Abstract
Background
Previous studies have demonstrated that P21 (WAF1/CIP1) is a valuable prognostic factor in several malignant tumors. However, it is not known whether P21 can predict the prognosis in patients with esophageal cancer (EC). The aim of this research was to investigate the contribution of P21 expression to the clinicopathological characteristics and of EC.
Methods
A systematic review and meta-analysis of study focusing on P21 expression, clinicopathological characteristics, and clinical outcomes in patients with EC was performed using seven databases (PubMed, Embase, Web of Science, and four Chinese databases). Pooled hazard ratios and odds ratios were used to explore the association between P21 expression, clinicopathological characteristics, and outcomes in patients with EC. The heterogeneity of the studies was classified by the I2 statistic. The sensitivity analysis was then utilized to assess the robustness of the results. Finally, the funnel plot and Begg's test were used to evaluate the publication bias.
Results
Forty-five studies with 3098 patients were eligible for inclusion in the meta-analysis. Thirty of these studies reported on clinicopathological characteristics and 15 on clinical outcomes. The pooled hazard ratio of 1.456 (95% confidence intervals 1.033–2.053, P = 0.032) for overall survival indicated that a low P21 expression level was an unfavorable prognostic factor for a clinical outcome in patients with EC. Furthermore, the pooled odds ratio confirmed an association between decreased P21 expression and poor clinicopathological characteristics, including differentiation, lymph node metastasis, invasion, and higher grade and clinical stage. Notably, high P21 expression was a significant predictor of a favorable response to chemotherapy. There was no evidence of publication bias.
Conclusion
Reduced P21 expression is associated with a poor outcome in patients with EC.
1. Introduction
Esophageal cancer (EC) is the seventh leading cause of cancer mortality worldwide and in 2016 accounted for 15,690 deaths in the United States alone [1]. EC is a complex disease that includes squamous cell carcinoma, adenocarcinoma, and other rarer histologic types. Risk factors are slightly different between the two major types but include sex, race, alcohol consumption, diet, and genetics [2–4]. Several genetic biomarkers are effective in predicting the prognosis of patients with EC, including TP53, CYCLIN D1, VEGF, COX-2, and HER-2 [5]. Moreover, treatment based on these molecular targets has improved survival outcomes in patients with this disease. For example, inhibitors of c-MET [6], EGFR [7], HER2 [8], and VEGR [9] have been demonstrated to extend survival in these patients. However, drug resistance remains a major concern, and not all patients benefit from targeted therapy. Therefore, novel biomarkers are required to provide insight into the molecular mechanism of EC, identify novel diagnostic methods, and increase the number of treatment options available.
P21 (WAF1/CIP1), a member of the P21/P27/P57 family, is a universal cell cycle inhibitor regulated by P53. P21 plays an essential role in the control of cell growth, terminal differentiation, stem cell phenotypes, apoptosis, and cellular stress response. P21 has also been reported to participate in the proliferation of all types of cells. The expression of P21 is altered by wild-type P53 when DNA is damaged, resulting in cell cycle arrest or apoptosis at the G1 checkpoint. P21 plays a vital role in limiting proliferation and tumor growth, and abnormal expression of this gene has been observed in various types of malignancy. Recent research by Xie and colleagues [10] suggests that overexpression of P21 is associated with a poor prognosis in patients with non-small-cell lung cancer, while the loss of P21 protein expression could be a significant predictor of disease progression in patients with pancreatic cancer [11]. A further study demonstrated that aberrant expression of the P21 protein is associated with vascular invasion, pathological disease stage, and overall survival in patients with gastric cancer [12]. Interestingly, Goan et al. reported that overexpression of P21 predicted an unfavorable survival outcome in patients with esophageal squamous cell carcinoma [13] while other researchers found a significant association of low P21 expression with shorter survival in patients with the disease [14, 15]. Furthermore, P21 was found to regulate apoptosis in acute myeloid leukemia cells and malignant glioma cells [16, 17]. Thus, although there is an association of P21 expression with various types of cancer, the impact of the P21 level on the disease progression and prognosis of EC remains controversial. Therefore, we performed a systematic review and meta-analysis to assess the potential contribution of P21 expression to the clinicopathological characteristics and prognosis of EC.
2. Method and Materials
2.1. Search Strategy
The PubMed, Embase, Web of Science, China National Knowledge Infrastructure, Chongqing VIP, SinoMed, and Wanfang databases were electronically searched up to 30 September 2019. The following search terms were used: (((((((((((((P21) OR CIP1) OR SDI1) OR WAF1) OR CAP20) OR CDKN1) OR CDKN1A) OR P21CIP1) OR MDA-6)) OR P21WAF1)) OR “cyclin-dependent kinase inhibitor P21”[Mesh])) AND ((“esophageal neoplasms”[MESH]) OR (((((esophageal cancer) OR esophageal carcinoma) OR esophageal tumor) OR esophageal malignan∗) OR esophageal neoplas∗).
2.2. Inclusion and Exclusion Criteria
Studies were eligible for inclusion in the meta-analysis if they met the following criteria: (1) the subjects were patients diagnosed with any type of EC; (2) P21 expression in tissue or serum was detected by Western blot, quantitative real-time polymerase chain reaction (PCR), immunohistochemistry, or RNA sequencing; (3) the association of the P21 expression level with clinicopathological characteristics or the prognosis of EC was investigated; (4) the study population included more than 20 patients with EC; and (5) publication was written in the Chinese or English language. The following exclusion criteria were applied: publication as a review, abstract, experimental study, or letter and no key data provided for the evaluation of the relationship between differential expression of P21 and the clinicopathological characteristics and survival outcomes in patients with EC.
2.3. Data Extraction and Quality Assessment
The following data were collected and tabulated: the surname of the first author, year of publication, histologic type, sample size, country, specimen type, P21 detection assay used, and the Newcastle-Ottawa Scale (NOS) score. The NOS score was used to assess the quality of the included studies as follows: >6, high quality; 5–6, medium quality; and <5, low quality.
2.4. Statistical Analysis
The pooled hazard ratio (HR) and 95% confidence intervals (CIs) were used to estimate the impact of P21 expression level on the survival outcome in patients with EC. The individual HRs and 95% CIs were extracted directly from the text by two investigators (JW and LL). A pooled HR > 1 and 95% CIs that did not overlap indicated a positive association between a lower P21 expression level and a poorer survival outcome. When the HR and 95% CIs for survival were not provided, estimates were calculated from the Kaplan-Meier curves according to the method described by Tierney et al. [18]. All data were extracted by two of the authors working independently (FW and LQ). The pooled ORs and associated 95% CIs were used to determine the association between the P21 expression level and the clinicopathological characteristics of patients with EC according to specimen type (tumor sample vs. normal control), age (younger vs. older), sex (male vs. female), differentiation (poor vs. well or moderate), tumor stage (III–IV vs. I–II), distant metastasis (yes vs. no), lymph node metastasis (yes vs. no), grade (G3–4 vs. G1–2), depth of invasion (III–IV vs. I–II), tumor size (large vs. small), tumor location (upper-middle vs. low), and clinical stage (III/IV vs. I/II). We also explored the relationship between P21 expression and other better-studied biomarkers of EC, including P53 and the apoptosis index. The ability of the P21 level to predict the efficacy of chemotherapy was analyzed by combining the ORs. As with the HRs, an OR > 1 indicated a positive correlation between decreased P21 expression and poor clinicopathological characteristics.
