Abstract
Acinetobacter baumannii (A. baumannii) is an important opportunistic pathogen causing serious nosocomial infections, which is considered as the most threatening Gram-negative bacteria (GNB). Outer membrane protein A (OmpA), a major component of outer membrane proteins (OMPs) in GNB, is a key virulence factor which mediates bacterial biofilm formation, eukaryotic cell infection, antibiotic resistance and immunomodulation. The characteristics of OmpA in Escherichia coli (E. coli) have been extensively studied since 1974, but only in recent years researchers started to clarify the functions of OmpA in A. baumannii. In this review, we summarized the structure and functions of OmpA in A. baumannii (AbOmpA), collected novel therapeutic strategies against it for treating A. baumannii infection, and emphasized the feasibility of using AbOmpA as a potential therapeutic target.
Keywords: A. baumannii, AbOmpA, Infection, Therapeutic target, Multidrug-resistant
Background
Acinetobacter baumannii (A. baumannii) causes hospital acquired infections (HAI), such as ventilator-associated pneumonia, bacteremia, urinary tract infections, meningitis, and surgical wound infections [1, 2], which leads to an increasing mortality in patients. Risk factors for these infections includes mechanical ventilation, usage of broad-spectrum antibiotics, ICU stay time and coma [3]. Statistically, about 1,000,000 people globally were infected with A. baumannii every year, and half of these infections were caused by multidrug-resistant (MDR) strains [4]. The mortality rate of A. baumannii infection in ICU was 45~60%, even reaching 84.3% when patients were infected with extensive drug resistance (XDR) A. baumannii [5, 6]. In 2017, Carbapenem-resistant A. baumannii (CRAB) ranked first in terms of threats to human health and urgency of developing relevant antibiotics, according to the drug-resistant bacteria list released by WHO [7].
However, colistin (CST) and tegacycline, the most commonly used antibiotics against carbapenemase-producing GNB, have become inefficient due to antibiotic resistance [8, 9]. In order to achieve clinical effectiveness, physicians had to increase the dose of CST and combine it with tegacycline [10]. Unfortunately, for bacteremia and serious respiratory tract infections caused by CRAB and XDR-A. baumannii respectively, combination therapy still did not work effectively [11, 12]. Facing the serious bacterial resistance, researchers have realized that it is urgent to develop new antibacterial strategies rather than rely on traditional antibiotics.
Generally, there are two ways to design novel antimicrobial agents. The one is to inhibit the production of essential substance for bacteria survival [13, 14]; the other is to inhibit virulence factors or antibiotic resistance genes of pathogenic bacteria in order to suppress pathogenicity or improve their sensitivity to antibiotics [15, 16]. However, inhibiting single essential component inevitably brings about great evolutionary pressure to bacteria and promotes the development of high-level drug-resistant strains [17]. Therefore, the novel intervention strategy targeting non-enssential processes is the key to overcome bacterial resistance [18]. For instance, inhibition of AbaI/AbaR quorum sensing systems with natural or synthetic inhibitor agent blocks the communication among bacteria and inhibits biofilms’ formation [19]. As a class of important virulence factors in bacteria, outer membrane proteins (OMPs) have attracted much more attention.
OMPs are a class of unique integral membrane proteins anchored in OM, whose β-barrel structures were formed by 8 to 26 strands. There are large, extend loops between the strands on the extracellular side and short loops on the periplasmic side. These characteristics give OMPs high stability in membrane and capability of fighting against extremely harsh environments [20]. Although different OMPs possess different sequences and functions, they share similar structure and biological properties [21]. OMPs of bacteria consist of even number strands, and importantly the function and stand shear number depend on their sequences. For example, as virulence related proteins, complement-binding protein OmpX in E.coli [22] and fibronectin- and heparin-binding protein Ail in Yersinia pestis [23] share comparable similar structure, but their sequence identity was lower than 45%. The diversity of OMPs sequence occurs at N terminal substantially more than C terminal, and the conserved β signal controls folding and correct assembly of OMPs [24].
However, up to now, the types of OMPs in A. baumannii have not been identified clearly, and only a few scattered reports were available, mainly including BamA, LptD, Omp33–36, OmpW. CarO and OprD. BamA itself as an OMP automatically can insert into OM and is responsible for the assemble of other OMPs [25]; LptD mediates the transportation of LPS to outer membrane (OM), loss of which can cause the accumulation of intermediates and fault location of LPS, and eventually lead to the disruption of bacterial membrane integrity [26]; Omp33–36 is a channel for the passage of water, which can induce apoptosis of host cells by activating caspase and regulate autophagy [27]; OmpW is a kind of hydrophobic porin existing in OM and cytoplasm, and plays an important role in modulating homeostasis of iron ion in bacteria [28]; CarO and OprD are related with resistance of carbapenem [29].
Among those OMPs of A. baumannii, OmpA is the most deeply studied virulence factor which plays key roles in regulating the adhesion, aggressiveness, and biofilm formation of A. baumannii and immune response of host. Overproduction of OmpA is an independent risk factor for the mortality rate of nosocomial pneumonia and bacteremia caused by A. baumannii [30]. Furthermore, the expression level of OmpA measured by qRT-PCR can be used as a rapid diagnostic index for antibiotic-resistant A. baumannii, by which the results are highly consistent with those through traditional MIC analysis [31].
This review overviewed the structure, function, and pathogenesis of AbOmpA, summarized therapeutic strategies targeting AbOmpA, and highlighted why AbOmpA is a potential target for the treatment of A. baumannii.
OmpA structure and function
OmpA was first identified as a heat-modifiable protein in Escherichia coli (E. coli) in 1974 [32] and originally purified in 1977. Its molecular mass ranges from 28 kDa to 36 kDa [33]. OmpA family is a group of surface-exposed, porin proteins with high-copy number in Omps of GNB. N-terminal domain of OmpA is an antiparallel β-barrel structure consisting of eight transmembrane strands so as to be embedded in the outer membrane. The eight strands are connected by four long loops on the surface of outer membrane and three short turns in periplasmic domain forming globular C-terminal [34]. Even in a specific bacterial strain, the amino acid sequences of OmpA are various among multiple subclasses [35].
