qin F. et al., 2008 [39] |
| DRIP2 | A. thaliana | RING-HC | Nucleus | Qin F. et al., 2008 [39] |
| EMR | A. thaliana | RING-HC | Cytoplasm | Park J.H. et al., 2018 [40] |
| FLY1 | A. thaliana | RING-H2 | Golgi apparatus | Voiniciuc C. et al., 2013 [41] |
| HOS1 | A. thaliana | RING-C2 | Nucleus | Lee H. et al.,2001, Dong C.H. et al., 2006, Kima J.H. et al., 2017 [42,43] |
| HUB1 | A. thaliana | RING-HC | Fleury D. et al., 2007 [3] | |
| MIEL1 | A. thaliana | RING-H2 | Nucleus | Marino D. et al., 2013 [44] |
| NERF | A. thaliana | RING-HC | Nucleus | Gao W. et al., 2015 [28] |
| RFI2 | A. thaliana | RING-H2 | Nucleus | Chen M.J. et al., 2006 [45] |
| RGLG2 | A. thaliana | RING-HC | Cell membrane | Cheng M.C. et al., 2012 [46] |
| Rma1 | A. thaliana | RING-HC | Endoplasmic network | Lee H.K. et al., 2009 [26] |
| SDIR1 | A. thaliana | RING-H2 | Cell membrane | Zhang Y.Y. et al., 2007 [47] |
| SINAT5 | A. thaliana | RING-HC | Nucleus | Xie Q. et al., 2002 [48] |
| STRF1 | A. thaliana | RING-H2 | Cytoplasm and cell membrane | Tian M.M. et al., 2015 [49] |
| XERICO | A. thaliana | RING-H2 | Ko J.H. et al., 2006 [50] | |
| OsBIRF1 | O. sativa | RING-H2 | Liu H.Z. et al., 2008 [51] | |
| OsCOIN | O. sativa | RING-C2 | Nuclear and cytoplasm | Liu K.M. et al., 2007 [24] |
| OsDIS1 | O. sativa | RING-H2 | Nucleus | Ning Y. et al., 2011 [52] |
| OsDSG1 | O. sativa | RING-H2 | Park G.G. et al., 2010 [53] | |
| OsHAF1 | O. sativa | RING-HC | Yang Y. et al., 2015 [5] | |
| OsHCI1 | O. sativa | RING-HC | Golgi apparatus | Lim S.D. et al., 2013 [27] |
| OsHIRP1 | O. sativa | RING-HC | Nucleus | Kim J.H. et al., 2019 [29] |
| OsHTAS | O. sativa | RING-H2 | Nuclear and cytoplasm | Liu J.P. et al., 2016 [54] |
| OsMAR1 | O. sativa | RING-H2 | Related to the microtubules | Park Y.C. et al., 2018 [55] |
| OsRDCP1 | O. sativa | RING-HC | Cell membrane | Bae H. et al., 2011 [56] |
| OsRZFP34 | O. sativa | RING-HC | Hus K.H. et al., 2014 [57] | |
| OsSADR1 | O. sativa | RING-H2 | Nucleus | Hwang S.G. et al., 2018 [58] |
| OsSIRH2-14 | O. sativa | RING-H2 | Cell membrane, cytoplasm, Golgi | Park Y.C. et al., 2019 [59] |
| OsSIRP1 | O. sativa | RING-HC | Cytoplasm | Hwang S.G. et al., 2016 [60] |
| CaAIRF1 | C. annuum L. | RING-HC | Nucleus | Lim C.W. et al., 2017 [61] |
| CaASRF1 | C. annuum L. | RING-H2 | Nuclear and cytoplasm | Joo H. et al., 2019 [62] |
| CaDSR1 | C. annuum L. | RING-H2 | Nuclear and cytoplasm | Lim C.W. et al., 2018 [63] |
| CaRFP1 | C. annuum L. | RING-HC | Hong J.K. et al., 2007 [13] | |
| CaRZFP1 | C. annuum L. | RING-HC | Zeba N. et al., 2009 [4] | |
| RmaIH1 | C. annuum L. | RING-HC | Endoplasmic network | Lee H.K. et al., 2009 [26] |
| ZmRFP1 | Z. mays L. | RING-H2 | Cell membrane | Xia Z.L. et al., 2012 [25] |
| ZmXerico1 | Z. mays L. | RING-H2 | Cytoplasm | Brugière N. et al., 2017 [64] |
| ZmXerico2 | Z. mays L. | RING-H2 | Cytoplasm | Brugière N. et al., 2017 [64] |
| EIRP1 | V. pseudoreticulata | RING-HC | Nucleus | Yu Y. et al., 2013 [65] |
| VpRH2 | V. pseudoreticulata | RING-H2 | Cytoplasm and cell membrane | Wan G.L. et al., 2017 [66] |
| TaDIS1 | T. aestivuml | RING-HC | Liu Y. et al., 2018 [67] | |
| TaRZF70 | T. aestivuml | RING-H2 | Kam J. et al., 2007 [68] | |
| AdZFP1 | A. dracunculus L. | RING-HC | YanG.X. et al., 2008 [69] | |
| BrRZFP1 | B. rapa | RING-HC | Cytoplasm and cell membrane | Jun Y.J. et al., 2013 [70] |
| GmARI1 | G. max | RING-HC | Nucleus | Zhang X.L. et al., 2014 [71] |
| LjCZF1 | L. japonicus | RING-HC | Cytoplasm and cell membrane | Cai K. et al., 2018 [72] |
| MsRH2-1 | M. sativa | RING-H2 | Karlowski W.M. et al., 2003 [73] | |
| MeRZF | M. esculenta | RING-H2 | Cell membrane | Reis S.P.D. et al., 2012 [74] |
| NbZFP1 | N. benthamiana | RING-HC | Chloroplast | Wu W.X. et al., 2014 [75] |
| NtRHF1 | N. tabacum | RING-H2 | Xia Z.L. et al., 2012 [76] | |
| SpRing | S. lycopersicum | RING-H2 | Endoplasmic network | Qi S.L. et al., 2016 [77] |
However, those located in the biofilm system mainly regulates the degradation and transportation of intracellular proteins, including signal transduction components, which may be controlled by ubiquitination [30,41]. Interestingly, some RING-finger proteins can enter the nucleus from the plasma membrane to participate in the regulation of nuclear transcription factors, such as the Arabidopsis RGLG2 transport from the plasma membrane to the nucleus under drought stress to participate in the degradation of ERF53 [46].
3. RING-Finger Protein Functions
The RING-finger domains may act as a substrate binding domain [2,7], which is essential for catalyzing the E3 ligase activity of RING-finger proteins [8]. In plants, a certain number of RING-finger proteins act as E3 ubiquitin ligase. They mainly direct target proteins or interact with other proteins to participate in the gene’s expression level to regulate its various physiological processes [37].
3.1. RING-Finger Proteins Are Involved in Plant Growth and Development
Currently, there are few studies on RING-finger proteins involved in plant growth and development. Mainly, these studies concentrate on the role of E3 ligase in the photoperiod, leaf, and root development (Table 2).
Table 2.