The I2 statistic was used to classify the heterogeneity of the studies as low (I2 < 30%), moderate (30% ≤ I2 < 60%), substantial (61% ≤ I2 < 75%), or high (I2 ≥ 75%) [19]. A P value for the I2 statistic less than 0.10 or I2 larger than 50% was defined as having statistically significant heterogeneity, and thus, a random-effect model was used. In contrast, a fixed-effect model was used when heterogeneity was not significant. Publication bias was quantified by Begg's test and funnel plot analyses [20]. All statistical analyses were performed using Stata (version 12, StataCorp, College Station, TX, USA).
3. Results
3.1. Eligible Studies
The literature search yielded 1523 citations in total. After removing 907 duplicates, 606 articles were deemed eligible for further evaluation. After screening the titles and abstracts, a further 539 studies were excluded, leaving 74 articles for full-text review. Finally, 45 studies involving 3098 patients with EC were included in the meta-analysis (Figure 1) [13–15, 21–62]. All studies were published between 1997 and 2016 and assessed the correlation between abnormal P21 expression and outcomes in patients with EC (Table 1). Thirty studies focused on the association of the P21 expression level with clinicopathological characteristics, and 15 assessed the ability of the P21 expression level to predict overall survival (Table 2). Twenty-seven studies were performed in China and 11 in Japan. Most of the included studies detected the P21 level by immunohistochemistry with cutoff values ranging from 1% to 50%, while the remaining studies used real-time PCR or Western blotting. Six studies with a score of 9 and 13 studies with a score of 8 were considered high quality, and 7 studies with NOS scores < 7 were considered low quality.
Figure 1.
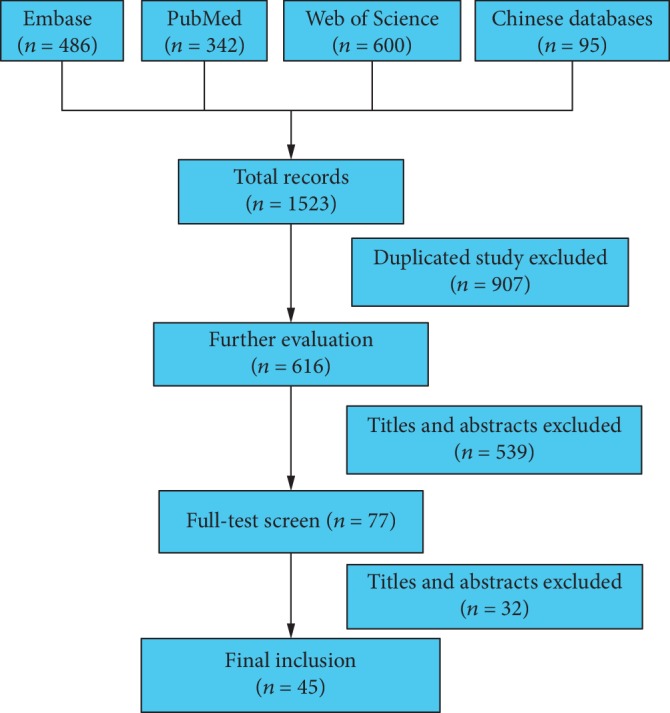
Flowchart of the study selection.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study and year | Year | Cancer | Sample size | Country | Specimen type | Cutoff value | Method | NOS quality score |
|---|---|---|---|---|---|---|---|---|
| Wu 1995 | 1995 | Esophageal cancer | 40 | China | Tissue | NA | Immunohistochemistry | 7 |
| Ohashi 1997 | 1997 | SCC | 25 | Japan | Tissue | NA | Immunohistochemistry | 6 |
| Toh 1997 | 1997 | SCC | 61 | Japan | Tissue | 10% | Immunohistochemistry | 7 |
| Jiang 1998 | 1998 | SCC | 46 | China | Tissue | 10% | Immunohistochemistry | 7 |
| Sarbia 1998 | 1998 | SCC (surgical treatment) | 149 | Germany | Tissue | 50% | Immunohistochemistry | 9 |
| Kuwahara 1999 | 1999 | SCC | 32 | Japan | Tissue | 10% | Immunohistochemistry | 8 |
| Lam 1999 | 1999 | SCC | 153 | Hong Kong | Tissue | 50% | Immunohistochemistry | 7 |
| Natsugoe 1999 | 1999 | SCC | 111 | Japan | Tissue | 10% | Immunohistochemistry | 8 |
| Nita 1999 | 1999 | SCC | 62 | Japan | Tissue | 14 | Immunohistochemistry | 9 |
| Shimada 1999 | 1999 | SCC | 116 | Japan | Tissue | 50% | Immunohistochemistry | 8 |
| Zhang 1999 | 1999 | Esophageal cancer | 38 | China | Tissue | 5% | Immunohistochemistry | 9 |
| Fan 2000 | 2000 | Esophageal cancer | 56 | China | Tissue | NA | Immunohistochemistry | 7 |
| Liu 2000 | 2000 | SCC | 80 | China | Tissue | 1% | Immunohistochemistry | 8 |
| Nakashida 2000 | 2000 | SCC | 30 | Japan | Tissue | 10% | Immunohistochemistry | 7 |
| Matsumoto 2001 | 2001 | SCC | 79 | Japan | Tissue | 10% | Immunohistochemistry | 7 |
| Zhan 2001 | 2001 | Esophageal cancer | 30 | China | Tissue | 10% | Immunohistochemistry | 6 |
| Li 2002 | 2002 | AD | 35 | China | Tissue | NA | Immunohistochemistry | 6 |
| Cui 2003 | 2003 | SCC | 72 | China | Tissue | 5% | Immunohistochemistry | 8 |
| Guner 2003 | 2003 | SCC | 63 | Germany | Tissue | 10% | Immunohistochemistry | 7 |
| Zhang 2003 | 2003 | SCC | 43 | China | Tissue | 0% | Immunohistochemistry | 7 |
| Li 2004 | 2004 | SCC | 80 | China | Tissue | 25% | Immunohistochemistry | 8 |
| Li Li 2004 | 2004 | SCC | 48 | China | Tissue | NA | Immunohistochemistry, ISH | 6 |
| Nakamura 2004 | 2004 | SCC | 76 | Japan | Tissue | 10% | Immunohistochemistry | 9 |
| Chang 2005 | 2005 | Esophageal cancer | 118 | Korea | Tissue | 10% | Immunohistochemistry | 7 |