In recent years, with clarifying the native structure of AbOmpA, researchers have found that the amino acids of AbOmpA from a variety of clinical isolates are highly conserved (> 89%), while they are not homologous to human proteome [36]. By comparing various OmpA-like proteins, the two conservative amino acids, R286 and Asp271, which are located in C-terminal domain of OmpA, were identified. Both amino acids non-covalently bind to diaminopimelate amino acid (DAP), a component of peptidoglycan (PGN) [37, 38]. This interaction suggests that OmpA plays a key role in maintaining bacterial surface integrity. Generally, in GNB species, OmpA stabilize their structure by self-dimerization, which prevents the bending connections between β-barrel structure and periplasmic from being lysed [39].
AbOmpA is essential for A.baumannii to adhere to and invade epithelial cells
A. baumannii is capable of entering and persisting inside host cells. Firstly, it adheres to host cells, then invades and translocates into nucleus. After killing host cells, it disseminates in bloodstream and tissues [40]. AbOmpA mediates the adhesion and invasion of A.baumannii to epithelial cells [41](Fig. 1a, upper panel). Compared with wild-type bacteria, the isogenic AbOmpA-mutant strain is more difficult to invade host cells. Pre-incubation with recombinant AbOmpA (rAbOmpA) dramatically inhibits adhesion and invasion of highly invasive A. baumannii 05KA103 to epithelial cells. And in vivo, the pathogenesis is delayed by the mutation of AbOmpA, as evidence showed that less bacterial burden in blood of a murine pneumonia model [42]. For the adhesion mechanism of OmpA, Smani et al. [43] found that it was more easier for A. baumannii to attach the 96-well plates coated with fibronection than that coated with BSA, and they identified the protein binding with fibronection was OmpA, indicating that the binding of OmpA to firbronectin was the first step of the interaction between A. baumannii with host cells.
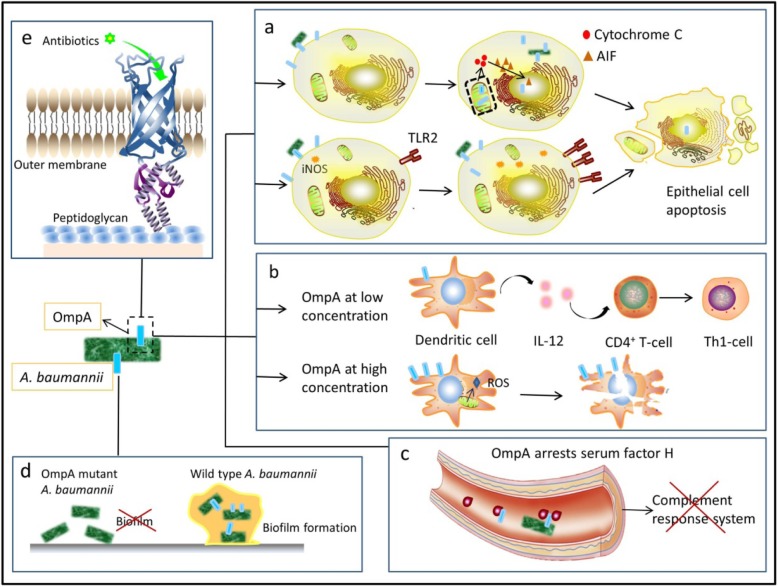
Fig. 1.
Functions of OmpA in A. baumannii. a upper panel. When contacting with epithelial cells, bacteria secrets OmpA into these cells. The OmpA are able to translocate in nucleus and mitochondria, and stimulate mitochondria to release cytochrome c. Then cytochrome c promotes apoptosis inducing factor (AIF) to translocate into nucleus and finally causes apoptosis of epithelial cells. Lower panel. OmpA increases the production of nitric oxide synthase (iNOS) and surface expression of Toll-like receptor 2 (TLR2) in epithelial cells, both of which trigger host cell death. b At low concentration, OmpA activates DCs which then stimulates CD4+T cells to exert Th1 response, while at high concentration; OmpA kills DCs by inducing mitochondria to release ROS. c AbOmpA can arrest serum factor H in serum, causing the paralysis of complement response. d AbOmpA play a dominant role in attaching abiotic surfaces and forming biofilm matrix. e AbOmpA is a porin protein which locates in outer membrane, selectively allowing the permeation of small molecular compounds
AbOmpA could directly cause host cells death if delivered to host cells via outer membrane vesicles (OMVs) [44]. As shown in Fig. 1a, after entering host cells, AbOmpA can localize to mitochondria, leading to the release of molecules (such as cytochrome C, apoptosis-inducing factor (AIF)) In early stage of A.baumannii infection, AIF degrades chromosome DNA and facilitates apoptosis of epithelial cells [45, 46]. In addition, OmpA can also translocate to nucleus of host cells depending on its monopartite nuclear localization signal (NLS)-“KTKEGRAMNRR”, which located between residues 320 and 330 of OmpA. This subcellular targeting of rAbOmpA can induce the apoptosis of host cells, but the specific underlying mechanism is not clear [46]. rAbOmpA purified from A.baumannii ATCC19606 at the concentration of 6 μg/mL is sufficient to exert cytotoxicity to human laryngeal epithelial HEp-2 cells [45]. AbOmpA promotes A. baumannii to adherent to and invade host cells, leading to their death by localizing in mitochondria and nucleus, but whether AbOmpA works in other organelles remains to be explored.
AbOmpA stimulates innate immune response
AbOmpA also influences host immune system. Although AbOmpA treatment does not influence the expression level of pro-inflammatory cytokines or chemokine, it increases the production of nitric oxide synthase (iNOS) and surface expression of Toll-like receptor 2 (TLR2) in HEp-2 cells [47](Fig. 1a, lower panel). Both of them are important in host-defense mechanism. Nitric oxide (NO) exerts bacteriostatic and bactericidal functions in pulmonary infection [48], and this oxidative stress provides another causal factor to cell death. And TLRs recognizes pathogen-associated molecular patterns (PAMPs) and triggers immune response [49, 50]. The effects of AbOmpA on bone marrow-derived dendritic cells (DCs) are illustrated in Fig. 1b. AbOmpA at a concentration of 200 ng/mL activates DCs via TLR2, MAPK and NF-κB pathway, stimulating CD4+T cells towards a Th1 response [51, 52]. However, AbOmpA tends to kill DCs at high concentrations (≥3 μg/mL) by increasing the reactive oxygen species (ROS) from mitochondria [51, 53]. Inoculation with rAbOmpA could induce stype 2 immune response in mice, which damaged the balance between IL-4 and INF-c and led to the occurrence of infection [54]. In addition, AbOmpA paralyzed the complement response system by arresting serum factor H [55, 56] (Fig. 1c).