RING-finger proteins involved in plant growth and development.
| Gene Name | AGI Loci | Protein | Function | References |
|---|---|---|---|---|
| BIG BROTHER | AT3g63530 | E3 ligase | regulator of Arabidopsis floral organ size | Dish S. et al., 2006 [34] |
| BRH1 | AT3g61460 | E3 ligase | alters rosette leaf shape | Wang X. et al., 2018 [35] |
| CIP8 | AT5g64920 | E3 ligase | regulator of photomorphogenesis | Hardtke C.S. et al., 2002 [36] |
| COP1 | AT2g32950 | E3 ligase | regulator of photomorphogenesis | Von Arnim A.G. et al., 1993 [38] |
| CaRZFP1 | regulator of root development | Zeba N. et al., 2009 [13] | ||
| EMR | AT4g26400 | E3 ligase | involved in the degradation of ER-associated protein | Park J.H. et al., 2018 [40] |
| FLY1 | AT4g28370 | E3 ligase | regulates the degree of pectin methylesterification in seed mucilage | Voiniciuc C. et al., 2013 [41] |
| HOS1 | AT2g39810 | E3 ligase | regulator of photomorphogenesis and flowering time | Lee H. et al. 2001, Kima J. Het al., 2017 [42,43] |
| HUB1 | AT2g44950 | E3 ligase | regulator of root development | Fleury D. et al., 2007 [3] |
| LjCZF1 | E3 ligase | a positive regulator of symbiotic nodulation | Cai K. et al., 2018 [72] | |
| MsRH2-1 | regulator of root and nodule development | Karlowski W.M. et al., 2003 [73] | ||
| NbZFP1 | regulator of fruit development, plant height, and leaf spacing | Wu W.X. et al., 2014 [75] | ||
| OsHAF1 | E3 ligase | regulator of photomorphogenesis | Park G.G. et al., 2010 [5] | |
| RFI2 | AT2g47700 | E3 ligase | regulator of flowering time | Chen M.J. et al., 2006 [45] |
| SINAT5 | AF480944 | E3 ligase | regulator of lateral root development | Xie Q. et al., 2002 [48] |
Photomorphogenesis is critical to plant flowering. Arabidopsis constitutively photomorhogenic (COP1) is a negative regulator of photomorphogenesis. It directly targets the bzip transcription factor hy 5 (HY5), a positive regulator of photomorphogenesis, for degradation via the proteasome pathway in the dark [27,38]. COP1 and its interactive partner COP1 interacting protein 8 (CIP8) both possess the RING-finger domain and activity of E3 ubiquitin ligase. CIP8 may be associated with the activation of nuclear localization signals of COP1, thereby affecting the localization of COP1 in dark conditions. Moreover, CIP8 has an ubiquitin ligase function in cooperation with an E2 enzyme, AtUBC8-CIP8. It is suggested that the AtUBC8-CIP8 module can degrade HY5 in the proteasome by direct interaction with COP1 [36].
The photoperiod phenomenon is an important factor for affecting flower formation, which is the core process of plant growth and development. Red and far-red insensitive 2 (RFI2) is a RING-finger protein that participates in the photoperiod flowering pathway. The rfi-2 promotes the expression of CONSTANS (CO), a central activator of photoperiodic flowering, and FLOWERING LOCUS T (FT) under long-day conditions, leading to an early flower phenotype. Moreover, under red and far-red light, the phenotype exhibits hypocotyl elongation [45]. However, Arabidopsis, a RING-finger protein with a high expression of osmotically responsive gene 1 (HOS1) (an E3 ubiquitin ligase), under intermittent cold stress treatment, leads to the degradation of CO via the ubiquitination pathway. A decrease of the CO protein leads to a delay in flowering. It was also found that HOS1 controls the transcriptional activity of phytochrome interacting factor 4 (PIF4) to participate in phyB-mediated signal light morphogenesis [42]. Another RING-finger protein heading date associated factor 1 (OsHAF1) in rice also participates in the photoperiod response process by the ubiquitination degradation of heading date 1 (HD1) [5].
RING-finger proteins are also involved in the growth and development of roots. The RING-finger drosophila protein sina (SINAT5), histone monoubiquitination1 (HUB1) in Arabidopsis thaliana, Medicago sativa RING-H2 zinc finger protein (MsRH2-1) in M. sativa and Capsicum annuum ring zinc finger protein 1 (CaRZFP1) in pepper are involved in plant root development. Overexpression of the SINAT5 gene in Arabidopsis showed fewer lateral roots, while the lateral roots of the sinat5 mutant plants increased. These lateral root phenotypes correlate with SINAT5, which can degrade the NAC domain containing protein 1 (NAC1) via the ubiquitin pathway [48]. The MsRH2-1 gene is closely related to the development of lateral roots and nodules, with the highest level of transcription in roots and nodules of M. sativa. The MsRH2-1 overexpression line shows inhibited development of lateral roots and suggests that MsRH2-1 may function as an E3 ligase and perform a function of the E3 ligase for substrate-specific degradation via the ubiquitin-proteasome system involved in auxin signaling [73]. Overexpression of the CaRZFP1 gene in tobacco revealed a phenotype with a larger primary root and more lateral roots in transgenic lines. CaRZFP1 was mainly related to the up-regulation of Root-hair-specific Cell Wall Proline-rich Protein (PRP) root hairs and lateral roots in overexpressing lines [4]. An lhk1-interacting protein (LjCZF1) from Lotus japonicus is a positive regulator of symbiotic nodulation, possibly through interaction with LHK1 (Lotus Histidine Kinase 1), which is essential for nodule formation [72]. However, whether CaRZFP1 and LjCZF1 are E3 ligase still needs to be verified. A mutant of the HUB1 gene in Arabidopsis exhibited slower growth of primary roots. HUB1 as a RING E3 ligase regulates the growth rate of plant roots [3].
The RING-finger protein is also involved in leaf and height development. Overexpression of the MsRH2-1 gene in alfalfa can degenerate leaves and inhibit leaf vein formation. However, overexpression of this gene in Arabidopsis leads to the discovery of rosettes [73]. Overexpression of the CaRZFP1 gene in tobacco leads to fast growth, size, an increased number of leaves, and heavier fresh vegetation [4]. When the brassinosteroid-responsive RING-H2 (BRH1) gene in Arabidopsis thaliana is overexpressed, the rosette leaves of the transgenic lines were extremely curled, suggesting that it may be involved in the Brassinosteroid (BR) signaling pathway to regulate the shape of the leaves [35]. However another RING finger protein, (the endoplasmic reticulum-associated degradation) ERAD-mediating RING finger protein (EMR), an E3 ligase, also affects the synthesis of BR signaling proteins on the endoplasmic reticulum to regulate plant height [40]. Silencing of the Nicotiana benthamiana zinc finger (NbZFP1) hampered fruit development. Compared to the WT (wild type), overexpression of NbZFP1 displayed a short internode length and a sturdy stem phenotype [75].
RING-finger proteins are also involved in fruit development. Flying saucer1 (FLY1) and big brother (BB) are used as E3 ligases for floral organs of the seed pectins and control the degree of methyl esterification. However, the regulation mechanisms are not clear for these two proteins [34,41]. It is believed that with continuous research, the mechanism of the RING-finger protein involved in the growth and development process will be more clearly revealed.
3.2. RING-Finger Proteins Are Involved in Plant Stress Resistance
Stress is an important limiting factor affecting plant growth and crop production. Over the long process of evolution, plants have produced many different biotic/abiotic stress responses and regulation methods. In recent years, many studies have shown that RING finger proteins are involved in responding to biotic and abiotic stress in plants (Table 3).