| Goan 2005 | 2005 | SCC | 36 | China | Tissue | 50% | Immunohistochemistry | 8 |
| Gu 2005 | 2005 | SCC (single surgery) | 50 | China | Tissue | 10% | Immunohistochemistry | 7 |
| Gu 2005 | 2005 | SCC (surgery+chemotherapy) | 50 | China | Tissue | 10% | Immunohistochemistry | 7 |
| Li 2005 | 2005 | SCC | 43 | China | Tissue | 10% | Immunohistochemistry | 9 |
| Fan 2006 | 2006 | SCC | 40 | China | Tissue | 10% | Immunohistochemistry | 7 |
| Liu 2006 | 2006 | SCC | 60 | China | Tissue | 25% | Immunohistochemistry | 6 |
| Han 2007 | 2007 | SCC | 40 | Turkey | Tissue | 10% | Immunohistochemistry | 7 |
| Ishida 2007 | 2007 | SCC | 32 | Japan | Tissue | 20% | Immunohistochemistry | 8 |
| Wang 2008 | 2008 | SCC | 48 | China | Tissue | 25% | Immunohistochemistry | 8 |
| Zhang 2008 | 2008 | SCC | 45 | China | Tissue | NA | RT-PCR | 7 |
| Lin 2010 | 2010 | SCC | 148 | China | Tissue | 10% | Immunohistochemistry | 8 |
| Taghavi 2010 | 2010 | SCC | 80 | Iran | Tissue | 50% | Immunohistochemistry | 8 |
| Zhang 2010 | 2010 | SCC | 90 | China | Tissue | NA | Immunohistochemistry | 7 |
| Arsenijevic 2012 | 2012 | SCC | 41 | Serbia | Tissue | NA | Immunohistochemistry | 6 |
| Liu 2012 | 2012 | SCC | 189 | China | Tissue | NA | Immunohistochemistry | 6 |
| Li 2013 | 2013 | SCC | 48 | China | Tissue | NA | RT-PCR | 7 |
| Shiozaki 2013 | 2013 | SCC | 69 | Japan | Tissue | 30% | Immunohistochemistry | 8 |
| Zhang 2013 | 2013 | SCC | 51 | China | Tissue | NA | Western blot | 7 |
| Zhang 2014 | 2014 | SCC | 62 | China | Tissue | 50% | Immunohistochemistry | 7 |
| Cheng 2015 | 2015 | SCC | 80 | China | Tissue | 25% | Immunohistochemistry | 8 |
| Lin 2016 | 2016 | SCC | 153 | China | Tissue | 10% | Immunohistochemistry | 9 |
Table 2.
The main characteristics of studies investigating the prognostic value of P21 and overall survival.
| Study | HR | LL | UL | Survival | Statistical method |
|---|---|---|---|---|---|
| Sarbia M 1998 | 0.556 | 0.347 | 0.885 | OS | Multivariable |
| Kuwahara M 1999 | 3.409 | 1.388 | 8.373 | OS | Multivariable |
| Natsugoe S 1999 | 1.920 | 1.065 | 3.460 | OS | Survival curve |
| Shimada Y 1999 | 2.398 | 1.477 | 3.906 | OS | Multivariable |
| Lam KY 1999 | 0.627 | 0.411 | 0.956 | OS | Survival curve |
| Nita ME 1999 | 1.713 | 1.022 | 2.871 | OS | Multivariable |
| GUNER D 2003 | 0.435 | 0.200 | 0.943 | OS | Multivariable |
| Nakamura T 2004 | 1.530 | 1.040 | 2.330 | OS | Multivariable |
| Goan YG 2005 | 0.549 | 0.308 | 0.980 | OS | Multivariable |
| Lin CD 2010 | 2.322 | 1.091 | 4.940 | OS | Univariate |
| Taghavi N 2010 | 3.946 | 0.430 | 30.860 | OS | Multivariable |
| Liu J 2012 | 2.139 | 1.199 | 3.816 | OS | Survival curve |
| Shiozaki A 2013 | 2.379 | 1.700 | 3.313 | OS | Multivariable |
| Zhang Y 2014 | 1.867 | 1.029 | 3.387 | OS | Multivariable |
| Lin Y 2016 | 2.623 | 1.005 | 8.147 | OS | Multivariable |
Note: HR = hazard ratio; LL = lower confidence interval limit; UL = upper confidence interval limit; OS = overall survival.
3.2. Prognostic Value of P21 in Patients with EC
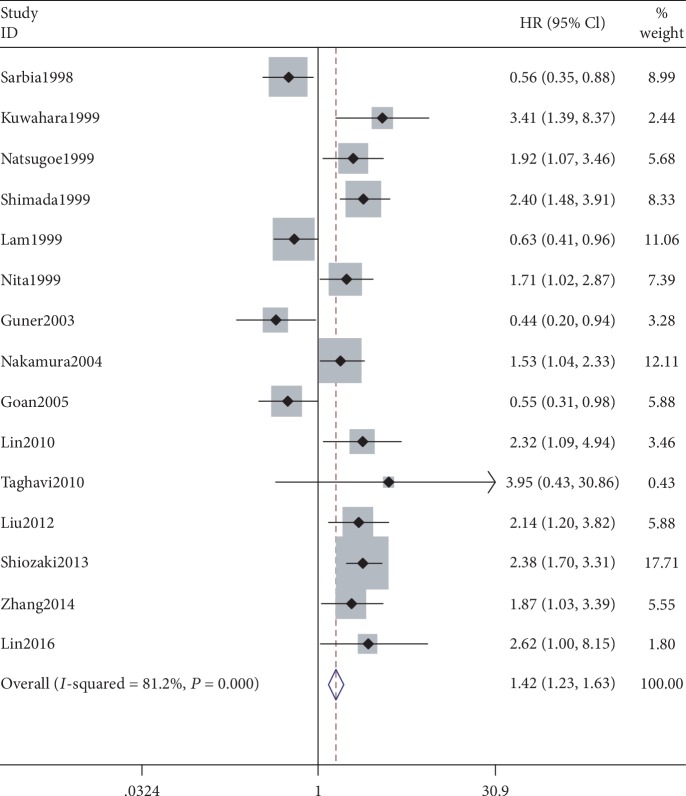
A meta-analysis of the 15 studies that reported overall survival yielded a pooled HR of 1.456 (95% CI: 1.033–2.053, P = 0.032, z = 2.14), indicating a significant association between the P21-negative group and decreased survival time when compared with the P21-positive group. Significant heterogeneity was noted across the studies (I2 = 81.2%, P < 0.05, Figure 2). Therefore, a random-effects model was used. Next, subgroup analyses of publication country, continent, sample size, cutoff value, and the statistical methods used to calculate the HRs were performed to explore the origin of the heterogeneity Ultimately, the country of publication might be a source of heterogeneity. The pooled HR of 2.05 indicated that a low P21 expression level was correlated with shorter survival time in the Japanese studies. Notably, the degree of heterogeneity in this group was reduced in the fixed-effects model (I2 = 0.0%, P = 0.425). However, obvious heterogeneity was also detected in the other subgroup analyses.