AbOmpA induces biofilm formation
Biofilm enables A.baumannii to survive under hostile conditions [57], mainly consisting of protein, extracellular DNA and polysaccharides. According to the statistics of National Institutes of Health and the Center for Disease and Prevention, 65–80% human infections were caused by biofilm-forming bacteria [58]. Based on the multi-functional characteristics of biofilm formation, a series of genes associated with bacterial adhesion and biofilm formation were identified, such as AbOmpA, beta-lactamase PER-1 (blaPER-1) and biofilm-associated protein (Bap) [59]. Indeed, the OmpA of A. baumannii ATCC19606 plays a dominant role in forming stable biofilm on plastic surface (Fig. 1d). AbOmpA mutant strains fail to form biofilm, while replenishment of AbOmpA allele efficiently restores the ability [41].
Permeation of small molecular antibiotics through AbOmpA
Porins are only β-barrel proteins that have been found in outer membrane of GNB. AbOmpA is the most abundant porin associated with drug resistance, epithelial cells attachment and biofilm formation [60, 61]. Smani et al. first demonstrated the effect of AbOmpA on the phenotype of multi-resistant A. baumannii, and found that the depletion of OmpA gene decreased the MICs of chloramphenicol, aztreonam, and nalidixic by 8, 8 and 2.67 fold, respectively. This data suggested that OmpA may participate in extrusion of antibacterial compound from periplasmic region and couple with efflux systems in inner membrane [62]. The transmembrane transport controlled by porins is an important way for delivering nutrients and small molecular hydrophilic antibacterial compound to bacteria [63]. Ramkumar et al. [64] found that AbOmpA selectively allowed the passage of small molecular antibiotics (Fig. 1e). For instance, ETX2514, the broad-spectrum β-lactamase inhibitor, penetrates through AbOmpA and enhances the antibacterial activity of sulbactam in an AbOmpA dependent way. In the future, clarifying AbOmpA crystal structure will reveal the relationship among preliminary structure of substrate, porin protein and permeation, providing more information for design of small molecular substrate [64].
Regulation of AbOmpA expression
Among GNB, the factors influencing OmpA expression are mostly characterized in Escherichia coli, such as nutrients, culture conditions, bacteriophage infection and metabolic enzymes [65]. However, the OmpA-regulating mechanisms of A. baumannii are still being explored. Although OmpA in GNB possess a similar two-part structure, amino acid sequences located on the outer surface of bacteria are various among different genus [35]. Besides learning from the studies of E. coli, we are supposed to explore characteristics of OmpA in A. baumannii. In recent years, it has been shown that AbOmpA is a stress-related outer membrane protein, the expression of which is affected by internal and external environment of bacteria [66]. During studying the effects of temperature, dryness and nutrient deprivation on the long-term survival of sensitive strain ATCC19606 and clinical isolates, Bravo Z [67] et al. found that adhesion and biofilm formation related genes, OmpA, bfmR and csuAB were all down-regulated in starved cells, leading to difficulties in forming biofilms and spreading of A. baumannii in blood [67]. Hfq protein, a host bacterial factor first discovered in E.coi, is indispensable for RNA synthesis of bacteriophage Qb, [68], and now it is considered as a transcriptional regulator related to stress responses. Hfq deficiency retards cell growth and enhances cellular sensitivity to environment stress. The expression level of OmpA in Hfq mutant strain is dramatically lower than that in a wild type strain [69]. In addition, there is a causal correlation between biofilm formation and AbOmpA expression in A.baumannii under the treatment of antibiotics at sub-minimum inhibitory concentrations (MIC). Yoshinori et al. [70] incubated ATCC19606 and clinical isolates with 1/2 MIC of polymyxin B (PMB) and colistin (CST) for 24 h, respectively, and found that there was a positive correlation between the mRNA level of AbOmpA and the number of biofilm cells in CST treated ATCC19606 strain. The same results were observed in R3 clinical isolates in the presence of PMB [70]. Meropenem at the concentration of 64 μg/mL and 128 μg/mL increased AbOmpA expression by 1.81 and 1.63 folds, respectively [71].
Overall, there are three factors that mainly involved in the regulation of AbOmpA in recent studies. Firstly, disadvantaged environments such as starvation, decreases the production of AbOmpA, making it difficult for A. baumannii to form biofilm and adhere to host cells; Secondly, Hfq, a transcriptional regulator, whose expression level is closely related to that of AbOmpA; Finally, some antibiotics at sub-MIC promote AbOmpA expression and biofilm formation. Although several factors have been proved associated with OmpA exppresion in A. baumannii, the underlying mechanisms remain to be explored.
Therapeutic strategies targeting AbOmpA
Polypeptide
Nowadays, the synthetic small polypeptide which specifically binds to OmpA has been designed to prevent A. baumannii from contacting with host cells. AOA-2, a cyclic hexapeptide as a blocking agent of AbOmpA without bactericidal activity decreases the adhesion of A. baumannii, Pseudomonas aeruginosa and E. coli to the surfaces of biotic and abiotic, and significantly enhances the sensitivity of A. baumannii to CST at the concentration of 125 μg/mL. In vivo, the intraperitoneal injection of AOA-2 (10 mg/kg) in combination with CST (10 mg/kg) improved the survival rate of mice with bacteraemia by 20% [72, 73]. In addition, some classic antimicrobial peptides (AMPs) interacting with AbOmpA have been gradually discovered, which are a series of endogenous defense peptides to kill bacteria and fungus [74–76]. For example, bovine myeloid antimicrobial peptide (BMAP-28) and its analog peptides killed MDR-A. baumannii by interacting with AbOmpA. These compounds started to destroy A. baumannii at the concentration of 40 μg/mL within only 15 min, and after 30 min, the bacterial cells were obviously damaged with leaking cytoplasm [77]. In addition, LL-37 interacted with the amino acid residues 74–84 of AbOmpA in a dose dependent way, decreasing the adhesion of A. baumannii to host cells. However, this inhibitory effect of LL-37 on adhesion was greatly reduced after AbOmpA deletion [78]. Human defensing-5 (HD5) is an endogenous peptide which kills MDR-A. baumannii. In order to enhance the antibacterial activity of HD5, non-cationic and non-hydrophobic residues were replaced by positively charged arginine to obtain a derivative named HD5d5, which could strongly bind to AbOmpA and then exerted its toxin-neutralizing function [79]. Although there is evidence that MDR-Staphylococcus aureus are resistant to LL-37 by producing catabolic enzymes [80], whether A. baumannii could develop resistance to natural AMPs has not been reported. The synthetic small peptide, specifically targeting AbOmpA without bactericidal activity, may avoid triggering bacterial evolution pressure and could be used alone or combined with other antibacterial compounds to exert synergistic effects.
