Abstract
Reactive oxygen species (ROS), associated with oxidative stress, are involved in many biological processes such as apoptosis, necrosis, and autophagy. Oxidative stress might induce neuronal damage via ROS generation, causing neurodegenerative diseases. Erigeron annuus (EA) has antioxidant properties and could protect neurons from oxidative stress. In this study, we investigated the protective effect of the aerial parts (EAA) and flowers (EAF) from EA on ROS-mediated apoptosis in pheochromocytoma 12 cells. We quantified 18 types of phenolic compounds using high-performance liquid chromatography. Pretreatment of the cells with EAA and EAF attenuated ROS generation and induced the expression of antioxidant enzymes such as superoxide dismutase 2, catalase, and glutathione peroxidase. In addition, EAF reduced the expression of apoptotic proteins such as Bax/Bcl-xL, caspase-3, and caspase-8 to a greater extent than that with EAA. These results suggested that the protective effect of EAF against oxidative stress-induced apoptosis might be due to the prevention of ROS generation mediated by oxidative enzymes.
1. Introduction
Reactive oxygen species (ROS) play an important role in regulating normal physiological and developmental functions such as cell cycle progression, proliferation, differentiation, migration, and cell death. ROS are generated in the mitochondria as byproducts of cellular metabolism [1]. Oxidative stress induced by ROS, such as superoxide (O2) or hydrogen peroxide (H2O2), has been associated with several pathologies and diseases such as diabetes, arthrosis, and Alzheimer's and Parkinson's diseases [2]. When the production of ROS surpasses the cellular antioxidant capacity, damage to macromolecules such as protein and DNA contributes to cell toxicity or apoptosis directly or indirectly [1–3]. Among the enzymes that are involved in ROS generation, catalase (CAT) and glutathione peroxidase (GPx) convert H2O2 to H2O; meanwhile, superoxide dismutase (SOD) converts O2 to H2O2 [4, 5]. Additionally, the SOS response has the effect of eliminating the ROS reaction, only if its enzymatic activity interacts with that of CAT and/or CAT.
Apoptosis is controlled by extrinsic and intrinsic pathways (mitochondrial pathway) [6]. ROS-mediated mechanisms drive apoptosis through intrinsic pathways to regulate cell death [3]. The intrinsic apoptosis pathway consists of intracellular signaling between proapoptotic proteins. For example, the Bcl-2 family includes proteins such as the antiapoptotic activator Bcl-xL and the proapoptotic effector Bax, which interacts with other proteins [7]. Additionally, overexpression of the antiapoptotic protein Bcl-xL, which appears to be bound to the mitochondrial membrane, can block apoptosis [8]. Conversely, Bax causes apoptosis by inducing the release of cytochrome-c, a key component of the mitochondria electron transport chain, into the cytoplasm [8, 9]. This intrinsic pathway induces caspase-independent cell death, as it is controlled by Bcl-2 family proteins such as Bax and Bcl-xL [7]. In contrast, procaspase-8 is the apical caspase in the extrinsic pathway, for which activation occurs within the DISC (death-inducing signaling complex), and proceeds by directly activating procaspase-3 [6]. Procaspase-3 exists in the cytosol as an inactivated dimer, and the activation of procaspase-3 takes places after the cleavage of caspase-8 [10]. Cleaved caspase-3 acts as a direct executioner of apoptosis [10].
Erigeron annuus (EA), which belongs to the Asteraceae family, has white flowers and is commonly found in grasslands and roadsides. In addition, EA has traditionally been used as a medicinal plant for dyspepsia, abdominal pain, urine bleeding, and hypoglycemic effects [11]. Many compounds such as flavanone, erigeroflavanone, sesquiterpenoids, ergosterol peroxide, caffeic acid, and pyromeconic acid can be derived from the aerial part (EAA) and flowers (EAF) of EA [12–17]. It has been reported that these compounds have several activities such as reductase inhibitory in aldose, antiatherosclerotic, neuroprotective, antioxidant, and cytoprotective effects [12, 14–17]. Although several studies have demonstrated the effect of EA as an antioxidant and neuroprotective agent, studies on its effect against damage to neuronal cells due to oxidative stress are scarce. In this study, we demonstrated that EAA and EAF can effectively block the intrinsic and extrinsic apoptosis pathways via ROS-mediated signaling. Our data suggest that EAA and EAF could inhibit ROS mediated-apoptosis in PC12 cells under oxidative stress by upregulating the expression of antioxidant enzymes and downregulating apoptotic proteins.
2. Materials and Methods
2.1. Chemicals, Antibodies, and Apparatus
All reagents were purchased from Sigma Aldrich (Saint Louis, MO, USA), unless otherwise indicated. CellTiter 96® AQueous One Solution (MTS) was obtained from Promega (Madison, WI, USA). Pheochromocytoma (PC12) cells were purchased from the ATCC (Manassas, VA, USA). All cell culture reagents were obtained from Gibco (Burlington, ON, Canada). Radio immunoprecipitation (RIPA) cell lysis buffer was purchased from GenDepot (Katy, TX, USA). Bradford and enhanced chemiluminescence (ECL) reagents for protein assays were from Bio-Rad (CA, USA). All antibodies were from Abcam (Cambridge, UK), unless otherwise stated. Antibodies against β-actin, cleaved caspase-8, and caspase-8 were purchased from Santa Cruz (Dallas, TX, USA). Meanwhile, anticleaved caspase-3 was purchased from Cell Signaling (Danvers, MA, USA). Evaporation was conducted using an evaporator system under reflux in vacuo from BÜCHI (Sankt Gallen, Swiss). Multimode-plate reading was performed with a Synergy H1 Hybrid Reader from BioTek Instruments (Winooski, VT, USA). The confocal microscope for fluorescent imaging was purchased from Zeiss (Oberkochen, German). Protein expression levels were assessed with a chemiluminator from Davinch-K (Seoul, Korea). Analysis was performed using a high-performance liquid chromatography (HPLC) 2790/5 system equipped with a photodiode array (PDA) 2996 from Waters (Milford, MA, USA). The INNO column was obtained from Young Jin Biochrom Co., Ltd., (Seoul, Korea). Acetonitrile and water labeled as HPLC grade solvents were purchased from Fisher Scientific Ltd., (Sunnyvale, CA, USA).