Table 3.
RING-finger proteins involved in plant stress resistance.
| Gene Name | AGI Loci | Protein | Function | References |
|---|---|---|---|---|
| AdZFP1 | regulator of plant tolerance to drought stress | Yang X. et al., 2008 [69] | ||
| AtAIRP1 | AT4G23450 | E3 ligase | regulator of plant tolerance to drought stress | Ryu M.Y. et al., 2010 [30] |
| AtAIRP4 | AT5G58787 | E3 ligase | regulator of plant tolerance to drought stress | Yang L. et al., 2016 [31] |
| AtATRF1 | E3 ligase | regulator of plant tolerance to drought stress | Qin X. et al., 2017 [32] | |
| ATL9 | AT2g35000 | E3 ligase | regulator of plant resistance to viable nutrient pathogens | Beerocaccobo M. et al., 2010 [33] |
| BrRZFP1 | regulator of plant tolerance to drought and cold stress | Jung Y.J. et al., 2013 [70] | ||
| CaAIRF1 | E3 ligase | regulator of plant tolerance to drought stress | Lim C.W. et al., 2017 [61] | |
| CaASRF1 | E3 ligase | regulator of plant tolerance to drought stress | Joo H. et al., 2019 [62] | |
| CaDSR1 | E3 ligase | regulator of plant tolerance to drought stress | Lim C.W. et al., 2018 [63] | |
| CaRFP1 | E3 ligase | regulator of plant tolerance to salt stress | Hong J.K. et al., 2007 [13] | |
| DRIP1 | E3 ligase | regulator of plant tolerance to drought stress | Qin F. et al., 2008 [39] | |
| DRIP2 | E3 ligase | regulator of plant tolerance to drought stress | Qin F. et al., 2008 [39] | |
| EIRP1 | E3 ligase | involved in pathogen defense | Yu Y. et al., 2013 [65] | |
| GmARI1 | E3 ligase | regulator of plant tolerance to Aluminium stress | Zhang X.L. et al., 2014 [73] | |
| HOS1 | AT2G39810 | E3 ligase | regulator of plant tolerance to cold stress | Dong C.H. et al., 2006 [78] |
| MeRZF | regulator of plant tolerance to salt stress | Reis S.P.D. et al., 2012 [74] | ||
| MIEL1 | AT5G18650 | E3 ligase | regulator of plant tolerance to biotic stress | Marino D. et al., 2013 [44] |
| NbZFP1 | regulator of plant tolerance to tobacco mosaic virus | Wu W.X. et al., 2014 [75] | ||
| NERF | E3 ligase | regulator of plant tolerance to drought stress | Gao W. et al., 2015 [28] | |
| NtRHF1 | E3 ligase | regulator of plant tolerance to drought stress | Xia Z.L. et al., 2012 [76] | |
| OsBIRF1 | regulator of plant tolerance to drought stress | Liu H.Z. et al., 2008 [51] | ||
| OsCOIN | regulator of plant tolerance to cold stress | Liu K.M. et al., 2007 [24] | ||
| OsDSG1 | E3 ligase | regulator of plant tolerance to drought stress | Liu K.M. et al., 2007 [53] | |
| OsHCI1 | E3 ligase | regulator of plant tolerance to high temperature stress | Lim S.D. et al., 2013 [27] | |
| OsHIRP1 | E3 ligase | regulator of plant tolerance to high temperature stress | Kim J.H. et al., 2019 [29] | |
| OsHTAS | E3 ligase | regulator of plant tolerance to high temperature stress | Liu J.P. et al., 2016 [54] | |
| OsMAR1 | E3 ligase | regulator of plant tolerance to salt stress | Park Y.C. et al., 2018 [55] | |
| OsRDCP1 | E3 ligase | regulator of plant tolerance to drought stress | Bae H. et al., 2011 [56] | |
| OsRZFP34 | regulator of plant tolerance to high temperature stress | Hus K.H. et al., 2014 [57] | ||
| OsSADR1 | E3 ligase | regulator of plant tolerance to drought stress | Hwang S.G. et al., 2018 [58] | |
| OsSIRH2-14 | E3 ligase | regulator of plant tolerance to salt stress | Park Y.C. et al., 2019 [59] | |
| OsSIRP1 | E3 ligase | regulator of plant tolerance to salt stress | Hwang S.G. et al., 2016 [60] | |
| RGLG2 | AT5G14420 | E3 ligase | regulator of plant tolerance to drought stress | Cheng M.C. et al., 2012 [46] |
| Rma1 | AT4G03510 | E3 ligase | regulator of plant tolerance to drought stress | Lee H.K. et al., 2009 [26] |
| RmaIH1 | E3 ligase | regulator of plant tolerance to drought stress | Lee H.K. et al., 2009 [26] | |
| SDIR1 | AT3G55530 | E3 ligase | regulator of plant tolerance to salt stress | Lee H.K. et al., 2009 [47] |
| SpRing | regulator of plant tolerance to salt stress | Qi S.L. et al., 2016 [77] | ||
| STRF1 | E3 ligase | regulator of plant tolerance to salt stress | Tian M.M. et al., 2015 [49] | |
| TaDIS1 | regulator of plant tolerance to drought stress | Liu Y. et al., 2018 [67] | ||
| TaRZF70 | regulator of plant tolerance to drought stress | Kam J. et al., 2007 [68] | ||
| VpRH2 | KU296022 | improves resistance to powdery mildew fungus | Wang L. et al., 2017 [66] | |
| XERICO | AT2G04240 | E3 ligase | regulator of plant tolerance to drought stress | Ko J.H. et al., 2006 [50] |
| ZmRFP1 | E3 ligase | regulator of plant tolerance to drought stress | Xia Z.L. et al., 2012 [25] | |
| ZmXerico1 | E3 ligase | regulator of plant tolerance to drought stress | Brugière N. et al., 2017 [64] | |
| ZmXerico2 | E3 ligase | regulator of plant tolerance to drought stress | Brugière N. et al., 2017 [64] |
3.2.1. RING-Finger Proteins Are Involved in Plant Drought Resistance
Drought is a major abiotic stress factor affecting plant survival. It is necessary to analyze drought resistance genes and analyze their drought resistance mechanisms. Recently, it has been reported that several RING-containing proteins function as E3 ligases in response to the Abscisic Acid (ABA) dependent defense mechanism against drought stress. A study found that XERICO in Arabidopsis, and its homologous genes ZmXerico1/2 in Zea mays, are overexpressed in Arabidopsis and improve resistance to drought. However, XERICO raises the biosynthesis of ABA by degrading the ASK1-interacting F-box protein (AtTLP9) in the proteasome system, while ZmXerico1/2 makes the ABA more stable via the ubiquitin of ABA 8′-hydroxylases to improve drought resistance [50,64]. In the rice osdsg1 mutant line and Delayed Seed Germination 1 (OsDSG1)-RNAi plants, the expression level of ABA signaling-related genes was significantly increased and lead to greater resistance to drought than the wild-type. It was estimated that OsDSG1 degrades aba insensitive 3 (OsAIB3) via the 26S proteasome system and negatively regulates plant drought resistance by participating in the ABA-pathway [53]. The Capsicum annuum ADIP1 interacting ring finger protein 1 (CaAIRF1) degrades Capsicum annuum Type 2C Protein Phosphatase (CaADP1) through the ubiquitination pathway by changing the sensitivity to ABA to improve drought resistance [61]. The Capsicum annuum drought sensitive RING finger protein 1(CaDSR1) exhibited E3 ligase activity and promoted CaDILZ1 expression through the 26S proteasome pathway to alter ABA content in the modulation of drought tolerance [63]. Capsicum annuum ABA sensitive RING finger E3 ligase 1 (CaASRF1) positively modulates ABA signaling via modulation of CaAIBZ1’s stability to drought stress [62]. Many RING genes in different species are induced by ABA to