Figure 2.
Meta-analysis comparing P21 expression and overall survival in esophageal cancer patients in 15 studies reporting prognosis of esophageal cancers.
3.3. Correlation between P21 and Clinicopathological Characteristics of Patients with EC
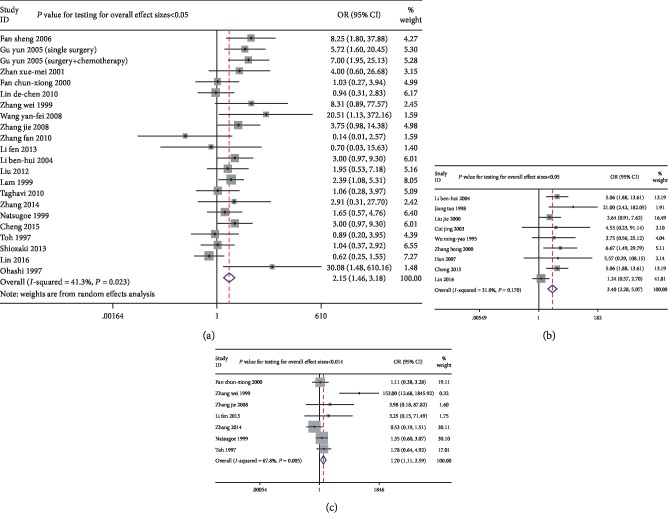
Decreased P21 expression was observed in tumors with poorer differentiation (pooled OR = 2.153, 95% CIs 1.455–3.184). Significant heterogeneity was found between the studies (I2 = 41.30%, P = 0.023, Figure 3(a)), so a random-effect model was used. There was a significant association of lower P21 expression with a higher tumor grade (pooled OR = 3.399, 95% CI 2.278–5.071, P < 0.05, z = 5.99). No significant heterogeneity was found between the studies (I2 = 31.00%, P = 0.17, Figure 3(b)). Significant heterogeneity was found between the studies reporting on the clinical stage (I2 = 53.4% and P = 0.002), so a random-effect model was used. There was a significant correlation between decreased P21 expression and an advanced clinical stage (pooled OR = 1.697, 95% CI 1.111–2.594, P = 0.014, Figure 3(c)). There was also a significant correlation between P21 expression and lymph node metastasis (pooled OR = 1.691, 95% CI 1.165–2.455, P = 0.006, z = 2.76) in 23 studies, in which there was slight heterogeneity (I2 = 57.70%, P < 0.05, Figure 4(a)). Lower P21 expression was significantly associated with a higher risk of invasion (pooled OR = 1.939, 95% CI 1.328–2.83, P = 0.001; I2 = 0.00%, P = 0.589, Figure 4(b)). Moreover, a significant correlation was found between low P21 expression and a low apoptosis index (pooled OR = 0.131, 95% CI 0.064–0.269, P < 0.05, z = 5.55; I2 = 0.00%, P = 0.656, Figure 4(c)). Importantly, there was a significant association between a high P21 expression level and a favorable response to chemotherapy (pooled OR = 5.987, 95% CI 2.930–12.234, P < 0.05, z = 4.91; I2 = 0.00%, P = 0.443, Figure 5). However, there was no significant association between P21 expression and any other clinical parameters (Table 3).
Figure 3.
Forest plots of odds ratios for P21 expression and clinicopathological parameters including differentiation, grading, and clinical stage in esophageal cancer patients: (a) differentiation (OR = 2.153, 95% CIs: 1.455-3.184, P < 0.05); (b) grading (OR = 3.399, 95% CIs: 2.278-5.071, P < 0.05); (c) clinical stage (OR = 1.697, 95% CIs: 1.111-2.594, P = 0.014).
Figure 4.
Forest plots of odds ratios for P21 expression and clinicopathological parameters including lymph node metastasis, invasion, and apoptosis index in esophageal cancer patients: (a) lymph node metastasis (OR = 1.691, 95% CIs: 1.165~2.455, P = 0.006); (b) invasion (OR = 1.939, 95% CIs: 1.328-2.830, P = 0.001); (c) apoptosis index (OR = 0.131, 95% CIs: 0.064-0.269, P < 0.05).
Figure 5.
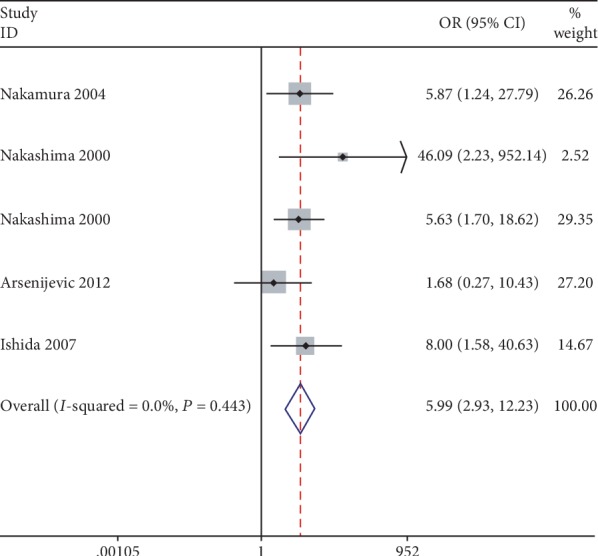
Forest plots of odds ratios for P21 expression and clinicopathological parameters including chemotherapy effectivity in esophageal cancer patients.
Table 3.