2.2. Plant Materials, Extraction, and Yields
EA samples were collected from Eumsung (Chungcheongbuk-do, Korea) in 2018. The plant material was identified by Jeong Hoon Lee, PhD. (Department of Herbal Crop Research), and the voucher specimen (NIBRVP0000456433) was deposited at National Institute of Biological Resources. They were then divided into two groups, the aerial part (stem and leaves, EAA) and flower part (EAF). For the preparation of extracts, EAA and EAF (100 g, each) were grinded, sifted through a testing sieve (aperture 1.40 mm, wire 0.71 mm), and extracted three times with 70% ethanol at a 1 : 10 (v : v) ratio for 24 h, at room temperature. After filtration, all extracts were evaporated in vacuo, freeze-dried (20 mTorr, −40°C, 1 week), and stored at −80°C. Extraction yields were calculated as previously described [18] as follows: yields (%) = total extract dried weight/raw material weight × 100.
2.3. Analysis of Antioxidant Components
2.3.1. Determination of Total Phenolic Contents (TPC)
TPCs of extracts were determined by the Folin–Denis's phenol method, with slight modifications [19]. Specifically, 500 μL of each extract (2 mg/mL) was mixed with 50 μL of 1 N Folin–Ciocalteu reagent for 3 min. Then, 100 μL of 20% sodium carbonate solution was added. After 1 h, the absorbance was measured at 725 nm using a multimode-plate reader. TPC was calculated from the calibration curve using gallic acid, and the results were expressed as gallic acid equivalents (y = 0.064x + 0.024).
2.3.2. Determination of Total Flavonoid Contents (TFC)
TFCs of each extract were determined by the aluminum chloride colorimetric method, with slight modifications [20]. Briefly, 150 μL of each extract (2 mg/mL) was mixed with 10 μL of 10% aluminum chloride solution, 10 μL of 1 M potassium acetate, and 280 μL of distilled water. The mixture was kept at room temperature for 30 min and then measured at 415 nm using a multimode-plate reader. TFC was expressed as quercetin equivalents (y = 0.001x + 0.015), which reflected the amount of quercetin (μg/mL).
2.4. Antioxidant Activity Assay
2.4.1. ABTS+ Radical Scavenging Assay
ABTS+ (2,2′-azino-bis 3-ethylbenzothiazolin-6-sulfonic acid) radical scavenging activity was measured as described by Van den Berg, with some modifications [21]. ABTS+ solutions containing 7.4 mM 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) ammonium salt and 2.6 mM potassium persulfate were prepared in distilled water for 24 h. The absorbance of the solution was adjusted to 0.70 ± 0.05 at 732 nm. Next, 20 μL samples were mixed with 180 μL of ABTS+ solution and incubated for 30 min in the dark at room temperature. Absorbance values of ABTS radicals were measured for the different samples using a multimode-plate reader at 732 nm. The following samples were tested: Treatment, Blank1, Blank2, and Control corresponded to the sample, H2O, H2O + sample, respectively. An ABTS radical scavenging assay (RSA) was performed for each concentration according to the following equation: ABTS+ RSA (%) = [(Acontrol − ABlankl1) − (ATreatment − ABlank2)/Acontrol − ABlankl1] × 100. Additionally, the half-maximal inhibitory concentration (IC50) was calculated for each extract.
2.4.2. DPPH Radical Scavenging Assay
DPPH (2,2-diphenyl-1-picryllydrazyl) radical scavenging activity was measured according to the Bondet method, with some modifications [22]. DPPH solution containing 300 μM of DPPH in 95% ethanol was prepared. The absorbance of the solution was adjusted to 1.00 ± 0.05 at 515 nm. Next, 25 μL of samples was mixed with 225 μL of DPPH solution and incubated for 30 min in the dark covered with aluminum foil at room temperature. Absorbance was measured using a multimode-plate reader at 515 nm. Among the samples tested were the following: Treatment, Blank1, Blank2, and Control corresponding to the sample, H2O, and H2O + sample, respectively. DPPH radical scavenging activity (RSA) was calculated for each concentration using the following equation: DPPH RSA (%) = [(Acontrol − ABlankl1) − (ATreatment − ABlank2)/Acontrol − ABlankl1] × 100. The half-maximal inhibitory concentration (IC50) was also calculated for each extract.
2.5. HPLC Analysis of Antioxidant Compounds
2.5.1. Sample Preparation for HPLC
To investigate phenolic compounds in EAA and EAF (5 g), the modified method of Ahn et al. was applied [23]. Each extract was redissolved in water and fractionated with ethyl acetate/ether (1 : 1 = v/v) to obtain the phenol-rich fraction. Each fraction was concentrated under reduced pressure, dissolved in methanol (10 mg/mL each), filtered through a 0.22 μM polyvinylidine difluoride (PVDF) membrane, and analyzed by HPLC.
2.5.2. HPLC for Phenolic Compound Analysis
Antioxidant components and phenolic compounds were analyzed by a Waters HPLC 2790/5 system equipped with PDA by Waters 2996. HPLC separation of compounds for qualitative and quantitative analysis was performed using a reverse phase system with an INNO column at 35°C. Homogentisic acid, gallic acid, protocatechuic acid, chlorogenic acid, (+)-catechin, caffeic acid, phloretic acid, p-coumaric acid, ferulic acid, veratric acid, salicylic acid, naringin, hesperidin, quercetin, cinnamic acid, naringenin, kaempferol, and hesperidin were used as phenolic compound standards. All standards were prepared in methanol and filtered through a 0.22 μM PVDF membrane. The mobile phase consisted of solvent A (0.5% acetic acid in water) and solvent B (0.5% acetic acid in acetonitrile). The gradient program with the mobile phase was modified [23]. The elution program was as follows (B %): 8–10%, 2 min; 10–30%, 27 min; 30–90%, 50 min; 90–100%, 51 min; 100%, 60 min; 100–8%, 62 min; and 8%, 70 min. The injection volume was 10 μL, the flow rate was 1.0 mL/min, and phenols were detected at 280 nm using UV light.
2.6. Cell Culture
PC12 cells were cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37°C in a 5% CO2 incubator. The medium was replaced every 2 days for subculture. Cells from passages 5–10 were used in all experiments.