respond to drought stress, such as Arabidopsis ABA-insensitive RING protein 1 (AtAIRP1), AtAIRP4, Nicotiana tabacum RING-H2 Finger Gene 1 (NtRHF1), OsBIRF1, TaRZF70, and AdZFP1 [30,31,51,68,69,76]. Furthermore, AtAIRP1 and NtRHF1 have been proven to be E3 ligases. However, OsBIRF1, TaRZF70, and AdZFP1 have not been proven to be E3 ligases. Some RING-finger protein independent ABA-pathways also respond to drought stress responses. DREB2A-interacting protein1 (DRIP1) and DRIP2, isolated from Arabidopsis thaliana, acts as a negative regulator in drought-responsive gene expression by targeting dehydration-responsive element binding protein 2 (ADREB2A) to facilitate 26S proteasome proteolysis [39]. NUCLEAR FACTORY A 5 (NFY5), a key drought-induced transcription factor, can be degraded by NFYA5 enhancing RING finger (NERF) in the proteasome pathway, which is important for controlling stomatal closure and drought resistance in Arabidopsis thaliana [28]. RING membrane-anchor 1 (Rma1) in Arabidopsis thaliana, and its homologous Rma1H1 in pepper, can mediate the ubiquitination of plasma membrane aquaporin (PIP2), which positively regulates plant drought resistance [26]. RING domain ligase 2 (RGLG2) negatively regulates drought stress response via the ubiquitin ethylene response factor 53 (AtERF53) in Arabidopsis [46]. The Oryza sativa RING domain-containing protein OsRDCP1, as a RING E3 ligase, may be involved in the transportation or degradation of a negative transcription factor or factors that inhibit(s) the expression of water stress-induced genes [56]. The Oryza sativa drought-induced SINA protein OsDIS1, via the 26S proteasome-dependent pathway, degrades Oryza sativa NIMA-related kinase 6 (OsNek6), plaing a negative role in drought stress tolerance. The orthologue protein in wheat (Triticum aestivum L.) TaDIS1 may perform a negatively function in drought stress by regulating the stress response-related genes [52,67].
3.2.2. RING-Finger Proteins Are Involved in Salt and Aluminium Resistance
Some RING-finger proteins are also involved in salt stress. The salt and drought-induced ring finger 1 (SDIR1) in Arabidopsis thaliana and its homologous protein ZmRFP1 are found to participate in the regulation of drought’s stress response. However, SDIR1 also acts as an E3 ligase to ubiquitinate the modification of SDAIR-interacting protein 1 (SDIR1P1) to regulate the expression of transcription factor ABA-INSENSITIVE5 (ABI5), a key ABA-pathway gene, thereby participating in the salt response process of Arabidopsis thaliana [47]. Oryza sativa salt, ABA, and drought stress-induced RING finger protein 1 (OsSADR1) act as E3 ligases and function negatively in drought and salt stress [58]. CaRFP1 in pepper is an E3 ligase and directly targets the basic PR-1 protein (CaBPR1) to ubiquitinate the modification involved in the signaling pathway of ABA in response to salt stress [13]. Salt tolerance RING finger 1 (STRF1) from Arabidopsis and Oryza sativa salt-induced RING finger protein 1 (OsSIRP1), as E3 ligases, participate in the response of salt stress. STRF1 mainly regulates the expression of membrane transport-related proteins. OsSIRP1 is a negative regulator of salt tolerance, and its target protein needs to be further studied [49,60]. The microtubule-associated RING finger protein 1 (OsMAR1), an E3 ligase, acts as a negative regulator for salt-stress response through the regulation of the O. sativa chymotrypsin protease inhibitor 2 (OsCPI2), but anther rice RING H2-type E3 ligase, OsSIRH2-14 (previously named OsRFPH2-14), plays a positive role in salinity tolerance by regulating salt-related proteins, including an HKT-type Na+ transporter (OsHKT2; 1) [55,59]. The RING-finger proteins M. esculenta RZF (MeRZF) and SpRing were found to respond to salt stress in cassava and wild tomato, respectively. However, the mechanism by which they play a role remains to be further studied [74,77].
Aluminum (Al) toxicity is a major limiting factor in the production of acid soil crops. In recent studies, a series of E3 ubiquitin ligases have been discovered to regulate plant Al tolerance or resistance. Arabidopsis thaliana Al tolerance RING finger 1 (AtATRF1), acting as an E3 ligase, mediates the aluminum tolerance of Arabidopsis thaliana. Studies have shown that plants overexpressing AtATRF1 enhance tolerance to Al, while Al can induce the expression of AtATRF1. AtATRF1 is located in the nucleus and may interact and ubiquitinate Ataxia telangiectasia-mutated and RAD3-like Protein (AtATR), a transcriptional regulator, which plays an important role in plant growth and development [32]. Soybean ariadne-like ubiquitin ligase protein (GmARI1) functions as an E3 ligase and might mediate soybean responses to the tolerance of Al stress through oxidative species signals, which may overlap with plant hormone signaling pathways [71].
3.2.3. RING-Finger Proteins Are Involved in Temperature Stress
Low-temperature or high-temperature stress affect the normal life metabolism of plants, thereby affecting their growth and development. Recently, there have been many reports about RING-finger proteins in response to low-temperature stress. The RING-finger protein HOS1 in Arabidopsis thaliana acts as a negative regulator of low-temperature response gene transcription and can function as an E3 ubiquitin ligase. Under low temperature conditions, HOS1 can degrade an inducer of cbf expression 1 (ICE1) through the 26S protease pathway, while overexpression of HOS1 makes plants sensitive to low temperatures [43,78]. Oryza sativa cold-inducible (OsCOIN) could respond to low-temperature stress, relying on the ABA pathway [24]. Furthermore, the expression of cold stress-related genes, such as OsLti6b and OsP5CS, could be induced in the overexpression line of OsCOIN, making it useful for enhancing tolerance to low temperatures [24]. The expression of the BrRZFP gene in Brassica rapa can be induced by low temperatures; the ABA, drought, and salt stress resistance of tobacco plants heterologously expressing this gene are also enhanced [70]. However, OsCOIN and BrRZFP have not been shown to function as E3 ligases. There are few reports on the response of RING finger proteins, which are mainly concentrated in rice, to high temperature stress. Oryza sativa heat and cold induced 1(OsHCI1) and Oryza sativa heat-induced RING finger protein 1(OsHIRP1) both act as E3 ligases to positively regulate heat stress responses [27,29]. The rice OsRZFP34 gene and HEAT TOLERANCE AT SEEDLING STAGE (OsHTAS) gene can participate in the ABA pathway, change the stomatal switch state in leaves, and improve high-temperature tolerance. However, only OsHTAS was proven to be an E3 ligase [54,57].