Vaccine
As an ideal antigen of vaccine, AbOmpA is conserved among various clinical strains with the genome that is different from human genome [54, 81, 82]. Luo G et al. found that immunizing mice with 3 μg rOmpA in 0.1% aluminum hydroxide (Al (OH)3) significantly improved the survival rate of diabetic animals infected by A. baumannii HUMC1 by 40%, and decreased the counts of bacterial colonies of all the organs (except lung) by ten-fold, compared with control. In addition, the IgG antibody against rOmpA in serum was also dramatically enhanced [83]. Badmasti F et al. demonstrated that the survival rate of mice with disseminated sepsis induced by ATCC19606 was remarkably improved (70%) after treating with recombinant conserved immunodominant region of AbOmpA (8-346aa), and in addition, the combination with Bap(1-487aa) also increased the survival rate (> 80%) of mice infected by MDR AB-44 [84]. Furthermore, some derivatives of AbOmpA with higher antigenicity and lower toxicity were designed by bioinformatic and immunoinformatic tools. For example, a novel immunogenic model with 12 strands was obtained by modifying amino acid sequences of OmpA, in which K320 and K322 were substituted by Alanine, “NADEEFWN” was replaced by “YKYDFDGVNRGTRGTSEEGTL”, “VVQPGQEAAAPAAAQ” located at C-terminal and position 1–24 of N- terminal were removed. This AbOmpA-derived antigen was capable of triggering the production of antibodies which kills Pseudomonas aeruginosa and A. baumannii [36]. Another way is to develop DNA vaccine, which has attracted more attention owing to the effectiveness and durability. DNA vaccine is considerably safe and tolerable because it does not contain weakened or dead pathogenic during its production [85]. Hossein et al. cloned AbOmpA gene and inserted it into an eukaryotic expression vector pBudCE4.1 to obtain the recombinant pBudCE4.1–ompA. After transfected with this recombinant plasmid, human dermal fibroblast cells (HDF) effectively expressed AbOmpA [86]. Next, they evaluated the immunogenic potential of pBudCE4.1–ompA in mice model. After immunization with this vaccine, IL-2, IL-4, IL-12, IgM, IgG, and INF-γ all dramatically increased in serum and more animals survived compared to the control group [87]. However, we should not ignore the oncogenic potential of recombinant molecules due to its random integration into host genome.
Monoclonal antibodies (mAbs)
It is widely accepted that antibodies can be used to defense against microbial infections. Passive immunization induced by antibodies targeting AbOmpA used to be considered as a potential therapeutic method for MDR and XDR-A. baumannii infections [83]. However, treatment with polyclonal anti-OmpA sera has displayed many inevitable shortcomings, such as immune complex hypersensitivity, low content of specific antibodies and the potential danger of infectious diseases spread [88, 89]. Recently, monoclonal antibody (mAb) technology contributes to the development of antibacterial mAbs. Compared with polyclonal anti-OmpA sera, mAbs possess more advantages, such as higher safety, better homology and more specific targets [90]. The mAbs targeting OmpA promotes macrophages to kill A. baumannii 307.30 (AB307.30), except those covered with thick capsule, especially XDR-A.baumannii. Evidence showed that the binding of mAbs to clinical isolates was much weaker than that between mABs and ATCC19606. Whether the capsule over the cell wall prevents mAbs from binding to XDR-A.baumannii? The K1 capsule-negative mutant strain (AB307.30) strongly combined with Anti-OmpA mAbs, suggesting capsule polysaccharides may shield the binding sites of OmpA [91]. The poor combination between mAbs and XDR-A. baumannii needs to be solved in the further, and mAbs can also be used for other conserved epitopes of A. baumannii.
Conclusions
With the emergence of MDR, XDR and pan-drug-resistant A. baumannii [92, 93], the traditional therapies have already failed to defeat complex infections caused by these pathogens. It is urgent to find new methods for treating drug-resistant A. baumannii infection. AbOmpA is becoming a potential therapeutic target for the following reasons. Firstly, AbOmpA is the most abundant and highly conserved porin in A. baumannii, which closely associated with virulence and bacteria survival under the hostile conditions. In addition, since it is not homologous to human genome, inhibition of it will not bring unnecessary damages to host cells. More importantly, AbOmpA is not essential for bacteria survival, suppression of which may not induce bacteria resistance. Although the studies about therapeutic strategies for A.baumannii infections are still limited and some even accompanied with certain risks, AbOmpA is a promising therapeutic target for the treatment of A.baumanii infections.
Acknowledgements
None.
Abbreviations
- A. baumannii
Acinetobacter baumannii
- AbOmpA
OmpA in A. baumannii
- Bap
Biofilm-associated protein
- blaPER-1
beta-lactamase PER-1
- BMAP
Bovine myeloid antimicrobial peptide
- CRAB
Carbapenem-resistant A. baumannii
- CST
Colistin
- DAP
Diaminopimelate amino acid
- DCs
Dendritic cells
- E. coli
Escherichia coli
- GNB
Gram-negative bacteria
- HAI
Hospital acquired infections
- HD5
Human defensing-5
- HDF
Dermal fibroblast cells
- iNOS
Nitric oxide synthase
- mAbs
monoclonal antibodies
- MDR
Multidrug-resistant
- MIC
Minimum inhibitory concentrations
- NLS
Nuclear localization signal
- NO
Nitric oxide
- OM
Outer membrane
- OmpA
Outer membrane protein A
- OMPs
Outer membrane proteins
- OMVs
Outer membrane vesicles
- PAMPs
Pathogen-associated molecular patterns
- PGN
Peptidoglycan
- PMB
Polymyxin B
- rAbOmpA
recombinant AbOmpA
- TLR2
Toll-like receptor 2
- XDR
Extensive drug resistance
Authors’ contributions
XX initiated the idea, guided the article structure, and improved the final manuscript. DN and YH reviewed the published studies and composed the draft of the manuscript. ZC, ML, ZH, XM and XL provided critical feedback on drafts of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81972359) and the fund from Fourth Military Medical University (No. 2018JSTS05).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dan Nie and Yue Hu contributed equally to this work.