Main results for meta-analysis between p21 and clinicopathological features in esophageal cancer.
| Clinical parameters | OR [95% CI] | P | z | I 2 | P | Begg's test |
|---|---|---|---|---|---|---|
| Tissue | 2.210 [0.811-6.022] | 0.121 | 1.550 | 90.9% | P < 0.05 | 0.373 |
| Age | 0.969 [0.716-1.312] | 0.840 | 0.210 | 81.8% | P < 0.05 | 0.732 |
| Gender | 0.758 [0.566-1.015] | 0.063 | 1.860 | 85.4% | P < 0.05 | 0.484 |
| Differentiation | 2.153 [1.455-3.184] | P < 0.05 | 3.840 | 41.3% | 0.023 | 0.185 |
| Clinical stage | 1.616 [1.068-2.446] | 0.023 | 0.002 | 53.4% | 0.002 | 0.096 |
| Tumor stage | 0.885 [0.541-1.448] | 0.627 | 0.490 | 93.7% | P < 0.05 | 0.089 |
| Lymph node | 1.691 [1.165-2.455] | 0.006 | 2.760 | 57.7% | P < 0.05 | 1.000 |
| Metastasis | 0.637 [0.356-1.140] | 0.129 | 1.520 | 76.7% | P < 0.05 | 0.296 |
| Grading | 3.399 [2.278-5.071] | P < 0.05 | 5.990 | 31.0% | 0.170 | 0.532 |
| Invasion | 1.939 [1.328-2.830] | 0.001 | 3.430 | 58.9% | P < 0.05 | 0.788 |
| Tumor size | 1.005 [0.602-1.675] | 0.986 | 0.020 | 36.0% | 0.181 | 0.806 |
| Tumor location | 0.971 [0.553-1.706] | 0.918 | 0.100 | 51.8% | 0.034 | 0.917 |
| P53 | 1.700 [0.918-3.148] | 0.092 | 1.690 | 63.6% | 0.003 | 0.210 |
| AI (apoptosis index) | 0.131 [0.064-0.269] | P < 0.05 | 5.550 | 65.6% | P < 0.05 | 1.000 |
| Chemotherapy effectivity | 5.987 [2.930-12.234] | P < 0.05 | 4.910 | 44.3% | P < 0.05 | 0.462 |
OR = odds ratio; CI = confidence interval.
3.4. Sensitivity Analysis and Publication Bias
A sensitivity analysis confirmed that the results were not obviously impacted by any individual study, suggesting that the meta-analysis had good stability. The results of Begg's test and the funnel plot did not indicate any publication bias (Figure 6, Table 3).
Figure 6.
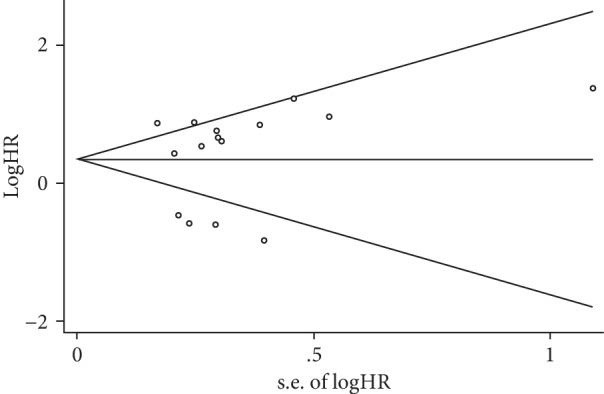
Funnel plot for the publication bias test of the analysis between P21 expression and overall survival in esophageal cancer patients. The horizontal line means the pooled effect estimate.
4. Discussion
There is accumulating evidence to suggest that abnormal expression of P21 is present in various types of malignancy, including gastric [63], lung [64], and tonsillar [65] cancers. However, the results of studies that have investigated the potential role of P21 expression are not consistent. To our knowledge, this meta-analysis contains the largest number of studies that have evaluated the association between P21 expression and the clinicopathological characteristics and outcomes in patients with EC. We found that overall survival in patients with EC was likely to be longer in those with higher P21 expression than in those with lower P21 expression. Our finding that decreased P21 expression was correlated with disease progression, that is, differentiation, lymph node metastasis, and invasion, as well as an advanced disease grade and clinical stage, indicates that P21 has a suppressor role in EC. A particularly important finding in this study was that P21 might be a valuable predictor of the effectiveness of chemotherapy.
In this study, there was a significant association between low P21 expression and a poorer outcome of EC. In contrast, high P21 expression has been reported to be an unfavorable prognostic factor in patients with prostate cancer [66] and breast cancer [67]. However, the results of yet other studies in patients with cervical adenocarcinoma [68] and bladder cancer [69] are consistent with our finding that P21 might act as a tumor suppressor. Like in our study, previous research showed significant associations between a low P21 level and advanced clinical stage and grade of bladder cancer, indicating that P21 has an important role in tumor progression [70]. A previous study in prostate cancer showed that P21 inhibits cell growth by targeting E2F1 [71]. It has been confirmed that P21 expression could be reduced by DDX3 in lung cancer, leading to inhibition of the growth of cancer cells. Wu and colleagues demonstrated that inhibition of P21 via the P53-DDX3 pathway may promote the proliferation of cancer cells and tumor growth in vitro and in vivo [69]. Moreover, it has been shown that P21 interacts with subunits of cyclin-dependent kinases [72], resulting in inhibition of tumor growth and progression. Finally, the tumor suppressor activity of P21 can be promoted by interaction with tumor-related factors like MYC, proliferating cell nuclear antigen (PCNA), and signal transducer and activator of transcription (STAT3) [73–76].
This study has several limitations that should be taken into account when interpreting its results. First, according to the NOS criteria, the quality of the included studies was variable (ranging from a score of 6 to a score of 9). Second, several HR values and their respective 95% CIs were obtained from Kaplan-Meier curves, potentially leading to inaccurate results. The inclusion of univariate HRs without adjustment could also have contributed to heterogeneity. Third, the methodological differences between the studies may have resulted in the underestimation of the effect size. For example, most of the studies detected P21 expression by immunohistochemistry, but some used diverse methods, including Western blot and real-time quantitative PCR. The use of the streptavidin-peroxidase conjugate in some studies and the streptavidin-biotin complex method in others was also a potential source of heterogeneity. Another methodological difference was that the most common cutoff values for the detection of P21 were 10% and 50%, but this was not completely consistent across the studies. Inclusion of research published only in Chinese or English may have been another source of bias, given that negative results tend to be published in local journals. Furthermore, the number of studies included in this analysis was limited and we only restrict the patient number for the enrolled studies by a threshold of 20.
5. Conclusion
In this study, the results suggest that low P21 expression has a clinically important negative clinicopathological and prognostic impact in patients with EC. Well-designed studies that include larger patient cohorts are required to identify the mechanisms underlying how P21 is involved in the tumorigenesis and progression of EC.