3.2.4. RING-Finger Proteins Are Involved in Biotic Stress
Biotic stress is general term for various biological factors that are unfavorable to survival and development, including pests, fungi, bacteria, and viruses. Recent studies have shown that RING-finger proteins are involved in biotic stress responses in many species. The RING-finger protein MYB30-interacting E3 ligase 1 (MIEL1) in Arabidopsis thaliana acts as an E3 ligase ubiquitinating transcription factor Myb domain protein 30 (MYB30) and degrades transgenic MYB30, thereby reducing the expression of disease-resistant genes and reducing the plant’s immune response [44]. Arabidopsis toxicos en levadura 9 (ATL9) is also involved as an E3 ligase in regulating plant resistance to viable nutrient pathogens. When a pathogen infects a plant, it can induce the expression of ATL9 and ATL9 to resist the inhibitory protein of the pathogen by ubiquitination hydrolysis, thereby promoting an immune response [33]. When the CaRFP1 gene was overexpressed in Arabidopsis thaliana, the transgenic plants became more sensitive to tomato bacterial spot disease. This may be due to the fact that the protein acts as an E3 ligase, degrading the expression levels of disease-related genes such as (synthesis of pathogenesis-related) PR-2 and PR-5 [13]. The Erysiphe necator-induced RING finger protein 1 (EIRP) is also involved in the pathogen defense response in Vitis pseudoreticulata by degrading the transcription factor VpWRKY11 through the ubiquitin proteasomal system, which enhances the ability of East China Grapes to resist pathogens [65]. Another RING finger protein in Vitis pseudoreticulata VpRH2, as an E3 ligase, improves resistance to powdery mildew by interacting with VpGRP2A [66]. Heterologous expression of the OsBIRF1 gene and overexpression of the NbZFP1 gene in tobacco could enhance resistance to the tobacco mosaic virus, and these two RING-finger proteins may enhance disease resistance by regulating the expression of PR genes [75].
4. Conclusions
The growth and development of plants and their ability to adapt to a variety of stresses are mainly realized by changing their protein expression and metabolic pathways. It is of great significance to study the expression and function of these proteins in order to improve plant growth and tolerance to stress [79,80,81]. Protein ubiquitination is one of the most important modifications after protein translation in plants, and the ubiquitin ligase E3 determines the specific selection of substrate proteins. Our study demonstrates that the plant RING-finger family of E3 ligases is quite diverse. In addition to the previously defined types of RING-finger domains, the types we identified include modified RING-finger domains that display variation in spacing between, or have amino acids substitutions at, conserved zinc-coordinating residues. Therefore, searches for RING-finger proteins should not be limited to known types. However, a greater characterization of plant RING-finger domain types is needed to further define the requirements for functional RING-finger proteins. The presence of various types of RING-finger domains are involved in the ubiquitination pathway, and each type of RING-finger domain may correspond to multiple E2 enzymes. Further biochemical analyses utilizing different families of plant E2s to define functional E2–E3 combinations would give insight into the specific requirements for E2–E3 interactions. The number of RING-finger proteins, the different types of RING-finger domains, and the presence of a variety of protein–protein interaction domains in the RING-finger proteins suggest a role for the RING-type E3 ligase in different cellular processes via the targeted regulation of numerous substrates. At present, RING-finger protein research is mainly focused on plant growth and development, as well as related studies. These RING-fingers are mainly used as ubiquitin ligase E3 to degrade other proteins through the 26 proteasomes. Most of them are involved in the ABA pathway and participate in anti-stress, such as XERICO and CaDSR1 [50,63]. However, it remains to be determined how E3 proteins are interacted with substrate proteins, whether they recognize substrate proteins with the same characteristics, and whether E3 ubiquitin ligase can modify substrate proteins by ubiquitin or poly-ubiquitin. In addition, current research is mainly concentrated in Arabidopsis and rice, while little is known about other species, such as wheat and corn. The function of a large number of RING-finger proteins still needs to be discovered and studied in different species. With the continuous development of genome sequencing technology, more plant RING-finger proteins will be identified, which will be comprehensively studied by bioinformatics analysis, functional genomics, transcriptomics, proteomics, and metabolomics. This will greatly facilitate the study of the function and mechanism of RING-fingers and accelerate the process of genetic engineering to create excellent new germplasms.
Author Contributions
M.X. and J.S. conceived the review. M.X. and J.S. wrote the manuscript. R.I.A., Y.S., and A.R. edited the manuscript. J.S. produced the figures. All authors read and approved the manuscript.
Funding
This work was supported by the Science Foundation for Young Scholars of the Tobacco Research Institute of the Chinese Academy of Agricultural Sciences (2018B02).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Glickman M.H., Ciechanover A. The ubiquitin-proteasome proteolytic pathway: Destruction for the sake of construction. Physiol. Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 2.Borden K.L. RING domains: Master builders of molecular scaffolds? J. Mol. Biol. 2000;295:1103–1112. doi: 10.1006/jmbi.1999.3429. [DOI] [PubMed] [Google Scholar]
- 3.Fleury D., Himanen K., Cnops G., Nelissen H., Boccardi T.M., Maere S., Beemster G.T., Neyt P., Anami S., Robles P., et al. The Arabidopsis thaliana homolog of yeast BRE1 has a function in cell cycle regulation during early leaf and root growth. Plant Cell. 2007;19:417–432. doi: 10.1105/tpc.106.041319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeba N., Isbat M., Kwon N.J., Lee M.O., Kim S.R., Hong C.B. Heat-inducible C3HC4 type RING zinc finger protein gene from Capsicum annuum enhances growth of transgenic tobacco. Planta. 2009;229:861–871. doi: 10.1007/s00425-008-0884-0. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Fu D., Zhu C., He Y., Zhang H., Liu T., Li X., Wu C. The RING-Finger Ubiquitin Ligase HAF1 Mediates Heading date 1 Degradation during Photoperiodic Flowering in Rice. Plant Cell. 2015;27:2455–2468. doi: 10.1105/tpc.15.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W.T., He M., Wang J., Wang Y.P. Zinc finger protein (ZFP) in plants-A review. Plant Omics. 2013;6:474–480. doi: 10.3835/plantgenome2013.05.0018. [DOI] [Google Scholar]