Contributor Information
Dan Nie, Email: niedan890604@126.com.
Yue Hu, Email: 564243373@qq.com.
Zhou Chen, Email: chenzhou_cky@163.com.
Mingkai Li, Email: mingkai@fmmu.edu.cn.
Zheng Hou, Email: zhenghou79@gmail.com.
Xiaoxing Luo, Email: xxluo3@fmmu.edu.cn.
Xinggang Mao, Email: xinggmao@163.com.
Xiaoyan Xue, Email: xxy.0707@163.com.
References
- 1.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalopoulos A, Falagas ME. Treatment of Acinetobacter infections. Expert Opin Pharmacother. 2010;11:779–788. doi: 10.1517/14656561003596350. [DOI] [PubMed] [Google Scholar]
- 3.Ma MY, Xu J, Yu N, Huang GM. Analysis of drug resistance of Acinetobacter baumannii and its related factors in ICU. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:686–689. doi: 10.3760/cma.j.issn.2095-4352.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Rex JH. The value of single-pathogen antibacterial agents. Nat Rev Drug Discov. 2013;12:963. doi: 10.1038/nrd3957-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inchai J, Pothirat C, Bumroongkit C, Limsukon A, Khositsakulchai W, Liwsrisakun C. Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care. 2015;3:9. doi: 10.1186/s40560-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uwingabiye J, Frikh M, Lemnouer A, Bssaibis F, Belefquih B, Maleb A, Dahraoui S, Belyamani L, Bait A, Haimeur C, Louzi L, Ibrahimi A, Elouennass M. Acinetobacter infections prevalence and frequency of the antibiotics resistance: comparative study of intensive care units versus other hospital units. Pan Afr Med J. 2016;23:191. doi: 10.11604/pamj.2016.23.191.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543:15. doi: 10.1038/nature.2017.21550. [DOI] [PubMed] [Google Scholar]
- 8.Kontopoulou K, Protonotariou E, Vasilakos K, Kriti M, Koteli A, Antoniadou E, Sofianou D. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 beta-lactamase resistant to colistin. J Hosp Infect. 2010;76:70–73. doi: 10.1016/j.jhin.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Spanu T, De Angelis G, Cipriani M, Pedruzzi B, D'Inzeo T, Cataldo MA, Sganga G, Tacconelli E. In vivo emergence of tigecycline resistance in multidrug-resistant Klebsiella pneumoniae and Escherichia coli. Antimicrob Agents Chemother. 2012;56:4516–4518. doi: 10.1128/AAC.00234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Patel G, Huprikar S, Calfee DP, Jenkins SG. Decreased susceptibility to polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J Clin Microbiol. 2009;47:1611–1612. doi: 10.1128/JCM.02466-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee NY, Lee JC, Li MC, Li CW, Ko WC. Empirical antimicrobial therapy for critically ill patients with Acinetobacter baumannii bacteremia: combination is better. J Microbiol Immunol Infect. 2013;46:397–398. doi: 10.1016/j.jmii.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Shields RK, Clancy CJ, Gillis LM, Kwak EJ, Silveira FP, Massih RC, Eschenauer GA, Potoski BA, Nguyen MH. Epidemiology, clinical characteristics and outcomes of extensively drug-resistant Acinetobacter baumannii infections among solid organ transplant recipients. PLoS One. 2012;7:e52349. doi: 10.1371/journal.pone.0052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Zhou, Nie Dan, Hu Yue, Li Mingkai, Hou Zheng, Mao Xinggang, Luo Xiaoxing, Xue Xiaoyan. Efficient Delivery of Antisense Oligonucleotides by an Amphipathic Cell-Penetrating Peptide in Acinetobacter baumannii. Current Drug Delivery. 2019;16(8):728–736. doi: 10.2174/1567201816666190627141931. [DOI] [PubMed] [Google Scholar]
- 14.Kawai Yoshikazu, Mercier Romain, Mickiewicz Katarzyna, Serafini Agnese, Sório de Carvalho Luiz Pedro, Errington Jeff. Crucial role for central carbon metabolism in the bacterial L-form switch and killing by β-lactam antibiotics. Nature Microbiology. 2019;4(10):1716–1726. doi: 10.1038/s41564-019-0497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krueger E, Brown AC. Inhibition of bacterial toxin recognition of membrane components as an anti-virulence strategy. J Biol Eng. 2019;13:4. doi: 10.1186/s13036-018-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelbicova T, Barakova A, Florianova M, Karpiskova R. Detection of colistin-resistant Acinetobacter baumannii with the mcr-4 gene. Klinicka mikrobiologie a infekcni lekarstvi. 2019;25:4–6. [PubMed] [Google Scholar]
- 17.Monserrat-Martinez Ana, Gambin Yann, Sierecki Emma. Thinking Outside the Bug: Molecular Targets and Strategies to Overcome Antibiotic Resistance. International Journal of Molecular Sciences. 2019;20(6):1255. doi: 10.3390/ijms20061255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muhlen S, Dersch P. Anti-virulence strategies to target bacterial infections. Curr Top Microbiol Immunol. 2016;398:147–183. doi: 10.1007/82_2015_490. [DOI] [PubMed] [Google Scholar]
- 19.Saipriya K., Swathi C.H., Ratnakar K.S., Sritharan V. Quorum‐sensing system in Acinetobacter baumannii : a potential target for new drug development. Journal of Applied Microbiology. 2019;128(1):15–27. doi: 10.1111/jam.14330. [DOI] [PubMed] [Google Scholar]
- 20.Rollauer Sarah E., Sooreshjani Moloud A., Noinaj Nicholas, Buchanan Susan K. Outer membrane protein biogenesis in Gram-negative bacteria. Philosophical Transactions of the Royal Society B: Biological Sciences. 2015;370(1679):20150023. doi: 10.1098/rstb.2015.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi D, Mahalakshmi R. Transmembrane beta-barrels: evolution, folding and energetics. Biochim Biophys Acta Biomembr. 1859;2017:2467–2482. doi: 10.1016/j.bbamem.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogt J, Schulz GE. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure. 1999;7:1301–1309. doi: 10.1016/S0969-2126(00)80063-5. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita S, Lukacik P, Barnard TJ, Noinaj N, Felek S, Tsang TM, Krukonis ES, Hinnebusch BJ, Buchanan SK. Structural insights into ail-mediated adhesion in Yersinia pestis. Structure. 2011;19:1672–1682. doi: 10.1016/j.str.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gessmann D, Chung YH, Danoff EJ, Plummer AM, Sandlin CW, Zaccai NR, Fleming KG. Outer membrane beta-barrel protein folding is physically controlled by periplasmic lipid head groups and BamA. Proc Natl Acad Sci U S A. 2014;111:5878–5883. doi: 10.1073/pnas.1322473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi U, Lee CR. Antimicrobial agents that inhibit the outer membrane assembly Machines of Gram-Negative Bacteria. J Microbiol Biotechnol. 2019;29:1–10. doi: 10.4014/jmb.1804.03051. [DOI] [PubMed] [Google Scholar]