Acknowledgments
This work was supported by the National Natural Scientific Foundation of China (Nos. 81602391, 81502666, and 81802997), the Natural Science Foundation of Hubei Province of China (No. 2019CFA034), the Scientific and Technological Project of Shiyan City of Hubei Province (No. 17Y12), and the project of Shiyan Taihe Hospital (No. 2017JJXM006, 2017JJXM066).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Junbo Wu, Liang Liu, and Feng Wu contributed equally to this work.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2017. CA: a Cancer Journal for Clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal N., Jiao Y., Bettegowda C., et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discovery. 2012;2(10):899–905. doi: 10.1158/2159-8290.cd-12-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariette C., Finzi L., Piessen G., van Seuningen I., Triboulet J. P. Esophageal carcinoma: prognostic differences between squamous cell carcinoma and adenocarcinoma. World Journal of Surgery. 2005;29(1):39–45. doi: 10.1007/s00268-004-7542-x. [DOI] [PubMed] [Google Scholar]
- 4.Siewert J. R., Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Seminars in Radiation Oncology. 2007;17(1):38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Chen M., Huang J., Zhu Z., Zhang J., Li K. Systematic review and meta-analysis of tumor biomarkers in predicting prognosis in esophageal cancer. BMC Cancer. 2013;13(1):p. 539. doi: 10.1186/1471-2407-13-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson G. A., Zhang X., Stang M. T., et al. Inhibition of c-Met as a therapeutic strategy for esophageal adenocarcinoma. Neoplasia. 2006;8(11):949–955. doi: 10.1593/neo.06499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato F., Kubota Y., Natsuizaka M., et al. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biology & Therapy. 2015;16(6):933–940. doi: 10.1080/15384047.2015.1040959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo X. F., Zhu X. F., Zhong G. S., Deng B. G., Gao Z. T., Wang H. Lapatinib, a dual inhibitor of EGFR and HER2, has synergistic effects with 5-fluorouracil on esophageal carcinoma. Oncology Reports. 2012;27(5):1639–1645. doi: 10.3892/or.2012.1659. [DOI] [PubMed] [Google Scholar]
- 9.Burris H. A., 3rd, Dowlati A., Moss R. A., et al. Phase I study of pazopanib in combination with paclitaxel and carboplatin given every 21 days in patients with advanced solid tumors. Molecular Cancer Therapeutics. 2012;11(8):1820–1828. doi: 10.1158/1535-7163.MCT-11-0997. [DOI] [PubMed] [Google Scholar]
- 10.Xie D., Lan L., Huang K., et al. Association of p53/p21 expression and cigarette smoking with tumor progression and poor prognosis in non-small cell lung cancer patients. Oncology Reports. 2014;32(6):2517–2526. doi: 10.3892/or.2014.3538. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y., Yang S., Sun N., Chen J. Differential expression of STAT1 and p21 proteins predicts pancreatic cancer progression and prognosis. Pancreas. 2014;43(4):619–623. doi: 10.1097/MPA.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 12.Liu X., Yu H., Cai H., Wang Y. Expression of CD24, p21, p53, and c-myc in alpha-fetoprotein-producing gastric cancer: correlation with clinicopathologic characteristics and survival. Journal of Surgical Oncology. 2014;109(8):859–864. doi: 10.1002/jso.23599. [DOI] [PubMed] [Google Scholar]
- 13.Goan Y. G., Hsu H. K., Chang H. C., Chou Y. P., Chiang K. H., Cheng J. T. Deregulated p21WAF1 overexpression impacts survival of surgically resected esophageal squamous cell carcinoma patients. The Annals of Thoracic Surgery. 2005;80(3):1007–1016. doi: 10.1016/j.athoracsur.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 14.Lin Y., Shen L. Y., Fu H., et al. P21, COX-2, and E-cadherin are potential prognostic factors for esophageal squamous cell carcinoma. Diseases of the Esophagus. 2017;30(2):1–10. doi: 10.1111/dote.12522. [DOI] [PubMed] [Google Scholar]
- 15.Natsugoe S., Nakashima S., Matsumoto M., et al. Expression of p21WAF1/Cip1 in the p53-dependent pathway is related to prognosis in patients with advanced esophageal carcinoma. Clinical Cancer Research. 1999;5(9):2445–2449. [PubMed] [Google Scholar]
- 16.Wu X., Yang N., Zhou W.-H., et al. Up-regulation of P21 inhibits TRAIL-mediated extrinsic apoptosis, contributing resistance to SAHA in acute myeloid leukemia cells. Cellular Physiology and Biochemistry. 2014;34(2):506–518. doi: 10.1159/000363018. [DOI] [PubMed] [Google Scholar]
- 17.Wagenknecht B., Hermisson M., Eitel K., Weller M. Proteasome inhibitors induce p53/p21-independent apoptosis in human glioma cells. Cellular Physiology and Biochemistry. 1999;9(3):117–125. doi: 10.1159/000016308. [DOI] [PubMed] [Google Scholar]
- 18.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):p. 16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt G. H., Oxman A. D., Kunz R., et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. Journal of Clinical Epidemiology. 2011;64(12):1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 21.Nita M. E., Nagawa H., Tominaga O., et al. p21Waf1/Cip1 expression is a prognostic marker in curatively resected esophageal squamous cell carcinoma, but not p27Kip1, p53, or Rb. Annals of Surgical Oncology. 1999;6(5):481–488. doi: 10.1007/s10434-999-0481-x. [DOI] [PubMed] [Google Scholar]
- 22.Kuwahara M., Hirai T., Yoshida K., et al. p53, p21(Waf1/Cip1) and cyclin D1 protein expression and prognosis in esophageal cancer. Diseases of the Esophagus. 1999;12(2):116–119. doi: 10.1046/j.1442-2050.1999.00034.x. [DOI] [PubMed] [Google Scholar]
- 23.Lam K. Y., Law S., Tin L., Tung P. H., Wong J. The clinicopathological significance of p21 and p53 expression in esophageal squamous cell carcinoma: an analysis of 153 patients. The American Journal of Gastroenterology. 1999;94(8):2060–2068. doi: 10.1111/j.1572-0241.1999.01278.x. [DOI] [PubMed] [Google Scholar]
- 24.Ishida M., Morita M., Saeki H., et al. Expression of p53 and p21 and the clinical response for hyperthermochemoradiotherapy in patients with squamous cell carcinoma of the esophagus. Anticancer Research. 2007;27(5B):3501–3506. [PubMed] [Google Scholar]