- 7.Kosarev P., Mayer K.F., Hardtke C.S. Evaluation and classification of RING-finger domains encoded by the Arabidopsis genome. Genome Biol. 2002;3:0016.1–0016.12. doi: 10.1186/gb-2002-3-4-research0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metzger M.B., Pruneda J.N., Klevit R.E., Weissman A.M. RING-type E3 ligases: Master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim. Biophys. Acta. 2014;1843:47–60. doi: 10.1016/j.bbamcr.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone S.L., Hauksdottir H., Troy A., Herschleb J., Kraft E., Callis J. Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol. 2005;137:13–30. doi: 10.1104/pp.104.052423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freemont P.S., Hanson I.M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991;64:483–484. doi: 10.1016/0092-8674(91)90229-R. [DOI] [PubMed] [Google Scholar]
- 11.Borden K.L., Freemont P.S. The RING finger domain: A recent example of a sequence-structure family. Curr. Opin. Struct. Biol. 1996;6:395–401. doi: 10.1016/S0959-440X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Lopez D., Munoz-Belman F., Gonzalez-Prieto J.M., Aguilar-Hernandez V., Guzman P. Repertoire of plant ring e3 ubiquitin ligases revisited: New groups counting gene families and single genes. PLoS ONE. 2018;13:e0203442. doi: 10.1371/journal.pone.0203442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong J.K., Choi H.W., Hwang I.S., Hwang B.K. Role of a novel pathogen-induced pepper C3-H-C4 type RING-finger protein gene, CaRFPI, in disease susceptibility and osmotic stress tolerance. Plant Mol. Biol. 2007;63:571–588. doi: 10.1007/s11103-006-9110-2. [DOI] [PubMed] [Google Scholar]
- 14.Guzman P. ATLs and BTLs, plant-specific and general eukaryotic structurally-related E3 ubiquitin ligases. Plant Sci. Int. J. Exp. Plant Biol. 2014;215:69–75. doi: 10.1016/j.plantsci.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Lim S.D., Yim W.C., Moon J.C., Kim D.S., Lee B.M., Jang C.S. A gene family encoding RING finger proteins in rice: Their expansion, expression diversity, and co-expressed genes. Plant Mol. Biol. 2010;72:369–380. doi: 10.1007/s11103-009-9576-9. [DOI] [PubMed] [Google Scholar]
- 16.Lim S.D., Hwang J.G., Jung C.G., Hwang S.G., Moon J.C., Jang C.S. Comprehensive analysis of the rice RING E3 ligase family reveals their functional diversity in response to abiotic stress. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes. 2013;20:299–314. doi: 10.1093/dnares/dst011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q.G., Yang J.L., Wang Z.C., Xu X.M., Mao X.L., Li D.D., Hu X.Q., Jin D.C., Li C.H. Genome-wide Classification, Identification and Expression Profile of the C3HC4-type RING Finger Gene Family in Poplar (Populus trichocarpa) Plant Mol. Biol. Rep. 2015;33:1740–1754. doi: 10.1007/s11105-015-0870-1. [DOI] [Google Scholar]
- 18.Alam I., Yang Y.Q., Wang Y., Zhu M.L., Wang H.B., Chalhoub B., Lu Y.H. Genome-wide identification, evolution and expression analysis of RING finger protein genes in Brassica rapa. Sci. Rep. 2017:7. doi: 10.1038/srep40690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y.Q., Lu Y.H. Genome-wide survey, characterization, and expression analysis of RING finger protein genes in Brassica oleracea and their syntenic comparison to Brassica rapa and Arabidopsis thaliana. Genome. 2018;61:685–697. doi: 10.1139/gen-2018-0046. [DOI] [PubMed] [Google Scholar]
- 20.Li Y., Wu B., Yu Y., Yang G., Wu C., Zheng C. Genome-wide analysis of the RING finger gene family in apple. Mol. Genet. Genom. MGG. 2011;286:81–94. doi: 10.1007/s00438-011-0625-0. [DOI] [PubMed] [Google Scholar]
- 21.Yang L., Miao M., Lyu H., Cao X., Li J., Li Y., Li Z., Chang W. Genome wide indentification, evolution and expression analysis of RING-finger gene family in Solanum lycopersicum. Int. J. Mol. Sci. 2019;30:4864. doi: 10.3390/ijms20194864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y., Li M.Y., Zhao J., Zhang Y.C., Xie Q.J., Chen D.H. Genome-wide analysis of RING finger proteins in the smallest free-living photosynthetic eukaryote Ostreococus tauri. Mar. Genom. 2016;26:51–61. doi: 10.1016/j.margen.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Chen M., Ni M. RED AND FAR-RED INSENSITIVE 2, a RING-domain zinc finger protein, mediates phytochrome-controlled seedling deetiolation responses. Plant Physiol. 2006;140:457–465. doi: 10.1104/pp.105.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu K., Wang L., Xu Y., Chen N., Ma Q., Li F., Chong K. Overexpression of OsCOIN, a putative cold inducible zinc finger protein, increased tolerance to chilling, salt and drought, and enhanced proline level in rice. Planta. 2007;226:1007–1016. doi: 10.1007/s00425-007-0548-5. [DOI] [PubMed] [Google Scholar]
- 25.Xia Z., Liu Q., Wu J., Ding J. ZmRFP1, the putative ortholog of SDIR1, encodes a RING-H2 E3 ubiquitin ligase and responds to drought stress in an ABA-dependent manner in maize. Gene. 2012;495:146–153. doi: 10.1016/j.gene.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Lee H.K., Cho S.K., Son O., Xu Z., Hwang I., Kim W.T. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell. 2009;21:622–641. doi: 10.1105/tpc.108.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim S.D., Cho H.Y., Park Y.C., Ham D.J., Lee J.K., Jang C.S. The rice RING finger E3 ligase, OsHCI1, drives nuclear export of multiple substrate proteins and its heterogeneous overexpression enhances acquired thermotolerance. J. Exp. Bot. 2013;64:2899–2914. doi: 10.1093/jxb/ert143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao W., Liu W., Zhao M., Li W.X. NERF encodes a RING E3 ligase important for drought resistance and enhances the expression of its antisense gene NFYA5 in Arabidopsis. Nucleic Acids Res. 2015;43:607–617. doi: 10.1093/nar/gku1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.H., Lim S.D., Jang C.S. Oryza sativa heat-induced RING finger protein 1 (OsHIRP1) positively regulates plant response to heat stress. Plant Mol. Biol. 2019;99:545–559. doi: 10.1007/s11103-019-00835-9. [DOI] [PubMed] [Google Scholar]
- 30.Ryu M.Y., Cho S.K., Kim W.T. The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiol. 2010;154:1983–1997. doi: 10.1104/pp.110.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L., Liu Q., Liu Z., Yang H., Wang J., Li X., Yang Y. Arabidopsis C3HC4-RING finger E3 ubiquitin ligase AtAIRP4 positively regulates stress-responsive abscisic acid signaling. J. Integr. Plant Biol. 2016;58:67–80. doi: 10.1111/jipb.12364. [DOI] [PubMed] [Google Scholar]