2.7. Intracellular ROS Generation
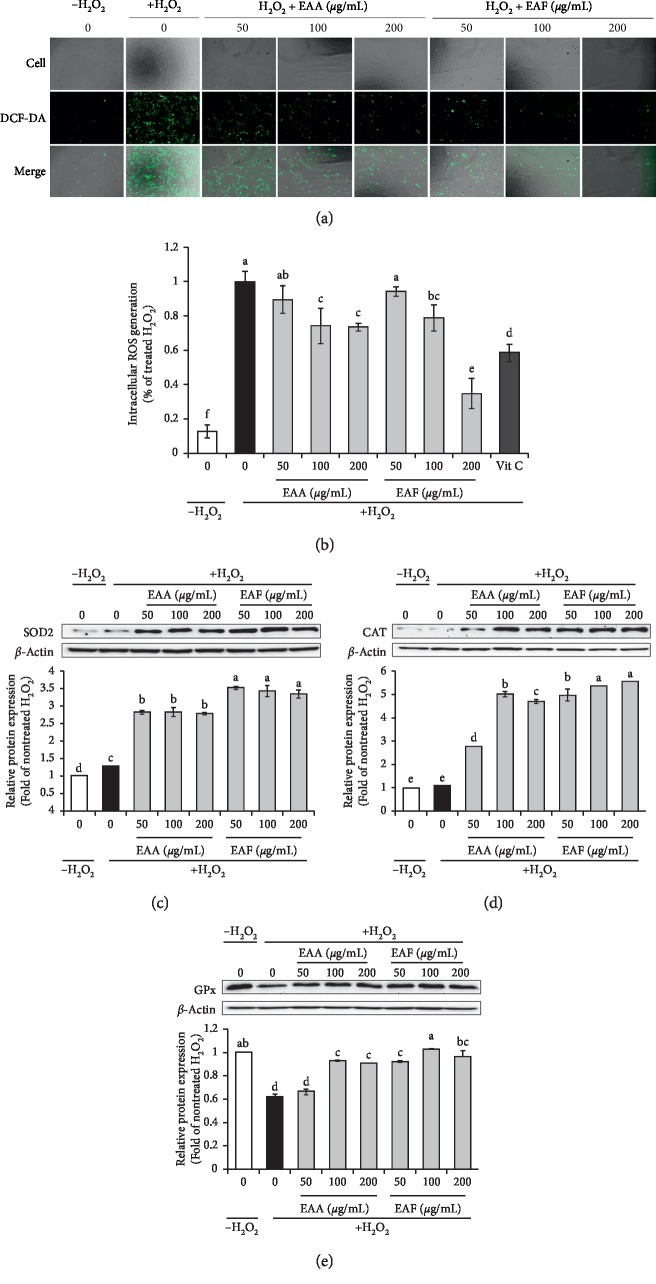
Intracellular ROS generation was measured by a modified dichloro-dihydro-fluorescein diacetate (DCFH-DA) method [24]. PC12 cells were cultured with EAA and EAF in black 96-well plates at a density of 1.0 × 104 cells/well. After 24 h, the culture cells were treated with 50 μM H2O2 in SFM for 20 min and then with 20 μM DCF-DA in serum-free medium for 30 min; samples were then washed, and 100 μL Dulbecco's phosphate buffered saline (DPBS) was added to each well. Vitamin C (10 μg/mL) was used as a positive control. Vitamin C is well known as an antioxidant and is widely used as a positive control for antioxidant studies. The fluorescence was measured with a multiplate reader at 485 nm/535 nm (excitation/emission). In addition, fluorescent micrographs were obtained with a confocal microscope.
2.8. Preparation of Protein Samples
PC12 cells were pretreated with EAA and EAF (50, 100, and 200 μg/mL) for 24 h and with H2O2 (50 μM) for 20 min. Then, cells were rinsed, scraped off, and collected with DPBS on ice. After centrifugation at 3000 rpm, the DPBS was removed completely, and cells were lysed with RIPA buffer for total protein extraction, following the manufacturer's instructions. Protein amounts were determined using the Bradford assay. Protein samples were mixed with 5x loading buffer, boiled for 5–10 min, and stored at −80°C.
2.9. Western Blotting
Western blotting was performed with 10–15% tris-HCl gels for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred onto PVDF membranes, washed three times, and subsequently incubated with primary antibodies (at a 1 : 1000 dilution) at 4°C overnight. The membranes were washed three times again and incubated with horseradish peroxidase-conjugated secondary antibody (at a 1 : 2000 dilution) for 1 h at room temperature [25]. Protein expression was visualized using the ECL reagent, following the manufacturer's instructions.
2.10. Statistical Analysis
All experimental data were expressed as means ± standard deviations (SDs) of three independent experiments. The statistical analyses in this study were performed using a one-way analysis of variance (ANOVA) with a Duncan's test, t-tests and correlation analysis based on Pearson's correlation coefficient (Statistical Package for the Social Sciences, ver. 21.0 for Window ver. 10).
3. Results
3.1. Antioxidant Components and Activities of EAA and EAF
It has been reported that 70% ethanol is the most effective solution to extract triterpenoids from Jatropha curcas leaves and phenols from Moringa oleifera and Curcuma longa extractions [26]. Therefore, the extraction of EAA and EAF rendered yields of 25.16% and 25.84%, respectively (Table 1). The compound contents were analyzed by measuring TPC and TFC. The concentrations of the phenolic compounds, namely, TPC and TFC, with antioxidant activity [27] were determined for each EA part, specifically for EAA (9.8 and 20.8 mg/g) and EAF (8.9 and 10.1 mg/g) (Table 1). Moreover, we calculated the levels of DPPH and ABTS+, indicative of antioxidant activity, by simple colorimetric methods [28]. The antioxidant activities of EAA and EAF were examined using ascorbic acid (AA) as the positive control [29]. IC50 values of EAA and EAF were 11.0 and 17.5 μg/mL for ABTS+ and 114.0 and 112.2 5 μg/mL for the DPPH-scavenging effect, respectively (Table 1).
Table 1.