- 25.Han U., Can O. I., Han S., Kayhan B., Onal B. U. Expressions of p53, VEGF C, p21: could they be used in preoperative evaluation of lymph node metastasis of esophageal squamous cell carcinoma? Diseases of the Esophagus. 2007;20(5):379–385. doi: 10.1111/j.1442-2050.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 26.Taghavi N., Biramijamal F., Sotoudeh M., et al. Association of p53/p21 expression with cigarette smoking and prognosis in esophageal squamous cell carcinoma patients. World Journal of Gastroenterology. 2010;16(39):4958–4967. doi: 10.3748/wjg.v16.i39.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Zhang Y., Yun H., Lai R., Su M. Correlation of STAT1 with apoptosis and cell-cycle markers in esophageal squamous cell carcinoma. PLoS One. 2014;9(12, article e113928) doi: 10.1371/journal.pone.0113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T., Hayashi K., Ota M., Ide H., Takasaki K., Mitsuhashi M. Expression of p21(Waf1/Cip1) predicts response and survival of esophageal cancer patients treated by chemoradiotherapy. Diseases of the Esophagus. 2004;17(4):315–321. doi: 10.1111/j.1442-2050.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheng H., Chen C., Liu L. U., Zhan N. A., Li B. Expression of Smad4, TGF-βRII, and p21waf1 in esophageal squamous cell carcinoma tissue. Oncology Letters. 2015;9(6):2847–2853. doi: 10.3892/ol.2015.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumoto M., Natsugoe S., Nakashima S., et al. Clinical significance and prognostic value of apoptosis related proteins in superficial esophageal squamous cell carcinoma. Annals of Surgical Oncology. 2001;8(7):598–604. doi: 10.1007/s10434-001-0598-z. [DOI] [PubMed] [Google Scholar]
- 31.Shiozaki A., Nakashima S., Ichikawa D., et al. Prognostic significance of p21 expression in patients with esophageal squamous cell carcinoma. Anticancer Research. 2013;33(10):4329–4335. [PubMed] [Google Scholar]
- 32.Toh Y., Kuwano H., Sonoda K., et al. Correlation between reduced p21(WAF1/CIP1) expression and abnormal p53 expression in esophageal carcinomas. International Journal of Oncology. 1997;11(4):703–708. doi: 10.3892/ijo.11.4.703. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima S., Natsugoe S., Matsumoto M., et al. Expression of p53 and p21 is useful for the prediction of preoperative chemotherapeutic effects in esophageal carcinoma. Anticancer Research. 2000;20(3B):1933–1937. [PubMed] [Google Scholar]
- 34.Arsenijevic T., Micev M., Nikolic V., Gavrilovic D., Radulovic S., Pesko P. Is there a correlation between molecular markers and response to neoadjuvant chemoradiotherapy in locally advanced squamous cell esophageal cancer? Journal of BUON. 2012;17(4):706–711. [PubMed] [Google Scholar]
- 35.Guner D., Sturm I., Hemmati P., et al. Multigene analysis of Rb pathway and apoptosis control in esophageal squamous cell carcinoma identifies patients with good prognosis. International Journal of Cancer. 2003;103(4):445–454. doi: 10.1002/ijc.10850. [DOI] [PubMed] [Google Scholar]
- 36.Liu J. M., Liu W. M., Feng L., Fu G. Analysis of the relationship between P~(21), nm23-H1 and invasion and metastasis of esophageal cancer. China Medical Herald. 2006;3(23):73–74. [Google Scholar]
- 37.Gu Y., Sun D. Q., Qin J., Feng Z. Q., Zhang W. M. The apoptosis and the expression of apoptosis-related proteins in esophageal cancers with and without preoperative chemotherapy. Acta Academiae Medicinae Xuzhou. 2005;25(1):14–18. [Google Scholar]
- 38.Zhang N., Liu J. F. Association of P53/P21 expression with cigarette smoking, drinking and clinical characteristics in patients with squamous cell carcinoma of the esophagus. Journal of Esophageal Surgery. 2013;1(1):6–11. [Google Scholar]
- 39.Wang L. D., Yang W. C., Zhou Q., Li Y. X., Gao S. S. Changes of WAF-1 and P53 expression in esophageal and precancerous lesions. Journal of Henan Medical University. 1997;32(1):15–18. [Google Scholar]
- 40.Fang F., Fang J. N., Hao X. C. The clinical significance of apoptosis-related genes in esophageal cancer tissues. Journal of Baotou Medicine. 2006;30(4):1–2. [Google Scholar]
- 41.Li L. M., Wang J. W., Ji C. X., Liu Q. H. Correlation analysis and expression of P53, P21waf, MDM2 and BAX in human esophageal squamous cell carcinoma. Chinese Journal of Anatomy. 2005;28(1):26–28. [Google Scholar]
- 42.Li B. H., Mao Z. F., Wu Z. Y., Wang Z. Q. Expression of Smad4, TpRII and P21 waf1 in esophageal squamous cancer tissue and its biological significance. Medical Journal of Wuhan University. 2004;1(1):453–455. [Google Scholar]
- 43.Wang Y. F., Liu Y. Z., Li L. N. Expression of P21 proteins in tissues of esophageal squamous carcinoma. Journal of Practical Diagnosis and Therapy. 2008;22(2):108–109. [Google Scholar]
- 44.Jiang T., Zhao Y., Liu K. The expression and relationship of WAF/CIP1 and p53 in esophageal carcinoma. Chinese Journal of Experimental Surgery. 1998;15(3):218–219. [Google Scholar]
- 45.Zhang F., Peng J., Li B. L. Expression and significance of p21 protein in esophageal cancer and esophageal benign hyperplasia. Journal of Bengbu Medical College. 2010;35(4):372–373. [Google Scholar]
- 46.Zhang H., Niu Y. Y., Hu M. P. Expression and significance of p53, p21, p16, cyclinD1 and CDK4 in esophageal squamous carcinoma. Immunological Journal. 2003;19(4):312–314. [Google Scholar]
- 47.Lin D. C., Shi Z. Z., Xue L. Y., et al. Expression of cell cycle related proteins cyclin D1, p53 and p21WAF1/Cip1 in esophageal squamous cell carcinoma. Hereditas. 2010;32(5):455–460. doi: 10.3724/SP.J.1005.2010.00455. [DOI] [PubMed] [Google Scholar]
- 48.Cui J., Li S., Xu C. The expression of cyclinD1 and p21waf1 in esophageal suquamous cell carcinogenesis. Journal of Xinxiang Medical College. 2003;20(3):166–168. [Google Scholar]
- 49.Zhan X. M., Wang G. X., Sun S. W., Wang J. Y., Li L. Expression of p16, p21, p53, cyclin D1 and their siginificance on esophageal carcinoma. The Practical Journal of Cancer. 2001;16(1):36–38. [Google Scholar]
- 50.Zhang J., Wei L. Y. Expressions of p21, cyclin D1 mRNA in esophageal carcinoma and its significance. Medical Information Operations Sciences Fascicule. 2008;21(6):534–536. [Google Scholar]
- 51.Li F., Jiang X. F., Wang H. J., Pang Z. L., Gulinuer M. H. Y., Li H. W. Expressions of PTEN and p21 genes in Hazak patients with esophageal cancer. Tianjin Medical Journal. 2013;1(9):852–854. [Google Scholar]
- 52.Fang C. X., Deng Y. P., Xie Z. F. Immunohistochemistry analysis of p53, p21 and nm23 expression in esophageal cancer. Chinese Journal of Laboratory Diagnosis. 2000;1(4):22–23. [Google Scholar]