- 32.Qin X.M., Huang S., Liu Y.Q., Bian M.D., Shi W.L., Zuo Z.C., Yang Z.M. Overexpression of A RING finger ubiquitin ligase gene AtATRF1 enhances aluminium tolerance in Arabidopsis thaliana. J. Plant Biol. 2017;60:66–74. doi: 10.1007/s12374-016-0903-9. [DOI] [Google Scholar]
- 33.Berrocal-Lobo M., Stone S., Yang X., Antico J., Callis J., Ramonell K.M., Somerville S. ATL9, a RING zinc finger protein with E3 ubiquitin ligase activity implicated in chitin- and NADPH oxidase-mediated defense responses. PLoS ONE. 2010;5:e14426. doi: 10.1371/journal.pone.0014426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Disch S., Anastasiou E., Sharma V.K., Laux T., Fletcher J.C., Lenhard M. The E3 ubiquitin ligase BIG BROTHER controls arabidopsis organ size in a dosage-dependent manner. Curr. Biol. 2006;16:272–279. doi: 10.1016/j.cub.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Wang X., Chen E., Ge X., Gong Q., Butt H., Zhang C., Yang Z., Li F., Zhang X. Overexpressed BRH1, a RING finger gene, alters rosette leaf shape in Arabidopsis thaliana. Sci. China Life Sci. 2018;61:79–87. doi: 10.1007/s11427-017-9133-8. [DOI] [PubMed] [Google Scholar]
- 36.Hardtke C.S., Okamoto H., Stoop-Myer C., Deng X.W. Biochemical evidence for ubiquitin ligase activity of the Arabidopsis COP1 interacting protein 8 (CIP8) Plant J. Cell Mol. Biol. 2002;30:385–394. doi: 10.1046/j.1365-313X.2002.01298.x. [DOI] [PubMed] [Google Scholar]
- 37.Deng X.W., Caspar T., Quail P.H. cop1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991;5:1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- 38.Von Arnim A.G., Deng X.W. Ring finger motif of Arabidopsis thaliana COP1 defines a new class of zinc-binding domain. J. Biol. Chem. 1993;268:19626–19631. [PubMed] [Google Scholar]
- 39.Qin F., Sakuma Y., Tran L.S., Maruyama K., Kidokoro S., Fujita Y., Fujita M., Umezawa T., Sawano Y., Miyazono K., et al. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell. 2008;20:1693–1707. doi: 10.1105/tpc.107.057380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J.H., Kang C.H., Nawkar G.M., Lee E.S., Paeng S.K., Chae H.B., Chi Y.H., Kim W.Y., Yun D.J., Lee S.Y. EMR, a cytosolic-abundant ring finger E3 ligase, mediates ER-associated protein degradation in Arabidopsis. New Phytol. 2018;220:163–177. doi: 10.1111/nph.15279. [DOI] [PubMed] [Google Scholar]
- 41.Voiniciuc C., Dean G.H., Griffiths J.S., Kirchsteiger K., Hwang Y.T., Gillett A., Dow G., Western T.L., Estelle M., Haughn G.W. Flying saucer1 is a transmembrane RING E3 ubiquitin ligase that regulates the degree of pectin methylesterification in Arabidopsis seed mucilage. Plant Cell. 2013;25:944–959. doi: 10.1105/tpc.112.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.H., Lee H.J., Park C.M. HOS1 acts as a key modulator of hypocotyl photomorphogenesis. Plant Signal. Behav. 2017;12:e1315497. doi: 10.1080/15592324.2017.1315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H., Xiong L., Gong Z., Ishitani M., Stevenson B., Zhu J.K. The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo--cytoplasmic partitioning. Genes Dev. 2001;15:912–924. doi: 10.1101/gad.866801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marino D., Froidure S., Canonne J., Ben Khaled S., Khafif M., Pouzet C., Jauneau A., Roby D., Rivas S. Arabidopsis ubiquitin ligase MIEL1 mediates degradation of the transcription factor MYB30 weakening plant defence. Nat. Commun. 2013;4:1476. doi: 10.1038/ncomms2479. [DOI] [PubMed] [Google Scholar]
- 45.Chen M., Ni M. RFI2, a RING-domain zinc finger protein, negatively regulates CONSTANS expression and photoperiodic flowering. Plant J. Cell Mol. Biol. 2006;46:823–833. doi: 10.1111/j.1365-313X.2006.02740.x. [DOI] [PubMed] [Google Scholar]
- 46.Cheng M.C., Hsieh E.J., Chen J.H., Chen H.Y., Lin T.P. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 2012;158:363–375. doi: 10.1104/pp.111.189738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Yang C., Li Y., Zheng N., Chen H., Zhao Q., Gao T., Guo H., Xie Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell. 2007;19:1912–1929. doi: 10.1105/tpc.106.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Q., Guo H.S., Dallman G., Fang S., Weissman A.M., Chua N.H. SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals. Nature. 2002;419:167–170. doi: 10.1038/nature00998. [DOI] [PubMed] [Google Scholar]
- 49.Tian M., Lou L., Liu L., Yu F., Zhao Q., Zhang H., Wu Y., Tang S., Xia R., Zhu B., et al. The RING finger E3 ligase STRF1 is involved in membrane trafficking and modulates salt-stress response in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2015;82:81–92. doi: 10.1111/tpj.12797. [DOI] [PubMed] [Google Scholar]
- 50.Ko J.H., Yang S.H., Han K.H. Upregulation of an Arabidopsis RING-H2 gene, XERICO, confers drought tolerance through increased abscisic acid biosynthesis. Plant J. Cell Mol. Biol. 2006;47:343–355. doi: 10.1111/j.1365-313X.2006.02782.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu H., Zhang H., Yang Y., Li G., Yang Y., Wang X., Basnayake B.M., Li D., Song F. Functional analysis reveals pleiotropic effects of rice RING-H2 finger protein gene OsBIRF1 on regulation of growth and defense responses against abiotic and biotic stresses. Plant Mol. Biol. 2008;68:17–30. doi: 10.1007/s11103-008-9349-x. [DOI] [PubMed] [Google Scholar]
- 52.Ning Y., Jantasuriyarat C., Zhao Q., Zhang H., Chen S., Liu J., Liu L., Tang S., Park C.H., Wang X., et al. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 2011;157:242–255. doi: 10.1104/pp.111.180893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park G.G., Park J.J., Yoon J., Yu S.N., An G. A RING finger E3 ligase gene, Oryza sativa Delayed Seed Germination 1 (OsDSG1), controls seed germination and stress responses in rice. Plant Mol. Biol. 2010;74:467–478. doi: 10.1007/s11103-010-9687-3. [DOI] [PubMed] [Google Scholar]
- 54.Hsu K.H., Liu C.C., Wu S.J., Kuo Y.Y., Lu C.A., Wu C.R., Lian P.J., Hong C.Y., Ke Y.T., Huang J.H., et al. Expression of a gene encoding a rice RING zinc-finger protein, OsRZFP34, enhances stomata opening. Plant Mol. Biol. 2014;86:125–137. doi: 10.1007/s11103-014-0217-6. [DOI] [PubMed] [Google Scholar]
- 55.Park Y.C., Chapagain S., Jang C.S. The microtubule-associated RING finger protein 1 (OsMAR1) acts as a negative regulator for salt-stress response through the regulation of OCPI2 (O. sativa chymotrypsin protease inhibitor 2) Planta. 2018;247:875–886. doi: 10.1007/s00425-017-2834-1. [DOI] [PubMed] [Google Scholar]