Antioxidant components, activities, and yields of EAA and EAF.
| Samplesa | TPCb (GAE mg/g) | TFCc (QUE mg/g) | ABTS+ (IC50, μg/mL) | DPPH (IC50, μg/mL) | Yields (%) |
|---|---|---|---|---|---|
| EAA | 9.8 ± 0.1b | 8.9 ± 0.6b | 11.0 ± 0.6b | 114.0 ± 5.4a | 25.16 ± 0.86a |
| EAF | 20.8 ± 0.1a | 10.1 ± 0.1a | 17.5 ± 0.6b | 112.2 ± 2.9a | 25.84 ± 2.29a |
| AA | — | — | 34.1 ± 0.3a | 10.2 ± 0.3b | — |
aEAA: aerial parts of Erigeron annuus; EAF: flowers of Erigeron annuus; AA: ascorbic acid. bTPC: total phenol contents. cTFC: total flavonoid contents. All values are means ± SD. Means with different letters on the same column are significantly different at p < 0.05 by t-tests (for TPC, TFC, and yields) and Tukey's test (for ABTS+ and DPPH).
3.2. Quantification of Phenolic Compounds from EAA and EAF by HPLC
Phenolic compounds, which are known to be responsible for the beneficial effects of EAA and EAF, were analyzed by HPLC using a PDA detector. For the determination of phenolic compounds, EAA and EAF were reextracted from phenol-rich fractions. The chromatograms of phenolic compounds and phenol-rich fractions of EAA and EAF are shown in Figure 1. The homogentisic acid peak was the highest for both EAA and EAF and the peak of kaempferol was higher with EAF than with EAA. In addition, gallic, phloretic, and ferulic acids were not detected, but veratric and cinnamic acids were detected specifically in EAF. Phenolic compound contents in EAA and EAF were identified based on the calibration curve (Tables 2 and 3). The total phenolic compound (TP) contents of EAF (97.32 mg/g, 1.43-fold), as analyzed by HPLC, were higher than those of EAA, and these results were similar for TPC and TFC. The contents of homogentisic acid (1.11-fold), protocatechuic acid (1.18-fold), (+)-catechin (3.03-fold), naringin (2.36-fold), and naringenin (1.79-folds) were higher in EAA than in EAF. Conversely, the contents of chlorogenic acid (2.06-fold), p-coumaric acid (1.47-fold), salicylic acid (5.89-fold), hesperidin (2.68-fold), quercetin (3.60-fold), and kaempferol (21.31-fold) were higher in EAF than in EAA. As a result, EAA and EAF might have higher scavenging effects because of these phenolic compounds.
Figure 1.
The chromatograms of phenolic compounds from EAA and EAF based on HPLC analysis. Peak identification: 1: homogentisic acid, 2: gallic acid, 3: protocatechuic acid, 4: chlorogenic acid, 5: (+)-catechin, 6: caffeic acid, 7: phloretic acid, 8: p-coumaric acid, 9: ferulic acid, 10: veratric acid, 11: salicylic acid, 12: naringin, 13: hesperidin, 14: quercetin, 15: cinnamic acid, 16: naringenin, 17: kaempferol, and 18: hesperidin. Chromatogram of (a) standard solution used for phenolic compound analysis (100 μg/mL each), (b) EAA (10 mg/mL), and (c) EAF (10 mg/mL). EAA: Erigeron annuus aerial parts; EAF: E. annuus flowers.
Table 2.
Phenolic compounds contents of EAA and EAF analyzed by HPLC (n = 3).
| Number | Phenolic compounds | Contents (mg/g dried weight) | |
|---|---|---|---|
| EAAa | EAFb | ||
| 1 | Homogentisic acid | 53.75 ± 1.51a | 48.42 ± 4.15a |
| 2 | Gallic acid | N.D.c | N.D. |
| 3 | Protocatechnic acid | 0.73 ± 0.01e | 0.62 ± 0.00d |
| 4 | Chlorogenic acid | 2.02 ± 0.02c | 0.98 ± 0.01d |
| 5 | (+)-Catechin | 2.09 ± 0.26c | 0.69 ± 0.02d |
| 6 | Caffeic acid | 0.64 ± 0.01e | 0.92 ± 0.02d |
| 7 | Phloretic acid | N.D. | N.D. |
| 8 | p-Coumaric acid | 0.61 ± 0.02e | 0.90 ± 0.02d |
| 9 | Ferulic acid | N.D. | N.D. |
| 10 | Veratric acid | N.D. | 0.85 ± 0.03d |
| 11 | Salicylic acid | 3.63 ± 0.18b | 21.40 ± 1.48b |
| 12 | Naringin | 1.74 ± 0.07cd | 0.74 ± 0.03d |
| 13 | Hesperidin | 1.00 ± 0.03de | 2.69 ± 0.21d |
| 14 | Quercetin | 0.52 ± 0.00e | 1.88 ± 0.02d |
| 15 | Cinnamic acid | N.D. | 0.89 ± 0.07d |
| 16 | Naringenin | 0.54 ± 0.00e | 0.42 ± 0.00d |
| 17 | Kaempferol | 0.75 ± 0.01e | 15.94 ± 1.47c |
| 18 | Hesperitin | N.D. | N.D. |
|
| |||
| Total contents | 68.02 ± 1.90 | 97.21 ± 7.52 | |
aEAA: aerial parts of Erigeron annuus, bEAF: flowers of Erigeron annuus, cN.D.: not detected. All values are means ± SD. Means with different letters on the same line (to analyze EAA and EAF) are significantly different at p < 0.05 by Duncan's test.
Table 3.
Retention time and calibration curves of standards (n = 3).