- 26.Bojkovic J, Richie DL, Six DA, Rath CM, Sawyer WS, Hu Q, Dean CR. Characterization of an Acinetobacter baumannii lptD deletion strain: permeability defects and response to inhibition of lipopolysaccharide and fatty acid biosynthesis. J Bacteriol. 2015;198:731–741. doi: 10.1128/JB.00639-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rumbo C, Tomas M, Fernandez Moreira E, Soares NC, Carvajal M, Santillana E, Beceiro A, Romero A, Bou G. The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells. Infect Immun. 2014;82:4666–4680. doi: 10.1128/IAI.02034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catel-Ferreira M, Marti S, Guillon L, Jara L, Coadou G, Molle V, Bouffartigues E, Bou G, Shalk I, Jouenne T, Vila-Farres X, De E. The outer membrane porin OmpW of Acinetobacter baumannii is involved in iron uptake and colistin binding. FEBS Lett. 2016;590:224–231. doi: 10.1002/1873-3468.12050. [DOI] [PubMed] [Google Scholar]
- 29.Zhu LJ, Chen XY, Hou PF. Mutation of CarO participates in drug resistance in imipenem-resistant Acinetobacter baumannii. J Clin Lab Anal. 2019;33:e22976. doi: 10.1002/jcla.22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Encinales V, Alvarez-Marin R, Pachon-Ibanez ME, Fernandez-Cuenca F, Pascual A, Garnacho-Montero J, Martinez-Martinez L, Vila J, Tomas MM, Cisneros JM, Bou G, Rodriguez-Bano J, Pachon J, Smani Y. Overproduction of outer membrane protein a by Acinetobacter baumannii as a risk factor for nosocomial pneumonia, bacteremia, and mortality rate increase. J Infect Dis. 2017;215:966–974. doi: 10.1093/infdis/jix010. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Pena R, Dominguez-Herrera J, Pachon J, McConnell MJ. Rapid detection of antibiotic resistance in Acinetobacter baumannii using quantitative real-time PCR. J Antimicrob Chemother. 2013;68:1572–1575. doi: 10.1093/jac/dkt057. [DOI] [PubMed] [Google Scholar]
- 32.Foulds J, Chai TJ. Defeat of colicin tolerance in Escherichia coli ompA mutants: evidence for interaction between colicin L-JF246 and the cytoplasmic membrane. J Bacteriol. 1978;133:158–164. doi: 10.1128/JB.133.1.158-164.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai TJ, Foulds J. Purification of protein a, an outer membrane component missing in Escherichia coli K-12 ompA mutants. Biochim Biophys Acta. 1977;493:210–215. doi: 10.1016/0005-2795(77)90274-4. [DOI] [PubMed] [Google Scholar]
- 34.Confer AW, Ayalew S. The OmpA family of proteins: roles in bacterial pathogenesis and immunity. Vet Microbiol. 2013;163:207–222. doi: 10.1016/j.vetmic.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Hounsome JD, Baillie S, Noofeli M, Riboldi-Tunnicliffe A, Burchmore RJ, Isaacs NW, Davies RL. Outer membrane protein a of bovine and ovine isolates of Mannheimia haemolytica is surface exposed and contains host species-specific epitopes. Infect Immun. 2011;79:4332–4341. doi: 10.1128/IAI.05469-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahangiri A, Rasooli I, Owlia P, Fooladi AA, Salimian J. In silico design of an immunogen against Acinetobacter baumannii based on a novel model for native structure of outer membrane protein a. Microb Pathog. 2017;105:201–210. doi: 10.1016/j.micpath.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Lee WC, Yeo KJ, Ryu KS, Kumarasiri M, Hesek D, Lee M, Mobashery S, Song JH, Kim SI, Lee JC, Cheong C, Jeon YH, Kim HY. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012;26:219–228. doi: 10.1096/fj.11-188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samsudin F, Ortiz-Suarez ML, Piggot TJ, Bond PJ, Khalid S. OmpA: a flexible clamp for bacterial Cell Wall attachment. Structure. 2016;24:2227–2235. doi: 10.1016/j.str.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Marcoux J, Politis A, Rinehart D, Marshall DP, Wallace MI, Tamm LK, Robinson CV. Mass spectrometry defines the C-terminal dimerization domain and enables modeling of the structure of full-length OmpA. Structure. 2014;22:781–790. doi: 10.1016/j.str.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parra-Millan R, Guerrero-Gomez D, Ayerbe-Algaba R, Pachon-Ibanez ME, Miranda-Vizuete A, Pachon J, Smani Y. Intracellular Trafficking and Persistence of Acinetobacter baumannii Requires Transcription Factor EB. mSphere. 2018;3:e00106–18. [DOI] [PMC free article] [PubMed]