- 56.Bae H., Kim S.K., Cho S.K., Kang B.G., Kim W.T. Overexpression of OsRDCP1, a rice RING domain-containing E3 ubiquitin ligase, increased tolerance to drought stress in rice (Oryza sativa L.) Plant Sci. Int. J. Exp. Plant Biol. 2011;180:775–782. doi: 10.1016/j.plantsci.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Liu J., Zhang C., Wei C., Liu X., Wang M., Yu F., Xie Q., Tu J. The RING Finger Ubiquitin E3 Ligase OsHTAS Enhances Heat Tolerance by Promoting H2O2-Induced Stomatal Closure in Rice. Plant Physiol. 2016;170:429–443. doi: 10.1104/pp.15.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park Y.C., Chapagain S., Jang C.S. A Negative Regulator in Response to Salinity in Rice: Oryza sativa Salt-, ABA- and Drought-Induced RING Finger Protein 1 (OsSADR1) Plant Cell Physiol. 2018;59:575–589. doi: 10.1093/pcp/pcy009. [DOI] [PubMed] [Google Scholar]
- 59.Park Y.C., Lim S.D., Moon J.C., Jang C.S. A rice really interesting new gene H2-type E3 ligase, OsSIRH2-14, enhances salinity tolerance via ubiquitin/26S proteasome-mediated degradation of salt-related proteins. Plant Cell Environ. 2019;42:3061–3076. doi: 10.1111/pce.13619. [DOI] [PubMed] [Google Scholar]
- 60.Hwang S.G., Kim J.J., Lim S.D., Park Y.C., Moon J.C., Jang C.S. Molecular dissection of Oryza sativa salt-induced RING Finger Protein 1 (OsSIRP1): Possible involvement in the sensitivity response to salinity stress. Physiol. Plant. 2016;158:168–179. doi: 10.1111/ppl.12459. [DOI] [PubMed] [Google Scholar]
- 61.Lim C.W., Baek W., Lee S.C. The Pepper RING-Type E3 Ligase CaAIRF1 Regulates ABA and Drought Signaling via CaADIP1 Protein Phosphatase Degradation. Plant Physiol. 2017;173:2323–2339. doi: 10.1104/pp.16.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joo H., Lim C.W., Lee S.C. A pepper RING-type E3 ligase, CaASRF1, plays a positive role in drought tolerance via modulation of CaAIBZ1 stability. Plant J. Cell Mol. Biol. 2019;98:5–18. doi: 10.1111/tpj.14191. [DOI] [PubMed] [Google Scholar]
- 63.Lim C.W., Baek W., Lee S.C. Roles of pepper bZIP protein CaDILZ1 and its interacting partner RING-type E3 ligase CaDSR1 in modulation of drought tolerance. Plant J. Cell Mol. Biol. 2018;96:452–467. doi: 10.1111/tpj.14046. [DOI] [PubMed] [Google Scholar]
- 64.Brugiere N., Zhang W., Xu Q., Scolaro E.J., Lu C., Kahsay R.Y., Kise R., Trecker L., Williams R.W., Hakimi S., et al. Overexpression of RING Domain E3 Ligase ZmXerico1 Confers Drought Tolerance through Regulation of ABA Homeostasis. Plant Physiol. 2017;175:1350–1369. doi: 10.1104/pp.17.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu Y., Xu W., Wang J., Wang L., Yao W., Yang Y., Xu Y., Ma F., Du Y., Wang Y. The Chinese wild grapevine (Vitis pseudoreticulata) E3 ubiquitin ligase Erysiphe necator-induced RING finger protein 1 (EIRP1) activates plant defense responses by inducing proteolysis of the VpWRKY11 transcription factor. New Phytol. 2013;200:834–846. doi: 10.1111/nph.12418. [DOI] [PubMed] [Google Scholar]
- 66.Wang L., Xie X., Yao W., Wang J., Ma F., Wang C., Yang Y., Tong W., Zhang J., Xu Y., et al. RING-H2-type E3 gene VpRH2 from Vitis pseudoreticulata improves resistance to powdery mildew by interacting with VpGRP2A. J. Exp. Bot. 2017;68:1669–1687. doi: 10.1093/jxb/erx033. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Li L., Zhang L., Lv Q., Zhao Y., Li X. Isolation and identification of wheat gene TaDIS1 encoding a RING finger domain protein, which negatively regulates drought stress tolerance in transgenic Arabidopsis. Plant Sci. Int. J. Exp. Plant Biol. 2018;275:49–59. doi: 10.1016/j.plantsci.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Kam J., Gresshoff P., Shorter R., Xue G.P. Expression analysis of RING zinc finger genes from Triticum aestivum and identification of TaRZF70 that contains four RING-H2 domains and differentially responds to water deficit between leaf and root. Plant Sci. 2007;173:650–659. doi: 10.1016/j.plantsci.2007.09.001. [DOI] [Google Scholar]
- 69.Yang X., Sun C., Hu Y., Lin Z. Molecular cloning and characterization of a gene encoding RING zinc finger ankyrin protein from drought-tolerant Artemisia desertorum. J. Biosci. 2008;33:103–112. doi: 10.1007/s12038-008-0026-7. [DOI] [PubMed] [Google Scholar]
- 70.Jung Y.J., Lee I.H., Nou I.S., Lee K.D., Rashotte A.M., Kang K.K. BrRZFP1 a Brassica rapa C3HC4-type RING zinc finger protein involved in cold, salt and dehydration stress. Plant Biol. 2013;15:274–283. doi: 10.1111/j.1438-8677.2012.00631.x. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X., Wang N., Chen P., Gao M., Liu J., Wang Y., Zhao T., Li Y., Gai J. Overexpression of a soybean ariadne-like ubiquitin ligase gene GmARI1 enhances aluminum tolerance in Arabidopsis. PLoS ONE. 2014;9:e111120. doi: 10.1371/journal.pone.0111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai K., Yin J., Chao H., Ren Y., Jin L., Cao Y., Duanmu D., Zhang Z. A C3HC4-type RING finger protein regulates rhizobial infection and nodule organogenesis in Lotus japonicus. J. Integr. Plant Biol. 2018;60:878–896. doi: 10.1111/jipb.12703. [DOI] [PubMed] [Google Scholar]
- 73.Karlowski W.M., Hirsch A.M. The over-expression of an alfalfa RING-H2 gene induces pleiotropic effects on plant growth and development. Plant Mol. Biol. 2003;52:121–133. doi: 10.1023/A:1023916701669. [DOI] [PubMed] [Google Scholar]
- 74.Dos Reis S.P., Tavares Lde S., Costa Cde N., Brigida A.B., de Souza C.R. Molecular cloning and characterization of a novel RING zinc-finger protein gene up-regulated under in vitro salt stress in cassava. Mol. Biol. Rep. 2012;39:6513–6519. doi: 10.1007/s11033-012-1479-1. [DOI] [PubMed] [Google Scholar]
- 75.Wu W.X., Cheng Z.W., Liu M.J., Yang X.F., Qiu D.W. C3HC4-Type RING Finger Protein NbZFP1 Is Involved in Growth and Fruit Development in Nicotiana benthamiana. PLoS ONE. 2014:9. doi: 10.1371/journal.pone.0099352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia Z., Su X., Liu J., Wang M. The RING-H2 finger gene 1 (RHF1) encodes an E3 ubiquitin ligase and participates in drought stress response in Nicotiana tabacum. Genetica. 2013;141:11–21. doi: 10.1007/s10709-013-9702-0. [DOI] [PubMed] [Google Scholar]
- 77.Qi S.L., Lin Q.F., Zhu H.S., Gao F.H., Zhang W.H., Hua X.J. The RING Finger E3 Ligase SpRing is a Positive Regulator of Salt Stress Signaling in Salt-Tolerant Wild Tomato Species. Plant Cell Physiol. 2016;57:528–539. doi: 10.1093/pcp/pcw006. [DOI] [PubMed] [Google Scholar]
- 78.Dong C.H., Agarwal M., Zhang Y., Xie Q., Zhu J.K. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA. 2006;103:8281–8286. doi: 10.1073/pnas.0602874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shinozaki K., Yamaguchi-Shinozaki K. Gene Expression and Signal Transduction in Water-Stress Response. Plant Physiol. 1997;115:327–334. doi: 10.1104/pp.115.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finkelstein R.R., Gampala S.S., Rock C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14:S15–S45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]