| Number | Phenolic compounds | Rta (min) | R2b | Calibration curvec (Y = aX + b) |
|---|---|---|---|---|
| 1 | Homogentisic acid | 5.07 | 1.00 | Y = 15266X − 261028 |
| 2 | Gallic acid | 6.37 | 0.99 | Y = 12344X − 257389 |
| 3 | Protocatechnic acid | 7.67 | 1.00 | Y = 17658X − 339681 |
| 4 | Chlorogenic acid | 10.16 | 1.00 | Y = 21148X − 461992 |
| 5 | (+)-Catechin | 10.26 | 1.00 | Y = 3427.1X − 41775 |
| 6 | Caffeic acid | 12.49 | 1.00 | Y = 45912X − 904096 |
| 7 | Phloretic acid | 15.58 | 0.99 | Y = 6986.6X − 173117 |
| 8 | p-Coumaric acid | 16.95 | 1.00 | Y = 109086X − 2095025 |
| 9 | Ferulic acid | 18.92 | 1.00 | Y = 52036X − 996550 |
| 10 | Veratric acid | 19.76 | 1.00 | Y = 12550X − 234385 |
| 11 | Salicylic acid | 21.13 | 1.00 | Y = 5350X − 102288 |
| 12 | Naringin | 22.25 | 0.99 | Y = 11740X − 158668 |
| 13 | Hesperidin | 23.12 | 1.00 | Y = 3197X − 57859 |
| 14 | Quercetin | 30.61 | 1.00 | Y = 11756X − 257156 |
| 15 | Cinnamic acid | 31.90 | 1.00 | Y = 112897S − 2E + 06 |
| 16 | Naringenin | 33.88 | 0.99 | Y = 47325X − 691049 |
| 17 | Kaempferol | 34.69 | 1.00 | Y = 27333X − 605965 |
| 18 | Hesperitin | 34.97 | 0.99 | Y = 31446X − 426773 |
aRt: retention time; bcorrelation coefficients for three data points in the calibration curve; cwhere the Y and X are the peak area and concentration of the analyses (μg/mL), respectively.
3.3. EAA and EAF Reduce Intracellular ROS Generation in PC12 Cells
ROS production was analyzed by staining with DCFH-DA and detecting and quantitatively measuring the fluorescence by confocal microscopy (Figure 2(a)). Incubation of PC12 cells with H2O2 led to an increase in DCF fluorescence intensity, which was proportional to the amount of ROS generated. However, after treatment with EAA and EAF, the green fluorescence signal of H2O2-treated cells decreased compared with that observed for the controls. The addition of vitamin C (50 μg/mL) served as a positive control with an inhibition rate of approximately 60%. EAF (200 μg/mL) resulted in a more pronounced reduction than the positive control. These results showed that ROS generation was significantly elevated in a dose-dependent manner (Figure 2(b)). Intracellular ROS generation was reduced to a greater extent after pretreating PC12 cells, specifically up to 7.87-fold. The levels of intracellular ROS generated in cells treated with EAA and EAF (50, 100, and 200 μg/mL) decreased in a concentration-dependent manner compared with those in cells treated with H2O2 only (EAA, 74.27 and 73.62%; EAF, 78.81 and 34.92%).
Figure 2.
EAA and EAF protect PC12 cells from H2O2-induced oxidative injury. (a) The cells were treated with EAA and EAF (50, 100, and 200 μg/mL) for 24 h and then treated with DCFH-DA (20 μM) for 30 min. The intracellular levels of ROS were visualized using fluorescent confocal microscopy. (b) Quantitative measurement of intracellular ROS generation. (c–e) Cells were treated with EAA and EA (50, 100, and 200 μg/mL) for 24 h. Vitamin C (ascorbic acid) was positive control (50 μg/mL). The expression levels of SOD2 (c), CAT (d), and GPx (e) were detected by western blot analysis in PC12 cells. All protein expression levels were quantified by normalizing to β-actin levels. All columns are means ± SD (n = 3). Means with different letters on the all-color columns were significantly different at p < 0.05 based on Duncan's test. EAA: Erigeron annuus aerial parts; EAF: E. annuus flowers.
3.4. EAA and EAF Reduce Levels of Oxidative Stress-Related Proteins
The antioxidant protein levels of SOD2, CAT, and GPx correlate with reductions in ROS [30]. Therefore, we investigated the effect of EAA and EAF on the expression levels of these oxidative proteins in H2O2-treated PC12 cells (Figures 2(c)–2(e)). SOD2 activity was significantly increased by H2O2 (1.28-fold) and further increased in the presence of EAA and EAF (2.78–2.83- and 3.35–3.52-fold, respectively) in H2O2-treated PC12 cells. In contrast, H2O2 significantly maintained or reduced the levels of the antioxidant enzymes CAT and GPx (1.07- and 0.61-fold, respectively). Meanwhile, EAA and EAF enhanced the activities of CAT (2.79–4.70- and 4.96–5.54-fold, respectively) and GPx (0.66–0.92- and 0.92–1.02-fold, respectively) in H2O2-treated PC12 cells.
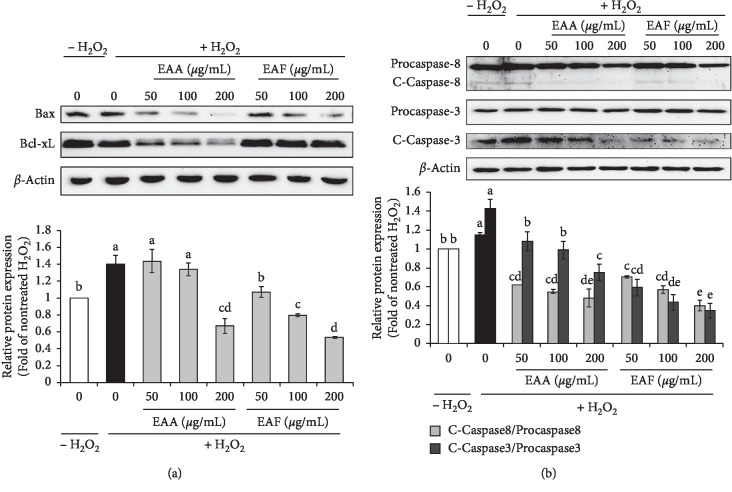
3.5. Inhibitory Effects of EAA and EAF on Apoptosis in H2O2-Treated PC12 Cells
We finally investigated whether ROS affects EAA- and EAF-induced apoptosis based on the two major pathways (intrinsic and extrinsic). To determine the effect of EAA and EAF on apoptosis in H2O2-treated PC12 cells, protein levels of components of the intrinsic and extrinsic pathways were evaluated by western blotting. Results from the intrinsic pathway are shown in Figure 3(a). Specifically, treatment with H2O2 only upregulated the expression of Bax/Bcl-xL compared with that in untreated cells. Exposure of H2O2-treated PC12 cells to EAA and EAF reduced the expression of Bax/Bcl-xL (0.09–0.67-fold) in a dose-dependent manner. Results of the extrinsic pathway are shown in Figure 3(b). In this investigation, the cells treated with H2O2 only showed increased levels of cleaved-caspase8 and cleaved-caspase 3 (1.20- and 1.31-fold, respectively) compared with those in untreated cells. However, our results showed that EAA and EAF reduced pro- and cleaved-caspase8 and cleaved-caspase 3 levels in a dose-dependent manner.