- 41.Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77:3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi CH, Lee JS, Lee YC, Park TI, Lee JC. Acinetobacter baumannii invades epithelial cells and outer membrane protein a mediates interactions with epithelial cells. BMC Microbiol. 2008;8:216. doi: 10.1186/1471-2180-8-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smani Y, McConnell MJ, Pachon J. Role of fibronectin in the adhesion of Acinetobacter baumannii to host cells. PLoS One. 2012;7:e33073. doi: 10.1371/journal.pone.0033073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin JS, Kwon SO, Moon DC, Gurung M, Lee JH, Kim SI, Lee JC. Acinetobacter baumannii secretes cytotoxic outer membrane protein a via outer membrane vesicles. PLoS One. 2011;6:e17027. doi: 10.1371/journal.pone.0017027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, Kim SA, Lee SK, Lee JC. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7:1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 46.Choi CH, Hyun SH, Lee JY, Lee JS, Lee YS, Kim SA, Chae JP, Yoo SM, Lee JC. Acinetobacter baumannii outer membrane protein a targets the nucleus and induces cytotoxicity. Cell Microbiol. 2008;10:309–319. doi: 10.1111/j.1462-5822.2007.01041.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim SA, Yoo SM, Hyun SH, Choi CH, Yang SY, Kim HJ, Jang BC, Suh SI, Lee JC. Global gene expression patterns and induction of innate immune response in human laryngeal epithelial cells in response to Acinetobacter baumannii outer membrane protein a. FEMS Immunol Med Microbiol. 2008;54:45–52. doi: 10.1111/j.1574-695X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 48.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/S0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 50.Trinchieri G, Sher A. Cooperation of toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 51.Lee JS, Lee JC, Lee CM, Jung ID, Jeong YI, Seong EY, Chung HY, Park YM. Outer membrane protein a of Acinetobacter baumannii induces differentiation of CD4+ T cells toward a Th1 polarizing phenotype through the activation of dendritic cells. Biochem Pharmacol. 2007;74:86–97. doi: 10.1016/j.bcp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Mortensen BL, Skaar EP. Host-microbe interactions that shape the pathogenesis of Acinetobacter baumannii infection. Cell Microbiol. 2012;14:1336–1344. doi: 10.1111/j.1462-5822.2012.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JS, Choi CH, Kim JW, Lee JC. Acinetobacter baumannii outer membrane protein a induces dendritic cell death through mitochondrial targeting. J Microbiol. 2010;48:387–392. doi: 10.1007/s12275-010-0155-1. [DOI] [PubMed] [Google Scholar]
- 54.Lin L, Tan B, Pantapalangkoor P, Ho T, Hujer AM, Taracila MA, Bonomo RA, Spellberg B. Acinetobacter baumannii rOmpA vaccine dose alters immune polarization and immunodominant epitopes. Vaccine. 2013;31:313–318. doi: 10.1016/j.vaccine.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SW, Choi CH, Moon DC, Jin JS, Lee JH, Shin JH, Kim JM, Lee YC, Seol SY, Cho DT, Lee JC. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett. 2009;301:224–231. doi: 10.1111/j.1574-6968.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- 56.King LB, Swiatlo E, Swiatlo A, McDaniel LS. Serum resistance and biofilm formation in clinical isolates of Acinetobacter baumannii. FEMS Immunol Med Microbiol. 2009;55:414–421. doi: 10.1111/j.1574-695X.2009.00538.x. [DOI] [PubMed] [Google Scholar]
- 57.Gunn JS, Bakaletz LO, Wozniak DJ. What's on the outside matters: the role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J Biol Chem. 2016;291:12538–12546. doi: 10.1074/jbc.R115.707547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramos-Gallardo G. Chronic wounds in burn injury: a case report on importance of biofilms. World J Plastic Surg. 2016;5:175–180. [PMC free article] [PubMed] [Google Scholar]
- 59.Badmasti F, Siadat SD, Bouzari S, Ajdary S, Shahcheraghi F. Molecular detection of genes related to biofilm formation in multidrug-resistant Acinetobacter baumannii isolated from clinical settings. J Med Microbiol. 2015;64:559–564. doi: 10.1099/jmm.0.000058. [DOI] [PubMed] [Google Scholar]
- 60.Eze EC, Chenia HY, El Zowalaty ME. Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infect Drug Resist. 2018;11:2277–2299. doi: 10.2147/IDR.S169894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smani Y, Fabrega A, Roca I, Sanchez-Encinales V, Vila J, Pachon J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob Agents Chemother. 2014;58:1806–1808. doi: 10.1128/AAC.02101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masi M, Winterhalter M, Pages JM. Outer Membrane Porins. Subcell Biochem. 2019;92:79–123. doi: 10.1007/978-3-030-18768-2_4. [DOI] [PubMed] [Google Scholar]
- 64.Iyer R, Moussa SH, Durand-Reville TF, Tommasi R, Miller A. Acinetobacter baumannii OmpA is a selective antibiotic Permeant Porin. ACS infect Dis. 2018;4:373–381. doi: 10.1021/acsinfecdis.7b00168. [DOI] [PubMed] [Google Scholar]
- 65.Smith SG, Mahon V, Lambert MA, Fagan RP. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol Lett. 2007;273:1–11. doi: 10.1111/j.1574-6968.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 66.Soares NC, Cabral MP, Gayoso C, Mallo S, Rodriguez-Velo P, Fernandez-Moreira E, Bou G. Associating growth-phase-related changes in the proteome of Acinetobacter baumannii with increased resistance to oxidative stress. J Proteome Res. 2010;9:1951–1964. doi: 10.1021/pr901116r. [DOI] [PubMed] [Google Scholar]
- 67.Bravo Z, Orruno M, Parada C, Kaberdin VR, Barcina I, Arana I. The long-term survival of Acinetobacter baumannii ATCC 19606(T) under nutrient-deprived conditions does not require the entry into the viable but non-culturable state. Arch Microbiol. 2016;198:399–407. doi: 10.1007/s00203-016-1200-1. [DOI] [PubMed] [Google Scholar]
- 68.Franze de Fernandez MT, Eoyang L, August JT. Factor fraction required for the synthesis of bacteriophage Qbeta-RNA. Nature. 1968;219:588–590. doi: 10.1038/219588a0. [DOI] [PubMed] [Google Scholar]