- 53.Liu J., Chen S. L., Zhang W., Su Q. P21 WAF1 gene expression with P53 mutation in esophageal carcinoma. World Chinese Journal of Digestology. 2000;8(12):1350–1353. [Google Scholar]
- 54.Li W. J., Li Y. J., Li J. M. The significance of cyclin E and p21 exrepssion in Barrett esopealgus and and adenocarcinoma. Chinese Journal of Internal Medicine. 2002;41(12):50–51. [Google Scholar]
- 55.Li L., Qi F. Y., Zuo L. F., Li J. R., Zhang D. H. The significance of cyclinE and P21 waf1 expression in the esophageal epithelial carcinogenesis. Journal of Practical Oncology. 2004;19(6):504–507. [Google Scholar]
- 56.Chang M. S., Seung Lee H., Lan Lee B., Tae Kim Y., Sang Lee J., Kim W. H. Differential protein expression between esophageal squamous cell carcinoma and dysplasia, and prognostic significance of protein markers. Pathology - Research and Practice. 2005;201(6):417–425. doi: 10.1016/j.prp.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Sarbia M., Stahl M., zur Hausen A., et al. Expression of p21WAF1 predicts outcome of esophageal cancer patients treated by surgery alone or by combined therapy modalities. Clinical Cancer Research. 1998;4(11):2615–2623. [PubMed] [Google Scholar]
- 58.Ohashi K., Nemoto T., Eishi Y., Matsuno A., Nakamura K., Hirokawa K. Expression of the cyclin dependent kinase inhibitor p21WAF1/CIP1 in oesophageal squamous cell carcinomas. Virchows Archiv. 1997;430(5):389–395. doi: 10.1007/s004280050048. [DOI] [PubMed] [Google Scholar]
- 59.Shimada Y., Imamura M., Watanabe G., et al. Prognostic factors of oesophageal squamous cell carcinoma from the perspective of molecular biology. British Journal of Cancer. 1999;80(8):1281–1288. doi: 10.1038/sj.bjc.6990499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J., Hu Y., Hu W., et al. Expression and prognostic relevance of p21WAF1 in stage III esophageal squamous cell carcinoma. Diseases of the Esophagus. 2012;25(1):67–71. doi: 10.1111/j.1442-2050.2011.01217.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W., Jing M. J., Li S. J. Prognostic significance of tumor supressor P21CIP1/WAF1 expression in esophageal carcinoma. Chinese Journal of Clinical Gastroenterology. 1999;11(4):147–148. [Google Scholar]
- 62.Wu M. Y., Zheng R. M., Shen J. The expression of P21 CIP1/WAF1 in carcinoma of the esophagus and its relation to prognosis. Cancer. 1995;11(4):97–98. [Google Scholar]
- 63.Y-W L., Xia R., Lu K., et al. LincRNAFEZF1-AS1 represses p21 expression to promote gastric cancer proliferation through LSD1-Mediated H3K4me2 demethylation. Molecular Cancer. 2017;16:p. 39. doi: 10.1186/s12943-017-0588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin D., Lu X., Su J., et al. Long noncoding RNA AFAP1-AS1 predicts a poor prognosis and regulates non–small cell lung cancer cell proliferation by epigenetically repressing p21 expression. Molecular Cancer. 2018;17(1, article 92) doi: 10.1186/s12943-018-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hafkamp H. C., Mooren J. J., Claessen S. M., et al. P21Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Modern Pathology. 2009;22(5):686–698. doi: 10.1038/modpathol.2009.23. [DOI] [PubMed] [Google Scholar]
- 66.Aaltomaa S., Lipponen P., Eskelinen M., Ala-Opas M., Kosma V. M. Prognostic value and expression of p21(waf1/cip1) protein in prostate cancer. The Prostate. 1999;39:8–15. doi: 10.1002/(SICI)1097-0045(19990401)39:1<8::AID-PROS2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 67.Winters Z., Hunt N., Bradburn M., et al. Subcellular localisation of cyclin B, Cdc2 and p21WAF1/CIP1 in breast cancer: association with prognosis. European Journal of Cancer. 2001;37(18, article S0959804901003276):2405–2412. doi: 10.1016/S0959-8049(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 68.Lu X., Toki T., Konishi I., Nikaido T., Fujii S. Expression of p21WAF1/CIP1 in adenocarcinoma of the uterine cervix: a possible immunohistochemical marker of a favorable prognosis. Cancer. 1998;82(12):2409–2417. doi: 10.1002/(sici)1097-0142(19980615)82:12<2409::aid-cncr15>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 69.Wu D. W., Liu W. S., Wang J., Chen C. Y., Cheng Y. W., Lee H. Reduced p21 (WAF1/CIP1) via alteration of p53-DDX3 pathway is associated with poor relapse-free survival in early-stage human papillomavirus-associated lung cancer. Clinical Cancer Research. 2011;17(7):1895–1905. doi: 10.1158/1078-0432.CCR-10-2316. [DOI] [PubMed] [Google Scholar]
- 70.Puhalla H., Wrba F., Kandioler D., et al. Expression of p21 (Wafl/Cip1), p57(Kip2) and HER2/neu in patients with gallbladder cancer. Anticancer Research. 2007;27(3B):1679–1684. [PubMed] [Google Scholar]
- 71.Hu Z., Zhang D., Hao J., et al. Induction of DNA damage and p21-dependent senescence by Riccardin D is a novel mechanism contributing to its growth suppression in prostate cancer cells in vitro and in vivo. Cancer Chemotherapy and Pharmacology. 2014;73(2):397–407. doi: 10.1007/s00280-013-2365-9. [DOI] [PubMed] [Google Scholar]
- 72.Sherr C. J., Roberts J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes & Development. 1999;13(12):1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 73.Kitaura H., Shinshi M., Uchikoshi Y., Ono T., Iguchi-Ariga S. M., Ariga H. Reciprocal regulation via protein-protein interaction between c-Myc and p21cip1/waf1/sdi1 in DNA replication and transcription. Journal of Biological Chemistry. 2000;275(14):10477–10483. doi: 10.1074/jbc.275.14.10477. [DOI] [PubMed] [Google Scholar]
- 74.Wei Z., Jiang X., Qiao H., et al. STAT3 interacts with Skp2/p27/p21 pathway to regulate the motility and invasion of gastric cancer cells. Cellular Signalling. 2013;25(4):931–938. doi: 10.1016/j.cellsig.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Liao X. H., Lu D. L., Wang N., et al. Estrogen receptor α mediates proliferation of breast cancer MCF-7 cells via a p21/PCNA/E2F1-dependent pathway. The FEBS Journal. 2014;281(3):927–942. doi: 10.1111/febs.12658. [DOI] [PubMed] [Google Scholar]
- 76.Inoue S., Hao Z., Elia A. J., et al. Mule/Huwe1/Arf-BP1 suppresses Ras-driven tumorigenesis by preventing c-Myc/Miz1-mediated down-regulation of p21 and p15. Genes & Development. 2013;27(10):1101–1114. doi: 10.1101/gad.214577.113. [DOI] [PMC free article] [PubMed] [Google Scholar]