Figure 3.
EAA and EAF protect PC12 cells from H2O2-induced oxidative apoptosis. (a) Protein expression related to the intrinsic pathway of apoptosis (Bax/Bcl-xL). (b) Protein expression related to the extrinsic pathway (cleaved- (C-) pro-caspase-8 and -3). Cells were treated with EAA and EA (50, 100, and 200 μg/mL) for 24 h. Protein expression levels were detected by western blot analysis in PC12 cells. Protein expression was quantified based on normalized β-actin levels. All columns are means ± SD (n = 3). Means with different letters on the all-color column were significantly different at p < 0.05 based on Duncan's test. EAA: Erigeron annuus aerial parts; EAF: E. annuus flowers.
4. Discussion
Air pollution caused by ambient air particulate matter such as diesel exhausts particles, and nitrate induces oxidative stress that can trigger ROS [31, 32]. ROS generates toxic byproducts that cause damage to the cell by producing oxidative stress; however, the cell expresses several antioxidant enzymes to protect against this effect [1]. The overexpression of ROS causes an imbalance between oxidant production and antioxidant capacity and leads to the onset of neurodegenerative diseases such as Alzheimer's and Parkinson's diseases [2]. ROS can decrease through nonenzymatic and enzymatic reactions [30]. Nonenzymatic antioxidants such as phenolic acids, flavonoids, and other compounds increase the activity of glutathione peroxidase GSH (which reduces H2O2 to H2O) [30].
Phenolic compounds are present in various medicinal plants [33]. The phenolic hydroxyl groups in phenolic compounds play an important role in antioxidant properties by donating hydrogens and scavenging radicals [34]. Therefore, we identified antioxidants in the phenol-rich fractions of EAA and EAF by HPLC analysis. Linearity of the calibration curve with a correlation coefficient >0.99 was obtained from the plot of a minimum of five standard concentrations (10, 25, 50, 100, and 200 μg/mL) by using the least square method (Table 3) [35]. Homogentisic and salicylic acids, which were abundant in both EAA and EAF, confer beneficial effects such as the inhibition of oxidation, anti-inflammation [36–38], and disease tolerance in plants [39]. Kaempferol, which was significantly present in EAF, exerts other beneficial effects such as anticancer cell proliferation, in vitro antioxidation, and the inhibition of autophagy [40–43]. These phenolic and flavonoid compounds produced by photosynthesis are stored in plant leaves and accumulate in the vacuoles of flowers [44]. Indeed, phenols and flavonoids in plants have antioxidant effects by transferring hydroxyl groups to the radicals in ABTS+ and DPPH and participating in electron delocalization, thus providing stability [45]. Our results suggested that the antioxidative effects of EAA and EAF might be due to their TPC and TFC contents. Importantly, antioxidant contents and activities were not significantly different between EA parts. However, EAA and EAF have stronger antioxidant activities than AA in ABTS+ scavenging (3.1- and 1.9-fold, respectively). The correlation between antioxidant activities (ABTS+ and DPPH) and the content of TPs and phenolic compounds (homogentisic acid, salicylic acid, and kaempferol) in EAA and EAF was also investigated (Table 4). The antioxidant effect of ABTS+ was significantly correlated with TPAs (−0.921), salicylic acid (−0.975), and kaempferol (−0.0695). These results showed that the antioxidant effects on ABTS+ and DPPH were due to TPs, salicylic acid, and kaempferol contained in EAA and EAF.
Table 4.
Correlation analysis of antioxidant components and activities.
| Factorsa | ABTS | DPPH | TP | Homogentisic acid | Salicylic acid | Kaempferol | ROS |
|---|---|---|---|---|---|---|---|
| ABTS | 1.000 | −0.127 | −0.921∗∗ | −0.764 | −0.975∗∗ | −0.0968∗∗ | 0.929∗∗ |
| DPPH | 1.000 | 0.326 | −0.056 | 0.255 | 0.300 | −0.469 | |
| TP | 1.000 | −0.489 | 0.979∗∗ | −0.982∗∗ | −0.949∗∗ | ||
| Homogentic acid | 1.000 | −0.656 | −0.641 | 0.637 | |||
| Salicylic acid | 1.000 | −0.999∗∗ | −0.965∗∗ | ||||
| Kaempferol | 1.000 | −0.976∗∗ | |||||
| ROS | 1.000 |
aFactors: ABTS and DPPH was analyzed by IC50 value; TP, total contents of phenolic compounds; ROS, intracellular reactive oxygen species. Significance was determined using SPSS by Pierson's correlation coefficient; ∗p < 0.05 and ∗∗p < 0.01.
Enzymatic antioxidants, which decrease ROS generation (such as SOD2 (which reduces O2− to H2O2) and CAT and GPx (which reduce H2O2 to H2O)), are usually secreted into the cytosol to protect tissue damage due to oxidative stress [3, 30]. In addition, H2O2 generates intracellular ROS which regulate apoptosis [3]. To measure the inhibition of intracellular ROS generation in the presence of H2O2, we tested the application of various concentrations of EAA and EAF (50, 100, and 200 μg/mL) to PC12 cells after treating the cells with 50 μM of H2O2 for 20 min, as those conditions resulted in the highest ROS generation (data not shown). Our results showed that intracellular ROS generation was reduced in a dose-dependent manner after pretreatment with EAA and EAF, compared with that in PC12 cells only treated with H2O2 (Figures 2(a) and 2(b)). The maintenance of homeostasis in multicellular organisms depends on complex networks of intracellular signals [1]. Cell organelles generate intracellular ROS via oxidative stress injury [2]. When ROS are overproduced in cells, they cause various diseases in humans, including neurological diseases [1, 2]. However, ROS can be inhibited by antioxidant components [2]. For example, caffeic acid isolated from Erigeron annuus leaves reduced H2O2-induced ROS in PC12 cells. In addition, salicylic acid increases levels of antioxidant enzymes that reduce ROS generation such as CAT, SOD, and GPx. Meanwhile, kaempferol was found to protect HIT-T15 cells from oxidative damage [15, 42, 46]. Our results showed higher levels of TPC, TPF, and phenolic compounds, which have antioxidant effects, following the treatment of PC12 cells with EAF, compared with those observed with EAA treatment. Moreover, among TPs, salicylic acid and kaempferol were significantly correlated with ROS generation as shown in Table 4 (−0.965 and −0.976). Our study indicated that EAF contained more antioxidant compounds than EAA, and thus EAF further prevented oxidation in H2O2-treated PC12 cells.