- 69.Kuo HY, Chao HH, Liao PC, Hsu L, Chang KC, Tung CH, Chen CH, Liou ML. Functional characterization of Acinetobacter baumannii lacking the RNA chaperone Hfq. Front Microbiol. 2017;8:2068. doi: 10.3389/fmicb.2017.02068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato Y, Unno Y, Ubagai T, Ono Y. Sub-minimum inhibitory concentrations of colistin and polymyxin B promote Acinetobacter baumannii biofilm formation. PLoS One. 2018;13:e0194556. doi: 10.1371/journal.pone.0194556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Navidifar T, Amin M, Rashno M. Effects of sub-inhibitory concentrations of meropenem and tigecycline on the expression of genes regulating pili, efflux pumps and virulence factors involved in biofilm formation by Acinetobacter baumannii. Infect Drug Resist. 2019;12:1099–1111. doi: 10.2147/IDR.S199993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parra-Millan R, Vila-Farres X, Ayerbe-Algaba R, Varese M, Sanchez-Encinales V, Bayo N, Pachon-Ibanez ME, Teixido M, Vila J, Pachon J, Giralt E, Smani Y. Synergistic activity of an OmpA inhibitor and colistin against colistin-resistant Acinetobacter baumannii: mechanistic analysis and in vivo efficacy. J Antimicrob Chemother. 2018;73:3405–3412. doi: 10.1093/jac/dky343. [DOI] [PubMed] [Google Scholar]
- 73.Vila-Farres X, Parra-Millan R, Sanchez-Encinales V, Varese M, Ayerbe-Algaba R, Bayo N, Guardiola S, Pachon-Ibanez ME, Kotev M, Garcia J, Teixido M, Vila J, Pachon J, Giralt E, Smani Y. Combating virulence of gram-negative bacilli by OmpA inhibition. Sci Rep. 2017;7:14683. doi: 10.1038/s41598-017-14972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 75.Storici P, Tossi A, Lenarcic B, Romeo D. Purification and structural characterization of bovine cathelicidins, precursors of antimicrobial peptides. Eur J Biochem. 1996;238:769–776. doi: 10.1111/j.1432-1033.1996.0769w.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang S, Yan C, Zhang X, Shi D, Chi L, Luo G, Deng J. Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomat Sci. 2018;6:2757–2772. doi: 10.1039/C8BM00807H. [DOI] [PubMed] [Google Scholar]
- 77.Guo Y, Xun M, Han J. A bovine myeloid antimicrobial peptide (BMAP-28) and its analogs kill pan-drug-resistant Acinetobacter baumannii by interacting with outer membrane protein a (OmpA) Medicine. 2018;97:e12832. doi: 10.1097/MD.0000000000012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin MF, Tsai PW, Chen JY, Lin YY, Lan CY. OmpA binding mediates the effect of antimicrobial peptide LL-37 on Acinetobacter baumannii. PLoS One. 2015;10:e0141107. doi: 10.1371/journal.pone.0141107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang C, Zhao G, Wang S, Chen Y, Gong Y, Chen S, Xu Y, Hu M, Wang X, Zeng H, Wang A, Liu D, Su Y, Cheng T, Chen F, Wang J. A simplified derivative of human Defensin 5 with potent and efficient activity against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2018;62. [DOI] [PMC free article] [PubMed]
- 80.Sieprawska-Lupa M, Mydel P, Krawczyk K, Wojcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrob Agents Chemother. 2004;48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen W. Current advances and challenges in the development of Acinetobacter vaccines. Hum Vaccines Immunother. 2015;11:2495–2500. doi: 10.1080/21645515.2015.1052354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fajardo Bonin R, Chapeaurouge A, Perales J, da Silva JG, Jr, do Nascimento HJ, D’Alincourt Carvalho Assef AP, Moreno Senna JP. Identification of immunogenic proteins of the bacterium Acinetobacter baumannii using a proteomic approach. Proteomics Clin Appl. 2014;8:916–923. doi: 10.1002/prca.201300133. [DOI] [PubMed] [Google Scholar]
- 83.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, Doi Y, Adams MD, Russo TA, Spellberg B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS One. 2012;7:e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Badmasti F, Ajdary S, Bouzari S, Fooladi AA, Shahcheraghi F, Siadat SD. Immunological evaluation of OMV (PagL)+bap(1-487aa) and AbOmpA(8-346aa)+bap(1-487aa) as vaccine candidates against Acinetobacter baumannii sepsis infection. Mol Immunol. 2015;67:552–558. doi: 10.1016/j.molimm.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 85.Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012;162:171–182. doi: 10.1016/j.jbiotec.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ansari H, Doosti A, Kargar M, Bijanzadeh M, Jaafarinia M. Cloning of ompA gene from Acinetobacter baumannii into the eukaryotic expression vector pBudCE4.1 as DNA vaccine. Indian J Microbiol. 2018;58:174–181. doi: 10.1007/s12088-017-0705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ansari H, Tahmasebi-Birgani M, Bijanzadeh M, Doosti A, Kargar M. Study of the immunogenicity of outer membrane protein a (ompA) gene from Acinetobacter baumannii as DNA vaccine candidate in vivo. Iran J Basic Med Sci. 2019;22:669–675. doi: 10.22038/ijbms.2019.30799.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Casadevall A. Antibody-based therapies for emerging infectious diseases. Emerg Infect Dis. 1996;2:200–208. doi: 10.3201/eid0203.960306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weisman LE, Cruess DF, Fischer GW. Opsonic activity of commercially available standard intravenous immunoglobulin preparations. Pediatr Infect Dis J. 1994;13:1122–1125. doi: 10.1097/00006454-199412000-00010. [DOI] [PubMed] [Google Scholar]
- 90.McConnell MJ. Where are we with monoclonal antibodies for multidrug-resistant infections? Drug Discov Today. 2019;24:1132–1138. doi: 10.1016/j.drudis.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 91.Wang-Lin SX, Olson R, Beanan JM, MacDonald U, Balthasar JP, Russo TA. The capsular polysaccharide of Acinetobacter baumannii is an obstacle for therapeutic passive immunization strategies. Infect Immun. 2017;85. [DOI] [PMC free article] [PubMed]
- 92.Karaiskos I, Lagou S, Pontikis K, Rapti V, Poulakou G. The “old” and the “new” antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front Public Health. 2019;7:151. doi: 10.3389/fpubh.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang W, Zhang Q, Li W, Chen Y, Shu C, Li Q, Zhou J, Ye C, Bai H, Sun W, Yang X, Ma Y. Anti-outer membrane vesicle antibodies increase antibiotic sensitivity of Pan-drug-resistant Acinetobacter baumannii. Front Microbiol. 2019;10:1379. doi: 10.3389/fmicb.2019.01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.