Apoptosis-induced ROS comprises two major pathways, the intrinsic and extrinsic [6]. The proapoptotic members (Bcl-xL and Bax) release cytochrome c to the mitochondrial membrane through the intrinsic pathway [3]. In the extrinsic pathway, cleaved-caspase-8 cleaves and activates procaspase-3 [10]. Through these two pathways, caspase 3 directs cell death directly [10]. In our investigation, EAA and EAF reduced the levels of apoptosis-activating factors such as caspase-3 and caspase-8, as well as the Bax/Bcl-xL ratio. Specifically, these apoptotic factors were further decreased with EAF treatment compared with that with EAA. Antioxidant enzymes are known to prevent apoptosis via ROS and directly reduce ROS generation [3, 30]. EAF reduced apoptotic protein levels, and its antioxidant effect was higher than that of EAA, even in the presence of nonenzymatic antioxidants such as phenolic acid and flavonoid. Further, EAF downregulated the intracellular apoptosis pathways induced by ROS generation (Figures 2(c)–2(e) and 3).
5. Conclusions
The present study demonstrates that EAA and EAF have neuroprotective effects on PC12 cells. EAA and EAF exerted protective effects against apoptosis by targeting oxidant and antioxidant agents such as SOD2, CAT, and the GPx system. Specifically, EAF contained abundant phenolic compounds and had a higher antioxidant effect than EAA. EAF could exert its antiapoptotic effect by reducing the number of ROS and enzymatic factors. Therefore, it could be a good source of natural antioxidants to prevent neurodegenerative diseases arising from oxidative stress-induced neuronal damage by regulating the expression of apoptotic proteins.
Acknowledgments
This study was supported by the Cooperative Research Program for Agriculture Science and Technology Development (grant no. PJ01415802), the Rural Development Administration, Republic of Korea.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Ji Yeon Lee and Jeong-Yong Park contributed equally to this work.
References
- 1.Thannickal V. J., Fanburg B. L. Reactive oxygen species in cell signaling. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000;279(6):1005–1028. doi: 10.1152/ajplung.2000.279.6.l1005. [DOI] [PubMed] [Google Scholar]
- 2.Uttara B., Singh A., Zamboni P., Mahajan R. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon H.-U., Haj-Yehia A., Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5(5):415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B., Gutteridge J. M., Cross C. E. Free radicals, antioxidants, and human disease: where are we now? The Journal of Laboratory and Clinical Medicine. 1992;119(6):598–620. [PubMed] [Google Scholar]
- 5.Yu H., Ge Y., Wang Y., et al. A fused selenium-containing protein with both GPx and SOD activities. Biochemical and Biophysical Research Communications. 2007;358(3):873–878. doi: 10.1016/j.bbrc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Hongmei Z. Apoptosis and Medicine. London, UK: Intech; 2012. Extrinsic and intrinsic apoptosis signal pathway review. [DOI] [Google Scholar]
- 7.Lindenboim L., Yuan J., Stein R. Bcl-xS and Bax induce different apoptotic pathways in PC12 cells. Oncogene. 2000;19(14):1783–1793. doi: 10.1038/sj.onc.1203495. [DOI] [PubMed] [Google Scholar]
- 8.Desagher S., Martinou J.-C. Mitochondria as the central control point of apoptosis. Trends in Cell Biology. 2000;10(9):369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 9.Finucane D. M., Bossy-Wetzel E., Waterhouse N. J., Cotter T. G., Green D. R. Bax-induced caspase activation and apoptosis via CytochromecRelease from mitochondria is inhibitable by Bcl-xL. Journal of Biological Chemistry. 1999;274(4):2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 10.Boatright K. M., Salvesen G. S. Mechanisms of caspase activation. Current Opinion in Cell Biology. 2003;15(6):725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Jeong C. H., Nam E. K., Shim K. H. Chemical components in different parts of Erigeron annuus. Journal of the Korean Society of Food Science and Nutrition. 2005;34(6):857–861. doi: 10.3746/jkfn.2005.34.6.857. [DOI] [Google Scholar]
- 12.Yoo N. H., Jang D. S., Yoo J. L., et al. Erigeroflavanone, a flavanone derivative from the flowers ofErigeron annuuswith protein glycation and aldose reductase inhibitory activity. Journal of Natural Products. 2008;71(4):713–715. doi: 10.1021/np070489a. [DOI] [PubMed] [Google Scholar]
- 13.Iijima T., Yaoita Y., Kikuchi M. Five new sesquiterpenoids and a new diterpenoid from Erigeron annuus (L.) PERS., Erigeron philadelphicus L. and Erigeron sumatrensis RETZ. Chemical & Pharmaceutical Bulletin. 2003;51(5):545–549. doi: 10.1248/cpb.51.545. [DOI] [PubMed] [Google Scholar]
- 14.Kim D.-H., Jung S. J., Chung I.-S., et al. Ergosterol peroxide from flowers of Erigeron annuus L. as an anti-atherosclerosis agent. Archives of Pharmacal Research. 2005;28(5):541–545. doi: 10.1007/bf02977755. [DOI] [PubMed] [Google Scholar]
- 15.Jeong C.-H., Jeong H. R., Choi G. N., Kim D.-O., Lee U., Heo H. J. Neuroprotective and anti-oxidant effects of caffeic acid isolated from Erigeron annuus leaf. Chinese Medicine. 2011;6(1):p. 25. doi: 10.1186/1749-8546-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashidoko Y. Pyromeconic acid and its glucosidic derivatives from leaves of Erigeron annuus, and the siderophile activity of pyromeconic acid. Bioscience, Biotechnology, and Biochemistry. 1995;59(5):886–890. doi: 10.1271/bbb.59.886. [DOI] [PubMed] [Google Scholar]
- 17.Kim O. S., Kim Y. S., Jang D. S., Yoo N. H., Kim J. S. Cytoprotection against hydrogen peroxide-induced cell death in cultured mouse mesangial cells by erigeroflavanone, a novel compound from the flowers of Erigeron annuus. Chemico-Biological Interactions. 2009;180(3):414–420. doi: 10.1016/j.cbi.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Veggi P. C., Cavalcanti R. N., Meireles M. A. A. Production of phenolic-rich extracts from Brazilian plants using supercritical and subcritical fluid extraction: experimental data and economic evaluation. Journal of Food Engineering. 2014;131:96–109. doi: 10.1016/j.jfoodeng.2014.01.027. [DOI] [Google Scholar]
- 19.Fazio A., Plastina P., Meijerink J., Witkamp R. F., Gabriele B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chemistry. 2013;140(4):817–824. doi: 10.1016/j.foodchem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Chang C. C., Yang M. H., Wen H. M., Chern J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis. 2002;10(3):178–182. [Google Scholar]
- 21.van den Berg R., Haenen G. R. M. M., van den Berg H., Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chemistry. 1999;66(4):511–517. doi: 10.1016/s0308-8146(99)00089-8. [DOI] [Google Scholar]
- 22.Bondet V., Brand-Williams W., Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH.free radical method. LWT—Food Science and Technology. 1997;30(6):609–615. doi: 10.1006/fstl.1997.0240. [DOI] [Google Scholar]
- 23.Ahn Y. S., Lee E. S., Kim J. T. History of Korean Gardening. South Korea: The Korean Society of Horticulture; 2013. [Google Scholar]
- 24.Kim H.-J., Park C.-G., Varghese R., Lee J. Y., Kim Y., Sung G.-H. In-vitro antioxidative, antiinflammatory properties of Aurea helianthus leaf extract a Korean traditional medicinal plant. Saudi Journal of Biological Sciences. 2017;24(8):1943–1947. doi: 10.1016/j.sjbs.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood T., Yang P. C. Western blot: technique, theory, and trouble shooting. North American Journal of Medical Sciences. 2012;4(4):429–34. doi: 10.4103/1947-2714.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azwanida N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Medicinal & Aromatic Plants. 2015;4(3):3–8. doi: 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- 27.Nicholson R. L., Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annual Review of Phytopathology. 1992;30(1):369–389. doi: 10.1146/annurev.py.30.090192.002101. [DOI] [Google Scholar]
- 28.Park S. J. Antioxidant and anti-adipogenic effects of ethanolic extracts from Ixeris dentata Nakai. Culinary Science & Hospitality Research. 2014;20(1) doi: 10.20878/cshr.2014.20.1.011. [DOI] [Google Scholar]
- 29.Arrigoni O., De Tullio M. C. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta (BBA)—General Subjects. 2002;1569(1–3):1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 30.Apel K., Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55(1):373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 31.Hartz A. M. S., Bauer B., Block M. L., Hong J.-S., Miller D. S. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. The FASEB Journal. 2008;22(8):2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Risom L., Møller P., Blo L. Oxidative stress-induced DNA damage by particulate air pollution. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005;592(1-2):119–137. doi: 10.1016/j.mrfmmm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Heleno S. A., Martins A., Queiroz M. J. R. P., Ferreira I. C. F. R. Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chemistry. 2015;173:501–513. doi: 10.1016/j.foodchem.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 34.Van Hung P. Phenolic compounds of cereals and their antioxidant capacity. Critical Reviews in Food Science and Nutrition. 2016;56(1):25–35. doi: 10.1080/10408398.2012.708909. [DOI] [PubMed] [Google Scholar]
- 35.Guideline I. H. T. Validation of analytical procedures: text and methodology Q2 (R1). Proceedings of the International Conference on Harmonization; 2005; Geneva, Switzerland. pp. 11–12. [Google Scholar]
- 36.Rosa A., Tuberoso C. I. G., Atzeri A., Melis M. P., Bifulco E., Dessì M. A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chemistry. 2011;129(3):1045–1053. doi: 10.1016/j.foodchem.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 37.Wang S.-L., Li H.-T., Zhang L.-J., Lin Z.-H., Kuo Y.-H. Conversion of squid pen to homogentisic acid via Paenibacillus sp. TKU036 and the antioxidant and anti-inflammatory activities of homogentisic acid. Marine Drugs. 2016;14(10):p. 183. doi: 10.3390/md14100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z., Ma L., Zhang X., Xu L., Cao J., Jiang W. The effect of exogenous salicylic acid on antioxidant activity, bioactive compounds and antioxidant system in apricot fruit. Scientia Horticulturae. 2015;181:113–120. doi: 10.1016/j.scienta.2014.10.055. [DOI] [Google Scholar]
- 39.Malamy J., Klessig D. F. Salicylic acid and plant disease resistance. The Plant Journal. 1992;2(5):643–654. doi: 10.1111/j.1365-313x.1992.tb00133.x. [DOI] [Google Scholar]
- 40.Zhang Y., Chen A. Y., Li M., Chen C., Yao Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. Journal of Surgical Research. 2008;148(1):17–23. doi: 10.1016/j.jss.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyake M., Arai N., Ushio S., Iwaki K., Ikeda M., Kurimoto M. Promoting effect of kaempferol on the differentiation and mineralization of murine pre-osteoblastic cell line MC3T3-E1. Bioscience, Biotechnology, and Biochemistry. 2003;67(6):1199–1205. doi: 10.1271/bbb.67.1199. [DOI] [PubMed] [Google Scholar]
- 42.Lee Y. J., Suh K. S., Choi M. C., et al. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative damage. Phytotherapy Research. 2010;24(3):419–423. doi: 10.1002/ptr.2983. [DOI] [PubMed] [Google Scholar]
- 43.Kim C.-J., Shin S.-H., Kim B.-J., et al. The effects of kaempferol-inhibited autophagy on osteoclast formation. International Journal of Molecular Sciences. 2018;19(1):p. 125. doi: 10.3390/ijms19010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghasemzadeh A., Ghasemzadeh N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. Journal of Medicinal Plants Research. 2011;5(31):6697–6703. doi: 10.5897/jmpr11.1404. [DOI] [Google Scholar]
- 45.Rice-Evans C. A., Miller N. J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal S., Sairam R. K., Srivastava G. C., Meena R. C. Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biologia Plantarum. 2005;49(4):541–550. doi: 10.1007/s10535-005-0048-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.